Abstract
Background & Aims:
We evaluated the efficacy and safety of diet-modulated autologous fecal microbiota transplantation (aFMT) for treatment of weight regain after the weight loss phase.
Methods:
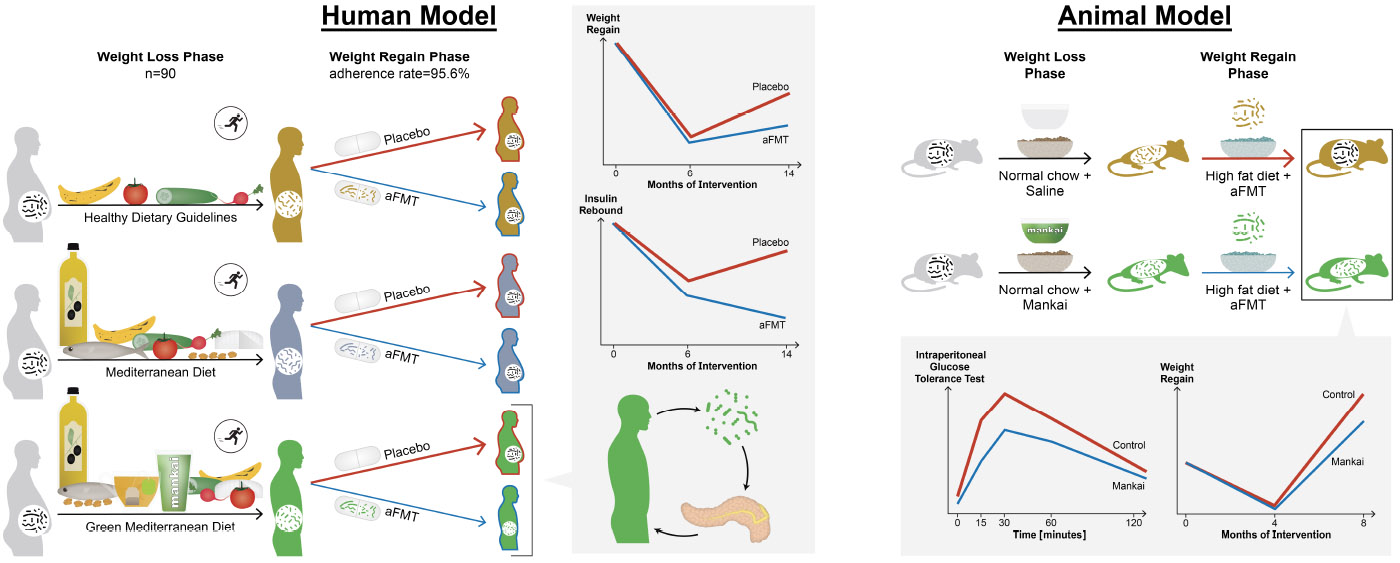
In the DIRECT-PLUS weight loss trial (May 2017 through July 2018), abdominally obese or dyslipidemic participants in Israel were randomly assigned to (1)healthy dietary guidelines, (2)Mediterranean diet, and (3)green-Mediterranean diet weight-loss groups. All groups received free gym membership and physical activity guidelines. Both iso-caloric Mediterranean groups consumed 28g/day walnuts (+440mg/d polyphenols provided). The green-Mediterranean dieters further consumed green tea (3-4 cups/day) and a Wolffia-globosa (Mankai strain;100g/day) green shake (+800mg/day polyphenols provided). After 6 months (weight-loss phase), 90 eligible participants (mean age, 52 years; mean weight loss, 8.3 kg) provided a fecal sample that was processed into aFMT by frozen, opaque and odorless capsules. The participants were then randomly assigned to groups that received 100 capsules containing their own fecal microbiota or placebo until month 14. The primary outcome was regain of the lost weight over the expected weight regain phase (months 6–14). Secondary outcomes were gastrointestinal symptoms, waist-circumference, glycemic status and changes in the gut microbiome, as measured by metagenomic sequencing and 16s-rRNA. We validated the results in a parallel in-vivo study of mice specifically fed with Mankai, as compared to control chow diet.
Results:
Of the 90 participants in the aFMT trial, 96% ingested at least 80 of 100 oral aFMT or placebo frozen capsules over the transplantation period. No aFMT-related adverse events or symptoms were observed. For the primary outcome, although no significant differences in weight regain were observed among the participants in the different lifestyle interventions during months 6–14 (aFMT, 30.4% vs. placebo, 40.6%;P=.28), aFMT significantly attenuated weight regain in the green-Mediterranean group (aFMT, 17.1%, vs placebo, 50%; P=.02), but not in the dietary guidelines (P=.57) or Mediterranean diet (P=.64) groups (P for the interaction=.03). Accordingly, aFMT attenuated waist circumference gain (aFMT, 1.89cm vs placebo, 5.05cm;P=.01) and insulin rebound (aFMT, 1.46±3.6μIU/ml vs placebo, 1.64±4.7μIU/ml;P=.04) in the green Mediterranean group but not in the dietary guidelines or Mediterranean diet (P for the interaction=.04 and .03, respectively). The green-Mediterranean diet was the only intervention to induce a significant change in microbiome composition during the weight loss phase, and to prompt preservation of weight loss-associated specific bacteria and microbial metabolic pathways (mainly microbial sugar transport) following the aFMT. In mice, Mankai-modulated aFMT in the weight loss phase, compared with control diet aFMT, significantly prevented weight regain, and resulted in better glucose tolerance, during a high-fat-diet induced regain phase (P<.05 for all).
Conclusions:
Autologous FMT, collected during the weight loss phase and administrated in the regain phase, might preserve weight loss and glycemic control and is associated with specific microbiome signatures. High-polyphenols, green plant-based or Mankai diet better optimizes the microbiome for an aFMT procedure. (ClinicalTrials.gov number, NCT03020186)
Keywords: Autologous FMT, obesity, weight regain after diet, diabetes
Graphical Abstract
Lay Summary:
This study found that participants who lost weight on a healthy diet and were then fed capsules containing fecal material collected during the diet period for months after the maximal weight loss, regained less weight than participants given placebo tablets, by modulating the intestinal microbiota. A plant-based diet (in participants) or Mankai diet (in mice) produced the optimal fecal microbiome for preventing weight regain and for retaining glycemic control.
INTRODUCTION
Weight regain and rebound of cardiometabolic risk factors following initial rapid weight-loss has long been a major challenge of durable dieting1, a phenomenon that was also observed in ours and others previous long-term weight loss trials.2-4 Several mechanisms may explain the regain in body weight and cardiometabolic risk5,6, with the gut microbiota potentially serving as a modifiable treatment target.7
Fecal microbiota transplantation (FMT), reconstitution of the gut microbiota by transplantation of stool from a healthy individual, offers a potent therapeutic approach in diseases mediated by gut dysbiosis, and is now considered standard of care in treatment of recurrent Clostridioides difficile infection.8-10 As previously reported by us, oral, capsulized, FMT as a route of administration, can offer a safe FMT procedure in the outpatient setting.11 The gut microbiota has repeatedly been associated with metabolic functions, and FMT from lean individuals mitigated obesity and detrimental metabolic traits in several animal experiments.7,12-17 Preliminary studies suggest that transfer of a ‘lean microbiome’ by FMT might modulate glycemic control, without inducing weight-loss in obese individuals.9,10,18 However, human studies are sparse, possibly due to safety concerns and practical barriers associated with FMT.19,20 Autologous FMT (aFMT) might serve as a viable alternative, as it was recently shown to improve post-antibiotic microbiome reconstitution in six individuals.21
Increased consumption of plants, along with reduced consumption of red and processed meat, were linked to lower risk of obesity, type 2 diabetes and all-cause mortality.22-24 These favorable effects of plant-based diets were previously attributed to their increased fraction of plant polyphenols and dietary fibers,25,26 with both components shown to have a prebiotic effect.27
The Dietary Intervention RandomizEd Controlled Trial PoLyphenols-UnproceSsed (DIRECT PLUS), aimed to examine whether the potential efficacy and safety of diet-modulated aFMT on weight regain attenuation is differently affected by distinct weight-loss interventions. We hypothesized that green-Mediterranean/high polyphenols diet, enriched with green-tea and Wolfia-globose (Mankai) green plant, could be potent in optimizing the microbiome as the platform of successful subsequent aFMT.
