Abstract
Background
We undertook this study to evaluate the association between hyperglycemia and outcomes in patients with coronavirus disease 2019 (COVID‐19) admitted to the intensive care unit (ICU).
Methods
We conducted a multicenter retrospective study involving all adults with COVID‐19 admitted to the ICU between March and May 2020. Patients were divided into normoglycemic (average blood glucose <140 mg/dL) and hyperglycemic (average blood glucose ≥140 mg/dL) groups. Outcomes such as mortality, need and duration of mechanical ventilation, and length of hospital and ICU stays were measured.
Results
Among 495 patients, 58.4% were male with a median age of 68 years (interquartile range [IQR]: 58.00‐77.00), and baseline average blood glucose was 186.6 (SD ± 130.8). Preexisting diabetes was present in 35.8% of the studied cohort. Combined ICU and hospital mortality rates were 23.8%; mortality and mechanical ventilation rates were significantly higher in the hyperglycemic group with 31.4% vs 16.6% (P = .001) and 50.0% vs 37.2% (P = .004), respectively. Age above 60 years (hazard ratio [HR] 3.21; 95% CI 1.78, 5.78) and hyperglycemia (HR 1.79; 95% CI 1.14, 2.82) were the only significant predictors of in‐hospital mortality. Increased risk for hyperglycemia was found in patients with steroid use (odds ratio [OR] 1.521; 95% CI 1.054, 2.194), triglycerides ≥150 mg/dL (OR 1.62; 95% CI 1.109, 2.379), and African American race (OR 0.79; 95% CI 0.65, 0.95).
Conclusions
Hyperglycemia in patients with COVID‐19 is significantly associated with a prolonged ICU length of stay, higher need of mechanical ventilation, and increased risk of mortality in the critical care setting. Tighter blood glucose control (≤140 mg/dL) might improve outcomes in COVID‐19 critically ill patients; evidence from ongoing clinical trials is needed.
Keywords: COVID‐19, critical care unit, glucose control, hyperglycemia, mortality
Highlights
Hyperglycemia has been reported in almost half of the patients infected with coronavirus disease 2019.
In critically ill patients, hyperglycemia is associated with increased morbidity and mortality, irrespective of preexisting diabetes.
In our study, patients with blood glucose ≥140 mg/dL and admitted to the intensive care unit were found to have the worst outcomes in terms of mortality, mechanical ventilation need, and intensive care unit length of stay.
Further studies are needed to clarify if outcomes significantly improve with tighter glucose targets.
摘要
背景
我们开展了这项研究, 以评估入住重症监护病房(ICU)的新型冠状病毒肺炎(COVID‐19)患者高血糖与预后的关系。
方法
我们进行了一项多中心回顾性研究, 研究对象为2020年3月至5月间入住ICU的所有患有COVID‐19的成年人。将患者分为正常血糖组(平均血糖<140 mg/dL)和高血糖组(平均血糖≥140 mg/dL)。观察结果包括死亡率, 需要机械通气的时间, 住院和ICU的时间。
结果
495例患者中, 男性占58.4%, 中位年龄68岁(四分位数范围:58.00~77.00), 基线平均血糖为186.6(SD±130.8)。35.8%的研究队列中存在既往糖尿病。ICU和住院综合死亡率为23.8%, 高血糖组与正常血糖组相比, 死亡率为31.4%比16.6%(P=0.001), 机械通气率为50.0%比37.2%(P=0.004)。60岁以上(危险比HR 3.21; 95% Cl 1.78, 5.78)和高血糖(HR 1.79; 95% Cl 1.14, 2.82)是住院死亡率的唯一有意义的预测因素。使用类固醇(OR 1.521; 95%CI 1.054, 2.194), 三酰甘油≥150 mg/dL(OR 1.62; 95%CI 1.109, 2.379)和非洲裔美国人(OR 0.79; 95%CI为0.65, 0.95)的患者高血糖风险增加。
结论
COVID‐19患者的高血糖与ICU住院时间延长, 更高的机械通气需求以及重症监护环境中死亡风险的增加显著相关。更严格的血糖控制(≤140mg/dL)可能会改善COVID‐19危重患者的预后; 还需要正在进行的临床试验证据进一步证明该结论。
Keywords: COVID‐19, 重症监护病房, 血糖控制, 高血糖, 死亡率
1. INTRODUCTION
Since the World Health Organization (WHO) declared the coronavirus disease 2019 (COVID‐19) outbreak a pandemic, approximately 821 909 deaths have been confirmed globally. 1 COVID‐19 can result in severe disease with admissions to the intensive care unit (ICU), especially among older adults with severe underlying health conditions. 2 Multiple studies have attempted to analyze the different factors associated with mortality. 3 Age, gender, obesity, and other medical comorbidities (eg, chronic cardiac and pulmonary conditions, hypertension, diabetes, and chronic kidney disease) were the most common factors that might predict mortality in patients with COVID‐19. 4 Hyperglycemia was one of the modifiable factors that has been shown to be associated with an increased risk of mortality. 5 Studies showed that laboratory values such as d‐dimer, prolonged prothrombin time/activated partial thromboplastin time, lymphopenia, and neutrophilia might help in predicting disease severity and outcomes. 3 , 6
Few studies examined the effect of hyperglycemia in COVID‐19 critically ill patients. Results suggested that preexisting diabetes is associated with an increased risk of mortality, but also uncontrolled hyperglycemia is strongly associated with a prolonged in‐hospital and ICU length of stay (LOS), and an increased risk of mortality. 5 , 7
