Abstract
Research about heart failure (HF) has made major progress in the last years. We give here an update on the most recent findings. Landmark trials have established new treatments for HF with reduced ejection fraction. Sacubitril/valsartan was superior to enalapril in PARADIGM‐HF trial, and its initiation during hospitalization for acute HF or early after discharge can now be considered. More recently, new therapeutic pathways have been developed. In the DAPA‐HF and EMPEROR‐Reduced trials, dapagliflozin and empagliflozin reduced the risk of the primary composite endpoint, compared with placebo [hazard ratio (HR) 0.74; 95% confidence interval (CI) 0.65–0.85; P < 0.001 and HR 0.75; 95% CI 0.65–0.86; P < 0.001, respectively]. Second, vericiguat, an oral soluble guanylate cyclase stimulator, reduced the composite endpoint of cardiovascular death or HF hospitalization vs. placebo (HR 0.90; 95% CI 0.82–0.98; P = 0.02). On the other hand, both the diagnosis and treatment of HF with preserved ejection fraction, as well as management of advanced HF and acute HF, remain challenging. A better phenotyping of patients with HF would be helpful for prognostic stratification and treatment selection. Further aspects, such as the use of devices, treatment of arrhythmias, and percutaneous treatment of valvular heart disease in patients with HF, are also discussed and reviewed in this article.
Keywords: Heart failure, Acute heart failure, HfpEF, HFrEF, Diagnosis, Treatment
Introduction
Heart failure (HF) is a major health and economic burden worldwide. 1 , 2 , 3 , 4 , 5 Because mortality remains high and the quality of life is poor, it is an area of active research. 6 , 7 , 8 , 9 , 10 In this article, we update recent published data and findings.
Epidemiology: geographic and temporal trends
Data concerning the epidemiology of HF remain insufficient. 11 , 12 , 13 The Heart Failure Association (HFA) Atlas is a novel European HF data set with a major aim to provide information about HF epidemiology, resource, and reimbursement policies for HF management. 14
In a recent meta‐analysis, Jones et al. presented long‐term outcomes in 1.5 million ambulatory patients with chronic HF, including 60 non‐interventional studies. Despite an improvement in 5 year survival rates between 1970–1979 and 2000–2009 from 29.1% to 59.7%, mortality rates remain unacceptably high and larger than for most types of cancer (Figure 1 ). 11 , 15 Trends in HF hospitalizations from 2000 to 2014 were examined in Norway. Age‐standardized rates of incident HF hospitalization declined on average 1.9% and 1.8% per year for men and women, respectively. Although mortality was reduced, HF rehospitalizations increased. 16
Figure 1.
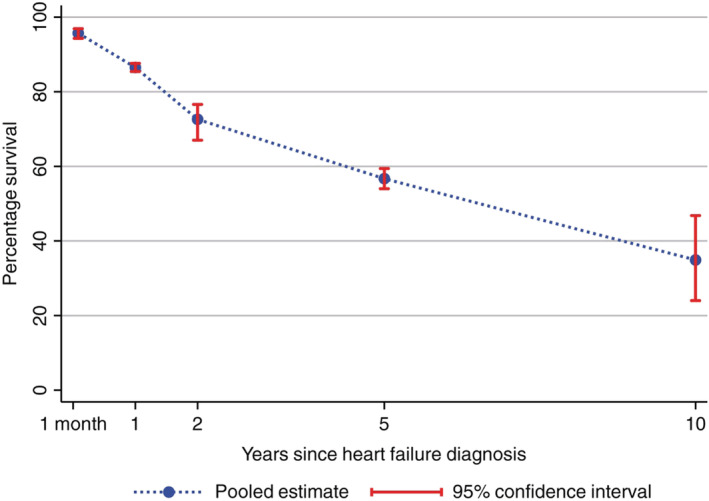
Combined survival rates for people with heart failure over time. Adapted from Jones et al. 11
Several studies have highlighted the regional heterogeneity of HF populations, patients' characteristics, and outcomes, with important implications for global trial design. 12 , 17 Dewan et al. compared heart failure with reduced ejection fraction (HFrEF) patients enrolled in different continents in two large trials: Prospective comparison of angiotensin receptor neprilysin inhibitors (ARNI) with angiotensin‐converting enzyme inhibitors to Determine Impact on Global Mortality and morbidity in HF (PARADIGM‐HF) and Aliskiren Trial to Minimize OutcomeS in Patients with HEart failuRE (ATMOSPHERE). They found that Asian patients were younger (55.0–63.9 years) than those in Western Europe (67.9 years) and North America (66.6 years). The adjusted risk of cardiovascular (CV) death was higher in many Asian countries, except Japan. Using Western Europe as the reference group, the adjusted hazard ratio (HR) for China, India, Thailand, and Japan was 1.89 (1.58–2.27), 1.76 (1.49–2.09), 1.87 (1.18–2.96), and 0.77 (0.53–1.12), respectively. 18
Prospective multinational data from Asia showed that heart failure with preserved ejection fraction (HFpEF) affects relatively young patients with high prevalence of co‐morbidities, most commonly hypertension, anaemia, chronic kidney disease, diabetes, coronary heart disease, and atrial fibrillation (AF). 12 Gender may also impact these international differences in HFrEF. 19 Similar risk factors for incident HF were reported in the UK population. 20 Novel risk factors for worse status continue to be discovered, including nutritional deficiencies even in the developed world. 21
Because outcomes are different worldwide, designing a risk prediction model remains challenging and might underestimate or overestimate mortality in some countries. 22
Specific phenotypes
Among the specific causes of HF, cardiomyopathies are a heterogeneous group of heart muscle diseases. 23 , 24 , 25 , 26 Current knowledge regarding the incidence and prevalence of cardiomyopathies and HF has been summarized in a recent position paper by the HFA of the European Society of Cardiology (ESC). 27 Genetic phenotyping of cardiomyopathies is helpful to understand the clinical course of the disease and to address aetiology‐based therapy. 28 , 29 , 30 Desmoglein‐2 mutation carriers were found to be at high risk of end‐stage HF compared with plakophilin‐2 mutation carriers in arrhythmogenic right ventricular (RV) cardiomyopathy. 31 , 32
A specific phenotype of HF is that caused by amyloidosis. Cardiac amyloidosis is a peculiar cardiomyopathy, characterized by extracellular deposition of misfolded proteins that leads to increased biventricular wall thickness and increased myocardial stiffness. 33 , 34 Different types of protein deposition cause different types of amyloidosis: light‐chain amyloidosis and transthyretin amyloidosis are the most frequent, and the transthyretin stabilizer tafamidis has recently been introduced to improve the prognosis of affected patients. 35 Amyloidosis may be a major cause of severe symptoms and poor outcomes. 33 , 36 , 37 Transthyretin amyloidosis is an underdiagnosed cause of HF, especially in HFpEF, hypertrophic or restrictive cardiomyopathy, and aortic stenosis. Compared with wild‐type transthyretin amyloidosis, transthyretin‐related hereditary amyloidosis more often affects men and is characterized by an early onset with concomitant neurological involvement. 38 Such patients need to be screened, and specific treatments can be considered. 39
Much recent interest has also focused on other cardiomyopathies including peripartum cardiomyopathy. 40
Co‐morbidities
Co‐morbidities play a major role in the clinical presentation and outcomes of HF. 41 , 42 , 43 More than 70% of patients with HF are burdened by co‐morbidities, and they have an independent effect on mortality. 44 , 45
Type 2 diabetes mellitus confers a greater risk of new onset HF, HF rehospitalization, and CV and all‐cause mortality, particularly when patients require insulin treatment and/or have concomitant conditions. 46 , 47 , 48 , 49 , 50 , 51
Further common co‐morbidities associated with HF are chronic kidney disease, 52 , 53 chronic obstructive pulmonary disease, 54 central nervous system abnormalities, 55 , 56 sleep disordered breathing, 57 iron deficiency, cancer, 58 , 59 , 60 , 61 cachexia, 62 , 63 , 64 muscle wasting (sarcopenia), 51 , 65 , 66 , 67 , 68 , 69 and frailty. 70 , 71 , 72 , 73 The prevalence of frailty is increased in HF and is associated with worse outcome. 74 Its relation with outcome is independent from other variables in most of the studies. Frailty seems to be more common in HFpEF patients than in HFrEF due to the greater burden in cardiac and non‐cardiac co‐morbidities in the second condition. 75 , 76 Recently, a new Frailty Score was developed by the HFA. It considers four main domains: clinical, physical–functional, cognitive–psychological, and social. Each domain covers different variables, including co‐morbidities (clinical domain); cognitive impairment and mood disturbances, such as depression (cognitive–psychological); physical impairment, sarcopenia, or cachexia (functional); and isolation or the absence of a caregiver (social). This score should identify high‐risk patients (Figure 2 ). 72 The prognostic role of social and economic factors has been shown in recent studies, 20 , 77 and also, sex differences are important to consider in the treatment of patients. 78 , 79 , 80 , 81 , 82 , 83
Figure 2.
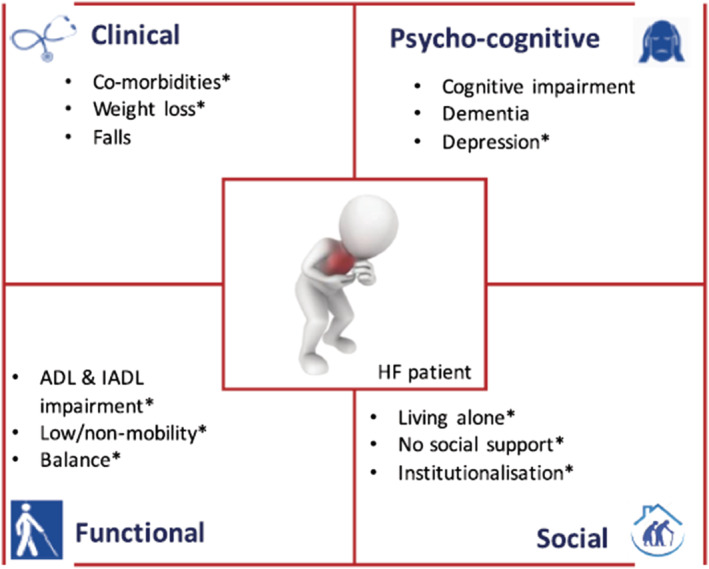
The four main domains—clinical, physical–functional, cognitive–psychological, and social—defining Heart Failure Association Frailty Score. Reversible and/or treatable variables are identified by asterisks. ADL, activities of daily living; HF, heart failure; IADL, instrumental activities of daily living. From Vitale et al. 72
Heart failure and cancer
There is a complex and intriguing relationship between HF and cancer. 70 , 84 , 85 , 86 , 87 On one hand, patients with HF have a higher occurrence of malignancy due to a combination of underlying shared mechanisms and risk factors. 58 , 59 , 88 On the other hand, cancer patients frequently develop HF, due to cardiotoxicity. 60 , 89 , 90 , 91 , 92 , 93 In breast cancer patients, increased levels of N‐terminal pro B‐type natriuretic peptide (NT‐proBNP) and impaired global longitudinal strain (GLS) were found in 34% and 23% of the patients, respectively. GLS and left ventricular ejection fraction (LVEF) declined with increasing cumulative anthracycline dose. 94 Both left ventricular (LV) and RV GLS impairment predicted cardiotoxicity also in patients receiving trastuzumab. 95 Also, plasma troponin levels may identify patients at higher risk of CV complications. 96 In a recent experimental model, the protective effect of phenylalanine‐butyramide against doxorubicin‐induced cardiotoxicity has been shown. 97
Diagnosis and prognosis
New scores and risk prediction models are continuously developed to stratify risk in HF patients. 98 , 99 , 100 , 101
Clinical signs
Clinical signs have important prognostic value. Low systolic blood pressure and elevated heart rate are associated with poorer outcomes. 102 , 103 The role of heart rate is controversial in patients with concomitant AF. No relation with outcomes was shown in one meta‐analysis. 104 A study by Sartipy et al., including HFpEF patients from the Swedish Heart Failure Registry, showed that in those with concomitant AF, higher heart rates were associated with poorer outcomes at short term (1 year) but had no prognostic value in the long term. 105
Biomarkers
Plasma levels of natriuretic peptides (NPs) are related with LV wall stress and are surrogates for intracardiac filling pressures. They are useful to discriminate HF from non‐cardiac breathlessness in patients presenting to the emergency department. Lower levels of NPs have a very high negative predictive value for the diagnosis of HF. In patients with chronic HF, NPs may be persistently elevated, and an increase of 100% or more may suggest an acute decompensation. 106 , 107 Moreover, sex and several co‐morbidities may influence NP levels, requiring adjusted cut‐off: for instance, obese patients have lower values of NT‐proBNP. 108 NPs, along with troponin, are the most useful biomarkers to predict outcomes in both chronic and acute HF to date. 109 , 110 , 111 , 112 NT‐proBNP is also predictive for non‐CV death. 113 The role of mid‐regional pro‐atrial natriuretic peptide has been recently investigated not only in the acute setting but also in chronic HF, and it has a diagnostic value similar to NT‐proBNP. 114
An analysis of the Aliskiren Trial on Acute Heart Failure Outcome (ASTRONAUT) failed to show a role of plasma renin activity for the selection of patients with the highest likelihood to respond to aliskiren, even if it has a negative prognostic value. 115 , 116 In PARADIGM‐HF, elevated levels of growth factor 15 were associated with mortality and CV outcomes, though they were not changed by assigned treatment. 117
Given the central role of inflammation in the pathophysiology of HF, pro‐inflammatory cytokines, including interleukin 6 (IL‐6), raised interest in the scientific community. 118 , 119 , 120 , 121 Over 50% of the patients with HFrEF in A systems BIOlogy Study to TAilored Treatment in Chronic Heart Failure (BIOSTAT‐CHF) had elevated IL‐6 levels, associated with iron deficiency, AF, and poorer clinical outcome. IL‐6 could become a potential therapeutic target, despite trials targeting another marker of inflammation, tumour necrosis factor alpha, were largely unsuccessful. 122
Circulating microRNAs continue to be of major interest. Different levels of plasma microRNAs could help in discriminating the aetiology behind HF (ischaemic vs. non‐ischaemic). 123 Such levels might change after treatment and could represent a tool to monitor patients' clinical status, although evidence is lacking and further research is needed. 124 , 125 , 126
There is also increasing interest in biomarkers that may simultaneously be markers, disease process mediators, and therapeutic targets. 127 , 128 , 129
The role of imaging, exercise testing, and invasive haemodynamic measurement
Echocardiography allows the assessment of LV volumes and LVEF as well as an estimate of LV filling pressure, RV size and function, valvular disease, and pulmonary artery pressure. 130 , 131 LV systolic ejection time (SET) is shorter in HF and is an independent predictor of incident HF (HR 1.07; 95% CI 1.02–1.14, per 10 ms decrease). 132 Interestingly, a longer SET was associated with improved outcomes among HFrEF but not HFpEF patients. Hence, an increase in SET seems a promising pathway to improve systolic function in these patients. 133
Another major area of research regards the left atrium. Left atrial structure and function has been shown to predict outcomes in patients with HF and AF. 134 Left atrial strain provided better diagnostic accuracy than conventional echocardiographic measures to discriminate HFpEF from non‐cardiac causes of dyspnoea 135 and was associated with impaired haemodynamics both at rest and during exercise. 136 , 137
Cardiac magnetic resonance (CMR) may be helpful in tissue characterization, detection, and quantification of myocardial fibrosis and adipose tissue, which are associated with HF development and progression. 138 , 139 Myocardial adipose deposition and epicardial fat, both of which can be carefully measured by CMR, may play a major role in the development of HFpEF. 139 , 140
The 6 min walk test is a valid tool to assess exercise capacity. In BIOSTAT‐CHF, both a reduced walked distance at baseline and a decline at 9 month follow‐up were associated with a worse prognosis and were not modified by the up‐titration of drugs. 141
Cardiopulmonary exercise test‐derived parameters, such as peak exercise oxygen uptake (peak VO2) and minute ventilation/carbon dioxide relationship slope (VE/VCO2 slope), have a major role for the assessment of the patients with advanced HF and a possible indication to heart transplantation. 43 HF prognosis has improved in the last years, suggesting the need of update prognostic threshold of these parameters. Paolillo et al. analysed the metabolic exercise cardiac kidney index (MECKI) score database and divided patients in four groups by enrolment year: Group 1 1993–2000, Group 2 2001–2005, Group 3 2006–2010, and Group 4 2011–2015. In Groups 1, 2, 3, and 4, a 20% overall risk [CV death, urgent heart transplantation, or left ventricular assist device (LVAD) implantation] was observed for peak VO2: 15 mL/min/kg (95% CI 16–13), 9 (11–8), 4 (4–2), and 5 (7–4), respectively. The VE/VCO2 slope value for a 20% risk was 32 (37–29), 47 (51–43), 59 (64–55), and 57 (63–52), respectively. 142 Exercise oscillatory ventilation is another pivotal parameter in cardiopulmonary exercise test, and it has a similar prognostic value in both HFrEF and heart failure with mid‐range ejection fraction patients. 143
Right heart catheterization maintains a major role for the prognostic stratification of patients with severe symptoms. 43 It discriminates between patients with isolated postcapillary pulmonary hypertension and those with combined postcapillary and precapillary pulmonary hypertension who have worse outcomes both in HFrEF and valve disease. 144 , 145 , 146
Medical treatment of heart failure with reduced ejection fraction
Neurohormonal antagonists
Neurohormonal antagonists, including angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, beta‐blockers, and mineralocorticoid receptor antagonists, play a pivotal role in the treatment of HFrEF, improving the clinical course of the disease. 147 , 148 , 149 In the more recent PARADIGM‐HF trial, sacubitril/valsartan was superior to enalapril in reducing the risks of death and of hospitalization for HF. 150 , 151 A similar efficacy was shown in the reduction of recurrent events (Wei, Lin, and Weissfeld HR in the sacubitril/valsartan group 0.79; 95% CI 0.71–0.89). 152 Moreover, treatment with sacubitril/valsartan allows a greater reduction in loop diuretic doses compared with enalapril. 153 Results from the Comparison of Pre‐ and Post‐discharge Initiation of LCZ696 Therapy in HFrEF Patients After an Acute Decompensation Event (TRANSITION) study suggest that initiation of ARNI in HFrEF patients stabilized after an acute HF event, either in hospital or shortly after discharge, is feasible. 154 De novo HFrEF patients had also major benefits compared with those with prior diagnosis, showing faster and greater decreases in NT‐proBNP and high‐sensitivity troponin T and lower rates of HF and all‐cause rehospitalization. 155
In the ESC‐EORP‐HFA Heart Failure Long‐Term Registry, 84% of outpatients were eligible for sacubitril/valsartan based on European Medicines Agency/Food and Drug Administration label, but only 12–28% met the criteria used in guidelines. 156 Data from Germany showed a still insufficient rate of initiation and dose up‐titration of this agent. 157 Reasons behind this under‐prescription must be explored to ensure guideline‐directed medical therapy (GDMT). Indeed, physicians and patients' adherence to GDMT is associated with improved outcomes over both the short term and longer term. 158 , 159 Many factors, including older age, hypotension, and impaired renal function, may contribute to underuse of GDMT. 160 , 161 , 162 A slow titration of ARNI is associated with better treatment success also in patients with slow systolic blood pressure. 163 In EMPHASIS‐HF (Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure), renal function did not influence beneficial effects of eplerenone, even if patients with impaired renal function were more susceptible to adverse events (hyperkalaemia and renal failure events) and drug discontinuation. 164 Hyperkalaemia represents another limiting factor in the prescription of renin–angiotensin–aldosterone system inhibitors. 165 , 166 , 167 Novel potassium‐lowering agents, such as patiromer and sodium zirconium cyclosilicate, may be helpful in achieving optimization of therapy, but further studies are needed. 39
Sodium–glucose co‐transporter 2 inhibitors
In the last years, major advances occurred concerning antidiabetic drugs and CV risk, and a new pathway of HF treatment—different from the neurohormonal one—has been opened (Figure 3 ). 168 , 169
Figure 3.
