Background – Thymus and activation‐regulated chemokine (TARC/CCL17) has been implicated in the pathogenesis of canine atopic dermatitis (cAD). Serum TARC concentrations are a reliable biomarker for human atopic dermatitis; however, their potential as a biomarker for cAD has not been investigated. Hypothesis/Objectives – To investigate whether serum TARC concentrations correlate with disease severity and therapeutic responses for cAD. Conclusions and clinical relevance – Serum TARC concentrations have potential as a clinical and research tool for the objective evaluation of disease severity and therapeutic responses for cAD.

Abstract
Background
Thymus and activation‐regulated chemokine (TARC/CCL17) has been implicated in the pathogenesis of canine atopic dermatitis (cAD). Serum TARC concentrations are a reliable biomarker for human atopic dermatitis; however, their potential as a biomarker for cAD has not been investigated.
Hypothesis/Objectives
To investigate whether serum TARC concentrations correlate with disease severity and therapeutic responses for cAD.
Animals
Thirty‐nine dogs with cAD and 42 healthy dogs were recruited.
Methods and materials
Serum TARC concentrations in dogs with cAD and healthy dogs were measured by sandwich ELISA with anti‐canine TARC antibodies. The clinical severity of cAD was scored using the validated Canine Atopic Dermatitis Extent and Severity Index, 4th iteration (CADESI‐04). Serum TARC concentrations were compared between dogs with cAD and healthy controls, and their relationship with CADESI‐04 was examined. Serum TARC concentrations also were measured in 20 dogs with cAD treated with prednisolone or oclacitinib for four weeks.
Results
Serum TARC concentrations were significantly higher in dogs with cAD than in healthy dogs (P < 0.001). In dogs with cAD, serum TARC concentrations correlated with CADESI‐04 scores (ρ = 0.457, P < 0.01). Furthermore, serum TARC concentrations significantly decreased in treated dogs with the attenuation of clinical signs (P < 0.001). Changes in serum TARC concentrations before and after treatment correlated with those in CADESI‐04 scores (ρ = 0.746, P < 0.001).
Conclusions and clinical relevance
Serum TARC concentrations have potential as a clinical and research tool for the objective evaluation of disease severity and therapeutic responses for cAD.
Résumé
Contexte
La TARC/CCL17 (thymus and activation‐regulated chemokine) a été impliquée dans la pathogénie de la dermatite atopique canine (cAD). Les concentrations sériques de TARC sont un biomarqueur fiable chez l’homme atopique ; cependant, leur potentiel en tant que biomarqueur pour cAD n’a pas été étudié.
Hypothèses/Objectifs
Etudier si les concentrations sériques de TARC corrèlent avec la sévérité de la maladie et les réponses thérapeutiques pour cAD.
Sujets
Trente – neuf chiens avec cAD et 42 chiens sains ont été inclus.
Matériels et méthodes
Les concentrations sériques en TARC chez les chiens avec cAD et les chiens sains ont été mesurées par ELISA avec anticorps anti‐TARC canin. La sévérité clinique de cAD a été notée par CADESI‐04 (Canine Atopic Dermatitis Extent and Severity Index, 4th iteration). Les concentrations de TARC sériques ont été comparées entre les chiens avec cAD et les chiens sains contrôles, et leur relation avec CADESI‐04 a été étudiée. Les concentrations sériques de TARC ont aussi été mesurées chez 20 chiens avec cAD traités par prednisolone ou oclacitinib pendant quatre semaines.
Résultats
Les concentrations sériques de TARC étaient significativement plus élevées chez les chiens avec cAD que chez les chiens sains (P < 0.001). Chez les chiens avec cAD, les concentrations sériques de TARC corrélaient avec les scores de CADESI‐04 (ρ = 0.457, P< 0.01). En outre, les concentrations sériques de TARC diminuaient significativement chez les chiens traités avec atténuation des signes cliniques (P < 0.001). Les changements de concentrations sériques de TARC avant et après traitement corrélaient avec ceux des scores CADESI‐04 (ρ = 0.746, P < 0.001).
Conclusions et importance clinique
Les concentrations sériques de TARC ont le potentiel d’être un outil clinique et de recherche pour l’évaluation objective de l’intensité de la maladie et de la réponse thérapeutique de la CAD.
RESUMEN
Introducción
el timo y la quimiocina regulada por activación (TARC/CCL17) se han implicado en la patogénesis de la dermatitis atópica canina (cAD). Las concentraciones séricas de TARC son un biomarcador fiable en la dermatitis atópica humana; sin embargo, no se ha investigado su potencial como biomarcador en cAD.
Hipótesis/Objetivos
investigar si las concentraciones séricas de TARC se correlacionan con la gravedad de la enfermedad y las respuestas terapéuticas en cAD.
Animales
se incluyeron treinta y nueve perros con cAD y 42 perros sanos.
Métodos y materiales
Las concentraciones séricas de TARC en perros con cAD y perros sanos se midieron mediante ELISA sándwich con anticuerpos anti‐TARC caninos. La gravedad clínica de la cAD se valoró utilizando el índice validado de extensión y gravedad de la dermatitis atópica canina, cuarta versión (CADESI‐04). Se compararon las concentraciones de TARC en suero entre perros con cAD y controles sanos, y se examinó su relación con CADESI‐04. También se midieron las concentraciones séricas de TARC en 20 perros con cAD tratados con prednisolona u oclacitinib durante cuatro semanas.
Resultados
las concentraciones séricas de TARC fueron significativamente más altas en perros con cAD que en perros sanos (P <0,001). En perros con cAD, las concentraciones séricas de TARC se correlacionaron con las puntuaciones de CADESI‐04 (ρ = 0,457, P <0,01). Además, las concentraciones séricas de TARC disminuyeron significativamente en los perros tratados con la disminución de los signos clínicos (P <0,001). Los cambios en las concentraciones séricas de TARC antes y después del tratamiento se correlacionaron con los de los valores de CADESI‐04 (ρ = 0,746, P <0,001).
Conclusiones y relevancia clínica
las concentraciones séricas de TARC tienen potencial como herramienta clínica y de investigación para la evaluación objetiva de la gravedad de la enfermedad y las respuestas terapéuticas en cAD.
Zusammenfassung
Hintergrund
Dem Thymus und Activation‐regulated Chemokine (TARC/CCL17) wird in der Pathogenese der caninen atopischen Dermatitis (cAD) eine Rolle zugeschrieben. Serum TARC Konzentrationen sind ein verlässlicher Biomarker bei der atopischen Dermatitis des Menschen; ihr Potential als Biomarker für die cAD wurde jedoch noch nicht untersucht.
Tiere
Neununddreißig Hunde mit cAD und 42 gesunde Hunde wurden rekrutiert.
Methoden und Materialien
Serum TARC Konzentrationen bei Hunden mit cAD und bei gesunden Hunden wurden mittels Sandwich ELISA mit anti‐caninen TARC Antikörpern gemessen. Der klinische Schweregrad der cAD wurde mittels validiertem Canine Atopic Dermatitis Extent and Severity Index, 4te Ausgabe (CADESI‐04) beurteilt. Die Serum TARC Konzentrationen wurden zwischen den Hunden mit cAD und den gesunden Kontrollen verglichen und ihre Beziehung mittels CADESI‐04 untersucht. Es wurden auch die TARC Konzentrationen bei 20 Hunden mit cAD, die mit Prednisolon oder Oclacitinib vier Wochen lang behandelt worden waren, gemessen.
Ergebnisse
Die Serum TARC Konzentrationen waren bei Hunden mit cAD signifikant höher als bei den gesunden Hunden (P < 0,001). Bei Hunden mit cAD korrelierten die Serum TARC Konzentrationen mit den CADESI‐04 Werten (ρ = 0.746, P < 0.001). Weiters nahmen die Serum TARC Konzentrationen bei behandelten Hunden mit Abnahme der klinischen Zeichen ab (P < 0,001). Veränderungen bei den Serum TARC Konzentrationen vor und nach der Behandlung korrelierten mit denen der CADESI‐04 Werte (ρ = 0.746, P < 0.001).
