Abstract
Lower gastrointestinal endoscopy has evolved over time, fulfilling a widening diagnostic and therapeutic remit. As our understanding of colorectal cancer and its prevention has improved, endoscopy has progressed with improved diagnostic technologies and advancing endoscopic therapies. Despite this, the fundamental design of the endoscope has remained similar since its inception. This review presents the important role lower gastrointestinal endoscopy serves in the prevention of colorectal cancer and the desirable characteristics of the endoscope that would enhance this. A brief history of the endoscope is presented. Current and future robotic endoscopic platforms, which may fulfil these desirable characteristics, are discussed. The incorporation of new technologies from allied scientific disciplines will help the endoscope fulfil its maximum potential in preventing the increasing global burden of colorectal cancer. There are a number of endoscopic platforms under development, which show significant promise.
Keywords: colorectal cancer, disposable endoscopes, endoscopic mucosal resection, endoscopic submucosal dissection, robotic endoscopy
Lower gastrointestinal endoscopy (LGI) has evolved from a diagnostic adjunct to a complex subspecialty in its own right, with an evolving therapeutic role.
Endoscopy plays a major role in the prevention, diagnosis and increasingly the management of colorectal cancer. Colorectal cancer is the third most common cancer worldwide, representing 10.2% of all cancers in 2018.1 By 2030, cases are expected to increase by 60%, to 2.2 million new cases and 1.1 million deaths.2 Adenomas are the predominant precursor lesion, and identification and removal of these is a key function of endoscopy in preventing colorectal cancer.3 Every 1% increase in adenoma detection rate (ADR) reduces cancer risk by 3%.4 The introduction of screening programmes plays an important role in mitigating this risk, with case–control studies in Germany and the United States suggesting such programmes reduce 10-year colorectal cancer risk.5,6
Endoscopic mucosal resection (EMR) has been used conventionally to remove large mucosal lesions in piecemeal fashion. Endoscopic submucosal dissection (ESD) allows en bloc resection of early gastrointestinal (GI) cancer and has been shown to reduce recurrence rates and the need for surgery. ESD is a challenging technique that requires significant upskilling to achieve competence and is associated with increased risk of complications. Although well established in East Asia, its uptake has been relatively slow among Western endoscopists.
Endoscopic therapeutic capabilities continue to progress beyond the lumen. Access to the submucosal ‘third space’ offers the potential to endoscopically treat achalasia (Peri-oral endomyotomy – POEM)), subepithelial tumours (Submucosal tunelling endoscopic resection - STER/Per-oral endoscopic tunnel resection - POET), refractory gastroparesis (Gastric per-oral endomyotomy- G-POEM/Per-oral pyloromyotomy - POP), Zenker’s diverticulum (Zencker’s per-oral endomyotomy – Z-POEM/Submuocal tunnelling endoscopic septum division – STESD) and oesophageal strictures (Per-oral endoscopic tunnel resection for restoration of the oesophagus – POETRE).7
The ability to safely breach the lumen endoscopically has opened up the exciting potential of full-thickness resection and natural orifice transluminal endoscopic surgery (NOTES), with potential improvements in cosmesis, recovery time and complication rates compared with traditional open surgery.8
The basic endoscope, other than small changes and developments, has remained similar in fundamental design since its early adoption. Incorporation of new technologies from other scientific disciplines and collaborative work with the engineering community has brought innovative ideas to this relatively unchanged instrument. These exciting developments will allow the endoscope to safely and effectively fulfil its evolving remit.
This review begins with a brief history of gastroenterological endoscopy. The desired characteristics of lower GI endoscopes and the limitations of current endoscopy are discussed. Platforms with robotic-driven locomotion followed by platforms with both robotic-driven locomotion and robotic-driven instrumentation are described.
Reaching the modern era of endoscopy
Bozzini first developed the candle and mirror–illuminated ‘Lichtleiter’ endoscope in 1806,9 with a variety of speculae for different orifices (Figure 1). Edison’s incandescent bulb allowed better illumination which Mikulicz used in the tip of his gastroscope in 1881.10 In 1932, Schindler’s semiflexible gastroscope achieved 32° of flexibility using multiple lenses housed in bronze wire coiling and protective rubber tubing.11,12
Figure 1.
Evolution of the endoscope: (a) Lichtleiter endoscope (1806), (b) Mikulicz gastroscope (1881), (c) Schindler’s gastroscope (1932) and (d) Hirschowitz fibrescope (1957).
The introduction of fibre-optic technology allowed non-linear transmission of images, enabling Hirschowitz to present the first fully flexible ‘fibrescope’ at the American Gastric Annual Meeting in 1957.13 The addition of insufflation, suction and instrument channels with a second fibreoptic cable for light transmission and a four-way deflectable tip, controlled by antagonistic cables running down the endoscope, resulted in the Olympus colonoscope in 1970.14 Miniaturisation of a charged couple device allowed Welch Allyn to release the first video endoscope in 1983, closely resembling the endoscope we are familiar with today.15
Modern adaptations of the basic endoscope design have allowed visualisation of the entire GI tract through balloon and spiral endoscopes. Incorporation of ultrasound probes has enabled diagnostic and therapeutic hepatopancreaticobiliary work, with accurate assessments of depth of invasion of luminal dysplastic lesions.
Miniature GI endoscopy, a term proposed by Sami and colleagues, including ultrathin endoscopy, colon capsule endoscopy and scanning single-fibre endoscopy, can improve tolerability and aid therapeutic access to traditionally poorly accessible parts of the alimentary canal. Portable nasoendoscopy allows simpler and cheaper diagnostic examinations in clinic settings.16
Desirable endoscopic characteristics
Missed adenomas on diagnostic endoscopy are well documented and are associated with interval colorectal cancer.17 The ideal endoscope would facilitate complete mucosal visualisation and polyp detection with intra-procedural characterisation (‘optical biopsy’).
Precision endoscope tip and instrument control, required for therapeutic work, can be compromised by the length and flexibility of the endoscope. As opposed to laparoscopic instrumentation, the endoscope and instruments are deployed in a tortuous configuration. Instruments deployed in a non-linear flexible endoscope suffer from a delay in transmission of user inputs and loss of manipulation forces. Fine control of the endoscope tip is similarly compromised by the cable-controlled system due to friction and elongation of cables across the bending joints, an issue known as ‘backlash hysteresis’. The operative field is narrow and unstable, which compromises accurate triangulation of instruments.18 The ideal endoscope would allow accurate control of the endoscope tip and triangulation of instruments within a stable operative field.
Control of the endoscope using torque and dial control can be ergonomically taxing and has an associated steep learning curve. A favourably ergonomic endoscope is important to facilitate complicated therapeutic interventions such as ESD. The ideal endoscope would be ergonomically optimised.