Management of hyperglycemia in critically ill patients is challenging. According to the American Diabetes Association (ADA), blood glucose levels should be maintained between 140 and 180 mg/dL in patients admitted to the ICU. 8 However, some studies suggested that tighter glucose control (<140 mg/dL) might provide some benefit in the outcome of patients with COVID‐19. 9 It has been challenging to advocate strong evidence‐based recommendations for the best treatment protocol for hyperglycemia in COVID‐19 infection, considering the expanding nature of the disease and the lack of available data. Therefore, the purpose of this study is to explore the effect of hyperglycemia on the outcomes of critically ill COVID‐19 adult patients in an attempt to identify the blood glucose target that could improve outcomes in this population in the future.
2. PARTICIPANTS AND METHODS
We conducted a multicenter retrospective cohort study across the Cleveland Clinic Enterprise, a 10‐hospital health care system in northeast Ohio, serving an over 2.7 million population. The study was reviewed and approved by the Cleveland Clinic Institutional Review Board.
The study included all critically ill patients admitted to all ICUs between 15 March and 30 May 2020. The diagnosis of COVID‐19 infection was established by reverse transcriptase polymerase chain reaction detection of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) RNA nasopharyngeal swab. All our patients were classified to have severe disease according to the national protocol published by the Chinese National Health Commission. 10 Mean blood glucose was defined as the average blood glucose levels from the date of admission until the date of ICU discharge or death. Based on the American Association of Clinical Endocrinologists and American Diabetes Association Consensus Statement on Inpatient Glycemic Control, hyperglycemia in hospitalized patients was defined as any blood glucose value >140 mg/dL (>7.8 mmol/L). Therefore, patients were grouped as normoglycemic with a mean blood glucose <140 mg/dL or hyperglycemic with a mean blood glucose level of ≥140 mg/dL. 8 , 9 Overweight and obesity were defined as body mass index (BMI) ≥ 25 and 30 kg/m2 respectively.
The primary end point included mortality difference between hyperglycemic and normoglycemic patients with COVID‐19 admitted to the ICU. The need for mechanical ventilation, duration of mechanical ventilation, LOS in the ICU and hospital, and risk factors associated with hyperglycemia were the secondary end points of interest. Patients were followed and data collected till hospital discharge, or ICU or hospital mortality.
2.1. Data collection
Data were obtained from the Quality Data Registries with the support of electronic research associates. Collected data included baseline characteristics, demographics, clinical information, and laboratory values. Outcome variables including mortality, hospital and ICU LOS, the need of mechanical ventilation, and the duration of mechanical ventilation were obtained as well. A medical record number was used to identify patients. No personal information was collected.
2.2. Statistical analysis
Descriptive statistics were used to characterize the cohort. Demographic variables were described using mean ± SD for continuous variables if they were normally distributed or as median (interquartile range, IQR) if they were not, and counts with percentages for categorical variables. The two‐sample t test or Wilcoxon rank sum test was used to compare continuous variables between groups. In contrast, the chi‐square test with continuity correction was applied to compare categorical variables. For the analysis of mortality, a multivariate Cox regression analysis to assess the effects of age, sex, and hyperglycemia was done. The optimal mean blood glucose cutoff points were evaluated by receiver operator characteristic (ROC) curve. Optimum cutoff values for laboratory tests were determined depending on sensitivity and specificity. Finally, univariate and multivariate logistic regression analysis was used to evaluate risk factors for developing hyperglycemia. All analyses were performed at a significance level of .05. IBM SPSS software version 20.0 (Armonk, NY: IBM Corp) (Statistical Package for the Social Sciences), R version 3.5.0 (The R Foundation for Statistical Computing, Vienna, Austria), and SAS 9.4 software (SAS Institute, Cary, North Carolina) were used for the above analyses.