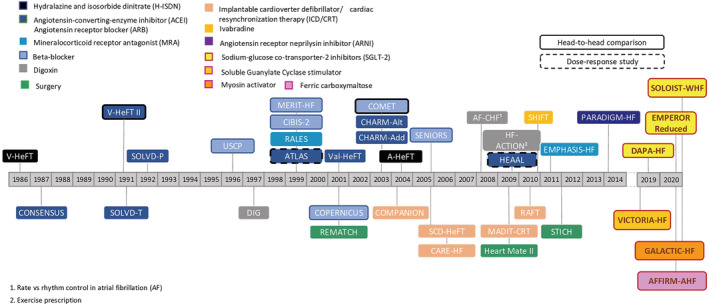
Positive trials in the treatment of heart failure with reduced ejection fraction from 1986 to 2020. Modified from McMurray. 168
Sodium–glucose co‐transporter 2 (SGLT‐2) inhibitors—empagliflozin, canagliflozin, and dapagliflozin—have consistently shown a reduced risk of HF hospitalization or CV death in diabetic patients regardless of baseline CV disease and previous history of HF. 170 , 171 Dapagliflozin And Prevention of Adverse outcome in Heart Failure (DAPA‐HF) is the first trial proving a significant benefit of an SGLT‐2 inhibitor—dapagliflozin—with a reduction in the risk of the composite endpoint of CV death or worsening HF (hospitalization or an urgent visit requiring intravenous therapy for HF) in HFrEF patients with or without diabetes (HR 0.74; 95% CI 0.65–0.85). Each of the three components of the composite outcome was less frequent in the dapagliflozin group. 172 , 173 The baseline characteristics of DAPA‐HF patients were similar to those in contemporary HFrEF registries and trials. 174 The Empagliflozin Outcome Trial in Patients with Chronic Heart Failure and a Reduced Ejection Fraction (EMPEROR‐Reduced) included patients with a more severe LV systolic dysfunction, higher levels of NPs, and lower estimated glomerular filtration rate, as compared with the patients in the DAPA‐HF trial. Empagliflozin reduced the combined risk for CV death or hospitalization for HF compared with placebo (HR 0.75; 95% CI 0.65–0.86; P < 0.001), a difference that was primarily related to a reduction in hospitalization for HF. Indeed, CV death was not significantly reduced probably because EMPEROR‐Reduced trial had less statistical power than DAPA‐HF (less number of events in a smaller size of the trial and shorter follow‐up). The beneficial effects of empagliflozin were consistent in subgroup analyses (diabetes vs. non‐diabetes; ARNI treatment vs. no ARNI treatment). Furthermore, empagliflozin was associated with a slower rate of decline in the estimated glomerular filtration rate and with a lower risk of serious renal outcomes. Thus, the EMPEROR‐Reduced trial extends the benefits of SGLT‐2 inhibitors in stable, more advanced HF population. 175 , 176 In the Effect of Sotagliflozin on Cardiovascular Events in Patients with Type 2 Diabetes Post Worsening Heart Failure (SOLOIST‐WHF) trial, sotagliflozin showed beneficial effects in patients with diabetes and recent worsening HF. 177 Further studies will assess the efficacy of SGLT‐2 inhibitors in other settings (i.e. HFpEF or acute HF). 178 , 179
The mechanisms behind the beneficial effects of SGLT‐2 inhibitors are largely unknown. Besides glycosuric and natriuretic effects, empagliflozin seems to have a direct pleiotropic effect on cardiomyocytes. It improves adenosine triphosphate production and myocardium metabolism, diastolic function, and cardiac remodelling and has favourable effects. 180 , 181 , 182 , 183
Treatment of iron deficiency
Iron deficiency is common in HF patients and is associated with poor exercise capacity, directly affecting mitochondrial respiration and the function of skeletal muscle and cardiomyocytes. 184 , 185 , 186 , 187 , 188 Indeed, cellular oxidative metabolism relies largely on iron availability in skeletal muscle. 189 , 190 , 191 , 192 Iron depletion is also associated with reduced quality of life and survival. Treatment with intravenous ferric carboxymaltose (FCM) has been shown to lead to improvements of functional capacity, symptoms, and quality of life in chronic HF patients. 193 A recent meta‐analysis including four major randomized trials showed a reduction of HF hospitalizations and CV mortality in iron deficiency patients treated with FCM. 194 AFFIRM‐AHF (Study to Compare Ferric Carboxymaltose with Placebo in Patients with Acute Heart Failure and Iron Deficiency) evaluated the effects of intravenous FCM in patients hospitalized for acute heart failure. 195 It showed that treatment with FCM was safe and reduced the risk of HF hospitalisations. 196
Other options
Vericiguat Global Study in Subjects with Heart Failure with Reduced Ejection Fraction (VICTORIA) was a randomized, placebo‐controlled trial evaluating safety and efficacy of vericiguat in HFrEF patients with recent worsening HF. Vericiguat was superior to placebo in the reduction of composite endpoint of CV death or HF hospitalization (HR 0.90; 95% CI 0.82–0.98; P = 0.02). 197 Baseline characteristics of VICTORIA‐enrolled subjects showed a high‐risk population when compared with PARADIGM‐HF. The median MAGGIC (Meta‐Analysis Global Group in Chronic Heart Failure) risk score was 23 (interquartile range 18–27) in VICTORIA vs. 20 (interquartile range 16–24) in PARADIGM‐HF trial. 198 In the GALACTIC‐HF (Global Approach to Lowering Adverse Cardiac outcomes Through Improving Contractility in Heart Failure) trial, the selective cardiac myosin activator omecamtiv mecarbil showed a reduction in the primary composite endpoint of a first HF event or death from CV causes. 199
Neladenoson bialanate, a partial adenosine A1 receptor agonist, failed to demonstrate favourable changes in NT‐proBNP, LVEF, high‐sensitivity troponin T, or CV mortality and HF hospitalization in PANTHEON trial. On the other hand, a decrease in renal function was observed. 200
Standard treatment of advanced HF remains unsatisfactory. Positive inotropes may be used as bridge strategy and palliative care, 43 while no positive inotrope is currently approved for long‐term treatment in chronic HF. 201 However, intermittent administration of levosimendan in ambulatory patients has been associated with reduction in NT‐proBNP levels and HF hospitalization. 202
The DIGIT‐HF (DIGitoxin to Improve ouTcomes in patients with advanced chronic Heart Failure) trial has been designed to demonstrate a role of digitoxin on the top of standard care in improving mortality and morbidity in advanced HFrEF. 203
Devices
Cardiac resynchronization therapy
Cardiac resynchronization therapy (CRT) is less frequently used than expected, even when indicated by guidelines. 204 , 205 , 206 , 207
In an individual patient data meta‐analysis of five randomized controlled trials, QRS duration was the only independent predictor of CRT benefit on mortality. Along with QRS duration, lower height but not sex played a role in the composite endpoint of all‐cause mortality or first hospitalization for HF. 208 In another study, body mass index was associated with outcome: overweight or obese patients receiving CRT with defibrillator were at a lower risk of death compared with underweight subjects. 209
According to an analysis from the ESC CRT Survey II, the benefit and complication rates from CRT upgrading, in implantable cardioverter defibrillator (ICD) or pacemaker carriers, are the same as for de novo CRT patients. 210 Therefore, patients with a pacing‐induced cardiomyopathy must be closely monitored, and an upgrade to CRT or His bundle pacing device might be considered. 211
Cardiac resynchronization therapy may be less effective in patients with AF because both atrioventricular (AV) and biventricular resynchronization are required. However, AV junction ablation might represent a safe option: in a small trial, CRT with defibrillator patients with permanent AF, who underwent AV junction ablation, experienced less ICD shocks and a lower incidence of HF hospitalization, compared with patients with AF medical therapy alone. 212
A study on neonatal rat ventricular cardiomyocytes showed that irregular pacing induces pro‐fibrotic signalling with paracrine effects and oxidative stress, eventually leading to a remodelling process. A similar mechanism might be involved in AF‐related HF with arrhythmic ventricular contractions and increased morbidity and mortality. 213
Implantable cardioverter defibrillator
The selection of patients who might benefit from ICD implantation is often challenging. 214 , 215 Myocardial infarction survivors with LVEF > 35% burdened by diabetes and/or kidney dysfunction have a high risk of sudden cardiac death (SCD), but the risk of a non‐SCD event is even higher, suggesting that the extension of ICD implantation in such patients might not be worthwhile. 52 A combined analysis of four major primary prevention trials in HFrEF patients assessed the effects of ICD implantation in diabetic vs. non‐diabetic patients. The use of ICD was associated with a reduced risk of mortality in non‐diabetic patients (HR 0.56; 95% CI 0.46–0.67) but not among those with diabetes. 216
Percutaneous treatment of mitral and tricuspid regurgitation
Transcatheter mitral valve interventions are spreading as treatment options in patients with HF and severe secondary mitral regurgitation. 39 , 217 , 218 Two randomized controlled trials investigated the prognostic impact of percutaneous edge‐to‐edge mitral valve repair by MitraClip in HF patients. Percutaneous Repair with the MitraClip Device for Severe Functional/Secondary Mitral Regurgitation (MITRA‐FR) showed no reduction in HF hospitalizations or mortality in patients undergoing MitraClip compared with those receiving conservative management up to 2 year follow‐up. 219 On the other hand, Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation (COAPT) demonstrated an impressive reduction in both 2 year HF hospitalizations, which was the primary endpoint, and 2 year all‐cause death. Such a discrepancy might be due to the different characteristics of the patients included, suggesting that only carefully selected patients may derive a prognostic benefit from secondary mitral regurgitation correction. 39 , 220 Further data, coming from observational studies, showed safety and efficacy of MitraClip in improving symptoms and clinical status also in patients with advanced HF, such as those with low ejection fraction (EF) and pulmonary hypertension. 146 , 221
Orban et al. showed a significant improvement in New York Heart Association class, 6 min walk test distance, and quality of life in 50 patients undergoing percutaneous edge‐to‐edge repair on the tricuspid valve. 222 Schlotter et al. reported outcomes of 159 patients with severe functional tricuspid regurgitation, who underwent transcatheter tricuspid valve repair. Patients were stratified into four aetiology‐based clinical scenarios: patients receiving chronic haemodialysis, patients with significant mitral regurgitation, patients with severe pulmonary hypertension, and patients with a history of AF/flutter. Patients with pulmonary hypertension had the highest rates of the primary composite endpoint (death, HF hospitalization, or reintervention), while the highest mortality rate was observed in haemodialysis patients (33.3%). 223
Mechanical circulatory support
Left ventricular assist device is a promising option for patients with advanced HF, either as a bridge to transplant or as a lifelong treatment. 224 , 225 , 226 In a Spanish retrospective study, 291 patients waiting for cardiac transplant received LVAD. They had a better outcome, compared with those undergoing temporary biventricular assist devices or extracorporeal membrane oxygenation as bridge to heart transplantation. 227 LVAD implantation may also favour a recovery of LV function especially when associated with administration of neurohormonal antagonists. 228 Data from the Postgraduate Course in Heart Failure (PCHF)‐VAD registry showed a better survival in LVAD patients who also received a cardiac implantable electronic device with a defibrillator component (HR 0.64; 95% CI 0.46–0.91; P = 0.012) compared with those who only had LVAD support. 229 Also, exercise training may provide incremental benefits in LVAD carriers. The rationale behind the Exercise training in patients with a LVAD (Ex‐VAD) trial is to assess if a 12 week supervised exercise training could improve the quality of life and the functional capacity in LVAD patients. 230 However, among patients with a reduced EF and New York Heart Association Classes III–IV eligible for heart transplantation or LVAD, more than half declines the indication. 231 Infections, as well as bleeding and thrombo‐embolic events, are the most common and severe complications of LVAD. The platelet activity state may predict the risk of thrombo‐embolic complications after LVAD. 232 LVAD design continues to improve and will be subject to ongoing evaluation. 233
Telemedicine and disease management
Home telemonitoring is a useful tool for the management of HF patients. 39 TIM‐HF2 (Telemedical Interventional Management in Heart Failure II study) trial showed a positive impact of telemedicine on unplanned CV hospitalization and mortality. 234 , 235 , 236 The HOME‐HF study was designed to assess the feasibility and efficacy of BNP home measurement in reduction of HF‐related events. It was early interrupted because of slow enrolment, low event rate, and the need of standardize BNP spontaneous fluctuation. 237 The HFA has developed a well‐visited tool to assist in patient communication and education, a crucial part of ongoing disease management, 238 which the development of e‐health strategies will likely accelerate, 239 as will better mechanisms to enhance dosing choices in guideline‐directed HF medication. 240 , 241
Novel perspectives
New therapeutic strategies are emerging for patients with HF of ischaemic aetiology.
BioVentrix Revivent TC System is a transcatheter technique that aims at limiting myocardial scar on the beating heart of HF patients. At 12 month follow‐up, symptomatic patients with previous anterior myocardial infarction who received this treatment had an improvement of LV function, symptoms, and quality of life. 242
The Autologous Mesenchymal Stromal Cell Therapy in Heart Failure (MSC‐HF) trial randomized patients with ischaemic HF to receive either intramyocardial injections of bone marrow‐derived mesenchymal stromal cells or placebo. At 4 year follow‐up, patients experienced improved myocardial function and myocardial mass. 243 The Stem Cell therapy in IschaEmic Non‐treatable Cardiac disease (SCIENCE) trial will assess the efficacy and safety of intramyocardial cell therapy of adipose‐derived stromal cells from healthy donors (allogeneic donation) in patients with ischaemic HF. 244 Cardiac contractility modulation has been shown to have the potential to improve functional capacity, especially in those with HF and mildly reduced LVEF (25–45%). 245
Central sleep apnoea (CSA) is a predictor of CV morbidity and mortality in HF patients. The Treatment of Predominant Central Sleep Apnoea by Adaptive Servo Ventilation in Patients with Heart Failure (SERVE‐HF) trial compared adaptive servo‐ventilation and medical therapy in patients with HF and CSA. The primary endpoint, changes in LVEF at 1 year, was similar between the two groups. However, CV mortality was higher in the adaptive servo‐ventilation vs. control arm. Furthermore, other endpoints, such as changes in LV dimensions and cardiac biomarkers, were not affected by the adaptive servo‐ventilation. 246
A post hoc analysis from the remedē System Pivotal Trial including patients with CSA and baseline HF showed an improvement in the quality of life and a reduction in HF hospitalizations in patients undergoing phrenic nerve stimulation compared with control group (inactive system). 247
Epigenetic processes and aberrant gene expression are important mechanisms in HF. A study by Berulava et al. showed that the methylation of m6A RNA can modify the physiological processes leading to HF. Thus, epitranscriptomic pathways could be potential therapeutic targets. 248 Proteomic processes also represent a field of interest and could be targets for the future. A recent study demonstrated that glutathione, arginine and proline, and pyruvate pathways were activated in HF patients who died or were rehospitalized. 249 The ubiquitous lysosomal protease cathepsin D is secreted as a response to oxidative stress, but its role in HF was unknown. It has been recently discovered that elevated levels of cathepsin D are associated with HF severity and poor outcome. 250
Heart failure with preserved ejection fraction
Clinical phenotypes and pathophysiology
Heart failure with preserved ejection fraction is a heterogeneous syndrome with several clinical manifestations. 251 , 252 , 253
Using a machine learning‐based cluster analysis, Segar et al. identified three phenogroups of patients with HFpEF enrolled in the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist Trial (TOPCAT) trial. Patients in the first phenogroup had a higher burden of co‐morbidities, increased levels of NPs, and LV impairment; the second phenogroup had less co‐morbidities but a worst diastolic dysfunction; the third phenogroup had lower levels of NPs, intermediate co‐morbidities, and the most favourable diastolic profile. Phenogroup 1 had higher rates of HF rehospitalizations and mortality, when compared with Phenogroup 3. Phenogroups 2 and 3 shared the same risk of mortality. A greater risk of rehospitalization was observed in Phenogroup 2 vs. 3, 254 a feature also seen with the increased risk associated with co‐morbid pulmonary disease in this syndrome. 255
The pathophysiology of HFpEF remains largely unknown. Coronary microvascular dysfunction might play a central role in the development of HFpEF. It can be equally caused by both endothelium‐based and endothelium‐independent mechanisms. When the microvascular dysfunction is not endothelium related, patients show a worse diastolic function and prognosis. 256
Another possible mechanism is the systemic and intramyocardial inflammation. 119 Obesity and type 2 diabetes mellitus are common in HFpEF patients and often coexist. They both cause inflammation and expansion of epicardial adipose tissue, leading to atrial damage and LV fibrosis/stiffness. 257 , 258 AF often represents the first manifestation of HFpEF and can be a consequence of atrial myopathy. 258
A study by Wu et al. investigated the role of myocardial steatosis in diastolic dysfunction. Intramyocardial fat deposition was measured using CMR in 305 subjects (34 patients with HFrEF, 163 with HFpEF, and 108 non‐HF controls). HFpEF patients display a more pronounced intramyocardial fat deposition, when compared with HFrEF patients or controls, leading to diastolic impairment. 139 Such phenomenon is—once again—more clear in patients burdened by obesity and metabolic syndrome, in whom adiposity is greater and is associated with myocardial injury and AF. 140 Differences between Asia and western countries are also seen in the presentation of HFpEF. 259
Diagnosis and prognosis: a challenge for the medical community
The diagnosis of HFpEF remains challenging. Recently, two independently derived algorithms for the HFpEF diagnosis have been published: the H2FPEF score from the Mayo Clinic (Rochester, MN, USA) and the European HFA‐PEFF 4‐step algorithm. 260 , 261 The American H2FPEF score was based on clinical and echocardiographic characteristics and validated with invasive haemodynamic testing as gold standard. It identified six variables as HFpEF predictors: obesity (body mass index > 30 kg/m2), AF, age > 60 years, treatment with two or more antihypertensive drugs, E/e' > 9, and pulmonary artery systolic pressure > 35 mmHg. The resultant total H2FPEF score ranged from 0 to 9, with the scores <2 suggesting a low likelihood and scores ≥6 reflecting a high likelihood of HFpEF. The European HFA‐PEFF 4‐step algorithm is a new stepwise diagnostic tool. It starts from pretest assessment (based on signs, symptoms, electrocardiographic alterations, and laboratory tests), going through risk stratification with rest imaging, analysing specific functional and morphological echocardiographic parameters. The HFA‐PEFF score is the sum of points from functional, morphological, and biomarker domains (2 points for each major criteria and 1 for each minor criteria). A total score ≥5 is considered diagnostic for HFpEF, while a total score <1 determines a very low probability of HFpEF. The intermediate values will need further investigation, with stress echocardiography and invasive haemodynamics. The diagnostic pathway could be finally completed with aetiological workup. Barandiarán Aizpurua et al. tried to validate the second step of the HFA‐PEFF algorithm, based on echocardiographic findings and NPs. 262 However, these results might be overestimated due to the high HFpEF case–control ratio and to the low diagnostic support with invasive measurements.
Diagnostic tools aiming to assess tolerance to exercise are mandatory in HFpEF patients. 263 , 264 , 265 However, co‐morbidities could limit exercise capacity. Elderly patients with elevated left atrial pressure and impaired reservoir present an abnormal exercise haemodynamics. 136 The evaluation of left atrial reservoir strain could be helpful to discriminate HFpEF from non‐cardiac dyspnoea. 135 In patients who are capable of performing exercise, oxygen consumption trajectory has been suggested as a predictor of disease severity. 266 Estimated plasma volume status has been also proposed as a tool for the prediction of long‐term outcome in HFpEF patients. 267
Treatment: lack of favourable results
Despite the growing impact of HFpEF, there is still no established pharmacological therapy. In the Prospective comparison of ARNI with angiotensin receptor blockers Global Outcomes in HFpEF (PARAGON‐HF) trial, sacubitril/valsartan did not reduce the incidence of HF hospitalizations or CV death. However, the subgroup analysis showed that women and patients with lower EF should have a benefit from the treatment. 268
In obesity‐related HFpEF, underlying pathophysiological abnormalities may be related to derangements in beta‐adrenergic drive and to increased aldosterone and neprylisin activity. 120 , 180 Thus, drugs acting against these pathways could be beneficial. 269
In patients with HFpEF and diabetes, insulin treatment is associated with poor outcome. 270 Moreover, neither furosemide nor torasemide was associated with effect on myocardial fibrosis in these patients. 271 Given their anti‐inflammatory and anti‐fibrotic actions, SGLT‐2 inhibitors might ameliorate cardiac remodelling also in HFpEF patients. Ongoing trials will assess whether such drugs will be extended to HFpEF. 178
Novel specific Na+/Ca2+ exchanger inhibitor ORM‐11035 is able to reduce cardiac remodelling and diastolic dysfunction with no effects on systemic blood pressure in rats. Considering the promising results, future trials are needed to evaluate the feasibility of such treatment also in humans. 272
Acute heart failure
Epidemiology
Several precipitating factors may lead to acute HF, namely, arrhythmia, respiratory infections, and other non‐CV factors. 273 Worsening HF may develop in an outpatient or an inpatient setting, with a similar drastic increase in event rates. 274 , 275 The Heart Failure Registry of Patient Outcomes (HERO) study is a prospective, longitudinal multicentre registry including patients hospitalized with acute HF in China. In‐hospital or 3 day post‐discharge mortality was 3.2%. Death or readmission rate from the 4th day post‐discharge to first follow‐up was 22.4%. 276 In the first 30 days after admission for acute HF, 2% of patients experience SCD or resuscitated SCD or ventricular tachycardia/fibrillation. 277 In an Italian series, the 1 year mortality rate was 20%, with the highest risk of death during the index hospitalization. 278 The admission department seems to play a role in the natural history of HF, with the general medicine department associated with a poor prognosis, probably due to different characteristics of patients (older age and several co‐morbidities). 278 , 279
Management
Clinical signs, biomarkers, and imaging are essential in the diagnostic process, in‐hospital monitoring, and pre‐discharge evaluation of acute HF. 280 The clinical classification of acute HF into four different profiles, defined by the presence of congestion and/or peripheral hypoperfusion, 147 provides information on both early and long‐term outcomes. 281 , 282 Signs and symptoms of congestion represent the major cause of HF hospitalization both in HFrEF and in HFpEF patients. 283 Clinical residual congestion at discharge was detected in 30.9% of patients in the ESC‐EORP‐HFA Heart Failure Long‐Term Registry, and it was associated with increased 1 year mortality (Figure 4 ). 281 The Reprieve System, a device that continuously measures urine output and supplies intravenous fluid to maintain fluid balance, may represent a useful tool to control decongestion. 284
Figure 4.
