ABSTRACT
We report the adaptability of rat islets vitrified-warmed on nylon mesh (NM) device or silk fibroin (SF) sponge disc for the normalization of the blood glucose level in rat models of diabetes. One-hundred rat islets were cryopreserved according to a minimum volume cooling protocol on an NM device or a solid surface vitrification protocol on an SF sponge disc. The recovery rate (97.1% vs. 93.8%), the viability (77.9% vs. 74.4%), and the stimulation index (4.7 vs. 4.2) in glucose-stimulated insulin secretion (GSIS) assay of the post-warm islets were comparable between the NM vitrification and the SF vitrification groups. The viability and the stimulation index of the fresh control islets were identified to be 97.5% and 6.5, respectively. Eight hundred islets from the NM or the SF vitrification group or the fresh control group were transplanted beneath the kidney capsule of a streptozotocin-induced diabetic rat (blood glucose level > 350 mg/dl). Within 3 weeks after transplantation, the acquisition of euglycemia (< 200 mg/dl) was observed in recipient rats (80.0–83.3%). An intraperitoneal glucose tolerance test on Day-30 and Day-60 showed similar 2-h responses to the glucose uptake of cured rats among the compared groups. Moreover, the successful engraftment of transplants was confirmed by the Day-70 nephrectomy through the subsequent diabetes reversal and histological evaluation. Thus, large quantities of rat islets vitrified-warmed on an NM device or an SF sponge disc were proven to be fully functional both in vitro and in vivo, due to the GSIS and syngeneic transplantation, respectively.
KEYWORDS: Rat islets, silk fibroin sponge, nylon mesh, vitrification, subrenal transplantation, GSIS
Introduction
Type I diabetes mellitus (T1D) is a consequence of the destruction of insulin-producing pancreatic β-cells due to an autoimmune disorder, which leads to chronic hyperglycemia, affecting the functionality of various organs. The transplantation of pancreatic islets has been considered useful for the clinical treatment of T1D since the Edmonton protocol for islet transplantation made the long-term insulin independence of T1D patients possible using a glucocorticoid-free immunosuppressive regimen.1 However, multiple transplantation treatments are required by most patients to achieve insulin independence. As the islet number required for a transplantation treatment exceeds the total number of islets isolated from a single donor, the severe shortage of islet donors is a significant limitation of the global dissemination of islet transplantation.2 Furthermore, damages of the islet and extracellular matrix due to enzymatic digestion during the islet isolation process3 as well as an instant blood-mediated inflammatory reaction4 lead to the loss of transplanted islets, causing an increase in the islet number required for successful transplantation.
The cryopreservation of islets without functional loss can overcome the inevitable shortage of donors as well as allow time to investigate immunological tissue matching between donors and recipients. A slow-freezing method that had been originally developed for mammalian embryos was employed by an earlier study for islet cryopreservation.5 This method is applicable to cryopreserve a large quantity of islets in a single operation, but a detrimental ice crystal formation during the freeze-thaw process resulted in the impaired insulin secretion of the post-thaw islets.6 Vitrification is the most promising method for the prevention of ice crystal formation and the improvement of oocyte/embryo cryosurvival.7 The vitrification of rat islets using Cryotop® as a cryodevice was reported in our study to be superior to conventional freezing in regard of the viability, the insulin secretion, and the expression of the β-cell function regulatory gene.8 Several cryodevices, including Cryotop®,8 open-pulled straw,9 cryoloop,9 hollow fiber,10 and nylon mesh (NM),11,12 have been applied in association with the vitrification of rodent islets. Among them, the hollow fiber device and the NM device made the handling of a large quantity of islets possible by skipping the time-consuming and labor-intensive glass capillary operations (25−35 mouse islets10 and 50−100 rat islets,11,12 respectively).
It has also been recently reported by us that the silk fibroin (SF) protein has an excellent property to form a novel cryodevice for the vitrification of bovine oocytes, with similar perceived blastocyst yields after in vitro fertilization when compared to the NM or the Cryotop® device.13 The SF protein extracted from the cocoon of the silkworm Bombyx mori is mechanically robust due to its unique β-sheet structure attributing to subdomains that are rich in glycine, alanine, serine, and tyrosine, and can be processed easily into gels, films, or sponges.14,15 The high biocompatibility of the SF is favorable for the preparation of surgical sutures or scaffold materials,16-18 and may contribute to the future development of a transplantable islet cryodevice/scaffold. For example, the functionality of the SF sponge can be reinforced by fusing the peptide sequence with biological activity through the production of transgenic silkworms.19,20 Besides, the SF sponge may serve as an extracellular matrix-like structure, which is essential to maintain the vascularization of the islets, by leaving SF fragments on the surface of the islets.
The present study aimed to investigate the functionality of rat islets vitrified-warmed as a large quantity group (100 islets per NM device or SF sponge disc). The revivability of the post-warm islets was evaluated by assays in vitro (membrane integrity-based viability and glucose-stimulated insulin secretion [GSIS] ability) and in vivo (euglycemia after subrenal transplantation and intraperitoneal glucose tolerance test [IPGTT]), as shown in Figure 1.
Figure 1.
