Graphical abstract
Abbreviations: IL-18, Interleukin-18; IL-1β, Interleukin-1 beta; GSDMD, Gasdermin D; NLRP3, Nucleotide-binding domain-like receptor protein 3; ASC, apoptosis-associated speck-like protein; ROS, Reactive oxygen species; NF-κB, Nuclear factor-kappa B; LPS, Lipopolysaccharide; ATP, Adenosine triphosphate; PAMPs, Pathogen-associated molecular patterns; PRRs, Pattern recognition receptors; DAMPs, Damage-associated molecular patterns; NLRP1, NOD-like receptor protein 1; CNS, central nervous system; TXNIP, Thioredoxin-interacting protein; AIM2, Absent in melanoma 2; double stranded DNAIR, Ischemia reperfusion; OPCs, Oligodendrocyte progenitor cells; JOA, Japanese orthopedics association; NDI, Neck data index; NLRP1b, NOD-like receptor protein 1b; CCK-8, Cell Counting Kit-8; CORM-3, Carbon monoxide releasing molecle-3; CO, Carbon monoxide; IRE1, Inositol requiring enzyme 1; si-RNA, Small interfering RNA; Nrf2, Nuclear factor erythroid 2-related factor 2; HO-1, Heme oxygenase-1; H2O2, Hydrogen peroxide; ECH, Echinacoside; Gal-3, Galectin-3; BBG, Brilliant blue G; Cx43, Connexin 43; TLR4, Toll-like receptor 4; DRD1, Dopamine Receptor D1
Keywords: Spinal cord injury, Neuroinflammation, Pyroptosis, NLRP3, Caspase-1, Therapeutic implications
Highlights
-
•
Neuroinflammation plays a key role in the secondary injury of acute SCI.
-
•
Pyroptosis is defined as a newly programmed cell death.
-
•
Recent studies show that pyroptosis play a key role in SCI.
-
•
Some molecules can significantly inhibit pyroptosis process in SCI.
-
•
Targeting pyroptosis and inflammasome components can be novel therapeutic strategies for SCI.
Abstract
Background
Currently, spinal cord injury (SCI) is a pathological incident that triggers several neuropathological conditions, leading to the initiation of neuronal damage with several pro-inflammatory mediators' release. However, pyroptosis is recognized as a new programmed cell death mechanism regulated by the stimulation of caspase-1 and/or caspase-11/-4/-5 signaling pathways with a series of inflammatory responses.
Aim
Our current review concisely summarizes the potential role of pyroptosis-regulated programmed cell death in SCI, according to several molecular and pathophysiological mechanisms. This review also highlights the targeting of pyroptosis signaling pathways and inflammasome components and its therapeutic implications for the treatment of SCI.
Key scientific concepts
Multiple pieces of evidence have illustrated that pyroptosis plays significant roles in cell swelling, plasma membrane lysis, chromatin fragmentation and intracellular pro-inflammatory factors including IL-18 and IL-1β release. In addition, pyroptosis is directly mediated by the recently discovered family of pore-forming protein known as GSDMD. Current investigations have documented that pyroptosis-regulated cell death plays a critical role in the pathogenesis of multiple neurological disorders as well as SCI. Our narrative article suggests that inhibiting the pyroptosis-regulated cell death and inflammasome components could be a promising therapeutic approach for the treatment of SCI in the near future.
Introduction
Nowadays, spinal cord injury (SCI) is an extreme injury, that is still a challenging clinical and public health concern worldwide [1]. It causes serious motor and neurological dysfunctions and exceedingly reduces the quality of life every year [1]. Recently, SCI is seriously raising the burden of social health care in the world. The prevalence rate of SCI is nearly 20.7–83 per million in United States and approximately 8.0–130.6 per million in Europe [2]. Multiple studies have documented that the similar incidence rates in China as well as developed countries including United States [3], [4]. After damage, spinal cord causes some major and minor pathophysiological changes induced by several traumas such as shear stress, torsion and contusion compression [5], [6]. Moreover, several studies have documented that inflammation, tissue ischemia, cell edema, apoptosis, intracellular ion homeostasis imbalance and other secondary pathophysiological changes can largely enhance the level of acute SCI which results in some various neurological dysfunctions after the injury [7], [8].
Neuroinflammation hugely triggers a series of secondary injury which finally leads to neuronal death process [9]. Furthermore, the cascade of secondary injuries triggers enormous inflammatory responses in the damaged area. Several studies have illustrated that inflammation can widely extend to the adjacent tissues, driving to cell death process and largely inhibit axonal regeneration and functional outcomes after SCI [10], [11]. Current evidences have illustrated that the activation of inflammasome signaling pathway plays significant roles in cell pyrophosphorylation and stimulates the secretion of several inflammatory factors [12], [13]. Besides, neuroinflammatory responses play crucial roles in the secretion and release of pro-inflammatory factors such as IL-1β and IL-18, leading to the initiation of cell death mechanisms [14], [15]. The NLRP3 activation and ASC recruitment subsequently mediate the caspase-1 and regulate the maturation and secretion of pro-IL-1β and pro-IL-18 inflammatory factors [16]. However, inflammasomes usually contain NLRP3, ASC and caspase-1, which are clustered after various endogenous 'risk' signals [17]. In addition, several stimulating factors including ROS synthesis can largely trigger NLRP3 inflammasome and other inflammasome components [18], [19]. Targeting the NLRP3 and other inflammasome components can significantly expend neuroprotection in SCI mice model.
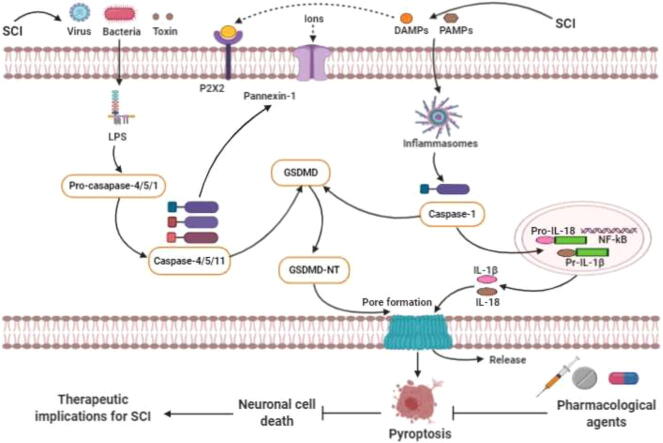
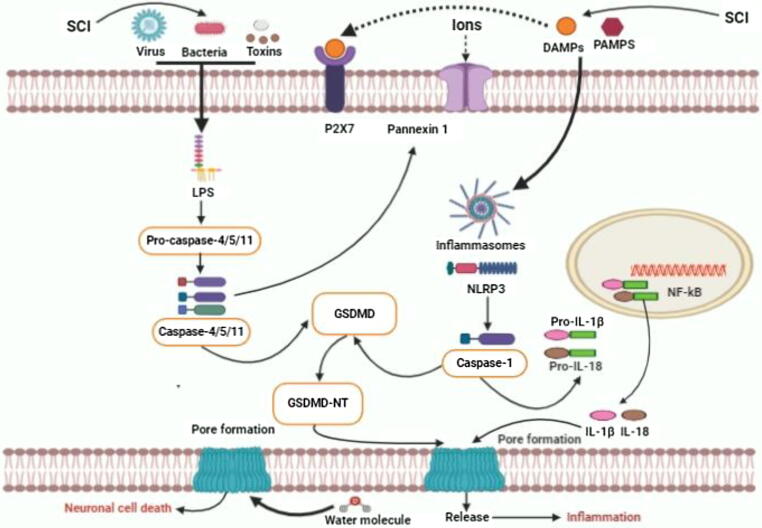
The morphological characteristics and basic functions of pyroptosis-regulated cell death were initially established in macrophages infected with Gram-negative bacteria known as Shigella flexneri in 1992 [20]. Unpredictably, similar findings were first notated in macrophages infected with Salmonella bacteria in 1999 [21]. Pyroptosis is called as a recently identified programmed cell death regulated by the activation of caspase-1, caspase-4/5/11 and GSDMD regulated signaling pathways and release of several inflammatory mediators such as IL-1β and IL-18 [22], [23], [24], [25]. Mounting pieces of evidence illustrate that GSDMD indicates a large gasdermin family bearing a novel cellular membrane pore-forming activity [26], [27]. Thus, pyroptosis is generally redefined as gasdermin-mediated programmed necrosis [28]. The gasdermins are considered as a family of recently discovered pore-forming effector protein molecules, which lead to the cellular membrane permeabilization and pyroptosis-regulated cell death initiation [29]. Emerging evidence also demonstrates that gasdermins are usually associated with numerous genetic diseases [30], [31]. In addition, recent studies demonstrate that GSDMD-mediated pyroptosis plays crucial roles in the pathogenesis of multiple neurological diseases [32]. Furthermore, current studies have illustrated that pyroptosis signaling pathways play vital roles in CNS disorders [33]. Inflammasomes formation through caspase-1-canonical and/or caspase-4/5/11-non-canonical pyroptosis signaling pathways activation may be triggered via intracellular and extracellular signaling pathways during several noxious stimuli [34]. Indeed, LPS derived from several pathogens including bacteria, virus and toxins senses and stimulates caspase-4/5/11 activation, which leads to pyroptosis-regulated cell death initiation [35], [36]. Previous studies demonstrate that the p10-form autoprocessed caspase-4/11 binds the GSDMD-C domain with a high affinity [36], [37]. Structural comparison of autoprocessed and unprocessed capase-11 identifies a β sheet-induced autoprocessing. The binding enhances dimerization-induced caspases initiation, interpreting a cleavage autonomously of the cleavage-site tetrapeptide sequence [38]. During the activation of pyroptosis-signaling pathways, the pro-caspase-1 can be indirectly connected with the adaptor protein ASC, which is combined with a pattern recognition receptor to form a macromolecular complex and these are designated as inflammasomes [20], [39]. After the activation of pro-caspase-1, it is then cleaved by inflammatory responses to form active caspase-1. Then, the active caspase-1 may cleave the GSDMD protein molecule to form active N- and C-terminal kinase portions [39], [40]. Furthermore, N-terminal products noticeably stimulate cell membrane perforation and cell death mechanisms and largely trigger a series of inflammatory responses. The GSDMD-NT strongly binds to phosphatidic acid, phosphatidylserine and phosphatidylinositol on the cell membrane, activating oligomerization and leading to pores formation [34], [41], [42]. The diameters of the membrane pores can be 15 nm inner and 32 nm outer, whereas the diameter of the IL-18 is 4.5 nm, which is readily capable of transcending through the membrane pores. The GSDMD-CT is usually soluble and can return to the GSDMD-NT, impeding its activation process. Moreover, recent evidence has documented that caspase-1/4/5/11 pathway activation can induce pyroptotic-cell death mechanism by cleaved GSDMD. In contrast, caspase-3 has traditionally been regarded as a significant hallmark of apoptosis for a long time [43], [44], [45], [46]. Recent investigations have also illustrated that caspase-3 activation may influence and regulate GSDME, which highly leads to triggering pyroptosis mechanisms [46]. GSDME is commonly found in different organs in the body including fetal cochlea, heart as well as kidneys [26]. Growing evidence suggests that GSDME-gene mutation plays a crucial role in the non-syndromic hearing impairment [47]. Caspase-3 is generally considered as a crucial effector of apoptosis-mediated cell death, which can stimulate GSDME [48]. Notably, the activated caspase-3 leads to the cleavage of GSDME and N- and C-terminal domains formation [49]. GSDME-NT can be stimulated by caspase-3, which is similar to the GSDMD-NT, leading to the formation of pores in the cellular membrane region (Fig. 1) [50], [51]. The actual role of GSDME in stimulating pyroptosis upon apoptosis has largely been constrained in the extensive investigations [51]. During the mitochondrial apoptotic process, GSDME plays a redundant role in channel formation. The study has illustrated that GSDME induces cellular lysis in macrophages of the ripoptosome in the mitochondrial apoptotic process [47]. Tixeira et al. also demonstrated that GSDME plays a dispensable role in pyroptosis regulation of the human T cells and monocytes [49]. Caspase-1/11-gene knockout bone marrow-derived macrophages are generally stimulated with flagellin, cytochrome-c and Fas ligand [52]. Similar to the GSDMD-regulated pyroptosis, GSDME-independent secondary necrotic-mediated cell death can also induce pyroptosis [53]. Emerging evidence reports that caspase-8 activation induces the NLRP3 inflammasome, indicating that caspases-8 can also trigger pyroptosis-regulated cell death [54]. Amazingly, Oring and colleagues reported that the inhibition of tak1 kinase by the Yersinia effector protein yopJ attributed caspase-8-dependent cleavage of GSDMD and resulted in pyroptosis, suggesting that caspase-8 as a key inducer of pyroptosis-regulated cell death [55]. These remarkable findings indicate that the caspases-8/GSDMD signaling pathway could be another initiator of pyroptosis. Unfortunately, the actual role of casapase-8 dependent pyroptosis in SCI is still unclear. Further investigations in more detail are highly demanded to explore the actual mechanism and potential role of casapase-8 dependent pyroptosis in SCI in future. Therefore, GSDME and GSDMD regulated pyroptosis can open a new window for the inflammatory pattern of cell death that also requires more investigations on the actual mechanism and significance in numerous diseases progression including CNS disorders, SCI and cancers in future [30].