Reprocessing and maintenance costs have been estimated to be between US$101.16 and US$238.7119 per colonoscopy. Endoscopy-associated infection has been predominantly reported with duodenoscope, but there is evidence of infection associated with colonoscopy.20 The cost of hospitalisation secondary to colonoscopy-related infection has been estimated to be between US$20.12 and US$46.52 per procedure.19 Disposable endoscopes may mitigate these issues. The ideal endoscope would be cost-efficient and minimises infection risk.
The optimal endoscope would allow uncompromised and technologically supported visualisation of the entire mucosa with ergonomic and intuitive controls of both the endoscope and instruments. It should be well tolerated by patients, have a low infection risk and be as inexpensive to maintain as feasible.
Improving lesion recognition with imaging techniques and attachable devices
High-definition white light endoscopy and magnification have allowed ever more detailed mucosal assessment. Endocytoscopy is a commercially available magnification technology, allowing cellular-level vision at 1000× magnification.
Optical filters and post-processing of images have allowed even greater differentiation of lesions than white light allows. Technologies include narrow band imaging (Olympus, Tokyo, Japan), I-Scan (Pentax, Tokyo, Japan), Flexible Spectral Imaging Colour Enhancement (FICE) (Fujinon, Tokyo, Japan), blue laser imaging endoscopy (Fujinon, Tokyo, Japan) and linked colour imaging (Fujinon, Tokyo, Japan).21 These technologies have shown significant improvements in ADR and have been incorporated into the everyday practice of many endoscopists.
Further technologies such as optical coherence tomography, confocal laser endomicroscopy, elastic scattering spectroscopy, autofluorescence imaging and endoscopic polarised scanning spectroscopy may move us closer to optical rather than histological diagnosis, but these are predominantly research techniques currently.
Accurate optical diagnosis would allow a resect-and-discard approach of diminutive lesions with attractive potential cost savings. A simulated model of such a strategy in the United States suggested US$33 million of annual savings,22 which projected worldwide would have significant global cost-saving implications.
Improving lesion detection
Attachable devices such as EndoCuff and purpose-built scopes such as G-Eye™ (Pentax Medical, Tokyo, Japan) improve mucosal visualisation by mechanically opening up haustral folds on withdrawal. EndoCuff (Arc Medical Ltd., Leeds, UK), shown in Figure 2, is a disposable plastic cap that is attached to the endoscope tip with plastic soft branches that extend on withdrawal, holding colonic folds open. Evidence has been mixed, with some meta-analyses showing improved ADR [41.3% versus 34.2%,23 relative risk (RR) = 1.18,24 odds ratio = 1.3725]. The prospective randomised controlled trial (RCT; ADENOMA TRIAL) showed an ADR of 40.9% versus 36.2% in the bowel cancer screening programme;26 however, a prospective RCT in bowel scope patients (B- ADENOMA) showed no improvement compared with conventional endoscopy.27
Figure 2.
EndoRings (left) and ENDOCUFF VISION.
A recent systematic review and meta-analysis looking at ‘behind fold visualising techniques’ including cap-assisted ENDOCUFF VISION, EndoRings and FUSE showed no improvement in ADR compared with conventional colonoscopy although there was a significant improvement with G-Eye (Pentax, Japan) with an RR of 1.3.28
Although colonoscopy remains the gold standard for investigation of the colon, there are those who are unable to tolerate complete optical colonoscopy. Other options for luminal screening include computed tomographic (CT) colonography and colon capsule endoscopy. Colon capsule endoscopy, with the second-generation PillCamTM2, involves swallowing a less than 3 g capsule which wirelessly transmits data to an external recorder worn by the patient.29 It is a safe and well-tolerated examination.30 This second-generation device has sensitivities for polyps greater than 5 mm in size, approaching that of optical colonoscopy, but comparison with CT colonography is less clear with conflicting recent evidence.31
Computer-aided diagnosis (CAD) through convolutional neural networks offer potential benefits in both polyp detection and optical diagnosis. CAD through deep learning has been shown to match or even exceed expert clinicians in other areas such as pathology and oncology. Application of CAD to colon capsule endoscopy may help address the significant time commitment of interpreting capsule images. Clinical trials will determine the optimal use of CAD in luminal imaging,32 with the technology having exciting potential applicability.
Robotic-driven locomotion endoscope platforms
Motorised spiral endoscopy (MSE): Power Spiral (Olympus)
Balloon enteroscopy and video capsule endoscopy have been the preferred modalities to examine small bowel mucosa.33 Capsule endoscopy does not allow for therapeutic work. Balloon enteroscopy can be cumbersome.
Spiral enteroscopy allows for deep enteroscopy through the continuous corkscrewing motion of the endoscope tip and pleating of the bowel.34
A meta-analysis comparing spiral enteroscopy (the two-person controlled Spirus Medical Endo-Ease Overtube) versus balloon enteroscopy showed similar diagnostic and therapeutic outcomes but with shorter procedural times (mean reduction of 11.26 min, p = 0.010).35
Olympus developed Power Spiral (Figure 3) in 2015: a single-operator spiral enteroscope with a built-in rotating motor, a disposable short overtube with atraumatic soft spiral fins, foot pedal controls and a visual force gauge.36
Figure 3.
Power Spiral (Olympus, Tokyo, Japan).33
A recent small prospective clinical trial in 30 patients showed 100% success in anterograde enteroscopy (beyond the ligament of Trietz) and retrograde enteroscopy (proximal to the ileocaecal valve) with 70% total enteroscopy rate. There was a 20% adverse event rate (mucosal tears, jejunal haematoma and oesophageal erosion), but no serious events occurred.37
A feasibility trial looking at the use of MSE in colonoscopy demonstrated a caecal and terminal ileal intubation rate of 96.7% with an ADR of 46.6%. The soft fins theoretically flatten mucosal folds on withdrawal to increase lesion detection.36
The potential for improved caecal intubation rates and better visualisation, with single-operator spiral intubation, is potentially interesting but needs further validation.38
Aer-O-Scope (GI View Ltd, Tel Aviv, Israel)
Aer-O-Scope (GI View Ltd), CE-marked and FDA (US Food and Drug Adminstration) approved39 (Figure 4), was designed to improve visualisation, reduce perforation, improve comfort, decrease infection risk and reduce the learning curve for operators.40
Figure 4.
Aer-O-Scope. Images show whole setup, insertion portion and close up view of tip with demonstration of vieiwing angles below. (GI View Ltd, Tel Aviv, Israel).40
The novel platform is a joystick-controlled disposable pneumatic self-propelling and navigating endoscope. The endoscope has a rectal balloon and hourglass-shaped scanning balloon. Both balloons are inflated on insertion, creating an airtight seal between them. A computer algorithm inflates the space, with carbon dioxide creating positive pressure and thus gentle propulsion of the proximal scanning balloon.
