Summary
BTN3A molecules—BTN3A1 in particular—emerged as important mediators of Vγ9Vδ2 T cell activation by phosphoantigens. These metabolites can originate from infections, e.g. with Mycobacterium tuberculosis, or by alterations in cellular metabolism. Despite the growing interest in the BTN3A genes and their high expression in immune cells and various cancers, little is known about their transcriptional regulation. Here we show that these genes are induced by NLRC5, a regulator of MHC class I gene transcription, through an atypical regulatory motif found in their promoters. Accordingly, a robust correlation between NLRC5 and BTN3A gene expression was found in healthy, in M. tuberculosis-infected donors' blood cells, and in primary tumors. Moreover, forcing NLRC5 expression promoted Vγ9Vδ2 T-cell-mediated killing of tumor cells in a BTN3A-dependent manner. Altogether, these findings indicate that NLRC5 regulates the expression of BTN3A genes and hence open opportunities to modulate antimicrobial and anticancer immunity.
Subject Areas: Immunology, Microbiology, Cell Biology
Graphical Abstract
Highlights
-
•
BTN3A promoters contain a unique regulatory motif occupied by overexpressed NLRC5
-
•
NLRC5 and BTN3A mRNA levels correlate in healthy and diseased cells
-
•
NLRC5 overexpression increases susceptibility to Vγ9Vδ2 T-cell-mediated elimination
Immunology; Microbiology; Cell Biology
Introduction
Butyrophilins (BTNs) are an emerging family of molecules fulfilling immune and non-immune functions. Human BTNs comprise BTN1A1, BTN2A1, and BTN2A2, as well as BTN3A1, BTN3A2, and BTN3A3. The genes encoding these proteins are located in the extended major histocompatibility complex (MHC) locus on chromosome 6 (Abeler-Dorner et al., 2012; Arnett and Viney, 2014). Structurally, BTNs are membrane proteins sharing similarity with the B7 immunoglobulin superfamily of costimulatory/coinhibitory molecules (Arnett and Viney, 2014). In fact, BTN2A2 and BTN1A1 have been shown to act as co-inhibitory ligands hindering T cell activation and proliferation, and Btn2a2 knockout mice exhibit exacerbated T-cell-mediated autoimmunity (Sarter et al., 2016; Smith et al., 2010).
Human BTN3A1-3 proteins, which have no murine homologs, are composed of two extracellular immunoglobulin-like domains and a transmembrane region linked—in BTN3A1 and BTN3A3—to an intracellular B30.2 domain (Harly et al., 2012; Sandstrom et al., 2014; Vavassori et al., 2013). Despite this divergence in the intracellular portion, BTN3A1, BTN3A2, and BTN3A3 show >95% homology in the extracellular domain, suggesting that they are the products of recent duplications. Although not sufficient, BTN3A1 is necessary for the activation of Vγ9Vδ2 T cells (Riano et al., 2014; Sandstrom et al., 2014; Vantourout et al., 2018; Vavassori et al., 2013). Although the expression of BTN3A2 and BTN3A3 can support BTN3A1's function, it recently became clear that BTN2A1 is the second critical molecule to stimulate Vγ9Vδ2 T cells (Karunakaran et al., 2020; Rigau et al., 2020; Vantourout et al., 2018). BTN2A1 presents the intracellular B30.2 domain and the two extracellular immunoglobulin-like domains (Abeler-Dorner et al., 2012; Arnett and Viney, 2014). Vγ9Vδ2 T cells are activated by phosphorylated metabolites, also called phosphoantigens (PAgs), that derive from a dysfunctional mevalonate pathway, such as isopentenyl pyrophosphate (IPP), or from microorganisms, as for instance Mycobacterium tuberculosis-derived (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMBPP) (Vantourout and Hayday, 2013). PAgs have been proposed to bind either the immunoglobulin-like domain or the B30.2 domain of BTN3A1, inducing a conformational change and/or stabilizing surface BTN3A1 to engage the γδ T cell receptor, whereas BTN2A1 interacts with the germline region of the Vγ9 chain (Gu et al., 2017; Harly et al., 2012; Karunakaran et al., 2020; Rigau et al., 2020; Sandstrom et al., 2014; Vavassori et al., 2013). Increased levels of PAgs from metabolically stressed, transformed, and infected cells are thus sensed by Vγ9Vδ2 T cells, leading to their activation, expansion, and participation in the immune response (De Libero et al., 2014). For instance, this subset of unconventional T cells is significantly expanded during M. tuberculosis infection (Cheng et al., 2018; Kabelitz et al., 1991). Furthermore, intratumoral γδ T cells emerged as the most significant favorable cancer-wide prognostic population, and their potential role in immunotherapy is being increasingly investigated (Benyamine et al., 2016, 2017; Gentles et al., 2015; Le Page et al., 2012; Peedicayil et al., 2010; Zocchi et al., 2017).
The transcriptional regulation of BTN genes remains poorly characterized. Recently, it has been shown that Btn2a2 induction is regulated by the transcriptional regulator CIITA (class II major histocompatibility complex transactivator) (Sarter et al., 2016). This factor belongs to the nucleotide-binding oligomerization domain (NOD)-like receptor (NLR) family of proteins and, together with its closest homolog NLRC5 (NLR family CARD domain containing 5), is known to control the transcription of MHC and related genes (Chelbi et al., 2017; Jongsma et al., 2019; Reith and Mach, 2001; Sarter et al., 2016). CIITA is the master transcriptional regulator of MHC class II genes, whereas we and others showed that NLRC5 is an important transcriptional regulator of MHC class I genes, markedly in T lymphocytes (Chelbi et al., 2017; Meissner et al., 2010; Neerincx et al., 2013; Robbins et al., 2012; Staehli et al., 2012; Yao et al., 2012). These two NLRs, CIITA and NLRC5, are recruited to their respective target gene promoter by a multiprotein complex known as “enhanceosome” (Ludigs et al., 2015; Meissner et al., 2012a; Neerincx et al., 2012). This complex assembles on the promoter sequence called “SXY” module, which is composed of four individual elements (S, X1, X2, and Y) oriented and spaced in a specific manner (Anderson et al., 2017; Ludigs et al., 2015; Masternak et al., 2003; Meissner et al., 2012b; Neerincx et al., 2012 (Krawczyk et al., 2004)). Although we still do not know which factor recognizes the S-box, the X1-box is bound by the regulatory factor X (RFX) complex, the X2-box by cAMP-responsive element binding protein (CREB)/activating transcription factor (ATF) family members, and the Y-box by the nuclear transcription factor Y (NFY)-complex (Chelbi et al., 2017). Taken together, the finding that Btn2a2 is a target of CIITA and the localization of BTN genes close to the MHC locus suggest the existence of common evolutionary links. This prompted us to investigate the transcriptional regulation of BTN genes and to hypothesize that they represent a novel set of NLRC5 or CIITA targets.
We found that BTN3A1-3 genes exhibited an atypical SXY module in their proximal promoter region. This regulatory motif presented a reverse complement Y-box at an altered spacing from the X-motif. Using chromatin immunoprecipitation and gene reporter assays, we demonstrated that overexpressed NLRC5 occupies and transactivates this atypical module. We showed that forcing NLRC5 expression led to increased levels of BTN3A mRNA and protein. Data mining in transcriptome datasets of M. tuberculosis-infected and uninfected individuals' blood as well as of various cancers revealed a strong correlation between NLRC5 and BTN3A1 and BTN3A2 gene expression. Furthermore, loss of both NLRC5 copies was associated with significantly diminished expression of BTN3A1 and BTN3A2 in cancer cells. On a functional level, we observed that overexpression of NLRC5 enhanced Vγ9Vδ2 T-cell-mediated elimination of target cells, which was mediated by BTN3A molecules as demonstrated using a loss-of-function approach. Altogether, these findings indicate NLRC5 modulation as a possible way of targeting Vγ9Vδ2 T-cell-mediated immunity.