Fig. 1.
The overview of the critical roles of pyroptosis-regulated cell death in SCI.
Role of pyroptosis-regulated cell death mechanisms in SCI
Current shreds of evidence have been demonstrating that pyroptosis signaling pathways play critical roles in neurological disorder as well as SCI [32], [56]. J. Wang et al. demonstrated that the pyroptosis signaling pathways-related proteins such as NLRP3, ASC, caspase-1 and GSDMD expression levels were remarkably enhanced in the injured spinal cord rat model compared to the sham animal model groups [57]. The author and colleagues have also demonstrated that pro-inflammatory cytokines levels are augmented in the SCI model group compared to the sham groups [57]. Current investigations have demonstrated that the NF-κB molecular signaling pathway plays important roles in inducing the NLRP3 inflammasome and pro-inflammatory factors release such as IL-1β and IL-18 in multiple disorders as well as neurological disorders [58], [59], [60], [61]. The authors have also indicated that the NF-kB expression level was highly enhanced in the SCI model group. Indeed, the authors explored a correlation between inflammatory responses and pyroptosis signaling pathways in SCI animal models and microglia [57]. The NLRP3, ASC, caspase-1, GSDMD, IL-1β and IL-18 protein expression levels were up-regulated in SCI rats; also the NLRP3, caspase-1 and GSDMD expression levels were enormously up-regulated in LPS + ATP-induced BV-2 cells. The GSDMD-mediated neuronal cell death was markedly found in the previous study. Notably, immunofluorescence findings demonstrated that the GSDMD protein expression significantly enhanced in both spinal cord tissues and BV-2 cells in the previous study [57]. Furthermore, prior evidence have documented that NLRP3 inflammasome considerably subsists in microglia [59], [62], [63]. The NLRP3 inflammasome can be activated by PAMPs and PRRs [64], [65]. PAMPs activate the NF-kB signaling pathway and induce the NLRP3, other inflammasomes as well as inflammatory cytokines including pro-IL-1β and pro-IL-18 release. Moreover, DAMPs activate the NLRP3 inflammasome by recruiting pro-caspase-1 in large quantities via ASC adaptor protein activation, which results in the formation of an autocatalytic process that predominantly stimulates the caspase-1 in SCI animal models [66], [67], [68]. Mounting pieces of evidence have documented that NF-κB pathway can tremendously trigger the NLRP3 inflammasome activation and inflammatory factors release after SCI [69]. Zhang et al. illustrated that pyroptosis plays a critical role in SCI [70]. Owing to, the author initially detected the mRNA expression level of pyroptosis signaling pathway-regulated key genes after SCI. It has also been documented that caspase-1, IL-1β and IL-18 mRNA expression levels are sharply increased on several days of post-injury in SCI model. In addition, double immunofluorescence staining distinctly illustrated that the cleaved-caspase-1 positive neuron significantly diminished the lesion size in the spinal cord anterior horn of the injured spinal cord rats group. The pyroptosis-related protein marker GSDMD, cleaved-caspase-1, cleaved-caspase-11 and inflammatory mediators such as IL-1β and IL-18 protein expression levels were augmented mainly after SCI. The RT-PCR results in the previous study have pointed that the time course of the NLRP1 and NLRP3 mRNA expression was generally persistent with caspase-1 after SCI, illustrating pyroptosis-regulated neuronal cell death was markedly involved with several inflammasomes signaling pathway initiation in SCI animal model. The pyroptosis-related key proteins including GSDMD, NLRP1, NLRP3, cleaved-caspase-1, IL-1β, expression levels were remarkably enhanced in rat neuronal cells. Furthermore, the TXNIP protein expression levels were notably up-regulated in the SCI animal group than the control animal group. Prior evidences have illustrated that TXNIP molecule can activate the NLRP3 inflammasome and pyroptosis signaling pathways [70], [71], [72]. In addition, current evidence has noted the potential role of AIM2 in the formation of an inflammasome after massive exposure to an exogenous pathogen or host ectopic nucleic acids [73], [74], [75]. Therefore, the NLRP3 induced pyroptosis-regulated cell death plays key roles in SCI.
Moreover, current evidence has illustrated that AIM2 is tremendously expressed in neurons after SCI in rodent models [76]. AIM2 inflammasome can be activated in spinal neurons after SCI [77]. In addition, growing evidence has also documented that neuronal loss and inflammasomes formation were prevented in the AIM2, ASC and caspase-1 gene-knockout mice model compared to the wild-type mice model [78]. Authors from a study documented that the AIM2 expression level was tremendously enhanced at 4 h and 8 h post I/R-induced spinal tissue in C57BL/6 mice model [79]. The quantitative results strongly indicated that the dsDNA released in the serum and CSF both pointed at 4 h post-IR induced mice injured spinal model. In addition, AIM2 inflammasome elements such as ASC, cleaved caspase-1 and IL-1β were sharply increased in the beginning at 4 h post-IR and remained constant with time during the 24-h reperfusion period. Authors have also ascertained the specific cellular distribution of the AIM2 molecule in the injured spinal cord tissues to investigate its pharmacological mechanism of actions after SCI [79]. Immunofluorescence staining illustrated that AIM2 molecules were initially augmented in neuronal cells in IR-induced SCI model. It has also been demonstrated that the AIM2 expression levels were considerably high in dsDNA in the CSF from IR-induced SCI mice model compared to the CSF from non-injured mice model, indicating the strong immunogenicity. Moreover, the protein expression of AIM2, ASC and cleaved caspase-1 levels were largely increased in IR injury-challenged CSF induced models, meaning that dsDNA can significantly activate the AIM2 inflammasome. Major inflammasome called NLRP3 is a mostly higher level in the SCI mice model. Transitionally enhanced AIM2 molecule and dsDNA levels were found early as 4 h post-injured SCI model, even though the most severe and persistent symptoms of motor dysfunction were found at 12 h or later. Thus, this outstanding evidence strongly concludes that AIM2 inflammasome and other inflammasomes are rapidly activated in their common components, leading to augmented ASC and cleaved caspase-1 expression levels after SCI. Therefore, the dominance of the rapid pyroptosis process at high concentrations of dsDNA at 4 h is the promoter of consequent neuronal death.
Yanagisawa et al. performed studies to investigate the significant role of inflammasomes in SCI animal model [80]. Authors of a study clearly reported that the NLRP3 protein expression levels were significantly high in the low impact and high impact SCI groups on day 1, 3 and 7 after injuries [80]. Studies have also illustrated that caspase-2 and ASC expression levels were also considerably high in SCI model on day 1, 3 and 7 of post injuries than that of the sham model, though there was no substantial distinction between the low impact and high impact SCI model groups. Immunofluorescence staining clearly indicated that the NLRP3, ASC and caspase-2 in OPCs expression levels are hugely increased in the low impact and high impact SCI model groups compared to the sham group on day 1, 3 and 7 of post injuries [80]. Furthermore, NLRP3 and ASC expression levels in astrocytes were also increased in the low impact group on day 3 after the injury but not on day 1, 7 and 14 compared to the control animal group.
Regarding the severity of the injury, the expression of ASC in OPCs was increased on the first day after injury in the high impact and low impact model groups compared to the sham operation group. The comparison of NLRP3 protein expression level between OPCs and astrocytes demonstrated that OPCs expression was at a higher level than astrocytes on day 1 after the injury [80]. The Impedance of the astrocytes to pyroptosis-regulated cell death process can be due to other reasons for astrocytes survival after SCI, leading to glial scar formation. Moreover, the higher expression levels of inflammasome components were notably found in astrocytes on day 3 after SCI. Although its expression level is lower than that of OPCs, these critical findings finally illustrate that inflammasome-mediated cell death mechanisms occur in astrocytes. Extensive studies have documented that TXNIP overexpression triggers NLRP3 and caspase-1 activation to initiate pyroptosis mechanisms [81], [82]. Emerging evidence shows that TXNIP overexpression highly induces the NLRP3 inflammasome activation in the injured spinal cord areas and triggers pyroptosis-regulated neuronal cell death mechanisms [80].
S. Xu et al. investigated whether NLRP3/GSDMD could trigger neuroinflammation after SCI or not [56]. Hence, the author divided the 20 SCI patients into high-expression and low-expression groups based on the fact, whether NLRP3/GSDMD gene expression reached an average level or not. The NLRP3/GSDMD high-expression group had a lower JOA score and NDI compared to the NLRP3/GSDMD low-expression group. The NLRP3/GSDMD protein expression levels were high in the SCI patient samples compared to the normal patient samples. A positive correlation between NLRP3/GSDMD expression and JOA score was confirmed by linear regression analysis. The NLRP3 and AIM2 transcript levels were up-raising after SCI. Moreover, several inflammasome-related genes such as NLRP3, ASC, IL-1β, IL-18, CASP-1 and GSDMD and three inflammasome sensors such as NLRP3, NLRP1 and AIM2 demonstrated higher levels in the injured spinal cord mice at day 3 of post injured models [56]. Pro-inflammatory cytokines such as IL-1β, IL-18 release were also in high levels after SCI, besides, both GSDMD and N-terminal GSDMD (GSDMD-N, an active form of GSDMD) expression levels were also increased after SCI. The GSDMD expression level coincided with CD68 (a marker of macrophages/microglia) was increased by immunofluorescence staining analysis. The NLRP3, ASC, CASP-1 and GSDMD pyroptosis-related key genes in transcript levels were also enhanced in BV-2 cells [56]. The similar changes in NLRP3, GSDMD and ASC protein levels in response to changes in CD73 has been confirmed by both western blot analysis and immunofluorescence staining in vitro studies. The immunofluorescence staining clearly illustrated that GSDMD-overexpression could induce huge neuronal cell loss after SCI. These critical findings report that pyroptosis-regulated programmed cell death process plays critical roles in SCI progression.
It has been claimed from prior studies that the NLRP1b and NLRP3 mRNA expression levels increased within 6 h, while ASC remained low in SCI experimental animal model [70]. Previous studies have also recorded a further rise in NLRP3 and ASC within 72 h and a decline in 7 days [70]. Meanwhile, NLRP1b expression levels have decreased after 24 h and reached to a baseline value 72 h after the injury. Besides, the NLRP1b mRNA expression level has been transiently expressed in the sham operation group, then dramatically decreased to a minimum at 6 and 24 h and recovered slightly to the basal value at 72 h in SCI models. The expression of cleaved caspase-1, pro-caspase-1, pro-IL-1β and pro-IL-1β protein levels were significantly enhanced at 72 h after injury. The NLRP3 and ASC specific-gene expression levels were considerably higher after SCI. In the previous study, the author and colleagues also investigated the distribution of ASC+ cells and the co-localization of ASC+ and NLRP3+ cells in the epicenter of SCI lesion by both immunohistochemistry and immunofluorescence staining [70]. The number of ASC+ cells remarkably higher after SCI at 72 h compared to the sham animal models. These substantial shreds of evidence strongly indicate that pyroptosis plays a crucial role in SCI.