The pressure exerted by the endoscopist is dissipated over the balloons which are hydrophilic coated to lower friction, resulting in colonic wall pressure of 60 mbar versus 1200 mbar that can be seen in conventional colonoscopy.40
The fully steerable tip contains a high-definition camera with 57° field of view anteriorly and a novel ‘omniview’ 360° camera that allows panoramic views behind haustral folds. The tip contains an LED light source with irrigation, suction and a recently introduced therapeutic channel.41
Aer-O-Scope has achieved caecal intubation rates of 98.2%, but has only visualised 87.5% of polyps seen on conventional colonoscopy. This was despite a 94.9% lesion recognition on a simulated double-blinded porcine study.41 Caecal intubation took a mean of 13.38 min. Operators became proficient in this technique after 8–10 procedures.40
The latest version is anticipated to be released at a cost of US$250 for the disposable section and US$15,000 for the non-disposable platform. Conventional reusable colonoscopes range from US$80,000 to US$120,000 and reprocessing costs between US$140 and US$280. Disposable endoscope cost benefits are potentially attractive and disruptive.42
Its novel mechanism has potential benefits for patient tolerability, endoscope reprocessing costs and polyp detection. Further evaluation is required to validate these claims, and the low polyp detection rate must be interrogated and improved upon.
Endotics (Era Endoscopy, Peccioli, Italy)
Endotics (Era Endoscopy) (Figure 5) is a self-propelled, joystick-controlled endoscope. It consists of a disposable probe with a steerable tip and flexible body, a 3-mm working channel, a specialised electro-pneumatic tank and a separate workstation allowing handheld control with a joystick. The probe contains a camera with conventional LED light source, water and an air channel. The probe head is steerable 180° in all directions.
Figure 5.

Endotics (Era Endoscopy, Peccioli, Italy).43
Endotics has a novel semi-automatic self-propulsion mechanism. The probe advances using a semi-automatic sequence based on the inchworm. The probe can clamp onto the mucosa through both vacuum and mechanical grasping. The proximal end of the probe clamps onto the mucosa, the central part of the probe then elongates and the distal end then clamps onto the mucosa, allowing the proximal part to detach and the central part to shorten again, thus advancing the endoscope like an inchworm. Repetition of these steps self-propels the endoscope.
Conventional endoscopy relies on pressure on the bowel wall to allow navigation around corners, which can cause mesenteric stretch and thus pain. The novel mechanism of Endotics reduces lateral force and thus mesenteric stretch.
A simulated study with a porcine colon and load sensors showed a 90% reduction in stress pattern compared with standard colonoscopy. A trial in 40 patients showed a significant reduction in pain and discomfort scores compared with conventional endoscopy (0.9 and 1.1 versus 6.9 and 6.8, respectively).44
In a cohort of patients with clinical or familial risk of polyps and carcinoma, Endotics showed a 93.3% sensitivity and 100% specificity for the detection of polyps.45
Early experimentation showed initially poor caecal intubation rates: 27% in 200944 and 81.6% in 2010.45 A retrospective study, however, showed a 93.1% caecal intubation rate with Endotics in 276 patients with failed caecal intubation on conventional colonoscopy.46
The CE-marked Endotics system is commercially available.47 It may serve a role in failed conventional colonoscopy, with the additional benefits of a working channel to allow therapeutics, a disposable probe (preventing infection and reducing the associated costs and issues with endoscope reprocessing) and a user-friendly ergonomic design (reducing the learning curve).
Invendoscope (Ambu, Ballerup, Denmark)
The CE-marked and FDA-cleared48 Invendoscope (Ambu) (Figure 6) is a single-use ergonomically designed colonoscope. The former self-propulsion inverted sleeve mechanism seen in Invendo SC20 was abandoned. The current iteration, Invendo SC210, has a hydraulically actuated tip with electromechanical actuation rather than the more traditional Bowden cables.49 The tip can deflect 180° in all directions within a small working radius of 35 mm.50 The tip houses an HD camera with suction, irrigation and 3.2 mm working channels.
Figure 6.
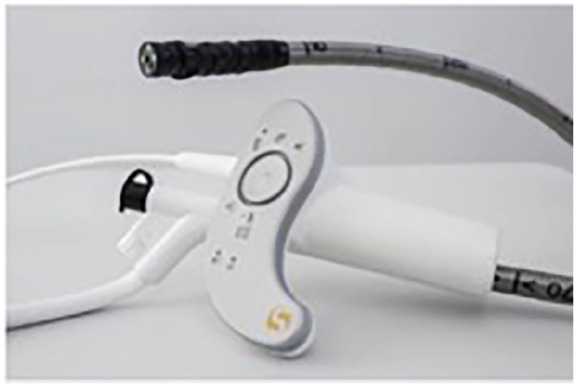
Invendoscope (Ambu, Ballerup, Denmark).49
The endoscope is controllable by a detachable gamepad-style joystick controller. This handheld controller purports to have a better ergonomic design which will reduce the musculoskeletal burden on the endoscopist.51
An early feasibility study in 2011 with Invendo SC20 showed a caecal intubation rate of 98.4% and a median time to caecum of 15 min. Sedation was only needed by 4.9%.52 More recently, SC210 underwent a feasibility study in 40 patients with a caecal intubation rate of 95%, time to caecum of 14.23 min and no major complications.53–55
The cost of the disposable component is US$350. The Invendoscope offers potential cost benefits in scope acquisition and reprocessing costs. It purports ergonomic benefit to the user and reduction in discomfort to the patient. The authors of the recent feasibility study acknowledge that comparative study against conventional colonoscopy is now needed.53–55
Robotic-driven endoscope locomotion and instrumentation platforms
Endoluminal Assistant for Surgical Endoscopy (ICube Laboratory, Strasbourg, France)
Endoluminal Assistant for Surgical Endoscopy (EASE; ICube laboratory) (Figure 7) is a single-user teleoperated master-slave system. The EASE platform initially began as the two-person operated Anubiscope;56 a tulip-shaped distal cap facilitated atraumatic insertion which opened at the target site to reveal two flexible arms with 4 degrees of freedom (DOFs),57 allowing a larger operative field.58
Figure 7.
Endoluminal Assistant for Surgical Endoscopy (EASE). a) slave component; b) master component (ICube laboratory, Strasbourg, France).59
Anubiscope developed into the teleoperated STRAS (Single-access Transluminal Robotic Assistant for Surgeons) system. Légner and colleagues60 completed 12 of 18 porcine colorectal ESDs with version 1 and Zorn and colleagues61 completed 12 of 12 porcine colorectal ESDs with version 2.
