Abstract
To investigate the effects of renin‐angiotensin‐aldosterone system (RAAS) inhibitors on the prognosis in patients with coronavirus disease 2019 (COVID‐19). A meta‐analysis was performed. We systematically searched PubMed, the Cochrane Library, the Web of Science, EMBASE, medRxiv, and bioRxiv database through October 30, 2020. The primary and secondary outcomes were mortality and severe COVID‐19, respectively. We included 25 studies with 22,734 COVID‐19 patients, and we compared the outcomes between patients who did and did not receive angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers (ACEIs/ARBs). The use of ACEIs/ARBs was not associated with higher risks of severe disease (odds ratio [OR] = 0.89; 95% confidence interval [CI]: 0.63, 1.15; I 2 = 38.55%), mechanical ventilation (OR = 0.89; 95% CI: 0.61, 1.16; I 2 = 3.19%), dialysis (OR = 1.24; 95% CI: 0.09, 2.39; I 2 = 0.00%), or the length of hospital stay (SMD = 0.05; 95% CI: −0.16, 0.26; I 2 = 84.43%) in COVID‐19 patients. The effect estimates showed an overall protective effect of ACEIs/ARBs against mortality (OR = 0.65; 95% CI: 0.46, 0.85; I 2 = 73.37%), severity/mortality (OR = 0.69; 95% CI: 0.43, 0.95; I 2 = 22.90%), transfer to the intensive care unit among COVID‐19 patients with hypertension (OR = 0.36, 95% CI: 0.19, 0.53, I 2 = 0.00%), hospitalization (OR = 0.79; 95% CI: 0.60, 0.98; I 2 = 0.00%), and acute respiratory distress syndrome (OR = 0.71; 95% CI: 0.46, 0.95; I 2 = 0.00%). The use of RAAS inhibitor was not associated with increased mortality or disease severity in COVID‐19 patients. This study supports the current guidelines that discourage the discontinuation of RAAS inhibitors in COVID‐19 patients.
Keywords: angiotensin receptor blockers (ARBs), angiotensin‐converting enzyme inhibitors (ACEIs), coronavirus disease 2019 (COVID‐19), meta‐analysis, mortality, severity
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) is caused by a newly identified coronavirus and ranges in severity from symptoms similar to those of the common cold to a terminal disease. This outbreak started in December 2019 in Wuhan, Hubei Province, China, and has since spread worldwide. Unfortunately, there is still no specific and effective treatment for COVID‐19.
Angiotensin‐converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) act on the renin‐angiotensin‐aldosterone system (RAAS) by attenuating the hypertensive effects of angiotensin II. 1 , 2 , 3 Angiotensin‐converting enzyme 2 (ACE2) is a carboxypeptidase that processes angiotensin II to the angiotensin (1–7) fragment, a vasodilatory, anti‐inflammatory peptide. 4 ACE2 expression is particularly high in lung epithelial cells but is also present in cardiac myocytes and endothelial cells. 4 , 5 , 6 High ACE2 and angiotensin 1–7 levels play beneficial roles in cardiovascular and pulmonary diseases, suggesting that this pathway is involved in the overall benefits observed in patients treated with ACEIs and/or ARBs.
There are many similarities between severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and SARS‐CoV. These similarities are important because ACE2 was previously identified as a functional SARS‐CoV receptor in vitro 7 and in vivo. 8 It is required for host cell entry and subsequent viral replication. 9 COVID‐19 patients with cardiovascular disease are at increased risk of mortality. Quite often, these patients are being treated with drugs targeting the RAAS. The upregulation of ACE2 by ACEIs and ARBs has led to the hypothesis that these antihypertensive drugs might increase a patient's susceptibility to contracting COVID‐19 and the likelihood of developing severe disease. Therefore, there is controversy regarding the clinical management of patients undergoing RAAS modulation by ACEIs/ARBs.
It is important to determine whether the RAAS inhibitor (ACEI/ARB) use is associated with increased risks of contracting COVID‐19 and developing severe disease. Thus, we performed a meta‐analysis of the available studies to explore whether the use of RAAS inhibitors was associated with severe disease and mortality in COVID‐19 patients.
2. METHODS
This study involved a systematic review of the existing observational cohort studies examining the effect of the use of ACEIs/ARBs on the outcome of COVID‐19. We followed the Meta‐analyses Of Observational Studies in Epidemiology (MOOSE) checklist.
The protocol for this systematic review was registered with PROSPERO (CRD42020209389).
2.1. Search strategy
The literature search identified all studies published from the time the COVID‐19 outbreak began (December 2019) until October 30, 2020, with no restrictions on the country. We systematically searched the PubMed, Cochrane Library, Web of Science, EMBASE, medRxiv, and bioRxiv databases with the following search keywords: (COVID‐19 OR 2019 novel coronavirus disease OR SARS‐CoV‐2 infection, etc.) AND (Angiotensin‐Converting Enzyme Inhibitors OR Inhibitors, Kininase II OR Inhibitors, ACE OR ACE Inhibitors, etc.) AND (Angiotensin Receptor Antagonists OR Angiotensin II Receptor Blockers, etc.).
Additionally, a manual search of the retrieved articles, related review articles, and meta‐analyses was conducted to identify other eligible studies.
2.2. Inclusion criteria
The inclusion criteria were as follows: (1) study design: observational cohort studies; (2) grouping method: ARBs/ACEIs and non‐ARBs/ACEIs group, according to their usage of antihypertensive drugs (the use of ACEIs and ARBs before admission and during hospital stay); and (3) availability of information needed to calculate odds ratios (ORs) or the adjusted data; (4) participants of studies are inpatients or outpatients. Editorials, correspondences, conference abstracts, and commentaries were excluded from our study. The preliminary screening and full‐text evaluation were independently performed by two reviewers.
