Abstract
Background
Fruit drinks are the most commonly consumed sugar-sweetened beverage among young children. Fruit drinks carry many nutrition-related claims on the front of package (FOP). Nutrition-related claims affect individuals’ perceptions of the healthfulness of products and purchase intentions, often creating a “health halo” effect.
Objective
The aims of this study were to describe the prevalence of FOP nutrition-related claims on fruit drinks purchased by households with young children and to examine the association between claims and the nutritional profile of fruit drinks.
Design
The sample included 2059 fruit drinks purchased by households with children 0 to 5 years old participating in Nielsen Homescan in 2017. FOP labels were obtained from 2 databases that contain bar code–level information on all printed material on product labels. A codebook was used to code for presence of FOP nutrition-related claims. The coded claims data were linked by bar code with Nutrition Facts label data. Claim type prevalence was calculated, and the association between claim types and median calories and total grams of sugar per 100 mL was analyzed using Wilcoxon rank-sum tests. The percentages of products containing noncaloric sweeteners (NCSs) with and without each claim type were also calculated and compared.
Results
Almost all (97%) fruit drinks sampled had at least 1 nutrition-related FOP claim. Implied natural claims such as “natural flavors” were the most common (55% of products), followed by claims about the presence of juice or nectar (49%). Claims about vitamin C (33%), sugar (29%), and calories (23%) were also common. Fruit drinks with vitamin C, juice or nectar, fruit or fruit flavor, and overt natural claims were higher in calories and sugar and less likely to contain NCSs compared with products without these claims. Fruit drinks with calorie, sugar, NCS, implied natural, and other claims were lower in calories and sugar and more likely to contain NCSs compared with products without these claims.
Conclusions
Claims are prevalent on fruit drinks purchased by households with young children. This is concerning given prior research demonstrating that claims can mislead consumers. Regulatory actions such as requiring a warning or disclosure on drinks that contain added sugars or NCSs should be considered.
Keywords: Sugar-sweetened beverage, Content analysis, Marketing, Nutrition claims, Fruit drink
ON ANY GIVEN DAY, ALMOST 85% OF INFANTS AND toddlers consume added sugar, and sugar-sweetened beverages (SSBs) are the top source of added sugar in the diets of young children.1,2 Consuming amounts of added sugar in early life that exceed thresholds set by current dietary guidance can lead to diet-related chronic diseases such as insulin resistance, obesity, and dental caries.3 Fruit drinks are the most commonly consumed type of SSB among 0- to 5-year-olds.4 There are disparities in SSB and fruit drink consumption between racial/ethnic groups, with non-Hispanic Black children being more likely to consume fruit drinks than non-Hispanic White and Hispanic children.5,6 In addition to containing added sugar, some fruit drink products also contain noncaloric sweeteners (NCSs). Consumption of NCSs is not recommended during early childhood due to their potential to predispose children to sweet taste preferences and the unknown long-term health consequences associated with consumption.7 However, the presence of NCSs in the food supply is growing exponentially.8 Strategies intended to reduce fruit drink consumption among young children may improve diet quality and reduce the prevalence of diet-related chronic diseases.
“Nutrition-related claims”–a term developed for the purposes of this article–are statements about the nutritional content or ingredients of a product, health claims, or messages about overall product healthfulness. Nutrition-related claims are particularly prevalent on the front of package (FOP) of fruit drinks. Some fruit drink varieties carry 4 or more claims per package.9 Nutrition-related claims may mislead parents about the healthfulness of products and increase their purchase intentions.10-12 Parents of young children may be particularly susceptible to nutrition-related claims as they are often motivated to provide healthful foods and beverages to their children.13 Claims have been shown to increase the perceived healthfulness of products and reduce the amount of time consumers spend searching for additional (and more complete) nutritional information on the package such as the Nutrition Facts label.10,11,14 Claims can also create a “health halo” effect whereby consumers mistakenly generalize the benefit touted by a claim to the overall healthfulness of the product.15 Nutrition-related claims can also be considered misleading because products carrying claims may be high in one beneficial nutrient or low in one harmful nutrient, but may also contain high levels of other nutrients of concern such as saturated fat, sodium, or sugar.11,16-19
Policy changes are needed to reduce exposure to FOP nutrition-related claims. These changes could occur through voluntary shifts by the food industry, although this is unlikely, regulatory action by government agencies such as the Food and Drug Administration (FDA), or action by Congress. For example, the FDA is considering new regulations for the use of specific nutrition-related claims such as “healthy” and “natural” as part of its Nutrition Innovation Strategy.20 Additionally, advocates have urged FDA to bar claims on foods and beverages that are high in added sugar, require premarket approval for structure/function claims, and strengthen their fortification policy by disallowing fortification of SSBs.21 Understanding the prevalence of a variety of claims on fruit drinks purchased by households with young children, the nutrients or ingredients most predominantly marketed on the FOP, and how claims relate to the nutritional content of these products can inform future labeling policy and other public health stakeholder efforts such as educating parents and caregivers about how to navigate food labels or improving manufacturer marketing practices.
Prior studies in other food categories have found that nutrition-related claims are prevalent, do not serve as reliable indicators of product healthfulness, and may be misleading.16-18,22 However, key gaps in knowledge remain to inform policy, legal, or regulatory action to reduce potentially misleading claims. For example, some of these studies focused exclusively on only a subset of types of claims, such as low-content claims (eg, low calorie, low sodium).18 Others focused solely on products marketed toward children; however, parents and caregivers are the primary food shoppers in a given household, making it important to examine a wider variety of products.16,22 One report documented the prevalence of what they called ingredient, health-related, and real claims on a sample of fruit drinks marketed to children.22 More research is needed to document the presence of a wide range of nutrition-related claims on a large sample of a key intervention target for improving the diets of young children, fruit drinks. Also, comparing the nutritional profiles of fruit drinks with and without nutrition-related claims can provide information about whether claims are in fact used to make unhealthy products appear healthy.
This study aims to fill previous gaps in the literature by documenting a comprehensive list of all claims present on fruit drinks, the most commonly consumed SSB among young children. Thus, the objectives of this study were to describe the prevalence of nutrition-related claims on the FOP of fruit drinks and to examine the association between those claims and the nutritional profile of fruit drinks in terms of grams of total sugar per 100 mL, calories per 100 mL, and presence of NCS using a large sample of fruit drinks purchased by households in the United States with young children.
