Objective:
To evaluate changes in weight and BMI in adults with HIV-1 at 1 and 2 years after starting an antiretroviral regimen that included doravirine, ritonavir-boosted darunavir, or efavirenz.
Design:
Post-hoc analysis of pooled data from three randomized controlled trials.
Methods:
We evaluated weight change from baseline, weight gain at least 10%, and increase in BMI after 48 and 96 weeks of treatment with doravirine, ritonavir-boosted darunavir, or efavirenz-based regimens. Risk factors for weight gain and metabolic outcomes associated with weight gain were also examined.
Results:
Mean (and median) weight changes were similar for doravirine [1.7 (1.0) kg] and ritonavir-boosted darunavir [1.4 (0.6) kg] and were lower for efavirenz [0.6 (0.0) kg] at week 48 but were similar across all treatment groups at week 96 [2.4 (1.5), 1.8 (0.7), and 1.6 (1.0) kg, respectively]. No significant differences between treatment groups were found in the proportion of participants with at least 10% weight gain or the proportion with BMI class increase at either time point. Low CD4+ T-cell count and high HIV-1 RNA at baseline were associated with at least 10% weight gain and BMI class increase at both timepoints, but treatment group, age, sex, and race were not.
Conclusion:
Weight gains over 96 weeks were low in all treatment groups and were similar to the average yearly change in adults without HIV-1. Significant weight gain and BMI class increase were similar across the treatment groups and were predicted by low baseline CD4+ T-cell count and high baseline HIV-1 RNA.
Keywords: darunavir, doravirine, efavirenz, HIV-1, weight gain
Introduction
Adults tend to gain weight progressively through middle age, with an average weight gain estimated at 0.5–1 kg/year [1]. Over time, this modest accumulation of weight can lead to obesity. The prevalence of obesity, defined as having a BMI of 30 or higher, has nearly tripled since 1975: in 2016, 39% of adults worldwide were overweight (BMI of 25 or higher) and 13% were obese [2]. In the United States, 72% of adults were overweight and 40% were obese in 2015–2016 [3]. Being overweight or obese increases the risk of all-cause mortality and other health problems including cardiovascular disease, diabetes, certain cancers, and nonalcoholic fatty liver disease [4,5].
For people living with HIV (PLWH), weight gain after starting antiretroviral therapy (ART) is often considered a return to health. Weight gain during the first year after ART initiation has been associated with lower mortality in underweight and normal-weight patients, but not in overweight or obese patients [6], whereas other studies have linked weight gain during HIV treatment to increased risk for diabetes, cardiovascular disease, hepatic steatosis, and liver fibrosis [7–10]. Since the introduction of more effective and better tolerated ART, the prevalence of being overweight or obese has increased among PLWH [11–16]. Women with HIV are at higher risk than men for weight gain and obesity following ART initiation [17,18]. Other risk factors for weight gain in PLWH include lower BMI, higher CD4+ T-cell count, and lower HIV RNA at diagnosis [12–15].
Data from observational studies and randomized trials suggest that regimens containing an integrase strand-transfer inhibitor (InSTI) are associated with greater weight gain than other regimens [19]. In a pooled analysis of eight randomized controlled trials [20], mean weight gain after 96 weeks was higher with InSTI-based regimens than with protease inhibitor or nonnucleoside reverse transcriptase inhibitor (NNRTI)-based regimens, and with tenofovir alafenamide/emtricitabine (FTC) compared with other NRTI pairs, in a population that was predominantly male (88%) and white (62%). Similar patterns have been observed in women [21] and in black PLWH [22,23]. The mechanism for this association is currently unknown; proposed explanations include improved gastrointestinal tolerability, faster virologic control, interference with the melanocortin 4 receptor [24], and promotion of adipocyte hypertrophy, fibrosis, and insulin resistance [25].
Doravirine (DOR) is a next-generation NNRTI for the treatment of HIV-1, available as a single entity and as a fixed combination with lamivudine and tenofovir disoproxil fumarate (DOR/3TC/TDF). Clinical trials in treatment-naïve adults have shown that DOR-based regimens have noninferior efficacy and a superior lipid profile compared with ritonavir-boosted darunavir (DRV+r) [26] and efavirenz (EFV)-based regimens [27], as well as significantly fewer neuropsychiatric events than EFV-based regimens [27,28]. In adults with HIV-1 who switched from a stable ART regimen, DOR/3TC/TDF maintained virologic suppression for 48 weeks and demonstrated a favorable safety and tolerability profile, which included improved lipid profiles compared with continuation of a boosted protease inhibitor regimen [29]. Using data from three randomized controlled trials of DOR, we conducted a post-hoc analysis of changes in weight and BMI in treatment-naive adults with HIV-1 who received combination therapy with two NRTIs and either DOR, DRV+r, or EFV for 96 weeks.
