Abstract
The Western Pacific Region is the largest and most diverse region in the world, made up of 37 countries and territories in the Pacific, Oceania and parts of Asia, with a population of more than 1.9 billion people stretching over an area from China and Mongolia in the north to New Zealand in the south. In 1999, 22 countries and territories in the Pacific joined together and launched the Pacific Programme to Eliminate Lymphatic Filariasis. Shortly after, the Global Programme to Eliminate Lymphatic Filariasis was launched in 2000. In 2004, 12 countries in the Asia subregion of the Western Pacific Region and Southeast Asian Region joined and developed the Mekong-Plus Strategic Plan for Elimination of Lymphatic Filariasis. Since then, significant efforts have been made by all endemic countries, with annual mass drug administration (MDA) as a principal strategy, through strong partnership with the WHO and other donors and partners. As a result, by the end of 2019, 10 of 22 endemic countries in the region, including 8 of 16 countries in the Pacific and 2 countries in the Asia subregion, achieved WHO validation for elimination of lymphatic filariasis (LF) as a public health problem. All the other countries are either progressing with post-MDA surveillance or accelerating efforts by adoption of the new triple drug therapy strategy and enhancement of MDA campaigns to tackle persistent transmission. Some 85% of the originally endemic implementation units have stopped MDA and the number of people requiring MDA for LF in the Western Pacific Region was reduced by 72% from 2000 to 2018. This paper reviews the progress, key success factors and remaining challenges and indicates the way forward to achieve LF elimination in the Western Pacific Region.
Introduction
The WHO Western Pacific Region is the largest and most diverse region in the world, made up of 37 countries and territories in the Pacific, Oceania and parts of Asia, with a population of more than 1.9 billion people from China and Mongolia in the north to New Zealand in the south. Lymphatic filariasis (LF) in the Western Pacific Region is also epidemiologically complex and diverse (Table 1).1Wuchereria bancrofti is the most widespread parasite in the region, is nocturnally periodic in the Asia subregion, Micronesia and part of Melanesia, but is diurnally subperiodic in Polynesia and the other part of Melanesia. Brugia malayi is also known to be present in Brunei Darussalam, Malaysia and parts of Cambodia, the Philippines and Vietnam in the region.
Table 1.
Filarial parasites and vectors in 22 LF-endemic countries in the WHO Western Pacific Region1
| Parasite | ||||
|---|---|---|---|---|
| Sub-region | Country and area | Species | Periodicity | Vector |
| Pacific (Polynesia) | American Samoa | W. bancrofti | Diurnally sub-periodic | Aedes |
| Cook Islands | W. bancrofti | Diurnally sub-periodic | Aedes | |
| French Polynesia | W. bancrofti | Diurnally sub-periodic | Aedes | |
| Niue | W. bancrofti | Diurnally sub-periodic | Aedes | |
| Samoa | W. bancrofti | Diurnally sub-periodic | Aedes | |
| Tonga | W. bancrofti | Diurnally sub-periodic | Aedes | |
| Tuvalu | W. bancrofti | Diurnally sub-periodic | Aedes | |
| Wallis and Futuna | W. bancrofti | Diurnally sub-periodic | Aedes | |
| Pacific (Micronesia) | Kiribati | W. bancrofti | Nocturnally periodic | Culex |
| Marshall Islands | W. bancrofti | Nocturnally periodic | Culex | |
| Micronesia, FS | W. bancrofti | Nocturnally periodic | Culex | |
| Palau | W. bancrofti | Nocturnally periodic | Culex | |
| Pacific (Melanesia) | Fiji | W. bancrofti | Diurnally sub-periodic | Aedes |
| New Caledonia | W. bancrofti | Diurnally sub-periodic | Aedes | |
| Papua New Guinea | W. bancrofti | Nocturnally periodic | Anopheles | |
| Vanuatu | W. bancrofti | Nocturnally periodic | Anopheles | |
| Asia | Brunei Darussalam | B. malayi | Nocturnally periodic | Mansonia |
| Cambodia | W. bancrofti | Nocturnally periodic | Anopheles | |
| Lao PDR | W. bancrofti | Nocturnally periodic | ||
| Malaysia | W. bancrofti | Nocturnally periodic | Anopheles | |
| B. malayi | Nocturnally periodic | Mansonia | ||
| Diurnally sub-periodic | Mansonia | |||
| Philippines | W. bancrofti | Nocturnally periodic | Anopheles | |
| Diurnally sub-periodic | Aedes | |||
| B. malayi | Nocturnally sub-periodic | Mansonia | ||
| Viet Nam | W. bancrofti | Nocturnally periodic | Culex | |
| B. malayi | Nocturnally periodic | Mansonia | ||
Historical context of LF elimination prior to the launch of the Pacific Programme to Eliminate Lymphatic Filariasis and the Global Programme to Eliminate Lymphatic Filariasis (before 1999)