METHODS
Fruit Drink Sample and Label Data
To create the sample, first all fruit drinks purchased by US households participating in the Nielsen Homescan Consumer Panel23 in 2017 (the most recent data available) were identified. Nielsen Homescan is a longitudinal data set that contains product-level information on food and beverage purchases from more than 60,000 households in the United States across 76 geographical markets. Panel households are provided scanning equipment and report all packaged food transactions. The methodology for how these household purchase data are collected is described elsewhere.23 The sample was then restricted to fruit drinks purchased by Nielsen households that had a direct match at the bar code level to existing product-level Nutrition Facts label data that our research group maintains (described later) (n = 3909). The sample was then limited to fruit drinks purchased by Nielsen households with children 0 to 5 years old (n = 2230) (Figure 1).
Figure 1.
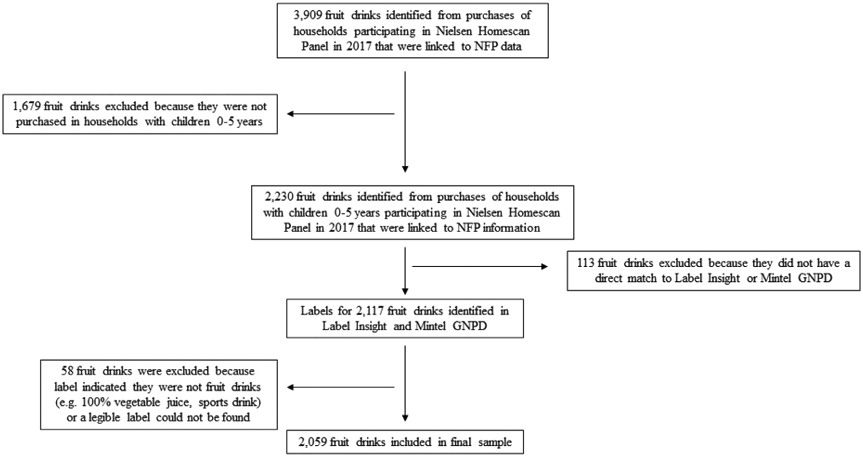
Flow chart detailing fruit drink product selection process from 2017 Nielsen Homescan Panel Data. GNPD = Global New Products Database; NFP = Nutrition Facts Panel.
Fruit drinks were defined as fruit-flavored juice cocktails, cordials, nectars, or other fruit-flavored drinks with added caloric sweeteners, NCSs, or both. Liquid ready-to-drink (RTD), frozen concentrate, liquid concentrate, and powder forms of fruit drinks were included. Fruit-flavored waters, sports drinks, energy drinks, and 100% juice were excluded from the sample. These exclusions are consistent with existing categorizations of fruit drinks used by national nutrition surveys.24,25
Finally, the sample was restricted to products that had a label in Label Insight or Mintel Global New Products Database (GNPD)26,27 (described later), which were used to obtain images of fruit drink package labels for coding (n = 2117). Fifty-eight drinks were excluded during coding because, upon viewing the label, it was determined the beverage did not meet inclusion criteria (eg, flavored milk, 100% vegetable juice) or because a legible photo of the label could not be identified. Out 2230 fruit drink products purchased by households with young children, 2059 were included in the final sample (Figure 1).
Photos of all fruit drink packages in the sample were obtained from Label Insight and Mintel GNPD for coding. The Label Insight database contains the ingredient list, brand names, and all other printed material on the entire label (eg, all types of added sweeteners and health or nutrition claims) and date stamp for when the data were collected. Mintel GNPD data captures nutrition, ingredient, label, and date-stamp information on new products entering the global market since 1996. Label Insight does not keep historical records of labels, so we were unable to find 60 products. In these cases, the manufacturer website or large retailer websites were searched for photos of the label using product information to find an acceptable substitute photo.
To examine whether the sample included all top-selling fruit drinks among United States households, brands in the sample were compared with 2017-2018 sales data from a global market research firm, Euromonitor International,28 for juice drinks (categorized by Euromonitor as containing 24% or less juice), nectars (25% or more juice), and concentrates. Thirty-two of the 34 top-selling national brands were included in the sample, with only 2 brands of liquid concentrates not included (Table 1, available at www.jandonline.org).
Coding and Categorization of Nutrition-Related Claims
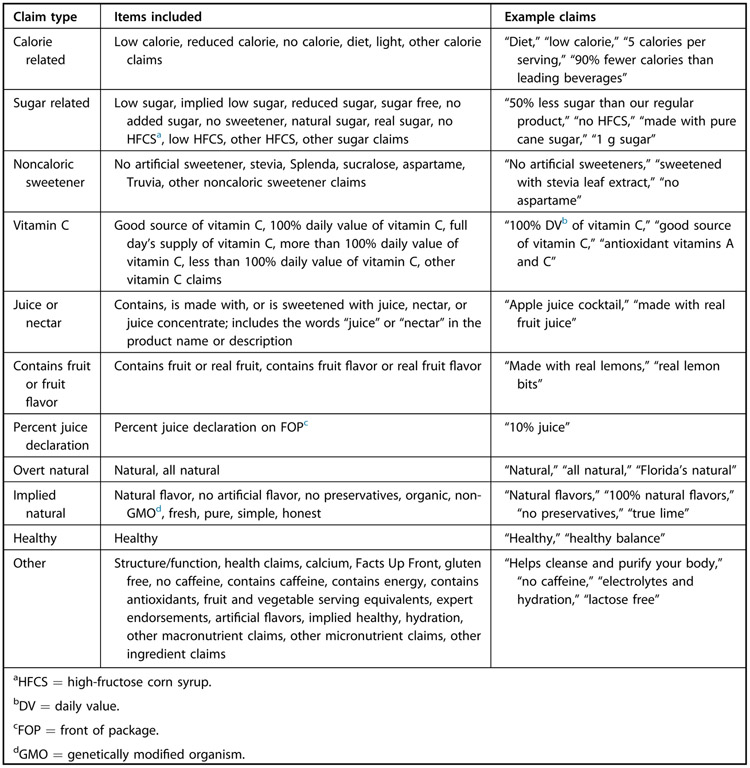
A codebook adapted from a study of FOP marketing on beverages was used to code for the presence or absence of various nutrition-related claims in any text on the product FOP including product names and brands.29 The codebook captured a wide variety of claim types, some of which are categories of claims currently defined and regulated by FDA and some of which are not, but FDA could or has proposed to regulate them in the future. The claim types included in the codebook that are currently regulated by FDA include nutrient-content claims (claims that directly or by implication characterize the level of a nutrient in the food), health claims (claims that directly or by implication characterize the relationship of any substance to a disease or health-related condition), and structure/function claims (claims that describe the effect that a substance has on the structure or function of the body and do not make reference to a disease).30 Products carrying nutrient-content claims must contain specific amounts defined by FDA of the nutrients listed in the claim.31 Health claims must be reviewed and approved by FDA prior to use on a product, and only specific health claims that meet FDA’s Significant Scientific Agreement standard may be used.32,33 By contrast, structure/function claims do not need FDA approval prior to use and do not need to be substantiated by evidence. However, FDA states structure/function claims must be truthful and not misleading.34,35 FDA also requires declarations of percent fruit juice on products resembling juice, but these need not appear on the FOP.30
Other claims that are not currently regulated by FDA were included in the codebook as well to inform future policy and regulatory efforts. These include claims such as “natural” or factual statements about ingredients (eg, “no high-fructose corn syrup”), which are only required to be truthful and not misleading. Because there were too many claim types captured in the codebook to present them individually, the claims captured by the codebook were then further condensed into the categories described in Figure 2 for the purposes of analysis. Claim categories for analysis were developed based on key nutrients or ingredients of public health interest, claim prevalence, and claim policy relevance. Detailed information on the prevalence of all claim types included in the codebook can be found in Table 2 (available at www.jandonline.org).