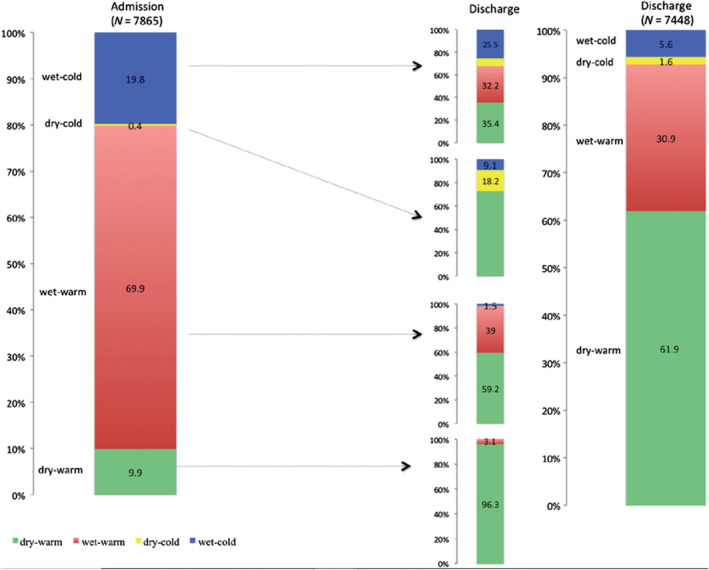
Classification based on congestion/hypoperfusion status assessed by clinical examination performed at admission and discharge. Classification at discharge was used in 7448 patients discharged alive. From Chioncel et al. 281
The serial assessment of spot urine sodium predicts effectiveness of decongestion and outcome, 285 as well as decrease in NT‐proBNP levels. 286 , 287 Higher levels of mid‐regional pro‐adrenomedullin and its active form, bio‐adrenomedullin, were observed in the presence of volume overload and have been proposed as markers of congestion. 288 Mid‐regional pro‐adrenomedullin and bio‐adrenomedullin provide a great accuracy in diagnosis of acute HF and detection of residual congestion. 289 , 290 Elevated levels of blood lactate, associated with hypoperfusion, and markers of multi‐organ injury/dysfunction are predictors of poorer outcomes. 291 , 292 , 293 Worsening renal function is only associated with adverse events in patients without decreased BNP. 286 We are seeing the importance of assessing adequacy of the decongestive therapies in acute HF. 294
Echocardiography estimates LV and RV filling pressure. Higher inferior vena cava diameter and lower jugular venous ratio are independently associated with poorer outcome also in outpatients. 287 Lung ultrasound, through the assessment of B‐lines, ensures an accurate tool for differential diagnosis of acute dyspnoea, 295 , 296 , 297 has a prognostic value, 287 , 298 and has been proposed to guide diuretic treatment in outpatients with benefits. 299 A recent expert consensus aims at standardizing approach in image acquisition methods and B‐line quantification. 300
Medical therapy
Treatment of acute HF may be divided into three phases—initial stabilization, after initial stabilization, and pre‐discharge and post‐discharge period. 301 Decongestion is the main goal of acute HF therapy. Loop diuretics are the first choice in order to achieve euvolaemia, and diuretic resistance is associated with poorer outcomes. 302 In patients at high risk for diuretic resistance, addition of acetazolamide may be useful. 303 , 304
Treatment of the acute phase failed to improve outcomes so far. Vasodilators seem to be neutral, although an excessive pressure drop is associated with kidney impairment and worse outcome. 305 Inotropes and/or vasopressors are associated with an increased risk of all‐cause death. Despite the neutral results of the second RELAX in Acute Heart Failure (RELAX‐AHF‐2) trial, 306 a recent meta‐analysis including serelaxin trials has shown that serelaxin reduces the risk of 5 day worsening HF and has beneficial effects on markers of renal function and cardiac damage. 307 Furthermore, in the RELAX‐AHF‐EU study, serelaxin showed a reduction of worsening HF and all‐cause death through Day 5 when added to standard of care therapy. 308 Ongoing studies will assess tolerability and efficacy of BMS‐986231, a novel nitroxyl donor with potential benefits on haemodynamics. 309
Meanwhile, the optimization of oral treatment in the pre‐discharge and post‐discharge period plays a pivotal role in improving survival. 310 Early initiation of sacubitril/valsartan before or shortly after discharge is safe and beneficial. 154 , 155 The STRONG‐HF (Safety, Tolerability and efficacy of Rapid Optimization, helped by NT‐proBNP and GDF‐15, of Heart Failure therapies) trial will assess whether fast up‐titration of GDMT can be a safe and feasible option in patients discharged after acute HF. 311
Conclusions
We have summarized the most recent findings in HF discussing many aspects such as epidemiology, diagnosis, co‐morbidities, and treatment. Despite improvements in treatment of HFrEF, HF is still a major cause of poor quality of life, morbidity, and mortality worldwide. However, major advances have been achieved recently in the medical management of HFrEF with the discovery of new therapeutic pathways, namely, SGLT‐2 inhibitors, and with better treatment of co‐morbidities. New devices are emerging for specific conditions such as sleep apnoea, and percutaneous treatment of mitral and tricuspid regurgitation may have a major impact on symptoms and clinical outcomes. HFpEF remains an unsolved issue, particularly in terms of diagnosis and treatment. Better patient phenotyping seems the next promising step. However, this hypothesis needs testing in properly designed clinical studies.
Conflict of interest
D.T. and M.A. declare that they have no conflict of interest. M.S.A. has received personal fees from Servier, outside the submitted work. SvH has been a paid consultant for and/or received honoraria payments from Bayer, Boehringer Ingelheim, BRAHMS, Chugai, Grünenthal, Helsinn, Hexal, Novartis, Pharmacosmos, Respicardia, Roche, Sorin, and Vifor. SvH owns shares in Actimed. SvH reports research support from IMI and the German Center for Cardiovascular Research (DZHK). M.M. has received in the last 3 years personal honoraria from Abbott Vascular, Actelion, Amgen, AstraZeneca, Bayer, LivaNova, Servier, Vifor Pharma, and Windtree Therapeutics for participation to trials' committees or advisory boards and speaker honoraria from Abbott Vascular, Edwards Therapeutics, and Servier.
Tomasoni, D. , Adamo, M. , Anker, M. S. , von Haehling, S. , Coats, A. J. S. , and Metra, M. (2020) Heart failure in the last year: progress and perspective. ESC Heart Failure, 7: 3505–3530. 10.1002/ehf2.13124.
References
- 1. Bundgaard JS, Mogensen UM, Christensen S, Ploug U, Rørth R, Ibsen R, Kjellberg J, Køber L. The economic burden of heart failure in Denmark from 1998 to 2016. Eur J Heart Fail 2019; 21: 1526–1531. [DOI] [PubMed] [Google Scholar]
- 2. Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev 2017; 3: 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cook C, Cole G, Asaria P, Jabbour R, Francis DP. The annual global economic burden of heart failure. Int J Cardiol 2014; 171: 368–376. [DOI] [PubMed] [Google Scholar]
- 4. Lesyuk W, Kriza C, Kolominsky‐Rabas P. Cost‐of‐illness studies in heart failure: a systematic review 2004–2016. BMC Cardiovasc Disord 2018; 18: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 2014; 63: 1123–1133. [DOI] [PubMed] [Google Scholar]
- 6. Gallagher AM, Lucas R, Cowie MR. Assessing health‐related quality of life in heart failure patients attending an outpatient clinic: a pragmatic approach. ESC Heart Fail 2019; 6: 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ketilsdottir A, Ingadottir B, Jaarsma T. Self‐reported health and quality of life outcomes of heart failure patients in the aftermath of a national economic crisis: a cross‐sectional study. ESC Heart Fail 2019; 6: 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Leon CF, Grady KL, Eaton C, Rucker‐Whitaker C, Janssen I, Calvin J, Powell LH. Quality of life in a diverse population of patients with heart failure: BASELINE FINDINGS FROM THE HEART FAILURE ADHERENCE AND RETENTION TRIAL (HART). J Cardiopulm Rehabil Prev 2009; 29: 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dokainish H, Teo K, Zhu J, Roy A, AlHabib KF, ElSayed A, Palileo‐Villaneuva L, Lopez‐Jaramillo P, Karaye K, Yusoff K, Orlandini A, Sliwa K, Mondo C, Lanas F, Prabhakaran D, Badr A, Elmaghawry M, Damasceno A, Tibazarwa K, Belley‐Cote E, Balasubramanian K, Islam S, Yacoub MH, Huffman MD, Harkness K, Grinvalds A, McKelvie R, Bangdiwala SI, Yusuf S, INTER‐CHF Investigators . Global mortality variations in patients with heart failure: results from the International Congestive Heart Failure (INTER‐CHF) prospective cohort study. Lancet Glob Health 2017; 5: e665–e672. [DOI] [PubMed] [Google Scholar]
- 10. Nieminen MS, Dickstein K, Fonseca C, Serrano JM, Parissis J, Fedele F, Wikström G, Agostoni P, Atar S, Baholli L, Brito D, Colet JC, Édes I, Gómez Mesa JE, Gorjup V, Garza EH, González Juanatey JR, Karanovic N, Karavidas A, Katsytadze I, Kivikko M, Matskeplishvili S, Merkely B, Morandi F, Novoa A, Oliva F, Ostadal P, Pereira‐Barretto A, Pollesello P, Rudiger A, Schwinger RH, Wieser M, Yavelov I, Zymliński R. The patient perspective: quality of life in advanced heart failure with frequent hospitalisations. Int J Cardiol 2015; 191: 256–264. [DOI] [PubMed] [Google Scholar]
- 11. Jones NR, Roalfe AK, Adoki I, Hobbs FDR, Taylor CJ. Survival of patients with chronic heart failure in the community: a systematic review and meta‐analysis. Eur J Heart Fail 2019; 21: 1306–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tromp J, Teng TH, Tay WT, Hung CL, Narasimhan C, Shimizu W, Park SW, Liew HB, Ngarmukos T, Reyes EB, Siswanto BB, Yu CM, Zhang S, Yap J, MacDonald M, Ling LH, Leineweber K, Richards AM, Zile MR, Anand IS, Lam CSP, Investigators ASIAN‐HF. Heart failure with preserved ejection fraction in Asia. Eur J Heart Fail 2019; 21: 23–36. [DOI] [PubMed] [Google Scholar]
- 13. Hao G, Wang X, Chen Z, Zhang L, Zhang Y, Wei B, Zheng C, Kang Y, Jiang L, Zhu Z, Zhang J, Wang Z, Gao R, China Hypertension Survey Investigators . Prevalence of heart failure and left ventricular dysfunction in China: the China Hypertension Survey, 2012–2015. Eur J Heart Fail 2019; 21: 1329–1337. [DOI] [PubMed] [Google Scholar]
- 14. Seferović PM, Jankowska E, Coats AJS, Maggioni AP, Lopatin Y, Milinković I, Polovina M, Lainščak M, Timmis A, Huculeci R, Vardas P, Task Force of the HFA Atlas, and the ESC Atlas of Cardiology leadership, developed in collaboration with the National Heart Failure Societies of the ESC member and ESC affiliated member countries . The Heart Failure Association Atlas: rationale, objectives, and methods. Eur J Heart Fail 2020; 22: 638–645. [DOI] [PubMed] [Google Scholar]
- 15. Mamas MA, Sperrin M, Watson MC, Coutts A, Wilde K, Burton C, Kadam UT, Kwok CS, Clark AB, Murchie P, Buchan I, Hannaford PC, Myint PK. Do patients have worse outcomes in heart failure than in cancer? A primary care‐based cohort study with 10‐year follow‐up in Scotland. Eur J Heart Fail 2017; 19: 1095–1104. [DOI] [PubMed] [Google Scholar]
- 16. Sulo G, Igland J, Øverland S, Egeland GM, Roth GA, Vollset SE, Tell GS. Heart failure in Norway, 2000–2014: analysing incident, total and readmission rates using data from the Cardiovascular Disease in Norway (CVDNOR) Project. Eur J Heart Fail 2020; 22: 241–248. [DOI] [PubMed] [Google Scholar]
- 17. Tromp J, Ferreira JP, Janwanishstaporn S, Shah M, Greenberg B, Zannad F, Lam CSP. Heart failure around the world. Eur J Heart Fail 2019; 21: 1187–1196. [DOI] [PubMed] [Google Scholar]
- 18. Dewan P, Jhund PS, Shen L, Petrie MC, Abraham WT, Atif Ali M, Chen CH, Desai AS, Dickstein K, Huang J, Kiatchoosakun S, Kim KS, Køber L, Lai WT, Liao Y, Mogensen UM, Oh BH, Packer M, Rouleau JL, Shi V, Sibulo AS Jr, Solomon SD, Sritara P, Swedberg K, Tsutsui H, Zile MR, McMurray JJV. Heart failure with reduced ejection fraction: comparison of patient characteristics and clinical outcomes within Asia and between Asia. Europe and the Americas Eur J Heart Fail 2019; 21: 577–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chandramouli C, Teng TK, Tay WT, Yap J, MacDonald MR, Tromp J, Yan L, Siswanto B, Reyes EB, Ngarmukos T, Yu CM, Hung CL, Anand I, Richards AM, Ling LH, Regensteiner JG, Lam CSP, ASIAN‐HF Investigators . Impact of diabetes and sex in heart failure with reduced ejection fraction patients from the ASIAN‐HF registry. Eur J Heart Fail 2019; 21: 297–307. [DOI] [PubMed] [Google Scholar]
- 20. Uijl A, Koudstaal S, Direk K, Denaxas S, Groenwold RHH, Banerjee A, Hoes AW, Hemingway H, Asselbergs FW. Risk factors for incident heart failure in age‐ and sex‐specific strata: a population‐based cohort using linked electronic health records. Eur J Heart Fail 2019; 21: 1197–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bomer N, Grote Beverborg N, Hoes MF, Streng KW, Vermeer M, Dokter MM, IJmker J, Anker SD, Cleland JGF, Hillege HL, Lang CC, Ng LL, Samani NJ, Tromp J, van Veldhuisen DJ, Touw DJ, Voors AA, van der Meer P. Selenium and outcome in heart failure. Eur J Heart Fail 2019; 22: 1415–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nagai T, Sundaram V, Shoaib A, Shiraishi Y, Kohsaka S, Rothnie KJ, Piper S, McDonagh TA, Hardman SMC, Goda A, Mizuno A, Sawano M, Rigby AS, Quint JK, Yoshikawa T, Clark AL, Anzai T, Cleland JGF. Validation of U.S. mortality prediction models for hospitalized heart failure in the United Kingdom and Japan. Eur J Heart Fail 2018; 20: 1179–1190. [DOI] [PubMed] [Google Scholar]
- 23. Doleeb S, Kratz A, Salter M, Thohan V. Strong muscles, weak heart: testosterone‐induced cardiomyopathy. ESC Heart Fail 2019; 6: 1000–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nagao K, Inada T, Tamura A, Kajitani K, Shimamura K, Yukawa H, Aida K, Sowa N, Nishiga M, Horie T, Makita T, Ono K, Tanaka M. Circulating markers of collagen types I, III, and IV in patients with dilated cardiomyopathy: relationships with myocardial collagen expression. ESC Heart Fail 2018; 5: 1044–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jääskeläinen P, Vangipurapu J, Raivo J, Kuulasmaa T, Heliö T, Aalto‐Setälä K, Kaartinen M, Ilveskoski E, Vanninen S, Hämäläinen L, Melin J, Kokkonen J, Nieminen MS, FinHCM Study Group , Laakso M, Kuusisto J. Genetic basis and outcome in a nationwide study of Finnish patients with hypertrophic cardiomyopathy. ESC Heart Fail 2019; 6: 436–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bocchi EA, Rassi S, Guimarães GV, Argentina, Chile, and Brazil SHIFT Investigators . Safety profile and efficacy of ivabradine in heart failure due to Chagas heart disease: a post hoc analysis of the SHIFT trial. ESC Heart Fail 2018; 5: 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Seferović PM, Polovina M, Bauersachs J, Arad M, Gal TB, Lund LH, Felix SB, Arbustini E, Caforio ALP, Farmakis D, Filippatos GS, Gialafos E, Kanjuh V, Krljanac G, Limongelli G, Linhart A, Lyon AR, Maksimović R, Miličić D, Milinković I, Noutsias M, Oto A, Oto Ö, Pavlović SU, Piepoli MF, Ristić AD, Rosano GMC, Seggewiss H, Ašanin M, Seferović JP, Ruschitzka F, Čelutkiene J, Jaarsma T, Mueller C, Moura B, Hill L, Volterrani M, Lopatin Y, Metra M, Backs J, Mullens W, Chioncel O, de Boer RA, Anker S, Rapezzi C, Coats AJS, Tschöpe C. Heart failure in cardiomyopathies: a position paper from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019; 21: 553–576. [DOI] [PubMed] [Google Scholar]
- 28. Al‐Saaidi RA, Rasmussen TB, Birkler RID, Palmfeldt J, Beqqali A, Pinto YM, Nissen PH, Baandrup U, Mølgaard H, Hey TM, Eiskjaer H, Bross P, Mogensen J. The clinical outcome of LMNA missense mutations can be associated with the amount of mutated protein in the nuclear envelope. Eur J Heart Fail 2018; 20: 1404–1412. [DOI] [PubMed] [Google Scholar]
- 29. Elliott PM, Anastasakis A, Asimaki A, Basso C, Bauce B, Brooke MA, Calkins H, Corrado D, Duru F, Green KJ, Judge DP, Kelsell D, Lambiase PD, McKenna WJ, Pilichou K, Protonotarios A, Saffitz JE, Syrris P, Tandri H, te Riele A, Thiene G, Tsatsopoulou A, van Tintelen JP. Definition and treatment of arrhythmogenic cardiomyopathy: an updated expert panel report. Eur J Heart Fail 2019; 21: 955–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Czepluch FS, Wollnik B, Hasenfuß G. Genetic determinants of heart failure: facts and numbers. ESC Heart Fail 2018; 5: 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hermida A, Fressart V, Hidden‐Lucet F, Donal E, Probst V, Deharo JC, Chevalier P, Klug D, Mansencal N, Delacretaz E, Cosnay P, Scanu P, Extramiana F, Keller DI, Rouanet S, Charron P, Gandjbakhch E. High risk of heart failure associated with desmoglein‐2 mutations compared to plakophilin‐2 mutations in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Eur J Heart Fail 2019; 21: 792–800. [DOI] [PubMed] [Google Scholar]
- 32. Pilichou K, Basso C. Heart failure in arrhythmogenic cardiomyopathy: is phenotypic variability just a matter of genetics? Eur J Heart Fail 2019; 21: 801–802. [DOI] [PubMed] [Google Scholar]
- 33. Yamamoto H, Yokochi T. Transthyretin cardiac amyloidosis: an update on diagnosis and treatment. ESC Heart Fail 2019; 6: 1128–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mints YY, Doros G, Berk JL, Connors LH, Ruberg FL. Features of atrial fibrillation in wild‐type transthyretin cardiac amyloidosis: a systematic review and clinical experience. ESC Heart Fail 2018; 5: 772–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shintani Y, Okada A, Morita Y, Hamatani Y, Amano M, Takahama H, Amaki M, Hasegawa T, Ohta‐Ogo K, Kanzaki H, Ishibashi‐Ueda H, Yasuda S, Shimazaki C, Yoshinaga T, Yazaki M, Sekijima Y, Izumi C. Monitoring treatment response to tafamidis by serial native T1 and extracellular volume in transthyretin amyloid cardiomyopathy. ESC Heart Fail 2019; 6: 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Quarta CC, Kruger JL, Falk RH. Cardiac amyloidosis. Circulation 2012; 126: e178–e182. [DOI] [PubMed] [Google Scholar]
- 37. Clemmensen TS, Mølgaard H, Sörensen J, Eiskjaer H, Andersen NF, Mellemkjaer S, Andersen MJ, Tolbod LP, Harms HJ, Poulsen SH. Inotropic myocardial reserve deficiency is the predominant feature of exercise haemodynamics in cardiac amyloidosis. Eur J Heart Fail 2017; 19: 1457–1465. [DOI] [PubMed] [Google Scholar]
- 38. Gagliardi C, Perfetto F, Lorenzini M, Ferlini A, Salvi F, Milandri A, Quarta CC, Taborchi G, Bartolini S, Frusconi S, Martone R, Cinelli MM, Foffi S, Reggiani MLB, Fabbri G, Cataldo P, Cappelli F, Rapezzi C. Phenotypic profile of Ile68Leu transthyretin amyloidosis: an underdiagnosed cause of heart failure. Eur J Heart Fail 2018; 20: 1417–1425. [DOI] [PubMed] [Google Scholar]
- 39. Seferovic PM, Ponikowski P, Anker SD, Bauersachs J, Chioncel O, Cleland JGF, de Boer RA, Drexel H, Ben Gal T, Hill L, Jaarsma T, Jankowska EA, Anker MS, Lainscak M, Lewis BS, McDonagh T, Metra M, Milicic D, Mullens W, Piepoli MF, Rosano G, Ruschitzka F, Volterrani M, Voors AA, Filippatos G, Coats AJS. Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019; 21: 1169–1186. [DOI] [PubMed] [Google Scholar]
- 40. Bauersachs J, König T, van der Meer P, Petrie MC, Hilfiker‐Kleiner D, Mbakwem A, Hamdan R, Jackson AM, Forsyth P, de Boer RA, Mueller C, Lyon AR, Lund LH, Piepoli MF, Heymans S, Chioncel O, Anker SD, Ponikowski P, Seferovic PM, Johnson MR, Mebazaa A, Sliwa K. Pathophysiology, diagnosis and management of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Study Group on peripartum cardiomyopathy. Eur J Heart Fail 2019; 21: 827–843. [DOI] [PubMed] [Google Scholar]
- 41. Iorio A, Senni M, Barbati G, Greene SJ, Poli S, Zambon E, di Nora C, Cioffi G, Tarantini L, Gavazzi A, Sinagra G, di Lenarda A. Prevalence and prognostic impact of non‐cardiac co‐morbidities in heart failure outpatients with preserved and reduced ejection fraction: a community‐based study. Eur J Heart Fail 2018; 20: 1257–1266. [DOI] [PubMed] [Google Scholar]
- 42. Wolsk E, Claggett B, Køber L, Pocock S, Yusuf S, Swedberg K, McMurray JJV, Granger CB, Pfeffer MA, Solomon SD. Contribution of cardiac and extra‐cardiac disease burden to risk of cardiovascular outcomes varies by ejection fraction in heart failure. Eur J Heart Fail 2018; 20: 504–510. [DOI] [PubMed] [Google Scholar]