Schlussfolgerungen und klinische Bedeutung
Die Serum TARC Konzentrationen haben Potential als klinisches und wissenschaftliches Werkzeug zur objektiven Evaluierung der Schwere der Erkrankung und der therapeutischen Resonanz bei cAD.
Resumo
Contexto
A quimiocina regulada pelo timo e ativação (TARC / CCL17) tem sido implicada na patogênese da dermatite atópica canina (DAC). As concentrações séricas de TARC são um biomarcador confiável para dermatite atópica humana; no entanto, seu potencial como biomarcador para CAD não foi investigado.
Hipótese/Objetivos
Investigar se as concentrações séricas de TARC se correlacionam com a gravidade da doença e as respostas terapêuticas para DAC.
Animais
Trinta e nove cães com DAC e 42 cães saudáveis foram recrutados.
Métodos e materiais
As concentrações séricas de TARC em cães com DAC e em cães saudáveis foram medidas por ELISA sanduíche com anticorpos anti‐TARC caninos. A gravidade clínica da DAC foi avaliada usando o índice de extensão e gravidade da dermatite atópica canina validado, 4ª iteração (CADESI‐04). As concentrações séricas de TARC foram comparadas entre cães com DAC e controles saudáveis, e sua relação com CADESI‐04 foi avaliada. As concentrações séricas de TARC também foram mensuradas em 20 cães com DAC tratados com prednisolona ou oclacitinib por quatro semanas.
Resultados
As concentrações séricas de TARC foram significativamente maiores em cães com DAC do que em cães saudáveis (P<0,001). Em cães com DAC, as concentrações séricas de TARC se correlacionaram com as pontuações de CADESI‐04 (ρ = 0,457, P<0,01). Além disso, as concentrações séricas de TARC diminuíram significativamente nos cães tratados, com a atenuação dos sinais clínicos (P <0,001). Mudanças nas concentrações séricas de TARC antes e depois do tratamento correlacionaram‐se com as dos escores CADESI‐04 (ρ = 0,746, P<0,001).
Conclusões e relevância clínica
As concentrações séricas de TARC têm potencial como uma ferramenta clínica e de pesquisa para a avaliação objetiva da gravidade da doença e das respostas terapêuticas para DAC.
要約
背景
胸腺および活性化調節ケモカイン(TARC / CCL17)は、犬アトピー性皮膚炎(cAD)の病因に関与している。血清TARC濃度は、ヒトアトピー性皮膚炎の信頼できるバイオマーカーである。ただし、cADに対するバイオマーカーとしての可能性は調査されていない。
仮説/目的
本研究の目的は、血清TARC濃度が疾患の重症度およびcADの治療反応と相関するかどうかを調査することであった。
供試動物
cAD犬39頭と健常犬42頭を募った。
材料と方法
cAD犬および健常犬の血清TARC濃度を、抗犬TARC抗体を用いたサンドイッチELISAで測定した。 cADの臨床的重症度は、検証済みの犬アトピー性皮膚炎の程度および重症度指数、4thインテレーション(CADESI‐04)を使用してスコア化された。cAD犬および健常犬間の血清TARC濃度を比較し、CADESI‐04との関係を調査した。血清TARC濃度も、プレドニゾロンまたはオクラシチニブで4週間治療されたcADの犬20頭で測定した。
結果
血清TARC濃度は、健常犬よりもcAD犬で有意に高かった(P <0.001)。 cAD犬では、血清TARC濃度がCADESI‐04スコアと相関していた(ρ= 0.457、P <0.01)。さらに、治療された犬の血清TARC濃度は、臨床徴候の減衰とともに有意に減少した(P <0.001)。治療前後の血清TARC濃度の変化は、CADESI‐04スコアの変化と相関していた(ρ= 0.746、P <0.001)。
結論と臨床的関連性
血清TARC濃度は、cADの疾患の重症度および治療反応を客観的に評価する臨床および研究ツールとしての可能性を秘めている。
摘要
背景
胸腺和活化调节趋化因子(TARC/CCL17)与犬异位性皮炎(cAD)的发病机制有关。血清TARC浓度是人类特应性皮炎的可靠生物标志物;然而,尚未有研究报道其作为cAD生物标志物的潜力。
假设/目的
研究血清TARC浓度是否与cAD的疾病严重程度和治疗反应相关。
动物
招募了39只cAD犬和42只健康犬。
方法和材料
通过夹心ELISA和抗犬TARC抗体测定cAD犬和健康犬的血清TARC浓度。使用犬异位性皮炎程度和严重指数第4版(CADESI‐04)对cAD的临床严重程度进行评分。比较cAD犬和健康对照犬的血清TARC浓度,并检查其与CADESI‐04的关系。还测定了泼尼松龙或奥拉替尼治疗4周的20只cAD犬,测定了血清TARC浓度。
结果
cAD犬血清TARC浓度显著高于健康犬(P<0.001)。对于cAD犬,血清TARC浓度与CADESI‐04评分相关(ρ=0.457,P<0.01)。此外,治疗犬的血清TARC浓度显著降低,临床症状减轻(P<0.001)。治疗前后血清TARC浓度变化与CADESI‐04评分相关(ρ=0.746,P<0.001)。
结论和临床相关性
检测血清TARC浓度,有可能作为客观评价疾病严重程度和cAD治疗反应的临床和研究工具。
Introduction
Canine atopic dermatitis (cAD) is a chronic inflammatory and pruritic skin disease. It shares many clinical characteristics with its counterpart in humans, such as a genetic predisposition, early age of onset, predilection sites of affected skin, a relationship with epidermal barrier defects and frequent colonization by Staphylococcus. 1 Furthermore, dogs with cAD show characteristic laboratory findings, with elevated allergen‐specific immunoglobulin (Ig)E concentrations and increased eosinophil counts in peripheral blood that may be attributed to excessive amounts of Type 2 helper T (Th2) cytokines. 2 , 3 , 4 Previous studies reported that concentrations of Th2 cytokines, including interleukin (IL)‐4, IL‐5 and IL‐13, in serum, 5 peripheral blood mononuclear cells 3 and lesional skin, 6 , 7 , 8 were higher in atopic dogs than in healthy dogs. One study also found elevated numbers of IL‐4‐expressing helper T cells in the peripheral blood of dogs with cAD. 9 Therefore, cAD is a Th2‐associated inflammatory disease, similar to human atopic dermatitis (AD).
Thymus and activation‐regulated chemokine (TARC/CCL17) is a functional ligand for CC chemokine receptor 4 (CCR4), which is selectively expressed on Th2 cells. 10 , 11 The number of CCR4+ helper T cells was reported to be elevated in the peripheral blood of dogs with cAD. 12 Furthermore, previous studies demonstrated the preferential transcription of CCR4 and its ligand TARC in the lesional skin of dogs with cAD, and not in nonlesional and healthy skin. 13 , 14 Immunohistochemical analyses revealed that TARC was expressed in the lesional keratinocytes of cAD. 11 In an in vitro study using canine keratinocytes, TARC was induced by an agonist of protease‐activated receptor‐2 that recognized proteases derived from house dust mites (HDM), a major allergen in cAD. 15 Previous studies showed that the transcription of TARC was upregulated, with the greatest increases being observed among the genes examined in the lesional skin of experimentally sensitized dogs with HDM antigens. 16 , 17 In another study using an experimental cAD model, a CCR4 antagonist reduced the infiltration of CCR4+ mononuclear cells into skin. 18 Therefore, TARC likely plays a crucial role in the pathogenesis of cAD by facilitating the migration of Th2 cells to lesional skin.
Clinical studies of human AD demonstrated that serum or plasma TARC concentrations correlated with disease severity. 19 , 20 , 21 , 22 , 23 Additionally, among several biomarkers, such as serum lactate dehydrogenase (LDH), total IgE concentrations and peripheral eosinophil counts, serum TARC concentrations more strongly correlated with clinical scores. 24 , 25 Serum TARC concentrations are currently the most sensitive biomarker for assessing the disease severity of human AD. Furthermore, serum TARC concentrations in patients with human AD decreased with corresponding improvements in clinical signs after treatments with topical agents, including corticosteroids and/or tacrolimus 19 , 22 , 26 and oral ciclosporin. 20 , 25 Accordingly, serum TARC concentrations also have been used as an objective marker to evaluate therapeutic responses in human AD. 24
Although increasing evidence implicates TARC in cAD, it currently remains unclear whether serum TARC concentrations have potential as a biomarker for cAD. In the present study, we investigated whether serum TARC concentrations correlate with disease severity and therapeutic responses for cAD using a canine TARC‐specific ELISA system.