Results
BTN3A genes have distinguishable S and X modules
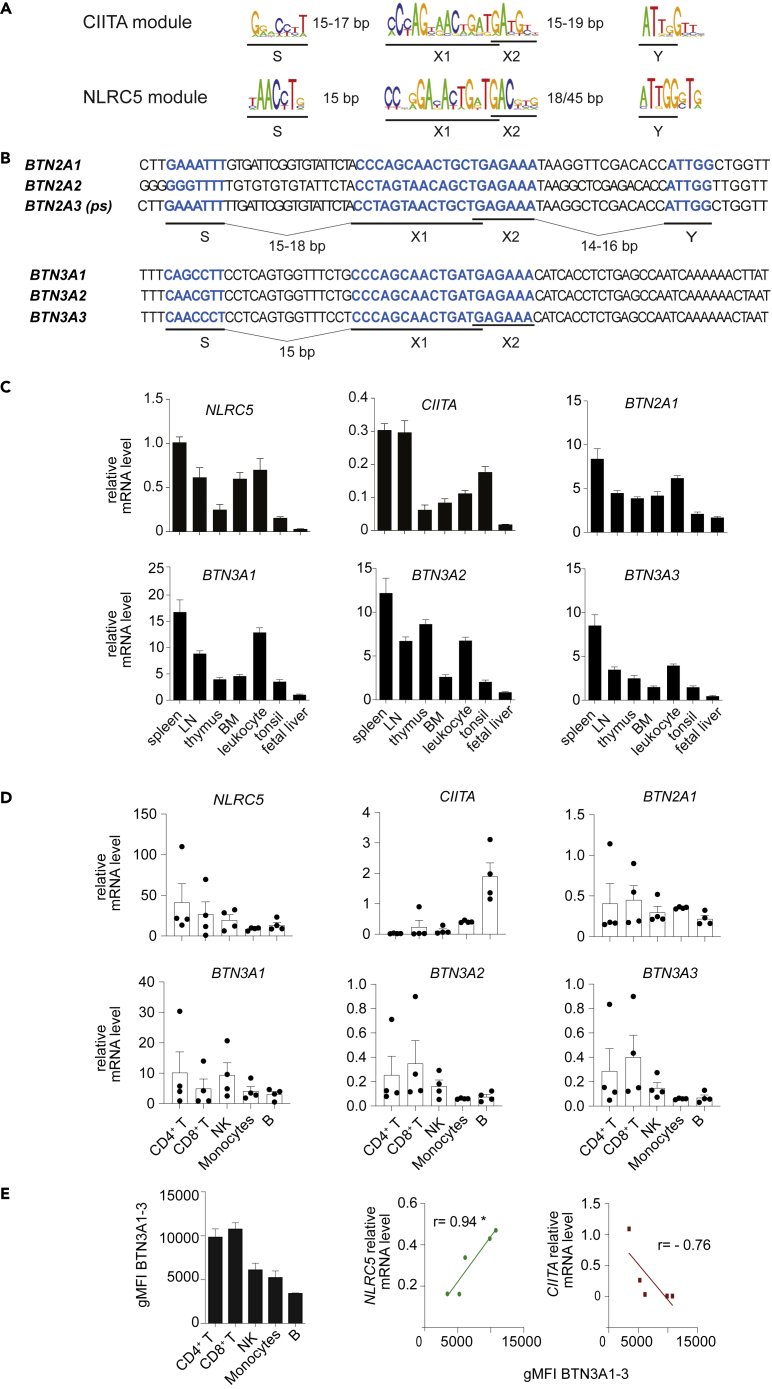
To understand how far the mechanisms governing MHC gene transcription might be common to BTN genes, we screened their promoter sequences for the presence of SXY modules (Figures 1A and 1B) (Krawczyk et al., 2008; Ludigs et al., 2015). We confirmed the presence of an SXY consensus in the BTN2A2 promoter (Figure 1B) (Sarter et al., 2016). Previous findings showed that the S-motif of NLRC5-occupied promoters is unique and strictly required for NLRC5 activity (Figure 1A) (Ludigs et al., 2015; Meissner et al., 2012a). The S-box sequence in both human and mouse BTN2A2 did not suggest regulation through NLRC5, but rather a role for CIITA (Figures 1B and S1A). Indeed, expression of murine Btn2a2 in various organs derived from Nlrc5-deficient mice was not significantly decreased (Figure S1A). We also noticed the presence of an X-motif in the promoters of BTN2A1, the pseudogene BTN2A3, BTN3A1, BTN3A2, and BTN3A3 genes (Figures 1A and 1B). Of note, 15 bp upstream of the X-box, the highly homologous promoter regions of the BTN3A genes, exhibited an S-box suggestive of NLRC5-mediated transcriptional regulation.
Figure 1.
BTN3A promoters present conserved S- and X-boxes
(A) The sequence logo for the human CIITA and mouse NLRC5 SXY consensus are shown.
(B) Sequence alignment of the S-, X-, and Y-motifs located within the proximal promoter region of human BTN2A1, BTN2A2, BTN2A3 (ps, pseudogene), BTN3A1, BTN3A2, and BTN3A3 genes. The sequences corresponding to the S-, X-, and Y-motifs are highlighted in blue and distances between them are indicated.
(C and D) NLRC5, CIITA, BTN2A1, and BTN3A1-3 mRNA levels (relative to POLR2A mRNA) were assessed by quantitative RT-PCR (qRT-PCR) in the indicated human tissues (C) and in blood-derived CD4+ T cells (CD4+CD3+), CD8+ T cells (CD8+CD3+), NK cells (CD56+CD3−), monocytes (CD14+CD3−), and B cells (CD19+CD3−) (D).
(E) Geometric mean fluorescence intensity (gMFI) of BTN3A1-3 (CD277) as measured by flow cytometry in the indicated blood-derived cell subsets (on the left) and the correlation of the mean value with those of NLRC5 and CIITA mRNA levels measured by qRT-PCR in the same cells (on the right). Spearman's correlation coefficient (R) and significance are indicated. (C–E) Results illustrate mean ± SD of n = 3 technical replicates (C) and mean ± SEM of n = 4 (D) and n = 3 (E) individual donors. Lymph node, LN; bone marrow, BM. ∗p < 0.05
BTN3A1-3, BTN2A1, NLRC5, and CIITA transcripts were abundant in most of the tested immune organs and cells (Figure 1C). Among the main blood cell subsets, the mRNA profile of BTN3A1-3 was reminiscent of the one of NLRC5, whereas it largely differed from the one of CIITA (Figure 1D). Instead, BTN2A1 presented a distinct pattern, characterized by higher expression levels in monocytes (Figure 1D) (Rigau et al., 2020). We also observed a marked similarity between the expression pattern of NLRC5 and the BTN3A protein display (by using a monoclonal antibody anti-CD277, which recognizes all three BTN3A isoforms; Figures 1E and S1B). We next took advantage of bare lymphocyte syndrome (BLS) patient-derived and in-vitro-generated B cell lines lacking expression of CIITA or the RFX complex subunit RFX5 or RFXAP (Ludigs et al., 2015, Tarantelli et al., 2018). The absence of the RFX factors affected the expression of BTN3A1-3 and BTN2A1 transcripts, in particular following interferon (IFN)γ treatment (Figure S1C). This was not observed in the absence of CIITA, indicating that this NLR was not necessary for the regulation of these genes (Figure S1C). Taken together, these data support the involvement of the enhanceosome platform and prompted us to perform further analyses on NLRC5 in the regulation of BTN3A1-3 genes.
NLRC5 overexpression induces transcription of BTN3A genes
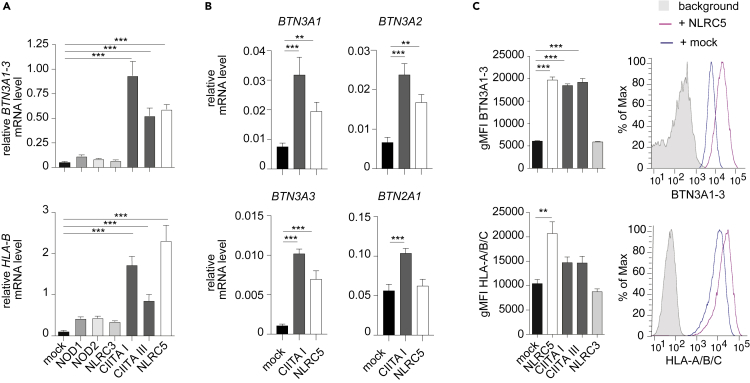
We next tested whether NLRC5 induced the transcription of BTN3A1-3 genes, first employing a primer pair that detects all three gene products. After 48 h, overexpression of NLRC5 in HEK293T cells led to increased transcript levels of BTN3A1-3 and HLA-B, the latter used here as a positive control (Figure 2A). To assess the specificity of the transcriptional effects of NLRC5, we overexpressed other NLR members. Although NOD1, NOD2, and NLRC3 had marginal effects on the expression of BTN3A1-3 and of HLA-B, two CIITA isoforms (CIITA I and CIITA III) induced the BTN3A1-3 and the HLA-B gene (Figure 2A). The induction in the levels of BTN3A1-3 and HLA-B transcripts by NLRC5 and CIITA I encoding plasmids was already detected 24 h following transfection (Figure S2A). Both NLRC5 and CIITA I induced the three BTN3A genes (Figure 2B). In contrast, only CIITA I moderately induced BTN2A1 mRNA (Figure 2B). Finally, we demonstrated that overexpression of NLRC5 and CIITA, but not of NLRC3, increased surface expression of BTN3A1-3 (Figure 2C). It is important to point out that although CIITA does not regulate MHC class I genes at the endogenous level, it is well established that its overexpression leads to their transactivation (Chang et al., 1996; Gobin et al., 1997; Ludigs et al., 2015; Martin et al., 1997; Robbins et al., 2012; Williams et al., 1998), as shown for HLA-B (Figure S2B). Conversely, the activity of NLRC5 maintains its specificity toward the HLA-B but not HLA-DRA genes even when overexpressed (Figure S2B). Therefore, these data encouraged us to further investigate whether BTN3A1-3 genes are regulated by mechanisms similar to the ones controlling MHC class I gene expression.
Figure 2.
NLRC5 overexpression increases BTN3A1-3 expression
(A and B) BTN3A1-3, BTN2A1, or HLA-B mRNA levels (relative to POLR2A mRNA) were measured by qRT-PCR 48 h following transfection of plasmids encoding the indicated NLR proteins or an empty vector (mock) in HEK293T.