- 43. Crespo‐Leiro MG, Metra M, Lund LH, Milicic D, Costanzo MR, Filippatos G, Gustafsson F, Tsui S, Barge‐Caballero E, De Jonge N, Frigerio M, Hamdan R, Hasin T, Hülsmann M, Nalbantgil S, Potena L, Bauersachs J, Gkouziouta A, Ruhparwar A, Ristic AD, Straburzynska‐Migaj E, McDonagh T, Seferovic P, Ruschitzka F. Advanced heart failure: a position statement of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2018; 20: 1505–1535. [DOI] [PubMed] [Google Scholar]
- 44. Ambrosy AP, Stevens SR, Al‐Khalidi HR, Rouleau JL, Bouabdallaoui N, Carson PE, Adlbrecht C, Cleland JGF, Dabrowski R, Golba KS, Pina IL, Sueta CA, Roy A, Sopko G, Bonow RO, Velazquez EJ, STICH Trial Investigators . Burden of medical co‐morbidities and benefit from surgical revascularization in patients with ischaemic cardiomyopathy. Eur J Heart Fail 2019; 21: 373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chamberlain AM, St Sauver JL, Gerber Y, Manemann SM, Boyd CM, Dunlay SM, Rocca WA, Finney Rutten LJ, Jiang R, Weston SA, Roger VL. Multimorbidity in heart failure: a community perspective. Am J Med 2015; 128: 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cooper LB, Yap J, Tay WT, Teng TK, MacDonald M, Anand IS, Sharma A, O'Connor CM, Kraus WE, Mentz RJ, Lam CS, HF‐ACTION and ASIAN‐HF Investigators . Multi‐ethnic comparisons of diabetes in heart failure with reduced ejection fraction: insights from the HF‐ACTION trial and the ASIAN‐HF registry. Eur J Heart Fail 2018; 20: 1281–1289. [DOI] [PubMed] [Google Scholar]
- 47. Polovina M, Lund LH, Đikić D, Petrović‐Đorđević I, Krljanac G, Milinković I, Veljić I, Piepoli MF, Rosano GMC, Ristić AD, Ašanin M, Seferović PM. Type 2 diabetes increases the long‐term risk of heart failure and mortality in patients with atrial fibrillation. Eur J Heart Fail 2020; 22: 113–125. [DOI] [PubMed] [Google Scholar]
- 48. Cosmi F, Shen L, Magnoli M, Abraham WT, Anand IS, Cleland JG, Cohn JN, Cosmi D, de Berardis G, Dickstein K, Franzosi MG, Gullestad L, Jhund PS, Kjekshus J, Køber L, Lepore V, Lucisano G, Maggioni AP, Masson S, McMurray JJV, Nicolucci A, Petrarolo V, Robusto F, Staszewsky L, Tavazzi L, Teli R, Tognoni G, Wikstrand J, Latini R. Treatment with insulin is associated with worse outcome in patients with chronic heart failure and diabetes. Eur J Heart Fail 2018; 20: 888–895. [DOI] [PubMed] [Google Scholar]
- 49. Kristensen SL, Rørth R, Jhund PS, Shen L, Lee MMY, Petrie MC, Køber L, McMurray JJV, BEST Investigators . Microvascular complications in diabetes patients with heart failure and reduced ejection fraction‐insights from the Beta‐blocker Evaluation of Survival Trial. Eur J Heart Fail 2018; 20: 1549–1556. [DOI] [PubMed] [Google Scholar]
- 50. Garnham JO, Roberts LD, Espino‐Gonzalez E, Whitehead A, Swoboda PP, Koshy A, Gierula J, Paton MF, Cubbon RM, Kearney MT, Egginton S, Bowen TS, Witte KK. Chronic heart failure with diabetes mellitus is characterized by a severe skeletal muscle pathology. J Cachexia Sarcopenia Muscle 2020; 11: 394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Niedziela JT, Hudzik B, Strojek K, Poloński L, Gąsior M, Rozentryt P. Weight loss in heart failure is associated with increased mortality only in non‐obese patients without diabetes. J Cachexia Sarcopenia Muscle 2019; 10: 1307–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Coiro S, Girerd N, Sharma A, Rossignol P, Tritto I, Pitt B, Pfeffer MA, McMurray JJV, Ambrosio G, Dickstein K, Moss A, Zannad F. Association of diabetes and kidney function according to age and systolic function with the incidence of sudden cardiac death and non‐sudden cardiac death in myocardial infarction survivors with heart failure. Eur J Heart Fail 2019; 21: 1248–1258. [DOI] [PubMed] [Google Scholar]
- 53. Koppe L, Fouque D, Kalantar‐Zadeh K. Kidney cachexia or protein‐energy wasting in chronic kidney disease: facts and numbers. J Cachexia Sarcopenia Muscle 2019; 10: 479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Canepa M, Straburzynska‐Migaj E, Drozdz J, Fernandez‐Vivancos C, Pinilla JMG, Nyolczas N, Temporelli PL, Mebazaa A, Lainscak M, Laroche C, Maggioni AP, Piepoli MF, Coats AJS, Ferrari R, Tavazzi L, ESC‐HFA Heart Failure Long‐Term Registry Investigators . Characteristics, treatments and 1‐year prognosis of hospitalized and ambulatory heart failure patients with chronic obstructive pulmonary disease in the European Society of Cardiology Heart Failure Long‐Term Registry. Eur J Heart Fail 2018; 20: 100–110. [DOI] [PubMed] [Google Scholar]
- 55. Doehner W, Ural D, Haeusler KG, Čelutkienė J, Bestetti R, Cavusoglu Y, Peña‐Duque MA, Glavas D, Iacoviello M, Laufs U, Alvear RM, Mbakwem A, Piepoli MF, Rosen SD, Tsivgoulis G, Vitale C, Yilmaz MB, Anker SD, Filippatos G, Seferovic P, Coats AJS, Ruschitzka F. Heart and brain interaction in patients with heart failure: overview and proposal for a taxonomy. A position paper from the Study Group on Heart and Brain Interaction of the Heart Failure Association. Eur J Heart Fail 2018; 20: 199–215. [DOI] [PubMed] [Google Scholar]
- 56. Parati G, Ochoa JE. Prognostic value of baroreflex sensitivity in heart failure. A 2018 reappraisal. Eur J Heart Fail 2019; 21: 59–62. [DOI] [PubMed] [Google Scholar]
- 57. Kokkinos P, Faselis C, Franklin B, Lavie CJ, Sidossis L, Moore H, Karasik P, Myers J. Cardiorespiratory fitness, body mass index and heart failure incidence. Eur J Heart Fail 2019; 21: 436–444. [DOI] [PubMed] [Google Scholar]
- 58. Moliner P, Lupón J, de Antonio M, Domingo M, Santiago‐Vacas E, Zamora E, Cediel G, Santesmases J, Díez‐Quevedo C, Troya MI, Boldó M, Altmir S, Alonso N, González B, Núñez J, Bayes‐Genis A. Trends in modes of death in heart failure over the last two decades: less sudden death but cancer deaths on the rise. Eur J Heart Fail 2019; 21: 1259–1266. [DOI] [PubMed] [Google Scholar]
- 59. de Boer RA, Meijers WC, van der Meer P, van Veldhuisen DJ. Cancer and heart disease: associations and relations. Eur J Heart Fail 2019; 21: 1515–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Banke A, Fosbøl EL, Møller JE, Gislason GH, Andersen M, Bernsdorf M, Jensen MB, Schou M, Ejlertsen B. Long‐term effect of epirubicin on incidence of heart failure in women with breast cancer: insight from a randomized clinical trial. Eur J Heart Fail 2018; 20: 1447–1453. [DOI] [PubMed] [Google Scholar]
- 61. Mansouri I, Allodji RS, Hill C, El‐Fayech C, Pein F, Diallo S, Schwartz B, Vu‐Bezin G, Veres C, Souchard V, Dumas A, Bolle S, Thomas‐Teinturier C, Pacquement H, Munzer M, Bondiau PY, Berchery D, Fresneau B, Oberlin O, Diallo I, de Vathaire F, Haddy N. The role of irradiated heart and left ventricular volumes in heart failure occurrence after childhood cancer. Eur J Heart Fail 201; 21: 509–518. [DOI] [PubMed] [Google Scholar]
- 62. Lena A, Ebner N, Anker MS. Cardiac cachexia. Eur Heart J Suppl 2019; 21: L24–L27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lena A, Ebner N, Coats AJS, Anker MS. Cardiac cachexia: the mandate to increase clinician awareness. Curr Opin Support Palliat Care 2019; 13: 298–304. [DOI] [PubMed] [Google Scholar]
- 64. Hulmi JJ, Nissinen TA, Räsänen M, Degerman J, Lautaoja JH, Hemanthakumar KA, Backman JT, Ritvos O, Silvennoinen M, Kivelä R. Prevention of chemotherapy‐induced cachexia by ACVR2B ligand blocking has different effects on heart and skeletal muscle. J Cachexia Sarcopenia Muscle 2018; 9: 417–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bauer J, Morley JE, Schols AMWJ, Ferrucci L, Cruz‐Jentoft AJ, Dent E, Baracos VE, Crawford JA, Doehner W, Heymsfield SB, Jatoi A, Kalantar‐Zadeh K, Lainscak M, Landi F, Laviano A, Mancuso M, Muscaritoli M, Prado CM, Strasser F, von Haehling S, Coats AJS, Anker SD. Sarcopenia: a time for action. An SCWD position paper. J Cachexia Sarcopenia Muscle 2019; 10: 956–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. von Haehling S. Muscle wasting and sarcopenia in heart failure: a brief overview of the current literature. ESC Heart Fail 2018; 5: 1074–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. von Haehling S, Garfias Macedo T, Valentova M, Anker MS, Ebner N, Bekfani T, Haarmann H, Schefold JC, Lainscak M, Cleland JGF, Doehner W, Hasenfuss G, Anker SD. Muscle wasting as an independent predictor of survival in patients with chronic heart failure. J Cachexia Sarcopenia Muscle 2020; 11: 1242–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Suzuki T, Palus S, Springer J. Skeletal muscle wasting in chronic heart failure. ESC Heart Fail 2018; 5: 1099–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lena A, Anker MS, Springer J. Muscle wasting and sarcopenia in heart failure–the current state of science. Int J Mol Sci 2020; 21: 6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lena A, Coats AJS, Anker MS. Metabolic disorders in heart failure and cancer. ESC Heart Fail 2018; 5: 1092–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Antunes‐Correa LM, Trevizan PF, Bacurau AVN, Ferreira‐Santos L, Gomes JLP, Urias U, Oliveira PA, Alves MJNN, de Almeida DR, Brum PC, Oliveira EM, Hajjar L, Kalil Filho R, Negrão CE. Effects of aerobic and inspiratory training on skeletal muscle microRNA‐1 and downstream‐associated pathways in patients with heart failure. J Cachexia Sarcopenia Muscle 2020; 11: 89–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Vitale C, Jankowska E, Hill L, Piepoli M, Doehner W, Anker SD, Lainscak M, Jaarsma T, Ponikowski P, Rosano GMC, Seferovic P, Coats AJ. Heart Failure Association/European Society of Cardiology position paper on frailty in patients with heart failure. Eur J Heart Fail 2019; 21: 1299–1305. [DOI] [PubMed] [Google Scholar]
- 73. Matsue Y, Kamiya K, Saito H, Saito K, Ogasahara Y, Maekawa E, Konishi M, Kitai T, Iwata K, Jujo K, Wada H, Kasai T, Nagamatsu H, Ozawa T, Izawa K, Yamamoto S, Aizawa N, Yonezawa R, Oka K, Momomura SI, Kagiyama N. Prevalence and prognostic impact of the coexistence of multiple frailty domains in elderly patients with heart failure: the FRAGILE‐HF cohort study. Eur J Heart Fail 2020. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 74. Bielecka‐Dabrowa A, Ebner N, dos Santos MR, Ishida J, Hasenfuss G, von Haehling S. Cachexia, muscle wasting, and frailty in cardiovascular disease. Eur J Heart Fail 2020. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 75. Ather S, Chan W, Bozkurt B, Aguilar D, Ramasubbu K, Zachariah AA, Wehrens XH, Deswal A. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol 2012; 59: 998–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sanders NA, Supiano MA, Lewis EF, Liu J, Claggett B, Pfeffer MA, Desai AS, Sweitzer NK, Solomon SD, Fang JC. The frailty syndrome and outcomes in the TOPCAT trial. Eur J Heart Fail 2018; 20: 1570–1577. [DOI] [PubMed] [Google Scholar]
- 77. Sokoreli I, Pauws SC, Steyerberg EW, de Vries GJ, Riistama JM, Tesanovic A, Kazmi S, Pellicori P, Cleland JG, Clark AL. Prognostic value of psychosocial factors for first and recurrent hospitalizations and mortality in heart failure patients: insights from the OPERA‐HF study. Eur J Heart Fail 2018; 20: 689–696. [DOI] [PubMed] [Google Scholar]
- 78. Yeung SSY, Reijnierse EM, Pham VK, Trappenburg MC, Lim WK, Meskers CGM, Maier AB. Sarcopenia and its association with falls and fractures in older adults: a systematic review and meta‐analysis. J Cachexia Sarcopenia Muscle 2019; 10: 485–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Suetta C, Haddock B, Alcazar J, Noerst T, Hansen OM, Ludvig H, Kamper RS, Schnohr P, Prescott E, Andersen LL, Frandsen U, Aagaard P, Bülow J, Hovind P, Simonsen L. The Copenhagen Sarcopenia Study: lean mass, strength, power, and physical function in a Danish cohort aged 20–93 years. J Cachexia Sarcopenia Muscle 2019; 10: 1316–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gutman SJ, Costello BT, Papapostolou S, Iles L, Ja J, Hare JL, Ellims A, Marwick TH, Taylor AJ. Impact of sex, socio‐economic status, and remoteness on therapy and survival in heart failure. ESC Heart Fail 2019; 6: 944–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Levinsson A, Dubé MP, Tardif JC, de Denus S. Sex, drugs, and heart failure: a sex‐sensitive review of the evidence base behind current heart failure clinical guidelines. ESC Heart Fail 2018; 5: 745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Palau P, Domínguez E, Núñez J. Sex differences on peak oxygen uptake in heart failure. ESC Heart Fail 2019; 6: 921–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Dewan P, Rørth R, Raparelli V, Campbell RT, Shen L, Jhund PS, Petrie MC, Anand IS, Carson PE, Desai AS, Granger CB, Køber L, Komajda M, McKelvie RS, O'Meara E, Pfeffer MA, Pitt B, Solomon SD, Swedberg K, Zile MR, McMurray JJV. Sex‐related differences in heart failure with preserved ejection fraction. Circ Heart Fail 2019; 12: e006539. [DOI] [PubMed] [Google Scholar]
- 84. Anker MS, von Haehling S, Landmesser U, Coats AJS, Anker SD. Cancer and heart failure–more than meets the eye: common risk factors and co‐morbidities. Eur J Heart Fail 2018; 20: 1382–1384. [DOI] [PubMed] [Google Scholar]
- 85. Anker MS, Lena A, Hadzibegovic S, Belenkov Y, Bergler‐Klein J, de Boer RA, Cohen‐Solal A, Farmakis D, von Haehling S, López‐Fernández T, Pudil R, Suter T, Tocchetti CG, Lyon AR, Heart Failure Association Cardio‐Oncology Study Group of the European Society of Cardiology . Modern‐day cardio‐oncology: a report from the ‘Heart Failure and World Congress on Acute Heart Failure 2018’. ESC Heart Fail 2018; 5: 1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Anker MS , Hadzibegovic S, Lena A, Belenkov Y, Bergler‐Klein J, de Boer RA, Farmakis D, von Haehling S, Iakobishvili Z, Maack C, Pudil R, Skouri H, Cohen‐Solal A, Tocchetti CG, Coats AJS, Seferović PM, Lyon AR, Heart Failure Association Cardio‐Oncology Study Group of the European Society of Cardiology . Recent advances in cardio‐oncology: a report from the ‘Heart Failure Association 2019 and World Congress on Acute Heart Failure 2019’. ESC Heart Fail 2019; 6: 1140–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Anker MS, Ebner N, Hildebrandt B, Springer J, Sinn M, Riess H, Anker SD, Landmesser U, Haverkamp W, von Haehling S. Resting heart rate is an independent predictor of death in patients with colorectal, pancreatic, and non‐small cell lung cancer: results of a prospective cardiovascular long‐term study. Eur J Heart Fail 2016; 18: 1524–1534. [DOI] [PubMed] [Google Scholar]
- 88. Ameri P, Canepa M, Anker MS, Belenkov Y, Bergler‐Klein J, Cohen‐Solal A, Farmakis D, López‐Fernández T, Lainscak M, Pudil R, Ruschitska F, Seferovic P, Filippatos G, Coats A, Suter T, von Haehling S, Ciardiello F, de Boer RA, Lyon AR, Tocchetti CG, Heart Failure Association Cardio‐Oncology Study Group of the European Society of Cardiology . Cancer diagnosis in patients with heart failure: epidemiology, clinical implications and gaps in knowledge. Eur J Heart Fail 2018; 20: 879–887. [DOI] [PubMed] [Google Scholar]
- 89. Fornaro A, Olivotto I, Rigacci L, Ciaccheri M, Tomberli B, Ferrantini C, Coppini R, Girolami F, Mazzarotto F, Chiostri M, Milli M, Marchionni N, Castelli G. Comparison of long‐term outcome in anthracycline‐related versus idiopathic dilated cardiomyopathy: a single centre experience. Eur J Heart Fail 2018; 20: 898–906. [DOI] [PubMed] [Google Scholar]
- 90. Pareek N, Cevallos J, Moliner P, Shah M, Tan LL, Chambers V, Baksi AJ, Khattar RS, Sharma R, Rosen SD, Lyon AR. Activity and outcomes of a cardio‐oncology service in the United Kingdom–a five‐year experience. Eur J Heart Fail 2018; 20: 1721–1731. [DOI] [PubMed] [Google Scholar]
- 91. Totzeck M, Mincu RI, Heusch G, Rassaf T. Heart failure from cancer therapy: can we prevent it? ESC Heart Fail 2019; 6: 856–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Muehlberg F, Funk S, Zange L, von Knobelsdorff‐Brenkenhoff F, Blaszczyk E, Schulz A, Ghani S, Reichardt A, Reichardt P, Schulz‐Menger J. Native myocardial T1 time can predict development of subsequent anthracycline‐induced cardiomyopathy. ESC Heart Fail 2018; 5: 620–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GYH, Lyon AR, Lopez Fernandez T, Mohty D, Piepoli MF, Tamargo J, Torbicki A, Suter TM, ESC Scientific Document Group . 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J 2016; 37: 2768–2801. [DOI] [PubMed] [Google Scholar]
- 94. Jacobse JN, Steggink LC, Sonke GS, Schaapveld M, Hummel YM, Steenbruggen TG, Lefrandt JD, Nuver J, Crijns APG, Aleman BMP, van der Meer P, Gietema JA, van Leeuwen FE. Myocardial dysfunction in long‐term breast cancer survivors treated at ages 40–50 years. Eur J Heart Fail 2020; 22: 338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Keramida K, Farmakis D, Bingcang J, Sulemane S, Sutherland S, Bingcang RA, Ramachandran K, Tzavara C, Charalampopoulos G, Filippiadis D, Kouris N, Nihoyannopoulos P. Longitudinal changes of right ventricular deformation mechanics during trastuzumab therapy in breast cancer patients. Eur J Heart Fail 2019; 21: 529–535. [DOI] [PubMed] [Google Scholar]
- 96. Michel L, Mincu RI, Mahabadi AA, Settelmeier S, Al‐Rashid F, Rassaf T, Totzeck M. Troponins and brain natriuretic peptides for the prediction of cardiotoxicity in cancer patients: a meta‐analysis. Eur J Heart Fail 2020; 22: 350–361. [DOI] [PubMed] [Google Scholar]
- 97. Russo M, Guida F, Paparo L, Trinchese G, Aitoro R, Avagliano C, Fiordelisi A, Napolitano F, Mercurio V, Sala V, Li M, Sorriento D, Ciccarelli M, Ghigo A, Hirsch E, Bianco R, Iaccarino G, Abete P, Bonaduce D, Calignano A, Berni Canani R, Tocchetti CG. The novel butyrate derivative phenylalanine‐butyramide protects from doxorubicin‐induced cardiotoxicity. Eur J Heart Fail 2019; 21: 519–528. [DOI] [PubMed] [Google Scholar]
- 98. Sawano M, Shiraishi Y, Kohsaka S, Nagai T, Goda A, Mizuno A, Sujino Y, Nagatomo Y, Kohno T, Anzai T, Fukuda K, Yoshikawa T. Performance of the MAGGIC heart failure risk score and its modification with the addition of discharge natriuretic peptides. ESC Heart Fail 2018; 5: 610–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Agostoni P, Paolillo S, Mapelli M, Gentile P, Salvioni E, Veglia F, Bonomi A, Corrà U, Lagioia R, Limongelli G, Sinagra G, Cattadori G, Scardovi AB, Metra M, Carubelli V, Scrutinio D, Raimondo R, Emdin M, Piepoli M, Magrì D, Parati G, Caravita S, Re F, Cicoira M, Minà C, Correale M, Frigerio M, Bussotti M, Oliva F, Battaia E, Belardinelli R, Mezzani A, Pastormerlo L, Guazzi M, Badagliacca R, di Lenarda A, Passino C, Sciomer S, Zambon E, Pacileo G, Ricci R, Apostolo A, Palermo P, Contini M, Clemenza F, Marchese G, Gargiulo P, Binno S, Lombardi C, Passantino A, Filardi PP. Multiparametric prognostic scores in chronic heart failure with reduced ejection fraction: a long‐term comparison. Eur J Heart Fail 2018; 20: 700–710. [DOI] [PubMed] [Google Scholar]
- 100. O'Connor C, Fiuzat M, Mulder H, Coles A, Ahmad T, Ezekowitz JA, Adams KF, Piña IL, Anstrom KJ, Cooper LS, Mark DB, Whellan DJ, Januzzi JL Jr, Leifer ES, Felker GM. Clinical factors related to morbidity and mortality in high‐risk heart failure patients: the GUIDE‐IT predictive model and risk score. Eur J Heart Fail 2019; 21: 770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Adler ED, Voors AA, Klein L, Macheret F, Braun OO, Urey MA, Zhu W, Sama I, Tadel M, Campagnari C, Greenberg B, Yagil A. Improving risk prediction in heart failure using machine learning. Eur J Heart Fail 2020; 22: 139–147. [DOI] [PubMed] [Google Scholar]