The EASE is the most current iteration. The master controller consists of joysticks, thumb switches and triggers with two screens.59 The slave component consists of a cart containing actuation equipment connected to a 53.5-cm detachable endoscope. There are two 4.3 mm channels down which a variety of teleoperable instruments can be delivered and a 3.2-mm channel for conventional instruments to be deployed.
A non-randomised animal trial was conducted with a laparoscopic surgeon with no ESD or robotic experience using the EASE system and an expert endoscopist with over 1000 ESDs performing conventional ESD on porcine colonic pseudotumours. The robotic group achieved a 100% en bloc resection rate with a significantly reduced perforation rate (5% versus 33%) and total procedural time (33.36 versus 47.38 min; p = 0.011).59
This is a powerful demonstration of a robotic platform allowing quicker, safer and more successful endoluminal surgery to be performed by a novice versus current benchmark techniques performed by an expert. Further work will be needed before in vivo trials are possible.
I²Snake (Hamlyn Centre, London, UK)
The I²Snake (Intuitive Imaging Sensing Navigated and Kinematically Enhanced Robot; Hamlyn Centre) (Figure 8) is an evolution of the i-SNAKE platform: a multi-articulated device activated by embedded micromotors and local tendons with a flexible neck actuated by two pairs of antagonistic tendons. This was held by a robotic arm giving 7 DOFs. Benchtop trials suggested more accurate control of tip movement compared with conventional endoscopy.62
Figure 8.

I²Snake (Hamlyn Centre, London, UK).63
I²Snake’s body consists of 13 three-dimensional (3D) printed steel vertebrae, divided into three sections, with orthogonally arranged rolling joints which are steel tendon–driven. This allows benchtop manipulation forces of 5.6 N64 and 7 DOFs and a broad range of configurations including S shapes and retroflexion, biomimicking a snake. There are four channels allowing deployment of a camera and light source, two 3.8 mm diameter instruments, and suction and irrigation and incorporated graspers which are also externally actuated with 5 DOFs.
An LBR iiwa 14 robotic arm (KUKA, Germany) holds the body and works in three modes. The ‘global positioning mode’ allows direct control of the arm to orient the robot to the insertion point. The ‘teleoperation mode’ allows combined control of the I²Snake and robotic arm using inverse kinematics. Movements of the device and instruments are controlled with two electromagnetically tracked hand grippers with motion scaling to increase precision. Use of a robotic arm allows for future work on dynamic motion compensation, which could improve stability during precise instrumentation.63
External tendon actuation has issues with ‘backlash hysteresis’, but artificial intelligence (AI)-based software to compensate for this is being developed.65 Future ex vivo trials are required.
Endomaster EASE system (EndoMASTER Pte, Singapore)
The Master and Slave Transluminal Endoscopic Robot (MASTER; EndoMASTER Pte) (Figure 9) was a master-slave platform initially designed for NOTES procedures66 but pivoted into ESD. Early versions had a cable and motion sensor hand-controlled master device with haptic feedback. The slave system consisted of a grasper and electrocautery hook with elbow and wrist joints, allowing 7 DOFs. These were mounted externally onto a conventional dual-channel endoscope. The instruments were externally actuated through tendon sheaths running through the instrument channels, allowing manipulation forces of 3–5 N.67 Two operators were required,68 the tools were fixed and an overtube was required for use.
Figure 9.
Master (EndoMASTER Pte, Singapore).
Initial feasibility studies showed success in porcine hepatic wedge resection,69 gastric ESD68 and gastric full-thickness resection.70 A human feasibility study was successful in achieving R0 resection in five patients with gastric ESD with no complication and a mean dissection time of 18.6 min.71 A small trial demonstrated comparable results between novices and non-experts when using MASTER for ESD and a short learning curve for novices.72
The most recent iteration, the Endomaster EASE system, has a purpose-built ‘slave’ flexible endoscope with two instrument channels down which a variety of cable-actuated robotic instruments can be deployed interchangeably with 7 DOFs. A recent preclinical feasibility live porcine trial showed 100% (5/5) en bloc resection in porcine colorectal ESD without serious adverse events.73
The Endomaster Platform shows exciting promise but currently has no CE or FDA approval.43
Flex Colorectal Drive (Medrobotics Corp, Raynham, Massachusetts, USA)
The Flex Robotic System (Medrobotics Corp) (Figure 10) is a single-operator endoluminal surgical platform that was originally designed to access anatomically difficult areas in head and neck surgery.
Figure 10.

Flex Colorectal Drive (Medrobotics Corp, Raynam, Massachusetts, USA).74
The endoscope has two concentric overtube systems made up of multiple segments that articulate at a single point. The outer tube advances first with the inner tube following. The endoscope is flexible on insertion but can be stiffened in a non-linear shape to provide a stable base for intervention.75
A range of flexible wristed instruments pass down two accessory channels mounted on the sides of the endoscope.51 They are controlled directly in a similar manner to laparoscopic instruments, allowing direct mechanical feedback. This differs from the electro-mechanically decoupled master-slave systems seen in other robotic platforms such as EASE, i-SNAKE and Endomaster EASE.74 The endoscope itself is controlled by a simple joystick style platform based on the OMEGA 3 force dimension, allowing intuitive control.56
A multicentre, prospective single-armed trial showed success in transoral oropharyngeal surgery. Access and visualisation of the intended lesion were successful in 95% (75/80), with successful surgery in 91.1% (72/79).76
The system was modified to maintain rectal insufflation for transanal surgery. A human cadaveric trial showed feasibility of Flex for transanal total mesorectal excision (taTME).74 A retrospective case series of rectal local excision in 11 patients with Flex Colorectal Drive showed successful R0 resection in 10 of the patients, with 1 being abandoned due to poor patient positioning rather than robot error.77
A recent study compared Flex-assisted ESD with traditional ESD by five inexperienced operators (<5 ex vivo conventional ESD). Hundred percent of en bloc resection was completed with Flex (10/10) and 50% with conventional ESD (5/10, p < 0.0001). The total procedure time was significantly lower (34.1 versus 88.6 min) in the Flex group, and there was a trend towards higher perforation rate (p = 0.18) in the traditional ESD group. Technical workload (using NASA TLX scores: a quantitative scoring system of workload) was also significantly lower in the Flex group (28.4 versus 47).78
The Flex colorectal drive has great potential in endoscopic intraluminal and transluminal surgery. It was cleared for use by the FDA in 2017. It currently only has a 25-cm reach. Increasing the length of the device is an exciting prospect.
