Abstract
Aim
(1) To systematically review the literature on developmental outcomes from infancy to adolescence of children with complex congenital heart disease (CHD) who underwent early surgery; (2) to run a meta‐regression analysis on the Bayley Scales of Infant Development, Second Edition Mental Developmental Index and Psychomotor Developmental Index (PDI) of infants up to 24 months and IQs of preschool‐aged children to adolescents; (3) to assess associations between perioperative risk factors and outcomes.
Method
We searched pertinent literature (January 1990 to January 2019) in PubMed, Embase, CINAHL, and PsycINFO. Selection criteria included infants with complex CHD who had primary surgery within the first 9 weeks of life. Methodological quality, including risk of bias and internal validity, were assessed.
Results
In total, 185 papers met the inclusion criteria; the 100 with high to moderate methodological quality were analysed in detail. Substantial heterogeneity in the group with CHD and in methodology existed. The outcome of infants with single‐ventricle CHD was inferior to those with two‐ventricle CHD (respectively: average scores for PDI 77 and 88; intelligence scores 92 and 98). Perioperative risk factors were inconsistently associated with developmental outcomes.
Interpretation
The literature on children undergoing surgery in early infancy suggests that infants with a single ventricle are at highest risk of adverse developmental outcomes.
Short abstract
This article is commented on by Ilardi et al. on pages 8‐9 of this issue.
Abbreviations
- BSID‐II
Bayley Scales of Infant Development, Second Edition
- BSID‐III
Bayley Scales of Infant and Toddler Development, Third Edition
- CHD
Congenital heart disease
- HLHS
Hypoplastic left heart syndrome
- MDI
Mental Developmental Index
- PDI
Psychomotor Developmental Index
- STAT
Society of Thoracic Surgeons and the European Association for Cardio‐thoracic Surgery Congenital Heart Surgery
- TGA
Transposition of the great arteries
What this paper adds.
Children with complex congenital heart disease (CHD) are at increased risk of impaired developmental outcome.
Children with single‐ventricle CHD have worse outcomes than children with two‐ventricle CHD.
Children with two‐ventricle CHD gradually grow out of their initial developmental impairment.
Perioperative factors are inconsistently associated with outcome.
Congenital heart disease (CHD) is the most common birth defect worldwide, affecting millions of newborns every year.1 The mean prevalence of CHD between 1970 and 2017 globally was 8.22 per 1000. During this period, the overall prevalence of CHD globally increased by 10% every 5 years.1 Owing in part to earlier detection, the reported prevalence of ‘mild lesions’ such as ventricular septal defect, atrial septal defect, or patent ductus arteriosus increased almost threefold, whereas the prevalence of severe disorders, including hypoplastic left heart syndrome (HLHS), decreased.1 With advances in diagnostic technologies, surgical management, and postoperative care, over 90% of children with critical or complex CHD are expected to survive to adulthood in the current era. The significant success of these advances has exposed a heightened risk of brain injury and developmental disorders and disabilities.2 Multiple neuroimaging studies have demonstrated that children with CHD are at increased risk of congenital and acquired brain lesions,3, 4, 5, 6 and delayed neural maturation,7, 8 which both may originate in utero.8, 9
As survival has increased over the past few decades, it has allowed clinicians to document long‐term developmental follow‐up of these children. A growing body of literature indicates that outcomes of infants with complex CHD, namely children with HLHS, other single‐ventricle pathology, or d‐transposition of the great arteries (TGA), has shifted from mortality to developmental disability. Developmental outcomes of survivors of complex CHD are dependent upon a multitude of disease‐specific, treatment‐related, and individual patient‐specific factors that are interrelated and cumulative.2 On the one hand, follow‐up of survivors of complex CHD indicates that these infants generally develop reasonably well; but on the other hand, most studies also suggest that developmental outcome of these children is somewhat less favorable than that of children without complex CHD.
To improve insight into developmental outcome of infants with complex CHD, we performed a systematic review and meta‐analysis. Previously, Karsdorp et al.10 reviewed psychological and cognitive outcomes in children with mild to severe CHD reported in the literature up to 2005, and Snookes et al.11 reviewed motor and cognitive development in infants undergoing cardiac surgery during the first 6 months of life reported in the literature up to 2008. We focused on surviving children with complex CHD who underwent surgery within the first 9 weeks of life – as these are the children with the most complex forms of CHD – and on developmental outcomes across the continuum of life from infancy to adolescence. We addressed the following questions. (1) Do survivors with complex CHD have worse motor, cognitive, language, and behavioral outcomes than children without CHD? We hypothesized that motor, cognitive, language, and behavioral outcomes of children with complex CHD are worse than that of children without CHD. (2) Does the period in which the infants had their primary surgery, for example the 1980s and 1990s or the early part of the current century, affect developmental outcomes? We hypothesized that developmental outcomes of infants who had surgery in the 1980s and 1990s, when surgical techniques were less advanced, are less favorable than developmental outcomes of infants who had surgery more recently. (3) Do developmental outcomes of children who had surgery in early infancy for single‐ventricle CHD differ from those of children who had surgery in early infancy for two‐ventricle CHD? We hypothesized that children with single‐ventricle CHD have less favorable developmental outcomes than children with two‐ventricle CHD. (4) Which perioperative risk factors put children with complex CHD who have surgery in early infancy at risk of unfavorable developmental outcomes? We hypothesized that (a) the degree of preoperative and/or postoperative hypoxemia, (b) the application and duration of cerebral protection procedures, such as deep hypothermic cardiac arrest or regional low‐flow perfusion during cardiopulmonary bypass, and (c) two parameters of severity of clinical illness in the phase of primary surgery, namely length of mechanical ventilation and length of hospitalization, are all associated with worse developmental outcome.
METHOD
Search strategy
We searched the literature for studies on developmental outcomes of children born with CHD who underwent complete or staged surgery within the first 9 weeks of life. Electronic databases searched were PubMed, EMBASE, CINAHL, and PsycINFO to identify studies published from January 1990 to January 2019. Each database was searched with combinations of terms and medical subject headings adapted to the specific database filter. We also searched the reference list of original articles for additional relevant articles. The search consisted of many variations of the combination of terms describing (1) CHD, (2) surgery, (3) age, and (4) development (Appendix S1, online supporting information).
We included all randomized controlled trials and prospective cohort observational studies that addressed developmental outcomes in gross and/or fine motor development, cognition, speech and language development, and behavior in children and adolescents (newborn to 19y) with CHD that required surgery within the first 9 weeks of life. We excluded studies that evaluated: (1) children with CHD that required primary surgery after 9 weeks of life; (2) participants who received a heart transplant or extracorporeal membrane oxygenation; (3) studies that excluded HLHS in the sample; (4) groups where more than 25% of individuals were infants born preterm with low birthweight or extreme low birthweight; (5) outcomes only dealing with quality of life, stress, social–emotional measures, health‐related outcomes, or excluding motor outcomes specifically. In addition, we excluded (6) reviews, surveys, case reports or case studies, editorials, anecdotal letters, and papers with a non‐peer‐reviewed source, and (7) papers not published in English. The review protocol was registered in the International Prospective Register of Systematic Reviews, PROSPERO (identification number CRD42017051226).
Evaluation procedure
The first author and a medical information specialist from the medical library at the University Medical Center Groningen, the Netherlands, developed the search string and adapted it to specific database filters. One person (DH) searched the databases and retrieved full texts of the studies that met the inclusion criteria and, when necessary for clarity about inclusion, consulted another author (MH‐A). Two reviewers (DH and MH‐A) read the papers independently and summarized the findings on data extraction forms to assess internal validity and risk bias. Disagreements or discrepancies in scoring were discussed until a consensus was reached with the involvement of a third reviewer (AVB) if necessary. The consensus is reported in Tables [Link], [Link], [Link], [Link] (online supporting information).
Each critical review included geographical region in which the study was performed, surgical period, age of the participant at the time of developmental testing, and disposition of the participant group based on type of heart lesion at birth and subsequent type of surgery, STAT scores (severity score of the Society of Thoracic Surgeons and the European Association for Cardio‐thoracic Surgery Congenital Heart Surgery mortality score and the concomitant congenital heart surgery mortality categories),12, 13 whether the participant was born with single‐ or two‐ventricle physiology, and the mortality rate in the study groups. We also noted whether studies took the following confounding factors into account: genetic defects, preterm birth, neurological abnormalities, or heart transplant.
Study quality and publication bias (‘small study bias’) were assessed. The procedures to assess internal validity and risk of bias were in line with the Patients, Interventions, Comparators, Outcomes (PICO) approach as emphasized in Preferred Reporting Items of Systematic Reviews and Meta‐Analysis Protocols (PRISMA‐P).14 The quality appraisal began with an assessment of inclusion and exclusion criteria. Next, we analysed: (1) the studies’ internal validity and (2) methodological quality based on the criteria of Mallen et al.15 Internal validity scores were calculated on the basis of study design (prospective design: 1 credit); presence of a typically developing comparison group without a CHD or statistics comparing the study sample with the norm reference group (present: 1 credit); number of eligible participants after those excluded on the basis of relevant criteria (present: 1 credit); attrition before enrollment for not being consented or parent refusal (≤15%: 1 credit); attrition between enrollment and the time of developmental testing (<15%: 2 credits, 16–30%: 1 credit); assessors masked to the presence of a CHD diagnosis (present: 1 credit); and validity of tests used to evaluate development (valid tests: 1 credit). Internal validity was based on the sum of the credits; 8 to 6 points denoted high validity, 5 to 4 points moderate, and less than 4 points low.
The Mallen score was used to evaluate methodological quality and risk of bias in more detail; this score is designed to assess observational studies.15 We selected 14 of the 30 Mallen criteria as they met the specific needs of this review. Each item had a dichotomous score (‘yes’ for criterion present and ‘no’ for criterion not present). The number of items fulfilling each criterion were added and resulted in the Mallen score. On the basis of face value and their distribution, the Mallen scores were interpreted as follows: 12 to 14 points denoted a high methodological quality, 8 to 11 points moderate, and <8 points low.
Developmental outcome data were summarized into four separate tables by age group: infant (birth to ≤24mo), preschool (25mo–≤4y 6mo), school age (4y 7mo–≤12y), and adolescent (13y–≤19y). Each study was ranked according to its internal validity and Mallen scores, and summarized by type of heart lesion, age of the child at the time of testing, neurological examination and test, and developmental outcomes. The outcomes were organized as the domain of development, namely motor, cognition, speech/language, and behavioral (including activities of daily life), test used, reported means and standard deviations, and a summary of the results.
Meta‐analysis and evaluation of associations with risk factors
To combine results from different studies into summarized statistics, we performed a meta‐regression analysis. From all developmental outcomes described, only the Bayley Psychomotor Developmental Index (PDI) and Mental Developmental Index (MDI; infant group), and the full‐scale IQ, verbal IQ, and performance IQ (preschool, school age, and adolescent combined group), were reported sufficiently to allow for meta‐analysis. Only studies that reported means and standard deviations were included. If these data were lacking in the paper, authors were contacted for missing data. When studies generated more than one paper in a certain age period (e.g. three papers on outcome in infancy), only the paper that reported outcome at the oldest age was included in the meta‐analysis.
We focused on the effects of: (1) surgical period, (2) age, and (3) differences in outcomes between children with a single‐ or two‐ventricle CHD. For this, we related the a priori chosen study level characteristics ‘age’ and ‘surgical period effect’ of the studies to the outcome variables of interest. Age in infancy was coded in months and in the older children was coded in years. Surgical period was coded as the median year of the period during which the cohort had their primary surgery. This a priori choice allowed us to investigate whether these characteristics explained any of the heterogeneity of the outcome estimates between studies. As we still expected residual heterogeneity to remain, we chose to perform a random‐effects meta‐regression and used restricted maximum likelihood estimation, hence estimating the mean of a distribution of effects across studies. Residual heterogeneity was expressed as I 2, the percentage of total variability due to heterogeneity across studies (with >75% denoting high heterogeneity) and as τ 2, denoting the estimated amount of total heterogeneity among the true effects (i.e. the variability among the true effects that is not accounted for by the moderators included in the model). To evaluate the effect of ventricular group (single‐ vs two‐ventricle CHD), we expanded the meta‐regression model with the dichotomous moderator ventricular group. For this, we used only the data provided by the studies separately for children with single‐ or two‐ventricle CHD.
The meta‐regression analyses of the infant group also took into consideration different editions of the Bayley Scales of Infant Development, Second Edition (BSID‐II) and Bayley Scales of Infant and Toddler Development, Third Edition (BSID‐III). We used the algorithm from Jary et al.16 to convert the BSID‐III scores to the BSID‐II scores to compare the PDI and MDI scores across studies. All analyses were performed with the metafor library in R version 3.3.2 (31st October 2016; R Foundation for Statistical Computing, Vienna, Austria); p≤0.05 was considered statistically significant. We used funnel plots and Egger’s linear regression test to assess the presence of publication bias for the infant data set PDI, MDI; preschool, school age, and adolescent IQs; and mortality. Authors of various studies were contacted to request information for missing data items as necessary.