Figure 2.
Definitions and examples of claims found on fruit drinks’ front of package.
Two coders participated in a 5-day codebook training, which consisted of familiarizing themselves with the codebook, coding products with similar claim profiles (eg, fruit-flavored snacks), and making modifications to improve the clarity of codebook items. After the training, the 2 coders independently coded the same subsample of 15% of the products (n = 331) drawn at random from the full sample to assess interrater reliability. Percentage agreement for all items ranged from 87% to 100%. Gwet’s AC1 reliability coefficients36 were calculated using AgreeStat and found to be within an acceptable range across items (0.82-1.0). One item relating to the size of the statement of identity on fruit drink products was dropped because it had a reliability below 0.8. Using all other items, coders split the remaining 85% of the sample and coded their portions independently. The full codebook can be made available upon request.
Linking Claims Data to Nutrition Facts Label Information
Nutrition Facts label data from products in Nielsen Homescan have previously been collected at the bar code level from images of labels from Label Insight and Mintel GNPD by a team of trained nutritionists using a process described elsewhere.23 To understand the association between the presence of claims and each product’s nutritional profile, the coded claims data for each product were linked through each fruit drink’s bar code to this existing product-level Nutrition Facts label information. Nutrition Facts label information includes each product’s serving size, package size, calories per serving and per 100 mL, grams of total sugar per serving and per 100 mL, and ingredients list. Label Insight continuously updates their database with images of new labels from products and does not keep historical records. Therefore, to ensure the image we coded for claims matched the image previously used for Nutrition Facts label information, the post dates (the dates the images were uploaded to Label Insight and Mintel GNPD) for the label images used to code for claims and the label image used to collect Nutrition Facts label data were compared. If there was a difference greater than 3 months in the post dates, new Nutrition Facts label information was collected using the images of the labels from Label Insight and Mintel that were coded for claims because of possible reformulation over this period (n = 74 products). As described previously, Label Insight does not keep historical records, so substitute label photos were obtained for 60 fruit drink products from manufacturer or other retailer websites. For these 60 products, Nutrition Facts label information from the manufacturer website was used if it was available because this Nutrition Facts label information was a direct match with the label that was coded for claims. If Nutrition Facts label information was not available from the manufacturer, the Nutrition Facts label data from the existing Nutrition Facts label data set was used.
To calculate the nutritional content of as-consumed frozen and liquid concentrates and powders, reconstitution factors were created and applied. Unique reconstitution factors were created based on the preparation methods on the product packaging for powders with caloric sweeteners, powders with NCSs, powders with both caloric sweeteners and NCSs, frozen concentrates, liquid concentrates with NCSs, and liquid concentrates with caloric sweeteners due to differences in preparation methods for these products.
Data Analysis
Percentages were used to describe the composition of the sample in terms of how many of the fruit drinks were in liquid RTD, powder, or concentrate form with caloric sweeteners and/or NCSs. The average number of claims on the FOP was determined by calculating the number of claims on each fruit drink product (including all specific claim types listed in Table 2) summing those values, and dividing by the total number of fruit drink products. The average number of claims of the FOP of a variety of package sizes was calculated to determine if there was a relationship between size of the fruit juice package and presence of claims. The percentage of products with each claim type was calculated among all fruit drinks and among forms of fruit drinks (ie, liquid RTDs, powder, liquid and frozen concentrate). The sample was not weighted by household purchases for these analyses, because it is important to characterize and monitor claims and other attributes of key food and beverage categories of the food supply as it is available.29,37-39 Cross-tabulations were used to determine the most common combinations of claim types present on fruit drinks; however, these results are not presented because they did not provide any novel information beyond what is already presented. To understand whether claims were associated with more favorable nutrition profiles, the relationship between presence or absence of each claim type and calories per 100 mL as well as total grams of sugar per 100 mL was examined using Wilcoxon rank-sum tests. A P value of <.047 was considered statistically significant based on the Benjamini-Hochberg procedure to adjust for multiple comparisons.40 Statistical tests were not conducted when there were fewer than 30 observations in one or both categories. The proportion of products with and without each nutrition-related claim that contained NCSs was also calculated and compared using a 2-sample test of proportions. All statistical analyses were conducted using STATA version 16 (StataCorp, June 2019). This study was deemed nonhuman subjects research by University of North Carolina at Chapel Hill Institutional Review Board.
RESULTS
General Sample Characteristics
The majority (76%) of the 2059 fruit drinks were in liquid RTD form, 18% were powders, and 6% were either frozen or liquid concentrates (Table 3, available at www.jandonline.org). Roughly half (52%) of the fruit drink products contained added caloric sweeteners, 21% contained NCSs, and 27% contained both caloric sweeteners and NCSs (Table 3, available at www.jandonline.org). Only 23% of all products had fruit or juice as 1 of the first 2 ingredients in the ingredients list (Table 3, available at www.jandonline.org).
Prevalence of Nutrition-Related Claims
Almost all (97%) of the fruit drink products purchased by households with young children had at least 1 FOP nutrition-related claim (Figure 3), with an average of 3.6 claims (range 0-16). The average number of claims did not differ considerably based on package size, with the exception of products with a volume less than 100 mL. These products had an average of 4.8 FOP claims compared with 3.0 to 3.3 FOP claims on products greater than 100 mL (Table 4, available at www.jandonline.org). This difference can be attributed to powdered fruit drinks, which are smaller in size and have more claims on average than liquid RTD fruit drinks or frozen or liquid concentrates. About one-quarter (29%) of all products had at least 1 nutrient-content claim (eg, “low calorie,” “high in vitamin C”) (Table 2, available at www.jandonline.org). At least 1 factual ingredient claim such as “contains vitamin C” or “no high-fructose corn syrup” was present on 55% of fruit drinks (Table 2, available at www.jandonline.org).