Methods and materials
Animals
Thirty‐nine client‐owned dogs with spontaneous cAD in 17 veterinary hospitals were recruited consecutively for the present study. The age of animals ranged between one and 15 years (median age eight years); there were 22 females (16 neutered) and 17 males (nine neutered). Breeds included Shiba inus (n = 19), mongrels (n = 8), French bulldogs (n = 4) and one each of the following breeds: beagle, Border collie, Labrador retriever, Maltese, miniature dachshund, Samoyed, toy poodle and West Highland white terrier. The diagnosis of cAD was based on the combination of compatible clinical features 27 and the fulfilment of at least five of eight criteria. 28 Other pruritic skin diseases, such as flea allergy dermatitis, scabies, demodicosis, and bacterial or Malassezia dermatitis, were excluded based on routine dermatological examinations and therapeutic trials. In cases exhibiting year‐round clinical signs, an elimination diet trial for a minimum of eight weeks was performed to rule out a cutaneous adverse food reaction. All affected dogs had serological evidence of allergen‐specific IgE. Tests for Dermatophagoides farinae (Der f) HDM or Der f 2 were performed on all dogs (Nippon Zenyaku Kogyo, Zenoaq; Fukushima, Japan). In cases in which these tests were negative, other serum allergen‐specific IgE tests for environmental allergens, such as Der f HDM, Der f 2, grasses and pollens, were performed (at Animal Allergy Clinical Laboratories; Kanagawa, Japan). 29
Forty‐two healthy dogs were used as control samples. Dogs were considered to be healthy based on their medical history and a physical examination. The age of animals ranged between one and 13 years (median age 4.5 years); there were 24 females (15 neutered) and 18 males (seven neutered). They originated from a research beagle colony (n = 12) or were privately owned (n = 30). The privately owned dog breeds included Shiba inu (n = 21), golden retriever (n = 5) and one each of the following breeds: cavalier King Charles spaniel, French bulldog, Shetland sheepdog and toy poodle. Research beagles were kept for experimental purposes under a protocol approved by the Animal Care and Use Committee of our institute (#17014). In Japan, ethics committees are not available for private‐practice animal hospitals. Even so, this study was conducted in accordance with the ethical codes of the Japan Veterinary Medical Association. 30
Clinical score assessment and treatment for cAD
The validated Canine Atopic Dermatitis Extent and Severity Index, 4th iteration (CADESI‐04) was used to evaluate the severity of lesions. 31 Twenty of 39 affected dogs had been treated with oral prednisolone (Predonin, Shionogi & Co., Ltd.; Osaka, Japan; 0.5–1.0 mg/kg once daily for six days, then every other day; n = 11) or oral oclacitinib (Apoquel, Zoetis Inc.; Florham Park, NJ, USA; 0.4–0.6 mg/kg twice daily for 14 days, then once daily; n = 9) for 28 days, according to a previous study. 32 CADESI‐04 scores were evaluated before and after treatment.
Blood samples
Blood samples were collected from dogs with cAD and client‐owned healthy dogs that visited the clinic for routine check‐ups or vaccinations; the owners of all dogs consented to sample collection for the purpose of this study. One millilitre blood samples were obtained from each dog and centrifuged to separate serum. For all recruited dogs, any anti‐inflammatory agents, including glucocorticoids, ciclosporin, oclacitinib or antihistamines were withdrawn for at least two weeks. 33 Blood collection was performed on all dogs with cAD. Twenty of 39 atopic dogs also underwent blood sampling after four weeks of treatment with oral prednisolone or oclacitinib. Serum samples were stored at −30°C until analysed.
Recombinant canine TARC
The recombinant canine TARC protein was produced using an Escherichia coli expression system. The cDNA sequence, excluding the signal sequence, was inserted into an E. coli expression vector (pGEX‐4T‐1, 28954549, GE Healthcare Life Sciences; Little Chalfont, UK). The recombinant canine TARC protein was purified as a fusion protein with glutathione S‐transferase (GST); the mature protein and GST protein were cleaved using thrombin (GE Healthcare Life Sciences; 27‐0846‐01) and only the mature protein was purified.
Anti‐canine TARC monoclonal and polyclonal antibodies
The cDNA of canine TARC was inserted into a mammalian expression vector (pcDNA3.1, V79020, Thermo Fisher Scientific; Waltham, MA, USA). Mice (BALB/c) were immunized with plasmid DNA to establish a monoclonal antibody. The recombinant protein was used for monoclonal antibody screening. Rabbits were immunized with the same plasmid DNA that was used to develop the monoclonal antibody in order to generate a polyclonal antibody. After DNA immunization, an increase in the antibody titre was confirmed using a recombinant protein and blood was collected to obtain a polyclonal antibody. The production of anti‐canine TARC antibodies using mice and rabbits was approved by the Animal Care and Use Committee of our institute (#Z120720, #Z130413).
ELISA
The anti‐TARC monoclonal antibody with carbonate buffer (0.25 μg/mL) was immobilized on ELISA plates (Thermo Scientific Nunc, 439454, Thermo Fisher Scientific) in 100 μL, and incubated at 4°C overnight. ELISA plates were washed using PBST (Phosphate buffered saline containing 0.05% Tween‐20), 100 μL of sera diluted two‐fold with a blocking buffer (four‐fold Block Ace in PBST, UK‐B80, Yukijirushi; Tokyo, Japan) was added and then plates were kept at room temperature for 2 h. After washing with washing buffer, the anti‐TARC polyclonal antibody, 400‐fold diluted with blocking buffer, was added in 100 μL increments and the plates were kept at room temperature for 2 h. After washing, horseradish peroxidase (HRP)‐conjugated goat anti‐rabbit IgG (656120, Invitrogen; Carlsbad, CA, USA), 2,000‐fold diluted with blocking buffer, was added to the plates, which were then kept at room temperature for 2 h. After washing, 3,3‐,5,5‐tetramethylbenzidine (3405‐100TAB, Sigma‐Aldrich, St Louis, MO, USA) solution was added to the plates in 100 μL increments and plates were then kept at room temperature for 10 min. To stop the reaction, 50 μL of 2nH2SO4 was added to the plates. Plates were read at a dual wavelength of 450–570 nm with a micro plate leader (model 680, 168‐1000, Bio‐Rad; Hercules, CA, USA). The assay was performed in duplicate on the same day. Diluted recombinant TARC protein from 98 to 50,000 pg/mL, instead of sera, was added to the plate in duplicate to prepare a calibration curve. Optical density values were increased proportionally with concentrations of the recombinant canine TARC (R 2 = 0.9961, P < 0.001; Figure S1). The workflow of ELISA is shown in Figure S2.
Statistical analysis
Serum TARC concentrations were measured as continuous variables. The results of serum TARC concentrations were represented as medians with interquartile ranges (IQR: 25–75th percentiles) owing to the non‐normal data distribution. Differences in serum TARC concentrations as the dependent variables were analysed using the Wilcoxon–Mann–Whitney U‐test for comparisons between the independent variables of group – dogs with cAD and healthy dogs. The Wilcoxon signed rank test was used to compare paired serum TARC concentrations before and after treatment. Similarly, CADESI‐04 scores were collected as continuous variables. The relationship between serum TARC concentrations and CADESI‐04 scores was analysed by the Spearman rank correlation test. A value of P < 0.05 was considered to be significant. Statistical analyses were performed using prism 8 software (GraphPad; San Diego, CA, USA).