METHODS
Eligibility and recruitment
The DIRECT PLUS (ClinicalTrials.gov Identifier: NCT03020186) weight-loss trial was a two-phased randomized controlled trial involving overweight sedentary adults. The trial was conducted between May 2017 and July 2018 among employees of the nuclear research center in Dimona, Israel, an isolated facility with an on-site clinic and monitored provided lunch. Eligibility included age above 30 years, abdominal obesity [waist circumference: men>102 cm, women>88 cm] or dyslipidemia (TG>150 and HDL-c≤40 for men; ≤50 for women). Exclusion criteria are listed in Supplementary Appendix 1. The study was approved and monitored by the Soroka University Medical Center human subject committee. All participants provided written informed consent and received no financial compensation or gifts.
Randomization and study design
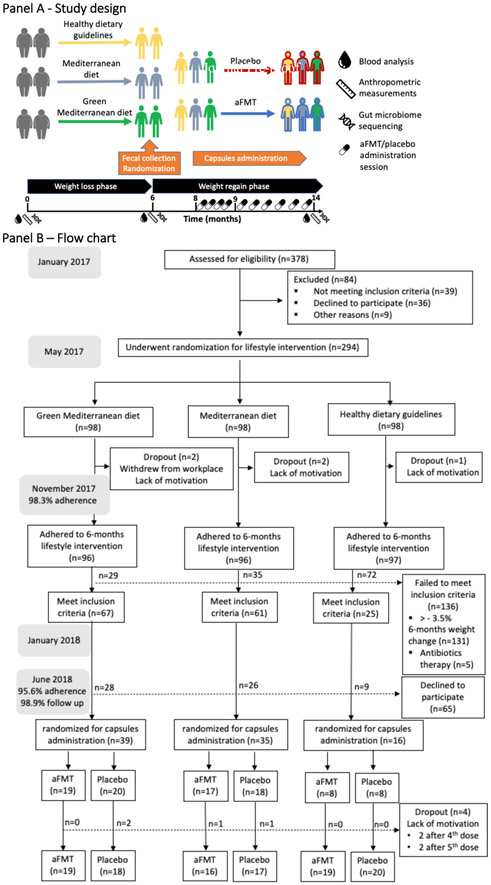
The trial included two phases: (1) a randomized, open-label, lifestyle intervention (2) a randomized, double-blind, aFMT augmentation. In the first phase, participants were randomly assigned in a 1:1:1 ratio to one of three lifestyle intervention groups: physical activity and healthy dietary guidelines; physical activity and Mediterranean diet or physical activity and green-Mediterranean diet, as detailed below. Following 6-months of lifestyle intervention, the expected weight nadir based on our previous dietary interventional trials2,3, participants who lost at least 3.5% body-weight were recruited to the double blind, placebo controlled, aFMT intervention as an augmentation to their assigned lifestyle intervention. The 3.5% cut-off was chosen by an estimation aimed to maximize statistical power, accounting for both expected sample size and weight difference, based on our previous CENTRAL trial (Supplementary Appendix 2).2 Participants prescribed antibiotic therapy 2 months prior to randomization were excluded. Eligible participants were asked to deliver a full fecal sample that was processed into aFMT capsules. The participants were simultaneously randomized, in a 1:1 ratio within sex and lifestyle intervention strata (Supplementary Appendix 3), to receive 10 grams of aFMT (consumed as ten 1 gram capsules) or identical placebo capsules delivered 10 times starting at 8-months following initiation of lifestyle intervention (2 months following fecal collection), over a 6-month period. Thus, a total of ~100g fecal matter was consumed between months 8 and 14, as detailed below.
The initial protocol timed the administration period to months 10-12. However, in an attempt to robustly, and more persistently, counteract weight regain, the protocol was amended prior to intervention initiation to a 6-month administration period (months 8-14).
Lifestyle intervention
All groups received free gym memberships and instructions to engage in moderate-intensity physical activity, ~80% of which was aerobic. The workout program included 45-60 minutes of aerobic training 3-4 times per week, and 2-3 sessions of resistance training per week.
Healthy dietary guidelines:
In addition to the workout program, participants received standard nutritional counseling to promote a healthy diet, and to achieve a similar intervention intensity (defined as the intensity of group and personal guidance during the trial) to that of the other two arms.
Mediterranean diet:
In addition to the workout program, participants were instructed to adopt a calorie-restricted Mediterranean diet as described in our previous trials2,3 supplemented with 28g/day of walnuts (containing 440 mg polyphenols) that was provided by the study team.
Green-Mediterranean diet:
In addition to the Mediterranean intervention (including the provided walnuts), the green-Mediterranean diet was designed to contain less red and processed meat compared to the Mediterranean diet, while being richer in plants and polyphenols. Participants were provided with the following items: 4 cups per day of green tea and 100g frozen cubes of Wolfia-globose duckweed (Mankai cultivated strain) aquatic plant28,29 consumed as a 500ml green shake. Thus, the green-Mediterranean diet contained an additional 800mg/day of polyphenols beyond that provided in the Mediterranean diet. Both Mediterranean and green-Mediterranean diets were equally calorie restricted (iso-caloric), containing 1500-1800 kcal for men and 1200-1400 kcal for women.
All lifestyle interventions included 90-minute nutritional and physical activity sessions in the workplace. Sessions were weekly during the first month, once a month during the subsequent 6 months, and every other month thereafter (lifestyle interventions are detailed in Supplementary Appendix 4). Adherence to the diet was assessed by monitoring attendance in the sessions and quantified by a self-administered validated electronic 127-item food-frequency questionnaire.30 Adherence to the exercise intervention was monitored during the group meetings and quantified using an electronic self-reported validated physical activity questionnaire.31 Physical activity intensity levels were subsequently measured using Metabolic Equivalent for Task (MET) units per week, where each unit is defined as the ratio of work metabolic rate to the standard resting metabolic rate, and MET levels can range from 0.9 (sleeping) up to 18 METs (fast running).32
aFMT capsule processing and administration
Full fecal samples were collected at 6-months at the study site, immediately frozen to −20°C for 1-3 days, and then transferred to −80°C pending processing at the Center for Microbiome Research at Shamir Medical Center. Following randomization, samples from participants allocated to the aFMT group were processed to aFMT capsules as previously described11 and as reported in Supplementary Appendix 5. Each batch was divided in 10 equal doses of 10 capsules, transferred to the study site and stored at −80°C pending administration. aFMT and placebo frozen capsules were opaque and odorless and identical in appearance. Placebo capsules consisted of agarose in normal saline/glycerol (the same vehicle as in aFMT capsules). The participants and investigators were blinded to the treatment group allocation. As the capsules were host-specific, a strict identification protocol was applied during each administration session. Administration sessions were held weekly for the first month, and every 3 weeks thereafter for a total of 10 sessions. Each individual administration was directly observed by an investigator.