2.3. Data extraction
The following data were extracted: (1) study information: author name, country, publication journal, study design, study population, sample source, confounder adjustments, and study quality. (2) Participant information: age, sex, comorbidity, and ACEI/ARB use. (3) Outcomes: the primary outcome was mortality in patients with COVID‐19. The secondary outcomes were severe COVID‐19, acute respiratory distress syndrome (ARDS), the need for mechanical ventilation, the need for dialysis, transfer to the intensive care unit (ICU), length of hospital stay, and hospitalization due to COVID‐19. The full texts of the articles selected by one or both of the assessors were retrieved for the final evaluation. Two assessors read the full‐text articles and independently extracted the information from the selected studies. A third assessor reviewed the data extraction, and any disagreement was resolved through consensus.
2.4. Data synthesis and analysis
The meta‐analysis was performed using Stata 16.0 software. Heterogeneity was assessed using I 2 statistics. χ 2 tests were used to assess the homogeneity of the studies. The I 2 statistic reflects the proportion of the total variation observed between trials that is attributable to differences between the trials rather than to sampling error (chance). Random‐effects models were used for the pooled analyses, regardless of the degree of heterogeneity. To provide a quantitative estimate of the associations between ACEI/ARB use and outcomes in COVID‐19 patients, the ORs and corresponding 95% CIs were extracted from the published articles. When the OR was not given, tabular data were used to calculate the OR. Because the participants differed across the studies with regard to whether they had hypertension, subgroup analyses were performed. Publication bias was estimated visually based on funnel plots and quantitatively with Egger's test. A single study was used to analyze the source of heterogeneity. Furthermore, a meta‐regression model was conducted to explore the potential modulators for ACEI/ARB treatment effects.
2.5. Appraisal of the quality of studies
The Newcastle Ottawa Scale was used to assess article quality. The quality of the studies was qualitatively evaluated by two independent assessors. Any disagreement with regard to the quality assessment was resolved through consensus. Studies with scores >7 were considered at low risk of bias, those with scores of 5–7 had a moderate risk of bias, and those with scores <5 had a high risk of bias. Articles with a high risk of bias were excluded. 10
3. RESULTS
3.1. Literature retrieval
Through the literature search, we retrieved 3204 articles. In total, 1448 articles were screened after duplicates were removed. After the titles and abstracts were read, 1233 articles were excluded. Among the remaining 215 papers, 25 articles were included in our study after the full texts were reviewed (Figure 1).
Figure 1.
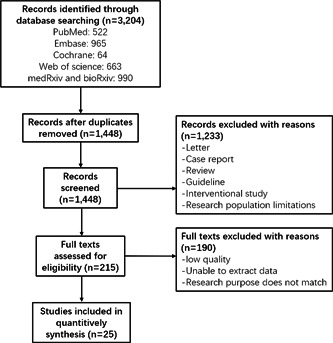
Flow chart of the literature screening process
3.2. Study description
A total of 22,734 participants were enrolled in the 25 included studies. The study sample size ranged from 36 to 7933 participants, with a mean age ranging from 52 to 78 years. Most studies included participants with hypertension, and 10 studies included the general population. Most of the studies were carried out in China (n = 11) and the United States (n = 4), and other studies were conducted in Italy, the UK, Spain, France, South Korea, and Turkey 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 (Tables 1–2).
Table 1.
Characteristics of the included studies (1)
| Author | Country | Journal | Study design | Sample size | Male | Age (years) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACEI/ARB | Non‐ACEI/ARB | ACEI/ARB | Non‐ACEI/ARB | ACEI/ARB | Non‐ACEI/ARB | |||||||
| Mean | SD | Mean | SD | NOS | ||||||||
| Bean, D M | UK | European Journal of Heart Failure | A retrospective cohort study | 399 | 801 | 231 | 455 | 73.02 | 13.46 | 65.45 | 18.1 | 8 |
| Bae, D J | USA | The American Journal of Cardiology | A propensity score‐matched analysis | 78 | 71 | 47 | 36 | 65 | 14.81 | 64 | 19.26 | 7 |
| Senkal, N | Turkey | Anatolian Journal of Cardiology | A propensity score‐matched analysis | 104 | 52 | 53 | 30 | 63 | 11.98 | 65 | 12 | 7 |
| Khera, R (Outpatient Study) | USA | medRxiv: the preprint server for health sciences | A propensity score‐matched analysis (outpatient Study) | 1453 | 810 | 796 | 331 | 68.50 | 13.35 | 71.0 | 14.81 | 8 |
| Khera, R (Inpatient Study) | USA | medRxiv: the preprint server for health sciences | A propensity score‐matched analysis (inpatient Study) | 4587 | 3346 | 2170 | 1431 | 76 | 11.11 | 78.0 | 11.85 | 8 |
| Gao, C | China | European Heart Journal | A retrospective cohort study | 183 | 527 | 104 | 266 | 62.64 | 11 | 64.84 | 11.19 | 8 |
| Zhang, P | China | Circulation Research | A propensity score‐matched analysis | 174 | 348 | 94 | 197 | 64 | 8.89 | 64 | 9.63 | 8 |
| Li, J | China | JAMA Cardiology | A single‐center retrospective cohort study | 115 | 247 | 68 | 121 | 65.0 | 11.85 | 67.0 | 11.11 | 5 |
| Jung, S Y | Korea | Clinical Infectious Diseases | A nationwide population‐based cohort study | 377 | 1577 | 8 | ||||||
| Priyank, S | USA | Journal of Hypertension | A retrospective cohort study | 207 | 324 | 87 | 131 | 64.0 | 12.4 | 57.6 | 17.8 | 7 |
| Zhou, X | China | Clinical and Experimental Hypertension | A single‐center retrospective cohort study | 15 | 21 | 9 | 10 | 58.5 | 10.1 | 69.2 | 7.5 | 6 |
| Pan, W | China | Hypertension (Dallas, Tex.: 1979) | A single‐center retrospective cohort study | 41 | 241 | 16 | 127 | 70 | 9.63 | 69 | 10.37 | 6 |