The Western Pacific Region has a long history of fighting LF. The first record of elephantiasis was made by Captain James Cook in Tonga in 1785; in 1877, Manson described in Amoy (now Xiamen), China, the involvement of an insect in the transmission of an infectious agent, W. bancrofti by Culex mosquitoes.2,3 This was a landmark discovery that later led Ross to demonstrate that mosquitoes were vectors of malaria parasites and that Aedes aegypti transmitted yellow fever in the Americas.4 These discoveries led to the emergence of the discipline of medical entomology. Microfilariae were observed in blood films as early as 1896 in Fiji, Tonga and Samoa.2 The first attempt at mass control in the Pacific began in Fiji in 1944, focusing on vector control. During the 1950s, mass treatment using diethylcarbamazine citrate (DEC) was implemented at scale in many Pacific island countries, namely, American Samoa, Fiji, French Polynesia, Samoa and Wallis and Futuna and on a more limited scale in the Cook Islands, Niue, Palau, Papua New Guinea, Tokelau, Tonga and Tuvalu; this followed extensive WHO surveys by Iyengar in the region.2,5 In the absence of a standard recommended regimen, various mass drug administration (MDA) strategies (different doses, frequency and duration) were attempted; however, multiple rounds of MDA using DEC with high coverage was found to be highly effective in reducing the prevalence of infection overall.2,5
In the Asia subregion, the first description of microfilariae in the blood of a patient was in Japan and China in 1876, followed by the Philippines, Malaysia and Korea in the early 1900s.5–9 Prior to World War II, several epidemiological surveys on filariasis were conducted and the geographical distribution was elucidated in these countries; these studies were extended after 1945 and various intervention chemotherapy strategies tested. A trial treatment with DEC was initiated in 1951 in Japan, which was soon followed in Malaysia, Korea, China and the Philippines, initially for individual case treatment then later for mass treatment.5-10 Following blood surveys and DEC treatment of microfilaria-positive cases, Japan finally celebrated the elimination of LF in 1988.6,7 China also reached the basic elimination criteria (<1% microfilaremia prevalence at village level) in the 1990s using a DEC-fortified salt strategy and was acknowledged by the WHO for having eliminated LF as a public health problem in 2007.8 Korea was also validated as having eliminated LF in 2008.9 Malaysia and the Philippines launched national filariasis control programmes in 1960s, which reduced the nationwide prevalence by the 1990s.10,11 The epidemiological situation was also known in other endemic countries in Asia by the 1970s but no significant LF control interventions were carried out prior to the launch of the Global Programme to Eliminate Lymphatic Filariasis (GPELF).
Progress of LF elimination after the launch of the Pacific Programme to Eliminate Lymphatic Filariasis and GPELF (after 1999)
In 1997, the World Health Assembly adopted a resolution to call for global elimination of LF as a public health problem. In March 1999, the Meeting of the Ministers of Health for the Pacific Island Countries convened by the WHO Western Pacific Regional Office (WPRO) acted on this resolution, requesting the WPRO to make elimination of LF a priority for these countries. This resulted in the launch of the Pacific Programme to Eliminate Lymphatic Filariasis (PacELF) as an alliance of 22 Pacific island countries and areas.2,12 In 2000, the WHO launched GPELF, with a goal to achieve global elimination of LF as a public health problem by 2020.
Table 2 shows the progress of LF elimination programmes in the Western Pacific Region to date, with reported national MDA coverage. Many countries in the Pacific began LF remapping in 1999–2001 using the standard protocol and, consequently, 17 countries including Papua New Guinea were formally classified as endemic.2 Countries that showed high baseline antigenemia prevalence included American Samoa (16.5%), Fiji (16.6%), French Polynesia (10.8% in Leeward islands and 17.7% in Marquesas islands) and Tuvalu (22.3%).2 The majority of Pacific island countries had the entire country as one implementation unit (IU), with the exception of relatively large countries such as Fiji and French Polynesia. In 1999, Samoa became the first country to implement MDA since the launch of the PacELF.2 Subsequently, 9 out of 17 endemic countries in the Pacific began implementing MDA during 2000 and 2001. By 2003, the total number of people treated peaked at >1.2 million people in 13 countries against the estimated 1.9 million people requiring MDA (equivalent to 63.2% treatment coverage across the PacELF).13
Table 2.