In the analysis of the association between cardiovascular risk factors and developmental outcome we categorized studies as either two‐ventricle studies, indicating that most children had two‐ventricle CHD, or single‐ventricle studies, implying that most children had single‐ventricle CHD.
Finally, to see whether potential changes in morbidity over the years were associated with changes in mortality over time, we performed a meta‐regression analysis on the mortality data reported in the studies included in the review. As not all studies included followed their cohorts into adolescence, we used only the mortality data from the infant set of papers for this meta‐regression analysis, modelling the log of odds of the reported mortality proportions. Specific attention was paid to the effect of the type of cardiac lesion (single‐ventricle vs two‐ventricle).
RESULTS
Study selection
Figure S1 (online supporting information) shows the selection of papers. The database searches yielded 5291 papers, of which 4816 were excluded on the basis of duplication and screening the title or abstract. We assessed the full text of the remaining 475 papers. We excluded 290 papers as most did not meet the inclusion criteria of age at primary surgery before 9 weeks of life or did not report developmental outcomes. The remaining 185 papers were reviewed in detail and categorized according to age. We analysed the studies’ methodological quality by assessing internal validity and the Mallen score.
Methodological quality
The Mallen scores of the 185 included papers ranged from 3 to 13. The cut‐off for low Mallen quality was set at not more than 7, a score that was assigned to 46 papers (25%); the cut‐off for high Mallen quality was set to at least 12, with 16 papers (9%) meeting this criterion. One hundred papers (54%) met the criteria for overall high or moderate methodological quality (infant 45 of 75; preschool age 18 of 32, school age 31 of 59, and adolescent 6 of 19). The other 85 papers had a low methodological quality and were excluded from further analysis (Tables [Link], [Link], [Link], [Link], [Link], [Link], [Link], [Link], online supporting information). The reasons attributing to low methodological quality most frequently were ‘absence of a control group’ (134 of 185 [72%]), ‘lack of an evaluator blind to the presence of a cardiac defect’ (176 of 185 [95%]), ‘lack of a reliable assessment of disease state’ (170 of 185 [92%]), and attrition. The last three factors were common sources of risk of bias. The absence of information on the number of eligible infants – and therewith on selection bias – and a relatively high attrition often reduced internal validity and explained much of the difference between high, moderate, and low methodological quality. In 80 of 185 (43%) papers, no information on selection bias at enrollment was present; and 84 of 185 (45%) reported attrition at the time of developmental testing was at least 30%. The methodological quality criteria most often met were ‘prospective study design’ (138 out of 185 [74%]), ‘validity of developmental test’ (100%), and ‘adjustment for confounding’ (128 of 185 [69%]), with the last two reducing the risk of bias. The papers with the highest methodological quality (30 of 185 [16%]) had the lowest selection bias, attrition no more than 15%, adjusted for confounding, and had either ‘developmental evaluator blind to the presence of cardiac diagnosis’ or ‘presence of a control group with CHD’.
Study characteristics and developmental outcome
The surgical era in this review was 1981 to 2015. Thus, the era spanned the period ranging from the earliest phases of the arterial switch operation and palliated surgeries for HLHS to recent times with the advancement of these techniques. Heart transplantation was mentioned from 2000 onwards. Most studies (138 out of 185 [75%]) excluded children with chromosomal abnormalities.
The characteristics and developmental outcome measures of the 100 papers with moderate to high methodological quality are summarized in four age‐specific tables, rank ordered on the basis of methodological quality (Tables [Link], [Link], [Link], [Link], [Link], [Link], [Link], [Link]). The 100 papers included 15 studies that generated 58 papers, some of which were longitudinal, and 42 individual studies. Two investigator groups, one from Boston, USA,17 the other from Aachen, Germany,18 tracked cohorts of children with d‐TGA from birth to 16 years of age and from 3 years to 21 years of age respectively. These studies represented the longest follow‐up and both cohorts of children had primary surgery between the mid‐1980s and the early 1990s. Thirty‐five of the 100 papers compared developmental outcomes in children with CHD with typically developing groups or the general population, whereas the remaining 65 papers addressed the association between specific variables, such as the complexity of the cardiac lesion, surgical approach, perfusion methods to support vital organs during surgery, sedation and anesthetic medications, perioperative seizures, neuroimaging, and developmental outcome. Many studies focused on factors associated with developmental outcome within the group of children with CHD and did not focus on developmental outcome of children with CHD compared with the general population.
Mortality
We addressed mortality during the surgical periods reported in 30 papers of the infant set (1983–2015) to determine: (1) the temporal trend of mortality and (2) whether a difference in mortality existed between children with single‐ and two‐ventricle CHD. In 23 of the 30 studies, surgery was performed in the years 2000 to 2009; only four studies included surgeries performed from 1980 to 1999.
The meta‐regression analysis showed that overall mortality did not change over the years (estimated log odds −1.90 [95% confidence interval {CI} −2.23 to −1.57]). Back‐transformation indicated that mortality had an estimated median rate of 13.0% (95% CI 9.7–17.2). The meta‐regression model for the smaller number of studies (n=16) that reported mortality in children with single‐ and two‐ventricle disorders separately indicated that the type of cardiac lesion was a significant moderator (p<0.001). The model showed that mortality in infants with single‐ventricle CHD was 25.2% (95% CI 14.2–40.6) compared with 5.8% (95% CI 2.9–11.2) mortality in infants with two‐ventricle CHD.
Infancy: birth to 2 years
The 45 papers in the infant set represented nearly half of the papers included in the review. Eighteen of these papers were rated with high internal validity (four with a score of 7, 12 with a score of 6); the remaining 27 papers were rated as moderate internal validity (12 with a score of 5; 15 with a score of 4). The Mallen score ranged from 13 to 7. Individual papers represented infants from North America (33 papers), Australia and New Zealand (seven papers), and Europe (five papers). White children were the most represented ethnicity (Tables S1 and S5). Within the set of 45 papers, four papers were derived from one study,19, 20, 21, 22 10 papers belonged to three studies, one study17 produced four papers,17, 23, 24, 25 two studies26, 27produced three papers each,26, 27, 28, 29, 30, 31 and eight papers were the result of four studies32, 34, 35, 36, 38 that generated two papers each.32, 33, 34, 35, 36, 37, 38, 39 Group size ranged from 11 to 359 children (median 54).
The surgical era of the infants evaluated in the 45 papers was 1983 to 2015. All papers studied infants with CHD with STAT categories 3 to 5 at the primary surgery (5 corresponding to the most complex surgeries). Twelve papers addressed outcomes of infants born with single‐ventricle physiology, mostly HLHS or less frequently with other structural cardiac abnormalities associated with a single‐ventricle physiology.40 Eleven papers focused on outcomes of infants born with two‐ventricle defects, including TGA, total anomalous pulmonary venous connection, truncus arteriosus, and interrupted aortic arch (all forms of complex CHD). TGA was the most frequent two‐ventricle defect evaluated. We excluded infants with tetralogy of Fallot in the developmental results analyses, as not all of these infants met the surgical age criteria for this review. The remaining 22 papers addressed a mixed sample of infants with either single‐ or two‐ventricle defects.
The infants’ ages at the time of developmental testing varied: between newborn and 6 months (seven papers), 7 to 12 months (14 papers), 13 to 17 months (three papers), and 18 to 24 months (26 papers). Four of the last group of papers also included older children, hence these papers return in the preschool‐age section. Most papers in the 18‐ to 24‐month range were from the Western Canada Registry (16 of 26 [62%]).
Motor development and cognitive development were assessed most frequently (40 and 37 papers respectively), with language development a close third. Various forms of behavioral outcomes were assessed. BSID‐II and BSID‐III were used as the primary instruments to measure motor, cognition, and language development in 38 of the 45 papers (84%). Table S5 outlines all the other developmental measures used.
Meta‐analysis of Bayley PDI and MDI
Twenty‐one papers generated 31 data entries that could be included in the meta‐analysis of motor and cognitive outcomes measured by an edition of the Bayley scales. Some papers generated more than one outcome; for example, Garcia Guerra et al.41 evaluated half of their sample with BSID‐II and the other half with BSID‐III; Atallah et al.34 reported BSID‐II scores from two different surgical shunt eras; while others reported scores from two, four, and five different CHD lesions respectively.42, 43, 44
Figure 1a depicts a forest plot of the PDI scores of the studies included in the meta‐analysis.22, 23, 27, 30, 32, 34, 37, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55 The estimated mean PDI resulting from the random‐effects meta‐analysis was 78.72 (95% CI 76.09–81.36). In the meta‐regression model that included both covariates, the testing age of the infants was not associated with PDI, while the period of surgery was associated with the PDI (Fig. 1b). Over the years, the PDI of the infants decreased: an increase of 1 year in the surgery period resulted in an average decrease of −1.16 points PDI (p<0.01, 95% CI −1.90 to −0.42). Finally, we investigated whether infants with single‐ventricle CHD had worse PDI scores than those with two‐ventricle CHD. The type of cardiac lesion was a significant moderator (p<0.001). The model estimating PDI for a fixed age of 21 months and a fixed surgical period at the year 2000 yielded scores for infants with single‐ventricle defects (77.16, 95% CI 71.69–82.62) that were significantly lower than those for infants with two‐ventricle defects (88.22, 95% CI 84.18–92.25).
Figure 1.
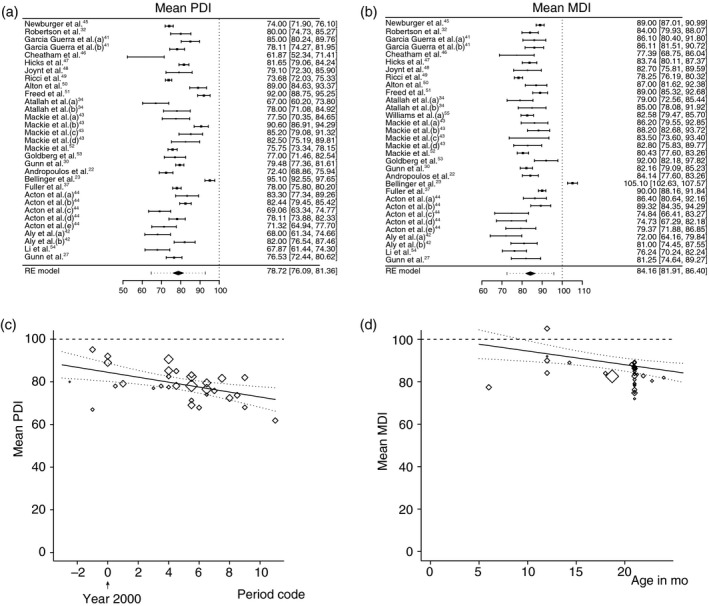
Meta‐analyses of Bayley Psychomotor Developmental Index (PDI) and Mental Developmental Index (MDI). Forest plots of (a) PDI, (b) MDI. The studies are listed by the author’s last name and year, and ranked according to methodological quality (high at the top). The black boxes indicate the means, the lines the confidence intervals (CIs), and the dotted lines the prediction intervals. The black diamond indicates the estimated means from the random‐effects (RE) meta‐analysis. Heterogeneity remained: (PDI: τ 2 =49.47 [p<0.001], I 2 =94%; MDI: τ 2 =34.03 [p<0.001]). I 2 =89%). (c) Bubble plot regression graph of the association between surgery era and mean PDI of the individual studies. The symbol sizes are related to the weights of the studies in the analysis; larger diamonds indicate larger/more precise studies. Based on the meta‐regression RE model with adjustment for age, the predicted average mean PDI as a function of the covariate period (with age fixed at 21mo) is also added to the plot (line) with 95% CI (dotted lines). Surgery era code is indicated as the median year of the surgery period. (d) Bubble plot regression graph of the association between assessment age of the infant and MDI. For explanation, see the description of (c).
Figure 1c depicts a forest plot of the MDI. The estimated mean MDI resulting from the random‐effects meta‐analysis was 84.16 (95% CI 81.91–86.40). Both the testing age and the period of surgery were associated with MDI scores (Fig. 1d). Adjusted for surgery period, an increase of 1 month in infant age resulted in an average decrease of −0.64 points MDI (p=0.005, 95% CI −1.09 to −0.20); adjusted for the infant’s age, an increase of 1 year in the surgery period resulted in an average decrease of −0.94 points MDI (p<0.001, 95% CI −1.47 to −0.41). The type of cardiac lesion was not a significant moderator for MDI (p=0.07). The model estimating MDI for a fixed average age of 21 months and a fixed surgical period at the year 2000 yielded scores of 85.42 (95% CI 80.18–90.67) for infants with single‐ventricle CHD and 89.77 (95% CI 85.90–93.64) for those with two‐ventricle CHD.
