Abstract
Background
Recent data suggests an association between blood hyperviscosity and both propensity for thrombosis and disease severity in patients with COVID‐19. This raises the possibility that increased viscosity may contribute to endothelial damage and multiorgan failure in COVID‐19, and that therapeutic plasma exchange (TPE) to decrease viscosity may improve patient outcomes.
Here we sought to share our experience using TPE in the first 6 patients treated for COVID‐19‐associated hyperviscosity.
Study Design and Methods
Six critically ill COVID‐19 patients with plasma viscosity levels ranging from 2.6 to 4.2 centipoise (cP; normal range, 1.4‐1.8 cP) underwent daily TPE for 2‐3 treatments.
Results
TPE decreased plasma viscosity in all six patients (Pre‐TPE median 3.75 cP, range 2.6‐4.2 cP; Post‐TPE median 1.6 cP, range 1.5‐1.9 cP). TPE also decreased fibrinogen levels in all five patients for whom results were available (Pre‐TPE median 739 mg/dL, range 601‐1188 mg/dL; Post‐TPE median 359 mg/dL, range 235‐461 mg/dL); D‐dimer levels in all six patients (Pre‐TPE median 5921 ng/mL, range 1134‐60 000 ng/mL; Post‐TPE median 4893 ng/mL, range 620‐7518 ng/mL); and CRP levels in five of six patients (Pre‐TPE median 292 mg/L, range 136‐329 mg/L; Post‐TPE median 84 mg/L, range 31‐211 mg/L). While the two sickest patients died, significant improvement in clinical status was observed in four of six patients shortly after TPE.
Conclusions
This series demonstrates the utility of TPE to rapidly correct increased blood viscosity in patients with COVID‐19‐associated hyperviscosity. Large randomized trials are needed to determine whether TPE may improve clinical outcomes for patients with COVID‐19.
1. INTRODUCTION
Hypercoagulability is increasingly recognized as a significant contributor to morbidity and mortality among critically ill patients with coronavirus disease 2019 (COVID‐19). Early studies reported deep vein thrombosis (DVT) incidence of 20%‐46% in patients with COVID‐19. 1 , 2 Another study found a more than 5‐fold increase in the incidence of pulmonary embolism (PE) among patients admitted to the intensive care unit (ICU) with COVID‐19 as compared to historical admissions for acute respiratory distress syndrome (ARDS). 3 Autopsy series have confirmed the presence of both microvascular and macrovascular thrombi, including in patients receiving therapeutic anticoagulation, with one series reporting nine‐times more alveolar capillary microthrombi in patients who died from COVID‐19 than in patients who died from H1N1 influenza. 4 , 5 , 6 , 7
Amid efforts to understand the etiology of hypercoagulability in COVID‐19, we measured plasma viscosity in critically ill COVID‐19 patients admitted to the ICU. 8 Notably, all fifteen of the first patients tested had elevated plasma viscosity, and those with the highest levels had the highest Sequential Organ Failure Assessment (SOFA) scores, a mortality prediction score based on objective measures of 6 organ systems, and were experiencing an acute thrombotic complication. 9 Given that therapeutic plasma exchange (TPE) is an established treatment for other conditions characterized by hyperviscosity, including Waldenstrom macroglobulinemia and hypergammaglobulinemia from multiple myeloma, we performed TPE in six critically ill patients with COVID‐19‐associated hyperviscosity. 10
2. CASE REPORTS
All six patients were male, ranging in age from 44 to 75 years old, and hospitalized for an average of 17 days (range 6‐43 days) before TPE. Importantly, all patients were failing to improve despite standard supportive care and were determined by their primary teams to have symptomatic hyperviscosity, as evidenced by clotting complications despite anticoagulation. Table 1 summarizes patient characteristics and SOFA scores just before TPE was initiated.
TABLE 1.
