Abstract
In the peripheral nervous system (PNS), proper development of Schwann cells (SCs) contributing to axonal myelination is critical for neuronal function. Impairments of SCs or neuronal axons give rise to several myelin‐related disorders, including dysmyelinating and demyelinating diseases. Pathological mechanisms, however, have been understood at the elementary level and targeted therapeutics has remained undeveloped. Here, we identify Fibulin 5 (FBLN5), an extracellular matrix (ECM) protein, as a key paracrine factor of human Wharton's jelly‐derived mesenchymal stem cells (WJ‐MSCs) to control the development of SCs. We show that co‐culture with WJ‐MSCs or treatment of recombinant FBLN5 promotes the proliferation of SCs through ERK activation, whereas FBLN5‐depleted WJ‐MSCs do not. We further reveal that during myelination of SCs, FBLN5 binds to Integrin and modulates actin remodeling, such as the formation of lamellipodia and filopodia, through RAC1 activity. Finally, we show that FBLN5 effectively restores the myelination defects of SCs in the zebrafish model of Charcot‐Marie‐Tooth (CMT) type 1, a representative demyelinating disease. Overall, our data propose human WJ‐MSCs or FBLN5 protein as a potential treatment for myelin‐related diseases, including CMT.
Keywords: actin remodeling, Charcot‐Marie‐Tooth disease, Fibulin 5, mesenchymal stem cell, Schwann cell myelination
The common pathological symptom of dysmyelinating and demyelinating diseases is uncompact myelin ensheathments of the axons. Our findings suggest that FBLN5 binds to the Integrin receptors and promotes actin remodeling through RAC1 activation in the Schwann cells. Then, the Schwann cells are recovered by an activated actin remodeling mechanism and induce the compact myelination of the diseased axons.
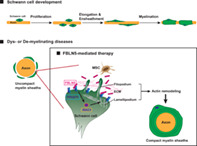
Significance statement.
No effective treatments for any type of Charcot‐Marie‐Tooth (CMTs) have been developed to date, thus this study considered mesenchymal stem cells‐based therapies. FBLN5 was identified as a key paracrine factor, important for the proliferation of Schwann cells (SCs). The RGD motif of FBLN5 was revealed to be necessary for RAC1 activation through binding to Integrin. It was further found that the FBLN5‐RAC1 molecular axis modulates actin remodeling, which is essential for SCs myelination in vivo utilizing zebrafish. Finally, the results of this study demonstrated the efficiency of FBLN5 in the recovery of CMT type 1 zebrafish model.
1. INTRODUCTION
The myelin sheaths of axons in the vertebrate nervous system are important for the fast and proper transmission of nerve impulses, and for the axonal homeostasis and survival. 1 , 2 Myelin is a lipoprotein structure produced by specific types of cells: Schwann cells (SCs) in the PNS and oligodendrocytes in the central nervous system. In the PNS, myelination occurs as the SCs form myelins from multiple layers of the membrane and enwrap axons. 3 , 4 Thus, impairment of SCs or neuronal axons results in severe myelin‐related disorders, referred to as dysmyelinating and demyelinating diseases. 5 , 6
CMT disease is one of the most common inherited neuropathies, with a prevalence of 1:2500 worldwide. 7 , 8 CMTs are genetically heterogeneous and mainly associated with mutations in genes that encode proteins functioning in neurons or SCs. 9 , 10 The disease includes numerous subtypes (types 1 through 7 and X‐linked forms), with common symptoms being distal weakness and muscle atrophy in the early state, foot deformities and hand atrophy in the late state. 11 , 12 CMT type 1 is the most common type and majority of the causative genes are involved in various cellular functions such as endosomal sorting, mitochondrial dynamics, basal membrane adhesion, cytoskeleton stability, myelin formation in SCs. 13 , 14
Although persistent identification of CMT causative genes has accelerated understanding of the genetic basis and the cellular function underpinning disease mechanisms, no effective treatments for any type of CMTs have been developed. Thus, there has been a great need for new treatment strategies for CMTs, and cell‐based therapies such as using mesenchymal stem cells (MSCs) has been considered as an alternative approach to treatment. 15 , 16 This is because MSCs are effective in repairing damaged cells by promoting cell proliferation or preventing cell death in various types of cells. 17 , 18 Indeed, previous clinical trials have reported that human‐derived MSCs can be applied safely and efficiently to cure neurological diseases. 19 , 20 The therapeutic effects of MSCs are mainly due to paracrine factors acting on disease‐related targets or adjacent cells. Recent studies supporting this have identified significant proteins among paracrine factors by proteomic analysis, and have demonstrated that recombinant proteins have therapeutic effects comparable to MSCs in several disease model systems. 21 , 22 From human MSCs, thus, uncovering key paracrine factors affecting SCs may be an alternative approach to developing targeted treatment of myelin‐related diseases including CMTs.
In this study, we identified FBLN5 as a crucial paracrine factor from human Wharton's jelly‐derived mesenchymal stem cells (WJ‐MSCs) that affect the development of SCs. The secreted FBLN5 plays as a composite of the extracellular matrix (ECM) molecules in various types of cells including neurons, SCs, endothelial cells, fibroblasts, and smooth muscle cells. 23 The cell‐matrix communication is important in diverse cellular processes, such as organogenesis and angiogenesis, particularly the ECM of SCs is critical for the proliferation and myelination. 24 , 25 Previously, a mutation in FBLN5 (c.1117C>T) was identified in CMT type 1, and the effect of mutation on the conductivities of neurons and muscles was reported. 26 , 27 However, the molecular mechanism of FBLN5 in CMT pathology has been veiled to date. Here, we have investigated role of FBLN5 in the developmental processes of SCs including proliferation and myelination, and revealed that the tripeptide Arg‐Gly‐Asp (RGD) motif of FBLN5, highly conserved across species, is necessary for Rac Family Small GTPase 1 (RAC1) activation through binding to Integrin. In addition, we demonstrated the efficiency of FBLN5 in the recovery of defective SC myelination in a CMT type 1 zebrafish model. We hence suggest that WJ‐MSCs or FBLN5 may be a potential therapeutic target for myelin‐associated diseases such as CMTs.
2. RESULTS
2.1. Identification of FBLN5 from human WJ‐MSCs affecting the proliferation of SCs
To determine the effects of WJ‐MSCs on SC development, S16 cells derived from the rat sciatic nerves and utilized as an immortalized SC line 28 , 29 were co‐cultivated with human WJ‐MSCs using a transwell culture system (Figure 1A). S16 cells express less myelin‐related proteins including glycoprotein and galactocerebroside than the in vivo system, 28 whereas the expression of myelinating SCs markers such as SOX10, S100β, peripheral myelin protein 22 (PMP22), and myelin protein zero (MPZ) is comparable to the in vivo. 28 , 29 , 30 , 31 , 32 Thus, S16 cells may not fully reflect the physiological properties of SCs, but their fundamental features are sufficient to be utilized to examine SC development in vitro. The proliferation of co‐cultured S16 cells was compared with that of S16 cells cultured in the absence of WJ‐MSCs by counting the number of cells 48 hours after cultivating. The number of S16 cells was increased in the presence of WJ‐MSCs (Figure 1B,C), thereby suggesting that WJ‐MSCs might affect the proliferation of SCs.
FIGURE 1.