(C) HEK293T cells were co-transfected with vectors coding for the indicated NLRs or empty vector (mock) and a GFP-encoding plasmid to identify transfected cells. Graphs depict the quantification of HLA-A/B/C and BTN3A1-3 geometric MFI (gMFI) gating on GFP+ cells 48 h post-transfection. Histogram overlays show HLA-A/B/C and BTN3A1-3 expression for background (gray), mock- (blue), and NLRC5-transfected (pink) HEK293T cells. (A–C) Results are depicted as mean ± SD (n = 3 technical replicates) and are representative of at least two independent experiments. Statistical differences were determined by one-way ANOVA followed by comparison of the experimental conditions to the corresponding mock transfection and were corrected for multiple testing using the Dunnett method. Only statistically significant differences are illustrated. ∗∗p < 0.01; ∗∗∗p < 0.001.
BTN3A genes have an atypical SXY module
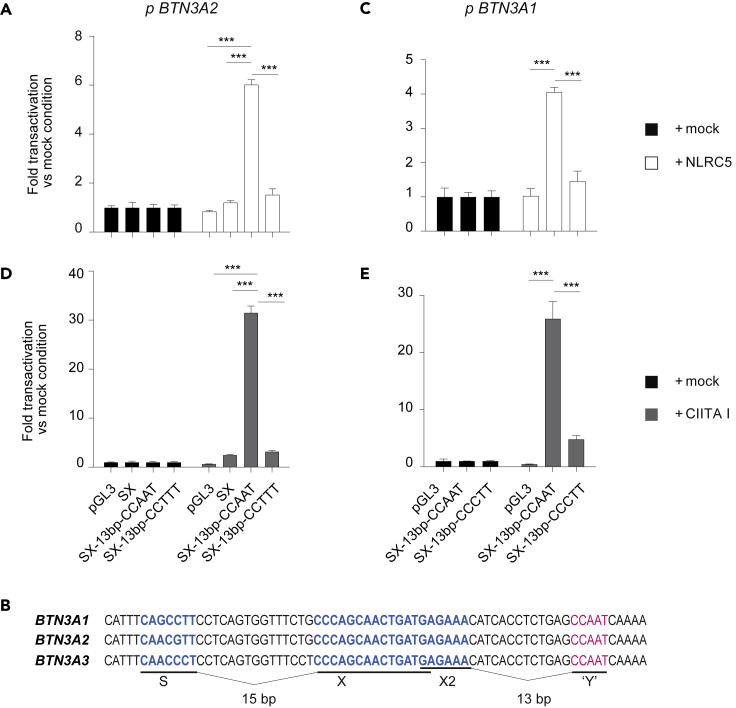
In order to substantiate the hypothesis that BTN3A1-3 are direct targets of NLRC5, we cloned the proximal promoter region, containing the S- and X-motif, of BTN3A2—as a representative BTN3A promoter—into a luciferase reporter plasmid (referred to as “SX”). As we did not identify an “ATTGG” Y-box sequence nearby, we hypothesized that it could be dispensable for NLRC5-dependent transactivation of these genes (Figure 1B). However, we observed that these promoters were not transactivated by NLRC5 (Figure 3A). We therefore took a closer look at the promoters of BTN3A1-3 genes and identified the reverse complement of the “ATTGG” Y-box, “CCAAT,” 13 bp downstream of the X-box (Figure 3B). As the latter constitutes the canonical NFY-binding site, we cloned an extended portion of the promoters, which included this motif, into a luciferase reporter plasmid (referred to as “SX-13bp-CCAAT”). This enabled transactivation by NLRC5 (Figure 3A). Moreover, scrambling of the “CCAAT” sequence (referred to as “SX-13bp-CCTTT”) abrogated NLRC5-mediated transactivation, pinpointing the importance of this unique “Y” box (Figure 3A). NLRC5 was also able to transactivate the “SX-13bp-CCAAT” BTN3A1 promoter construct, which diverges from the “SX-13bp-CCAAT” BTN3A2 reporter by only 2 bp in the S-box, in a CCAAT-dependent manner (Figure 3C). Not surprisingly, similar results were observed when overexpressing CIITA I (Figures 3D and 3E). These results reveal the presence of an unconventional SXY module in the BTN3A promoters.
Figure 3.
BTN3A genes harbor an atypical but functional SXY module
(A, C–E) Luciferase reporter assays were performed in HEK293T cells co-transfected with the parental pGL3 backbone or the indicated BTN3A2 (A, D) or BTN3A1 (C, E) promoter constructs, and an empty (mock) vector, or a vector coding for NLRC5 (A, C) or CIITA (D, E). SX-13bp-CCAAT contains the BTN3A1 or BTN3A2 promoter region, as indicated, with S-, X-, and 13 bp downstream CCAAT-box; where indicated, the CCAAT-box was mutated. (B) Alignment of the proximal BTN3A1-3 promoter region containing S- and X-motifs highlighted in blue and a 13 bp downstream “Y” CCAAT-box highlighted in pink. Distances between motifs are indicated. (A and C–E) Data are expressed as fold transactivation as compared with the mock condition. Results represent mean ± SD of n = 3 (A and D) and n = 4 (C and E) technical replicates and are representative of at least two independent experiments. Statistical differences were determined by performing a two-way ANOVA followed by comparison of the SX-13bp-CCAAT condition to the others transfected with the same NLR and were corrected for multiple testing using the Holm-Sidak method. Only statistically significant differences are illustrated. ∗∗∗p < 0.001.
The S-box and the distance of the CCAAT-box are key for NLRC5-mediated transactivation
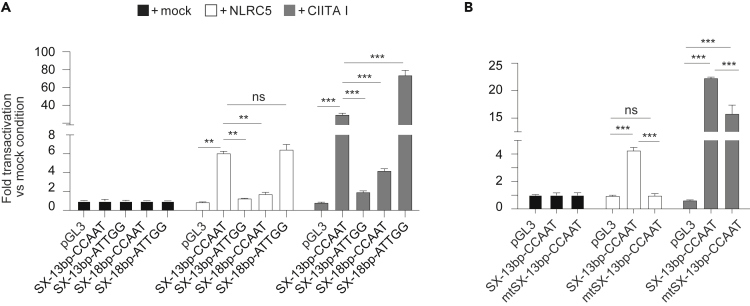
One intriguing feature of the BTN3A promoters is the fact that the spacing between the X-box and the CCAAT sequence (13 bp) is shorter than the usual one found at the MHC promoters between the X-box and the ATTGG (usually 17–18 bp). To assess whether the unusual orientation and distance were important, we generated a luciferase reporter plasmid in which the CCAAT sequence of BTN3A2 promoter was substituted by an ATTGG (referred to as “SX-13bp-ATTGG”) at the 13 bp distance from the X-box. Interestingly, this promoter was transactivated neither by NLRC5 nor by CIITA (Figure 4A). We also moved the CCAAT sequence 18 bp downstream of the X-box (referred to as “SX-18bp-CCAAT”). Again, NLRC5- and CIITA-mediated transactivation were impaired (Figure 4A). Yet, insertion of an ATTGG sequence at 18 bp distance from the X-box (referred to as “SX-18bp-ATTGG”), which mirrors the classical organization of the HLA promoters, restored transactivation by NLRC5 and CIITA (Figure 4A). These results show that both orientation and distance of the NFY-binding site are crucial for transcriptional induction by these NLRs.
Figure 4.
S-box sequence and CCAAT-box position are important for NLRC5 transactivation
(A and B) Luciferase reporter assays were performed in HEK293T cells co-transfected with the parental pGL3 backbone or the indicated BTN3A2 promoter constructs, and a vector coding for NLRC5 or CIITA I, or an empty (mock) vector; mtSX indicates a scrambled S-box sequence (the CAACGTT sequence was substituted by CCAGAGT) (B). Data are expressed as fold transactivation as compared to the mock condition. Results represent mean ± SD of n = 3 technical replicates and are representative of at least three independent experiments. Statistical differences were determined by performing a two-way ANOVA followed by comparison of the indicated conditions and were corrected for multiple testing using the Holm-Sidak method. ∗∗p < 0.01; ∗∗∗p < 0.001; ns: not significant.
As the S-box found in the promoters of the BTN3A genes exhibits similarity to the one required for NLRC5-mediated transactivation, we next assessed its contribution to the transactivation of the BTN3A2 promoter. In line with results from the MHC class I gene promoter, scrambling the S-box sequence severely compromised NLRC5-mediated, but not CIITA-mediated, transactivation (Figure 4B). This underlines the importance of the S-box for NLRC5-mediated regulation.
BTN3A genes are direct targets of NLRC5
In order to prove that BTN3A genes are direct targets of NLRC5, we performed chromatin immunoprecipitation (ChIP) experiments. In the absence of an antibody against endogenous human NLRC5, we generated an HA-tagged version of wild-type NLRC5 (wt NLRC5) and of a mutant of the Walker A motif (mt NLRC5), which prevents NLRC5 nuclear translocation and transcriptional activity (Meissner et al., 2010). We then co-transfected HEK293T cells with plasmids coding for wt NLRC5 or mt NLRC5, and for the surface protein CD72, thus enabling the enrichment of transfected cells. ChIP using chromatin from wt NLRC5-transfected cells led to a substantial enrichment of the promoter region of HLA-B but also of BTN3A1 and BTN3A2 genes as compared with mt NLRC5-transfected cells (Figure 5). By contrast, the promoter of HOXC8, a gene not known to be controlled by NLRC5, was not enriched (Figure 5). Although we were unable to design primers specific for BTN3A3 promoter due to the high homology of the regulatory regions of these genes, these data demonstrate occupation of BTN3A1 and A2 promoters by NLRC5 (Figure 5).
Figure 5.