Methods
Data were pooled from three double-blind, randomized controlled trials of DOR in treatment-naive adults with HIV-1: MK-1439A Protocol 007 (P007; NCT01632345), DRIVE-FORWARD (NCT02275780), and DRIVE-AHEAD (NCT02403674). P007 [28] was a phase 2b dose-ranging study of DOR 25–200 mg and EFV 600 mg, each given in combination with FTC/TDF. DRIVE-FORWARD [26] is a phase 3 noninferiority trial comparing DOR and DRV+r, each given in combination with 2 NRTIs, and DRIVE-AHEAD [27] is a phase 3 noninferiority trial comparing DOR/3TC/TDF and EFV/FTC/TDF. The 96-week blinded phase of both phase 3 trials has completed, and the open-label extension phases are ongoing. All three trials were conducted in compliance with Good Clinical Practice requirements and applicable statutes and regulations regarding ethical committee review, informed consent, and the protection of humans participating in biomedical research.
Double-blind data through week 96 of each trial were combined by treatment group. The combined DOR group included participants who received DOR 100 mg with two NRTIs in P007 or DRIVE-FORWARD and those who received DOR/3TC/TDF in DRIVE-AHEAD. The DRV+r group included participants who received DRV+r with two NRTIs in DRIVE-FORWARD, and the combined EFV group included participants who received EFV 600 mg with two NRTIs in P007 or EFV/FTC/TDF in DRIVE-AHEAD. All participants who received at least one dose of study medication were included in the analyses.
Statistical analysis
The endpoints of interest were the change in weight from baseline, the proportion of participants with at least 10% weight gain, and the proportion of participants with an increase in BMI class. The change in weight from baseline was calculated as the mean change with 95% confidence interval (CI), and as the median change with interquartile range, at weeks 48 and 96 for each treatment group.
For the proportion of participants with at least 10% weight gain, the treatment groups were compared using generalized linear models with binomial distribution and identity link. Explanatory variables included in the model were treatment group, region, sex, indicator for race (black/nonblack), interaction terms of treatment by sex and by race indicator, and baseline values of age, weight, BMI, log10 CD4+ T-cell count, and log10 HIV-1 RNA. To ensure that the estimates for treatment effects and treatment differences reflected the study population, observed marginal proportions for region, sex, and black/nonblack were used in the model.
BMI categories were defined as follows: underweight, BMI less than 18.5; normal weight, BMI at least 18.5 to less than 25; overweight, BMI at least 25 to less than 30; and obese, BMI at least 30. An increase in BMI class was defined as a change from underweight or normal at baseline to overweight or obese at week 48 or 96, or a change from overweight at baseline to obese at week 48 or 96. A generalized linear model with binomial distribution and identity link was used to estimate the proportions in each treatment group with BMI class increase and the differences between DOR and the comparators, adjusting for region, sex, race group, and baseline values of age, BMI, log10 CD4+ T-cell counts, and log10 HIV-1 RNA. Three race groups with small numbers of participants (American Indian or Alaska Native, Multiracial, and Native Hawaiian or Pacific Islander) were combined for model feasibility.
We also examined the relationship between weight gain status and the following metabolic endpoints: change from baseline in total cholesterol (TC), LDL cholesterol (LDL-C), HDL cholesterol, and triglycerides; development of hypertension or diabetes; and new use of antihypertensive, antidiabetic, or lipid-lowering medication. Information regarding new diagnosis of hypertension or diabetes was not specifically collected in the DOR trials; for this analysis, these ‘new diagnoses’ were identified by reviewing the adverse events spontaneously reported by the participants, which may or may not reflect a true medical diagnosis. Antihypertensive medications included angiotensin-converting enzyme inhibitors, angiotensin II antagonists, beta-blockers, calcium channel blockers, and diuretics; these medications are sometimes used to treat conditions other than hypertension.
Participants were classified into two weight-gain groups according to their percentage weight gain at week 96; the high-gain group included participants with at least 10% weight gain, and the low-gain group included those with less than 10% weight gain. Changes from baseline in lipid endpoints, the proportions of participants with new hypertension or diabetes, and the proportions with new medication use were summarized by weight-gain group within each treatment group. Realizing that the observed differences between the two weight-gain groups could be confounded by differences in their baseline characteristics, we further analyzed the data adjusting for baseline variables.
The change from baseline in lipid endpoints was analyzed using a repeated measure mixed model, and the proportion of participants with new medication use was analyzed using generalized linear models with binomial distribution and identity link. The explanatory variables included treatment group, weight-gain group, their interaction, and other significant variables selected based on their statistical significance in models with these variables individually or in combination, depending on the endpoint being analyzed. The potential explanatory variables to enter the models were age, sex, black or African-American race, baseline weight, baseline CD4+ cell counts, and log of baseline viral load.