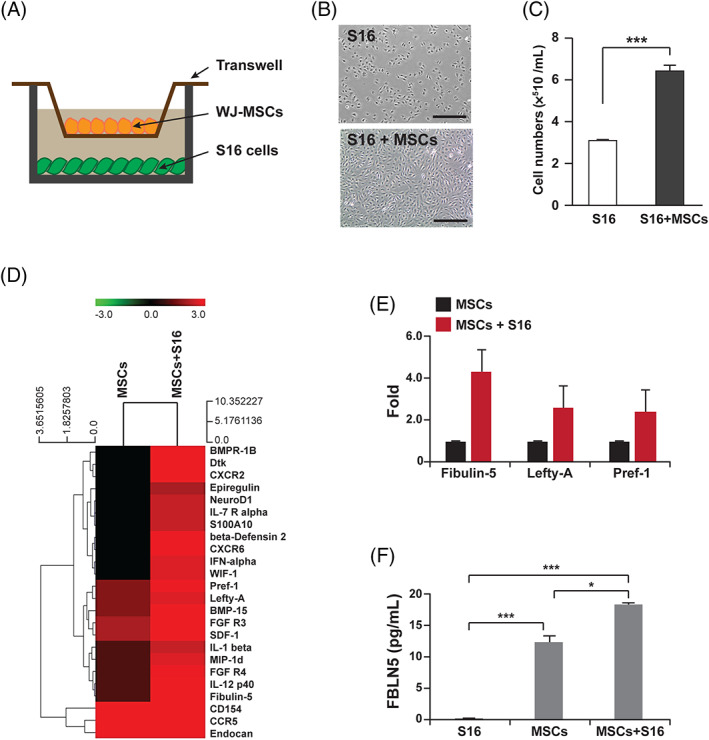
Identification of WJ‐MSCs derived paracrine factors affecting Schwann cell proliferation. A, A schematic diagram for the co‐cultivation system of MSCs and S16 cells. B, Images of S16 cells after 24 hours of cultivation with or without WJ‐MSCs. Scale bars, 400 μm. C, Quantification of total number of S16 cells counted at each indicated condition. Statistical significance was determined using the unpaired Student's t‐test with Welch's correction (***P < .001). D, A heatmap comparing the expression of secreted proteins in the media collected from the indicated culture conditions. E, Quantifications of fold changes in spot intensities for Fibulin 5, Lefty‐A, and Pref‐1. F, The concentration of FBLN5 contained in the culture media collected from each indicated condition was measured using ELISA. Statistical significance was determined using one‐way ANOVA followed by Tukey's post hoc test (*P < .005, ***P < .001)
Next, to identify major paracrine molecules associated with the proliferation of SCs from the WJ‐MSCs, antibody microarrays were performed in the co‐culture system. The antibody array was designed to detect 507 human proteins using conditioned media collected from cell culture dishes. The data analyzed by duplicated arrays showed that cytokines containing several interleukin family members are mainly secreted from WJ‐MSCs under co‐cultivation with S16 cells (Figure 1D). As reported, cytokines are involved in the development of SCs 33 , 34 ; we have paid attention to proteins whose role in SCs is relatively unknown. Accordingly, FBLN5, LEFTY A, and PREF 1 were selected for further validation, and all three proteins revealed elevated secretion under co‐culture with S16 cells (Figure 1E and Supplementary Figure 1). In particular, we focused on FBLN5 based on the identification of mutations in FBLN5 in CMT type 1 patients. 35 , 36 Enzyme‐linked immunosorbent assay (ELISA) for FBLN5 confirmed that WJ‐MSCs cultured with S16 cells secreted more FBLN5 than the cells cultured alone (Figure 1F). The concentration of FBLN5 secreted from single cultured WJ‐MSCs was 12.5 ± 0.99 pg/mL, while the concentration secreted under co‐culture with S16 cells increased to 18.41 ± 0.26 pg/mL (Figure 1F).
To determine if FBLN5 is a primary regulator of SC proliferation, S16 cells were treated with recombinant FBLN5 protein in a dose‐dependent manner followed by cell counting kit‐8 (CCK‐8) analysis (Figure 2A). The results revealed that 10 ng/mL of recombinant FBLN5 was sufficient to facilitate the proliferation of S16 cells (Figure 2A‐C). Next, WJ‐MSCs were transfected with two kinds of verified siRNAs for FBLN5 (Supplementary Figure 2) or with control siRNAs. Subsequently, the transfected cells were then co‐cultured with S16 cells to examine the effect of the presence or absence of FBLN5 on SC proliferation. The S16 cells cultivated with FBLN5‐depleted MSCs showed fewer cells than S16 cells cultured with control MSCs (Figure 2B,C). In order to clarify the role of FBLN5 in SC proliferation, 5‐bromo‐2′‐deoxyuridine (BrdU) assay was performed in S16 cells with or without recombinant FBLN5. The assay data showed that the proliferation of FBLN5‐treated S16 cells was more accelerated (Figure 2D,E). Given that the FBLN family proteins increase cell proliferation through the TGF‐β/ERK/MAPK pathway in 3 T3‐L1 cells, 37 we determined whether FBLN5 is also involved in the proliferation of S16 cells though ERK activity. We performed immunoblot assays for phosphorylated ERK1/2 in the recombinant FBLN5‐treated S16 cells and revealed that FBLN5 is associated with the activation of ERK in SCs (Supplementary Figure 3). Furthermore, to assess the role of FBLN5 in cell migration, characteristic of SCs at the proliferation phase, an in vitro wound‐healing assay was performed. For 48 hours after wound induction by scratching, the S16 cells exhibited approximately 20% closure of the wounds, whereas the cells co‐cultured with WJ‐MSCs or treated with recombinant FBLN5 exhibited approximately 2.5 times more closure extent of the controls (Supplementary Figure 4). Taken together, these data suggest that WJ‐MSCs, which secrete several proteins including FBLN5, play an important role in the development of SCs and that the recombinant FBLN5 protein is sufficient to control the proliferation of SCs.
FIGURE 2.
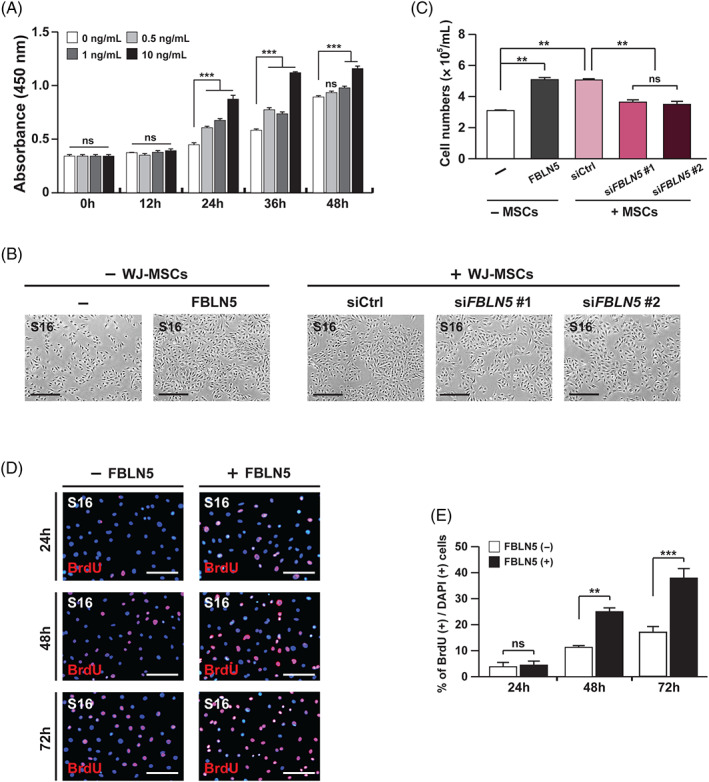
FBLN5 promotes the proliferation of Schwann cells. A, Proliferating cells in the S16 cells treated with FBLN5 at the indicated concentrations were counted by CCK‐8 assay at the indicated incubation times. Statistical significance was determined using 2‐way ANOVA followed by Bonferroni's post hoc test (***P < .001, ns: non‐significant). B, Images of S16 cells after 24 hours of cultivation with nothing, recombinant FBLN5, or with siRNAs‐transfected WJ‐MSCs. Scale bars, 400 μm. C, Quantification of total S16 cell numbers counted for each indicated condition. Statistical significance was determined using one‐way ANOVA followed by Tukey's post hoc test (**P < 0.005, ns: non‐significant). D, Images of S16 cells with or without recombinant FBLN5, comparing proliferating cells by BrdU assay. Cellular nuclei were stained with DAPI (blue). Scale bars, 400 μm. E, Quantification of proliferative cells in (D) from each field of view at the indicated time points. Statistical significance was determined using 2‐way ANOVA followed by Bonferroni's post hoc test (**P < .005, ***P < .001, ns: non‐significant). The data are shown as the mean ± SD of three independent experiments per condition
2.2. FBLN5 is involved in the myelination of SCs
To establish the role of FBLN5 during SC development in vivo, we performed a loss‐of‐function study using a zebrafish model system. The Tg(claudin K:gal4‐vp16;uas:egfp) 38 , 39 zebrafish line was used to monitor SC development, in which myelinating SCs were labeled with enhanced GFP. We generated fbln5‐knockdown zebrafish using verified translation‐blocking morpholinos (MOs) (Supplementary Figure 5). Consistent with the results from S16 cells, in the zebrafish lateral line containing SCs, the lack of fbln5 led to a decrease in cell proliferation, whereas the exogenous fbln5 increased cell proliferation (Figure 3A‐C). Thus, these data support that FBLN5 is essential for the proliferation of SCs. As confirming the effect of FBLN5 on cell proliferation in physiological states, we examined the potential role of FBLN5 in the myelination of SCs. The GFP signal indicating myelinating SCs was analyzed by comparison between Tg(claudin K:gal4‐vp16;uas:egfp) zebrafish injected with control MOs‐injected or fbln5 MOs‐injected at 5 days postfertilization (dpf). The results showed that the knockdown of fbln5 results in myelination defects in the zebrafish PNS without gross morphological defects depending on the concentration of MOs (Figure 3D,E, Supplementary Figure 5A). In demyelinating disease, reduced conductivity in the nervous system is one of the pathophysiological symptoms. Thus, we examined the startle response in zebrafish injected with control MOs or fbln5 MOs and revealed that control zebrafish quickly responded to stimuli, while the fbln5 knockdown zebrafish rarely responded to stimuli (Supplementary Figure 6). Together, our data suggest that FBLN5 is crucial for the myelination of SCs and its function has been evolutionally conserved.