Complementary mice model
We utilized an obese mouse model, comparable to the human aFMT trial. The model was achieved by a 4-week high-fat diet feeding of Swiss-Webster mice. The obese mice subsequently underwent a 4-week weight-loss phase, induced by normal-chow feeding, with an added daily Mankai gavage equivalent in quantity to the daily intake in the human trial (0.2gr/kg/day). Controls were fed with the same normal-chow diet, with the sole difference being daily saline gavage replacing the Mankai. Following the weight-loss phase, stool samples were collected and processed to aFMT inocula. Next, both Mankai and control groups losing at least 8% underwent a weight-regain phase, induced by a 4-week high-fat diet, with each mouse receiving bi-weekly aFMT from its post-weight-loss fecal sample. Mice body weight was measured along the study, and insulin sensitivity was measured following the weight regain by Intraperitoneal glucose tolerance test.
Blood, fecal and clinical measurements
Participants were weighed without shoes to the nearest 0.1 kg at baseline, 6 and 14-months. Waist circumference was measured halfway between the last rib and the iliac crest to the nearest millimeter at baseline, 6, and 14-months. An online symptoms questionnaire, based on Common Terminology Criteria for Adverse Events version 5.0, was used to assess possible adverse effects 24-h after intake of capsules, following the 1st, 4th, 6th and 8th administration session. Blood samples were obtained after a 12-hour fast at baseline, 6 and 14 months, centrifuged and stored at −80°C until assayed (Supplementary Appendix 6). Presence of type 2 diabetes mellitus was defined for participants with baseline fasting plasma glucose levels ≥126 mg/dL or hemoglobin A1c (HbA1c) levels ≥6.5% or if regularly treated with oral antihyperglycemic medications or exogenous insulin. Pre-diabetes was defined as fasting plasma glucose levels between 100 and 125 mg/dL or HbA1c levels in the range of 5.7-6.4%. Fecal samples were collected at baseline, 6 and 14 months at the study site, immediately frozen to −20°C for 1-3 days, then transferred to −80°C pending DNA extraction for shotgun metagenomic sequencing and 16s rRNA, when appropriate. To characterize the microbiome, fecal DNA was extracted, sequenced and normalized with an average depth of 15.4±2.6 million reads per sample (mean±standard deviation). DNA sequences were aligned using an accelerated version of the Needleman-Wunsch algorithm to a curated database containing all representative genomes in RefSeq v86. Each input sequence was assigned the lowest common ancestor that was consistent across at least 80% of all reference sequences tied for best hit. The number of counts for each taxon was then normalized to the average genome length. Species accounting for less than 1X10−3 of all species-level markers were discarded. Kyoto Encyclopedia of Genes and Genomes (KEGG) Orthology groups were observed directly using alignment against a gene database derived from the strain database used above. Fecal samples were further sequenced on a MiSeq platform following amplification of V3-V4 hypervariable region of the 16S rRNA gene using the primer set 341F/806R, and processed by the DADA2 pipeline (see Supplementary Appendix 7-8). Participants prescribed antibiotic therapy 2 months prior to the delivery of baseline fecal samples were excluded from all microbiome analyses. Fecal samples handling and sequencing for the mice microbiome analysis is described in supplementary Appendix 8.
All blood biochemical assays were performed at the University of Leipzig, Germany. Fecal metagenomic sequencing was performed at CoreBiome, Minnesota, USA. 16s rRNA sequencing was performed at Fondazione Edmund Mach, Italy. Laboratory personnel were blinded to the randomized lifestyle and aFMT interventions.
Statistical analysis
The primary outcome was weight regain, defined as percent weight change between 6 and 14 months from the initial 6-month weight loss. Secondary outcomes were gastrointestinal symptoms, waist circumference rebound, glycemic control and gut microbiome changes. All outcomes were assessed by aFMT treatment effect, and by the interaction with green-Mediterranean diet.
The intention-to-treat analyses included all 90 participants by imputing the missing follow-up data (n=1) of the primary outcome using the multiple imputation technique (Supplementary Appendix 9). Continuous variables are presented as means ± standard deviations, and categorical variables are presented as total count. Differences between time points are expressed as absolute values, unless specified otherwise. To detect differences between treatment groups, t-tests were used for parametric variables. Nonparametric variables and data determined to be nonnormal after log-transformation were analyzed using the Mann-Whitney test. A linear regression model with analysis of variance (ANOVA) was applied to assess the interaction between the green-Mediterranean diet and aFMT, and to assess the interaction between green-Mediterranean-specific components at time 6-months and aFMT. Intake of each component (i.e. Mankai and green tea) was defined as high or low compared to the overall median intake. Microbial composition similarity between time points in each group was compared using permutational multivariate analysis of variance (PERMANOVA) of weighted UniFrac distances. To evaluate changes in specific bacteria and KEGG module relative abundance, weight-associated bacteria/pathways were identified by comparing relative abundance change between the baseline and the 6-month time point, discarding bacteria/pathways with mean relative abundance under 1X10−3. Next, aFMT-affected bacteria/pathways were screened by identifying those who remained significantly changed after 14 months in the aFMT group, excluding bacteria/pathways that changed in the placebo group as well. To assess to what extent microbial features were preserved by aFMT within lifestyle intervention strata, the number of features observed in the microbiome analysis were compared to a permuted null model with 1000 iterations, shuffling sample labeling at each iteration. Comparisons were made using Wilcoxon rank-sum test, applying Benjamini-Hochberg false-discovery-rate corrections for multiple comparisons. All microbiome analyses were validated using centered-log ratio transformation, accounting for data compositionality. Differences were considered significant for p<0.05. Statistical analyses were performed using R software, version 3.5.3. All authors had access to the study data and reviewed and approved the final manuscript.
RESULTS
Enrollment, baseline characteristics and adherence
Following 6 months of dietary intervention, 155 of 294 (52.7%) DIRECT-PLUS participants met the inclusion criteria of 6-month weight-loss with no recent antibiotic therapy. Of these, 90 subjects who consented were randomly assigned to aFMT (n=44) or placebo (n=46) (Figure 1). No significant differences in weight, anthropometric or metabolic characteristics were observed at 6 months and in 0-6-month changes between the 90 enrolled subjects to the 65 who declined. Baseline and 6-month characteristics of the participants, across treatment and lifestyle intervention groups, are presented in Table 1. At baseline, mean age was 52 years and mean body mass index (BMI) was 31.3 kg/m2. Of the study population, 12.2% had type 2 diabetes and 36.7% had pre-diabetes. Ninety one percent of participants were male, representing the workplace profile.
Figure 1: Study design, enrollment of the participants and completion of the Study.
Panel A: Experimental design; Panel B: Trial flowchart.
Table 1-.