3. RESULTS
3.1. Baseline characteristics
We identified 495 patients with severe COVID‐19 infection. Demographics, clinical features, and laboratory values are summarized in Table 1. Among the evaluated patients, 289 (58.4%) patients were males with a median age of 68 years (IQR: 58.00‐77.00). The mean value of blood glucose at the time of admission was 186.6 mg/dL (SD ± 130.8). White Caucasian race was the most prevalent in our cohort (54.9%). Preexistent diabetes, whether type 1 or type 2, was present in 177 (35.8%) of the studied cohort, being naturally more prevalent in the hyperglycemic group compared with the normoglycemic group (63.6% vs 9.1%, P < .001). Overweight was highly prevalent in our population, and the median BMI was 29.74 kg/m2 (IQR: 25.61‐34.92). Subcutaneous and intravenous insulin were used in 129 (26.0%) and 70 (14.1%) patients, respectively.
TABLE 1.
Baseline characteristics of study population
| Characteristics | All patients (N = 495) | Patients with hyperglycemia (n = 242) | Patients with normoglycemia (n = 253) | P value |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Male | 289 (58.4) | 142 (58.7) | 147 (58.1) | .897 |
| Female | 206 (41.6) | 100 (41.3) | 106 (41.9) | |
| Race, n (%) | ||||
| Caucasian | 272 (54.9) | 136 (56.2) | 136 (53.8) | .024 |
| African American | 192 (38.8) | 99 (40.9) | 93 (36.8) | |
| Others | 31 (6.3) | 7 (2.8) | 24 (9.5) | |
| Age, median (IQR), years | 68.00 (58.00‐77.00) | 69.00 (59.00‐77.25) | 66.00 (56.00‐77.00) | .231 |
| Age category, n (%) | ||||
| <60 years old | 145 (29.3) | 64 (26.4) | 81 (32.0) | .174 |
| >60 years old | 350 (70.7) | 178 (73.6) | 172 (68.0) | |
| BMI, median (IQR), kg/m2 | 29.74 (25.61‐34.92) | 29.50 (25.22‐34.79) | 30.01 (25.78‐35.26) | .653 |
| BMI, n (%) | ||||
| BMI < 30 kg/m2 | 254 (51.3) | 127 (52.5) | 127 (50.2) | .612 |
| BMI > 30 kg/m2 | 241 (48.7) | 115 (47.5) | 126 (49.8) | |
| Diabetes mellitus, n (%) | 177 (35.8%) | 154 (63.6%) | 23 (9.1%) | <.001 |
| Steroid use, n (%) | 205 (41.4%) | 114 (47.3%) | 91 (36.0%) | .011 |
| Types of insulin, n (%) | ||||
| SQ insulin | 129 (26.0) | 82 (33.9) | 47 (18.6) | <.001 |
| IV insulin | 70 (14.1) | 24 (9.9) | 46 (18.2) | |
| Multiple routes | 133 (26.9) | 112 (46.3) | 21 (8.3) | |
| APACHE III score, median (IQR) | 46.00 (25.00‐46.00) | 61.0 (46.00‐79.00) | 53.0 (39.00‐70.55) | .006 |
| Laboratories values | ||||
| HbA1c, median (IQR), mmol/mol | 64.00 (43.00‐72.00) | 61.0 (49.00‐85.00) | 42.00 (35.00‐49.00) | .002 |
| % | 8.0 (6.1‐8.7) | 7.7 (6.6‐9.9) | 6.0 (5.4‐6.6) | |
| C‐ reactive protein, median (IQR), mg/dL | 12.30 (6.20‐19.15) | 13.00 (6.68‐19.93) | 11.60 (5.53‐18.28) | .222 |
| Ferritin, median (IQR), ng/mL | 836.05 (444.35‐1069.50) | 896.80 (452.10‐1751.00) | 747.00 (435.60‐1522.00) | .100 |
| Fibrinogen, median (IQR), mg/dL | 546.00 (427.75‐683.75) | 526.00 (427.00‐690.00) | 560.00 (427.00‐690.00) | .396 |
| d‐dimer median (IQR), ng/mL | 1465 (840.00‐3062.50) | 1410.00 (795.00‐2900.00) | 1530.00 (855.00‐3325.00) | .498 |
| Triglycerides, median (IQR), mg/dL | 136.0 (103.00‐202.25) | 148.00 (108.25‐23.25) | 122.50 (92.75‐184.25) | .033 |
| White blood cell count, median (IQR), per mm3 | 7.97(5.65‐11.55) | 8.5 (5.52‐12.38) | 7.35 (5.68‐10.49) | .269 |
| Interleukin‐6, median (IQR), pg/mL | 56.20 (22.40‐139.50) | 56.30 (26.55‐121.70) | 52.90 (17.75‐167.35) | .944 |
Note: Continuous variables are described as mean ± SD for parametric variables and median (IQR) for nonparametric variables unless otherwise indicated. The P values <.05 were considered as statistically significant.
