Abstract
Background
The aims of this systematic review are to (a) evaluate the current literature on the impact of postoperative therapy for resected squamous cell carcinoma of the head and neck (SCCHN) on oncologic and non‐oncologic outcomes and (b) identify the optimal evidence‐based postoperative therapy recommendations for commonly encountered clinical scenarios.
Methods
An analysis of the medical literature from peer‐reviewed journals was conducted using the Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) guideline. Prospective studies and methodology‐based systematic reviews and meta‐analyses of postoperative therapy for SCCHN were identified by searching Medline (OVID) and EMBASE (Elsevier) using controlled vocabulary terms (ie, National Library of Medicine Medical Subject Headings [MeSH], EMTREE). Study screening and selection was performed with Covidence software and full‐text review. The RAND/UCLA appropriateness method was used by the expert panel to rate the appropriate use of postoperative therapy, and the modified Delphi method was used to come to consensus.
Results
A total of 5660 studies were identified and screened using the title and abstract, leading to 201 studies assessed for relevance using full‐text review. After limitation to the eligibility criteria, 101 studies from 1977 to 2020 were identified, including 77 with oncologic endpoints and 24 with function and quality of life endpoints. All studies reported staging prior to the implementation of American Joint Committee on Cancer (AJCC‐8).
Conclusions
Prospective clinical studies and systematic reviews identified through the PRISMA systematic review provided good evidence for consensus statements regarding the appropriate use of postoperative therapy for resected SCCHN. Further research is needed in domains where consensus by the expert panel could not be achieved for the appropriateness of specific postoperative therapeutic interventions.
Keywords: adjuvant therapy, appropriate use, guidelines, head and neck cancer, postoperative
1. INTRODUCTION
There have been substantial changes in the management of squamous cell carcinoma of the head and neck (SCCHN) since publication of the clinical studies that form the basis of postoperative management for resected SCCHN. First, the epidemiology, causative factors, and prognosis of SCCHN have changed, particularly in the USA and regions of Europe. Oropharyngeal cancers may not only be caused by cigarette smoking and alcohol consumption but may be virally induced. It has been established that human papillomavirus (HPV)‐mediated oropharyngeal cancer has an improved prognosis compared to HPV‐negative oropharyngeal cancer. 1 , 2 Second, treatments themselves have evolved. The common clinical use of intensity modulated radiation therapy (IMRT) facilitates flexibility of radiation targeting. The use of transoral laser and transoral robotic surgery (TORS) has increased over the last decade, allowing for less morbid surgical approaches in the head and neck. 3 Third, modern radiologic imaging allows more accurate and sensitive preoperative staging. Lastly, the prognostic power of additional pathologic risk factors such as depth of invasion (DOI) for oral cavity tumors, 4 , 5 and extranodal extension (ENE) have been incorporated into the American Joint Committee on Cancer (AJCC) 8th edition staging classification. 6 While changes in staging do not themselves constitute changes in management, they provide prognostic information and potential additional criterion for eligibility criteria in future clinical trials.
The aims of this systematic review were to (a) evaluate the current literature on the impact of postoperative therapy for SCCHN on oncologic and non‐oncologic outcomes and (b) identify the optimal evidence‐based postoperative therapy recommendations for commonly encountered clinical scenarios. The primary objective was to identify studies with quantitative outcomes after postoperative radiation therapy (PORT), postoperative radiation with chemotherapy (chemo‐PORT), or postoperative chemotherapy with endpoints of overall survival (OS), and local, regional, and distant control. The secondary objective was to identify studies that describe quantitative outcomes related to toxicity, quality of life (QOL), or measurements of function.
2. METHODS
The American Radium Society (ARS) Appropriate Use Criteria presented in this manuscript are evidence‐based guidelines for postoperative treatment of squamous cell carcinoma of the head and neck (SCCHN). Since the ARS and the American College of Radiology (ACR) last published a literature review and expert consensus guideline on postoperative therapy in 2011, 7 additional relevant clinical trials have been published. The objectives of this systematic review are 2‐fold: first, to comprehensively evaluate existing prospective clinical studies of postoperative therapy for resected SCCHN using a formalized methodologic approach described by the Preferred Reporting Items for Systematic Review and Meta‐Analyses Protocol (PRISMA‐P), 8 and second, to provide general treatment recommendations to assist clinical decision‐making, highlighting areas of controversy and uncertainty.
The literature was reviewed for quality of study design, cohort size, selection bias, evaluation of participants in relation to time from exposure, and methods of assessments. The RAND/UCLA appropriateness method 9 was used by the expert panel to rate the appropriate use of procedures and the modified Delphi 10 was used to come to consensus. The expert panel is composed of multidisciplinary radiation, medical, surgical oncologists, and an academic librarian.
2.1. Systematic review strategy
An analysis of the medical literature from peer‐reviewed journals was conducted using the Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) 8 , 11 guidelines to search and retrieve a comprehensive set of relevant articles. Studies discussing adjuvant therapy following surgery for squamous cell carcinoma of the head and neck were identified by searching Medline (OVID) and EMBASE (Elsevier) on January 10, 2019. Controlled vocabulary terms (ie, National Library of Medicine Medical Subject Headings [MeSH], EMTREE) were included when available and appropriate. The search strategies were designed and executed by a librarian (CM). No language limits or year restrictions were applied. The exact search terms used for each of the databases are provided in the Supporting Information.
2.2. Study selection
Selection was performed using Covidence software. Title and abstract screening were performed by one author (DNM) using the PICO (participants, interventions, comparators, outcomes) inclusion and exclusion criteria outlined below. Full text was obtained for all titles/abstracts that met inclusion criteria and for studies where there was uncertainty based on the title/abstract screening. Full text review was performed by two independent reviewers (DNM, AGS) blinded to each other's judgment. Disagreement was resolved through discussion regarding study relevance related to the inclusion/exclusion criteria and concordance was obtained. Each study was graded using the Oxford Centre for Evidence‐Based Medicine 2011 Levels of Evidence Table. 12
The PICO framework was used to identify relevant studies. 8 Studies were included if they were published randomized controlled trials (RCT), randomized trials, single and multi‐arm non‐randomized prospective clinical trials, systematic reviews and meta‐analyses that adhered to a published methodology such as Cochrane Review, PRISMA, or QUOROM guidelines. 13 Longitudinal prospective cohort studies were included only if there was a baseline pre‐intervention assessment. Studies comparing postoperative vs definitive therapy were not included as this was not the primary objective of the systematic review. The study population included adult patients (age 18 years or older) with stage I‐IVB SCCHN and no distant metastases (DM), who had no prior head and neck RT and were treated with curative‐intent surgery. The following disease sites were included: oral cavity, oropharynx, hypopharynx, and larynx. Studies were excluded if they did not include at least 20 patients treated with surgery and postoperative therapy or the population was predominantly composed of nasopharyngeal carcinoma, paranasal sinus cancer, cancer of unknown primary, nasal cavity cancer, recurrent head and neck cancer, or patients treated with re‐irradiation with or without chemotherapy. Details of the PICO specifications are provided in the Supporting Information.
2.3. Consensus voting
Clinical variants with corresponding treatment options were created to represent commonly encountered clinical scenarios, including those for which management is controversial (Tables 1A, 2A, 3A). These were reviewed by all panelists prior to voting. Variants were circulated for voting whereby panelists rated each treatment using a score of “1 to 9,” representing “usually not appropriate” (1‐3), “may be appropriate (4‐6), and “usually appropriate” (7‐9). Panelists were blinded to each other's votes. The results were reviewed and discussed, maintaining anonymity of voting. A second round of voting was performed, and results were again reviewed and discussed prior to finalizing votes. The median score was determined and agreement was determined as per the BIOMED Concerted Action on Appropriateness definition outlined in the RAND/UCLA methodology 9 whereby agreement was defined as ≤3 votes outside the 3‐point region containing the median (1‐3;4‐6;7‐9), for a panel of 11 to 13, and ≤4 votes outside the 3‐point region containing the median for a panel of 14 to 16. Detailed voting results for the variant Tables 1A, 2A, 3A are provided in the Supporting Information. The strength of recommendations was graded using the GRADE system. 14
TABLE 1A.
Clinical condition: Resected early‐stage SCC of the oral cavity with deep depth of invasion (DOI)
| Variant 1: | pT2 N0 M0 moderately differentiated SCC of the right lateral oral tongue treated with a partial glossectomy and ipsilateral neck dissection (levels IA‐B, II, III, IV). Pathology showed a 1.5 cm SCC, 1.5 cm DOI, closest margin of 0.5 cm, 24 negative lymph nodes, no perineural invasion or lymphovascular invasion. |
| Question 1: Is DOI a factor that prompts any additional therapy? | |||||
|---|---|---|---|---|---|
| Treatment | Rating Category | Group Median Rating | Disagreement | SOE | SOR |
| Observation (No additional therapy) | M* | 5 | X | L | — |
| Contralateral neck dissection—no postoperative therapy if pN0 | U | 2 | EC | ↑ | |
| PORT | A | 7 | EO | — | |
| PORT + concurrent systemic therapy | U | 1 | EC | ↑ | |
| Question 2: If postoperative RT is administered, what volume is appropriate? | |||||
|---|---|---|---|---|---|
| Radiation volume | Rating category | Group median rating | Disagreement | SOE | SOR |
| PORT to primary site only | U | 3 | EC | ↑ | |
| PORT to primary site + ipsilateral neck | M* | 5 | X | EO | — |
| PORT to primary site + bilateral neck a | M* | 5 | X | EO | — |
Notes: Rating: A‐Usually appropriate; M‐May be appropriate; U‐Usually not appropriate. Disagreement, that is, the variation of the individual ratings from the median rating indicates panel disagreement on the final recommendation (see narrative text). When there was disagreement, the group median rating is set automatically to 5. Strength of Evidence: S‐Strong; M‐Moderate; L‐Limited; EC‐Expert consensus; EO‐Expert opinion. Strength of Recommendation: ↑ Strong Recommendation; ↓ Weak Recommendation; — Additional considerations do not strengthen or weaken the panel's recommendation.
Abbreviations: DOI, depth of invasion; PORT, postoperative radiation therapy; SCC, squamous cell carcinoma of the head and neck.
For this specific variant, “bilateral neck” refers to RT of any contralateral nodal region including contralateral level IB‐alone. Of note, these variants do not provide granularity regarding specific nodal chains of treatment.
TABLE 2A.
Clinical condition: Resected locally advanced SCC of the larynx with extranodal extension (ENE)
| Variant 3: | pT3 N2a M0 moderately differentiated SCC of the supraglottic larynx treated with total laryngectomy and bilateral neck dissections (levels II‐IV). Pathology showed a 4.5 cm SCC and a 0.5 cm closest margin. The right neck dissection showed 1 out of 22 left neck nodes with 1.5 cm of SCC from level II with 2 mm of ENE; the left neck dissection showed 0 involved out of 19 neck nodes. |
| Question 1: What postoperative therapy is recommended in the presence of ENE? | |||||
|---|---|---|---|---|---|
| Treatment | Rating category | Group median rating | Disagreement | SOE | SOR |
| Observation (No additional therapy) | U | 1 | S | ↑ | |
| PORT‐alone | M* | 5 | X | S | ↑ |
| PORT‐alone altered fractionation a | M* | 5 | X | M | — |
| PORT conventional fractionation b + systemic therapy | A | 8 | S | ↑ | |
| Question 2: What are appropriate radiation volumes? | |||||
|---|---|---|---|---|---|
| Radiation volume | Rating category | Group median rating | Disagreement | SOE | SOR |
| PORT to primary site + bilateral neck | A | 8 | M | ↑ | |
| PORT to primary site + pathological node‐positive neck only | M* | 5 | X | M | ↓ |
| PORT to bilateral neck (no primary site) | U | 3 | L | ↑ | |
| PORT to pathological node‐positive neck only | U | 3 | L | ↑ | |
| Question 3: Which systemic therapy is appropriate? | |||||
|---|---|---|---|---|---|
| Systemic therapy | Rating category | Group median rating | Disagreement | SOE | SOR |
| No systemic therapy | U | 2 | S | ↑ | |
| Cisplatin 100 mg/m2 for 2 to 3 cycles | A | 9 | S | ↑ | |
| Cisplatin 40 mg/m2 weekly | M* | 5 | X | L | ↑ |
| Carboplatin AUC2 weekly | M* | 5 | X | L | — |
| Carboplatin AUC2 + paclitaxel weekly | M* | 5 | X | L | — |
| Cetuximab weekly | M* | 5 | X | L | — |
| Cetuximab + docetaxel 15 mg/m2 weekly | M* | 5 | X | M | — |
Notes: Rating: A‐Usually appropriate; M‐May be appropriate; U‐Usually not appropriate. Disagreement, that is, the variation of the individual ratings from the median rating indicates panel disagreement on the final recommendation (see narrative text). When there was disagreement, the group median rating is set automatically to 5. Strength of Evidence: S‐Strong; M‐Moderate; L‐Limited; EC‐Expert consensus; EO‐Expert opinion. Strength of Recommendation: ↑ Strong Recommendation; ↓ Weak Recommendation; — Additional considerations do not strengthen or weaken the panel's recommendation.
Altered fractionation refers to >2.0 Gy per fraction and/or >5 fractions per week. This excludes the practice of simultaneous integrated boost of >2.0 Gy per fraction to a limited high‐risk area.
Conventional fractionation refers to 1.8 to 2.0 Gy per fraction, given once daily, 5 days per week.
TABLE 3A.
Clinical condition: Resected p16‐positive SCC of the oropharynx
| Variant 5: | A lifetime non‐smoker with a pT1 N1 M0 p16‐positive SCC of the tonsil treated with transoral radical resection and ipsilateral neck dissection (levels II‐IV). The pathology showed a 1.5 cm tonsil‐confined tumor, 0.5 cm closest margin. There were 1 of 22 nodes with a 4.0 cm deposit of SCC at level II with no extranodal extension. |
| Question 1: What additional therapy is recommended in the presence of a single involved lymph node > 3 cm? | |||||
|---|---|---|---|---|---|
| Treatment | Rating Category | Group Median Rating | Disagreement | SOE | SOR |
| Observation (No additional therapy) | M* | 5 | X | EO | — |
| PORT alone, de‐escalation 50 to 54 Gy | M* | 5 | X | EC | ↓ |
| PORT‐alone, 60 to 66 Gy | A | 7 | L | ↓ | |
| PORT conventional fractionation a + systemic therapy | U | 1 | EC | ↑ | |
| Question 2: If PORT is recommended what are appropriate treatment volumes? | |||||
|---|---|---|---|---|---|
| Treatment radiation volume | Rating category | Group median rating | Disagreement | SOE | SOR |
| PORT to primary site + ipsilateral neck | A | 8 | M | ↑ | |
| PORT to primary site + bilateral neck | M* | 5 | X | L | — |
| PORT to ipsilateral neck only | M* | 5 | X | EC | — |
Notes: Rating: A‐Usually appropriate; M‐May be appropriate; U‐Usually not appropriate. Disagreement, that is, the variation of the individual ratings from the median rating indicates panel disagreement on the final recommendation (see narrative text). When there was disagreement, the group median rating was set automatically to 5. Strength of Evidence: S‐Strong; M‐Moderate; L‐Limited; EC‐Expert consensus; EO‐Expert opinion. Strength of Recommendation: ↑ Strong Recommendation; ↓ Weak Recommendation; — Additional considerations do not strengthen or weaken the panel's recommendation.
