Immune-related adverse events (irAEs) are critical limitations to cancer immunotherapy. Understanding mechanisms driving irAEs is critical to their prevention. Here, the authors discuss several hypotheses, the testing of which may improve patient outcomes.
Abstract
The treatment of many cancers has been revolutionized by immune checkpoint blockade (ICB) as a standard-of-care therapeutic. Despite many successes, a large proportion of patients treated with ICB agents experience immune-related adverse events (irAEs) in the form of clinical autoimmunity, ranging from mild to life threatening, that can limit cancer treatment. A mechanistic understanding of these irAEs is required to better treat or prevent irAEs and to predict those patients who are susceptible to irAEs. We propose several mechanisms that may contribute to the generation of irAEs: (1) preexisting susceptibility to autoimmunity, (2) aberrant presentation of “self” by the tumor, and (3) loss of tolerance driven by the tumor or tissue microenvironment.
Introduction
Immune checkpoint blockade (ICB) has revolutionized cancer therapy with several US Food and Drug Administration–approved treatments targeting the inhibitory receptors (IRs) cytotoxic T lymphocyte antigen 4 (CTLA-4), programmed cell death protein 1 (PD-1), and programmed death ligand 1 (PD-L1). Therapies targeting several other IRs are under clinical investigation. These IRs and their ligands play a critical role in maintaining immune homeostasis and resolving inflammation. Indeed, genetic deletion or antibody-mediated blockade of IRs/ligands in mouse models exacerbates existing autoimmunity or even precipitates spontaneous autoimmunity, highlighting the importance of IRs in immune homeostasis (reviewed in Zhang and Vignali, 2016).
In a subset of patients, treatment with ICB leads to aberrant immune activation against specific organs, known as “immune-related adverse events” (irAEs). Generally, irAEs colocalize around barrier (gut, lungs, and skin) or endocrine tissues (pancreas and thyroid; June et al., 2017), but importantly, the most common irAEs differ with different immunotherapeutic targets (Pauken et al., 2019b). This implies differing mechanisms of autoimmune reactivity between different ICBs, complicating the study of irAEs. irAEs are quite common, ranging from mild to life threatening, and can occur in up to 85% of patients treated with anti–CTLA-4 and in up to 37% and 24% of those treated with anti–PD-1 and anti–PD-L1, respectively (Brahmer et al., 2018; Pauken et al., 2019b). The timing of immune toxicity onset is variable, with skin toxicities often manifesting early, followed later by pulmonary or gastrointestinal manifestations, such as colitis, hepatitis, or endocrinopathies. Dual blockade of the CTLA-4 and PD-1 pathways leads to both increased frequency and severity of irAEs (Brahmer et al., 2018).
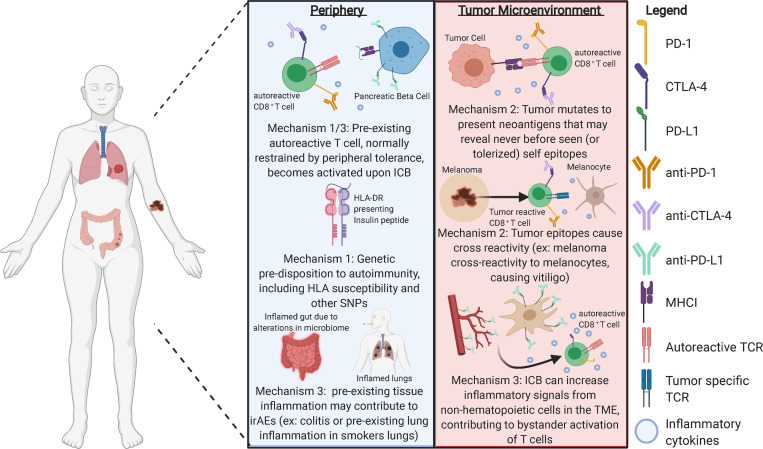
ICB-induced irAEs are similar in symptoms to spontaneous autoimmunity, though disease pathology may be quite different. Most spontaneous autoimmune diseases are likely induced by a combination of environmental and genetic factors, where the precise inciting event leading to symptoms is often unknown. In contrast, with ICB-induced irAEs, the inciting event is known. Other reviews have covered clinical management of irAEs (Brahmer et al., 2018). Here we focus on three potential mechanisms leading to the development of irAEs. We hypothesize that the development of irAEs represents a break in tolerance and occurs through multiple, often overlapping mechanisms (Fig. 1). While three distinct mechanisms are discussed, more than one may contribute to the irAEs experienced by each patient.