Abbreviations: APACHE III, Acute Physiology and Chronic Health Evaluation III; BMI, body mass index; HbA1c, glycosylated hemoglobin; IQR, interquartile range.
Most patients had normal leukocyte count at admission with a median of 7.97/mm3 (IQR: 5.65‐11.55). The COVID‐19 patients with hyperglycemia had a higher median leukocyte count as compared with patients without hyperglycemia (8.5 [IQR: 5.52‐12.38] vs 7.5 [IQR: 5.68‐10.49]). Although not statistically significant, inflammatory markers such as ferritin, C‐reactive protein, and interleukin‐6 were higher in patients with hyperglycemia compared with patients in the normoglycemia group.
The median baseline glycosylated hemoglobin (HbA1c) levels (61.0 vs 42.0 mmol/mol, 7.7% vs 6.0%; P = .002), triglycerides levels (148 vs 122 mg/dL, P = .033), and APACHE III (Acute Physiology and Chronic Health Evaluation III) score (61 vs 53, P = .006) were higher in the ICU hyperglycemic group compared with the normoglycemic group. Also, as expected, patients requiring glucocorticoid treatment were more likely to have hyperglycemia: 114 (47.3%) patients in the hyperglycemia cohort were treated with glucocorticoid compared with 91 (36.0%) patients in the normoglycemia cohort. No statistical differences were found between the hyperglycemic and the normoglycemic groups for all other baseline characteristics.
3.2. Outcomes
Regarding the outcomes described in Table 2, the combined ICU and in‐hospital mortality rate was 23.8%. A total of 118 deaths, out of the 495 ICU patients with COVID‐19 occurred across our health care system. The optimum cutoff value for mean blood glucose to predict mortality in critically ill COVID‐19 patients was 140 mg/dL with a sensitivity of 70.3% and specificity of 55.5% (Figure S1 and Table S1). Compared with the normoglycemic group, the mortality rate was statistically higher in the group with hyperglycemia (31.4% vs 16.6%, P < .001). Also, mechanical ventilation was more prevalent in patients with hyperglycemia (50.0% vs 37.2%, P = .004), but no statistically significant difference was found regarding the duration of mechanical ventilation (6.8 vs 7.1, P = .564) between the two groups. Although the hyperglycemic cohort had a statistically significant higher ICU LOS compared with the normoglycemic cohort (5.5 vs 3.5, P < .001), no significant differences were appreciated in the overall median hospital LOS between both groups (15.9 vs 15.8, P = .754).
TABLE 2.
Outcomes in patients with COVID‐19 admitted to ICU
| Variables | Patients with hyperglycemia (n = 242) | Patients with normoglycemia (n = 253) | P value |
|---|---|---|---|
| Intensive care unit length of stay, median (IQR), days | 5.53 (5.50‐16.41) | 3.40 (6.36‐16.39) | <.001 |
| Hospital length of stay, median (IQR), days | 15.85 (10.25‐22.73) | 15.83 (11.06‐24.03) | .754 |
| Mechanical ventilation, n (%) | 121 (50.0) | 94 (37.2) | .004 |
| Duration of mechanical ventilation, median (IQR), days | 6.81 (2.93‐13.37) | 7.08 (3.49‐11.62) | .564 |
| Disposition at discharge, n (%) | |||
| Alive | 166 (68.6) | 211 (83.4) | <.001 |
| Dead | 76 (31.4) | 42 (16.6) |
Note: P values <.05 were considered as statistically significant.
Abbreviations: COVID‐19, coronavirus disease 2019; ICU, intensive care unit; IQR, interquartile range.
Univariate and multivariate linear regression analyses were performed to explore the factors predicting hyperglycemia among our patients (Figure 1). The results showed that glucocorticoid use (odds ratio [OR] 1.52; 95% CI 1.05, 2.19), triglycerides ≥ 150 mg/dL (OR 1.62; 95% CI 1.11, 2.38), and Caucasian vs African American race (OR 0.79; 95% CI 0.65, 0.95) were statistically significant predictors for increased risk of hyperglycemia in critically ill patients with COVID‐19 infection (Table 3). Also, history of diabetes mellitus was a significant predictor of hyperglycemia (OR 17.5; 95% CI 10.59, 28.91).
