Abstract
Purpose of review:
The aim of this review is to describe the latest studies on sarcoidosis incidence, prevalence and risk factors with a special focus on reports in the last two years. The potential biases affecting these studies are discussed.
Recent findings:
The prevalence and incidence of sarcoidosis vary greatly depending on region of the world. Variations in data sources and settings can affect estimates of the burden of sarcoidosis, sometimes making them difficult to compare across countries. It is not well understood how the distribution of sarcoidosis phenotypes differs across populations. Age, sex and race are the most important sources of variation in incidence and prevalence. Recent epidemiological studies provide new insights on the role of genetic and non-genetic risk factors for sarcoidosis.
Summary:
High quality and systematically collected data, with depth (detailed information per individual) and breadth (many individuals), is needed to further understand the complexity and heterogeneity of sarcoidosis.
Keywords: Sarcoidosis, epidemiology, incidence, prevalence, risk
Introduction
Sarcoidosis is a challenging disease to study due to its heterogeneity across patients and around the world. Deciphering the reasons for variation in the incidence and prevalence of sarcoidosis between different populations can help to reveal risk factors associated with its development. In recent years, secondary analyses of readily available data routinely collected as part of clinical health care have provided a contemporary view of the disease based on large samples. Bigger numbers are not always better, though, and these data often lack the finer details that are needed to identify sarcoidosis phenotypes and minimize misclassification. This review will summarize the literature published in the last two years on sarcoidosis incidence, prevalence and risk factors.
Incidence and prevalence of sarcoidosis across the world
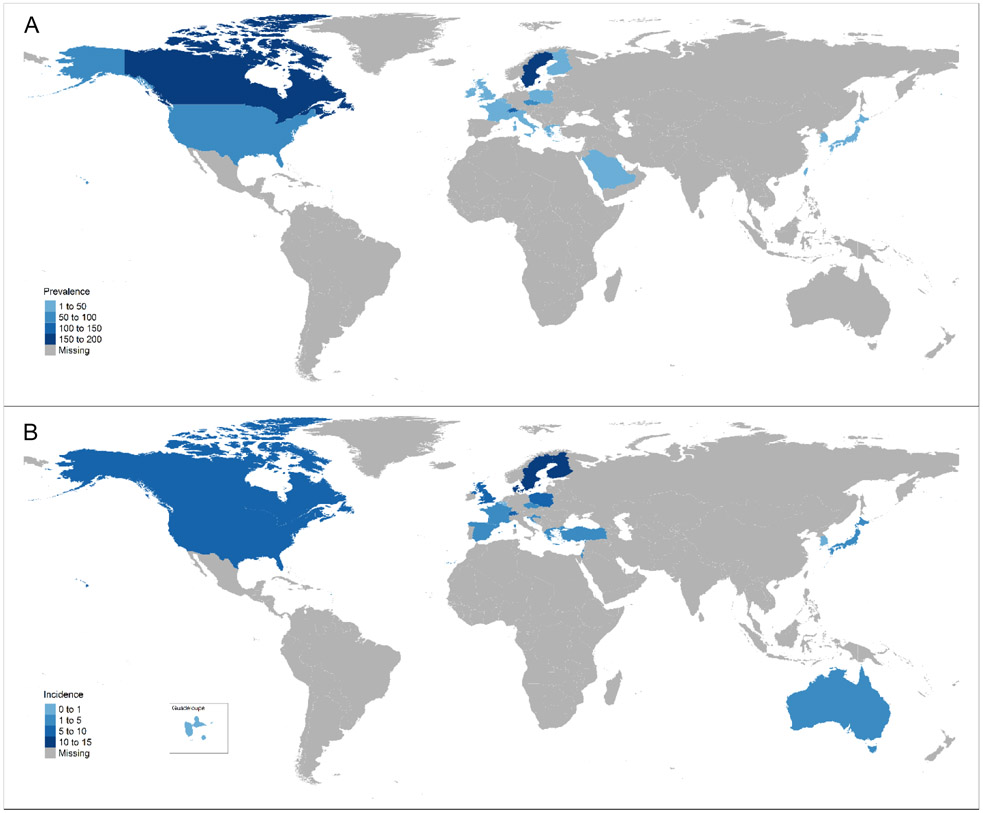
The prevalence of sarcoidosis varies greatly depending on region of the world, from 1-5 per 100,000 in South Korea(1, 2), Taiwan(3) and Japan(4) to 140-160 per 100,000 in Sweden(5) and Canada(6) (Figure; Table 1). There is still much we do not know about the occurrence of sarcoidosis in other parts of the world, as is evident from the large areas with missing information (Figure). The derivation of estimates from different sources in different settings make comparisons difficult. Administrative datasets from countries with universal access to healthcare provide a picture of the clinically significant disease burden on the population. However, these data sources often vary in the number of years of coverage which can affect the prevalence estimates. A recent study from Ontario, Canada used administrative data comprised of inpatient and outpatient visits listing an international classification of diseases (ICD) code for sarcoidosis (6). They show how increasing the number of years of observation from 5 years to 24 years (due to increased data availability) increases the prevalence over twofold from 66 to 143 per 100,000. Many years of data coverage could overestimate the true disease burden if patients have disease which has resolved but are still counted as cases decades later. Furthermore, some reports utilize only hospitalization data, which underestimates of the true number of sarcoidosis cases, missing many who are seen in outpatient care only (7).