Figure 1.
There are several possible mechanisms contributing to irAEs in response to ICB. Importantly, these may occur distal to the tumor, in the periphery, or within the TME. It is still unclear which mechanism, if any, is the main driver of irAEs or to what degree interplay of these mechanisms contributes to disease. As irAEs are a critical limitation to cancer immunotherapy, understanding these mechanisms and how to prevent them is essential to the future of cancer immunotherapeutics.
Mechanism 1: Preexisting susceptibility to autoimmunity
In spontaneous autoimmunity, inherited susceptibilities, such as HLA haplotypes or single-nucleotide polymorphisms (SNPs), are often highly predictive of the risk of developing specific autoimmune syndromes, such as HLA-DR4 enrichment in type 1 diabetes patients. In a case series of ICB-induced diabetes, HLA-DR4 was similarly enriched among affected individuals (Marchand et al., 2019). Furthermore, SNPs in IRs are commonly associated with autoimmune conditions (Theofilopoulos et al., 2017), positing the theory that patients experiencing irAEs may have a genetic predisposition for autoimmunity and yet never experience an inciting incident. Studies linking SNPs to irAEs are ongoing.
Additionally, ICB may reveal preexisting, subclinical autoimmunity. Recent studies estimate that, in healthy mice, as many as 30% of CD4+ Foxp3– cells may recognize “self” (Cebula et al., 2019). These cells may be poised for activation following ICB. Furthermore, a study in non–small cell lung carcinoma found a positive correlation between nonautoimmune patients with preexisting autoantibodies and both response to anti-PD1 therapy and the development of irAEs (Toi et al., 2019), supporting the hypothesis that ICB may exacerbate preexisting autoimmunity. Studies linking subclinical autoimmunity or genetic predisposition to irAEs are incomplete and represent an active area of research.
Data regarding patients with preexisting clinical autoimmune conditions and treatment with ICB are limited, as initial clinical trials excluded individuals with a history of autoimmunity. Retrospective studies show that only a subset of autoimmune patients treated with ICB developed flares after ICB treatment, with the exception of those with preexisting gastrointestinal or neurological autoimmune diseases (Menzies et al., 2017), the reason for which is unknown. Interestingly, a subset of these patients developed new autoimmune symptoms (Menzies et al., 2017). Taken together, these observations suggest that mechanisms other than simply genetic predisposition or subclinical autoimmunity contribute to the development of irAEs. Understanding these clinical observations may reveal the impact genetics play as well as new mechanisms that contribute to the development of irAEs.
Mechanism 2: Aberrant presentation of “self” by the tumor
Tumor cells may break peripheral tolerance by aberrantly expressing self-peptides that are generally restricted during development (e.g., cancer testis antigens) or to immune-privileged sites such as the central nervous system, testes, or eye. In this mechanism, it is the tumor cells that intrinsically drive the break in tolerance. Though cancer testis antigens can be exploited to generate an immune response to a tumor (Wei et al., 2019), it is conceivable that autoreactive T cells may be activated after ICB treatment and traffic to the physiological site of antigen expression. For example, vitiligo is associated with increased response to ICB in melanoma and may represent a tissue-specific response against a skin antigen that was triggered by the primary immune response to the tumor (Byrne and Turk, 2011). Similarly, uveitis, in which inflammation of the anterior or posterior compartment of the eye occurs after ICB treatment (Cunningham et al., 2020; Dow et al., 2020), may reflect aberrant expression of ocular antigens by tumor cells.