FIGURE 3.
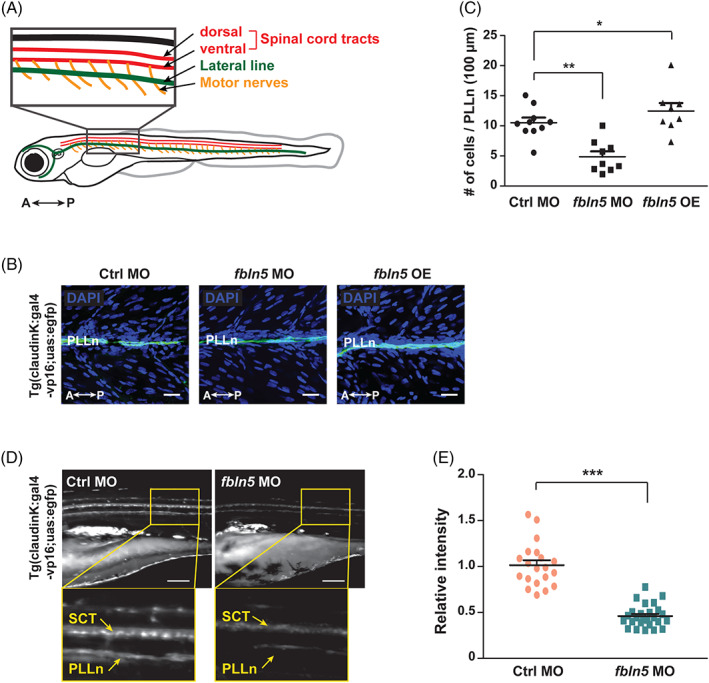
FBLN5 is required for myelination of Schwann cells. A, A diagram of zebrafish larvae identifying the simplified peripheral nervous system, consisting of the spinal cord tracts, lateral lines, and the spinal motor nerves. A, anterior; P, posterior. B, Lateral view images of the whole‐mounted Tg(claudin K:gal4‐vp16;uas:egfp) zebrafish injected with control‐, fbln5‐MOs, or fbln5 mRNA after DAPI staining at 4 dpf. PLLn (green), posterior lateral line; A, anterior; P, posterior. OE, overexpression. Scale bars, 20 μm. C, Quantification of cell numbers counted within the PLLn of (B) images. Statistical significance was determined using one‐way ANOVA followed by Dunnett's post hoc test (*P < .05, **P < .005). D, Lateral view images of the Tg(claudin K:gal4‐vp16;uas:egfp) zebrafish injected with control‐MOs or fbln5‐MOs at 5 dpf. The images within the rectangles are magnified in the bottom panels. SCT, spinal cord tracts; PLLn, posterior lateral line; A, anterior; P, posterior. Scale bars, 100 μm. E, Quantification of the relative intensity of PLLn in equivalent fields of view in the images of (D). Statistical significance was determined using the unpaired Student's t‐test with Welch's correction (***P < .001). The data are shown as the mean ± SD of three independent experiments with ≥20 embryos per condition (C and E)
2.3. FBLN5 regulates actin remodeling through RAC1 activation in SC myelination
Integrin plays an important role in several dynamics of SCs, such as cytoskeletal rearrangement, proliferation, and survival. 23 , 40 , 41 Based on the structure of FBLN5 with RGD motif involved in Integrin binding, 23 , 41 we investigated the potential role of FBLN5 in cytoskeletal dynamics during SC myelination. First, primary SCs were isolated from the sciatic nerves of postnatal mice and myelinated in vitro. We confirmed the primary cells by immunostaining for Sox10, a molecular marker of SCs 42 (Figure 4A). Consistent with the previous reports that Sox10 is a nucleocytoplasmic shuttling protein localizing in both the cytoplasm and nucleus, 43 , 44 our data showed that Sox10 is expressed mainly in the nucleus of long‐cultivated SCs (Supplementary Figure 7). Then, actin remodeling of SCs was analyzed after treatment with recombinant FBLN5 protein, because the protrusion of lamellipodia and filopodia is a prior process of myelination. The FBLN5‐treated SCs gave rise to more and longer lamellipodia/filopodia protrusions than PBS‐treated control SCs (Figure 4A‐D). However, treatment of the Integrin inhibitor, RGD peptide, significantly repressed the enhancement of lamellipodia/filopodia generation by FBLN5 in the primary SCs (Figure 4A‐D). Hence, these data suggest that FBLN5 plays a role in actin remodeling through Integrin‐mediated signaling in SCs.
FIGURE 4.