Baseline characteristics of the study population
| All subjects | Healthy
dietary guidelines |
Mediterranean diet | Green
Mediterranean diet |
|||||
|---|---|---|---|---|---|---|---|---|
| Treatment | aFMT | Placebo | aFMT | Placebo | aFMT | Placebo | aFMT | Placebo |
| Subjects - no. | 44 | 46 | 8 | 8 | 17 | 18 | 19 | 20 |
| Male sex - no. | 42 | 40 | 7 | 8 | 16 | 15 | 19 | 17 |
| Diabetes - no.(%) | 8 (18) | 3 (7) | 2 (25) | 0 (0) | 0 (0) | 1 (6) | 6 (2) | 2 (10) |
| Pre-diabetes - no.(%) | 15 (34) | 18 (39) | 4 (50) | 2 (25) | 5 (25) | 6 (33) | 6 (32) | 10 (50) |
| Age - yr (sd) | 53.14 (9.97) | 51.63 (11.65) | 52.43 (7.55) | 52.05 (12.17) | 54.49 (9.88) | 52.61 (10.75) | 52.24 (11.20) | 50.57 (12.71) |
| Baseline Body mass index (sd) | 30.89 (3.45) | 31.39 (4.09) | 30.57 (3.81) | 29.66 (2.11) | 30.93 (3.77) | 31.92 (5.28) | 30.97 (3.19) | 31.60 (3.40) |
| Baseline Waist circumference- cm (sd) | 108.91 (7.44) | 109.59 (10.55) | 108.25 (7.63) | 102.38 (5.37) | 109.18 (7.44) | 113.17 (13.72) | 108.95 (7.76) | 109.25 (7.12) |
| Baseline Weight-kg (sd) | 93.74 (14.12) | 92.20 (14.45) | 90.41 (15.44) | 83.22 (9.82) | 93.12 (13.58) | 96.24 (17.24) | 95.70 (14.50) | 92.16 (11.98) |
| Baseline fasting plasma glucose-mg/dl | 101.66 (19.22) | 100.16 (15.75) | 101.17 (16.19) | 95.78 (13.96) | 97.86 (7.02) | 99.25 (11.04) | 105.24 (26.52) | 102.73 (19.77) |
| Baseline serum triglycerides-mg/dL | 144.30 (57.82) | 131.56 (68.75) | 149.68 (58.79) | 191.38 (123.11) | 147.00 (62.76) | 129.39 (53.90) | 144.30 (57.82) | 131.56 (68.75) |
| Baseline serum HDL cholesterol-mg/dL | 44.61 (10.30) | 47.78 (13.02) | 41.94 (7.71) | 49.37 (15.32) | 42.65 (9.61) | 42.37 (8.67) | 44.61 (10.30) | 47.78 (13.02) |
| 6-months weight-loss - kg (sd) | −8.28 (5.16) | −8.25 (4.85) | −6.20 (3.06) | −4.65 (2.26) | −8.23 (4.01) | −9.38 (5.27) | −9.21 (6.54) | −8.68 (4.71) |
| 6-months characteristics | ||||||||
| Body mass index (sd) | 28.17 (2.95) | 28.60 (3.36) | 28.47 (3.56) | 28.03 (1.86) | 28.19 (3.35) | 28.84 (4.03) | 28.02 (2.42) | 28.62 (3.29) |
| Weight - kg (sd) | 85.46 (12.30) | 83.95 (11.74) | 84.21 (14.62) | 78.58 (8.25) | 84.89 (12.54) | 86.86 (12.87) | 86.49 (11.67) | 83.48 (11.52) |
| Waist circumference- cm (sd) | 99.30 (7.32) | 99.22 (8.02) | 98.38 (8.25) | 97.75 (6.80) | 99.76 (8.50) | 101.89 (6.94) | 99.26 (6.06) | 97.40 (9.02) |
| Fasting plasma glucose- mg/dl (sd) | 97.12 (14.78) | 94.71 (8.30) | 93.39 (19.90) | 92.17 (6.72) | 94.63 (7.18) | 94.17 (8.07) | 101.14 (17.26) | 96.22 (9.10) |
| Fasting plasma insulin- microU/ml (sd) | 10.17 (5.08) | 10.63 (5.12) | 9.38 (5.54) | 11.99 (5.36) | 9.18 (3.54) | 10.09 (3.50) | 11.54 (6.06) | 10.57 (6.28) |
| HOMA-IR (sd) | 2.56 (1.63) | 2.49 (1.22) | 2.37 (1.97) | 2.77 (1.33) | 2.15 (0.85) | 2.34 (0.82) | 3.06 (1.98) | 2.52 (1.50) |
Values are presented as means (standard deviation) for continues variables and total number (percent) for categorical variables. No significant differences were observed between placebo or aFMT group in the measured baseline characteristics, overall and across lifestyle interventions. HOMA-IR denotes homeostasis model assessment of insulin resistance
Following six months of lifestyle intervention mean weight-loss was −8.27±5Kg. No significant differences were observed between the randomized treatment groups in anthropometric or laboratory measures at six months, in total or within lifestyle intervention group strata. As previously reported28, the green-Mediterranean group was distinguished by decreased intake of red meat and poultry and increased intake of fish, green tea, and Wolfia-globose (Mankai), compared with the Mediterranean group. At 6-months, both Mediterranean diets reported lower carbohydrate and higher protein intake than the dietary guidelines group (P<.01 for all), with no significant difference in fat intake (P=.10). At the end of the trial, no significant differences in the macronutrient intake were observed between the aFMT and placebo groups, across lifestyle intervention strata. All three intervention groups similarly increased their physical activity level compared to baseline. By the end of the trial, no significant difference was observed in the reported physical activity between the aFMT and placebo group, within all three lifestyle interventions (supplementary Tables 3-4). The overall treatment compliance rate, defined as intake of >80 capsules, was 95.6%
Safety and symptom monitoring
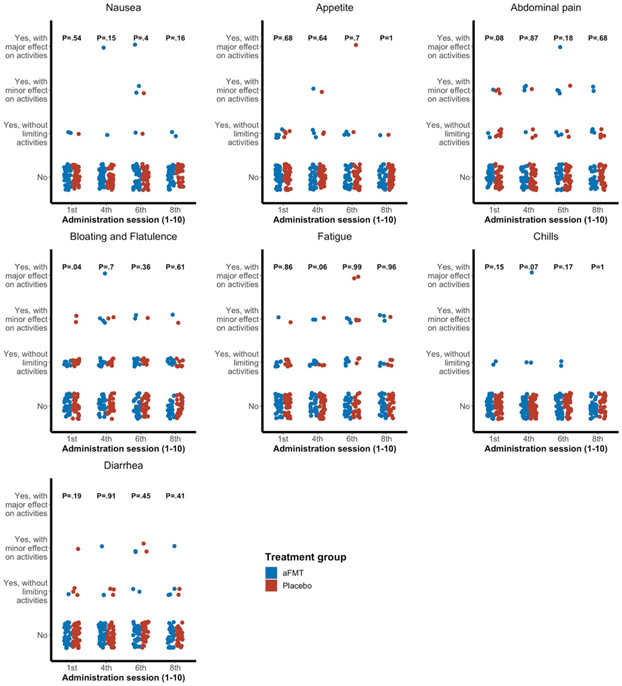
No severe adverse events were reported during the study period. Following the first administration session, more participants in the placebo group reported bloating and flatulence compared with the aFMT group (P=.04). During the remainder of the study, no significant differences between groups were observed for any of a variety of symptoms (Figure 2).
Figure 2: Symptom monitoring.
Comparison between aFMT and placebo treatment groups in monitored symptoms.
P values represent comparisons between treatment groups at each timepoint.
Dietary and aFMT effects on weight regain, and the interaction with green-Mediterranean diet
The green-Mediterranean and Mediterranean groups exhibited a similar 0 to 6-month weight loss (i.e., baseline), with both showing significantly greater reduction than the dietary guidelines group (−8.9±5.6kg, −8.8±4.7 kg and −5.4±2.7kg respectively, P=.03 for both Mediterranean groups compared with the dietary guidelines group). Across treatment groups, the aFMT and placebo groups experienced a similar 0 to 6-month weight-loss, before the capsule administration (aFMT= −8.3±5.1Kg vs. placebo=−8.3±4.8 Kg, P=.92).