All tumors arise as a series of mutations from normal healthy cells, though tumors that are most likely to respond to ICB are often associated with increased mutational burden and predicted neoantigens (Miao et al., 2018). Indeed, increased diversity of the T cell repertoire has been associated with increased irAEs after ipilimumab (anti–CTLA-4; Oh et al., 2017). Importantly, as irAEs are not necessarily specific to the organ of the primary or metastatic sites of the tumor, immune-mediated destruction may unleash presentation of self-antigens not previously seen. One method by which neoantigens may be generated is through alternative reading frames, leading to expression of defective ribosomal products, which have been described in viral infections such as influenza, as a source of T cell epitopes (Zanker et al., 2019). These alternative reading frames generating new antigens might activate autoreactive T cells.
Similarly, epitope spreading, defined as an immune response to a new epitope distinct from the primary epitope of immune reactivity, often triggers autoimmunity in response to tissue trauma and propagates existing autoimmunity (Powell and Black, 2001; Vanderlugt and Miller, 2002). Interestingly, epitope spreading has been cited as a mechanism of action in many immunotherapies (Brossart, 2020). While epitope spreading toward tumor antigens is clearly beneficial, it is conceivable that T cell–mediated tumor destruction may reveal previously unexposed epitopes, some of which may trigger autoreactivity. Further studies are required to ensure tumor-specific epitope spreading occurs while limiting nonspecific T cell activation that may propagate autoimmunity.
In this vein, autoimmune syndromes might be expected to arise in the setting of malignancy. Indeed, the association between certain cancers and autoimmune syndromes is well documented. Small cell lung cancer or thymoma is associated with specific paraneoplastic syndromes (Graus and Dalmau, 2019), and the onset of scleroderma with autoantibodies directed against RNA polymerase III has been linked to the development of cancer (Shah and Casciola-Rosen, 2015). The mechanisms linking cancers and autoimmunity are unclear but raise the question whether the process of tumorigenesis, at least for certain tumors, can drive immunity directed toward autoantigens. Taken together, these observations suggest that, by nature, a tumor may promote the aberrant expression and recognition of self-antigens in an inflammatory setting that would otherwise induce a tolerogenic response. Future studies aimed at identifying T cells in tumors with specificities matching self-antigens expressed in other organs would confirm these hypotheses.
Mechanism 3: Loss of tolerance driven by the tissue or tumor microenvironment (TME)
The TME uniquely contributes to successful immune evasion and tumor growth (Junttila and de Sauvage, 2013) and may itself contribute to the loss of tolerance seen in irAEs. In this mechanism, it is the TME that drives the break in tolerance extrinsically from the tumor cells. It is also possible that certain tissue locations may impart unique environmental characteristics that could impact the probability of loss of tolerance (e.g., sites of environmental interface, such as the colon). Single-cell RNA sequencing of immune cells in ICB-associated colitis demonstrated shared TCRs between a cytotoxic effector CD8+ population and CD8+ resident memory T cells, suggesting that these CD8+ resident memory T cells may contribute to early-onset colitis either directly or through the recruitment of other T cell populations (Luoma et al., 2020). Indeed, the generally harsh and inflammatory environment of the TME may contribute to immune activation and breaks in peripheral tolerance.
Furthermore, within the TME, nonhematopoietic cells may contribute to irAEs such as cancer-associated fibroblasts (CAFs) and tumor vasculature. While vital to supporting the tumor, CAFs and tumor vasculature also interact with and influence immune cell function (Hendry et al., 2016; Monteran and Erez, 2019). Importantly, CAFs and tumor vasculature directly express PD-L1 and PD-L2 to suppress effector T cells in the TME (Hendry et al., 2016; Monteran and Erez, 2019; Skowera et al., 2015). Treatment with ICB anti–PD-L1 may then enhance generalized inflammation and T cell effector function, providing an optimal environment for nonspecific T cell activation, possibly against autoantigens.