Conventional fractionation refers to 1.8 to 2.0 Gy per fraction, given once daily, 5 days per week.
TABLE 1B.
Clinical condition: Resected early‐stage SCC of the oral cavity with a close margin.
| Variant 2: | pT2 N0 M0 moderately differentiated SCC of the right lateral oral tongue treated with a partial glossectomy and ipsilateral neck dissection (levels IA‐B, II, III, IV). Pathology showed a 2.5 cm SCC, 0.4 cm depth of invasion, <0.1 cm deep margin, 24 negative lymph nodes, no perineural invasion or lymphovascular invasion. |
| Question 1: What additional therapy is recommended for the close margin? | |||||
|---|---|---|---|---|---|
| Treatment | Rating Category | Group Median Rating | Disagreement | SOE | SOR |
| Observation (No additional therapy) | U | 2 | L | ↑ | |
| Re‐excision and no additional therapy if no residual SCC | M* | 5 | X | L | ↑ |
| PORT alone 60 to 66 Gy | A | 7 | L | ↑ | |
| PORT alone altered fractionation a | M* | 5 | X | L | — |
| PORT conventional fractionation b + systemic therapy | M* | 5 | X | L | — |
| Question 2: If radiation is recommended, what volumes are appropriate? | |||||
|---|---|---|---|---|---|
| Treatment | Rating category | Group median rating | Disagreement | SOE | SOR |
| Radiation volume | |||||
| PORT to primary site only | M* | 5 | X | L | — |
| PORT to primary site + ipsilateral neck | M* | 5 | X | L | — |
| PORT to primary site + bilateral neck c | M* | 5 | X | L | — |
Notes: Rating: A‐Usually appropriate; M‐May be appropriate; U‐Usually not appropriate. Disagreement, that is, the variation of the individual ratings from the median rating indicates panel disagreement on the final recommendation (see narrative text). When there was disagreement, the group median rating was set automatically to 5. Strength of Evidence: S‐Strong; M‐Moderate; L‐Limited; EC‐Expert consensus; EO‐Expert opinion. Strength of Recommendation: ↑ Strong Recommendation; ↓ Weak Recommendation; — Additional considerations do not strengthen or weaken the panel's recommendation.
Abbreviations: PORT, postoperative radiation therapy; SCC, squamous cell carcinoma of the head and neck; RT, Radiation Therapy.
Altered fractionation refers to >2.0 Gy per fraction and/or >5 fractions per week. This excludes the practice of simultaneous integrated boost of >2.0 Gy per fraction to a limited high‐risk area.
Conventional fractionation refers to 1.8 to 2.0 Gy per fraction, given once daily, 5 days per week.
For this specific variant, “bilateral neck” refers to RT of any contralateral nodal region including contralateral level IB. Of note, these guidelines do not provide granularity regarding specific nodal chains of treatment.
TABLE 2B.
Clinical condition: Resected locally advanced SCC of the larynx with intermediate risk factors
| Variant 4: | pT3 N2a M0 moderately differentiated SCC of the supraglottic larynx treated with total laryngectomy and bilateral neck dissections (levels II‐IV). Pathology showed a 4.5 cm SCC with perineural invasion (PNI) and lymphovascular invasion (LVI); 0.5 cm closest margin. The right neck dissection showed 1 out of 22 left neck nodes with 3.5 cm of SCC from level II without extranodal extension; the left neck dissection showed 0 out of 19 involved neck nodes. |
| Question 1: What postoperative therapy is recommended in the presence of multiple intermediate risk factors? | |||||
|---|---|---|---|---|---|
| Treatment | Rating category | Group median rating | Disagreement | SOE | SOR |
| Observation (No additional therapy) | U | 2 | S | ↑ | |
| PORT‐alone 60 to 66 Gy | A | 8 | M | ↑ | |
| PORT‐alone altered fractionation a | M* | 5 | X | M | ↓ |
| PORT conventional fractionation b + systemic therapy | M* | 5 | X | M | ↓ |
Notes: Rating: A‐Usually appropriate; M‐May be appropriate; U‐Usually not appropriate. Disagreement, that is, the variation of the individual ratings from the median rating indicates panel disagreement on the final recommendation (see narrative text). When there was disagreement, the group median rating was set automatically to 5. Strength of Evidence: S‐Strong; M‐Moderate; L‐Limited; EC‐Expert consensus; EO‐Expert opinion. Strength of Recommendation: ↑ Strong Recommendation; ↓ Weak Recommendation; — Additional considerations do not strengthen or weaken the panel's recommendation.
Altered fractionation refers to >2.0 Gy per fraction and/or >5 fractions per week. This excludes the practice of simultaneous integrated boost of >2.0 Gy per fraction to a limited high‐risk area.
Conventional fractionation refers to 1.8 to 2.0 Gy per fraction, given once daily, 5 days per week.
TABLE 3B.
Clinical condition: Resected p16‐positive SCC of the oropharynx with extranodal extension (ENE).
| Variant 6: | A lifetime non‐smoker with a pT1 N1 M0 p16‐positive SCC of the tonsil treated with transoral radical resection and ipsilateral neck dissection (levels II‐IV). The tumor was 1.5 cm, tonsil‐confined, with a 0.5 cm closest margin. The ipsilateral neck dissection showed 1 out of 22 left neck nodes with a 4.0 cm SCC deposit at level II with 0.2 cm ENE. |
| Question 1: In the presence of ENE, what postoperative therapy is recommended? | |||||
|---|---|---|---|---|---|
| Treatment | Rating category | Group median rating | Disagreement | SOE | SOR |
| Observation (No additional therapy) | U | 1 | EC | ↑ | |
| PORT alone, de‐escalation 50 to 54 Gy | U | 2 | M | ↑ | |
| PORT alone, 60 to 66 Gy | M* | 5 | X | M | — |
| PORT conventional fractionation a + systemic therapy | A | 8 | M | ↓ | |
| Question 2: What radiation volume is appropriate? | |||||
|---|---|---|---|---|---|
| Treatment radiation volume | Rating category | Group median rating | Disagreement | SOE | SOR |
| PORT to primary site + ipsilateral neck | A | 7 | EC | ↑ | |
| PORT to primary site + bilateral neck | A | 7 | EC | ↓ | |
| PORT to ipsilateral neck only | M* | 5 | X | EO | — |
| Question 3: What concurrent systemic therapy is appropriate, if recommended? | |||||
|---|---|---|---|---|---|
| Treatment radiation volume | Rating category | Group median rating | Disagreement | SOE | SOR |
| No systemic therapy | M* | 5 | X | M | ↓ |
| Cisplatin 100 mg/m2 for 2 to 3 cycles | A | 8 | M | ↑ | |
| Cisplatin 40 mg/m2 weekly | M* | 5 | X | L | ↑ |
| Carboplatin AUC2 weekly | M* | 5 | X | L | ↑ |
| Carboplatin AUC2 + paclitaxel weekly | M* | 5 | X | EC | — |
| Cetuximab weekly | M* | 5 | X | EC | — |
| Cetuximab + docetaxel 15 mg/m2 weekly | M* | 5 | X | EO | ↑ |
Notes: Rating: A‐Usually appropriate; M‐May be appropriate; U‐Usually not appropriate. Disagreement, that is, the variation of the individual ratings from the median rating indicates panel disagreement on the final recommendation (see narrative text). When there was disagreement, the group median rating was set automatically to 5. Strength of Evidence: S‐Strong; M‐Moderate; L‐Limited; EC‐Expert consensus; EO‐Expert opinion. Strength of Recommendation: ↑ Strong Recommendation; ↓ Weak Recommendation; — Additional considerations do not strengthen or weaken the panel's recommendation.
Conventional fractionation refers to 1.8 to 2.0 Gy.
Select retrospective studies are referenced only to provide context for specific topics but are not included in the evidence Tables or as the supporting evidence for oncologic intervention. Such studies are also described as retrospective in the body of the text.
3. RESULTS
3.1. Characteristics of studies identified in the literature search
The process of study identification is summarized in Figure 1. A total of 5689 studies were identified using Ovid Medline and Embase. After removal of duplicates, 5660 studies were screened using the title and abstract leading to 201 studies assessed for relevance using full‐text review. After limitation to the eligibility criteria, 96 studies were identified. An additional five eligible studies were added, including four that were published after the cut‐off date of 01/10/2019 15 , 16 , 17 , 18 and one randomized trial that was not identified through the literature search. 19
FIGURE 1.
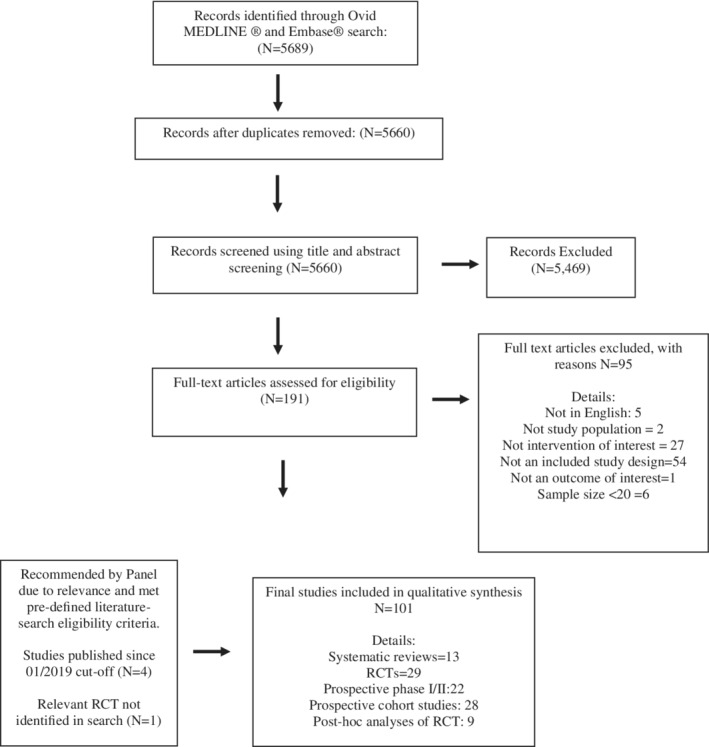
Study selection QUOROM flow diagram
Studies with primary oncologic endpoints are shown in Table S1 and included 11 systematic reviews, 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 24 randomized trials with four long‐term updates, 19 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 29 non‐randomized clinical trials, 15 , 16 , 17 , 18 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 and nine post hoc analyses of randomized trials. 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 The randomized trials are presented in Table 4. Studies that focused on non‐oncologic endpoints are shown in Table S2 and included two systematic reviews, 92 , 93 one randomized trial, 94 and 21 non‐randomized clinical trials. 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 All studies reported staging prior to the implementation of AJCC‐8.
TABLE 4.