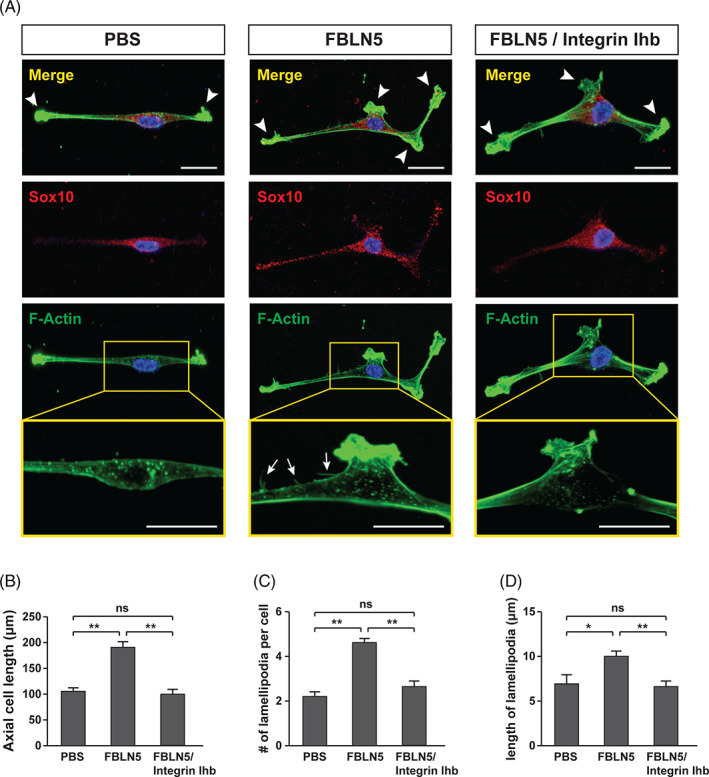
FBLN5 regulates Integrin‐mediated actin dynamics in Schwann cells. A, Images of mouse primary Schwann cells double immunostained with Sox10 and F‐actin antibodies after treatment with recombinant FBLN5 alone or with an integrin inhibitor (Integrin Ihb). PBS‐treated Schwann cells were used as controls. Arrowheads and arrows indicate lamellipodia and filopodia, respectively. Scale bars, 20 μm. B‐D, Quantification of axial cell length in (B), lamellipodia number/cell in (C), and lamellipodia length/cell in (D) from data examined using the ImageJ software within equivalent fields of view in the images of (A). The data are shown as the mean ± SD of three independent experiments. Statistical significance was determined using one‐way ANOVA followed by Tukey's post hoc test (*P < .05, **P < .005; ns, nonsignificant)
Next, we examined the involvement of RAC1 in an FBLN5‐mediated mechanism that modulates actin remodeling of SCs, because RAC1 induces actin polymerization for extensive protrusion of cell membranes during myelination. The dorsal root ganglion (DRG) neurons were isolated from the mice spinal cord to induce myelination of the primary SCs by a co‐cultivation system (Figure 5A). Then, the recombinant FBLN5 protein was treated 6 days after the co‐cultivation, followed by immunostaining for the myelination marker myelin basic protein (MBP) (Figure 5A). FBLN5 promoted the myelination of primary SCs as much as that of the Laminin‐treated controls SCs, whereas the Integrin inhibitor suppressed the reinforcement of FBLN5‐dependent myelination (Figure 5B,C). Next, we generated the constitutive active form of mouse Rac1 (Q61L) 45 , 46 , 47 and introduced it exogenously into the primary SCs. Notably, expression of Rac1 (Q61L) prevented the repression of SC myelination by the Integrin inhibitor (Figure 5B,C). We also produced a zebrafish fbln5 (ΔRGD) construct with a highly conserved RGD motif deleted, and assumed that its overexpression would interfere with Integrin‐mediated RAC1 activation in zebrafish (Figure 5D). Remarkably, the Tg(claudin K:gal4‐vp16;uas:egfp) zebrafish overexpressing the fbln5 (ΔRGD) construct showed drastically reduced myelination of SCs at 5 dpf (Figure 5E,F). Furthermore, we performed a pull‐down assay to examine rac1 activation in zebrafish. We found that a lack of fbln5 decreased rac1 activation, whereas overexpression of fbln5 sufficiently activated rac1 in the fbln5‐depleted zebrafish (Figure 5G). Finally, we monitored the extent of myelination in zebrafish to determine whether RAC1 activity is essential for the role of FBLN5 in SC myelination. First, we cross‐sectioned the control MOs‐injected or fbln5 MOs‐injected zebrafish at 5 dpf and observed them using a transmission electron microscope (TEM). We examined the extent of myelination, focusing mainly on the Mauthner neuronal axons and the reticulospinal tracts, and found that the fbln5‐deficient zebrafish had much less myelinated axons (Figure 5H‐J and Supplementary Figure 8). Then, we produced a zebrafish rac1 (Q61L) and overexpressed the construct in the fbln5 MOs‐injected zebrafish. The data revealed that expression of rac1 (Q61L) relieved the suppression of myelination in the fbln5‐knockdown zebrafish PNS (Figure 5H‐J). Taken together, our results suggest that activation of RAC1 through binding of FBLN5 with Integrin is essential for actin remodeling to modulate SC myelination.
FIGURE 5.
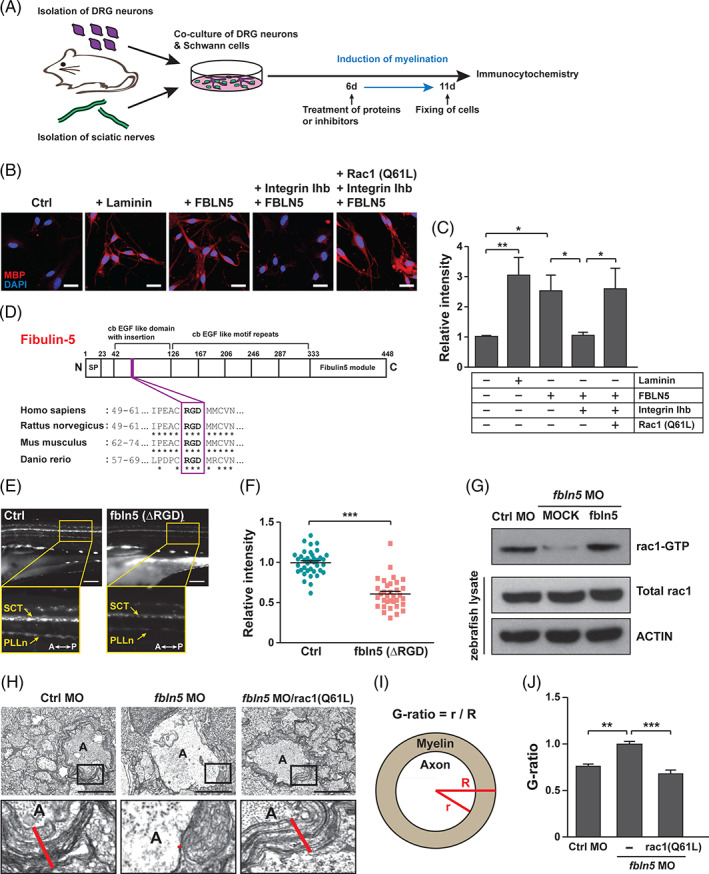
FBLN5 regulates myelination through RAC1 activation in Schwann cells. A, A schematic diagram of the induction of axonal myelination by the co‐culturing of mouse primary DRG neurons and Schwann cells isolated from sciatic nerves. The cells were treated with proteins or inhibitors at 6 days after co‐cultivation and fixed for immunostaining 5 days after the treatment. B, Images of mouse primary Schwann cells immunostained with anti‐MBP antibodies after co‐cultivation with primary DRG neurons under the indicated conditions. Laminin‐treated Schwann cells were a positive control of myelination‐induced cells. Scale bars, 20 μm. C, Quantification of the relative intensities of MBP staining in the images of (B). Statistical significance was determined using one‐way ANOVA followed by Tukey's post hoc test (*P < .05, **P < .005). D, A schematic diagram of the human FBLN5 protein structure identifying the RGD motif conserved in rat, mouse, and zebrafish fbln5 protein. E, Lateral view images of the Tg(claudin K:gal4‐vp16;uas:egfp) zebrafish injected with MOCK or fbln5 (ΔRGD) mRNAs at 5 dpf. The images within the rectangles are magnified in the bottom panels. SCT, spinal cord tracts; PLLn, posterior lateral line; A, anterior; P, posterior. Scale bars, 100 μm. F, Quantification of the relative intensity of PLLn in equivalent fields of view in the images of (E). The data are shown as the mean ± SD of three independent experiments with ≥20 embryos per condition. Statistical significance was determined using unpaired Student's t‐test with Welch's correction (***P < .001). G, Results of pull‐down assay for active rac1 in the indicated zebrafish tissues using PAK‐PBP beads. Protein levels were normalized against β‐actin in the same blots. H, TEM images of cross‐sectioned zebrafish of the indicated genotype at 5 dpf. The images within the rectangles are magnified in the bottom panels. The red lines indicate the thicknesses of the myelin sheaths in the Mauthner axons. A, axon. Scale bars, 2 μm. I, A schematic diagram of how the G‐ratio is obtained: the G‐ratio is calculated by dividing the radius of the axon (r) by the total radius of the axon and myelin (R). J, Quantification of G‐ratios calculated in the Mauthner axons of the indicated zebrafish. Statistical significance was determined using one‐way ANOVA followed by Dunnett's post hoc test (**P < .005, ***P < .001). The data are shown as the mean ± SD (C, F, J)
2.4. FBLN5 is effective in restoring the demyelinating peripheral neuropathy
We next considered whether FBLN5 could be a potential treatment for demyelinating peripheral neuropathy, including CMT. It has been reported that duplication and mutation of the PMP22 gene is involved in CMT type 1. 48 , 49 Thus, we have generated a CMT zebrafish model overexpressing zebrafish pmp22α and found that the CMT zebrafish have less myelination in the peripheral lateral line (Figure 6A,B). Then, we overexpressed fbln5 in the CMT zebrafish model and found that the exogenous fbln5 rescues the myelination defects to normal levels (Figure 6A,B). Further TEM analysis revealed that the uncompact myelin sheaths of the Mauthner neuronal axons and reticulospinal tracts in the CMT zebrafish are significantly recovered by exogenous fbln5 (Figure 6C,D). Collectively, these results suggest that FBLN5 can be a potential target to treat demyelinating peripheral neuropathy, such as CMT.
