Abstract
Background
Few studies have explored the work of sterile processing departments (SPD) from a systems perspective. Effective decontamination is critical for removing organic matter and reducing microbial levels from used surgical instruments prior to disinfection or sterilisation and is delivered through a combination of human work and supporting technologies and processes.
Objective
In this paper we report the results of a work systems analysis that sought to identify the complex multilevel interdependencies that create performance variation in decontamination and identify potential improvement interventions.
Methods
The research was conducted at a 700-bed academic hospital with two reprocessing facilities decontaminating approximately 23 000 units each month. Mixed methods, including 56 hours of observations of work as done, formal and informal interviews with relevant stakeholders and analysis of data collected about the system, were used to iteratively develop a process map, task analysis, abstraction hierarchy and a variance matrix.
Results
We identified 21 different performance shaping factors, 30 potential failures, 16 types of process variance, and 10 outcome variances in decontamination. Approximately 2% of trays were returned to decontamination from assembly, while decontamination problems were found in about 1% of surgical cases. Staff knowledge, production pressures, instrument design, tray composition and workstation design contributed to outcomes such as reduced throughput, tray defects, staff injuries, increased inventory and equipment costs, and patient injuries.
Conclusions
Ensuring patients and technicians’ safety and efficient SPD operation requires improved design of instruments and the decontamination area, skilled staff, proper equipment maintenance and effective coordination of reprocessing tasks.
INTRODUCTION
Healthcare-associated infections (HAI) affect 5%–10% of hospitalised patients, with 1.7 million HAIs per year resulting in 99 000 deaths and costing $20 billion1 per year in the USA. Surgical site infections account for 20% of these HAIs,2,3 some of which have been associated with deficiencies in sterile processing.4–7 The cleaning of medical equipment ranks among the top 10 most common compliance issues, and Centers for Medicare and Medicaid Services and the Joint Commission report one-third of hospitals have reprocessing deficiencies.8 Reports from the Food and Drug Administration have identified cases in which instruments have been reused without being reprocessed, and where contaminated instruments have been discovered in the operating room (OR) both prior to and after the produre.9,10 A number of highly publicised reprocessing failures, such as the potential exposure of over 3700 patients to HIV, and hepatitis B and C in New Jersey, USA, have also resulted in public scrutiny and a loss of trust.4,5,11
Despite considerable interest in systems approaches to addressing central line infections,12 urinary tract infections13 and clinician behaviour,14,15 few studies have explored the work of sterile processing departments (SPD). SPDs receive used instruments directly from ORs and other clinical areas, and through a combination of human work, defined processes and supporting technologies: decontaminate (remove, lower and limit human tissue, bone, bodily fluids and other organic material); assemble (pack instruments in trays and prepare them for sterilisation); sterilise (select the appropriate sterilisation method and parameters); and store (establish and implement controls to maintain the sterility of sterilised items until used) instruments for future use.16 Effective decontamination is critical for removing organic matter and reducing microbial levels prior to disinfection or sterilisation of surgical instruments, with failures leading to HAIs.17,18 Training, managerial or facility-related causes are usually cited as the causes of these failures.19,20 Our initial exploration of SPD work led us to propose that performance was shaped at multiple levels within the system of work and that it would be possible to identify many improvement opportunities.21–23
Multilevel systems engineering approaches have been useful for understanding the complex interactions between systems components. Different methods have different advantages and weaknesses, for example, regarding precision, the expertise required for them, process clarity and repeatability.24–26 To understand the inputs, transformation processes, interactions and outputs27 that contribute to performance variations in sterile processing, we used a work systems analysis (WSA) approach which combined qualitative and quantitative techniques, including observations of work as done, formal and informal interviews with relevant stakeholders and analysis of data collected about the system.28 WSA approaches have been successfully used within a sterile processing context as a tool to understand the implementation of guideline use29 and in other complex healthcare contexts including management of chronic illnesses30; safety in anaesthesiology31; outpatient healthcare32; and Clostridium difficile prevention.33
In this paper, we report the results of our WSA of the decontamination process. Our goal was to reveal complex interdependencies and trade-offs required for successful SPD work. The product of this would be a variance matrix describing key tasks and the key performance shaping factors (PSF) within the system. By reporting the different stages of our methodology we also aimed to make this analysis transparent in a way that would allow future replication or refinement. As one of the first studies to explore the function of SPD work in detail, we hoped to provide a replicable approach that would identify sources of variation and could serve as a framework for future investigation and intervention.
METHODS
Setting
The research was conducted at a 700-bed academic hospital with two reprocessing facilities employing 89 full-time staff, serving 56 onsite clinics, 31 ORs and 9 ambulatory centres, and decontaminating approximately 23 000 units (trays, sets, wraps) each month. Three additional reprocessing facilities at different health systems across the USA were also observed to inform the generalisability of our findings and identify potential controls.