Progress of LF elimination in the Western Pacific Region 1999-2020 (the number in cells are reported national MDA coverage)12
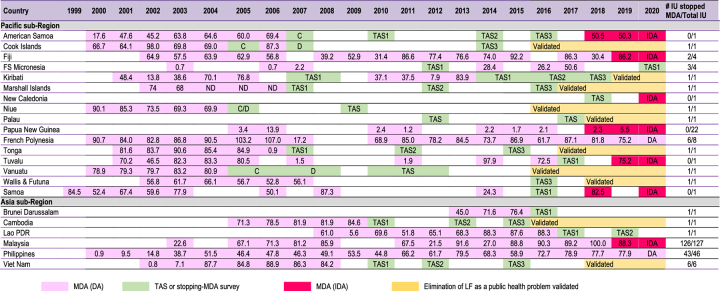
|
IU: implementation unit; FS Micronesia.: Federated states of Micronesia; Lao PDR: Lao People's Democratic Republic; MDA: mass drug administration; PNG: Papua New Guinea; TAS: transmission assessment survey; DA: DEC and albendazole; IDA: ivermectin, DEC and albendazole.
During 2005–2007, several countries began to stop MDA and conduct impact assessment after the five rounds of MDA.2 The Cook Islands, Niue, Tonga and Vanuatu were found to have achieved the criteria for stopping MDA of <1% antigenemia prevalence from the baseline antigenaemia prevalence, in the range of 2.7–8.6% at the launch of the PacELF after five rounds of MDA with effective coverage.2,14-17 All these countries stopped MDA in 2007. In the Marshall Islands, only Mejit island and Ailuk Atoll were found to have a high LF prevalence in 2002 (44.2% and 29.1%, respectively) and thus MDA was only targeted at these two locations.2 After five consecutive rounds of MDA with effective coverage, these two IUs also reduced their antigenaemia prevalence to <1% and, consequently, the Marshall Islands also stopped MDA in 2007.2 In 2011, the WHO published the GPELF manual on transmission assessment surveys (TAS) methodology to assess if a series of rounds of MDA had successfully reduced the prevalence of infection to levels equal to or below the critical cut-off threshold and to decide whether MDA could be stopped.18 American Samoa, Wallis and Futuna, and Kiribati passed TAS1 and formally moved to the post-MDA surveillance phase in 2011, 2012 and 2013, respectively, despite not fully achieving five consecutive rounds of effective MDA.13 Countries that entered the post-MDA surveillance phase continued with implementation of at least three rounds of TAS every 2 years.
In 2016, the WHO established a standard operating procedure for validating the national elimination of LF as a public health problem.19 Following this, the WHO validated elimination status in the Cook Islands, Niue and Vanuatu in 2016, the Marshall Islands and Tonga in 2017, Palau and Wallis and Futuna in 2018 and Kiribati in 2019. Hence, by 2020, 8 of 17 PacELF countries had reached the 2020 elimination target.
After the launch of the GPELF, the endemic countries in the Asia subregion revived efforts for LF elimination using the standardised GPELF protocols for MDA, monitoring and evaluation. Furthermore, following the example of the PacELF as a subregional alliance, eight endemic countries in the Asia subregion and four countries in the WHO Southeast Asia Region joined to create the Mekong-Plus group and developed the Mekong-Plus Strategic Plan for Elimination of LF 2004–2010.20 All endemic countries began remapping LF using nationwide surveys or historical data on endemicity and clinical cases. The Philippines became the first country to start MDA in the Asia subregion.13 MDA was initiated in five provinces in 2001, then was progressively scaled up to reach 100% geographical coverage in 2003.13 Because of logistic difficulties in a nation of several thousand islands, the presence of many indigenous populations and areas of armed conflict, the Philippines struggled to reach national coverage of 65% until 2011. Nonetheless, the national and subnational programmes continued to demonstrate commitment to progressing LF elimination and, in 2008, the first stopping-MDA survey was implemented and three provinces successfully stopped MDA. As of August 2020, 43 of 46 endemic IUs (93.4%) have moved to post-MDA surveillance (unpublished data). Cambodia identified only one province with >1% antigen prevalence; however, a decision was made to take a conservative approach by not only including another province with some microfilaria positives, but also to map two suspected provinces at district level and concluding two provinces and an additional four districts from two other provinces as endemic IUs.21 Five consecutive rounds of MDA were implemented covering all endemic IUs during 2005–2009, which achieved >70% epidemiological coverage.21 After a stopping-MDA survey was passed in 2010, Cambodia continued to pass TAS 2 and 3 criteria and become the first country in the Asia subregion to be validated for LF elimination in 2016.21 Vietnam also found LF was focalised to six districts. MDA was started there in 2003, then was gradually scaled up and each IU completed five consecutive rounds of MDA by the end of 2008, with all achieving >80% epidemiological coverage.22 Stopping-MDA surveys were passed in 2010–2011, TAS 2 and 3 were also passed and Vietnam was validated by the WHO as having achieved LF elimination in 2016.22