Emerging evidence has reported that spinal cord neurons can substantially express P2X7 purinergic receptors and exposure to ATP led to high-frequency spiking, irreversible enhance cytosolic calcium and cell death [83]. P2X4 receptors are usually found in spinal neurons and regulate inflammasomes initiation after SCI [83]. SCI promotes ATP release in peritraumatic areas [83]. Thus, the excessive ATP release triggers these purinergic receptors to stimulate NLRP1 inflammasome formation. Lin and colleagues reported that NLRP3, NLRP1, ASC and pro-caspase-1 activation induces neuronal cell death after SCI [84]. Immunofluorescence staining indicated that the NLRP1 inflammasome overexpression could cause spinal neuronal cell death. In addition, Zendedel et al. demonstrated that SCI stimulates a complex scenario of inflammasome-induced neuronal cell death [81]. These notable findings suggest that pyroptosis-regulated cell death plays critical roles in SCI. Besides, pyroptosis-regulated cell death can be extremely pathological term and lead to human diseases progression. However, further investigations are highly demanded to explore the actual role of pyroptosis and inflammasome signaling pathways in SCI in future.
Inhibiting the pyroptosis-regulated cell death and inflammasome components for the therapeutic implications of SCI
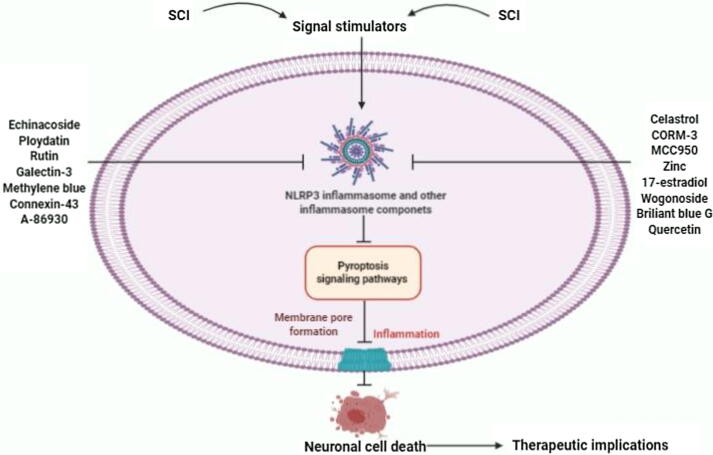
Inhibiting pyroptosis and inflammasome components is a novel therapeutic strategy for SCI therapy, which has recently been receiving greater attention to the researchers and clinicians. The term, celastrol (CSL) is known as a triterpenoid methyl triterpene [85]. It is widely used to treat the cerebral ischemia and Parkinson’s disease patients and has substantial anti-inflammatory effects in CNS [86], [87]. In a study, the authors have found a significant relationship between CSL and pyroptosis process in SCI animal model [57]. Prior evidence has indicated that CSL administration can efficaciously decline the GSDMD, NLRP3, ASC and caspase-1 expression levels in injured spinal rat models [57]. In addition, CSL administration could tremendously inhibit pyroptosis-regulated neuronal cell death and enhance hindlimb motor function recovery in SCI rats [57]. Therefore, it had been clearly confirmed to explore the potential effect of CSL on LPS+ ATP-induced BV-2 cells death in the previous study [57]. The authors also illustrated that CSL treatments in various concentrations largely reduced the NLRP3, ASC, pro-caspase-1, caspase-1 and GSDMD protein expression levels in LPS+ ATP-induced BV-2 cells. In addition, the authors reported that CSL treatment could significantly impede pyroptosis-regulated neuronal cell death after SCI. Besides, previous evidence confirmed that the serum levels of IL-1β and IL-18 were considerably declined, after CSL administration in the SCI model group [57]. The IL-1β and IL-18 protein expression levels were decreased in the CSL treated group. Besides, the CCK-8 results have confirmed that CSL treatments can significantly impede the death of microglia. Furthermore, CSL treatment significantly declines the expression of NF-kB/P-P65 levels in LPS+ ATP-induced BV-2 cells. More importantly, CSL treatments can largely reduce inflammatory mediators through inhibiting the pyroptosis and inflammasomes signaling pathways in microglial cells in vitro experiments [57]. The authors markedly demonstrated that CSL treatment could impede microglia cell death and improve axonal regeneration via suppressing pyroptosis signaling pathways and inflammasome components at the injured spinal cord region. Notably, the histopathological analysis clearly indicated that CSL treatment at 7 days could tremendously reduce cavity formation in the injured spinal cord region. These significant findings suggest that CSL could be a promising therapeutic candidate in targeting pyroptosis signaling pathways for the therapeutic implications of SCI in the near future.
MCC950 is regarded as a selective NLRP3 inflammasome blocker, which has recently been documented to suppress the canonical and non-canonical NLRP3 inflammasome activation in several experimental investigations [88], [89], [90], [91], [92]. Recently, Jiao and colleagues conducted a study on the potential effects of MCC950 treatment in the experimental SCI mice models and in-vitro experiments [93]. Importantly, the authors demonstrated that MCC950 administration could significantly enhance the hind limb movements, grip strength, spinal cord edema and histopathological scores in the SCI mice models. The authors documented that MCC950 administration could tremendously impede the NLRP3, ASC and caspase-1 signaling pathways and pro-inflammatory factors including TNF-α, IL-1β and IL-18 expression levels in the injured spinal cord tissue areas. In addition, MCC950 treatments could decline spinal neuron injury and NLRP3 inflammasome induction in both LPS and OGD-induced neuronal cells [93]. Therefore, these significant findings suggest that MCC950 could be a promising therapeutic candidate by suppressing the NLRP3 inflammasome and other inflammasomes for the treatment of SCI in the near future.
Zheng and colleagues have reported that carbon monoxide releasing molecule-3 (CORM-3) has substantial neuroprotective effects in SCI animal model (Table 1) [70]. However, inflammasomes play critical roles in inflammatory innate immune responses after SCI [94]. Accumulating shreds of evidence have clearly demonstrated that CO has significant anti-inflammatory effects in SCI. The author and colleagues have explained the potential pharmacological effects of CO to inhibit the pyroptosis as well as inflammasome signaling pathways in SCI animal model. The authors have demonstrated that CORM-3 administration can efficaciously impede the activation of caspase-1 and caspase-11 as well as the release of pro-inflammatory factors such as IL-1β and IL-18 [70]. The authors also confirmed that the serum IL-1β and IL-18 levels are lower in the CORM-3 treated rats group compared to the SCI model group at 3 days of post-injury by ELISA analysis [70]. After SCI, the caspase-1 mRNA expression levels were noticeably increased at day 1 and it was at a constant level till day 7. After SCI, CORM-3 treatment efficaciously reduces the NLRP1 and NLRP3 expression levels. Consequently, these important findings illustrate that CORM-3 and inhalable CO can significantly reduce the NLRP1 and NLRP3 inflammasomes activation after SCI. However, inflammasome-mediated pyroptosis programmed cell death mechanism was firstly observed in neurons in CNS [32]. CORM-3 efficaciously inhibits the pyroptosis-related protein marker called GSDMD expression level, indicating that this potent molecule inhibits pyroptosis signaling pathways after SCI. CORM-3 treatments effectively inhibit the expression of cleaved GSDMD pro-IL-1β, pro-IL-18 protein levels in the drug-treated injured spinal cord group and CORM-3 therapy substantially reduces the OGD-stimulated GSDMD, cleaved-caspase-1, IL-1β, NLRP1 and NLRP3 expression levels in neurons. Previous investigations reported that endoplasmic reticulum (ER) stress triggers TXNIP-induced NLRP3 inflammasome and other inflammasome components leading to cell death initiation [95], [96]. The authors demonstrated that COMR-3 treatments could tremendously inhibit IRE1-mediated inflammasome activation and neuronal cell death after SCI [70]. The authors also demonstrated that inhibiting the IRE1 pathway could impede the initiation of inflammasomes in OGD neuron in-vitro studies. IRE1 inhibition by si-RNA interdicted the NLRP1, NLRP3, GSDMD, cleaved caspase-1 and IL-1β expressions in OGD-exposed neuron. Recent investigations have illustrated that the pharmacological inhibition of IRE1 can efficaciously reduce the activation of inflammasome components and pyroptosis signaling pathways in SCI animal model [70]. Studies have also documented that STF-083010 administration mainly alleviates the IRE1 phosphorylation in SCI model. STF-083010 administration can reduce the fluorescence intensity of cleaved caspase-1 in the neuron at post-SCI and reduce the GSDMD, cleaved GSDMD, cleaved-caspase1, cleaved-caspase-11, NLRP1, NLRP3 as well as pro-inflammatory factors such as IL-1β, IL-18, pro-IL-1β and pro-IL-18 expression levels. CORM-3 treatment remarkably improves the histopathological and functional outcomes and increases neuronal survival resulting from decreased pyroptosis cell death process (Fig. 2). Thus, these substantial findings indicate that CORM-3 significantly inhibits pyroptosis process and inflammasome components in both SCI rat and in-vitro model. Apoptosis has traditionally been recognized as the most studied cell death mechanism in CNS and SCI; previous studies illustrated the relevance of targeting pyroptosis and inflammasome components to improve neurological outcomes after SCI [97], [98]. Therefore, these findings also distinctly prove that the exogenous CORM-3 administration enhances CO concentrations in spinal tissues and attenuates the neuronal pyroptotic death mechanisms after SCI. This evidence also suggests that the cell death mechanism might be a potential target in the IRE1-mediated inflammasome signaling regulation for SCI therapy in the near future.
Table 1.
Compounds suppressing pyroptosis and inflammasome components to treat SCI.
| Compounds | Mechanisms of pyroptosis and inflammasomes inhibition | Pharmacological effects | Effective dose (kg/B.W) | References |
|---|---|---|---|---|
| Celastrol | NLRP3/ASC/ Caspase-1/GSDMD/IL-1β/IL-18 | Impedes neuronal cell death Promotes axonal regeneration and functional outcomes | 1 mg/kg | [57] |
| MCC950 | NLRP3/ASC/Caspase-1/TNF-α, IL-1β/IL-18 | Enhances neuroprotection and inhibits neuronal loss Promotes axonal regeneration and functional recovery | 10 or 50 mg/kg | [93] |
| CORM-3 | TXNIP/NLRP1/NLRP3/GSDMD/Cleaved caspase-1 | Inhibits neuronal cell death Promotes axonal regeneration and functional recovery | 8 mg/kg | [70] |
| Zinc | NLRP3/IL-1β/IL-18 via promoting Nrf2/HO-1/NQO-1 | Promotes axonal regeneration Improves functional recovery | 30 mg/kg | [100] |
| Reduces neuronal loss and cavity formation | ||||
| Echinacoside | NEK7/NLRP3/ASC/Caspase-1/NF-κB /IL-1β/IL-18 | Reduces neuroinflammation and spinal edema Improves functional recovery | 20 mg/kg | [68] |
| Polydatin | NLRP3/ASC/Caspase-1/GSDMD//IL-1β/18 | Inhibits neuronal cell deathand cavity formation Promotes axonal regeneration and functional outcomes | 20, 40 mg/kg | [106] |
| Rutin | NLRP1/NLRP3/GSDMD/Cleaved caspase-1/ IL-1β/18 | Reduces neuronal loss and cavity formation Promotes neuroprotection and functional recovery | 100 mg/kg | [110], [111], [69] |
| Promotes axonal regeneration and neuronal tissue protection | ||||
| 17-estradiol (E2) | NLRP1b/NLRP3/ASC/Caspase-1/ IL-1β/18 | Inhibits neuronal loss Improves functional recovery | 4 mg/kg | [112], [113], [114], [115], [116] |
| Gal-3 | TXNIP/NLRP3/ IL-1β/IL-18 | Enhances tissue protection Reduces neuronal loss and neuroinflammation Improves functional recovery | 10 mg/kg | [118] |
| Methylene blue | NLRP3/NLRC4/ASC/Caspase-1 | Inhibits neuronal lossand reduces cavity formation Promotes axonal regeneration and functional recovery | 2 mg/kg | [122] |
| Brilliant blue | GP2X/NLRP3/ASC/Cleaved XIAP/Caspase-1/Caspase-11/IL-1β/IL-18 | Reduces neuronal loss and cavity formation Promotes axonal regeneration and functional outcomes | 50 mg/kg | [123], [124], [125] |
| Peptide-5 | NLRP3/ASC/Caspase-1/IL-18 | Maintains neuropathic pain after peripheral nerve injury Promotes neuronal protection and prevents from neuronal cell death Enhances neuroprotection | 2.5, 5, 10 or 20 mg/kg | [126], [127], [128], [129] |
| Wogonoside | NLRP3/NF-κB /TLR4 | Reduces neuronal loss Improves functional recovery | 12, 25 or 50 mg/kg | [135] |
| A-68930 | NLRP3/Caspase-1/IL-1β/IL-18 | Promotes neuroprotectionand axonal regeneration Reduces neuronal loss and improves functional recovery | 5 mg/kg | [140] |
| Quercetin | ROS/NLRP3/IL-1β/IL-18 | Promotes neuroprotection axonal regeneration Reduces neuronal loss and cavity formation | 100 mg/kg | [145] |
Fig. 2.