Figure 3.
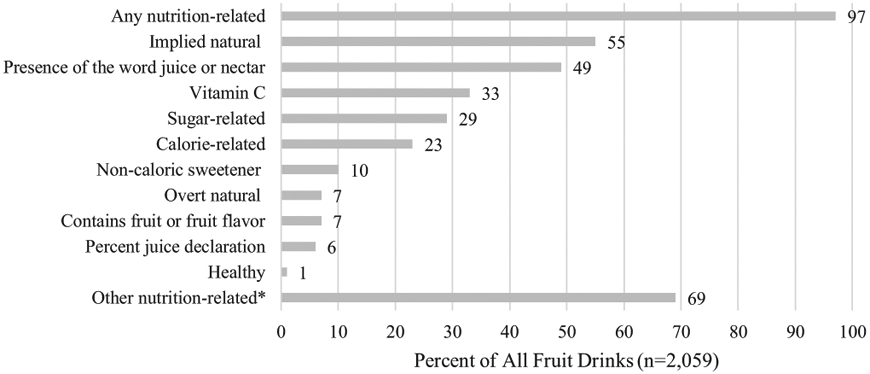
Percent of all fruit drink products purchased by Nielsen Homescan Households with children 0 to 5 years old in 2017 with at least 1 nutrition-related claim on the front of package by claim type. *Other nutrition-related claims include structure/function claims; health claims; claims regarding calcium; Facts Up Front; gluten free claims; no caffeine claims; claims regarding containing caffeine, energy, antioxidants; fruit and vegetable serving equivalent claims; expert endorsements; claims regarding artificial flavors; claims implying healthy; claims regarding hydration; other macronutrient, micronutrient, or ingredient claims.
Implied natural claims such as “natural flavors,” “organic,” or “no preservatives” were present on 55% of products, making them the most common type of claim on fruit drinks. The most prevalent type of implied natural claim was “natural flavors,” present on 41% of products. Overt natural claims that include uses of the word “natural” alone or the phrase “all natural” were less common, present on only 7% of products (Figure 3).
Claims or text about the presence of juice or nectar in fruit drinks, such as “apple juice cocktail,” were the second most common category, present on 49% of fruit drinks. Juice claims were less common on powders and concentrates compared with liquid RTD fruit drinks (Table 5, available at www.jandonline.org). Only 6% of fruit drinks had a percent juice declaration on the FOP. Claims about vitamin C content such as “100% DV of vitamin C” or “good source of vitamin C” were found on 33% of products (Figure 3). About one-quarter of these vitamin C claims were nutrient-content claims, and the remainder were factual ingredient claims (Table 2, available at www.jandonline.org).
Calorie- and sugar-related claims were present on 23% and 29% of fruit drinks, respectively (Figure 3). Two types of nutrient-content claims, reduced or low calorie claims and “light” or “diet” claims, were the most common type of calorie-related claims, followed by factual claims about calorie content such as “5 calories per serving” (Table 2, available at www.jandonline.org). The most common sugar-related claim was “sugar free,” followed by a type of nutrient-content claim, reduced sugar claims, and claims about having “no high-fructose corn syrup” (Table 2, available at www.jandonline.org). Claims about the absence of sugar such as “sugar free” were more common than claims about presence of sugar such as “real sugar” (Table 5, available at www.jandonline.org). Overall, powdered forms of fruit drinks had a greater proportion of sugar- and calorie-related FOP claims than liquid RTD fruit drinks or liquid or frozen concentrates (Table 5, available at www.jandonline.org).
NCS claims were present on 10% of fruit drink products in the sample. The most common NCS claim was “no artificial sweeteners” (Table 2, available at www.jandonline.org). Thirty-two percent of products with the “no artificial sweeteners” claim contained caloric sweeteners and 68% contained NCSs such as stevia (data not shown). Claims about the presence of NCSs (eg, “contains Splenda”), were essentially equally as prevalent as claims about the absence of any artificial sweeteners or of specific types of NCSs (Table 5, available at www.jandonline.org). NCS claims were more common on powders than on concentrates or liquid RTD fruit drinks (Table 5, available at www.jandonline.org).
Use of the word “healthy” on the FOP of fruit drinks was rare; only 1% of products in the sample contained these claims. No health claims as defined by FDA were found on fruit drinks in the sample. Very few products (1%) had structure/function claims as defined by FDA on the FOP. More detailed information on the prevalence of specific claim categories can be found in Table 2 (available at www.jandonline.org). The claim category, other nutrition-related claims, was very heterogeneous and the prevalence for each specific claim type included in this category (eg, no caffeine, energy, hydration) was low.
Presence of Claims and Nutritional Profile
The findings presented in Figure 4 suggest that products with a given claim type (eg, calorie-related) are different from products without that specific claim type in terms of calorie and total sugar content per 100 mL as well as presence of NCSs. There appears to be 2 broad patterns of how claim types are associated with nutritional profile. Fruit drinks with some claim types (vitamin C, juice or nectar, fruit or fruit flavor, overt natural) are higher in calories and grams of total sugar per 100 mL than products without these claims (Figure 4). Products with these claim types are also less likely to contain NCSs than products without those claims (Figure 4). Fruit drinks with other claim types (calorie-related, sugar-related, NCS, implied natural, other nutrition-related) are lower in calories and grams of total sugar per 100 mL (Figure 4) than products without these claims. Products with these claim types are also more likely to contain NCSs than products without these claims (Figure 4).
Figure 4.