FIGURE 6.
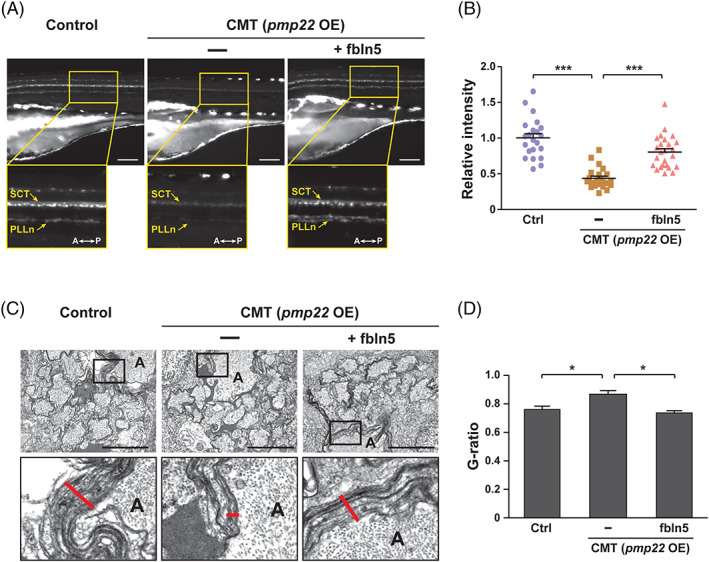
FBLN5 restores the myelination defects in the CMT zebrafish model. A, Lateral view images of the Tg(claudin K:gal4‐vp16;uas:egfp) control and zebrafish injected with pmp22 mRNAs alone or with fbln5 mRNAs at 5 dpf. The images within the rectangles are magnified in the bottom panels. SCT, spinal cord tracts; PLLn, posterior lateral line; A, anterior; P, posterior; OE, overexpression. Scale bars, 100 μm. B, Quantification of the relative intensities of PLLn in equivalent fields of view in the images of (A). Statistical significance was determined using the one‐way ANOVA followed by Dunnett's post hoc test (***P < .001). C, TEM images of cross‐sectioned zebrafish of the indicated genotype at 5 dpf. The images within the rectangles are magnified in the bottom panels. The red lines indicate the thicknesses of the myelin sheaths in the Mauthner axons. A, axon. Scale bars, 2 μm. D, Quantification of the G‐ratios calculated in mauthner axons of the indicated zebrafish. Statistical significance was determined using the one‐way ANOVA followed by Dunnett's post hoc test (*P < .05). The data are shown as the mean ± SD (B, D)
3. DISCUSSION
Here, we identified FBLN5 as a pivotal protein secreted from the WJ‐MSCs that affected the development of SCs. We demonstrated that FBLN5 promoted SC proliferation in vitro and in vivo systems and further clarified that it was necessary for SC myelination. We found that FBLN5 bound to Integrin through the RGD motif and that the interaction was crucial for the activation of RAC1 to modulate actin remodeling (Figure 7). Notably, we revealed that recombinant FBLN5 protein effectively restored the myelination defects in the CMT zebrafish model generated by overexpressing the CMT type1 causative gene pmp22. Thus, these data imply that WJ‐MSCs and the paracrine factor, FBLN5, can be applied as a targeted therapy for myelin‐related disorders including CMTs.
FIGURE 7.
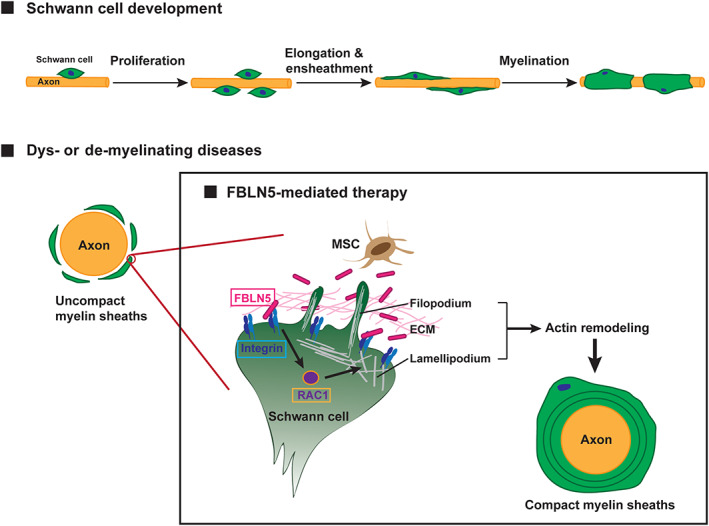
Overall proposed model of the therapeutic mechanism of FBLN5 in myelin‐related diseases. Proper development of Schwann cells through the processes of proliferation, elongation, and myelination is essential for the functioning of the PNS. The common pathological symptom of dysmyelinating and demyelinating diseases is uncompact myelin ensheathments of the axons. Our findings suggest that FBLN5 secreted from MSCs or recombinant FBLN5 present in the ECM binds to the Integrin receptors and promotes actin remodeling through RAC1 activation in the Schwann cells. Then, the Schwann cells are recovered by an activated actin remodeling mechanism and induce the compact myelination of the diseased axons
Reportedly, FBLN5 is associated with the proliferation of several types of cells, such as 3T3L1 line and smooth muscle cells. 23 , 50 , 51 In this study, we suggest that the proliferation of SCs is also controlled by FBLN5. Given that ERK activity is dependent on the concentration of recombinant FBLN5 (Supplementary Figure 3), we have postulated that ERK activation is critical for the FBLN5‐mediated development of SCs, including proliferation and myelination. Indeed, signal transduction through ERK is involved in the development of SCs, including proliferation, differentiation, and myelination. 52 , 53 , 54 However, the involvement of ERK signaling in the myelination processes of SCs is complex. For instance, sustained activation of the MEK–ERK pathway in SCs results in hypermyelination as well as a decrease in regenerative axons and loose density of intraepithelial nerve fibers. 55 , 56 Thus, the investigations to uncover the mechanism by which FBLN5 regulates ERK activation, and particularly to explore role of FBLN5 in the ERK‐dependent recovery of impaired SCs will be followed.
During the myelin sheathing of axons in the PNS, Integrin‐mediated signal transductions in the ECM composed of Laminin, Collagen, and Fibronectin are important in SCs. 25 , 57 This signaling is mainly involved in the cytoskeletal dynamics of SCs during myelination, 58 , 59 but the molecular mechanism has not yet been understood. As we have identified one of the ECM molecules, FBLN5, as an important regulator of SC myelination, we suggest a novel molecular mechanism that modulates actin dynamics by supporting the importance of ECM signaling in the process. Our data revealed that the binding of FBLN5 to the Integrin receptors was essential for RAC1 activation to promote actin protrusion, such as lamellipodia/filopodia generation. It has been previously suggested that RAC1 signaling through Integrin β‐1 is essential for the myelination of SCs. 60 , 61 RAC1 activates the Arp2/3 complex and induces lamellipodia formation through actin branching at the membrane of SCs. 62 In addition, PAK, an effector of RAC1, is also involved in the control of SCs myelination. PAK induces the expressions of Krox‐20, a transcriptional enhancer of several myelination‐related genes, and Galactocerebroside, a general marker of mature SCs, through activation of cAMP. 60 , 63 The expression of Krox‐20 is also regulated by YAP/TAZ in the myelination of SCs. 64 Previous studies have reported that FBLN5 is associated with YAP/TAZ signaling in the proliferation and migration of the airway smooth muscle cells. 65 Based on these data, we hypothesize that FBLN5 may be important for the regulation of genes critical for myelination such as Krox‐20 and YAP/TAZ, and/or for the activation of actin‐associated proteins such as Arp2/3 and PAK in the myelination of SCs. Thus, the relationship between FBLN5 and these molecules will be further investigated to better understand the FBLN5‐mediated mechanism.