Results
Animals and clinical data
Forty‐two healthy dogs (male:female ratio 18:24; median age 4.5 years; median weight 10.0 kg) and 39 dogs with cAD (17:22; 8 years; 9.0 kg) were recruited. No significant differences were observed in age, sex or weight between healthy and atopic dogs (data not shown). In both groups, Shiba inus accounted for approximately half of the cases (21 of 42 healthy dogs, 19 of 39 atopic dogs). Index scores in 39 atopic dogs ranged between 4 and 139 (median 40) on the CADESI‐04 scale, which has a maximum score of 180. 31 The severity of cAD included mild (CADESI‐04 index 0–34, 17 dogs), moderate (35–59, 15 dogs) or severe (≥60, seven dogs) according to the CADESI‐04 severity categories. 31 CADESI‐04 scores of all healthy dogs were 0. Twenty dogs with cAD were treated with prednisolone (11 dogs) or oclacitinib (nine dogs). The median CADESI‐04 score was 41 (range 7–139) before treatment and decreased to 11 (range 0–117) after treatment. Breed, age, sex, weight, treatment and CADESI‐04 scores at baseline and after treatment in dogs with cAD are listed in Table 1. The breed, age, sex and weight of healthy dogs are shown in Table 2.
Table 1.
Breed, age, sex, weight, Canine Atopic Dermatitis Extent and Severity Index, 4th iteration (CADESI‐04) scores and serum thymus and activation‐regulated chemokine (TARC) concentrations at baseline and after treatment in dogs with atopic dermatitis
| Case | Breed | Age (years) | Sex | Weight (kg) | Baseline | After treatment | Treatment | ||
|---|---|---|---|---|---|---|---|---|---|
| CADESI‐04 score | Serum TARC concentration (pg/mL) | CADESI‐04 score | Serum TARC concentration (pg/mL) | ||||||
| 1 | Shiba inu | 6 | SF | 8 | 41 | 3,143 | 19 | 1,693 | Prednisolone |
| 2 | Shiba inu | 6 | CM | 11 | 50 | 2,415 | 25 | 346 | Prednisolone |
| 3 | Shiba inu | 7 | F | 8 | 40 | 1,591 | 10 | 310 | Prednisolone |
| 4 | Shiba inu | 7 | CM | 11 | 7 | 663 | 4 | 309 | Prednisolone |
| 5 | Shiba inu | 8 | SF | 8 | 54 | 2,113 | 10 | 189 | Oclacitinib |
| 6 | Shiba inu | 8 | SF | 10 | 37 | 2,447 | 9 | 1,532 | Prednisolone |
| 7 | Shiba inu | 11 | SF | 14 | 25 | 4,563 | 17 | 3,937 | Prednisolone |
| 8 | Shiba inu | 13 | SF | 8 | 105 | 5,469 | 53 | 564 | Prednisolone |
| 9 | Shiba inu | 13 | SF | 7.2 | 32 | 2,135 | 2 | 195 | Oclacitinib |
| 10 | Shiba inu | 13 | CM | 10 | 41 | 1,753 | 12 | 183 | Prednisolone |
| 11 | French bulldog | 9 | SF | 11 | 65 | 5,006 | 35 | 1,825 | Oclacitinib |
| 12 | French bulldog | 7 | SF | 10.6 | 22 | 3,275 | 2 | 251 | Oclacitinib |
| 13 | French bulldog | 11 | F | 8.2 | 139 | 3,173 | 117 | 1,376 | Oclacitinib |
| 14 | Mixed breed | 5 | M | 13 | 43 | 6,502 | 7 | 3,564 | Prednisolone |
| 15 | Mixed breed | 11 | M | 23 | 48 | 4,406 | 15 | 2,052 | Oclacitinib |
| 16 | Beagle | 12 | SF | 9 | 19 | 953 | 5 | 150 | Prednisolone |
| 17 | Maltese | 11 | SF | 6.5 | 53 | 1,558 | 32 | 884 | Oclacitinib |
| 18 | Miniature dachshund | 10 | SF | 3 | 13 | 4,060 | 0 | 2,708 | Oclacitinib |
| 19 | Toy poodle | 2 | CM | 4.2 | 14 | 1,338 | 4 | 632 | Prednisolone |
| 20 | West Highland white terrier | 9 | M | 8 | 46 | 2,195 | 17 | 177 | Oclacitinib |
| 21 | Shiba inu | 1 | M | 8.1 | 4 | 2,540 | |||
| 22 | Shiba inu | 3 | M | 11 | 65 | 7,152 | |||
| 23 | Shiba inu | 8 | F | 10.5 | 34 | 2,040 | |||
| 24 | Shiba inu | 8 | M | 24 | 103 | 6,372 | |||
| 25 | Shiba inu | 10 | M | 9 | 46 | 3,626 | |||
| 26 | Shiba inu | 11 | F | 9 | 15 | 1,553 | |||
| 27 | Shiba inu | 13 | F | 8 | 28 | 1,443 | |||
| 28 | Shiba inu | 14 | SF | 15.2 | 68 | 2,153 | |||
| 29 | Shiba inu | 15 | SF | 7.4 | 69 | 6,816 | |||
| 30 | Mixed breed | 3 | SF | 5.7 | 10 | 3,261 | |||
| 31 | Mixed breed | 3 | CM | 19 | 18 | 1,300 | |||
| 32 | Mixed breed | 5 | F | 9 | 42 | 769 | |||
| 33 | Mixed breed | 6 | CM | 6.8 | 20 | 2,057 | |||
| 34 | Mixed breed | 6 | CM | 11 | 44 | 5,078 | |||
| 35 | Mixed breed | 8 | M | 8.6 | 59 | 1,287 | |||
| 36 | Border collie | 10 | CM | 17.4 | 36 | 3,760 | |||
| 37 | French bulldog | 1 | SF | 8 | 4 | 945 | |||
| 38 | Labrador retriever | 11 | CM | 33 | 24 | 1,535 | |||
| 39 | Samoyed | 7 | SF | 23 | 15 | 2,227 | |||
CM, castrated male; F, female; M, male; SF, spayed female.
Table 2.
Breed, age, sex, weight and serum thymus and activation‐regulated chemokine (TARC) concentrations in healthy dogs
| Case | Breed | Age (years) | Sex | Weight (kg) | Serum TARC concentration (pg/mL) |
|---|---|---|---|---|---|
| 1 | Shiba inu | 0.6 | M | 6.4 | 1,330 |
| 2 | Shiba inu | 1 | CM | 7.9 | 180 |
| 3 | Shiba inu | 1 | CM | 13 | 205 |
| 4 | Shiba inu | 2 | SF | 11.2 | 183 |
| 5 | Shiba inu | 2 | SF | 13.2 | 416 |
| 6 | Shiba inu | 2 | SF | 11.5 | 117 |
| 7 | Shiba inu | 3 | SF | 8.7 | 283 |
| 8 | Shiba inu | 4 | M | 8 | 249 |
| 9 | Shiba inu | 6 | F | 9.2 | 0 |
| 10 | Shiba inu | 6 | SF | 8.5 | 150 |
| 11 | Shiba inu | 6 | M | 10.5 | 2,243 |
| 12 | Shiba inu | 6 | M | 10.2 | 123 |
| 13 | Shiba inu | 7 | F | 6.6 | 0 |
| 14 | Shiba inu | 7 | SF | 12.9 | 409 |
| 15 | Shiba inu | 7 | SF | 9.9 | 192 |
| 16 | Shiba inu | 8 | SF | 12.3 | 164 |
| 17 | Shiba inu | 8 | M | 8.7 | 343 |
| 18 | Shiba inu | 9 | SF | 10.3 | 201 |
| 19 | Shiba inu | 11 | CM | 10.4 | 157 |
| 20 | Shiba inu | 12 | SF | 9.7 | 614 |
| 21 | Shiba inu | 13 | CM | 11 | 146 |
| 22 | Beagle | 2 | M | 10 | 164 |
| 23 | Beagle | 3 | F | 10 | 245 |
| 24 | Beagle | 3 | F | 10 | 178 |
| 25 | Beagle | 3 | F | 10 | 194 |
| 26 | Beagle | 3 | M | 10 | 132 |
| 27 | Beagle | 3 | M | 10 | 149 |
| 28 | Beagle | 3 | M | 10 | 124 |
| 29 | Beagle | 4 | F | 10 | 189 |
| 30 | Beagle | 4 | F | 10 | 229 |
| 31 | Beagle | 4 | M | 10 | 281 |
| 32 | Beagle | 5 | M | 10 | 322 |
| 33 | Beagle | 9 | F | 10 | 146 |
| 34 | Golden retriever | 3 | CM | 33.6 | 346 |
| 35 | Golden retriever | 4 | SF | 28 | 205 |
| 36 | Golden retriever | 8 | SF | 24.5 | 250 |
| 37 | Golden retriever | 9 | SF | 29 | 172 |
| 38 | Golden retriever | 10 | SF | 28.7 | 127 |
| 39 | Cavalier King Charles spaniel | 5 | SF | 8.5 | 0 |
| 40 | French bulldog | 1 | F | 12.8 | 391 |
| 41 | Shetland sheepdog | 8 | CM | 13.5 | 122 |
| 42 | Toy poodle | 5 | CM | 3.4 | 82 |
CM, castrated male; F, female; M, male; SF, spayed female.