Additional outcomes
Studies that used tests other than the BSID‐II and BSID‐III also suggested impaired motor development in infancy.26, 28, 39, 56 Additional outcomes included an increased risk of poorly regulated behavioral states, reduced visual orienting, jitteriness, hypotonia, motor asymmetries, and feeding difficulties. These additional outcomes could be associated with impaired brain growth already present before surgery in infants with cyanosis.57, 58, 59 Parents reported increased emotional problems in infants with HLHS, and increased parenting stress,27 yet behavioral skills comparable to typically developing infants.49, 60 One study reported that language development was delayed in one‐third of infants at 18 to 24 months on the Language Development Survey.41
Preschool age: 25 months up to and including 4 years 6 months
For the preschool age group, 18 out of 32 papers were rated with high or moderate methodological quality. Of the 18, seven papers were rated with high internal validity (one paper with a score of 7, six with a score of 6); the remaining 11 papers were rated with moderate internal validity (four papers with a score of 5, seven with a score of 4). The Mallen score ranged from 7 to 12. Individual papers represented preschool‐age children from North America (12 papers) and Europe (six papers). White children were the most represented ethnicity (Table S2). Within the set of 18 papers, three24, 61, 62 were derived from a single infant study;17 two papers63, 64 were derived from another infant study;26 another two papers from a single study;65, 66 and one was a follow‐up paper67 from an infant study.45 Group size ranged from 7 to 420 children (median 36). The surgical era of the preschool children evaluated was 1983 to 2015. STAT category at primary surgery was 3 to 5. Seven papers addressed outcomes of infants born with single‐ventricle physiology, mostly HLHS. Seven papers focused on outcomes in infants born with two‐ventricle defects, which included TGA and miscellaneous two‐ventricle repairs that met the criteria of surgery before 9 weeks of life. The remaining four papers addressed a mixed sample of single‐ and two‐ventricle defects.
The children’s age at developmental testing varied: between 25 and 30 months (four papers), 31 and 36 months (five papers), and 47 and 54 months (six papers). Three papers had a wide age range, which when combined covered children aged 7 to 67 months.
Nine areas of development were addressed in 18 papers by 20 different tests, excluding different versions of the same test (see Table S5 for the other tests used).
Cognitive development was the primary interest of outcome at preschool age. Nine papers reported outcomes; six used BSID‐II or BSID‐III as at least one instrument to measure cognitive development, two used the Wechsler Preschool and Primary Scale of Intelligence to measure intelligence (see Table S6 for the other tests). Children with single‐ventricle defects generally had cognitive development within the average range, yet children with HLHS scored in low average ranges. Rogers et al.68 reported moderate to profound cognitive delay in seven of 11 children (64%) with HLHS, but contemporary papers reported better outcomes. Children with two‐ventricle defects, primarily TGA, had cognitive skills in the typical range, yet the data indicated their intelligence scores were lower than the population mean. The IQ data of preschool age are included in the meta‐analysis of IQ scores which also covers school age and adolescent age.
Motor development and language development were each addressed in four papers. The BSID‐II or BSID‐III was used in three papers to measure motor development and one for language development. Motor development was measured in one paper with the Peabody Developmental Motor Scales, Second Edition and the grooved pegboard test for children with two‐ventricle CHD.62 Sarajuuri et al.63 reported that children with single‐ventricle defects had significantly lower motor development than a comparison group; yet their scores were comparable to those of typically developing children in general.65 Bellinger et al.62 reported worse balance, manual dexterity, and stationary skills in children with TGA than the population mean at 4 years, while Toet et al.69 reported that children with TGA had average motor development. Bellinger et al.62 also reported an increased risk of speech errors, apraxia of speech, and abnormalities of volitional oral movements in children with two‐ventricle CHD. Language development, primarily expressive language, was measured by six different tests or parts of tests, including the BSID‐III. Children with single‐ventricle defects, especially those with HLHS, had significantly lower language developmental scores than a comparison group64 and the population mean.65
Other areas of development, namely visual–motor and global development, were addressed in a few papers with varied results. Two studies reported that functional skills of children with single‐ventricle defects were significantly worse than those of typically developing children in general.67, 68
School age: 4 years 7 months up to and including 12 years
Thirty‐one of 59 papers (53%) were rated with high or moderate methodological quality. Of the 31, five papers were rated with high internal validity (five papers with a score of 6); the remaining 26 papers were rated with moderate internal validity (12 with a score of 5, 14 with a score of 4). The Mallen scores ranged from 5 to 12. Individual papers represented school‐age children from Europe (18 papers), North America (12 papers), and Australia (one paper). White children were the most represented ethnicity among these papers (Table S3). Within the set of 31 papers, eight70, 71, 72, 73, 74, 75, 76, 77 were derived from one single infant study;17 two papers78, 79 from one infant study;26 two separate infant studies32, 38 each produced one paper respectively;80, 81 two separate studies17, 82 each produced three papers;18, 83, 84, 85, 86 and one study87 produced two papers.87, 88 Group size ranged from 15 to 155 children (median 37). The surgical era of school‐age children was 1981 to 2012. STAT category at primary surgery was 3 to 5. Six papers addressed outcomes of infants born with a single ventricle; samples included HLHS. Nineteen papers focused on infants born with two ventricles; samples included TGA. The remaining six papers addressed a mixed sample of single‐ and two‐ventricle defects.
The children’s age at the time of developmental testing varied: 4 years 7 months to 6 years (nine papers), 7 to 9 years (14 papers), and 10 to 12 years (five papers). Three papers covered children aged 5 to 7 years.
Eight areas of development were assessed by approximately 30 different tests, excluding different versions of the same test (Table S7). Cognitive development was the primary outcome measured at school age. Intelligence was addressed in 19 papers. Data from 10 of these papers that used the Wechsler Preschool and Primary Scale of Intelligence or Wechsler Intelligence Scale for Children were included in the meta‐analysis of IQ scores, which follows in a later section. Papers that did not use the Wechsler Preschool and Primary Scale of Intelligence or Wechsler Intelligence Scale for Children but instead used other tests reported comparable intelligence between children with TGA and siblings,89 population or reference mean,18, 83 or comparison group;84, 85, 86 and lower intelligence of children with HLHS compared with the population mean or control children.90, 91 Other areas of cognition described in papers in the school‐age set were academic achievement, executive function, memory, learning, processing speed, concentration, attention, and social cognition. Outcomes indicated that children with single‐ventricle defects were at increased risk of having shortcomings in auditory and visual attention, processing speed, specific memory tasks, and fine motor skills.78, 91, 92, 93 Children with two‐ventricle defects, primarily TGA, had an increased risk of deficits in reading and mathematics, acquired abilities, processing speed, aspects of memory, inhibition and cognitive flexibility, and aspects of social cognition.18, 71, 77, 83, 84, 85, 86, 94
Behavioral and emotional outcomes were also an area of high interest. Twelve papers reporting findings, of which seven used the Child Behavior Checklist. The results were mixed but suggested that children with complex CHD are at increased risk of problems in aspects of externalizing and internalizing behavior, especially anxiety, and somatic complaints.76, 82, 87, 93, 95 Interestingly, a similar risk was not present when the children rated themselves.87 Results of the Teacher Report Form suggested that the children with CHD were at increased risk of problematic adaptive functioning, social relationships, and school performance.76 Studies indicated that although attention‐deficit/hyperactivity disorder was reported to be three to four times higher at school age than in the general population, a diagnosis of autism was less common yet still two times higher than in the general population.96, 97, 98
Other areas of development were addressed to a lesser extent. Motor development, visual–motor integration, and language were each assessed in seven papers and speech was assessed in three papers. Tests for motor development and language varied; the Beery–Buktenica Developmental Test of Visual–Motor Integration was the primary test to assess visual–motor integration. The results indicated that balance and gross motor deficits were more prevalent in children with single‐ and two‐ventricle hearts defects than in typical developing children78, 79, 80 and that impairment in visual–motor integration might be a significant problem associated with academic performance.70, 78, 81, 89, 92, 99 Finally, the studies concluded that children with CHD may be at increased risk of language deficits71, 83, 86, 92, 100 and worse adaptive function.92, 95
Adolescent age: 13 years up to and including 19 years
Six of the 19 papers were rated with moderate methodological quality (three papers with a score of 5, three papers with a score of 4), and none with high methodological quality. The Mallen scores ranged from 7 to 11. Individual papers represented adolescents from North America (four papers) and Europe (two papers). White children were the most represented ethnicity among these papers (Table S4).
Within the six papers, two101, 102 were derived from one infant follow‐up study;17 one paper0103 was derived from another infant study;18 two papers were based on the same adolescent study;104, 105 while the sixth paper was a stand‐alone adolescent study.0106 Group size ranged from 54 to 220 children (median 149). Study methods varied among the papers: one cross sectional design,0106 one randomized controlled trial,0101 two prospective designs,102, 103 and two secondary analyses.104, 105 The surgical era represented by these papers was 1981 to 2009. One paper focused on adolescents with single‐ventricle defects only;0106 three papers focused on two‐ventricle defects (TGA);101, 102, 103 and two papers addressed outcome in mixed samples of single‐ and two‐ventricle defects.104, 105 The adolescents’ ages at the time of developmental testing was 13 to 16 years (five papers), while one paper covered the ages of 10 to 19 years. The median age was 15 years.
Of the six papers, four addressed aspects of cognition,101, 103, 104, 106 one addressed psychiatric status,0102 and two103, 105 addressed both cognition and psychiatric or psychological status. The studies used 20 different testing modalities (Table S8).
The results of the studies suggested that adolescents with CHD had IQs within normal ranges but an increased risk of having impairments in processing speed, reading, mathematics, attention, memory, and aspects of executive function, such as cognitive flexibility, problem solving, and inhibition. Additionally, the studies implied that adolescents with CHD, in particular adolescents with single‐ventricle defects, were at increased risk of lifetime psychiatric diagnoses, attention‐deficit/hyperactivity disorder, and self‐reported anxiety.101, 102, 103, 104, 105, 106
Meta‐analysis of IQ scores
The data of the papers addressing IQ outcomes at preschool age, school age, and adolescence using the Wechsler Preschool and Primary Scale of Intelligence or Wechsler Intelligence Scale for Children evaluations (full‐scale IQ, verbal IQ, or performance IQ scores) were entered into the meta‐analysis. Figure 2a depicts a forest plot of papers included in the meta‐analysis for full‐scale IQ scores.62, 71, 81, 88, 92, 93, 94, 99, 100, 107, 108, 109 Fourteen papers generated 22 entries that could be included in this meta‐analysis. Some papers generated more than one outcome: Creighton et al.81 and Ryberg et al.0109 each compared outcomes of four groups of children with different CHD in two different studies; Sterken et al.99 compared outcomes of children with complex CHD and typically developing groups at two different periods; and Sarajuuri et al.92 compared two groups of children with single‐ventricle defects and typically developing groups.
Figure 2.
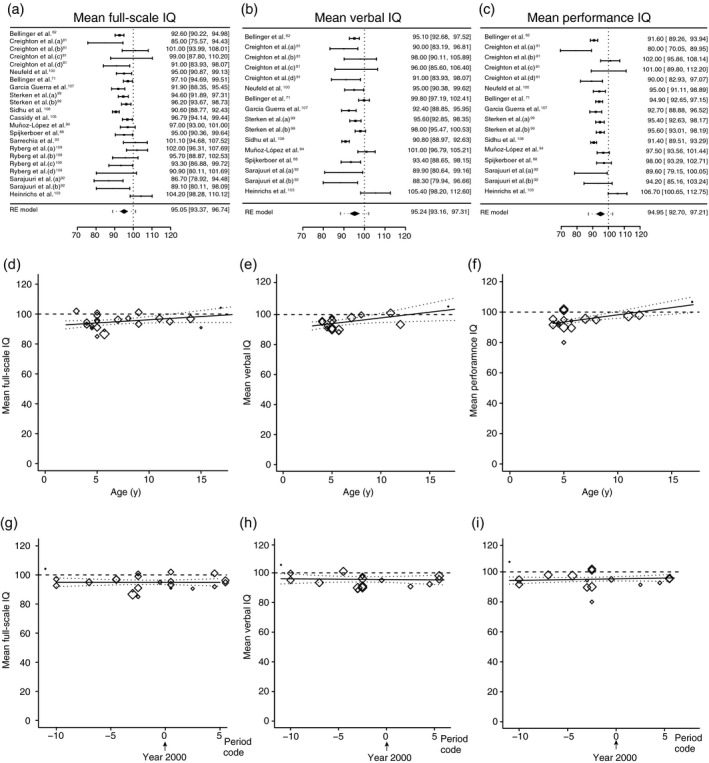
Meta‐analyses of IQ scores. (a–c) Forest plots of full‐scale IQ, verbal IQ, and performance IQ respectively. The studies are listed by the author’s last name and year, and ranked according to methodological quality (high at the top). The black boxes indicate the means, the lines the confidence intervals, and the dotted lines the prediction intervals. The black diamond indicates the estimated means from the random‐effects (RE) meta‐analysis. Heterogeneity remained: (full‐scale IQ: τ 2=9.21 [p<0.001], I 2=70.3%; verbal IQ: τ 2 =11.2 [p<0.001], I 2=75.7%; performance IQ: τ 2=14.1 [p<0.001], I 2 =80.4%). (d–f) Bubble plot regression graphs of the association between assessment age of the child and full‐scale IQ, verbal IQ, and performance IQ respectively. (g–i) Bubble plot regression graphs of the association between surgery era and full‐scale IQ, verbal IQ, and performance IQ respectively. For explanation, see the description of Figure 1.