Malaysia defined mukims (equivalent to subdistricts) or submukims (equivalent to communities) as IUs, in contrast to other countries where provinces or districts were defined as IUs. Malaysia began a series of high-coverage MDA and extensive surveillance activities throughout endemic areas in 2003.11 In 2010–2011, TAS 1 was carried out using antibody-based test kit in Peninsular Malaysia and consequently 63 of 67 IUs achieved <2% antibody prevalence and stopped MDA. Since then, many IUs have progressed with TAS, although some failed pre-TAS or TAS and continued or resumed MDA. By 2018, all 127 IUs stopped MDA and 105 IUs even passed TAS 3 (82.7%). However, one IU in Sarawak State failed TAS 2 in 2018. In addition, a series of hotspots with >2% antibody prevalence at village level have been discovered through intensive surveillance activities in IUs that have already stopped MDA, many of which are aboriginal communities.
Lao People's Democratic Republic identified only one province as endemic. MDA was started in one of the districts in the endemic province in 2008 then was gradually scaled up to cover all five districts in 2010; effective coverage continued annually until 2015 (except for one round in 2011). The province passed TAS 1 in 2016 and TAS 2 in 2019, each recording zero antigen positives (unpublished data). Lao People's Democratic Republic is scheduled for TAS 3 in 2021. Brunei Darussalam found that all districts had <1% antigenemia prevalence, while some subdistricts had focal transmission. Three consecutive rounds of annual MDA were implemented in 2013–2015, all with effective coverage. Brunei Darussalam passed TAS 1 in 2016.23
In November 2017, the WHO issued guidelines recommending the triple therapy of ivermectin, DEC and Albendazole (IDA) as an alternative regimen to accelerate the elimination of LF.24 This followed initial work on the efficacy and safety of IDA in Papua New Guinea over the previous decade.25-27 This was regarded not only as a game-changer to reduce the timeline to elimination but also as an opportunity to revive LF elimination efforts in the remaining endemic countries in the Western Pacific Region. Samoa was the first country in the world to implement IDA in August 2018. This was followed by American Samoa, Fiji and Papua New Guinea in the same year, followed by Malaysia and Tuvalu in 2019 (Table 2). Extensive efforts were made to retrain the health workforce to renew their knowledge on LF transmission and elimination to ensure that the IDA strategy was safely implemented. To date, all countries adopting IDA in the region have achieved effective coverage. All the remaining endemic countries are preparing to adopt IDA in 2021. With this momentum, all countries in the region (with the exception of Papua New Guinea) are expected to stop MDA and move to post-MDA surveillance by the end of 2023. Papua New Guinea plans to stop MDA in major island provinces and scale up its efforts to highland provinces with IDA over the coming years.
Key success factors and remaining challenges of LF elimination in the Western Pacific Region
Efforts to eliminate LF, which started in the early 1900s in the Western Pacific Region, have both reduced the overall prevalence over time and also generated valuable experience with which to guide current global strategies. The PacELF and GPELF have standardised control and monitoring strategies and supported all endemic countries to accelerate the elimination programme. Consequently, half of the endemic countries in the region have achieved the elimination of LF as a public health problem by the initial target date of 2020. By 2020, 85% of the originally endemic IUs stopped MDA and the number of people requiring MDA for LF in the Western Pacific Region was reduced by 72% from 2000 to 2018.28 The success and progress of LF elimination in the region to date is attributed to a combination of strategic, programmatic and epidemiological factors, as described below.
Country ownership and leadership and programmatic capacity built at all levels in countries
The PacELF, which preceded the launch of the GPELF, strived to develop simple, evidence-based and practical programmatic steps, standard operating protocols and achievable goals that could be understood, followed and implemented by each country regardless of differences in size, culture, language and religion.29 Country ownership and leadership is at the core of the PacELF and GPELF. Historically, most countries in the region had few implementing partners to support operations inside their countries, other than the WHO, academic institutions and international volunteers. Therefore, efforts were made to ensure that the capacity to plan and implement LF elimination activities was built in countries, with support of the global and regional partnership. This enabled countries to assume the leadership of programmes and maintain a strong commitment facilitated by supporting agencies.