- 102. Ferreira JP, Duarte K, Pfeffer MA, McMurray JJV, Pitt B, Dickstein K, Zannad F, Rossignol P, High‐Risk Myocardial Infarction Database Initiative . Association between mean systolic and diastolic blood pressure throughout the follow‐up and cardiovascular events in acute myocardial infarction patients with systolic dysfunction and/or heart failure: an analysis from the High‐Risk Myocardial Infarction Database Initiative. Eur J Heart Fail 2018; 20: 323–331. [DOI] [PubMed] [Google Scholar]
- 103. Böhm M, Cammann VL, Ghadri JR, Ukena C, Gili S, di Vece D, Kato K, Ding KJ, Szawan KA, Micek J, Jurisic S, D'Ascenzo F, Frangieh AH, Rechsteiner D, Seifert B, Ruschitzka F, Lüscher T, Templin C, Collaborators ITAK. Interaction of systolic blood pressure and resting heart rate with clinical outcomes in takotsubo syndrome: insights from the International Takotsubo Registry. Eur J Heart Fail 2018; 20: 1021–1030. [DOI] [PubMed] [Google Scholar]
- 104. Cleland JGF, Bunting KV, Flather MD, Altman DG, Holmes J, Coats AJS, Manzano L, McMurray JJV, Ruschitzka F, van Veldhuisen DJ, von Lueder TG, Böhm M, Andersson B, Kjekshus J, Packer M, Rigby AS, Rosano G, Wedel H, Hjalmarson Å, Wikstrand J, Kotecha D, Beta‐blockers in Heart Failure Collaborative Group . Beta‐blockers for heart failure with reduced, mid‐range, and preserved ejection fraction: an individual patient‐level analysis of double‐blind randomized trials. Eur Heart J 2018; 39: 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Sartipy U, Savarese G, Dahlström U, Fu M, Lund LH. Association of heart rate with mortality in sinus rhythm and atrial fibrillation in heart failure with preserved ejection fraction. Eur J Heart Fail 2019; 21: 471–479. [DOI] [PubMed] [Google Scholar]
- 106. Mueller C, McDonald K, de Boer RA, Maisel A, Cleland JGF, Kozhuharov N, Coats AJS, Metra M, Mebazaa A, Ruschitzka F, Lainscak M, Filippatos G, Seferovic PM, Meijers WC, Bayes‐Genis A, Mueller T, Richards M, Januzzi JL Jr, Heart Failure Association of the European Society of Cardiology . Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail 2019; 21: 715–731. [DOI] [PubMed] [Google Scholar]
- 107. Anker MS, von Haehling S, Anker SD. Novel biomarkers in heart failure and cardio‐oncology. Kardiol Pol 2019; 77: 329–330. [DOI] [PubMed] [Google Scholar]
- 108. Suthahar N, Meijers WC, Ho JE, Gansevoort RT, Voors AA, van der Meer P, Bakker SJL, Heymans S, van Empel V, Schroen B, van der Harst P, van Veldhuisen DJ, de Boer RA. Sex‐ specific associations of obesity and N‐terminal pro‐B‐type natriuretic peptide levels in the general population. Eur J Heart Fail 2018; 20: 1205–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Welsh P, Kou L, Yu C, Anand I, van Veldhuisen DJ, Maggioni AP, Desai AS, Solomon SD, Pfeffer MA, Cheng S, Gullestad L, Aukrust P, Ueland T, Swedberg K, Young JB, Kattan MW, Sattar N, McMurray JJV. Prognostic importance of emerging cardiac, inflammatory, and renal biomarkers in chronic heart failure patients with reduced ejection fraction and anaemia: RED‐HF study. Eur J Heart Fail 2018; 20: 268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Campbell DJ, Gong FF, Jelinek MV, Castro JM, Coller JM, McGrady M, Boffa U, Shiel L, Wang BH, Liew D, Wolfe R, Stewart S, Owen AJ, Krum H, Reid CM, Prior DL. Prediction of incident heart failure by serum amino‐terminal pro‐B‐type natriuretic peptide level in a community‐based cohort. Eur J Heart Fail 2019; 21: 449–459. [DOI] [PubMed] [Google Scholar]
- 111. Greene SJ, Butler J, Fonarow GC, Subacius HP, Ambrosy AP, Vaduganathan M, Triggiani M, Solomon SD, Lewis EF, Maggioni AP, Böhm M, Chioncel O, Nodari S, Senni M, Zannad F, Gheorghiade M, ASTRONAUT Investigators and Coordinators . Pre‐discharge and early post‐discharge troponin elevation among patients hospitalized for heart failure with reduced ejection fraction: findings from the ASTRONAUT trial. Eur J Heart Fail 2018; 20: 281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Rørth R, Jhund PS, Kristensen SL, Desai AS, Køber L, Rouleau JL, Solomon SD, Swedberg K, Zile MR, Packer M, McMurray JJV. The prognostic value of troponin T and N‐terminal pro B‐type natriuretic peptide, alone and in combination, in heart failure patients with and without diabetes. Eur J Heart Fail 2019; 21: 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Ferreira JP, Ouwerkerk W, Tromp J, Ng L, Dickstein K, Anker S, Filippatos G, Cleland JG, Metra M, van Veldhuisen DJ, Voors AA, Zannad F. Cardiovascular and non‐cardiovascular death distinction: the utility of troponin beyond N‐terminal pro‐B‐type natriuretic peptide. Findings from the BIOSTAT‐CHF study. Eur J Heart Fail 2020; 22: 81–89. [DOI] [PubMed] [Google Scholar]
- 114. Gohar A, Rutten FH, den Ruijter H, Kelder JC, von Haehling S, Anker SD, Möckel M, Hoes AW. Mid‐regional pro‐atrial natriuretic peptide for the early detection of non‐acute heart failure. Eur J Heart Fail 2019; 21: 1219–1227. [DOI] [PubMed] [Google Scholar]
- 115. Vaduganathan M, Cheema B, Cleveland E, Sankar K, Subacius H, Fonarow GC, Solomon SD, Lewis EF, Greene SJ, Maggioni AP, Böhm M, Zannad F, Butler J, Gheorghiade M. Plasma renin activity, response to aliskiren, and clinical outcomes in patients hospitalized for heart failure: the ASTRONAUT trial. Eur J Heart Fail 2018; 20: 677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Rachwan RJ, Butler J, Collins SP, Cotter G, Davison BA, Senger S, Ezekowitz JA, Filippatos G, Levy PD, Metra M, Ponikowski P, Teerlink JR, Voors AA, de Boer RA, Soergel DG, Felker GM, Pang PS. Is plasma renin activity associated with worse outcomes in acute heart failure? A secondary analysis from the BLAST‐AHF trial. Eur J Heart Fail 2019; 21: 1561–1570. [DOI] [PubMed] [Google Scholar]
- 117. Bouabdallaoui N, Claggett B, Zile MR, McMurray JJV, O'Meara E, Packer M, Prescott MF, Swedberg K, Solomon SD, Rouleau JL, PARADIGM‐HF Investigators and Committees . Growth differentiation factor‐15 is not modified by sacubitril/valsartan and is an independent marker of risk in patients with heart failure and reduced ejection fraction: the PARADIGM‐HF trial. Eur J Heart Fail 2018; 20: 1701–1709. [DOI] [PubMed] [Google Scholar]
- 118. Hanberg JS, Rao VS, Ahmad T, Chunara Z, Mahoney D, Jackson K, Jacoby D, Chen M, Wilson FP, Tang WHW, Kakkar R, Testani JM. Inflammation and cardio‐renal interactions in heart failure: a potential role for interleukin‐6. Eur J Heart Fail 2018; 20: 933–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Siegismund CS, Escher F, Lassner D, Kühl U, Gross U, Fruhwald F, Wenzel P, Münzel T, Frey N, Linke RP, Schultheiss HP. Intramyocardial inflammation predicts adverse outcome in patients with cardiac AL amyloidosis. Eur J Heart Fail 2018; 20: 751–757. [DOI] [PubMed] [Google Scholar]
- 120. Packer M. Derangements in adrenergic‐adipokine signalling establish a neurohormonal basis for obesity‐related heart failure with a preserved ejection fraction. Eur J Heart Fail 2018; 20: 873–878. [DOI] [PubMed] [Google Scholar]
- 121. van der Pol A, van Gilst WH, Voors AA, van der Meer P. Treating oxidative stress in heart failure: past, present and future. Eur J Heart Fail 2019; 21: 425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Markousis‐Mavrogenis G, Tromp J, Ouwerkerk W, Devalaraja M, Anker SD, Cleland JG, Dickstein K, Filippatos GS, van der Harst P, Lang CC, Metra M, Ng LL, Ponikowski P, Samani NJ, Zannad F, Zwinderman AH, Hillege HL, van Veldhuisen DJ, Kakkar R, Voors AA, van der Meer P. The clinical significance of interleukin‐6 in heart failure: results from the BIOSTAT‐CHF study. Eur J Heart Fail 2019; 21: 965–973. [DOI] [PubMed] [Google Scholar]
- 123. De Rosa S, Eposito F, Carella C, Strangio A, Ammirati G, Sabatino J, Abbate FG, Iaconetti C, Liguori V, Pergola V, Polimeni A, Coletta S, Gareri C, Trimarco B, Stabile G, Curcio A, Indolfi C, Rapacciuolo A. Transcoronary concentration gradients of circulating microRNAs in heart failure. Eur J Heart Fail 2018; 20: 1000–1010. [DOI] [PubMed] [Google Scholar]
- 124. van Boven N, Kardys I, van Vark LC, Akkerhuis KM, de Ronde MWJ, Khan MAF, Merkus D, Liu Z, Voors AA, Asselbergs FW, van den Bos EJ, Boersma E, Hillege H, Duncker DJ, Pinto YM, Postmus D. Serially measured circulating microRNAs and adverse clinical outcomes in patients with acute heart failure. Eur J Heart Fail 2018; 20: 89–96. [DOI] [PubMed] [Google Scholar]
- 125. Bayés‐Genis A, Lanfear DE, de Ronde MWJ, Lupón J, Leenders JJ, Liu Z, Zuithoff NPA, Eijkemans MJC, Zamora E, de Antonio M, Zwinderman AH, Pinto‐Sietsma SJ, Pinto YM. Prognostic value of circulating microRNAs on heart failure‐related morbidity and mortality in two large diverse cohorts of general heart failure patients. Eur J Heart Fail 2018; 20: 67–75. [DOI] [PubMed] [Google Scholar]
- 126. Masson S, Batkai S, Beermann J, Bär C, Pfanne A, Thum S, Magnoli M, Balconi G, Nicolosi GL, Tavazzi L, Latini R, Thum T. Circulating microRNA‐132 levels improve risk prediction for heart failure hospitalization in patients with chronic heart failure. Eur J Heart Fail 2018; 20: 78–85. [DOI] [PubMed] [Google Scholar]
- 127. Voors AA, Kremer D, Geven C, ter Maaten JM, Struck J, Bergmann A, Pickkers P, Metra M, Mebazaa A, Düngen HD, Butler J. Adrenomedullin in heart failure: pathophysiology and therapeutic application. Eur J Heart Fail 2019; 21: 163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Nougué H, Pezel T, Picard F, Sadoune M, Arrigo M, Beauvais F, Launay JM, Cohen‐Solal A, Vodovar N, Logeart D. Effects of sacubitril/valsartan on neprilysin targets and the metabolism of natriuretic peptides in chronic heart failure: a mechanistic clinical study. Eur J Heart Fail 2019; 21: 598–605. [DOI] [PubMed] [Google Scholar]
- 129. Chioncel O, Ambrosy AP. Trimethylamine N‐oxide and risk of heart failure progression: marker or mediator of disease. Eur J Heart Fail 2019; 21: 887–890. [DOI] [PubMed] [Google Scholar]
- 130. Nagueh SF. Non‐invasive assessment of left ventricular filling pressure. Eur J Heart Fail 2018; 20: 38–48. [DOI] [PubMed] [Google Scholar]
- 131. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28: 1–39 e14. [DOI] [PubMed] [Google Scholar]
- 132. Biering‐Sørensen T, Querejeta Roca G, Hegde SM, Shah AM, Claggett B, Mosley TH Jr, Butler KR Jr, Solomon SD. Left ventricular ejection time is an independent predictor of incident heart failure in a community‐based cohort. Eur J Heart Fail 2018; 20: 1106–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Patel PA, Ambrosy AP, Phelan M, Alenezi F, Chiswell K, van Dyke MK, Tomfohr J, Honarpour N, Velazquez EJ. Association between systolic ejection time and outcomes in heart failure by ejection fraction. Eur J Heart Fail 2020; 22: 1174–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Inciardi RM, Giugliano RP, Claggett B, Gupta DK, Chandra A, Ruff CT, Antman EM, Mercuri MF, Grosso MA, Braunwald E, Solomon SD, ENGAGE AF‐TIMI 48 Investigators . Left atrial structure and function and the risk of death or heart failure in atrial fibrillation. Eur J Heart Fail 2019; 21: 1571–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Reddy YNV, Obokata M, Egbe A, Yang JH, Pislaru S, Lin G, Carter R, Borlaug BA. Left atrial strain and compliance in the diagnostic evaluation of heart failure with preserved ejection fraction. Eur J Heart Fail 2019; 21: 891–900. [DOI] [PubMed] [Google Scholar]
- 136. Telles F, Nanayakkara S, Evans S, Patel HC, Mariani JA, Vizi D, William J, Marwick TH, Kaye DM. Impaired left atrial strain predicts abnormal exercise haemodynamics in heart failure with preserved ejection fraction. Eur J Heart Fail 2019; 21: 495–505. [DOI] [PubMed] [Google Scholar]
- 137. Inciardi RM, Rossi A, Bergamini C, Benfari G, Maffeis C, Greco C, Drago A, Guazzi M, Ribichini FL, Cicoira M. Mitral regurgitation, left atrial structural and functional remodelling and the effect on pulmonary haemodynamics. Eur J Heart Fail 2020; 22: 499–506. [DOI] [PubMed] [Google Scholar]
- 138. de Boer RA, de Keulenaer G, Bauersachs J, Brutsaert D, Cleland JG, Diez J, Du XJ, Ford P, Heinzel FR, Lipson KE, McDonagh T, Lopez‐Andres N, Lunde IG, Lyon AR, Pollesello P, Prasad SK, Tocchetti CG, Mayr M, Sluijter JPG, Thum T, Tschöpe C, Zannad F, Zimmermann WH, Ruschitzka F, Filippatos G, Lindsey ML, Maack C, Heymans S. Towards better definition, quantification and treatment of fibrosis in heart failure. A scientific roadmap by the Committee of Translational Research of the Heart Failure Association (HFA) of the European Society of Cardiology. Eur J Heart Fail 2019; 21: 272–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Wu CK, Lee JK, Hsu JC, Su MM, Wu YF, Lin TT, Lan CW, Hwang JJ, Lin LY. Myocardial adipose deposition and the development of heart failure with preserved ejection fraction. Eur J Heart Fail 2020; 22: 445–454. [DOI] [PubMed] [Google Scholar]
- 140. van Woerden G, Gorter TM, Westenbrink BD, Willems TP, van Veldhuisen DJ, Rienstra M. Epicardial fat in heart failure patients with mid‐range and preserved ejection fraction. Eur J Heart Fail 2018; 20: 1559–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Ferreira JP, Metra M, Anker SD, Dickstein K, Lang CC, Ng L, Samani NJ, Cleland JG, van Veldhuisen DJ, Voors AA, Zannad F. Clinical correlates and outcome associated with changes in 6‐minute walking distance in patients with heart failure: findings from the BIOSTAT‐CHF study. Eur J Heart Fail 2019; 21: 218–226. [DOI] [PubMed] [Google Scholar]
- 142. Paolillo S, Veglia F, Salvioni E, Corrà U, Piepoli M, Lagioia R, Limongelli G, Sinagra G, Cattadori G, Scardovi AB, Metra M, Senni M, Bonomi A, Scrutinio D, Raimondo R, Emdin M, Magrì D, Parati G, Re F, Cicoira M, Minà C, Correale M, Frigerio M, Bussotti M, Battaia E, Guazzi M, Badagliacca R, di Lenarda A, Maggioni A, Passino C, Sciomer S, Pacileo G, Mapelli M, Vignati C, Clemenza F, Binno S, Lombardi C, Filardi PP, Agostoni P, MECKI Score Research Group (see Appendix) . Heart failure prognosis over time: how the prognostic role of oxygen consumption and ventilatory efficiency during exercise has changed in the last 20 years. Eur J Heart Fail 2019; 21: 208–217. [DOI] [PubMed] [Google Scholar]
- 143. Rovai S, Corrà U, Piepoli M, Vignati C, Salvioni E, Bonomi A, Mattavelli I, Arcari L, Scardovi AB, Perrone Filardi P, Lagioia R, Paolillo S, Magrì D, Limongelli G, Metra M, Senni M, Scrutinio D, Raimondo R, Emdin M, Lombardi C, Cattadori G, Parati G, Re F, Cicoira M, Villani GQ, Minà C, Correale M, Frigerio M, Perna E, Mapelli M, Magini A, Clemenza F, Bussotti M, Battaia E, Guazzi M, Bandera F, Badagliacca R, di Lenarda A, Pacileo G, Maggioni A, Passino C, Sciomer S, Sinagra G, Agostoni P, MECKI Score Research Group (see Appendix 1) . Exercise oscillatory ventilation and prognosis in heart failure patients with reduced and mid‐range ejection fraction. Eur J Heart Fail 2019; 21: 1586–1595. [DOI] [PubMed] [Google Scholar]
- 144. Palazzini M, Dardi F, Manes A, Bacchi Reggiani ML, Gotti E, Rinaldi A, Albini A, Monti E, Galiè N. Pulmonary hypertension due to left heart disease: analysis of survival according to the haemodynamic classification of the 2015 ESC/ERS guidelines and insights for future changes. Eur J Heart Fail 2018; 20: 248–255. [DOI] [PubMed] [Google Scholar]
- 145. Weber L, Rickli H, Haager PK, Joerg L, Weilenmann D, Brenner R, Taramasso M, Baier P, Maisano F, Maeder MT. Haemodynamic mechanisms and long‐term prognostic impact of pulmonary hypertension in patients with severe aortic stenosis undergoing valve replacement. Eur J Heart Fail 2019; 21: 172–181. [DOI] [PubMed] [Google Scholar]
- 146. Tigges E, Blankenberg S, von Bardeleben RS, Zürn C, Bekeredjian R, Ouarrak T, Sievert H, Nickenig G, Boekstegers P, Senges J, Schillinger W, Lubos E. Implication of pulmonary hypertension in patients undergoing MitraClip therapy: results from the German transcatheter mitral valve interventions (TRAMI) registry. Eur J Heart Fail 2018; 20: 585–594. [DOI] [PubMed] [Google Scholar]
- 147. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 148. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017; 136: e137–e161. [DOI] [PubMed] [Google Scholar]
- 149. Komajda M, Böhm M, Borer JS, Ford I, Tavazzi L, Pannaux M, Swedberg K. Incremental benefit of drug therapies for chronic heart failure with reduced ejection fraction: a network meta‐analysis. Eur J Heart Fail 2018; 20: 1315–1322. [DOI] [PubMed] [Google Scholar]
- 150. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, PARADIGM‐HF Investigators and Committees . Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 151. Ambrosy AP, Mentz RJ, Fiuzat M, Cleland JGF, Greene SJ, O'Connor CM, Teerlink JR, Zannad F, Solomon SD. The role of angiotensin receptor‐neprilysin inhibitors in cardiovascular disease–existing evidence, knowledge gaps, and future directions. Eur J Heart Fail 2018; 20: 963–972. [DOI] [PubMed] [Google Scholar]
- 152. Mogensen UM, Gong J, Jhund PS, Shen L, Køber L, Desai AS, Lefkowitz MP, Packer M, Rouleau JL, Solomon SD, Claggett BL, Swedberg K, Zile MR, Mueller‐Velten G, McMurray JJV. Effect of sacubitril/valsartan on recurrent events in the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM‐HF). Eur J Heart Fail 2018; 20: 760–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Vardeny O, Claggett B, Kachadourian J, Desai AS, Packer M, Rouleau J, Zile MR, Swedberg K, Lefkowitz M, Shi V, McMurray JJV, Solomon SD. Reduced loop diuretic use in patients taking sacubitril/valsartan compared with enalapril: the PARADIGM‐HF trial. Eur J Heart Fail 2019; 21: 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Wachter R, Senni M, Belohlavek J, Straburzynska‐Migaj E, Witte KK, Kobalava Z, Fonseca C, Goncalvesova E, Cavusoglu Y, Fernandez A, Chaaban S, Bøhmer E, Pouleur AC, Mueller C, Tribouilloy C, Lonn E, Buraiki AL, Gniot J, Mozheiko M, Lelonek M, Noè A, Schwende H, Bao W, Butylin D, Pascual‐Figal D, TRANSITION Investigators . Initiation of sacubitril/valsartan in haemodynamically stabilised heart failure patients in hospital or early after discharge: primary results of the randomised TRANSITION study. Eur J Heart Fail 2019; 21: 998–1007. [DOI] [PubMed] [Google Scholar]
- 155. Senni M, Wachter R, Witte KK, Straburzynska‐Migaj E, Belohlavek J, Fonseca C, Mueller C, Lonn E, Chakrabarti A, Bao W, Noe A, Schwende H, Butylin D, Pascual‐Figal D, Investigators TRANSITION. Initiation of sacubitril/valsartan shortly after hospitalisation for acutely decompensated heart failure in patients with newly diagnosed (de novo) heart failure: a subgroup analysis of the TRANSITION study. Eur J Heart Fail 2020; 22: 303–312. [DOI] [PubMed] [Google Scholar]
- 156. Kapelios CJ, Lainscak M, Savarese G, Laroche C, Seferovic P, Ruschitzka F, Coats A, Anker SD, Crespo‐Leiro MG, Filippatos G, Piepoli MF, Rosano G, Zanolla L, Aguiar C, Murin J, Leszek P, McDonagh T, Maggioni AP, Lund LH, Heart Failure Long‐Term Registry Investigators . Sacubitril/valsartan eligibility and outcomes in the ESC‐EORP‐HFA Heart Failure Long‐Term Registry: bridging between European Medicines Agency/Food and Drug Administration label, the PARADIGM‐HF trial, ESC guidelines, and real world. Eur J Heart Fail 2019; 21: 1383–1397. [DOI] [PubMed] [Google Scholar]