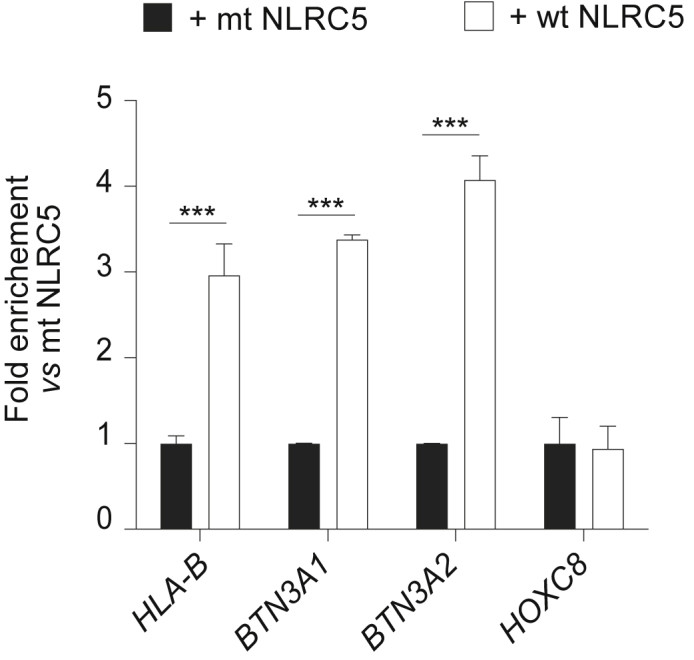
NLRC5 directly binds to BTN3A promoter regions
Vectors encoding human wild-type NLRC5 (wt NLRC5) or Walker A mutant (mt NLRC5) were cotransfected with CD72 into HEK293T. Forty-eight hours later, CD72-positive cells were MACS enriched and chromatin prepared. Binding of NLRC5 to the indicated promoters was assessed by chromatin immunoprecipitation (ChIP) followed by quantitative PCR. Results are expressed as fold enrichment as compared with the mt NLRC5 condition. Results are depicted as the mean ± SD of n = 3 technical replicates and are representative of at least two independent experiments. Statistical differences were determined by performing a two-way ANOVA followed by comparison of the wt NLRC5 with mt NLRC5 transfection and were corrected for multiple testing using the Holm-Sidak method. ∗∗∗p < 0.001.
NLRC5 and BTN3A genes are co-regulated in health and disease
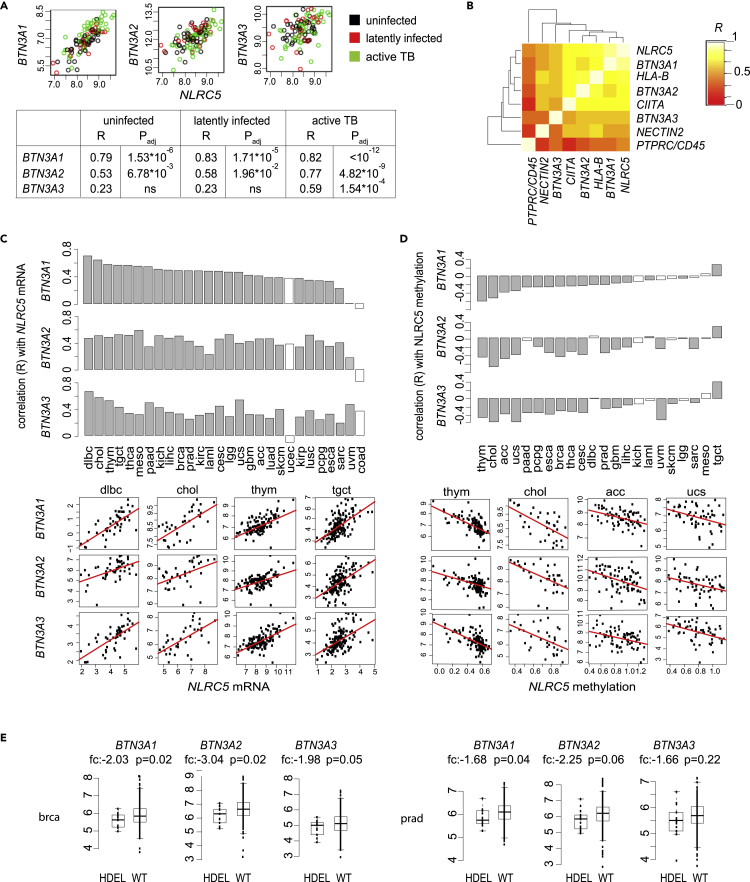
We next checked the correlation between NLRC5 and BTN3A1-3 expression in human pathological conditions in which Vγ9Vδ2 T cells are considered relevant. First, we analyzed transcriptomic data from a Gambia tuberculosis cohort study (Maertzdorf et al., 2011). A significant correlation of NLRC5 with BTN3A1 and BTN3A2 expression was observed in healthy uninfected donors (Figure 6A). These correlations were stronger in donors latently infected with M. tuberculosis and in active tuberculosis (TB) patients (Figure 6A). For BTN3A3, a significant correlation with NLRC5 was observed only in active TB patients (Figure 6A). Of note, the correlations of BTN3A genes with CIITA expression were lower, but still significant in most cases, whereas BTN2A1 levels correlated with neither of the two NLRs (Figure S3A). Hierarchical clustering of the correlations for the entire cohort (i.e. all three groups) highlighted the close proximity of NLRC5 to BTN3A1, BTN3A2, and HLA-B, whereas BTN3A3 was more distant (Figure 6B). Corroborating the specificity of these results, NECTIN2 and PTPRC/CD45, two genes not expected to be regulated by NLRC5 or CIITA, were clustering at farther distances (Figure 6B). Taken together, these data highlight a potential role for NLRC5 in anti-mycobacterial immunity.
Figure 6.
BTN3A1-3 expression correlates with NLRC5 levels
(A and B) Pairwise correlation of NLRC5 and BTN3A1, BTN3A2, BTN3A3 expression are visualized. Analyses were performed using transcriptome datasets of the Gambia M. tuberculosis (TB) cohort study (GSE28623). (A) Scatterplots of gene expression are shown. The samples are divided in the groups “uninfected” (black), “latently infected” (red), and “active TB” (green). The table displays the Spearman's correlation coefficient (R), and Bonferroni adjusted p values (p.adj) for the 3 groups. (B) Pairwise correlations of NLRC5 and selected genes are visualized using a heatmap and hierarchical clustering tree of Spearman's rank correlations. Correlations were calculated on the entire cohort.
(C–E) Data from The Cancer Genome Atlas (TCGA) provisional dataset collections were analyzed after adjustment for CD45 expression. (C) Spearman's correlation coefficient (R values) for NLRC5 and BTN3A1, BTN3A2, or BTN3A3 mRNA expression across cancer types. Scatterplots are shown for the top-ranked cancer types. (D) Spearman's correlation coefficient for NLRC5 promoter methylation and BTN3A1, BTN3A2, or BTN3A3 mRNA expression across cancer types. Scatterplots are shown for the best-ranked cancer types. (C, D) Gray bars indicate significant correlations (p < 0.05 after Bonferroni correction) and white bars non-significant ones. (E) BTN3A1, BTN3A2, and BTN3A3 mRNA abundance is plotted according to NLRC5 copy number status. fc: fold change of expression in HDEL (n = 14 and n = 13 for brca and prad, respectively) over WT group (n = 272 and n = 361 for brca and prad, respectively). HDEL: homozygous deletion; WT: wild type. Two group comparisons were performed using unpaired t tests, two-tailed, unequal variance, and p values are indicated. Cancer types' abbreviations are described in the Transparent Methods section.
We next investigated the correlation between NLRC5 and BTN3A1-3 mRNA levels across cancers in “The Cancer Genome Atlas” (TCGA; https://www.cancer.gov/tcga) provisional datasets. Given the very high expression of these genes in immune cells, we corrected for CD45 mRNA prior to sample analysis to reduce the confounding effect by infiltrating leukocytes (Figures 1C–1E) (Ludigs et al., 2016; Staehli et al., 2012). In most cancers, we found a significant correlation between NLRC5 and BTN3A1-3 transcript abundance (Figure 6C), whereas the correlation with NECTIN2 was low and mostly non-significant (Figure S3B). The correlation with HLA-B was generally good, with the exception of the dlbc dataset (Figure S3B) (Yoshihama et al., 2016). We therefore tested an independent cohort of DLBCL samples (GSE10846; Figure S3C), which corroborated a generally weaker correlation between NLRC5 and HLA-B than with the BTN3A genes. Across cancers, BTN3A1-3 correlated better with NLRC5 than with CIITA expression, whereas the profile of BTN2A1 was quite independent from the transcript levels of both NLRs (Figure S3D).
We next investigated whether methylation of the NLRC5 promoter inversely correlated with the expression of the BTN3A genes. Data supported this hypothesis, including for HLA-B, whereas barely significant and variable results were observed for NECTIN2 (Figures 6D and S3E). We also interrogated whether structural alterations leading to NLRC5 copy number loss were concomitant with BTN3A gene expression decrease. We thus focused on breast (brca) and prostate (prad) cancer, the only tumor sets having more than five samples lacking both NLRC5 copies, and compared BTN3A expression of samples harboring loss of NLRC5 (homozygous deletion, HDEL) with samples having normal copy number (WT). Despite the small sample size, BTN3A1, BTN3A2, as well as HLA-B gene expression were reduced in the absence of NLRC5 (Figures 6E and S3F). As for most correlations tested in Figure 6, a similar trend was observed also for BTN3A3, although less robust (Figure 6E). We wondered whether the presence of a “C” instead of the conserved “T” residue in the S-box of this gene's promoter might underlie this observation (Figure S3G). We thus substituted this T with a C in the BTN3A2 SX-13bp-CCAAT reporter plasmid. Supporting our hypothesis, this modification reduced NLRC5-mediated transactivation (Figure S3G). Taken together, these data underscore the robust correlation of NLRC5 with BTN3A1 and BTN3A2 transcript abundance in healthy and diseased conditions in which Vγ9Vδ2 T cells are considered relevant.