The estimated mean full‐scale IQ resulting from the random‐effects meta‐analysis was 95.1 (95% CI 93.4–96.7). In a meta‐regression model including both covariates, neither the age of testing of the children nor the period of surgery was associated with full‐scale IQ (Fig. 2d,g). We also investigated whether children with single‐ventricle CHD had worse full‐scale IQ than those with two‐ventricle CHD. The type of cardiac lesion was a significant moderator (p=0.03). The model estimating full‐scale IQ for a fixed average age of 7 years and a fixed surgical period at the year 2000 yielded scores of 92.42 (95% CI 88.7–96.1) for children with single‐ventricle CHD and 97.75 (95% CI 93.8–101.7) for children with two‐ventricle CHD.
Figure 2b,c depicts forest plots of the papers included in the meta‐analysis for verbal IQ and performance IQ respectively. Eleven papers generated 16 entries for verbal IQ and performance IQ; not all papers that reported full‐scale IQ outcomes also reported verbal IQ and performance IQ outcomes. Some papers generated more than one outcome for the reasons listed above.81, 92, 99
The estimated mean verbal IQ resulting from the random‐effects meta‐analysis was 95.2 (95% CI 93.2–97.3). In the meta‐regression model that included both covariates, the period of surgery was not associated with verbal IQ (Fig. 2h), while the testing age of the child was associated with verbal IQ (Fig. 2e). As the child aged, the verbal IQ increased: an increase of 1 year in age resulted in an average increase of 0.76 points of verbal IQ (p=0.02, 95% CI 0.13–1.40). We investigated whether children with single‐ventricle CHD had worse verbal IQ than those with two‐ventricle CHD and found the type of cardiac lesion was not a significant moderator (p=0.07), indicating no significant difference between the groups.
The estimated performance IQ resulting from the random‐effects meta‐analysis was 95.0 (95% CI 92.7–97.2). In the meta‐regression model including both covariates, the period of surgery was not associated with performance IQ (Fig. 2i), while the testing age of the child was associated with performance IQ (Fig. 2f). Similar to verbal IQ, as the child aged, the performance IQ increased: an increase of 1 year in age resulted in an average increase of 0.95 points of performance IQ (p<0.001, 95% CI 0.49–1.41). We investigated the effect of single‐ and two‐ventricle defects on performance IQ and found the type of cardiac lesion was a significant moderator (p=0.05). The model estimating performance IQ for a fixed average age of 7 years and a fixed surgical period at the year 2000 yielded scores of 90.43 (95% CI 83.69–97.18) for children with single‐ventricle CHD and 97.48 (95% CI 92.93–102.03) for those with two‐ventricle CHD.
Publication bias
All meta‐analyses showed considerable heterogeneity. This made examining potential ‘small study effect’ (through visual inspection of funnel plots and Egger’s test) a challenge, as true heterogeneity in itself may also cause funnel plot asymmetry.110, 111 Keeping in mind that the results of funnel plots and Egger’s test are less reliable, we examined all funnel plots and performed Egger’s tests. Overall, the results did not suggest the presence of publication bias (Appendix S2, online supporting information).
Perioperative risk factors and developmental outcome
Preoperative, intraoperative, and postoperative hypoxemia
The most frequently used indicators of hypoxemia consisted of invasive arterial blood gas measurements, including partial pressure of oxygen in arterial blood, partial pressure of carbon dioxide and lactate levels, and non‐invasive variables, such as regional cerebral oxygen saturation using near‐infrared spectroscopy. The studies used preoperative, intraoperative, and postoperative measurements in the first week after surgery to report the degree of hypoxemia. In a preliminary analysis, we evaluated whether the period during which hypoxemia was assessed mattered. It revealed that the period of hypoxemia (pre‐, intra‐, or postoperative) did not seem to affect the associations between hypoxemia and developmental outcome. We therefore pooled the periods (Table S9, online supporting information).
Twelve infant studies addressed associations between one or more hypoxemia variables and developmental outcomes (four single‐ventricle studies, six two‐ventricle studies, two mixed studies). Of the four single‐ventricle studies, one reported a significant association between lactate levels and worse developmental outcome,26 one reported mixed results between worse cognition with a variable of hypoxemia and no association with another variable of hypoxemia,34 and two studies did not find significant associations.21, 112 Of the two‐ventricle studies, one found a significant association between hypoxemia and worse motor and cognitive outcome,19 four reported inconsistent results of worse motor and behavioral adaptive skills yet preserved cognition,22, 41, 50, 51 and the remaining study reported low predictive value of hypoxemia and disability.33 Of the studies with mixed lesions, one reported varied results,30 and the other reported no association between hypoxemia and developmental outcome.52
Of the preschool set of papers, six (four two‐ventricle studies, two mixed studies) addressed associations between one or more hypoxemia variables and developmental outcome. Of the two‐ventricle studies, three reported inconsistent results69, 106, 113 and the other reported no association between hypoxemia and development.114 Of the mixed studies, one reported a significant association between hypoxemia and worse cognitive outcome0107 and the other one reported varied results.115
Of the school age and adolescent set of papers, five (all two‐ventricle studies) addressed associations between one or more hypoxemia variables and development. Three papers reported significant associations between hypoxemia and worse developmental outcome: one with worse motor, speech function, and impaired neurological function,83 one with parent‐reported worse behavior and social development,82 and one with worse neurological dysfunction.0103 One study reported mixed results dependent upon the trend of lactate levels before and after surgery.100 The remaining study reported no association between hypoxemia and cognitive outcome.0101
In conclusion, 23 of the 101 papers addressed associations between hypoxemia variables and developmental outcome (15 of which included mainly children with two‐ventricle CHD). A significant association between hypoxemia and adverse development outcome existed in 25% of papers, no association existed in 25% of papers, and the remaining studies demonstrated varied results. These results suggested that hypoxemia was significantly associated with some aspect of development in 75% of the papers that addressed the association; however, no clear pattern of association between hypoxemia and developmental outcome emerged.
Variables of cerebral protection during cardiopulmonary bypass
We addressed the question of associations between variables of cerebral protection during cardiopulmonary bypass and developmental outcome by evaluating: (1) the use of deep hypothermic cardiac arrest with or without regional low‐flow perfusion or antegrade cerebral perfusion and (2) the duration of cerebral protection on cardiopulmonary bypass during the index cardiac surgery. Our review indicated the effect of both variables was similar, therefore only associations between presence and/or duration of cerebral protection and developmental outcome are reported (Table S10, online supporting information).
Of the infant set of papers, 25 (nine single‐ventricle studies, 15 two‐ventricle studies, and one mixed study) reported associations between the variables of cerebral protection and developmental outcome. None of the single‐ventricle studies reported consistent associations between cerebral protection variables and developmental outcomes. Four reported mixed results between the variables of cerebral protection and developmental outcome without a specific pattern of associations emerging.19, 21, 34 The remaining five studies reported no association between the variables of cerebral protection and developmental outcomes.26, 45, 53, 54, 110 Of the 15 two‐ventricle studies, three found significant associations between the variables of cerebral protection and developmental outcome: one reported associations with increased prevalence of disability;32 one with worse language development;47 and one with suboptimal neurological signs.17 Mixed results were present in four studies without a specific pattern of association present.23, 25, 41, 48 The remaining eight studies reported no association with developmental outcome.22, 30, 31, 37, 50, 51, 52, 60 The two studies with mixed lesions reported no association between the variables of cerebral protection and developmental outcome.52, 56
Of the preschool set of papers, 11 (four single‐ventricle studies, seven two‐ventricle studies) reported associations between the variables of cerebral protection and developmental outcome. Of the four single‐ventricle studies, none reported significant associations between the variables of cerebral protection and developmental outcome. One described mixed results; one stated the variables were worse in some aspects of cognition and not in others;116 while the remaining three papers reported no association between the variables of cerebral protection and developmental outcome.63, 65, 67 Of the seven two‐ventricle studies, two revealed significant associations between cerebral protection variables and developmental outcome: one reported worse full‐scale IQ and impaired visual–motor integration,0106 the other reported worse motor development and speech functions.62 Two of the studies stated mixed results,107, 117 and the remaining three studies reported no association with the variables of cerebral protection and developmental outcomes.113, 114, 115
Of the school and adolescent age set of papers, 17 (three single‐ventricle studies, 13 two‐ventricle studies, one mixed study) reported associations between the variables of cerebral protection and developmental outcomes. Six of the 17 papers presented outcomes on adolescents. Of the three single‐ventricle studies, one stated significant associations: longer durations of cerebral protection, particularly with deep hypothermic cardiac arrest, were associated with worse full‐scale IQ;92 one reported worse visual and verbal long‐term memory tasks, decreased processing speed, and low average IQ.91 The remaining single‐ventricle study stated no association between the variables of cerebral protection and motor or neuropsychological outcome.78 Of the two‐ventricle studies, none reported a significant association between the variables of cerebral protection and developmental outcome. Seven studies stated inconsistent results including impaired visual–spatial skills, impaired speech function and social development, decreased processing speed, and increased impulsive responsiveness; yet they also reported, in most studies, no associations of the parameters of cerebral protection and intelligence, some aspects of academic achievement, or autism.18, 70, 71, 73, 82, 83 The remaining six two‐ventricle studies stated no association between the variables of cerebral oxygenation and intelligence, parent‐ and teacher‐rated behavior, and global psychosocial functioning.76, 81, 100, 102, 103, 118 The single mixed study reported no association with the duration of cardiopulmonary bypass and motor development.80
In conclusion, 53 of the 100 of the papers with high or moderate methodological quality addressed associations between cerebral protection variables and developmental outcome. Only six of these papers (11%) reported a significant association between the variables of cerebral protection and worse developmental outcomes; however, not one domain of development was affected consistently. More than 30% of the papers (19 out of 53) reported mixed results with no association between the variables of cerebral protection and aspects of intelligence yet a significant association with impaired visual–spatial skills, decreased processing speed, and aspects of behavior was apparent. Most of the papers (28 out of 53) reported no association. The studies suggest that methods of vital organ support, namely with or without some blood flow to the brain during cardiopulmonary bypass, are not clearly associated with better or worse developmental outcomes at any age. Thus, developmental outcome from both methods of vital organ support seem to be associated with nearly equal neuroprotection.
Variables of severity of clinical illness: length of mechanical ventilation and hospitalization
We addressed the question of associations between variables of severity of clinical illness by evaluating the association between: (1) number of days of mechanical ventilation, (2) number of days in the intensive care unit, and (3) length of stay (days) in the hospital and developmental outcome. For the infant set of papers, we addressed these variables in the phase of primary surgery and for the preschool, school age, and adolescent sets of papers in terms of overall days after one or more surgeries. We first addressed the effect of the clinical illness variables on developmental outcome separately. As preliminary analysis indicated that the associations between the variables of clinical illness and developmental outcome were similar, we summarized the results by reporting the associations between ‘clinical illness’ (implying that one or more of the above variables indicated more severe illness) and developmental outcomes. For details, see Table S11 (online supporting information).
Of the infant set of papers, 17 (six single‐ventricle studies, eight two‐ventricle studies, three mixed studies) reported the associations of ‘clinical illness’ and developmental outcome. Of the single‐ventricle studies, two stated significant associations between ‘clinical illness’ and developmental outcome: one reported worse motor and cognitive outcome,45 the other stated worse cognition and language;112 one study reported no association between ‘clinical illness’ and developmental outcome;54 and three stated inconsistent results.21, 34, 119
Of the eight two‐ventricle studies, five reported a significant association between ‘clinical illness’ and adverse developmental outcome: two studies identified worse motor, cognitive, and language development;19, 22 one decreased motor and cognitive development;51 one poorer language development;47 and one reported an overall increase in adverse outcome.32 Two two‐ventricle studies stated inconsistent associations between ‘clinical illness’ and developmental outcome,37, 41 while one study showed no association.52 Of the three studies with mixed lesions, one reported a significant association ‘clinical illness’ and worse motor development32 while the other two showed inconsistent associations between ‘clinical illness’ and developmental outcome.30, 56
Of the preschool set of papers, seven (three single‐ventricle studies, three two‐ventricle studies, and one mixed study) reported the associations of ‘clinical illness’ and developmental outcome. One of the single‐ventricle studies identified significant associations between ‘clinical illness’ and worse motor and cognitive development,63 while the other two stated inconsistent associations between ‘clinical illness’ and developmental outcome.65, 67 Of the three two‐ventricle studies, one described no associations between ‘clinical illness’ and developmental outcome,114 and two with inconsistent associations.106, 113 The one study with mixed lesions also reported inconsistent associations between ‘clinical illness’ and developmental outcome.114
Of the school age and adolescent set of papers, 11 (three single‐ventricle studies, seven two‐ventricle studies, one mixed study) reported the associations of ‘clinical illness’ and developmental outcome. While one of three single‐ventricle studies identified significant associations between ‘clinical illness’ and worse cognitive outcome,92 one stated inconsistent associations,93 and one reported no association between ‘clinical illness’ and developmental outcome.78 Of the two‐ventricle studies, two reported a significant association between clinical illness and developmental outcome: one showed decreased cognition and visual–motor integration,100 the other reported lower parent‐reported adaptive skills.95 Three identified no associations between ‘clinical illness’ and developmental outcome71, 102, 118 and two described inconsistent associations.72, 76 The mixed lesion study reported worse motor performance.80
In conclusion, 35 of the 100 papers addressed associations between ‘clinical illness’ and developmental outcome. Thirteen (37%) reported significant associations while fifteen (43%) reported inconsistent associations.