Results
A total of 1710 participants were included in the pooled analyses: 855 received DOR, 383 received DRV+r, and 472 received EFV. The study population was 85% male, 63% white, and 20% black or African-American. At baseline, the majority of participants (55.8%) were in the normal weight category (BMI ≥ 18.5–<25), 3.9% were underweight (BMI < 18.5), 29.1% were overweight (BMI ≥ 25–<30), and 11.2% were obese (BMI ≥ 30) (refer to Table, Supplemental Digital Content 1, for additional participant characteristics).
Change in body weight
At week 48, mean weight gain was similar in the DOR group [1.7 kg, 95% CI (1.4, 2.1)] and the DRV+r group [1.4 kg (0.6, 2.1)] and slightly lower in the EFV group [0.6 kg (−0.1, 1.2)]. Greater weight gains were observed in all treatment groups at week 96, and the mean change was similar across the groups [2.4 (1.9, 2.8), 1.8 (0.8, 2.7), and 1.6 (0.9, 2.3) kg, respectively]. Median weight gains were consistently lower than the mean values at week 48: DOR 1.0 kg (interquartile range: −1.2, 3.9), DRV+r 0.6 kg (−1.9, 3.4), and EFV 0.0 kg (−2.6, 2.8); and at week 96: DOR 1.5 kg (−1.0, 4.9), DRV+r 0.7 kg (−1.9, 5.1), and EFV 1.0 kg (−2.2, 4.6).
The majority of participants in each treatment group experienced less than 5% weight gain (Fig. 1). The proportion of participants with at least 5% weight gain at week 48 was similar in the DOR and DRV+r groups (26.5 and 23.1%, respectively) and was lower in the EFV group (20.6%); by week 96 the proportion with at least 5% weight gain was similar across all treatment groups (DOR 31.8%, DRV+r 32.8%, and EFV 32.0%). Weight gain of 10% or more was observed in 8–10% of participants at week 48 and in 14–16% of participants at week 96 (Fig. 1) and was not significantly different between the treatment groups at either time point after adjusting for baseline factors (Fig. 2).
Fig. 1.
Distribution of participants by percentage change in weight (<5, ≥5 to <10, ≥10%).
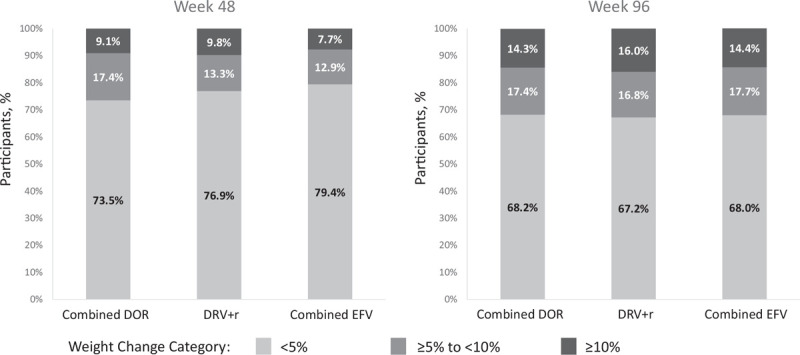
DOR, doravirine; DRV+r, ritonavir-boosted darunavir; EFV, efavirenz.
Fig. 2.
Proportion of participants with at least 10% weight gain.
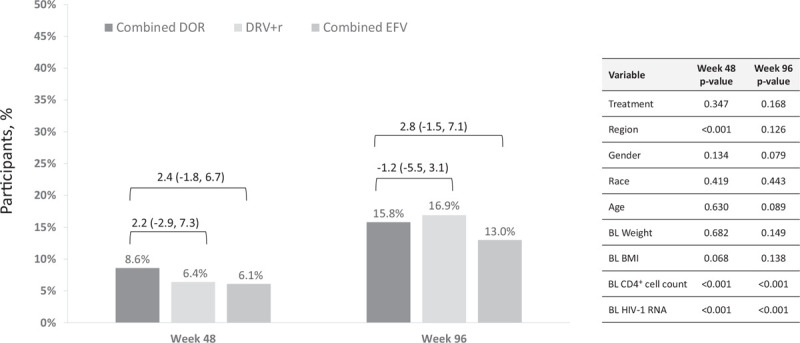
Estimated proportions and 95% confidence interval for treatment group differences (doravirine minus comparator) are from the generalized linear model. DOR, doravirine; DRV+r, ritonavir-boosted darunavir; EFV, efavirenz.
Change in BMI
The majority of participants in each treatment group remained in their baseline BMI class at weeks 48 and 96 (Table 1). Among participants who were not obese at baseline, the proportion who were overweight increased over time in all treatment groups (Fig. 3), and a small proportion became obese by week 96 (DOR 7%, DRV+r 6%, and EFV 5%). The proportion of participants whose BMI class increased to overweight or obese was not significantly different between the DOR group and either of the comparator groups at week 48 or 96 (Fig. 4).