Importantly, it is worth noting that many nonhematopoietic tissues throughout the body express IR ligands as a mechanism of peripheral tolerance, such as pancreatic islets expressing PD-L1 (Pauken et al., 2019a). Treatment with anti–PD-L1 (pembrolizumab, nivolumab, or cemiplimab) therefore represents a direct break in peripheral tolerance in which previously restrained autoantigen-specific T cells may become activated. Alternatively, IRs expressed on nonhematopoietic tissues may lead to toxicities; for example, CTLA-4 expression on the pituitary gland likely contributes to hypophysitis after anti–CTLA-4 (ipilimumab) treatment (Iwama et al., 2014). In this scenario, ICB anti–CTLA-4 bound to CTLA-4 on the pituitary gland was found to form immune complexes and activate the classical complement pathway, thereby propagating tissue damage (Iwama et al., 2014).
Preexisting nonautoimmune inflammation in the underlying organ tissue may also predispose patients to irAEs. For example, in lung cancer patients, a population enriched for smoking-related inflammation before cancer diagnosis, higher rates of pneumonitis have been documented after ICB (Nishino et al., 2016).
Finally, similar to traditional autoimmunity (Zhang et al., 2020), the antitumor response to both anti–CTLA-4 and anti–PD-1 may be influenced by the microbiome (Gopalakrishnan et al., 2018); studies have linked a relationship between irAE colitis and specific bacterial phyla, such as Firmicutes. It is unclear whether the effect of the microbiome separately drives the antitumor response or irAEs, but it is clear the microbiome plays a role in driving both systemic and local autoimmunity (Zhang et al., 2020). As the gut is a known modulator of specific T cell subsets (such as skewing toward T regulatory cell versus helper T cell type 17 phenotype; Gopalakrishnan et al., 2018), altering the microbiome may alter the naturally tolerogenic environment of the gut. Ultimately, understanding how the environment of the tumor or distal sites, such as the gut, impacts the development of irAEs could lead to approaches that can predict and/or limit this process.
Conclusions and future directions
Although posited as separate mechanisms, overlap exists among these hypotheses. For example, a genetic predisposition, such as a particular HLA haplotype, may be sufficient on its own or may also require the inflammatory milieu of the TME to induce autoreactive T cells or epitope spreading. Furthermore, more than one of these mechanisms may contribute to the irAEs observed in each individual patient. As irAEs are a critical limitation of cancer immunotherapy, it is paramount to understand the pathogenesis and treatment of irAEs. Future studies should focus on the questions described in the following paragraphs.
Who is at risk? As genetic predisposition to spontaneous autoimmunity is incompletely predictive of irAEs (mechanism 1), larger epidemiological studies may elucidate novel susceptibilities. Likewise, developing tumor models in autoimmune-prone mice may provide insight into mechanisms related to genetic susceptibility or the role of epitope spreading.
What are the mechanisms that lead to irAEs, and are they unique or overlapping? High-resolution characterization of human samples both before and after irAEs through multiparametric flow cytometry, single-cell RNA sequencing, and TCR or B cell receptor sequencing is important to elucidate potential mechanisms and to pinpoint the contributions of specific cell populations.
Is there a relationship between antitumor immunity and autoimmunity? irAEs and other published evidence suggest this relationship exists. There are many shared features between autoimmunity and antitumor immunity, including several common phenotypes in immune cells. Of particular interest is the similarity in transcriptional signature observed in two cell types: tumor-derived exhausted CD8+ T cells and activated helper T cell type 17 cells that drive an autoimmune phenotype (Schnell et al., 2020). Furthermore, some single cells in this study show enrichment for both signatures, which suggests that shared transcriptional programs are involved in the development of these T cell fates and may be subject to similar immunoregulation (Chihara et al., 2018). Dissecting these differences may potentially provide a therapeutic strategy to enhance antitumor immunity without increasing autoimmunity. Research is ongoing to better understand this relationship. Deep immune and transcriptomic analysis will aid in our understanding of this relationship and possibly suggest novel approaches for the treatment of cancer and autoimmunity.