Figure.
The a) prevalence and b) incidence of sarcoidosis reported globally since 1990.
Table 1.
Studies reporting the prevalence and incidence of sarcoidosis since 1990 used to create the Figure.
| Country | Study | Prevalence per 100,000 |
Incidence per 100,000 per year |
|---|---|---|---|
| Australia | Gillman 2007(49) | 4.7 | |
| Belgium | Thomeer 2001(50) | 2 | 0.3 |
| Canada | Fidler 2019(6) | 143 | 6.8 |
| Croatia | Mise 2011(51) | 3.3 | |
| Czech | Kolek 1994(52) | 63.1 | 4.4 |
| Denmark | Byg 2003(53) | 7.2 | |
| Finland | Pietinalho 1995(4) | 28 | 11.4 |
| France | Duchemann 2017(54) | 30 | 4.9 |
| France (Guadeloupe, an overseas region in the Caribbean) | Coquart 2015(55) | 21 | 2.3 |
| Greece | Karakatsani 2009(56) | 5.9 | 1.07 |
| Ireland | Nicholson 2010(57) | 28 | |
| Israel | Yigla 2006(58) | 2 | |
| Italy | Beghe 2017(59) | 49 | |
| Japan | Pietinalho 1995(4) | 3.7 | 1 |
| Poland | Kowalska 2014(36) | 6.5 | 5.9 |
| Saudi Arabia | Al-Khouzaie 2011(60) | 13 | |
| Serbia | Pesut 2005(61) | 1.9 | |
| South Korea | Yoon 2018(1) | 4.69 | 0.48 |
| Spain | Fite 1996(62) | 1.4 | |
| Sweden | Arkema 2016(5) | 160 | 11.5 |
| Switzerland | Deubelbeiss 2010(35) | 121 | 7 |
| Taiwan | Wu 2017(3) | 2.2 | |
| Turkey | Musellim 2009(63) | 4 | |
| United Kingdom (N. Ireland only) | Nicholson 2010(57) | 11 | |
| United Kingdom | Gribbin 2006(64) | 5 | |
| United States | Baughman 2016(8) | 60 | 8.3 |
It is often stated that the incidence is highest in Scandinavian countries, but it should be noted that there have been no contemporary estimates from any Scandinavian countries other than Sweden in the last 20 years (5). Using Sweden’s nationwide register data, the incidence of sarcoidosis was 11.5 per 100,000 (5), which is slightly higher than estimates from the U.S. (about 8-11 per 100,000 (8-10)) and Canada (6.8 per 100,000 per year (6)). Estimates from East Asia are consistently lower, with the most recent data from South Korea (incidence was 0.5-1.3 per 100,000, depending on definition and study (1, 2)) (Figure).
Comparisons across countries can be useful to generate hypotheses but should be made with caution. The data sources and methods of analysis can lead to an over- or underestimation of incidence and prevalence and decisions made by investigators can affect results (Table 2). Two studies from Korea using similar data sources reported different estimates of prevalence and incidence, likely due to differences in how sarcoidosis was defined (1, 2). There are variations between countries in thresholds for hospital admission, guidelines for diagnosis, and the occurrence of other competing diseases such as tuberculosis (TB). Differences in the distribution of age and sex between source populations may make comparisons difficult. There is not only variation between countries in these factors, but also within countries. For example, in Sweden, the regional variation in the prevalence of sarcoidosis partially reflects differences in diagnosis and treatment patterns as well as demographics across healthcare regions (11). In the Stockholm region, patients were more likely to be younger, female and have higher education compared to the other regions. In some studies, an observed increase in newly diagnosed cases may be explained by the increase in availability of better imaging, as seen in cardiac sarcoidosis (12).
Table 2.