| Lam, K W | USA | The Journal of Infectious Diseases | A single‐center retrospective cohort study | 335 | 279 | 189 | 149 | 68 | 15.56 | 73 | 15.56 | 7 |
| Yang, G | China | Hypertension (Dallas, Tex.: 1979) | A single‐center retrospective cohort study | 43 | 83 | 21 | 41 | 65 | 11.11 | 67 | 9.63 | 7 |
| Zeng, Z H | China | medRxiv | A single‐center retrospective cohort study | 28 | 47 | 12 | 23 | 64 | 12 | 69 | 10 | 6 |
| Selcuk, M | Turkey | Clinical and Experimental Hypertension | A retrospective cohort study | 74 | 39 | 36 | 23 | 67 | 11 | 58 | 10 | 5 |
| Chen, C | China | Journal of the American Heart Association | A single‐center retrospective cohort study | 355 | 827 | 176 | 404 | 68 | 11.85 | 68 | 10.37 | 7 |
| Huang, Z | China | Annals of Translational Medicine | A retrospective cohort study | 20 | 30 | 10 | 17 | 52.65 | 13.12 | 67.77 | 12.84 | 5 |
| Feng, Z | China | medRxiv | A multicenter, retrospective cohort study | 16 | 49 | 10 | 23 | 57 | 10.37 | 63 | 11.85 | 8 |
| Felice, C | Italy | American Journal of Hypertension | A single‐center retrospective cohort study | 82 | 51 | 59 | 27 | 71 | 12.60 | 76.2 | 11.9 | 7 |
| Wang, Z C | China | Medical Science Monitor | A propensity score‐matched analysis | 62 | 62 | 33 | 30 | 68.5 | 12.68 | 67 | 10.74 | 7 |
| Yahyavi, A | Iran | Internal and Emergency Medicine | A retrospective cohort study | 500 | 2053 | 272 | 1226 | 66.8 | 12.3 | 55.9 | 18.4 | 6 |
| Covino, M | Italy | Internal Medicine Journal | A retrospective cohort study | 111 | 55 | 78 | 31 | 72 | 11.11 | 77 | 12.59 | 5 |
| Palazzuoli, A | Italy | Journal of the American Heart Association | A multicenter, retrospective cohort study | 304 | 477 | 193 | 305 | 72.4 | 10.4 | 66 | 14.8 | 5 |
| Negreira‐Caamano, M | Spain | High Blood Pressure & Cardiovascular Prevention | A single‐center retrospective cohort study | 392 | 153 | 206 | 77 | 75.9 | 12.1 | 78 | 12.9 | 6 |
| Lafaurie, M | France | Fundamental & Clinical Pharmacology | A retrospective cohort study | 73 | 36 | 39 | 20 | 73 | 12.59 | 77 | 13.33 | 5 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; NOS, Newcastle Ottawa Scale.
Table 2.
Characteristics of the included studies (2)
| Author | Diagnosis of COVID‐19 | Data sources | Study population | Follow‐up time | Adjustment factors |
|---|---|---|---|---|---|
| Bean, D M | Real‐time RT‐PCR | Extracted from clinical notes, outpatient clinic letters and inpatient medication orders | General population | 21 days | Age, sex, hypertension, diabetes mellitus, chronic kidney disease, ischemic heart disease, heart failure |
| Bae, D J | RT‐PCR of a nasopharyngeal swab or a bronchoalveolar lavage | Extracted from the electronic medical record and the index healthcare COVID‐19 contact (a patient's first interaction with a healthcare system to discuss COVID‐19 symptoms and testing via phone call, telemedicine visit, outpatient clinic visit, or emergency room visit was defined as the index healthcare COVID‐19 contact) | General population | Age, hypertension, dyslipidemia, diabetes/pre‐diabetes, CAD, CHF, CVA, chronic lung disease, and CKD/ESRD | |
| Senkal, N | RT‐PCR of a nasopharyngeal swab and an ultra low‐dose spiral CT of the chest | Extracted from patient charts | General population | Age, sex, sick days before hospital admission, comorbidities (diabetes mellitus, COPD/asthma, CAD, CHF, and CKD), current smoking status, number of antihypertensives used, furosemide use, doxazosin use, and serum creatinine level) | |
| Khera, R (Outpatient Study) | NA | A research database from a single large US health insurance provider | People with hypertension | Age, gender, race, insurance type, conditions, diabetes, myocardial infarction, heart failure and chronic kidney disease, each of the comorbidities in the Charlson Comorbidity Index, and the number of antihypertensive agents used for the patient | |
| Khera, R (Inpatient Study) | NA | A research database from a single large US health insurance provider | People with hypertension | Age, gender, race, insurance type, conditions, diabetes, myocardial infarction, heart failure and chronic kidney disease, each of the comorbidities in the Charlson Comorbidity Index, and the number of anti‐hypertensive agents used for the patient | |
| Gao, C | According to WHO interim guidance and diagnosis and treatment protocol for novel coronavirus pneumonia from the National Health Commission of China | Extracted from electronic medical records | General population | The final date of follow‐up was 1 April 2020 and the median duration of follow‐up (hospitalization) was 21 (12– 32) days. | |
| Zhang, P | CT manifestations and RT‐PCR according to the New Coronavirus Pneumonia Prevention and Control Program (5th edition) | Extracted from the electronic medical system, picture achieving and communication system, laboratory information system, medical history and doctor advices | People with hypertension | The final date of follow‐up was March 7, 2020 | Imbalanced variables (D‐dimer, procalcitonin, and unilateral lesion) and in‐hospital medications (antiviral drug and lipid‐lowering drug) between ACEI/ARB versus non‐ACEI/ARB groups in following mixed‐effect Cox model |
| Li, J | RT‐PCR | Extracted from electronic medical records | General population | ||
| Jung, S Y | RT‐PCR of a nasopharyngeal swab | The Korean Health Insurance Review and Assessment database | General population | All patients were followed until the first instance of death or 8 April 2020. | Age, sex, Charlson comorbidity index, immunosuppression, and hospital type |