Of note, the actin dynamics of SCs is essential throughout the processes of myelination. 66 , 67 , 68 In the initial stages of the myelination, actin polymerization is necessary for SCs to wrap and elongate the axons. 61 , 69 Then, SCs express MBP and widely distribute them for myelin sheathing of axons by forcing actin depolymerization. 70 Moreover, the role of molecules involved in actin polymerization/depolymerization during myelination is very complex. For instance, actin depolymerization‐associated factors including Cofilin‐1 and Gelsolin induce the initiation of SCs myelination, 71 whereas actin polymerization‐related factors such as Arp 2/3 do not appear to be critical for myelin wrapping. 70 Intriguingly, in the oligodendrocytes, MBP is highly expressed during the depolymerization of actin, and its lack leads to the accumulation of actin filaments. 72 , 73 In the present study, we showed that the treatment of recombinant FBLN5 protein induces the expression of MBP and subsequent actin protrusions in SCs. Thus, further investigations to better understand the role of FBLN5‐mediated MBP and its regulatory mechanisms in actin dynamics will be followed.
In the process of demyelination that occurs due to the failure to remove the myelin debris resulting from damages of axons or SCs, actin polymerization is required for fragmentation of myelin sheaths. 74 , 75 , 76 , 77 Accordingly, inhibition of RAC1 prevents actin polymerization, which leads to suppression of myelin fragmentation and subsequent axonal remyelination. 78 , 79 Here, we showed that exogenous FBLN5 effectively rescues the myelination defects in the PNS of the CMT type 1 zebrafish model. Nevertheless, this study is limited to the function of FBLN5 in the early stages of axonal myelination such as SCs proliferation, migration, and myelin formation. Thus, further investigation is needed to demonstrate the involvement of FBLN5‐RAC1 molecular axis in axonal demyelination and remyelination after injury in the PNS.
Several reports have suggested that the therapies using stem cells including MSCs are potential treatments for intractable diseases. 80 , 81 , 82 Although MSCs have limitations such as difficulty in transplantation, including postimplantation immune rejection and possibility of becoming cancer cells, it is gaining attention as an alternative treatment because of the abilities to differentiate into various types of cells and to suppress inflammation‐induced immune response. 83 Notably, it has been suggested that the application of extracellular vesicles (EVs) of MSCs instead of the entire cells may be a direct treatment by overcoming these limitations. However, the key molecules released from the EVs of MSCs and the involved mechanisms have been poorly understood in many disorders. In this study, we have investigated the effect of human WJ‐MSCs on the myelination of SCs and identified paracrine factors that play a crucial role in the proliferation, cytoskeletal rearrangement, and migration of SCs. Thus our study suggests that WJ‐MSCs can be a potent treatment for the myelin‐related peripheral neuropathy including CMTs.
4. MATERIALS AND METHODS
4.1. Cell culture
S16 cells (ATCC CRL‐2941, American Type Culture Collection, Rockville, Maryland), the rat SC line, were cultured in Dulbecco's Modified Eagle's medium (Biowest S.A.S, Nuaillé, France) supplemented with 10% fetal bovine serum (FBS) (Gibco, Carlsbad, California), 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco) in 5% CO2 at 37°C. Human WJ‐MSCs were isolated according to the previously specified procedure 84 and cultured in Alpha Minimum Essential Medium (Gibco) supplemented with 10% FBS and 50 μg/mL gentamicin (Gibco) in 5% CO2 at 37°C. For co‐cultivation, the WJ‐MSCs (1.5 × 105 cells) were seeded into the upper chamber of transwell inserts (pore size of 1 μm, BD Biosciences, Franklin Lakes, New Jersey) when the S16 cells reached a confluence of almost 70%. These cells were co‐cultured for 24 hours in a condition of serum‐free media in 5% CO2 at 37°C. Human cell subject research was reviewed and approved by the Institutional Review Board of Samsung Medical Center, and informed consent was obtained from pregnant mothers (IRB#2016‐07‐102).
4.2. Characterization of WJ‐MSCs
According to the MSC criteria of the International Society for Cell Therapy (ISCT), 85 immunophenotypic analysis of WJ‐MSCs was performed by flow cytometry to determine the expression of the following markers: CD44, CD73, CD90, CD105, CD14, CD11b, HLA‐DR (MHC‐II), CD34, CD45, and CD19 (BD Biosciences). At least 10 000 events were acquired on a BD FACSVerse (BD Biosciences, New Jersey), and the results were analyzed with BD FACSuite software version 10 (BD Biosciences). The differentiation of WJ‐MSCs was tested according to the procedure outlined in a previous report. 86
4.3. Antibody array
The RayBio Biotin Label‐based Human Antibody Array (#AAH‐BLG‐1‐4), which is available to detect a total of 507 of human proteins, was applied for the analysis of secreted proteins from the conditioned media for the co‐cultivation of MSCs and S16 cells. All array data obtained using the Axon GenePix 4000B microarray scanner was analyzed with GenePix Pro 6.0 software. The average score of protein expression was normalized with that of internal biotinylated controls in duplicated arrays. The clustering analysis of hits was performed using the MeV software (Dana‐Farber Cancer Institute, Boston, Massachusetts). The heatmap was generated by candidates with log 2 (fold change) >1.5.
4.4. Enzyme‐linked immunosorbent assay
The ELISA kit (LSBio, LS‐F6448) was used for the analysis of the expression of proteins selected from the antibody array. The protein concentration was calculated from the intensity value measured at a wavelength of 450 nm.
4.5. CCK‐8 assay
The CCK‐8 (CCK‐8, Dojindo, Tokyo, Japan) was applied for analysis of the proliferation of S16 cells. For the assay, S16 cells (1 × 104 cells/well) were seeded into 96‐well cell plates, including 100 μL of conditioned culture medium, and incubated overnight. The CCK‐8 reagent of 10 μL was added to each well and incubated for 1 hour at 37°C. Then a microplate reader (xMark Microplate Absorbance Spectrophotometer) was used to analyze the cellular absorbance at a wavelength of 450 nm.
4.6. BrdU assay
S16 cells, cultured on round glass coverslips in 12‐well plates, were pretreated with BrdU (10 μM) for 1 hour, and then treated with 10 ng/mL of recombinant human FBLN5 protein in a serum‐free state. The cells were fixed in 3% formaldehyde and treated with 2 N HCl for 30 minutes at 37°C for antigen retrieval followed by neutralization with boric acid (pH 8.4). After blocking for 1 hour at room temperature, the cells were stained with anti‐BrdU antibody (#ab6326, Abcam), secondary (#112‐165‐143, Jackson ImmunoResearch Laboratories), and Hoechst 33342 (#H1399, Life Technologies Corporation) antibodies. The immunostained images were acquired using an LSM 700 confocal microscope (Carl Zeiss AG, Jena, Germany). The images were analyzed with ImageJ software.
4.7. Transfection
The verified siRNAs for FBLN5 (Supplementary Table 1) were transfected to WJ‐MSCs using Lipofectamine 3000 (Invitrogen, Carlsbad, California) according to the manufacturer's instructions. The non‐targeting control siRNAs (Bioneer, SN‐1001, Korea), which have at least four mismatch nucleotides to the human, mouse, and rat Fibulin genes, were transfected as a control. The transfected cells were applied for the next experiments after 24 hours of incubation in a complete medium.
4.8. In vitro wound healing assay
S16 cells reached to 90% confluence in 6‐well plates were treated with mitomycin (10 μg/mL) for 1 hour to inhibit cell proliferation. Confluent monolayers of the cells then were scratched using a 200 μL pipette tip to generate the wound. The cells were washed with PBS and cultured with nothing, WJ‐MSCs, or recombinant FBLN5 for 48 hours. To determine the extent of wound closure, photographs of the wound were taken 0, 24, and 48 hours later using a phase‐contrast microscope (Olympus, CKX41, Japan). Wound closure was measured proximate cells length using ImageJ software (NIH).