Randomized trials evaluating oncologic outcomes after postoperative therapy for resected squamous cell carcinoma of the head and neck (N = 24)
| First author (country) | Arms | Patients (N) | Median f/u | Results | LOE | |
|---|---|---|---|---|---|---|
| Sites | ||||||
| Stage | Years accrued | |||||
| Definition of risk (if applicable) | Regimen details | |||||
| Observation vs postoperative RT (PORT) | ||||||
|
Kokal (USA) 41 |
Observation vs PORT |
51, OC, Pharynx, Lx Stage III/IV |
1981 to 1984 RT was 50 Gy |
30.3 mo |
Decreased “overall recurrence rate” in PORT arm (55.6% vs 36.5%, P = “NS”) |
2 2 |
|
Mishra (India) 45 |
Observation vs PORT |
140, OC (all buccal) Stage III/IV |
1990 to 1994 RT: cobalt‐60, mean dose of 60 Gy |
NR | DFS improved with PORT 38% and 68% (P < .005). | 2 |
| PORT timing, dose, and fractionation | ||||||
|
Ang (USA) 31 |
Adjuvant treatment by pathologic risk stratification. Low risk: no PORT Intermediate: PORT High‐risk: PORT vs accelerated PORT |
213, OC, OPX, LX, HPX Stage III (48%), Stage IV(38%) ECE in 49% Risk group definition: Low risk: no RF; intermediate risk = 1 RF other than ECE: high‐risk = ECE or ≥2 risk factors. Risk factors: ECE, >1 nodal group, ≥2positive nodes >3 cm node, OC site, microscopic +margin and PNI. |
1991 to 1995 Low risk: no PORT Intermediate risk: 57.6 Gy in 32 f over 6.5 wk High risk: 63 Gy in 1.8 Gy/f over 7 wk vs 63 Gy 1.8 Gy/d × 3 wk then 2 fractions per day for last 2 wk (5 wk total) |
59 months. |
Patients with low or intermediate risks had higher LRC and OS than those with high‐risk features (P = .003 and P = .0001, respectively), despite receiving no PORT or lower dose PORT. For high‐risk patients, there was a trend toward higher LRC and OS when PORT was delivered in 5 rather than 7 wk. Prolonged interval between surgery and PORT in the 7‐week schedule was associated with lower LRC (P = .03) and OS (P = .01). |
1 |
| Awwad (Egypt) 33 |
Accelerated hyperfractionation (AHF) vs conventional fractionation (CF) |
56 OPX, OC, LX, HPX, Nodal metastases T3/4, N0‐2 postoperative. 31% “inadequate margin” Fresh tissue labeled with in vitro 3H‐thymidine labeling index (TLI) as marker of fast vs slower growing tumors. |
1987 to 1989. AHF: 42 Gy/30 F/11 d (3 F/d, interval 4 h) CF: conventional 50 Gy/25 F/5 wk All with telecobalt. Chemo: none |
NR |
NSD in 3‐y DFS (39% ± 9% CF vs 54% ± 10% AHF) or OS (36% ± 9% CF vs 54% ± 10% AHF). Higher late complications in CFX arm (87% vs 63%). AHF associated with a significant DFS benefit in faster growing tumors (especially for TLI 10.4% corresponding to T pot of <4.5 d). No benefit to AHF for slower growing tumors. |
2 |
|
Awwad (Egypt) 34 |
Accelerated hyperfractionation (AHF) vs conventional fractionation (CF) |
70 OC, LX, HPX T2/N1‐2N2 or T3‐4/any N 48% “inadequate margin” |
1995 to 1997. AHF: 3 fractions per day at 1.4 Gy/fraction to 46.2 Gy/33 fractions in 12 d. 6 d per week CF: 60 Gy in 30 fx in 6 wk, 5 d a week. |
NR |
3‐y LRC was significantly better in the accelerated hyperfractionation (88 + 4%) than in the CF (57 + 9%) group, P = .01. NSD in OS Late normal tissue reactions were worse in AFX group (either significant or “trend.”) Overall treatment time (time between surgery and the end of radiotherapy) longer than 10 wk had a significantly unfavorable effect on the LRC for all patients.) (P = .005) |
2 |
|
Kramer (USA) 42 RTOG 73‐03 10 y update: Tupchong 55 |
Preoperative RT vs PORT |
277 OC, OPX, SGL or HPX Stage III/IV: >85% |
1973 to 1979 SGL/HPX: 2 arms randomized to preoperative 50 Gy or postoperative 60 Gy OC/OPX: 3 arms randomized to preoperative 50 Gy or postoperative 60 Gy or definitive RT 65 to 70 Gy w surgery for salvage if residual at 6 wk post RT. RT dose: preoperative RT (50 Gy) or postoperative RT (60 Gy) |
10 y |
RC was significantly better for PORT patients than for preoperative RT patients (P = .04), but absolute survival was not affected (P = .15). 31% (18/58) of preoperative patients failed locally within 2 y vs 18% (11/60) in surgery + PORT group. After 2 y, distant metastases and second primaries were main failure types, particularly in surgery + PORT group. |
1 |
|
Peters (USA) 46 Update: Rosenthal 50 |
Stratified by risk factors and randomized: Lower risk group: 57.6 vs 63 Gy Higher risk group: 63 vs 68.4 Gy, all at 1.8 Gy per fraction. |
240 OC, OPX, HPX, LX T3/4:72% Very few early stage pts with risk factors: T1: 5 pts T2: 42 pts (26 T2N+) Risk group based on a point‐system. Patients' primary sites and involved necks were independently assigned to higher‐ or lower‐risk categories based on a cumulative point score representing increasing risk of recurrence. |
1983 to 1991. RT: Cobalt. 54 Gy given surgically undisturbed sites of potential disease; 57.6 Gy to pathologically uninvolved parts of surgical bed, including dissected region with negative nodes. Lower risk: 57.6 Gy vs 63 Gy (1.8 Gy/d) Higher risk: 63 Gy vs 68.4 Gy (1.8 Gy/d) Note: lower risk group initially was 52.2 to 54 Gy but due to early recurrences, minimal dose was changed to 57.6 Gy. |
>20 y |
Patients who received a dose of ≤54 Gy had a higher primary failure rate than those receiving ≥57.6 Gy (P = .02). For patients with ECE, recurrence was higher at 57.6 than 63 Gy or more. The long‐term report clarified that for low‐risk patients 57.6 Gy was sufficient. For high risk patients, doses >63 Gy did not have a SS benefit (they did not receive lower than 63 Gy). Two or more of the following risk factors were associated with an increased risk of recurrence: oral cavity primary, mucosal margins close or positive, nerve invasion, ≥2 positive lymph nodes, largest node >3 cm, treatment delay greater than 6 wk, and ≥ Zubrod performance status 2. Moderate to severe complications of combined treatment occurred in 7.1% of patients; these were more frequent in patients who received >63 Gy. |
1 |
|
Sanguinetti (Italy) 51 |
Conventional fractionation (CF) vs accelerated fractionation (AF) |
226, OC, OPX, HPX, Lx Stage III: 9.3% Stage IV: 87.6% Patients with at least 1 high‐risk feature: pT4, positive margin, pN >1, perineural/ lymphovascular invasion, ENE, subglottic extension |
1994 to 2000 CF: 2 Gy fraction per day (60 Gy in 6 wk) AF: (64 Gy in 5 wk) with a biphasic concomitant boost delivered during the first and last weeks of treatment. |
30.6 months. |
NSD in 2‐y LRC [80% ± 4% for CF, 78% ± 5% for AF (P = .52)] or 2‐y OS [67% ± 5% for CF, 64% ± 5% for AF (P = .84)]. Trend for improved LRC for patients who had a delay in starting RT (≥6.9 wk) and were treated with AF compared with CF (HR = 0.5, 95% CI 0.2‐1.1). |
2 |
|
Suwinski (Poland) 54 |
Conventional fractionation CF) vs accelerated fractionation (AF) |
279, OC, OPX: 121 LX: 158 Median time from surgery to RT was 9 wk. Risk group based on MDACC described in Peters et al 46 High‐risk patients: score of ≥3 at primary site and/or neck. In brief, score of 3 for neck would be any ENE, 2 or more involved nodes, grade 3 tumor and a positive node. For primary site score of 3 would be non‐larynx site except T1N0, microscopic positive margin, or a combination of LVI, grade 3 tumor, + <5 mm margin. |
2001 to 2004 No chemotherapy RT: 3D‐RT with parallel opposed fields: adjuvant CF: 63 Gy in 35 fractions of 1.8 Gy given 5 d per week AF: same RT but given 7 d a week (postoperative continuous accelerated irradiation: p‐CAIR). 45 to 50 Gy in 25 fractions given to the elective areas (undissected areas and dissected pathologically negative). |
48 months. |
Actuarial 3‐y LRC was 64% vs 70% for CF and p‐CAIR (P = .32). Subset analysis demonstrated significant improvement of 3‐y LRC in patients with OPX/OC tumors receiving p‐CAIR vs CF (74% vs 53%, P = .02). No improvement in LRC for Lx patients (P = .46). Acute/late toxicity was acceptable, although more patients had confluent mucositis with p‐CAIR (60% vs 33%). Limit: ENE not routinely reported (approximately 30% unknown ENE status), delay in RT start (approximately 50% >9 wk from surgery to RT), lack of systemic radiosensitizer |
2 |
|
Vandenbrouck (France) 56 |
Preoperative RT vs PORT |
49, HPX Stage III/IV (74%) |
1967 to 1969 RT: preoperative was 5500 cGy over 5.5 wk; postoperative was 5500 cGy over 6 wk with interval to start less than 4 wk postoperative. |
Trial stopped early due to unexpected high rate of postoperative deaths in patients who received preoperative RT. PORT improved OS (56% vs 20%, P < .01) |
2 | |
| Addition of concurrent chemotherapy to PORT | ||||||
|
Argiris (USA) 32 |
PORT vs PORT + weekly carboplatin 100 mg/m2 |
76, OC, OPX, HPX, LX Stage IV 92% All high‐risk High‐risk features: ECE (75%) or + margin (0.5 mm or less), LVI, PNI, ≥3 + LN |
1994 to 2002 RT: 59.4 Gy/1.8 Gy/fraction, 50.4 Gy to clinically uninvolved. Carboplatin: 6 cycles |
5.3 y | No statistically significant differences in DFS (HR 0.82, P = .60) or OS (HR 0.90, P = .73). The 2‐y DFS (primary endpoint) was 58% (95% CI 39%‐76%) in arm A (radiation alone) vs 71% (95% CI, 56%‐ 88%) in arm B (radiation and carboplatin). Terminated early due to slow accrual. | 2 |
|
Bachaud (France) 35 |
PORT vs PORT + weekly cisplatin 50 mg/m2 |
83, OC OPX, LX, HPX, CUP Stage III/IV All with ECE |
1984 to 1988 RT: All Cobalt‐60 Cisplatin: weekly 50 mg/m2 (7‐9 cycles) |
NR | Improved OS in chemo‐PORT group (P < .01). The RT group displayed a higher rate of LRF compared to chemotherapy group (41 vs 23%; P = .08). Survival without LRF was better in the chemotherapy group, the difference being close to the level of significance (P = .05). | 1 |
|
Bandyopadhyay (India) 36 |
PORT vs PORT + cisplatin 35 to 40 mg/m2 weekly |
54, OC All had high‐risk factors defined as: Stage III or IV (n = 43,80%), 2 or more +LN, ECE, PNI or LVI, positive margin (n = 8, 15%), level IV/V involvement. |
2006 to 2008 PORT (60 Gy/30 F/6 wk) |
47 mo | LRC 51.4% with chemo‐PORT vs 35.6% in PORT (P = .39). 5‐y OS 56.4% in CRT vs 51.3% PORT with significant increase in grade 3‐4 toxicity. | 2 |
|
Bernier (Europe) 37 |
PORT vs PORT + cisplatin 100 mg/m2 |
334, OC, OPX, HPX, LX Stage III/IV ENE 57% High‐risk defined as: pT3 or pT4 and any N‐stage except pT3N0 larynx with negative margins; T1‐2N2‐3; T1‐2N0‐01 with ≥1: ENE, positive margins, PNI, or vascular tumor embolism; or OC/OPX with nodes at level IV or V. |
1994 to 2000 RT: 66 Gy over 6 1/2 wk Cisplatin 100 mg/m2 on days 1, 22, and 43 |
60 mo |
PFS higher in the chemo‐PORT vs PORT (HR 0.75; 95% CI, 0.56 to 0.99, P = .04), with 5‐y PFS of 47% and 36%, respectively. The OS higher in chemo‐PORT group p (P = .02 by the log‐rank test; HR for death, 0.70; 95% CI, 0.52 to 0.95). Severe (grade 3 or higher) adverse effects were more frequent after combined therapy (41%) than after RT (21%, P = .001). |
1 |
|
Cooper (USA) 38 Update: Cooper 39 |
PORT vs PORT + cisplatin 100 mg/m2 |
459 OC, OPX, HPX, LX High risk defined as ≥1 factor: 2 or more involved nodes, ECE, microscopically involved mucosal margins (n = 73, 18%). |
1995 to 2000 PORT was 60 to 66 Gy in 30 to 33 fractions over a period of 6 to 6.6 wk. Cisplatin: 100 mg/m2 q3 wk × 3 cycles |
45.9 mo |
Improved LRC in chemo‐PORT group than in the PORT‐alone group (HR for local or regional recurrence, 0.61; 95% CI, 0.41 to 0.91; P = .01). OS did not reach statistical significance in initial report and with longer follow‐up (HR for death, 0.84; 95% CI, 0.65 to 1.09; P = .19). The incidence of acute adverse effects of grade 3 or greater was 34% in the RT group and 77% in the combined‐therapy group (P < .001). |
1 |
| Noronha (India) 19 |
PORT + cisplatin 30 mg/m2 weekly vs PORT + cisplatin 100 mg/m2 every 3 wk |
N = 300 Stage III/IV OC, OPX, HPX, LX, CUP 90% ENE Adjuvant therapy in 93% (7% were definitive) If adjuvant, patients had ≥1 of: ENE, ≤5 mm margin or positive margin, more than 2 positive lymph nodes, T4 primary |
2013 to 2017 RT: 60 Gy for adjuvant to high risk areas (tumor bed and involved nodal area). Cobalt or 2D (conventional simulator) |
22 mo | 2‐y LRC 58.5% in once a week vs 73.1% in every‐3‐week arm (P = .14). Acute grade 3 or higher 71.6% in once‐a‐week vs 84.6% in once‐every‐3‐wk (P = .006) | 1 |
|
Racadot (France) 47 |
PORT vs PORT + carboplatin 50 mg/m2 twice weekly |
144, OPX, HPX, LX Patients: Resected node positive with or without ECE. |
1994 to 2002 PORT (54‐72 Gy, 30‐40 fractions, 6‐8 wk) ± concomitant Carboplatin (50 mg/m2 administered by IV infusion twice weekly × 6 wk). |
106 mo |
2‐y rate of LRC was 73% (95% CI: 0.61‐0.84) in the combined treatment group and 68% (95% CI: 0.57‐0.80) in the radiotherapy group (P = .26). OS did not differ significantly between groups (HR, 1.05; 95% CI: 0.69‐1.60; P = .81) Stopped early due to publication of preliminary results of EORTC. |
2 |
|
Smid 52 (Slovenia) Update: Zakotnik 57 |
PORT vs PORT + mitomycin C and bleomycin |
114, OC, OPX, HPX, LX, NC, PNS Stage III/IV ENE 53% 59% ≥1 high‐risk factor High‐risk factors: ENE, PNI, LVI, microscopic or macroscopic residual disease). |
1997 to 2001 Total RT dose (56‐70 Gy). |
32.2 mo, Update: 76 mo |
At 5‐y in the PORT and postoperative CRT arms, LRC was 65% and 88% (P = .03), DFS 33% and 53% (P = .04), and OS 37% and 55% (P = .09). On planned subgroup analysis, benefit of chemotherapy appeared restricted to those with high‐risk factors. |
1 |
| Adjuvant chemotherapy regimens | ||||||
|
Harari (USA) 40 |
PORT + cetuximab + cisplatin 30 mg/m2 vs PORT + cetuximab + docetaxel 15 mg/m2 |
238, OC, OPX, HPX, LX Stage III/IV All patients had ≥1: Positive margins and/or ENE and/or two or more nodal metastases. |
2004 to 2006 | 4.4 y | Delivery of chemo‐PORT and cetuximab is feasible and tolerated. 2‐y OS was 69% for the cisplatin arm and 79% for the docetaxel arm; 2‐y DFS was 57% and 66%, respectively. Grade 3 to 4 myelosuppression was observed in 28% of patients in the cisplatin arm and 14% in the docetaxel arm; mucositis was observed in 56% and 54%, respectively. |
2 2 |
|
Head and Neck Contracts Program Study, National Cancer Institute (USA) 53 |
Induction cisplatin + bleomycin × 1 cycle followed by S + PORT vs induction cisplatin and bleomycin followed by S + PORT and adjuvant monthly cisplatin 80 mg/m2 × 6 mo vs PORT |
462 OC, LX, HPX Stage III/IV Stage II pyriform sinus |
1978 to 1982 RT: during week 3 or 4 after surgery. 60 Gy in 5 to 5.5 wk for clear margins. 60 Gy for microscopic residual or close margins (<0.5 cm on permanent), positive frozen followed by re‐excision or ENE. 70 Gy to gross residual. |
61 mo | While OS and DFS were NS different between 3 groups, adjuvant chemotherapy group had a lower rate of distant relapse (P < .03). | 1 |
|
Lam (Hong Kong) 43 |
No postoperative chemotherapy vs Postoperative levamisole and UFT (uracil/tegafur) |