FIGURE 1.
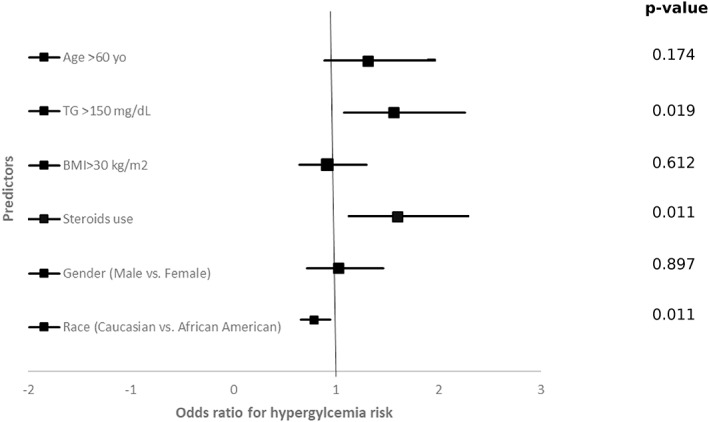
Forest Plot showing univariate logistic regression analysis with odds ratios (95% confidence interval) of different predictors for hyperglycemia in patients admitted to ICU with COVID‐19. ICU, Intensive Care Unit; TG, Triglycerides; BMI, Body mass Index
TABLE 3.
Univariate and multivariate analyses for predictors of hyperglycemia in patients with COVID‐19 admitted to ICU
| Variables | Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| P value | OR | 95% CI | P value | OR | 95% CI | |||
| Lower | Upper | Lower | Upper | |||||
| Age ≥ 60 years | .174 | 1.310 | 0.888 | 1.933 | ||||
| Triglycerides ≥ 150 mg/dL | .019 | 1.559 | 1.076 | 2.259 | .013 | 1.625 | 1.109 | 2.379 |
| BMI ≥ 30 kg/m2 | .612 | 0.913 | 0.641 | 1.299 | ||||
| Steroid use | .011 | 1.598 | 1.114 | 2.291 | .025 | 1.521 | 1.054 | 2.194 |
| Gender (male vs female) | .897 | 1.024 | 0.716 | 1.464 | ||||
| Race (Caucasian vs African American) | .011 | 0.788 | 0.655 | 0.948 | .015 | 0.791 | 0.655 | 0.956 |
Abbreviations: BMI, body mass index; COVID‐19, coronavirus disease 2019; ICU, intensive care unit; OR, odds ratio.
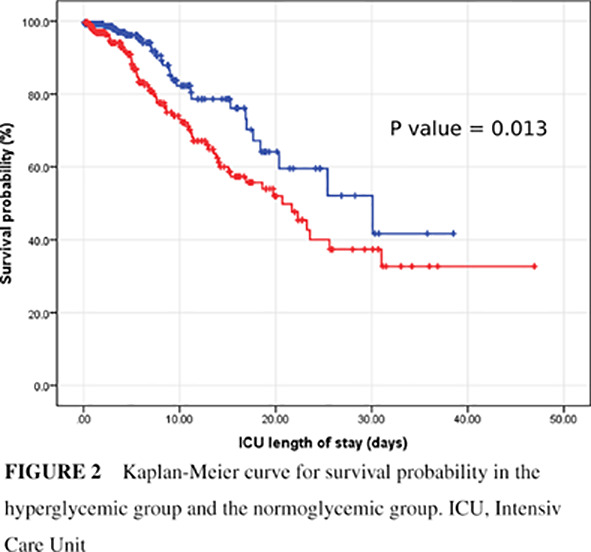
A multivariable Cox regression analysis was conducted to assess predictors of ICU mortality. Age older than 60 (HR 3.21; 95% CI 1.78, 5.78) and hyperglycemia with mean ICU glucose ≥140 mg/dL (HR 1.79; 95% CI 1.14, 2.82) were associated with increased ICU mortality (Figure 2). On the other hand, diagnosis of diabetes mellitus, whether type 1 or type 2, gender, race, BMI, or being on mechanical ventilation were not predictors for increased ICU mortality (Table 4).
FIGURE 2.
