Summary
The current COVID‐19 pandemic is caused by the SARS‐CoV‐2 coronavirus. The initial recognized symptoms were respiratory, sometimes culminating in severe respiratory distress requiring ventilation, and causing death in a percentage of those infected. As time has passed, other symptoms have been recognized. The initial reports of cutaneous manifestations were from Italian dermatologists, probably because Italy was the first European country to be heavily affected by the pandemic. The overall clinical presentation, course and outcome of SARS‐CoV‐2 infection in children differ from those in adults as do the cutaneous manifestations of childhood. In this review, we summarize the current knowledge on the cutaneous manifestations of COVID‐19 in children after thorough and critical review of articles published in the literature and from the personal experience of a large panel of paediatric dermatologists in Europe. In Part 1, we discuss one of the first and most widespread cutaneous manifestations of COVID‐19, chilblain‐like lesions, and in Part 2 we expanded to other manifestations, including erythema multiforme, urticaria and Kawasaki disease‐like inflammatory multisystemic syndrome. In this part of the review, we discuss the histological findings of COVID‐19 manifestations, and the testing and management of infected children for both COVID‐19 and any other pre‐existing conditions.
Abstract
Click here for the corresponding questions to this CME article.
Introduction
The current COVID‐19 pandemic has affected almost all countries worldwide. The overall clinical presentation, course and outcome of SARS‐CoV‐2 infection, as well as the cutaneous manifestations of childhood COVID‐19 differ from those of adults. Below we describe the histological findings of COVID‐19 manifestations, and the testing and management of infected children.
Dermatopathology of cutaneous COVID‐19 infection in children
A systematic review found that skin lesions were present in only 0.25% of 2445 pediatric patients in 119 published studies.1 In addition, biopsies from skin lesions in children with confirmed or suspected COVID‐19 have rarely been described in the literature. Most of the histopathological descriptions come from isolated cases or small series, and some lesion types have been biopsied only in adults. As cutaneous lesions may be related to the direct effect of the virus and/or to the immune responses secondary to the infection, histological findings may reflect the direct cytopathic effect of the virus or secondary inflammatory responses. Histology often follows usual patterns (Table 1).
Table 1.
Histological appearances of COVID‐19‐associated rashes in children.
| Presentation | Histological appearance | Immunohistochemistry | Virus present in biopsied tissue? |
| Chilblains (Fig. 2)16, 17, 56, 57 | Moderate to dense superficial and deep perivascular lymphocytic infiltrate; exocytosis to the epidermis and acrosyringia; perieccrine accentuation; necrotic keratinocytes; mild vacuolar degeneration of the basal layer; papillary dermal oedema and spongiosis; lymphocytic vasculitis with fibrinoid necrosis; endothelialitis and thrombosis; red cell extravasation and dermal oedema | T‐cell infiltrate with a predominance of CD4 over CD8 T lymphocytes. Scattered CD30+ cells are occasionally observed. Mature B cells represent a minor proportion of the infiltrate | Presence of cytoplasmic granular positivity for SARS‐CoV‐2 spike protein in endothelial cells in upper dermis and epithelial cells of the secretory portion of eccrine glands in one study. Round membrane‐bound structures within the cytoplasm of endothelial cells showing an electrolucent centre and surrounded by tiny spikes in keeping with viral particles in one case |
| Maculopapular eruptions3, 58 | Superficial perivascular dermatitis with slight exocytosis; swollen thrombosed vessels with neutrophils; eosinophils and nuclear debris. Cuffs of lymphocytes surrounding blood vessels; focal acantholytic suprabasal clefts with dyskeratotic and ballooning herpes‐like keratinocytes; groups of apoptotic keratinocytes in the epidermis58 | – | – |
| Erythema multiforme18 | Normal epidermis; spongiosis and mild vacuolar interface damage and exocytosis of lymphocytes; superficial and deep perivascular and perieccrine lymphocytic infiltrate reaching the adipose tissue; no eosinophils; vascular ectasia and mild features of lymphocytic vasculitis; but fibrinoid necrosis and thrombosis were absent | T‐cell infiltrate with a predominance of CD4 over CD8 T lymphocytes; scattered CD30+ cells occasionally observed; mature B cells represented a minor proportion of the infiltrate | Positivity for SARS‐CoV/SARS‐CoV‐2 spike protein was demonstrated in endothelial cells and epithelial cells of eccrine glands in 2 cases |
| Purpuric and livedoid patterns8, 9, 59 | Dilated superficial dermal vessels lined by swollen endothelial cells and significant red cell extravasation around the vessels, multiple thrombi occluding most small‐sized vessels of the superficial and mid‐dermis, pauci‐inflammatory thrombogenic vasculopathy were demonstrated | IgM, C3 and fibrinogen within dermal vessel; C9 deposition with deposition of C5b‐9 and C4d in the cutaneous microvasculature | Cytoplasmic granular positivity for SARS‐CoV/SARS‐CoV‐2 spike protein was detected in the cytoplasm of endothelial cells and epithelial cells of eccrine glands in one case; COVID‐19 spike glycoproteins in the cutaneous microvasculature |
Urticarial rashes
To our knowledge, no reports on histological features of urticarial rashes in children have been published. Histology in adults reveal perivascular infiltrates of lymphocytes, eosinophils and upper dermal oedema,2 and vacuolar‐type interface dermatitis with occasional necrotic keratinocytes without the presence of eosinophils, resembling erythema multiforme (EM).3
Vesicular exanthem
Biopsies from vesicular lesions in adults with COVID‐19 have shown suprabasal intraepidermal unilocular vesicles with prominent nonballooning acantholysis and eosinophilic dyskeratosis, with a striking ‘pomegranate‐like’ aspect. No nuclear atypia or large multinucleated cells were noted, and no vasculitis was seen. Direct immunofluorescence and SARS‐CoV‐2 PCR tests performed on vesicles were reported to be negative.4 The findings described can mimic other acantholytic disorders such as autoimmune or familial pemphigus or Grover transient acantholytic dermatosis5 and it is important to exclude herpetic infection.6, 7
Purpuric and livedoid lesions
In a case series of adult patients with COVID‐19 and purpuric skin lesions, a pauci‐inflammatory thrombogenic vasculopathy, with deposition of C5b‐9 and C4d and colocalization of COVID‐19 spike glycoproteins and C5b‐9 and C4d in the cutaneous microvasculature was demonstrated.8, 9
Pityriasis rosea‐like lesions
The histology of a digitate papulosquamous eruption reminiscent of pityriasis rosea in an adult patient revealed mild diffuse epidermal spongiosis, along with spongiotic vesicles containing lymphocytes and Langerhans cells. The papillary dermis was slightly oedematous, and a mild lymphohistiocytic infiltrate was seen in the upper dermis.10
Testing for COVID‐19 and other exclusion tests
There is currently no test with high sensitivity and specificity for diagnosing COVID‐19 featuring skin lesions in the paediatric population. Therefore, the epidemiological context, possibility of exposure to COVID‐19, history of flu signs and the characteristics of cutaneous signs (acute signs, but these are not very specific) are key points that allow for a tentative diagnosis of COVID‐19‐related skin lesions in children.