The primary outcome was weight regain, defined as percent weight change between 6 and 14 months from the initial 6-month weight loss. Following the capsule administration phase, there was no significant difference in weight regain between the dietary groups (dietary guidelines=39.1±50.3%, Mediterranean= 36.1±40.2% and green-Mediterranean= 33.6±45.4%; P=.92). Overall, regain was 30.4±44% (+2.2kg) in the total aFMT groups vs. 40.6±43% (+2.6kg) regain in the total placebo groups (P=.28).
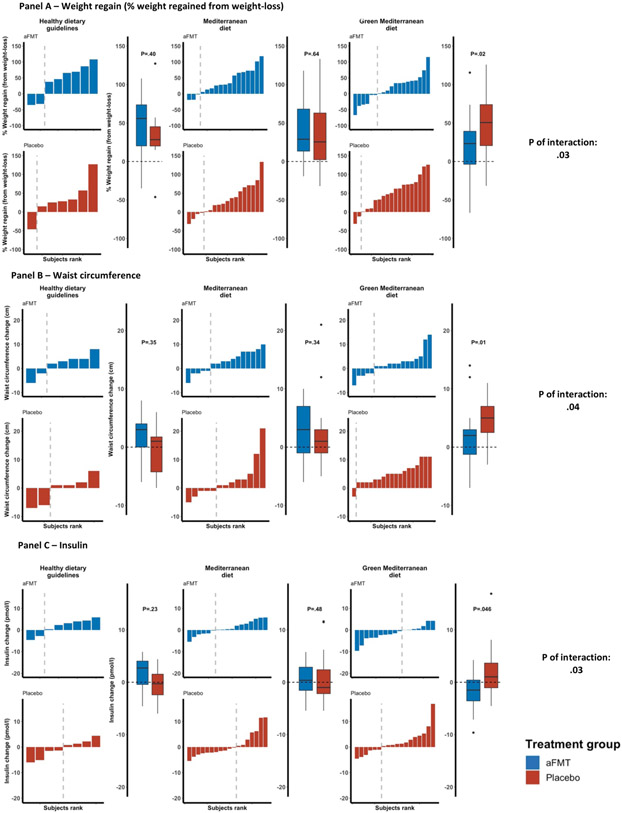
Examining the interaction between diet and treatment, aFMT significantly attenuated weight regain in the green-Mediterranean diet group [aFMT= 17.1±42.8% (+1.6Kg ) vs. placebo=50±42.9% (+3.6kg); P=.02], but not in the dietary guidelines and Mediterranean groups (P=.57 and P=.64, respectively; P of interaction with green-Mediterranean diet =.03) (Figure 3A).
Figure 3: aFMT effect across lifestyle interventions; 6-14 months changes in anthropometric measures and glycemic state.
Body weight (A), waist circumference (B) and insulin (C) changes between 6 and 14-months.
* Regain % was calculated as 100 * (14-months – 6-months) / (6-months – 0-months).
In an exploratory analysis, evaluating the interaction between aFMT and the intake of green-Mediterranean-specific components, i.e. Mankai and green tea, on weight regain, increased frequency of Mankai intake at 6 months was associated with lower subsequent weight regain in the aFMT group as compared to placebo (P of interaction=.04). A similar, though marginally significant, pattern was found in participants with increased green tea intake at 6-months (P of interaction=.06).
Dietary and aFMT effects on waist circumference and glycemic status, and the interactions with green-Mediterranean diet
The secondary outcomes were rebound of waist circumference and glycemic control. Waist circumference change between 6-14 months was not different across dietary groups (dietary guidelines=0.7±4.4cm, Mediterranean=2.4±5.1cm and green-Mediterranean=3.5±4.5cm; P=.16), nor between treatment groups (aFMT=2.2±4.5cm vs. placebo=3±5.1cm; P=.58). However, a significant attenuation was observed by aFMT in the green-Mediterranean group (aFMT=1.89±4.9cm vs. placebo=5.05±3.6cm; P=.01), and not in the dietary guidelines and Mediterranean groups (P=.56, P=.34, respectively; P of interaction=.04) (Figure 3B). Similarly, no significant difference was observed in 6 to 14-months change in fasting insulin levels across dietary groups (dietary guidelines=0.5±3.7μIU/ml, Mediterranean=0.8±4.1μIU/ml and green-Mediterranean=0.2±4.5μIU/ml; P=.82) or between treatment groups (aFMT=0.06±3.6μIU/ml vs. placebo=0.9±4.6μIU/ml; P=.71). However, we observed a significant difference in the 6 to 14-months change in fasting insulin levels between the aFMT (−1.46±3.6 μIU/ml) and placebo (+1.64±4.7 μIU/ml) groups in the green-Mediterranean group (P between treatment groups=.04), while no effect was observed within the dietary guidelines and Mediterranean groups (P=.21 and P=.47; P of interaction=.03) (Figure 3C). A similar pattern could be observed in HOMA-IR (P between dietary group=.72; P between treatment groups=.69; P aFMT vs. Placebo within the green Mediterranean group=.08; P of interaction=.07) (supplementary figures S1). No differences were observed in 6 to 14-months fasting plasma glucose change across dietary groups (P=.38), treatment groups (P=1) and the interaction between the two (P=.97).
Microbiome functional and compositional changes
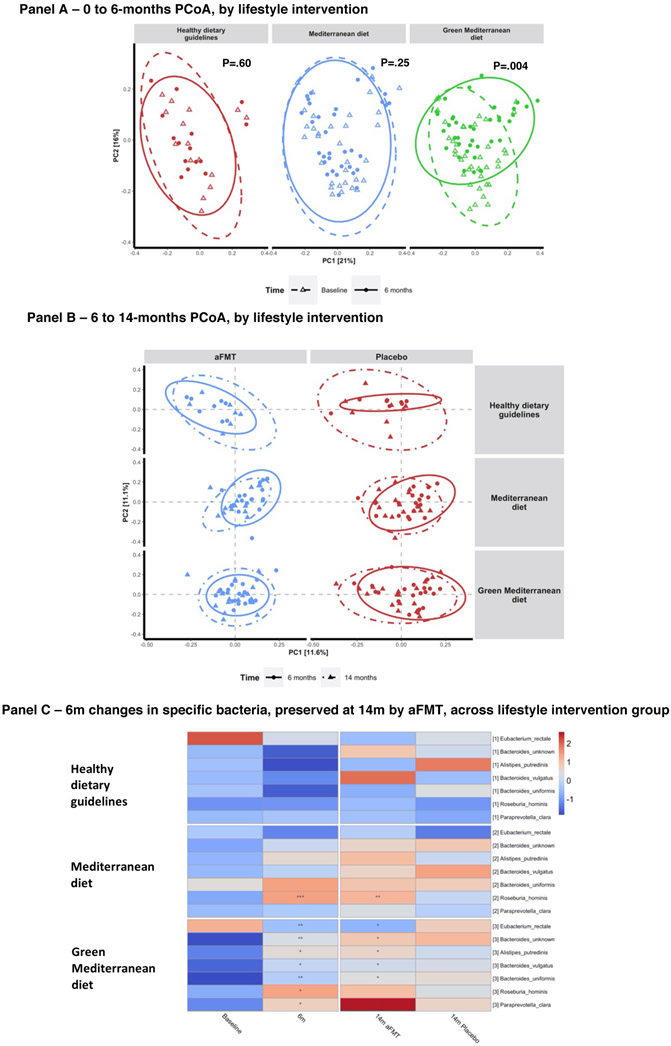
Comparing microbiome composition change at the weight-loss phase (0-6 months) across lifestyle intervention groups, a significant shift could be observed between 0 and 6-month fecal samples in the green Mediterranean group (P=.004), but not in the Mediterranean (P=.25) and dietary guidelines (P=.63) groups (Figure 4A). Following the weight regain phase (6-14 months) the aFMT-Green-Mediterranean group had the most stable gut microbiome composition, though no significant differences were present between 6 and 14-month samples within any of the subgroups (Figure 4B).