65 OC, OPX, HPX, LX Stage III/IV |
1993 to 1995 Not clear how chemotherapy sequenced with PORT (majority received PORT) |
30 mo | A non‐statistically significant difference in DM (10% chemo vs 32% no chemo, P = .06) and 5‐y DFS (57% with chemo and 39% without). | 3 |
|
Laramore (USA) 44 Aka INT 0034/RTOG 85‐03) |
PORT alone vs 3 cycles of adjuvant cisplatin/5FU followed by PORT |
448, OC/OPX/LX: stage III/IV HPX: Stage II High risk if ENE, margins <5 mm or CIS at margins. Low risk: no ENE and margins ≥5 mm |
1985 to 1990. PORT dose risk‐stratified based on pathology: low risk: 50 Gy/25 F high risk: additional 10 Gy to high risk region. RT was 1.8‐2.0 Gy/d Chemotherapy: cisplatin 100 mg/m2 on d1and 5‐FU 1 g/m2 24hr infusion d1‐5 q 21 d for 3 cycles |
45.7 mo |
The 4‐y OS was 44% in the RT arm and 48% in the CT/RT arm (P = n.s.). 4‐y DFS was 38% in the RT arm compared to 46% in the CT/RT arm (P = n.s.). At 4 y the local/regional failure rate was 29% vs 26% for the RT and CT/RT arms, respectively (P = n.s.). The incidence of first failure in the neck nodes was 10% on the RT arm compared to 5% on the CT/RT arm (P = .03 without adjusting for multiple testing) and the overall incidence of distant metastases was 23% on the RT arm compared to 15% on the CT/RT arm (P = .03). “Risk group” status was a significant risk factor for LRC. |
1 |
|
Rao (India) 48 |
Observation without PORT vs MTX on postoperative day 3, 10, 17. |
135, OC (alveolobuccal) Stage III/IV |
1987 to 1989 No patients received RT |
Minimum 12 mo. (median NR) |
DFS of 71% in the MTX arm vs 45% in the control arm, (P < .01) with improved LC at primary site in MTX arm (P < .01) |
2 |
|
Rentschler (USA) 49 |
PORT or PORT and adjuvant MTX. |
60 Lx, OC, OPX, HPX, NPX Stage III/IV |
1979 to 1983 All received surgery and PORT. 5 taken off due to unresectable disease at surgery. MTX dose‐escalated from 40 mg/m2 and escalated 10 mg/m2 weekly. Given as 4 doses preoperative, 4 doses postoperative pre‐RT and 8 doses post‐RT. |
43 mo | Closed due to slow accrual. Median peak MTX dose was 80 mg/m2. No statistically significant difference in actuarial DFS (P = 1.0) or OS (P = .61). Although patients on the MTX arm appeared to have less local and regional recurrences at first recurrence (thus more distant metastases), this did not reach statistical significance (P = .06). There was NSD between the sites of recurrence at death or last follow‐up (P = .38). | 3 |
Abbreviations: Key: 5‐FU, fluorouracil; AF, accelerated fractionation; CF, conventional fractionation; CI, confidence interval; CRT, chemoradiation; CUP, carcinoma of unknown primary site; DFS, disease‐free survival; DSS, disease‐specific survival; DM, distant metastases; EBRT, external beam radiation therapy; ECS, extracapsular spread; ENE, extranodal spread; HNSCC, head and neck squamous cell carcinoma; HPX, hypopharynx; HR, hazard ratio; LOE, level of evidence; LRC, local‐regional control; LX, larynx; LVI, lymphovascular invasion; MTX, methotrexate; NC, nasal cavity; NPX, nasopharynx; NR, not reported; NSD, no significant difference; OC, oral cavity; OPX, oropharynx; OS, overall survival; PNI, perineural invasion; PNS, paranasal sinus; PORT, postoperative radiation therapy; QOL, quality of life; RCT, randomized controlled trial; RFS, recurrence‐free survival; RT, radiation therapy; S, surgery; SCC, squamous cell carcinoma; TORS, transoral robotic surgery.
4. TOPIC 1: SUMMARY OF STUDIES WITH ONCOLOGIC ENDPOINTS
4.1. The role of postoperative radiation therapy (PORT)
There are two randomized trials comparing observation to PORT. One trial showed a disease‐free survival (DFS) benefit with PORT for patients with stage III/IV buccal mucosa cancers. 45 The other randomized trial demonstrated a non‐statistically significant difference in overall recurrence (55.6% vs 36.5%, P = NS, exact value not reported). 41 Single arm phase I and phase II studies showed that PORT can be sequenced after minimally invasive surgery including transoral laser supraglottic laryngectomy, 58 and transoral robotic surgery. 62 , 69 , 82
The regular use of PORT for resected stage III/IV SCCHN is based on both retrospective and prospective studies. These demonstrated worse local‐regional control after surgery alone or after PORT, for patients with pathologic risk factors for recurrence, compared to patients without risk factors. Individual pathologic risk factors for recurrence include: T3 or T4 tumors, multiple involved nodes, lymphovascular invasion, anatomic location (eg, oral cavity vs oropharynx), low neck location of lymph nodes, extranodal extension (ENE), perineural invasion, and close/positive margins. 42 , 46 , 55 , 64 , 65 , 85 , 116 An increasing number of risk factors is associated with an increased risk of recurrence. 31 , 46
Additional risk factors for recurrence identified in retrospective studies that are not regularly incorporated as selection criteria in prospective studies of PORT or chemo‐PORT include poorly differentiated tumors 117 , 118 and, for oral cavity cancers, depth of invasion (DOI). 4 , 5 The AJCC‐8th edition staging incorporated DOI in the staging for oral cavity cancers in recognition of its prognostic significance.
4.2. Radiation therapy‐sequence and timing
PORT is typically preferred over preoperative RT. This practice reflects results of clinical trials. A randomized trial, RTOG 7303, demonstrated an improvement in local‐regional control (LRC) for patients treated with 60 Gy postoperatively compared to 50 Gy preoperatively (LRC, 65% vs 48%, P = .04). 42 , 55 Another study of patients with resected hypopharynx SCCHN compared PORT to preoperative RT using 55 Gy in each arm. The study was stopped early due to an unexpectedly high rate of postoperative deaths in the preoperative RT arm. Additionally, the 5‐year OS was better in the PORT group (5‐year OS 56% vs 20%, P < .10—no other P‐value given). 56
Timely initiation and completion of PORT are important for optimal LRC and OS. 21 , 31 , 46 , 51 , 81 There are several metrics for the optimal initiation and duration of postoperative therapy. 21 These include time from diagnosis to treatment initiation (DTI), 21 surgery to PORT‐initiation, 21 , 31 , 46 , 81 and surgery to end of PORT. 21 , 31 In general, a ≤6‐week interval from surgery to starting PORT is optimal. This metric is derived principally from studies of patients treated with radiation alone, yet retrospective studies suggest that timely initiation of adjuvant radiation is also associated with better OS amongst cohorts treated with chemoPORT. 21 For patients treated with PORT alone, accelerated radiation may improve LRC for patients who had a delay in starting RT as compared to conventional fractionation. 51 , 54
4.3. PORT dose and fractionation
One RCT evaluated radiation dose amongst patients stratified by pathologic risk factors for recurrence 46 for patients with stage III/IV SCCHN treated with PORT without chemotherapy. Doses of ≤54 Gy, given in 1.8 Gy fractions, to the primary site and/or pathologically involved neck were associated with higher local‐regional relapse rates (LRR). There was no significant dose response detected above 57.6 Gy except for patients with ENE in the neck who had improved LRR at doses ≥63 Gy. 46 , 50 A range of conventionally fractionated doses were used in prospective phase II and III clinical trials. In general, the high‐risk region of the postoperative bed was given 60 to 66 Gy in 1.8 to 2.0 Gy per fraction, the higher dose given for concerns regarding margin‐status or ENE. Lower doses ranging from 46 to 54 Gy in 1.8 to 2.0 Gy were generally given to electively treated regions.
Simultaneous integrated boosts, or “dose‐painting” are frequently used with IMRT resulting in lower fraction sizes of <1.8 Gy to a low‐risk elective nodal coverage. Prospective clinical trials should analyze the frequency of in‐field regional failures when treated at such low‐dose fraction sizes to determine the impact of “dose‐migration” to lower fraction sizes with the common use of IMRT dose‐painting.
The MARCH meta‐analysis of both definitive and postoperative patients showed that altered fractionation improved OS and secondary endpoints of progression‐free survival (PFS) and LRC. While all forms of altered fractionation improved local control, only hyperfractionation improved OS compared to conventional fractionation. 23
However, in a subsequent meta‐analysis restricted to postoperative randomized trials (n = 6), there was no improvement in OS, PFS, or LRC with postoperative accelerated fractionation compared to conventional fractionation. 24 The study was limited by the heterogeneity in accelerated fractionation regimens of the RCTs and a smaller population, reducing the ability to identify potential subgroups that may benefit most from accelerated regimens.
Even though individual RCTs have not demonstrated an OS benefit 33 , 34 , 51 with postoperative accelerated fractionation, some have shown a DFS or LRC benefit in specific subgroups such as those with stage III/IV tumors and a prolonged interval from surgery to RT 51 ; rapidly proliferating tumors 33 , 34 ; or oropharynx/oral cavity tumors. 54 A RCT from Poland compared 7 days a week accelerated fractionation (“P‐CAIR” postoperative‐continuous accelerated irradiation) with conventional fraction given 5 days a week using 1.8 Gy per day to 63 Gy. There was no benefit to accelerated fractionation in the total population and the rate of acute mucositis was doubled. There was, however, a LRC benefit in the oral cavity and oropharynx subgroup (74% p‐CAIR vs 53% CF, P = .02) in a population with a long median time from surgery to PORT of 9 weeks. 54 A post hoc analysis stratified by HPV‐status showed 100% 5‐year LRC for HPV‐positive patients and no statistically significant difference in outcomes for HPV‐negative (5‐year LRC 50.3% in CF vs 65.2% in p‐CAIR, P = .37). 91 The differential impact of accelerated fractionation by HPV status further supports the observation that improved risk‐group stratification by pathologic factors such as HPV‐status can better determine which patients may benefit from intensification with accelerated radiation (ie, HPV‐negative tumors).
Single‐arm prospective 76 , 79 , 81 and randomized studies 23 , 24 , 51 demonstrated that altered fractionation increases acute toxicities, such as mucositis, dermatitis and feeding‐tube use during treatment. The effects on late toxicity have varied, but at least one study showed an increase in late toxicity. 23 , 24 , 34
4.4. Altered fractionation and chemotherapy
In the definitive setting, the combination of chemotherapy and conventionally fractionated RT improved OS compared to altered fractionation. 23 In the postoperative setting, there are limited data comparing postoperative altered fractionation with or without chemotherapy.
The committee favors concurrent chemoradiation schedules over altered fractionation regimens for chemotherapy‐eligible patients in view of the strength of evidence for the addition of chemotherapy to conventional fractionation for high‐risk patients.
4.5. Radiation volume
Most clinical trials summarized in Tables 4 and S1 were completed in the pre‐IMRT era and describe techniques treating the entire postoperative bed and, with some exceptions such as smaller lateralized buccal/alveolar ridge tumors, treating both sides of the neck to elective doses with higher dose to involved regions.
Reducing the volume of irradiated tissue decreases acute and late toxicity. This was shown in two phase II studies that reduced radiation dose and/or volume to the pathologically negative neck or margin‐negative primary site; both demonstrated good LRC compared to historical controls. 15 , 70 The first prospective phase II study eliminated radiation from the surgically treated pathologically node‐negative neck in a cohort of patients with Stage III/IV SCCHN of whom 47% also received concurrent chemotherapy. At a median follow‐up of 53 months, there were no isolated recurrences in the un‐irradiated surgically‐treated neck. 15 The other phase II study was the AVOID trial from the University of Pennsylvania. They omitted radiation to the resected primary site of 60 patients with at ≥2 mm margins after TORS and no lymphovascular or perineural invasion. The 2‐year local recurrence free survival was 97.9% at 2.4‐year follow‐up. 18 While there may be an impact of systemic therapy on potential microscopic disease in the un‐irradiated neck, existing studies have not demonstrated a role for systemic therapy in treating the unirradiated neck. In the absence of PORT, adjuvant chemotherapy (methotrexate) improved disease‐free survival (DFS) by reducing local recurrence in a population of resected alveolobuccal HNSCC, 49 supporting the concept that postoperative chemotherapy may have an effect on local‐regional microscopic disease in some cases of resected SCCHN. Currently there is no demonstrated role for chemotherapy as a substitute for PORT when there are pathologic risk factors for recurrence.
4.6. Role of proton therapy
The role of postoperative proton therapy currently is not well‐defined and has not been compared in a randomized clinical trial to standard photon PORT delivered using IMRT and IGRT (Image Guided Radiation Therapy). Dosimetric studies suggest that with appropriate treatment‐planning a reduction in dose to normal tissue, such as the mucosa of the oral cavity (for non‐oral‐cavity cancers), may translate into reduced mucositis and dry mouth. 119 At present, there is insufficient published evidence of either improved local control or decreased morbidity to recommend postoperative proton therapy for resected SCCHN.
4.7. Addition of chemotherapy to PORT
Suboptimal outcomes with PORT alone served as the impetus to study the addition of chemotherapy to PORT in phase II 59 and randomized phase III clinical trials, 19 , 32 , 35 , 38 , 43 , 47 , 52 , 57 also summarized in systematic reviews and meta‐analyses. 20 , 26 , 29 , 30
Early single‐arm prospective clinical trials evaluated the optimal sequencing of postoperative chemotherapy before or after PORT. 64 , 65 Randomized trials compared PORT alone to either postoperative chemotherapy before PORT 44 or after PORT. 49 , 53 None showed a statistically significant improvement in OS or LRC, yet two trials showed a statistically significant reduction in DM with the addition of three cycles of postoperative cisplatin and 5FU given before PORT 44 or six cycles of maintenance monthly cisplatin 80 mg/m2 after PORT. 53 Subsequent clinical trials studied the benefit of concurrent chemotherapy with PORT.