Approach
The WSA moved through four stages: (1) process map (2), task analysis, (3) abstraction hierarchy, and (4) variance matrix, with each stage building on the last. This study employed observations, interviews and the study of administrative data and documentation to develop a WSA of the decontamination process.28 At each stage we validated and refined our models based on feedback obtained from an expert group of SPD technicians, supervisors, and managers, safety professionals, quality improvement experts and other hospital administrators.
Direct observations were conducted at the main and secondary SPDs as well as the three external SPDs. A total of 56 hours of observations were conducted in 22 weeks by a postdoctoral researcher (MA) with a background in human factors, supported by an engineering undergraduate (EH). Approximately 48 hours of observation were conducted at the main site and 2–3 hours were conducted at each additional site to facilitate the development of a work systems model of instrument reprocessing and identify sociotechnical challenges in the decontamination process. The observations methodology specified a ‘thicker’ note-taking approach that emphasised capturing broader range of variances, rather than specific events.34 Semistructured interviews were conducted by two researchers (MA and KC) with sterile processing technicians (18), SPD supervisors (3), SPD administrators (2), SPD educators (2), safety and risk management staff (2), quality improvement staff (2), infection control staff (2) and an executive in perioperative services (1). As individuals or dyads we asked for feedback and explored current and past challenges in instrument reprocessing, coordination of reprocessing efforts across the hospital, process and outcome data used and improvement efforts. Front-line SPD staff were asked about their daily work, issues they frequently encounter, and sources of performance variation, and feedback. SPD educators were interviewed together about orientation and training of technicians and preceptors. At the three external sites, only SPD managers were interviewed about the challenges their SPDs experience, how technicians are trained and the data they use to support decision-making. Interviews were not audio recorded, but extensive notes were taken during both the observations and structured interviews.
Process map and task analysis
An initial process map was drafted to define the flow of trays (a set of instruments used for a specific procedure or surgeon) through the decontamination process.35 Further process detail was represented in a hierarchical task analysis (HTA), which identifies the human actions required to complete tasks and reveals the non-linearity of these tasks.25,36 Ad hoc interviews (where necessity and opportunity allowed further detailed conversation about specific topics) were conducted with sterile processing technicians and supervisors by the observers, to gather more specific information about decontamination processes and iterate our models. We used pictures and videos to capture workstation layouts, to document how decontamination processes were performed (‘work as done’) and to capture examples of the variations in instrument trays received from the OR. We also reviewed standard operating procedures (SOP), organisational policies and training materials, and conducted additional observations to explore how these were enacted. When discrepancies were identified, usually between SOPs and observations (‘work as imagined vs work as done’), observations were given precedence and the discrepancy was discussed with a technician for clarification.
Abstraction hierarchy
The notes from our observations and interviews were compiled into a comprehensive system description of decontamination, which identified the stakeholders, boundaries and a range of systems-related dimensions.28 These themes were grouped into an abstraction hierarchy using the Systems Engineering Initiative for Patient Safety (SEIPS)37 for the multilevel systems framework. Each lane in the abstraction hierarchy represented a level of the SEIPS model (tasks, tools and technologies internal environment, organisation, and external environment), and specific factors such as support tools, equipment and maintenance schedules, policies and SOPs, reward and feedback mechanisms, and environmental factors—lighting, noise and temperature—were identified in each level.
Variance matrix
Combining the key tasks from the HTA with the abstraction hierarchy, we identified PSFs, potential failure modes, and process and outcome variances for each key decontamination task. These PSFs included people (knowledge, skills and abilities; KSA), tools and technology (instrument and workstation), internal environment (lighting) and organisation (production pressure). Next, we identified how the resulting task failures might be observed or measured as variance in the process, and finally how these process variances have or could lead to undesired outcomes.28 Failure points in the process were identified by observation, interviews, review of SOPs and training documents, and implied through various administrative databases. We also noted different controls implemented at the main and secondary sites to help prevent or reduce undesired outcomes but did not collect data on the effectiveness of these controls.
Data sampling
Administrative databases were used to collect data on the number of returns from assembly to decontamination (reflecting instruments identified during repacking that had not been properly cleaned), and tray defects reported in the OR (reflecting a range of instrument problems, including decontamination issues). Analysis was based on availability and accuracy (as indicated by SPD administrators), which varied between data sets.
Returns to decontamination were available from September 2016 to March 2018 and were analysed over time and by tray type, using the overall number of trays processed per month as a denominator. Tray defect data, derived from routinely collected self-reports of OR staff for July 2016 to December 2017, were analysed by defect type, grouped into the phase where the defect most likely arose (assembly, decontamination, sterilisation and case cart preparation), with the total number of cases performed during the period providing a denominator. Defects were categorised by phase according to the consensus of the two coders (KC and MA). These categorisations were largely based on existing SPD classifications.
