Abstract
Aims
The associations between potassium level and outcomes, the effect of sacubitril–valsartan on potassium level, and whether potassium level modified the effect of sacubitril–valsartan in patients with heart failure and a reduced ejection fraction were studied in PARADIGM‐HF. Several outcomes, including cardiovascular death, sudden death, pump failure death, non‐cardiovascular death and heart failure hospitalization, were examined.
Methods and results
A total of 8399 patients were randomized to either enalapril or sacubitril–valsartan. Potassium level at randomization and follow‐up was examined as a continuous and categorical variable (≤3.5, 3.6–4.0, 4.1–4.9, 5.0–5.4 and ≥5.5 mmol/L) in various statistical models. Hyperkalaemia was defined as K+ ≥5.5 mmol/L and hypokalaemia as K+ ≤3.5 mmol/L. Compared with potassium 4.1–4.9 mmol/L, both hypokalaemia [hazard ratio (HR) 2.40, 95% confidence interval (CI) 1.84–3.14] and hyperkalaemia (HR 1.42, 95% CI 1.10–1.83) were associated with a higher risk for cardiovascular death. However, potassium abnormalities were similarly associated with sudden death and pump failure death, as well as non‐cardiovascular death and heart failure hospitalization. Sacubitril–valsartan had no effect on potassium overall. The benefit of sacubitril–valsartan over enalapril was consistent across the range of baseline potassium levels.
Conclusions
Although both higher and lower potassium levels were independent predictors of cardiovascular death, potassium abnormalities may mainly be markers rather than mediators of risk for death.
Keywords: Potassium, Outcomes, Hypokalaemia, Hyperkalaemia, Sacubitril–valsartan, Mineralocorticoid receptor antagonists
Introduction
Potassium is essential for normal cellular function and alterations in its regulation can lead to gastrointestinal, neuromuscular and cardiac abnormalities, some of which can be life‐threatening. 1 The treatments used in patients with heart failure and reduced ejection fraction (HFrEF) commonly cause potassium disturbances; hypokalaemia is induced by loop and thiazide diuretics, and hyperkalaemia results from the use of renin–angiotensin–aldosterone system (RAAS) inhibitors. 2 , 3 Although the clinical significance, and even the definition, of hyperkalaemia is a matter of debate, higher potassium concentrations often lead to the withholding or withdrawal of RAAS inhibitors, which are life‐saving therapies in patients with HFrEF. 4 However, hypokalaemia may be of as much concern as hyperkalaemia in HFrEF because in other groups of individuals (e.g. the general population and patients with myocardial infarction, hypertension and chronic kidney disease 5 , 6 , 7 , 8 , 9 ) the relationship between potassium concentration and clinical outcomes is U‐shaped, and both low and high potassium levels are associated with worse outcomes. 10 , 11 , 12
The effects of sacubitril–valsartan on potassium have not been described in detail and may differ from those of enalapril as sacubitril–valsartan reduces aldosterone, a key regulator of potassium. 13 The sacubitril–valsartan combination probably lowers aldosterone because natriuretic peptides are powerful inhibitors of aldosterone secretion and neprilysin inhibition (sacubitril) increases natriuretic peptide levels. 14 , 15 The Prospective Comparison of ARNI with an ACE‐Inhibitor to Determine Impact on Global Mortality and Morbidity in Heart Failure trial (PARADIGM‐HF) 13 represents one of the largest and most contemporary HFrEF cohorts, in which most patients received each of the aforementioned drugs known to affect potassium levels.
The aims of the present study were to examine the range of plasma potassium concentrations found in patients with HFrEF, the relationships between potassium concentration and outcomes, and the effect of sacubitril–valsartan on potassium.
Methods
PARADIGM‐HF
The design, methods and principal results of the PARADIGM‐HF trial have been previously reported. 13 In brief, the trial was a randomized, double‐blind, prospective comparison of the effect of sacubitril (97 mg) and valsartan (103 mg) administered twice daily (LCZ696, 200 mg twice daily) and that of enalapril (10 mg twice daily) in 8399 patients with chronic HF [New York Heart Association (NYHA) classes II–IV] and a left ventricular ejection fraction (LVEF) of ≤40%. Prior to randomization, all participants underwent single‐blind, sequential run‐in periods to ensure the tolerability of both study drugs at target doses. Eligible patients were those treated with an appropriate regimen of background HF medications at stable doses for at least 4 weeks, including an angiotensin‐converting enzyme inhibitor (ACEi) or an angiotensin receptor blocker (ARB) (at a dose equivalent to enalapril 10 mg/day or greater) and a beta‐blocker (unless not tolerated). The use of mineralocorticoid receptor antagonists (MRAs) was left to the discretion of the investigators but was encouraged if tolerated. Key exclusion criteria included symptomatic hypotension, a systolic blood pressure (SBP) of <100 mmHg at screening or 95 mmHg at randomization, an estimated glomerular filtration rate (eGFR) of <30 mL/min/1.73m2 at screening/randomization or a decrease in the eGFR of >25% (amended to 35%) between screening and randomization, and a serum K+ level of >5.2 mmol/L at screening or >5.4 mmol/L at randomization. Serum K+ was measured at every study visit (Supplementary material online, Table S1 ). The protocol recommended that any patient with serum potassium of >5.3 mmol/L after randomization required regular, repeated checks of potassium concentration (beyond that prescribed in the protocol) until it was clear that the potassium concentration was stable and not rising to levels of concern (≥5.5 mmol/L and <6.0 mmol/L) or potential danger (≥6.0 mmol/L). 13 No recommendation for dose adjustment in the setting of hypokalaemia was given. The primary composite endpoint was death from cardiovascular (CV) causes or first hospitalization for HF (CV death/HF hospitalization). The present analysis also examines CV, all‐cause and sudden cardiac death (or resuscitated cardiac arrest). The median follow‐up time was 810 (range: 564–1069) days or 116 (range: 81–153) weeks. The trial was approved by the institutional review board or ethics committee at each participating site, and all participants provided written informed consent prior to participation.