Data published by the Chinese Epidemiology Group of Emergency Response Mechanism of New Coronavirus Pneumonia showed that about 2% of the patients infected with COVID‐19 or SARS‐CoV‐2 (out of 72 314 patients) were children aged 0–9 years.11 The incubation period in children is usually about 2 days, with a range of 2–10 days. Children usually undergo more asymptomatic forms, less severe symptoms and better prognosis than adults.11–13 Because > 90% of the children have asymptomatic, mild or moderate disease, the diagnosis of COVID‐19 may be overlooked. However, it may be necessary to better characterize the cases of COVID‐19 in children to allow better control of the pandemic.
Some indications for the proper testing and diagnosis of children with suspected COVID‐19 are provided, along with an algorithm (Fig. 1) that can help clinicians to investigate children with skin rashes suspected of COVID‐19 infection.
Figure 1.
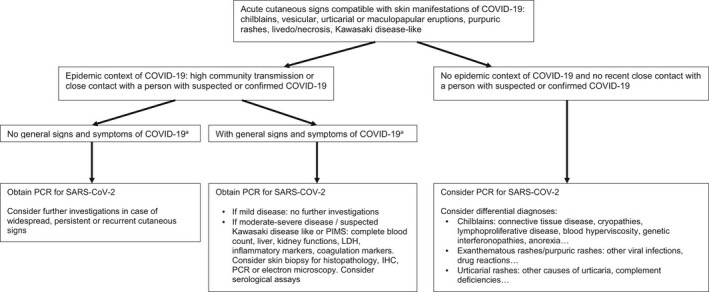
Algorithm for diagnosis of COVID‐19 in children with a skin eruption. IHC, immunohistochemistry; LDH, lactate dehydrogenase; PIMS, paediatric inflammatory multisystem syndrome. *Fever > 38 °C, muscle pain, headaches, asthenia, cough, dyspnoea, nausea/vomiting/diarrhoea and anosmia/agueusia.
SARS‐CoV‐2 reverse transcription PCR
The gold standard for confirmation of COVID‐19 in subjects with clinical symptoms consists of testing samples taken from the respiratory tract to assess for the presence of nucleic acid targets specific to SARS‐CoV‐2. Nasopharyngeal swabs are the preferred choice for testing, although oropharyngeal swabs are also acceptable.13 Reverse transcription (RT)‐PCR was shown to be very specific, but varied in sensitivity, thus negative results do exclude the possibility of infection. RT‐PCR results vary over time, and testing is more likely to be positive if performed during the first days of symptoms.14
RT‐PCR testing was reported in children presenting with COVID‐19‐related cutaneous signs, such as chilblains (Fig. 2).15–21 Of these, 68 had pseudo‐chilblains, 4 had EM, and 1 was a neonate with diffuse livedo associated with acute respiratory signs. Of the 69 patients, 61 underwent nasopharyngeal or oropharyngeal swabs for RT‐PCR testing, and 59 (97%) of these were negative. The two positive cases included a neonate with livedo,20 and a child with chilblains and associated EM who had gastrointestinal signs 2 days before occurrence of the chilblains.18
Figure 2.