PORT and cisplatin: Two large randomized controlled trials, Radiation Therapy Oncology Group (RTOG) 9501 and the European Organization Research and Treatment of Cancer (EORTC) 22 931 demonstrated a statistically significant LRC benefit with the addition of cisplatin 100 mg/m2 to PORT, given every 3 weeks for three cycles 37 , 38 for patients with “high‐risk” features. A statistically significant OS improvement was shown in the EORTC study. 37 In RTOG 9501, while the difference in 3‐year OS was 56% vs 47% (P = .09), a statistically significant OS improvement was not demonstrated in long‐term follow‐up. 38 , 39 The trials had different definitions of “high‐risk” features. The EORTC trial enrolled patients with tumor ≤5 mm from the surgical section margins, ENE, involvement of lymph nodes at levels 4 or 5 from oral cavity or oropharynx cancer, perineural disease, and/or vascular embolism. The RTOG trial enrolled high‐risk patients with ENE, tumor at the surgical section margins, or two or more involved lymph nodes. The rate of grade ≥3 toxicity was more than doubled with the addition of chemotherapy. The rate of DM did not differ between arms. Subsequent single arm phase II studies showed the regimen could be combined with a postoperative accelerated weekly concomitant boost regimen 74 and could be tolerated by populations outside of North America and Europe, such as in Japan. 67 , 73
Two trials compared weekly dosing of cisplatin with PORT compared to PORT‐alone in patients with stage III/IV SCCHN. 35 , 36 An OS benefit was demonstrated when cisplatin 50 mg/m2 weekly was added to PORT for stage III/IV SCCHN with extranodal extension; there was a non‐statistically significant improvement in LRC. 35 A smaller study with a wider definition of “high‐risk” used weekly cisplatin at a lower dose of 35 to 40 mg/m2 and did not show a statistically significant difference in OS or LRC. 36
PORT and mitomycin‐C (MMC): MMC and bleomycin conferred a LRC and OS benefit when added to PORT for resected stage III/IV SCCHN. 52 A subsequent pooled analysis showed MMC added to PORT improved LRC and DFS but not OS or DM. 86 , 90 This benefit persisted with longer follow‐up accompanied by an increase in late toxicity such as hypothyroidism. 57
PORT and carboplatin: Two RCTs showed no DFS or OS benefit with the addition of carboplatin 50 mg/m2 twice weekly 47 or 100 mg/m2 weekly 32 to PORT in a population of stage III/IV resected SCCHN with high‐risk features. However, both studies closed prematurely prior to completing the anticipated enrollment.
Other randomized studies: One prospective study of postoperative levamisole and uracil/tegafur did not show an OS or DFS benefit but did demonstrate a non‐statistically significant decrease in DM in the chemotherapy group (10% vs 32%, P = .06). 43 In this study, approximately 78% of patients received PORT, but the timing of chemotherapy in relation to PORT, whether concurrent or otherwise, was not described.
A pooled analysis 29 of the results from four randomized studies 35 , 37 , 38 , 52 demonstrated a LRC benefit and OS benefit with the addition of concurrent chemotherapy to PORT. At least half of patients in each trial had ENE, and they predominantly had T3 or T4 disease. Other systematic reviews and meta‐analyses confirmed a benefit of postoperative CRT, with a trade‐off of increased acute toxicity. 26 The effect on late toxicity from the addition of chemotherapy to PORT is not well‐defined in prospective studies. Several trials demonstrated LRC and/or OS benefits with the addition of chemotherapy to PORT, but none of them confirmed a reduction in DM. 37 , 38 , 52 , 57
A Cochrane review of chemotherapy for oral cavity and oropharyngeal cancer concluded that postoperative chemotherapy is associated with improved OS compared to surgery with or without PORT and that this improvement may be greater with concurrent chemotherapy compared to sequential therapy. 20 This is consistent with meta‐analyses of definitive treatment, which show the greatest benefit of chemotherapy when administered concurrently with radiation therapy. 120
4.8. Optimal concurrent chemotherapy
The majority of evidence supporting concurrent chemotherapy is with concurrent cisplatin, driven by the two largest trials, RTOG 9501 38 and EORTC 22931. 37 Reduced‐dose three‐weekly or weekly regimens were explored to improve the tolerability beyond that three cycles of cisplatin 100 mg/m2. 61 A systematic review and meta‐analysis compared every 3‐week high‐dose cisplatin (100 mg/m2, three doses) to weekly lower‐dose cisplatin (≤50 mg/m2, ≥ six doses) included 11 clinical trials from the postoperative setting; it showed no OS difference with similar rates of compliance and slightly higher grade 3 to 4 dysphagia and weight loss with the weekly regimen. 27 The review included a randomized trial of PORT with either cisplatin 30 mg/m2 weekly or three‐weekly cisplatin 100 mg/m2, 19 confirming superior LRC (primary endpoint) with three‐weekly cisplatin. It is noteworthy that the 30 mg/m2 is lower than the 40 mg/m2 commonly employed in clinical practice and the 50 mg/m2 weekly cisplatin dose that tested favorably in the RCT comparing it to PORT‐alone, 35 discussed above. At the time of this publication, the Japanese Clinical Oncology Group (JCOG) 1008 trial has been published in abstract form and showed non‐inferiority of weekly cisplatin 40 mg/m2 to three‐weekly cisplatin in high‐risk patients with microscopically positive margin and/or extranodal extension. 121
4.8.1. PORT combined with EGFR‐inhibition
While phase I and II studies have evaluated the feasibility and efficacy of EGFR inhibition combined with PORT, it has not been directly compared with cisplatin and PORT. RTOG 0234 tested the addition of cetuximab to weekly cisplatin or weekly docetaxel with favorable results compared to historical controls of the RTOG 9501 trial of PORT and three‐weekly cisplatin. 38 , 40 The combination of PORT with panitumumab and weekly cisplatin 30 mg/m2 was feasible in a stage III/IV high‐risk resected cohort. 60 PORT combined with the irreversible EGFR/HER inhibitor, afatinib, with or without docetaxel was not tolerated in an oral cavity cancer population. 17 PORT with weekly cisplatin will be directly compared to PORT with docetaxel and PORT with docetaxel/cetuximab in the first phase of RTOG 1216. The trial will reopen and include an arm of PORT and weekly cisplatin with the checkpoint inhibitor, atezolizumab, to be compared to PORT and cisplatin, and PORT and docetaxel/cetuximab (NCT01810913).
4.8.2. PORT and other chemotherapy regimens
Additional published postoperative regimens were focused on intensification and do not directly compare PORT with concurrent chemotherapy. 63 , 66 , 68 , 72 , 75 , 77 , 78 Studies have shown that PORT with or without chemotherapy therapy can be delivered after transoral resection with good oncologic outcomes. 80 , 82
4.9. Margins—Defining an optimal margin
A “close” or microscopically positive margin is a traditional indication for PORT or chemoPORT, having been used as an eligibility criterion for multiple prospective clinical trials. There is controversy regarding the margin sufficient to omit PORT, or to warrant intensification with chemoPORT. One of the reasons for this is that margin status was defined differently in clinical trials. For example, in RTOG 9501 a “high‐risk” feature was a microscopically positive margin. Yet in the EORTC study, patients were considered “high‐risk” for a close margin defined as <5 mm. 37 , 38 A large retrospective study of SCC of the oral tongue (n = 381), of whom 25% received postoperative therapy, proposed redefining the cut‐off for close margin to ≤2.2 mm. 122
While a microscopically positive margin is a clear indication for postoperative therapy, the optimal cut‐off to consider a margin “negative” (not close), is still subject to debate and may well be different for different primary sites and p16‐positive (HPV‐mediated) oropharynx cancers (as opposed to p16‐negative).
Further uncertainty has arisen in the context of transoral surgical management of oropharynx cancer where the superior constrictor margin is typically no more than 2 mm, representing the average thickness of the constrictor muscle. Current trials such as ECOG 3311 defined a negative margin at the deep constrictor as “no tumor on ink,” highlighting the importance of careful intraoperative labeling to enable pathologists to interpret the location of the margins such that the limited margin can be placed in anatomical context. Lastly, piece‐meal resection or separately submitted margins can make it challenging to quantify the distance of tumor to the margin.
Brachytherapy: Postoperative brachytherapy is used by some institutions to minimize off‐target integral dose to the adjacent tissue. There are few prospective studies evaluating outcomes with postoperative brachytherapy and none that compare brachytherapy boost to full‐course external beam RT. Two prospective studies incorporated high‐dose rate brachytherapy and EBRT demonstrating feasibility of the combination 71 , 77 ; there were increased severe complications in posteriorly located implants compared to anterior implants. 71 Even when brachytherapy is given to the surgical bed, an R1 resection was associated with worse local control than an R0 resection 88 ; a brachytherapy boost should not be used as a substitute for a margin‐negative resection. The timing of brachytherapy (whether before postoperative day five or after) did not seem to have a statistically significant impact on patients with newly diagnosed SCCHN. 89
5. TOPIC 2: SUMMARY OF STUDIES WITH QUALITY OF LIFE AND FUNCTION OUTCOMES
Monographs shown in Table S2 reported quality of life and functional assessments after postoperative therapy. Two systematic reviews summarized clinical studies with questionnaire‐based endpoints 93 and observer‐rated speech and swallow functional assessments. 92 A single‐randomized trial studied the effect of radiation to normal tissue and observer‐rated mucositis. 94 The remaining studies either included patient‐questionnaire endpoints 95 , 96 , 97 , 98 , 99 , 100 , 101 , 103 , 104 , 105 , 106 , 109 , 110 , 113 , 115 or observer‐rated measurements of head and shoulder mobility, 102 video fluoroscopy and speech assessment using standardized testing, 107 lymphedema and fibrosis measurement, 108 oral bite force and masticatory function, 111 tongue sensation and mobility 112 and maximal mouth opening. 114
5.1. Pretreatment factors and function after postoperative therapy
Baseline patient and tumor‐related factors prior to surgery are predictive of outcomes after resection and postoperative therapy. Patient‐related factors include baseline comorbidity, 92 depression, 98 socioeconomic status, and advanced age. 115 Tumor‐related predictors of poor QOL and functional outcomes include higher T‐category, 92 N‐category, 110 and tumor location. 96 For example, one prospective study of mostly locally advanced oral cavity SCCHN patients showed worse patient reported QOL outcomes for floor of mouth tumors relative to other tumors including oral tongue, alveolar ridge, and hard palate. 96
Prior to postoperative therapy, patients can sustain postsurgical changes (such as lymphedema and fibrosis) that impact QOL and function 108 ; altered mastication 111 ; reduced tongue mobility and sensation 112 ; decreased maximal mouth opening 114 ; and shoulder dysfunction. 113 Surgical factors associated with worse QOL and/or function include the extent of resection, the site of resection, and the type of reconstruction or prostheses required. 92 , 93 , 96 , 104 , 109
Such postoperative changes should be considered as baseline assessments in the course of clinical trials prior to initiation of postoperative therapy. Posttreatment function and QOL reflect both surgical and postsurgical treatments and may not solely be related to postoperative treatments.
6. TOPIC 3: RESECTED EARLY‐STAGE SCCHN
The role of PORT in stage I‐II SCCHN is not well‐defined; these patients are not well‐represented in randomized trials (see Table 1 and 4). A small percentage of patients with involved margins were included in studies of PORT and/or chemo‐PORT. An increased number of adverse features (eg, perineural invasion, depth of invasion, margin status, lymphovascular invasion, poor differentiation, etc) is likely to influence the risk of recurrence for patients with resected Stage I‐II SCCHN, 122 as suggested by literature review. When there are uninvolved margins, the benefit of postoperative therapy for patients with single risk factors such as perineural invasion 28 or combinations thereof, is not well‐defined.
Retrospective data show the importance of DOI as a risk factor for LRR. 4 , 5 As a result, recent studies, such as RTOG 0920 (NCT00956007), which compare PORT to PORT with cetuximab include depth of invasion (DOI) in the eligibility criteria, specifically including AJCC‐7 pT2 N0 oral cavity (OC) cancers >2 cm but ≤4 cm, with >5 mm. There is insufficient prospective evidence to recommend regular application of PORT for patients with OC tumors that are ≤4 cm but with >5 mm DOI.
7. TOPIC 4: RESECTED LOCALLY‐ADVANCED SCCHN, NEGATIVE MARGINS AND NO EXTRANODAL EXTENSION
Most patients included in prospective studies of postoperative therapy have pathologic stage III or IV cancers. Even within the group of stage III‐IV SCCHN, there may be low‐risk groups than may not require postoperative therapy. A systematic review of randomized and non‐randomized studies sought to determine the benefit of PORT in patients with pT1‐2 oropharynx cancer and a single involved ipsilateral node ≤3 cm (AJCC‐7 pN1). 25 Due to the limitations and heterogeneity of existing data, the strength of evidence was considered low for making recommendations for patients with pT1‐2 N1 oropharynx cancer with a single ipsilateral node and no ENE with tumor‐free resection margins. There is also limited evidence to support use of PORT for patients with a single lymph node >3 cm and ≤6 cm (AJCC‐7 N‐category N2a).
A post hoc subgroup analysis of two randomized trials confirmed a LRC and OS benefit from adding chemotherapy to PORT for patients with microscopically positive margins and ENE. 78 , 84 Aside from the risk factors of positive microscopically involved margins and ENE, there is uncertainty regarding risk factors that warrant the addition of chemotherapy to PORT. The absence of a statistically significant OS benefit in subgroups without ENE or positive margins does not define lack of benefit. It may have been related to lack of statistical power or relatively smaller benefit than for patients who had the high‐risk features of ENE or positive margins. The decision to provide postoperative chemo‐PORT should be made on a case‐by‐case basis (Table 2B).