Different dataset characteristics or analysis methods and their effect on estimating incidence and prevalence.
| Measure | Characteristic of dataset and/or analysis method | Effect on estimate |
|---|---|---|
| Incidence | No washout period to exclude prevalent cases | Overestimation |
| Prevalence | Few years of coverage | Underestimation |
| Many years of coverage | Overestimation | |
| Incidence and Prevalence | Hospitalization data only | Underestimation |
| Not standardized by age, sex | Underestimation or overestimation |
Distribution of sarcoidosis phenotypes
A limitation of many administrative database studies is the lack of information on sarcoidosis phenotype, which is an important factor related to the incidence and prevalence. Some studies suggest that the prevalence of different clinical phenotypes vary by region, age and sex (13, 14). If there is a relationship between a risk factor and a specific sarcoidosis phenotype, and the phenotype is not examined separately, investigators will not detect the association. Löfgren syndrome is a distinct disease subset, with specific genetic markers and a different prognosis compared to non-Löfgren (15), however few studies of incidence and prevalence report it separately. There are other ways to define sarcoidosis phenotypes, such as by pulmonary and extra-pulmonary or by specific organ manifestations. In a study from the GenPhenReSa (Genotype-Pheotype Relationship in Sarcoidosis) project, investigators combined data from 31 study centers in Europe and used data-driven methods to identify five distinct subgroups based on organ involvement (16). These new clinical phenotypes can be used to stratify patients into more homogenous groups (17). This is an excellent example of a large collaboration using the power of big data. Collaborations require standardized or “harmonized” data, not only on sarcoidosis characteristics but also on outcomes. A “standard set” of outcomes has been developed and tested to see whether it could be used across centers (18). The investigators found that it was feasible to collect data on mortality, pulmonary function, weight/body mass index (BMI) and clinical outcome status but patient-reported values such as quality of life were not yet feasible to obtain.
The role of race in the incidence and prevalence of sarcoidosis
Race is an important factor when considering the rate of sarcoidosis, since the diversity of the population sample may lead to different incidence and prevalence estimates. The highest incidence and prevalence of sarcoidosis in the U.S. is consistently observed in non-Hispanic Blacks (Blacks), with Black American women having the highest rates (19). The age-adjusted sarcoidosis-specific mortality rate is higher for Black Americans compared to non-Hispanic White Americans, indicating a worse disease prognosis (20, 21). Race is often used as a proxy for genetics, but it does not capture the full complexity of a person’s genetic risk including interaction with social and environmental determinants. Furthermore, other factors are related to race which can be associated with an increased burden of disease. For example, systemic and structural racism in the U.S. influences Blacks’ environmental exposures through housing and occupation (22, 23), and acts as a barrier to timely diagnosis and treatment, affecting health (24). To better understand the role of race in sarcoidosis diagnosis and prognosis, information is needed on how long patients had symptoms before they received their diagnosis and whether this differs by race. Routine incorporation of measures of the social determinants of health (e.g., experiences of racism/discrimination, socioeconomic status, barriers/facilitators to care) into clinical records would also be an important step towards this goal.
Variation in sarcoidosis onset across the sexes
Some studies report that sarcoidosis is more common in women than in men, and others observe no difference between the sexes (13). Small clinical samples of highly selected prevalent cases (who are more likely to have chronic disease and need specialized care) are not representative of the larger sarcoidosis population. For example, if the sample is older there will be more women because women tend to be diagnosed with sarcoidosis at a later age. The later age at diagnosis in women compared to men may be related to the decreased lung function associated with menopause (25). The relationship between sex hormones and sarcoidosis risk has only been investigated in one study in women (26). Researchers used indicators for estrogen exposure (older age at menopause, age at first and last birth) and found a protective effect of estrogen on sarcoidosis risk. In a recent cross-sectional study of 13 men, testosterone levels did not differ between patients with sarcoidosis and controls, but higher testosterone levels were correlated with a decreased forced vital capacity (27). Larger samples are needed to examine the role between sex hormones both in women and men and sarcoidosis onset and severity.
The latest studies on risk factors for sarcoidosis
Genetic predisposition has been investigated in several studies of candidate genes, genome wide associations (GWA), whole exome sequencing and familial aggregation (28). Most familial aggregation studies have relied on questionnaires to determine family history of sarcoidosis which can be misclassified (Table 3). A study from Sweden did not rely on self-reported family history, but instead linked relatives to healthcare visits for sarcoidosis (29). There was a 3.7-fold increased risk associated with having at least one first degree relative with sarcoidosis and the heritability was 39% (29). GWA studies have identified susceptibility genes but a large proportion of the heritability remains unexplained (30). A large, ethnically diverse GWA study of ocular sarcoidosis found that the association with HLA-DRB1*04:01 was ancestry-specific and observed a novel association with MAGI1 (31). This highlights the importance of investigating the genetic determinants of disease heterogeneity in diverse patient samples.