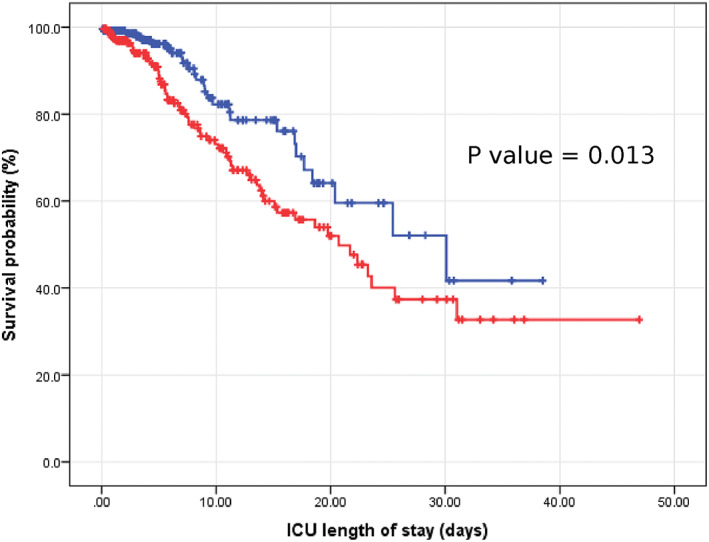
Kaplan‐Meier curve for survival probability in the hyperglycemic group and the normoglycemic group. ICU, Intensive Care Unit
TABLE 4.
Multivariate Cox regression analysis for survival of patients with COVID‐19 admitted to ICU
| Variables | Coefficient | SE | P value | HR | 95% CI | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Age (≥60 vs <60 years) | 1.165 | 0.299 | <.001 | 3.215 | 1.788 | 5.780 |
| Gender (male vs female) | −0.317 | 0.189 | .093 | 0.728 | 0.503 | 1.054 |
| Weight (BMI ≥ 30 vs <30 kg/m2) | −0.238 | 0.189 | .206 | 0.788 | 0.544 | 1.140 |
| Average blood glucose | ||||||
| (≥140 vs <140 mg/dL) | 0.586 | 0.232 | .011 | 1.796 | 1.141 | 2.828 |
| Race (Caucasian vs African American) | 0.055 | 0.108 | .662 | 1.057 | 0.854 | 1.307 |
| Diabetes mellitus | −0.194 | 0.221 | .383 | 0.823 | 0.535 | 1.275 |
| Mechanical ventilation | 0.092 | 0.194 | .635 | 1.102 | 0.753 | 1.613 |
Abbreviations: BMI, body mass index; COVID‐19, coronavirus disease 2019; HR, hazard ratio; ICU, intensive care unit.
4. DISCUSSION
Our retrospective study aimed to evaluate the association between hyperglycemia and outcomes in patients with severe COVID‐19. In our cohort, 49% of all patients with severe COVID‐19 admitted to the medical ICU were found to have hyperglycemia. Our results revealed that blood glucose ≥140 mg/dL and age above 60 years were associated with worst outcomes determined by an increase in mortality rate, severe APACHE III scores, prolonged ICU LOS, and higher need for mechanical ventilation.
Hyperglycemia is a common complication in critically ill patients and is associated with increased morbidity and mortality, irrespective of preexisting diabetes. 11 Hyperglycemia was reported in half of the patients with COVID‐19 in a preliminary report from Wuhan, China. 12 In the study reported by Sardu et al, 25 out of 59 (42%) patients with COVID‐19 were hyperglycemic during admission. 9 In our study, we noticed a higher percentage of hyperglycemic events among our patients. This can be explained by the fact that all patients included in our study were critically ill and managed in the ICU. Also, 41% of our cohort required steroids during admission.
Diabetes has been described as a risk factor for worse prognosis and mortality in patients with COVID‐19. 13 Among 7162 patients with COVID‐19 reported to the Centers for Disease Control and Prevention (CDC) from 12 February to 28 March 2020, diabetes was present in 32% of ICU patients, 14 similar to the percentage seen in our study population (35%). A retrospective study with 7337 patients with COVID‐19, including 952 (12.9%) with preexisting diabetes, revealed significantly higher mortality (7.8% vs 2.7%, adjusted HR: 1.49) and increased medical intervention rates in patients with diabetes vs those without diabetes. 15 A meta‐analysis of 16 003 patients also reported that diabetes was associated with a two‐fold increase in mortality and severity of COVID‐19 presentation when compared with patients without diabetes. 16 The CORONADO study, a multicenter French prospective observational study, revealed no association between HbA1c level and mortality. 17 , 18 A retrospective study from China comparing patients with COVID‐19 with and without diabetes revealed diabetes was not significantly associated with higher in‐hospital mortality (HR 1.58; 95% CI, 0.84‐2.99). 19 Moreover, age ≥70 years and underlying hypertension were independent risk factors for in‐hospital mortality in patients with diabetes. Despite the conflicting evidence supporting that diabetes can increase the risk of mortality, our study did not show a statistically significant association between preexisting diabetes and mortality.