Graphical description of targeting pyroptosis-regulated cell death for the therapeutic implications in SCI.
Zinc (Zn) plays essential roles in maintaining several cell functions [99]. Mei et al. clearly illustrated the potential effect of Zn on NLRP3 inflammasome and Nrf2/HO-1 signaling pathways after SCI [100]. After Zn administration, the NLRP3, ASC and caspase-1 expression levels were found high on day 3 after the injury in SCI mice model. Immunofluorescence staining indicated that Zn administration could efficaciously reduce the IL-1β positive neuron in the spinal cord anterior horn in SCI animal model. Zn administration can efficaciously block the NLRP3 inflammasome activation in SCI. Author and colleagues have assumed that the NLRP3 inflammasome is regulated by Zn ion through the Nrf2/HO-1 molecular signaling pathways activation [100]. The authors injected Zn intraperitoneally at 2 h and concurrently injected ML385, called an Nrf2/HO-1 signaling pathway inhibitor in SCI mice models in the previous investigation. Zn treatment could substantially up-regulate the nuclear Nrf2, HO-1 and NQO-1-related protein expression levels. Inflammasome-related protein expressions in the SCI-ZnG-ml385 group were opposite to the Zn-treated SCI model group and the NLRP3 protein expression levels remained unchanged. The authors have also documented that the Nrf2 protein expression level increased and the NLRP3 protein expression level decreased in the Zn-treated injured mice group compared with the SCI model group [100]. Studies have demonstrated that short-term Zn therapy may substantially enhance neuronal survival and functional recovery through inhibiting the NLRP3 activation and facilitating the Nrf2 signaling pathway. The authors investigated the substantial therapeutic role in VSC4.1 cells to investigate the Nrf2 signaling pathway in regulating Zn’s activation of NLRP3 inflammasome [100]. Besides, Zn treatment could considerably increase the Nrf2, HO-1, NQO-1 protein expression levels in VSC4.1 cells induced with H2O2 and ATP for a particular time and inhibit the NLRP3 inflammasome initiation [100]. Likewise, ML385 treatment reversed the potential effect of Zn treatments in H2O2 and ATP-induced VSC4.1 cells. ML385 treatment also enhanced the proportion of NLRP3-positive cells; unfortunately, Zn-therapy could not substantially inverse this event. These findings indicated that Zn treatment could efficaciously impede the NLRP3 inflammasome activation through the Nrf2/HO-1 signaling pathways in both in-vivo and in-vitro studies. Therefore, further investigations should be performed to elucidate in more detail on the potential role of this agent to inhibit the pyroptosis process as well as inflammasome components for the therapeutic implications of SCI in the near future.
ECH is known as phenylethanoid glycoside isolated from Cistanches used as a herbal medicine in whole China [101]. Further investigations have documented that ECH has significant neuroprotective activities [102], [103]. In a study, Gao and colleagues focused on exploring the potential therapeutic effects of ECH to impede the NLRP3 and other inflammasome components in microglia injury and SCI rat model [68]. The authors demonstrated that ECH administration efficaciously blocked the NEK7, NLRP3, caspase-1, ASC, as well as inflammatory mediators such as IL-1β, and IL-18 protein expression levels in the injured spinal cord on day 3 after the injury [68]. Administration of ECH in various concentrations largely impeded the expression of NEK7, NLRP3, ASC, caspase-1 as well as pro-inflammatory factors including IL-18 and IL-1β levels in LPS/ATP-induced BV-2 cells. Moreover, immunofluorescence staining distinctly illustrated that the NLRP3 expression level was notably enhanced in the injured spinal cord tissue sample and BV-2 cells [68]. ECH treatments in a concentration-dependent manner reduced the fluorescent intensity, demonstrating that ECH evidently reduced the NLRP3 protein expression in BV-2 cells. Several studies have referred that NF-κB signaling pathway can actively trigger the NLRP3 and other inflammasomes [68]. ECH treatment could impede the NF-κB signaling pathway-mediated protein expression in LPS/ATP-induced BV-2 cells. Administration of ECH in a concentration-dependent manner inverted the p-p65 and p-IκBα ratios. In addition, previous pieces of evidence have reported that ROS production plays a significant role in inflammasomes activation [104], [105]. ECH treatment could effectively reduce ROS production in PC-12 cells [68]. Therefore, these significant findings indicate that ECH can efficaciously reduce inflammasomes activation in SCI.
Polydatin (PD) is known as a glucoside of resveratrol which plays important roles in various pharmacological activities including anti-inflammation as well as anti-oxidation [106], [107]. Authors in a study have found the potential pharmacological effects of PD in the SCI model [106]. The authors demonstrated that PD treatment substantially reduces the NLRP3, ASC, cleaved caspase-1, caspase-1 and pro-inflammatory mediators including IL-1β and IL-18 expression levels in SCI animal model and LPS-induced BV-2 microglia in-vitro model [106]. After incubation with PD in different concentrations, the phosphorylation levels of NF-κBp65 were noticeably decreased and there was a sharp decline in phosphorylated IκB [106]. PD treatments substantially reduced the LPS-induced NF-κB signaling pathway in BV-2 microglia and impeded the initiation of NLRP3 inflammasome and iNOS in microglia [106], though authors did not identify the pyroptosis marker protein GSDMD and other signaling pathways in the previous study. In consequence, this molecule can be a potent drug for targeting pyroptosis signaling pathways in the therapeutic implications of the SCI treatment in the near future.
Rutin (RT) is a known flavonoid found in foods and plants which has significant anti-inflammatory effects [108]. Researchers have found that RT can efficaciously reduce neuronal damage after intracerebral hemorrhage in rat model [109] and has significant neuroprotective effects by enhancing neurotrophy after SCI [110], [111]. Another investigation has reported that RT administration also decreases the NLRP3, ASC and active-caspase-1 expression levels in SCI animal model. Besides, RT administration can substantially minimize the release of pro-inflammatory factors including IL-1β and IL-18 levels and reduce ROS production after SCI. Evidence illustrated that ROS could markedly regulate the NLRP3 inflammasome and other inflammasome components activation in CNS disorders [69]. The author and colleagues could not clearly demonstrate the pyroptosis-related protein GSDMD expression in the previous study. Thus, future investigations are highly essential on this novel agent to inhibit the pyroptosis signaling pathways in SCI in the near future. However, these outstanding findings conclude that this molecule can be a potential pyroptosis inhibitor for the SCI treatment in the near future.
Previous investigations have demonstrated that estrogen and progesterone or its metabolites improve functional outcomes after SCI [112], [113]. 17-estradiol (E2) is known as a neuroprotective hormone, plays a significant role as an anti-inflammatory activity in various neurological disorders as well as SCI [114], [115]. A study also demonstrated that the inflammatory mediators including IL-1β and IL-18 protein expression as well as serum levels were substantially attenuated by the E2 administration in the SCI treated rat group. More importantly, E2 administration largely attenuates the NLRP1b, NLRP3, ASC and caspase-1 protein expression levels in the SCI model group. After SCI, the protein and mRNA levels of NLRP3 inflammasome were expressed at 72 h [116]. Furthermore, the NLRP3 and ASC were significantly associated with oligodendrocytes, whereas an inflammasome component is not markedly expressed in APC+ cells. E2 and progesterone moderately impeded the demyelination of corpus callosum in a cuprizone induced experimental SCI animal model. Moreover, E2 treatment effectively inhibits the inflammasomes components and reduces local inflammatory responses in the injured spinal cord [116]. Therefore, these investigations indicate that this agent can effectively inhibit the pyroptosis phenomena and further investigations must be implemented to analyze the potential effects of this potent agent to inhibit the pyroptosis process for the intervention of SCI in future.
Gal-3 is a member of the galectin family, contains a sugar recognition domain with a specific affinity for β-galactoside [117]. W. Sheng et al. documented that the Gal-3 inhibition could substantially attenuate the TXNIP/NLRP3 over-expression levels in the injured spinal rat model [118]. Moreover, Gal-3 efficaciously suppressed the Gal-3, TXNIP, NLRP3 and IL-1β proteins expression level in SCI model, compared to the LPS model group. It has also been documented that si-TXNIP largely suppresses TXNIP and NLRP3 expression levels and reduces IL-1β and IL-18 levels in LPS-induced group and si-NLRP3 considerably down-regulates the NLRP3 and IL-1β protein expression in-vitro experiment [118]. Therefore, these substantial findings conclude that inhibiting the TXNIP molecule and NLRP3 inflammasome can be a potential therapeutic target to prevent the pyroptosis -regulated cell death after SCI in the near future.
Methylene blue (MB) plays an important role as a neuroprotective agent in several neurological disorders [119], [120], [121]. Lin and colleagues revealed that MB has significantly impeded the IL-1β and IL-18 expression levels instead of their mRNA levels in activated microglia [122]. Further investigations have documented that MB administration can significantly inhibit both NLRP3 and NLRC4 inflammasomes activation in microglia and the similar effect of MB treatment has been found in the rat SCI microglia, except that the NLRC4 inflammasome activation has not significantly found [122]. MB treatment markedly reduces the cleaved caspase-1 and IL-1β expression levels under LPS+ATP stimulation [122]. The authors then verified the most inflammasomes including NLRP3, NLRC4 and AIM2 protein expression levels in microglia [122]. Similarly, MB treatments sharply declined the NLRP3 and NLRC4 protein expression levels in stimulated microglia [122]. In addition, co-immuno-precipitation assay clearly illustrated that MB treatment could induce the strong binding of ASC to NLRP3 and NLRC4 in stimulated microglia. Moreover, MB treatment enormously minimizes the ASC to NLRP3 and NLRC4 binding, indicating that this agent can inhibit the activation of inflammasome components at the injured spinal cord areas [122]. Although the authors did not clearly explain the pyroptosis marker including GSDMD and other signaling pathways, further investigations must be carried out to clarify more detail on the potential effects of this molecule to inhibit the pyroptotic neuronal cell death mechanism for the novel therapies of SCI in the near future. Thus, these notable findings suggested that MB largely suppressed the inflammatory responses through inhibiting inflammasomes activation after SCI.
BBG is called a P2X7 receptor inhibitor, which notably enhances functional recoveries after SCI [123], [124]. Zhou et al. studied the potential effect of BBG on inflammasomes-related protein expression in SCI model [125]. The study also reported that the P2X7, NLRP3, ASC, cleaved XIAP, caspase-11 and caspase-1 expression levels were noticeably enhanced in the SCI animal group than the sham model group. In addition, the P2X7, NLRP3, ASC, cleaved XIAP, caspase-11 and caspase-1 protein levels were declined in the BBG treated SCI animal model. Moreover, NLRP1 protein expression levels did not significantly differ among the SCI model groups and immunohistochemistry staining also illustrated that the NLRP3 and ASC protein expression levels were notably decreased in the BBG treated SCI animal model [125]. BBG administration can efficaciously reduce the pro-inflammatory factors including IL-1β and IL-18 expression levels after SCI. Consequently, these critical findings conclude that BBG administration can significantly inhibit the inflammasomes-related proteins expression and neuronal cell death in SCI animal model. Therefore, further studies are required to explain in more detail on the potential effects of this molecule in inhibiting the pyroptosis-signaling pathways for the novel therapies of SCI in future.