| Priyank, S | RT‐PCR of a nasopharyngeal swab | Extracted from electronic medical records | General population | Age, sex, BMI, baseline comorbidities, and presenting illness severity | |
| Zhou, X | According to the COVID19 diagnosis and treatment program issued by the Chinese National Health Committee | Extracted from electronic medical records | People with hypertension | Age, sex, hospitalization time, time from onset to hospital admission | |
| Pan, W | According to the Diagnosis and Treatment of Novel Coronavirus Pneumonia (sixth edition) guidelines published by the National Health Commission of China | Extracted from electronic medical records | People with hypertension | The clinical outcomes were recorded until February 24, 2020. | |
| Lam, K W | RT‐PCR of a nasopharyngeal swab | Extracted from electronic medical records | People with hypertension | Age, gender, history of heart failure, chronic obstructive pulmonary disease, and asthma (comorbidities that were significantly different between groups) | |
| Yang, G | According to the guideline of SARS‐CoV‐2 (The Fifth Trial V ersion of the Chinese National Health Commission) | Extracted from electronic medical records | People with hypertension | The clinical outcomes were monitored up to March 3, 2020, the final date of follow‐up. | |
| Zeng, Z H | According to the criteria previously established by the WHO | Extracted from clinical and laboratory records | People with hypertension | Follow‐up was cutoff on March 8, 2020. | |
| Selcuk, M | RT‐PCR | Extracted from electronic medical records | People with hypertension | Age, D‐dimer, LDH | |
| Chen, C | According to symptoms, RT‐PCR of a nasopharyngeal swab and radiological findings of interstitial pneumonia on CT scan | Extracted from patients' electronic medical records | People with hypertension | The clinical follow‐up was terminated on April 24, 2020, when the last COVID‐19 patient was discharged. | |
| Huang, Z | According to the Novel Coronavirus Pneumonia Diagnosis and Treatment Guideline (5th ed.) (in Chinese) published by the National Health Commission of China | Extracted from electronic nursing and medical records | People with hypertension | ||
| Feng, Z | RT‐PCR of nasal and pharyngeal swab specimens | Extracted from electronic medical records | People with hypertension | The final date of follow‐up was March 15, 2020. | Age |
| Felice, C | RT‐PCR of a nasopharyngeal swab | Patients' demographics and clinical characteristics were collected by medical records and entered into an anonymous database | People with hypertension | Age, gender, body mass index, days with symptoms before admission, previous cardiovascular events, diabetes, and cancer | |
| Wang, Z C | RT‐PCR of a nasopharyngeal swab | Extracted from electronic medical records | People with hypertension | Age, sex, BMI, previous comorbidities, vital signs, disease severity, ion concentration, hepatic and renal function, blood cell count, CRP, and IL‐6 on the clinical outcomes | |
| Yahyavi, A | Patients diagnosed with COVID‐19 according to World Health Organization interim guidance | The data were collected from the SEPAS system, a national integrated care electronic health record system | General population | Patients were followed after discharge for at least 120 days. | |
| Covino, M | According to the WHO interim guidance | Extracted from electronic medical records | People with hypertension | ||
| Palazzuoli, A | RT‐PCR of a nasopharyngeal swab | Extracted from electronic medical records | General population | ||
| Negreira‐Caamano, M | NA | Extracted from electronic medical records | People with hypertension | The follow‐up period was measured in days from hospital admission to the date of the clinical event or to hospital discharge if no events were registered. | |
| Lafaurie, M | According to the WHO guidance | Extracted from electronic medical records | General population |
Abbreviations: CT, computed tomography; RT‐PCR, reverse transcriptase polymerase chain reaction; WHO, World Health Organization.
3.3. Meta‐analysis
3.3.1. ACEI/ARB use and the risk of mortality in COVID‐19 patients
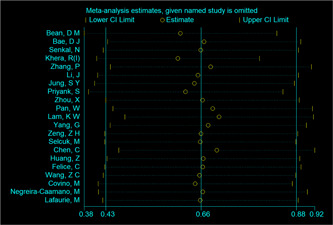
The overall analysis of mortality included 21 studies. The association between ACEI/ARB use and the risk of mortality was estimated. Overall, the risk of mortality was significantly lower in COVID‐19 patients taking ACEIs/ARBs than in those not taking ACEIs/ARBs (OR = 0.65; 95% CI: 0.46, 0.85; Figure 2). However, there was substantial heterogeneity among the studies (I 2 = 73.37%, p < .05). A subgroup analysis was performed based on whether the participants had hypertension. In the general population, the risk of mortality in patients taking ACEIs/ARBs was similar to that in patients not taking ACEIs/ARBs (OR = 0.98; 95% CI: 0.75, 1.22; I 2 = 13.62%; Figure 2). In the studies performed with patients with hypertension, the risk of mortality was significantly lower in patients taking ACEIs/ARBs than in those not taking ACEIs/ARBs (OR = 0.51; 95% CI: 0.29, 0.73; I 2 = 73.37%; Figure 2). No significant publication bias was observed (p value of the Egger's test = 0.65, Table 3). Meta‐regression analysis showed that asthma (p = .00) and cerebral vascular diseases (p = .00) have significant modulating effect of ACEIs/ARBs treatment on the mortality of COVID‐19 patients (Table 4). A single study was used to analyze the source of heterogeneity. However, no study is considered a source of heterogeneity (Appendix Figure A1).
Figure 2.
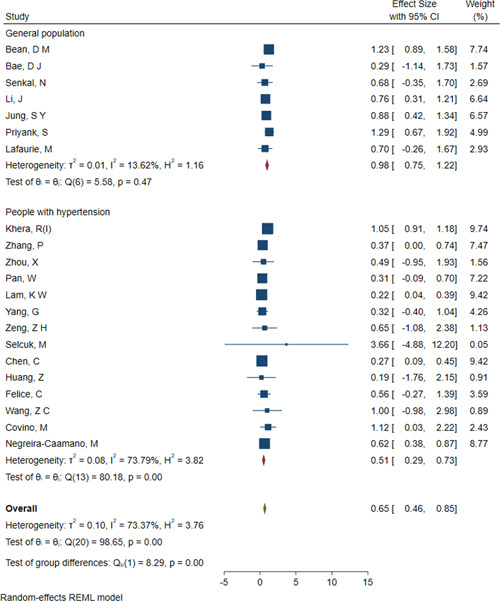
Forest plot of ACEI/ARB use and the risk of mortality in COVID‐19 patients. ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; COVID‐19, coronavirus disease 2019
Table 3.