DISCUSSION
We reviewed developmental outcome of children with complex CHD in 100 papers with moderate to high methodological quality spanning a surgical era of almost 30 years. The results from the meta‐analyses indicate that infants with CHD have significantly lower scores on the BSID‐II PDI and MDI, with overall averages below one standard deviation of the population mean (79 and 84 respectively). After infancy, outcomes are better and differ for children with single‐ and two‐ventricle CHD. This finding is illustrated by the average full‐scale IQs, approaching in children with two‐ventricle CHD the population mean (98), but being half a standard deviation below the mean in children with single‐ventricle CHD (92). The analysis of the risk factors suggested that, in general, perioperative hypoxemia, the type of cerebral protection procedure used, and severity of clinical illness were not consistently associated with developmental outcome.
Methodological considerations
This systematic review and meta‐analysis was the result of literature from worldwide electronic databases. The 100 papers selected with moderate to high methodological quality were predominantly from Europe and North America; none were from Asia or Africa, which suggests that we understand little about the developmental outcomes in children with CHD in a large part of the world. Of the 100 papers, 30 had high methodological quality while the remaining 70 had moderate methodological quality, the latter implying that the studies had some risk of bias. To check whether methodological quality affected the results of our meta‐analyses, we performed post hoc analyses in which we added methodological quality as an effect moderator. The results showed that methodological quality played a significant role as moderator in none of the five models for the outcome variables PDI, MDI (infants), full‐scale IQ, verbal IQ, and performance IQ (preschool, school age, and adolescents) (p‐values 0.99, 0.91, 0.47, 0.50, and 0.40 respectively). Nevertheless, it should be acknowledged that the studies included in the meta‐analyses showed substantial heterogeneity, which could not always be eliminated by the inclusion of covariates. Therefore, the results should be interpreted with appropriate caution. The heterogeneity interfered with a proper analysis of the effect of ‘small studies’; therefore evidence of small study bias could not be conclusively determined.
Nearly half of the papers reported findings on children below the age of 2 years, and one‐third of the papers reported outcomes of school‐aged children. Only four studies addressed developmental outcomes longitudinally over an extended period, two from birth to 5 years,26, 38 one from birth to adolescent age,17 and one from 3 years to young adulthood.18 The two most extensive studies followed a group of infants with TGA (two‐ventricle CHD). The low number of protracted studies illustrates the difficulty in understanding the true course of development in children with complex CHD, especially those with single‐ventricle CHD, as perhaps only the least affected children with surgically corrected defects participate in research or survive into adolescence. The difficulty in long‐term follow‐up of children with CHD is also reflected in the decrease in the papers’ methodological quality with increasing duration of follow‐up: 60% of the infant set of papers met our criteria of moderate to high quality compared with 32% of the adolescent set of papers.
Important sources of reduction of methodological quality were attrition, non‐standardized report of the state of disease, absence of a comparison group, and blinding to the presence of CHD. Most of these methodological problems can be attributed to the difference in the studies’ original aim and the aim of our study. Many studies focused on within‐group associations and did not have the comparison of developmental outcome of children with/without CHD as a primary goal, which was our aim. Yet, our understanding of long‐term developmental outcome of children with complex CHD would benefit from studies that either include a comparison group or test whether developmental outcome of children with CHD differs significantly from published population norms. Attrition is inevitable in long‐term follow‐up studies. When it occurs, reasons for attrition should be reported and selection bias by attrition should be checked.120 Means to reduce attrition are the use of child‐ and family‐friendly evaluation methods, a home assessment of the children, and the use of newsletters to regularly update the families on the study’s progress.121 Finally, research in the field would benefit from the use of standardized references to classify the severity of CHD, such as the Risk Adjustment for Congenital Heart Surgery, the Aristotle Basic Complexity scores, or the STAT mortality score. Of these three scores, the STAT score was reported to be the best predictor of both length of stay in the intensive care unit and hospital mortality.122
Developmental outcome
Cognitive development
Cognitive development was evaluated from infancy to adolescent age, with the BSID‐II and BSID‐III most commonly used in early childhood and the Wechsler Scales being most frequently applied at older ages. The meta‐analyses showed that MDI in children with CHD below 2 years of age was on average one standard deviation lower than that of peers without CHD, whereas IQ at older ages in general was in the typical range, albeit slightly lower than that of peers without CHD. Disease complexity (single‐ vs two‐ventricle pathology) was not directly associated with cognitive development delay early in life. It did, however, influence cognition beyond infancy; children with single‐ventricle CHD had lower IQ scores than those with two‐ventricle CHD. Our data indicate that children with two‐ventricle CHD have IQ scores within the typical range; in other words, they experience catch up growth in cognitive milestones. On the other hand, children with single‐ventricle CHD continued to have cognitive impairments. This delay was not only reflected in their IQ scores, but also in specific cognitive functions, such as impaired visuo‐spatial skills and reduced executive function, including impaired attention and working memory, which in turn contributed to lower academic achievement. The pattern of cognitive impairments of children with CHD mimics that of infants born very preterm.2, 123 Evidence is accumulating that the cognitive impairments in infants born preterm are associated with reduced brain volumes and altered brain microstructure, including altered connectivity.124, 125 Children with CHD are known to have an increased risk of similar forms of altered brain structure and brain injury,6, 7, 8, 9, 126, 127, 128 especially children with single‐ventricle CHD,129, 130, 131 probably explaining their increased risk of cognitive impairments.
The data in the literature suggest that as time progressed and medical care of infants with complex CHD improved, the infants’ MDI decreased. We wondered whether this decrease in cognitive function over the years could be attributed to a reduction in mortality; that is, with an increased survival of the infants with the most complex forms of CHD who may not have made it as far as surgery in the earlier years but who are now doing so. The analysis of the mortality data reported in our set of infant papers covering surgical eras from 1983 to 2015 did not support this explanation. However, our literature search was not designed to answer the question on changes in mortality over time. A quick review of outcome data in the literature demonstrated a substantial decline in CHD mortality from 1987 to 2005, especially with severe forms of CHD.132 In addition, Liu et al.1 confirmed improved survival of children with severe forms of CHD between 1970 and 2000; survival stabilized thereafter.
Motor development
Motor outcome was evaluated primarily during infancy, with the BSID‐II and BSID‐III. Motor development was studied infrequently after 2 years 6 months. The meta‐analysis demonstrated that motor development of infants with CHD was considerably impaired compared with peers; especially for children with single‐ventricle CHD. In contrast to the MDI, PDI was not affected by the infant’s age. The PDI decreased as time of surgical period moved from the mid‐1980s to 2015, similar to the MDI. The few studies that addressed motor development after infancy suggested that children with complex CHD, in particular children with single‐ventricle CHD, continued to be at increased risk of motor difficulties, resulting in an increased risk of impaired balance, reduced manual dexterity, and decreased strength. The profile of motor impairments of children with CHD – like that of cognitive impairments – resembles that of infants born very preterm.2, 123 In infants born preterm, this profile is associated with a substantially increased risk of developmental coordination disorder.133 The motor impairments in infants born very preterm are attributed to reduced brain volumes and altered white matter microstructural organization at term‐equivalent age.134 The motor impairments in children with CHD may result from similar changes in brain development. To test this hypothesis, studies to measure long‐term motor outcome in children with CHD are needed, especially as it is well known that motor impairments may impact functional skills, self‐esteem, and participation in activities with peers.135, 136, 137, 138
Additional developmental outcomes
Language was evaluated from infancy to school age with a variety of assessment tools. This review suggested that children with CHD, especially those with single‐ventricle CHD, were at greater risk of language impairment than peers, especially expressive language. Part of the language impairment may be attributed to hearing loss, which is known to have a higher prevalence in children with complex CHD.139, 140 The data indicated that speech disorders, such as apraxia or abnormalities of volitional oral movements, emerged during preschool age and persisted in primary school age. Whether children with CHD outgrow these impairments was not clear, as language and speech were not assessed specifically in adolescence. Like the higher prevalence of cognitive and motor impairments in children with CHD, the higher prevalence of speech and language impairments may also be explained by their increased risk of altered brain structure.
Behavior was evaluated frequently from later preschool age to adolescence, primarily with the Child Behavior Checklist. The review suggests that school‐age children with CHD have similar problems as children with other chronic diseases. Although children with CHD are reported to be at increased risk of depression and anxiety, they may be less self‐aware of these emotions compared with assessment from their parents or teachers. Adolescents with CHD, on the other hand, seem to acknowledge increased feelings of anxiety and depression. The data indicate that children with CHD have an increased risk of attention‐deficit/hyperactivity disorder compared with peers. A similar pattern exists in infants born very preterm.125
Perioperative risk factors
Review of the current literature reveals that the association between perioperative risk factors and developmental outcomes is inconsistent. The factor that predicted outcome best – but still inconsistently – was the length of stay in the intensive care unit or hospital. Like other groups of infants at risk (infants born preterm), length of stay in the hospital is one of the best predictors of developmental outcome;141, 142, 143 as it integrates the clinical net effect of risk and resilience. Our finding of inconsistent relationships between perioperative risk factors and developmental outcome indicates that other factors outweigh the contribution of the factors that we evaluated. Most probably, developmental outcome is largely determined by alterations in brain development or brain lesions occurring before cardiac surgery, prenatally or postnatally, as denoted by the review of Mebius et al.5 The transition from fetal to postnatal circulation and the preoperative period may be particular periods of hemodynamic instability that may predispose the brain to further injury, particularly if the time to surgery is protracted.144, 145 The etiology of impaired developmental outcome in children with CHD most likely results from an accumulation of events, with prenatally occurring microstructural changes in the white matter leading to an increased vulnerability for additional brain damage perinatally.3 The increased vulnerability stresses the need for careful monitoring of brain function in children with complex CHD from early postnatal age onwards. Early identification of infants with CHD at high risk of adverse developmental outcomes allows for referral and initiation of early intervention.
Strengths and limitations
The strengths of this systematic review are that: (1) it covered the literature on developmental outcome of infants with complex CHD over a period of almost 30 years; (2) it used a stringent analysis of methodological quality; and (3) it included meta‐analyses of motor and cognitive outcomes. To counteract heterogeneity in children with TGA, we excluded studies published before 1990, as the arterial switch operation was the standard of care from 1990 onwards.
It may be considered a limitation that the studies reviewed were heterogeneous in nature, for example showing heterogeneity in cardiac diagnosis, age at follow‐up, assessment tools, and follow‐up rate. The heterogeneity was also present in the meta‐analysis (Figs 1 and 2). It precluded the determination of evidence of small study bias. The studies included in this review were observational studies. This type of study is associated with possible over‐ or under‐reporting of the outcome measure. Finally, specific attention was not directed to the role of chromosomal abnormalities and the social context of the child. We recognized the presence of chromosomal abnormalities in the methodological quality appraisal and found that most studies excluded infants with known chromosomal abnormalities because these infants are at increased risk of developmental disorders regardless of their CHD.146, 147 We therefore could not address the effect of chromosomal anomalies on developmental outcome. However, studies2, 148 suggest that developmental outcomes of infants with CHD and chromosomal anomalies are worse than those of infants with CHD without these anomalies. Likewise, it is known that the infant’s social context is a powerful modifier of developmental outcome and may act as an effect moderator.149
Conclusion
Children with complex CHD are at increased risk of impaired developmental outcome, particularly those with single‐ventricle CHD. We therefore recommend careful monitoring of infants with complex CHD to detect those at high risk of a developmental disorder to allow early referral for developmental intervention.
This extensive review of the literature has demonstrated that developmental outcome of infants subjected to early surgery for complex CHD in the last two decades of the 20th century was not worse than that of children who had surgery in the contemporary era. Rather, developmental scores for cognitive and motor development in infancy tended to decrease in more recent years, which may have been caused by an increased survival of the most vulnerable children.