In Malaysia and the Philippines, despite their geographical and epidemiological complexity with multiple vectors and parasites, numerous indigenous populations, islands or extensive forest habitats, >90% of the endemic areas have currently moved to the post-MDA surveillance phase and are expected to move to post-MDA surveillance nationwide in the coming years. This was also made possible by a strong commitment of LF elimination programme staff at all levels of the countries to implement MDA campaigns under difficult circumstances and in the local context by utilising opportunities, despite the absence of external donors and limited in-country implementing partners.
Despite the current COVID-19 pandemic, countries continue to execute the programme, demonstrating commitment and flexibility. The majority of countries and areas in the Western Pacific Region have so far managed to keep COVID-19 under control through strict travel restrictions, border control and quarantine measures. Despite restrictions on international travel, many countries continue to implement planned MDA and deworming campaigns while mitigating protective measures against COVID-19 are in place. Vanuatu, having built a strong capacity to engage communities through LF elimination efforts, continues to deliver treatment for yaws, scabies and soil-transmitted helminthiases integrated with a COVID-19 awareness campaign initiated at the beginning of the pandemic.
Five consecutive rounds of MDA with effective coverage through strong engagement of communities and health workforce
Cambodia, the Cook Islands, Niue, Tonga, Vanuatu and Vietnam all implemented and sustained high coverage MDA for five to six consecutive years in line with the GPELF guidance. In these countries, extensive social mobilisation and communication campaigns were organised using TV, radio, newspapers, leaflets, schools, community volunteers, churches and temples while various in-country partners were engaged in the delivery of medicines house-to-house, via schools, markets, health facilities and workplaces to ensure directly observed treatment and safety of MDA.12-16,20 All health staff and health workers involved in the MDA campaigns were thoroughly trained and jointly planned MDA preparation and implementation, including monitoring of potential adverse events and how to prevent and manage them, and led social mobilisation and health education campaigns targeting local policymakers, community and religious leaders, school principals and community members.13-16,20 It was emphasised at the Programme Managers Meeting to Accelerate Control and Elimination of Neglected Tropical Diseases in the Western Pacific Region (held virtually on 1–4 September 2020) that listening to local leaders, health service providers and beneficiaries, engaging them in MDA planning, preparation and implementation, creating regular opportunities for local implementers to review data and jointly discuss achievements and failure to improve MDA coverage, has been the key to success to date.
Vector transmission efficiency and effective vector control
The principal vectors of W. bancrofti and B. malayi are predominantly night-biting Anopheles, Mansonia or Culex mosquitoes. LF-endemic areas in Cambodia were also endemic for malaria and vector control against malaria was extensively implemented.21 In the Red River Delta of Vietnam, improvement in housing and infrastructure appears to have contributed to a reduction in Culex mosquito abundance (unpublished data). These factors might have led to the focalised transmission of both parasites in just a few districts in Cambodia and Vietnam at the programme’s inception. In Vanuatu, where the vector of W. bancrofti was Anopheles mosquitoes, intensive malaria vector control interventions have long been implemented.16 In early studies by Webber, effective vector control for malaria elimination was considered to be the major factor in eliminating LF from the Solomon Islands without LF-specific interventions.30,31
In Kiribati, the Marshall Islands and Palau in Micronesia, W. bancrofti is nocturnally periodic and transmitted by Culex spp. Culex mosquitoes, however, are the least efficient vectors among all four vector mosquito genera and thus lower intensities of transmission are observed in the extensive endemic areas where Culex acts as vector.32,33
Small populations with limited population mobility within country and from other endemic countries
The majority of countries that continue to experience persistent transmission in the Pacific are where highly efficient and day-biting Aedes mosquitoes are the principal vectors of W. bancrofti. However, despite sharing Aedes vectors, the Cook Islands, Niue, Tonga, Wallis and Futuna were able to achieve the elimination goal. Their success might additionally be attributed to their population sizes, with limited population mobility. The population of each of the Cook Islands, Niue, Wallis and Futuna is <18 000 (Tonga is an exception with a population of >103 000). By contrast, those countries with persistent transmission, such as Fiji, French Polynesia and Samoa, have populations of 200 000–900 000 as well as significant movement of peoples both within country and from other endemic countries. The extent of population mobility between American Samoa villages, Samoa districts and other countries has been investigated and is indicated to be one of the potential factors contributing to persistent transmission of LF in both countries, although this needs further investigation.34,35
The remaining challenges and the way forward—ending the fight against LF in the Western Pacific Region
Despite remarkable progress and success to date, the remaining journey to complete the last mile to the regional elimination of LF remains a challenge.