Point-of-use reprocessing (reflecting the quality of organisation and cleaning of instruments by OR staff prior to arrival in decontamination) was not collected systematically, so was sampled via direct observation at the point of arrival for used trays. Data were collected by two research assistants according to three classifications: good (organised and cleaned); fair (disorganised and clean, organised not cleaned); and poor (disorganised and not cleaned). Observations were conducted on four different weekdays in the mornings (09:00–12:00) and afternoons (13:00–16:00) at each of the SPD facilities for a total of 24 hours’ observations. The research assistants were trained by the primary observer (MA) and provided sample pictures of contaminated trays for each of the classifications. Inter-rater reliability (IRR) was not calculated.
RESULTS
System description
Decontamination begins with point-of-use reprocessing, where the instruments are wiped, rinsed with sterile water, returned to their trays and sprayed with an enzymatic solution.7 This process needs to be timely to prevent contaminants from drying; to prevent the formation of biofilm (a thin layer of bacteria); to reduce the risks of sharps injuries; and to ensure the instruments remain in the correct trays for the next procedure. For longer procedures where there may be a delay in reprocessing, instruments should be wrapped in moist towels. Trays of these pretreated instruments are then delivered to the decontamination room in SPD, using a dumb waiter or transport carts, for comprehensive cleaning. Contaminated trays are received predominantly from the OR but are also from vendors, other units within the hospital and ambulatory centres.
On arrival into decontamination, each tray is bar code scanned, and is usually placed in a holding area to await processing. Trays may be organised by more experienced staff into groups representing different cleaning demands (mode, specialty) and priorities. Decontamination requires surgical instruments to be removed from their tray, inspected, opened, disassembled, cleaned and returned to their tray (online supplementary figure 1). SPDs contain several decontamination workstations, composed of two to three sinks, several small cleaning instruments, a large magnifier to inspect instruments, a dispensing mechanism for enzymatic or non-enzymatic cleaning fluid and a thermometer to provide water temperature readings. High-level disinfectant machines may also be operated in the decontamination area. Several different cleaning modes are employed such as manual washing (employing brushes and syringes), soaking, ultrasonic cleaning and machine wash using the washer-disinfector (online supplementary figure 2). The cleaning mode is primarily defined by the type of instrument and the manufacturer’s instructions for use (IFU) but may also depend on the availability of ultrasonic cleaners, the level of soiling and time pressure. Orthopaedic instruments usually undergo comprehensive cleaning including manual washing, soaking, ultrasonic cleaning and machine wash using the washer-disinfector (online supplementary figure 2). Other instruments, such as scopes and lenses, may only be manually washed using a non-enzymatic solution. Laparoscopic instruments might be manually washed, and then placed in the ultrasonic cleaner, time permitting.
Once instruments are cleaned, the trays are scanned out of decontamination and transferred into the ‘clean’ room for assembly and sterilisation. Most trays are transferred from decontamination through the washer-disinfector. Other trays, which include instruments that cannot be submerged, are transferred through a window. Contaminated tray containers (the outer protective ‘shell’ that houses each tray) and case carts (which contain the multiple trays required for each surgery) are washed and transferred to the ‘clean’ room using the cart washers. There were no appreciable differences in the decontamination process among the four sites.
Discrepancies between SOPs and observed work
Instrument trays were frequently left untreated, with bioburden on them for several hours and were rarely cleaned according to the specific IFUs, which were usually unavailable. Sometimes instruments were not always carefully laid out or appropriately disassembled before being cleaned, and were sometimes insufficiently separated once hand-washed, potentially allowing the mixing of clean and unclean instruments within a tray (eg, instruments were moved from one end of the tray to the other as they were cleaned, rather than being removed from the tray then replaced). Ultrasonic cleaning of instruments was regularly skipped either because the machines were not in service, or because rapid turnover did not allow the necessary ultrasonic cleaning cycle. Personal protective equipment was not always worn appropriately.
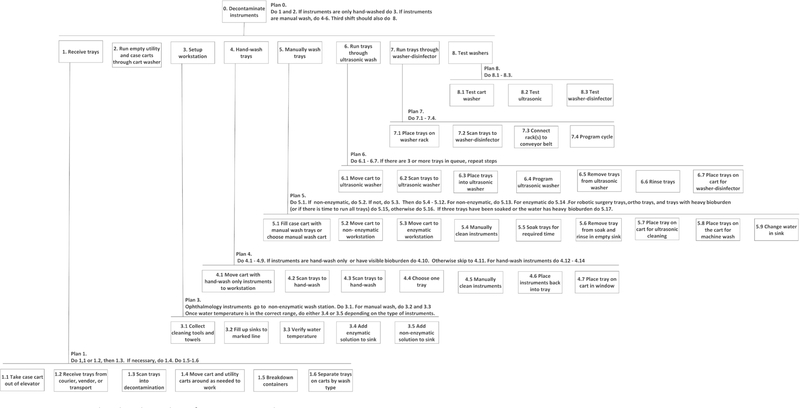
Hierarchical task analysis
The HTA illustrated all of the major tasks and subtasks performed by the sterile processing technician during decontamination including receiving trays, running the cart washer, preparing the workstation, cleaning instruments and testing the decontamination equipment (figure 1). Once the trays are received, they are scanned, treated with enzymatic fluid, if appropriate, and separated by cleaning mode by a technician. Technicians will then run the containers and case carts through the washer and prepare their workstation. To prepare their workstation, technicians gather the required tools and materials (eg, brushes, towels and syringes), fill the sink to the correct depth, verify the water temperature and add the correct dosage of cleaning fluid (figure 1). Some tasks, such as filling the sink and receiving trays, are performed concurrently. Technicians disassemble instruments, check them for bioburden, manually scrub them, flush cannulated instruments with a syringe or flushing device, then return the instruments to the tray for soaking and rinsing. Technicians may also run trays through the ultrasonic cleaner and washer-disinfector. Once per day, technicians also test the ultrasonic cleaner, cart washer and washer-disinfectors to ensure they are functioning correctly.