Statistical analysis
Continuous variables are expressed as the mean ± standard deviation or median (percentile25‐75). Categorical variables are presented as absolute numbers (n) and percentages. Baseline characteristics were compared between the following K+ categories of ≤3.5, 3.6–4.0, 4.1–4.9, 5.0–5.4 and ≥5.5 mmol/L, using analysis of variance (ANOVA) or Kruskal–Wallis tests as appropriate. Potassium levels below and above the optimal range of 4–5 mmol/L were examined in order to better elucidate which patient characteristics were associated with ‘non‐optimal’ potassium concentrations and how clinical outcomes related to potassium levels above and below the optimal range. The additional categories created were ≤3.5 mmol/L and 3.6–4.0 mmol/L, as well as 5.0–5.4 mmol/L and ≥5.5 mmol/L, as these included sufficient numbers of patients and events to allow for valid statistical analysis. Several models were used to study the association of K+ with the outcomes. For baseline K+, Cox proportional hazards models were used with K+ as a categorical or continuous variable (using a fractional polynomial ‘spline’ with five knots as described by Harrell 16 ). For K+ throughout the follow‐up, time‐updated (‘repeated measures’) Cox models were used with ‘start’ and ‘stop’ times between each K+ measurement. Multi‐level survival analysis (a two‐stage ‘joint model’) was also used to combine the longitudinal (repeated‐measures) and survival (time‐to‐event endpoint) aspects of the data. A two‐level model with patient identification as a random effect and the log (follow‐up time) as a random coefficient was fitted. For the survival portion of the model, a Weibull distribution was used, with the continuous K+ (fractional polynomial) as the main exposure variable. Landmark analyses were performed to assess the association between the cumulative plus K+ changes at 2 months and subsequent outcomes. The 2‐month landmark was chosen to avoid excessive missing K+ values (Supplementary material online, Table S1 ). Regression estimates are presented as hazard ratios (HR) with 95% confidence intervals (CIs). The models were adjusted for all the characteristics presented in Table 1 , including age, diabetes, race, region, NYHA class, N‐terminal pro brain natriuretic peptide (NT‐proBNP), concomitant medications and eGFR (time‐updated eGFR whenever appropriate). Potential unmeasured confounding was examined by calculating E‐values. 17 All statistical analyses were conducted in STATA® Version 16 (StataCorp LP, College Station, TX, USA). A P‐value of <0.05 was accepted as the threshold for statistical significance without correction for multiplicity of tests given the exploratory nature of this analysis.
Table 1.
Baseline characteristics of PARADIGM‐HF patients by potassium categories at randomization
| Characteristics by K+ categories | ≤3.5 mmol/L | 3.6–4.0 mmol/L | 4.1–4.9 mmol/L | 5.0–5.4 mmol/L | ≥5.5 mmol/L | P‐value |
|---|---|---|---|---|---|---|
| n | 164 | 1069 | 5625 | 1168 | 197 | |
| Age, years, mean ± SD | 62.0 ± 13.0 | 61.7 ± 12.2 | 63.9 ± 11.3 | 65.4 ± 10.7 | 63.4 ± 10.8 | <0.001 |
| Age >70 years, n (%) | 42 (25.6) | 258 (24.1) | 1704 (30.3) | 400 (34.3) | 58 (29.4) | <0.001 |
| Female sex, n (%) | 39 (23.8) | 251 (23.5) | 1213 (21.6) | 251 (21.5) | 45 (22.8) | 0.65 |
| Race or ethnic group, n (%) | ||||||
| White | 70 (42.7) | 530 (49.6) | 3796 (67.5) | 858 (73.5) | 150 (76.1) | <0.001 |
| Black | 24 (14.6) | 95 (8.9) | 261 (4.6) | 37 (3.2) | 5 (2.5) | |
| Asian | 44 (26.8) | 294 (27.5) | 988 (17.6) | 152 (13.0) | 23 (11.7) | |
| Other | 26 (15.9) | 150 (14.0) | 580 (10.3) | 121 (10.4) | 19 (9.6) | |
| Region, n (%) | ||||||
| North America | 26 (15.9) | 132 (12.3) | 379 (6.7) | 45 (3.9) | 6 (3.0) | <0.001 |
| Latin America | 33 (20.1) | 227 (21.2) | 928 (16.5) | 188 (16.1) | 32 (16.2) | |
| Western Europe and other | 36 (22.0) | 179 (16.7) | 1436 (25.5) | 297 (25.4) | 38 (19.3) | |
| Central Europe | 27 (16.5) | 243 (22.7) | 1906 (33.9) | 490 (42.0) | 97 (49.2) | |
| Asia Pacific | 42 (25.6) | 288 (26.9) | 976 (17.4) | 148 (12.7) | 24 (12.2) | |
| Systolic blood pressure, mmHg, mean ± SD | 125.0 ± 17.0 | 122.5 ± 16.1 | 121.3 ± 15.3 | 120.1 ± 14.4 | 119.9 ± 13.3 | <0.001 |
| Heart rate, bpm, mean ± SD | 75.5 ± 13.3 | 73.5 ± 12.0 | 72.1 ± 11.9 | 72.2 ± 12.3 | 72.8 ± 12.8 | <0.001 |
| BMI, kg/m2, mean ± SD | 28.2 ± 7.5 | 27.9 ± 5.8 | 28.2 ± 5.4 | 28.3 ± 5.4 | 28.0 ± 5.7 | 0.42 |
| eGFR, mL/min/1.73 m2, mean ± SD | 72.4 ± 34.1 | 72.4 ± 20.8 | 68.0 ± 19.5 | 62.6 ± 18.5 | 60.3 ± 18.9 | <0.001 |
| eGFR ≤60, n (%) | 62 (37.8) | 312 (29.2) | 2083 (37.0) | 582 (49.8) | 108 (54.8) | <0.001 |
| Ischaemic cardiomyopathy, n (%) | 90 (54.9) | 588 (55.0) | 3347 (59.5) | 757 (64.8) | 135 (68.5) | <0.001 |
| Left ventricular ejection fraction, %, mean ± SD | 29.3 ± 6.4 | 29.4 ± 6.4 | 29.5 ± 6.2 | 29.6 ± 6.1 | 30.1 ± 6.4 | 0.59 |
| BNP, pg/mL, median (range) | 311 (184–717) | 291 (169–611) | 243 (150–447) | 260 (160–468) | 271 (171–530) | <0.001 |
| BNP in AF+, median (range) | 360 (184–692) | 291 (181–544) | 245 (157–434) | 251 (159–453) | 273 (185–588) | <0.001 |
| BNP in AF‐, median (range) | 302 (183–783) | 291 (161–643) | 241 (145–455) | 269 (160–471) | 267 (153–491) | <0.001 |