First, the majority of countries that continue to experience persistent transmission in the Pacific—American Samoa, Fiji, French Polynesia, Samoa and Tuvalu—are where highly efficient and day-biting Aedes mosquitoes are the principal vectors of W. bancrofti. The prevalence of LF in these countries was historically as high as >40% when mass treatment against LF started in the 1950s.2 At the inception of the PacELF in 1999–2001, baseline prevalence was 13.8–22.3%.2 The long history of MDA over several decades results in fatigue in continuing MDA among communities and the health workforce, making achieving consecutive rounds of high-coverage MDA difficult. Despite continuous efforts that invariably reduced the overall prevalence of LF and led some areas in these countries to move to post-MDA surveillance, recrudescence of transmission or persistent transmission in some foci has been observed. The timely introduction and WHO approval of the triple therapy IDA regimen has emerged as a catalyst to revive national efforts. The WHO's recommendation on integrated vector management to complement MDA in areas of insufficient MDA impact should also be revisited.1,36-38
Second, all remaining countries and areas in the Western Pacific Region have geographic, logistic and security challenges, ranging from the outer islands of Tuvalu, which are hundreds of kilometres apart from the main island, to pristine forests in the highlands of Papua New Guinea and areas of armed conflict in the Philippines. Reaching these communities and delivering essential medication or test kits on an annual basis is a hugely expensive and dangerous undertaking. Tuvalu failed TAS in 2017, with all the detected antigenaemia-positive individuals found on an outer island. Sufficient resources need to be mobilised, and the efficiency, effectiveness and safety of delivery of each intervention needs to be maximised through innovation and integration where feasible. Traditionally, there were limited donors and partners committed to support countries in the region with sustained financial and operational support. However, the overall success is motivating new donors and partners to advocate for further support from domestic and international sources.
Third, several countries in the Pacific have small health ministries with limited health system capacities and are vulnerable to outbreaks of epidemics (dengue, measles or polio) and natural disasters (typhoons, earthquakes or landslides), while COVID-19 will potentially disrupt ongoing programmes, as LF programmes are frequently deprioritised in the face of other public health priorities or emergencies. The first IDA campaign implemented in Papua New Guinea in 2018 was exemplary. Preparation to launch the MDA campaign using the IDA strategy for the first time in New Ireland Province started in early 2018. However, the National Department of Health declared a polio outbreak in June 2018 and decided to launch a nationwide mass polio vaccination campaign in October 2018 as a national public health priority, the same week the launch of the IDA campaign was scheduled. Despite numerous logistic, human resource and coordination challenges, national and provincial health departments were able to implement the IDA campaign between semimonthly rounds of the polio vaccination campaign from December 2018 to January 2019.
Finally, there is a need to establish a system to detect and prevent recrudescence of transmission and to sustain care for patients with LF-associated morbidity in the postvalidation phase. Recrudescence of transmission of W. bancrofti is possible when highly efficient mosquito vectors, particularly Aedes species, are abundant. Residual morbidities and disabilities remain after countries have achieved elimination targets. Advocating for governments to invest in LF activities after the goal of LF elimination as a public health problem has been achieved is challenging, especially when many of the countries in the region have limited health system capacity and other competing public health priorities. Support from external partners to assist countries to explore and determine the most feasible and cost-effective options to integrate activities into other existing platforms is essential in resource-limited countries.
In 2018, the WHO Regional Committee for the Western Pacific Region endorsed the Regional Action Framework for Control and Elimination of Neglected Tropical Diseases in the Western Pacific (WPR/RC69/5).39 Achieving and sustaining the status of elimination of LF and alleviating suffering from associated morbidities and disabilities is one of the goals of the Framework. Noting all the above-mentioned regional challenges, the Framework identified four strategic pillars to further strengthen various programmatic and health system components and accelerate the control and elimination of LF and other neglected tropical diseases (NTDs), namely (i) catalysing coordinated multi-sectoral actions through strategic planning, advocacy and partnership, (ii) enhancing NTD intervention and service delivery for safety and efficiency, (iii) engaging and empowering communities, and (iv) measuring impacts and generating evidence through enhanced surveillance, research and innovation.