Can treatment of irAEs be achieved without reducing the beneficial effects of ICB on antitumor immunity? Most treatments for autoimmunity or irAEs involve immunosuppression, such as corticosteroids (Brahmer et al., 2018). However, data are accumulating to suggest that corticosteroid treatment for irAEs may be detrimental to eliciting an antitumor response (Pauken et al., 2019b). Can irAEs be effectively treated without compromising the efficacy of ICB? Development of novel immunotherapeutic approaches for the treatment of autoimmunity may provide alternative treatments for irAEs. In fact, immunotherapeutic prophylactic anti-TNF has been shown to be effective in diminishing irAEs without compromising antitumor immunity in mice, thereby showing that irAEs can be uncoupled from antitumor immunity (Perez-Ruiz et al., 2019). Further studies are warranted to translate these findings to patients.
To conclude, ICB has revolutionized the current treatment and outlook for multiple cancer types, though therapeutic toxicity is restricted by irAEs. Ongoing efforts to better understand mechanisms driving irAEs and to treat them will undoubtedly advance cancer immunotherapy and allow the treatment of more patients.
Acknowledgments
We thank everyone in the Vignali (https://www.vignali-lab.com/; @Vignali_Lab) and Sharpe (https://sharpelab.hms.harvard.edu/) laboratories for all their constructive comments and advice. We apologize to authors whose work could not be cited due to space constraints.
This work was supported by National Institutes of Health grants F31 AI147638 (to S. Grebinoski), T32 AI089443 (to S. Grebinoski and D.A.A. Vignali), T32 HL116324 (to K.P. Burke), P01 AI108545 (to D.A.A. Vignali and A.H. Sharpe), R01 DK089125 (to D.A.A. Vignali), and P50 CA127003 (to A.H. Sharpe).
Author contributions: S. Grebinoski, K.P. Burke, A.H. Sharpe, and D.A.A. Vignali conceived of the project and wrote the manuscript. S. Grebinoski generated the figure.
References
- Brahmer J.R., Lacchetti C., Schneider B.J., Atkins M.B., Brassil K.J., Caterino J.M., Chau I., Ernstoff M.S., Gardner J.M., Ginex P., et al. National Comprehensive Cancer Network . 2018. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 36:1714–1768. 10.1200/JCO.2017.77.6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brossart P. 2020. The role of antigen spreading in the efficacy of immunotherapies. Clin. Cancer Res. 26:4442–4447. 10.1158/1078-0432.CCR-20-0305 [DOI] [PubMed] [Google Scholar]
- Byrne K.T., and Turk M.J.. 2011. New perspectives on the role of vitiligo in immune responses to melanoma. Oncotarget. 2:684–694. 10.18632/oncotarget.323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebula A., Kuczma M., Szurek E., Pietrzak M., Savage N., Elhefnawy W.R., Rempala G., Kraj P., and Ignatowicz L.. 2019. Dormant pathogenic CD4+ T cells are prevalent in the peripheral repertoire of healthy mice. Nat. Commun. 10:4882 10.1038/s41467-019-12820-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chihara N., Madi A., Kondo T., Zhang H., Acharya N., Singer M., Nyman J., Marjanovic N.D., Kowalczyk M.S., Wang C., et al. 2018. Induction and transcriptional regulation of the co-inhibitory gene module in T cells. Nature. 558:454–459. 10.1038/s41586-018-0206-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham E.T., Moorthy R.S., and Zierhut M.. 2020. Immune checkpoint inhibitor-induced uveitis. Ocul. Immunol. Inflamm. 28:847–849. 10.1080/09273948.2020.1801286 [DOI] [PubMed] [Google Scholar]