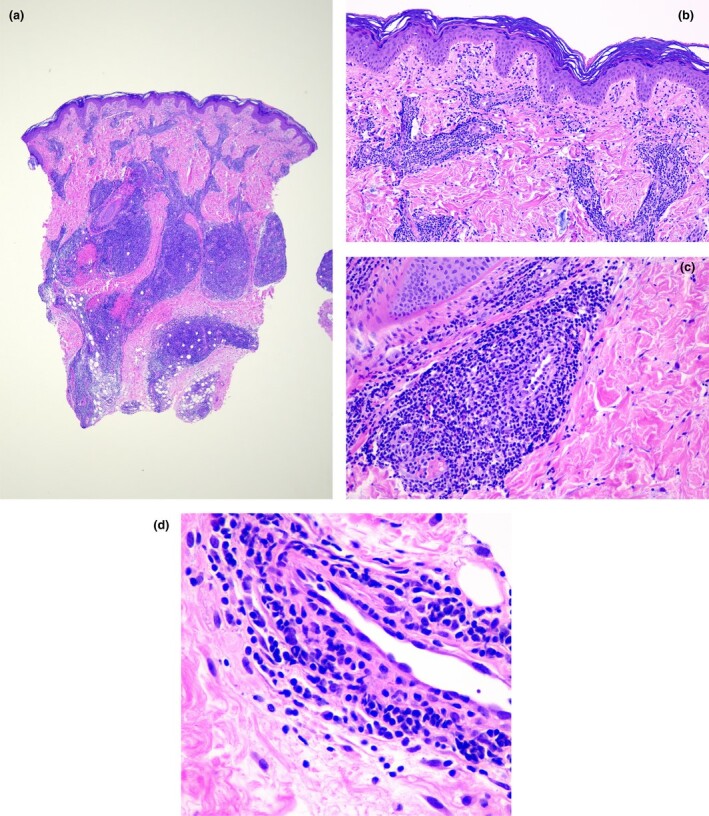
(a,b) Histopathology of chilblains in COVID‐19: (a) intense perivascular and perieccrine infiltrates; (b) oedema in the papillary dermis with both dermoepidermal and perivascular infiltrates; (c,d) prominent lymphocytic vasculitis with vessel wall damage in dermal vessels. Haematoxylin and eosin, original magnification (a) × 2; (b) × 10; (c) × 40; (d) × 100.
The published data suggest that when skin manifestations are concomitant with general signs of viral infection, PCR might be positive and is thus recommended (however, sensitivity is low), whereas when signs appear several days later, as is the case with pseudo‐chilblains, testing is usually negative.
Search for other infectious agents
Among most of the adult patients diagnosed with COVID‐19 with acute respiratory symptoms, the occurrence of concomitant respiratory viral infections (influenza viruses A and B, Mycoplasma pneumoniae, Streptococcus pneumoniae and Legionella pneumophila, among others) has been reported in > 50% cases.22 In children with isolated cutaneous signs, especially pseudo‐chilblains, immunoassays for parvovirus B19 were performed in 31 cases, and were either negative or in favour of an old infection.17, 19 However, the study did not look for associations with other infections.
The published data suggest that there is no need to search for parvovirus B19 antibodies by serological immunoassays in children having COVID‐19‐related cutaneous signs.
Serological immunoassays (COVID‐19 IgM, IgG and IgA antibodies)
Various serological assays, such as ELISA to determine antibodies against COVID‐19 are currently available, and provide information about the development of immunity against reinfection. With the available data, the utility of serological assays for diagnosing acute COVID‐19 infection seems limited. It has been shown that seroconversion occurs with a median time of 5–12 days for IgM antibodies and after 14 days for IgG and IgA.14, 23 Crossreactivity is also a potential problem,24 and a crossreactive antibody response between SARS‐CoV‐2 and SARS‐CoV infection has been shown.25 Interestingly, SARS‐CoV‐2 CD4+ cells are detected in 40%–60% of unexposed individuals. This result suggests that crossreactive T‐cell recognition might exist between previous common cold coronavirus infection and SARS‐CoV‐2.26
There are very limited data on use of serological assays in children with cutaneous COVID manifestations, but when performed they were negative for IgG and IgM in all cases.17, 19, 21 Only one patient showed positive IgA against COVID‐19.19 In an Italian series, specific serology testing against the S1 domain of SARS‐Cov‐2 spike protein was positive in 6 of 19 cases for IgA, and in 1 of 19 for IgG.17
The relevance of serological assay in dermatological conditions needs to be confirmed.27 The published data suggest that the presence of IgM and IgG by immunoassays has very low diagnostic relevance to cutaneous signs of COVID‐19 in children. The observations related to presence of IgA antibody responses need to be investigated further.
Routine laboratory tests, inflammation markers and coagulation panels
Laboratory tests are necessary in patients with severe general symptoms of COVID‐19 and in cases of cytokine storm syndrome, and such patients may require hospitalization. This is usually not the case for patients with isolated skin lesions except when such lesions are associated with general symptoms or persist beyond the expected duration. Unwell patients may demonstrate decreased albumin (~75%), elevated C‐reactive protein (~60%) and elevated lactate dehydrogenase (~60%) levels and lymphopenia (~40%). There is currently no biomarker or combination of biomarkers that is sufficiently sensitive/specific to establish a diagnosis of COVID‐19, or to pragmatically predict its clinical course.13
In most reported children with common COVID‐19‐related skin lesions who underwent routine laboratory tests, no significant abnormalities in serum chemistry were observed. The various coagulation markers were observed to be within the normal range in children, except for D‐dimer levels, which were slightly raised in three cases, whereas most others had a value of < 1000 ng/mL (normal level < 500 ng/mL).15, 16, 19 Adult patients with acral cyanosis often have a hypercoagulable and disseminated intravascular coagulation.28
In children with the severe disease of paediatric inflammatory multisystem syndrome (PIMS), abnormalities in laboratory values have been extensively reported.
Markers of autoimmunity, including antinuclear antibodies and cryoglobulins, tested in 14 of the 69 published cases of COVID‐19 in children, showed no significant anomalies, except in one child with COVID‐19‐related chilblains, who had raised anticardiolipin IgG antibodies.19
Management of children with COVID‐19 skin disease
Interference of COVID‐19 with treatment of chronic skin diseases in children
During the COVID‐19 pandemic, follow‐up for children affected by chronic diseases has been interrupted or moved to remote methods, especially in heavily affected countries. Outdoor play, activities and even schooling in most cases has been interrupted.
