Abstract
Background/Objective:
To evaluate the association between the duration of the latent phase of labor and subsequent processes and outcomes.
Methods:
Secondary analysis of prospectively collected data among 1,189 women with low-risk pregnancies and spontaneous labor.
Results:
Longer latent phase duration was associated with labor dystocia (eg, nulliparous ≥ mean [compared with < mean] aOR 3.95 [2.70–5.79]; multiparous ≥ mean [compared with < mean] aOR 5.45 [3.43–8.65]), interventions to ameliorate dystocia, and epidurals to cope or rest (eg, oxytocin augmentation: nulliparous > 80th% [compared with < 80th%] aOR 6.39 [4.04–10.12]; multiparous ≥ 80th% [compared with < 80th%] aOR 6.35 [3.79–10.64]). Longer latent phase duration was also associated with longer active phase and second stage. There were no associations between latent phase duration and risk for cesarean delivery or postpartum hemorrhage in a practice setting with relatively low rates of primary cesarean. Newborns born to multiparous women with latent phase of labor durations at and beyond the 80th% were more frequently admitted to the NICU (≥80th% [compared with < 80th%] aOR 2.7 [1.22–5.84]); however, two-thirds of these NICU admissions were likely for observation only.
Conclusions:
Longer duration of the spontaneous latent phase of labor among women with low-risk pregnancies may signal longer total labor processes, leading to an increase in diagnosis of dystocia, interventions to manage dystocia, and epidural use. Apart from multiparous neonatal NICU admission, no other maternal or child morbidity outcomes were elevated with longer duration of the latent phase of labor.
Keywords: active phase of labor, labor duration, labor dystocia, latent phase of labor
1 |. INTRODUCTION
There is a long-standing belief in a relationship between the duration of labor and perinatal risk.1 Definitions of normal labor duration signal the threshold of time beyond which labor is considered dystocic.2 Dystocic labor is frequently viewed as an abnormal stressor associated with risk for the laboring woman and her fetus or newborn.1,3 There is evidence to support this concern; women in environments with poor access to medical care are at risk for serious morbidity such as fistula, prolapse,4 and maternal-fetal-neonatal mortality after dystocic labor.5,6 Thus, when labor dystocia is diagnosed, it is considered appropriate to intervene in order to optimize outcomes. However, in high-resource countries, obstetric providers may overuse interventions to speed or curtail labor, incurring different perinatal risks.7–9 For these reasons, a thorough understanding of durations of each phase of labor and associated risks or benefits is important for determining the appropriate threshold of time in labor to transition from supportive care to intervention.
Although recent research suggests association between duration of time at the end of latent phase >90th percentile (vs. <90th percentile) and poor outcomes,10 little is known about the full duration of the latent phase of labor or whether the full duration influences or predicts labor processes and birth outcomes. This is a result of several factors, including the inherent challenges of measuring latent labor duration and that prior studies either marked onset of the latent phase of labor as the time of hospital admission or did not clearly define latent phase of labor onset. To address this question, recently published research described latent phase of labor duration and associated characteristics in a sample of low-risk women in spontaneous labor, marking latent phase of labor onset by the time that laboring women reported the start of labor symptoms.11 This study included women who chose hospital care with certified nurse-midwives (CNMs) and found that the duration of the latent phase was longer than previously estimated in the United States population.1,3,11–14 Among nulliparous women, duration of the latent phase was 9.0 hours at the median and 11.8 hours at the mean; among multiparous women, duration of the latent phase was 6.8 hours at the median and 9.3 hours at the mean.11 These findings are similar to recent European studies on labor duration that used analogous latent phase onset definitions.15,16 We suspect that these longer latent phase duration estimates predominantly relate to differences in the measurement of latent phase labor onset and contemporary understanding of active labor onset as beginning at six centimeters of cervical dilation11,15,16 Building on these findings, the purpose of this paper was to evaluate the association between the duration of the latent phase and perinatal processes and outcomes that occurred during active phase, second stage, birth, and the immediate postpartum period. Informed by prior labor duration research,17 we hypothesized that longer durations of the latent phase of labor would be associated with longer durations of both the active and second stages of labor, greater intervention use, and equivalent morbidity outcomes.
2 |. MATERIALS AND METHODS
2.1 |. Sample
The sample included women cared for by certified nurse-midwives (CNMs) at Oregon Health & Science University (OHSU) in Portland, Oregon. CNMs in this setting are licensed providers who independently care for low-risk women and consult, co-manage, or transfer as needed with obstetrical or perinatal colleagues. Maternity care team culture has been linked to variation in intervention rates18 and may importantly influence when a diagnosis of labor dystocia leads to cesarean.19 During the study period of 2012–17, the overall OHSU unscheduled primary cesarean delivery rate was 11.8%. In addition, several other characteristics signal a culture favoring vaginal birth; for example, nurses frequently remain with women during labor, and doulas are routinely part of many women’s labor support teams.19
In 2012, the OHSU CNM practice received IRB approval to prospectively collect observational data. More detailed data collection, validation steps, exclusions, and human subjects research permission have been previously described.11 Our sample (n = 1,189) included women choosing and receiving intrapartum CNM care who were ≥21 years of age and in spontaneous labor with a term (37+ weeks’ gestation), nonanomalous, singleton, live, and vertex fetus. We excluded women with a history of cesarean. Women with antenatal complications outside the CNM practice scope are transferred to physician care before labor onset, further decreasing the risk profile of the sample.
2.2 |. Analysis
Maternal demographic, health, and neonatal characteristics of the sample are described in Table 1, stratified by parity. Continuous variables (eg, gestational age in weeks) were transformed into clinically relevant categorical variables (eg, early term vs. term gestational age). Our primary independent variable was the duration of the latent phase of labor, defined as the time from the onset of latent phase symptoms (identified by the laboring woman) to the time of the onset of active labor (defined by the attending CNM). To explore how longer durations of the latent phase were associated with perinatal processes and outcomes, we considered latent phase duration at or longer than several time points of the distribution (as compared to less than each time point): ≥mean, median, 80th percentile, 90th percentile, and 95th percentile. These comparisons (eg, < vs. ≥ the median) created our dichotomous definitions. We chose these time points to explore how latent phase durations in the averageto-longer range were associated with outcomes. Our primary outcomes were as follows: amniotomy, oxytocin augmentation, epidural use, labor dystocia diagnosis (as determined by the CNM providing intrapartum care), mode of delivery, postpartum hemorrhage, neonatal intensive care unit (NICU) admission, and Apgar < 7 at 5 minutes.
TABLE 1.