Whether acute hyperglycemia and target glycemic management play a role in COVID‐19 severity and outcome is still to be determined. In the study published by Bode et al 5 among 493 patients discharged alive, the median LOS was longer in patients with diabetes and/or uncontrolled hyperglycemia (≥180 mg/dL) compared with patients without diabetes or hyperglycemia (5.7 vs 4.3 days, P < .001). Another study reported that higher admission fasting blood glucose levels in COVID‐19 was an independent predictor of ICU admission and poor outcomes, irrespective of steroid use. 20 Also, tight glycemic control has shown to have a protective effect with improved outcomes in patients with COVID‐19 with hyperglycemia. Sardu et al reported that a more substantial drop in glucose levels, obtained by insulin infusion, is associated with better outcomes in patients with COVID‐19. 9 Our study results came to the same conclusion that critically ill patients with COVID‐19 with uncontrolled blood glucose ≥140 mg/dL had the worst overall outcome in terms of disease severity, mortality, need for mechanical ventilation, and LOS. On the other hand, the use of glucocorticoid therapy, history of diabetes, BMI, and mechanical ventilation were not predictors of mortality in our study. This can be explained by the severity of the disease in the included population compared with other studies that compared patients with mild, moderate, and severe disease.
Preliminary results from the Randomized Evaluation of COVID‐19 Therapy (RECOVERY) trial showed that dexamethasone decreased mortality in patients with COVID‐19 requiring mechanical ventilation or oxygen support. 21 However, further reports have highlighted the potentially harmful effects of systemic corticosteroids in these patients. 22 Steroid‐induced hyperglycemia is a significant side effect in patients with and without diabetes that may result in worse outcomes and should be considered in the management of patients with COVID‐19. 23 Our results revealed a statistically significant increased risk in the development of uncontrolled hyperglycemia with steroid use. Moreover, our results revealed that a high triglyceride level (≥150 mg/dL) was significantly associated with hyperglycemia.
Despite being a multicenter cohort, our study has multiple limitations. The primary limitation is the retrospective and observational nature of the study, which did not allow us to generalize our results, especially in those with less severe disease. Secondly, patients with severe disease received various treatment modalities that might have a significant effect on glycemic levels.
Our study successfully analyzed the association of hyperglycemia in critically ill patients with COVID‐19 using mean glucose levels above 140 mg/dL with outcomes such as LOS (ICU and hospital), need and length of ventilation, and mortality. The fact that our study used mean average glucose levels during the entire ICU stay and not only portrayed admission glucose levels, allowed us to examine the effects of hyperglycemia in critically ill patients with COVID‐19 in a more precise manner.
Based on our results, patients with blood glucose ≥140 mg/dL have an increased mortality rate and a longer ICU LOS. Over the past two decades, there have been considerable changes in the management of blood glucose control in the acute care setting from tight glycemic control (80‐110 mg/dL) to a more moderate glycemic control (140‐180 mg/dL). 24 , 25 There are currently no clear guidelines or recommendations regarding optimal glycemic targets in patients with COVID‐19, especially in the critical care setting. Given the findings mentioned above, randomized clinical trials assessing specialized insulin protocols and the optimal target of tighter glucose control are needed. Further studies are also needed to assess the effect of triglyceride levels on blood glucose control and to evaluate if better control of triglyceride levels with the use of statins may optimize glucose control and improve mortality in patients with COVID‐19.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
Supporting information
Figure S1 Receiver operator curve for mean blood glucose level to predict overall mortality. The optimum cutoff value is marked by circle (140 mg/dL). Area under the curve for predicting mortality was 0.677, P‐value: .000001.
Table S1. Sensitivity and Specificity of Mean Blood Glucose Cutoff Levels for Predicting Mortality in Severe COVID‐19.
ACKNOWLEDGEMENTS
We want to express our gratitude to all the Fairview Hospital health care workers for their invaluable work. This research did not receive any funding.
Saand AR, Flores M, Kewan T, et al. Does inpatient hyperglycemia predict a worse outcome in COVID‐19 intensive care unit patients? Journal of Diabetes. 2021;13:253–260. 10.1111/1753-0407.13137
REFERENCES
- 1. Hopkins, J. Mortality analyses; 2020. https://coronavirus.jhu.edu/data/mortality. Accessed September 8, 2020.