- Dow E.R., Yung M., and Tsui E.. 2020. Immune checkpoint inhibitor-associated uveitis: review of treatments and outcomes. Ocul. Immunol. Inflamm.:1–9. 10.1080/09273948.2020.1781902 [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan V., Helmink B.A., Spencer C.N., Reuben A., and Wargo J.A.. 2018. The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell. 33:570–580. 10.1016/j.ccell.2018.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graus F., and Dalmau J.. 2019. Paraneoplastic neurological syndromes in the era of immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 16:535–548. 10.1038/s41571-019-0194-4 [DOI] [PubMed] [Google Scholar]
- Hendry S.A., Farnsworth R.H., Solomon B., Achen M.G., Stacker S.A., and Fox S.B.. 2016. The role of the tumor vasculature in the host immune response: implications for therapeutic strategies targeting the tumor microenvironment. Front. Immunol. 7:621 10.3389/fimmu.2016.00621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwama S., De Remigis A., Callahan M.K., Slovin S.F., Wolchok J.D., and Caturegli P.. 2014. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci. Transl. Med. 6:230ra45 10.1126/scitranslmed.3008002 [DOI] [PubMed] [Google Scholar]
- June C.H., Warshauer J.T., and Bluestone J.A.. 2017. Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat. Med. 23:540–547. 10.1038/nm.4321 [DOI] [PubMed] [Google Scholar]
- Junttila M.R., and de Sauvage F.J.. 2013. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 501:346–354. 10.1038/nature12626 [DOI] [PubMed] [Google Scholar]
- Luoma A.M., Suo S., Williams H.L., Sharova T., Sullivan K., Manos M., Bowling P., Hodi F.S., Rahma O., Sullivan R.J., et al. 2020. Molecular pathways of colon inflammation induced by cancer immunotherapy. Cell. 182:655–671.e22. 10.1016/j.cell.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand L., Disse E., Dalle S., Reffet S., Vouillarmet J., Fabien N., Thivolet C., and Cugnet-Anceau C.. 2019. The multifaceted nature of diabetes mellitus induced by checkpoint inhibitors. Acta Diabetol. 56:1239–1245. 10.1007/s00592-019-01402-w [DOI] [PubMed] [Google Scholar]
- Menzies A.M., Johnson D.B., Ramanujam S., Atkinson V.G., Wong A.N.M., Park J.J., McQuade J.L., Shoushtari A.N., Tsai K.K., Eroglu Z., et al. 2017. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann. Oncol. 28:368–376. 10.1093/annonc/mdw443 [DOI] [PubMed] [Google Scholar]
- Miao D., Margolis C.A., Vokes N.I., Liu D., Taylor-Weiner A., Wankowicz S.M., Adeegbe D., Keliher D., Schilling B., Tracy A., et al. 2018. Genomic correlates of response to immune checkpoint blockade in microsatellite-stable solid tumors. Nat. Genet. 50:1271–1281. 10.1038/s41588-018-0200-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteran L., and Erez N.. 2019. The dark side of fibroblasts: cancer-associated fibroblasts as mediators of immunosuppression in the tumor microenvironment. Front. Immunol. 10:1835 10.3389/fimmu.2019.01835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino M., Giobbie-Hurder A., Hatabu H., Ramaiya N.H., and Hodi F.S.. 2016. Incidence of programmed cell death 1 inhibitor–related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncol. 2:1607–1616. 10.1001/jamaoncol.2016.2453 [DOI] [PubMed] [Google Scholar]