A study to evaluate the influence of COVID‐19 on hospitalizations in a tertiary dermatology department in southwest Poland compared the number of hospitalized patients during the COVID‐19 pandemic with the same period in 2019.29 The number of hospitalized children (2–18 years old) decreased from 81 (12.9%) in 2019 to 12 (6.8%) during the pandemic, especially for patients with chronic inflammatory diseases [atopic dermatitis (AD), lichen planus, eczema, psoriasis, urticaria and pityriasis rubra pilaris].29 Similar data have been reported from Italy.30, 31 One study described the management of 6890 patients with psoriasis, including 120 children, during the pandemic in the three major reference centres for psoriasis in Sardinia, Italy; about 23% of the patients had severe psoriasis. The Italian drug agency (Agenzia Italiana del Farmaco) declared an automatic renewal of the therapeutic plan of patients with rare diseases or those under treatment with biologics. However, the dermatologists found difficulties including limitations to face‐to‐face consultations for severe cases, high infection rate among dermatologists (40% compared with the national average of 7%–8%), and cancellation of outpatient clinics.31
Dermatological consultations in Konya (Turkey) during the pandemic were mainly carried out by teledermatology (72.8% of consultations), which was reported to reduce the risk of disease transmission.32
A task force of 37 expert paediatric dermatologists completed a survey concerning the management of systemic immunosuppressive therapies for children during SARS‐CoV‐2 infection spread and developed educational contents for patients, caregivers and providers accessible online.33 Three main areas were analysed: (i) initiation of treatment, (ii) continuation of treatment and (iii) laboratory monitoring of systemic therapies. COVID‐19 significantly affected the management of immunosuppressive medications (97% of the respondents), depending on the drug (87%), the condition being treated (78%), family/patient preferences (62%) and risks of COVID‐19 vs. not treating the skin disease (84%). The management of specific immunosuppressive medications was analysed based on various clinical circumstances: (i) asymptomatic patients, (ii) patients with upper respiratory tract infection (URI) with unknown COVID‐19 status, (iii) patients with confirmed exposure to COVID‐19, and (iv) patients with confirmed SARS‐CoV‐2 infection. Selected drugs were classified into systemic therapy (methotrexate, mycophenolate mofetil, azathioprine, ciclosporin, systemic steroids, apremilast, JAK inhibitors) and biologic therapies [anti‐tumour necrosis factor‐α, anti‐interleukin (IL)‐17, anti‐IL‐12/23, anti‐IL‐23 and dupilumab].
The majority of dermatologists agreed that for SARS‐CoV‐2‐negative patients, all treatment should be continued or adapted to the context.33 In patients with a URI, most drugs were temporarily discontinued, except for systemic steroids, which were often decreased in dosage (30% of respondents), while apremilast and dupilumab were mostly continued (46% and 68%, respectively). In patients exposed to confirmed households and with confirmed COVID‐19 infection, all the selected drugs were mostly discontinued or decreased in dose, including systemic steroids (24% and 19% of respondents, respectively), apremilast (30% and 24%, respectively) and dupilumab (49% and 16%, respectively). In addition, the frequency of laboratory monitoring was also reduced. Notably, discontinuance of a biologic therapy could lead to failure after reintroduction, and tapering of corticosteroids may need to be assessed with regard to adrenal suppression, especially in cases of infection when stress‐dose steroids may be required. In summary, a final statement for the management of immunosuppressive medications was not achieved, and it was advised that decisions regarding treatment were regularly discussed with the patients and their caregivers.33
COVID‐19 and atopic dermatitis
The European Task Force on Atopic Dermatitis (ETFAD) published a statement on SARS‐CoV‐2 and AD.34 ETFAD suggested advising patients to continue all ongoing medications to prevent the disease worsening, to observe hygienic procedures and to moisturize their skin regularly. In addition, if systemic treatment should be stopped, it should be replaced by a topical therapy. Dupilumab is preferred over other medications, because it does not increase the risk of viral infection.
Another evaluation of the use of systemic drugs during the COVID outbreak suggested that dupilumab, apremilast and acitretin are not associated with increased risk of infection and seem to be safe during the COVID‐19 pandemic.35 Other suggestions to optimize the care of patients with AD during the outbreak included encouraging good skin care (e.g. hygiene, moisturizing), providing access to telehealth and following published indications on systemic drugs in this setting.36
Immunosuppressive medications
Similar advice has been advocated for the use of immunosuppressive medications in psoriasis, autoimmune bullous diseases and AD.37–40 Treatment of autoimmune bullous diseases should be maintained, owing to the high rate of morbidity and mortality of this disease group.41 In patients infected with SARS‐CoV‐2, azathioprine, mycophenolate mofetil/sodium, cyclophosphamide, methotrexate and ciclosporin may be stopped, whereas topical corticosteroids, dapsone/sulfapyridine, low‐dose prednisone/prednisolone, doxycycline/tetracycline, colchicine and intravenous immunoglobulin can be continued. Systemic corticosteroids should be tapered, not suddenly interrupted. Regular updated through World Health Organization (WHO)/Centers for Disease Control homepage and the European Academy of Dermatology and Venereology, which has published specific recommendations on its website, are recommended.41