CYCLOPS (Hamlyn Centre, London, UK)
CYCLOPS (Hamlyn Centre) (Figure 11) is a bimanual robotic platform that can be fitted onto a conventional endoscope to facilitate ESD. A collapsible scaffold allows insertion of the endoscope and subsequent deployment of the system when the point of therapy is reached.79 This is covered in a soft silicone sleeve for insertion. Two 4 mm aluminium overtubes contain the deployed instruments. A network of tendons, six pairs, actuate the overtubes and thus the instruments, allowing 5 DOFs.18 These in turn are actuated via Bowden cables by an external motor unit.
Figure 11.

CYCLOPS (Hamlyn Centre, London, UK).79
This novel use of parallel robotics allows high manipulation forces of 46 N, significantly higher than seen in many other robotic platforms. The operator controls the system through two haptic feedback Geomagic® Touch TM controllers. An early prototype showed a mean error of only 0.217 mm during an ellipse-tracing task.80
Benchtop ex vivo trials showed a significantly quicker dissection time in simulated ESD in chicken fillets when using CYCLOPS versus conventional ESD techniques. In vivo trials are underway.18
CYCLOPS has several advantages over comparative platforms, including its compatibility with conventional endoscopes, its novel parallel robotic actuation method delivering high manipulation forces and its accurate bimanual control. There are only ex vivo data so far, and it remains to be seen whether these benefits can be replicated during in vivo trials.
Future
Eye Gaze (Hamlyn Centre, London, UK)
Endoscopy controlled by eye movements has been successfully trialled in a simulated upper GI tract. This uses custom eye gaze software with a robotised arm and 3D printed cogwheels attached to the normal steering wheels of a Karl Storz endoscope (Figure 12). Four experts and one novice were able to successfully intubate a simulated upper GI tract and locate 10 targets.81
Figure 12.
Eye Gaze: robotised system (left) and simulated trial (right).
As endoscopic therapy becomes more complex, the potential for hands-free motion control of the endoscope may allow greater control of therapeutic instruments and potential ergonomic benefits.
Shared autonomy and AI
In 2017, Yang and colleagues82 suggest a six-level framework of autonomy in medical robots ranging from level 0 (no autonomy) to level 5 (full autonomy). Surgical robots have in some cases reached level 3 (conditional autonomy: system-generated tasks which the operator approves). However, in endoluminal surgery such as ESD, the robotic systems still lie at level 0, with some of the above projects discussed demonstrating level 1 autonomy (robot assistance but human has continuous control).
Endoluminal surgery involves circumferential target dissection as one of its key steps. Ma and colleagues proposed that this key step could be automated. They published their early work on a framework involving an operator-defined trajectory and adaptive control of a flexible manipulator with 3 DOFs. The system’s dynamic response to material deformation and external payloads allowed ongoing accuracy during dissection. This demonstration shows the potential for shared autonomy in the control of instruments and the potential to automate a key step of ESD.83
Deep learning and, more specifically, convolutional neuronal networks have allowed significant advancement in image and video processing. This has resulted in many commercially available lesion detection and characterisation systems being recently released.
Utilisation of AI beyond lesion detection and recognition in luminal endoscopy is hampered by the deformability of the gut. For intelligent navigation of the lumen by an endoscope, both the highly malleable shape of the gut and the position of the camera must be estimated together, which remains a challenge to the engineering community.84 Although simple surgical tasks have been successfully automated through AI, these as yet are not safely achievable on deformable tissue. This is a rapidly evolving field, and the continuing advancement of AI-driven endoluminal techniques should be expected both in navigation and in therapeutics.
Soft robotics
Soft robotics uses highly elastic material compared with biological tissues.85 Soft robots are able to adjust their stiffness. This would allow soft robots to navigate the lumen in a soft atraumatic configuration and stiffen at the point of intervention to allow stability during therapeutic intervention.50
Common soft robotic actuators still have limitations preventing their use currently for therapeutic endoscopy. Shape memory actuators change shape on heating, but the heat required currently could cause damage to surrounding tissues. Dielectric elastomers deform with electric current but currently generate low and delayed forces.86
Bernth and colleagues designed the 55-cm silicone mesh, earthworm-inspired ‘meshworm’ soft robot (Figure 13) with a USB camera in the tip. Its three segments are actuated by antagonistic tendons driven by small pulley motors. In a prototyped plastic colon, the meshworm averaged a speed of 1.21 ms/s, which equates to 30 min for an average caecal intubation.87
Figure 13.

Meshworm.50
STIFF-FLOP is a silicon-based soft robot inspired by octopus tentacles. It is actuated by a combination of pneumatics and granular jamming. Granular jamming is where devices can take on liquid, solid or in between states, producing variable stiffness.88 Pneumatic actuation pressurises three chambers in different combinations to produce a variety of movements.65 This combination allows navigation, elongation and stiffening. STIFF-FLOP has been successfully demonstrated in two cadaveric total mesorectal excisions, acquiring superior visual angles of the surgical field.89
Soft robotics offers exciting benefits including variable stiffness, minimised trauma and increased manoeuvrability with magnetic resonance imaging compatibility for endoscope localisation, but substantial technological progress is required before it can be incorporated into mainstream use.
Conclusion
The potential for flexible endoscopy is rapidly expanding with incorporation of technology from other scientific disciplines. This review has shown how improvements in the long-standing endoscopic platform can produce a safer and more tolerable endoscopic examination with greater diagnostic potential, incredibly important given the significance of lesion recognition and characterisation in preventing the increasing global burden of colorectal cancer.
The therapeutic potential of the endoscope is being increasingly recognised, and collaboration with engineers is helping to address the technical issues faced in fine instrument control and manipulation through a flexible device. This review has looked at some exciting platforms that tackle these issues, although they are all still some way from mainstream incorporation or cost-effectiveness. Future robotic and engineering technologies will continue to improve the capabilities of the endoscope, so their full potential can be harnessed to deliver the broadening remit of endoscopic therapy in a safe and effective way.
Footnotes
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
ORCID iD: Arun Sivananthan  https://orcid.org/0000-0002-1649-0150
https://orcid.org/0000-0002-1649-0150
Contributor Information
Arun Sivananthan, Imperial College NHS Healthcare Trust, London W2 1NY, UK.
Ben Glover, Imperial College, London, UK.
Lakshmana Ayaru, Imperial College NHS Healthcare Trust, London, UK.
Kinesh Patel, Chelsea and Westminster NHS Healthcare Trust, UK.
Ara Darzi, Imperial College, London, UK.
Nisha Patel, Imperial College, London, UK.