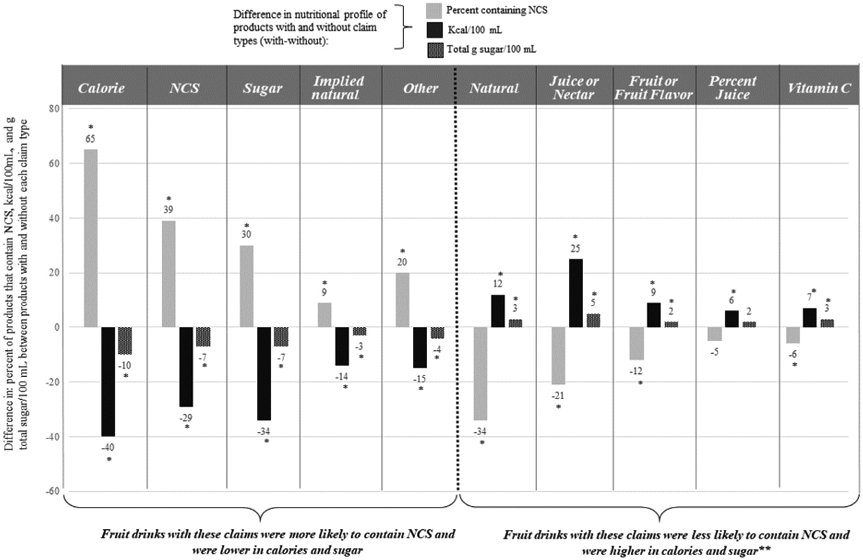
Differences in nutritional profile between fruit drinks with and without specific nutrition-related claims. NCS = noncaloric sweetener. *Statistically significantly different at P < .047. **Products with a percent juice disclosure trended toward significance but did not have a statistically significantly higher amount of total sugar than products without this claim or a significantly lower proportion of products with NCS. The claim type “healthy” was not included due to sample size.
DISCUSSION
A wide range of nutrition-related claims on a large sample of fruit drinks, an important contributor to SSB intake in children, were systematically examined. The findings suggest that nutrition-related claims are prevalent on fruit drinks purchased by households with young children. Based on current dietary recommendations, none of the products in the sample would be considered part of a healthy diet for young children because they either contain added sugars or NCSs, yet 97% of products had a nutrition-related claim. These claims may be contributing to confusion and excess consumption of fruit drinks because previous studies have generally demonstrated health and nutrition claims increase perceived product healthfulness and may increase purchase intentions,12,14,41-45 particularly among parents.
The most common categories of claims on fruit drinks were implied natural claims, text about juice or nectar, sugar, calorie, and vitamin C claims. The high prevalence of implied natural claims is perhaps not surprising, since “clean labels”– labels implying that products are minimally processed or free of additives–have become increasingly popular over the last decade.46 These claims have been shown to elicit an optimism bias or health halo effect in consumers where a positive perception of an ingredient is extended to a positive assessment of the entire food or product.15,46 For example, if a single ingredient is labeled as “natural” (eg, “natural flavor”), then the consumer may generalize that claim to the entire product.46 More generally, the word “natural” can improve consumers’ perceptions of a product’s taste, healthfulness, and environmental sustainability.46 The FDA currently does not define or regulate the use of the claim “natural” but has indicated a willingness to do so.20 However, the phrase “natural flavors” may not be subject to these new regulations and may continue to create confusion among consumers.47 This is particularly problematic in light of the fact that “natural flavors” was the most common implied natural claim found on fruit drinks.
The presence of the word juice on the FOP of fruit drinks was the second most common claim type. Juice was often found in the fruit drink product’s name, such as grape juice cocktail.30 This is not a claim by FDA’s definitions, rather it is defined by FDA as the product’s statement of identity, but this text has the potential to create confusion and a health halo effect among consumers in similar ways as other nutrition-related claims. For example, 60% of products that contained the word “juice” or “nectar” on the FOP did not have juice or fruit as 1 of the first 2 ingredients in the ingredient list. This may, in part, be due to the exclusion of 100% juice products. Additionally, only 11% of fruit drinks that contained the words “juice” or “fruit” also contained a percent juice disclosure on the FOP. The impact of juice claims or text on perceptions or purchases has not been examined to our knowledge. Given the potential of this text to confuse or mislead consumers and the prevalence of this claim type, future studies should examine the impact of juice claims on parental perceptions of healthfulness. Additionally, FDA should consider strengthening its existing regulations about percent juice disclosures on products that purport to contain fruit.30
Vitamin C claims including “100% DV [daily value] of vitamin C” or “good source of vitamin C” were present on approximately one-third of fruit drink labels. A recent publication showed, in 3 experiments, that a “100% vitamin C” claim on a fruit drink led to greater perceived product healthfulness and interest in consuming the product.48 Notably, products with a vitamin C claim in this sample had a median of 10.1 g of total sugar per 100 mL. The FDA should consider strengthening their fortification policy by banning fortification of SSBs with vitamin C or other vitamins. Additionally, the fortification of fruit drinks with vitamin C and use of vitamin C claims on fruit drinks, particularly on products targeted toward children, has little public health rationale, because fewer than 5% of young children consume less than the recommended amount of vitamin C.2
Sugar-related and calorie-related claims were present on approximately one-quarter of all fruit drink FOPs. Sugar-related claims included claims that were presence-framed (eg, “real cane sugar”) and absence-framed (eg, “less sugar than other leading juice drinks”). Studies generally demonstrate that for ingredients considered to be harmful, absence-framed claims have a stronger influence on consumer behavior.11 This is potentially concerning, because the results show that the majority, and for some claim types all, of the products containing one of the absence-framed sugar claims (eg, “sugar free,” “no added sugar,” “less sugar”) contained NCSs, which are currently not recommended for consumption among young children.7 Current regulations do not require NCSs to be disclosed anywhere on product packaging except in the ingredients list and the FDA has no regulations around NCS content or disclosure for products carrying “sugar free” and “no sugar added” claims.30 Regulatory actions to prevent potentially misleading consumers could include requiring NCSs to be disclosed on the FOP as well as prohibiting certain claims, such as “sugar free,” on products containing NCSs. Claims about NCSs were less common (10%) and were more evenly balanced between presence and absence framing. Future research should examine parent and caregiver perceptions of sugar absence claims, particularly as they relate to NCS content.
Several claim categories were not prevalent on fruit drinks. Health and structure/function claims were essentially absent from fruit drink packages in this sample. The lack of health claims is unsurprising given the strong evidence thresholds necessary for health claims to be displayed on product packaging. Given that fruit drinks are generally considered SSBs, there is likely insufficient evidence of any potential health benefits associated with consumption or with any of the ingredients in SSBs for products to carry FDA-reviewed and -approved health claims. The majority of the 16 structure/function claims found on fruit drinks focused on benefits for digestion and immunity. Given the relatively low prevalence of health and structure/function claims on products in our sample, the FDA should prioritize strengthening regulations for more common claim types like implied natural and nutrient-content claims.