The COVID‐19 pandemic has had psychosocial impacts on healthcare providers and patients with chronic skin disorders.42 Additionally, quarantine may have negative effects on chronic inflammatory conditions. Immobilization, decrease in physical activity and lockdown induce higher caloric intake, obesity and a negative impact on mood. All these factors worsen conditions such as psoriasis and hidradenitis suppurativa, and decrease adherence to treatment.43
Remote medicine and use of dermoscopy
Teledermatology and telemedicine offer an opportunity for continuing healthcare delivery.44–46 Some suggestions on using dermoscopy during the COVID‐19 pandemic have been proposed: (i) avoid dermoscopy at specific body sites: mucosa, hands, nails, face and eyes; (ii) patient and doctors should respect distancing in the waiting room, wear masks, and wash or sanitize hands before and after the examination; (iii) the dermoscope should sanitized before and after each patient; (iv) the examined skin should also be sanitized before and after the examination; and (v) digital reports should be encouraged in preference to printed reports.47
Recommendations for paediatric dermatologists attending children with suspected COVID‐19 skin disease
The following advice may continue to change over the following months. Only 1%–5% of confirmed COVID‐19‐positive cases are children. Overall, children have milder disease, and deaths in children are exceptional.48
As for adults, the initial evaluation of children should be carried out in the department at which the patient is first attended (e.g. emergency room, inpatient room), to avoid unnecessary patient movement in the hospital and avoid contact with patients in waiting areas. Dermatologists attending children with skin lesions who are suspected of having COVID‐19 should wear proper personal protective equipment, which may include mask, goggles, protective suits, head coverings and gloves.49
A thorough evaluation by an experienced paediatrician is highly recommended. Most children with SARS‐CoV‐2 infection should have a good general status and normal vital signs; poor general status and tachycardia are early signs of shock in COVID‐19.50, 51
Severe gastrointestinal symptoms appear to be more frequent than respiratory symptoms in severely ill children, and can indicate the possibility of PIMS.51 However, most patients with cutaneous lesions do not present with systemic symptoms or, if present, these are mild, and usually occur days to weeks before the appearance of the skin lesions.16
If history and clinical examination suggest SARS‐CoV‐2 infection, it is recommended to test by PCR and to follow up household contacts according to local health authority protocols. In children with mild symptoms, the likelihood of transmission of the infection beyond the first week after the onset of symptoms is very low,52, 53 so home lockdown for 10 days is recommended. If an infectious child requires consultation, then the healthcare practitioner should try to see them in a COVID‐19 area, as late as possible during the day to allow cleaning, and caregivers with symptoms must not attend. Informative announcements in the dermatology clinics must clearly explain medical requirements, such as fever screening at the clinic entrance, questionnaires about presence of symptoms in the previous 2 weeks or close contact with suspected or confirmed COVID‐19 cases, use of alcohol‐based hand sanitizer, wearing of surgical masks by children and caregivers, and maintaining appropriate distancing. Proper cleaning and disinfecting of the room should be carried out after every suspected or confirmed child is attended. Surfaces should be cleaned and disinfected regularly, such as doorknobs, light switches, computer keyboards, phones and examination tools such as dermoscopes.54 Surfaces should be disinfected with 1% sodium hypochlorite solutions, and electronic gadgets with the alcohol‐based disinfectant.
After an initial in‐person evaluation, teledermatology is a good choice for follow‐up of children with COVID‐19 until the skin lesions are completely resolved.55
All health professionals involved in the care of children should try to stay updated, in spite of the increasing number of articles appearing in the literature. Up until the 11 June 2020, only 3 months after the WHO declared the pandemic, 38 903 articles about COVID‐19 had been published in PubMed, with 1502 of these related to the paediatric population.
Learning points
The histopathology of COVID‐19‐related chilblains is similar to that of classic primary chilblains.
Sensitivity and specificity of PCR and serology tests are not high in children, and epidemiological data are important in the diagnosis of COVID‐19 in children.
More than 90% of children have asymptomatic, mild or moderate COVID‐19, and the diagnosis may thus be overlooked.
COVID‐19 may interfere with the course of chronic skin diseases and with the access of patients to specialized care; teledermatology may partly overcome this latter problem.
COVID‐19 may interfere with the use of immunosuppressive drugs for skin diseases; the continuation of these drugs should be balanced with the risk of withdrawal and worsening of the skin condition.
Dupilumab appears to be safe and not associated with increased risk of infection.
Contributor Information
D. Andina, Department of Dermatology Hospital Infantil Universitario Niño Jesús Madrid Spain
A. Belloni‐Fortina, Pediatric Dermatology Unit Department of Medicine DIMED University of Padua Padua Italy
C. Bodemer, Department of Dermatology Hospital Necker Enfants MaladesParis Centre University Paris France
E. Bonifazi, Dermatologia Pediatrica Association Bari Italy
A. Chiriac, Nicolina Medical Center Iasi Romania
I. Colmenero, Department of Pathology Hospital Infantil Universitario Niño Jesús Madrid Spain
A. Diociaiuti, Dermatology Unit Bambino Gesù Children's HospitalIRCCS Rome Italy
M. El‐Hachem, Dermatology Unit Bambino Gesù Children's HospitalIRCCS Rome Italy
L. Fertitta, Department of Dermatology Hospital Necker Enfants MaladesParis Centre University Paris France
van D. Gysel, Department of Pediatrics O. L. Vrouw Hospital Aalst Belgium.