Cx43 is known as hemichannels in spinal astrocytes, which is actively involved in maintaining neuropathic pain after peripheral nerve injury [126]. Peptide5 is known as a Cx43 mimetic peptide, plays significant roles in blocking hemichannels [127], [128]. Tonkin et al. demonstrated that peptide5, a Cx43 mimetic peptide treatment can markedly attenuate the NLRP3 inflammasome, ASC and caspase-1 protein expression levels in SCI mice model [129]. Moreover, the NLRP3 overexpression has been reported in the primary cultures of rat astrocytes, mouse cortical neurons and mouse microglia but not in the primary human neurons and purified microglia and the astrocyte cultures from fetal tissues [129], [130], [131], [132]. Dual immunofluorescence staining clearly illustrated that NLRP3 was mostly co-localized with NeuN + neurons and IBA-1 + microglia and only a small amount of GFAP + stellate cells were co-labeled dual immunofluorescence staining markedly pointed that NLRP3 was mainly co-localized with NeuN + neurons and IBA-1 + microglia. Blocking the Cx43 hemichannels with peptide5 significantly ameliorated the mechanical pain hypersensitivity by inhibiting the NLRP3 inflammasome in the injured spinal cord [129]. Although the authors did not investigate the pyroptosis signaling pathway-related proteins, more investigations are urgently essential to explain the potential effects of this molecule to inhibit the novel cell death program for the SCI therapy in the near future.
Wogonoside (WG) is derived from Scutellaria baicalensis, plays vital roles as an antithrombotic and antihypertensive agent, which is used for the treatment of coronary heart disease [133], [134]. In a study, author and colleagues investigated whether WG agent could inhibit the inflammatory responses induced by NF-κB and NLRP3 signaling pathways activation in SCI rat model or not [135]. The authors reported that treatment with 12.5 mg/kg of WG did not cause notable distinction in the caspase-1 or TLR4 expression levels, whereas 25 or 50 mg/kg WG enormously reduced the caspase-1 expression levels in SCI rat model. Besides, WG treatment can considerably reduce the NF-κB and NLRP3 expression levels [135]. A previous study also illustrated that the caspase-1 and TLR4 expression levels were dramatically augmented in the SCI group model, relative to the control group treatment with 12.5 mg/kg WG induced no remarkable variation in the caspase-1 or TLR4 expression levels, whereas 25 or 50 mg/kg WG treatment notably down-regulated the caspase-1 expression after SCI [135]. More importantly, the authors reported that WG administration could tremendously promote axonal regeneration and improve functional outcomes after SCI via suppressing caspases-1-mediated neuronal cell death [135]. Thus, these substantial findings conclude that WG can be a novel molecule for targeting pyroptosis in the therapeutic implications of SCI in the near future.
A-68930 stimulates DRD1, plays important roles as an anti-stress, anti-inflammatory and neuroprotective agent [136], [137], [138]. Previous studies have documented that dopamine significantly reduces the NLRP3 inflammasome activation through DRD1 signaling, and DRD1 can induce the NLRP3 ubiquitination and autophagy-mediated degradation in the experimental studies [139]. Moreover, DRD1 signaling can substantially mitigate LPS-induced inflammatory responses and monosodium urate crystal-stimulated peritoneal inflammatory responses through inhibiting the NLRP3 inflammasome [139]. A-68930 can regulate the neurotoxin 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced neuroinflammation and diminish huge neurons loss through inhibiting the NLRP3 inflammasome in mice model [139]. W Jiang et al. documented that A-68930 effectively minimized the activation of NLRP3 inflammasome and inflammatory cytokines release [140]. The author has also documented that A-68930 administration substantially reduced the protein expression of active caspase-1 levels in the drug-treated SCI model group [140]. Importantly, the authors illustrated that A-68930 treatments could promote axonal regeneration and impede neuronal loss in the injured spinal cord area [140]. A-68930 has significant effects on SCI by inhibiting the inflammasome components and reducing inflammatory responses [140]. Therefore, these notable shreds of evidence decisively indicate that A-68930 can be potent molecule to inhibit the pyroptosis-signaling pathways as well as inflammasome components for the unique therapeutic strategy of SCI in the future.
Quercetin (QUE) is known as a flavonoid available in abundance. Previous studies have reported that QUE can promote neurological recovery in experimental animal models [141], [142], [143], [144]. W Jiang et al. demonstrated that QUE could considerably impede the ROS synthesis and NLRP3 activation in SCI animal model [145]. The author and colleagues have also documented that after QUE administration, the pro-inflammatory factors including IL-1β and IL-18 protein expression levels were noticeably down-regulated at 72 h [145]. Thus, these critical shreds of evidence conclude that this potent molecule can considerably impede the NLRP3 inflammasome in SCI. Further investigations are highly demanded to elucidate the potential effects of this agent in inhibiting the pyroptosis process for the novel therapies of SCI.
Conclusion and future directions
Multiple pieces of evidence have documented that caspase-1 caspase-4/5/11 signaling pathways play crucial roles in SCI [56], [94]. Besides, emerging evidence has also documented that NLRP3 inflammasome activation plays key roles in pyroptosis process in SCI [146]. In our review, we have briefly described the critical functions of the activation of NLRP3, other inflammasomes and pyroptosis process, which are involved in SCI. Furthermore, current investigations strongly suggest that some essential molecules can be applied to inhibit those pathways for the novel pharmacological therapies of SCI in the near future. The actual mechanism of the discussed potential molecules has remained elusive. In addition, the investigations on the potential role of pyroptosis-regulated cell death in SCI are still limited to the experimental studies. Further investigations or clinical studies in more detail should be conducted to explore the actual mechanism and its potential role of pyroptosis-regulated cell death in SCI in the near future. Clinical studies of those agents on stem cells/exosomes/microvesicles can open a new gateway to find a potential therapeutic approach for the treatment of SCI in future [56], [57], [84]. Therefore, our narrative article suggests that inhibiting the pyroptosis-regulated cell death and inflammasome components could be a promising therapeutic approach for the treatment of SCI in the near future.
Compliance with Ethics Requirements
Our article does not contain any studies with human or animal subjects.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This review work was supported by the Natural Science Foundation of China (81972150, 81722028, 81801233); Natural Science Foundation of Zhejiang Province (LR18H50001), Research Unit of Research and Clinical Translation of Cell Growth Factors and Diseases of Chinese Academy of Medical Science (2019RU010).
Biographies

Abdullah Al Mamun is a doctoral degree research scholar at School of Pharmaceutical Sciences, Wenzhou Medical University, Wenzhou 325035, Zhejiang Province, China. He completed his bachelor degree from Southeast University, Banani, Dhaka-1213, Bangladesh. He is currently doing research on pyroptosis in various diseases including liver diseases and spinal cord injury under Professor, Jian Xiao direct research supervision. His doctoral degree research area is on the role of pyroptosis in spinal cord injury. Already he published some research and review articles on the prestigious journals with high impact factor. E-mail: pharmaalmamun@yahoo.com.

Yanqing Wu is a young research scholar at Institute of Life Sciences, Wenzhou University, Wenzhou 325035, Zhejiang Province, China. Her current research areas are on growth factors and diseases such as diabetes, peripheral nerve injury. She has published lots of research articles on the spinal cord injury in the international reputed peer-review journals. Currently, she is doing research on FGF1 in diabetes-induced spinal cord injury. She has also reviewed so many research and review articles in the reputed locally and internationally scientific and clinical peer-review journals. Email: yqwu220946@yeah.net.

Ilma Monalisa completed her bachelor degree (B. Pharm) from Department of Pharmacy, Southeast University, Banani, Dhaka-1213, Bangladesh. She is currently doing researcher on pyroptosis in various diseases including liver diseases and spinal cord injury. She already published lots of scientific and clinical research articles on the international recognized peer-review journal with high impact factors. E-mail: ilma.ruf08@gmail.com.

Chang Jia is a research scholar in the pediatric research fields focusing on the role of pyroptosis in cardiovascular diseases, kawashaki disease as well as various infectious diseases. She completed her doctoral degree from Nankai University, Tianjin, China. She already published lots of scientific and clinical research articles on the international recognized peer-review journal with high impact factors. E-mail: jiashang0802@163.com.

Kailiang Zhou is a research scholar in the orthopedics research field focusing on the role of autophagy in various diseases including spinal cord injury. He completed his doctoral degree from the department of Orthopaedics, The Second Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University, Wenzhou 325027, Zhejiang Province, China. He already published lot of scientific and clinical research articles on the international recognized peer-review journal with high impact factors. E-mail: zhoukailiang@wmu.edu.cn.

Fahad Munir completed his doctoral degree from the Department of Hepatobiliary Surgery, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou 325000, Zhejiang Province, China. He is currently doing research on novel cell death program of hepatocytes, immunological therapies for the treatment of liver diseases. Recently he published an article on the role of novel programmed cell death in the liver diseases. He already published some scientific and clinical research articles in the international recognized peer-review journal with high impact factors. He has reviewed lots of articles in the recognized scientific and clinical journals. E-mail: 1460907287@qq.com.