| NT‐proBNP, pg/mL, median (range) | 2476 (1199–5093) | 1884 (941–4165) | 1541 (862–3024) | 1705 (946–3380) | 1747 (926–3392) | <0.001 |
| NT‐proBNP in AF+, median (range) | 3019 (1561–6083) | 2131 (1114–4520) | 1812 (1059–3420) | 1961 (1172–3611) | 2155 (1412–4781) | <0.001 |
| NT‐proBNP in AF‐, median (range) | 2276 (1090–4565) | 1759 (847–3863) | 1383 (804–2782) | 1532 (856–3161) | 1547 (747–2424) | <0.001 |
| NYHA functional class, n (%) | ||||||
| I | 6 (3.7) | 50 (4.7) | 270 (4.8) | 46 (4.0) | 9 (4.6) | 0.37 |
| II | 120 (73.2) | 774 (72.5) | 3971 (70.7) | 801 (68.8) | 131 (67.2) | |
| III | 36 (22.0) | 235 (22.0) | 1342 (23.9) | 306 (26.3) | 55 (28.2) | |
| IV | 2 (1.2) | 9 (0.8) | 36 (0.6) | 11 (0.9) | 0 (0.0) | |
| Hypertension, n (%) | 116 (70.7) | 758 (70.9) | 3928 (69.8) | 866 (74.1) | 140 (71.1) | 0.068 |
| Diabetes, n (%) | 54 (32.9) | 334 (31.2) | 1915 (34.0) | 458 (39.2) | 79 (40.1) | <0.001 |
| Atrial fibrillation, n (%) | 60 (36.6) | 360 (33.7) | 2075 (36.9) | 445 (38.1) | 82 (41.6) | 0.12 |
| Prior HF hospitalization, n (%) | 119 (72.6) | 669 (62.6) | 3542 (63.0) | 715 (61.2) | 119 (60.4) | 0.074 |
| Prior MI, n (%) | 61 (37.2) | 411 (38.4) | 2420 (43.0) | 554 (47.4) | 99 (50.3) | <0.001 |
| Prior stroke, n (%) | 15 (9.1) | 77 (7.2) | 509 (9.0) | 95 (8.1) | 11 (5.6) | 0.15 |
| Prior use of ACEi, n (%) | 115 (70.1) | 772 (72.2) | 4395 (78.1) | 945 (80.9) | 162 (82.2) | <0.001 |
| Prior use of ARB, n (%) | 51 (31.1) | 298 (27.9) | 1249 (22.2) | 225 (19.3) | 36 (18.3) | <0.001 |
| Diuretic, n (%) | 141 (86.0) | 892 (83.4) | 4482 (79.7) | 920 (78.8) | 156 (79.2) | 0.011 |
| Digoxin, n (%) | 50 (30.5) | 334 (31.2) | 1718 (30.5) | 341 (29.2) | 57 (28.9) | 0.84 |
| Beta‐blocker, n (%) | 154 (93.9) | 968 (90.6) | 5247 (93.3) | 1098 (94.0) | 182 (92.4) | 0.013 |
| MRA, n (%) | 75 (45.7) | 509 (47.6) | 3179 (56.5) | 704 (60.3) | 117 (59.4) | <0.001 |
| ICD (incl. CRT‐D), n (%) | 18 (11.0) | 139 (13.0) | 868 (15.4) | 176 (15.1) | 18 (9.1) | 0.022 |
| CRT, n (%) | 9 (5.5) | 66 (6.2) | 398 (7.1) | 83 (7.1) | 9 (4.6) | 0.49 |
| Randomized to sacubitril–valsartan, n (%) | 84 (51.2) | 527 (49.3) | 2787 (49.5) | 605 (51.8) | 94 (47.7) | 0.63 |
ACEi, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BMI, body mass index; BNP, brain natriuretic peptide; CRT, cardiac resynchronization therapy; eGFR, estimated glomerular filtration rate; HF, heart failure; ICD, implantable cardioverter‐defibrillator; MI, myocardial infarction; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro brain natriuretic peptide; SD, standard deviation.
Results
Baseline characteristics
The clinical characteristics of patients, according to serum potassium category at randomization, are provided in Table 1 . Compared with patients with potassium within the normal range (4.1–4.9 mmol/L), those with lower potassium levels were younger, less often White, and had higher SBP and heart rate, better renal function, higher NT‐proBNP levels, a higher proportion of prior HF hospitalization, and a lower proportion of treatment with an ACEi/ARB and MRAs. In contrast, those with higher potassium levels were more often White, were of similar age, had similar SBP and heart rate, but had poorer renal function, slightly higher NT‐proBNP levels, and were more likely to have diabetes and to be treated with ACEi/ARBs and MRAs (Table 1 ).
Change in potassium level
A median of 8.5 (range: 1–16) central laboratory potassium measurements were performed per patient after randomization, averaging 13.6 measurements per year of follow‐up. During the follow‐up, serum potassium was available on 79 688 occasions (39 699 in the enalapril group and 39 989 in the sacubitril–valsartan group); there were 8473 occasions (10.6% of all occasions) when potassium was <4 mmol/L and 1601 (2.0%) occasions when potassium was ≤3.5 mmol/L. A potassium value of <4 mmol/L was observed on 3942 (9.9%) occasions in the enalapril group and on 4531 (11.3%) occasions in the sacubitril–valsartan group (sacubitril–valsartan vs. enalapril, P < 0.001). A potassium level of <3.5 mmol/L was observed on 722 (1.8%) occasions in the enalapril group and 879 (2.2%) occasions in the sacubitril–valsartan group (sacubitril–valsartan vs. enalapril, P < 0.001). During the follow‐up, a potassium level of >5 mmol/L was observed on 5368 (13.5%) occasions in the enalapril group and 4915 (12.3%) occasions in the sacubitril–valsartan group (sacubitril–valsartan vs. enalapril, P < 0.001). A potassium level of >5.5 mmol/L was observed on 974 (2.5%) occasions in the enalapril group and on 896 (2.2%) occasions in the sacubitril–valsartan group (sacubitril–valsartan vs. enalapril, P = 0.047) (Figure 1 ). Supplementary material online, Figure S1 presents the incidences of both hypo‐ and hyperkalaemia over time by treatment group.
Figure 1.
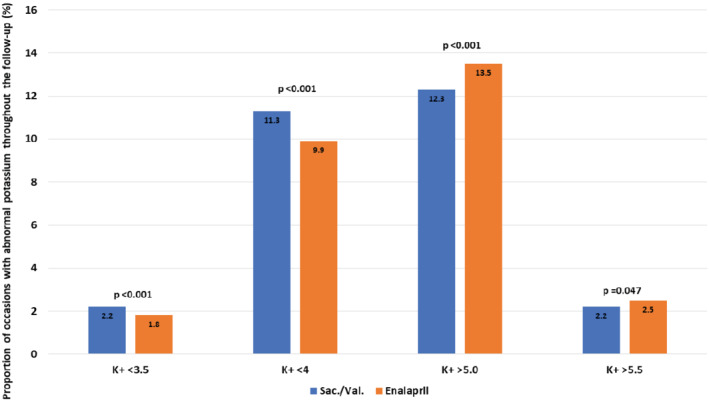
Proportion of occasions/visits in which an abnormal value of potassium was detected separated by treatment group. Sac/Val, sacubitril–valsartan.
Compared with enalapril, sacubitril–valsartan reduced potassium concentration slightly [mean change, compared with enalapril, at week 80: −0.07 mmol/L (range: −0.09 to −0.04 mmol/L); P < 0.001].