Table 3.
Risk factors for sarcoidosis reported in the past two years and examples of biases potentially affecting their investigation.
| Risk factor | Bias |
|---|---|
| Family history |
|
| Infection |
|
| Rural exposures |
|
| Occupational exposures |
|
Infection has long been hypothesized as a risk factor for sarcoidosis due to the histological similarities between sarcoidosis and granulomatous infectious diseases such as TB. One study using ICD codes from the Taiwan National Health Insurance Database observed an 8-fold increased risk of sarcoidosis associated with a history of TB. The risk of TB was also increased after sarcoidosis diagnosis (hazard ratio 1.85). Due to the reliance on ICD codes in this study, it cannot be ruled out that TB or sarcoidosis was misclassified. In a case-control study nested within Swedish registers (also using ICD codes), there was only a slightly increased sarcoidosis risk (odds ratio 1.19; 95%CI 1.09, 1.29) associated with a history of infection (32). In sensitivity analyses, the association could be completely explained by not-yet-diagnosed preclinical sarcoidosis (i.e. reverse causation, Table 3). We do not know how long before diagnosis the biological mechanisms underlying sarcoidosis pathogenesis began. Epidemiological studies often use the date of diagnosis as a proxy for disease onset, but this could be months to years after true onset. It is critical that the temporality of exposure and disease is established when making causal claims. If it is not possible to determine whether exposure occurred before disease onset, quantitative bias analyses can be used to communicate the uncertainty in estimates (33).
Environmental risk factors for sarcoidosis proposed in the literature are intriguing but replications of findings are lacking. The hypothesis that a rural exposure increases sarcoidosis risk is based on observations of associations with agricultural employment and lower population density (34). Some studies suggest that exposure to agriculture and metallurgy, which are located in rural areas, are linked to sarcoidosis however these studies assessed regional rather than individual-level exposures (35, 36). In a retrospective case-control study from Alberta, Canada, having lived on a farm was not associated with sarcoidosis (37). However, there was an association with un-piped water compared to piped and farm milk compared to store bought milk. This study attempted to minimize selection bias by including controls with other chronic respiratory diseases identified from the same internal medicine and pulmonary clinics as the cases. The choice of these controls could be problematic, though, if the exposure is also associated with other chronic respiratory diseases.
Finding a suitable comparison group can be challenging, especially when studying occupational exposures. A recent review calculated the proportion of sarcoidosis cases which were attributable to occupation and reported a pooled estimate from seven studies of 30% (the original studies ranged from 0% to 54%) (38). An understanding of the exposure in the source population, not only the sarcoidosis cases, is needed to make causal inferences. Previous occupational studies investigated exposed groups who experienced more screening (i.e. chest x-rays) compared to unexposed, which causes the rate to be overestimated in the exposed (detection bias). A recent study reported that there was no association between military deployment and sarcoidosis, which is in contrast to previous studies from the U.S. military (39). One explanation for this discrepancy is that chest x-ray is no longer a screening requirement for military personnel, thus including fewer asymptomatic cases (39). Another occupational exposure of interest is crystalline silica dust, which is a well-known cause of lung disease and associated with autoimmune diseases (40). A study of construction workers observed an association between silica dust and sarcoidosis, but only among ever-smokers (41). Misclassification of silicosis as sarcoidosis cannot be ruled out, as the sarcoidosis cases were identified through ICD-coded inpatient hospitalizations and not validated.