- Oh D.Y., Cham J., Zhang L., Fong G., Kwek S.S., Klinger M., Faham M., and Fong L.. 2017. Immune toxicities elicted by CTLA-4 blockade in cancer patients are associated with early diversification of the T-cell repertoire. Cancer Res. 77:1322–1330. 10.1158/0008-5472.CAN-16-2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauken C.M., Heyes R., and Lott D.G.. 2019a Mechanical, cellular, and proteomic properties of laryngotracheal cartilage. Cartilage. 10:321–328. 10.1177/1947603517749921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauken K.E., Dougan M., Rose N.R., Lichtman A.H., and Sharpe A.H.. 2019b Adverse events following cancer immunotherapy: obstacles and opportunities. Trends Immunol. 40:511–523. 10.1016/j.it.2019.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Ruiz E., Minute L., Otano I., Alvarez M., Ochoa M.C., Belsue V., de Andrea C., Rodriguez-Ruiz M.E., Perez-Gracia J.L., Marquez-Rodas I., et al. 2019. Prophylactic TNF blockade uncouples efficacy and toxicity in dual CTLA-4 and PD-1 immunotherapy. Nature. 569:428–432. 10.1038/s41586-019-1162-y [DOI] [PubMed] [Google Scholar]
- Powell A.M., and Black M.M.. 2001. Epitope spreading: protection from pathogens, but propagation of autoimmunity? Clin. Exp. Dermatol. 26:427–433. 10.1046/j.1365-2230.2001.00852.x [DOI] [PubMed] [Google Scholar]
- Schnell A., Bod L., Madi A., and Kuchroo V.K.. 2020. The yin and yang of co-inhibitory receptors: toward anti-tumor immunity without autoimmunity. Cell Res. 30:285–299. 10.1038/s41422-020-0277-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A.A., and Casciola-Rosen L.. 2015. Cancer and scleroderma: a paraneoplastic disease with implications for malignancy screening. Curr. Opin. Rheumatol. 27:563–570. 10.1097/BOR.0000000000000222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowera A., Ladell K., McLaren J.E., Dolton G., Matthews K.K., Gostick E., Kronenberg-Versteeg D., Eichmann M., Knight R.R., Heck S., et al. 2015. β-cell-specific CD8 T cell phenotype in type 1 diabetes reflects chronic autoantigen exposure. Diabetes. 64:916–925. 10.2337/db14-0332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theofilopoulos A.N., Kono D.H., and Baccala R.. 2017. The multiple pathways to autoimmunity. Nat. Immunol. 18:716–724. 10.1038/ni.3731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toi Y., Sugawara S., Sugisaka J., Ono H., Kawashima Y., Aiba T., Kawana S., Saito R., Aso M., Tsurumi K., et al. 2019. Profiling preexisting antibodies in patients treated with anti-PD-1 therapy for advanced non-small cell lung cancer. JAMA Oncol. 5:376–383. 10.1001/jamaoncol.2018.5860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderlugt C.L., and Miller S.D.. 2002. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nat. Rev. Immunol. 2:85–95. 10.1038/nri724 [DOI] [PubMed] [Google Scholar]
- Wei X., Chen F., Xin K., Wang Q., Yu L., Liu B., and Liu Q.. 2019. Cancer-testis antigen peptide vaccine for cancer immunotherapy: progress and prospects. Transl. Oncol. 12:733–738. 10.1016/j.tranon.2019.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanker D.J., Oveissi S., Tscharke D.C., Duan M., Wan S., Zhang X., Xiao K., Mifsud N.A., Gibbs J., Izzard L., et al. 2019. Influenza A virus infection induces viral and cellular defective ribosomal products encoded by alternative reading frames. J. Immunol. 202:3370–3380. 10.4049/jimmunol.1900070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., and Vignali D.A.. 2016. Co-stimulatory and co-inhibitory pathways in autoimmunity. Immunity. 44:1034–1051. 10.1016/j.immuni.2016.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Chen B.D., Zhao L.D., and Li H.. 2020. The gut microbiota: emerging evidence in autoimmune diseases. Trends Mol. Med. 26:862–873. 10.1016/j.molmed.2020.04.001 [DOI] [PubMed] [Google Scholar]