Patient demographic and clinical characteristics at the time of therapeutic plasma exchange (n = 6)
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | |
|---|---|---|---|---|---|---|
| Age | 56 | 44 | 75 | 49 | 52 | 57 |
| Sex | M | M | M | M | M | M |
| Race | African American | African American | African American | African American | African American | White |
| Comorbidities | HTN | Seizure disorder | COPD, HTN | HTN, DM | DM | CAD, cirrhosis, COPD |
| ICU day | 22 | 14 | 13 | 43 | 12 | 6 |
| SOFA score | 17 | 15 | 15 | 14 | 8 | 5 |
| CRP (mg/L) | 246 | 286 | 329 | 312 | 297 | 136 |
| D‐dimer (ng/mL FEU) | 6972 | 4346 | 6771 | >60 000 | 15 335 | 1130 |
| Fibrinogen (mg/dL) | 1188 | 717 | 601 | a | 947 | 739 |
| Plasma viscosity (cP) | 4.2 | 3.9 | 3.7 | 3.8 | 2.6 | 3.1 |
| Mechanical ventilation | Yes | Yes | Yes | Yes | Yes | Yes |
| Ventilator day | 19 | 14 | 13 | 39 | 12 | 1 |
| P/F ratio | 65 | 232 | 128 | 210 | 111 | 121 |
| PEEP | 14 | 12 | 14 | 6 | 14 | 10 |
| FiO2 | 80% | 40% | 90% | 40% | 70% | 70% |
| Vasopressors | Yes | Yes | Yes | Yes | No | No |
| Renal replacement therapy | Yes | Yes | Yes | Yes | No | No |
| Thrombosis | CRRT circuit clots | Vascular access and CRRT circuit clots | Radial artery line thrombosis | CRRT circuit clots, presumed PE | None known, suspected microthrombi | Left femoral DVT |
| Venous ultrasound | POCUS examination negative | Not done | Formal ultrasound negative | Not done | POCUS with SEC in bilateral femoral veins | POCUS with L femoral DVT and R femoral SEC |
| Anticoagulation | Heparin | Heparin | Argatroban | Bivalirudin | Heparin | Enoxaparin |
| Final disposition (hospital day) | Death (D24) | Discharged to LTAC (D34) | Death (D15) | Death (D84) | Discharged home (D29) | Discharged home (D16) |
Abbreviations: CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CRRT, continuous renal replacement therapy; DM diabetes mellitus; HTN, hypertension; ICU, intensive care unit; LTAC, long‐term acute‐care facility; PEEP, positive end‐expiratory pressure; P/F ratio, PaO2/FiO2 ratio; POCUS, point of care ultrasound; SEC, spontaneous echo contrast; SOFA, sequential organ failure assessment.
Accurate fibrinogen levels were unavailable for this patient due to assay interference, likely related to direct thrombin inhibitor anticoagulation 16 .
Patient 1 is a 56‐year‐old man admitted with a 7‐day history of shortness of breath (SOB). He was transferred to the ICU on hospital day (D) 4 and intubated for hypoxic respiratory failure. He developed acute kidney injury (AKI) requiring continuous renal replacement therapy (CRRT), encephalopathy, septic shock requiring vasopressors, and ARDS with superimposed Klebsiella pneumoniae ventilator‐associated pneumonia. The patientʼs CRRT circuit had recurrent clots despite high intensity heparin drip (starting dose 18 units/kg/h, monitored with anti‐Xa levels, titrated goal range 0.5‐0.7 units/mL) and use of a 2.2% citrate solution (rate 280 mL/h). On D22 plasma viscosity was critically elevated at 4.2 cP and the first of 2 TPE procedures initiated. Despite decreases in plasma viscosity, fibrinogen, D‐dimer, and CRP levels after TPE, as well as resolution of CRRT‐related clots, the patient continued to decline. On D24, he developed worsening lactic acidosis, refractory hypotension, and asystolic cardiac arrest.