Sample demographic, health characteristics, labor processes, and outcomes
| Nulliparous (n = 665) | Multiparous (n = 616) | |
|---|---|---|
| Demographic characteristics | n (%) | n (%) |
| Race | ||
| Caucasian | 580 (89.1) | 541 (89.6) |
| African American | 7 (1.1) | 13 (2.2) |
| Asian | 46 (7.1) | 35 (5.9) |
| Multiracial | 13 (2.0) | 8 (1.3) |
| Native American | 1 (0.2) | 4 (0.7) |
| Native Hawaiian | 4 (0.6) | 3 (0.5) |
| Married or partnered | 641 (97.7) | 597 (97.1) |
| ≥35 y of age | 148 (22.8) | 164 (27.0) |
| Gestational diabetes | 51 (7.7) | 39 (6.3) |
| Maternal height | ||
| <10th% | 50 (7.5) | 50 (8.2) |
| 10th%–90th% | 548 (82.4) | 490 (79.9) |
| >90th% | 67 (10.1) | 73 (11.9) |
| BMI | ||
| Underweight (<18) | 25 (3.8) | 23 (3.8) |
| Normal (18–25) | 442 (66.6) | 392 (63.8) |
| Overweight (25–30) | 131 (19.7) | 132 (21.5) |
| Obese (≥30) | 66 (9.9) | 67 (10.9) |
| Excess pregnancy weight gain by IOM guidelines | 19 (2.9) | 18 (3.0) |
| GBS vaginal colonization | 154 (23.5) | 147 (24.3) |
| Prelabor rupture of membranes | 21 (3.2) | 21 (3.4) |
| Gestational age at delivery | ||
| Early term (37–38 6/7 wk) | 84 (12.8) | 101 (16.6) |
| Term (39–40 6/7 wk) | 413 (62.7) | 371 (60.9) |
| Late term (41 w-41 6/7 wk) | 143 (21.7) | 112 (18.4) |
| Post-term (42 w +) | 19 (2.9) | 25 (4.1) |
| Birthweight by gestational age* | ||
| Small for gestational age | 30 (5.2) | 25 (4.6) |
| At gestational age | 472 (82.4) | 460 (84.4) |
| Large for gestational age | 71 (12.4) | 60 (11.0) |
| Fetal position at birth | ||
| Occiput anterior | 537 (90.9) | 544 (93.5) |
| Occiput posterior/occiput transverse | 54 (9.1) | 38 (6.5) |
| Intrapartum and postpartum processes and outcomes | ||
| Labor dystocia | 240 (36.1) | 130 (21.1) |
| Oxytocin augmentation | 176 (26.5) | 100 (16.2) |
| Amniotomy | 94 (14.1) | 59 (9.6) |
| Cesarean birth | 96 (14.6) | 36 (5.9) |
| Postpartum hemorrhage | 105 (16.1) | 76 (12.5) |
| Neonatal intensive care unit admission | 57 (8.7) | 36 (5.9) |
| Apgar < 7 at 5 min | 13 (1.9) | 6 (1.0) |
Note:
Infants were designated small for gestational age if their birthweight was in the bottom 10th percentile for gestational age; infants were considered large for gestational age if their birthweight was at or above the 90th percentile for their gestational age.
Abbreviations: BMI, body mass index; IOM, Institute of Medicine.
We compared < vs. ≥ duration at each time point (in hours) of latent phase labor among women with vs. without a given outcome using t tests. Logistic regression models were specified to examine the association between the duration of the latent phase of labor and each outcome variable. We compared each point of the distribution of the latent phase duration to determine the adjusted association between duration of the latent phase at each time point and the outcome. We chose one point of central tendency (mean) for regression analysis. We adjusted for factors that have been independently associated with labor duration or the primary outcomes examined in this analysis. Covariates included the following: maternal age, maternal height, BMI category, pregnancy weight gain, gestational diabetes, prelabor rupture of membranes, group beta streptococcus colonization status, neonatal birthweight, gestational age at delivery, and partner status.11,20–28 The above confounders precede latent phase duration in time (eg, age, BMI, and birthweight [birthweight is established at the time of labor onset although cannot be measured until after]) so cannot be on the causal pathway between the independent variable and the outcome; therefore, they are confounders.29 In contrast, fetal position (eg, OP)30 and chorioamnionitis31,32 are potentially on the causal pathway between duration of the latent phase of labor and subsequent labor processes and outcomes; for example, chorioamnionitis may result from a longer labor duration, thus occurring after labor onset and therefore be caused by the independent variable, rather than being upstream from it, as required of a confounder33 For this reason and because controlling for causal pathway variables results in bias,34 these two variables (fetal position and chorioamnionitis) were not controlled during analysis.33,35
We employed survival analysis for time-to-event outcomes. Survival analysis enables evaluation of the effect of one variable (here, latent phase duration) on a dependent variable that is measured as duration of time (eg, active phase labor). To examine the potential relationship between the duration of the latent phase of labor and the duration of the subsequent active phase and second stage of labor, we fit Cox proportional hazards models, adjusting for the same confounders. The Cox proportional hazards models used a binary exposure definition, comparing women with latent phase labor under a certain distribution point (eg, <80th%, referent) to women with latent phase labor over the distribution point (eg, ≥80th%). These models estimated hazard ratios describing how latent phase (eg, duration ≥90th%) was associated with duration of active phase and second stage. We generated survival curves from the models comparing active phase and second-stage labor among women with latent phase labor duration above and below the mean as a visual aid for interpretation. In addition, to provide clinically meaningful and more readily interpretable results, we used model-based prediction of the outcomes (hours in active phase labor and hours in the second stage) among women above and below the mean of latent phase duration.36 We predicted both outcomes under common covariate patterns in our sample, enabling comparison of usual results in each exposure group. All analyses were stratified by parity.
NICU admission varies by institution and individual provider practices and is commonly indicated for a period of neonatal observation rather than true morbidity. Although our NICU variable does not differentiate NICU admission for observation vs. morbidity, our data set does capture markers of neonatal morbidity at birth, before hospital discharge, and at 6 weeks postpartum. Though neonatal morbidity is certainly the more important outcome, we do not wish to disregard consequences of NICU admission for observation that might include separating a newborn from their family, interference with breastfeeding initiation and bonding, and provoking parental anxiety. Thus, we chose to analyze both NICU admission and neonatal morbidity by 6 weeks postpartum as important outcomes. To differentiate NICU clinical practices vs. morbidity, we conducted a sensitivity analysis comparing NICU admission with a composite neonatal morbidity marker that included inpatient diagnoses before postdelivery discharge and at 6 weeks postpartum.
3 |. RESULTS
After excluding observations (cases) that did not meet inclusion criteria, we analyzed a final sample of 599 nulliparous and 590 multiparous women (total n = 1189).11 We observed a low (<4%) rate of missing data for most variables, the exception being fetal position (9% missing); however, this variable was not used in multivariate modeling. Consistent with the practice population, the women in this sample were predominantly white, partnered (in a committed relationship) or married, and with normal BMI (Table 1).