Figure 4: Microbiome composition and species-level changes.
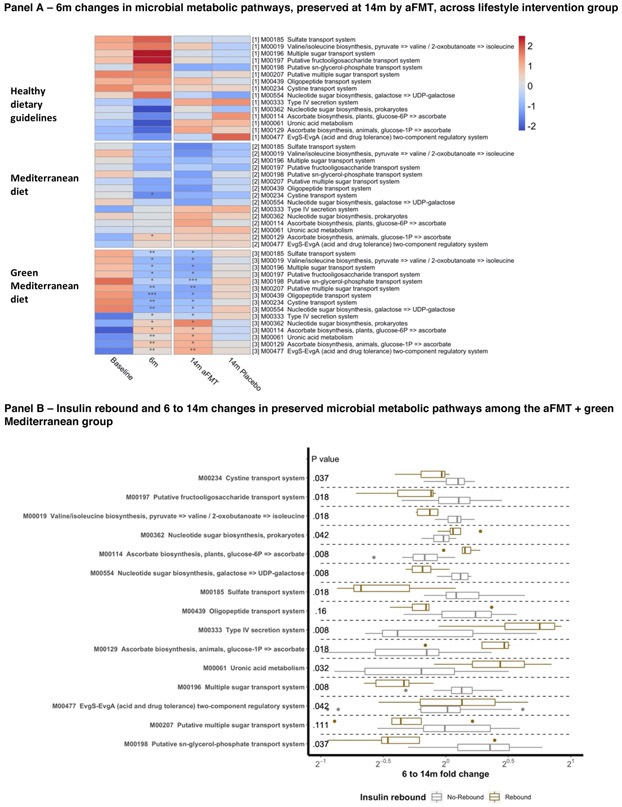
Panel A: Principal coordinate analysis (PCoA) of weighted UniFrac distances of microbiome composition measured by 16s rRNA sequencing. Each sub-plot displays the distances between individual microbiome samples within the distances of the indicated lifestyle intervention, at baseline and 6-months. Panel B: Principal coordinate analysis (PCoA) of Bray-Curtis distances of microbiome composition, each sub-plot displays the distances between individual microbiome samples within the indicated lifestyle intervention and treatment group, at 6 and 14-months. 95% standard error ellipses are shown for each sub-group; Panel C: Shotgun metagenomics assessed species (B) that were significantly changed by weight loss and maintained by the aFMT treatment, across lifestyle intervention groups.
No species/metabolic pathways were observed to be affected by diet and aFMT in the dietary guidelines group.
During the weight-loss phase, among the Mediterranean group, 64 microbial metabolic pathways and 17 species were significantly changed. The most prominent changes were the increased abundance of Akkermansia muciniphila and two sulfate degradation pathways, along with the decrease in Lactobacillus ruminis and the oxidative phase of the pentose phosphate pathway. A single bacterial species, Roseburia hominis, was observed to retain the Mediterranean group’s induced change by aFMT.
In the green Mediterranean group, 47 microbial metabolic pathways and 18 species were significantly altered. The most prominent changes were the increase in abundance of Bacteroides massiliensis and Paraprevotella clara, along with the increase in LPS biosynthesis and type IV secretion system pathways. Of these, following the regain phase, 15 pathways and 6 species remained significantly changed among the green Mediterranean participants in the aFMT group alone, not retaining the weight-loss-induced changes in the placebo group. Among the green Mediterranean group, the species Alistipes putredinis and Bacteroides vulgatus increased in abundance and several microbial sugar transport pathways were downregulated during the weight-loss phase, preserving the changes only in the aFMT group. (Figure 4C, Figure 5A, supplementary figures S2, S3, S5 and S6). The number of pathways and bacteria preserving the weight-loss-induced changes by aFMT, compared to the expected number by a permuted null model, was significantly higher in the green Mediterranean group (taxa: P=.002; KEGG modules: P=.03), but not in the dietary guidelines group (taxa: P=1; KEGG modules: P=1) and the Mediterranean group (taxa: P=.08; KEGG modules: P=1). As several sugar-related pathways were among the preserved KEGG pathways, we evaluated whether the 6-14 months changes in these pathways were associated with insulin rebound, within the aFMT-green Mediterranean group. Insulin rebound was found to be significantly associated with 13 of 15 preserved pathways (Figure 5B).
Figure 5: KEGG pathway - changes and association with insulin rebound.
Panel A: Shotgun metagenomics assessed metabolic pathways (KEGG modules) that were significantly changed by weight loss and maintained by the aFMT treatment, across lifestyle intervention groups. Panel B: Fold change of the same metabolic pathways as in ‘A’, comparing individuals with and without insulin rebound (defined as the median change between 6 and 14-months) among the aFMT+green Mediterranean group participants.
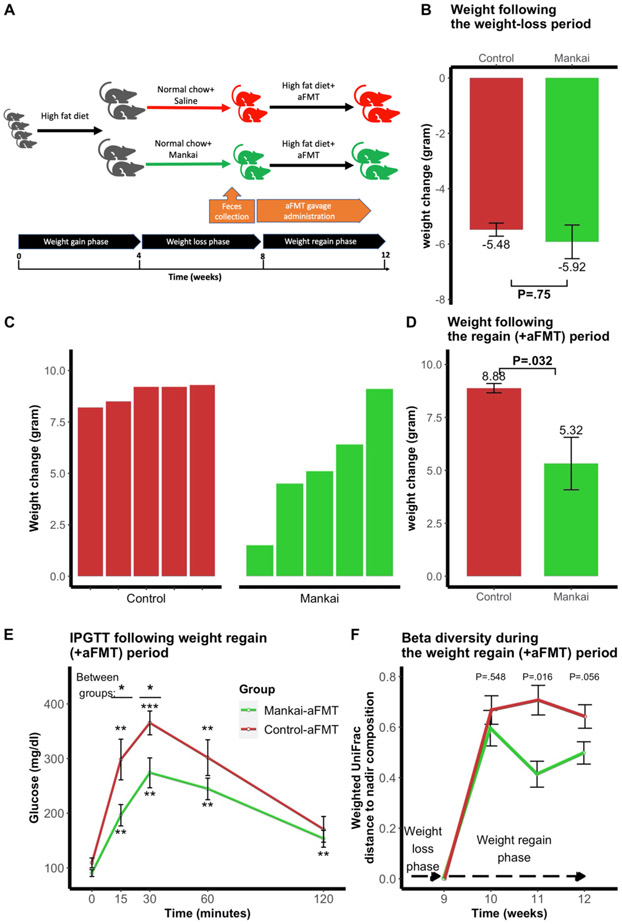
Mankai-modulated aFMT effect on weight regain and glucose in mice
During the weight-loss phase both mice groups had a similar weight-loss pattern (n=10; Mankai-fed=−5.92g vs. control =−5.48; P=.75) (Figure 6B). However, in the microbiome administration phase the Mankai-aFMT group attenuated the high-fat diet-induced weight regain, compared to control mice (Mankai=5.32g vs. control=8.88g; P=.03)(Figure 6C- D). Comparing glucose tolerance of both aFMT groups following the regain phase, the Mankai-aFMT group had significantly lower glucose levels at 15 minutes and 30 minutes in a 120-minute Intraperitoneal glucose tolerance test (Figure 6E). Assessing the mice gut microbiome composition change during the weight regain phase, both groups had undergone a prominent composition shift following the administration of high fat diet. However, the Mankai-aFMT group tended to preserve the weight-nadir composition, compared to the control (Week 11, P=.016; Week 12, P=.056; Figure 6F).