Serum TARC concentrations in dogs with cAD and healthy dogs
Serum TARC concentrations were >10‐fold higher in dogs with cAD than in healthy dogs. The median serum TARC concentration of dogs with cAD was 2,227 pg/mL (IQR 1,556–3,910), whereas that of healthy dogs was 183 pg/mL (IQR 146–273). Serum TARC concentrations were significantly higher in dogs with cAD than in healthy dogs (P < 0.001; Figure 1).
Figure 1.
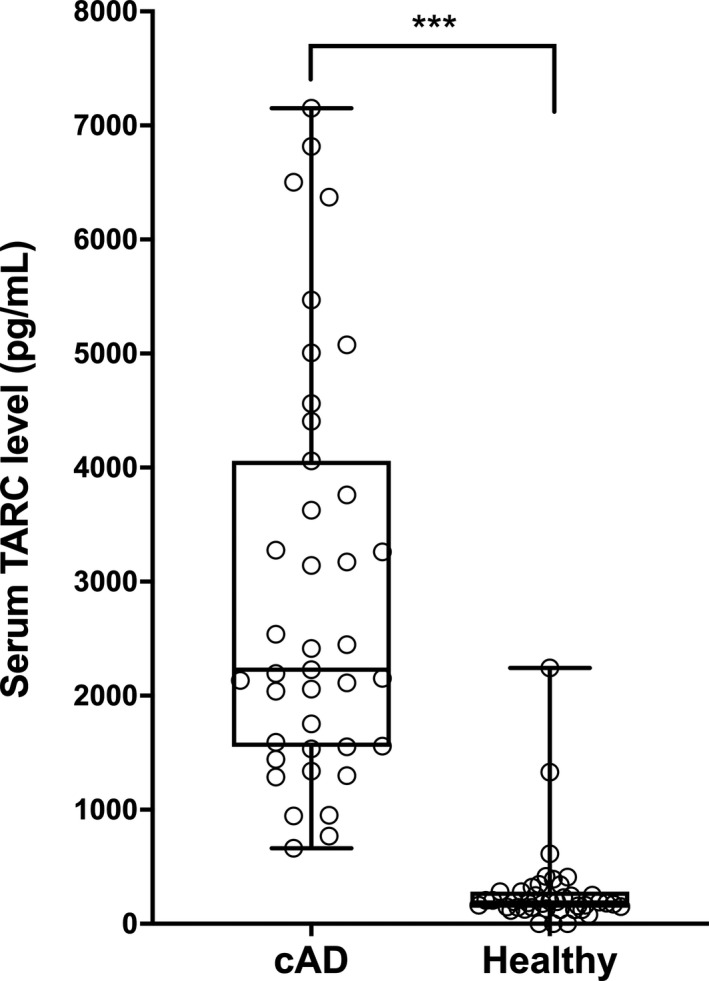
Serum thymus and activation‐regulated chemokine (TARC) concentrations in dogs with atopic dermatitis (cAD) (n = 39) and healthy dogs (n = 42).
Serum TARC concentrations were significantly higher in dogs with atopic dermatitis than in healthy controls (P < 0.001). The lines within the boxes indicate the median serum TARC concentrations and the upper and lower boundaries of the boxes represent the 25th and 75th percentiles, respectively. ***P < 0.001 by the Wilcoxon–Mann–Whitney U‐test.
Relationship between serum TARC concentrations and CADESI‐04 scores in dogs with cAD
In order to assess the utility of serum TARC concentrations as a marker for clinical severity, we examined the relationship between serum TARC concentrations and CADESI‐04 scores. Serum TARC concentrations in dogs with cAD correlated with CADESI‐04 scores (ρ = 0.457, P < 0.01; Figure 2).
Figure 2.
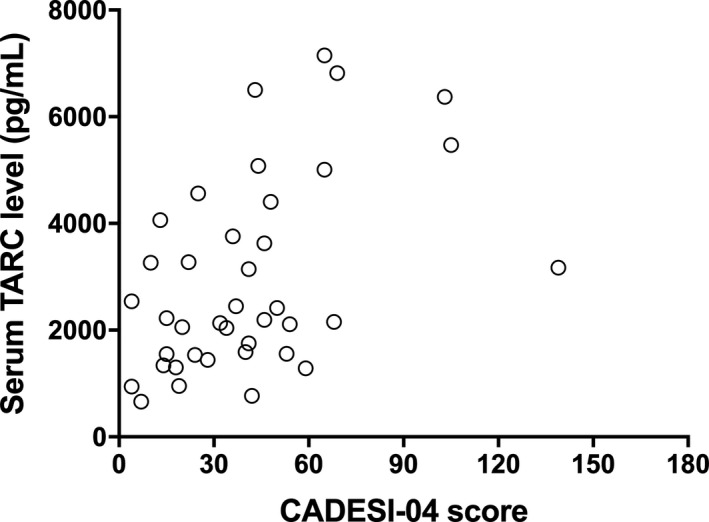
Relationship between serum thymus and activation‐regulated chemokine (TARC) concentrations and Canine Atopic Dermatitis Extent and Severity Index, 4th iteration (CADESI‐04) scores in dogs with atopic dermatitis (n = 39).
Serum TARC concentrations in dogs positively correlated with CADESI‐04 scores (ρ = 0.457, P < 0.01). **P < 0.01 by the Spearman rank correlation test.
Serum TARC concentrations before and after treatment in dogs with cAD
We measured serum TARC concentrations in 20 dogs with cAD before and after systemic treatment with oral prednisolone or oclacitinib. In all 20 dogs, serum TARC concentrations significantly decreased after four weeks of treatment in accordance with the amelioration of skin lesions (P < 0.001). Serum TARC concentrations decreased from 2,431 pg/mL (IQR 1,713–4,147) before treatment to 598 pg/mL (IQR 237–1,726) after treatment (Figure 3).
Figure 3.
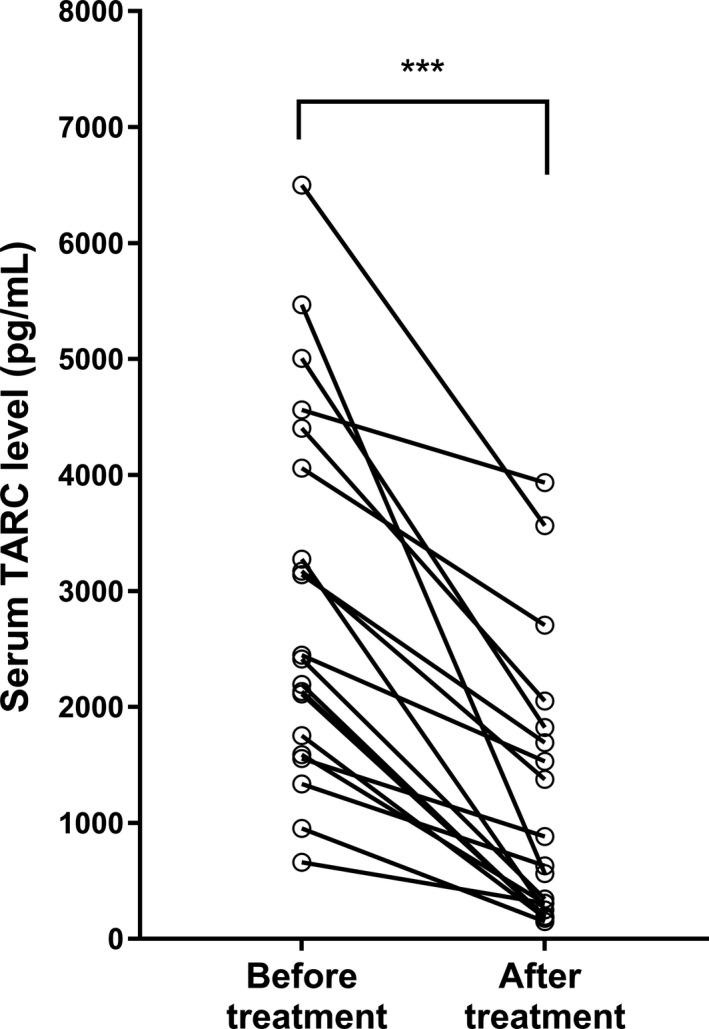
Serum thymus and activation‐regulated chemokine (TARC) concentrations before and after treatment in dogs with atopic dermatitis (cAD) (n = 20).