In crude analyses, we observed significant associations between the duration of latent phase of labor (independent variable) beyond the mean and several dependent variables: (a) dystocia, (b) interventions to ameliorate labor dystocia (oxytocin augmentation and amniotomy), and (c) use of epidural analgesia during active or second stages of labor. These relationships were consistent among both nulliparous (Table 2) and multiparous (Table 3) women. For example, women who were diagnosed with labor dystocia (vs. those not diagnosed with labor dystocia) had significantly longer latent phase of labor durations at multiple distribution points (eg, nulliparous: median = 13.2 hours vs. median = 7.2 hours, P < .001; multiparous: median = 13.3 hours vs. 5.9 hours, P < .001). We also observed associations of latent phase labor beyond the mean with both NICU admission and low Apgar scores at 5 minutes in multiparous women. No other comparisons showed significant differences.
TABLE 2.
Outcomes and distribution of latent phase labor, nulliparous women (hours [n])
| Outcome | No/Yes | Point of distribution | P valuesa | ||||
|---|---|---|---|---|---|---|---|
| Mean | Median | 80th | 90th | 95th | |||
| Amniotomy | N | 10.9 (194) | 8.3 (256) | 16.0 (103) | 22.0 (58) | 28.2 (26) | <.001 |
| Y | 17.5 (29) | 13.5 (39) | 24.3 (16) | 30.0 (9) | 41.0 (4) | ||
| Oxytocin augmentation | N | 9.4 (187) | 7.5 (227) | 14.2 (89) | 18.5 (46) | 23.3 (23) | <.001 |
| Y | 18.9 (56) | 15.0 (75) | 26.0 (30) | 35.5 (15) | 53.8 (8) | ||
| Labor dystocia diagnosis | N | 9.0 (155) | 7.2 (196) | 14.0 (83) | 18.0 (40) | 22.2 (20) | <.001 |
| Y | 17.2 (74) | 13.2 (99) | 24.6 (40) | 30.7 (20) | 51.3 (10) | ||
| Epidural | N | 9.0 (139) | 7.2 (175) | 14.0 (72) | 17.9 (35) | 22.0 (21) | <.001 |
| Y | 15.8 (82) | 12.1 (116) | 23.8 (47) | 30.0 (26) | 42.1 (12) | ||
| Cesarean delivery | N | 11.6 (205) | 9.0 (279) | 16.8 (111) | 23.2 (56) | 29.0 (29) | .081 |
| Y | 14.8 (14) | 11.8 (18) | 24.0 (8) | 29.5 (4) | 32.5 (2) | ||
| Postpartum hemorrhage | N | 11.5 (183) | 9.0 (251) | 17.0 (100) | 23.6 (50) | 30.0 (26) | .259 |
| Y | 12.9 (34) | 10.0 (48) | 22.0 (19) | 25.3 (10) | 29.3 (5) | ||
| NICU admission | N | 11.8 (208) | 9.1 (273) | 17.0 (116) | 24.0 (56) | 30.0 (29) | .248 |
| Y | 9.9 (19) | 8.5 (22) | 14.0 (10) | 22.8 (5) | 26.9 (3) | ||
| Apgar < 7 at 5 min | N | 11.8 (219) | 9.2 (291) | 17.0 (123) | 24.0 (61) | 30.0 (30) | .904 |
| Y | 11.3 (1) | 5.3 (4) | 8.5 (2) | 22.1 (1) | 37.4 (1) | ||
P value from t test comparing subjects with latent phase labor < mean to subjects with latent phase labor ≥ mean.
TABLE 3.
Outcomes and distribution of latent phase labor, multiparous women (hours [n])
| Outcome | No/Yes | Point of distribution | P valuea | ||||
|---|---|---|---|---|---|---|---|
| Mean | Median | 80th | 90th | 95th | |||
| Amniotomy | N | 8.8 (198) | 6.5 (266) | 13.0 (110) | 18.1 (52) | 23.1 (26) | <.001 |
| Y | 13.7 (16) | 8.3 (27) | 17.4 (11) | 32.5 (6) | 52.4 (3) | ||
| Oxytocin augmentation | N | 8.0 (185) | 6.0 (249) | 12.0 (101) | 15.5 (51) | 20.5 (26) | <.001 |
| Y | 16.4 (36) | 13.5 (45) | 23.1 (18) | 31.1 (9) | 43.2 (5) | ||
| Labor dystocia diagnosis | N | 7.7 (172) | 5.9 (229) | 11.5 (92) | 14.9 (46) | 20.1 (23) | <.001 |
| Y | 15.6 (47) | 13.3 (58) | 22.4 (23) | 29.6 (12) | 39.0 (6) | ||
| Epidural | N | 7.4 (156) | 5.5 (205) | 11.5 (82) | 14.8 (41) | 18.3 (21) | <.001 |
| Y | 13.7 (63) | 10.9 (84) | 20.7 (34) | 25.5 (17) | 39.5 (9) | ||
| Cesarean delivery | N | 9.2 (205) | 6.8 (278) | 13.0 (111) | 18.2 (56) | 24.2 (28) | .147 |
| Y | 12.6 (6) | 7.3 (9) | 22.1 (4) | 23.9 (2) | 30.0 (1) | ||
| Postpartum hemorrhage | N | 9.2 (189) | 7.0 (251) | 13.5 (101) | 18.8 (50) | 23.0 (25) | .374 |
| Y | 10.3 (22) | 6.7 (36) | 14.4 (15) | 24.5 (8) | 28.0 (4) | ||
| NICU admission | N | 9.0 (206) | 6.6 (269) | 13.0 (114) | 17.3 (56) | 23.8 (27) | .006 |
| Y | 13.7 (14) | 7.5 (18) | 22.2 (7) | 28.2 (4) | 40.6 (2) | ||
| Apgar < 7 at 5 min | N | 9.2 (208) | 6.7 (284) | 13.1 (114) | 18.4 (57) | 24.1 (29) | .014 |
| Y | 19.9 (4) | 20.5 (3) | 25.2 (1) | 27.6 (1) | 28.8 (1) | ||
P value from t test comparing subjects with latent phase labor < mean to subjects with latent phase labor ≥ mean.
After adjusting for confounders, associations between durations of latent phase labor beyond the mean and labor dystocia, oxytocin augmentation, amniotomy, and epidural use persisted, regardless of parity. We observed these associations at the mean, 80th, 90th, and 95th percentiles of the distribution of latent phase duration among nulliparous women (Table 4) and multiparous women (Table 5). For example, labor dystocia diagnosis: nulliparous ≥ 80th percentile (compared with < 80th percentile) aOR 5.38 (3.44–8.40); multiparous ≥ 80th percentile (compared with < 80th percentile) aOR 7.53 (4.60–12.31). Among nulliparous women, there was no significant relationship between duration of the latent phase and maternal or neonatal morbidity markers (eg, postpartum hemorrhage: ≥mean (compared with < mean) aOR 1.21 (0.75–1.96)]. Among multiparous women, those with latent phase duration longer than the 80th% experienced higher odds of their newborns being admitted to the NICU (≥80th% [compared with < 80th%] aOR 2.67 [1.22–5.84]). We were unable to model Apgar < 7 at 5 minutes among multiparous women because of the low number of infants with low Apgar scores.