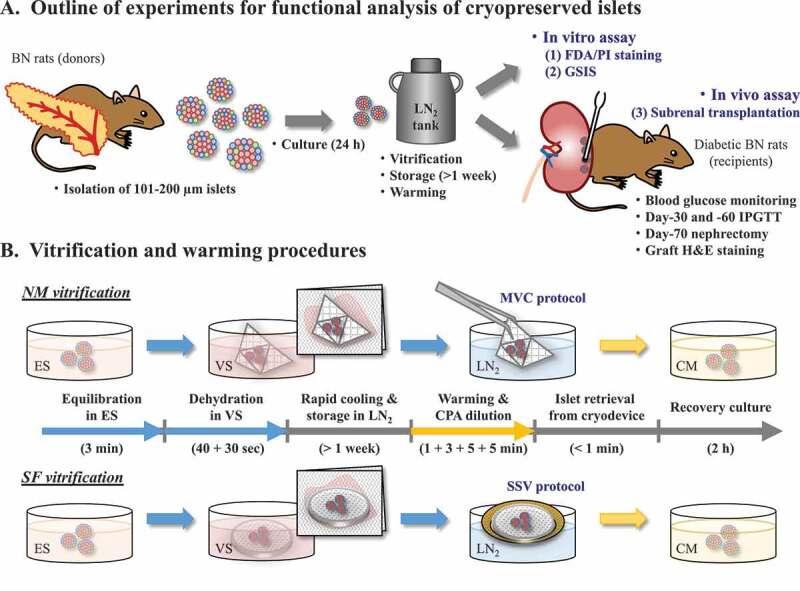
Schematic diagrams to show (A) research outline and (B) two vitrification procedures employed. (A) Functional revivability of cryopreserved rat pancreatic islets is evaluated by both in vitro and in vivo assays. Pancreatic islets are isolated by liberase digestion from Brown-Norway (BN) donor rats, and cultured for 24 h to remove debris damaged by the enzymatic digestion. Islets with a diameter of 101–200 µm are vitrified, stored in liquid nitrogen (LN2), and warmed-up. The post-warm islets are stained with fluorescent diacetate (FDA) and propidium iodide (PI) for estimating the in vitro viability, or are subjected to glucose-stimulated insulin secretion (GSIS) assay for evaluating their insulin secretion ability. Furthermore, the post-warm islets are transplanted beneath the kidney capsule of diabetes model BN rats (Day-0), and blood glucose levels of the recipients are monitored. Recipient rats achieved normoglycemia are subjected to an intraperitoneal glucose tolerance test (IPGTT) on Day-30 and -60. The cured recipients are nephrectomized on Day-70 and checked whether they can regain the diabetic hyperglycemia. Extracted grafts are sectioned and stained with hematoxylin/eosin (H&E) for histological evaluation. (B) Rat islets are vitrified-warmed using either an NM device or an SF sponge disc. Islets are exposed for 3 min to equilibration solution (ES) containing the membrane-permeable cryoprotective agents (CPAs) at low concentrations, and then loaded on NM or SF device in vitrification solution (VS) containing the permeable CPAs at high concentrations and the non-permeable disaccharide, sucrose. Absorption by a paper towel is used to minimize the VS volume surrounding the islets in both procedures for islet vitrification. Within 70 sec, islets on the NM device can be cooled by direct immersion into LN2 (minimum volume cooling [MVC] protocol), while islets on the SF sponge disc are cooled by placing on an aluminum boat floating on LN2 (solid surface vitrification [SSV] protocol). After rapid warming and stepwise CPA dilutions, islets are retrieved from the cryodevice and kept for 2 h in culture medium (CM)
Results
Characterization of cryodevice materials
The appearance of the NM device resembled to the developed figure of the triangular pyramid (Figure 2A). Due to the commercial availability of the NM sheet, some characteristics (pore size and thickness) of the device material were calculated from the product standards (Table 1). Three different batches of the SF sponge (1 mm × 50 mm × 50 mm) were also used for the quantification of the above characteristics (Figure 2B). Porosity was found to show a higher absorbent property of the SF sponge than the NM sheet.
Figure 2.
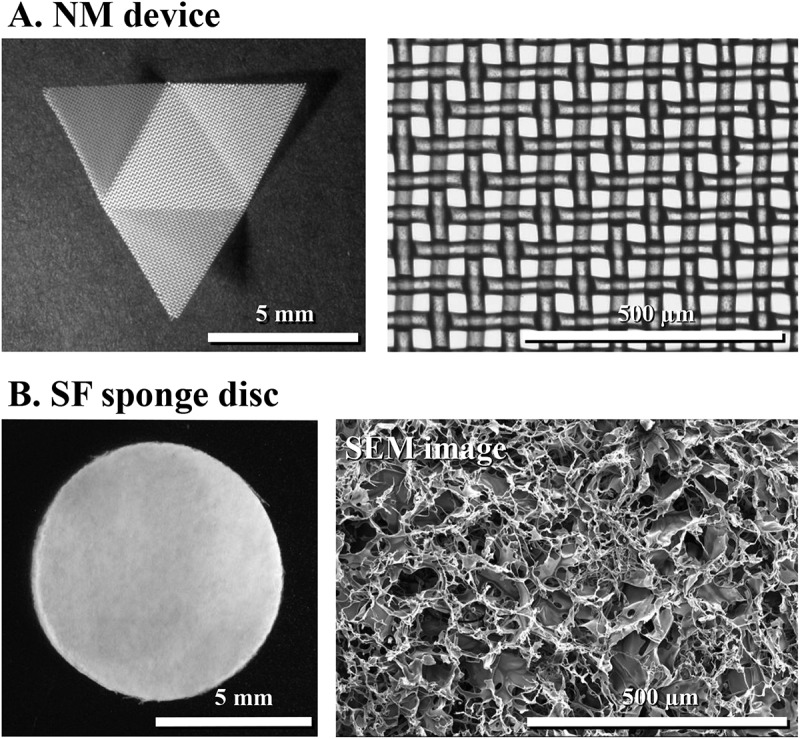
Cryodevices employed for vitrification of a hundred of rat pancreatic islets. (A) Nylon mesh device, processed as a developed figure of a triangular pyramid, with a square opening of 37 µm on a side length and nylon 66A-fiber diameter of 35 µm. (B) Silk fibroin sponge disc, with 8 mm in diameter and 1 mm in height
Table 1.