The dedication, commitment and capacity of all endemic countries in the Western Pacific Region, continuous support from global and regional partnerships to assist countries to address the remaining challenges and introduction of the IDA strategy, represents an opportunity to revive LF elimination efforts; we are confident that the historic achievement of regional elimination of LF will be within our reach, notwithstanding the challenges outlined.
Acknowledgements
We sincerely would like to acknowledge all former and current programme managers, policymakers, scientists, researchers and health staff/workers in the health ministries, and international volunteers, donors and partner agencies and the WHO at all levels as well as pharmaceutical donors who have committed and contributed to the progress of LF elimination in the Western Pacific Region to date.
Contributor Information
Aya Yajima, Division of Programmes of Disease Control, World Health Organization Western Pacific Regional Office, Manila, Philippines.
Kazuyo Ichimori, Institute of Tropical Medicine, Nagasaki University, Nagasaki, Japan.
Authors’ contributions
AY and KI designed the outline of the manuscript; AY drafted the manuscript, KI critically revised the manuscript; AY and KI finalised the manuscript.
Funding
The publication of the papers within this supplement were supported by MSD, GSK and Eisai through the Mectizan Donation Program (MDP) and the Global Alliance for LF Elimination (GAELF).
Competing interests
The authors declare that they have no competing interests.
Ethical approval
Not required.
Data availability
None.
References
- 1. World Health Organization Lymphatic filariasis: a handbook of practical entomology for national lymphatic filariasis elimination programmes. Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- 2. World Health Organization The PacELF Way: towards the elimination of lymphatic filariasis from the Pacific, 1999–2005. Manila, Philippines: World Health Organization Western Pacific Regional Office; 2006. [Google Scholar]
- 3. Cook GC, Zumla AI, eds. Manson's Tropical Diseases, 22nd ed Edinburgh, UK: Elsevier Science and WB Saunders; 2003. [Google Scholar]
- 4. Cox FEG, ed. The Wellcome Trust Illustrated History of Tropical Diseases. London, UK: The Wellcome Trust; 1996. [Google Scholar]
- 5. Sassa M. Human filariasis: A global survey of epidemiology and control. Tokyo, Japan: University of Tokyo Press; 1976. [Google Scholar]
- 6. Itoh M. Filariasis in Japan some 25 years after its eradication. Trop Med Health. 2011;39(1 Suppl 2):57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tada I. Lymphatic filariasis and its control in Japan - the background of success. Trop Med Health. 2011;39(1 Suppl 2):15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De-jian S, Xu-li D, Ji-hui D. The history of the elimination of lymphatic filariasis in China. Infect Dis Poverty. 2013;2(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cheun HI, Kong Y, Cho S et al. Successful control of lymphatic filariasis in the Republic of Korea. Korean J Parasitol. 2009;47(4):323–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kron M, Walker E, Hernandez L et al. Lymphatic filariasis in the Philippines. Parasitol Today. 2000;16(8):329–33. [DOI] [PubMed] [Google Scholar]
- 11. World Health Organization Meeting Report of the Consultation to Accelerate Elimination of Brugia malayi Transmission in Indonesia and Malaysia, 13–15 December 2016, Kota Kinabalu, Malaysia. Manila, Philippines: World Health Organization Western Pacific Regional Office; 2017. [Google Scholar]
- 12. Ichimori K, Graves PM.. Overview of PacELF - The Pacific Programme for the Elimination of Lymphatic Filariasis. Trop Med Health. 2017;45(34). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World Health Organization PCT Databank. Geneva, Switzerland: World Health Organization; Available at https://www.who.int/neglected_diseases/preventive_chemotherapy/lf/en [accessed May 16, 2020]. [Google Scholar]
- 14. Ave C, Kapa DR, Ottesen E. Elimination of lymphatic filariasis as a public health problem from the Cook Islands. Trop Med Health. 2018;46(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carlingford CN, Melrose W, Mokoia G et al. Elimination of lymphatic filariasis as a public health problem in Niue under PacELF, 1999-2016. Trop Med Health. 2019:47(20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Allen T, Taleo F, Graves PM et al. Impact of the Lymphatic Filariasis Control Program towards elimination of filariasis in Vanuatu, 1997–2006. Trop Med Health. 2017;45(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ofanoa R, Ofa T, Padmasiri EA et al. Elimination of lymphatic filariasis as a public health problem from Tonga. Trop Med Health. 2019;47(43). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. World Health Organization Monitoring and epidemiological assessment of mass drug administration in the Global Programme to Eliminate Lymphatic Filariasis. A manual for national elimination programmes. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 19. World Health Organization Validation of elimination of lymphatic filariasis as a public health problem. Geneva, Switzerland: World Health Organization; 2017. [Google Scholar]