Figure 1.
Hierarchical task analysis for instrument decontamination.
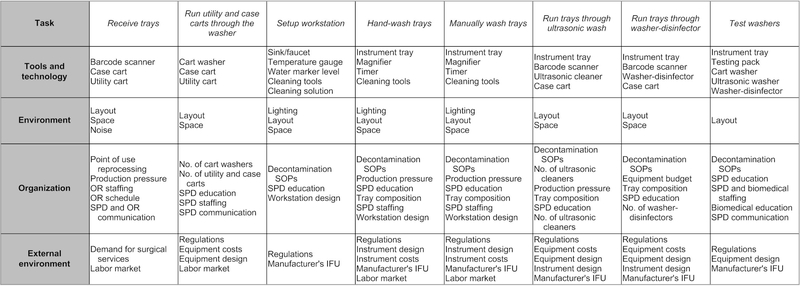
Abstraction hierarchy
The abstraction hierarchy is shown in figure 2. The decontamination task is affected by the tools and technology used to do it (eg, water level marker, sink/ faucet, brushes and syringes) and by the person using that technology when performing physical activities (eg, fill sink), monitoring and awareness (eg, verify temperature) and choosing a cleaning strategy (eg, soak instruments in tray). The internal working environment is created by the space and layout, lighting, temperature and noise. Organisational influences are related to financial constraints for instruments, equipment, staff and training, and the production pressures set by surgical schedules. The external environment factors include instrument and equipment designs and costs, regulations and external demands, including labour and surgical procedures. The regulation and recommendations for decontamination processes—such as soaking temperature and dosage of cleaning fluid—are promulgated by external organisations, such as the Association for the Advancement of Medical Instrumentation. These organisations also provide best practices and standards that inform SOPs and training for technicians.
Figure 2.
Abstraction hierarchy for instrument cleaning task in decontamination. IFU, instructions for use; OR, operating room; SOP, standard operating procedure; SPD, sterile processing department.
Variance matrix
Decontamination was divided into eight key tasks: retrieve trays; run carts and containers through the washer; choose the correct cleaning mode; manually scrub instrument; soak trays; run trays through the ultrasonic washer; run trays through washer-disinfector; and test the washers (ie, ultrasonic cleaner, cart washer, washer-disinfector). Although point-of-use reprocessing is performed outside the SPD, it was included in the matrix as it is the first step in the decontamination process. We identified 21 different PSFs, 30 potential failures, 16 types of process variance and 10 outcome variances (online supplementary table). Description of the PSFs is listed in table 1.
Table 1.
PSF descriptions and examples
| PSF | Description | Example(s) of failure |
|---|---|---|
| Staff KSAs (person) | KSAs of staff involved in reprocessing work, including SPD, OR and biomedical engineering | Technician fails to disassemble instrument during decontamination |
| Production pressure (organisation) | Pressure to perform higher numbers of cases, reduce OR turnover time and reduce reprocessing time | Scrub techs do not have time to perform point-of-use reprocessing |
| Demand variation (task) | The timing and rate at which trays are received from point-of-use areas (usually sporadically and in influxes) | Technician constantly interrupted by dumb waiter while cleaning instruments |
| Staffing (organisation) | Staffing and turnover in SPD, OR and biomedical engineering | Backlog of trays created due to understaffing in SPD |
| Workstation (tools/technology environment) | Lighting, number and depth of sinks, surface space, and placement and availability of decontamination tools and supplies (timer, temperature gauge, brushes and flushing aid) | Poor workstation design leads to staff fatigue or injury |
| Instrument design (external environment) | The features (metal, finishes, channels, and so on...) of an instrument that facilitate or hinder reprocessing | Contamination in interior channel of instrument missed during decontamination |
| Instrument IFU (external environment) | Instructions created by the manufacturer detailing the steps for properly cleaning and sterilising an instrument | IFUs unavailable, difficult to access or out of date |
| Tray composition (organisation) | The number and type of instruments in a tray | Instrument inappropriately exposed to immersion or high temperature |
| Equipment design (external) | Features, capacity and usability of the decontamination equipment (ultrasonic cleaner, cart washer and washer-disinfector) | Incorrect cycle selected for tray |
| Number of equipment (organisation) | Number of decontamination equipment available for reprocessing | Limited number of ultrasonic cleaners increases reprocessing time for orthopaedic instruments |
| Maintenance schedule (organisation) | Plan for preventative maintenance and prioritisation of corrective maintenance | Ultrasonic cleaner spends several days in disrepair |
| Communication (organisation) | Communication within SPD and between SPD and the OR, and biomedical engineering | Third shifts fail to inform incoming shift that tests were not conducted |
IFU, instructions for use; KSA, knowledge, skills and abilities; OR, operating room; PSF, performance shaping factor; SPD, sterile processing department.