Occupation is also related to neighborhood socioeconomic position, which is associated with sarcoidosis severity (42). In a study from Sweden, high neighborhood deprivation (an index created using information on income, education, unemployment and social welfare assistance) was associated with a 20% increased odds of sarcoidosis (43). There are several factors that comprise neighborhood deprivation, including psychological stress, decreased use of preventative healthcare, decreased exercise, and increased obesity. Obesity is an important factor, which has traditionally been viewed as a result of systemic corticosteroid therapy to treat sarcoidosis. Yet, several recent studies, mostly in women (44-47), have shown 50-250% increased associations between obesity (BMI≥30 kg/m2) and incident sarcoidosis. In a large study of 75,000 women (140 cases of sarcoidosis) in the Danish National Birth Cohort from 1996 to 2005, self-reported, pre-pregnancy obesity (BMI ≥30 kg/m2) was positively associated with sarcoidosis [hazard ratio: 3.59; 95% confidence interval (CI), 2.31–5.57] compared to low/normal BMI (18–25 kg/m2) (44). The Black Women’s Health Study (BWHS), a prospective follow-up of 59 000 US black women also found an association among 454 cases reported over a 16-year follow-up period (1995–2011). Obesity at baseline (1995) was associated with a 42% increased incidence of sarcoidosis (incidence rate ratio (IRR): 1.42; 95% CI, 1.07–1.89). Obesity at age 18 was similarly associated but did not reach statistical significance (IRR 1.40; 95% CI, 0.88–2.25) (45). An analysis of the Nurses’ Health Study II cohort of over 116 000 US (>98% white), female registered nurses yielded consistent findings. Both BMI at age 18 and current obesity (reported in the 2-year period prior to diagnosis) were respectively associated with a 50% (HR 1.50; 95% CI, 0.80–2.79) and 74% (HR 1.74; 95% CI, 1.26–2.40) increased incidence of sarcoidosis (272 cases) over a 24-year period (1989-2013) (46). Finally, a case–control study conducted among residents of Olmsted County, MN, USA (>90% white) analyzed 345 cases of sarcoidosis and their age- and sex-matched controls reported between 1976 and 2013. The authors reported an odds ratio of 2.54 (95% CI, 1.58–4.06) among obese compared to normal/low BMI patients (47). Taken together, these studies support the possible role of obesity as a risk factor for sarcoidosis. While the exact mechanism is unclear, there is evidence that immune cells in the lungs of obese individuals convert toward a pro-inflammatory phenotype, likely in response to metabolic and immune changes in adipose tissue (48). Future studies should focus on obesity in men, distribution of body fat (anthropometry), and on the distribution of phenotypes according to BMI. Such studies may further elucidate disease mechanism(s) and provide insight into possible treatment pathways and prevention strategies.
Conclusion
Recent findings from incidence, prevalence and risk factor studies have provided new insights into the distribution of sarcoidosis across populations. This heterogeneous and difficult-to-diagnose disease poses many challenges to epidemiological research. Large administrative datasets are useful for a big picture view and smaller clinical registries offer more detailed information but limited power. Ideally, we would have the best of both worlds in the same investigation – data on many patients with a lot of clinical information at baseline and over time, collected in a systematic way, and representative of the patient population. Successful collaborations will aim for this goal through the harmonization of data collection and pooling data carefully. The identification of risk factors for different clinical phenotypes will advance our understanding of the causes of sarcoidosis heterogeneity. Misclassification, selection bias and confounding are often unavoidable and make it challenging to study risk factors for sarcoidosis. Bias quantification can be used to evaluate the effect of these biases on results and help with the interpretation results in the presence of bias.
Key points.
The incidence and prevalence of sarcoidosis varies greatly around the world, and some of the variation may be due to differences between data sources leading to under- or overestimations.
Recently published studies provide new insights on the role of genetic and non-genetic risk factors for sarcoidosis.
Sarcoidosis phenotypes are likely caused by different biological mechanisms, therefore evaluations of risk factors should study phenotypes as separate outcomes.
Misclassification, selection bias and confounding affect epidemiological studies of sarcoidosis risk factors, and the effect of these biases should be carefully evaluated.
The success of future sarcoidosis research depends on the collaboration between researchers, which necessitates collecting high quality data in a systematic way.
Acknowledgements
We would like to thank Marios Rossides for his assistance with making the Figure.
Financial support and sponsorship
EVA is supported by SFO Epidemiology at Karolinska Institutet, The Swedish Research Council (Vetenskapsrådet 2017-01548) and The Swedish Heart-Lung Foundation (Hjärt-Lungfonden 20170412). YCC is supported by the National Cancer Institute (Grant No. U01-CA164974), and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (Grant No. R01AR057327).