Jian Xiao is a professor of Molecular Pharmacology Research Center, School of Pharmaceutical Sciences, Wenzhou Medical University, Wenzhou 325035, Zhejiang Province, China. He has more than 200 research and review articles in the global recognized peer-review journal with high impact factor. He has numbers of Chinese and international students in his research lab. He is currently focusing research on growth factors in various diseases including spinal cord injury, diabetes and peripheral nerve injury. He has reviewed lots of articles in the recognized international scientific and clinical journals. E-mail: xjian@wzmc.edu.cn, xfxj2000@126.com.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Burns A.S., O'Connell C. The challenge of spinal cord injury care in the developing world. J Spinal Cord Med. 2012;35(1):3–8. doi: 10.1179/2045772311Y.0000000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuan S., Shi Z., Cao F., Li J., Feng S. Epidemiological features of spinal cord injury in China: a systematic review. Front Neurol. 2018;9:683. doi: 10.3389/fneur.2018.00683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ning G.Z., Mu Z.P., Shangguan L., Tang Y., Li C.Q., Zhang Z.F. Epidemiological features of traumatic spinal cord injury in Chongqing, China. J Spinal Cord Med. 2016;39(4):455–460. doi: 10.1080/10790268.2015.1101982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Y., Wang X.B., Kan S.L., Ning G.Z., Li Y.L., Yang B. Traumatic spinal cord injury in Tianjin, China: a single-center report of 354 cases. Spinal Cord. 2016;54(9):670–674. doi: 10.1038/sc.2015.173. [DOI] [PubMed] [Google Scholar]
- 5.Reier P.J. Cellular transplantation strategies for spinal cord injury and translational neurobiology. NeuroRx: J Am Soc Exp NeuroTherapeut. 2004;1(4):424–451. doi: 10.1602/neurorx.1.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahuja C.S., Nori S., Tetreault L., Wilson J., Kwon B., Harrop J. Traumatic spinal cord injury-repair and regeneration. Neurosurgery. 2017;80(3s):S9–s22. doi: 10.1093/neuros/nyw080. [DOI] [PubMed] [Google Scholar]
- 7.Alizadeh A., Dyck S.M., Karimi-Abdolrezaee S. Traumatic spinal cord injury: an overview of pathophysiology, models and acute injury mechanisms. Front Neurol. 2019;10:282. doi: 10.3389/fneur.2019.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alizadeh A, Dyck SM, Kataria H, Shahriary GM, Nguyen DH, Santhosh KT. Neuregulin-1 positively modulates glial response and improves neurological recovery following traumatic spinal cord injury. Glia. 2017;65(7):1152–1175. doi: 10.1002/glia.23150. [DOI] [PubMed] [Google Scholar]
- 9.Simon D.W., McGeachy M.J., Bayır H., Clark R.S., Loane D.J., Kochanek P.M. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat Rev Neurol. 2017;13(3):171–191. doi: 10.1038/nrneurol.2017.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen L., Deng H., Cui H., Fang J., Zuo Z., Deng J. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9(6):7204–7218. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okada S. The pathophysiological role of acute inflammation after spinal cord injury. Inflamm Regener. 2016;36:20. doi: 10.1186/s41232-016-0026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Y., Wang H., Kouadir M., Song H., Shi F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019;10(2):128. doi: 10.1038/s41419-019-1413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song N., Li T. Regulation of NLRP3 inflammasome by phosphorylation. Front Immunol. 2018;9:2305. doi: 10.3389/fimmu.2018.02305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang W.Y., Tan M.S., Yu J.T., Tan L. Role of pro-inflammatory cytokines released from microglia in Alzheimer's disease. Ann Transl Med. 2015;3(10):136. doi: 10.3978/j.issn.2305-5839.2015.03.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaur D, Sharma V, Deshmukh R. Activation of microglia and astrocytes: a roadway to neuroinflammation and Alzheimer’s disease. Inflammopharmacology. 2019;27(4):663–677. doi: 10.1007/s10787-019-00580-x. [DOI] [PubMed] [Google Scholar]
- 16.Latz E., Xiao T.S., Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13(6):397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groslambert M., Py B.F. Spotlight on the NLRP3 inflammasome pathway. J Inflamm Res. 2018;11:359–374. doi: 10.2147/JIR.S141220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swanson KV, Deng M. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19(8):477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groslambert M., Py B.F. Regulation of the NLRP3 inflammasome. Med Sci: M/S. 2018;34(1):47–53. doi: 10.1051/medsci/20183401013. [DOI] [PubMed] [Google Scholar]
- 20.Man SM, Karki R, Kanneganti TD. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev. 2017;277(1):61–75. doi: 10.1111/imr.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Achouri S., Wright J.A., Evans L., Macleod C., Fraser G., Cicuta P. The frequency and duration of Salmonella-macrophage adhesion events determines infection efficiency. Philos Trans R Soc Lond B Biol Sci. 2015;370(1661):20140033. doi: 10.1098/rstb.2014.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ringel-Scaia V.M., McDaniel D.K., Allen I.C. The Goldilocks conundrum: NLR inflammasome modulation of gastrointestinal inflammation during inflammatory bowel disease. Crit Rev Immunol. 2016;36(4):283–314. doi: 10.1615/CritRevImmunol.2017019158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He W.T., Wan H., Hu L., Chen P., Wang X., Huang Z. Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res. 2015;25(12):1285–1298. doi: 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jorgensen I., Miao E.A. Pyroptotic cell death defends against intracellular pathogens. Immunol Rev. 2015;265(1):130–142. doi: 10.1111/imr.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruan J. Structural insight of gasdermin family driving pyroptotic cell death. Adv Exp Med Biol. 2019;1172:189–205. doi: 10.1007/978-981-13-9367-9_9. [DOI] [PubMed] [Google Scholar]
- 26.Kovacs S.B., Miao E.A. Gasdermins: effectors of pyroptosis. Trends Cell Biol. 2017;27(9):673–684. doi: 10.1016/j.tcb.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi J., Zhao Y., Wang K., Shi X., Wang Y., Huang H. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526(7575):660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 28.He W.T., Wan H., Hu L., Chen P., Wang X., Huang Z. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015;25(12):1285–1298. doi: 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneider K.S., Groß C.J., Dreier R.F., Saller B.S., Mishra R., Gorka O. The inflammasome drives GSDMD-independent secondary pyroptosis and IL-1 release in the absence of caspase-1 protease activity. Cell Rep. 2017;21(13):3846–3859. doi: 10.1016/j.celrep.2017.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Broz P, Pelegrín P. The gasdermins, a protein family executing cell death and inflammation. Nat Rev Immunol. 2020;20(3):143–157. doi: 10.1038/s41577-019-0228-2. [DOI] [PubMed] [Google Scholar]
- 31.Lu F., Lan Z., Xin Z., He C., Guo Z., Xia X. Emerging insights into molecular mechanisms underlying pyroptosis and functions of inflammasomes in diseases. J Cell Physiol. 2020;235(4):3207–3221. doi: 10.1002/jcp.29268. [DOI] [PubMed] [Google Scholar]
- 32.McKenzie B.A., Dixit V.M., Power C. Fiery cell death: pyroptosis in the central nervous system. Trends Neurosci. 2020;43(1):55–73. doi: 10.1016/j.tins.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 33.Shi J., Gao W., Shao F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci. 2017;42(4):245–254. doi: 10.1016/j.tibs.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Liu X., Zhang Z., Ruan J., Pan Y., Magupalli V.G., Wu H. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535(7610):153–158. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu X., Lieberman J. A mechanistic understanding of pyroptosis: the fiery death triggered by invasive infection. Adv Immunol. 2017;135:81–117. doi: 10.1016/bs.ai.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yokoyama S, Cai Y, Murata M, Tomita T, Yoneda M, Xu L. A novel pathway of LPS uptake through syndecan-1 leading to pyroptotic cell death. Elife. 2018;7(7):e37854. doi: 10.7554/eLife.37854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang J, Liu Z, Wang C, Yang R, Rathkey JK, Pinkard OW. Mechanism of gasdermin D recognition by inflammatory caspases and their inhibition by a gasdermin D-derived peptide inhibitor. Proc Natl Acad Sci U S A. 2018;115(26):6792–6797. doi: 10.1073/pnas.1800562115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang K., Sun Q., Zhong X., Zeng M., Zeng H., Shi X. Structural mechanism for GSDMD targeting by autoprocessed caspases in pyroptosis. Cell. 2020;180(5):941–955.e20. doi: 10.1016/j.cell.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 39.Aachoui Y., Sagulenko V., Miao E.A., Stacey K.J. Inflammasome-mediated pyroptotic and apoptotic cell death, and defense against infection. Curr Opin Microbiol. 2013;16(3):319–326. doi: 10.1016/j.mib.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kayagaki N., Stowe I.B., Lee B.L., O'Rourke K., Anderson K., Warming S. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526(7575):666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 41.Yuan Y.Y., Xie K.X., Wang S.L., Yuan L.W. Inflammatory caspase-related pyroptosis: mechanism, regulation and therapeutic potential for inflammatory bowel disease. Gastroenterol Rep. 2018;6(3):167–176. doi: 10.1093/gastro/goy011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ding J., Wang K., Liu W., She Y., Sun Q., Shi J. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535(7610):111–116. doi: 10.1038/nature18590. [DOI] [PubMed] [Google Scholar]
- 43.Cheng K.T., Xiong S., Ye Z., Hong Z., Di A., Tsang K.M. Caspase-11-mediated endothelial pyroptosis underlies endotoxemia-induced lung injury. J Clin Investig. 2017;127(11):4124–4135. doi: 10.1172/JCI94495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fang Y., Tian S., Pan Y., Li W., Wang Q., Tang Y. A new frontier in cancer. Biomed Pharmacother = Biomedecine pharmacotherapie. 2020;121 doi: 10.1016/j.biopha.2019.109595. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y.Y., Liu X.L., Zhao R. Induction of pyroptosis and its implications in cancer management. Front Oncol. 2019;9:971. doi: 10.3389/fonc.2019.00971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rogers C., Fernandes-Alnemri T., Mayes L., Alnemri D., Cingolani G., Alnemri E.S. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat Commun. 2017;8:14128. doi: 10.1038/ncomms14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Y.-Q., Peng J.-J., Peng J., Luo X.-J. The deafness gene GSDME: its involvement in cell apoptosis, secondary necrosis, and cancers. Naunyn-Schmiedeberg's Arch Pharmacol. 2019;392(9):1043–1048. doi: 10.1007/s00210-019-01674-7. [DOI] [PubMed] [Google Scholar]
- 48.Rogers C., Erkes D.A., Nardone A., Aplin A.E., Fernandes-Alnemri T., Alnemri E.S. Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation. Nat Commun. 2019;10(1):1689. doi: 10.1038/s41467-019-09397-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tixeira R., Shi B., Parkes M.A.F., Hodge A.L., Caruso S., Hulett M.D. Gasdermin E does not limit apoptotic cell disassembly by promoting early onset of secondary necrosis in Jurkat T cells and THP-1 monocytes. Front Immunol. 2018;9:2842. doi: 10.3389/fimmu.2018.02842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang CC, Li CG, Wang YF, Xu LH, He XH, Zeng QZ. Chemotherapeutic paclitaxel and cisplatin differentially induce pyroptosis in A549 lung cancer cells via caspase-3/GSDME activation. Apoptosis. 2019;24(3–4):312–325. doi: 10.1007/s10495-019-01515-1. [DOI] [PubMed] [Google Scholar]