4.9. Cultivation of DRG and primary SCs
The DRG and sciatic nerves were isolated from c57/BL6NJ mice at postnatal day 15 (P15) and transferred to the conical tube containing trypsin of 0.25% (25‐053‐CI, Corning, New York) and 1 mg/mL of collagenase (C2139, Sigma‐Aldrich, Saint Louis, Missouri). The primary tissues were incubated for 30 minutes at 37°C for digestion followed by centrifugation. The cell pellets were suspended in DMEM (10‐013‐CVR, Corning) containing fetal bovine serum of 15% (35‐016‐CV, Corning) and penicillin‐streptomycin of 1% (30‐002‐CI, Corning) after being washed with DPBS (21‐030‐CVR, Corning). The primary cells were cultivated on the coverslips in 35‐mm dishes or 6‐well plates coated with poly‐d‐lysine hydrobromide (P6407, Sigma‐Aldrich) in 5% CO2 at 37°C.
4.10. Zebrafish housing and manipulations
Adult Tg(claudin K:gal4‐vp16;uas:egfp) zebrafish were maintained with a cycle of 13 hours of light and 11 hours of dark in an automatic system (Genomic‐Design, Korea) at 28.5°C and pH 7.0‐7.9. The zebrafish embryos were collected by natural breeding and incubated in clean petri dishes containing E3 medium (297.7 mM NaCl, 10.7 mM KCl, 26.1 mM CaCl2, and 24.1 mM MgCl2) with 1% methylene blue (M2662, Samchun Chemicals, Korea) at 28.5°C. To inhibit the formation of melanin, which interferes with immunostaining, the zebrafish larvae were raised in E3 medium containing 0.2 mM N‐phenylthiourea (P7629, Sigma Aldrich). Animal subject research was reviewed and approved by the Institutional Animal Care and Use Committee at the Samsung Biomedical Research Institute and the Sungkyunkwan University (IACUC#20191204002).
4.11. DNA plasmid construction
The zebrafish fbln5, pmp22α, and rac1 were amplified with cDNAs obtained from zebrafish larvae at 5 days postfertilization (dpf). The coding sequences of fbln5, pmp22α, and rac1 were subcloned into pCS2+ vectors for mRNA expression in zebrafish. The cloned rac1 and fbln5 plasmids were mutated using a QuickChange II Site‐Directed Mutagenesis Kit (#200524, Aglient) to generate rac1 (Q61L) and fbln5 (ΔRGD) constructs, respectively. The mouse Rac1 coding sequences were subcloned into a pCMV vector and mutagenized to generate Rac1 (Q61L). The designed oligos for the above subclonings are presented in Supplementary Table 2.
4.12. Microinjection into zebrafish
To block the expression of zebrafish fbln5, translation blocking antisense morpholino oligonucleotides (MOs) (Supplementary Table 3) were designed and synthesized by GeneTools (Philomath, Oregon). The MOs were dissolved in nuclease‐free water and microinjected into zebrafish embryos using a gas‐powered microinjection system (PV83 Pneumatic PicoPump, SYS‐PV830, World Precision Instruments, Sarasota, Florida). The capped mRNAs of zebrafish genes (fbln5, pmp22, and rac1) were synthesized using a mMESSAGE mMACHIN T3 kit (AM1340, Ambion). The mRNAs synthesized in vitro (600‐800 ng/μL) were injected into zebrafish embryos with or without fbln5 MOs.
4.13. Immunoblot assay
The cell extracts were prepared by ultrasonication (Branson Ultrasonic, Danbury, CT) in a buffer (9.8 mol/L of UREA, 4% of CHAPS, 130 mmol/L of dithiothreitol, 40 mmol/L of Tris‐HCl, 0.1% of sodium dodecyl sulfate, 1 mmol/L of EDT, and a protease/phosphatase inhibitor cocktail). The zebrafish extracts were prepared at 5 dpf. The yolks of zebrafish larvae anesthetized with 0.02% of Tricaine (A5040, Sigma) were removed by gentle pipetting, and homogenized with a 1‐mL syringe in T‐PER buffer (78 510, Thermo Fisher Scientific, Massachusetts). Zebrafish or cellular protein extracts (20‐40 μg) were separated by SDS‐PAGE, and the resolved proteins were transferred to a 0.45‐μm PVDF membrane (IPVH00010, Millipore). After blocking in Tris‐buffered saline pH 7.5 with 0.1% Tween‐20 (TBST) including 5% skim milk (Cat.232100, BD Biosciences) for 30 to 60 minutes at room temperature (RT), each membrane was blotted with a primary antibody (mouse anti‐Rac1 [ARC03], Cytoskeleton, Colorado), rabbit (anti‐Fibulin5 [A9961], ABclonal, Massachusetts, 1:2000), or mouse (anti‐Actin [sc‐8432], Santa Cruz Biotechnology, California, 1:1000) overnight at 4°C. The membranes were then washed three times with TBST and incubated with a secondary antibody (goat anti mouse IgG horseradish peroxidase (HRP)‐conjugated antibody [GTX213111‐01], GeneTex, California, 1:5000) or goat anti‐rabbit IgG HRP‐conjugated antibody (GTX213110‐01, GeneTex, 1:5000) for 1 hour at RT. Nest, the membranes were washed three times with TBST and enhanced with chemiluminescence substrate (Nel104001EA, PerkinElmer, Massachusetts) to visualize specific proteins through film exposure.
4.14. RAC1 activity assay
Four hundred micrograms of cell lysates were incubated with 10 μg of P21‐associated kinase (PAK)‐PBD beads (BK035‐S, Cytoskeleton, Colorado) on the rotator for 1 hour at 4°C. The beads were then washed in a buffer (25 mM Tris pH 7.5, 30 mM MgCl2, and 40 mM NaCl). Finally, the bead‐bound proteins were denaturized by boiling in a 5× sample buffer for 2 minutes and separated by 8% SDS‐polyacrylamide gel electrophoresis.
4.15. Immunocytochemistry
Cells fixed in 4% PFA at RT for 30 minutes were permeabilized with 0.1% triton X‐100 in PBS for 5 minutes after washing with 1× PBS. Then, the cells were washed 2 to 3 times and blocked in PBS with 1% BSA at RT for 1 hour. The cells were incubated with primary antibodies: mouse anti‐Sox10 (sc‐365 692, Santa Cruz, 1:200), mouse anti‐MBP (sc‐271 524, Santa Cruz, 1:200) antibodies at 4°C overnight in blocking solution. After washing with 1x PBS, the cells were incubated with Alexa Flour 594‐conjugated secondary antibodies (mouse A11005, Invitrogen, 1:500) or Phalloidin‐iFluor 488 reagent (ab176753, Abcam) at RT for 1 h. For generating imaging data, randomly selected fields (≥3) were photographed using a confocal microscope (Carl Zeiss, LSM 700, Germany) and analyzed using ImageJ software (NIH) in all of imaging data.
4.16. Zebrafish motility analysis
Using Daniovision (Noldus, Wageningen, The Netherlands), the velocities of the zebrafish were analyzed by a tap test. Individual zebrafish larvae injected with MOs were transferred into 24‐well plates, with each well with 1 mL of E3 media. After the zebrafish acclimatized in the chamber for 30 minutes, double tapping stimuli (first at level 4 and second at level 8) were applied for the zebrafish every 10 seconds. The movements of the zebrafish were recorded and analyzed using Ethovision XT software (Noldus, Wageningen, The Netherlands).