Footnotes
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1.Yoon H-Y, Kim HM, Kim Y-J, et al. Prevalence and incidence of sarcoidosis in Korea: a nationwide population-based study. Respiratory Research. 2018;19(1):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park JE, Kim YS, Kang MJ, et al. Prevalence, incidence, and mortality of sarcoidosis in Korea, 2003-2015: A nationwide population-based study. Respir Med. 2018;144S:S28–S34. [DOI] [PubMed] [Google Scholar]
- 3.Wu CH, Chung PI, Wu CY, et al. Comorbid autoimmune diseases in patients with sarcoidosis: A nationwide case-control study in Taiwan. Journal of Dermatology. 2017;44(4):423–30. [DOI] [PubMed] [Google Scholar]
- 4.Pietinalho A, Hiraga Y, Hosoda Y, et al. The frequency of sarcoidosis in Finland and Hokkaido, Japan. A comparative epidemiological study. Sarcoidosis. 1995;12(1):61–7. [PubMed] [Google Scholar]
- 5.Arkema EV, Grunewald J, Kullberg S, et al. Sarcoidosis incidence and prevalence: a nationwide register-based assessment in Sweden. European Respiratory Journal. 2016;48(6):1690–9. [DOI] [PubMed] [Google Scholar]
- *6.Fidler LM, Balter M, Fisher JH, et al. Epidemiology and health outcomes of sarcoidosis in a universal healthcare population: a cohort study. Eur Respir J. 2019;54(4).The first study to report the incidence and prevalence of sarcoidosis from a province in Canada
- 7.Bogdan M, Nitsch-Osuch A, Kanecki K, et al. Sarcoidosis among hospitalized patients in Poland: a study based on a national hospital registry. Pol Arch Intern Med. 2019;129(9):580–5. [DOI] [PubMed] [Google Scholar]
- 8.Baughman RP, Field S, Costabel U, et al. Sarcoidosis in America. Analysis Based on Health Care Use. Annals of the American Thoracic Society. 2016;13(8):1244–52. [DOI] [PubMed] [Google Scholar]
- 9.Ungprasert P, Carmona EM, Utz JP, et al. Epidemiology of Sarcoidosis 1946-2013: A Population-Based Study. Mayo Clin Proc. 2016;91(2):183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dumas O, Abramovitz L, Wiley AS, et al. Epidemiology of Sarcoidosis in a Prospective Cohort Study of U.S. Women. Ann Am Thorac Soc. 2016;13(1):67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rossides M, Kullberg S, Eklund A, et al. Sarcoidosis diagnosis and treatment in Sweden: A register-based assessment of variations by region and calendar period. Respir Med. 2020;161:105846. [DOI] [PubMed] [Google Scholar]
- 12.Ekstrom K, Lehtonen J, Nordenswan HK, et al. Sudden death in cardiac sarcoidosis: an analysis of nationwide clinical and cause-of-death registries. Eur Heart J. 2019;40(37):3121–8. [DOI] [PubMed] [Google Scholar]
- *13.Brito-Zeron P, Kostov B, Superville D, et al. Geoepidemiological big data approach to sarcoidosis: geographical and ethnic determinants. Clin Exp Rheumatol. 2019;37(6):1052–64.A thorough review of the variation in sarcoidosis clinical characteristics from cohorts around the world
- 14.Hattori T, Konno S, Shijubo N, et al. Nationwide survey on the organ-specific prevalence and its interaction with sarcoidosis in Japan. Sci Rep. 2018;8(1):9440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubio-Rivas M, Franco J, Corbella X. Sarcoidosis presenting with and without Lofgren's syndrome: Clinical, radiological and behavioral differences observed in a group of 691patients. Joint Bone Spine. 2020;87(2):141–7. [DOI] [PubMed] [Google Scholar]
- **16.Schupp JC, Freitag-Wolf S, Bargagli E, et al. Phenotypes of organ involvement in sarcoidosis. Eur Respir J. 2018;51(1).A large multicenter study using data-driven methods to identify sarcoidosis phenotypes.
- 17.Bennett D, Cameli P, Lanzarone N, et al. Chitotriosidase: a biomarker of activity and severity in patients with sarcoidosis. Respir Res. 2020;21(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kampstra NA, van der Nat PB, Dijksman LM, et al. Results of the standard set for pulmonary sarcoidosis: feasibility and multicentre outcomes. ERJ Open Res. 2019;5(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wills AB, Adjemian J, Fontana JR, et al. Sarcoidosis-associated Hospitalizations in the United States, 2002 to 2012. Ann Am Thorac Soc. 2018;15(12):1490–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kearney GD, Obi ON, Maddipati V, et al. Sarcoidosis deaths in the United States: 1999-2016. Respir Med. 2019;149:30–5. [DOI] [PubMed] [Google Scholar]
- 21.Ogundipe F, Mehari A, Gillum R. Disparities in Sarcoidosis Mortality by Region, Urbanization, and Race in the United States: A Multiple Cause of Death Analysis. Am J Med. 2019;132(9):1062–8 e3. [DOI] [PubMed] [Google Scholar]