Serum TARC concentrations showed a significant decrease after four weeks of treatment with either oral prednisolone (n = 11) or oclacitinib (n = 9) (P < 0.001). ***P < 0.001 by the Wilcoxon–Mann–Whitney U‐test.
Relationship between changes in serum TARC concentrations and CADESI‐04 scores
In order to assess the utility of serum TARC concentrations as a marker for treatment responses, we examined the relationship between changes in TARC concentrations and CADESI‐04 scores after treatment. Changes in serum TARC concentrations correlated with those in CADESI‐04 scores (ρ = 0.746, P < 0.001; Figure 4).
Figure 4.
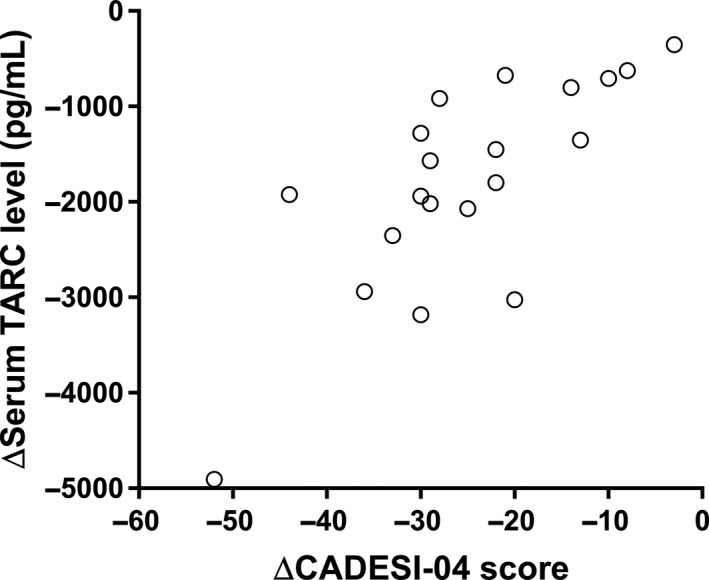
Relationship between changes in serum thymus and activation‐regulated chemokine (TARC) concentrations and Canine Atopic Dermatitis Extent and Severity Index, 4th iteration (CADESI‐04) scores (n = 20).
Changes in serum TARC concentrations before and after treatment with either oral prednisolone (n = 11) or oclacitinib (n = 9) positively correlated with the CADESI‐04 scores (ρ = 0.746, P < 0.001). ***P < 0.001 by the Spearman rank correlation test.
Discussion
To the best of the authors’ knowledge, this is the first study to investigate the relationship between serum TARC concentrations and the disease severity of cAD. To evaluate the usefulness of serum TARC concentrations as a biomarker for cAD, we established a sandwich ELISA system for quantifying canine TARC. Previous studies demonstrated that plasma and serum TARC concentrations in human patients with AD correlated with disease severity. 19 , 20 , 21 , 22 , 23 Serum TARC concentrations were found to be markedly higher than plasma TARC concentrations in patients with AD because platelets in serum released large amounts of TARC. 19 Furthermore, serum TARC concentrations were stable independent of the freeze–thaw process, whereas those in plasma contaminated with platelets were elevated after freeze–thaw due to the release of TARC. 34 A meta‐analysis proposed serum TARC concentrations as the most reliable biomarker for the disease severity of human AD. 35 Therefore, herein we used serum samples to quantify canine TARC.
The results obtained showed that serum TARC concentrations were >10‐fold higher in dogs with cAD than in healthy controls, which is consistent with previous findings on human AD. 19 , 22 The median serum TARC concentrations in dogs with cAD and healthy controls were 2,227 pg/mL (IQR 1,556–3,910) and 183 pg/mL (IQR 146–273), respectively. These concentrations closely corresponded to previously reported values from human counterparts in the literature with an average serum TARC concentration of 1,480 pg/mL (IQR 640–3,540) in patients with AD and 250 pg/mL (IQR 240–260) in healthy controls. 25 However, a previous study also showed that a high serum TARC concentration was not specific to human AD. An elevated serum TARC concentration also has been reported in human patients with other skin diseases, such as bullous pemphigoid, scabies, polymorphic prurigo, cutaneous T‐cell lymphoma, drug eruption, pustular dermatosis and other internal disorders. 24 Based on these findings, serum TARC concentrations have not been solely used to establish a diagnosis of human AD. Thus, additional studies that evaluate TARC concentrations in dogs with other diseases, besides cAD, are needed to assess the effect of other inflammatory or neoplastic skin conditions on TARC concentrations.
In the present study, serum TARC concentrations in two healthy dogs were >1,000 pg/mL (cases 1 and 11; Table 2). This anomaly may be partially due to an age‐related difference in TARC concentrations. One of the two dogs with a high serum TARC concentration (Case 1) was only 7 months old; the mean age of the healthy controls with lower serum TARC concentrations was 4.5 years. A previous study on healthy human subjects reported that serum TARC concentrations were higher in infants (<1 year old) than in children (>2–5 and 6–16 years old) and adults (>16 years old). 36 The same study also found that to achieve the same diagnostic accuracy of human AD, different cut‐off values were required for different age groups. 36 Because serum TARC concentrations in healthy human subjects differ among infants, children and adults, healthy thresholds also vary according to age, based on current evidence. 24 Therefore, further studies are needed to investigate age‐related differences in serum TARC concentrations in healthy and affected dogs in order to establish appropriate reference ranges. Additionally, it is important to note that one (Case 1) of these two outliers subsequently developed the typical clinical signs of cAD, suggesting that TARC concentrations became elevated before the onset of cAD. This hypothesis is supported by previous findings indicating the potential of serum TARC concentrations to detect subclinical dermal inflammation or the early stages of human AD. 37 , 38 Because the remaining outlier (Case 11) was lost to follow‐up, we were unable to confirm whether this subject eventually developed cAD or another disease that may have caused an elevated TARC concentration. Although it may be of clinical significance, it was not possible to confirm the utility of TARC concentrations in predicting the onset of cAD in the present study. Thus, further studies are necessary to evaluate that serum TARC concentrations would be a good marker to predict onset of cAD, which may lead to prophylactic administration of anti‐inflammatory agents.
The present results showed that serum TARC concentrations correlated with CADESI‐04 scores, suggesting the potential of TARC as a useful objective measure for the disease severity of cAD, similar to human AD. 20 , 21 , 22 In previous studies on human AD, among available markers, including total IgE concentrations, peripheral eosinophil counts and serum LDH concentrations, serum TARC more strongly correlated with clinical scores. 24 , 25 In studies on cAD, although total IgE concentrations were significantly higher in dogs with cAD than in healthy controls, they did not correlate with clinical severity according to the Canine Atopic Dermatitis Lesion Index. 2 Furthermore, the lesional severity scores of dogs with cAD did not correlate with the serum concentrations of other cytokines, including IL‐17, IL‐31 and macrophage migration inhibitory factor. 39 Therefore, serum TARC concentrations appear to be a more accurate marker than previously studied biomarkers and correspond well with the lesional severity of cAD.