References
- 1. World Health Organization and International Agency for Research on Cancer. Cancer Today Globocan 2018, http://gco.iarc.fr/today/fact-sheets-cancers (accessed 20 October 2019)
- 2. Arnold M, Sierra MS, Laversanne M, et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017; 66: 683–691. [DOI] [PubMed] [Google Scholar]
- 3. Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012; 366: 687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer. N Engl J Med 2014; 370: 1298–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brenner H, Chang-Claude J, Jansen L, et al. Reduced risk of colorectal cancer up to 10 years after screening, surveillance, or diagnostic colonoscopy. Gastroenterology 2014; 146: 709–717. [DOI] [PubMed] [Google Scholar]
- 6. Kahi CJ, Myers LJ, Slaven JE, et al. Lower endoscopy reduces colorectal cancer incidence in older individuals. Gastroenterology 2014; 146: 718–725.e3. [DOI] [PubMed] [Google Scholar]
- 7. Nabi Z, Reddy DN, Ramchandani M. Recent advances in third-space endoscopy. Gastroenterol Hepatol 2018; 14: 224–232. [PMC free article] [PubMed] [Google Scholar]
- 8. Patel N, Seneci C, Yang G-Z, et al. Flexible platforms for natural orifice transluminal and endoluminal surgery. Endosc Int Open 2014; 2: E117–E123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bozzini P. Lichtleiter, eine Erfindung zur Anschauung innerer Teile und Krankheiten, nebst der Abbildung [Light conductor, an invention for examining internal parts and diseases, together with illustrations]. J Der Pract Arzneykd Und Wundarzneykunst 1806; 24: 107–124. [Google Scholar]
- 10. Newell OK. The endoscopic instruments of Joseph Leiter of Vienna and the present development of endoscopy. Boston Med Surg J 1887; 117: 528–530. [Google Scholar]
- 11. Schindler R. The endoscopic study of gastric pathology. 2nd ed. Chicago, IL: University of Chicago, 1950. [Google Scholar]
- 12. Edmonson J. History of the instruments of gastrointestinal endoscopy. Gastrointest Endosc 1991; 37: S27–S56. [DOI] [PubMed] [Google Scholar]
- 13. Hirschowitz BI. Development and application of endoscopy. Gastroenterology 1993; 104: 337–342. [DOI] [PubMed] [Google Scholar]
- 14. Wolff W, Shinya H. Colonofiberoscopy. JAMA 1971; 217: 1609–1512. [PubMed] [Google Scholar]
- 15. Spurr C., Jr History of the instruments and techniques of gastrointestinal endoscopy. In: Sridhar S, Wu G. (eds) Diagnostic and therapeutic procedures in gastroenterology: an illustrated guide. 2nd ed. Totowa, NJ: Humana Press, 2018, pp. 3–13. [Google Scholar]
- 16. McGoran JJ, McAlindon ME, Iyer PG, et al. Miniature gastrointestinal endoscopy: now and the future. World J Gastroenterol 2019; 25: 4051–4060, https://www.wjgnet.com/1007-9327/full/v25/i30/4051.htm [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Gastrointest Endosc 2015; 81: 31–53. [DOI] [PubMed] [Google Scholar]
- 18. Patel NK. Advances in endoluminal surgery. London: Imperial College London, 2018. [Google Scholar]
- 19. Larsen S, Kalloo A, Hutfless S. The hidden cost of colonoscopy including cost of reprocessing and infection rate: the implications for disposable colonoscopes. Gut 2020; 69: 197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kovaleva J, Peters FTM, van der Mei HC, et al. Transmission of infection by flexible gastrointestinal endoscopy and bronchoscopy. Clin Microbiol Rev 2013; 26: 231–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Glover B, Patel N, Ashrafian H, et al. Diagnostic accuracy of i-scan image enhancement for real-time endoscopic diagnosis of small colorectal polyps: a meta-analysis. Therap Adv Gastroenterol 2018; 11: 1756284818814948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hassan C, Pickhardt PJ, Rex DK. A resect and discard strategy would improve cost-effectiveness of colorectal cancer screening. Clin Gastroenterol Hepatol 2010; 8: 865–9869. [DOI] [PubMed] [Google Scholar]
- 23. Williet N, Tournier Q, Vernet C, et al. Effect of Endocuff-assisted colonoscopy on adenoma detection rate: meta-analysis of randomized controlled trials. Endoscopy 2018; 50: 846–860. [DOI] [PubMed] [Google Scholar]
- 24. Triantafyllou K, Gkolfakis P, Tziatzios G, et al. Effect of Endocuff use on colonoscopy outcomes: a systematic review and meta-analysis. World J Gastroenterol 2019; 25: 1158–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jian HX, Feng BC, Zhang Y, et al. Endocuff-assisted colonoscopy could improve adenoma detection rate: a meta-analysis of randomized controlled trials. J Dig Dis 2019; 20: 578–588. [DOI] [PubMed] [Google Scholar]
- 26. Ngu WS, Bevan R, Tsiamoulos ZP, et al. Improved adenoma detection with Endocuff Vision: the ADENOMA randomised controlled trial. Gut 2019; 68: 280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Walls M, Brand A, Ngu WS, et al. The B-ADENOMA trial: a multicentre, randomised controlled trial of adenoma detection with Endocuff vision. Endoscopy Orals 2019; 68(Suppl. 2): A1–A269. [Google Scholar]
- 28. van Keulen KE, Soons E, Siersema PD. The role of behind folds visualizing techniques and technologies in improving adenoma detection rate. Curr Treat Options Gastroenterol 2019; 17: 394–407. [DOI] [PubMed] [Google Scholar]
- 29. MacLeod C, Monaghan E, Banerjee A, et al. Colon capsule endoscopy. Surgeon 2020; 18: 251–256. [DOI] [PubMed] [Google Scholar]
- 30. Postgate AJ, Burling D, Gupta A, et al. Safety, reliability and limitations of the given patency capsule in patients at risk of capsule retention: a 3-year technical review. Dig Dis Sci 2008; 53: 2732–2738. [DOI] [PubMed] [Google Scholar]
- 31. Hosoe N, Limpias Kamiya KJL, Hayashi Y, et al. Current status of colon capsule endoscopy. Dig Endosc. Epub ahead of print 16 June 2020. DOI: 10.1111/den.13769. [DOI] [PubMed] [Google Scholar]
- 32. Ahmad OF, Soares AS, Mazomenos E, et al. Artificial intelligence and computer-aided diagnosis in colonoscopy: current evidence and future directions. Lancet Gastroenterol Hepatol 2019; 4: 71–80. [DOI] [PubMed] [Google Scholar]