Structural characterization of cryodevice materials (nylon mesh sheet and silk fibroin sponge) employed for the vitrification of large quantity of rat islets
| Nylon mesh sheet | Silk fibroin sponge | |
|---|---|---|
| Pore size (µm) | 52.3 * | 101.1 ± 12.9 |
| Thickness (µm) | 35 or 70 * | 896.5 ± 39.5 |
| Porosity (%) | 19.7 ± 0.5 | 94.4 ± 1.0 |
*Calculated from product standards (Filter net N-No.355 T; Sansyo, Japan).
Mean ± SD, 3–5 replicates.
In vitro assessment of post-warm islets
The recovery rates of post-warm islets from the NM device and the SF sponge disc were 97.1% (5,550/5,715) and 93.8% (4,690/5,000), respectively. As shown in Table 2, the viability of post-warm islets was identified to be comparable between NM vitrification and SF vitrification groups (77.9% and 74.4%, respectively), although it was measured to be significantly lower in comparison with that of the fresh control group (97.5%). The GSIS assay of the post-warm islets indicated the presence of significant differences between the fresh control and the NM/SF vitrification groups in response to 3 mM glucose as well as between the fresh control and the SF vitrification groups in response to 20 mM glucose (Table 2). However, the stimulation index (SI), calculated by dividing the insulin levels in response to 20 mM glucose by the insulin levels in response to 3 mM glucose, did not differ among the three groups, ranging from 4.2 to 6.5.
Table 2.
In vitro viability and GSIS of rat islets vitrified-warmed on an NM device and an SF sponge disc
| Insulin secretion (ng/islet/h) |
||||
|---|---|---|---|---|
| Group | Viability (%) | 3 mM glucose | 20 mM glucose | Stimulation Index |
| Fresh control | 97.5 ± 0.5 a | 0.13 ± 0.02 a | 0.82 ± 0.04 a | 6.5 ± 0.6 |
| NM vitrification | 77.9 ± 3.0 b | 0.26 ± 0.05 b | 1.06 ± 0.06 ab | 4.7 ± 0.7 |
| SF vitrification | 74.4 ± 1.7 b | 0.30 ± 0.02 b | 1.23 ± 0.17 b | 4.2 ± 0.7 |
a,bDifferent superscripts within a column denote significant difference (P < 0.05).
Mean ± SE, 5 replicates (viability), and 6 replicates (GSIS).
In vivo assessment of post-warm islets
Islets vitrified-warmed using the NM device or the SF sponge disc (100 islets per device) as well as fresh control islets were transplanted beneath the kidney capsule of rat models of diabetes (800 islets per recipient, n = 5–6 each per group). Euglycemia was acquired by the recipient rats within 3 weeks post-transplantation at the proportions of 80.0% (4/5), 83.3% (5/6), and 80.0% (4/5) in the fresh control, the NM vitrification, and the SF vitrification groups, respectively (Figure 3A). Except for one recipient rat in the NM vitrification group that died accidentally during the Day-70 nephrectomy operation, the regained hyperglycemia of the other 12 recipients after the nephrectomy reflected the normal function of transplanted islets. Inhibited body weight gain after the nephrectomy of cured recipients (Figure 3B) may reflect their diabetic symptom, along with the effect of surgical operation.
Figure 3.
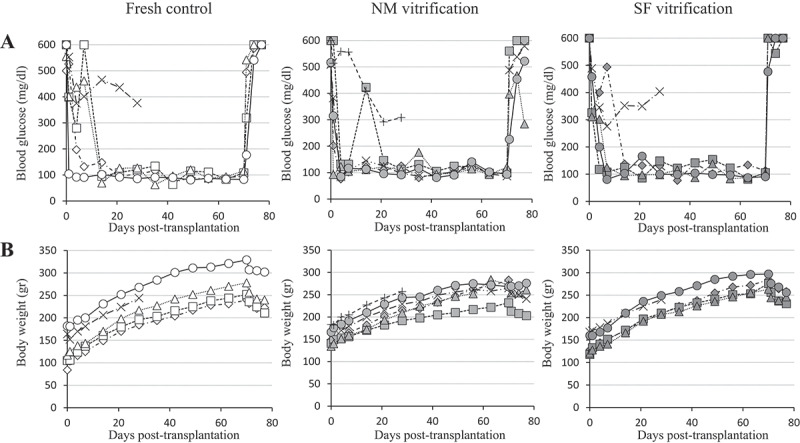
Glycemic control of individual diabetes model rats by syngeneic subrenal transplantation of fresh and vitrified-warmed islets. (A) Blood glucose level. (B) Body weight gain. Nephrectomy was performed 70 days after islet transplantation
The IPGTT performed on Day-30 (Figure 4A) and Day-60 (Figure 4B) showed similar responses to the glucose uptake of cured rats among the fresh control, the NM vitrification, and the SF vitrification groups, including the gradual glycemic control from 15 min to 120 min after the administration of glucose. The area under the curve (AUC) was also comparable among the fresh control, the NM vitrification, and the SF vitrification groups, as well as between the Day-30 IPGTT and the Day-60 IPGTT.
Figure 4.