- 20. World Health Organization Regional Strategic Plan for Elimination of Lymphatic Filariasis (2004-2007). New Delhi, India: World Health Organization Regional Office for Southeast Asia; 2004. [Google Scholar]
- 21. Khieu V, Or V, Tep C et al. How elimination of lymphatic filariasis as a public health problem in the Kingdom of Cambodia was achieved. Infect Dis Poverty. 2018;7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dung DT, Binh VTL, Worrell CM et al. Evaluation of a facility-based inspection tool to assess lymphedema management services in Vietnam. PLoS Negl Trop Dis. 2020;14(10):30008773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. World Health Organization The report of the seventeenth Meeting of the Western Pacific Regional Programme Review Group on Neglected Tropical Diseases, Siem Reap, Cambodia, 15-16 June 2017. Manila, Philippines: World Health Organization Western Pacific Regional Office; 2018. [Google Scholar]
- 24. World Health Organization Guideline: Alternative mass drug administration regimens to eliminate lymphatic filariasis. Geneva, Switzerland; World Health Organization; 2017. [PubMed] [Google Scholar]
- 25. Thomsen EK, Sanuke N, Baea M et al. Efficacy, safety, and pharmacokinetics of coadministered diethylcarbamazine, albendazole, and ivermectin for treatment of Bancroftian filariasis. Clin Infect Dis. 2016;62:334–41. [DOI] [PubMed] [Google Scholar]
- 26. King CL, Suamani J, Sanuku M et al. A trial of a triple-drug treatment for lymphatic filariasis. N Engl J Med. 2018;379:1801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weil GJ, Bogus J, Christian M et al. The safety of double- and triple-drug community mass drug administration for lymphatic filariasis: A multicenter, open-label, cluster-randomized study. PLoS Med. 2019;16(6):e1002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Local Burden of Disease 2019 Neglected Tropical Diseases Collaborators The global distribution of lymphatic filariasis, 2000-18: a geospatial analysis. Lancet Glob Health. 2020;8:e1186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ichimori K, King JD, Engels D et al. Global Programme to Eliminate Lymphatic Filariasis: The Processes Underlying Programme Success. PLoS Negl Trop Dis. 2014;8(12):e3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Webber RH. Vector control of filariasis in the Solomon Islands. The Southeast Asian J Trop Med Public Health. 1975;6(3):430–4. [PubMed] [Google Scholar]
- 31. Webber RH. The natural decline of Wuchereria bancrofti infection in a vector control situation in the Solomon Islands. Trans R Soc Trop Med Hyg. 1977;71(5):396–400. [DOI] [PubMed] [Google Scholar]
- 32. Chandra G. Nature limits filarial transmission. Parasit Vectors. 2008;1(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Snow LC, Michael E.. Transmission dynamics of lymphatic filariasis: Density-dependence in the uptake of Wuchereria bancrofti microfilariae by vector mosquitoes. Med Vet Entomol. 2002;16(4):409–23. [DOI] [PubMed] [Google Scholar]
- 34. Xu Z, Lau C, Zhou X et al. The extensive networks of frequent population mobility in the Samoan Islands and their implications for infectious disease transmission. Sci Rep. 2018;8:10136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Graves PM, Sheridan S, Fuimaono S et al. Demographic, socioeconomic and disease knowledge factors, but not population mobility, associated with lymphatic filariasis infection in adult workers in American Samoa in 2014. Parasit Vectors. 2020;13:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Webber R. Eradication of Wuchereria bancrofti infection through vector control. Trans R Soc Trop Med Hyg. 1979;73:722–4. [DOI] [PubMed] [Google Scholar]
- 37. David HM. Filariasis elimination, vector control and eradication challenges. Commentary on Webber, R. Eradication of Wuchereria bancrofti infection through vector control. Trans R Soc Trop Med Hyg. 1979;73:722–4. [DOI] [PubMed] [Google Scholar]
- 38. Bockarie MJ, Pedersen EM, White GB et al. Role of vector control in the global program to eliminate lymphatic filariasis. Ann Rev Entomol. 2009;54:469–87. [DOI] [PubMed] [Google Scholar]
- 39. World Health Organization The regional action framework for control and elimination of neglected tropical diseases in the Western Pacific. Manila, Philippines: World Health Organization Western Pacific Regional Office; 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
None.