Figure 6: Mankai supplementation during weight-loss and aFMT efficacy in mice.
Experimental design (A); Mankai enrichment during weight-loss and weight change during following the weight-loss period (B); Weight regain by high fat diet+aFMT following weight-loss with added Mankai/saline gavage (C+D) Intraperitoneal glucose tolerance test (IPGTT) following the weight regain phase (week 12) (E). Weighted UniFrac distances to the weight-nadir timepoint during the weight regain phase (F).
*P ≤.05, **P ≤.01, ***P ≤.001.
DISCUSSION
In this 14-month human trial including 90 participants, green Mediterranean/high polyphenols diet was the only lifestyle strategy that induced a significant change of the gut microbiome composition during the weight-loss phase, potentially optimizing the conditions for aFMT, derived from the maximal weight loss phase. Human aFMT appeared to be a safe procedure over 6 months of administration. The observed compositional change in the gut microbiome of the green Mediterranean group was subsequently associated with attenuated weight regain, waist circumference change and reduced insulin rebound, after repeated administration in the form of aFMT. The aFMT’s beneficial effect in the green Mediterranean group coincided with the stabilization of weight-loss associated changes in specific bacteria and microbial metabolic pathways, mainly related to sugar transport. In a mouse model, we were able to reproduce the effects of weight-nadir based aFMT on weight regain and insulin sensitivity, and to isolate the specific contribution of Mankai supplementation (one of the main components in the human intervention) to induce these effects.
The trial has several limitations. The study was conducted in a unique workplace with a vast majority of males. Combining three lifestyle strategies with aFMT treatment provided an opportunity to explore interactions between dietary strategies and aFMT intervention but reduced the sample size in each lifestyle group. Furthermore, due to the nature of the lifestyle interventions and eligibility criteria of the aFMT study, the sample size of the dietary guidelines group was lower than those from the two Mediterranean diets. For ethical and technical reasons, the gut microbiome was assessed in fecal matter, and not in bioactive sites along the gastrointestinal tract. Moreover, by administering minimally processed aFMT, specific bacteria that might have been more potent in this intervention were not isolated. Major strengths of this study include the simultaneous scheme, wherein all the trial phases were performed at the same time, the double-blinded placebo-controlled design, the strictly monitored capsule administrations, the high adherence rate and the relatively large sample size, the duration of intervention and follow-up and the validation of the results in a parallel mice model.
No significant adverse events or gastrointestinal symptoms were found to be related to the aFMT treatment. These safety data are critical, as FMT has garnered attention as a potential therapeutic option to promote weight-loss.9,10,12,20 Despite rigorous donor screening, safety concerns remain when using donor-derived biotherapeutics. As aFMT eliminates potential transmission of pathogens, it is likely to be a safe alternative.
The potential of FMT in human adiposity has already been established12, using FMT from twin pairs discordant for obesity into germ-free mice. The findings suggested that the lean/obese phenotype was at least partially transmissible, and that the ‘lean’ microbiome dominates the gut and attenuates the ‘obese’ microbiome's effects on adiposity.12 Consistent with the current study, randomized human studies that evaluated the effect of lean-to-obese allogenic FMT (n=189, n=3610) showed a beneficial response on insulin sensitivity.
We observed a sustained increase in specific microbial taxa in the green Mediterranean group by the aFMT treatment, including the species Alistipes putredinis, Bacteroides vulgatus and Bacteroides uniformis. Notably, all three were previously associated with a lean-state.12,33-35 Interestingly enough, Alistipes putredinis and Bacteroides vulgatus were associated not only with reduced body weight in the host, but with successful invasion from a lean host to an obese host.12 It is plausible that the metabolic effect of aFMT in the green Mediterranean group was partly mediated through the persistent colonization of these bacteria by aFMT, during the regain phase.
In addition, we observed a sustained change in several microbial metabolic pathways that were reported to be associated with body-weight, including ‘Valine, leucine and isoleucine degradation’, ‘Ascorbate biosynthesis, [animals; glucose–1P => ascorbate]’ and ‘Putative sugar transport system’.33 In an analysis aimed to evaluate the link between these pathways and insulin rebound, the first two were found to be associated, possibly affecting host insulin sensitivity. Based on these results, the green-Mediterranean diet likely influenced the host environment, altering the gut microbiome composition and facilitating metabolic memory retention as represented by the attenuation of body-weight and insulin rebound. Polyphenols are known for their prebiotic effect27, which might underlie the polyphenols’ contribution to the prevention of weight-regain as previously shown in mice.7 Notably, although no significant difference in weight regain was observed between the placebo groups across dietary interventions, the placebo-green Mediterranean group regained the greatest amount of weight.
This trial brings to light a novel approach to weight loss maintenance by microbiome optimization and conservation. However, this strategy should be further investigated as to which specific dietary components could modify the host microbiome potency.
In conclusion, the results suggest that beyond weight loss, dietary composition modifies the microbiota, and consequently, the therapeutic effect of aFMT by a combined ‘Prebiotics to Probiotics’ model, induced by specific microbiome-modulating diet, and administrated as aFMT.
Supplementary Material
What You Need to Know.
Background and Context: This study evaluated the efficacy and safety of diet-modulated autologous fecal microbiota transplantation (aFMT) in preventing weight regain in 90 obese participants over 14 months and mice fed a Mankai diet.
New Findings: aFMT, using fecal samples collected during a period of diet and weight loss, can prevent weight regain after the diet, and also increase glycemic control, possibly via specific microbiome signatures. This procedure is optimized by green, plant-based, weight loss diet.
Limitations: Most of the trial’s participants were men.
Impact: Diet induced weight-loss can be preserved, along with glycemic control, for months after a diet via aFMT capsules. A green, plant-based diet can optimize the fecal microbiota for this procedure.
ACKNOWLEDGEMENTS
We thank the DIRECT PLUS trial participants for their significant contributions. We thank Dr Ilan Shelef from Soroka University Medical Center for his contribution to the trial. We thank Dr Dov Brickner, Efrat Pupkin, Eyal Goshen, Avi Ben Shabat, and Benjamin Sarusi from the Nuclear Research Center for their valuable contributions. We thank Hodaya Hanya and Dr Nirit Keren from the Center for Microbiome Research at Shamir Medical Center for their contributions. At Fondazione Edmund Mach, we thank Drs Maddalena Sordo and Massimo Pindo and the Sequencing Platform team, for their contributions. We thank the California Walnuts Commission, Wissotsky tea, Ltd. and Hinoman, Ltd. for the specific products provided during the trial. All authors read and approved the final manuscript, had full access to all the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis. The investigators were responsible for the design and conduct of the study; for the collection, management, analysis, and interpretation of the data; for the preparation, review, and approval of the manuscript; and for the decision to submit the manuscript for publication. Ehud Rinott managed the data analysis. Drs Iris Shai and Ilan Youngster are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding sources: This work was supported by the Israeli Science Foundation (grant 1733/18), Israel Ministry of Health (grant no. 87472511), Israel Ministry of Science and Technology (grant 3-13604); the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Projektnummer 209933838 – SFB 1052; the Deutsche Forschungsgemeinschaft, Obesity Mechanisms; the Rosetrees trust (grant A2623); the Project “Cabala_diet&health” ( http://www.cabalaproject.eu/ ; – ERA-Net Cofund ERA-HDHL No 696295) “, and the California Walnuts Commission. Dr. Dong D. Wang’s research was supported by research grants from the National Institutes of Health (K99 DK119412) and the Boston Nutrition Obesity Research Center. None of the funders were involved in any stage of the design, conduct, or analysis of the study, and had no access to the study results before publication.