Overt natural (eg, “natural,” “all natural”) and “healthy” claims were also uncommon on fruit drinks. FDA is currently revising the definitions and nutritional thresholds for these claims. Despite the low prevalence in our sample, these claims are important to regulate, because prior research has shown they generally create confusion among consumers and can be misleading.46,49,50 Of interest, among the 14 products with a “healthy” claim, 13 products contained NCSs. There is interest in adding a sugar threshold to the current FDA definition of “healthy.” In addition to this threshold, FDA and advocates should consider not allowing products with NCSs to carry “healthy” claims. There is a growing body of evidence of potential health harms of NCS consumption, and if a sugar threshold is set for products carrying a “healthy” claim, manufacturers may reformulate high sugar products by substituting NCSs for caloric sweeteners.51-54
In addition to documenting the prevalence of specific claims on fruit drinks purchased by households with young children, this study also examined the relationship between the presence of nutrition-related claims and nutritional profile. The goal of these analyses was to examine whether the presence of a claim was consistently associated with a more favorable nutritional profile. Fruit drinks are in fact not healthy products despite the many FOP nutrition-related claims present on these products. Interestingly, these analyses found that, in terms of calorie, sugar, and NCS content, products with and without specific nutrition-related claims are, in fact, different from each other. However, the direction of the association between presence of a claim and calorie, sugar, and NCS content was inconsistent and depended on the claim type. Additionally, products with claim types that were associated with fewer calories and grams of sugar per 100 mL were also more likely to contain NCSs. These findings are consistent with other research that has generally shown that a high proportion of products with nutrition-related claims are high in nutrients of concern such as saturated fat, sodium, and added sugar and that claims are not reliable indicators of a product’s overall healthfulness.17,18,55,56 The utility of nutrition-related claims could be significantly improved if claims were limited to products that do not exceed nutrient thresholds established by nutrient profile models such as the Pan American Health Organization’s model.57 Additionally, other countries such as Chile have implemented FOP warning labels on products that contain excessive quantities of nutrients such as added sugar or saturated fat or ingredients such as NCSs. Future research could examine the extent to which FOP warning labels could counteract the potential confusion caused by nutrition-related claims.
Strengths and Limitations
A limitation to this study is that data on the proportion of total sugars that came from added sugars were not available. However, few of the products in this sample had juice or fruit as 1 of the first 2 ingredients, so it is likely that a substantial proportion of the total sugar content in this sample’s fruit drinks came from added sugars. This study also does not include information on the amounts of NCSs in fruit drinks, only whether NCSs were present or absent from each product. Additionally, this analysis does not include all fruit drink products in the US food supply nor does it weight the prevalence of claims by the amount of each fruit drink product purchased by households, so the values presented may not reflect consumers’ exposure to certain claim types. However, there is still value in understanding the unweighted presence of claims and other attributes of key product categories in the food supply. This information can be used to monitor changes in labeling and claims over time and advocate for labeling policy change.29,37-39 Future research should examine how the presence of specific claims is related to fruit drink purchases, examine which claims have the strongest association with purchases, and explore potential differences in purchases by parent or caregiver demographic characteristics. Our sample was also limited to products purchased among households with young children despite the fact that SSB consumption is a public health concern among children of all ages in the United States. Future work should explore if common claim types differ among SSBs purchased by households with older children. Using secondary label databases such as Mintel GNPD and Label Insight also present challenges because some of the product labels in these databases may be outdated.
Previous work has explored the nutritional quality of fruit drinks marketed specifically to children and the number and general type (eg, ingredient, nutrition-related, real) of claims on child-directed fruit drinks.22 Additionally, other studies have documented the presence of low/no calorie and sugar claims on juice and juice beverages.18 This work adds to the body of science by documenting claims with a high degree of specificity in a large sample of fruit drink products and by comparing the presence of claims with nutritional quality in this product category. This research can inform advocacy and policy efforts to prevent misleading industry practices by giving advocates information about what claim types are the most prevalent and the nutritional quality of products that currently carry claims. Another strength of this study is the large and comprehensive sample of 2059 fruit drink products that were identified from household purchases of families with children 0 to 5 years old.
CONCLUSION
This study found that nutrition-related claims are prevalent on fruit drink products purchased by households with young children. The presence of nutrition-related claims was not consistently associated with a more favorable nutritional profile. Given the previously documented influence of claims on consumer perceptions and the concerning levels of fruit drink consumption among young children in the United States, stakeholders such as public health advocates and the FDA should consider improvements to labeling regulation and policy. For example, the FDA could prohibit the use of certain claim types on sugar-sweetened fruit drinks or drinks with NCSs. Changes in FOP labeling and marketing will be one of many macro-level changes needed to bring young children’s dietary patterns closer in line with recommendations.
Supplementary Material
RESEARCH SNAPSHOT.
Research Questions: What is the prevalence of nutrition-related claims on fruit drinks purchased by households with young children (0-5 years old)? What is the relationship between nutrition-related claims and the nutritional profile (calories/100 mL, grams of sugar/100 mL, and presence of noncaloric sweeteners [NCSs]) of fruit drinks?
Key Findings: Nearly all (97%) fruit drinks sampled (n = 2059) had at least 1 nutrition-related claim. The most common claims were implied natural (eg, natural flavor), juice or nectar, vitamin C, calorie-related, and sugar-related claims. Presence of some claim types tested (calorie, sugar, NCS, implied natural, and other claims) was associated with lower calorie and sugar content but higher likelihood of containing NCS when compared with products without those claims.
ACKNOWLEDGEMENTS
The authors thank Shu Wen Ng, PhD Jessica Ostrowski, MPH and Emily Busey, MPH for their assistance in the nutritional profile analysis and the design of figures and tables. The authors also thank Sarah Sorscher, JD, MPH for her input on the policy implications of this work.
FUNDING/SUPPORT
This research was supported by grant #76337 from the Robert Wood Johnson Foundation. K01HL147713 from the National Heart, Lung, and Blood Institute of the National Institutes of Health supported M. G. Hall’s time writing the paper. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We are grateful to the Carolina Population Center and its NIH Center grant (P2C HD050924) for general support.
Footnotes
STATEMENT OF POTENTIAL CONFLICT OF INTEREST
No potential conflict of interest was reported by the authors.
Contributor Information
Emily W. Duffy, Gillings School of Global Public Health, University of North Carolina at Chapel Hill..
Marissa G. Hall, Gillings School of Global Public Health, University of North Carolina at Chapel Hill; Lineberger Comprehensive Cancer Center, Chapel Hill, NC..
Francesca R. Dillman Carpentier, Hussman School of Journalism and Media, University of North Carolina at Chapel Hill..
Aviva A. Musicus, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA..