A. Hernández‐Martín, Department of Dermatology Hospital Infantil Universitario Niño Jesús Madrid Spain
T. Hubiche, Department of Dermatology Université Côte d'Azur Nice France
C. Luca, Nicolina Medical Center Iasi Romania
L. Martos‐Cabrera, Department of Dermatology Hospital Infantil Universitario Niño Jesús Madrid Spain
A. Maruani, Department of Dermatology Unit of Pediatric Dermatology University of ToursSPHERE‐INSERM1246, CHRU Tours Tours France
F. Mazzotta, Dermatologia Pediatrica Association Bari Italy
A. D. Akkaya, Department of Dermatology Ulus Liv Hospital Istanbul Turkey
M. Casals, Department of Dermatology Hospital Universitari de Sabadell Barcelona Spain
J. Ferrando, Department of Dermatology Hospital Clìnic Barcelona Spain
R. Grimalt, Faculty of Medicine and Health Sciences Universitat Internacional de Catalunya Barcelona Spain
I. Grozdev, Department of Dermatology Children’s University Hospital Queen Fabiola Brussels Belgium
V. Kinsler, Department of Paediatric Dermatology Great Ormond Street Hospital for Children NHS Foundation Trust London UK
M. A. Morren, Pediatric Dermatology Unit Department of Pediatrics and Dermato‐Venereology University Hospital Lausanne and University of Lausanne Lausanne Switzerland
M. Munisami, Department of Dermatology and Sexually Transmitted Diseases Jawaharlal Institute Of Postgraduate Medical Education and Research (JIPMER) Puducherry India
A. Nanda, As'ad Al‐Hamad Dermatology Center Kuwait City Kuwait
M. P. Novoa, Department of Dermatology Hospital San Jose Bogota Colombia
H. Ott, Division of Pediatric Dermatology Children’s Hospital Auf der Bult Hannover Germany
S. Pasmans, Erasmus MC University Medical Center RotterdamSophia Children’s Hospital Rotterdam The Netherlands
C. Salavastru, Department of Paediatric Dermatology Colentina Clinical HospitalCarol Davila University of Medicine and Pharmacy Bucharest Romania
V. Zawar, Department of Dermatology Dr Vasantrao Pawar Medical College Nashik India
A. Torrelo, Department of Dermatology Hospital Infantil Universitario Niño Jesús Madrid Spain.
CPD questions
Learning objective
To gain up‐to‐date knowledge on the histological features of COVID‐19‐related skin diseases and the treatments for other skin diseases during the pandemic.
Question 1
Which of the following is not a histological feature of COVID‐19‐related chilblains?
(a) Lymphocytic vasculitis.
(b) Leucocytoclasis.
(c) Endothelialitis.
(d) Thrombosis.
(e) Red cell extravasation.
Question 2
What percentage of patients with confirmed COVID‐19 are children?
(a) < 5%.
(b) 10%–20%.
(c) 20%–40%.
(d) 40%–60%.
(e) 60%–80%.
Question 3
What is the most agreed‐upon option for children with atopic dermatitis treated with immunosuppressive drugs and no symptoms of COVID‐19?
(a) Continue therapy.
(b) Reduce dose to half.
(c) Taper for 2 weeks and then discontinue therapy.
(d) Discontinue therapy.
(e) Increase dose.
Question 4
Which of the following is the preferred systemic treatment for severe atopic dermatitis during COVID‐19 pandemic?
(a) Ciclosporin.
(b) Methotrexate.
(c) Azathioprine.
(d) Mycophenolate.
(e) Dupilumab.
Question 5
In a child with chilblains suspected to be related to COVID‐19, what of the following measures is correct?
(a) PCR is not necessary.
(b) The patient should be quarantined for 2 weeks regardless of the PCR result.
(c) The patient should be admitted to hospital.
(d) If PCR is negative, the likelihood of transmission of the infection is very low.
(e) Complete blood cell count, serum chemistry and coagulation studies should be obtained.
Instructions for answering questions
This learning activity is freely available online at http://www.wileyhealthlearning.com/ced
Users are encouraged to
Read the article in print or online, paying particular attention to the learning points and any author conflict of interest disclosures.
Reflect on the article.
Register or login online at http://www.wileyhealthlearning.com/ced and answer the CPD questions.
Complete the required evaluation component of the activity.
Once the test is passed, you will receive a certificate and the learning activity can be added to your RCP CPD diary as a self‐certified entry.
This activity will be available for CPD credit for 2 years following its publication date. At that time, it will be reviewed and potentially updated and extended for an additional period.
References
- Hoang A, Chorath K, Moreira A et al. COVID‐19 in 7780 pediatric patients: a systematic review. EClinicalMedicine 2020; 24: 100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez‐Jimenez P, Chicharro P, De Argila D et al. Urticaria‐like lesions in COVID‐19 patients are not really urticaria – a case with clinicopathologic correlation. J Eur Acad Dermatol Venereol 2020. 10.1111/jdv.16618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya G, Kaya A, Saurat JH. Clinical and histopathological features and potential pathological mechanisms of skin lesions in COVID‐19: review of the literature. Dermatopathology (Basel) 2020; 7: 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahé A, Birckel A, Merklen C et al. Histology of skin lesions establishes that the vesicular rash associated with COVID‐19 is not ‘varicella‐like’. J Eur Acad Dermatol Venereol 2020; 34: e559–61. 10.1111/jdv.16706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llamas‐Velasco M, Chicharro P, Rodríguez‐Jiménez P et al. Reply to "Clinical and histological characterization of vesicular COVID‐19 rashes: a prospective study in a tertiary care hospital": pseudoherpetic Grover disease seems to appear in patients with COVID‐19 infection. Clin Exp Dermatol 2020. 10.1111/ced.14305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsaie ML, Youssef EA, Nada HA. Herpes zoster might be an indicator for latent COVID 19 infection. Dermatol Ther 2020: e13666. 10.1111/dth.13666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsaie ML, Nada HA. Herpes zoster (shingles) complicating the course of COVID19 infection. J Dermatolog Treat 2020: 1–3. 10.1080/09546634.2020.1782823 [DOI] [PubMed] [Google Scholar]
- Magro C, Mulvey AJ, Berlin D et al. AGRO, complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID‐19 infection: a report of five cases. Transl Res 2020; 220: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch‐Amate X, Giavedoni P, Podlipnik S et al. Retiform purpura as a dermatological sign of covid‐19 coagulopathy. J Eur Acad Dermatol Venereol 2020; 34: e548–9. 10.1111/jdv.16689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez A, Sohier P, Benghanem S et al. Digitate papulosquamous eruption associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol 2020;156: 819. 10.1001/jamadermatol.2020.1704 [DOI] [PubMed] [Google Scholar]
- Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention. [The epidemiological characteristics of an outbreak of novel coronavirus diseases (COVID‐19) in China] (in Chinese). Zhonghua Liu Xing Bing Xue Za Zhi 2019; 2020(41): 141–51. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Interim guidelines for collecting, handling, and testing clinical specimens from persons for coronavirus disease 2019 (COVID‐19). Available at: https://www.cdc.gov/coronavirus/2019‐nCoV/lab/guidelines‐clinical‐specimens.html (accessed 5 June 2020).