The most frequent PSFs that contributed to failures included staff KSAs (5), production pressures (5), instrument design, tray composition and workstation design. Failures included unorganised instruments, lost instruments, instruments not treated with enzymatic fluid, lack of adherence to standards during manual wash and soaking, and technological issues such as choosing the incorrect machine cleaning cycle, and not maintaining equipment.
Process variation included less effective or prolonged cleaning, mixing of clean and contaminated instruments, inappropriate exposure to heat or immersion, and staff injury and fatigue. The most frequently appearing outcome variances were reduced throughput (11) and bioburden (12). However, we also identified staff injuries (sharps and musculoskeletal), inventory costs, instrument damage, tray defects (wrong instrument and missing instruments), damaged washer and patient injuries. Most of the observed controls, classified as tools and technology category, focused on placing knowledge in the world—SOP displays, access to IFUs—to help technicians clean the instruments effectively. Organisational controls sought to improve teamwork between SPD and the OR by assigning technicians to assist with point-of-use reprocessing and having OR staff work in SPD as part of their training or orientation. Improving coordination with biomedical engineering for equipment maintenance was another organisational intervention. Task controls varied from implementing double checks to incorporating tasks into a staff member’s workflow. The environmental controls attempted to improve the ergonomics of the decontamination work area.
Performance sampling
Return to decontamination
During the 19-month time period, data were available for 188311 reprocessed trays, of which 3958 were returned to decontamination from assembly for recleaning. Mean throughput was 9911 trays per month (SD=1061) with 208 trays (SD=52) returned back to decontamination. On average, 3.1% of trays (95%9 CI 2.7% to 3.4%) were returned to decontamination each month. Of the 59 specialty tray classifications used for these data, by far the highest number of returns was for power equipment (12% of trays), with most other trays around 4% (including transplant, oral surgery, general surgery, gynaecology and cardiothoracic) or 3% (neurosurgery, orthopaedics, urology, vascular, robotics).
Tray defect data
In the 41799 cases performed in the OR of the main site during the 18-month time frame, a total of 3900 defects were recorded (9.3% of cases). 9.8% (381) of these defects occurred during decontamination phase (bioburden, instrument not disassembled and foreign body in tray), with the rest occurring across assembly, sterilisation and case cart preparation. Specialties with the highest percentage of recorded decontamination defects included otolaryngology (3% of cases) and transplant (2%).
Point-of-use reprocessing
Of the 261 trays sampled arriving from OR and other clinical areas, 54% were received in good condition, 32% were received in fair condition and 15% were received in poor condition.
DISCUSSION
This WSA of decontamination in sterile processing revealed a range of multilevel sociotechnical factors that affect efficacy, efficiency and safety in decontamination. A reliable decontamination process is one that results in clean instruments that are correctly organised in the correct tray and passed on to assembly in a timely manner. Decontamination deficiencies result in undetected bioburden, missing and damaged instruments, and reduced throughput. These deficiencies have implications for HAIs, surgical durations, delays, cancellations, staff and patient injury, and the overall costs associated with delivering reliable surgical services. Approximately 1% of OR cases experienced a tray defect related to decontamination while approximately 2% of trays were returned from assembly to decontamination, demonstrating instances where decontamination failures were captured. The PSFs identified in this study demonstrated challenges in the decontamination process existed at multiple levels of the system. Improvement was often focused on individual performance issues, rather than system-rated control of variability, and was driven by OR-reported defect data, rather than process-related metrics that might offer additional insight for improvement. While some variance controls were also noted, these also tended to be person focused and, while our analysis of PSFs may not indicate the potential diversity of systems-based interventions, it suggests a broader range of targets for quality improvement than behavioural changes alone. Training and skill management, point-of-use processing, instrument and tray design, and the physical workspace all offer opportunities for improvement.