Meta‐analysis for studies included in the analysis
| Subgroup analysis | OR/SMD | 95% CI | I 2 (%) | p (the χ 2 test) | p (the Egger's test) | p (test of group differences) | |
|---|---|---|---|---|---|---|---|
| Mortality | 0.65 | 0.46, 0.85 | 73.37 | .00 | .65 | – | |
| General population | 0.98 | 0.75, 1.22 | 13.62 | – | – | .00 | |
| People with hypertension | 0.51 | 0.29, 0.73 | 73.37 | – | – | .00 | |
| Severe disease | 0.89 | 0.63, 1.15 | 38.55 | .13 | .72 | – | |
| Severity/mortality | 0.69 | 0.43, 0.95 | 22.90 | .24 | .59 | – | |
| Hospitalization | 0.79 | 0.60, 0.98 | 0.00 | .65 | .96 | – | |
| ICU* | 0.96 | 0.56, 1.37 | 88.31 | .00 | .07 | – | |
| General population | 1.14 | 0.57, 1.71 | 89.73 | – | – | .01 | |
| People with hypertension | 0.36 | 0.19, 0.53 | 0.00 | – | – | .01 | |
| Mechanical ventilation | 0.89 | 0.61, 1.16 | 3.19 | .35 | .11 | – | |
| ARDS | 0.71 | 0.46, 0.95 | 0.00 | .54 | .90 | – | |
| Dialysis | 1.24 | 0.09, 2.39 | 0.00 | .83 | .97 | – | |
| Length of hospital stay | 0.05 | ‐0.16, 0.26 | 84.43 | .00 | .01 | – | |
| General population | 0.10 | ‐0.32, 0.53 | 93.24 | – | – | .74 | |
| People with hypertension | 0.02 | ‐0.17, 0.21 | 44.20 | – | – | .74 |
Abbreviations: ARDS, acute respiratory distress syndrome; ICU, intensive care unit; OD, odds ration.
ICU: transfer to the intensive care unit.
Table 4.
P‐value of meta‐regression for the modulators
| Age | Male | Diabetes | Coronary heart disease | Heart failure | Chronic lung disease | COPD | Asthma | Cerebral vascular diseases | Chronic liver diseases | Chronic kidney disease | Malignancy | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mortality | 0.72 | 0.53 | 1.00 | 0.64 | 0.43 | 0.72 | 0.15 | 0.00 | 0.00 | 0.70 | 0.09 | 0.47 |
| Severe disease | 0.29 | 0.25 | 0.41 | 0.48 | 0.48 | 0.08 | 0.99 | 0.38 | 0.92 | 0.64 | 0.79 | 0.83 |
| ICU | 0.01 | 0.18 | 0.21 | 0.81 | 0.63 | 0.63 | 0.55 | 0.72 | 0.34 | 0.18 | 0.32 | 0.01 |
| Length of hospital stay | 0.06 | 0.63 | 0.35 | 1.00 | 0.53 | 0.48 | 0.01 | – | 0.20 | – | 0.46 | 0.57 |
Abbreviation: COPD, chronic obstructive pulmonary disease; ICU, intensive care unit.
3.3.2. Effect of ACEI/ARB use on COVID‐19 severity
The overall assessment with the random‐effects model showed that the use of ACEIs/ARBs was not associated with an elevated risk of severe COVID‐19 (OR = 0.89; 95% CI: 0.63, 1.15; I 2 = 38.55%), mechanical ventilation (OR = 0.89; 95% CI: 0.61, 1.16; I 2 = 3.19%), transfer to the ICU (OR = 0.96; 95% CI: 0.56, 1.37; I 2 = 88.31%; Figure 3) or dialysis (OR = 1.24; 95% CI: 0.09, 2.39; I 2 = 0.00%). Except for the analysis of transfer to the ICU, the other analyses had acceptable degrees of heterogeneity. The effect estimates showed an overall protective effect of the use of ACEIs/ARBs against severity/mortality (OR = 0.69; 95% CI: 0.43, 0.95; I 2 = 22.90%) and ARDS (OR = 0.71; 95% CI: 0.46, 0.95; I 2 = 0.00%), and all the analyses had acceptable degrees of heterogeneity (Table 3). In the analysis of the risk of transfer to the ICU, significant differences were observed between subgroups. In the studies involving people with hypertension, there was a significantly lower risk of transfer to the ICU in those taking ACEIs/ARBs than in those not taking ACEIs/ARBs (OR = 0.36; 95% CI: 0.19, 0.53; I 2 = 0.00%; Figure 3 and Table 3). Meta‐regression analysis showed that age (p = .01) and malignancy (p = .01) has a significant modulating effect of ACEIs/ARBs treatment on the risk of transfer to the ICU of COVID‐19 patients (Table 4). Furthermore, meta‐regression analysis showed that all the modulators have no significant modulating effect of ACEIs/ARBs treatment on the severity of COVID‐19 patients (p > .05, Table 4).
Figure 3.
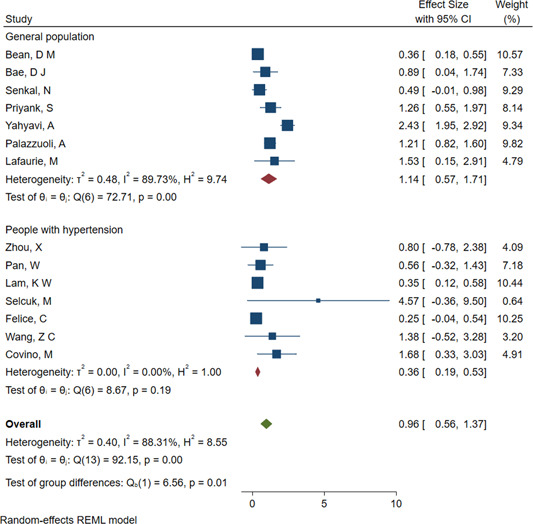
Forest plot of ACEI/ARB use and the risk of transfer to the ICU in COVID‐19 patients. ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; COVID‐19, coronavirus disease 2019; ICU, intensive care unit
3.3.3. Effect of ACEI/ARB use on the risk of hospitalization and length of hospital stay in COVID‐19 patients
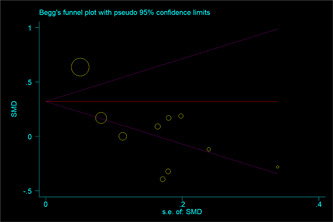
The effect estimates showed an overall protective effect of the use of ACEIs/ARBs against hospitalization (OR = 0.79; 95% CI: 0.60, 0.98; I 2 = 0.00%), with acceptable degrees of heterogeneity. The pooled analysis showed that the length of hospital stay (SMD = 0.05; 95% CI: −0.16, 0.26; I 2 = 84.43%) in COVID‐19 patients were not affected by the use of ACEIs/ARBs, although there was heterogeneity among the studies. No significant differences between subgroups were observed (Table 3). However, the analysis had a significant publication bias (Appendix Figure A2). Meta‐regression analysis showed that chronic obstructive pulmonary disease (COPD) has a significant modulating effect of ACEIs/ARBs treatment on the length of hospital stay of COVID‐19 patients (p = .01, Table 4).