- 2. Team CC‐R. Severe outcomes among patients with coronavirus disease 2019 (COVID‐19) ‐ United States, February 12‐March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gupta S, Hayek SS, Wang W, et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;180:1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grasselli G, Greco M, Zanella A, et al. Risk factors associated with mortality among patients with COVID‐19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180:1345‐1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bode B, Garrett V, Messler J, et al. Glycemic characteristics and clinical outcomes of COVID‐19 patients hospitalized in the United States. J Diabetes Sci Technol. 2020;14(4):813‐821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang S, Ma P, Zhang S, et al. Fasting blood glucose at admission is an independent predictor for 28‐day mortality in patients with COVID‐19 without previous diagnosis of diabetes: a multi‐Centre retrospective study. Diabetologia. 2020;63(10):2102‐2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. American Diabetes A. 15. Diabetes care in the hospital: standards of medical care in diabetes‐2020. Diabetes Care. 2020;43(suppl 1):S193‐S202. [DOI] [PubMed] [Google Scholar]
- 9. Sardu C, D'Onofrio N, Balestrieri ML, et al. Outcomes in patients with hyperglycemia affected by COVID‐19: can we do more on glycemic control? Diabetes Care. 2020;43(7):1408‐1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pei‐Fang W. Diagnosis and treatment protocol for novel coronavirus pneumonia (trial version 7). Chin Med J (Engl). 2020;133(9):1087‐1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Falciglia M. Causes and consequences of hyperglycemia in critical illness. Curr Opin Clin Nutr Metab Care. 2007;10(4):498‐503. [DOI] [PubMed] [Google Scholar]
- 12. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Apicella M, Campopiano MC, Mantuano M, Mazoni L, Coppelli A, del Prato S. COVID‐19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8(9):782‐792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Team CC‐R. Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 ‐ United States, February 12‐March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):382‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhu L, She Z‐G, Cheng X, et al. Association of blood glucose control and outcomes in patients with COVID‐19 and pre‐existing type 2 diabetes. Cell Metab. 2020;31(6):1068‐1077 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kumar A, Arora A, Sharma P, et al. Is diabetes mellitus associated with mortality and severity of COVID‐19? A meta‐analysis. Diabetes Metab Syndr. 2020;14(4):535‐545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cariou B, Hadjadj S, Wargny M, et al. Phenotypic characteristics and prognosis of inpatients with COVID‐19 and diabetes: the CORONADO study. Diabetologia. 2020;63(8):1500‐1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Scheen AJ, Marre M, Thivolet C. Prognostic factors in patients with diabetes hospitalized for COVID‐19: findings from the CORONADO study and other recent reports. Diabetes Metab. 2020;46:265‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shi Q, Zhang X, Jiang F, et al. Clinical characteristics and risk factors for mortality of COVID‐19 patients with Diabetes in Wuhan, China: a two‐center, retrospective study. Diabetes Care. 2020;43(7):1382‐1391. [DOI] [PubMed] [Google Scholar]
- 20. Liu SP, Zhang Q, Wang W, et al. Hyperglycemia is a strong predictor of poor prognosis in COVID‐19. Diabetes Res Clin Pract. 2020;167:108338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. RECOVERY Collaborative Group , Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid‐19 ‐ preliminary report. N Engl J Med. 2020. [Online ahead of print]. [Google Scholar]
- 22. Theoharides TC, Conti P. Dexamethasone for COVID‐19? Not so fast. J Biol Regul Homeost Agents. 2020;34(3):1241‐1243. [DOI] [PubMed] [Google Scholar]
- 23. Bonaventura A, Montecucco F. Steroid‐induced hyperglycemia: an underdiagnosed problem or clinical inertia? A narrative review. Diabetes Res Clin Pract. 2018;139:203‐220. [DOI] [PubMed] [Google Scholar]
- 24. NICE‐SUGAR Study Investigators for the Australian and New Zealand Intensive Care Society Clinical Trials Group and the Canadian Critical Care Trials Group , Finfer S, Chittock D, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283‐1297. [DOI] [PubMed] [Google Scholar]
- 25. Clain J, Ramar K, Surani SR. Glucose control in critical care. World J Diabetes. 2015;6(9):1082‐1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Receiver operator curve for mean blood glucose level to predict overall mortality. The optimum cutoff value is marked by circle (140 mg/dL). Area under the curve for predicting mortality was 0.677, P‐value: .000001.
Table S1. Sensitivity and Specificity of Mean Blood Glucose Cutoff Levels for Predicting Mortality in Severe COVID‐19.