8. TOPIC 5: RESECTED P16‐POSITIVE (HPV‐MEDIATED) SQUAMOUS CELL CARCINOMA OF THE OROPHARYNX
To date, there are no published RCTs that compare de‐intensified to standard postoperative therapy. 22 Single arm studies show good oncologic outcomes of de‐intensified regimens compared to historical controls. 16 , 18 A recent phase II study of 80 patients with HPV‐mediated oropharynx SCC showed a 2‐year PFS and LRC of 91.1% and 96.2%, respectively, with a postoperative regimen of 30 Gy given at 1.5 Gy twice daily with a simultaneous integrated boost of 1.8 Gy BID to areas of ENE (total dose 36 Gy) and concurrent weekly docetaxel 15 mg/m2. 16 The ECOG 3311 phase II clinical trial has completed follow‐up and will determine the efficacy of reduced‐ dose PORT (50 Gy) compared to standard PORT (60 Gy) for patients with intermediate‐risk cancers, negative margins, and ≤1 mm extranodal extension (NCT01898494). At the time of this publication, ECOG 3311 is published in abstract form 123 with a median follow‐up of 31.8 months and suggests that 50 Gy may be sufficient for such patients. The reduced dose arm will be carried forward into subsequent phase III trials. Other large multi‐institutional clinical trials are underway for postoperative de‐intensification studies for patients with favorable HPV‐positive oropharynx, including PATHOS (NCT02215265) and ORATOR‐II (NCT03210103) amongst many other single‐institutional phase II trials. De‐intensification of PORT should only be undertaken in the course of clinical trial (Table 3).
8.1. Summary of recommendations
The committee strongly recommends that postoperative therapy decisions be based on consideration of the factors that were used in the assignment of T‐ and N‐categories prior to the AJCC 8th edition as clinical trials that form the basis of the above recommendations employed staging prior to the recent AJCC staging criteria.
The committee identified several areas of controversy and subject for future research, as shown in the Clinical Variants (Tables 1A, 2A, 3A) and mentioned below, where there was insufficient agreement to provide a consensus recommendation.
The committee strongly recommends that it is usually appropriate that postoperative radiation therapy should be initiated within 6 weeks of surgery when postoperative healing permits.
The committee strongly recommends that concurrent systemic therapy is usually appropriate for relatively “fit” patients who have microscopically involved surgical margins and/or extranodal extension.
The committee strongly recommends concurrent cisplatin‐based therapy as usually appropriate. Specifically, cisplatin 100 mg/m2, is recommended as usually appropriate when concurrent therapy is indicated. There is insufficient evidence comparing three‐weekly cisplatin 100 mg/m2 to weekly cisplatin at doses of at least 40 mg/m2 weekly, which may be appropriate, to support adoption of the latter schedule.
The committee strongly recommends that altered fractionation should be considered usually appropriate for patients who are not medically eligible for chemotherapy but have risk factors that otherwise would warrant the addition of chemotherapy. The committee acknowledges that most studies showing the benefit of altered fractionation were performed in the pre‐IMRT era. There is insufficient evidence to recommend a preferred altered fractionation regimen.
The committee strongly recommends postoperative radiation therapy as usually appropriate for patients with intermediate risk factors for recurrence, including pT3‐4 tumors, multiple involved nodes, or a single node >6 cm.
The committee strongly recommends additional therapy as usually appropriate for early‐stage oral tongue cancer with a positive margin. PORT‐alone was strongly recommended as usually appropriate when re‐excision is not performed to clear the region of the close/positive margin. Yet there was insufficient agreement on the appropriateness of PORT to the primary‐site only or unilateral or bilateral neck irradiation. There was insufficient agreement on the role of concurrent systemic therapy or altered fractionation for this situation.
The committee recommends PORT as usually appropriate for early‐stage oral tongue cancer with increased depth of invasion (DOI); there is insufficient evidence to routinely recommend PORT for a specific DOI. While agreement was not reached on the appropriateness of unilateral or bilateral nodal irradiation, the committee recommended against PORT to the primary site only, which is usually not appropriate for oral cavity cancer with increased DOI.
The committee does not recommend de‐escalation of postoperative therapy, including reduced‐dose PORT or omission of chemotherapy for p16+ oropharynx cancer, in routine clinical practice until additional high‐quality prospective data is available including publication of ECOG 3311.
Clinical studies that compare standard of care postoperative therapy to de‐intensified therapy will be published in the upcoming years and may change the above recommendations.
8.2. Summary of evidence
Studies with primary oncologic endpoints are shown in the Table S1 and included 11 systematic reviews, 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 24 randomized trials with four long‐term updates, 19 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 29 non‐randomized clinical trials, 15 , 16 , 17 , 18 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 and nine post hoc analyses of randomized trials. 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 The randomized trials are presented in Table 4. Studies that focused on non‐oncologic endpoints are shown in the Table S2 and included two systematic reviews, 92 , 93 one randomized trial, 94 and 21 non‐randomized clinical trials. 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 All studies reported staging prior to the implementation of AJCC‐8.
The 101 references cited in the ARS Appropriate Use Criteria Head and Neck Cancer Postoperative Management Evidence Table were published from 1977 to 2020. Of the 101 references, 78 are categorized as therapeutic references including 11 systematic reviews/meta‐analyses, 10 well‐designed studies, 31 good quality studies, 21 quality studies that may have design limitations, and 1 of limited quality that may not be useful as primary evidence. Updates of randomized trials were included in the grading of the initial trial publication. There were 23 studies categorized as descriptive studies including 2 systematic reviews and 20 quality non‐randomized prospective cohort studies and 1 study of limited quality.
Although there are references that report on studies with design limitations, 41 well‐designed or good quality studies provide good evidence.
8.3. Supporting documents
For additional information on the ARS Appropriate Use Criteria methodology and other supporting documents go to http://www.americanradiumsociety.org/page/aucmethodology.
CONFLICT OF INTEREST
All panelists were required to declare all conflicts of interest for the previous 36 months prior to initiating work on this document. These complete disclosure forms are retained by the American Radium Society in perpetuity. The ARS Appropriate Use Criteria Steering Committee reviewed these disclosures with the chair and co‐chair of this document and approved participation of the panelists prior to starting development of this work. Disclosures potentially relevant to the content of this guideline are provided. Dr Bakst has nothing to disclose; Dr Beadle has nothing to disclose; Dr Beitler has nothing to disclose; Dr Chang has nothing to disclose; Dr Chen has nothing to disclose; Dr Cooper has nothing to disclose; Dr Galloway reports personal fees from Varian Medical Systems, personal fees from UpToDate Inc, outside the submitted work; Dr Koyfman reports grants from Merck, grants from Bristol Myers Squib, other from Varian Medical Systems, other from UpToDate, outside the submitted work; Dr Margalit reports personal fees from Galera Therapeutics in 2018, outside the submitted work; Dr Ridge has nothing to disclose; Dr Robbins has nothing to disclose; Dr Sacco reports other from Merck, outside the submitted work; Dr Siddiqui reports grants, personal fees and non‐financial support from Varian Medical Systems, Inc, other from American College of Radiology, other from Wayne State University School of Medicine, outside the submitted work; Dr Truong reports travel expenses from the American Board of Radiology and honoraria from the NCI PDQ Editorial Board; Dr Tsai reports consulting fees/honorarium from Varian; Dr Yom reports grants from Genentech, grants from Bristol‐Myers Squibb, grants from Merck, grants from BioMimetix, personal fees from Springer, personal fees from UpToDate, outside the submitted work.
Supporting information
Appendix S1: Supporting Information.
Table S1 Studies evaluating oncologic outcomes after adjuvant therapy for resected squamous cell carcinoma of the head and necks (n = 77).
Table S2 Studies evaluating the effect of adjuvant therapy on functional and toxicity outcomes (n = 24).
ACKNOWLEDGMENTS
The committee members extend our gratitude to Andrea Taylor for administrative support.
Margalit DN, Sacco AG, Cooper JS, et al. Systematic review of postoperative therapy for resected squamous cell carcinoma of the head and neck: Executive summary of the American Radium Society appropriate use criteria. Head & Neck. 2021;43:367–391. 10.1002/hed.26490
The American Radium Society Appropriate Use Criteria and its expert panels have developed criteria for determining appropriate procedures for diagnosis and treatment of specified medical condition(s). These criteria are intended to guide treating and referring physicians in making decisions regarding diagnosis and treatment of cancers and related or associated conditions. Generally, the complexity and severity of a patient's clinical condition should dictate the selection of appropriate diagnostic procedures or treatments. Only those examinations or treatments generally used for evaluation of the patient's condition are ranked. Other procedures necessary to evaluate or treat other co‐existent diseases or other medical consequences of this condition are not considered in this document. The availability of equipment or personnel may influence the selection of appropriate procedures or treatments. Procedures, techniques, or other interventions classified as investigational by the FDA have not been considered in developing these criteria; however, study of new equipment, applications, and treatment protocols should be encouraged. The ultimate decision regarding the appropriate use of any specific examination or treatment must be made by the referring physician and oncologist in light of all the circumstances presented in an individual examination. The ARS Appropriate Use Criteria seek and encourage collaboration with other organizations on the development of the Criteria through representation on expert panels. Participation by representatives from collaborating organizations on the expert panel does not necessarily imply individual or society endorsement of the final document. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or reflecting the views of the Uniformed Services University of the Health Sciences or the Department of Defense or the National Institutes of Health.
REFERENCES
- 1. Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mahal BA, Catalano PJ, Haddad RI, et al. Incidence and demographic burden of HPV‐associated Oropharyngeal head and Neck cancers in the United States. Cancer Epidemiol Biomarkers Prev. 2019;28(10):1660‐1667. [DOI] [PubMed] [Google Scholar]
- 3. Cracchiolo JR, Baxi SS, Morris LG, et al. Increase in primary surgical treatment of T1 and T2 oropharyngeal squamous cell carcinoma and rates of adverse pathologic features: National Cancer Data Base. Cancer. 2016;122(10):1523‐1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ganly I, Goldstein D, Carlson DL, et al. Long‐term regional control and survival in patients with "low‐risk," early stage oral tongue cancer managed by partial glossectomy and neck dissection without postoperative radiation: the importance of tumor thickness. Cancer. 2013;119(6):1168‐1176. [DOI] [PubMed] [Google Scholar]
- 5. International Consortium for Outcome Research in H , Neck C, Ebrahimi A, et al. Primary tumor staging for oral cancer and a proposed modification incorporating depth of invasion: an international multicenter retrospective study. JAMA Otolaryngol Head Neck Surg. 2014;140(12):1138‐1148. [DOI] [PubMed] [Google Scholar]
- 6. Amin MB, American Joint Committee on Cancer , American Cancer Society . AJCC Cancer Staging Manual. Eight edition/editor‐in‐chief, Mahul B. Amin, MD, FCAP; editors, Stephen B. Edge, MD, FACS and 16 others; Donna M. Gress, RHIT, CTR ‐ Technical editor; Laura R. Meyer, CAPM ‐ Managing editor. ed. Chicago IL: American Joint Committee on Cancer, Springer; 2017. [Google Scholar]
- 7. Salama JK, Saba N, Quon H, et al. ACR appropriateness criteria® adjuvant therapy for resected squamous cell carcinoma of the head and neck. Oral Oncol. 2011;47(7):554‐559. [DOI] [PubMed] [Google Scholar]
- 8. Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. [DOI] [PubMed] [Google Scholar]
- 9. Fitch K. The Rand/UCLA Appropriateness Method user's Manual. Rand: Santa Monica; 2001. [Google Scholar]
- 10. Dalkey NC, Helmer O. An experimental application of the DELPHI method to the use of experts. Manag Sci. 1963;9(3):458‐467. [Google Scholar]
- 11. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. OCEBM . Levels of Evidence Working Group. The Oxford 2011 Levels of Evidence. Oxford, England: Oxford Centre for Evidence‐Based Medicine; 2019. http://www.cebm.net/index.aspx?o=5653. Accessed June 1, 2019.