TABLE 4.
Adjusteda associations (95% confidence intervals) of outcomes by distribution point of latent phase of labor duration among nulliparous women
| Outcome | Measure of association | Point of distribution | |||||||
|---|---|---|---|---|---|---|---|---|---|
| <Mean (referent) | Mean | <80th (referent) | 80th | <90th (referent) | 90th | <95th (referent) | 95th | ||
| Amniotomy | aOR | ref | 4.45 (2.62, 7.57) | ref | 3.22 (1.88, 5.51) | ref | 3.18 (1.68, 6) | ref | 2.77 (1.18, 6.49) |
| Oxytocin augmentation | aOR | ref | 4.46 (2.92, 6.82) | ref | 6.39 (4.04, 10.12) | ref | 8.21 (4.57, 14.74) | ref | 11.28 (4.83, 26.36) |
| Labor dystocia diagnosis | aOR | ref | 3.95 (2.7, 5.79) | ref | 5.38 (3.44, 8.4) | ref | 7.51 (4.04, 13.97) | ref | 7.50 (3.13, 17.97) |
| Epidural | aOR | ref | 3.05 (2.12, 4.4) | ref | 3.51 (2.27, 5.44) | ref | 7.07 (3.63, 13.77) | ref | 8.89 (3.33, 23.72) |
| Cesarean delivery | aOR | ref | 1.6 (0.78, 3.29) | ref | 2.12 (0.98, 4.56) | ref | 2.22 (0.90, 5.48) | ref | 2.39 (0.75, 7.61) |
| Postpartum hemorrhage | aOR | ref | 1.21 (0.75, 1.96) | ref | 1.47 (0.85, 2.53) | ref | 1.24 (0.61, 2.52) | ref | 1.12 (0.41, 3.08) |
| NICU admission | aOR | ref | 0.56 (0.27, 1.2) | ref | 0.83 (0.35, 1.98) | ref | 1.18 (0.44, 3.19) | ref | 0.37 (0.05, 2.85) |
| Sensitivity analysis NICUa | aOR | ref | 1.19 (0.41, 3.46) | ref | 2.08 (0.67, 6.48) | ref | 2.03 (0.54, 7.59) | ref | 1.05 (0.13, 8.64) |
| Apgar < 7 at 5 min | aOR | ref | 0.16 (0.02, 1.57) | ref | 0.38 (0.04, 4.07) | ref | 0.75 (0.07, 8.49) | ref | 1.17 (0.08, 16.01) |
| Active phase of labor | aHR | ref | 0.66 (0.55, 0.79) | ref | 0.67 (0.54, 0.82) | ref | 0.58 (0.44, 0.77) | ref | 0.68 (0.47, 0.99) |
| Second-stage labor | aHR | ref | 0.77 (0.65, 0.93) | ref | 0.71 (0.58, 0.88) | ref | 0.77 (0.58, 1.01) | ref | 0.66 (0.45, 0.97) |
Abbreviations: aHR, adjusted hazard ratio; aOR, adjusted odds ratio; NICU, neonatal intensive care unit.
Adjusted for maternal age, maternal height, BMI category, pregnancy weight gain, partner status, gestational diabetes, spontaneous rupture of membranes, group beta streptococcus colonization status, neonatal birthweight, and gestational age.
Neonatal morbidity composite includes these diagnoses before hospital discharge postbirth or as reported by mother at 6 wk postpartum: meconium aspiration, infection, bronchopulmonary dysplasia, cardiac failure, hypovolemic shock, intraventricular hemorrhage, birth trauma, necrotizing enterocolitis, pneumonia, persistent pulmonary hypertension, renal failure, respiratory distress syndrome, Rh disease, seizures, sepsis, hyperbilirubinemia, hospital readmission, other neonatal complications.
TABLE 5.
Adjusteda associations (95% confidence intervals) of outcomes by distribution point of latent phase of labor duration among multiparous women
| Outcome | Measure of association | Point of distribution | |||||||
|---|---|---|---|---|---|---|---|---|---|
| <Mean (referent) | Mean | <80th (referent) | 80th | <90th (referent) | 90th | <95th (referent) | 95th | ||
| Amniotomy | aOR | ref | 1.68 (0.93, 3.04) | ref | 2.07 (1.07, 3.99) | ref | 2.27 (1.02, 5.07) | ref | 4.77 (1.83, 12.45) |
| Oxytocin augmentation | aOR | ref | 5.06 (3.05, 8.4) | ref | 6.35 (3.79, 10.64) | ref | 7.00 (3.81, 12.86) | ref | 6.44 (2.82, 14.72) |
| Labor dystocia diagnosis | aOR | ref | 5.45 (3.43, 8.65) | ref | 7.53 (4.6, 12.31) | ref | 5.85 (3.22, 10.62) | ref | 6.15 (2.68, 14.08) |
| Epidural | aOR | ref | 3.79 (2.54, 5.64) | ref | 4.69 (2.97, 7.4) | ref | 6.32 (3.46, 11.52) | ref | 7.39 (3.11, 17.59) |
| Cesarean delivery | aOR | ref | 1.01 (0.34, 2.95) | ref | 1.53 (0.47, 4.97) | ref | 3.42 (1.03, 11.33) | ref | 0.94 (0.08, 10.71) |
| Postpartum hemorrhage | aOR | ref | 0.76 (0.44, 1.32) | ref | 0.96 (0.5, 1.84) | ref | 1.22 (0.54, 2.75) | ref | 2.67 (1.06, 6.73) |
| NICU admissionb | aOR | ref | 1.35 (0.65, 2.81) | ref | 2.67 (1.22, 5.84) | ref | 4.86 (2.06, 11.45) | ref | 3.65 (1.12, 11.85) |
| Sensitivity analysis NICU | ref | 2.11 (0.61, 7.31) | ref | 3.01 (0.84, 10.85) | ref | 3.01 (0.84, 10.85) | ref | 2.77 (0.32, 24.04) | |
| Apgar < 7 at 5 min | aOR | ref | 5.46 (0.53, 56.65) | ref | 14.32 (1.28, 160.47) | ref | 35.33 (3.06, 407.38) | ref | 7.37 (0.59, 92.28) |
| Active phase of labor | aHR | ref | 0.59 (0.49, 0.71) | ref | 0.59 (0.47, 0.73) | ref | 0.55 (0.41, 0.74) | ref | 0.57 (0.38, 0.87) |
| Second-stage labor | aHR | ref | 0.58 (0.48, 0.7) | ref | 0.62 (0.5, 0.78) | ref | 0.53 (0.4, 0.71) | ref | 0.55 (0.37, 0.83) |
Abbreviations: aHR, adjusted hazard ratio; aOR, adjusted odds ratio; NICU, neonatal intensive care unit.