Patients treated with enalapril and an MRA had slightly higher potassium levels than patients treated with sacubitril–valsartan and an MRA. Patients treated with sacubitril–valsartan and an MRA had potassium levels similar to those in patients without MRA treatment (Figure 2 ).
Figure 2.
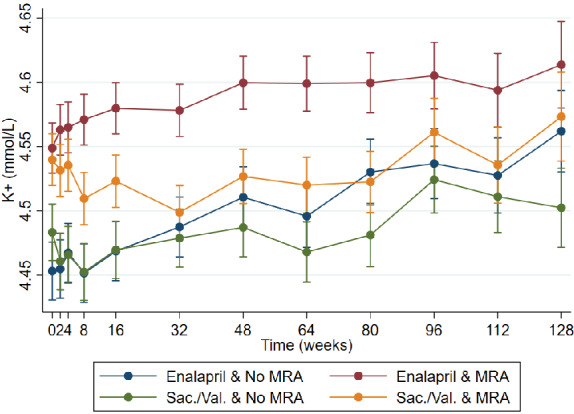
Potassium levels in patients allocated to sacubitril–valsartan or enalapril with or without a mineralocorticoid receptor antagonist (MRA). K+ change at week 80 sacubitril–valsartan vs. enalapril and no MRA: −0.05 (−0.08 to −0.01), P = 0.007. K+ change at week 80 sacubitril–valsartan vs. enalapril and MRA: −0.08 (−0.11 to −0.04), P < 0.001. Sac/Val, sacubitril–valsartan.
Clinical outcomes according to potassium level
There was a U‐shaped relationship between baseline potassium level and the clinical outcomes of interest. Patients in the lowest baseline potassium category (≤3.5 mmol/L) had higher event rates in comparison with patients with potassium in the normal range (4.1–4.9 mmol/L), even after adjustment for other prognostic variables (Table 2 ). This was also true for those in the highest potassium category (≥5.5 mmol/L) in relation to the mortality outcomes, although the magnitude of the elevation in risk was smaller than that seen in the lowest potassium category (Table 2 ).
Table 2.
Events, event rates and hazard ratios for baseline and time‐updated potassium levels for the various outcomes
| K+, mmol/L | Events, n (%) | Inc. rate, per 100 py | Baseline model | P‐value | Time‐updated model | P‐value | K+ by study drug interaction P‐value |
|---|---|---|---|---|---|---|---|
| Primary outcome | |||||||
| ≤3.5 | 70 (42.7) | 24.8 | 1.86 (1.46–2.37) | <0.001 | 1.91 (1.41–2.61) | <0.001 | 0.20 |
| 3.6–4.0 | 303 (28.3) | 14.1 | 1.25 (1.10–1.42) | 0.001 | 1.25 (1.06–1.47) | 0.009 | |
| 4.1–4.9 | 1277 (22.7) | 11 | Referent | – | Referent | – | |
| 5.0–5.4 | 292 (25.0) | 12.3 | 1.08 (0.95–1.23) | 0.25 | 1.08 (0.92–1.27) | 0.35 | |
| ≥5.5 | 47 (23.9) | 12.7 | 1.11 (0.83–1.48) | 0.50 | 1.38 (1.06–1.78) | 0.015 | |
| HF hospitalization | |||||||
| ≤3.5 | 40 (24.4) | 14.2 | 1.79 (1.29–2.47) | <0.001 | 2.01 (1.14–3.55) | 0.016 | 0.55 |
| 3.6–4.0 | 192 (18.0) | 8.9 | 1.39 (1.18–1.63) | <0.001 | 1.32 (9.98–1.77) | 0.063 | |
| 4.1–4.9 | 741 (13.2) | 6.4 | Referent | – | Referent | – | |
| 5.0–5.4 | 173 (14.8) | 7.3 | 1.08 (0.92–1.28) | 0.34 | 1.35 (1.03–1.77) | 0.028 | |
| ≥5.5 | 21 (10.7) | 5.7 | 0.85 (0.55–1.31) | 0.45 | 1.02 (0.50–1.08) | 0.95 | |
| CV death | |||||||
| ≤3.5 | 52 (31.7) | 16.1 | 2.25 (1.69–2.99) | <0.001 | 2.40 (1.84–3.14) | <0.001 | 0.45 |
| 3.6–4.0 | 177 (16.6) | 7.4 | 1.14 (0.97–1.35) | 0.11 | 1.27 (1.07–1.49) | 0.005 | |
| 4.1–4.9 | 778 (13.8) | 6.2 | Referent | – | Referent | – | |
| 5.0–5.4 | 186 (15.9) | 7.3 | 1.13 (0.97–1.34) | 0.11 | 1.19 (1.02–1.40) | 0.029 | |
| ≥5.5 | 37 (18.8) | 9.6 | 1.48 (1.06–2.06) | 0.021 | 1.42 (1.10–1.83) | 0.007 | |
| All‐cause death | |||||||
| ≤3.5 | 57 (34.8) | 17.6 | 2.00 (1.51–2.62) | <0.001 | 2.31 (1.80–2.96) | <0.001 | 0.41 |
| 3.6–4.0 | 215 (20.1) | 9 | 1.14 (0.98–1.33) | 0.084 | 1.26 (1.09–1.47) | 0.002 | |
| 4.1–4.9 | 963 (17.1) | 7.7 | Referent | – | Referent | – | |
| 5.0–5.4 | 235 (20.1) | 9.2 | 1.15 (1.00–1.33) | 0.052 | 1.26 (1.09–1.44) | 0.001 | |
| ≥5.5 | 47 (23.9) | 12.2 | 1.51 (1.12–2.02) | 0.006 | 1.37 (1.09–1.73) | 0.007 | |
| SCD or RCA | |||||||
| ≤3.5 | 21 (12.8) | 6.5 | 1.73 (1.11–2.70) | 0.015 | 2.45 (1.70–3.54) | <0.001 | 0.064 |
| 3.6–4.0 | 75 (7.0) | 3.1 | 0.90 (0.70–1.15) | 0.39 | 1.20 (0.95–1.52) | 0.13 | |
| 4.1–4.9 | 399 (7.1) | 3.2 | Referent | – | Referent | – | |
| 5.0–5.4 | 82 (7.0) | 3.2 | 1.03 (0.81–1.31) | 0.81 | 1.41 (1.13–1.75) | 0.002 | |
| ≥5.5 | 20 (10.2) | 5.3 | 1.65 (1.05–2.59) | 0.03 | 1.01 (0.64–1.59) | 0.97 | |
| Pump failure death | |||||||
| ≤3.5 | 15 (9.2) | 4.6 | 2.75 (1.61–4.70) | <0.001 | 3.43 (2.15–5.48) | <0.001 | 0.26 |
| 3.6–4.0 | 56 (5.2) | 2.3 | 1.54 (1.13–2.08) | 0.006 | 1.33 (0.96–1.85) | 0.087 | |
| 4.1–4.9 | 194 (3.5) | 1.6 | Referent | – | Referent | – | |
| 5.0–5.4 | 51 (4.4) | 2.0 | 1.18 (0.87–1.61) | 0.29 | 1.22 (0.91–1.65) | 0.19 | |
| ≥5.5 | 10 (5.1) | 2.6 | 1.54 (0.81–2.92) | 0.18 | 1.49 (0.93–1.37) | 0.095 | |
| Non‐CV death | |||||||
| ≤3.5 | 5 (3.1) | 1.5 | 0.83 (0.30–2.24) | 0.71 | 1.86 (0.95–3.66) | 0.072 | 0.46 |
| 3.6–4.0 | 38 (3.6) | 1.6 | 1.15 (0.80–1.64) | 0.45 | 1.24 (0.87–1.77) | 0.23 | |
| 4.1–4.9 | 185 (3.3) | 1.5 | Referent | – | Referent | – | |
| 5.0–5.4 | 49 (4.2) | 1.9 | 1.22 (0.88–1.67) | 0.23 | 1.54 (1.15–2.08) | 0.004 | |
| ≥5.5 | 10 (5.1) | 2.6 | 1.66 (0.87–3.15) | 0.12 | 1.17 (0.66–2.07) | 0.59 |
All models are adjusted on age, sex, race, region, systolic blood pressure, heart rate, body mass index, estimated glomerular filtration rate, ischaemic cardiomyopathy, left ventricular ejection fraction, N‐terminal pro brain natriuretic peptide, New York Heart Association class, hypertension, diabetes, atrial fibrillation, prior HF hospitalization, prior myocardial infarction, prior stroke, use of diuretics, digoxin, beta‐blockers, MRAs, cardiac devices, and treatment group allocation (sacubitril–valsartan or enalapril).