Table 1.
Shift in BMI class at weeks 48 and 96 by baseline BMI category and treatment group.
| Study week | Baseline BMI group | Study week BMI group | Combined DOR, n (%) | Main DRV+r, n (%) | Combined EFV, n (%) |
| Week 48 | Underweight | Total | 26 | 13 | 20 |
| Underweight | 16 (61.5) | 8 (61.5) | 14 (70.0) | ||
| Normal | 9 (34.6) | 4 (30.8) | 5 (25.0) | ||
| Overweight | 0 | 1 (7.7) | 1 (5.0) | ||
| Obese | 1 (3.8) | 0 | 0 | ||
| Normal | Total | 412 | 168 | 231 | |
| Underweight | 13 (3.2) | 1 (0.6) | 8 (3.5) | ||
| Normal | 348 (84.5) | 141 (83.9) | 201 (87.0) | ||
| Overweight | 48 (11.7) | 24 (14.3) | 21 (9.1) | ||
| Obese | 3 (0.7) | 2 (1.2) | 1 (0.4) | ||
| Overweight | Total | 229 | 102 | 111 | |
| Underweight | 0 | 0 | 1 (0.9) | ||
| Normal | 14 (6.1) | 15 (14.7) | 21 (18.9) | ||
| Overweight | 183 (79.9) | 79 (77.5) | 79 (71.2) | ||
| Obese | 32 (14.0) | 8 (7.8) | 10 (9.0) | ||
| Obese | Total | 84 | 33 | 40 | |
| Overweight | 9 (10.7) | 5 (15.2) | 6 (15.0) | ||
| Obese | 75 (89.3) | 28 (84.8) | 34 (85.0) | ||
| Week 96 | Underweight | Total | 24 | 11 | 19 |
| Underweight | 13 (54.2) | 7 (63.6) | 8 (42.1) | ||
| Normal | 10 (41.7) | 4 (36.4) | 10 (52.6) | ||
| Obese | 1 (4.2) | 0 | 1 (5.3) | ||
| Normal | Total | 377 | 140 | 211 | |
| Underweight | 7 (1.9) | 4 (2.9) | 3 (1.4) | ||
| Normal | 301 (79.8) | 108 (77.1) | 172 (81.5) | ||
| Overweight | 65 (17.2) | 26 (18.6) | 35 (16.6) | ||
| Obese | 4 (1.1) | 2 (1.4) | 1 (0.5) | ||
| Overweight | Total | 200 | 91 | 93 | |
| Normal | 21 (10.5) | 10 (11.0) | 15 (16.1) | ||
| Overweight | 140 (70.0) | 68 (74.7) | 65 (69.9) | ||
| Obese | 39 (19.5) | 13 (14.3) | 13 (14.0) | ||
| Obese | Total | 76 | 26 | 39 | |
| Normal | 0 | 1 (3.8) | 0 | ||
| Overweight | 8 (10.5) | 9 (34.6) | 3 (7.7) | ||
| Obese | 68 (89.5) | 16 (61.5) | 36 (92.3) |
BMI groups: underweight is less than 18.5, normal is at least 18.5 to less than 25, overweight is at least 25 to less than 30, obese is at least 30. Values in bold are the proportion of participants whose BMI on treatment remained in the baseline BMI category. DOR, doravirine; DRV+r, ritonavir-boosted darunavir; EFV, efavirenz.
Fig. 3.
Overall change in BMI class through week 96 (obese at baseline excluded).
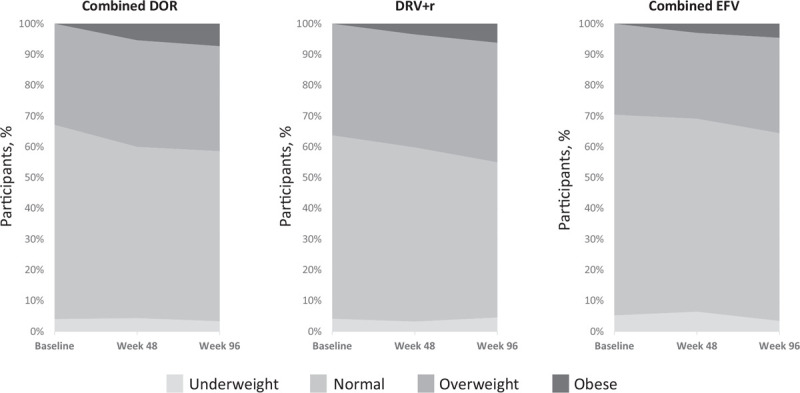
DOR, doravirine; DRV+r, ritonavir-boosted darunavir; EFV, efavirenz.
Fig. 4.
Proportion of participants with BMI class increase to overweight or obese.