4. DISCUSSION
This meta‐analysis included 25 articles that included more than 22,000 COVID‐19 patients. In summary, we presented evidence showing that there is no association between the use of RAAS inhibitors and a higher risk of severe disease, mechanical ventilation, dialysis, or the length of hospital stay. In fact, the use of ACEI/ARB therapy is associated with reduced risks of mortality, severity/mortality, hospitalization, transfer to the ICU and ARDS, especially among COVID‐19 patients with hypertension. The use of ACEIs/ARBs is associated with potentially protective effects against poor outcomes of COVID‐19.
Since the identification of the novel SARS‐CoV‐2 virus, there has been significant interest in whether the use of antihypertensives, specifically ACEIs/ARBs, increases mortality in patients infected with SARS‐CoV‐2. Clinical trials are underway to test the safety and efficacy of RAAS inhibitors in COVID‐19 patients. 36 , 37 Researchers have identified advanced age and comorbidities, such as hypertension, diabetes mellitus, and heart failure as risk factors for hospitalization in COVID‐19 patients and as negative prognostic factors. 38 Patients with these conditions are often treated with ACEIs/ARBs. Based on the fact that the ACE2 receptor allows SARS‐CoV‐2 to enter cells, 39 some initial publications suggested that the use of ACEIs and ARBs could be a potential risk factor for mortality due to COVID‐19. 40 , 41 But a recent study by Lee et al. 42 found that ACE2 localizes to the respiratory cilia and is not increased by ACEIs or ARBs. Despite the theoretical association with the increased risk of SARS‐CoV‐2 infection, there is currently no evidence to support a causal relationship between ACE2 upregulation and COVID‐19‐associated mortality. Furthermore, ACE2 expression may not be correlated with the severity of the disease.
ACEIs and ARBs are the cornerstone of a prognostically beneficial heart failure therapy with the highest level of evidence with regard to the reduction in mortality. 43 These drugs all have in common the inhibition of the adverse cardiovascular effects arising from the interaction of angiotensin II with angiotensin II receptor type 1. Discontinuation of heart failure therapy leads to the deterioration of cardiac function and heart failure within days to weeks, with a possible consequent increase in mortality. 44 Mortality due to COVID‐19 appears to be driven by the development of acute lung injury and ARDS due to a cytokine storm. Previous work has suggested a significant role of the RAAS in the development of ARDS. ARDS may be linked to enhancements of the vasoconstrictive, fibroproliferative, and proinflammatory effects of the ACE/angiotensin II pathway and reductions in the vasodilatory, anti‐inflammatory, and antifibrotic effects of the ACE2/angiotensin 1–7 pathway. 45 Conversely, a recent study showed that candesartan could ameliorate the COVID‐19 cytokine storm. 46 A series of observational studies have provided valuable insights into the question of whether ACEI/ARB therapy influences the risk of contracting COVID‐19 or experiencing an adverse outcome. Our meta‐analysis of these observational cohort studies showed that ACEI/ARB therapy was associated with reduced risks of mortality, severity/mortality, hospitalization, transfer to the ICU and ARDS, especially among COVID‐19 patients with hypertension. Several studies 11 , 12 , 13 , 15 , 17 , 18 , 19 , 31 , 33 , 35 included patients without hypertension or CVD in the non‐ACEI/ARB group, which may have led to an underestimation of the protective effect of ACEI/ARB use against adverse outcomes in COVID‐19 patients. Consistent with our conclusion, national/international scientific societies (e.g., the European Society of Cardiology, 47 Italian Society of Pharmacology, 48 Heart Failure Society of America, 49 and International Society of Hypertension 50 ) recommend that patients do not discontinue treatment with ACEIs or ARBs and that there is no need to switch to other medicines.
Most indicators in the meta‐analysis show good homogeneity between studies. Nonetheless, there was heterogeneity among the studies included in the meta‐analysis for three indicators (mortality, transfer to the ICU, and the length of hospital stay). To reduce the heterogeneity between the studies, we only included studies in which the subjects were outpatients or inpatients. A subgroup analysis was performed based on whether the participants had hypertension, and significant differences in mortality and transfer to the ICU were observed between the subgroups. A significant publication bias was observed in the length of hospital stay. Meta‐regression analysis showed that asthma, age, malignancy, COPD, and cerebral vascular diseases have a significant modulating effect of ACEIs/ARBs treatment on mortality, the risk of transfer to the ICU, and the length of hospital stay of COVID‐19 patients. Furthermore, the characteristics of the studies (e.g., methodological differences in the study design and variables used for adjustment), or even differences in recruitment, the timing of outcome measurements and the population (such as unknown environmental factors and/or underlying comorbidities), were certainly very important variables that may explain the heterogeneity of the data set as a whole. Another limitation is that the measurement of ACEI/ARB exposure was through medical record review or prescription, which is less reliable than other methods. Third, the definitions of COVID‐19 severity and outcomes were inconsistent among the included studies.