Finally, our review showed that perioperative factors were not consistently associated with developmental outcome. The impact of the conditions acting before surgery, in particular those acting during the fetal life and perinatal period, outweighs the developmental consequences of perioperative conditions. The finding that developmental outcome of infants with complex CHD mimics that of infants born very preterm suggests that the brain of children with complex CHD is especially vulnerable during the third trimester of gestation and throughout the perioperative period.6, 9, 129 We therefore recommend that careful monitoring of children with complex CHD starts in the third trimester of pregnancy.144, 150, 151
Our literature review showed a proliferation of papers related to developmental outcomes after the year 2000. However, very few prospective studies followed children beyond school age, and those that did followed children with two‐ventricle CHD, particularly TGA. This finding stresses the need for longitudinal studies, including children with single‐ventricle CHD that not only evaluate cognitive outcome but also motor and language development.
Supporting information
Appendix S1: Search string.
Appendix S2: Funnel plots of and Egger’s tests.
Table S1: Methodological quality table – infant
Table S2: Methodological quality table – preschool
Table S3: Methodological quality table – school age
Table S4: Methodological quality table – adolescent age
Table S5: Developmental outcome table – infant
Table S6: Developmental outcome table – preschool
Table S7: Developmental outcome – school age
Table S8: Developmental outcome table – adolescent age
Table S9: Risk factor variables of hypoxemia table
Table S10: Risk factor variables of cerebral protection table
Table S11: Risk factor variables of severity of illness table
Figure S1: Inclusion and exclusion of articles found in the search strategy.
Acknowledgements
We acknowledge Anneke Kracht for her assistance in the design of the figures. This review received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors. The authors have stated that they had no interests that could be perceived as posing a conflict or bias.
References
- 1. Liu Y, Chen S, Zühlke L, et al. Global birth prevalence of congenital heart defects 1970–2017: updated systematic review and meta‐analysis of 260 studies. Int J Epidemiol 2019; 48: 455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marelli A, Miller SP, Marino BS, Jefferson AL, Newburger JW. Brain in congenital heart disease across the lifespan: the cumulative burden of injury. Circulation 2016; 133: 1951–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Claessens NH, Kelly CJ, Counsell SJ, Benders M. Neuroimaging, cardiovascular physiology, and functional outcomes in infants with congenital heart disease. Dev Med Child Neurol 2017; 59: 894–902. [DOI] [PubMed] [Google Scholar]
- 4. Latal B. Neurodevelopmental outcomes of the child with congenital heart disease. Clin Perinatol 2016; 43: 173–85. [DOI] [PubMed] [Google Scholar]
- 5. Mebius MJ, Kooi EMW, Bilardo CM, Bos AF. Brain injury and neurodevelopmental outcome in congenital heart disease: a systematic review. Pediatrics 2017; 140: 1–21. [DOI] [PubMed] [Google Scholar]
- 6. Peyvandi S, Latal B, Miller SP, McQuillen PS. The neonatal brain in critical congenital heart disease: insights and future directions. Neuroimage 2019; 185: 776–82. [DOI] [PubMed] [Google Scholar]
- 7. Miller SP, McQuillen PS. Neurology of congenital heart disease: insight from brain imaging. Arch Dis Child Fetal Neonatal Ed 2007; 92: 435–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Licht DJ, Shera DM, Clancy RR, et al. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg 2009; 137: 529–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Limperopoulos C, Tworetzky W, McElhinney DB, et al. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation 2010; 121: 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karsdorp PA, Everaerd W, Kindt M, Mulder BJ. Psychological and cognitive functioning in children and adolescents with congenital heart disease: a meta‐analysis. J Pediatr Psychol 2007; 32: 527–41. [DOI] [PubMed] [Google Scholar]
- 11. Snookes SH, Gunn JK, Eldridge BJ, et al. A systematic review of motor and cognitive outcomes after early surgery for congenital heart disease. Pediatrics 2010; 125: e818–27. [DOI] [PubMed] [Google Scholar]
- 12. O’Brien SM, Clarke DR, Jacobs JP, et al. An empirically based tool for analyzing mortality associated with congenital heart surgery. J Thorac Cardiovasc Surg 2009; 138: 1139–53. [DOI] [PubMed] [Google Scholar]
- 13. Jacobs JP, Jacobs ML, Maruszewski B, et al. Initial application in the EACTS and STS Congenital Heart Surgery Databases of an empirically derived methodology of complexity adjustment to evaluate surgical case mix and results. Eur J Cardiothorac Surg 2012; 42: 775–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev 2015; 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mallen C, Peat G, Croft P. Quality assessment of observational studies is not commonplace in systematic reviews. J Clin Epidemiol 2006; 59: 765–9. [DOI] [PubMed] [Google Scholar]
- 16. Jary SW, Whitelaw A, Walløe L, Thoresen M. Comparison of Bayley 2 and Bayley 3 scores at 18 months in tern infants following neonatal encephalopathy and therapeutic hypothermia. Dev Med Child Neurol 2013; 55: 1053–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Newburger JW, Jonas RA, Wernovsky G, et al. A comparison of the perioperative neurologic effects of hypothermic circulatory arrest versus low‐flow cardiopulmonary bypass in infant heart surgery. N Engl J Med 1993; 329: 1057–64. [DOI] [PubMed] [Google Scholar]
- 18. Hövels‐Gürich HH, Seghaye MC, Däbritz S, Messmer BJ, von Bernuth G. Cognitive and motor development in preschool and school‐aged children after neonatal arterial switch operation. J Thorac Cardiovasc Surg 1997; 114: 578–85. [DOI] [PubMed] [Google Scholar]
- 19. Andropoulos DB, Easley RB, Brady K, et al. Changing expectations for neurological outcomes after the neonatal arterial switch operation. Ann Thorac Surg 2012; 94: 1250–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Andropoulos DB, Brady K, Easley RB, et al. Erythropoietin neuroprotection in neonatal cardiac surgery: a phase I/II safety and efficacy trial. J Thorac Cardiovasc Surg 2013; 146: 124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Andropoulos DB, Easley RB, Brady K, et al. Neurodevelopmental outcomes after regional cerebral perfusion with neuromonitoring for neonatal aortic arch reconstruction. Ann Thorac Surg 2013; 95: 648–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Andropoulos DB, Ahmad HB, Haq T, et al. The association between brain injury, perioperative anesthetic exposure, and 12‐month neurodevelopmental outcomes after neonatal cardiac surgery: a retrospective cohort study. Paediatr Anaesth 2014; 24: 266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bellinger DC, Jonas RA, Rappaport LA, et al. Developmental and neurologic status of children after heart surgery with hypothermic circulatory arrest or low‐flow cardiopulmonary bypass. New Engl J Med 1995; 332: 549–55. [DOI] [PubMed] [Google Scholar]
- 24. Bellinger DC, Rappaport LA, Wypij D, Wernovsky G, Newburger JW. Patterns of developmental dysfunction after surgery during infancy to correct transposition of the great arteries. J Dev Behav Pediatr 1997; 18: 75–83. [DOI] [PubMed] [Google Scholar]
- 25. Bartlett JM, Wypij D, Bellinger DC, et al. Effect of prenatal diagnosis on outcomes in D‐transposition of the great arteries. Pediatrics 2004; 113: e335–40. [DOI] [PubMed] [Google Scholar]
- 26. Sarajuuri A, Lönnqvist T, Mildh L, et al. Prospective follow‐up study of children with univentricular heart: Neurodevelopmental outcome at age 12 months. J Thorac Cardiovasc Surg 2009; 137: 139–45. [DOI] [PubMed] [Google Scholar]
- 27. Gunn JK, Beca J, Hunt RW, Olischar M, Shekerdemian LS. Perioperative amplitude‐integrated EEG and neurodevelopment in infants with congenital heart disease. Intensive Care Med 2012; 38: 1539–47. [DOI] [PubMed] [Google Scholar]
- 28. Rajantie I, Laurila M, Pollari K, et al. Motor development of infants with univentricular heart at the ages of 16 and 52 weeks. Pediatr Phys Ther 2013; 25: 444–50. [DOI] [PubMed] [Google Scholar]
- 29. Sarajuuri A, Lönnqvist T, Schmitt F, Almqvist F, Jokinen E. Patients with univentricular heart in early childhood: parenting stress and child behaviour. Acta Paediatr 2012; 101: 252–7. [DOI] [PubMed] [Google Scholar]
- 30. Gunn JK, Beca J, Hunt RW, et al. Perioperative risk factors for impaired neurodevelopment after cardiac surgery in early infancy. Arch Dis Child 2016; 101: 1010–6. [DOI] [PubMed] [Google Scholar]
- 31. Goldsworthy M, Franich‐Ray C, Kinney S, Shekerdemian L, Beca J, Gunn J. Relationship between social‐emotional and neurodevelopment of 2‐year‐old children with congenital heart disease. Congenit Heart Dis 2016; 11: 378–85. [DOI] [PubMed] [Google Scholar]
- 32. Robertson CM, Joffe AR, Sauve RS, et al. Outcomes from an interprovincial program of newborn open heart surgery. J Pediatr 2004; 144: 86–92. [DOI] [PubMed] [Google Scholar]
- 33. Cheung PY, Chui N, Joffe AR, et al. Postoperative lactate concentrations predict the outcome of infants aged 6 weeks or less after intracardiac surgery: a cohort follow‐up to 18 months. J Thorac Cardiovasc Surg 2005; 130: 837–43. [DOI] [PubMed] [Google Scholar]
- 34. Atallah J, Dinu IA, Joffe AR, et al. Two‐year survival and mental and psychomotor outcomes after the Norwood procedure: an analysis of the modified Blalock‐Taussig shunt and right ventricle‐to‐pulmonary artery shunt surgical eras. Circulation 2008; 118: 1410–8. [DOI] [PubMed] [Google Scholar]
- 35. Blackwood J, Joffe AR, Robertson CM, et al. Association of hemoglobin and transfusion with outcome after operations for hypoplastic left heart. Ann Thorac Surg 2010; 89: 1378–84. [DOI] [PubMed] [Google Scholar]
- 36. Gaynor JW, Gerdes M, Zackai EH, et al. Apolipoprotein E genotype and neurodevelopmental sequelae of infant cardiac surgery. J Thorac Cardiovasc Surg 2003; 126: 1736–45. [DOI] [PubMed] [Google Scholar]
- 37. Fuller S, Nord AS, Gerdes M, et al. Predictors of impaired neurodevelopmental outcomes at one year of age after infant cardiac surgery. Eur J Cardiothorac Surg 2009; 36: 40–7. [DOI] [PubMed] [Google Scholar]
- 38. Long SH, Galea MP, Eldridge BJ, Harris SR. Performance of 2‐year‐old children after early surgery for congenital heart disease on the Bayley Scales of Infant and Toddler Development, Third Edition. Early Hum Dev 2012; 88: 603–7. [DOI] [PubMed] [Google Scholar]
- 39. Long SH, Harris SR, Eldridge BJ, Galea MP. Gross motor development is delayed following early cardiac surgery. Cardiol Young 2012; 22: 574–82. [DOI] [PubMed] [Google Scholar]
- 40. Anderson R, Cook A. Morphology of the functional univentricular heart. Cardiol Young 2004; 14(Suppl. 1): 3–12. [DOI] [PubMed] [Google Scholar]
- 41. Garcia Guerra G, Robertson CMT, Alton GY, et al. Neurodevelopmental outcome following exposure to sedative and analgesic drugs for complex cardiac surgery in infancy. Paediatr Anaesth 2011; 21: 932–41. [DOI] [PubMed] [Google Scholar]
- 42. Aly SA, Zurakowski D, Glass P, Skurow‐Todd K, Jonas RA, Donofrio MT. Cerebral tissue oxygenation index and lactate at 24 hours postoperative predict survival and neurodevelopmental outcome after neonatal cardiac surgery. Congenit Heart Dis 2017; 12: 188–95. [DOI] [PubMed] [Google Scholar]
- 43. Mackie AS, Alton GY, Dinu IA, et al. Clinical outcome score predicts the need for neurodevelopmental intervention after infant heart surgery. J Thorac Cardiovasc Surg 2013; 145: 1248–54. [DOI] [PubMed] [Google Scholar]
- 44. Acton BV, Biggs WS, Creighton DE, et al. Overestimating neurodevelopment using the Bayley‐III after early complex cardiac surgery. Pediatrics 2011; 128: e794–800. [DOI] [PubMed] [Google Scholar]
- 45. Newburger JW, Sleeper LA, Bellinger DC, et al. Early developmental outcome in children with hypoplastic left heart syndrome and related anomalies: the single ventricle reconstruction trial. Circulation 2012; 125: 2081–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cheatham SL, Carey H, Chisolm JL, Heathcock JC, Steward D. Early results of neurodevelopment following hybrid stage I for hypoplastic left heart syndrome. Pediatr Cardiol 2015; 36: 685–91. [DOI] [PubMed] [Google Scholar]