Adjusted for maternal age, maternal height, BMI category, pregnancy weight gain, partner status, gestational diabetes, spontaneous rupture of membranes, group beta streptococcus colonization status, neonatal birthweight, and gestational age.
Neonatal morbidity composite includes these diagnoses before hospital discharge postbirth or as reported by mother at 6 wk postpartum: meconium aspiration, infection, bronchopulmonary dysplasia, cardiac failure, hypovolemic shock, intraventricular hemorrhage, birth trauma, necrotizing enterocolitis, pneumonia, persistent pulmonary hypertension, renal failure, respiratory distress syndrome, Rh disease, seizures, sepsis, hyperbilirubinemia, hospital readmission, other neonatal complications.
3.1 |. Sensitivity analysis
Among newborns admitted to the NICU, approximately onethird received a morbidity diagnosis by 6 weeks postpartum (nulliparous: 35.9%; multiparous: 29.6%), suggesting that approximately two-thirds of NICU admissions observed in this sample were for observation. We found that point estimates for the adjusted relationship between duration of the latent phase of labor and composite neonatal morbidity mimicked those for NICU admission but only became significant among multiparous women with latent phase duration beyond the 90th% (Tables 4 and 5).
3.2 |. Durations of active phase and second stage of labor
Regardless of parity, women with longer durations of the latent phase of labor experienced significantly longer durations of the active phase and the second stage of labor as illustrated by the lower relative hazards of active and second stages of labor (Tables 4 and 5). The associated survival curves (Figures 1 and 2) show that at any given point in time, save the extremes, a lower proportion of women with longer latent phase of labor have completed the given stage of labor compared with women with shorter latent phase of labor. For example, in the left panel of Figure 1, we can see that at hour 10, approximately 90% (1–0.10) of women with shorter latent phase have completed the active phase of labor. By contrast, only 80% of women with longer latent phase of labor have completed the active phase of labor by hour 10. To provide more clinically applicable results, we used model-based prediction of active phase labor above and below the mean of latent phase for women with common sample characteristics (eg, normal BMI, no GBS vaginal colonization). We found that among nulliparous women with latent phase duration less than the mean, 12 hours, the median duration of active phase labor was 5.75 hours, whereas for those with latent phase duration equal to or greater than the mean, the median duration of active phase labor was 8 hours (Table 6).
FIGURE 1.
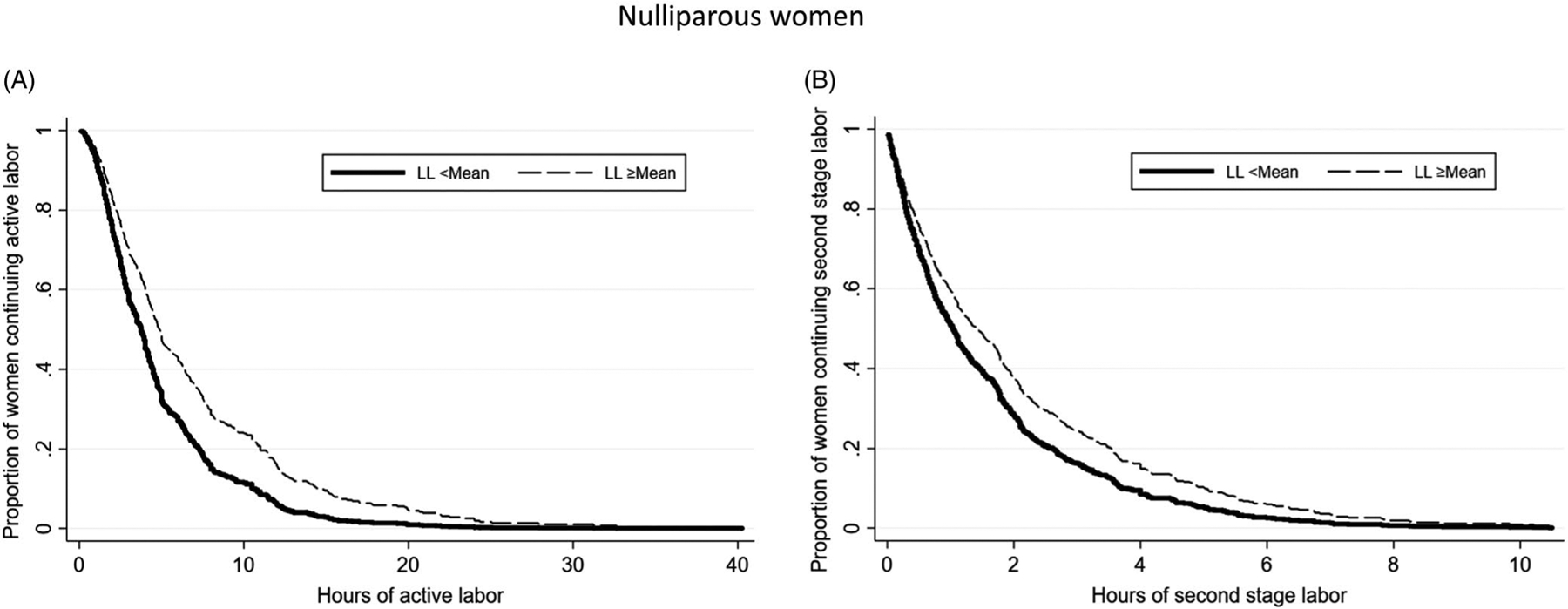
Survival curves from Cox proportional hazards models of (A) active labor; (B) second-stage labor among nulliparous women with latent phase labor <mean and ≥greater than the mean
FIGURE 2.