- 157. Wachter R, Fonseca AF, Balas B, Kap E, Engelhard J, Schlienger R, Klebs S, Wirta SB, Kostev K. Real‐world treatment patterns of sacubitril/valsartan: a longitudinal cohort study in Germany. Eur J Heart Fail 2019; 21: 588–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Komajda M, Schöpe J, Wagenpfeil S, Tavazzi L, Böhm M, Ponikowski P, Anker SD, Filippatos GS, Cowie MR, Investigators QUALIFY. Physicians' guideline adherence is associated with long‐term heart failure mortality in outpatients with heart failure with reduced ejection fraction: the QUALIFY international registry. Eur J Heart Fail 2019; 21: 921–929. [DOI] [PubMed] [Google Scholar]
- 159. Komajda M, Cowie MR, Tavazzi L, Ponikowski P, Anker SD, Filippatos GS, Investigators QUALIFY. Physicians' guideline adherence is associated with better prognosis in outpatients with heart failure with reduced ejection fraction: the QUALIFY international registry. Eur J Heart Fail 2017; 19: 1414–1423. [DOI] [PubMed] [Google Scholar]
- 160. Martens P, Beliën H, Dupont M, Mullens W. Insights into implementation of sacubitril/valsartan into clinical practice. ESC Heart Fail 2018; 5: 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Lainščak M, Milinković I, Polovina M, Crespo‐Leiro MG, Lund LH, Anker SD, Laroche C, Ferrari R, Coats AJS, McDonagh T, Filippatos G, Maggioni AP, Piepoli MF, Rosano GMC, Ruschitzka F, Simić D, Ašanin M, Eicher JC, Yilmaz MB, Seferović PM, European Society of Cardiology Heart Failure Long‐Term Registry Investigators Group . Sex‐ and age‐related differences in the management and outcomes of chronic heart failure: an analysis of patients from the ESC HFA EORP Heart Failure Long‐Term Registry. Eur J Heart Fail 2020; 22: 92–102. [DOI] [PubMed] [Google Scholar]
- 162. Savarese G, Carrero JJ, Pitt B, Anker SD, Rosano GMC, Dahlström U, Lund LH. Factors associated with underuse of mineralocorticoid receptor antagonists in heart failure with reduced ejection fraction: an analysis of 11 215 patients from the Swedish Heart Failure Registry. Eur J Heart Fail 2018; 20: 1326–1334. [DOI] [PubMed] [Google Scholar]
- 163. Senni M, McMurray JJV, Wachter R, McIntyre HF, Anand IS, Duino V, Sarkar A, Shi V, Charney A. Impact of systolic blood pressure on the safety and tolerability of initiating and up‐titrating sacubitril/valsartan in patients with heart failure and reduced ejection fraction: insights from the TITRATION study. Eur J Heart Fail 2018; 20: 491–500. [DOI] [PubMed] [Google Scholar]
- 164. Ferreira JP, Abreu P, McMurray JJV, van Veldhuisen DJ, Swedberg K, Pocock SJ, Vincent J, Lins K, Rossignol P, Pitt B, Zannad F. Renal function stratified dose comparisons of eplerenone versus placebo in the EMPHASIS‐HF trial. Eur J Heart Fail 2019; 21: 345–351. [DOI] [PubMed] [Google Scholar]
- 165. Butler J, Vijayakumar S, Pitt B. Need to revisit heart failure treatment guidelines for hyperkalaemia management during the use of mineralocorticoid receptor antagonists. Eur J Heart Fail 2018; 20: 1247–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Beusekamp JC, Tromp J, van der Wal HH, Anker SD, Cleland JG, Dickstein K, Filippatos G, van der Harst P, Hillege HL, Lang CC, Metra M, Ng LL, Ponikowski P, Samani NJ, van Veldhuisen DJ, Zwinderman AH, Rossignol P, Zannad F, Voors AA, van der Meer P. Potassium and the use of renin–angiotensin–aldosterone system inhibitors in heart failure with reduced ejection fraction: data from BIOSTAT‐CHF. Eur J Heart Fail 2018; 20: 923–930. [DOI] [PubMed] [Google Scholar]
- 167. Trevisan M, de Deco P, Xu H, Evans M, Lindholm B, Bellocco R, Barany P, Jernberg T, Lund LH, Carrero JJ. Incidence, predictors and clinical management of hyperkalaemia in new users of mineralocorticoid receptor antagonists. Eur J Heart Fail 2018; 20: 1217–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. McMurray JJ. Improving outcomes in heart failure: a personal perspective. Eur Heart J 2015; 36: 3467–3470. [DOI] [PubMed] [Google Scholar]
- 169. Seferović PM, Petrie MC, Filippatos GS, Anker SD, Rosano G, Bauersachs J, Paulus WJ, Komajda M, Cosentino F, de Boer RA, Farmakis D, Doehner W, Lambrinou E, Lopatin Y, Piepoli MF, Theodorakis MJ, Wiggers H, Lekakis J, Mebazaa A, Mamas MA, Tschöpe C, Hoes AW, Seferović JP, Logue J, McDonagh T, Riley JP, Milinković I, Polovina M, van Veldhuisen DJ, Lainscak M, Maggioni AP, Ruschitzka F, McMurray JJV. Type 2 diabetes mellitus and heart failure: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2018; 20: 853–872. [DOI] [PubMed] [Google Scholar]
- 170. Seferović PM, Coats AJS, Ponikowski P, Filippatos G, Huelsmann M, Jhund PS, Polovina MM, Komajda M, Seferović J, Sari I, Cosentino F, Ambrosio G, Metra M, Piepoli M, Chioncel O, Lund LH, Thum T, de Boer RA, Mullens W, Lopatin Y, Volterrani M, Hill L, Bauersachs J, Lyon A, Petrie MC, Anker S, Rosano GMC. European Society of Cardiology/Heart Failure Association position paper on the role and safety of new glucose‐lowering drugs in patients with heart failure. Eur J Heart Fail 2020; 22: 196–213. [DOI] [PubMed] [Google Scholar]
- 171. Böhm M, Slawik J, Brueckmann M, Mattheus M, George JT, Ofstad AP, Inzucchi SE, Fitchett D, Anker SD, Marx N, Wanner C, Zinman B, Verma S. Efficacy of empagliflozin on heart failure and renal outcomes in patients with atrial fibrillation: data from the EMPA‐REG OUTCOME trial. Eur J Heart Fail 2020; 22: 126–135. [DOI] [PubMed] [Google Scholar]
- 172. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM, DAPA‐HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 173. McMurray JJV, DeMets DL, Inzucchi SE, Køber L, Kosiborod MN, Langkilde AM, Martinez FA, Bengtsson O, Ponikowski P, Sabatine MS, Sjöstrand M, Solomon SD, DAPA‐HF Committees and Investigators . A trial to evaluate the effect of the sodium–glucose co‐transporter 2 inhibitor dapagliflozin on morbidity and mortality in patients with heart failure and reduced left ventricular ejection fraction (DAPA‐HF). Eur J Heart Fail 2019; 21: 665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. McMurray JJV, DeMets DL, Inzucchi SE, Køber L, Kosiborod MN, Langkilde AM, Martinez FA, Bengtsson O, Ponikowski P, Sabatine MS, Sjöstrand M, Solomon SD, DAPA‐HF Committees and Investigators . The Dapagliflozin And Prevention of Adverse‐outcomes in Heart Failure (DAPA‐HF) trial: baseline characteristics. Eur J Heart Fail 2019; 21: 1402–1411. [DOI] [PubMed] [Google Scholar]
- 175. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner‐La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F, EMPEROR‐Reduced Trial Investigators . Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020; 383: 1413–1424. [DOI] [PubMed] [Google Scholar]
- 176. Packer M, Butler J, Filippatos GS, Jamal W, Salsali A, Schnee J, Kimura K, Zeller C, George J, Brueckmann M, Anker SD, Zannad F, EMPEROR‐Reduced Trial Committees and Investigators . Evaluation of the effect of sodium–glucose co‐transporter 2 inhibition with empagliflozin on morbidity and mortality of patients with chronic heart failure and a reduced ejection fraction: rationale for and design of the EMPEROR‐Reduced trial. Eur J Heart Fail 2019; 21: 1270–1278. [DOI] [PubMed] [Google Scholar]
- 177. Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Voors AA, Metra M, Lund LH, Komajda M, Testani JM, Wilcox CS, Ponikowski P, Lopes RD, Verma S, Lapuerta P, Pitt B; SOLOIST‐WHF Trial Investigators . Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 2020. 10.1056/NEJMoa2030183. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 178. Anker SD, Butler J, Filippatos GS, Jamal W, Salsali A, Schnee J, Kimura K, Zeller C, George J, Brueckmann M, Zannad F, Packer M, EMPEROR‐Preserved Trial Committees and Investigators . Evaluation of the effects of sodium–glucose co‐transporter 2 inhibition with empagliflozin on morbidity and mortality in patients with chronic heart failure and a preserved ejection fraction: rationale for and design of the EMPEROR‐Preserved Trial. Eur J Heart Fail 2019; 21: 1279–1287. [DOI] [PubMed] [Google Scholar]
- 179. Abraham WT, Ponikowski P, Brueckmann M, Zeller C, Macesic H, Peil B, Brun M, Ustyugova A, Jamal W, Salsali A, Lindenfeld J, Anker SD, EMPERIAL Investigators and National Coordinators . Rationale and design of the EMPERIAL‐Preserved and EMPERIAL‐Reduced trials of empagliflozin in patients with chronic heart failure. Eur J Heart Fail 2019; 21: 932–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Packer M. Augmentation of glucagon‐like peptide‐1 receptor signalling by neprilysin inhibition: potential implications for patients with heart failure. Eur J Heart Fail 2018; 20: 973–977. [DOI] [PubMed] [Google Scholar]
- 181. Lan NSR, Fegan PG, Yeap BB, Dwivedi G. The effects of sodium–glucose cotransporter 2 inhibitors on left ventricular function: current evidence and future directions. ESC Heart Fail 2019; 6: 927–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Pabel S, Wagner S, Bollenberg H, Bengel P, Kovács Á, Schach C, Tirilomis P, Mustroph J, Renner A, Gummert J, Fischer T, van Linthout S, Tschöpe C, Streckfuss‐Bömeke K, Hasenfuss G, Maier LS, Hamdani N, Sossalla S. Empagliflozin directly improves diastolic function in human heart failure. Eur J Heart Fail 2018; 20: 1690–1700. [DOI] [PubMed] [Google Scholar]
- 183. Yurista SR, Silljé HHW, Oberdorf‐Maass SU, Schouten EM, Pavez Giani MG, Hillebrands JL, van Goor H, van Veldhuisen DJ, de Boer RA, Westenbrink BD. Sodium–glucose co‐transporter 2 inhibition with empagliflozin improves cardiac function in non‐diabetic rats with left ventricular dysfunction after myocardial infarction. Eur J Heart Fail 2019; 21: 862–873. [DOI] [PubMed] [Google Scholar]
- 184. Lam CSP, Doehner W, Comin‐Colet J, IRON CORE Group . Iron deficiency in chronic heart failure: case‐based practical guidance. ESC Heart Fail 2018; 5: 764–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Martens P, Verbrugge FH, Nijst P, Dupont M, Mullens W. Limited contractile reserve contributes to poor peak exercise capacity in iron‐deficient heart failure. Eur J Heart Fail 2018; 20: 806–808. [DOI] [PubMed] [Google Scholar]
- 186. Hoes MF, Grote Beverborg N, Kijlstra JD, Kuipers J, Swinkels DW, Giepmans BNG, Rodenburg RJ, van Veldhuisen DJ, de Boer RA, van der Meer P. Iron deficiency impairs contractility of human cardiomyocytes through decreased mitochondrial function. Eur J Heart Fail 2018; 20: 910–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Lüscher TF, Bart B, Banasiak W, Niegowska J, Kirwan BA, Mori C, von Eisenhart RB, Pocock SJ, Poole‐Wilson PA, Ponikowski P, FAIR‐HF Trial Investigators . Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009; 361: 2436–2448. [DOI] [PubMed] [Google Scholar]
- 188. Ponikowski P, van Veldhuisen DJ, Comin‐Colet J, Ertl G, Komajda M, Mareev V, McDonagh T, Parkhomenko A, Tavazzi L, Levesque V, Mori C, Roubert B, Filippatos G, Ruschitzka F, Anker SD, CONFIRM‐HF Investigators . Beneficial effects of long‐term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J 2015; 36: 657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Dziegala M, Josiak K, Kasztura M, Kobak K, von Haehling S, Banasiak W, Anker SD, Ponikowski P, Jankowska E. Iron deficiency as energetic insult to skeletal muscle in chronic diseases. J Cachexia Sarcopenia Muscle 2018; 9: 802–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Tkaczyszyn M, Drozd M, Węgrzynowska‐Teodorczyk K, Flinta I, Kobak K, Banasiak W, Ponikowski P, Jankowska EA. Depleted iron stores are associated with inspiratory muscle weakness independently of skeletal muscle mass in men with systolic chronic heart failure. J Cachexia Sarcopenia Muscle 2018; 9: 547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Halon‐Golabek M, Borkowska A, Kaczor JJ, Ziolkowski W, Flis DJ, Knap N, Kasperuk K, Antosiewicz J. hmSOD1 gene mutation‐induced disturbance in iron metabolism is mediated by impairment of Akt signalling pathway. J Cachexia Sarcopenia Muscle 2018; 9: 557–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Sedaghat‐Hamedani F, Kayvanpour E, Hamed S, Frankenstein L, Riffel J, Gi WT, Amr A, Shirvani Samani O, Haas J, Miersch T, Herpel E, Kreusser MM, Ehlermann P, Katus HA, Meder B. The chameleon of cardiology: cardiac sarcoidosis before and after heart transplantation. ESC Heart Fail 2020; 7: 692–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. McDonagh T, Damy T, Doehner W, Lam CSP, Sindone A, van der Meer P, Cohen‐Solal A, Kindermann I, Manito N, Pfister O, Pohjantähti‐Maaroos H, Taylor J, Comin‐Colet J. Screening, diagnosis and treatment of iron deficiency in chronic heart failure: putting the 2016 European Society of Cardiology heart failure guidelines into clinical practice. Eur J Heart Fail 2018; 20: 1664–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Anker SD, Kirwan BA, van Veldhuisen DJ, Filippatos G, Comin‐Colet J, Ruschitzka F, Lüscher TF, Arutyunov GP, Motro M, Mori C, Roubert B, Pocock SJ, Ponikowski P. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron‐deficient heart failure patients: an individual patient data meta‐analysis. Eur J Heart Fail 2018; 20: 125–133. [DOI] [PubMed] [Google Scholar]
- 195. Ponikowski P, Kirwan BA, Anker SD, Dorobantu M, Drozdz J, Fabien V, Filippatos G, Haboubi T, Keren A, Khintibidze I, Kragten H, Martinez FA, McDonagh T, Metra M, Milicic D, Nicolau JC, Ohlsson M, Parhomenko A, Pascual‐Figal DA, Ruschitzka F, Sim D, Skouri H, van der Meer P, Jankowska EA. Rationale and design of the AFFIRM‐AHF trial: a randomised, double‐blind, placebo‐controlled trial comparing the effect of intravenous ferric carboxymaltose on hospitalisations and mortality in iron‐deficient patients admitted for acute heart failure. Eur J Heart Fail 2019; 21: 1651–1658. [DOI] [PubMed] [Google Scholar]
- 196. Ponikowski P, Kirwan BA, Anker SD, McDonagh T, Dorobantu M, Drozdz J, Fabien V, Filippatos G, Göhring UM, Keren A, Khintibidze I, Kragten H, Martinez FA, Metra M, Milicic D, Nicolau JC, Ohlsson M, Parkhomenko A, Pascual‐Figal DA, Ruschitzka F, Sim D, Skouri H, van der Meer P, Lewis BS, Comin‐Colet J, von Haehling S, Cohen‐Solal A, Danchin N, Doehner W, Dargie HJ, Motro M, Butler J, Friede T, Jensen KH, Pocock S, Jankowska EA; AFFIRM‐AHF investigators . Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: a multicentre, double‐blind, randomised, controlled trial. Lancet 2020: S0140‐6736(20)32339‐4. 10.1016/S0140-6736(20)32339-4. Epub ahead of print. PMID: 33197395. [DOI] [PubMed] [Google Scholar]
- 197. Armstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, Lam CSP, Ponikowski P, Voors AA, Jia G, McNulty SE, Patel MJ, Roessig L, Koglin J, O'Connor CM, VICTORIA Study Group . Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med 2020; 382: 1883–1893. [DOI] [PubMed] [Google Scholar]
- 198. Pieske B, Patel MJ, Westerhout CM, Anstrom KJ, Butler J, Ezekowitz J, Hernandez AF, Koglin J, Lam CSP, Ponikowski P, Roessig L, Voors AA, O'Connor CM, Armstrong PW, VICTORIA Study Group . Baseline features of the VICTORIA (Vericiguat Global Study in Subjects with Heart Failure with Reduced Ejection Fraction) trial. Eur J Heart Fail 2019; 21: 1596–1604. [DOI] [PubMed] [Google Scholar]
- 199. Teerlink JR, Diaz R, Felker GM, McMurray JJV, Metra M, Solomon SD, Adams KF, Anand I, Arias‐Mendoza A, Biering‐Sørensen T, Bühm M, Bonderman D, Cleland JGF, Corbalan R, Crespo‐Leiro MG, Dahlstrüm U, Echeverria LE, Fang JC, Filippatos G, Fonseca C, Goncalvesova E, Goudev AR, Howlett JG, Lanfear DE, Li J, Lund M, Macdonald P, Mareev V, Momomura SI, O'Meara E, Parkhomenko A, Ponikowski P, Ramires FJA, Serpytis P, Sliwa K, Spinar J, Suter TM, Tomcsanyi J, Vandekerckhove H, Vinereanu D, Voors AA, Yilmaz MB, Zannad F, Sharpsten L, Legg JC, Varin C, Honarpour N, Abbasi SA, Malik FI, Kurtz CE; GALACTIC‐HF Investigators . Cardiac myosin activation with omecamtiv mecarbil in systolic heart failure. N Engl J Med 2020. 10.1056/NEJMoa2025797. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 200. Voors AA, Bax JJ, Hernandez AF, Wirtz AB, Pap AF, Ferreira AC, Senni M, van der Laan M, Butler J, PANTHEON Investigators . Safety and efficacy of the partial adenosine A1 receptor agonist neladenoson bialanate in patients with chronic heart failure with reduced ejection fraction: a phase IIb, randomized, double‐blind, placebo‐controlled trial. Eur J Heart Fail 2019; 21: 1426–1433. [DOI] [PubMed] [Google Scholar]
- 201. Ahmad T, Miller PE, McCullough M, Desai NR, Riello R, Psotka M, Böhm M, Allen LA, Teerlink JR, Rosano GMC, Lindenfeld J. Why has positive inotropy failed in chronic heart failure? Lessons from prior inotrope trials. Eur J Heart Fail 2019; 21: 1064–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Comín‐Colet J, Manito N, Segovia‐Cubero J, Delgado J, García Pinilla JM, Almenar L, Crespo‐Leiro MG, Sionis A, Blasco T, Pascual‐Figal D, Gonzalez‐Vilchez F, Lambert‐Rodríguez JL, Grau M, Bruguera J, LION‐HEART Study Investigators . Efficacy and safety of intermittent intravenous outpatient administration of levosimendan in patients with advanced heart failure: the LION‐HEART multicentre randomised trial. Eur J Heart Fail 2018; 20: 1128–1136. [DOI] [PubMed] [Google Scholar]
- 203. Bavendiek U, Berliner D, Dávila LA, Schwab J, Maier L, Philipp SA, Rieth A, Westenfeld R, Piorkowski C, Weber K, Hänselmann A, Oldhafer M, Schallhorn S, von der Leyen H, Schröder C, Veltmann C, Störk S, Böhm M, Koch A, Bauersachs J, DIGIT‐HF Investigators and Committees (see Appendix) . Rationale and design of the DIGIT‐HF trial (DIGitoxin to Improve ouTcomes in patients with advanced chronic Heart Failure): a randomized, double‐blind, placebo‐controlled study. Eur J Heart Fail 2019; 21: 676–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Lund LH, Svennblad B, Dahlström U, Ståhlberg M. Effect of expanding evidence and evolving clinical guidelines on the prevalence of indication for cardiac resynchronization therapy in patients with heart failure. Eur J Heart Fail 2018; 20: 769–777. [DOI] [PubMed] [Google Scholar]
- 205. Dickstein K, Normand C, Auricchio A, Bogale N, Cleland JG, Gitt AK, Stellbrink C, Anker SD, Filippatos G, Gasparini M, Hindricks G, Blomström Lundqvist C, Ponikowski P, Ruschitzka F, Botto GL, Bulava A, Duray G, Israel C, Leclercq C, Margitfalvi P, Cano Ó, Plummer C, Sarigul NU, Sterlinski M, Linde C. CRT Survey II: a European Society of Cardiology survey of cardiac resynchronisation therapy in 11 088 patients–who is doing what to whom and how? Eur J Heart Fail 2018; 20: 1039–1051. [DOI] [PubMed] [Google Scholar]
- 206. Boriani G, Diemberger I. Cardiac resynchronization therapy in the real world: need to focus on implant rates, patient selection, co‐morbidities, type of devices, and complications. Eur Heart J 2017; 38: 2129–2131. [DOI] [PubMed] [Google Scholar]
- 207. Linde C, Ellenbogen K, McAlister FA. Cardiac resynchronization therapy (CRT): clinical trials, guidelines, and target populations. Heart Rhythm 2012; 9: S3–S13. [DOI] [PubMed] [Google Scholar]