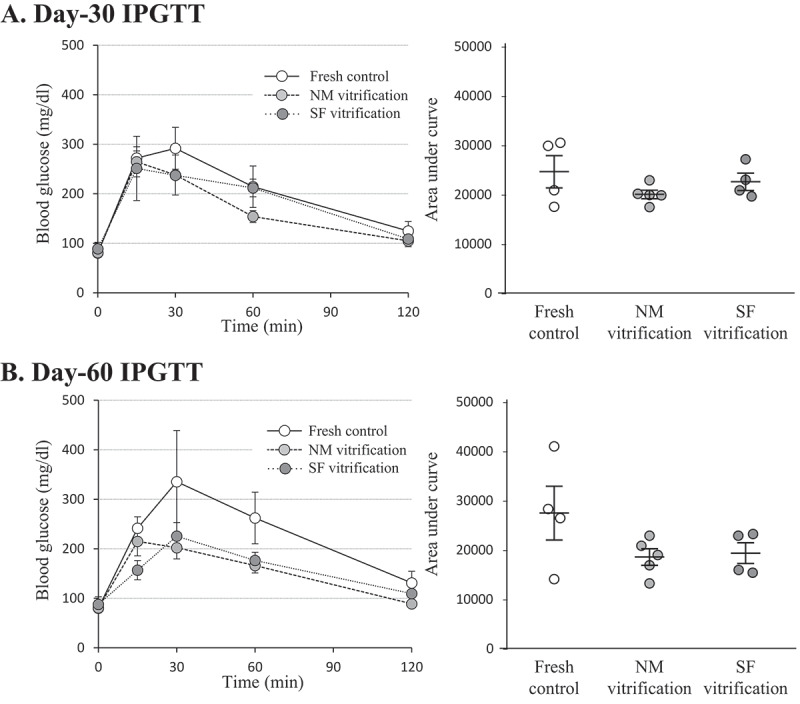
IPGTT and corresponding AUC of cured recipient rats. (A) Day-30. (B) Day-60. Mean ± SE of 4 or 5 individuals in each group. Individual AUC data were plotted on the Mean ± SE and compared by Tukey’s Honest Significant Difference test for multiple comparisons
In all groups, the islet grafts beneath the kidney capsule, that were retrieved from cured recipient rats on Day-70 after transplantation, had a well-developing blood vessel network (Figure 5A). Angiogenesis (red blood cells) were also observed inside the islet transplants in the hematoxylin/eosin (H&E)-stained graft sections (Figure 5B).
Figure 5.
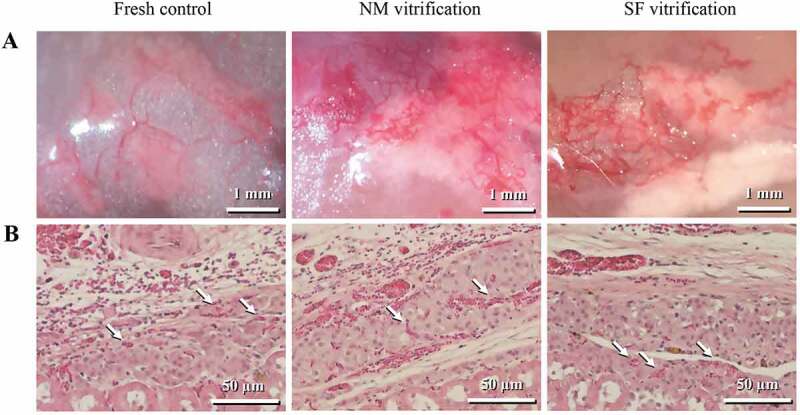
Islet grafts beneath the kidney capsule, retrieved from cured recipient rats on Day-70 after transplantation. (A) Islet grafts, along with angiogenesis. (B) Histological sections of grafts stained with H&E, showing kidney tissues (bottom part), blood vessels (arrows) and islets
Discussion
Rat pancreatic islets vitrified-warmed using the NM device and the SF sponge disc in a unit of 100 were proven to be functional for the glycemic control of streptozotocin-induced diabetic rats (Figure 3). As 800 islets are often transplanted beneath the kidney capsule of diabetic recipients in allogeneic rat models,9,21 the preparation of post-warm islets using conventional vitrification cryodevices, including Cryotop,8 open-pulled straw,9 and cryoloop9 (optimal sample size per device is approximately 10) would not be practical even in a rat model system. In contrast to these conventional vitrification protocols, the use of an NM device or SF sponge disc saves time and labor for the single islet transplantation because the replicate number of the warming processes can be reduced to 8 times. However, the scale-up merit in association with 100 islets per cryodevice is still inadequate when an application to clinical islet transplantation is considered (a dosage of 10,000–12,000 IEQ/kg is needed1), requiring the further improvement of the vitrification protocols.
A larger number of islets was transplanted to diabetes model animals when the equal functionality of cryopreserved islets could not be demonstrated compared to their fresh counterparts.22,23 However, such an islet number adjustment for transplantation was not necessary for our study, probably due to that the vitrified-warmed islets carried enough function for the secretion of insulin (SI range 4.2 to 4.7; Table 2). Therefore, in vitro assays, including the islet viability and the GSIS are important tools for the determination of the utility of cryopreserved islets for transplantation. The islets with an SI value of larger than 3 in the GSIS are considered suitable for the transplantation.24 The normal in vivo functionality of vitrified-warmed islets was further identified based on the regained hyperglycemia in recipients after Day-70 nephrectomy (Figure 3) as well as the comparable AUC in the IPGTT not only between the fresh control and the vitrification groups but also between Day-30 and Day-60 values (Figure 4), suggesting that the grafts of rat islets were responsible for the euglycemic state. Furthermore, the angiogenesis observed in the grafts (Figure 5) would contribute to the maintenance of the islet viability due to the supply of nutrients/oxygen as well as state of euglycemia due to insulin release.
The islets were vitrified-warmed on either the NM device by minimum volume cooling (MVC) protocol or the SF sponge disc by solid surface vitrification (SSV) protocol. Sasamoto et al.8 reported that rat islets vitrified-warmed in vitrification solution (VS) (EDT324; 30% ethylene glycol, 20% dimethyl sulfoxide, and 0.4 M trehalose) by device-free SSV protocol had an SI value of 6.4 and the potential to cure streptozotocin-induced diabetic rats of hyperglycemia. On the other hand, functionalities of mouse islets vitrified-warmed in EDT324 by SSV protocol were reported by Nagaya et al.9 to be inferior to those of the hollow fiber vitrification protocol, suggesting the rodent species-dependent difference. The most suitable cryopreservation protocol including the VS composition and/or the cryodevice type may also have differences between rat islets8 and hamster islets.25 Cryoinjury was previously reported to occurs at the periphery of vitrified-warmed rat islets,8 and β-cells were identified to be more likely to distribute centrally in rodent islets when compared with human islets.26 For the cryopreservation of human islets, a simple and large-scale vitrification protocol without a considerable loss of viability would be preferable.