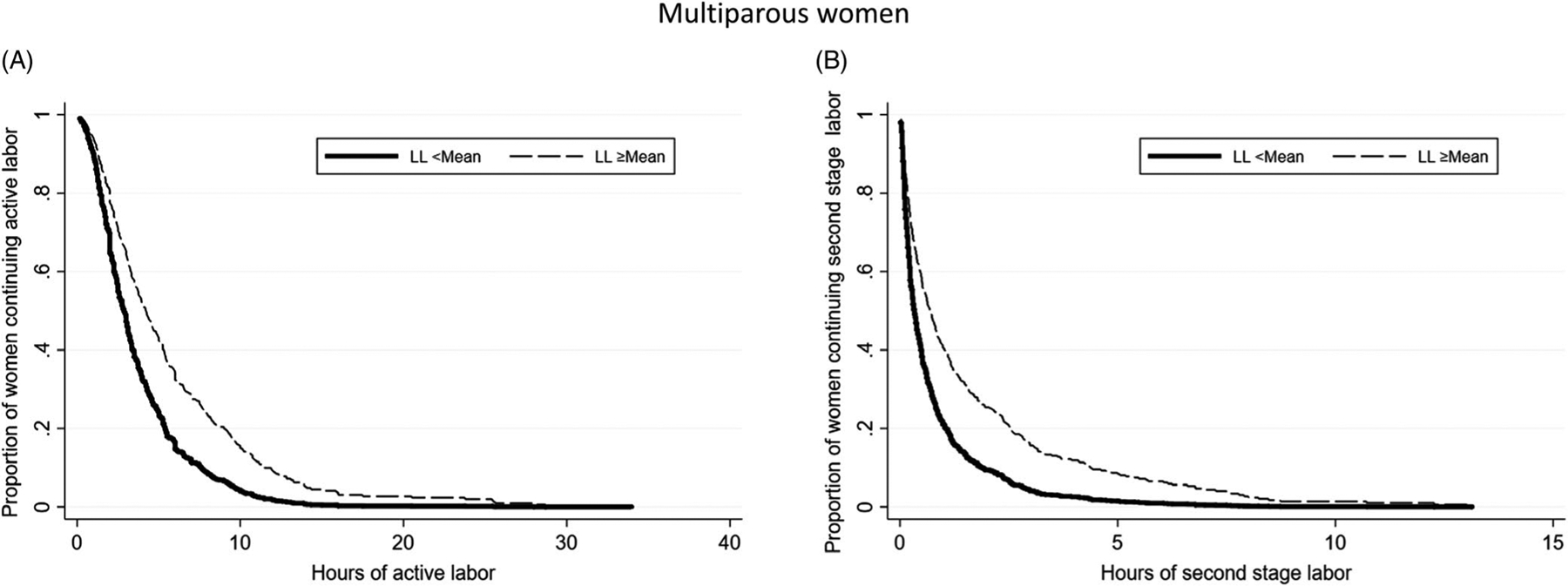
Survival curves from Cox proportional hazards models of (A) hours of active labor; (B) hours of second-stage labor among multiparous women with latent phase labor <mean and ≥greater than the mean
TABLE 6.
Estimate* of median active phase and second stage of labor in hours by length of latent labor
| Nulliparous women | Multiparous Women | |||
|---|---|---|---|---|
| Stage of labor | Latent labor duration < mean | Latent labor duration ≥ mean | Latent labor duration < mean | Latent labor duration ≥ mean |
| Active labor | 5.75 h | 8.00 h | 5.25 h | 8.25 h |
| Second-stage labor | 1.48 h | 1.90 h | 0.53 h | 1.17 h |
Note:
Estimate from model-based prediction for women: <35 y of age, middle 80th percentile of height, normal BMI, weight gain within CDC guidelines, partnered, no diabetes, no PROM, no GBS, birthweight AGA, term delivery.
4 |. DISCUSSION
4.1 |. Principal findings
In this sample of low-risk, United States women in spontaneous labor receiving in-hospital, CNM care, those with longer durations of the latent phase of labor were more likely to be diagnosed with labor dystocia and receive interventions to ameliorate dystocia (amniotomy, oxytocin augmentation) and interventions to cope or rest (epidural) during the active phase or second stage. In addition, we found that women with longer latent phase durations also experienced longer durations of both active phase and second stage. We found no association between the duration of the latent phase and risk for cesarean delivery or postpartum hemorrhage, but we did find that NICU admission was more common after longer (>80th and 95th percentile) latent phase in multiparous women. Sensitivity analysis using a neonatal morbidity composite variable suggested that 70% of these NICU admissions were for observation only, and risk for neonatal morbidity only reached significance when multiparous women’s latent phase durations exceeded the 90th%.
4.2 |. Clinical implications
Longer duration of the spontaneous latent phase of labor among women with low-risk pregnancies may signal longer total labor processes leading to an increase in diagnosis of dystocia, interventions to manage dystocia, and epidural use.17 It is possible that this pattern is related to abnormal or underdeveloped biological processes, but other factors may be involved. For example, longer latent phase may contribute to maternal exhaustion, increasing the desire or need for interventions to expedite rest and birth. Thus, increasing women’s opportunities to rest and receive support during the latent phase of labor are appropriate clinical actions.
Findings suggesting a potential association between longer latent phase duration and NICU admission among multiparous women are interesting as the duration in hours at the 80th, 90th, and 95th percentiles is shorter among multiparous than nulliparous women (eg, 24.5 hours vs. 30.0 hours at the 95th%),11 suggesting it may not be the number of hours in latent phase that is the fetal stressor. Instead, a longer latent phase of labor in multiparous women may signal underlying complications. Given prior findings that multiparous women with latent phase durations at the mean and beyond had significantly higher rates of chorioamnionitis,11 it is also possible that chorioamnionitis is a mediator between latent phase duration and NICU admission.32,33 Similarly, fetal malposition might be a mediator among nulliparous women. These findings add to knowledge from prior work signaling potential association and mediators between the duration of the latent phase of labor and worse neonatal outcomes.10
Because labor dystocia is the leading indicator for primary cesarean in the United States,7 it was interesting that there was no association between longer latent phase and increased risk for cesarean, despite the clear association between longer latent phase and labor dystocia in this sample. Given the relatively low unscheduled primary cesarean rates in the practice environment of this study, it is likely that multiple factors contributed to facilitation of vaginal birth after dystocia diagnosis.11,19,37 It is also worth noting that many women birthing in this setting experienced safe vaginal delivery even when labor durations were longer and included labor dystocia diagnoses. These incidental findings could signal that greater patience with longer labor durations may be appropriate for this population.
4.3 |. Research implications
It will be valuable to study if underlying physiologic or pathophysiologic factors might trigger both longer latent phase of labor and maternal or neonatal morbidity. We also endorse use of neonatal morbidity markers in conjunction with or in place of NICU admission for future research.
Future research should also explore these questions using more diverse samples. It has been noted that subpopulations of women have different labor patterns. For example, there is evidence that maternal adiposity affects cervical ripening, labor onset, and the efficiency of uterine contractions.20,38 As the latent phase of labor is the time when cervical effacement and initial dilation take place, these findings linking maternal adiposity to delays in cervical preparation and ripening could have implications for latent phase duration in women with obesity. Large databases are needed to study labor patterns across diverse populations of women and to enable robust subanalyses by maternal characteristics.33
Decades of research show that hospital admission of low-risk women in the latent phase of labor is associated with increased interventions, especially cesarean delivery.39,40 The association between total duration of latent phase with longer durations of active and second stage of labor (similar to correlations observed between the first and second stages of labor)17 raises new questions about the hospital admission-intervention association. If this association holds true in other populations, it may be that hospital admission at a shorter duration of latent phase (eg, earlier in the labor process) is a mediator on the pathway between latent phase of labor duration and intervention rather than a cause of intervention overuse. Future research should address this uncertainty by collecting data about the full duration of the latent phase, including the time that women experience latent phase of labor symptoms before hospital admission and comparing women with similar latent phase durations who were admitted to the hospital before vs. after active labor onset.