SPD technicians conduct complex cleaning tasks largely from memory while working in a hot, humid, noisy and distraction-prone environment.38 Training time ranged from 3 to 12 months across different sites, depending on state-wide legislation and previous experience.39 A large hospital can have 250 000 unique instruments, so it is unlikely that even the most experienced and highly trained SPD technician will be familiar with decontamination for every single instrument. Observation of a 20 min ‘in-service’ update focusing on two instruments from one equipment manufacturer suggested incongruency of training with the working environment and production pressure. SPD staff tend to be poorly paid and have low status within the organisation.39,40 High turnover creates challenges for day-to-day staffing, retaining expertise and developing preceptors and better training. Attempts to retain staff included career ladders for technicians to attain certifications, increased compensation and development of instrumentation expertise. To support technicians’ development, SPD educators suggested a need for more skilled preceptors and training41 that combines classroom, simulation, use in the OR, disassembly, soiling locations and improved in-service training for new instruments, with more emphasis on collaboration with ORs to improve point-of-use reprocessing.5,16
Ineffective point-of-use reprocessing by OR staff impedes cleaning, increases sharps risks and increases the likelihood of missing, wrong and damaged instruments,3,4 so effectiveness needs to be maintained in the face of OR production pressures. Though data were not routinely collected, our sampling suggested point-of-use processing was effective for only 54% of instrument trays. To improve point-of-use reprocessing, new OR staff sometimes spend time working in the SPD. Point-of-use displays or instructions may also be beneficial. Facilitating better capture and feedback to the OR of the condition of trays that arrive in SPD could support compliance and accountability42 while SPD technicians assigned to the point-of-use area can facilitate communication between SPD and the OR.
Instruments would ideally be designed where disassembly is intuitive and error tolerant, and inspection is easy,43,44 but increasingly complex designs can have the opposite effect.20 Interior channels, hinges and valves hide contamination, and require disassembly and additional processing time45 while IFUs for cleaning may be lengthy and unclear.46 The type and organisation of instruments in a tray also creates performance variation. Our data suggest neurosurgery, orthopaedics, otolaryngology and ear, nose and throat instruments and trays may present particular challenges. Newer technologies support cleaning of complex instruments (such as those with cannulation or interior channels) or inspection. However, efficacy evaluations are not always available and value proposition may be unclear47 making investment difficult to justify, while routine maintenance of these technologies may not be prioritised by biomedical engineering.48 During our observations, we noticed one of the two ultrasonic cleaning machines and one of the four washer-disinfectors spent several days in disrepair before they were repaired.
The individual workstations in decontamination create postural stressors, while insufficient numbers of sinks or surfaces require technicians to move back and forth, slowing down the process and creating opportunities to mix scrubbed and non-scrubbed instruments. Poor lighting can affect bioburden detection. Congestion of case carts resulted from a slow cart washing process and inadequate holding space. Some of the facilities observed were not designed to accommodate an increasing reprocessing volume or newer technologies. Newer facilities may benefit from more space to accommodate new technologies, workstations streamlined for specific cleaning modes, higher, shallower sinks, better lighting, chairs for technicians and cart washers that automatically load and unload carts.49
Rather than simply focusing on training, management or culture, performance improvement in decontamination benefits from a range of broader systems considerations. To our knowledge, this is the first attempt to explore the complex interactions between different systems components within decontamination in sterile processing. Our WSA approach, based on Karsh and Alper,28 provides a range of opportunities to intervene at multiple levels to shape performance and control variation. By combining commonly used systems analysis approaches (process map, HTA) with a multilevel systems framework (abstraction hierarchy based on SEIPS), we arrived at the variance matrix, which predicts relationships between inputs, processes and outputs at different levels in the system. In the absence of an ability to fully explore the reliability of our approach, by presenting each stage of our results, we aimed to demonstrate how each built from the last to provide sufficient methodological precision to allow rigorous replication or future refinement. In turn, we hope this demonstrates a transparent approach to systems analysis that facilitates scrutiny and wider application. Similarly, while we were unable to fully explore generalisability, our models generally held during visits to other sites. At the very least, we hope we have demonstrated some of the complexity of work in decontamination, and a range of opportunities for improvement beyond behavioural or managerial interventions.
Limitations and future research
Further research aims to use larger data sets to identify causal relationships between decontamination process measures, and investigate additional controls to alleviate undesired variation. Implementation of specific interventions would benefit from additional investigation and iterative testing across multiple SPDs. A larger multisite study could uncover more nuanced differences and challenges across different sites, allow us to obtain additional data and offer opportunities for comparison of processes, outcomes and proposed interventions.
IRR was not calculated for the observations. While IRR would have supported the rigour of our methodology, our primary concern regarding the observations was their overall consistency with the decontamination processes being modelled. We used the stakeholder interviews to help verify the models and the challenges identified accurately represented the system. We would also welcome a comparison of our findings with alternative strategies to investigate instrument reprocessing.
CONCLUSIONS
Ensuring patients and technicians’ safety, efficient SPD operation and reliable delivery of surgical services requires improved design of instruments and the decontamination area, skilled staff, proper equipment maintenance and effective communication and coordination of reprocessing tasks. Our WSA revealed a range of systems components that created variations in SPD processes and outcomes and illustrated potential interventions whose effects may be modelled and predicted. WSA also demonstrated the value of methods that link conceptual systems engineering models with design parameters, human performance and measurable outcomes in complex sociotechnical systems.