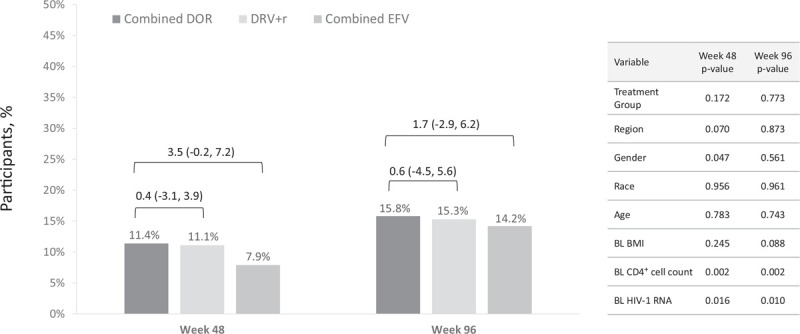
Estimated proportions and 95% confidence interval for treatment group differences (doravirine minus comparator) are from the generalized linear model. DOR, doravirine; DRV+r, ritonavir-boosted darunavir; EFV, efavirenz.
Risk factors for weight gain and BMI class increase
Low baseline CD4+ T-cell count and high baseline HIV-1 RNA were significant factors for at least 10% weight gain at weeks 48 and 96 (all P < 0.001) and for BMI class increase at week 48 (P = 0.002 and 0.016, respectively) and week 96 (P = 0.002 and 0.010, respectively). We did not find significant associations between treatment group, demographic characteristics (sex, race, age), or other baseline characteristics (weight, BMI, region) and at least 10% weight gain or BMI class increase in the multivariate model. However, mean weight gain was numerically higher in women than in men, and in black participants compared with nonblack participants, in all treatment groups at both time points (refer to Table, Supplemental Digital Content 2). Similarly, the proportion of participants with weight gain at least 10% was numerically higher in women than in men in all treatment groups at both time points, and in black participants compared with nonblack participants except in the DRV+r group at week 96 (refer to Table, Supplemental Digital Content 3).
Metabolic changes associated with weight gain
In all treatment groups, participants with high weight gain had larger mean increases in TC and LDL-C compared with those with low weight gain (refer to Figure, Supplemental Digital Content 4), although the 95% CIs overlapped in most cases. The mean changes in triglycerides followed a similar pattern in the DOR and EFV groups. New use of lipid-lowering therapy was generally low and similar between participants with high weight gain and those with low weight gain (refer to Table, Supplemental Digital Content 5). In all treatment groups, new diagnosis of hypertension and new use of antihypertensive medication were more common in participants with high weight gain than in those with low weight gain, even after adjusting for statistically significant variables of age, black race, and baseline weight, although the 95% CIs for the differences were wide and included zero (refer to Table, Supplemental Digital Content 6). New diagnosis of diabetes and new use of diabetes medication were low and similar between participants with high weight gain and those with low weight gain in all treatment groups (refer to Table, Supplemental Digital Content 7).
Discussion
The current post-hoc analysis of three randomized controlled trials is the first examination of weight changes during treatment with DOR, a next-generation NNRTI first approved in 2018 for the treatment of HIV-1. In this analysis, ART-naive participants who received DOR, DRV+r, or EFV-based regimens experienced weight gains that were similar to the average yearly change of 0.5–1.0 kg in adults without HIV-1 [1]: mean weight gain after ∼2 years of treatment was 2.4 kg in the DOR group, 1.8 kg in the DRV+r group, and 1.6 kg in the EFV group. Significant weight gain (≥10%) occurred in 15% of participants overall by week 96 and was not significantly different between the DOR, DRV+r, and EFV groups (16, 17, and 13%, respectively). The proportion of participants whose BMI class increased also was similar across the treatment groups (16, 15, and 14%, respectively).
Our results are consistent with a recent meta-analysis of 8 clinical trials that found weight gains of similar magnitude after 96 weeks of treatment with an NNRTI-based regimen [1.9 kg (95% CI 1.6–2.3)] or a boosted atazanavir regimen [1.7 kg (95% CI 1.0–2.4)] [20]. In a retrospective cohort study of 1152 ART-naive PLWH, weight gain after 18 months of treatment also was comparable for regimens containing an NNRTI (EFV or rilpivirine) [2.6 kg (95% CI 1.5–3.6)] and those containing a boosted protease inhibitor (DRV or atazanavir) [4.1 kg (95% CI 3.2–5.0)] [30]. In contrast, other studies have found higher weight gain with protease inhibitor-based regimens than with NNRTI-based regimens: a post hoc exploratory analysis of ACTG study A5224 s found significantly greater weight gain with boosted atazanavir than with EFV (mean difference 3.4 kg, P = 0.02) after 96 weeks of treatment [31], and the Swiss HIV Cohort Study found a larger BMI increase with atazanavir/ritonavir compared with EFV (P = 0.03) after 4 years of treatment [12]. In a recent observational study of 22,972 ART-naive adults who initiated treatment during 2007–2016, those starting an NNRTI-based regimen had lower 2-year mean weight gain (3.1 kg) compared with InSTI and protease inhibitor-based regimens (each 4.9 kg) [32]. Since the DOR trials did not include an InSTI-based regimen as a comparator, it is not clear how the weight changes we observed with DOR compare with those recently reported for InSTI-based regimens [20–23,30,32].