Michele L. Meyer, Hussman School of Journalism and Media, University of North Carolina at Chapel Hill..
Eric Rimm, Harvard T.H. Chan School of Public Health, Boston, MA..
Lindsey Smith Taillie, Gillings School of Global Public Health, University of North Carolina at Chapel Hill..
References
- 1.Herrick KA, Fryar CD, Hamner HC, Park S, Ogden CL. Added sugars intake among US infants and toddlers. J Acad Nutr Diet. 2020;120(1): 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey RL, Fulgoni VL, Cowan AE, Gaine PC. Sources of added sugars in young children, adolescents, and adults with low and high intakes of added sugars. Nutrients. 2018;10(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bleich SN, Vercammen KA. The negative impact of sugar-sweetened beverages on children’s health: an update of the literature. BMC Obes. 2018;5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kay MC, Welker EB, Jacquier EF, Story MT. Beverage consumption patterns among infants and young children (0(-)47.9 months): Data from the Feeding Infants and Toddlers Study, 2016. Nutrients. 2018;10(7):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bleich SN, Wolfson JA. Trends in SSBs and snack consumption among children by age, body weight, and race/ethnicity. Obesity (Silver Spring). 2015;23(5):1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welker EB, Jacquier EF, Catellier DJ, Anater AS, Story MT. Room for improvement remains in food consumption patterns of young children aged 2-4 years. J Nutr. 2018;148(9s):1536s–1546s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lott M, Callahan E, Welker Duffy E, Story M, Daniels S. Healthy Beverage Consumption in Early Childhood: Recommendations From Key National Health and Nutrition Organizations Technical scientific report. Durham, NC: Healthy Eating Research; 2019. [Google Scholar]
- 8.Yang Q Gain weight by “going diet?” Artificial sweeteners and the neurobiology of sugar cravings: Neuroscience 2010. Yale J Biol Med. 2010;83(2):101–108. [PMC free article] [PubMed] [Google Scholar]
- 9.Harris J, LoDolce M, Munsell C, Fleming-Milici F. Sugary Drink Facts 2014: Some Progress but Much Room for Improvement in Marketing to Youth. New Haven, CT: Rudd Center for Food Policy and Obesity; 2014. [Google Scholar]
- 10.Bech-Larsen T, Grunert KG. The perceived healthiness of functional foods. A conjoint study of Danish, Finnish and American consumers’ perception of functional foods. Appetite. 2003;40(1):9–14. [DOI] [PubMed] [Google Scholar]
- 11.André Q, Chandon P, Haws K. Healthy through presence or absence, nature or science? A framework for understanding front-of-package food claims. J Public Policy Mark. 2019;38(2):172–191. [Google Scholar]
- 12.Aschemann-Witzel J, Hamm U. Do consumers prefer foods with nutrition and health claims? Results of a purchase simulation. J Mark Comm. 2010;16(1-2):47–58. [Google Scholar]
- 13.Sherry B, McDivitt J, Birch LL, et al. Attitudes, practices, and concerns about child feeding and child weight status among socioeconomically diverse white, Hispanic, and African-American mothers. J Am Diet Assoc. 2004;104(2):215–221. [DOI] [PubMed] [Google Scholar]
- 14.Verrill L, Wood D, Cates S, Lando A, Zhang Y. Vitamin-fortified snack food may lead consumers to make poor dietary decisions. J Acad Nutr Diet. 2017;117(3):376–385. [DOI] [PubMed] [Google Scholar]
- 15.Burton S, Cook LA, Howlett E, Newman CL. Broken halos and shattered horns: Overcoming the biasing effects of prior expectations through objective information disclosure. J Acad Mark Sci. 2015;43(2):240–256. [Google Scholar]
- 16.Elliott C Packaging health: Examining “better-for-you” foods targeted at children. Can Public Policy. 2012;38(2):265–281. [Google Scholar]
- 17.Colby SE, Johnson L, Scheett A, Hoverson B. Nutrition marketing on food labels. J Nutr Educ Behav. 2010;42(2):92–98. [DOI] [PubMed] [Google Scholar]
- 18.Taillie LS, Ng SW, Xue Y, Busey E, Harding M. No fat, no sugar, no salt … No problem? Prevalence of “low-content” nutrient claims and their associations with the nutritional profile of food and beverage purchases in the United States. J Acad Nutr Diet. 2017;117(9):1366–1374.e1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Institute of Medicine. Front-of-Package Nutrition Rating Systems and Symbols: Phase I Report. Washington, DC: The National Academies Press; 2010. [PubMed] [Google Scholar]
- 20.US Food and Drug Administration. FDA nutrition innovation strategy. https://www.fda.gov/food/food-labeling-nutrition/fda-nutrition-innovation-strategy. Updated October 25, 2019 Accessed October 15, 2019.
- 21.Center for Science in the Public Interest. Re: FDA-2018-N-238; The Food and Drug Administration’s comprehensive, multi-year nutrition innovation strategy; public meeting; request for comments. https://cspinet.org/sites/default/files/attachment/nis-cspi-comments-with-appendix_0.pdf. Published October 11, 2018. Accessed June 12, 2020.
- 22.Harris JL, Romo-Palafox M, Yoon-Young C, Kibwana A. Children’s Drink Facts 2019: Sales, Nutrition, and Marketing of Children’s Drinks. Storrs, CT: UConn Rudd Center for Food Policy & Obesity; 2019. [Google Scholar]
- 23.Slining MM, Ng SW, Popkin BM. Food companies’ calorie-reduction pledges to improve U.S. diet. Am J Prev Med. 2013;44(2):174–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.US Department of Agriculture Agricultural Research Service. What we eat in America. Beltsville, MD; 2016. https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/1314/food_category_list.pdf. Accessed November 4, 2019. [Google Scholar]
- 25.Anater AS, Catellier DJ, Levine BA, et al. The Feeding Infants and Toddlers Study (FITS) 2016: Study design and methods. J Nutr. 2018;148(9s):1516s–1524s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Label Insight. Chicago, Illinois: https://www.labelinsight.com/. Last modified January 2020 Accessed March 20, 2020. [Google Scholar]
- 27.Mintel Global New Products Database. London, UK: https://www.mintel.com/global-new-products-database. Last modified January 2020 Accessed March 20, 2020. [Google Scholar]
- 28.Euromonitor International. Passport Global Market Information Database. Last modified November 2019 https://go.euromonitor.com/passport.html. Accessed August 7, 2020.