CV, cardiovascular; HF, heart failure; MRA, mineralocorticoid receptor antagonist; RCA, resuscitated cardiac arrest; SCD, sudden cardiac death or pump failure death.
The K+ group by study drug interaction P‐value refers to the interaction between the time‐updated potassium levels and the study drug (sacubitril–valsartan or enalapril). The interaction between the time‐updated potassium levels and eGFR (≤60 vs. >60 mL/min/1.73 m2) was also tested and none was significant (interaction P ≥ 0.1 for all the studied outcomes).
This overall pattern was generally more pronounced in the time‐updated models. For the primary outcome, the HR for a potassium level of 3.6–4.0 mmol/L was 1.25 (95% CI 1.06–1.47) and the HR for a potassium concentration of ≤3.5 mmol/L was 1.91 (95% CI 1.41–2.61), compared with the reference category (potassium 4.1–4.9 mmol/L). The HR for a potassium level of 5.0–5.4 mmol/L was 1.08 (95% CI 0.92–1.27) and for a potassium level of ≥5.5 mmol/L was 1.38 (95% CI 1.06–1.78).
Similar results were obtained for the individual components of the primary outcome and all‐cause death. The associations for sudden death and pump failure death were statistically significant for potassium levels of ≤3.5 mmol/L. Non‐CV death also appeared to show a U‐shaped association with potassium, but this was not statistically significant for potassium levels of either ≤3.5 mmol/L or ≥5.5 mmol/L, possibly as a result of the small number of events (Table 2 ).
The associations between time‐updated potassium level, modelled as a continuous variable, and the outcomes of interest are shown in Figure 3 and reinforce the findings of the categorical analyses described above. The same is shown for baseline potassium in supplementary material online, Figure S2 . The landmark analysis and joint models are illustrated in supplementary material online, Figures S3 and S4 . To offset the association between hypokalaemia (≤3.5 mmol/L) and risk for the primary outcome, an ‘unmeasured’ confounder would need to have an incremental HR of ≥2.5 on top of the adjusted analysis described (Supplementary material online, Figure S5 ).
Figure 3.
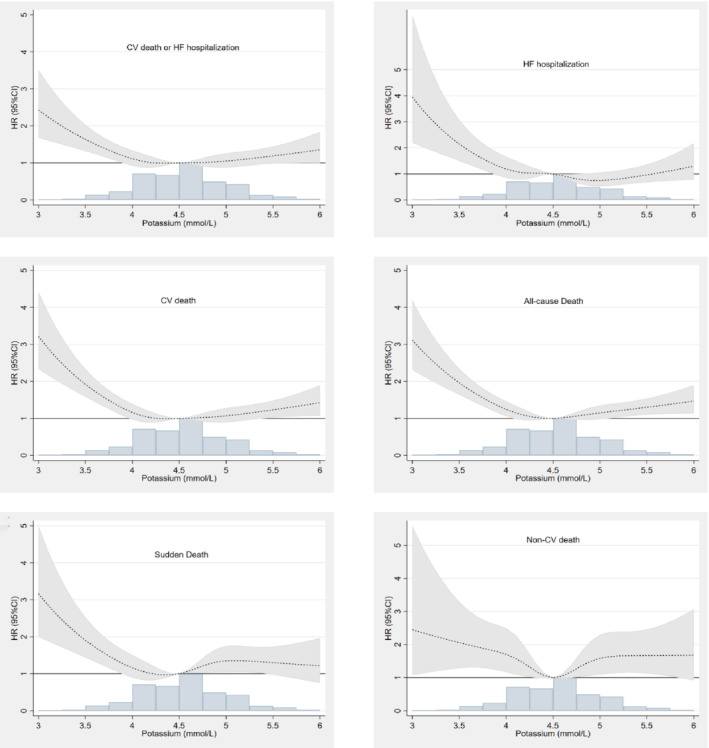
Time‐updated potassium and associations with the studied outcomes. The primary outcome was a composite of cardiovascular (CV) death or heart failure (HF) hospitalization. CI, confidence interval; HR, hazard ratio.
Outcomes related to change in potassium category
Landmark analysis, looking at outcomes according to change in potassium category, showed that patients moving from either the lowest or highest potassium category to the normal category had better outcomes than those remaining in the two extreme categories (Supplementary material online, Table S2 ). For example, the persistence of either hypokalaemia or hyperkalaemia was associated with a higher risk for subsequent CV death [HR 1.61 (95% CI 1.25–2.09), P < 0.001 for persistence of hypokalaemia; HR 1.32 (95% CI 1.01–1.72), P = 0.041 for persistence of hyperkalaemia].
Effect of sacubitril–valsartan according to potassium level
The benefit of sacubitril–valsartan over enalapril was consistent across baseline potassium categories and using potassium as a continuous time‐updated variable (Figure 4 ).
Figure 4.