- 51.Zheng Z, Bian Y, Zhang Y, Ren G, Li G. Metformin activates AMPK/SIRT1/NF-κB pathway and induces mitochondrial dysfunction to drive caspase3/GSDME-mediated cancer cell pyroptosis. Cell Cycle. 2020;19(10):1089–1104. doi: 10.1080/15384101.2020.1743911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee B.L., Mirrashidi K.M., Stowe I.B., Kummerfeld S.K., Watanabe C., Haley B. ASC- and caspase-8-dependent apoptotic pathway diverges from the NLRC4 inflammasome in macrophages. Sci Rep. 2018;8(1):3788. doi: 10.1038/s41598-018-21998-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aizawa E., Karasawa T., Watanabe S., Komada T., Kimura H., Kamata R. GSDME-dependent incomplete pyroptosis permits selective IL-1α release under caspase-1 inhibition. iScience. 2020;23(5) doi: 10.1016/j.isci.2020.101070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vince J.E., Silke J. The intersection of cell death and inflammasome activation. Cell Molecular Life Sci: CMLS. 2016;73(11–12):2349–2367. doi: 10.1007/s00018-016-2205-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Orning P, Weng D, Starheim K, Ratner D. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science. 2018;362(6418):1064–1069. doi: 10.1126/science.aau2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu S, Shao M, Ma X, Jiang J, Zhang F, Xu H. CD73 alleviates GSDMD-mediated pyroptosis in spinal cord injury through PI3K/AKT/Foxo1 signaling. Neurobiol Dis. 2020 doi: 10.21203/rs.2.18409/v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dai W., Wang X., Teng H., Li C., Wang B., Wang J. Celastrol inhibits microglial pyroptosis and attenuates inflammatory reaction in acute spinal cord injury rats. Int Immunopharmacol. 2019;66:215–223. doi: 10.1016/j.intimp.2018.11.029. [DOI] [PubMed] [Google Scholar]
- 58.Shao B.Z., Cao Q., Liu C. Targeting NLRP3 inflammasome in the treatment of CNS diseases. Front Mol Neurosci. 2018;11:320. doi: 10.3389/fnmol.2018.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Song L., Pei L., Yao S., Wu Y., Shang Y. NLRP3 inflammasome in neurological diseases, from functions to therapies. Front Cell Neurosci. 2017;11:63. doi: 10.3389/fncel.2017.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lippai D., Bala S., Petrasek J., Csak T., Levin I., Kurt-Jones E.A. Alcohol-induced IL-1beta in the brain is mediated by NLRP3/ASC inflammasome activation that amplifies neuroinflammation. J Leukoc Biol. 2013;94(1):171–182. doi: 10.1189/jlb.1212659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu Z.W., Zhang J., Li X., Wang Y., Fu Y.H., Gao X.Y. A new research hot spot: the role of NLRP3 inflammasome activation, a key step in pyroptosis, in diabetes and diabetic complications. Life Sci. 2020;240 doi: 10.1016/j.lfs.2019.117138. [DOI] [PubMed] [Google Scholar]
- 62.Gustin A., Kirchmeyer M., Koncina E., Felten P., Losciuto S., Heurtaux T. NLRP3 inflammasome is expressed and functional in mouse brain microglia but not in astrocytes. PLoS ONE. 2015;10(6) doi: 10.1371/journal.pone.0130624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Molagoda I.M.N., Lee S., Jayasooriya R., Jin C.Y., Choi Y.H., Kim G.Y. Deoxynivalenol enhances IL-1ss expression in BV2 microglial cells through activation of the NF-?B pathway and the ASC/NLRP3 inflammasome. EXCLI J. 2019;18:356–369. doi: 10.17179/excli2018-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mogensen T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009;22(2):240–273. doi: 10.1128/CMR.00046-08. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brubaker S.W., Bonham K.S., Zanoni I., Kagan J.C. Innate immune pattern recognition: a cell biological perspective. Annu Rev Immunol. 2015;33:257–290. doi: 10.1146/annurev-immunol-032414-112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kelley N., Jeltema D., Duan Y., He Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20(13) doi: 10.3390/ijms20133328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bauernfeind F.G., Horvath G., Stutz A., Alnemri E.S., MacDonald K., Speert D. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183(2):787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gao S., Xu T., Guo H., Deng Q., Xun C., Liang W. Ameliorative effects of echinacoside against spinal cord injury via inhibiting NLRP3 inflammasome signaling pathway. Life Sci. 2019;237 doi: 10.1016/j.lfs.2019.116978. [DOI] [PubMed] [Google Scholar]
- 69.Zhou K, Shi L, Wang Y, Chen S. Recent advances of the NLRP3 inflammasome in central nervous system disorders. J Immunol Res. 2016;2016:9238290. doi: 10.1155/2016/9238290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zheng G., Zhan Y., Wang H., Luo Z., Zheng F., Zhou Y. Carbon monoxide releasing molecule-3 alleviates neuron death after spinal cord injury via inflammasome regulation. EBioMed. 2019;40:643–654. doi: 10.1016/j.ebiom.2018.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nasoohi S, Ismael S, Ishrat T. Thioredoxin-Interacting Protein (TXNIP) in Cerebrovascular and Neurodegenerative Diseases: Regulation and Implication. Mol Neurobiol. 2018;55(10):7900–7920. doi: 10.1007/s12035-018-0917-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ishrat T., Mohamed I.N., Pillai B., Soliman S., Fouda A.Y., Ergul A. Thioredoxin-interacting protein: a novel target for neuroprotection in experimental thromboembolic stroke in mice. Mol Neurobiol. 2015;51(2):766–778. doi: 10.1007/s12035-014-8766-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hornung V., Ablasser A., Charrel-Dennis M., Bauernfeind F., Horvath G., Caffrey D.R. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458(7237):514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sagulenko V., Thygesen S.J., Sester D.P., Idris A., Cridland J.A., Vajjhala P.R. AIM2 and NLRP3 inflammasomes activate both apoptotic and pyroptotic death pathways via ASC. Cell Death Differ. 2013;20(9):1149–1160. doi: 10.1038/cdd.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rathinam V.A., Jiang Z., Waggoner S.N., Sharma S., Cole L.E., Waggoner L. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 2010;11(5):395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang S.N., Guo X.Y., Tang J., Ding S.Q., Shen L., Wang R. Expression and localization of absent in melanoma 2 in the injured spinal cord. Neural Regener Res. 2019;14(3):542–552. doi: 10.4103/1673-5374.245481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Adamczak S.E., de Rivero Vaccari J.P., Dale G., Brand F.J., 3rd, Nonner D., Bullock M.R., Dahl G.P., Dietrich W.D., Keane R.W. Pyroptotic neuronal cell death mediated by the AIM2 inflammasome. J Cereb Blood Flow Metab: Off J Int Soc Cerebral Blood Flow Metab. 2014;34(4):621–629. doi: 10.1038/jcbfm.2013.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kaushal V., Dye R., Pakavathkumar P., Foveau B., Flores J., Hyman B. Neuronal NLRP1 inflammasome activation of Caspase-1 coordinately regulates inflammatory interleukin-1-beta production and axonal degeneration-associated Caspase-6 activation. Cell Death Differ. 2015;22(10):1676–1686. doi: 10.1038/cdd.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li X.Q., Yu Q., Fang B., Zhang Z.L., Ma H. Knockdown of the AIM2 molecule attenuates ischemia-reperfusion-induced spinal neuronal pyroptosis by inhibiting AIM2 inflammasome activation and subsequent release of cleaved caspase-1 and IL-1beta. Neuropharmacology. 2019;160 doi: 10.1016/j.neuropharm.2019.05.038. [DOI] [PubMed] [Google Scholar]
- 80.Yanagisawa S., Katoh H., Imai T., Nomura S., Watanabe M. The relationship between inflammasomes and the endoplasmic reticulum stress response in the injured spinal cord. Neurosci Lett. 2019;705:54–59. doi: 10.1016/j.neulet.2019.04.033. [DOI] [PubMed] [Google Scholar]
- 81.Zendedel A., Johann S., Mehrabi S., Joghataei M.T., Hassanzadeh G., Kipp M. Activation and regulation of NLRP3 inflammasome by intrathecal application of SDF-1a in a spinal cord injury model. Mol Neurobiol. 2016;53(5):3063–3075. doi: 10.1007/s12035-015-9203-5. [DOI] [PubMed] [Google Scholar]
- 82.Jiang W., Li M., He F., Zhou S., Zhu L. Targeting the NLRP3 inflammasome to attenuate spinal cord injury in mice. J Neuroinflamm. 2017;14(1):207. doi: 10.1186/s12974-017-0980-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang X., Arcuino G., Takano T., Lin J., Peng W.G., Wan P. P2X7 receptor inhibition improves recovery after spinal cord injury. Nat Med. 2004;10(8):821–827. doi: 10.1038/nm1082. [DOI] [PubMed] [Google Scholar]
- 84.Lin W.-P., Xiong G.-P., Lin Q., Chen X.-W., Zhang L.-Q., Shi J.-X. Heme oxygenase-1 promotes neuron survival through down-regulation of neuronal NLRP1 expression after spinal cord injury. J Neuroinflamm. 2016;13(1):52. doi: 10.1186/s12974-016-0521-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bishayee A., Ahmed S., Brankov N., Perloff M. Triterpenoids as potential agents for the chemoprevention and therapy of breast cancer. Front Biosci (Landmark edition) 2011;16:980–996. doi: 10.2741/3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li Y., He D., Zhang X., Liu Z., Zhang X., Dong L. Protective effect of celastrol in rat cerebral ischemia model: down-regulating p-JNK, p-c-Jun and NF-kappaB. Brain Res. 2012;1464:8–13. doi: 10.1016/j.brainres.2012.04.054. [DOI] [PubMed] [Google Scholar]
- 87.Konieczny J., Jantas D., Lenda T., Domin H., Czarnecka A., Kuter K. Lack of neuroprotective effect of celastrol under conditions of proteasome inhibition by lactacystin in in vitro and in vivo studies: implications for Parkinson's disease. Neurotox Res. 2014;26(3):255–273. doi: 10.1007/s12640-014-9477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang Y., Lv X., Hu Z., Ye X., Zheng X., Ding Y., Xie P., Liu Q. Protection of Mcc950 against high-glucose-induced human retinal endothelial cell dysfunction. Cell Death Disease. 2017;8(7):e2941. doi: 10.1038/cddis.2017.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ismael S., Nasoohi S., Ishrat T. MCC950, the selective inhibitor of nucleotide oligomerization domain-like receptor protein-3 inflammasome, protects mice against traumatic brain injury. J Neurotrauma. 2018;35(11):1294–1303. doi: 10.1089/neu.2017.5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu X., Yin D., Ren H., Gao W., Li F., Sun D. Selective NLRP3 inflammasome inhibitor reduces neuroinflammation and improves long-term neurological outcomes in a murine model of traumatic brain injury. Neurobiol Disease. 2018;117:15–27. doi: 10.1016/j.nbd.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 91.Perera AP, Fernando R, Shinde T, Gundamaraju R, Southam B, Sohal SS. MCC950, a specific small molecule inhibitor of NLRP3 inflammasome attenuates colonic inflammation in spontaneous colitis mice. Sci Rep. 2018;8(1):8618. doi: 10.1038/s41598-018-26775-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen Y., Meng J., Xu Q., Long T., Bi F., Chang C. Rapamycin improves the neuroprotection effect of inhibition of NLRP3 inflammasome activation after TBI. Brain Res. 2019;1710:163–172. doi: 10.1016/j.brainres.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 93.Jiao J., Zhao G., Wang Y., Ren P., Wu M. MCC950, a selective inhibitor of NLRP3 inflammasome, reduces the inflammatory response and improves neurological outcomes in mice model of spinal cord injury. Front Molecular Biosci. 2020;7:37. doi: 10.3389/fmolb.2020.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mortezaee K., Khanlarkhani N., Beyer C., Zendedel A. Inflammasome: its role in traumatic brain and spinal cord injury. J Cell Physiol. 2018;233(7):5160–5169. doi: 10.1002/jcp.26287. [DOI] [PubMed] [Google Scholar]
- 95.Oslowski C.M., Hara T., O'Sullivan-Murphy B., Kanekura K., Lu S., Hara M. Thioredoxin-interacting protein mediates ER stress-induced β cell death through initiation of the inflammasome. Cell Metab. 2012;16(2):265–273. doi: 10.1016/j.cmet.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lerner A.G., Upton J.P., Praveen P.V., Ghosh R., Nakagawa Y., Igbaria A. IRE1α induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metab. 2012;16(2):250–264. doi: 10.1016/j.cmet.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yuan B., Zhou X.M., You Z.Q., Xu W.D., Fan J.M., Chen S.J. Inhibition of AIM2 inflammasome activation alleviates GSDMD-induced pyroptosis in early brain injury after subarachnoid haemorrhage. Cell Death Dis. 2020;11(1):76. doi: 10.1038/s41419-020-2248-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.de Rivero Vaccari J.P. Carbon monoxide releasing molecule-3 inhibits inflammasome activation: a potential therapy for spinal cord injury. EBioMedicine. 2019;40:17–18. doi: 10.1016/j.ebiom.2019.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cortese-Krott M.M., Kulakov L., Oplander C., Kolb-Bachofen V., Kroncke K.D., Suschek C.V. Zinc regulates iNOS-derived nitric oxide formation in endothelial cells. Redox Biol. 2014;2:945–954. doi: 10.1016/j.redox.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li D., Tian H., Li X., Mao L., Zhao X., Lin J. Zinc promotes functional recovery after spinal cord injury by activating Nrf2/HO-1 defense pathway and inhibiting inflammation of NLRP3 in nerve cells. Life Sci. 2020;245 doi: 10.1016/j.lfs.2020.117351. [DOI] [PubMed] [Google Scholar]