- 22.Williams DR. Race, socioeconomic status, and health. The added effects of racism and discrimination. Ann N Y Acad Sci. 1999;896:173–88. [DOI] [PubMed] [Google Scholar]
- 23.Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep. 2001;116(5):404–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelson A. Unequal treatment: confronting racial and ethnic disparities in health care. J Natl Med Assoc. 2002;94(8):666–8. [PMC free article] [PubMed] [Google Scholar]
- 25.Triebner K, Matulonga B, Johannessen A, et al. Menopause Is Associated with Accelerated Lung Function Decline. American Journal of Respiratory and Critical Care Medicine. 2017;195(8):1058–65. [DOI] [PubMed] [Google Scholar]
- 26.Cozier YC, Berman JS, Palmer JR, et al. Reproductive and hormonal factors in relation to incidence of sarcoidosis in US Black women: The Black Women's Health Study. Am J Epidemiol. 2012;176(7):635–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Manen MJG, Wester VL, van Rossum EFC, et al. Scalp hair cortisol and testosterone levels in patients with sarcoidosis. PLoS One. 2019;14(6):e0215763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spagnolo P. Chapter 4 - Genetics of Sarcoidosis In: Baughman RP, Valeyre D, editors. Sarcoidosis. Philadelphia: Elsevier; 2019. p. 55–66. [Google Scholar]
- 29.Rossides M, Grunewald J, Eklund A, et al. Familial aggregation and heritability of sarcoidosis: a Swedish nested case-control study. Eur Respir J. 2018;52(2). [DOI] [PubMed] [Google Scholar]
- 30.Manolio TA, Collins FS, Cox NJ, et al. Finding the missing heritability of complex diseases. Nature. 2009;461(7265):747–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *31.Garman L, Pezant N, Pastori A, et al. Genome-Wide Association Study of Ocular Sarcoidosis Confirms HLA Associations and Implicates Barrier Function and Autoimmunity in African Americans. Ocul Immunol Inflamm. 2020:1–6.This genome-wide association study of ocular sarcoidosis shows the importance of examining sarcoidosis phenotypes separately and the need for including diverse populations in resesarch
- **32.Rossides M, Kullberg S, Askling J, et al. Are infectious diseases risk factors for sarcoidosis or a result of reverse causation? Findings from a population-based nested case-control study. Eur J Epidemiol. 2020.This was the first study to examine infection diagnosed years before sarcoidosis using a large study population with prospectively collected data. They did not confirm a strong assocaition between infection and sarcoidosis but suggest that reverse causation inflates the association.
- 33.Lash TL, Fox MP, MacLehose RF, et al. Good practices for quantitative bias analysis. Int J Epidemiol. 2014;43(6):1969–85. [DOI] [PubMed] [Google Scholar]
- 34.Ramos-Casals M, Kostov B, Brito-Zeron P, et al. How the Frequency and Phenotype of Sarcoidosis is Driven by Environmental Determinants. Lung. 2019;197(4):427–36. [DOI] [PubMed] [Google Scholar]
- 35.Deubelbeiss U, Gemperli A, Schindler C, et al. Prevalence of sarcoidosis in Switzerland is associated with environmental factors. Eur Respir J. 2010;35(5):1088–97. [DOI] [PubMed] [Google Scholar]
- 36.Kowalska M, Niewiadomska E, Zejda JE. Epidemiology of sarcoidosis recorded in 2006-2010 in the Silesian voivodeship on the basis of routine medical reporting. Ann Agric Environ Med. 2014;21(1):55–8. [PubMed] [Google Scholar]
- 37.Schouten J, Beach J, Burstyn I, et al. Is Farm Milk a Risk Factor for Sarcoidosis? The Role of Farm Residence, Unpiped Water and Untreated Milk in Sarcoidosis: A Case-Referent Study in Alberta, Canada. Int J Environ Res Public Health. 2018;15(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blanc PD, Annesi-Maesano I, Balmes JR, et al. The Occupational Burden of Nonmalignant Respiratory Diseases. An Official American Thoracic Society and European Respiratory Society Statement. Am J Respir Crit Care Med. 2019;199(11):1312–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forbes DA, Anderson JT, Hamilton JA, et al. Relationship to Deployment on Sarcoidosis Staging and Severity in Military Personnel. Mil Med. 2020. [DOI] [PubMed] [Google Scholar]
- 40.Hoy RF, Chambers DC. Silica-related diseases in the modern world. Allergy. 2020. [DOI] [PubMed] [Google Scholar]