- 29.Mediano Stoltze F, Barker JO, Kanter R, et al. Prevalence of child-directed and general audience marketing strategies on the front of beverage packaging: the case of Chile. Public Health Nutr. 2018;21(3):454–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.US Food and Drug Administration Center for Food Safety and Applied Nutrition. A Food Labeling Guide: Guidance for Industry. College Park, MD: Food and Drug Administration, 2013. [Google Scholar]
- 31.Cornell Law School. Code of Federal Regulations Title 21 Subpart D §101.54-101.69. Specific requirements for nutrient content claims. https://www.law.cornell.edu/cfr/text/21/part-101/subpart-D. Published 2019. Accessed June 12, 2020. [Google Scholar]
- 32.Cornell Law School. Code of Federal Regulations Title 21 Subpart A §101.14. Health claims: General requirements. https://www.law.cornell.edu/cfr/text/21/101.14. Published 2019. Accessed June 12, 2020. [Google Scholar]
- 33.Cornell Law School. Code of Federal Regulations Title 21 Subpart E §101.70-101.83. Specific requirements for health claims. https://www.law.cornell.edu/cfr/text/21/part-101/subpart-E. Published 2019. Accessed June 12, 2020. [Google Scholar]
- 34.Cornell Law School. Code of Federal Regulations Title 21 §101.93. Certain types of statements for dietary supplements. https://www.law.cornell.edu/cfr/text/21/101.93. Published 2019. Accessed June 12, 2020. [Google Scholar]
- 35.Cornell Law School. U.S. Code §343 (r) (6). Misbranded food. https://www.law.cornell.edu/uscode/text/21/343. Published 2011. Accessed June 12, 2020.
- 36.Gwet KL. Computing inter-rater reliability and its variance in the presence of high agreement. Br J Math Stat Psychol. 2008;61(Pt 1):29–48. [DOI] [PubMed] [Google Scholar]
- 37.Stoltze FM, Reyes M, Taillie LS, Correa T, Corvalan C, Carpentier FD. Prevalence of health and nutrient content marketing strategies on breakfast cereal packages before and after a countrywide marketing and labeling regulation: A focus on Chile. Curr Dev Nutr. 2020;4(Suppl). 1723–1723. [Google Scholar]
- 38.Bernstein JT, Schermel A, Mills CM, L’Abbé MR. Total and free sugar content of Canadian prepackaged foods and beverages. Nutrients. 2016;8(9):582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duran AC, Ricardo CZ, Mais LA, Martins APB, Taillie LS. Conflicting messages on food and beverage packages: Front-of-package nutritional labeling, health and nutrition claims in brazil. Nutrients. 2019;11(12):2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57(1):289–300. [Google Scholar]
- 41.Dixon H, Scully M, Niven P, et al. Effects of nutrient content claims, sports celebrity endorsements and premium offers on pre-adolescent children’s food preferences: Experimental research. Pediatr Obes. 2014;9(2):e47–e57. [DOI] [PubMed] [Google Scholar]
- 42.Chandon P, Wansink B. Does food marketing need to make us fat? A review and solutions. Nutr Rev. 2012;70(10):571–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abrams KM, Evans C, Duff BR. Ignorance is bliss. How parents of preschool children make sense of front-of-package visuals and claims on food. Appetite. 2015;87:20–29. [DOI] [PubMed] [Google Scholar]
- 44.Iles IA, Nan X, Verrill L. Nutrient content claims: How they impact perceived healthfulness of fortified snack foods and the moderating effects of nutrition facts labels. Health Commun. 2018;33(10):1308–1316. [DOI] [PubMed] [Google Scholar]
- 45.Munsell CR, Harris JL, Sarda V, Schwartz MB. Parents’ beliefs about the healthfulness of sugary drink options: Opportunities to address misperceptions. Public Health Nutr. 2016;19(1):46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Asioli D, Aschemann-Witzel J, Caputo V, et al. Making sense of the “clean label” trends: A review of consumer food choice behavior and discussion of industry implications. Food Res Int. 2017;99(Pt 1):58–71. [DOI] [PubMed] [Google Scholar]
- 47.Goodman MJ. The “natural” vs. “natural flavors” conflict in food labeling: A regulatory viewpoint. Food Drug Law J. 2017;72(1):78–102. [PubMed] [Google Scholar]
- 48.Hall MG, Lazard AJ, Grummon AH, Mendel JR, Taillie LS. The impact of front-of-package claims, fruit images, and health warnings on consumers’ perceptions of sugar-sweetened fruit drinks: Three randomized experiments. Prev Med. 2020;132:105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu R, Hooker NH, Parasidis E, Simons CT. A natural experiment: Using immersive technologies to study the impact of “all-natural” labeling on perceived food quality, nutritional content, and liking. J Food Sci. 2017;82(3):825–833. [DOI] [PubMed] [Google Scholar]
- 50.Lusk JL. Consumer perceptions of healthy and natural food labels. https://corn.org/wp-content/uploads/2019/01/Consumer-Perception-Survey-on-Food-Labeling-Cover-Letter-and-Report-of-Findings-2019-01-23.pdf. Published 2019. Accessed April 4, 2020.
- 51.Baker-Smith CM, de Ferranti SD, Cochran WJ. The use of nonnutritive sweeteners in children. Pediatrics. 2019;144(5):1–18. [DOI] [PubMed] [Google Scholar]
- 52.Bian X, Chi L, Gao B, Tu P, Ru H, Lu K. Gut microbiome response to sucralose and its potential role in inducing liver inflammation in mice. Front Physiol. 2017;8:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suez J, Korem T, Zeevi D, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514(7521): 181–186. [DOI] [PubMed] [Google Scholar]
- 54.Higgins KA, Mattes RD. A randomized controlled trial contrasting the effects of 4 low-calorie sweeteners and sucrose on body weight in adults with overweight or obesity. Am J Clin Nutr. 2019;109(5):1288–1301. [DOI] [PubMed] [Google Scholar]
- 55.Devi A, Eyles H, Rayner M, et al. Nutritional quality, labelling and promotion of breakfast cereals on the New Zealand market. Appetite. 2014;81:253–260. [DOI] [PubMed] [Google Scholar]
- 56.Franco-Arellano B, Labonte ME, Bernstein JT, L’Abbe MR. Examining the nutritional quality of Canadian packaged foods and beverages with and without nutrition claims. Nutrients. 2018;10(7):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pan American Health Organization. Pan American Health Organization nutrient profile model. Washington, DC: https://iris.paho.org/bitstream/handle/10665.2/18621/9789275118733_eng.pdf?sequence=9&isAllowed=y Published 2016. Accessed December 3, 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.