- 33. Mans L, Arvanitakis M, Neuhaus H, et al. Motorized spiral enteroscopy for occult bleeding. Dig Dis 2018; 36: 325–327. [DOI] [PubMed] [Google Scholar]
- 34. Morgan D, Upchurch B, Draganov P, et al. Spiral enteroscopy: prospective U.S. multicenter study in patients with small-bowel disorders. Gastrointest Endosc 2014; 72: 992–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baniya R, Upadhaya S, Subedi SC, et al. Balloon enteroscopy versus spiral enteroscopy for small-bowel disorders: a systematic review and meta-analysis. Gastrointest Endosc 2017; 86: 997–1005. [DOI] [PubMed] [Google Scholar]
- 36. Beyna T, Schneider M, Pullmann D, et al. Motorized spiral colonoscopy: a first single-center feasibility trial. Endoscopy 2018; 50: 518–523. [DOI] [PubMed] [Google Scholar]
- 37. Beyna T, Arvanitakis M, Schneider M, et al. First prospective clinical trial on total motorized spiral enteroscopy (TMSET). Gastrointest Endosc 2019; 89: AB48. [DOI] [PubMed] [Google Scholar]
- 38. Spada C, Hassan C, Cesaro P, et al. Spiraling your insertion: a glimpse into the future of colonoscopy. Endoscopy 2018; 50: 469–470. [DOI] [PubMed] [Google Scholar]
- 39. GI View. FDA Aer-o-scope. IOSR J Econ Fin. 2016, https://www.accessdata.fda.gov/cdrh_docs/pdf14/K141286.pdf
- 40. Gluck N, Melhem A, Halpern Z, et al. A novel self-propelled disposable colonoscope is effective for colonoscopy in humans (with video). Gastrointest Endosc 2016; 83: 998–1004. [DOI] [PubMed] [Google Scholar]
- 41. Gluck N, Fishman S, Melhem A, et al. A novel colonoscope with panoramic visualization detected more simulated polyps than conventional colonoscopy in a live swine model. Endosc Int Open 2015; 3: E642–E645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ciocîrlan M. Low-cost disposable endoscope: pros and cons. Endosc Int Open 2019; 7: E1184–E1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Boškoski I, Costamagna G. Endoscopy robotics: current and future applications. Dig Endosc 2019; 31: 119–124. [DOI] [PubMed] [Google Scholar]
- 44. Cosentino F, Tumino E, Passoni GR, et al. Functional evaluation of the Endotics System, a new disposable self-propelled robotic colonoscope: in vitro tests and clinical trial. Int J Artif Organs 2009; 32: 517–527. [DOI] [PubMed] [Google Scholar]
- 45. Tumino E, Sacco R, Bertini M, et al. Endotics system vs colonoscopy for the detection of polyps. World J Gastroenterol 2010; 16: 5452–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tumino E, Parisi G, Bertoni M, et al. Use of robotic colonoscopy in patients with previous incomplete colonoscopy. Eur Rev Med Pharmacol Sci 2017; 21: 819–826. [PubMed] [Google Scholar]
- 47. Wong JYY, Ho KY. Robotics for advanced therapeutic colonoscopy. Clin Endosc 2018; 51: 552–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. U.S. Food and Drug Administration. K173085 Invendoscopy E210 System. Silver Spring, MD: U.S. Food and Drug Administration, 2018. [Google Scholar]
- 49. Khanicheh A, Shergill AK. Endoscope design for the future. Tech Gastrointest Endosc 2019; 21: 167–173. [Google Scholar]
- 50. Gifari MW, Naghibi H, Stramigioli S, et al. A review on recent advances in soft surgical robots for endoscopic applications. Int J Med Robot 2019; 15: e2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Peters BS, Armijo PR, Krause C, et al. Review of emerging surgical robotic technology. Surg Endosc 2018; 32: 1636–1655. [DOI] [PubMed] [Google Scholar]
- 52. Groth S, Rex DK, Rösch T, et al. High cecal intubation rates with a new computer-assisted colonoscope: a feasibility study. Am J Gastroenterol 2011; 106: 1075–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Straulino F, Genthner A, Kiesslich R, et al. Erratum: colonoscopy with the single use endoscope Invendoscope SC210. Gastrointest Endosc 2019; 89: AB718. [Google Scholar]
- 54. Straulino F, Genthner A, Kiesslich R, et al. Colonoscopy with the sterile single use endoscope Invendoscope Sc210. Gastrointest Endosc 2018; 87: AB257. [Google Scholar]
- 55. Straulino F, Genthner A, Ralf K, et al. Colonoscopy with the single use endoscope Invendoscope Sc210 in routine clinical practice. Endoscopy 2019; 51: S101. [Google Scholar]
- 56. Mathelin MDe. Single port and transluminal robotic assistant for surgeons: a modular and flexible telemanipulated robotic device for intraluminal surgery. In: Abedin-Nasab MH. (ed.) Handbook of robotic and image-guided surgery. Amsterdam: Elsevier Inc, 2020, pp. 123–146. [Google Scholar]
- 57. Patel N, Darzi A, Teare J. The endoscopy evolution: ‘the superscope era’. Frontline Gastroenterol 2015; 6: 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wai P, Chiu Y. Applications of flexible robots. In: Abedin-Nasab MH. (ed.) Handbook of robotic and image-guided surgery. Amsterdam: Elsevier Inc, 2020, pp. 303–322. [Google Scholar]
- 59. Mascagni P, Lim SG, Fiorillo C, et al. Democratizing endoscopic submucosal dissection: single-operator fully robotic colorectal endoscopic submucosal dissection in a pig model. Gastroenterology 2019; 156: 1569–1571. [DOI] [PubMed] [Google Scholar]
- 60. Légner A, Diana M, Halvax P, et al. Endoluminal surgical triangulation 2.0: a new flexible surgical robot. Preliminary pre-clinical results with colonic submucosal dissection. Int J Med Robot 2017; 13: e1819. [DOI] [PubMed] [Google Scholar]
- 61. Zorn L, Nageotte F, Zanne P, et al. A novel telemanipulated robotic assistant for surgical endoscopy: preclinical application to ESD. IEEE Trans Biomed Eng 2018; 65: 797–808. [DOI] [PubMed] [Google Scholar]
- 62. Patel N, Seneci CA, Shang J, et al. Evaluation of a novel flexible snake robot for endoluminal surgery. Surg Endosc 2015; 29: 3349–3355. [DOI] [PubMed] [Google Scholar]