NLRC5 overexpression confers susceptibility to γδ T-cell-mediated killing
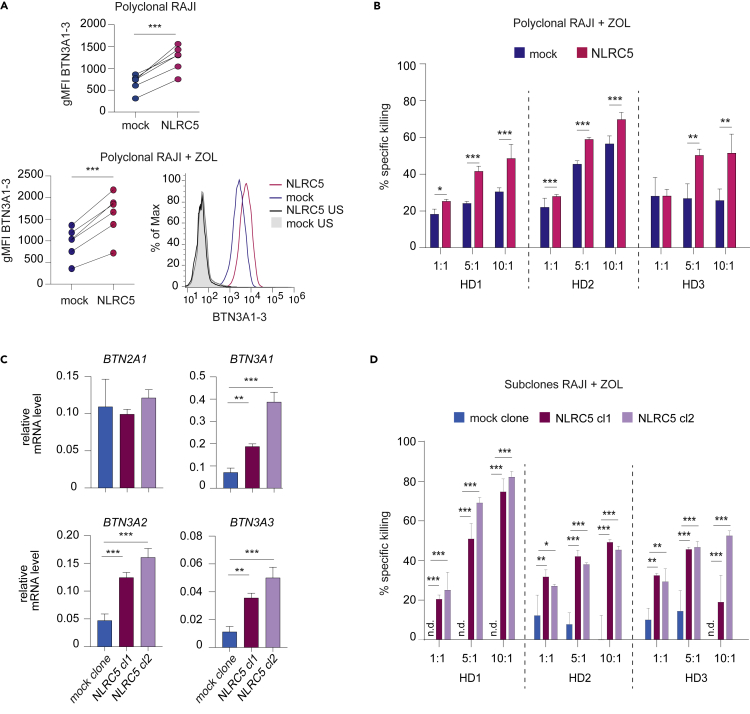
We thus overexpressed NLRC5 in Raji cells, a Burkitt lymphoma cell line known to trigger poor recognition by Vγ9Vδ2 T cells (Harly et al., 2012). As our results predicted, polyclonal NLRC5-transduced Raji cells presented increased levels of BTN3A1-3 surface expression as compared with mock-transduced control cells (Figure 7A). This was observed both in the absence and in the presence of zoledronate, a pharmacological inhibitor of the mevalonate pathway that increases IPP concentrations (Figure 7A). A similar trend was observed for HLA-A/B/C (Figure S4A). In a first set of experiments, killing of mock- or NLRC5-transduced polyclonal Raji cells was measured after 24 h of co-culture with in-vitro-expanded, primary Vγ9Vδ2 T cells in the presence of zoledronate. NLRC5 overexpression significantly enhanced the specific killing of Raji cells (Figure 7B). In these experiments, polyclonal populations of target cells with a transduction efficiency of roughly 68% were used. As this could have masked the effect of NLRC5 overexpression on Vγ9Vδ2 T-cell-mediated killing, we next generated subclones of mock- and NLRC5-transduced Raji cells. In line with data presented in Figure 2B, NLRC5-overexpressing subclones exhibited increased levels of BTN3A1-3 transcripts, but no difference in BTN2A1 mRNA (Figure 7C); in agreement, BTN3A1-3 surface expression was augmented (Figure S4B). Co-culture of Vγ9Vδ2 T cells with these subclones led to significantly increased IFNγ production (Figure S4C) and killing (Figure 7D). To demonstrate that this enhanced killing was mediated by the BTN3A molecules, we knocked out BTN3A1 by CRISPR/Cas9 in the NLRC5-overexpressing subclone 2. This loss-of-function strategy led to a decrease in the transcript levels of BTN3A1 and—due to the high homology of these two genes—of BTN3A2 (Figure S4D). As expected, these cells exhibited significantly reduced susceptibility to be killed by Vγ9Vδ2 T cells (Figure S4E). Therefore, NLRC5 overexpression not only increases the levels of BTN3A1-3 but also functionally promotes Vγ9Vδ2 T cell activation and killing.
Figure 7.
NLRC5 favors the activation of Vγ9Vδ2 T cells
(A) Polyclonal Raji cells were transduced with NLRC5-coding or mock vector and treated with zoledronate (+ZOL) for 24 h (bottom panel) or left untreated (top panel). BTN3A1-3 surface expression was analyzed by flow cytometry. Graphs depict a quantification of BTN3A1-3 geometric MFI (gMFI; top and bottom left), and the histogram overlays (bottom right) show BTN3A1-3 expression for unstained (US; black, gray), mock- (blue) and NLRC5-transfected polyclonal Raji cells (pink). Results represent six independent measurements.
(B) Specific killing of NLRC5- (pink) or mock-transduced polyclonal Raji cells (blue) was measured after 24 h of co-culture with Vγ9Vδ2 T cells at 3 effector-to-target ratios (1:1, 5:1, and 10:1) in the presence of ZOL. Depicted are the results for three independent healthy donors (HD) as mean ± SD (n = 3 technical replicates). The results are representative of four independent healthy donors and three independent experiments.
(C) BTN2A1 and BTN3A1, A2, and A3 mRNA levels (relative to POLR2A mRNA) were assessed by qRT-PCR in the NLRC5- (red and violet) or mock-transduced Raji subclones (blue). The results depict mean ± SD (n = 3 technical replicates) and are representative of 2 independent experiments.
(D) Specific killing of the NLRC5- or mock-transduced Raji subclones was measured after 24 h of co-culture with Vγ9Vδ2 T cells at 3 effector-to-target ratios in the presence of ZOL. n.d. correspond to conditions where no specific lysis of the target cells by the Vγ9Vδ2 T cells was detected. Depicted are the results for three independent healthy donors (HD) as mean ± SD (n = 3 technical replicates). The results are representative of six independent healthy donors and three independent experiments. (A–D) Statistical differences between the condition with and without NLRC5 overexpression were calculated using paired Student's t test (A) or post hoc Student's t tests adjusted for multiple comparisons using the Holm-Sidak method (B–D). ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001. Only statistically significant differences are illustrated.
Discussion
The role of NLRC5 as a transcriptional regulator of MHC class I genes has been rapidly unveiled over the past years (Chelbi et al., 2017; Ludigs et al., 2015; Meissner et al., 2010, 2012a; Neerincx et al., 2012, 2013; Robbins et al., 2012; Staehli et al., 2012; Yao et al., 2012). Yet, our knowledge of its global transcriptional targets in humans remains limited. This work provides the first evidence for the transcriptional regulation of BTN3A1-3 genes by NLRC5, broadening our understanding of its role in humans.
Analysis of promoter sequences of BTN genes that are clustered in the adjacent MHC locus highlighted the presence of SXY modules. One of these modules, in the BTN2A2 promoter, is transactivated by CIITA (Sarter et al., 2016). In contrast, CIITA's closest homolog NLRC5 did not contribute to the expression of Btn2a2 in the tested immune organs, adding further evidence for the role of CIITA in regulating this gene. We also identified an SXY module in the promoter of BTN2A1, and our results indicate that its expression is largely dependent on the RFX complex. However, BTN2A1 transcript levels, which were not increased by NLRC5 and moderately by CIITA, poorly correlated with these NLRs. Additional analyses are therefore needed to understand the transcriptional regulation of this gene. Further, data from BLS-derived B cell lines indicate that the induction of the BTN3A genes is also largely dependent on the presence of an enhanceosome, raising new questions on γδ T cell subsets in BLS patients.
Here, we identified an atypical SXY module in the promoters of BTN3A1-3 genes. Classical, non-classical, and selected MHC-related gene promoters contain—next to the S- and X-motif—an ATTGG Y-box (Krawczyk et al., 2008; Ludigs et al., 2015). Instead, the module of BTN3A1-3 promoters corresponds to the consensus sequence occupied by NLRC5 with regard to the S- and X-boxes but contains the reverse complement of the Y-box. This “CCAAT” sequence corresponds to the canonical regulatory motif occupied by the trimeric NFY complex (Dolfini et al., 2012). Interestingly, the promoter of the MHC-class II-associated invariant chain (li, also called CD74) also contains an atypical SXY, with the CCAAT motif at a reduced distance from the X-box (Brown et al., 1991; Doyle et al., 1990; Zhu and Jones, 1990). This is in line with our observations in the BTN3A promoter, in which both orientation and spacing of the Y-box are crucial for NLRC5-mediated transactivation, presumably through formation of an alternative enhanceosome complex. Finally, we substantiate the importance of the S-box by showing that the substitution of a single conserved position affects NLRC5-mediated transactivation, possibly contributing to differences in the expression of individual BTN3A genes.