- 13. Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta‐analyses. Lancet. 1999;354(9193):1896‐1900. [DOI] [PubMed] [Google Scholar]
- 14. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Contreras JA, Spencer C, DeWees T, et al. Eliminating postoperative radiation to the pathologically node‐negative neck: long‐term results of a prospective phase II study. J Clin Oncol. 2019;37(28):2548‐2555. [DOI] [PubMed] [Google Scholar]
- 16. Ma DJ, Price KA, Moore EJ, et al. Phase II evaluation of aggressive dose de‐escalation for adjuvant chemoradiotherapy in human papillomavirus‐associated oropharynx squamous cell carcinoma. J Clin Oncol. 2019;37(22):1909‐1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Margalit DN, Haddad RI, Tishler RB, et al. A phase 1 study of Afatinib in combination with postoperative radiation therapy with and without weekly Docetaxel in intermediate‐ and high‐risk patients with resected squamous cell carcinoma of the head and Neck. Int J Radiat Oncol Biol Phys. 2019;105:132‐139. [DOI] [PubMed] [Google Scholar]
- 18. Swisher‐McClure S, Lukens JN, Aggarwal C, et al. A phase 2 trial of alternative volumes of Oropharyngeal irradiation for de‐intensification (AVOID): omission of the resected primary tumor bed after transoral robotic surgery for human papilloma virus‐related squamous cell carcinoma of the oropharynx. Int J Radiat Oncol Biol Phys. 2020;106(4):725‐732. [DOI] [PubMed] [Google Scholar]
- 19. Noronha V, Joshi A, Patil VM, et al. Once‐a‐week versus once‐every‐3‐weeks cisplatin chemoradiation for locally advanced head and Neck cancer: a phase III randomized noninferiority trial. J Clin Oncol. 2018;36(11):1064‐1072. [DOI] [PubMed] [Google Scholar]
- 20. Furness S, Glenny AM, Worthington HV, et al. Interventions for the treatment of oral cavity and oropharyngeal cancer: chemotherapy. Cochrane Database Syst Rev. 2011;4:CD006386. [DOI] [PubMed] [Google Scholar]
- 21. Graboyes EM, Kompelli AR, Neskey DM, et al. Association of treatment delays with survival for patients with head and neck cancer: a systematic review. JAMA Otolaryngol Head Neck Surg. 2018;01:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Howard J, Dwivedi RC, Masterson L, Kothari P, Quon H, Holsinger FC. De‐intensified adjuvant (chemo)radiotherapy versus standard adjuvant chemoradiotherapy post transoral minimally invasive surgery for resectable HPV‐positive oropharyngeal carcinoma. Cochrane Database Syst Rev. 2018;12:CD012939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lacas B, Bourhis J, Overgaard J, et al. Role of radiotherapy fractionation in head and neck cancers (MARCH): an updated meta‐analysis. Lancet Oncol. 2017;18(9):1221‐1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Matuschek C, Haussmann J, Bolke E, et al. Accelerated vs. conventionally fractionated adjuvant radiotherapy in high‐risk head and neck cancer: a meta‐analysis. Radiat Oncol. 2018;13(1):195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moergel M, Meurer P, Ingel K, Wendt TG, Al‐Nawas B. Effectiveness of postoperative radiotherapy in patients with small oral and oropharyngeal squamous cell carcinoma and concomitant ipsilateral singular cervical lymph node metastasis (pN1): a meta‐analysis. Strahlenther Onkol. 2011;187(6):337‐343. [DOI] [PubMed] [Google Scholar]
- 26. Shang J, Gu J, Han Q, Xu Y, Yu X, Wang K. Chemoradiotherapy is superior to radiotherapy alone after surgery in advanced squamous cell carcinoma of the head and neck: a systematic review and meta‐analysis. Int J Clin Exp Med. 2014;7(9):2478‐2487. [PMC free article] [PubMed] [Google Scholar]
- 27. Szturz P, Wouters K, Kiyota N, et al. Weekly low‐dose versus three‐weekly high‐dose cisplatin for concurrent chemoradiation in locoregionally advanced non‐nasopharyngeal head and neck cancer: a systematic review and meta‐analysis of aggregate data. Oncologist. 2017;22(9):1056‐1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vonk J, Smit KA, Roodenburg JLN, et al. Effect of adjuvant radiotherapy on the local recurrence of oral squamous cell carcinoma with perineural invasion: a systematic review. Clin Otolaryngol. 2018;08:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Winquist E, Oliver T, Gilbert R. Postoperative chemoradiotherapy for advanced squamous cell carcinoma of the head and neck: a systematic review with meta‐analysis. Head Neck. 2007;29(1):38‐46. [DOI] [PubMed] [Google Scholar]
- 30. Zackrisson B, Mercke C, Strander H, Wennerberg J, Cavallin‐Stahl E. A systematic overview of radiation therapy effects in head and neck cancer. Acta Oncol. 2003;42(5–6):443‐461. [DOI] [PubMed] [Google Scholar]
- 31. Ang KK, Trotti A, Brown BW, et al. Randomized trial addressing risk features and time factors of surgery plus radiotherapy in advanced head‐and‐neck cancer. Int J Radiat Oncol Biol Phys. 2001;51(3):571‐578. [DOI] [PubMed] [Google Scholar]
- 32. Argiris A, Karamouzis MV, Johnson JT, et al. Long‐term results of a phase III randomized trial of postoperative radiotherapy with or without carboplatin in patients with high‐risk head and neck cancer. Laryngoscope. 2008;118(3):444‐449. [DOI] [PubMed] [Google Scholar]
- 33. Awwad HK, Khafagy Y, Barsoum M, et al. Accelerated versus conventional fractionation in the postoperative irradiation of locally advanced head and neck cancer: influence of tumour proliferation. Radiother Oncol. 1992;25(4):261‐266. [DOI] [PubMed] [Google Scholar]
- 34. Awwad HK, Lotayef M, Shouman T, et al. Accelerated hyperfractionation (AHF) compared to conventional fractionation (CF) in the postoperative radiotherapy of locally advanced head and neck cancer: influence of proliferation. Br J Cancer. 2002;86(4):517‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bachaud JM, Cohen‐Jonathan E, Alzieu C, David JM, Serrano E, Daly‐Schveitzer N. Combined postoperative radiotherapy and weekly cisplatin infusion for locally advanced head and neck carcinoma: final report of a randomized trial. Int J Radiat Oncol Biol Phys. 1996;36(5):999‐1004. [DOI] [PubMed] [Google Scholar]
- 36. Bandyopadhyay A, Senapati S, Samanta DR, Mohanty S, Das PK. Concurrent cisplatin‐based chemotherapy versus radiotherapy alone as adjuvant therapy for squamous cell carcinoma of the oral cavity bearing high‐risk features. Clin Cancer Investig J. 2015;4(5):610‐616. [Google Scholar]
- 37. Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350(19):1945‐1952. [DOI] [PubMed] [Google Scholar]
- 38. Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high‐risk squamous‐cell carcinoma of the head and neck. N Engl J Med. 2004;350(19):1937‐1944. [DOI] [PubMed] [Google Scholar]
- 39. Cooper JS, Zhang Q, Pajak TF, et al. Long‐term follow‐up of the RTOG 9501/intergroup phase III trial: postoperative concurrent radiation therapy and chemotherapy in high‐risk squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2012;84(5):1198‐1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Harari PM, Harris J, Kies MS, et al. Postoperative chemoradiotherapy and cetuximab for high‐risk squamous cell carcinoma of the head and neck: radiation therapy oncology group RTOG‐0234. J Clin Oncol. 2014;32(23):2486‐2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kokal WA, Neifeld JP, Eisert D, et al. Postoperative radiation as adjuvant treatment for carcinoma of the oral cavity, larynx, and pharynx: preliminary report of a prospective randomized trial. J Surg Oncol. 1988;38(2):71‐76. [DOI] [PubMed] [Google Scholar]
- 42. Kramer S, Gelber RD, Snow JB, et al. Combined radiation therapy and surgery in the management of advanced head and neck cancer: final report of study 73‐03 of the Radiation Therapy Oncology Group. Head Neck Surg. 1987;10(1):19‐30. [DOI] [PubMed] [Google Scholar]
- 43. Lam P, Yuen AP, Ho CM, Ho WK, Wei WI. Prospective randomized study of post‐operative chemotherapy with levamisole and UFT for head and neck carcinoma. Eur J Surg Oncol. 2001;27(8):750‐753. [DOI] [PubMed] [Google Scholar]
- 44. Laramore GE, Scott CB, al‐Sarraf M, et al. Adjuvant chemotherapy for resectable squamous cell carcinomas of the head and neck: report on intergroup study 0034. Int J Radiat Oncol Biol Phys. 1992;23(4):705‐713. [DOI] [PubMed] [Google Scholar]
- 45. Mishra RC, Singh DN, Mishra TK. Post‐operative radiotherapy in carcinoma of buccal mucosa, a prospective randomized trial. Eur J Surg Oncol. 1996;22(5):502‐504. [DOI] [PubMed] [Google Scholar]
- 46. Peters LJ, Goepfert H, Ang KK, et al. Evaluation of the dose for postoperative radiation therapy of head and neck cancer: first report of a prospective randomized trial. Int J Radiat Oncol Biol Phys. 1993;26(1):3‐11. [DOI] [PubMed] [Google Scholar]
- 47. Racadot S, Mercier M, Dussart S, et al. Randomized clinical trial of post‐operative radiotherapy versus concomitant carboplatin and radiotherapy for head and neck cancers with lymph node involvement. Radiother Oncol. 2008;87(2):164‐172. [DOI] [PubMed] [Google Scholar]
- 48. Rao RS, Parikh DM, Parikh HK, Bhansali MB, Fakih AR. Perioperative chemotherapy in oral cancer. J Surg Oncol. 1991;47(1):21‐26. [DOI] [PubMed] [Google Scholar]
- 49. Rentschler RE, Wilbur DW, Petti GH, et al. Adjuvant methotrexate escalated to toxicity for resectable stage III and IV squamous head and neck carcinomas—a prospective, randomized study. J Clin Oncol. 1987;5(2):278‐285. [DOI] [PubMed] [Google Scholar]
- 50. Rosenthal DI, Mohamed ASR, Garden AS, et al. Final report of a prospective randomized trial to evaluate the dose‐response relationship for postoperative radiation therapy and pathologic risk groups in patients with head and neck cancer. Int J Radiat Oncol Biol Phys. 2017;98(5):1002‐1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sanguineti G, Richetti A, Bignardi M, et al. Accelerated versus conventional fractionated postoperative radiotherapy for advanced head and neck cancer: results of a multicenter phase III study. Int J Radiat Oncol Biol Phys. 2005;61(3):762‐771. [DOI] [PubMed] [Google Scholar]
- 52. Smid L, Budihna M, Zakotnik B, et al. Postoperative concomitant irradiation and chemotherapy with mitomycin C and bleomycin for advanced head‐and‐neck carcinoma. Int J Radiat Oncol Biol Phys. 2003;56(4):1055‐1062. [DOI] [PubMed] [Google Scholar]
- 53. Study HaNCP . Adjuvant chemotherapy for advanced head and neck squamous carcinoma. Final report of the head and neck contracts program. Cancer. 1987;60(3):301‐311. [DOI] [PubMed] [Google Scholar]
- 54. Suwinski R, Bankowska‐Wozniak M, Majewski W, et al. Randomized clinical trial on 7‐days‐a‐week postoperative radiotherapy for high‐risk squamous cell head and neck cancer. Radiother Oncol. 2008;87(2):155‐163. [DOI] [PubMed] [Google Scholar]
- 55. Tupchong L, Scott CB, Blitzer PH, et al. Randomized study of preoperative versus postoperative radiation therapy in advanced head and neck carcinoma: long‐term follow‐up of RTOG study 73‐03. Int J Radiat Oncol Biol Phys. 1991;20(1):21‐28. [DOI] [PubMed] [Google Scholar]
- 56. Vandenbrouck C, Sancho H, Le Fur R, Richard JM, Cachin Y. Results of a randomized clinical trial of preoperative irradiation versus postoperative in treatment of tumors of the hypopharynx. Cancer. 1977;39(4):1445‐1449. [DOI] [PubMed] [Google Scholar]
- 57. Zakotnik B, Budihna M, Smid L, et al. Patterns of failure in patients with locally advanced head and neck cancer treated postoperatively with irradiation or concomitant irradiation with Mitomycin C and Bleomycin. Int J Radiat Oncol Biol Phys. 2007;67(3):685‐690. [DOI] [PubMed] [Google Scholar]
- 58. Agrawal A, Moon J, Davis RK, et al. Transoral carbon dioxide laser supraglottic laryngectomy and irradiation in stage I, II, and III squamous cell carcinoma of the supraglottic larynx: report of Southwest Oncology Group Phase 2 Trial S9709. Arch Otolaryngol Head Neck Surg. 2007;133(10):1044‐1050. [DOI] [PubMed] [Google Scholar]
- 59. Al‐Sarraf M, Pajak TF, Byhardt RW, Beitler JJ, Salter MM, Cooper JS. Postoperative radiotherapy with concurrent cisplatin appears to improve locoregional control of advanced, resectable head and neck cancers: RTOG 88‐24. Int J Radiat Oncol Biol Phys. 1997;37(4):777‐782. [DOI] [PubMed] [Google Scholar]
- 60. Ferris RL, Geiger JL, Trivedi S, et al. Phase II trial of post‐operative radiotherapy with concurrent cisplatin plus panitumumab in patients with high‐risk, resected head and neck cancer. Ann Oncol. 2016;27(12):2257‐2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Franchin G, Minatel E, Politi D, et al. Postoperative reduced dose of cisplatin concomitant with radiation therapy in high‐ risk head and neck squamous cell carcinoma. Cancer. 2009;115(11):2464‐2471. [DOI] [PubMed] [Google Scholar]
- 62. Genden EM, Kotz T, Tong CCL, et al. Transoral robotic resection and reconstruction for head and neck cancer. Laryngoscope. 2011;121(8):1668‐1674. [DOI] [PubMed] [Google Scholar]
- 63. Grecula JC, Schuller DE, Rhoades CA, et al. Intensification regimen 2 for advanced head and neck squamous cell carcinomas. 1999;125(12):1313‐1318. [DOI] [PubMed] [Google Scholar]
- 64. Jacobs JR, Pajak TF, al‐Sarraf M, et al. Chemotherapy following surgery for head and neck cancer. A radiation therapy oncology group study. Am J Clin Oncol. 1989;12(3):185‐189. [DOI] [PubMed] [Google Scholar]
- 65. Johnson JT, Myers EN, Mayernik DG, Nolan TA, Sigler BA, Wagner RL. Adjuvant methotrexate‐5‐fluorouracil for extracapsular squamous cell carcinoma in cervical metastasis. Laryngoscope. 1990;100(6):590‐592. [DOI] [PubMed] [Google Scholar]
- 66. Kish JA, Benedetti JK, Balcerzak SP, et al. Feasibility trial of postoperative radiotherapy and cisplatin followed by three courses of 5‐FU and cisplatin in patients with resected head and neck cancer: a Southwest Oncology Group study. Cancer J Sci Am. 1999;5(5):307‐311. [PubMed] [Google Scholar]
- 67. Kiyota N, Tahara M, Okano S, et al. Phase II feasibility trial of adjuvant chemoradiotherapy with 3‐weekly cisplatin for Japanese patients with post‐operative high‐risk squamous cell carcinoma of the head and neck. Jpn J Clin Oncol. 2012;42(10):927‐933. [DOI] [PubMed] [Google Scholar]
- 68. Kovacs AF, Schiemann M, Turowski B. Combined modality treatment of oral and oropharyngeal cancer including neoadjuvant intraarterial cisplatin and radical surgery followed by concurrent radiation and chemotherapy with weekly docetaxel ‐ three year results of a pilot study. J Craniomaxillofac Surg. 2002;30(2):112‐120. [DOI] [PubMed] [Google Scholar]