Abbreviations:
- aFMT
autologous fecal microbiota transplantation
- BMI
body mass index
Footnotes
Disclosures: IS advises the nutritional committee of Hinoman, Ltd. IY is an advisor for Mybiotics Ltd. DK is CEO of, and holds equity in, CoreBiome, Inc.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFRENCES
- 1.Anderson JW, Konz EC, Frederich RC, et al. Long-term weight-loss maintenance: a meta-analysis of US studies. Am J Clin Nutr 2001;74:579–584. [DOI] [PubMed] [Google Scholar]
- 2.Gepner Y, Shelef I, Schwarzfuchs D, et al. Effect of Distinct Lifestyle Interventions on Mobilization of Fat Storage Pools. Circulation 2018;137:1143–1157. [DOI] [PubMed] [Google Scholar]
- 3.Shai I, Schwarzfuchs D, Henkin Y, et al. Weight Loss with a Low-Carbohydrate, Mediterranean, or Low-Fat Diet. N Engl J Med 2008;359:229–241. [DOI] [PubMed] [Google Scholar]
- 4.Sacks FM, Bray GA, Carey VJ, et al. Comparison of Weight-Loss Diets with Different Compositions of Fat, Protein, and Carbohydrates. N Engl J Med 2009;360:859–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baak Van MA, Mariman ECM. Mechanisms of weight regain after weight loss — the role of adipose tissue. Nat Rev Endocrinol 2019:274–287. [DOI] [PubMed] [Google Scholar]
- 6.Erez G, Tirosh A, Rudich A, et al. Phenotypic and genetic variation in leptin as determinants of weight regain. Int J Obes 2011;35:785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thaiss CA, Itav S, Rothschild D, et al. Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature 2016:1–27. [DOI] [PubMed] [Google Scholar]
- 8.Mullish BH, Quraishi MN, Segal JP, et al. The use of faecal microbiota transplant as treatment for recurrent or refractory Clostridium difficile infection and other potential indications: joint British Society of Gastroenterology (BSG) and Healthcare Infection Society (HIS) guidelines. Gut 2018;67:1920–1941. [DOI] [PubMed] [Google Scholar]
- 9.Vrieze A, Nood E Van, Holleman F, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012;143:913–6.e7. [DOI] [PubMed] [Google Scholar]
- 10.Kootte RS, Levin E, Salojärvi J, et al. Improvement of Insulin Sensitivity after Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition. Cell Metab 2017;26:611–619.e6. [DOI] [PubMed] [Google Scholar]
- 11.Youngster I, Russell GH, Pindar C, et al. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. Jama 2014;312:1772–1778. [DOI] [PubMed] [Google Scholar]
- 12.Ridaura VK, Faith JJ, Rey FE, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013;341:1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lynch S V, Pedersen O. The Human Intestinal Microbiome in Health and Disease. N Engl J Med 2016;375:2369–2379. [DOI] [PubMed] [Google Scholar]
- 14.Aqel B, DiBaise JK. Role of the Gut Microbiome in Nonalcoholic Fatty Liver Disease. Nutr Clin Pract 2015;30:780–786. [DOI] [PubMed] [Google Scholar]
- 15.Harakeh SM, Khan I, Kumosani T, et al. Gut Microbiota: A Contributing Factor to Obesity. Front Cell Infect Microbiol 2016;6:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Zhao F, Wang Y, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 2017;5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shapiro H, Suez J, Elinav E. Personalized microbiome-based approaches to metabolic syndrome management and prevention. J Diabetes 2016. [DOI] [PubMed] [Google Scholar]
- 18.Allegretti JR, Kassam Z, Mullish BH, et al. Effects of Fecal Microbiota Transplantation With Oral Capsules in Obese Patients. Clin Gastroenterol Hepatol 2019. [DOI] [PubMed] [Google Scholar]
- 19.DeFilipp Z, Bloom PP, Torres Soto M, et al. Drug-Resistant E. coli Bacteremia Transmitted by Fecal Microbiota Transplant. N Engl J Med 2019:0028–4793. [DOI] [PubMed] [Google Scholar]
- 20.Wang S, Xu M, Wang W, et al. Systematic Review: Adverse Events of Fecal Microbiota Transplantation Grivennikov S, ed. PLoS One 2016;11:e0161174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suez J, Zmora N, Zilberman-Schapira G, et al. Post-Antibiotic Gut Mucosal Microbiome Reconstitution Is Impaired by Probiotics and Improved by Autologous FMT. Cell 2018;174:1406–1423.e16. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Ouyang Y, Liu J, et al. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2014;349:g4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Micha R, Wallace SK, Mozaffarian D. Red and Processed Meat Consumption and Risk of Incident Coronary Heart Disease, Stroke, and Diabetes Mellitus. Circulation 2010;121:2271–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwingshackl L, Hoffmann G, Lampousi A-M, et al. Food groups and risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol 2017;32:363–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Medina-Remón A, Casas R, Tressserra-Rimbau A, et al. Polyphenol intake from a Mediterranean diet decreases inflammatory biomarkers related to atherosclerosis: a substudy of the PREDIMED trial. Br J Clin Pharmacol 2017;83:114–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim Y, Je Y. Dietary Fiber Intake and Total Mortality: A Meta-Analysis of Prospective Cohort Studies. Am J Epidemiol 2014;180:565–573. [DOI] [PubMed] [Google Scholar]
- 27.Roopchand DE, Carmody RN, Kuhn P, et al. Dietary polyphenols promote growth of the gut bacterium akkermansia muciniphila and attenuate high-fat diet-induced metabolic syndrome. Diabetes 2015;64:2847–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yaskolka Meir A, Tsaban G, Zelicha H, et al. A Green-Mediterranean Diet, Supplemented with Mankai Duckweed, Preserves Iron-Homeostasis in Humans and Is Efficient in Reversal of Anemia in Rats. J Nutr 2019. [DOI] [PubMed] [Google Scholar]
- 29.Zelicha H, Kaplan A, Yaskolka Meir A, et al. The Effect of Wolffia globosa Mankai, a Green Aquatic Plant, on Postprandial Glycemic Response: A Randomized Crossover Controlled Trial. Diabetes Care 2019:dc182319. [DOI] [PubMed] [Google Scholar]
- 30.Shai I, Rosner BA, Shahar DR, et al. Dietary Evaluation and Attenuation of Relative Risk: Multiple Comparisons between Blood and Urinary Biomarkers, Food Frequency, and 24-Hour Recall Questionnaires: the DEARR Study. J Nutr 2005;135:573–579. [DOI] [PubMed] [Google Scholar]
- 31.Chasan-Taber S, Rimm EB, Stampfer MJ, et al. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology 1996;7:81–6. [DOI] [PubMed] [Google Scholar]
- 32.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 2000;32:S498–504. [DOI] [PubMed] [Google Scholar]
- 33.Liu R, Hong J, Xu X, et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat Med 2017;advance on. [DOI] [PubMed] [Google Scholar]
- 34.Zeevi D, Korem T, Zmora N, et al. Personalized Nutrition by Prediction of Glycemic Responses. Cell 2015;163:1079–1095. [DOI] [PubMed] [Google Scholar]
- 35.Gauffin Cano P, Santacruz A, Moya Á SY. Bacteroides Uniform is CECT 7771 Ameliorates Metabolic and Immunological Dysfunction in Mice With High-Fat-Diet Induced Obesity. PLoS One 2012;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.