Supplementary Material
Acknowledgments
Funding This study was supported by the Agency for Healthcare Research and Quality (1R03HS025538-01).
Footnotes
Competing interests None declared.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request.
REFERENCES
- 1.Centers for Disease Control and Prevention. Preventing healthcare-associated infections Available: http://www.cdc.gov/washington/~cdcatWork/pdf/infections.pdf [Accessed 30 Sep 2016].
- 2.Magill SS, Hellinger W, Cohen J, et al. Prevalence of healthcare-associated infections in acute care hospitals in Jacksonville, Florida. Infect Control Hosp Epidemiol 2012;33:283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 2014;370:1198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenblatt K. More than 3,000 patients at new Jersey surgery center possibly exposed to HIV, hepatitis; 2018. [Google Scholar]
- 5.Dancer SJ, Stewart M, Coulombe C, et al. Surgical site infections linked to contaminated surgical instruments. J Hosp Infect 2012;81:231–8. [DOI] [PubMed] [Google Scholar]
- 6.Hutzler L, Kraemer K, Iaboni L, et al. A hospital-wide initiative to eliminate preventable causes of immediate use steam sterilization. Aorn J 2013;98:597–607. [DOI] [PubMed] [Google Scholar]
- 7.Rutala WA, Weber DJ. Reprocessing semicritical items: current issues and new technologies. Am J Infect Control 2016;44:e53–62. [DOI] [PubMed] [Google Scholar]
- 8.Joint Commission identifies top standards compliance issues for 2011. Jt Comm Perspect 2012;32:1–6. [PubMed] [Google Scholar]
- 9.Schaefer M. Food and drug administration center for devices and radiological health public workshop on reprocessing of reusable medical devices. Silver Spring: (MD), 2011. [Google Scholar]
- 10.ECRI. Sterile processing departmenťs role in patient safety; 2012.
- 11.Doughton S. Seattle children's warns of potential infection risk Seattle Times; 2015: 27. [Google Scholar]
- 12.Andrioli ER, Furtado GHC, Medeiros EA. Catheter-Associated urinary tract infection after cardiovascular surgery: impact of a multifaceted intervention. Am J Infect Control 2016;44:289–93. [DOI] [PubMed] [Google Scholar]
- 13.Saint S, Olmsted RN, Fakih MG, et al. Translating health care-associated urinary tract infection prevention research into practice via the bladder bundle. Jt Comm J Qual Patient Saf 2009;35:449–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leotsakos A, Zheng H, Croteau R, et al. Standardization in patient safety: the who high 5S project. Int J Qual Health Care 2014;26:109–16. [DOI] [PubMed] [Google Scholar]
- 15.Srigley JA, Gardam M, Fernie G, et al. Hand hygiene monitoring technology: a systematic review of efficacy. J Hosp Infect 2015;89:51–60. [DOI] [PubMed] [Google Scholar]
- 16.Seavey RE. Collaboration between perioperative nurses and sterile processing department personnel. Aorn J 2010;91:454–62. [DOI] [PubMed] [Google Scholar]
- 17.Alfa MJ. Current issues result in a paradigm shift in reprocessing medical and surgical instruments. Am J Infect Control 2016;44:e41–5. [DOI] [PubMed] [Google Scholar]
- 18.Kovaleva J, Peters FTM, van der Mei HC, et al. Transmission of infection by flexible gastrointestinal endoscopy and bronchoscopy. Clin Microbiol Rev 2013;26:231–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seavey R. Taking the chaos out of accreditation surveys in sterile processing: high-level disinfection, sterilization, and antisepsis. Am J Infect Control 2016;44:e35–9. [DOI] [PubMed] [Google Scholar]
- 20.Stockert EW, Langerman A. Assessing the magnitude and costs of intraoperative inefficiencies attributable to surgical instrument trays. J Am Coll Surg 2014;219:646–55. [DOI] [PubMed] [Google Scholar]
- 21.Reason J. Managing the risks of organisational accidents. Aldershot: Ashgate, 1997. [Google Scholar]
- 22.Dekker SW. The field guide to human error investigations. Aldershot: Ashgate, 2002. [Google Scholar]
- 23.Alfred al Met. A work systems analysis of sterile processing: sterilization and case cart preparation. Structural Approaches to Address Issues in Patient Safety 2019;18:173–96. [DOI] [PubMed] [Google Scholar]
- 24.Igene OO, Johnson C. Analysis of medication dosing error related to computerised provider order entry system: a comparison of ECF, HFACS, stamp and AcciMap approaches. Health Informatics J;222:146045821985999. [DOI] [PubMed] [Google Scholar]
- 25.Colligan L, Anderson JE, Potts HWW, et al. Does the process MAP influence the outcome of quality improvement work? A comparison of a sequential flow diagram and a hierarchical task analysis diagram. BMC Health Serv Res 2010;10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jun GT, Ward J, Morris Z, et al. Health care process modelling: which method when? Int J Qual Health Care 2009;21:214–24. [DOI] [PubMed] [Google Scholar]