Patient 2 is a 44‐year‐old man admitted to the ICU with a 12 days history of fever and SOB. He was intubated on presentation for hypoxic respiratory failure. The patient developed AKI requiring CRRT, septic shock requiring vasopressors, encephalopathy, methicillin‐sensitive Staphylococcus aureus ventilator‐associated pneumonia, and ARDS. His course was further complicated by recurrent vascular access and CRRT circuit clots despite the use of 2.2% citrate solution and receiving low intensity (starting dose 15 units/kg/h, monitored with anti‐Xa levels, titrated for goal range 0.3‐0.5 units/mL) and high intensity heparin drip. On D14, plasma viscosity was 3.9 cP and the first of 3 TPE procedures initiated. After TPE the patient had no further clotting complications. His condition gradually improved, including improved renal and pulmonary function, allowing for tracheostomy on D26. He was transferred to a long‐term acute‐care facility on D34.
Patient 3 is a 75‐year‐old man admitted to the ICU with a 3 days history of worsening SOB. He was intubated on arrival for hypoxic respiratory failure. In the ICU the patient experienced AKI requiring CRRT, septic shock requiring vasopressors, cardiac arrhythmias, and markedly elevated liver enzymes. His course was further complicated by left upper extremity ischemia related to an arterial line thrombus that persisted after line removal and despite continuous argatroban drip (starting dose 0.5 mcg/kg/min, monitored with aPTT, titrated for goal range 60‐89 seconds). On D12 plasma viscosity was 3.7 cP and TPE performed on D13 and D14. Unfortunately, his clinical status further deteriorated and he died after a pulseless electrical activity arrest on D15.
Patient 4 is a 49‐year‐old man admitted to the ICU with a 10 days history of cough and SOB. On D4 he required intubation for hypoxic respiratory failure. His course was complicated by AKI requiring CRRT, septic shock requiring vasopressors, shock liver, encephalopathy, and prolonged respiratory failure necessitating tracheostomy on D33. On D37, despite continuous bivalirudin infusion (starting dose 0.05 mg/kg/h, monitored with aPTT, titrated for goal range 60‐80 seconds), he experienced a presumed massive PE, for which he received alteplase. On D43 plasma viscosity was 3.8 cP and he began the first of 2 TPE procedures. Following exchange, his mental status improved quickly and he was weaned from the ventilator and transferred out of the ICU. By D78 the patient was clinically stable and preparations for placement at a long‐term acute care facility were underway. Unfortunately, before transfer, he developed bacterial sepsis, likely related to a necrotic decubitus ulcer, with a cardiac arrest and death on D84.
Patient 5 is a 52‐year‐old man admitted to the ICU with a 9 days history of fever and SOB. He was intubated on admission. While in the ICU he developed AKI and severe ARDS. On D11, despite high intensity heparin drip, ultrasound revealed spontaneous echo contrast suggesting altered blood flow. 11 Viscosity testing was ordered out of concern for multi‐system organ failure related to microthrombi and hypercoagulable state. Initial viscosity testing clogged the viscometer; repeat testing was 2.6 cP and the first of 2 TPE procedures initiated on D12. After TPE the patient experienced renal and pulmonary recovery and was extubated on D17. He was discharged home without supplemental oxygen on D29.
Patient 6 is a 57‐year‐old man admitted to the hospital after a 5 days history of fevers, diarrhea, and syncope. He was transferred to the ICU and intubated on D6 for hypoxic respiratory failure. Despite standard prophylactic anticoagulation with 0.5 mg/kg/d enoxaparin, the patient developed a lower extremity deep venous thrombosis. Plasma viscosity was 3.1 cP and TPE performed on D6 and D7. After TPE the patientʼs oxygen requirements decreased and he was able to be extubated on D9. He was discharged home on D16 with supplemental oxygen.