- 47. Hicks MS, Sauve RS, Robertson CM, et al. Early childhood language outcomes after arterial switch operation: a prospective cohort study. SpringerPlus 2016; 5: 1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Joynt C, Robertson CM, Cheung PY, et al. Two‐year neurodevelopmental outcomes of infants undergoing neonatal cardiac surgery for interrupted aortic arch: a descriptive analysis. J Thorac Cardiovasc Surg 2009; 138: 924–32. [DOI] [PubMed] [Google Scholar]
- 49. Ricci MF, Alton GY, Ross DB, et al. Gastrostomy tube feeding after neonatal complex cardiac surgery identifies the need for early developmental intervention. J Pediatr 2016; 169: 160–5. [DOI] [PubMed] [Google Scholar]
- 50. Alton GY, Robertson CM, Sauve R, et al. Early childhood health, growth, and neurodevelopmental outcomes after complete repair of total anomalous pulmonary venous connection at 6 weeks or younger. J Thorac Cardiovasc Surg 2007; 133: 905–11. [DOI] [PubMed] [Google Scholar]
- 51. Freed DH, Robertson CM, Sauve RS, et al. Intermediate‐term outcomes of the arterial switch operation for transposition of great arteries in neonates: alive but well? J Thorac Cardiovasc Surg 2006; 132: 845–52. [DOI] [PubMed] [Google Scholar]
- 52. Mackie AS, Vatanpour S, Alton GY, et al. Clinical outcome score predicts adverse neurodevelopmental outcome after infant heart surgery. Ann Thorac Surg 2015; 99: 2124–32. [DOI] [PubMed] [Google Scholar]
- 53. Goldberg C. Neurocognitive outcomes for children with functional single ventricle malformations. Pediatr Cardiol 2007; 28: 443–7. [DOI] [PubMed] [Google Scholar]
- 54. Li X, Robertson CM, Yu X, et al. Early postoperative systemic inflammatory response is an important determinant for adverse 2‐year neurodevelopment‐associated outcomes after the Norwood procedure. J Thorac Cardiovasc Surg 2014; 148: 202–6. [DOI] [PubMed] [Google Scholar]
- 55. Williams IA, Tarullo AR, Grieve PG, et al. Fetal cerebrovascular resistance and neonatal EEG predict 18–month neurodevelopmental outcome in infants with congenital heart disease. Ultrsound Obstet Gynecol 2012; 40: 304–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Campbell MJ, Ziviani JM, Stocker CF, Khan A, Sakzewski L. Neuromotor performance in infants before and after early open‐heart surgery and risk factors for delayed development at 6 months of age. Cardiol Young 2019; 29: 100–9. [DOI] [PubMed] [Google Scholar]
- 57. Limperopoulos C, Majnemer A, Shevell MI, Rosenblatt B, Rohlicek C, Tchervenkov C. Neurologic status of newborns with congenital heart defects before open heart surgery. Pediatrics 1999; 103: 402–8. [DOI] [PubMed] [Google Scholar]
- 58. Limperopoulos C. Neurodevelopmental status of newborns and infants with congenital heart defects before and after open heart surgery. J Pediatr 2000; 137: 638–45. [DOI] [PubMed] [Google Scholar]
- 59. Owen M, Shevell M, Donofrio M, et al. Brain volume and neurobehavior in newborns with complex congenital heart defects. J Pediatr 2014; 164: 1121–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Martin BJ, Ross DB, Alton GY, et al. Clinical and functional developmental outcomes in neonates undergoing truncus arteriosus repair: a cohort study. Ann Thorac Surg 2016; 101: 1827–33. [DOI] [PubMed] [Google Scholar]
- 61. Rappaport LA, Wypij D, Bellinger DC, et al. Relation of seizures after cardiac surgery in early infancy to neurodevelopmental outcome. Boston Circulatory Arrest Study Group. Circulation 1998; 97: 773–9. [DOI] [PubMed] [Google Scholar]
- 62. Bellinger DC, Wypij D, Kuban KC, et al. Developmental and neurological status of children at 4 years of age after heart surgery with hypothermic circulatory arrest or low‐flow cardiopulmonary bypass. Circulation 1999; 100: 526–32. [DOI] [PubMed] [Google Scholar]
- 63. Sarajuuri A, Jokinen E, Puosi R, et al. Neurodevelopment in children with hypoplastic left heart syndrome. J Pediatr 2010; 157: 414–20. [DOI] [PubMed] [Google Scholar]
- 64. Puosi R, Korkman M, Sarajuuri A, et al. Neurocognitive development and behavioral outcome of 2‐year‐old children with univentricular heart. J Int Neuropsychol Soc 2011; 17: 1094–103. [DOI] [PubMed] [Google Scholar]
- 65. Reich B, Heye K, Tuura R, et al. Neurodevelopmental outcome and health related quality of life in children with single ventricle heart disease before Fontan procedure. Semin Thorac Cardiovasc Surg 2017; 29: 504–13. [DOI] [PubMed] [Google Scholar]
- 66. Knirsch W, Mayer KN, Scheer I, et al. Structural cerebral abnormalities and neurodevelopmental status in single ventricle congenital heart disease before Fontan procedure. Eur J Cardiothorac Surg 2017; 51: 740–6. [DOI] [PubMed] [Google Scholar]
- 67. Goldberg CS, Lu M, Sleeper LA, et al. Factors associated with neurodevelopment for children with single ventricle lesions. J Pediatr 2014; 165: 490–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rogers BT, Msall ME, Buck GM, et al. Neurodevelopmental outcome of infants with hypoplastic left heart syndrome. J Pediatr 1995; 126: 496–8. [DOI] [PubMed] [Google Scholar]
- 69. Toet MC, Flinterman A, Laar I, et al. Cerebral oxygen saturation and electrical brain activity before, during, and up to 36 hours after arterial switch procedure in neonates without pre‐existing brain damage: its relationship to neurodevelopmental outcome. Exp Brain Res 2005; 165: 343–50. [DOI] [PubMed] [Google Scholar]
- 70. Bellinger D, Bernstein JH, Kirkwood MW, Rappaport LA, Newburger JW. Visual‐spatial skills in children with open‐heart surgery. J Dev Behav Pediatr 2003; 24: 169–79. [DOI] [PubMed] [Google Scholar]
- 71. Bellinger DC, Wypij D, duPlessis AJ, et al. Neurodevelopmental status at eight years in children with dextro‐transposition of the great arteries: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg 2003; 126: 1385–96. [DOI] [PubMed] [Google Scholar]
- 72. Newburger JW, Wypij D, Bellinger DC, et al. Length of stay after infant heart surgery is related to cognitive outcome at age 8 years. J Pediatr 2003; 143: 67–73. [DOI] [PubMed] [Google Scholar]
- 73. Wypij D, Newburger JW, Rappaport LA, et al. The effect of duration of deep hypothermic circulatory arrest in infant heart surgery on late neurodevelopment: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg 2003; 126: 1397–403. [DOI] [PubMed] [Google Scholar]
- 74. de Ferranti S, Gauvreau K, Hickey PR, et al. Intraoperative hyperglycemia during infant cardiac surgery is not associated with adverse neurodevelopmental outcomes at 1, 4, and 8 years. Anesthesiology 2004; 100: 1345–52. [DOI] [PubMed] [Google Scholar]
- 75. McGrath E, Wypij D, Rappaport LA, Newburger JW, Bellinger DC. Prediction of IQ and achievement at age 8 years from neurodevelopmental status at age 1 year in children with D‐transposition of the great arteries. Pediatrics 2004; 114: e572–6. [DOI] [PubMed] [Google Scholar]
- 76. Bellinger DC, Newburger JW, Wypij D, Kuban KC, duPlesssis AJ, Rappaport LA. Behaviour at eight years in children with surgically corrected transposition: the Boston Circulatory Arrest Trial. Cardiol Young 2009; 19: 86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cassidy AR, White MT, DeMaso DR, Newburger JW, Bellinger DC. Processing speed, executive function, and academic achievement in children with dextro‐transposition of the great arteries: testing a longitudinal developmental cascade model. Neuropsychology 2016; 30: 874–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sarajuuri A, Jokinen E, Mildh L, et al. Neurodevelopmental burden at age 5 years in patients with univentricular heart. Pediatrics 2012; 130: 1636–46. [DOI] [PubMed] [Google Scholar]
- 79. Maenpaa H, Hakkinen A, Sarajuuri A. Changes in motor development during a 4‐year follow‐up on children with univentricular heart defects. Pediatr Phys Ther 2016; 28: 446–51. [DOI] [PubMed] [Google Scholar]
- 80. Long SH, Eldridge BJ, Harris SR, Cheung MM. Motor skills of 5‐year‐old children who underwent early cardiac surgery. Cardiol Young 2016; 26: 650–7. [DOI] [PubMed] [Google Scholar]
- 81. Creighton DE, Robertson CM, Sauve RS, et al. Neurocognitive, functional, and health outcomes at 5 years of age for children after complex cardiac surgery at 6 weeks of age or younger. Pediatrics 2007; 120: e478–86. [DOI] [PubMed] [Google Scholar]
- 82. Calderon J, Angeard N, Moutier S, et al. Impact of prenatal diagnosis on neurocognitive outcomes in children with transposition of the great arteries. J Pediatr 2012; 161: 94–8. [DOI] [PubMed] [Google Scholar]
- 83. Hovels‐Gurich HH, Konrad K, Wiesner M, et al. Long term behavioural outcome after neonatal arterial switch operation for transposition of the great arteries. Arch Dis Child 2002; 87: 506–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hovels‐Gurich HH, Seghaye MC, Schnitker R, et al. Long‐term neurodevelopmental outcomes in school‐aged children after neonatal arterial switch operation. J Thorac Cardiovasc Surg 2002; 124: 448–58. [DOI] [PubMed] [Google Scholar]
- 85. Calderon J, Angeard N, Pinabiaux C, et al. Facial expression recognition and emotion understanding in children after neonatal open‐heart surgery for transposition of the great arteries. Dev Med Child Neurol 2014; 56: 564–71. [DOI] [PubMed] [Google Scholar]
- 86. Calderon J, Jambaque I, Bonnet D, Angeard N. Executive functions development in 5– to 7‐year‐old children with transposition of the great arteries: a longitudinal study. Dev Neuropsychol 2014; 39: 365–84. [DOI] [PubMed] [Google Scholar]
- 87. Spijkerboer AW, Utens EM, Bogers AJ, et al. Long‐term behavioural and emotional problems in four cardiac diagnostic groups of children and adolescents after invasive treatment for congenital heart disease. Int J Cardiol 2008; 125: 66–73. [DOI] [PubMed] [Google Scholar]
- 88. Spijkerboer AW, Utens EM, Bogers AJ, et al. Long‐term intellectual functioning and school‐related behavioural outcomes in children and adolescents after invasive treatment for congenital heart disease. Br J Dev Psychol 2008; 26: 457–70. [Google Scholar]
- 89. Ellerbeck KA, Smith ML, Holden EW, et al. Neurodevelopmental outcomes in children surviving d‐transposition of the great arteries. J Dev Behav Pediatr 1998; 19: 335–41. [DOI] [PubMed] [Google Scholar]
- 90. Hagemo PS, Skarbo AB, Rasmussen M, et al. An extensive long term follow‐up of a cohort of patients with hypoplasia of the left heart. Cardiol Young 2007; 17: 51–5. [DOI] [PubMed] [Google Scholar]
- 91. Bergemann A, Hansen JH, Rotermann I, et al. Neuropsychological performance of school‐aged children after staged surgical palliation of hypoplastic left heart syndrome. Eur J Cardiothorac Surg 2015; 47: 803–11. [DOI] [PubMed] [Google Scholar]
- 92. Sarajuuri A, Jokinen E, Puosi R, et al. Neurodevelopmental and neuroradiologic outcomes in patients with univentricular heart aged 5 to 7 years: related risk factor analysis. J Thorac Cardiovasc Surg 2007; 133: 1524–32. [DOI] [PubMed] [Google Scholar]
- 93. Sarrechia I, Miatton M, De Wolf D, et al. Neurocognitive development and behaviour in school‐aged children after surgery for univentricular or biventricular congenital heart disease. Eur J Cardiothorac Surg 2016; 49: 167–74. [DOI] [PubMed] [Google Scholar]
- 94. Muñoz‐López M, Hoskote A, Chadwick MJ, et al. Hippocampal damage and memory impairment in congenital cyanotic heart disease. Hippocampus 2017; 27: 417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Laraja K, Sadhwani A, Tworetzky W, et al. Neurodevelopmental outcome in children after fetal cardiac intervention for aortic stenosis with evolving hypoplastic left heart syndrome. J Pediatr 2017; 184: 130–36.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Shillingford AJ, Glanzman MM, Ittenbach RF, et al. Inattention, hyperactivity, and school performance in a population of school‐age children with complex congenital heart disease. Pediatrics 2008; 121: e759–67. [DOI] [PubMed] [Google Scholar]
- 97. Bean Jaworski JL, Flynn T, Burnham N, et al. Rates of autism and potential risk factors in children with congenital heart defects. Congenit Heart Dis 2017; 12: 421–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Tsao PC, Lee YS, Jeng MJ, et al. Additive effect of congenital heart disease and early developmental disorders on attention‐deficit/hyperactivity disorder and autism spectrum disorder: a nationwide population‐based longitudinal study. Eur Child Adolesc Psychiatry 2017; 26: 1351–9. [DOI] [PubMed] [Google Scholar]