The practical use of the NM device and the SF sponge disc without any loss of the islet recovery, the viability, and the insulin secretion ability (Table 2) may be further emphasized for the suitability of the vitrification protocol in efficient islet transplantation. The NM device does not require the handling of individual islets with glass pipettes and facilitates the minimization of the VS volume surrounding the islets by absorption with a paper towel.11,12 The SF sponge disc is also suitable for the minimization of the VS volume due to the spontaneous absorption property of the multiporous structure (Figure 2B). Since the SF protein from silkworm cocoon is easy to be processed into gels, films, or sponges,14,15 the unique properties such as the biocompatibility, the controllable degradation rate and the mechanical strength of the SF may be favorable to designate scaffold for islet engraftment. The SF-based hydrogel was reported by Hamilton et al.27 to allow the sustainable function of the mouse islet grafts for 42 days, as well as an accelerated recovery from hyperglycemia by the existence of SF-based hydrogel as a scaffold. Moreover, Mao et al.18 also reported the effective use of a heparinized macroporous SF scaffold for the transplantation of syngeneic islets in a mouse model.
Since the direct immersion vitrification of the SF sponge disc and the excess boiling of the liquid nitrogen (LN2) resulted in severe fractures of the discs (data not shown), the SSV protocol28 was applied with minor modifications when the SF sponge disc was employed as a cryodevice. The mechanical parameters of cryodevice materials (Table 1) showed that the SF sponge fabricated from 4% SF solution had a high absorbent property due to the larger porosity when compared to the NM sheet, while the tensile elastic modulus of the SF sponge was observed to be substantially inferior compared to that of the NM sheet. The SSV protocol applied to the SF sponge disc caused intriguing results in the GSIS (Table 2) and the insulin secretion level to 20 mM glucose stimulation in the SF vitrification group was found to increased 1.5-folds in comparison with that in the fresh control group. This phenomenon may be explained by an impaired glucose responsiveness due to insulin leak from damaged islet cells (as negative effect) and/or islet hyperactivity due to adhesion to the SF protein (as a positive effect).
In conclusion, the large quantities of rat islets vitrified-warmed on NM device or SF sponge disc were proven to be fully functional by both in vitro assays (viability and GSIS) and in vivo assays (subrenal transplantation and IPGTT in rat models of diabetes).
Materials and methods
Study design
The functional revivability of vitrified-warmed rat pancreatic islets was evaluated by both in vitro and in vivo assays (Figure 1A). Fresh islets were selected to serve as the control. The in vitro assay included fluorescent diacetate (FDA)/propidium iodide (PI) double staining for the measurement of the viability and the GSIS for the insulin secretion level, while the in vivo assay included subrenal transplantation into streptozotocin-induced diabetic rats. Blood glucose levels were monitored for 80 days. Subsequently, IPGTT was conducted to recipient rats on Day-30 and Day-60, and nephrectomy on Day-70. Two vitrification procedures were compared (Figure 1B). The islets were vitrified-warmed on either the NM device by the MVC protocol or the SF sponge disc by the SSV protocol. In both procedures, the volume of the VS surrounding the islets was minimized by absorption with paper towel prior to rapid cooling into LN2.
Chemicals and animals
Unless otherwise indicated, the chemicals used in this study were purchased from Sigma-Aldrich Corp. (USA). The specific pathogen-free Brown-Norway (BN) rats were purchased from Japan SLC, Inc. (Japan) and housed in an environmentally controlled room with a 12-h dark/12-h light cycle at a temperature of 23 ± 3°C with free access to a laboratory diet (NMF; Oriental Yeast Co., Ltd., Japan) and tap water. Male rats between the age of 8 and 12 weeks were used as both islet donors and recipients. All procedures performed during the animal experiment were reviewed and approved by the Animal Care and Use Committee of the Shinshu University, Nagano, Japan.
Cryodevices
Preparation of the NM device
A 10-mm triangle sheet was cut from commercially available NM (autoclaved; a square opening of 37 µm on a side length, nylon 66A-fiber diameter of 35 µm; Filter net N-No.355 T; Sansyo, Japan) and processed in order to form a triangular pyramid by folding into quarters.
Preparation of the SF sponge disc
Degummed silk fiber of a Bombyx mori silkworm cocoon was dissolved in 9 M lithium bromide to yield a concentration of SF at 6–7% (wt/vol) by stirring at an ambient temperature (25 ± 2°C) for 12–18 h. The SF solution was subsequently dialyzed against pure water using a cellulose dialysis membrane (MWCO = 12,000–14,000; As One Corp., Japan) for 2 days, with exchanging dialysis water at 8-h intervals. The SF solution was concentrated as it was placed to stand in the cellulose dialysis membrane at ambient temperature to yield a 6–10% SF concentration. Insoluble debris from the silk fiber was separated by centrifugation for 30 min at 12,000 × g. The final concentration of the SF solution was subsequently determined from the weight of the residue after completely drying the aliquot solution.
The SF solution was diluted with pure water containing 1.0% dimethyl sulfoxide (Wako Pure Chemical Industries, Japan) to yield an SF concentration of 4.0% and poured into a square mold (1 mm height, 50 mm × 50 mm bottom). The solution was afterward frozen at −20°C for 14 h in a programmable low-temperature precision bath (JCB-3300; EYELA, Japan). Air thawing of the frozen solution resulted in the formation of a porous structure, and the remaining dimethyl sulfoxide was removed from the SF sponge as it was placed in pure water for 2 days with water exchanged every 8 h. Discs (8 mm in diameter, 1 mm in height) were cut out from the SF sponge. The SF sponge discs were afterward sterilized by autoclaving in pure water, and then kept overnight at 4°C in VS until use.