A related question is whether the duration of the latent phase influences subsequent labor dystocia or whether the duration of the latent phase is one of several components of labor dystocia. The phases of labor have traditionally been conceptualized and described as separate phenomenon in research as well as practice.1,3 Future research might examine perinatal outcomes related to duration of separate phases of labor vs. duration of labor as one unit.41
4.4 |. Strengths and limitations
The duration of the latent phase variable used for this analysis is likely shaped by variation in women’s identification of labor onset. Thus, it is possible that the duration of latent phase in this data set is more importantly related to women’s latent phase of labor symptom perception and/or capacity to cope with latent phase of labor symptoms than underlying physiologic processes.42,43 Coping in this study could have been influenced by factors such as doula and nursing care during labor. As well, antecedents, such as self-efficacy, may play a role in when women note spontaneous latent phase onset and progression of the latent phase of labor and what coping approaches lead to improved self-management during latent phase.41,44,45 Future research should explore symptoms that women experience during the onset and progression of latent labor and what coping approaches lead to improved self-management during latent labor.16,42,46–48 Future research should also examine whether women benefit from antenatal skill building to help them cope with latent phase symptoms,49 potentially shortening the period of perceived latent phase duration.
The diagnosis of labor dystocia is also fundamentally informed by the maternity care provider’s definition or perception of normal and abnormal labor length.2 Longer durations of the latent phase may prime maternity care providers to perceive the subsequent phases of labor as “slow” and intervene to hasten delivery. Factors shaping provider perception of normal vs. abnormal labor progress and the diagnosis of dystocia are important to explore in future research.
Study strengths include the use of a data set with a latent phase of labor duration variable that includes labor before hospital admission. This facilitates study of processes and outcomes related to the entire duration of spontaneous latent phase. Limitations of the study include sample homogeneity and size. Finally, as spontaneous latent phase of labor research is nascent, there was little guidance for specifying regression models.
4.5 |. Conclusions
This study contributes contemporary estimates of the correlation between the entire duration of the latent phase (including labor that occurred before hospital admission) and labor processes and outcomes during the active phase and second stage of labor. We noted significant association between longer durations of the spontaneous latent phase of labor and longer durations of subsequent labor phases, more frequent diagnosis of labor dystocia, and greater use of several interventions but not cesarean. We found no association between duration of the latent phase of labor and risk for postpartum hemorrhage, and we found no association between the duration of latent phase and increased morbidity risk for newborns of nulliparous women. The association between longer multiparous latent phase duration and neonatal morbidity is an important direction for future research, and use of markers other than NICU admission is recommended.
Funding information
During this research, Dr Ellen Tilden received support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and National Institutes of Health Office of Research on Women’s Health, Oregon BIRCWH Scholars in Women’s Health Research across the Lifespan (K12HD043488-14). This source of funding had no involvement in any aspects of the research presented in this manuscript. During this research,Dr Jonathan M. Snowden and Mekhala Dissanayake were supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant number R00 HD079658-03). During this research, Dr Julia C. Phillippi was supported by the Agency for Healthcare Research and Quality [grant number K08HS024733]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. Dr Nicole Carlson was supported by the National Institute of Nursing Research of the National Institutes of Health under Award Number K01NR016984 during research reported in this publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Friedman E. Primigravid labor: a graphicostatistical analysis. Obstet Gynecol. 1955;6(6):567–589. [DOI] [PubMed] [Google Scholar]
- 2.Neal JL, Ryan SL, Lowe NK et al. Labor dystocia: uses of related nomenclature. J Midwifery Women’s Health. 2015;60(5):485–498. [DOI] [PubMed] [Google Scholar]
- 3.Friedman EA. Labor in multiparas: a graphicostatistical analysis. Obstet Gynecol. 1956;8(6):686–703. [PubMed] [Google Scholar]
- 4.Cichowitz C, Watt MH, McHome B, Masenga GG. Delays contributing to the development and repair of obstetric fistula in northern Tanzania. Int Urogynecol J. 2018;29(3):397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alkema L, Chou D, Hogan D. Global, regional, and national levels and trends in maternal mortality between 1990 and 2015, with scenario-based projections to 2030: a systematic analysis by the UN Maternal Mortality Estimation Inter-Agency Group. Lancet. 2015;15:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu L, Oza S, Hogan D et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. The Lancet. 2016;388(10063):3027–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caughey A, Cahill AG, Guise J-M, Rouse DJ. Safe prevention of the primary cesarean delivery. Am J Obstet Gynecol. 2014;210:179–193. [DOI] [PubMed] [Google Scholar]
- 8.Silver RM. Implications of the first cesarean: perinatal and future reproductive health and subsequent cesareans, placentation issues, uterine rupture risk, morbidity, and mortality. Semin Perinatol. 2012;36(5):315–323. [DOI] [PubMed] [Google Scholar]
- 9.Yuan C, Gaskins AJ, Blaine AI et al. Association between cesarean birth and risk of obesity in offspring in childhood, adolescence, and early adulthood. JAMA Pediatrics. 2016;170(11);e162385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenbloom JI, Woolfolk CL, Wan L et al. The transition from latent to active labor and adverse obstetrical outcomes. Am J Obstet Gynecol. 2019;221(5):487.e1–487.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tilden EL, Phillippi JC, Ahlberg M et al. Describing latent phase duration and associated characteristics among 1281 low-risk women in spontaneous labor. Birth. 2019;46(4):592–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedman EA, Sachtleben MR. Dysfunctional labor. I. Prolonged latent phase in the nullipara. Obstet Gynecol. 1961;17:135–148. [PubMed] [Google Scholar]
- 13.Peisner DB, Rosen MG. Latent phase of labor in normal patients: a reassessment. Obstet Gynecol. 1985;66(5):644–648. [PubMed] [Google Scholar]
- 14.Peisner DB, Rosen MG. Transition from latent to active labor. Obstet Gynecol. 1986;68(4):448–451. [PubMed] [Google Scholar]