We found that indicators of more severe disease (low CD4+ T-cell count and high HIV-1 RNA at baseline) were significant factors for at least 10% weight gain and BMI class increase, consistent with results from other randomized controlled trials [20] as well as observational cohort studies [6,13,15,16], and with the return-to-health effect of HIV treatment. However, we did not find an association between baseline BMI and at least 10% weight gain or BMI class increase.
In our subgroup analyses, mean weight gain tended to be higher in women and in black participants, consistent with other reports [17,20–23]. However, sex and race were not consistently identified as significant factors for at least 10% weight gain or BMI class increase in the multivariate analyses. The low proportion of women (15%) and black participants (20%) in this study may have limited the ability to detect significant associations for these variables.
Being overweight or obese may increase the risk of metabolic and cardiovascular disorders (CVD) in PLWH [4,5], although the mechanism for these observations has not been elucidated. In our study population, participants with at least 10% weight gain appeared to be at higher risk for elevated cholesterol and LDL-C, as well as the development of hypertension and start of antihypertensive medication. Dyslipidemia and hypertension are among the traditional risk factors that contribute to increased risk of CVD in PLWH [33,34]. In addition, HIV infection is an independent risk factor for myocardial infarction and other CVD [35–37]; thus, maintaining normal weight may provide an important opportunity to avoid additional CVD risk in this population.
Our results should be interpreted with caution because these analyses were not prespecified in the original study plans. In addition, these analyses were not designed to evaluate potential mechanisms for weight gain in PLWH. Measurements of waist circumference and body fat distribution were not collected in the DOR trials, so we cannot determine whether the weight changes we observed were generalized or localized. Future clinical trials should include a more standardized approach to weight gain and related variables such as waist circumference, blood pressure, lipids, glucose, insulin, and hemoglobin A1C. In addition, longer follow-up is needed to understand the trajectory of weight and body composition changes during long-term ART and the impact on diabetes, cardiovascular disease, and nonalcoholic fatty liver disease in PLWH.
Conclusion
The current post-hoc analyses show that weight gain in treatment-naïve adults with HIV-1 was low and similar for DOR, DRV+r, and EFV-based regimens through 96 weeks of treatment, and that at least 10% weight gain was associated with low CD4+ T-cell count and high HIV-1 RNA at baseline and with an increased risk of hypertension and elevated plasma lipid levels at week 96. For patients who experience clinically meaningful weight gain on ART, studies evaluating switch strategies are needed to assist with HIV treatment selection. A post-hoc analysis of weight change after switching from a stable ART regimen to DOR/3TC/TDF in the DRIVE-SHIFT trial is currently ongoing.
Acknowledgements
Principal contributions of the authors: C.O. R.E., M.T., J.K.R.: data collection, interpretation of results; Z.J.X.: designed and conducted the statistical analyses; F.A.B., C.H., P.S., E.A.M.: study design, interpretation of results. All authors contributed to review and revision of the article and approved the final version for submission.
We thank all of the study participants and the clinical trial investigators and staff for their contributions to the doravirine clinical program. Funding for this research was provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Medical writing and editorial support were provided by Kim M. Strohmaier and Carol Zecca, employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Data sharing statement: Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA (MSD) is committed to providing qualified scientific researchers access to anonymized patient level data and clinical study reports from the company's clinical trials for the purpose of conducting legitimate scientific research. The company is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The process includes submission of data requests to the MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php). Data will be made available for request after product approval in the US and EU or after product development is discontinued. There are circumstances that may prevent MSD from sharing the requested data.
Conflicts of interest
C.O reports personal fees from MSD during the conduct of the study, grants and personal fees from GSK; grants and personal fees from Gilead Sciences: grants and personal fees from ViiV. R.E. reports personal fees from Gilead Sciences; personal fees from ViiV. M.T. reports that her institution, AIDS Research Consortium of Atlanta, received clinical trial support from MSD during the conduct of the study; clinical trial support from Bristol Myers Squibb, Cepheid, Inc., Cytodyn, Inc., Frontier Bioscience, Inc., Gilead Sciences, Inc., Glaxo Smith Kline, Taimed, Inc., and ViiV Healthcare. J.K.R. reports personal fees from Abbvie, Gilead, Janssen, Merck, Theratechnologies, and ViiV. F.A.B., Z.J.X., C.H., P.S., and E.M. are current or former employees of Merck Sharp & Dohme Corp, a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA (MSD).