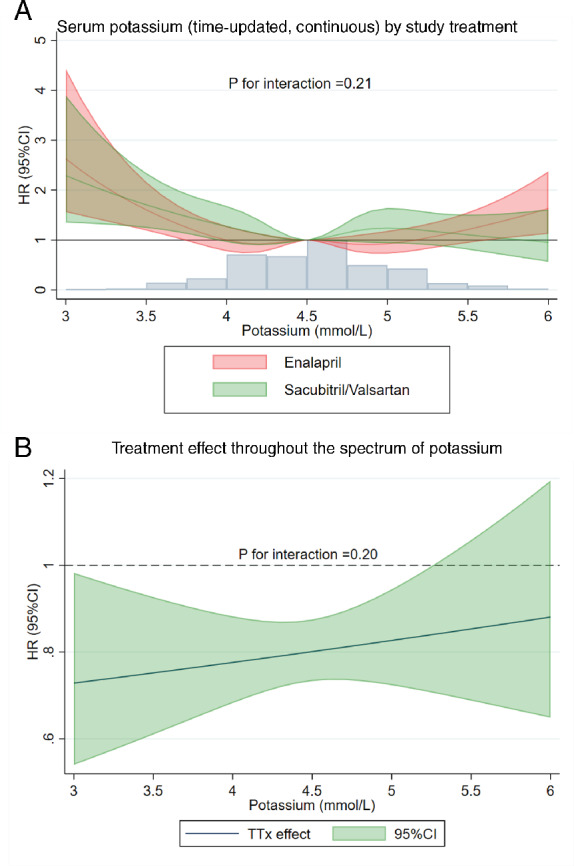
Interaction between serum potassium levels and the study treatment. (A) Serum potassium (time‐updated, continuous) by study treatment. (B) Treatment effect throughout the spectrum of potassium concentration. Example illustrating the primary outcome associations (similar results are obtained for the other outcomes) i.e. no potassium by study treatment interaction (see also Table 2 ). CI, confidence interval; HR, hazard ratio; TTx effect, treatment effect of sacubitril–valsartan vs. enalapril.
Discussion
The present study found that both hypo‐ and hyperkalaemia were associated with a higher risk for adverse clinical outcomes, compared with a normal potassium concentration. Although the risk was greater with hypokalaemia than with hyperkalaemia, the associations were largely non‐specific, suggesting that the risk related to potassium disturbances reflected disease severity, rather than a direct effect of potassium. Sacubitril–valsartan reduced potassium level slightly, on average, compared with enalapril. The beneficial effect of sacubitril–valsartan over enalapril was consistent across the spectrum of potassium levels studied in PARADIGM‐HF.
In keeping with prior studies in patients recently discharged after hospital admission for HF and in chronic HFrEF, the present study found a U‐shaped relationship between potassium level and mortality in the well‐treated ambulatory patients with chronic HFrEF and mainly mild symptoms in PARADIGM‐HF. 18 , 19 , 20 , 21 After adjusting for a wide range of clinical predictors, and NT‐proBNP, the elevation in risk for death associated with higher potassium was modest and less pronounced than in hypokalaemia. Although prior studies have suggested that the elevated risk for death associated with hyperkalaemia may reflect underdosing or even the withholding of RAAS blockers, 22 , 23 this is not likely to represent the explanation in the present trial given that patients started on the maximum dose of the study drug and every effort was made to maintain the optimal dose during follow‐up. Moreover, the associations between higher potassium and fatal outcomes were similar for all subtypes of death examined, including non‐CV death, which suggests a lack of any specific mechanistic direct effect of hyperkalaemia. This perspective is supported by the similar association between hyperkalaemia and risk for HF hospitalization, given the lack of obvious pathophysiological link between higher potassium and risk for HF hospitalization. Scrutiny of the baseline characteristics of participants with hyperkalaemia shows them to be older, to have more frequent ischaemic aetiology and greater comorbidity, including worse renal function. It is possible that the multivariable models used in the present study did not fully adjust for the measured and unmeasured differences between patients with hyperkalaemia and those with normal potassium levels. Therefore, a higher potassium concentration may generally represent a marker of a sicker patient, although, in individuals, occasionally, hyperkalaemia may still directly cause a fatal arrhythmia or conduction disturbance. 24 , 25 , 26
There was also an association between low potassium and higher mortality and the risk associated with low potassium was greater than that found for high potassium and was strengthened in the time‐updated model. Although all patients were on a full dose of enalapril or sacubitril–valsartan, MRA use was less frequent in individuals in the lowest potassium category, in contrast to those with high potassium concentrations. However, MRA use was included in the multivariable adjustment and therefore neither the lower use of MRAs nor the underuse of ACEi or ARBs seem to account for the association between hypokalaemia and higher risk for death. More surprisingly, perhaps, the association between low potassium and outcomes also appeared to lack specificity, with similarly elevated risk for various types of death, as seen with higher potassium concentrations. Moreover, the risk for HF hospitalization was elevated to the same degree as that for overall and cause‐specific mortality. This suggests that the association between hypokalaemia and worse outcomes is non‐specific in nature and low potassium may also be a marker of disease severity. At first sight, this conclusion may seem to be at variance with the finding that the ‘correction’ of hypokalaemia (and hyperkalaemia) was associated with a better outcome than a persisting abnormality of potassium. However, the patients who shifted from an abnormal to a normal potassium category may have been patients whose clinical status improved.
Another interesting observation was that the overall comorbidity profile of patients with hypokalaemia was generally of lower risk than that of patients with hyperkalaemia; patients in the lower potassium categories had better renal function, and less ischaemic cardiac disease, diabetes and atrial fibrillation than patients with higher potassium, although patients with low potassium had higher NT‐proBNP levels, greater use of diuretics and a more frequent history of HF hospitalization. One interpretation of these findings may be that low potassium, reflecting a greater use of diuretics and less use of MRAs in more recently hospitalized and inadequately decongested patients, is a marker of a patient with a greater probability of worsening that will lead to hospital admission or death. 27 This might suggest that the present multivariable adjustment did not fully account for some of the measured confounders discussed or that there was significant unmeasured confounding. For example, there were striking differences in geographic region of enrolment and race across potassium categories. North America (a region of low MRA use) was relatively over‐represented and Central and Eastern Europe (regions of high MRA use) were relatively under‐represented in the potassium ≤3.5 mmol/L category, although there are clearly many other demographic, social, cultural and environmental influences on health, and differences in health care provision, across these regions. There was also relative over‐representation of Black and Asian individuals in the lowest potassium category, compared with the normal and higher potassium groups. The explanation for this is not obvious. The use of MRAs was not lower in Blacks or Asians and the other factors mentioned in relation to geographical region plus additional genetic variables may be important. 28 Finally, it remains likely that a general indirect and non‐specific association between lower potassium level and worse outcome, as discussed, conceals a true direct biological link, at least in some patients, between profound hypokalaemia and cardiac mortality.