- 69. Lörincz BB, Möckelmann N, Busch CJ, et al. Two‐year survival analysis of 50 consecutive head and neck cancer patients treated with transoral robotic surgery in a single European Centre. Ann Surg Oncol. 2015;22:1028‐1033. [DOI] [PubMed] [Google Scholar]
- 70. Martinez Carrillo M, Tovar Martin I, Martinez Lara I, Ruiz de Almodovar Rivera JM, Del Moral Avila R. Selective use of postoperative neck radiotherapy in oral cavity and oropharynx cancer: a prospective clinical study. Radiat Oncol. 2013;8:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Martinez‐Monge R, Gomez‐Iturriaga A, Cambeiro M, et al. Phase I‐II trial of perioperative high‐dose‐rate brachytherapy in oral cavity and oropharyngeal cancer. Brachytherapy. 2009;8(1):26‐33. [DOI] [PubMed] [Google Scholar]
- 72. Matuschek C, Bölke E, Belka C, et al. Feasibility of 6‐month maintenance cetuximab after adjuvant concurrent chemoradiation plus cetuximab in squamous cell carcinoma of the head and neck. Strahlenther Onkol. 2013;189(8):625‐631. [DOI] [PubMed] [Google Scholar]
- 73. Otsuru M, Ota Y, Aoki T, et al. A study of adjuvant chemoradiotherapy with tri‐weekly cisplatin for postoperative high‐risk oral squamous cell carcinoma. Tokai J Exp Clin Med. 2017;42(1):19‐24. [PubMed] [Google Scholar]
- 74. Pehlivan B, Luthi F, Matzinger O, et al. Feasibility and efficacy of accelerated weekly concomitant boost postoperative radiation therapy combined with concomitant chemotherapy in patients with locally advanced head and neck cancer. Ann Surg Oncol. 2009;16(5):1337‐1343. [DOI] [PubMed] [Google Scholar]
- 75. Rosenthal DI, Harris J, Forastiere AA, et al. Early postoperative paclitaxel followed by concurrent paclitaxel and cisplatin with radiation therapy for patients with resected high‐risk head and neck squamous cell carcinoma: report of the phase II trial RTOG 0024. J Clin Oncol. 2009;27(28):4727‐4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sanguineti G, Corvo R, Vitale V, Lionetto R, Foppiano F. Postoperative radiotherapy for head and neck squamous cell carcinomas: feasibility of a biphasic accelerated treatment schedule. Int J Radiat Oncol Biol Phys. 1996;36(5):1147‐1153. [DOI] [PubMed] [Google Scholar]
- 77. Schuller DE, Grecula JC, Agrawal A, et al. Multimodal intensification therapy for previously untreated advanced resectable squamous cell carcinoma of the oral cavity, oropharynx, or hypopharynx. Cancer. 2002;94(12):3169‐3178. [DOI] [PubMed] [Google Scholar]
- 78. Schuller DE, Grecula JC, Gahbauer RA, et al. Intensified regimen for advanced head and neck squamous cell carcinomas. Arch Otolaryngol Head Neck Surg. 1997;123(2):139‐144. [DOI] [PubMed] [Google Scholar]
- 79. Shah N, Saunders MI, Dische S. A pilot study of postoperative CHART and CHARTWEL in head and neck cancer. Clin Oncol (R Coll Radiol). 2000;12(6):392‐396. [DOI] [PubMed] [Google Scholar]
- 80. Smith RV, Schiff BA, Garg M, Haigentz M. The impact of transoral robotic surgery on the overall treatment of oropharyngeal cancer patients. Laryngoscope. 2015;125:S1‐S15. [DOI] [PubMed] [Google Scholar]
- 81. Trotti A, Klotch D, Endicott J, Ridley M, Cantor A. Postoperative accelerated radiotherapy in high‐risk squamous cell carcinoma of the head and neck: long‐term results of a prospective trial. Head Neck. 1998;20(2):119‐123. [DOI] [PubMed] [Google Scholar]
- 82. Weinstein GS, Quon H, O'Malley BW Jr, Kim GG, Cohen MA. Selective neck dissection and deintensified postoperative radiation and chemotherapy for oropharyngeal cancer: a subset analysis of the University of Pennsylvania transoral robotic surgery trial. Laryngoscope. 2010;120(9):1749‐1755. [DOI] [PubMed] [Google Scholar]
- 83. Aref A, Berkey BA, Schwade JG, et al. The influence of beam energy on the outcome of postoperative radiotherapy in head and neck cancer patients: secondary analysis of RTOG 85‐03. Int J Radiat Oncol Biol Phys. 2000;47(2):389‐394. [DOI] [PubMed] [Google Scholar]
- 84. Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck. 2005;27(10):843‐850. [DOI] [PubMed] [Google Scholar]
- 85. Cooper JS, Pajak TF, Forastiere A, et al. Precisely defining high‐risk operable head and neck tumors based on RTOG #85‐03 and #88‐24: targets for postoperative radiochemotherapy? Head Neck. 1998;20(7):588‐594. [DOI] [PubMed] [Google Scholar]
- 86. Haffty BG, Son YH, Sasaki CT, et al. Mitomycin C as an adjunct to postoperative radiation therapy in squamous cell carcinoma of the head and neck: results from two randomized clinical trials. Int J Radiat Oncol Biol Phys. 1993;27(2):241‐250. [DOI] [PubMed] [Google Scholar]
- 87. Jacobs JR, Casiano RR, Schuller DE, Pajak TF, Laramore GE. al‐Sarraf M. chemotherapy as predictor of compliance. J Surg Oncol. 1994;55(3):143‐148. [DOI] [PubMed] [Google Scholar]
- 88. Martinez‐Monge R, Pagola Divasson M, Cambeiro M, et al. Determinants of complications and outcome in high‐risk squamous cell head‐and‐neck cancer treated with perioperative high‐dose rate brachytherapy (PHDRB). Int J Radiat Oncol Biol Phys. 2011;81(4):e245‐e254. [DOI] [PubMed] [Google Scholar]
- 89. Martínez‐Monge R, Valtueña G, Santisteban M, et al. Time to loading and locoregional control in perioperative high‐dose‐rate brachytherapy: the tumor bed effect revisited. Brachytherapy. 2015;14(4):565‐570. [DOI] [PubMed] [Google Scholar]
- 90. Rewari AN, Haffty BG, Wilson LD, et al. Postoperative concurrent chemoradiotherapy with mitomycin in advanced squamous cell carcinoma of the head and neck: results from three prospective randomized trials. Cancer J. 2006;12(2):123‐129. [PubMed] [Google Scholar]
- 91. Snietura M, Piglowski W, Jaworska M, et al. Impact of HPV infection on the clinical outcome of p‐CAIR trial in head and neck cancer. Eur Arch Otorhinolaryngol. 2011;268(5):721‐726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kreeft AM, Van Der Molen L, Hilgers FJ, Balm AJ. Speech and swallowing after surgical treatment of advanced oral and oropharyngeal carcinoma: a systematic review of the literature. Eur Arch Otorhinolaryngol. 2009;266(11):1687‐1698. [DOI] [PubMed] [Google Scholar]
- 93. Rathod S, Livergant J, Klein J, Witterick I, Ringash J. A systematic review of quality of life in head and neck cancer treated with surgery with or without adjuvant treatment. Oral Oncol. 2015;51(10):888‐900. [DOI] [PubMed] [Google Scholar]
- 94. Wang ZH, Zhang SZ, Zhang ZY, et al. Protecting the oral mucosa in patients with oral tongue squamous cell carcinoma treated postoperatively with intensity‐modulated radiotherapy: a randomized study. Laryngoscope. 2012;122(2):291‐298. [DOI] [PubMed] [Google Scholar]
- 95. Achim V, Bolognone RK, Palmer AD, et al. Long‐term functional and quality‐of‐life outcomes after transoral robotic surgery in patients with oropharyngeal cancer. JAMA Otolaryngol Head Neck Surg. 2018;144(1):18‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Breeze J, Rennie A, Dawson D, et al. Patient‐reported quality of life outcomes following treatment for oral cancer. Int J Oral Maxillofac Surg. 2018;47(3):296‐301. [DOI] [PubMed] [Google Scholar]
- 97. Ch'ng S, Oates J, Gao K, et al. Prospective quality of life assessment between treatment groups for oral cavity squamous cell carcinoma. Head Neck. 2014;36(6):834‐840. [DOI] [PubMed] [Google Scholar]
- 98. de Graeff A, de Leeuw JR, Ros WJ, Hordijk GJ, Blijham GH, Winnubst JA. Pretreatment factors predicting quality of life after treatment for head and neck cancer. Head Neck. 2000;22(4):398‐407. [DOI] [PubMed] [Google Scholar]
- 99. Fang FM, Tsai WL, Chien CY, Chiu HC, Wang CJ. Health‐related quality of life outcome for oral cancer survivors after surgery and postoperative radiotherapy. Jpn J Clin Oncol. 2004;34(11):641‐646. [DOI] [PubMed] [Google Scholar]
- 100. Kessler PA, Bloch‐Birkholz A, Leher A, Neukam FW, Wiltfang J. Evaluation of quality of life of patients with oral squamous cell carcinoma. Comparison of two treatment protocols in a prospective study. Radiother Oncol. 2004;70(3):275‐282. [DOI] [PubMed] [Google Scholar]
- 101. Li X, Sun Q, Guo S. Functional assessments in patients undergoing radial forearm flap following hemiglossectomy. J Craniofac Surg. 2016;27(2):e172‐e175. [DOI] [PubMed] [Google Scholar]
- 102. Nowak P, Parzuchowski J, Jacobs JR. Effects of combined modality therapy of head and neck carcinoma on shoulder and head mobility. J Surg Oncol. 1989;41(3):143‐147. [DOI] [PubMed] [Google Scholar]
- 103. Oates J, Davies S, Roydhouse JK, Fethney J, White K. The effect of cancer stage and treatment modality on quality of life in oropharyngeal cancer. Laryngoscope. 2014;124(1):151‐158. [DOI] [PubMed] [Google Scholar]
- 104. Ohkoshi A, Ogawa T, Nakanome A, et al. Predictors of chewing and swallowing disorders after surgery for locally advanced oral cancer with free flap reconstruction: a prospective, observational study. Surg Oncol. 2018;27(3):490‐494. [DOI] [PubMed] [Google Scholar]
- 105. Olthoff A, Steuer‐Vogt MK, Licht K, Sauer‐Goenen M, Werner C, Ambrosch P. Quality of life after treatment for laryngeal carcinomas. ORL J Oto‐Rhino‐Laryngol Relat Specialties. 2006;68(5):253‐258. [DOI] [PubMed] [Google Scholar]
- 106. Oskam IM, Verdonck‐de Leeuw IM, Aaronson NK, et al. Prospective evaluation of health‐related quality of life in long‐term oral and oropharyngeal cancer survivors and the perceived need for supportive care. Oral Oncol. 2013;49(5):443‐448. [DOI] [PubMed] [Google Scholar]
- 107. Pauloski BR, Logemann JA, Rademaker AW, et al. Speech and swallowing function after oral and oropharyngeal resections: one‐year follow‐up. Head Neck. 1994;16(4):313‐322. [DOI] [PubMed] [Google Scholar]
- 108. Ridner SH, Dietrich MS, Niermann K, Cmelak A, Mannion K, Murphy B. A prospective study of the lymphedema and fibrosis continuum in patients with head and neck cancer. Lymphat Res Biol. 2016;14(4):198‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Schoen PJ, Raghoebar GM, Bouma J, et al. Prosthodontic rehabilitation of oral function in head‐neck cancer patients with dental implants placed simultaneously during ablative tumour surgery: an assessment of treatment outcomes and quality of life. Int J Oral Maxillofac Surg. 2008;37(1):8‐16. [DOI] [PubMed] [Google Scholar]
- 110. Sinclair CF, McColloch NL, Carroll WR, Rosenthal EL, Desmond RA, Magnuson JS. Patient‐perceived and objective functional outcomes following transoral robotic surgery for early oropharyngeal carcinoma. Arch Otolaryngol Head Neck Surg. 2011;137(11):1112‐1116. [DOI] [PubMed] [Google Scholar]
- 111. Speksnijder CM, van der Bilt A, Abbink JH, Merkx MA, Koole R. Mastication in patients treated for malignancies in tongue and/or floor of mouth: a 1‐year prospective study. Head Neck. 2011;33(7):1013‐1020. [DOI] [PubMed] [Google Scholar]
- 112. Speksnijder CM, van der Bilt A, van der Glas HW, Koole R, Merkx MA. Tongue function in patients treated for malignancies in tongue and/or floor of mouth; a one year prospective study. Int J Oral Maxillofac Surg. 2011;40(12):1388‐1394. [DOI] [PubMed] [Google Scholar]
- 113. Sun Q, Guo S, Wang D, Xu N. Shoulder dysfunction after radiotherapy in surgically and nonsurgically treated necks: a prospective study. Medicine (United States). 2015;94(30):1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wetzels JW, Merkx MA, de Haan AF, Koole R, Speksnijder CM. Maximum mouth opening and trismus in 143 patients treated for oral cancer: a 1‐year prospective study. Head Neck. 2014;36(12):1754‐1762. [DOI] [PubMed] [Google Scholar]
- 115. Yang ZH, Chen WL, Huang HZ, Pan CB, Li JS. Quality of life of patients with tongue cancer 1 year after surgery. J Oral Maxillofac Surg. 2010;68(9):2164‐2168. [DOI] [PubMed] [Google Scholar]
- 116. Hosni A, Huang SH, Chiu K, et al. Predictors of early recurrence prior to planned postoperative radiation therapy for Oral cavity squamous cell carcinoma and outcomes following salvage intensified radiation therapy. Int J Radiat Oncol Biol Phys. 2019;103(2):363‐373. [DOI] [PubMed] [Google Scholar]
- 117. Liao CT, Lin CY, Fan KH, et al. Identification of a high‐risk group among patients with oral cavity squamous cell carcinoma and pT1‐2N0 disease. Int J Radiat Oncol Biol Phys. 2012;82(1):284‐290. [DOI] [PubMed] [Google Scholar]
- 118. Noble AR, Greskovich JF, Han J, et al. Risk factors associated with disease recurrence in patients with stage III/IV squamous cell carcinoma of the oral cavity treated with surgery and postoperative radiotherapy. Anticancer Res. 2016;36(2):785‐792. [PubMed] [Google Scholar]
- 119. Blanchard P, Gunn GB, Lin A, Foote RL, Lee NY, Frank SJ. Proton therapy for head and neck cancers. Semin Radiat Oncol. 2018;28(1):53‐63. [DOI] [PubMed] [Google Scholar]
- 120. Blanchard P, Baujat B, Holostenco V, et al. Meta‐analysis of chemotherapy in head and neck cancer (MACH‐NC): a comprehensive analysis by tumour site. Radiother Oncol. 2011;100(1):33‐40. [DOI] [PubMed] [Google Scholar]
- 121. Kiyota N, Tahara M, Fujii H, et al. Phase II/III trial of post‐operative chemoradiotherapy comparing 3‐weekly cisplatin with weekly cisplatin in high‐risk patients with squamous cell carcinoma of head and neck (JCOG1008). J Clin Oncol. 2020;38(15):6502. [DOI] [PubMed] [Google Scholar]
- 122. Subramaniam N, Balasubramanian D, Murthy S, Limbachiya S, Thankappan K, Iyer S. Adverse pathologic features in early oral squamous cell carcinoma and the role of postoperative radiotherapy‐a review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;124(1):24‐31. [DOI] [PubMed] [Google Scholar]
- 123. Ferris RL, Flamand Y, Weinstein G, Li S, Quon H, Mehra R. Transoral robotic surgical resection followed by randomization to low‐ or standard‐dose IMRT in resectable p16+ locally advanced oropharynx cancer: a trial of the ECOG‐ACRIN Cancer Research Group (E3311). J Clin Oncol. 2020;38:6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information.
Table S1 Studies evaluating oncologic outcomes after adjuvant therapy for resected squamous cell carcinoma of the head and necks (n = 77).
Table S2 Studies evaluating the effect of adjuvant therapy on functional and toxicity outcomes (n = 24).