Characterization of the device materials
The SF sponge was lyophilized for 24 h (FD-5N; EYELA, Japan) and the porous structure was observed under a scanning electron microscope (SEM; JSM-6010LA; JEOL, Japan) after sputter-coating with platinum. The ImageJ software (National Institutes of Health, USA; http://imagej.nih.gov/ij/) was applied for the quantitative characterization of the diameter of surface pores (n = 30) as well as the two-dimensional porosity. The thickness of the lyophilized SF disc was measured using a micrometer under a stereomicroscope (× 100 magnification). All measurements were replicated at least three times.
Isolation of rat islets
Pancreatic islets were isolated from BN male rats at the age of 8–12 weeks. Briefly, the bile duct of the rats was clamped and cannulated with a fine plastic tube and the pancreas was subsequently distended with approximately 8 ml of cold liberase thermolysin-low solution (1 WU/ml liberase in Hanks’ balanced salt solution, HBSS). The pancreas was excised, minced and incubated at 37°C for 30 min. The digested tissues were afterward suspended with Histopaque 1119 and purified by density gradient centrifugation with Histopaque 1077 and 2% fetal bovine serum (FBS; Thermo Fisher Scientific Inc., USA) in the HBSS. Islets with a size of 101–200 µm in mean longest and widest diameter were handpicked using capillary pipettes under a stereomicroscope and subsequently cultured for 24 h in RPMI-1640 (Life Technologies, Inc., USA) supplemented with 10% FBS and antibiotics (100 U/ml penicillin and 100 µg/ml streptomycin) at 37°C in a humidified atmosphere of 5% CO2 in the air until further use for vitrification.
Vitrification and warming
NM vitrification
The NM vitrification protocol was conducted as described previously. Briefly, 100 ± 10 islets were equilibrated with 7.5% ethylene glycol (Wako) and 7.5% dimethyl sulfoxide in RPMI-1640 containing 20% FBS (defined as the equilibration solution [ES]) for 3 min at ambient temperature. The islets were subsequently placed on the center of the NM triangle using a capillary pipette. Immediately afterward, the ES was removed by placing the device on a sterilized filter paper (Kimwipes®; Nippon Paper Crecia Co., Ltd., Japan) and the islets on the device were exposed to the VS comprised of 15% ethylene glycol, 15% dimethyl sulfoxide, and 0.5 M sucrose in RPMI-1640 containing 20% FBS for 70 sec at an ambient temperature using sterilized tweezers. Within this 70-sec interval, the device was placed on a new, sterilized filter paper to minimize the VS volume and subsequently quickly plunged into LN2.
After their storage in LN2-filled 1.0-ml cryotubes for at least 1 week, the islets were warmed by the immersion of the NM device into RPMI-1640 containing 20% FBS and 1.0 M sucrose at 38.5°C for 1 min. At this point, the post-warm islets on the NM device were transferred into 0.5, 0.25, and 0 M sucrose solution in a stepwise manner (3, 5, and 5 min, respectively). After the recovery of the islets from the NM device during the last 5 min using a capillary pipette, the islets were cultured in RPMI-1640/10% FBS for 2 h at 37°C in a humidified atmosphere of 5% CO2 in the air.
SF vitrification
Islets (100 ± 10) were equilibrated with the ES for 3 min at ambient temperature and subsequently placed onto the center of the VS-rinsed SF sponge disc using a capillary pipette. The SF sponge disc was afterward placed on a sterilized filter paper and gently pressed using sterilized tweezers to remove the excess VS while the islets remained on the surface of the SF sponge disc (up to 2 min). Then, the SF sponge disc was transferred into the VS for 40 sec at ambient temperature using sterilized tweezers and subsequently placed on a new sterilized filter paper for 30 sec. The SF sponge disc was rapidly cooled on the surface of an aluminum pan floating on LN2. The application of the SSV protocol prevented the destructive fracture of the SF sponge disc due to boiling (data not shown).
After storage in LN2-filled 1.0-ml cryotubes for at least 1 week, islets were warmed up, transferred to RPMI-1640/20% FBS, recovered from the SF sponge disc, and eventually cultured for 2 h according to the NM vitrification protocol.
In vitro assay
Islet viability
Islet viability was assessed by fluorescence double staining for the measurement of intracellular esterase activity and cell membrane integrity. An aliquot of 10 islets in each group was stained with 25 µg/ml of FDA and 25 µg/ml of PI for 30 sec under dark condition. After being washed three times in phosphate-buffered saline, the FDA (green)/PI (red) fluorescence images were acquired by the utilization of a fluorescence microscope (Keyence Corp., Japan). The fluorescent area was quantified using the ImageJ software. The viability was calculated as 100 × FDA-positive area divided by the total of FDA-positive + PI-positive area.
GSIS
The functionality of vitrified-warmed or fresh control islets was assessed by a static GSIS assay. Briefly, 10 islets per replicate in each group were washed three times with RPMI-1640/10% FBS containing 3 mM glucose and subsequently preincubated for 1 h at 37°C in a humidified atmosphere of 5% CO2 in the air. Afterward, the islets were stimulated by transferring them into RPMI-1640/10% FBS containing 20 mM glucose and incubation for 1 h as described above. At the end of the preincubation and incubation, the supernatants were collected and stored at –80°C until the conduction of insulin assay. The basal and stimulated insulin levels (ng/islet/h) were determined by an ELISA kit for rat insulin (MIoBS, Inc., Japan) and the SI was defined as the stimulated insulin level in response to 20 mM glucose divided by the basal level in response to 3 mM glucose.
In vivo assay
Subrenal transplantation
Male rat models for diabetes were prepared by a single intravenous injection of streptozotocin (Wako) with a dose of 65 mg/kg. Six days after the streptozotocin injection, blood was collected from the tail vein and the non-fasting blood glucose level was monitored with a commercial glucometer (GlucocardTM G Black; Arkray, Inc., Japan). Rats were considered diabetic in case of a blood glucose level of > 350 mg/dl and were subjected to islet transplantation the next day.