In conclusion, the use of an RAAS inhibitor was not associated with the risk of severe disease, mechanical ventilation, dialysis, or the length of hospital stay. However, ACEI/ARB use was associated with reduced risks of mortality, severity/mortality, hospitalization, transfer to the ICU among COVID‐19 patients with hypertension and ARDS. Our study supports the current guidelines that discourage the discontinuation of RAAS inhibitors in COVID‐19 patients. Prospective cohort studies with methodologically sound matching/adjustment of the analysis or randomized controlled trials are needed before a definite conclusion can be drawn.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Figure A1.
The single study of ACEI/ARB use and the risk of mortality in COVID‐19 patients. ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; COVID‐19, coronavirus disease 2019
Figure A2.
The result of Begg's test of ACEI/ARB use and the length of hospital stay in COVID‐19 patients. ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; COVID‐19, coronavirus disease 2019
Zhang G, Wu Y, Xu R, Du X. Effects of renin‐angiotensin‐aldosterone system inhibitors on disease severity and mortality in patients with COVID‐19: A meta‐analysis. J Med Virol. 2021;93:2287–2300. 10.1002/jmv.26695
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Gurwitz D. Angiotensin receptor blockers as tentative SARS‐CoV‐2 therapeutics. Drug Dev Res. 2020;81(5):537‐540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang P, Zhu L, Cai J, et al. Association of inpatient use of angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID‐19. Circ Res. 2020;126(12):1671‐1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sungnak W, Huang N, Bécavin C, et al. SARS‐CoV‐2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nature Med. 2020;26(5):681‐687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arendse LB, Danser AHJ, Poglitsch M, et al. Novel therapeutic approaches targeting the renin‐angiotensin system and associated peptides in hypertension and heart failure. Pharmacol Rev. 2019;71(4):539‐570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin‐converting enzyme. Cloning and functional expression as a captopril‐insensitive carboxypeptidase. J Biol Chem. 2000;275(43):33238‐43. [DOI] [PubMed] [Google Scholar]
- 6. Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single‐cell RNA‐seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019‐nCoV infection. Front Med. 2020;14(2):185‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li W, Moore MJ, Vasilieva N, et al. Angiotensin‐converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus‐induced lung injury. Nat Med. 2005;11(8):875‐879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol. 2010;25(9):603‐605. [DOI] [PubMed] [Google Scholar]
- 11. Bae DJ, Tehrani DM, Rabadia SV, et al. Angiotensin converting enzyme inhibitor and angiotensin II receptor blocker use among outpatients diagnosed with COVID‐19. Am J Cardiol. 2020;132:150‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bean DM, Kraljevic Z, Searle T, et al. Angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers are not associated with severe COVID‐19 infection in a multi‐site UK acute hospital trust. Eur J Heart Fail. 2020;22(6):967‐974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Senkal N, et al. Association between chronic ACE inhibitor exposure and decreased odds of severe disease in patients with COVID‐19. Anatol J Cardiol. 2020;24(1):21‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khera R, Clark C, Lu Y, et al. Association of angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers with the risk of hospitalization and death in hypertensive patients with coronavirus disease‐19 [published online ahead of print May 19, 2020]. medRxiv. 10.1101/2020.05.17.20104943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gao C, Cai Y, Zhang K, et al. Association of hypertension and antihypertensive treatment with COVID‐19 mortality: a retrospective observational study. Eur Heart J. 2020;41(22):2058‐2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang P, Zhu L, Cai J, et al. Association of inpatient use of angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID‐19. Circ Res. 2020;126(12):1671‐1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li J, Wang X, Chen J, Zhang H, Deng A. Association of renin‐angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID‐19) infection in Wuhan, China. JAMA Cardiol. 2020;5(7):825‐830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jung S, Choi JC, You S‐H, et al. Association of renin‐angiotensin‐aldosterone system inhibitors with COVID‐19‐related outcomes in Korea: a nationwide population‐based cohort study. Clin Infect Dis. 2020;71(16):2121‐2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shah P, Owens J, Franklin J, Jani Y, Kumar A, Doshi R. Baseline use of angiotensin‐converting enzyme inhibitor/AT1 blocker and outcomes in hospitalized coronavirus disease 2019 African‐American patients. J Hypertens. 2020;38(12):2537‐2541. [DOI] [PubMed] [Google Scholar]
- 20. Zhou X, Zhu J, Xu T. Clinical characteristics of coronavirus disease 2019 (COVID‐19) patients with hypertension on renin‐angiotensin system inhibitors. Clin Exp Hypertens. 2020;42(7):656‐660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pan W, Zhang J, Wang M, et al. Clinical features of COVID‐19 in patients with essential hypertension and the impacts of renin‐angiotensin‐aldosterone system inhibitors on the prognosis of COVID‐19 patients. Hypertension (Dallas, Tex.: 1979). 2020;76(3):732‐741. [DOI] [PubMed] [Google Scholar]
- 22. Lam KW, Chow KW, Vo J, et al. Continued in‐hospital ACE inhibitor and ARB Use in hypertensive COVID‐19 patients is associated with positive clinical outcomes. J Infect Dis. 2020;222(8):1256‐1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang G, Tan Z, Zhou L, et al. Effects of angiotensin II receptor blockers and ACE (angiotensin‐converting enzyme) inhibitors on virus infection, inflammatory status, and clinical outcomes in patients with COVID‐19 and hypertension: a single‐center retrospective study. Hypertension (Dallas, Tex.: 1979). 2020;76(1):51‐58. [DOI] [PubMed] [Google Scholar]
- 24. Zeng Z, Sha T, Zhang Y, et al. Hypertension in patients hospitalized with COVID‐19 in Wuhan China: a single‐center retrospective observational study [published online ahead of print April 11, 2020]. medRxiv. 10.1101/2020.04.06.20054825 [DOI] [Google Scholar]