- 99. Sterken C, Lemiere J, Van Den Berghe G, Mesotten D. Neurocognitive development after pediatric heart surgery. Pediatrics 2016; 137: e20154675. [DOI] [PubMed] [Google Scholar]
- 100. Neufeld RE, Clark BG, Robertson CM, et al. Five‐year neurocognitive and health outcomes after the neonatal arterial switch operation. J Thorac Cardiovasc Surg 2008; 136: 1413–21. [DOI] [PubMed] [Google Scholar]
- 101. Bellinger DC, Wypij D, Rivkin MJ, et al. Adolescents with d‐transposition of the great arteries corrected with the arterial switch procedure: neuropsychological assessment and structural brain imaging. Circulation 2011; 124: 1361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. DeMaso DR, Labella M, Taylor GA, et al. Psychiatric disorders and function in adolescents with d‐transposition of the great arteries. J Pediatr 2014; 165: 760–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Heinrichs AK, Holschen A, Krings T, et al. Neurologic and psycho‐intellectual outcome related to structural brain imaging in adolescents and young adults after neonatal arterial switch operation for transposition of the great arteries. J Thorac Cardiovasc Surg 2014; 148: 2190–9. [DOI] [PubMed] [Google Scholar]
- 104. Cassidy AR, White MT, DeMaso DR, et al. Executive function in children and adolescents with critical cyanotic congenital heart disease. J Int Neuropsychol Soc 2015; 21: 34–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Cassidy AR, Bernstein JH, Bellinger DC, et al. Visual‐spatial processing style is associated with psychopathology in adolescents with critical congenital heart disease. Child Neuropsychol 2018; 24: 451–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Idorn L, Jensen AS, Juul K, et al. Quality of life and cognitive function in Fontan patients, a population‐based study. Int J Cardiol 2013; 168: 3230–35. [DOI] [PubMed] [Google Scholar]
- 107. Garcia Guerra G, Robertson CM, Alton GY, et al. Neurotoxicity of sedative and analgesia drugs in young infants with congenital heart disease: 4‐year follow‐up. Paediatr Anaesth 2014; 24: 257–65. [DOI] [PubMed] [Google Scholar]
- 108. Sidhu N, Joffe AR, Doughty P, et al. Sepsis after cardiac surgery early in infancy and adverse 4.5‐year neurocognitive outcomes. J Am Heart Assoc 2015; 4: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ryberg C, Sunnegardh J, Thorson M, Broberg M. Intellectual functioning in children with congenital heart defects treated with surgery or by catheter interventions. Front Pediatr 2016; 4: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration 2011. www.handbook.cochrane.org (accessed 09 March 2011]
- 111. Terri N, Schmidt CH, Lau J. In an empirical evaluation of the funnel plot, researchers could not visually identify publication bias. J Clin Epidemiol 2005; 58: 894–901. [DOI] [PubMed] [Google Scholar]
- 112. Martin BJ, De Villiers Jonker I, Joffe AR, et al. Hypoplastic left heart syndrome is not associated with worse clinical or neurodevelopmental outcomes than other cardiac pathologies after the Norwood‐Sano operation. Pediatr Cardiol 2017; 38: 922–31. [DOI] [PubMed] [Google Scholar]
- 113. Alton GY, Taghados S, Joffe AR, Robertson CM, Dinu I. Prediction of preschool functional abilities after early complex cardiac surgery. Cardiol Young 2015; 25: 655–62. [DOI] [PubMed] [Google Scholar]
- 114. Bellinger DC, Wernovsky G, Rappaport LA, et al. Cognitive development of children following early repair of transposition of the great arteries using deep hypothermic circulatory arrest. Pediatrics 1991; 87: 701–7. [PubMed] [Google Scholar]
- 115. Ricci MF, Andersen JC, Joffe AR, et al. Chronic neuromotor disability after complex cardiac surgery in early life. Pediatrics 2015; 136: e922–33. [DOI] [PubMed] [Google Scholar]
- 116. Kern JH, Hinton VJ, Nereo NE, et al. Early developmental outcome after the norwood procedure for hypoplastic left heart syndrome. Pediatrics 1998; 102: 1148–52. [DOI] [PubMed] [Google Scholar]
- 117. Hovels‐Gurich HH, Seghaye MC, Sigler M, et al. Neurodevelopmental outcome related to cerebral risk factors in children after neonatal arterial switch operation. Ann Thorac Surg 2001; 71: 881–8. [DOI] [PubMed] [Google Scholar]
- 118. Spijkerboer AW, De Koning WB, Duivenvoorden HJ, et al. Medical predictors for long‐term behavioral and emotional outcomes in children and adolescents after invasive treatment of congenital heart disease. J Pediatr Surg 2010; 45: 2146–53. [DOI] [PubMed] [Google Scholar]
- 119. Knirsch W, Liamlahi R, Hug M, et al. Mortality and neurodevelopmental outcome at 1 year of age comparing hybrid and Norwood procedures. Eur J Cardiothorac Surg 2012; 42: 33–9. [DOI] [PubMed] [Google Scholar]
- 120. Fewtrell MS, Kennedy K, Singhal A, et al. How much loss to follow‐up is acceptable in long‐term randomised trials and prospective studies? Arch Dis Child 2008; 93: 458–61. [DOI] [PubMed] [Google Scholar]
- 121. Hadders‐Algra M. Early intervention: The challenge to find the best approach for infant and family. Austr Occup Ther J 2017; 64: E174. [DOI] [PubMed] [Google Scholar]
- 122. Alam S, Shalini A, Hedge R, et al. A comparative study of the risk stratification models for pediatric cardiac surgery. Egypt J Crit Care Physic 2018; 6: 5–8. [Google Scholar]
- 123. Morton PD, Ishibashi N, Jonas RA. Neurodevelopmental abnormalities and congenital heart disease: insights into altered brain maturation. Circ Res 2017; 120: 960–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Allotey J, Zamora J, Cheong‐See F, et al. Cognitive, motor, behavioural and academic performances of children born preterm: a meta‐analysis and systematic review involving 64 061 children. BJOG 2018; 125: 16–25. [DOI] [PubMed] [Google Scholar]
- 125. van Houdt CA, Oosterlaan J, van Wassenaer‐Leemhuis AG, et al. Executive function deficits in children born preterm or at low birthweight: a meta‐analysis. Dev Med Child Neurol 2019; 61: 1015–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Andropoulos DB, Brady KM, Easley RB, Fraser CD. Neuroprotection in pediatric cardiac surgery: What is on the horizon? Prog Pediatr Cardiol 2010; 29: 113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. von Rhein M, Buchmann A, Hagmann C, et al. Severe congenital heart defects are associated with global reduction of neonatal brain volumes. J Pediatr 2015; 167: 1259–63.e1251. [DOI] [PubMed] [Google Scholar]
- 128. Guo T, Chau V, Peyvandi S, et al. White matter injury in term neonates with congenital heart diseases: Topology & comparison with preterm newborns. Neuroimage 2019; 185: 742–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Clouchoux C, du Plessis AJ, Bouyssi‐Kobar M, et al. Delayed cortical development in fetuses with complex congenital heart disease. Cereb Cortex 2013; 23: 2932–43. [DOI] [PubMed] [Google Scholar]
- 130. Goff DA, Shera DM, Tang S, et al. Risk factors for preoperative periventricular leukomalacia in term neonates with hypoplastic left heart syndrome are patient related. J Thorac Cardiovasc Surg 2014; 147: 1312–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Rajagopalan R, Votava‐Smith J, Zhuang X, et al. Fetuses with single ventricle congenital heart disease manifest impairment of regional brain growth. Prenat Diagn 2018; 38: 1042–8. [DOI] [PubMed] [Google Scholar]
- 132. Khairy P, Ionescu‐Ittu R, Mackie AS, et al. Changing mortality in congenital heart disease. J Am Coll Cardiol 2010; 56: 1149–57. [DOI] [PubMed] [Google Scholar]
- 133. Williams J, Lee KJ, Anderson PJ. Prevalence of motor‐skill impairment in preterm children who do not develop cerebral palsy: a systematic review. Dev Med Child Neurol 2010; 52: 232–7. [DOI] [PubMed] [Google Scholar]
- 134. Dewey D, Thompson DK, Kelly CE, et al. Very preterm children at risk for developmental coordination disorder have brain alterations in motor areas. Acta Paediatr 2019; 108: 1649–60. [DOI] [PubMed] [Google Scholar]
- 135. Wuang Y, Wang C, Huang M. Health‐related quality of life in children with developmental coordination disorder and their parents. Occup Ther J Res Occup Participat Health 2012; 32: 142–50. [Google Scholar]
- 136. Wilson PH, Ruddock S, Smits‐Engelsman B, et al. Understanding performance deficits in developmental coordination disorder: a meta‐analysis of recent research. Dev Med Child Neur 2013; 55: 217–28. [DOI] [PubMed] [Google Scholar]
- 137. Geertsen SS, Thomas R, Larsen MN, et al. Motor skills and exercise capacity are associated with objective measures of cognitive functions and academic performance in preadolescent children. PLoS ONE 2016; 11: e0161960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Poole KL, Schmidt LA, Ferro MA, et al. Early developmental influences on self‐esteem trajectories from adolescence through adulthood: impact of birth weight and motor skills. Dev Psychopathol 2018; 30: 113–23. [DOI] [PubMed] [Google Scholar]
- 139. Robertson CM, Alton GY, Bork KT, et al. Bilateral sensory permanent hearing loss after palliative hypoplastic left heart syndrome operation. Ann Thorac Surg 2012; 93: 1248–53. [DOI] [PubMed] [Google Scholar]
- 140. Bork KT, To BP, Leonard NJ, et al. Prevalence of childhood permanent hearing loss after early complex cardiac surgery. J Pediatr 2018; 198: 104–9. [DOI] [PubMed] [Google Scholar]
- 141. Jaekel J, Bartmann P, Schneider W, Wolke D. Neurodevelopmental pathways to preterm children’s specific and general mathematic abilities. Early Hum Dev 2014; 90: 639–44. [DOI] [PubMed] [Google Scholar]
- 142. Lehner DC, Sadler LS. Toddler developmental delays after extensive hospitalization: primary care practitioner guidelines. Pediatr Nurs 2015; 41: 236–42. [PubMed] [Google Scholar]
- 143. So S, Patterson C, Gold A, et al. Early neurodevelopmental outcomes of infants with intestinal failure. Early Hum Dev 2016; 101: 11–6. [DOI] [PubMed] [Google Scholar]
- 144. Peyvandi S, Chau V, Guo T, et al. Neonatal brain injury and timing of neurodevelopmental assessment in patients with congenital heart disease. J Am Coll Cardiol 2018; 71: 1986–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Lynch JM, Gaynor JW, Licht DJ. Brain injury during transition in the newborn with congenital heart disease: hazards of the preoperative period. Semin Pediatr Neurol 2018; 28: 60–5. [DOI] [PubMed] [Google Scholar]
- 146. Watson CT, Marques‐Bonet T, Sharp AJ, Mefford HC. The genetics of microdeletion and microduplication syndromes: an update. Annu Rev Genom Hum Genet 2014; 15: 215–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Srour M, Shevell M. Genetics and the investigation of developmental delay/intellectual disability. Arch Dis Child 2014; 99: 386–9. [DOI] [PubMed] [Google Scholar]
- 148. Marino BS, Lipkin PH, Newburger JW, et al. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management. Circulation 2012; 126: 1143–72. [DOI] [PubMed] [Google Scholar]
- 149. Black MM, Walker SP, Fernald LCH, et al. Early childhood development coming of age: science through the life course. Lancet 2017; 389: 77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Jansen FA, Everwijn SM, Scheepjens R, et al. Fetal brain imaging in isolated congenital heart defects ‐ a systematic review and meta‐analysis. Prenat Diagn 2016; 36: 601–13. [DOI] [PubMed] [Google Scholar]
- 151. Khalil A, Suff N, Thilaganathan B, Hurrell A, Cooper D, Carvalho JS. Brain abnormalities and neurodevelopmental delay in congenital heart disease: systematic review and meta‐analysis. Ultrasound Obstet Gynecol 2014; 43: 14–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Search string.
Appendix S2: Funnel plots of and Egger’s tests.
Table S1: Methodological quality table – infant
Table S2: Methodological quality table – preschool
Table S3: Methodological quality table – school age
Table S4: Methodological quality table – adolescent age
Table S5: Developmental outcome table – infant
Table S6: Developmental outcome table – preschool
Table S7: Developmental outcome – school age
Table S8: Developmental outcome table – adolescent age
Table S9: Risk factor variables of hypoxemia table
Table S10: Risk factor variables of cerebral protection table
Table S11: Risk factor variables of severity of illness table
Figure S1: Inclusion and exclusion of articles found in the search strategy.