- 101.Wu C.J., Chien M.Y., Lin N.H., Lin Y.C., Chen W.Y., Chen C.H. Echinacoside isolated from cistanche tubulosa putatively stimulates growth hormone secretion via activation of the ghrelin receptor. Molecules. 2019;24(4) doi: 10.3390/molecules24040720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang J, Zhang Z, Xiang J, Cai M, Yu Z, Li X. Neuroprotective effects of echinacoside on regulating the stress-active p38MAPK and NF-kappaB p52 signals in the mice model of Parkinson’s disease. Neurochem Res. 2017;42(4):975–985. doi: 10.1007/s11064-016-2130-7. [DOI] [PubMed] [Google Scholar]
- 103.Liu J, Yang L, Dong Y, Zhang B, MaEchinacoside X. an Inestimable natural product in treatment of neurological and other disorders. Molecules. 2018;23(5) doi: 10.3390/molecules23051213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Harijith A., Ebenezer D.L., Natarajan V. Reactive oxygen species at the crossroads of inflammasome and inflammation. Front Physiol. 2014;5:352. doi: 10.3389/fphys.2014.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zheng Q., Ren Y., Reinach P.S., Xiao B., Lu H., Zhu Y. Reactive oxygen species activated NLRP3 inflammasomes initiate inflammation in hyperosmolarity stressed human corneal epithelial cells and environment-induced dry eye patients. Exp Eye Res. 2015;134:133–140. doi: 10.1016/j.exer.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 106.Lv R., Du L., Liu X., Zhou F., Zhang Z., Zhang L. Polydatin alleviates traumatic spinal cord injury by reducing microglial inflammation via regulation of iNOS and NLRP3 inflammasome pathway. Int Immunopharmacol. 2019;70:28–36. doi: 10.1016/j.intimp.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 107.Lv R., Du L., Zhang L., Zhang Z. Polydatin attenuates spinal cord injury in rats by inhibiting oxidative stress and microglia apoptosis via Nrf2/HO-1 pathway. Life Sci. 2019;217:119–127. doi: 10.1016/j.lfs.2018.11.053. [DOI] [PubMed] [Google Scholar]
- 108.Ginwala R., Bhavsar R., Chigbu D.I., Jain P., Khan Z.K. Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-inflammatory activity of apigenin. Antioxidants. 2019;8(2) doi: 10.3390/antiox8020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wu J., Maoqiang L., Fan H., Zhenyu B., Qifang H., Xuepeng W. Rutin attenuates neuroinflammation in spinal cord injury rats. J Surg Res. 2016;203(2):331–337. doi: 10.1016/j.jss.2016.02.041. [DOI] [PubMed] [Google Scholar]
- 110.Song H.L., Zhang X., Wang W.Z., Liu R.H., Zhao K., Liu M.Y. Neuroprotective mechanisms of rutin for spinal cord injury through anti-oxidation and anti-inflammation and inhibition of p38 mitogen activated protein kinase pathway. Neural Regen Res. 2018;13(1):128–134. doi: 10.4103/1673-5374.217349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang P., Ma X. Effect of rutin on spinal cord injury through inhibition of the expression of MIP-2 and activation of MMP-9, and downregulation of Akt phosphorylation. Mol Med Rep. 2015;12(5):7554–7560. doi: 10.3892/mmr.2015.4357. [DOI] [PubMed] [Google Scholar]
- 112.Späni C.B., Braun D.J., Van Eldik L.J. Sex-related responses after traumatic brain injury: considerations for preclinical modeling. Front Neuroendocrinol. 2018;50:52–66. doi: 10.1016/j.yfrne.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Godoy G., Gakis G., Smith C.L., Fahmy O. Effects of androgen and estrogen receptor signaling pathways on bladder cancer initiation and progression. Bladder cancer (Amsterdam, Netherlands) 2016;2(2):127–137. doi: 10.3233/BLC-160052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mosquera L., Colon J.M., Santiago J.M., Torrado A.I., Melendez M., Segarra A.C. Tamoxifen and estradiol improved locomotor function and increased spared tissue in rats after spinal cord injury: their antioxidant effect and role of estrogen receptor alpha. Brain Res. 2014;1561:11–22. doi: 10.1016/j.brainres.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Villa A., Vegeto E., Poletti A., Maggi A. Estrogens, neuroinflammation, and neurodegeneration. Endocr Rev. 2016;37(4):372–402. doi: 10.1210/er.2016-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zendedel A., Monnink F., Hassanzadeh G., Zaminy A., Ansar M.M., Habib P. Estrogen attenuates local inflammasome expression and activation after spinal cord injury. Mol Neurobiol. 2018;55(2):1364–1375. doi: 10.1007/s12035-017-0400-2. [DOI] [PubMed] [Google Scholar]
- 117.Vasta G.R. Galectins as pattern recognition receptors: structure, function, and evolution. Adv Exp Med Biol. 2012;946:21–36. doi: 10.1007/978-1-4614-0106-3_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ren Z., Liang W., Sheng J., Xun C., Xu T., Cao R. Gal-3 is a potential biomarker for spinal cord injury and Gal-3 deficiency attenuates neuroinflammation through ROS/TXNIP/NLRP3 signaling pathway. Biosci Rep. 2019;39(12) doi: 10.1042/BSR20192368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tsvetkov A.S., Miller J., Arrasate M., Wong J.S., Pleiss M.A., Finkbeiner S. A small-molecule scaffold induces autophagy in primary neurons and protects against toxicity in a Huntington disease model. Proc Natl Acad Sci USA. 2010;107(39):16982–16987. doi: 10.1073/pnas.1004498107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Poteet E., Winters A., Yan L.J., Shufelt K., Green K.N., Simpkins J.W. Neuroprotective actions of methylene blue and its derivatives. PLoS ONE. 2012;7(10) doi: 10.1371/journal.pone.0048279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shen Q., Du F., Huang S., Rodriguez P., Watts L.T., Duong T.Q. Neuroprotective efficacy of methylene blue in ischemic stroke: an MRI study. PLoS ONE. 2013;8(11) doi: 10.1371/journal.pone.0079833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ertunc M.E., Hotamisligil G.S. Lipid signaling and lipotoxicity in metaflammation: indications for metabolic disease pathogenesis and treatment. J Lipid Res. 2016;57(12):2099–2114. doi: 10.1194/jlr.R066514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ridderstrom M., Ohlsson M. Brilliant blue G treatment facilitates regeneration after optic nerve injury in the adult rat. NeuroReport. 2014;25(17):1405–1410. doi: 10.1097/WNR.0000000000000286. [DOI] [PubMed] [Google Scholar]
- 124.Peng W., Cotrina M.L., Han X., Yu H., Bekar L., Blum L. Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury. Proc Natl Acad Sci USA. 2009;106(30):12489–12493. doi: 10.1073/pnas.0902531106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhou X., Yang Y., Wu L., Wang Y., Du C., Li C. Brilliant Blue G inhibits inflammasome activation and reduces disruption of blood-spinal cord barrier induced by spinal cord injury in rats. Med Sci Monitor: Int Med J Exp Clin Res. 2019;25:6359–6366. doi: 10.12659/MSM.915865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xing L., Yang T., Cui S., Chen G. Connexin hemichannels in astrocytes: role in CNS disorders. Front Mol Neurosci. 2019;12:23. doi: 10.3389/fnmol.2019.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Abudara V., Bechberger J., Freitas-Andrade M., De Bock M., Wang N., Bultynck G. The connexin43 mimetic peptide Gap19 inhibits hemichannels without altering gap junctional communication in astrocytes. Front Cell Neurosci. 2014;8:306. doi: 10.3389/fncel.2014.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mao Y., Tonkin R.S., Nguyen T., O'Carroll S.J., Nicholson L.F., Green C.R. Systemic administration of Connexin43 mimetic peptide improves functional recovery after traumatic spinal cord injury in adult rats. J Neurotrauma. 2017;34(3):707–719. doi: 10.1089/neu.2016.4625. [DOI] [PubMed] [Google Scholar]
- 129.Tonkin R.S., Bowles C., Perera C.J., Keating B.A., Makker P.G.S., Duffy S.S. Attenuation of mechanical pain hypersensitivity by treatment with Peptide5, a connexin-43 mimetic peptide, involves inhibition of NLRP3 inflammasome in nerve-injured mice. Exp Neurol. 2018;300:1–12. doi: 10.1016/j.expneurol.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 130.Chavez C.E., Oyarzun J.E., Avendano B.C., Mellado L.A., Inostroza C.A., Alvear T.F. The opening of connexin 43 hemichannels alters hippocampal astrocyte function and neuronal survival in prenatally LPS-Exposed adult offspring. Front Cell Neurosci. 2019;13:460. doi: 10.3389/fncel.2019.00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Diaz EF, Labra VC, Alvear TF, Mellado LA, Inostroza CA, Oyarzun JE. Connexin 43 hemichannels and pannexin-1 channels contribute to the alpha-synuclein-induced dysfunction and death of astrocytes. Glia. 2019;67(8):1598–1619. doi: 10.1002/glia.23631. [DOI] [PubMed] [Google Scholar]
- 132.O'Carroll S., Gorrie C., Velamoor S., Green C., Nicholson L. J Neaurosci Res. 2013;75(3):256–267. doi: 10.1016/j.neures.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 133.Cao Y., Liang L., Xu J., Wu J., Yan Y., Lin P. The effect of Scutellaria baicalensis stem-leaf flavonoids on spatial learning and memory in chronic cerebral ischemia-induced vascular dementia of rats. Acta Biochim Biophy Sin. 2016;48(5):437–446. doi: 10.1093/abbs/gmw024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li H., Hui H., Xu J., Yang H., Zhang X., Liu X. Wogonoside induces growth inhibition and cell cycle arrest via promoting the expression and binding activity of GATA-1 in chronic myelogenous leukemia cells. Arch Toxicol. 2016;90(6):1507–1522. doi: 10.1007/s00204-015-1552-3. [DOI] [PubMed] [Google Scholar]
- 135.Zhu Y., Zhu H., Wang Z., Gao F., Wang J., Zhang W. Wogonoside alleviates inflammation induced by traumatic spinal cord injury by suppressing NF-κB and NLRP3 inflammasome activation. Exp Ther Med. 2017;14(4):3304–3308. doi: 10.3892/etm.2017.4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Salmi P., Ahlenius S. Sedative effects of the dopamine D1 receptor agonist A 68930 on rat open-field behavior. NeuroReport. 2000;11(6):1269–1272. doi: 10.1097/00001756-200004270-00025. [DOI] [PubMed] [Google Scholar]
- 137.Rasheed N., Ahmad A., Al-Sheeha M., Alghasham A., Palit G. Neuroprotective and anti-stress effect of A68930 in acute and chronic unpredictable stress model in rats. Neurosci Lett. 2011;504(2):151–155. doi: 10.1016/j.neulet.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 138.Yan Y., Jiang W., Liu L., Wang X., Ding C., Tian Z. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell. 2015;160(1–2):62–73. doi: 10.1016/j.cell.2014.11.047. [DOI] [PubMed] [Google Scholar]
- 139.Nakano K., Higashi T., Hashimoto K., Takagi R., Tanaka Y., Matsushita S. Antagonizing dopamine D1-like receptor inhibits Th17 cell differentiation: preventive and therapeutic effects on experimental autoimmune encephalomyelitis. Biochem Biophys Res Commun. 2008;373(2):286–291. doi: 10.1016/j.bbrc.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 140.Jiang W., Li M., He F., Bian Z., Liu J., He Q. Dopamine D1 receptor agonist A-68930 inhibits NLRP3 inflammasome activation and protects rats from spinal cord injury-induced acute lung injury. Spinal Cord. 2016;54(11):951–956. doi: 10.1038/sc.2016.52. [DOI] [PubMed] [Google Scholar]
- 141.Schultke E., Kamencic H., Skihar V.M., Griebel R., Juurlink B. Quercetin in an animal model of spinal cord compression injury: correlation of treatment duration with recovery of motor function. Spinal Cord. 2010;48(2):112–117. doi: 10.1038/sc.2009.111. [DOI] [PubMed] [Google Scholar]
- 142.Schultke E., Kendall E., Kamencic H., Ghong Z., Griebel R.W., Juurlink B.H. Quercetin promotes functional recovery following acute spinal cord injury. J Neurotrauma. 2003;20(6):583–591. doi: 10.1089/089771503767168500. [DOI] [PubMed] [Google Scholar]
- 143.Ocal O., Borcek A.O., Pasaoglu O., Gundogdu A.C., Kaplanoglu G.T., Baykaner M.K. Can quercetin be an option for treatment of spinal cord injury? An experimental study. Turk Neurosurg. 2019;29(2):247–253. doi: 10.5137/1019-5149.JTN.23799-18.1. [DOI] [PubMed] [Google Scholar]
- 144.Zahid A., Li B., Kombe A.J.K., Jin T., Tao J. Pharmacological Inhibitors of the NLRP3 Inflammasome. Front Immunol. 2019;10:2538. doi: 10.3389/fimmu.2019.02538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jiang W., Huang Y., Han N., He F., Li M., Bian Z. Quercetin suppresses NLRP3 inflammasome activation and attenuates histopathology in a rat model of spinal cord injury. Spinal Cord. 2016;54(8):592–596. doi: 10.1038/sc.2015.227. [DOI] [PubMed] [Google Scholar]
- 146.Jiang W, Li M, He F, Zhu L. Inhibition of NLRP3 inflammasome attenuates spinal cord injury-induced lung injury in mice. J Cell Physiol. 2019;234(5):6012–6022. doi: 10.1002/jcp.27233. [DOI] [PubMed] [Google Scholar]