Compared with enalapril, sacubitril–valsartan had little effect on potassium in PARADIGM‐HF, overall. However, in participants treated with an MRA, compared with enalapril, sacubitril–valsartan reduced potassium significantly, maintaining levels within the normal range (and similar to those in patients not treated with MRAs). 29 One possible (but merely speculative) explanation for the lower potassium levels in the sacubitril–valsartan group is that compared with enalapril, sacubitril–valsartan improved renal function and this may have led to greater distal delivery of loop diuretic and more potassium excretion as a result. A previous report demonstrated that sacubitril–valsartan reduced the risk for hyperkalaemia in patients treated with an MRA. 30 The present study expands those findings by comparing the effects of sacubitril–valsartan with those of enalapril across the full spectrum of potassium concentrations. Furthermore, the benefit of sacubitril–valsartan over enalapril was consistent across the range of baseline potassium levels among the patients randomized in PARADIGM‐HF.
Limitations
This was a post hoc analysis of the PARADIGM‐HF trial. The patients were highly selected, tolerating recommended doses of enalapril and sacubitril–valsartan in a run‐in period. Although this reduces the generalizability of the current findings, the trial design also addresses the limitations of earlier studies. Regular monitoring of potassium and protocol‐guided mitigation may have led to the correction of potassium abnormalities more frequently, or more quickly, than in routine practice (and perhaps in hyperkalaemia more often than in hypokalaemia, as treatment of only the former was addressed in the protocol). Moreover, a serum potassium level of >5.2 mmol/L at screening (or >5.4 mmol/L at randomization) was an exclusion criterion, which may have led to the selection of patients less prone to the development of hyperkalaemia. Although having an independent adjudication committee was a major advantage of the present study, some events, including sudden death and pump failure death, remain difficult to adjudicate and may be prone to misclassification.
Conclusions
In PARADIGM‐HF, both hyperkalaemia and hypokalaemia were associated with a higher risk for death, although the association was stronger for the latter. While both higher and lower potassium levels remained independent predictors of death after adjustment in multivariable models, they were also associated with non‐CV death and HF hospitalization, which suggests that potassium abnormalities are mainly markers of disease severity. Compared with enalapril, sacubitril–valsartan had no overall effect on potassium concentrations in PARADIGM‐HF, although it reduced potassium significantly in participants treated with an MRA, maintaining it in the normal range in these individuals. The benefit of sacubitril–valsartan over enalapril was consistent across the range of baseline potassium levels among the patients randomized in PARADIGM‐HF.
Funding
J.P.F. is funded by a European Society of Cardiology research grant for collaboration with the University of Glasgow. All other authors report no specific funding for this project. J.J.V.M. and P.S.J. are supported by a British Heart Foundation Centre of Research Excellence grant (RE/18/6/34217).
Conflict of interest: none declared.
Supporting information
Table S1. Missing values of potassium and estimated glomerular filtration rate (eGFR).
Table S2. Potassium changes from randomization to 2 months and subsequent outcomes.
Figure S1. Incidence of both hypo‐ and hyperkalaemia throughout the follow‐up and by randomized treatment group in the PARADIGM‐HF trial.
Figure S2. Baseline potassium and subsequent outcomes.
Figure S3. Landmark (2‐month) analysis and subsequent outcomes.
Figure S4. Joint model for potassium and subsequent outcomes.
Figure S5. E‐value (and corresponding hazard ratio) needed to offset the association of hypokalaemia (≤3.5 mmol/L) with the study's primary outcome.
References
- 1. Palmer BF. Managing hyperkalemia caused by inhibitors of the renin–angiotensin–aldosterone system. N Engl J Med 2004;351:585–592. [DOI] [PubMed] [Google Scholar]
- 2. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 3. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos G, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2016;68:1476–1488. [DOI] [PubMed] [Google Scholar]
- 4. Zannad F, Ferreira JP, Pitt B. Potassium binders for the prevention of hyperkalaemia in heart failure patients: implementation issues and future developments. Eur Heart J Suppl 2019;21 (Suppl. A):A55–A60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Collins AJ, Pitt B, Reaven N, Funk S, McGaughey K, Wilson D, Bushinsky DA. Association of serum potassium with all‐cause mortality in patients with and without heart failure chronic kidney disease and/or diabetes. Am J Nephrol 2017;46:213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Krogager ML, Torp‐Pedersen C, Mortensen RN, Kober L, Gislason G, Sogaard P, Aasbjerg K. Short‐term mortality risk of serum potassium levels in hypertension: a retrospective analysis of nationwide registry data. Eur Heart J 2016;38:104–112. [DOI] [PubMed] [Google Scholar]
- 7. Goyal A, Spertus JA, Gosch K, Venkitachalam L, Jones PG, Van den Berghe G, Kosiborod M. Serum potassium levels and mortality in acute myocardial infarction. JAMA 2012;307:157–164. [DOI] [PubMed] [Google Scholar]
- 8. Pitt B, Rossignol P. Serum potassium in patients with chronic heart failure: once we make a U‐turn where should we go? Eur Heart J 2017;38:2897–2899. [DOI] [PubMed] [Google Scholar]
- 9. Kovesdy CP, Matsushita K, Sang Y, Brunskill NJ, Carrero JJ, Chodick G, Hasegawa T, Heerspink HL, Hirayama A, Landman GWD, Levin A, Nitsch D, Wheeler DC, Coresh J, Hallan SI, Shalev V, Grams ME; CKD Prognosis Consortium. Serum potassium and adverse outcomes across the range of kidney function: a CKD Prognosis Consortium meta‐analysis. Eur Heart J 2018;39:1535–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hayes J, Kalantar‐Zadeh K, Lu JL, Turban S, Anderson JE, Kovesdy CP. Association of hypo‐ and hyperkalemia with disease progression and mortality in males with chronic kidney disease: the role of race. Nephron Clin Pract 2012;120:c8–c16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shiyovich A, Gilutz H, Plakht Y. Potassium fluctuations are associated with inhospital mortality from acute myocardial infarction. Soroka Acute Myocardial Infarction II (SAMI‐II) Project. Angiology 2018;69:709–717. [DOI] [PubMed] [Google Scholar]