In the present study, we also investigated whether serum TARC concentrations reflected therapeutic responses. To the best of our knowledge, a serum biomarker has not yet been used to assess spontaneous cAD before and after treatment in individual subjects. We demonstrated that serum TARC concentrations decreased with corresponding improvements in clinical signs in all dogs treated with prednisolone or oclacitinib, both of which are first‐line treatments in the management of cAD exacerbations. We also found a strong correlation between changes in TARC concentrations and CADESI‐04 scores after treatment. The present results show the potential of serum TARC concentrations as an objective marker for evaluating therapeutic responses in cAD treated with prednisolone or oclacitinib.
The comparison of TARC concentrations before and after treatment with prednisolone and oclacitinib would have been clinically interesting. However, because dogs were not randomized into each group, there was a risk of allocation bias leading to inaccurate interpretation. A previous study using a mouse model of allergic dermatitis reported that oclacitinib reduced the TARC concentration in skin. 40 In this model, oclacitinib also suppressed IL‐31, TNF‐α and TSLP, although abrupt withdrawal led to a rapid increase of these cytokines. 40 , 41 TNF‐α has been shown to be the mediator inducing TARC release from canine keratinocytes. 42 , 43 Thus, oclacitinib may reduce serum TARC concentrations by inhibiting TNF‐α in keratinocytes. Common treatment regimens for cAD also may include the use of topical glucocorticoids, oral ciclosporin or lokivetmab injections, which were not evaluated in the present study. In human patients with AD, serum TARC concentrations decreased after the treatments with topical glucocorticoids 19 , 22 , 26 or oral ciclosporin. 20 , 25 Additionally, an in vitro study revealed that IL‐31 induced TARC in human keratinocytes. 44 These studies suggest that serum TARC has the potential to act as a marker for evaluating therapeutic responses in cAD treated with other drugs, although the generalizability of our results cannot be assumed for these drugs. Therefore, specific interactions between serum TARC concentrations and other treatment modalities require further investigation.
In conclusion, serum TARC concentrations correlated with the lesional severity scores of cAD, indicating that serum TARC concentrations are an objective marker for assessing disease severity and therapeutic responses in cAD. The assessment of disease severity and efficacy of treatment for cAD has relied on traditional markers susceptible to various confounders. The use of TARC as a biomarker will allow standardized evaluations not only in clinical settings, but also importantly in research settings, thereby facilitating the cross‐comparison of trials and its use as a surrogate end‐point. Further studies are needed to confirm the potential of serum TARC concentrations as an objective and predictive marker in the treatment monitoring of cAD.
Supporting information
Figure S1. A standard curve of the sandwich ELISA with serial dilutions of purified recombinant canine TARC ranging from 98 to 50,000 pg/mL.
Figure S2. The workflow of the sandwich ELISA assay.
Acknowledgements
The authors would like to thank Takahiro Akita (Akita Animal Hospital), Daisuke Itsukaichi (Poplar Animal Hospital), Chiaki Kitanaka (SENA Animal Hospital Rakuhoku Animal Wellness Center), Jin Kozakai (Ai Animal Hospital Kashima), Yuko Machida (AZ Animal Hospital), Hisanori Mutoh (Miyaki Animal Clinic), Hiromi Oboso (Cookie Animal Hospital), Taichi Oshima (Taichi Animal Hospital) and Akihiro Sugeno (Sugeno Animal Hospital) for collecting blood samples.
Ryota Asahina and Kazunori Ueda are joint first authors.
Sources of Funding: This study was self‐funded.
Conflict of Interest: No conflicts of interest have been declared.
References
- 1. Marsella R, De Benedetto A. Atopic dermatitis in animals and people: an update and comparative review. Vet Sci 2017; 4: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chaudhary SK, Singh SK, Kumari P, et al. Alterations in circulating concentrations of IL‐17, IL‐31 and total IgE in dogs with atopic dermatitis. Vet Dermatol 2019; 30: 383‐e114. [DOI] [PubMed] [Google Scholar]
- 3. Hayashiya S, Tani K, Morimoto M, et al. Expression of T helper 1 and T helper 2 cytokine mRNAs in freshly isolated peripheral blood mononuclear cells from dogs with atopic dermatitis. J Vet Med A Physiol Pathol Clin Med 2002; 49: 27–31. [DOI] [PubMed] [Google Scholar]
- 4. Lian TM, Halliwell RE. Allergen‐specific IgE and IgGd antibodies in atopic and normal dogs. Vet Immunol Immunopathol 1998; 66: 203–223. [DOI] [PubMed] [Google Scholar]
- 5. Majewska A, Gajewska M, Dembele K, et al. Lymphocytic, cytokine and transcriptomic profiles in peripheral blood of dogs with atopic dermatitis. BMC Vet Res 2016; 12: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nuttall TJ, Knight PA, McAleese SM, et al. Expression of Th1, Th2 and immunosuppressive cytokine gene transcripts in canine atopic dermatitis. Clin Exp Allergy 2002; 32: 789–795. [DOI] [PubMed] [Google Scholar]
- 7. Olivry T, Dean GA, Tompkins MB, et al. Toward a canine model of atopic dermatitis: amplification of cytokine–gene transcripts in the skin of atopic dogs. Exp Dermatol 1999; 8: 204–211. [DOI] [PubMed] [Google Scholar]
- 8. Schlotter YM, Rutten VP, Riemers FM, et al. Lesional skin in atopic dogs shows a mixed Type‐1 and Type‐2 immune responsiveness. Vet Immunol Immunopathol 2011; 143: 20–26. [DOI] [PubMed] [Google Scholar]
- 9. Martins GC, de Oliveira Melo Júnior OA, Botoni LS, et al. Clinical‐pathological and immunological biomarkers in dogs with atopic dermatitis. Vet Immunol Immunopathol 2018; 205: 58–64. [DOI] [PubMed] [Google Scholar]
- 10. Iio A, Motohashi T, Kunisada T, et al. Preferential gene transcription of T helper 2 cytokines in peripheral CCR4(+) CD4(+) lymphocytes in dogs. Vet Dermatol 2014; 25: 199–e150. [DOI] [PubMed] [Google Scholar]
- 11. Maeda S, Tsukui T, Saze K, et al. Production of a monoclonal antibody to canine thymus and activation‐regulated chemokine (TARC) and detection of TARC in lesional skin from dogs with atopic dermatitis. Vet Immunol Immunopathol 2005; 103: 83–92. [DOI] [PubMed] [Google Scholar]
- 12. Maeda S, Ohmori K, Yasuda N, et al. Increase of CC chemokine receptor 4‐positive cells in the peripheral CD4 cells in dogs with atopic dermatitis or experimentally sensitized to Japanese cedar pollen. Clin Exp Allergy 2004; 34: 1,467–1,473. [DOI] [PubMed] [Google Scholar]
- 13. Maeda S, Fujiwara S, Omori K, et al. Lesional expression of thymus and activation‐regulated chemokine in canine atopic dermatitis. Vet Immunol Immunopathol 2002; 88: 79–87. [DOI] [PubMed] [Google Scholar]
- 14. Maeda S, Okayama T, Omori K, et al. Expression of CC chemokine receptor 4 (CCR4) mRNA in canine atopic skin lesion. Vet Immunol Immunopathol 2002; 90: 145–154. [DOI] [PubMed] [Google Scholar]
- 15. Maeda S, Maeda S, Ohno K, et al. Protease‐activated receptor‐2 induces proinflammatory cytokine and chemokine gene expression in canine keratinocytes. Vet Immunol Immunopathol 2013; 153: 17–25. [DOI] [PubMed] [Google Scholar]
- 16. Marsella R, Olivry T, Maeda S. Cellular and cytokine kinetics after epicutaneous allergen challenge (atopy patch testing) with house dust mites in high‐IgE beagles. Vet Dermatol 2006; 17: 111–120. [DOI] [PubMed] [Google Scholar]