- 208. Linde C, Cleland JGF, Gold MR, Claude Daubert J, Tang ASL, Young JB, Sherfesee L, Abraham WT. The interaction of sex, height, and QRS duration on the effects of cardiac resynchronization therapy on morbidity and mortality: an individual‐patient data meta‐analysis. Eur J Heart Fail 2018; 20: 780–791. [DOI] [PubMed] [Google Scholar]
- 209. Echouffo‐Tcheugui JB, Masoudi FA, Bao H, Curtis JP, Heidenreich PA, Fonarow GC. Body mass index and outcomes of cardiac resynchronization with implantable cardioverter‐defibrillator therapy in older patients with heart failure. Eur J Heart Fail 2019; 21: 1093–1102. [DOI] [PubMed] [Google Scholar]
- 210. Linde CM, Normand C, Bogale N, Auricchio A, Sterlinski M, Marinskis G, Sticherling C, Bulava A, Pérez ÓC, Maass AH, Witte KK, Rekvava R, Abdelali S, Dickstein K. Upgrades from a previous device compared to de novo cardiac resynchronization therapy in the European Society of Cardiology CRT Survey II. Eur J Heart Fail 2018; 20: 1457–1468. [DOI] [PubMed] [Google Scholar]
- 211. Cho SW, Gwag HB, Hwang JK, Chun KJ, Park KM, On YK, Kim JS, Park SJ. Clinical features, predictors, and long‐term prognosis of pacing‐induced cardiomyopathy. Eur J Heart Fail 2019; 21: 643–651. [DOI] [PubMed] [Google Scholar]
- 212. Gasparini M, Kloppe A, Lunati M, Anselme F, Landolina M, Martinez‐Ferrer JB, Proclemer A, Morani G, Biffi M, Ricci R, Rordorf R, Mangoni L, Manotta L, Grammatico A, Leyva F, Boriani G. Atrioventricular junction ablation in patients with atrial fibrillation treated with cardiac resynchronization therapy: positive impact on ventricular arrhythmias, implantable cardioverter‐defibrillator therapies and hospitalizations. Eur J Heart Fail 2018; 20: 1472–1481. [DOI] [PubMed] [Google Scholar]
- 213. Slawik J, Adrian L, Hohl M, Lothschütz S, Laufs U, Böhm M. Irregular pacing of ventricular cardiomyocytes induces pro‐fibrotic signalling involving paracrine effects of transforming growth factor beta and connective tissue growth factor. Eur J Heart Fail 2019; 21: 482–491. [DOI] [PubMed] [Google Scholar]
- 214. Lee TC, Qian M, Mu L, di Tullio MR, Graham S, Mann DL, Nakanishi K, Teerlink JR, Lip GYH, Freudenberger RS, Sacco RL, Mohr JP, Labovitz AJ, Ponikowski P, Lok DJ, Estol C, Anker SD, Pullicino PM, Buchsbaum R, Levin B, Thompson JLP, Homma S, Ye S, WARCEF Investigators . Association between mortality and implantable cardioverter‐defibrillators by aetiology of heart failure: a propensity‐matched analysis of the WARCEF trial. ESC Heart Fail 2019; 6: 297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML, Multicenter Automatic Defibrillator Implantation Trial II Investigators . Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346: 877–883. [DOI] [PubMed] [Google Scholar]
- 216. Sharma A, Al‐Khatib SM, Ezekowitz JA, Cooper LB, Fordyce CB, Michael Felker G, Bardy GH, Poole JE, Thomas Bigger J, Buxton AE, Moss AJ, Friedman DJ, Lee KL, Steinman R, Dorian P, Cappato R, Kadish AH, Kudenchuk PJ, Mark DB, Peterson ED, Inoue LYT, Sanders GD. Implantable cardioverter‐defibrillators in heart failure patients with reduced ejection fraction and diabetes. Eur J Heart Fail 2018; 20: 1031–1038. [DOI] [PubMed] [Google Scholar]
- 217. Lavall D, Hagendorff A, Schirmer SH, Böhm M, Borger MA, Laufs U. Mitral valve interventions in heart failure. ESC Heart Fail 2018; 5: 552–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Donato A, Elgin E. In HF with secondary mitral regurgitation, transcatheter mitral valve repair reduced HF hospitalizations at 2 years. Ann Intern Med 2019; 170: JC7. [DOI] [PubMed] [Google Scholar]
- 219. Iung B, Armoiry X, Vahanian A, Boutitie F, Mewton N, Trochu JN, Lefèvre T, Messika‐Zeitoun D, Guerin P, Cormier B, Brochet E, Thibault H, Himbert D, Thivolet S, Leurent G, Bonnet G, Donal E, Piriou N, Piot C, Habib G, Rouleau F, Carrié D, Nejjari M, Ohlmann P, Saint Etienne C, Leroux L, Gilard M, Samson G, Rioufol G, Maucort‐Boulch D, Obadia JF, MITRA‐FR Investigators . Percutaneous repair or medical treatment for secondary mitral regurgitation: outcomes at 2 years. Eur J Heart Fail 2019; 21: 1619–1627. [DOI] [PubMed] [Google Scholar]
- 220. Senni M, Adamo M, Metra M, Alfieri O, Vahanian A. Treatment of functional mitral regurgitation in chronic heart failure: can we get a ‘proof of concept’ from the MITRA‐FR and COAPT trials? Eur J Heart Fail 2019; 21: 852–861. [DOI] [PubMed] [Google Scholar]
- 221. Geis NA, Puls M, Lubos E, Zuern CS, Franke J, Schueler R, von Bardeleben RS, Boekstegers P, Ouarrak T, Zahn R, Ince H, Senges J, Katus HA, Bekeredjian R. Safety and efficacy of MitraClip™ therapy in patients with severely impaired left ventricular ejection fraction: results from the German transcatheter mitral valve interventions (TRAMI) registry. Eur J Heart Fail 2018; 20: 598–608. [DOI] [PubMed] [Google Scholar]
- 222. Orban M, Besler C, Braun D, Nabauer M, Zimmer M, Orban M, Noack T, Mehilli J, Hagl C, Seeburger J, Borger M, Linke A, Thiele H, Massberg S, Ender J, Lurz P, Hausleiter J. Six‐month outcome after transcatheter edge‐to‐edge repair of severe tricuspid regurgitation in patients with heart failure. Eur J Heart Fail 2018; 20: 1055–1062. [DOI] [PubMed] [Google Scholar]
- 223. Schlotter F, Orban M, Rommel KP, Besler C, von Roeder M, Braun D, Unterhuber M, Borger M, Hagl C, Orban M, Nabauer M, Massberg S, Thiele H, Hausleiter J, Lurz P. Aetiology‐based clinical scenarios predict outcomes of transcatheter edge‐to‐edge tricuspid valve repair of functional tricuspid regurgitation. Eur J Heart Fail 2019; 21: 1117–1125. [DOI] [PubMed] [Google Scholar]
- 224. Gustafsson F, Rogers JG. Left ventricular assist device therapy in advanced heart failure: patient selection and outcomes. Eur J Heart Fail 2017; 19: 595–602. [DOI] [PubMed] [Google Scholar]
- 225. Rogers JG, Pagani FD, Tatooles AJ, Bhat G, Slaughter MS, Birks EJ, Boyce SW, Najjar SS, Jeevanandam V, Anderson AS, Gregoric ID, Mallidi H, Leadley K, Aaronson KD, Frazier OH, Milano CA. Intrapericardial left ventricular assist device for advanced heart failure. N Engl J Med 2017; 376: 451–460. [DOI] [PubMed] [Google Scholar]
- 226. Jakovljevic DG, Yacoub MH, Schueler S, MacGowan GA, Velicki L, Seferovic PM, Hothi S, Tzeng BH, Brodie DA, Birks E, Tan LB. Left ventricular assist device as a bridge to recovery for patients with advanced heart failure. J Am Coll Cardiol 2017; 69: 1924–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Barge‐Caballero E, Almenar‐Bonet L, Gonzalez‐Vilchez F, Lambert‐Rodríguez JL, González‐Costello J, Segovia‐Cubero J, Castel‐Lavilla MA, Delgado‐Jiménez J, Garrido‐Bravo IP, Rangel‐Sousa D, Martínez‐Sellés M, de la Fuente‐Galan L, Rábago‐Juan‐Aracil G, Sanz‐Julve M, Hervás‐Sotomayor D, Mirabet‐Pérez S, Muñiz J, Crespo‐Leiro MG. Clinical outcomes of temporary mechanical circulatory support as a direct bridge to heart transplantation: a nationwide Spanish registry. Eur J Heart Fail 2018; 20: 178–186. [DOI] [PubMed] [Google Scholar]
- 228. Catino AB, Ferrin P, Wever‐Pinzon J, Horne BD, Wever‐Pinzon O, Kfoury AG, McCreath L, Diakos NA, McKellar S, Koliopoulou A, Bonios MJ, Al‐Sarie M, Taleb I, Dranow E, Fang JC, Drakos SG. Clinical and histopathological effects of heart failure drug therapy in advanced heart failure patients on chronic mechanical circulatory support. Eur J Heart Fail 2018; 20: 164–174. [DOI] [PubMed] [Google Scholar]
- 229. Cikes M, Jakus N, Claggett B, Brugts JJ, Timmermans P, Pouleur AC, Rubis P, van Craenenbroeck EM, Gaizauskas E, Grundmann S, Paolillo S, Barge‐Caballero E, D'Amario D, Gkouziouta A, Planinc I, Veenis JF, Jacquet LM, Houard L, Holcman K, Gigase A, Rega F, Rucinskas K, Adamopoulos S, Agostoni P, Biocina B, Gasparovic H, Lund LH, Flammer AJ, Metra M, Milicic D, Ruschitzka F, PCHF‐VAD registry . Cardiac implantable electronic devices with a defibrillator component and all‐cause mortality in left ventricular assist device carriers: results from the PCHF‐VAD registry. Eur J Heart Fail 2019; 21: 1129–1141. [DOI] [PubMed] [Google Scholar]
- 230. Bobenko A, Schoenrath F, Knierim JH, Friede T, Verheyen N, Mehra MR, Haykowsky M, Herrmann‐Lingen C, Duvinage A, Pieske‐Kraigher E, Halle M, Falk V, Pieske B, Edelmann F. Exercise training in patients with a left ventricular assist device (Ex‐VAD): rationale and design of a multicentre, prospective, assessor‐blinded, randomized, controlled trial. Eur J Heart Fail 2019; 21: 1152–1159. [DOI] [PubMed] [Google Scholar]
- 231. Lund LH, Trochu JN, Meyns B, Caliskan K, Shaw S, Schmitto JD, Schibilsky D, Damme L, Heatley J, Gustafsson F. Screening for heart transplantation and left ventricular assist system: results from the ScrEEning for advanced Heart Failure treatment (SEE‐HF) study. Eur J Heart Fail 2018; 20: 152–160. [DOI] [PubMed] [Google Scholar]
- 232. Consolo F, Sferrazza G, Motolone G, Contri R, Valerio L, Lembo R, Pozzi L, Della Valle P, de Bonis M, Zangrillo A, Fiore GB, Redaelli A, Slepian MJ, Pappalardo F. Platelet activation is a preoperative risk factor for the development of thromboembolic complications in patients with continuous‐flow left ventricular assist device. Eur J Heart Fail 2018; 20: 792–800. [DOI] [PubMed] [Google Scholar]
- 233. Schmitto JD, Pya Y, Zimpfer D, Krabatsch T, Garbade J, Rao V, Morshuis M, Beyersdorf F, Marasco S, Sood P, Damme L, Netuka I. Long‐term evaluation of a fully magnetically levitated circulatory support device for advanced heart failure–two‐year results from the HeartMate 3 CE Mark Study. Eur J Heart Fail 2019; 21: 90–97. [DOI] [PubMed] [Google Scholar]
- 234. Koehler F, Koehler K, Deckwart O, Prescher S, Wegscheider K, Winkler S, Vettorazzi E, Polze A, Stangl K, Hartmann O, Marx A, Neuhaus P, Scherf M, Kirwan BA, Anker SD. Telemedical Interventional Management in Heart Failure II (TIM‐HF2), a randomised, controlled trial investigating the impact of telemedicine on unplanned cardiovascular hospitalisations and mortality in heart failure patients: study design and description of the intervention. Eur J Heart Fail 2018; 20: 1485–1493. [DOI] [PubMed] [Google Scholar]
- 235. Koehler J, Stengel A, Hofmann T, Wegscheider K, Koehler K, Sehner S, Rose M, Deckwart O, Anker SD, Koehler F, Laufs U. Telemonitoring in patients with chronic heart failure and moderate depressed symptoms–results of the Telemedical Interventional Monitoring in Heart Failure (TIM‐HF) study. Eur J Heart Fail Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 236. Eurlings CGMJ, Boyne JJ, de Boer RA, Brunner‐La Rocca HP. Telemedicine in heart failure–more than nice to have? Neth Heart J 2019; 27: 5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. McDonald K, Troughton R, Dahlström U, Dargie H, Krum H, van der Meer P, McDonagh T, Atherton JJ, Kupfer K, San George RC, Richards M, Doughty R. Daily home BNP monitoring in heart failure for prediction of impending clinical deterioration: results from the HOME HF study. Eur J Heart Fail 2018; 20: 474–480. [DOI] [PubMed] [Google Scholar]
- 238. Wagenaar KP, Broekhuizen BDL, Jaarsma T, Kok I, Mosterd A, Willems FF, Linssen GCM, Agema WRP, Anneveldt S, Lucas CMHB, Mannaerts HFJ, Wajon EMCJ, Dickstein K, Cramer MJ, Landman MAJ, Hoes AW, Rutten FH. Effectiveness of the European Society of Cardiology/Heart Failure Association website ‘heartfailurematters.org’ and an e‐health adjusted care pathway in patients with stable heart failure: results of the ‘e‐Vita HF’ randomized controlled trial. Eur J Heart Fail 2019; 21: 238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Piepoli MF. E‐health in self‐care of heart failure patients: promises become reality. Eur J Heart Fail 2019; 21: 247–248. [DOI] [PubMed] [Google Scholar]
- 240. Marti CN, Fonarow GC, Anker SD, Yancy C, Vaduganathan M, Greene SJ, Ahmed A, Januzzi JL, Gheorghiade M, Filippatos G, Butler J. Medication dosing for heart failure with reduced ejection fraction–opportunities and challenges. Eur J Heart Fail 2019; 21: 286–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Schulz M, Griese‐Mammen N, Anker SD, Koehler F, Ihle P, Ruckes C, Schumacher PM, Trenk D, Böhm M, Laufs U, PHARM‐CHF Investigators . Pharmacy‐based interdisciplinary intervention for patients with chronic heart failure: results of the PHARM‐CHF randomized controlled trial. Eur J Heart Fail 2019; 21: 1012–1021. [DOI] [PubMed] [Google Scholar]
- 242. Klein P, Anker SD, Wechsler A, Skalsky I, Neuzil P, Annest LS, Bifi M, McDonagh T, Frerker C, Schmidt T, Sievert H, Demaria AN, Kelle S. Less invasive ventricular reconstruction for ischaemic heart failure. Eur J Heart Fail 2019; 21: 1638–1650. [DOI] [PubMed] [Google Scholar]
- 243. Mathiasen AB, Qayyum AA, Jørgensen E, Helqvist S, Kofoed KF, Haack‐Sørensen M, Ekblond A, Kastrup J. Bone marrow‐derived mesenchymal stromal cell treatment in patients with ischaemic heart failure: final 4‐year follow‐up of the MSC‐HF trial. Eur J Heart Fail 2020; 22: 884–892. [DOI] [PubMed] [Google Scholar]
- 244. Paitazoglou C, Bergmann MW, Vrtovec B, Chamuleau SAJ, van Klarenbosch B, Wojakowski W, Michalewska‐Włudarczyk A, Gyöngyösi M, Ekblond A, Haack‐Sørensen M, Jaquet K, Vrangbaek K, Kastrup J, SCIENCE Investigators . Rationale and design of the European multicentre study on Stem Cell therapy in IschEmic Non‐treatable Cardiac diseasE (SCIENCE). Eur J Heart Fail 2019; 21: 1032–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Tschöpe C, Kherad B, Klein O, Lipp A, Blaschke F, Gutterman D, Burkhoff D, Hamdani N, Spillmann F, van Linthout S. Cardiac contractility modulation: mechanisms of action in heart failure with reduced ejection fraction and beyond. Eur J Heart Fail 2019; 21: 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Cowie MR, Woehrle H, Wegscheider K, Vettorazzi E, Lezius S, Koenig W, Weidemann F, Smith G, Angermann C, d'Ortho MP, Erdmann E, Levy P, Simonds AK, Somers VK, Zannad F, Teschler H. Adaptive servo‐ventilation for central sleep apnoea in systolic heart failure: results of the major substudy of SERVE‐HF. Eur J Heart Fail 2018; 20: 536–544. [DOI] [PubMed] [Google Scholar]
- 247. Costanzo MR, Ponikowski P, Coats A, Javaheri S, Augostini R, Goldberg LR, Holcomb R, Kao A, Khayat RN, Oldenburg O, Stellbrink C, McKane S, Abraham WT, remedē® System Pivotal Trial Study Group . Phrenic nerve stimulation to treat patients with central sleep apnoea and heart failure. Eur J Heart Fail 2018; 20: 1746–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Berulava T, Buchholz E, Elerdashvili V, Pena T, Islam MR, Lbik D, Mohamed BA, Renner A, von Lewinski D, Sacherer M, Bohnsack KE, Bohnsack MT, Jain G, Capece V, Cleve N, Burkhardt S, Hasenfuss G, Fischer A, Toischer K. Changes in m6A RNA methylation contribute to heart failure progression by modulating translation. Eur J Heart Fail 2020; 22: 54–66. [DOI] [PubMed] [Google Scholar]
- 249. Cao TH, Jones DJL, Voors AA, Quinn PA, Sandhu JK, Chan DCS, Parry HM, Mohan M, Mordi IR, Sama IE, Anker SD, Cleland JG, Dickstein K, Filippatos G, Hillege HL, Metra M, Ponikowski P, Samani NJ, van Veldhuisen DJ, Zannad F, Lang CC, Ng LL. Plasma proteomic approach in patients with heart failure: insights into pathogenesis of disease progression and potential novel treatment targets. Eur J Heart Fail 2020; 22: 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Hoes MF, Tromp J, Ouwerkerk W, Bomer N, Oberdorf‐Maass SU, Samani NJ, Ng LL, Lang CC, van der Harst P, Hillege H, Anker SD, Metra M, van Veldhuisen DJ, Voors AA, van der Meer P. The role of cathepsin D in the pathophysiology of heart failure and its potentially beneficial properties: a translational approach. Eur J Heart Fail 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Shiga T, Suzuki A, Haruta S, Mori F, Ota Y, Yagi M, Oka T, Tanaka H, Murasaki S, Yamauchi T, Katoh J, Hattori H, Kikuchi N, Watanabe E, Yamada Y, Haruki S, Kogure T, Suzuki T, Uetsuka Y, Hagiwara N, HIJ‐HF II Investigators . Clinical characteristics of hospitalized heart failure patients with preserved, mid‐range, and reduced ejection fractions in Japan. ESC Heart Fail 2019; 6: 475–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Beldhuis IE, Myhre PL, Claggett B, Damman K, Fang JC, Lewis EF, O'Meara E, Pitt B, Shah SJ, Voors AA, Pfeffer MA, Solomon SD, Desai AS. Efficacy and safety of spironolactone in patients with HFpEF and chronic kidney disease. JACC Heart Fail 2019; 7: 25–32. [DOI] [PubMed] [Google Scholar]
- 253. Savji N, Meijers WC, Bartz TM, Bhambhani V, Cushman M, Nayor M, Kizer JR, Sarma A, Blaha MJ, Gansevoort RT, Gardin JM, Hillege HL, Ji F, Kop WJ, Lau ES, Lee DS, Sadreyev R, van Gilst WH, Wang TJ, Zanni MV, Vasan RS, Allen NB, Psaty BM, van der Harst P, Levy D, Larson M, Shah SJ, de Boer RA, Gottdiener JS, Ho JE. The association of obesity and cardiometabolic traits with incident HFpEF and HFrEF. JACC Heart Fail 2018; 6: 701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Segar MW, Patel KV, Ayers C, Basit M, Tang WHW, Willett D, Berry J, Grodin JL, Pandey A. Phenomapping of patients with heart failure with preserved ejection fraction using machine learning‐based unsupervised cluster analysis. Eur J Heart Fail 2020; 22: 148–158. [DOI] [PubMed] [Google Scholar]
- 255. Ramalho SHR, Claggett BL, Sweitzer NK, Fang JC, Shah SJ, Anand IS, Pitt B, Lewis EF, Pfeffer MA, Solomon SD, Shah AM. Impact of pulmonary disease on the prognosis in heart failure with preserved ejection fraction: the TOPCAT trial. Eur J Heart Fail 2020; 22: 557–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Yang JH, Obokata M, Reddy YNV, Redfield MM, Lerman A, Borlaug BA. Endothelium‐dependent and independent coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. Eur J Heart Fail 2020; 22: 432–441. [DOI] [PubMed] [Google Scholar]
- 257. Fernandes‐Silva MM, Shah AM, Claggett B, Cheng S, Tanaka H, Silvestre OM, Nadruz W, Borlaug BA, Solomon SD. Adiposity, body composition and ventricular‐arterial stiffness in the elderly: the Atherosclerosis Risk in Communities Study. Eur J Heart Fail 2018; 20: 1191–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Packer M. Do most patients with obesity or type 2 diabetes, and atrial fibrillation, also have undiagnosed heart failure? A critical conceptual framework for understanding mechanisms and improving diagnosis and treatment. Eur J Heart Fail 2020; 22: 214–227. [DOI] [PubMed] [Google Scholar]
- 259. Senni M, Iorio A, Seferović P. Heart failure with preserved ejection fraction in Asia: the far side of the moon? Eur J Heart Fail 2019; 21: 37–39. [DOI] [PubMed] [Google Scholar]
- 260. Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A simple, evidence‐based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation 2018; 138: 861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Pieske B, Tschöpe C, de Boer RA, Fraser AG, Anker SD, Donal E, Edelmann F, Fu M, Guazzi M, Lam CSP, Lancellotti P, Melenovsky V, Morris DA, Nagel E, Pieske‐Kraigher E, Ponikowski P, Solomon SD, Vasan RS, Rutten FH, Voors AA, Ruschitzka F, Paulus WJ, Seferovic P, Filippatos G. How to diagnose heart failure with preserved ejection fraction: the HFA‐PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J 2019; 40: 3297–3317. [DOI] [PubMed] [Google Scholar]
- 262. Barandiarán Aizpurua A, Sanders‐van Wijk S, Brunner‐La Rocca HP, Henkens M, Heymans S, Beussink‐Nelson L, Shah SJ, van Empel VPM. Validation of the HFA‐PEFF score for the diagnosis of heart failure with preserved ejection fraction. Eur J Heart Fail 2020; 22: 413–421. [DOI] [PubMed] [Google Scholar]