On the day of islet transplantation (defined as Day-0), the diabetic rats were anesthetized by the inhalation of isoflurane gas as well as the intramuscular injection of medetomidine hydrochloride (1.2–2.5 mg/kg; Kyoritsu Seiyaku Co., Japan) and butorphanol tartrate (0.8–1.7 mg/kg; Meiji Seika Pharma Co., Ltd., Japan), and the skin was shaved and swabbed with 70% ethanol and iodophors. The left kidney was subsequently exposed through a 1.5-cm lumbar incision. Eight hundred islets (size category: 101–200 µm in diameter) from the fresh control group, the NM vitrification group, or the SF vitrification group were transplanted into space beneath the kidney capsule of the diabetic rats using a glass capillary connected to a mouthpiece. The capsulotomy was left unsatured. The kidney was afterward placed back into its original position and the incision was closed with a surgical suture. The recipient rats were recovered from the anesthetic maintenance state by an intramuscular injection of atipamezole hydrochloride (6.0–12.5 mg/kg; Kyoritsu). The un-fasting blood glucose level, sampled by tail puncture, and the body weight gain of the recipient rats were monitored on Day-1, -4, -7, -14, -21, -28, -35, -42, -49, -56, -63, and -70 to assess the islet graft function. Rats that maintained a blood glucose level of < 200 mg/dl for two consecutive measurements were considered to have reversed diabetes (euglycemia). The left kidneys of the cured rats were removed on Day-70 under isoflurane anesthesia, and the blood glucose level as well as the body weight of the hemi-nephrectomized rats were monitored on Day-71, -74, and -77.
For histological evaluation, the grafts from the nephrectomized rats were fixed in 4% paraformaldehyde in phosphate-buffered saline and kept at 4°C until further use. They were embedded in paraffin and subsequently sliced into 4 µm in thickness. After deparaffinization, the sections were stained with H&E.
IPGTT
To conduct the IPGTT for the further assessment of the metabolic capacity of the islet grafts, the cured rats were fasted overnight before receiving an intraperitoneal glucose bolus (2 g/kg; administered as a 50% solution in water) on Day-30 and -60. Blood glucose levels, sampled by tail puncture, were monitored at baseline (time 0, 15, 30, 60, and 120 min) after the injection, allowing for the AUC to be calculated and analyzed among the fresh control group, the NM vitrification group and the SF vitrification group.
Statistical analysis
The percentage data of the viability were subjected to arcsine transformation before the application of the analysis of variance. In case the analysis of variance was significant, differences among the values retrieved from the groups were analyzed by Tukey’s Honest Significant Difference test for multiple comparisons. Data were considered statistically significant at P < .05.
Funding Statement
This study was supported by Grant-in-Aid for Scientific Research from Japan Society for the Promotion of Science [JSPS; 16K07985 and 20K06364 to S.H], a grant of General Collaborative Project from the National Institute for Physiological Science, Japan [NIPS; No.139 to S.H.], and a grant of Leading Advanced Projects for Medical Innovation from Japan Agency for Medical Research and Development [LEAP/AMED; JP18gm0010002 to M.H.]. K.N-I. and T.Y. were recipients from Kaneko-Hachiro Scholarship Foundation and Yoshida Scholarship Foundation, respectively.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Shapiro AM, Laley JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV.. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 2.Al-Adra DP, Gill RS, Imes S, O’Gorman D, Kin T, Axford SJ, Shi X, Senior PA, Shapiro AM. Single-donor islet transplantation and long-term insulin independence in select patients with type 1 diabetes mellitus. Transplantation. 2014;98(9):1007–1012. doi: 10.1097/TP.0000000000000217. [DOI] [PubMed] [Google Scholar]
- 3.Cheng JY, Raghunath M, Whitelock J, Poole-Warren L. Matrix components and scaffolds for sustained islet function. Tissue Eng Part B Rev. 2011;17(4):235–247. doi: 10.1089/ten.TEB.2011.0004. [DOI] [PubMed] [Google Scholar]
- 4.Bennet W, Groth CG, Larsson R, Nilsson B, Korsgren O. Isolated human islets trigger an instant blood mediated inflammatory reaction: implications for intraportal islet transplantation as a treatment for patients with type 1 diabetes. Ups J Med Sci. 2000;105(2):125–133. doi: 10.1517/03009734000000059. [DOI] [PubMed] [Google Scholar]
- 5.Bank HL. Cryobiology of isolated islets of Langerhans circa 1982. Cryobiology. 1983;20(2):119–128. doi: 10.1016/0011-2240(83)90001-9. [DOI] [PubMed] [Google Scholar]
- 6.Kneteman NM, Alderson D, Scharp DW, Lacy PE. Long-term cryogenic storage of purified adult human islets of Langerhans. Diabetes. 1989;38(3):386–396. doi: 10.2337/diab.38.3.386. [DOI] [PubMed] [Google Scholar]
- 7.Hwang IS, Hochi S. Recent progress in cryopreservation of bovine oocytes. BioMed Res Int. 2014;2014:570647. doi: 10.1155/2014/570647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamanaka T, Tashima K, Takashima S, Goto T, Hirabayashi M, Hochi S. Direct comparison of Cryotop® vitrification and Bicell® freezing on recovery of functional rat pancreatic islets. Cryobiology. 2016;73(3):376–382. doi: 10.1016/j.cryobiol.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Sasamoto H, Futami M, Ando Y, Nakaji S. Cryopreservation of rat islets of Langerhans by vitrification. J Artif Organs. 2012;15(3):283–289. doi: 10.1007/s10047-012-0635-7. [DOI] [PubMed] [Google Scholar]