- 15.Ängeby K, Wilde-Larsson B, Hildingsson I, Sandin-Bojö AK. Prevalence of prolonged latent phase and labor outcomes: review of birth records in a Swedish population. J Midwifery Women’s Health. 2018;63(1):33–44. [DOI] [PubMed] [Google Scholar]
- 16.Gross MM, Burian RA, Frömke C, Hecker H, Schippert C, Hillemanns P. Onset of labour: women’s experiences and midwives’ assessments in relation to first stage duration. Arch Gynecol Obstet. 2009;280(6):899–905. [DOI] [PubMed] [Google Scholar]
- 17.Nelson DB, McIntire DD, Leveno KJ. Relationship of the length of the first stage of labor to the length of the second stage. Obstet Gynecol. 2013;122(1):27–32. [DOI] [PubMed] [Google Scholar]
- 18.Neal JL, Carlson NS, Phillippi JC et al. Midwifery presence in United States medical centers and labor care and birth outcomes among low-risk nulliparous women: a Consortium on Safe Labor study. Birth. 2018;46(3):475–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White VanGompel E, Perez S, Datta A, Wang C, Cape V, Main E. Cesarean overuse and the culture of care. Health Serv Res. 2019;54(2):417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kominiarek MA, Zhang J, Vanveldhuisen P, Troendle J, Beaver J, Hibbard JU. Contemporary labor patterns: the impact of maternal body mass index. Am J Obstet Gynecol. 2011;205(3):244.e1–244. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young TK, Woodmansee B. Factors that are associated with cesarean delivery in a large private practice: the importance of prepregnancy body mass index and weight gain. Am J Obstet Gynecol. 2002;187(2):312–318. [DOI] [PubMed] [Google Scholar]
- 22.Olesen AW, Westergaard JG, Olsen J. Perinatal and maternal complications related to postterm delivery: a national register-based study, 1978–1993. Am J Obstet Gynecol. 2003;189(1):222–227. [DOI] [PubMed] [Google Scholar]
- 23.Mogren I, Lindqvist M, Petersson K et al. Maternal height and risk of caesarean section in singleton births in Sweden—a population-based study using data from the Swedish Pregnancy Register 2011 to 2016. PLOS ONE. 2018;13(5):e0198124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aiken CEM, Hone L, Murphy HR, Meek CL. Improving outcomes in gestational diabetes: does gestational weight gain matter? Diabet Med. 2019;36(2):167–176. [DOI] [PubMed] [Google Scholar]
- 25.Sebire NJ, Jolly M, Harris JP et al. Maternal obesity and pregnancy outcome: a study of 287 213 pregnancies in London. Int J Obesity. 2001;25(8):1175–1182. [DOI] [PubMed] [Google Scholar]
- 26.Cleary-Goldman J, Malone FD, Vidaver J et al. Impact of maternal age on obstetric outcome. Obstet Gynecol. 2005;105(5):983–990. [DOI] [PubMed] [Google Scholar]
- 27.Madan E, Meyer MP, Amortequi A. Chorioamnionitis: a study of organisms isolated in perinatal autopsies. Ann Clin Lab Sci. 1988;18(1):39–45. [PubMed] [Google Scholar]
- 28.Hannah ME, Hodnett ED, Willan A, Foster GA, Di Cecco R, Helewa M. Prelabor rupture of the membranes at term: expectant management at home or in hospital? Obstet Gynecol. 2000;96(4):533–538. [DOI] [PubMed] [Google Scholar]
- 29.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. New York, NY: Wolters Kluwer; 2008. [Google Scholar]
- 30.Pilliod RA, Caughey AB. Fetal malpresentation and malposition: diagnosis and management. Obstet Gynecol Clin North Am. 2017;44(4):631–643. [DOI] [PubMed] [Google Scholar]
- 31.Edwards RK. Chorioamnionitis and labor. Obstet Gynecol Clin North Am. 2005;32(2):287–296. [DOI] [PubMed] [Google Scholar]
- 32.Clapp MA, James KE, Bates SV, Kaimal AJ. Unexpected term NICU admissions: a marker of obstetrical care quality? Am J Obstet Gynecol. 2019;220(4):395.e391–395.e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tilden EL, Snowden JM. The causal inference framework: a primer on concepts and methods for improving the study of wellwoman childbearing processes. J Midwifery Women’s Health. 2018;63(6):700–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cole SR, Hernan MA. Fallibility in estimating direct effects. Int J Epidemiol. 2002;31(1):163–165. [DOI] [PubMed] [Google Scholar]
- 35.Snowden JM, Tilden EL. Further applications of advanced methods to infer causes in the study of physiologic childbirth. J Midwifery Women’s Health. 2018;63(6):710–720. [DOI] [PubMed] [Google Scholar]
- 36.Snowden JM, Rose S, Mortimer KM. Implementation of G-computation on a simulated data set: demonstration of a causal inference technique. Am J Epidemiol. 2011;173(7):731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tilden EL, Snowden JM, Caughey AB, Lowe NK. Reframing US maternity care: lessons learned from end-of-life care. J Midwifery Women’s Health. 2016;62(1):9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carlson NS, Hernandez TL, Hurt KJ. Parturition dysfunction in obesity: time to target the pathobiology. Reprod Biol Endocrinol. 2015;13(1):135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hemminki E, Simukka R. The timing of hospital admission and progress of labour. Eur J Obstetric Gynecol Reprod Biol. 1986;22:85–94. [DOI] [PubMed] [Google Scholar]
- 40.Iobst SE, Breman RB, Bingham D, Storr CL, Zhu S, Johantgen M. Associations among cervical dilatation at admission, intrapartum care, and birth mode in low-risk, nulliparous women. Birth. 2019;46(2):253–261. [DOI] [PubMed] [Google Scholar]
- 41.Tilden EL, Caughey AB, Lee CS, Emeis C. The effect of childbirth self-efficacy on perinatal outcomes. JOGNN - J Obstetric Gynecol Neonatal Nursing. 2016;45(4):465–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riegel B, Jaarsma T, Lee CS, Stromberg A. Integrating symptoms into the middle-range theory of self-care of chronic illness. Adv Nurs Sci. 2019;42(3):206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whitburn LY, Jones LE, Davey MA, McDonald S. The nature of labour pain: an updated review of the literature. Women Birth. 2019;32(1):28–38. [DOI] [PubMed] [Google Scholar]
- 44.Lowe N. The nature of labor pain. Am J Obstet Gynecol. 2002;186(5):S16–S24. [DOI] [PubMed] [Google Scholar]
- 45.Lowe N. Maternal confidence for labor: development of childbirth self-efficacy inventory. Res Nurs Health. 1993;16(2):141–149. [DOI] [PubMed] [Google Scholar]
- 46.Gross MM, Haunschild T, Stoexen T, Methner V, Guenter HH. Women’s recognition of the spontaneous onset of labor. Birth. 2003;30(4):267–271. [DOI] [PubMed] [Google Scholar]
- 47.Edmonds JK, Zabbo G. Women’s descriptions of labor onset and progression before hospital admission. Nurs Women’s Health. 2017;21(4):250–258. [DOI] [PubMed] [Google Scholar]
- 48.Eri TS, Bondas T, Gross MM, Janssen P, Green JM. A balancing act in an unknown territory: a metasynthesis of first-time mothers experiences in early labour. Midwifery. 2015;31(3):e58–e67. [DOI] [PubMed] [Google Scholar]
- 49.Tilden EL, Emeis CL, Caughey AB, Weinstein SR, Futernick SB, Lee CS. The influence of group versus individual prenatal care on phase of labor at hospital admission. J Midwifery Women’s Health. 2016;61(4):427–434. [DOI] [PubMed] [Google Scholar]