- 17. Olivry T, Mayhew D, Paps JS, et al. Early activation of Th2/Th22 Inflammatory and pruritogenic pathways in acute canine atopic dermatitis skin lesions. J Invest Dermatol 2016; 136: 1,961–1,969. [DOI] [PubMed] [Google Scholar]
- 18. Murray C, Ahrens K, Devalaraja M, et al. Use of a canine model of atopic dermatitis to investigate the efficacy of a CCR4 antagonist in allergen‐induced skin inflammation in a randomized study. J Invest Dermatol 2016; 136: 665–671. [DOI] [PubMed] [Google Scholar]
- 19. Fujisawa T, Fujisawa R, Kato Y, et al. Presence of high contents of thymus and activation‐regulated chemokine in platelets and elevated plasma levels of thymus and activation‐regulated chemokine and macrophage‐derived chemokine in patients with atopic dermatitis. J Allergy Clin Immunol 2002; 110: 139–146. [DOI] [PubMed] [Google Scholar]
- 20. Hijnen D, De Bruin‐Weller M, Oosting B, et al. Serum thymus and activation‐regulated chemokine (TARC) and cutaneous T cell‐ attracting chemokine (CTACK) levels in allergic diseases: TARC and CTACK are disease‐specific markers for atopic dermatitis. J Allergy Clin Immunol 2004; 113: 334–340. [DOI] [PubMed] [Google Scholar]
- 21. Jahnz‐Rozyk K, Targowski T, Paluchowska E, et al. Serum thymus and activation‐regulated chemokine, macrophage‐derived chemokine and eotaxin as markers of severity of atopic dermatitis. Allergy 2005; 60: 685–688. [DOI] [PubMed] [Google Scholar]
- 22. Kakinuma T, Nakamura K, Wakugawa M, et al. Thymus and activation‐regulated chemokine in atopic dermatitis: Serum thymus and activation‐regulated chemokine level is closely related with disease activity. J Allergy Clin Immunol 2001; 107: 535–541. [DOI] [PubMed] [Google Scholar]
- 23. Morita A, Kikuoka S, Horikawa T, et al. Evaluation of human thymus and activation‐regulated chemokine concentrations in blood using a new sandwich ELISA based on monoclonal antibodies. Clin Chim Acta 2002; 322: 67–75. [DOI] [PubMed] [Google Scholar]
- 24. Kataoka Y. Thymus and activation‐regulated chemokine as a clinical biomarker in atopic dermatitis. J Dermatol 2014; 41: 221–229. [DOI] [PubMed] [Google Scholar]
- 25. Kou K, Aihara M, Matsunaga T, et al. Association of serum interleukin‐18 and other biomarkers with disease severity in adults with atopic dermatitis. Arch Dermatol Res 2012; 304: 305–312. [DOI] [PubMed] [Google Scholar]
- 26. Yasukochi Y, Nakahara T, Abe T, et al. Reduction of serum TARC levels in atopic dermatitis by topical anti‐inflammatory treatments. Asian Pac J Allergy Immunol 2014; 32: 240–245. [DOI] [PubMed] [Google Scholar]
- 27. Terada Y, Nagata M, Murayama N, et al. Clinical comparison of human and canine atopic dermatitis using human diagnostic criteria (Japanese Dermatological Association, 2009): proposal of provisional diagnostic criteria for canine atopic dermatitis. J Dermatol 2011; 38: 784–790. [DOI] [PubMed] [Google Scholar]
- 28. Favrot C, Steffan J, Seewald W, et al. A prospective study on the clinical features of chronic canine atopic dermatitis and its diagnosis. Vet Dermatol 2010; 21: 23–31. [DOI] [PubMed] [Google Scholar]
- 29. Okayama T, Matsuno Y, Yasuda N, et al. Establishment of a quantitative ELISA for the measurement of allergen‐specific IgE in dogs using anti‐IgE antibody cross‐reactive to mouse and dog IgE. Vet Immunol Immunopathol 2011; 139: 99–106. [DOI] [PubMed] [Google Scholar]
- 30. Yuki M, Aoyama R, Nakagawa M, et al. A clinical investigation on serum Amyloid A concentration in client‐owned healthy and diseased cats in a primary care animal hospital. Vet Sci 2020; 7: E45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Olivry T, Saridomichelakis M, Nuttall T, et al. Validation of the Canine Atopic Dermatitis Extent and Severity Index (CADESI)‐4, a simplified severity scale for assessing skin lesions of atopic dermatitis in dogs. Vet Dermatol 2014; 25: 77–85, e25. [DOI] [PubMed] [Google Scholar]
- 32. Gadeyne C, Little P, King VL, et al. Efficacy of oclacitinib (Apoquel®) compared with prednisolone for the control of pruritus and clinical signs associated with allergic dermatitis in client‐owned dogs in Australia. Vet Dermatol 2014; 25: 512–518, e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Katayama M, Igarashi H, Tani K, et al. Effect of multiple oral dosing of fluconazole on the pharmacokinetics of cyclosporine in healthy beagles. J Vet Med Sci 2008; 70: 85–88. [DOI] [PubMed] [Google Scholar]
- 34. Zhao X, Delgado L, Weiner R, et al. Influence of pre‐analytical factors on thymus‐ and activation‐regulated chemokine quantitation in plasma. J Circ Biomark 2015; 4: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thijs J, Krastev T, Weidinger S, et al. Biomarkers for atopic dermatitis: a systematic review and meta‐analysis. Curr Opin Allergy Clin Immunol 2015; 15: 453–460. [DOI] [PubMed] [Google Scholar]
- 36. Fujisawa T, Nagao M, Hiraguchi Y, et al. Serum measurement of thymus and activation‐regulated chemokine/CCL17 in children with atopic dermatitis: elevated normal levels in infancy and age‐specific analysis in atopic dermatitis. Pediatr Allergy Immunol 2009; 20: 633–641. [DOI] [PubMed] [Google Scholar]
- 37. Furue M, Matsumoto T, Yamamoto T, et al. Correlation between serum thymus and activation‐regulated chemokine levels and stratum corneum barrier function in healthy individuals and patients with mild atopic dermatitis. J Dermatol Sci 2012; 66: 60–63. [DOI] [PubMed] [Google Scholar]
- 38. Shoda T, Futamura K, Kobayashi F, et al. Expression of thymus and activation‐regulated chemokine (TARC) by human dermal cells, but not epidermal keratinocytes. J Dermatol Sci 2014; 76: 90–95. [DOI] [PubMed] [Google Scholar]
- 39. Gow DJ, Jackson H, Forsythe P, et al. Measurement of serum macrophage migration inhibitory factor (MIF) and correlation with severity and pruritus scores in client owned dogs with atopic dermatitis. Vet Dermatol 2019; 30: 115–e32. [DOI] [PubMed] [Google Scholar]
- 40. Fukuyama T, Ehling S, Cook E, et al. Topically administered janus‐kinase inhibitors tofacitinib and oclacitinib display impressive antipruritic and anti‐inflammatory responses in a model of allergic dermatitis. J Pharmacol Exp Ther 2015; 354: 394–405. [DOI] [PubMed] [Google Scholar]
- 41. Fukuyama T, Ganchingco JR, Bäumer W. Demonstration of rebound phenomenon following abrupt withdrawal of the JAK1 inhibitor oclacitinib. Eur J Pharmacol 2017; 794: 20–26. [DOI] [PubMed] [Google Scholar]
- 42. Banovic F, Olivry T, Bäumer W, et al. Diluted sodium hypochlorite (bleach) in dogs: antiseptic efficacy, local tolerability and in vitro effect on skin barrier function and inflammation. Vet Dermatol 2018; 29: 6–e5. [DOI] [PubMed] [Google Scholar]
- 43. Shibata S, Maeda S, Kondo N, et al. Identification of the signaling pathway of TNF‐alpha‐induced CCL17/TARC transcription in a canine keratinocyte cell line. Vet Immunol Immunopathol 2011; 139: 90–98. [DOI] [PubMed] [Google Scholar]
- 44. Dillon SR, Sprecher C, Hammond A, et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol 2004; 5: 752–760. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. A standard curve of the sandwich ELISA with serial dilutions of purified recombinant canine TARC ranging from 98 to 50,000 pg/mL.
Figure S2. The workflow of the sandwich ELISA assay.