- 10.Nagaya M, Matsunari H, Kanai T, Maehara M, Nakano K, Umeki I, Katsumata Y, Kasai Y, Sakai R, Kobayashi M, et al. An effective new cryopreservation procedure for pancreatic islets using hollow fiber vitrification. Horm Metab Res. 2016;48(8):540–549. doi: 10.1055/s-0042-102628. [DOI] [PubMed] [Google Scholar]
- 11.Yamanaka T, Goto T, Hirabayashi M, Hochi S. Nylon mesh device for vitrification of large quantities of rat pancreatic islets. Biopreserv Biobank. 2017;15(5):457–462. doi: 10.1089/bio.2017.0044. [DOI] [PubMed] [Google Scholar]
- 12.Nakayama K, Yamanaka T, Tamada Y, Hirabayashi M, Hochi S. Supplementary cryoprotective effect of carboxylated ε-poly-l-lysine during vitrification of rat pancreatic islets. Cryobiology. 2019;88:70–74. doi: 10.1016/j.cryobiol.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Nakayama K, Chinen S, Teshima J, Tamada Y, Hirabayashi M, Hochi S. Slik fibroin sheet multilayer suitable for vitrification of in vitro-matured bovine oocytes. Theriogenology. 2020;145:109–114. doi: 10.1016/j.theriogenology.2020.01.052. [DOI] [PubMed] [Google Scholar]
- 14.Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, Kaplan DL. Silk-based biomaterials. Biomaterials. 2003;24(3):401–416. doi: 10.1016/s0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- 15.Vepari C, Kaplan DL. Silk as a biomaterial. Prog Polym Sci. 2007;32(8–9):991–1007. doi: 10.1016/j.progpolymsci.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stendahl JC, Kaufman DB, Stupp SI. Extracellular matrix in pancreatic islets: relevance to scaffold design and transplantation. Cell Transplant. 2009;18(1):1–12. doi: 10.3727/096368909788237195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meinel L, Karageorgiou V, Hofmann S, Fajardo R, Snyder B, Li C, Zichner L, Langer R, Vunjak-Novakovic G, Kaplan DL. Engineering bone-like tissue in vitro using human bone marrow stem cells and silk scaffolds. J Biomed Mater Res A. 2004;71(1):25–34. doi: 10.1002/jbm.a.30117. [DOI] [PubMed] [Google Scholar]
- 18.Mao D, Zhu M, Zhang X, Ma R, Yang X, Ke T, Wang L, Li Z, Kong D, Li C. A macroporous heparin-releasing silk fibroin scaffold improves islet transplantation outcome by promoting islet revascularisation and survival. Acta Biomater. 2017;59:210–220. doi: 10.1016/j.actbio.2017.06.039. [DOI] [PubMed] [Google Scholar]
- 19.Kambe Y, Yamamoto K, Kojima K, Tamada Y, Tomita N. Effects of RGDS sequence genetically interfused in the silk fibroin light chain protein on chondrocyte adhesion and cartilage synthesis. Biomaterials. 2010;31(29):7503–7511. doi: 10.1016/j.biomaterials.2010.06.045. [DOI] [PubMed] [Google Scholar]
- 20.Kambe Y, Kojima K, Tamada Y, Tomita N, Kameda T. Silk fibroin sponges with cell growth-promoting activity induced by genetically fused basic fibroblast growth factor. J Biomed Mater Res A. 2016;104(1):82–93. doi: 10.1002/jbm.a.35543. [DOI] [PubMed] [Google Scholar]
- 21.Ohnishi K, Murakami M, Morikawa M, Yamaguchi A. Effect of the silk protein sericin on cryopreserved rat islets. J Hepatobiliary Pancreat Sci. 2012;19(4):354–360. doi: 10.1007/s00534-011-0415-4. [DOI] [PubMed] [Google Scholar]
- 22.Corominola H, Mendola J, Esmatjes E, Sáenz A, Fernández-Cruz L, Gomis R. Cryopreservation of pancreatic islets prior to transplantation: a comparison between UW solution and RPMI culture medium. Cryobiology. 1998;37(2):110–118. doi: 10.1016/cryo.1998.2107. [DOI] [PubMed] [Google Scholar]
- 23.Mendola J, Corominola H, Gonzalez-Clemente JM, Esmatjes E, Saenz A, Fernandez-Cruz L, Gomis R. Follow-up study of the revascularization process of cryopreserved islets of Langerhans. Cryobiology. 1996;33(5):530–543. doi: 10.1016/cryo.1996.0057. [DOI] [PubMed] [Google Scholar]
- 24.Sakata N, Egawa S, Sumi S, Unno M. Optimization of glucose level to determine the stimulation index of isolated rat islets. Pancreas. 2008;36(4):417–423. doi: 10.1097/MPA.0b013e31815ccad2. [DOI] [PubMed] [Google Scholar]
- 25.Agudelo CA, Iwata H. The development of alternative vitrification solutions for microencapsulated islets. Biomaterials. 2008;29(9):1167–1176. doi: 10.1016/j.biomaterials.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 26.Dolensek J, Rupnik MS, Stozer A. Structural similarities and differences between the human and the mouse pancreas. Islets. 2015;7(1):e1024405. doi: 10.1080/19382014.2015.1024405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamilton DC, Shih HH, Schubert RA, Michie SA, Staats PN, Kaplan DL, Fontaine MJ. A silk-based encapsulation platform for pancreatic islet transplantation improves islet function in vivo. J Tissue Eng Regen Med. 2017;11(3):887–895. doi: 10.1002/term.1990. [DOI] [PubMed] [Google Scholar]
- 28.Dinnyés A, Dai Y, Jiang S, Yang X. High development rates of vitrified bovine oocytes following parthenogenetic activation, in vitro fertilization, and somatic cell nuclear transfer. Biol Reprod. 2000;63(2):513–518. doi: 10.1095/biolreprod63.2.513. [DOI] [PubMed] [Google Scholar]