- 27.Ayanian JZ, Markel H. Donabedian's lasting framework for health care quality. N Engl J Med 2016;375:205–7. [DOI] [PubMed] [Google Scholar]
- 28.Karsh B, Alper S. Advances in Patient Safety: From Research to Implementation (Volume 2: Concepts and Methodology). In: Henriksen K, ed. Work system analysis: the key to understanding health care systems, in advances in patient safety: from research to implementation Rockville, MD: Agency for Healthcare Research and Quality, 2005. [Google Scholar]
- 29.Hall-Andersen LB, Broberg O. Integrating Ergonomics into engineering design: the role of objects. Appl Ergon 2014;45:647–54. [DOI] [PubMed] [Google Scholar]
- 30.Holden RJ, Valdez RS, Schubert CC, et al. Macroergonomic factors in the patient work system: examining the context of patients with chronic illness. Ergonomics 2017;60:26–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Y, Rivera AJ, Fortier CR, et al. A human factors engineering study of the medication delivery process during an anesthetic: Self-filled syringes versus prefilled syringes. Anesthesiology 2016;124:795–803. [DOI] [PubMed] [Google Scholar]
- 32.Hallock ML, Alper SJ, Karsh B. A macro-ergonomic work system analysis of the diagnostic testing process in an outpatient health care facility for process improvement and patient safety. Ergonomics 2006;49:544–66. [DOI] [PubMed] [Google Scholar]
- 33.Yanke E, Zellmer C, Van Hoof S, et al. Understanding the current state of infection prevention to prevent Clostridium difficile infection: a human factors and systems engineering approach. Am J Infect Control 2015;43:241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Catchpole K, Neyens DM, Abernathy J, et al. Framework for direct observation of performance and safety in healthcare. BMJ Qual Saf 2017;26:1015–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McLaughlin N, Rodstein J, Burke MA, et al. Demystifying process mapping: a key step in neurosurgical quality improvement initiatives. Oxford University Press, 2014: 75 99–109. [DOI] [PubMed] [Google Scholar]
- 36.Phipps DL, Meakin GH, Beatty PCW. Extending hierarchical task analysis to identify cognitive demands and information design requirements. Appl Ergon 2011;42:741–8. [DOI] [PubMed] [Google Scholar]
- 37.Waterson P, Robertson MM, Cooke NJ, et al. Defining the methodological challenges and opportunities for an effective science of sociotechnical systems and safety. Ergonomics 2015;58:565–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grundgeiger T, Sanderson P. Interruptions in healthcare: theoretical views. Int J Med Inform 2009;78:293–307. [DOI] [PubMed] [Google Scholar]
- 39.Chobin N. The real costs of surgical instrument training in sterile processing revisited. Aorn J 2010;92:185–93. [DOI] [PubMed] [Google Scholar]
- 40.Swanson SC. Shifting the sterile processing department paradigm: a mandate for change. Aorn J 2008;88:241–7. [DOI] [PubMed] [Google Scholar]
- 41.De Meo M. The need for proper SPD training. Biomed Instrum Technol 2010;44:150–1. [DOI] [PubMed] [Google Scholar]
- 42.Jamtvedt G, Young JM, Kristoffersen DT, et al. Does telling people what they have been doing change what they do? A systematic review of the effects of audit and feedback. Qual Saf Health Care 2006;15:433–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.FDA. Reprocessing medical devices in health care settings: validation methods and labeling; 2015.
- 44.Branaghan R, Andre AD, Seraphina S. The dirty human factors of reprocessing: best practices in design, IFU, and testing in international Symposium of human factors and ergonomics in health care. Boston, MA, 2018. [Google Scholar]
- 45.FDA. Factors affecting quality of reprocessing, 2015. Available: http://www.fda.gov/medicaldevices/productsandmedicalprocedures/reprocessingofreusablemedicaldevices/ucm454622.htm [Accessed 2 Oct 2016].
- 46.Stephens A, Assang A. What do you mean you canť sterilize it? The reusable medical device matrix. Can Oper Room Nurs J 2010;28:6–11. [PubMed] [Google Scholar]
- 47.Dinakaran S, Kayarkar VV. Debris on processed ophthalmic instruments: a cause for concern. Eye 2002;16:281–4. [DOI] [PubMed] [Google Scholar]
- 48.Hamdi N, Oweis R, Abu Zraiq H, et al. An intelligent healthcare management system: a new approach in work-order prioritization for medical equipment maintenance requests. J Med Syst 2012;36:557–67. [DOI] [PubMed] [Google Scholar]
- 49.Joseph A, Rashid M. The architecture of safety: Hospital design. Curr Opin Crit Care 2007;13:714–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.