Laboratory trends demonstrating levels of fibrinogen, D‐dimer, and CRP in relation to TPE procedures are shown for one representative patient (patient 5, Figure 1A). TPE decreased plasma viscosity in all six patients (Pre‐TPE median 3.75 cp, range 2.6‐4.2 cP; Post‐TPE median 1.6 cP, range 1.5‐1.9 cP); fibrinogen levels in all five patients for whom levels were available (Pre‐TPE median 739 mg/dL, range 601‐1188 mg/dL; Post‐TPE median 359 mg/dL, range 235‐461 mg/dL); D‐dimer levels in all six patients (Pre‐TPE median 5921 ng/mL, range 1134‐60 000 ng/mL; Post‐TPE median 4893 ng/mL, range 620‐7518 ng/mL); and CRP levels in five of six patients (Pre‐TPE median 292 mg/L, range 136‐329 mg/L; Post‐TPE median 84 mg/L, range 31‐211 mg/L) (Figure 1B). Clinical status improved in four of six patients, whose SOFA scores went from 15, 14, 8, and 5 before TPE, to 10, 8, 5, and 4, respectively, after the procedure (Figure 1C).
FIGURE 1.
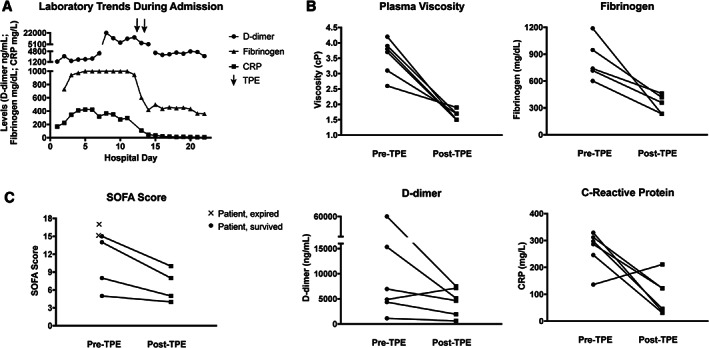
Clinical and laboratory parameters of critically ill patients with COVID‐19 before and after therapeutic plasma exchange (TPE). Laboratory values over the entire hospitalization are shown for one representative patient in relationship to TPE treatments (A). Plasma viscosity, fibrinogen, D‐dimer and CRP levels were measured within the 24 hours before initiating TPE (Pre‐TPE), and again within 24 hours after the final TPE session (Post‐TPE) in all patients as available (B). Sequential Organ Failure Assessment (SOFA) scores at the time of decision to initiate TPE (Pre‐TPE) in all six patients, and again 48 hours after the final TPE session (Post‐TPE) in the four surviving patients (C)
3. MATERIALS AND METHODS
The patients reported represent the first 6 patients with COVID‐19‐associated hyperviscosity that underwent TPE during ICU admission at an Emory Healthcare facility. Laboratory testing was performed in the clinical laboratory. All patients were positive for SARS‐CoV‐2 RNA by RT‐PCR on nasopharyngeal specimens. Decisions about clinical care were made by the clinical care teams, including whether to perform point‐of‐care ultrasound to evaluate for spontaneous echo contrast as an indication of low blood flow. 11 There were no defined exclusion criteria for TPE and four of the patients had SOFA scores ≥14, suggesting a high likelihood of impending mortality. Plasma viscosity was measured using traditional capillary viscometry (reference range 1.4‐1.8 centipoise, cP). Following informed consent, TPE was performed on two consecutive days following institutional protocols for a one plasma volume exchange with frozen plasma replacement, except for one patient who received three sessions. In all patients, plasma viscosity, fibrinogen, D‐dimer, and CRP levels were measured within 24 hours before initiating TPE, and again within 24 hours after the final TPE. Clinical and laboratory data were abstracted from electronic medical records in accordance with the Institutional Review Board determination.
4. DISCUSSION
Here we report our initial experience using TPE to lower plasma viscosity in six critically ill patients with COVID‐19‐associated hyperviscosity. We hypothesize that by lowering both viscosity, which alters blood flow, and fibrinogen, a key mediator of thrombi, TPE may help mitigate the propensity for thrombosis observed in COVID‐19, particularly in patients with hypercoagulability despite therapeutic anticoagulation. 1 , 2 , 3 , 4 , 5 , 6 Unfortunately, the two patients who were the most ill at the time of TPE initiation died within 48 hours of the procedure. Still, the brisk clinical improvement following TPE alongside the lack of any additional clotting complications in the remaining patients is promising. These results suggest that any benefit of TPE in treating critically ill COVID‐19 patients may be earlier in the disease course and additional studies assessing the optimal timing of intervention are warranted.