- 41.Jonsson E, Jarvholm B, Andersson M. Silica dust and sarcoidosis in Swedish construction workers. Occup Med (Lond). 2019;69(7):482–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harper LJ, Gerke AK, Wang XF, et al. Income and Other Contributors to Poor Outcomes in U.S. Patients with Sarcoidosis. Am J Respir Crit Care Med. 2020;201(8):955–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li X, Sundquist J, Hamano T, et al. Neighborhood Deprivation and Risks of Autoimmune Disorders: A National Cohort Study in Sweden. Int J Environ Res Public Health. 2019;16(20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harpsoe MC, Basit S, Andersson M, et al. Body mass index and risk of autoimmune diseases: a study within the Danish National Birth Cohort. Int J Epidemiol. 2014;43(3):843–55. [DOI] [PubMed] [Google Scholar]
- 45.Cozier YC, Coogan PF, Govender P, et al. Obesity and weight gain in relation to incidence of sarcoidosis in US black women: data from the Black Women's Health Study. Chest. 2015;147(4):1086–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dumas O, Boggs KM, Cozier YC, et al. Prospective study of body mass index and risk of sarcoidosis in US women. Eur Respir J. 2017;50(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ungprasert P, Crowson CS, Matteson EL. Smoking, obesity and risk of sarcoidosis: A population-based nested case-control study. Respir Med. 2016;120:87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lugogo NL, Hollingsworth JW, Howell DL, et al. Alveolar macrophages from overweight/obese subjects with asthma demonstrate a proinflammatory phenotype. Am J Respir Crit Care Med. 2012;186(5):404–11. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Gillman A, Steinfort C. Sarcoidosis in Australia. Intern Med J. 2007;37(6):356–9. [DOI] [PubMed] [Google Scholar]
- 50.Thomeer M, Demedts M, Vandeurzen K, et al. Registration of interstitial lung diseases by 20 centres of respiratory medicine in Flanders. Acta Clin Belg. 2001;56(3):163–72. [DOI] [PubMed] [Google Scholar]
- 51.Mise K, Jurcev-Savicevic A, Goic-Barisic I, et al. Sarcoidosis and tuberculosis in South Croatia: are there epidemiological similarities or not? Public Health. 2011;125(10):734–7. [DOI] [PubMed] [Google Scholar]
- 52.Kolek V. Epidemiological study on sarcoidosis in Moravia and Silesia. Sarcoidosis. 1994;11(2):110–2. [PubMed] [Google Scholar]
- 53.Byg KE, Milman N, Hansen S. Sarcoidosis in Denmark 1980-1994. A registry-based incidence study comprising 5536 patients. Sarcoidosis Vasc Diffuse Lung Dis. 2003;20(1):46–52. [PubMed] [Google Scholar]
- 54.Duchemann B, Annesi-Maesano I, Jacobe de Naurois C, et al. Prevalence and incidence of interstitial lung diseases in a multi-ethnic county of Greater Paris. Eur Respir J. 2017;50(2). [DOI] [PubMed] [Google Scholar]
- 55.Coquart N, Cadelis G, Tressieres B, et al. Epidemiology of sarcoidosis in Afro-Caribbean people: a 7-year retrospective study in Guadeloupe. Int J Dermatol. 2015;54(2):188–92. [DOI] [PubMed] [Google Scholar]
- 56.Karakatsani A, Papakosta D, Rapti A, et al. Epidemiology of interstitial lung diseases in Greece. Respir Med. 2009;103(8):1122–9. [DOI] [PubMed] [Google Scholar]
- 57.Nicholson TT, Plant BJ, Henry MT, et al. Sarcoidosis in Ireland: regional differences in prevalence and mortality from 1996-2005. Sarcoidosis Vasc Diffuse Lung Dis. 2010;27(2):111–20. [PubMed] [Google Scholar]
- 58.Yigla M, Badarna-Abu-Ria N, Tov N, et al. Sarcoidosis in northern Israel; clinical characteristics of 120 patients. Sarcoidosis Vasc Diffuse Lung Dis. 2002;19(3):220–6. [PubMed] [Google Scholar]
- 59.Beghe D, Dall'Asta L, Garavelli C, et al. Sarcoidosis in an Italian province. Prevalence and environmental risk factors. PLoS One. 2017;12(5):e0176859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Al-Khouzaie TH, Al-Tawfiq JA, Al Subhi FM. Sarcoidosis in the eastern region of Saudi Arabia. Ann Thorac Med. 2011;6(1):22–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pesut D. [The role of central registry in control and research of sarcoidosls in Serbia and Montenegro]. Med Pregl. 2005;58 Suppl 1:7–10. [PubMed] [Google Scholar]
- 62.Fite E, Alsina JM, Anto JM, et al. Sarcoidosis: family contact study. Respiration. 1998;65(1):34–9. [DOI] [PubMed] [Google Scholar]
- 63.Musellim B, Kumbasar OO, Ongen G, et al. Epidemiological features of Turkish patients with sarcoidosis. Respir Med. 2009;103(6):907–12. [DOI] [PubMed] [Google Scholar]
- 64.Gribbin J, Hubbard RB, Le Jeune I, et al. Incidence and mortality of idiopathic pulmonary fibrosis and sarcoidosis in the UK. Thorax. 2006;61(11):980–5. [DOI] [PMC free article] [PubMed] [Google Scholar]