Importantly, overexpressed, but not endogenous, CIITA transactivates MHC class I genes, questioning its physiological contribution to BTN3A1-3 gene transcription (Chang et al., 1996; Gobin et al., 1997; Ludigs et al., 2015; Martin et al., 1997; Robbins et al., 2012; Williams et al., 1998). This is corroborated by the observation that CIITA deficiency did not reduce BTN3A expression in B cell lines. Although the question on the physiological contribution to BTN3A1-3 transcription is open also for NLRC5, robust correlative data support this possibility. BTN3A1-3 expression is abundant in T and NK cells, similar to the profile of NLRC5 (Neerincx et al., 2010; Staehli et al., 2012; Wu et al., 2009). The S-box in BTN3A1-3 promoters strongly resembles the one of NLRC5-transactivated genes (Ludigs et al., 2015; Meissner et al., 2012b). In the blood of both uninfected and M. tuberculosis-infected individuals, expression of NLRC5 strongly correlated with the one of BTN3A1, which stimulates PAgs-mediated activation of Vγ9Vδ2 T cells (Harly et al., 2012). Finally, we observed a robust coregulation between BTN3A1/BTN3A2 and NLRC5 expression in various cancers, and NLRC5 homozygous deletion was associated with a decrease in the abundance of BTN3A1 and BTN3A2 mRNA (Maertzdorf et al., 2011). Therefore, although we do not exclude a contribution of CIITA or a redundant function by these NLRs in the regulation of these genes, our results support a role for NLRC5 in regulating BTN3A gene transcription in normal as well as pathological conditions such as TB or cancer.
Our data demonstrate that forcing the expression of NLRC5 in cancerous target cells significantly promotes their killing by Vγ9Vδ2 T cells, highlighting a novel functional link between NLRC5 expression and cytotoxicity by unconventional T cells. Because NLRC5 overexpression did not significantly impact BTN2A1 expression, and MHCI and B2M are known to be dispensable and even hinder the activation of these unconventional T cells by engaging surface inhibitory receptors typical of NK cells (Bakker et al., 1998; Carena et al., 1997; Fisch et al., 1997; Halary et al., 1997; Morita et al., 1995), BTN3A molecules constitute the best candidates responsible for the NLRC5-driven enhanced Vγ9Vδ2 T-cells-mediated killing. In accordance, CRISPR-mediated knockdown of BTN3A molecules nearly abrogated the killing enhancement induced by NLRC5 overexpressing cells. NLRC5 has already been linked to antitumor responses and patient prognosis (Farashi et al., 2019; Fernandez-Jimenez et al., 2019; Wang et al., 2019; Yoshihama et al., 2016). Although this has been attributed to its regulation of the MHC class I pathway, it will be important to consider the potential contribution of the hereby discovered NLRC5/BTN3A regulation axis because altered expression of BTN3A genes has been associated with cancer and other diseases (Benyamine et al., 2017; Blazquez et al., 2018; Le Page et al., 2012; Peedicayil et al., 2010; Viken et al., 2009). Indeed, intratumoral γδ T cells have emerged as the most significant favorable cancer-wide prognostic infiltrating immune subset, and γδ T-cell-based clinical trials are increasingly performed, expanding our portfolio of immunotherapeutical approaches (Gentles et al., 2015). In addition to its role in the activation of γδ T cells, recent findings illustrate how BTN3A1 can play an inhibitory function on αβ T cells, by hindering the segregation of the phosphatase CD45 from the immune synapse (Payne et al., 2020). Importantly, this inhibition is relieved upon zoledronate treatment or by the use of CD277-specific agonistic antibodies, which enable the concomitant activation of Vγ9Vδ2 T cells. Our discoveries indicate therefore that targeting the NLRC5 axis, possibly in combination with such treatments, might represent an attractive anti-cancer strategy leveraging on both αβ and γδ T cells.
Our results suggest that, in humans, NLRC5 regulates more genes than previously thought and challenge its role as a transactivator of MHC class I and related genes only (Ludigs et al., 2015). BTN3A1 molecules have recently been shown to mediate Vγ9Vδ2 T cell activation in response to host- or microbial-derived metabolites, suggesting an involvement of NLRC5 in the γδ T-cell-mediated host immunity (Benyamine et al., 2016; Harly et al., 2012; Sandstrom et al., 2014; Vavassori et al., 2013). This observation is in agreement with previous findings that NLRC5 regulates non-classical MHC class I genes, such as murine H2-T10/H2-T22, which are recognized by a fraction of γδ T cells (Crowley et al., 2000). Therefore, regulation of BTN3A1 transcription by NLRC5 shows strong parallels with its established function, reinforcing its role as a modulator of conventional and unconventional T cell immunity.
Limitations of the study
Even if BTN3A molecules are induced by NLRC5 and required for Vγ9Vδ2 T cell activation, we cannot rule out the possibility that other NLRC5 targets, known or unknown, might contribute to the observed effect. In addition, further experiments are required to prove the pathophysiological relevance of these findings in cancer or infection.
Resource availability
Lead contact
Requests for further information and reagents should be directed to and will be fulfilled by the Lead Contact Greta Guarda (greta.guarda@irb.usi.ch).
Materials availability
Materials generated in this study will be made available upon reasonable request and may require a material transfer agreement.
Data and code availability
This study did not generate datasets or analyze codes.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank S. Monticelli (IRB, Bellinzona) for critical reading of the manuscript and help. We thank R. Spaapen (Sanquin Research, Amsterdam), P. Van den Elsen (Leiden University, Leiden), M. Kornete and L. Jeker (University of Basel, Basel), R. Mantovani (Università degli Studi di Milano, Milan), F. Bertoni (IOR, Bellinzona), T. Kufer (University of Hohenheim, Hohenheim), D. Cohen and K. Ludigs (during their time at the University of Lausanne (UNIL), Lausanne), and M. Juilland and M. Thome (UNIL, Lausanne) for reagents and/or technical help. We thank the UNIL for sharing transgenic mice. This work was supported by the Swiss National Science Foundation [PP00P3_139094, PP00P3_165833, and 310030_185185 to G.G.], the European Research Council [ERC-2012-StG310890 to G.G.], and the Fondazione San Salvatore, Lugano. This work was partially supported by the German Research Foundation (DFG) [EXC-294 (BIOSS), EXC-2189 (CIBSS) and SFB850 (C10) to S.M.].
Author contributions
A.T.D., J.S., A.Z., H.J.K., S.M.B., G.C., and S.T.C. performed the experiments; L.L., S.T.C., P.V.E., S.K., W.R., and S.M. shared reagents, help, and advice; I. K. performed the bioinformatic analyses; A.T.D., J.S., I.K., S.T.C., S.M., and G.G. designed the research, analyzed the data, and wrote the manuscript.
Declaration of interests
Other projects in G.G. laboratory are supported by OM-Pharma, Meyrin, IFM Therapeutics, Boston, and Novartis Foundation. Unrelated projects in SM laboratory are supported by the Eurostars program (EUROPEAN UNION HORIZON, 2020 FRAMEWORK PROGRAM).
Published: January 22, 2021
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101900.
Contributor Information
Sonia T. Chelbi, Email: sonia.chelbi@irb.usi.ch.
Greta Guarda, Email: greta.guarda@irb.usi.ch.