- 12. Kovesdy CP, Appel LJ, Grams ME, Gutekunst L, McCullough PA, Palmer BF, Pitt B, Sica DA, Townsend RR. Potassium homeostasis in health and disease: a scientific workshop cosponsored by the National Kidney Foundation and the American Society of Hypertension. J Am Soc Hypertens 2017;11:783–800. [DOI] [PubMed] [Google Scholar]
- 13. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR; PARADIGM‐HF investigators and committees. Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 14. McMurray J, Coutie WJ, McFarlane L, Struthers AD. Atrial natriuretic factor inhibits ACTH stimulated aldosterone but not cortisol secretion in man. Eur J Clin Pharmacol 1988;35:409–412. [DOI] [PubMed] [Google Scholar]
- 15. Anderson JV, Struthers AD, Payne NN, Slater JD, Bloom SR. Atrial natriuretic peptide inhibits the aldosterone response to angiotensin II in man. Clin Sci (Lond) 1986;70:507–512. [DOI] [PubMed] [Google Scholar]
- 16. Harrell F. Regression Modeling Strategies: With Applications to Linear Models Logistic Regression and Survival Analysis. New York, NY: Springer; 2001. [Google Scholar]
- 17. Haneuse S, VanderWeele TJ, Arterburn D. Using the E‐value to assess the potential effect of unmeasured confounding in observational studies. JAMA 2019;321:602–603. [DOI] [PubMed] [Google Scholar]
- 18. Aldahl M, Jensen AC, Davidsen L, Eriksen MA, Moller Hansen S, Nielsen BJ, Krogager ML, Kober L, Torp‐Pedersen C, Sogaard P. Associations of serum potassium levels with mortality in chronic heart failure patients. Eur Heart J 2017;38:2890–2896. [DOI] [PubMed] [Google Scholar]
- 19. Nunez J, Bayes‐Genis A, Zannad F, Rossignol P, Nunez E, Bodi V, Minana G, Santas E, Chorro FJ, Mollar A, Carratala A, Navarro J, Gorriz JL, Lupon J, Husser O, Metra M, Sanchis J. Long‐term potassium monitoring and dynamics in heart failure and risk of mortality. Circulation 2018;137:1320–1330. [DOI] [PubMed] [Google Scholar]
- 20. Savarese G, Xu H, Trevisan M, Dahlstrom U, Rossignol P, Pitt B, Lund LH, Carrero JJ. Incidence predictors and outcome associations of dyskalemia in heart failure with preserved, mid‐range and reduced ejection fraction. JACC Heart Fail 2019;7:65–76. [DOI] [PubMed] [Google Scholar]
- 21. Ferreira JP, Butler J, Rossignol P, Pitt B, Anker SD, Kosiborod M, Lund LH, Bakris GL, Weir MR, Zannad F. Abnormalities of potassium in heart failure: JACC state‐of‐the‐art review. J Am Coll Cardiol 2020;75:2836–2850. [DOI] [PubMed] [Google Scholar]
- 22. Cooper LB, Benson L, Mentz RJ, Savarese G, DeVore AD, Carrero JJ, Dahlstrom U, Anker SD, Lainscak M, Hernandez AF, Pitt B, Lund LH. Association between potassium level and outcomes in heart failure with reduced ejection fraction: a cohort study from the Swedish Heart Failure Registry. Eur J Heart Fail 2020;22:1390–1398. [DOI] [PubMed] [Google Scholar]
- 23. Rossignol P, Lainscak M, Crespo‐Leiro MG, Laroche C, Piepoli MF, Filippatos G, Rosano GMC, Savarese G, Anker SD, Seferovic PM, Ruschitzka F, Coats AJS, Mebazaa A, McDonagh T, Sahuquillo A, Penco M, Maggioni AP, Lund LH; Heart Failure Long‐Term Registry Investigators Group. Unravelling the interplay between hyperkalaemia renin–angiotensin–aldosterone inhibitor use and clinical outcomes. Data from 9222 chronic heart failure patients of the ESC‐HFA‐EORP Heart Failure Long‐Term Registry. Eur J Heart Fail 2020;22:1378–1389. [DOI] [PubMed] [Google Scholar]
- 24. Fisch C, Knoebel SB, Feigenbaum H, Greenspan K. Potassium and the monophasic action potential electrocardiogram conduction and arrhythmias. Prog Cardiovasc Dis 1966;8:387–418. [DOI] [PubMed] [Google Scholar]
- 25. Macdonald JE, Struthers AD. What is the optimal serum potassium level in cardiovascular patients? J Am Coll Cardiol 2004;43:155–161. [DOI] [PubMed] [Google Scholar]
- 26. Weiss JN, Qu Z, Shivkumar K. Electrophysiology of hypokalemia and hyperkalemia. Circ Arrhythm Electrophysiol 2017;10:e004667 10.1002/ejhf.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rossignol P, Girerd N, Bakris G, Vardeny O, Claggett B, McMurray JJV, Swedberg K, Krum H, van Veldhuisen DJ, Shi H, Spanyers S, Vincent J, Fay R, Lamiral Z, Solomon SD, Zannad F, Pitt B. Impact of eplerenone on cardiovascular outcomes in heart failure patients with hypokalaemia. Eur J Heart Fail 2017;19:792–799. [DOI] [PubMed] [Google Scholar]
- 28. Kristensen SL, Martinez F, Jhund PS, Arango JL, Belohlavek J, Boytsov S, Cabrera W, Gomez E, Hagege AA, Huang J, Kiatchoosakun S, Kim KS, Mendoza I, Senni M, Squire IB, Vinereanu D, Wong RC, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, Packer M, McMurray JJ. Geographic variations in the PARADIGM‐HF heart failure trial. Eur Heart J 2016;37:3167–3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vaduganathan M, Claggett BL, Jhund PS, Cunningham JW, Pedro Ferreira J, Zannad F, Packer M, Fonarow GC, McMurray JJV, Solomon SD. Estimating lifetime benefits of comprehensive disease‐modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: a comparative analysis of three randomised controlled trials. Lancet 2020;396:121–128. [DOI] [PubMed] [Google Scholar]
- 30. Desai AS, Vardeny O, Claggett B, McMurray JJ, Packer M, Swedberg K, Rouleau JL, Zile MR, Lefkowitz M, Shi V, Solomon SD. Reduced risk of hyperkalemia during treatment of heart failure with mineralocorticoid receptor antagonists by use of sacubitril/valsartan compared with enalapril: a secondary analysis of the PARADIGM‐HF trial. JAMA Cardiol 2017;2:79–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Missing values of potassium and estimated glomerular filtration rate (eGFR).
Table S2. Potassium changes from randomization to 2 months and subsequent outcomes.
Figure S1. Incidence of both hypo‐ and hyperkalaemia throughout the follow‐up and by randomized treatment group in the PARADIGM‐HF trial.
Figure S2. Baseline potassium and subsequent outcomes.
Figure S3. Landmark (2‐month) analysis and subsequent outcomes.
Figure S4. Joint model for potassium and subsequent outcomes.
Figure S5. E‐value (and corresponding hazard ratio) needed to offset the association of hypokalaemia (≤3.5 mmol/L) with the study's primary outcome.