- Ludvigsson JF. Systematic review of COVID‐19 in children shows milder cases and a better prognosis than adults. Acta Paediatr 2020; 9: 1088–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethuraman N, Jeremiah SS, Ryo A. Interpreting diagnostic tests for SARS‐CoV‐2. JAMA 2020; 323: 2249. 10.1001/jama.2020.8259 [DOI] [PubMed] [Google Scholar]
- Colonna C, Monzani NA, Rocchi A et al. Chilblain‐like lesions in children following suspected COVID‐19 infection. Pediatr Dermatol 2020; 37: 437–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andina D, Noguera‐Morel L, Bascuas‐Arribas M et al. Chilblains in children in the setting of COVID‐19 pandemic. Pediatr Dermatol 2020; 37: 406–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hachem M, Diociaiuti A, Concato C et al. A clinical, histopathological and laboratory study of 19 consecutive Italian paediatric patients with chilblain‐like lesions: lights and shadows on the relationship with COVID‐19 infection. J Eur Acad Dermatol Venereol 2020. 10.1111/jdv.16682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrelo A, Andina D, Santonja C et al. Erythema multiforme‐like lesions in children and COVID‐19. Pediatr Dermatol 2020; 37: 442–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubiche T, Cardot‐Leccia N, Le Duff F et al. Chilblains appear as a manifestation of SARS‐CoV‐2 infection and reveal features of type I interferonopathy and micro‐vasculopathy (in press). Preprint available at: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3586683 (accessed 26 August 2020).
- Kamali Aghdam M, Jafari N, Eftekhari K. Novel coronavirus in a 15‐day‐old neonate with clinical signs of sepsis, a case report. Infect Dis (Lond) 2020; 52: 427–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordoro KM, Reynolds SD, Wattier R, Mccalmont TH. Clustered cases of acral perniosis: clinical features, histopathology and relationship to COVID‐19. Pediatr Dermatol 2020; 37: 419–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Q, Li G, Xing Y et al. Precautions are needed for COVID‐19 patients with coinfection of common respiratory pathogens. MedRxiv 2020. 10.1101/2020.02.29.2002769 [DOI] [Google Scholar]
- Wölfel R, Corman VM, Guggemos W et al. Virological assessment of hospitalized patients with COVID‐19. Nature 2020; 581: 465–9. [DOI] [PubMed] [Google Scholar]
- Patrick DM, Petric M, Skowronski DM et al. An outbreak of human coronavirus OC43 infection and serological cross‐reactivity with SARS coronavirus. Can J Infect Dis Med Microbiol 2006; 17: 330–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv H, Wu NC, Tsang OT et al. Cross‐reactive antibody response between SARS‐CoV‐2 and SARS‐CoV infections. Cell Rep 2020; 31: 107725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifoni A, Weiskopf D, Ramirez SI et al. Targets of T cell responses to SARS‐CoV‐2 coronavirus in humans with COVID‐19 disease and unexposed individuals. Cell 2020; 181: 1489–501.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jääskeläinen AJ, Kekäläinen E, Kallio‐Kokko H et al. Evaluation of commercial and automated SARS‐CoV‐2 IgG and IgA ELISAs using coronavirus disease (COVID‐19) patient samples. Euro Surveill 2020; 25: 2000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors JM, Levy JH. COVID‐19 and its implications for thrombosis and anticoagulation. Blood 2020; 135: 2033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialynicki‐Birula R, Siemasz I, Otlewska A et al. Influence of COVID‐19 pandemic on hospitalizations at the tertiary dermatology department in south‐west Poland. Dermatol Ther 2020: e13738. 10.1111/dth.13738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrizi A, Bardazzi F, Filippi F et al. The COVID‐19 outbreak in Italy: preventive and protective measures adopted by the Dermatology Unit of Bologna University Hospital. Dermatol Ther 2020; 33: e13469. 10.1111/dth.13469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzori L, Mugheddu C, Addis G et al. Psoriasis health care in the time of the coronavirus pandemic: insights from dedicated centers in Sardinia (Italy). J Eur Acad Dermatol Venereol 2020; 34: e247–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temiz SA, Dursun R, Daye M, Ataseven A. Evaluation of dermatology consultations in the era of COVID‐19. Dermatol Ther 2020; 33: e13642. 10.1111/dth.13642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SD, Mathur AN, Chiu YE et al. Systemic immunosuppressive therapy for inflammatory skin diseases in children: expert consensus‐based guidance for clinical decision‐making during the COVID‐19 pandemic. Pediatr Dermatol 2020; 37: 424–4. [DOI] [PubMed] [Google Scholar]
- Wollenberg A, Flohr C, Simon D et al. European Task Force on Atopic Dermatitis (ETFAD) statement on severe acute respiratory syndrome coronavirus 2 (SARS‐Cov‐2)‐infection and atopic dermatitis. J Eur Acad Dermatol Venereol 2020; 34: e241–2. [DOI] [PubMed] [Google Scholar]