Supplemental Information
References
- Abeler-Dorner L., Swamy M., Williams G., Hayday A.C., Bas A. Butyrophilins: an emerging family of immune regulators. Trends Immunol. 2012;33:34–41. doi: 10.1016/j.it.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Anderson D.A., 3rd, Grajales-Reyes G.E., Satpathy A.T., Vasquez Hueichucura C.E., Murphy T.L., Murphy K.M. Revisiting the specificity of the MHC class II transactivator CIITA in classical murine dendritic cells in vivo. Eur. J. Immunol. 2017;47:1317–1323. doi: 10.1002/eji.201747050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett H.A., Viney J.L. Immune modulation by butyrophilins. Nat. Rev. Immunol. 2014;14:559–569. doi: 10.1038/nri3715. [DOI] [PubMed] [Google Scholar]
- Bakker A.B., Phillips J.H., Figdor C.G., Lanier L.L. Killer cell inhibitory receptors for MHC class I molecules regulate lysis of melanoma cells mediated by NK cells, gamma delta T cells, and antigen-specific CTL. J. Immunol. 1998;160:5239–5245. [PubMed] [Google Scholar]
- Benyamine A., Le Roy A., Mamessier E., Gertner-Dardenne J., Castanier C., Orlanducci F., Pouyet L., Goubard A., Collette Y., Vey N. BTN3A molecules considerably improve Vgamma9Vdelta2T cells-based immunotherapy in acute myeloid leukemia. Oncoimmunology. 2016;5:e1146843. doi: 10.1080/2162402X.2016.1146843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyamine A., Loncle C., Foucher E., Blazquez J.L., Castanier C., Chretien A.S., Modesti M., Secq V., Chouaib S., Gironella M. BTN3A is a prognosis marker and a promising target for Vgamma9Vdelta2 T cells based-immunotherapy in pancreatic ductal adenocarcinoma (PDAC) Oncoimmunology. 2017;7:e1372080. doi: 10.1080/2162402X.2017.1372080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazquez J.L., Benyamine A., Pasero C., Olive D. New insights into the regulation of gammadelta T cells by BTN3A and other BTN/BTNL in tumor immunity. Front. Immunol. 2018;9:1601. doi: 10.3389/fimmu.2018.01601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A.M., Barr C.L., Ting J.P. Sequences homologous to class II MHC W, X, and Y elements mediate constitutive and IFN-gamma-induced expression of human class II-associated invariant chain gene. J. Immunol. 1991;146:3183–3189. [PubMed] [Google Scholar]
- Carena I., Shamshiev A., Donda A., Colonna M., Libero G.D. Major histocompatibility complex class I molecules modulate activation threshold and early signaling of T cell antigen receptor-gamma/delta stimulated by nonpeptidic ligands. J. Exp. Med. 1997;186:1769–1774. doi: 10.1084/jem.186.10.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.H., Guerder S., Hong S.C., van Ewijk W., Flavell R.A. Mice lacking the MHC class II transactivator (CIITA) show tissue-specific impairment of MHC class II expression. Immunity. 1996;4:167–178. doi: 10.1016/s1074-7613(00)80681-0. [DOI] [PubMed] [Google Scholar]
- Chelbi S.T., Dang A.T., Guarda G. Emerging major histocompatibility complex class I-related functions of NLRC5. Adv. Immunol. 2017;133:89–119. doi: 10.1016/bs.ai.2016.11.003. [DOI] [PubMed] [Google Scholar]
- Cheng C., Wang B., Gao L., Liu J., Chen X., Huang H., Zhao Z. Next generation sequencing reveals changes of the gammadelta T cell receptor repertoires in patients with pulmonary tuberculosis. Sci. Rep. 2018;8:3956. doi: 10.1038/s41598-018-22061-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley M.P., Fahrer A.M., Baumgarth N., Hampl J., Gutgemann I., Teyton L., Chien Y. A population of murine gammadelta T cells that recognize an inducible MHC class Ib molecule. Science. 2000;287:314–316. doi: 10.1126/science.287.5451.314. [DOI] [PubMed] [Google Scholar]
- De Libero G., Lau S.Y., Mori L. Phosphoantigen presentation to TCR gammadelta cells, a conundrum getting less gray zones. Front. Immunol. 2014;5:679. doi: 10.3389/fimmu.2014.00679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolfini D., Gatta R., Mantovani R. NF-Y and the transcriptional activation of CCAAT promoters. Crit. Rev. Biochem. Mol. Biol. 2012;47:29–49. doi: 10.3109/10409238.2011.628970. [DOI] [PubMed] [Google Scholar]
- Doyle C., Ford P.J., Ponath P.D., Spies T., Strominger J.L. Regulation of the class II-associated invariant chain gene in normal and mutant B lymphocytes. Proc. Natl. Acad. Sci. U S A. 1990;87:4590–4594. doi: 10.1073/pnas.87.12.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farashi S., Kryza T., Clements J., Batra J. Post-GWAS in prostate cancer: from genetic association to biological contribution. Nat. Rev. Cancer. 2019;19:46–59. doi: 10.1038/s41568-018-0087-3. [DOI] [PubMed] [Google Scholar]
- Fernandez-Jimenez N., Garcia-Etxebarria K., Plaza-Izurieta L., Romero-Garmendia I., Jauregi-Miguel A., Legarda M., Ecsedi S., Castellanos-Rubio A., Cahais V., Cuenin C. The methylome of the celiac intestinal epithelium harbours genotype-independent alterations in the HLA region. Sci. Rep. 2019;9:1298. doi: 10.1038/s41598-018-37746-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisch P., Meuer E., Pende D., Rothenfusser S., Viale O., Kock S., Ferrone S., Fradelizi D., Klein G., Moretta L. Control of B cell lymphoma recognition via natural killer inhibitory receptors implies a role for human Vgamma9/Vdelta2 T cells in tumor immunity. Eur. J. Immunol. 1997;27:3368–3379. doi: 10.1002/eji.1830271236. [DOI] [PubMed] [Google Scholar]
- Gentles A.J., Newman A.M., Liu C.L., Bratman S.V., Feng W., Kim D., Nair V.S., Xu Y., Khuong A., Hoang C.D. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 2015;21:938–945. doi: 10.1038/nm.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobin S.J., Peijnenburg A., Keijsers V., van den Elsen P.J. Site alpha is crucial for two routes of IFN gamma-induced MHC class I transactivation: the ISRE-mediated route and a novel pathway involving CIITA. Immunity. 1997;6:601–611. doi: 10.1016/s1074-7613(00)80348-9. [DOI] [PubMed] [Google Scholar]
- Gu S., Sachleben J.R., Boughter C.T., Nawrocka W.I., Borowska M.T., Tarrasch J.T., Skiniotis G., Roux B., Adams E.J. Phosphoantigen-induced conformational change of butyrophilin 3A1 (BTN3A1) and its implication on Vgamma9Vdelta2 T cell activation. Proc. Natl. Acad. Sci. U S A. 2017;114:E7311–E7320. doi: 10.1073/pnas.1707547114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halary F., Peyrat M.A., Champagne E., Lopez-Botet M., Moretta A., Moretta L., Vie H., Fournie J.J., Bonneville M. Control of self-reactive cytotoxic T lymphocytes expressing gamma delta T cell receptors by natural killer inhibitory receptors. Eur. J. Immunol. 1997;27:2812–2821. doi: 10.1002/eji.1830271111. [DOI] [PubMed] [Google Scholar]
- Harly C., Guillaume Y., Nedellec S., Peigne C.M., Monkkonen H., Monkkonen J., Li J., Kuball J., Adams E.J., Netzer S. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human gammadelta T-cell subset. Blood. 2012;120:2269–2279. doi: 10.1182/blood-2012-05-430470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongsma M.L.M., Guarda G., Spaapen R.M. The regulatory network behind MHC class I expression. Mol. Immunol. 2019;113:16–21. doi: 10.1016/j.molimm.2017.12.005. [DOI] [PubMed] [Google Scholar]
- Kabelitz D., Bender A., Prospero T., Wesselborg S., Janssen O., Pechhold K. The primary response of human gamma/delta + T cells to Mycobacterium tuberculosis is restricted to V gamma 9-bearing cells. J. Exp. Med. 1991;173:1331–1338. doi: 10.1084/jem.173.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunakaran M.M., Willcox C.R., Salim M., Paletta D., Fichtner A.S., Noll A., Starick L., Nohren A., Begley C.R., Berwick K.A. Butyrophilin-2A1 directly binds germline-encoded regions of the Vgamma9Vdelta2 TCR and is essential for phosphoantigen sensing. Immunity. 2020;52:487–498 e486. doi: 10.1016/j.immuni.2020.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk M., Peyraud N., Rybtsova N., Masternak K., Bucher P., Barras E., Reith W. Long distance control of MHC class II expression by multiple distal enhancers regulated by regulatory factor X complex and CIITA. J. Immunol. 2004;173:6200–6210. doi: 10.4049/jimmunol.173.10.6200. [DOI] [PubMed] [Google Scholar]
- Krawczyk M., Seguin-Estevez Q., Leimgruber E., Sperisen P., Schmid C., Bucher P., Reith W. Identification of CIITA regulated genetic module dedicated for antigen presentation. PLoS Genet. 2008;4:e1000058. doi: 10.1371/journal.pgen.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Page C., Marineau A., Bonza P.K., Rahimi K., Cyr L., Labouba I., Madore J., Delvoye N., Mes-Masson A.M., Provencher D.M. BTN3A2 expression in epithelial ovarian cancer is associated with higher tumor infiltrating T cells and a better prognosis. PLoS one. 2012;7:e38541. doi: 10.1371/journal.pone.0038541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludigs K., Jandus C., Utzschneider D.T., Staehli F., Bessoles S., Dang A.T., Rota G., Castro W., Zehn D., Vivier E. NLRC5 shields T lymphocytes from NK-cell-mediated elimination under inflammatory conditions. Nat. Commun. 2016;7:10554. doi: 10.1038/ncomms10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludigs K., Seguin-Estevez Q., Lemeille S., Ferrero I., Rota G., Chelbi S., Mattmann C., MacDonald H.R., Reith W., Guarda G. NLRC5 exclusively transactivates MHC class I and related genes through a distinctive SXY module. PLoS Genet. 2015;11:e1005088. doi: 10.1371/journal.pgen.1005088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maertzdorf J., Ota M., Repsilber D., Mollenkopf H.J., Weiner J., Hill P.C., Kaufmann S.H. Functional correlations of pathogenesis-driven gene expression signatures in tuberculosis. PLoS one. 2011;6:e26938. doi: 10.1371/journal.pone.0026938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B.K., Chin K.C., Olsen J.C., Skinner C.A., Dey A., Ozato K., Ting J.P. Induction of MHC class I expression by the MHC class II transactivator CIITA. Immunity. 1997;6:591–600. doi: 10.1016/s1074-7613(00)80347-7. [DOI] [PubMed] [Google Scholar]