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
References
- 1.Hutfless S, Maruthur NM, Wilson RF, Gudzune KA, Brown R, Lau B, et al. Strategies to prevent weight gain among adults. Rockville, MD: Agency for Healthcare Research and Quality (US); 2013. [PubMed] [Google Scholar]
- 2.World Health Organization. Overweight and obesity, key facts. Available at: www.who.int/news-room/fact-sheets/detail/obesity-and-overweight [accessed January 2020]. [Google Scholar]
- 3.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity among adults and youth: United States, 2015–2016. Available at: https://www.cdc.gov/nchs/data/databriefs/db288.pdf [accessed Feb 2020]. [PubMed] [Google Scholar]
- 4.Haslam CM, James WP. Obesity. Lancet 2005; 366:1197–1209. [DOI] [PubMed] [Google Scholar]
- 5.Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology 2010; 51:679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuh B, Tate J, Butt AA, Crothers K, Freiberg M, Leaf D, et al. Weight change after antiretroviral therapy and mortality. Clin Infect Dis 2015; 60:1852–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Achhra AC, Mocroft A, Reiss P, Sabin C, Ryom L, de Wit S, et al. Short-term weight gain after antiretroviral therapy initiation and subsequent risk of cardiovascular disease and diabetes: the D:A:D study. HIV Med 2016; 17:255–268. [DOI] [PubMed] [Google Scholar]
- 8.Kumar S, Samaras K. The impact of weight gain during HIV treatment on risk of prediabetes, diabetes mellitus, cardiovascular disease, and mortality. Front Endocrinol (Lausanne) 2018; 9:705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohr R, Boesecke C, Dold L, Schierwagen R, Schwarze-Zander C, Wasmuth JC, et al. Return-to-health effect of modern combined antiretroviral therapy potentially predisposes HIV patients to hepatic steatosis. Medicine (Baltimore) 2018; 97:e0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lemoine M, Lacombe K, Bastard JP, Sébire M, Fonquernie L, Valin N, et al. Metabolic syndrome and obesity are the cornerstones of liver fibrosis in HIV-monoinfected patients. AIDS 2017; 31:1955–1964. [DOI] [PubMed] [Google Scholar]
- 11.Koethe JR, Jenkins CA, Lau B, Shepherd BE, Justice AC, Tate JP, et al. Rising obesity prevalence and weight gain among adults starting antiretroviral therapy in the United States and Canada. AIDS Res Hum Retroviruses 2016; 32:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hasse B, Iff M, Ledergerber B, Calmy A, Schmid P, Hauser C, et al. Obesity trends and body mass index changes after starting antiretroviral treatment: the Swiss HIV Cohort Study. Open Forum Infect Dis 2014; 32:ofu040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tate T, Willig AL, Willig JH, Raper JL, Moneyham L, Kempf M-C, et al. HIV infection and obesity: where did all the wasting go?. Antivir Ther 2012; 17:1281–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crum-Cianflone N, Roediger MP, Eberly L, Headd M, Marconi V, Ganesan A, et al. Increasing rates of obesity among HIV-infected persons during the HIV epidemic. PLoS One 2010; 5:e10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bakal DR, Coelho LE, Luz PM, Clark JL, De Boni RB, Cardoso SW, et al. Obesity following ART initiation is common and influenced by both traditional and HIV-/ART-specific risk factors. J Antimicrob Chemother 2018; 73:2177–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lakey W, Yang LY, Yancy W, Chow SC, Hicks C. Short communication: from wasting to obesity: initial antiretroviral therapy and weight gain in HIV-infected persons. AIDS Res Hum Retroviruses 2013; 29:435–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bares SH, Smeaton LM, Xu A, Godfrey C, McComsey GA. HIV-infected women gain more weight than HIV-infected men following the initiation of antiretroviral therapy. J Womens Health (Larchmt) 2018; 27:1162–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson-Paul AM, Wei SC, Mattson CL, Robertson M, Hernandez-Romieu AC, Bell TK, Skarbinski J. Obesity among HIV-infected adults receiving medical care in the United States: data from the cross-sectional medical monitoring project and national health and nutrition examination survey. Medicine (Baltimore) 2015; 94:e1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill A, Waters L, Pozniak A. Are new antiretroviral treatments increasing the risks of clinical obesity?. J Virus Erad 2019; 5:41–43. [PMC free article] [PubMed] [Google Scholar]