- 25. Selçuk M, Çınar T, Keskin M, et al. Is the use of ACE inb/ARBs associated with higher in‐hospital mortality in Covid‐19 pneumonia patients? Clin Exp Hyperten (New York, N.Y.: 1993). 2020;42(8):738‐742. [DOI] [PubMed] [Google Scholar]
- 26. Chen C, Wang F, Chen P, et al. Mortality and pre‐hospitalization use of renin‐angiotensin system inhibitors in hypertensive COVID‐19 patients [published online ahead of print August 18, 2020]. J Am Heart Assoc. 9:e017736. 10.1101/2020.04.08.20057539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huang Z, Cao J, Yao Y, et al. The effect of RAS blockers on the clinical characteristics of COVID‐19 patients with hypertension. Ann Transl Med. 2020;8:4307‐1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Feng Z, Li J, Yao S, et al. The use of adjuvant therapy in preventing progression to severe pneumonia in patients with coronavirus disease 2019: a multicenter data analysis [published online ahead of print April 10, 2020]. medRxiv. [Google Scholar]
- 29. Felice C, Nardin C, Di Tanna GL, et al. Use of RAAS inhibitors and risk of clinical deterioration in COVID‐19: results from an Italian cohort of 133 hypertensives. Am J Hypertens. 2020;33(10):944‐948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang Z, Zhang D, Wang S, et al. A retrospective study from 2 centers in China on the effects of continued use of angiotensin‐converting enzyme inhibitors and angiotensin ii receptor blockers in patients with hypertension and COVID‐19. Med Sci Monit. 2020;26:e926651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yahyavi A, Hemmati N, Derakhshan P, et al. Angiotensin enzyme inhibitors and angiotensin receptor blockers as protective factors in COVID‐19 mortality: a retrospective cohort study [published online ahead of print October 21, 2020]. Intern Emerg Med. 1‐11. 10.1007/s11739-020-02523-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Covino M, De Matteis G, Burzo ML, et al. Angiotensin‐converting enzyme inhibitors or angiotensin Ii receptor blockers and prognosis of hypertensive patients hospitalized with COVID‐19 [published online ahead of print October 6, 2020]. Internal Med J. 10.1111/imj.15078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Palazzuoli A, Mancone M, De Ferrari GM, et al. Antecedent administration of angiotensin converting enzyme inhibitors or angiotensin II receptor antagonists and survival after hospitalization for SARS‐CoV‐2 (COVID‐19). J Am Heart Assoc. 2020;9:22 e017364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Negreira‐Caamaño M, Piqueras‐Flores J, Martínez‐DelRi J, et al. Impact of treatment with renin‐angiotensin system inhibitors on clinical outcomes in hypertensive patients hospitalized with COVID‐19. High Blood Press Cardiovasc Prev. 2020;27(6):561‐568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lafaurie M, Martin‐Blondel G, Delobel P, Charpentier S, Sommet A, Moulis G. Outcome of patients hospitalized for COVID‐19 and exposure to angiotensin‐converting enzyme inhibitors and angiotensin‐receptor blockers in France: results of the ACE‐CoV study [published online ahead of print October 28, 2020]. Fundamental Clin Pharmacol. 10.1111/fcp.12613 [DOI] [PubMed] [Google Scholar]
- 36. Danser AHJ, Epstein M, Batlle D. Renin‐angiotensin system blockers and the COVID‐19 pandemic: at present there is no evidence to abandon renin‐angiotensin system blockers. Hypertension (Dallas, Tex.: 1979). 2020;75:1382‐1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin‐angiotensin‐aldosterone system inhibitors in patients with COVID‐19. N Engl J Med. 2020;382(17):1653‐1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239‐1242. [DOI] [PubMed] [Google Scholar]
- 39. Zheng YY, Ma YT, Zhang JY, Xie X. COVID‐19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID‐19 infection? Lancet Respir Med. 2020;8(4):e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sommerstein R, Kochen MM, Messerli FH, Gräni C, et al. Coronavirus disease 2019 (COVID‐19): do angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers have a biphasic effect? J Am Heart Assoc. 2020;9(7):e016509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lee IT, Nakayama T, Wu CT, et al. ACE2 localizes to the respiratory cilia and is not increased by ACE inhibitors or ARBs. Nat Commun. 2020;11(1):5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18(8):891‐975. [DOI] [PubMed] [Google Scholar]
- 44. Halliday BP, Wassall R, Lota AS, et al. Withdrawal of pharmacological treatment for heart failure in patients with recovered dilated cardiomyopathy (TRED‐HF): an open‐label, pilot, randomised trial. Lancet (London, England). 2019;393(10166):61‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Skurk T, van Harmelen V, Hauner H. Angiotensin II stimulates the release of interleukin‐6 and interleukin‐8 from cultured human adipocytes by activation of NF‐kappaB. Arterioscler Thromb Vasc Biol. 2004;24(7):1199‐203. [DOI] [PubMed] [Google Scholar]
- 46. Elkahloun AG, Saavedra JM. Candesartan could ameliorate the COVID‐19 cytokine storm. Biomed Pharmacother. 2020;131:110653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.European Societies of Cardiology. Position Statement of the ESC Council on Hypertension on ACE‐Inhibitors and Angiotensin Receptor Blockers. https://www.escardio.org/Councils/Council-onHypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-andang. Accessed April 16, 2020.
- 48.Italian Society of Pharmacology. Documento Informativo della Società Italiana di Farmacologia. Uso di Ace‐Inibitori/Sartani ed infezione da COVID‐19. https://www.sifweb.org/documenti/document_2020-03-13_documento-informativo-della-societa-italiana-di-farmacologia-uso-di-ace-inibitori-sartani-ed-infezione-da-covid-19. Accessed March 13, 2020.
- 49.HFSA/ACC/AHA statement addresses concerns Re: using RAAS antagonists in COVID‐19. https://www.acc.org/latest-in-cardiology/articles/2020/03/17/08/59/hfsa-acc-aha-statement-addressesconcerns-re-using-raas-antagonists-in-covid-19. Accessed April 16, 2020. [DOI] [PMC free article] [PubMed]
- 50.International Society of Hypertension. A statement from the International Society of Hypertension on COVID‐19 | The International Society of Hypertension. https://ish-world.com/news/a/A-statement-from-the-International-Society-of-Hypertension-on-COVID-19/. Accessed March 16, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