TPE has been used for decades to treat patients with symptomatic hyperviscosity from excessive immunoglobulin production. 10 However, unlike diseases hallmarked by hypergammaglobulinemia, the drivers of increased plasma viscosity in COVID‐19 are unknown. Acute phase proteins, stimulated by systemic inflammatory mediators, are likely candidates. Fibrinogen, which is significantly elevated in some patients with COVID‐19, seems uniquely poised to contribute to thrombotic risk both by increasing viscosity and providing the substrate for clot formation. 12 Whether increased viscosity is unique to COVID‐19 or similarly occurs in other inflammatory or infectious diseases requires further investigation.
While others have proposed a beneficial role of TPE in COVID‐19 by reducing inflammatory cytokines and mediators, this is the first report to describe TPE in COVID‐19 with the primary goal of reducing viscosity. 13 , 14 We propose that normalization of viscosity, coupled with removal of fibrinogen, cytokines, and other acute phase proteins following TPE, may enhance patient recovery by improving blood flow, decreasing thrombotic risk, and reducing inflammation‐induced end‐organ injury. Still, a significant limitation of TPE is the potential for concomitant removal of anti‐SARS‐CoV‐2‐specific antibodies that may delay or hamper the resolution of infection. As a result, optimal approaches to TPE may include testing SARS‐CoV‐2‐specific antibody titers before and after the procedure and using convalescent donor plasma in the final exchange to deliver high‐titer neutralizing antibodies. 15 Nevertheless, TPE is generally considered a safe and well‐tolerated therapy, and none of our patients experienced any adverse events associated with the procedure.
It is important to note that this report, as a case series, is significantly limited by both the small number of patients and the lack of predefined inclusion and exclusion criteria. Here we report our experience using TPE in the first 6 patients identified by their primary care teams as having symptomatic COVID‐19‐associated hyperviscosity, including 2 patients in whom TPE was attempted despite expected impending mortality. Future studies with larger numbers of patients will be necessary to demonstrate therapeutic efficacy of TPE in COVID‐19 and the specific patient population most likely to benefit from treatment. A randomized controlled trial (RCT) designed to determine the safety and efficacy of TPE in lowering plasma viscosity and impacting patient outcomes in COVID‐19 is underway (NCT04441996).
In summary, our series demonstrates that TPE can correct COVID‐19‐associated hyperviscosity and suggests a potential benefit in patient outcomes when utilized earlier in patients with severe disease, prior to the onset of multi‐system organ failure. Systematic study of TPE through RCTs is needed to establish the best protocols and determine any true benefit of this intervention in COVID‐19 management.
CONFLICT OF INTEREST
All of the authors have no conflicts to disclose.
AUTHOR CONTRIBUTIONS
Alexander D. Truong, Sara C. Auld, Nicholas A. Barker, Sarah Friend, A. Thanushi Wynn, Jason Cobb, Roman M. Sniecinski, Christin‐Lauren Tanksley, Derek M. Polly, Manila Gaddh, Michael Connor, Hirotomo Nakahara, H. Clifford Sullivan, Christine Kempton, and Cheryl L. Maier designed and executed this work. Alexander D. Truong, Sara C. Auld, Nicholas A. Barker, Sarah Friend, A. Thanushi Wynn, Jason Cobb, and Christin‐Lauren Tanksley collected data. Cheryl L. Maier, Roman M. Sniecinski, Jeannette Guarner, Alexander Duncan, Cassandra D. Josephson, Sean R. Stowell and John D. Roback analyzed data. Alexander D. Truong, Sara C. Auld, Sean R. Stowell and Cheryl L. Maier wrote the initial draft, which was commented on and edited by all authors.