- 63. Berthet-Rayne P, Gras G, Leibrandt K, et al. The i 2 snake robotic platform for endoscopic surgery. Ann Biomed Eng 2018; 46: 1663–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Berthet-Rayne P, Leibrandt K, Kim K, et al. Rolling-joint design optimization for tendon driven snake-like surgical robots. In: 2018 IEEE/RSJ international conference on intelligent robots and systems (IROS), Madrid, 1–5 October 2018; pp. 4964–4971. New York: IEEE. [Google Scholar]
- 65. Berthet-Rayne P, Yang GZ. Vision based shape reconstruction of tendon driven snake-like surgical robots. 2017, pp. 69–70, https://www.ukras.org/wp-content/uploads/2018/10/HSMR17_proceedings-FINAL-1-reduced.pdf
- 66. Low SC, Tang SW, Thant ZM, et al. Master-slave robotic system for therapeutic gastrointestinal endoscopie procedures. Conf Proc IEEE Eng Med Biol Soc 2006; 2006: pp. 3850–3853. [DOI] [PubMed] [Google Scholar]
- 67. Yang K, Sun ZL, Kencana AP, et al. Enhancement of spatial orientation and haptic perception for master-slave robotic Natural Orifice Transluminal Endoscopic Surgery (NOTES). In: 2010 IEEE conference on robotics, automation and mechatronics, Singapore, 28–30 June 2010, pp. 15–18. New York: IEEE. [Google Scholar]
- 68. Wang Z, Phee SJ, Lomanto D, et al. Endoscopic submucosal dissection of gastric lesions by using a master and slave transluminal endoscopic robot: an animal survival study. Endoscopy 2012; 44: 690–694. [DOI] [PubMed] [Google Scholar]
- 69. Phee SJ, Ho KY, Lomanto D, et al. Natural orifice transgastric endoscopic wedge hepatic resection in an experimental model using an intuitively controlled master and slave transluminal endoscopic robot (MASTER). Surg Endosc 2010; 24: 2293–2298. [DOI] [PubMed] [Google Scholar]
- 70. Chiu PWY, Phee SJ, Wang Z, et al. Feasibility of full-thickness gastric resection using master and slave transluminal endoscopic robot and closure by overstitch: a preclinical study. Surg Endosc 2014; 28: 319–324. [DOI] [PubMed] [Google Scholar]
- 71. Phee SJ, Reddy N, Chiu PWY, et al. Robot-assisted endoscopic submucosal dissection is effective in treating patients with early-stage gastric neoplasia. Clin Gastroenterol Hepatol 2012; 10: 1117–1121. [DOI] [PubMed] [Google Scholar]
- 72. Chiu P, Phee S, Bhandari P, et al. Enhancing proficiency in performing endoscopic submucosal dissection (ESD) by using a prototype robotic endoscope. Endosc Int Open 2015; 3: E439–E442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chiu PWYW, Phee SJ, Ho K-Y. Colonic endoscopic submucosal dissection using ease robotic system: a preclinical study. Gastrointest Endosc 2019; 89: AB658–AB659. [DOI] [PubMed] [Google Scholar]
- 74. Carmichael H, D’Andrea AP, Skancke M, et al. Feasibility of transanal total mesorectal excision (taTME) using the Medrobotics Flex® System. Surg Endosc 2020; 34: 485–491. [DOI] [PubMed] [Google Scholar]
- 75. Cengiz TB, Steele SR, Gorgun E. Implementation of novel robotic systems in colorectal surgery. In: Abedin-Nasab MH. (ed.) Handbook of robotic and image-guided surgery. Amsterdam: Elsevier Inc, 2020, pp. 147–158. [Google Scholar]
- 76. Lang S, Mattheis S, Hasskamp P, et al. A European multicenter study evaluating the flex robotic system in transoral robotic surgery. Laryngoscope 2017; 127: 391–395. [DOI] [PubMed] [Google Scholar]
- 77. Paull JO, Graham A, Parascandola SA, et al. The outcomes of two robotic platforms performing transanal minimally invasive surgery for rectal neoplasia : a case series of 21 patients. J Robot Surg 2020; 14: 573–578. [DOI] [PubMed] [Google Scholar]
- 78. Turiani Hourneaux de Moura D, Aihara H, Jirapinyo P, et al. Robot-assisted endoscopic submucosal dissection versus conventional ESD for colorectal lesions: outcomes of a randomized pilot study in endoscopists without prior ESD experience (with video). Gastrointest Endosc 2019; 90: 290–298. [DOI] [PubMed] [Google Scholar]
- 79. Mylonas GP, Patel N, Teare J, et al. CYCLOPS: an endoscope attachment for endoscopic submucosal dissection. In: SAGES 2017 annual meeting, Houston, TX, 23 March 2017. [Google Scholar]
- 80. Vrielink TJCO, Zhao M, Darzi A, et al. ESD CYCLOPS: a new robotic surgical system for GI surgery. In: 2018 IEEE international conference on robotics and automation (ICRA), Brisbane, QLD, Australia, 21–25 May 2018, pp. 150–157. New York: IEEE. [Google Scholar]
- 81. Patel N, Kogkas A, Darzi A, et al. Eye gaze-controlled robotic flexible endoscopy: a feasibility study. Gut 2019; 68(Suppl. 2): 38–39. [Google Scholar]
- 82. Yang GZ, Cambias J, Cleary K, et al. Medical robotics – regulatory, ethical, and legal considerations for increasing levels of autonomy. Sci Robot 2017; 2: eaam8638. [DOI] [PubMed] [Google Scholar]
- 83. Ma X, Wang P, Ye M, et al. Shared autonomy of a flexible manipulator in constrained endoluminal surgical tasks. IEEE Robot Autom Lett 2019; 4: 3106–3112. [Google Scholar]
- 84. Ciuti G, Skonieczna-Żydecka K, Marlicz W, et al. Frontiers of robotic colonoscopy: a comprehensive review of robotic colonoscopes and technologies. J Clin Med 2020; 9: 1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Heung H, Chiu PWY, Li Z. Design and prototyping of a soft earthworm-like robot targeted for GI tract inspection. In: 2016 IEEE international conference on robotics and biomimetics (ROBIO), Qingdao, China, 3–7 December 2016, pp. 497–502. New York: IEEE. [Google Scholar]
- 86. Miriyev A, Stack K, Lipson H. Soft material for soft actuators. Nat Commun 2017; 8: 596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bernth JE, Arezzo A, Liu H. A novel robotic meshworm with segment-bending anchoring for colonoscopy. IEEE Robot Autom Lett 2017; 2: 17–18. [Google Scholar]
- 88. Putzu F, Konstantinova J, Althoefer K. Soft Particles for Granular Jamming. In: Althoefer K, Konstantinova J, Zhang K. (eds) Towards autonomous robotic systems. Cham: Springer International Publishing, 2019, pp. 65–74. [Google Scholar]
- 89. Arezzo A, Mintz Y, Allaix ME, et al. Total mesorectal excision using a soft and flexible robotic arm: a feasibility study in cadaver models. Surg Endosc 2017; 31: 264–273. [DOI] [PubMed] [Google Scholar]