- 20.Sax PE, Erlandson KM, Lake JE, Mccomsey GA, Orkin C, Esser S, et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis 2019; 71:1379–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerchberger AM, Sheth AN, Angert CD, Mehta CC, Summers NA, Ofotokun I, et al. Weight gain associated with integrase stand transfer inhibitor use in women. Clin Infect Dis 2019; 71:593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venter WDF, Moorhouse M, Sokhela S, Fairlie L, Mashabane N, Masenya M, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med 2019; 381:803–815. [DOI] [PubMed] [Google Scholar]
- 23.Kouanfack C, Mpoudi-Etame M, Omgba Bassega P, Eymard-Duvernay S, Leroy S, Boyer S, et al. NAMSAL ANRS 12313 Study Group. Dolutegravir-based or low-dose efavirenz-based regimen for the treatment of HIV-1. N Engl J Med 2019; 381:816–826. [DOI] [PubMed] [Google Scholar]
- 24.McMahon C, Trevaskis JL, Carter CC, Holsapple K, White K, Das M, et al. Lack of an association between clinical INSTI-related body weight gain and direct interference with MC4 receptor (MC4R), a key central regulator of body weight. PLoS One 2020; 15:e0229617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gorwood J, Bourgeois C, Pourcher V, Pourcher G, Charlotte F, Mantecon M, et al. The integrase inhibitors dolutegravir and raltegravir exert pro-adipogenic and profibrotic effects and induce insulin resistance in human/simian adipose tissue and human adipocytes. Clin Infect Dis 2020. ciaa259.doi: 10.1093/cid/ciaa259. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 26.Molina JM, Squires K, Sax PE, Cahn P, Lombaard J, DeJesus E, et al. Doravirine versus ritonavir-boosted darunavir in antiretroviral-naive adults with HIV-1 (DRIVE-FORWARD): 48-week results of a randomised, double-blind, phase 3, noninferiority trial. Lancet HIV 2018; 5:e211–e220. [DOI] [PubMed] [Google Scholar]
- 27.Orkin C, Squires KE, Molina JM, Sax PE, Wong W-W, Sussmann O, et al. Doravirine/lamivudine/tenofovir disoproxil fumarate is noninferior to efavirenz/emtricitabine/tenofovir disoproxil fumarate in treatment-naive adults with human immunodeficiency virus-1 infection: week 48 results of the DRIVE-AHEAD trial. Clin Infect Dis 2019; 68:535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gatell JM, Morales-Ramirez JO, Hagins DP, Thompson M, Arastéh K, Hoffmann C, et al. Doravirine dose selection and 96-week safety and efficacy versus efavirenz in antiretroviral therapy-naive adults with HIV-1 infection in a Phase IIb trial. Antivir Ther 2019; 24:425–435. [DOI] [PubMed] [Google Scholar]
- 29.Johnson M, Kumar P, Molina JM, Rizzardini G, Cahn P, Bickel M, et al. Switching to doravirine/lamivudine/tenofovir disoproxil fumarate (DOR/3TC/TDF) maintains HIV-1 virologic suppression through 48 weeks: results of the DRIVE-SHIFT trial. J Acquir Immune Defic Syndr 2019; 81:463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bourgi K, Rebeiro PF, Turner M, Castilho JL, Hulgan T, Raffanti SP, et al. Greater weight gain in treatment-naive persons starting dolutegravir-based antiretroviral therapy. Clin Infect Dis 2020; 70:1267–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erlandson KM, Kitch D, Tierney C, Sax PE, Daar ES, Tebas P, et al. Weight and lean body mass change with antiretroviral initiation and impact on bone mineral density. AIDS 2013; 27:2069–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bourgi K, Jenkins CA, Rebeiro PF, Palella F, Moore RD, Altoff KN, et al. Weight gain among treatment-naive persons with HIV starting integrase inhibitors compared to nonnucleoside reverse transcriptase inhibitors or protease inhibitors in a large observational cohort in the United States and Canada. J Int AIDS Soc 2020; 23:e25484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paisible AL, Chang CC, So-Armah KA, Butt AA, Leaf DA, Budoff M, et al. HIV infection, cardiovascular disease risk factor profile, and risk for acute myocardial infarction. J Acquir Immune Defic Syndr 2015; 68:209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007; 92:2506–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saves M, Chene G, Ducimetiere P, Leport C, Le Moal G, Amouyel P, et al. Risk factors for coronary heart disease in patients treated for human immunodeficiency virus infection compared with the general population. Clin Infect Dis 2003; 37:292–298. [DOI] [PubMed] [Google Scholar]
- 36.Kaplan RC, Kingsley LA, Sharrett AR, Li X, Lazar J, Tien PC, et al. Ten-year predicted coronary heart disease risk in HIV-infected men and women. Clin Infect Dis 2007; 45:1074–1081. [DOI] [PubMed] [Google Scholar]
- 37.Currier JS, Taylor A, Boyd F, Dezii CM, Kawabata H, Burtcel B, et al. Coronary heart disease in HIV-infected individuals. J Acquir Immune Defic Syndr 2003; 33:506–512. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