ACKNOWLEDGEMENTS
The authors wish to thank Dr. Lisa Daniels for her astute clinical observations that aided in the presentation of this series, as well as Melanie Sherman, Stacian Reynolds, and the clinical immunology staff for viscometry testing, and the hemapheresis nurses for their dedication in caring for these patients. SCA is supported by NIH/NIAID K23 AI134182; CLM is supported by NIH/NHLBI K99 HL150626‐01.
Truong AD, Auld SC, Barker NA, et al. Therapeutic plasma exchange for COVID‐19‐associated hyperviscosity. Transfusion. 2021;61:1029–1034. 10.1111/trf.16218
Alexander D. Truong and Sara C. Auld contributed equally to this study, as did Sean R. Stowell and Cheryl L. Maier.
REFERENCES
- 1. Zhang L, Feng X, Zhang D, et al. Deep vein thrombosis in hospitalized patients with coronavirus disease 2019 (COVID‐19) in Wuhan, China: Prevalence, risk factors, and outcome. Circulation. 2020;142(2):114–128. [DOI] [PubMed] [Google Scholar]
- 2. Middeldorp S, Coppens M, van Haaps T, et al. Incidence of venous thromboembolism in hospitalized patients with COVID‐19. J Thromb Haem. 2020;18(8):1995–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS‐CoV‐2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wichmann D, Sperhake J, Lutgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID‐19. Ann Int Med. 2020;173(4):268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lax S, Skok K, Zechner P, et al. Pulmonary arterial thrombosis in COVID‐19 with fatal outcome: Results from a prospective, single‐center, clinicopathologic case series. Ann Int Med. 2020;173(5):350–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fox S, Akmatbekov A, Harbet J, et al. Pulmonary and cardiac pathology in African American patients with COVID‐19: An autopsy series from New Orleans. Lancet Resp Med. 2020;8(7):681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ackermann M, Verleden S, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID‐10. NEJM. 2020;383(2):120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maier C, Truong A, Auld S, et al. COVID‐19‐associated hyperviscosity: A link between inflammation and thrombophilia? Lancet. 2020;395(10239):1758–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vincent J, Moreno R, Takala J, et al. The SOFA score to describe organ dysfunction/failure. Intensive Care Med. 1996;22:707–710. [DOI] [PubMed] [Google Scholar]
- 10. Padmanabhan A, Connelly‐Smith L, Aqui N, et al. Guidelines on the use of therapeutic apheresis in clinical practice – evidence‐based approach from the Writing Committee of the American Society for Apheresis: The eighth special issue. J Clin Apher. 2019;34:171–354. [DOI] [PubMed] [Google Scholar]
- 11. Black I. Spontaneous echo contrast: Where thereʼs smoke thereʼs fire. Echocardiography. 2000;17(4):373–382. [DOI] [PubMed] [Google Scholar]
- 12. Ranucci M, Ballotta A, Di Dedda U, et al. The procoagulant pattern of patients with COVID‐19 acute respiratory distress syndrome. J Thromb Haem. 2020;18(7):1747–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Keith P, Day M, Perkins L, et al. A novel treatment approach to the novel coronavirus: An argument for the use of therapeutic plasma exchange for fulminant COVID‐19. Crit Care. 2020;24:128. 10.1186/s13054-020-2836-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kesici S, Yavuz S, Bayrakci B. Get rid of the bad first: Therapeutic plasma exchange with convalescent plasma for severe COVID‐19. Proc Natl Acad Sci USA. 2020;117(23):12526–12527. 10.1073/pnas.2006691117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID‐19 with convalescent plasma. JAMA. 2020;323(16):1582–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maier C, Barker N, Sniecinski R. Falsely low fibrinogen levels in COVID‐19 patients on direct thrombin inhibitors. Anesth Analg. 2020;131(2):e117–e119. 10.1213/ANE.0000000000004949. [DOI] [PMC free article] [PubMed] [Google Scholar]