- Masternak K., Peyraud N., Krawczyk M., Barras E., Reith W. Chromatin remodeling and extragenic transcription at the MHC class II locus control region. Nat. Immunol. 2003;4:132–137. doi: 10.1038/ni883. [DOI] [PubMed] [Google Scholar]
- Meissner T.B., Li A., Biswas A., Lee K.H., Liu Y.J., Bayir E., Iliopoulos D., van den Elsen P.J., Kobayashi K.S. NLR family member NLRC5 is a transcriptional regulator of MHC class I genes. Proc. Natl. Acad. Sci. U S A. 2010;107:13794–13799. doi: 10.1073/pnas.1008684107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner T.B., Li A., Kobayashi K.S. NLRC5: a newly discovered MHC class I transactivator (CITA) Microbes Infect. 2012;14:477–484. doi: 10.1016/j.micinf.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner T.B., Liu Y.J., Lee K.H., Li A., Biswas A., van Eggermond M.C., van den Elsen P.J., Kobayashi K.S. NLRC5 cooperates with the RFX transcription factor complex to induce MHC class I gene expression. J. Immunol. 2012;188:4951–4958. doi: 10.4049/jimmunol.1103160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita C.T., Beckman E.M., Bukowski J.F., Tanaka Y., Band H., Bloom B.R., Golan D.E., Brenner M.B. Direct presentation of nonpeptide prenyl pyrophosphate antigens to human gamma delta T cells. Immunity. 1995;3:495–507. doi: 10.1016/1074-7613(95)90178-7. [DOI] [PubMed] [Google Scholar]
- Neerincx A., Castro W., Guarda G., Kufer T.A. NLRC5, at the heart of antigen presentation. Front. Immunol. 2013;4:397. doi: 10.3389/fimmu.2013.00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neerincx A., Lautz K., Menning M., Kremmer E., Zigrino P., Hosel M., Buning H., Schwarzenbacher R., Kufer T.A. A role for the human nucleotide-binding domain, leucine-rich repeat-containing family member NLRC5 in antiviral responses. J. Biol. Chem. 2010;285:26223–26232. doi: 10.1074/jbc.M110.109736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neerincx A., Rodriguez G.M., Steimle V., Kufer T.A. NLRC5 controls basal MHC class I gene expression in an MHC enhanceosome-dependent manner. J. Immunol. 2012;188:4940–4950. doi: 10.4049/jimmunol.1103136. [DOI] [PubMed] [Google Scholar]
- Payne K.K., Mine J.A., Biswas S., Chaurio R.A., Perales-Puchalt A., Anadon C.M., Costich T.L., Harro C.M., Walrath J., Ming Q. BTN3A1 governs antitumor responses by coordinating alphabeta and gammadelta T cells. Science. 2020;369:942–949. doi: 10.1126/science.aay2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peedicayil A., Vierkant R.A., Hartmann L.C., Fridley B.L., Fredericksen Z.S., White K.L., Elliott E.A., Phelan C.M., Tsai Y.Y., Berchuck A. Risk of ovarian cancer and inherited variants in relapse-associated genes. PloS one. 2010;5:e8884. doi: 10.1371/journal.pone.0008884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith W., Mach B. The bare lymphocyte syndrome and the regulation of MHC expression. Annu. Rev. Immunol. 2001;19:331–373. doi: 10.1146/annurev.immunol.19.1.331. [DOI] [PubMed] [Google Scholar]
- Riano F., Karunakaran M.M., Starick L., Li J., Scholz C.J., Kunzmann V., Olive D., Amslinger S., Herrmann T. Vgamma9Vdelta2 TCR-activation by phosphorylated antigens requires butyrophilin 3 A1 (BTN3A1) and additional genes on human chromosome 6. Eur. J. Immunol. 2014;44:2571–2576. doi: 10.1002/eji.201444712. [DOI] [PubMed] [Google Scholar]
- Rigau M., Ostrouska S., Fulford T.S., Johnson D.N., Woods K., Ruan Z., McWilliam H.E.G., Hudson C., Tutuka C., Wheatley A.K. Butyrophilin 2A1 is essential for phosphoantigen reactivity by gammadelta T cells. Science. 2020;367:eaay5516. doi: 10.1126/science.aay5516. [DOI] [PubMed] [Google Scholar]
- Robbins G.R., Truax A.D., Davis B.K., Zhang L., Brickey W.J., Ting J.P. Regulation of class I major histocompatibility complex (MHC) by nucleotide-binding domain, leucine-rich repeat-containing (NLR) proteins. J. Biol. Chem. 2012;287:24294–24303. doi: 10.1074/jbc.M112.364604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom A., Peigne C.M., Leger A., Crooks J.E., Konczak F., Gesnel M.C., Breathnach R., Bonneville M., Scotet E., Adams E.J. The intracellular B30.2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vgamma9Vdelta2 T cells. Immunity. 2014;40:490–500. doi: 10.1016/j.immuni.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter K., Leimgruber E., Gobet F., Agrawal V., Dunand-Sauthier I., Barras E., Mastelic-Gavillet B., Kamath A., Fontannaz P., Guery L. Btn2a2, a T cell immunomodulatory molecule coregulated with MHC class II genes. J. Exp. Med. 2016;213:177–187. doi: 10.1084/jem.20150435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I.A., Knezevic B.R., Ammann J.U., Rhodes D.A., Aw D., Palmer D.B., Mather I.H., Trowsdale J. BTN1A1, the mammary gland butyrophilin, and BTN2A2 are both inhibitors of T cell activation. J. Immunol. 2010;184:3514–3525. doi: 10.4049/jimmunol.0900416. [DOI] [PubMed] [Google Scholar]
- Staehli F., Ludigs K., Heinz L.X., Seguin-Estevez Q., Ferrero I., Braun M., Schroder K., Rebsamen M., Tardivel A., Mattmann C. NLRC5 deficiency selectively impairs MHC class I- dependent lymphocyte killing by cytotoxic T cells. J. Immunol. 2012;188:3820–3828. doi: 10.4049/jimmunol.1102671. [DOI] [PubMed] [Google Scholar]
- Tarantelli C., Gaudio E., Arribas A.J., Kwee I., Hillmann P., Rinaldi A., Cascione L., Spriano F., Bernasconi E., Guidetti F. PQR309 is a novel dual PI3K/mTOR inhibitor with preclinical antitumor activity in lymphomas as a single agent and in combination therapy. Clin. Cancer Res. 2018;24:120–129. doi: 10.1158/1078-0432.CCR-17-1041. [DOI] [PubMed] [Google Scholar]
- Vantourout P., Hayday A. Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nat. Rev. Immunol. 2013;13:88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vantourout P., Laing A., Woodward M.J., Zlatareva I., Apolonia L., Jones A.W., Snijders A.P., Malim M.H., Hayday A.C. Heteromeric interactions regulate butyrophilin (BTN) and BTN-like molecules governing gammadelta T cell biology. Proc. Natl. Acad. Sci. U S A. 2018;115:1039–1044. doi: 10.1073/pnas.1701237115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavassori S., Kumar A., Wan G.S., Ramanjaneyulu G.S., Cavallari M., El Daker S., Beddoe T., Theodossis A., Williams N.K., Gostick E. Butyrophilin 3A1 binds phosphorylated antigens and stimulates human gammadelta T cells. Nat. Immunol. 2013;14:908–916. doi: 10.1038/ni.2665. [DOI] [PubMed] [Google Scholar]
- Viken M.K., Blomhoff A., Olsson M., Akselsen H.E., Pociot F., Nerup J., Kockum I., Cambon-Thomsen A., Thorsby E., Undlien D.E. Reproducible association with type 1 diabetes in the extended class I region of the major histocompatibility complex. Genes Immun. 2009;10:323–333. doi: 10.1038/gene.2009.13. [DOI] [PubMed] [Google Scholar]
- Wang Q., Ding H., He Y., Li X., Cheng Y., Xu Q., Yang Y., Liao G., Meng X., Huang C. NLRC5 mediates cell proliferation, migration, and invasion by regulating the Wnt/beta-catenin signalling pathway in clear cell renal cell carcinoma. Cancer Lett. 2019;444:9–19. doi: 10.1016/j.canlet.2018.11.024. [DOI] [PubMed] [Google Scholar]
- Williams G.S., Malin M., Vremec D., Chang C.H., Boyd R., Benoist C., Mathis D. Mice lacking the transcription factor CIITA--a second look. Int. Immunol. 1998;10:1957–1967. doi: 10.1093/intimm/10.12.1957. [DOI] [PubMed] [Google Scholar]
- Wu C., Orozco C., Boyer J., Leglise M., Goodale J., Batalov S., Hodge C.L., Haase J., Janes J., Huss J.W., 3rd BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome Biol. 2009;10:R130. doi: 10.1186/gb-2009-10-11-r130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Wang Y., Chen F., Huang Y., Zhu S., Leng Q., Wang H., Shi Y., Qian Y. NLRC5 regulates MHC class I antigen presentation in host defense against intracellular pathogens. Cell Res. 2012;22:836–847. doi: 10.1038/cr.2012.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihama S., Roszik J., Downs I., Meissner T.B., Vijayan S., Chapuy B., Sidiq T., Shipp M.A., Lizee G.A., Kobayashi K.S. NLRC5/MHC class I transactivator is a target for immune evasion in cancer. Proc. Natl. Acad. Sci. U S A. 2016;113:5999–6004. doi: 10.1073/pnas.1602069113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., Jones P.P. Transcriptional control of the invariant chain gene involves promoter and enhancer elements common to and distinct from major histocompatibility complex class II genes. Mol. Cell. Biol. 1990;10:3906–3916. doi: 10.1128/mcb.10.8.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zocchi M.R., Costa D., Vene R., Tosetti F., Ferrari N., Minghelli S., Benelli R., Scabini S., Romairone E., Catellani S. Zoledronate can induce colorectal cancer microenvironment expressing BTN3A1 to stimulate effector gammadelta T cells with antitumor activity. Oncoimmunology. 2017;6:e1278099. doi: 10.1080/2162402X.2016.1278099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate datasets or analyze codes.