- 263. Wolsk E, Kaye D, Borlaug BA, Burkhoff D, Kitzman DW, Komtebedde J, Lam CSP, Ponikowski P, Shah SJ, Gustafsson F. Resting and exercise haemodynamics in relation to six‐minute walk test in patients with heart failure and preserved ejection fraction. Eur J Heart Fail 2018; 20: 715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Corrà U, Agostoni PG, Anker SD, Coats AJS, Crespo Leiro MG, de Boer RA, Harjola VP, Hill L, Lainscak M, Lund LH, Metra M, Ponikowski P, Riley J, Seferović PM, Piepoli MF. Role of cardiopulmonary exercise testing in clinical stratification in heart failure. A position paper from the Committee on Exercise Physiology and Training of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2018; 20: 3–15. [DOI] [PubMed] [Google Scholar]
- 265. Van Iterson EH, Johnson BD, Borlaug BA, Olson TP. Physiological dead space and arterial carbon dioxide contributions to exercise ventilatory inefficiency in patients with reduced or preserved ejection fraction heart failure. Eur J Heart Fail 2017; 19: 1675–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Popovic D, Arena R, Guazzi M. A flattening oxygen consumption trajectory phenotypes disease severity and poor prognosis in patients with heart failure with reduced, mid‐range, and preserved ejection fraction. Eur J Heart Fail 2018; 20: 1115–1124. [DOI] [PubMed] [Google Scholar]
- 267. Grodin JL, Philips S, Mullens W, Nijst P, Martens P, Fang JC, Drazner MH, Tang WHW, Pandey A. Prognostic implications of plasma volume status estimates in heart failure with preserved ejection fraction: insights from TOPCAT. Eur J Heart Fail 2019; 21: 634–642. [DOI] [PubMed] [Google Scholar]
- 268. Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, van Veldhuisen DJ, Zannad F, Zile MR, Desai AS, Claggett B, Jhund PS, Boytsov SA, Comin‐Colet J, Cleland J, Düngen HD, Goncalvesova E, Katova T, Kerr Saraiva JF, Lelonek M, Merkely B, Senni M, Shah SJ, Zhou J, Rizkala AR, Gong J, Shi VC, Lefkowitz MP, PARAGON‐HF Investigators and Committees . Angiotensin‐neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med 2019; 381: 1609–1620. [DOI] [PubMed] [Google Scholar]
- 269. Ravassa S, Trippel T, Bach D, Bachran D, González A, López B, Wachter R, Hasenfuss G, Delles C, Dominiczak AF, Pieske B, Díez J, Edelmann F. Biomarker‐based phenotyping of myocardial fibrosis identifies patients with heart failure with preserved ejection fraction resistant to the beneficial effects of spironolactone: results from the Aldo‐DHF trial. Eur J Heart Fail 2018; 20: 1290–1299. [DOI] [PubMed] [Google Scholar]
- 270. Shen L, Rørth R, Cosmi D, Kristensen SL, Petrie MC, Cosmi F, Latini R, Køber L, Anand IS, Carson PE, Granger CB, Komajda M, McKelvie RS, Solomon SD, Staszewsky L, Swedberg K, Huynh T, Zile MR, Jhund PS, McMurray JJV. Insulin treatment and clinical outcomes in patients with diabetes and heart failure with preserved ejection fraction. Eur J Heart Fail 2019; 21: 974–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Trippel TD, Van Linthout S, Westermann D, Lindhorst R, Sandek A, Ernst S, Bobenko A, Kasner M, Spillmann F, González A, López B, Ravassa S, Pieske B, Paulus WJ, Díez J, Edelmann F, Tschöpe C. Investigating a biomarker‐driven approach to target collagen turnover in diabetic heart failure with preserved ejection fraction patients. Effect of torasemide versus furosemide on serum C‐terminal propeptide of procollagen type I (DROP‐PIP trial). Eur J Heart Fail 2018; 20: 460–470. [DOI] [PubMed] [Google Scholar]
- 272. Primessnig U, Bracic T, Levijoki J, Otsomaa L, Pollesello P, Falcke M, Pieske B, Heinzel FR. Long‐term effects of Na+/Ca2+ exchanger inhibition with ORM‐11035 improves cardiac function and remodelling without lowering blood pressure in a model of heart failure with preserved ejection fraction. Eur J Heart Fail 2019; 21: 1543–1552. [DOI] [PubMed] [Google Scholar]
- 273. Platz E, Jhund PS, Claggett BL, Pfeffer MA, Swedberg K, Granger CB, Yusuf S, Solomon SD, McMurray JJ. Prevalence and prognostic importance of precipitating factors leading to heart failure hospitalization: recurrent hospitalizations and mortality. Eur J Heart Fail 2018; 20: 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Ferreira JP, Metra M, Mordi I, Gregson J, ter Maaten JM, Tromp J, Anker SD, Dickstein K, Hillege HL, Ng LL, van Veldhuisen DJ, Lang CC, Voors AA, Zannad F. Heart failure in the outpatient versus inpatient setting: findings from the BIOSTAT‐CHF study. Eur J Heart Fail 2019; 21: 112–120. [DOI] [PubMed] [Google Scholar]
- 275. Greene SJ, Felker GM, Butler J. Outpatient versus inpatient worsening heart failure: distinguishing biology and risk from location of care. Eur J Heart Fail 2019; 21: 121–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Li L, Liu R, Jiang C, Du X, Huffman MD, Lam CSP, Patel A, Hillis GS, Anderson CS, Ma C, Zhao X, Wang X, Li L, Dong J. Assessing the evidence‐practice gap for heart failure in China: the Heart Failure Registry of Patient Outcomes (HERO) study design and baseline characteristics. Eur J Heart Fail 2020; 22: 646–660. [DOI] [PubMed] [Google Scholar]
- 277. Pokorney SD, Al‐Khatib SM, Sun JL, Schulte P, O'Connor CM, Teerlink JR, Armstrong PW, Ezekowitz JA, Starling RC, Voors AA, Velazquez EJ, Hernandez AF, Mentz RJ. Sudden cardiac death after acute heart failure hospital admission: insights from ASCEND‐HF. Eur J Heart Fail 2018; 20: 525–532. [DOI] [PubMed] [Google Scholar]
- 278. Lombardi C, Peveri G, Cani D, Latta F, Bonelli A, Tomasoni D, Sbolli M, Ravera A, Carubelli V, Saccani N, Specchia C, Metra M. In‐hospital and long‐term mortality for acute heart failure: analysis at the time of admission to the emergency department. ESC Heart Fail 2020; 7: 2650–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Miró Ò, García Sarasola A, Fuenzalida C, Calderón S, Jacob J, Aguirre A, Wu DM, Rizzi MA, Malchair P, Haro A, Herrera S, Gil V, Martín‐Sánchez FJ, Llorens P, Herrero Puente P, Bueno H, Domínguez Rodríguez A, Müller CE, Mebazaa A, Chioncel O, Alquézar‐Arbé A, ICA‐SEMES Research Group . Departments involved during the first episode of acute heart failure and subsequent emergency department revisits and rehospitalisations: an outlook through the NOVICA cohort. Eur J Heart Fail 2019; 21: 1231–1244. [DOI] [PubMed] [Google Scholar]
- 280. Harjola VP, Parissis J, Brunner‐La Rocca HP, Čelutkienė J, Chioncel O, Collins SP, de Backer D, Filippatos GS, Gayat E, Hill L, Lainscak M, Lassus J, Masip J, Mebazaa A, Miró Ò, Mortara A, Mueller C, Mullens W, Nieminen MS, Rudiger A, Ruschitzka F, Seferovic PM, Sionis A, Vieillard‐Baron A, Weinstein JM, de Boer RA, Crespo‐Leiro MG, Piepoli M, Riley JP. Comprehensive in‐hospital monitoring in acute heart failure: applications for clinical practice and future directions for research. A statement from the Acute Heart Failure Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail 2018; 20: 1081–1099. [DOI] [PubMed] [Google Scholar]
- 281. Chioncel O, Mebazaa A, Maggioni AP, Harjola VP, Rosano G, Laroche C, Piepoli MF, Crespo‐Leiro MG, Lainscak M, Ponikowski P, Filippatos G, Ruschitzka F, Seferovic P, Coats AJS, Lund LH, ESC‐EORP‐HFA Heart Failure Long‐Term Registry Investigators . Acute heart failure congestion and perfusion status–impact of the clinical classification on in‐hospital and long‐term outcomes; insights from the ESC‐EORP‐HFA Heart Failure Long‐Term Registry. Eur J Heart Fail 2019; 21: 1338–1352. [DOI] [PubMed] [Google Scholar]
- 282. Javaloyes P, Miró Ò, Gil V, Martín‐Sánchez FJ, Jacob J, Herrero P, Takagi K, Alquézar‐Arbé A, López Díez MP, Martín E, Bibiano C, Escoda R, Gil C, Fuentes M, Llopis García G, Álvarez Pérez JM, Jerez A, Tost J, Llauger L, Romero R, Garrido JM, Rodríguez‐Adrada E, Sánchez C, Rossello X, Parissis J, Mebazaa A, Chioncel O, Llorens P, ICA‐SEMES Research Group . Clinical phenotypes of acute heart failure based on signs and symptoms of perfusion and congestion at emergency department presentation and their relationship with patient management and outcomes. Eur J Heart Fail 2019; 21: 1353–1365. [DOI] [PubMed] [Google Scholar]
- 283. van Aelst LNL, Arrigo M, Placido R, Akiyama E, Girerd N, Zannad F, Manivet P, Rossignol P, Badoz M, Sadoune M, Launay JM, Gayat E, Lam CSP, Cohen‐Solal A, Mebazaa A, Seronde MF. Acutely decompensated heart failure with preserved and reduced ejection fraction present with comparable haemodynamic congestion. Eur J Heart Fail 2018; 20: 738–747. [DOI] [PubMed] [Google Scholar]
- 284. Biegus J, Zymlinski R, Siwolowski P, Testani J, Szachniewicz J, Tycińska A, Banasiak W, Halpert A, Levin H, Ponikowski P. Controlled decongestion by Reprieve therapy in acute heart failure: results of the TARGET‐1 and TARGET‐2 studies. Eur J Heart Fail 2019; 21: 1079–1087. [DOI] [PubMed] [Google Scholar]
- 285. Biegus J, Zymliński R, Sokolski M, Todd J, Cotter G, Metra M, Jankowska EA, Banasiak W, Ponikowski P. Serial assessment of spot urine sodium predicts effectiveness of decongestion and outcome in patients with acute heart failure. Eur J Heart Fail 2019; 21: 624–633. [DOI] [PubMed] [Google Scholar]
- 286. Wettersten N, Horiuchi Y, van Veldhuisen DJ, Mueller C, Filippatos G, Nowak R, Hogan C, Kontos MC, Cannon CM, Müeller GA, Birkhahn R, Taub P, Vilke GM, Barnett O, McDonald K, Mahon N, Nuñez J, Briguori C, Passino C, Murray PT, Maisel A. B‐type natriuretic peptide trend predicts clinical significance of worsening renal function in acute heart failure. Eur J Heart Fail 2019; 21: 1553–1560. [DOI] [PubMed] [Google Scholar]
- 287. Pellicori P, Shah P, Cuthbert J, Urbinati A, Zhang J, Kallvikbacka‐Bennett A, Clark AL, Cleland JGF. Prevalence, pattern and clinical relevance of ultrasound indices of congestion in outpatients with heart failure. Eur J Heart Fail 2019; 21: 904–916. [DOI] [PubMed] [Google Scholar]
- 288. ter Maaten JM, Kremer D, Demissei BG, Struck J, Bergmann A, Anker SD, Ng LL, Dickstein K, Metra M, Samani NJ, Romaine SPR, Cleland J, Girerd N, Lang CC, van Veldhuisen DJ, Voors AA. Bio‐adrenomedullin as a marker of congestion in patients with new‐onset and worsening heart failure. Eur J Heart Fail 2019; 21: 732–743. [DOI] [PubMed] [Google Scholar]
- 289. Kuan WS, Ibrahim I, Chan SP, Li Z, Liew OW, Frampton C, Troughton R, Pemberton CJ, Chong JPC, Tan LL, Lin W, Ooi SBS, Richards AM. Mid‐regional pro‐adrenomedullin outperforms N‐terminal pro‐B‐type natriuretic peptide for the diagnosis of acute heart failure in the presence of atrial fibrillation. Eur J Heart Fail 2020; 22: 692–700. [DOI] [PubMed] [Google Scholar]
- 290. Pandhi P, Ter Maaten JM, Emmens JE, Struck J, Bergmann A, Cleland JG, Givertz MM, Metra M, O'Connor CM, Teerlink JR, Ponikowski P, Cotter G, Davison B, van Veldhuisen DJ, Voors AA. Clinical value of pre‐discharge bio‐adrenomedullin as a marker of residual congestion and high risk of heart failure hospital readmission. Eur J Heart Fail 2020; 22: 683–691. [DOI] [PubMed] [Google Scholar]
- 291. Zymliński R, Biegus J, Sokolski M, Siwołowski P, Nawrocka‐Millward S, Todd J, Jankowska EA, Banasiak W, Cotter G, Cleland JG, Ponikowski P. Increased blood lactate is prevalent and identifies poor prognosis in patients with acute heart failure without overt peripheral hypoperfusion. Eur J Heart Fail 2018; 20: 1011–1018. [DOI] [PubMed] [Google Scholar]
- 292. Zymliński R, Sokolski M, Biegus J, Siwołowski P, Nawrocka‐Millward S, Sokolska JM, Dudkowiak M, Marciniak D, Todd J, Jankowska EA, Banasiak W, Ponikowski P. Multi‐organ dysfunction/injury on admission identifies acute heart failure patients at high risk of poor outcome. Eur J Heart Fail 2019; 21: 744–750. [DOI] [PubMed] [Google Scholar]
- 293. Wettersten N, Horiuchi Y, van Veldhuisen DJ, Mueller C, Filippatos G, Nowak R, Hogan C, Kontos MC, Cannon CM, Müeller GA, Birkhahn R, Taub P, Vilke GM, Barnett O, McDonald K, Mahon N, Nuñez J, Briguori C, Passino C, Maisel A, Murray PT. Short‐term prognostic implications of serum and urine neutrophil gelatinase‐associated lipocalin in acute heart failure: findings from the AKINESIS study. Eur J Heart Fail 2020; 22: 251–263. [DOI] [PubMed] [Google Scholar]
- 294. Blumer V, Greene SJ, Sun JL, Bart BA, Ambrosy AP, Butler J, DeVore AD, Fudim M, Hernandez AF, McNulty SE, Felker GM, Mentz RJ. Haemoconcentration during treatment of acute heart failure with cardiorenal syndrome: from the CARRESS‐HF trial. Eur J Heart Fail 2019; 21: 1472–1476. [DOI] [PubMed] [Google Scholar]
- 295. Pivetta E, Goffi A, Nazerian P, Castagno D, Tozzetti C, Tizzani P, Tizzani M, Porrino G, Ferreri E, Busso V, Morello F, Paglieri C, Masoero M, Cassine E, Bovaro F, Grifoni S, Maule MM, Lupia E, Study Group on Lung Ultrasound from the Molinette and Careggi Hospitals . Lung ultrasound integrated with clinical assessment for the diagnosis of acute decompensated heart failure in the emergency department: a randomized controlled trial. Eur J Heart Fail 2019; 21: 754–766. [DOI] [PubMed] [Google Scholar]
- 296. Čelutkienė J, Lainscak M, Anderson L, Gayat E, Grapsa J, Harjola VP, Manka R, Nihoyannopoulos P, Filardi PP, Vrettou R, Anker SD, Filippatos G, Mebazaa A, Metra M, Piepoli M, Ruschitzka F, Zamorano JL, Rosano G, Seferovic P. Imaging in patients with suspected acute heart failure: timeline approach position statement on behalf of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2020; 22: 181–195. [DOI] [PubMed] [Google Scholar]
- 297. Bistola V, Polyzogopoulou E, Ikonomidis I, Parissis J. Lung ultrasound for the diagnosis of acute heart failure: time to upgrade current indication? Eur J Heart Fail 2019; 21: 767–769. [DOI] [PubMed] [Google Scholar]
- 298. Platz E, Merz AA, Jhund PS, Vazir A, Campbell R, McMurray JJ. Dynamic changes and prognostic value of pulmonary congestion by lung ultrasound in acute and chronic heart failure: a systematic review. Eur J Heart Fail 2017; 19: 1154–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Rivas‐Lasarte M, Álvarez‐García J, Fernández‐Martínez J, Maestro A, López‐López L, Solé‐González E, Pirla MJ, Mesado N, Mirabet S, Fluvià P, Brossa V, Sionis A, Roig E, Cinca J. Lung ultrasound‐guided treatment in ambulatory patients with heart failure: a randomized controlled clinical trial (LUS‐HF study). Eur J Heart Fail 2019; 21: 1605–1613. [DOI] [PubMed] [Google Scholar]
- 300. Platz E, Jhund PS, Girerd N, Pivetta E, McMurray JJV, Peacock WF, Masip J, Martin‐Sanchez FJ, Miró Ò, Price S, Cullen L, Maisel AS, Vrints C, Cowie MR, DiSssomma S, Bueno H, Mebazaa A, Gualandro DM, Tavares M, Metra M, Coats AJS, Ruschitzka F, Seferovic PM, Mueller C, Study Group on Acute Heart Failure of the Acute Cardiovascular Care Association and the Heart Failure Association of the European Society of Cardiology . Expert consensus document: Reporting checklist for quantification of pulmonary congestion by lung ultrasound in heart failure. Eur J Heart Fail 2019; 21: 844–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Tomasoni D, Lombardi CM, Sbolli M, Cotter G, Metra M. Acute heart failure: more questions than answers. Prog Cardiovasc Dis 2020. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 302. Mullens W, Damman K, Harjola VP, Mebazaa A, Brunner‐La Rocca HP, Martens P, Testani JM, Tang WHW, Orso F, Rossignol P, Metra M, Filippatos G, Seferovic PM, Ruschitzka F, Coats AJ. The use of diuretics in heart failure with congestion–a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019; 21: 137–155. [DOI] [PubMed] [Google Scholar]
- 303. Verbrugge FH, Martens P, Ameloot K, Haemels V, Penders J, Dupont M, Tang WHW, Droogné W, Mullens W. Acetazolamide to increase natriuresis in congestive heart failure at high risk for diuretic resistance. Eur J Heart Fail 2019; 21: 1415–1422. [DOI] [PubMed] [Google Scholar]
- 304. Mullens W, Verbrugge FH, Nijst P, Martens P, Tartaglia K, Theunissen E, Bruckers L, Droogne W, Troisfontaines P, Damman K, Lassus J, Mebazaa A, Filippatos G, Ruschitzka F, Dupont M. Rationale and design of the ADVOR (Acetazolamide in Decompensated Heart Failure with Volume Overload) trial. Eur J Heart Fail 2018; 20: 1591–1600. [DOI] [PubMed] [Google Scholar]
- 305. Cotter G, Metra M, Davison BA, Jondeau G, Cleland JGF, Bourge RC, Milo O, O'Connor CM, Parker JD, Torre‐Amione G, van Veldhuisen DJ, Kobrin I, Rainisio M, Senger S, Edwards C, McMurray JJV, Teerlink JR, VERITAS Investigators . Systolic blood pressure reduction during the first 24 h in acute heart failure admission: friend or foe? Eur J Heart Fail 2018; 20: 317–322. [DOI] [PubMed] [Google Scholar]
- 306. Metra M, Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Pang PS, Ponikowski P, Voors AA, Adams KF, Anker SD, Arias‐Mendoza A, Avendaño P, Bacal F, Böhm M, Bortman G, Cleland JGF, Cohen‐Solal A, Crespo‐Leiro MG, Dorobantu M, Echeverría LE, Ferrari R, Goland S, Goncalvesová E, Goudev A, Køber L, Lema‐Osores J, Levy PD, McDonald K, Manga P, Merkely B, Mueller C, Pieske B, Silva‐Cardoso J, Špinar J, Squire I, Stępińska J, van Mieghem W, von Lewinski D, Wikström G, Yilmaz MB, Hagner N, Holbro T, Hua TA, Sabarwal SV, Severin T, Szecsödy P, Gimpelewicz C, RELAX‐AHF‐2 Committees Investigators . Effects of serelaxin in patients with acute heart failure. N Engl J Med 2019; 381: 716–726.31433919 [Google Scholar]
- 307. Teerlink JR, Davison BA, Cotter G, Maggioni AP, Sato N, Chioncel O, Ertl G, Felker GM, Filippatos G, Greenberg BH, Pang PS, Ponikowski P, Edwards C, Senger S, Teichman SL, Nielsen OW, Voors AA, Metra M. Effects of serelaxin in patients admitted for acute heart failure: a meta‐analysis. Eur J Heart Fail 2020; 22: 315–329. [DOI] [PubMed] [Google Scholar]
- 308. Maggioni AP, López‐Sendón J, Nielsen OW, Hallén J, Aalamian‐Mattheis M, Wang Y, Ertl G. Efficacy and safety of serelaxin when added to standard of care in patients with acute heart failure: results from a PROBE study, RELAX‐AHF‐EU. Eur J Heart Fail 2019; 21: 322–333. [DOI] [PubMed] [Google Scholar]
- 309. Felker GM, Borentain M, Cleland JG, DeSouza MM, Kessler PD, O'Connor CM, Seiffert D, Teerlink JR, Voors AA, McMurray JJV. Rationale and design for the development of a novel nitroxyl donor in patients with acute heart failure. Eur J Heart Fail 2019; 21: 1022–1031. [DOI] [PubMed] [Google Scholar]
- 310. Gayat E, Arrigo M, Littnerova S, Sato N, Parenica J, Ishihara S, Spinar J, Müller C, Harjola VP, Lassus J, Miró Ò, Maggioni AP, AlHabib KF, Choi DJ, Park JJ, Zhang Y, Zhang J, Januzzi JL Jr, Kajimoto K, Cohen‐Solal A, Mebazaa A, GREAT Network . Heart failure oral therapies at discharge are associated with better outcome in acute heart failure: a propensity‐score matched study. Eur J Heart Fail 2018; 20: 345–354. [DOI] [PubMed] [Google Scholar]
- 311. Kimmoun A, Cotter G, Davison B, Takagi K, Addad F, Celutkiene J, Chioncel O, Solal AC, Diaz R, Damasceno A, Duengen HD, Filippatos G, Goncalvesova E, Merai I, Metra M, Ponikowski P, Privalov D, Sliwa K, Sani MU, Voors AA, Shogenov Z, Mebazaa A. Safety, Tolerability and efficacy of Rapid Optimization, helped by NT‐proBNP and GDF‐15, of Heart Failure therapies (STRONG‐HF): rationale and design for a multicentre, randomized, parallel‐group study. Eur J Heart Fail 2019; 21: 1459–1467. [DOI] [PubMed] [Google Scholar]