- Ricardo JW, Lipner SR. Considerations for safety in the use of systemic medications for psoriasis and atopic dermatitis during the COVID‐19 pandemic. Dermatol Ther 2020: e13687. 10.1111/dth.13687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah M, Sachdeva M, Alavi A et al. Optimizing care for atopic dermatitis patients during COVID‐19 pandemic. J Am Acad Dermatol 2020; 83: e165–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahil SK, Yiu ZZN, Mason KJ et al. Global reporting of cases of COVID‐19 in psoriasis and atopic dermatitis: an opportunity to inform care during a pandemic. Br J Dermatol 2020; 183: 404–6. 10.1111/bjd.19161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdollahimajd F, Shahidi‐Dadras M, Robati RM, Dadkhahfar S. Management of pemphigus in COVID‐19 pandemic era; a review article. Arch Acad Emerg Med 2020; 8: e51. [PMC free article] [PubMed] [Google Scholar]
- Di Altobrando A, Patrizi A, Bardazzi F. Should SARS‐CoV‐2 influence immunosuppressive therapy for autoimmune blistering diseases? J Eur Acad Dermatol Venereol 2020; 34: e295–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Rademaker M, Baker C, Foley P. COVID‐19 and the use of immunomodulatory and biologic agents for severe cutaneous disease: an Australian/New Zealand consensus statement. Australas J Dermatol 2020; 61: 210–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasperkiewicz M, Schmidt E, Fairley JA et al. Expert recommendations for the management of autoimmune bullous diseases during the COVID‐19 pandemic. J Eur Acad Dermatol Venereol 2020; 34: e302–3. 10.1111/jdv.16525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcovich S, Bersani FS, Chiricozzi A, De Simone C. Mass quarantine measures in the time of COVID‐19 pandemic: psychosocial implications for chronic skin conditions and a call for qualitative studies. J Eur Acad Dermatol Venereol 2020; 34: e293–4. 10.1111/jdv.16535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marasca C, Ruggiero A, Napolitano M et al. May COVID‐19 outbreaks lead to a worsening of skin chronic inflammatory conditions? Med Hypotheses 2020; 34: e302–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Zhang G, Zhan Y. Management strategies of autoimmune bullous diseases during the outbreak of novel coronavirus disease (COVID‐19). J Dermatolog Treat 2019: 1–2. 10.1080/09546634.2020.1771261 [DOI] [PubMed] [Google Scholar]
- Brunasso AMG, Massone C. Teledermatologic monitoring for chronic cutaneous autoimmune diseases with smartworking during Covid‐19 emergency in a tertiary center in Italy. Dermatol Ther 2020; 33: e13495. 10.1111/dth.13695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radi G, Simonetti O, Diotallevi F et al. How can I take care of you? The dermatologist meets patients' needs during the Covid19 pandemic. Dermatol Ther 2020; 33: e13740. 10.1111/dth.13740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakhar D, Kaur I, Kaul S. Art of performing dermoscopy during the times of coronavirus disease (COVID‐19): simple change in approach can save the day!. J Eur Acad Dermatol Venereol 2020; 34: e242–4. 10.1111/jdv.16412 [DOI] [PubMed] [Google Scholar]
- Devrim I, Byram N. Infection control practices in children during COVID‐19 pandemic: differences from adults. Am J Infect Control 2020; 48: 933–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Pradhan S, Xue S. What are we doing in the dermatology outpatient department amidst the raging of the 2019 novel coronavirus? J Am Acad Dermatol 2020; 82: 1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riphagen S, Gomez X, Gonzalez‐Martinez C et al. Hyperinflammatory shock in children during COVID‐19 pandemic. Lancet (Lond) Engl 2020; 39: 1607–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrero‐Hernández M, García‐Salido A, Leoz‐Gordillo I et al. Severe SARS‐CoV‐2 infection in children with suspected acute abdomen: a case series from a tertiary hospital in Spain. Pediatr Infect Dis J 2020; 39: e195–8. [DOI] [PubMed] [Google Scholar]
- Cheng HY, Jian SW, Liu DP et al. Contact tracing assessment of COVID‐19 transmission dynamics in Taiwan and risk at different exposure periods before and after symptom onset. JAMA Intern Med 2020; 180: 1156. 10.1001/jamainternmed.2020.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Yan LM, Wan L et al. Viral dynamics in mild and severe cases of COVID‐19. Lancet Infect Dis 2020; 20: 656–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahmy DH, El‐Amawy HS, El‐Samongy MA et al. COVID‐19 and dermatology: a comprehensive guide for dermatologists. J Eur Acad Dermatol Venereol 2020; 34: 1388–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villani A, Scalvenzi M, Fabbrocini G. Teledermatology: a useful tool to fight COVID‐19. J Dermatolog Treat 2020; 31: 325. [DOI] [PubMed] [Google Scholar]
- Fox SE, Akmatbekov A, Harbert JL et al. Pulmonary and cardiac pathology in African American patients with COVID‐19: an autopsy series from New Orleans. Lancet Respir Med 2020; 8: 681–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmenero I, Santonja C, Alonso‐Riano M et al. SARS‐COV‐2 endothelial infection causes COVID‐19 chilblains: histopathological, immunohistochemical and ultrastructural study of 7 paediatric patients. Br J Dermatol 2020; 183: 729–37. 10.1111/bjd.19491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S, Fernandez AP. Skin manifestations of COVID‐19. Cleve Clin J Med 2020. 10.3949/ccjm.87a.ccc031 [DOI] [PubMed] [Google Scholar]
- Andina D, Colmenero I, Santonja C. Suspected COVID‐19‐related reticulated purpura of the soles in an infant. Pediatr Dermatol. 2020; 10.1111/pde.14409 [DOI] [PubMed] [Google Scholar]