4.17. Transmission electron microscopy
Zebrafish larvae anesthetized with 0.02% tricaine (A5040, Sigma) were fixed in a buffer (2% glutaraldehyde and 2% paraformaldehyde in 0.1 M phosphate buffer at pH 7.4) for 24 hours. After being washed with 0.1 M phosphate buffer, the larva were postfixed in a buffer (1% osmium tetroxide [02236, Polysciences] in 0.1 M phosphate buffer) for 2 hours and then gradually dehydrated in ethanol (50%‐100%) and infiltrated with propylene oxide. The fixed zebrafish were embedded in a Poly/Bed 812 kit (#08791, Polysciences) and polymerized in an electron microscope oven (TD‐700, DOSAKA, Japan) for 24 hours at 65°C. The embedded zebrafish blocks were sectioned with an ultramicrotome (Leica EM UC‐7, Leica Microsystems, Austria) with a diamond knife (Diatome) at a thickness of 200 nm. The sectioned samples were stained with 1% toluidine blue (T3260, Sigma) and then observed by bright‐field microscopy. The regions for TEM were selected and re‐sectioned at a thickness of 70 nm. The ultrathin sectioned samples were transferred onto the copper/nickel grids for double staining with uranyl acetate (6%) and lead citrate. The stained samples were observed using TEM (JEM‐1011, JEOL, Japan).
4.18. Statistics
All statistical analyses were performed using the GraphPad Prism version 5 (GraphPad Software, La Jolla, California). Values were presented as the mean ± S.D. or fold changes relative to the mean controls. Differences between two groups were evaluated using unpaired Student's t‐tests with Welch's correction. Datasets having more than two groups or conditions were subjected to ANOVA followed by Tukey's, Dunnett's, or Bonferroni's post hoc test. A P‐value less than .05 was considered significant for any statistical test used.
5. CONCLUSION
To seek novel therapeutic targets for demyelinating diseases, we screened the paracrine factors secreted from MSCs co‐cultured with SCs and identified FBLN5 using antibody array analysis. We showed that after recombinant FBLN5 treatment, SCs proliferated more through activation of ERK. We further demonstrated that the RGD motif of FBLN5 is essential for binding to Integrin, and this interaction is involved in actin remodeling via RAC1 activity during myelination of SCs. Finally, we revealed that FBLN5 effectively restores myelination defects in the zebrafish model of demyelinating disease, suggesting that FBLN5 may be a potential target for myelin‐related disorders.
CONFLICT OF INTEREST
The authors declared no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
S.Y.W., S.K., H.S.J.: conducted experiments and analyzed data; K.W.C., B.‐O.C., J.W.C.: advised on experimental designs and commented on the manuscript; J.E.L.: designed the overall experiments and wrote the manuscript with substantial contribution from J.W.C., S.Y.W., and S.J.K.
Supporting information
Figure S1
Figure S2
Figure S3
Figure S4
Figure S5
Figure S6
Figure S7
Figure S8
Appendix S1. Tables
ACKNOWLEDGMENTS
This work was supported by National Research Foundation (2018R1A2A3074597 to J.E.L.; 2018R1A4A1024506 to K.W.C., B.‐O.C., and J.E.L.; and 2018R1D1A1B07046902 to J.W.C.) and Korea Health Technology R&D Project (HI14C3484 to J.W.C.) funded from the MSIP and KHIDI of the Korean government. We thank H.‐C. Park (Korea University) for Tg(claudin K:gal4‐vp16;uas:egfp) zebrafish and thank E.J. Kim and Yonsei Biomedical Research Institute (Yonsei University College of Medicine) for TEM imaging.
Won SY, Kwon S, Jeong HS, et al. Fibulin 5, a human Wharton's jelly‐derived mesenchymal stem cells‐secreted paracrine factor, attenuates peripheral nervous system myelination defects through the Integrin‐RAC1 signaling axis. Stem Cells. 2020;38:1578–1593. 10.1002/stem.3287
So Yeon Won and Soojin Kwon contributed equally to this study.
Funding information Korea Health Industry Development Institute, Grant/Award Number: HI14C3484; National Research Foundation of Korea, Grant/Award Numbers: 2018R1A2A3074597, 2018R1A4A1024506, 2018R1D1A1B07046902
Contributor Information
Jong Wook Chang, Email: jongwook.chang@samsung.com.
Ji Eun Lee, Email: jieun.lee@skku.edu.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- 1. Chamberlain KA, Sheng ZH. Mechanisms for the maintenance and regulation of axonal energy supply. J Neurosci Res. 2019;97:897‐913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marshall‐Phelps KLH, Kegel L, Baraban M, et al. Neuronal activity disrupts myelinated axon integrity in the absence of NKCC1b. J Cell Biol. 2020;219:e201909022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harty BL, Coelho F, Pease‐Raissi SE, et al. Myelinating Schwann cells ensheath multiple axons in the absence of E3 ligase component Fbxw7. Nat Commun. 2019;10:2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wolbert J, Li X, Heming M, et al. Redefining the heterogeneity of peripheral nerve cells in health and autoimmunity. Proc Natl Acad Sci U S A. 2020;117:9466‐9476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Duncan ID, Radcliff AB. Inherited and acquired disorders of myelin: the underlying myelin pathology. Exp Neurol. 2016;283:452‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fledrich R, Abdelaal T, Rasch L, et al. Targeting myelin lipid metabolism as a potential therapeutic strategy in a model of CMT1A neuropathy. Nat Commun. 2018;9:3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pareyson D, Saveri P, Pisciotta C. New developments in Charcot‐Marie‐Tooth neuropathy and related diseases. Curr Opin Neurol. 2017;30:471‐480. [DOI] [PubMed] [Google Scholar]
- 8. Theadom A, Roxburgh R, MacAulay E, et al. Prevalence of Charcot‐Marie‐Tooth disease across the lifespan: a population‐based epidemiological study. BMJ Open. 2019;9:e029240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barreto LC, Oliveira FS, Nunes PS, et al. Epidemiologic study of Charcot‐Marie‐Tooth disease: a systematic review. Neuroepidemiology. 2016;46:157‐165. [DOI] [PubMed] [Google Scholar]
- 10. Nagappa M, Sharma S, Govindaraj P, et al. PMP22 gene‐associated neuropathies: phenotypic spectrum in a cohort from India. J Mol Neurosci. 2020;70:778‐789. [DOI] [PubMed] [Google Scholar]
- 11. Pipis M, Rossor AM, Laura M, Reilly MM. Next‐generation sequencing in Charcot‐Marie‐Tooth disease: opportunities and challenges. Nat Rev Neurol. 2019;15:644‐656. [DOI] [PubMed] [Google Scholar]
- 12. Bird TD. In: Adam MP. et al., eds. Charcot‐Marie‐Tooth (CMT) Hereditary Neuropathy Overview. Seattle (WA): GeneReviews((R)); 1993. [PubMed] [Google Scholar]
- 13. Jerath NU, Shy ME. Hereditary motor and sensory neuropathies: understanding molecular pathogenesis could lead to future treatment strategies. Biochim Biophys Acta. 2015;1852:667‐678. [DOI] [PubMed] [Google Scholar]
- 14. Mittendorf KF, Marinko JT, Hampton CM, et al. Peripheral myelin protein 22 alters membrane architecture. Sci Adv. 2017;3:e1700220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bucan V, Vaslaitis D, Peck CT, Strauss S, Vogt PM, Radtke C. Effect of exosomes from rat adipose‐derived mesenchymal stem cells on neurite outgrowth and sciatic nerve regeneration after crush injury. Mol Neurobiol. 2019;56:1812‐1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qing L, Chen H, Tang J, Jia X. Exosomes and their MicroRNA cargo: new players in peripheral nerve regeneration. Neurorehabil Neural Repair. 2018;32:765‐776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fan XL, Zhang Y, Li X, Fu QL. Mechanisms underlying the protective effects of mesenchymal stem cell‐based therapy. Cell Mol Life Sci. 2020;77:2771‐2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Park BW, Jung SH, Das S, et al. In vivo priming of human mesenchymal stem cells with hepatocyte growth factor‐engineered mesenchymal stem cells promotes therapeutic potential for cardiac repair. Sci Adv. 2020;6:eaay6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee PH, Kim JW, Bang OY, Ahn YH, Joo IS, Huh K. Autologous mesenchymal stem cell therapy delays the progression of neurological deficits in patients with multiple system atrophy. Clin Pharmacol Ther. 2008;83:723‐730. [DOI] [PubMed] [Google Scholar]
- 20. Connick P, Kolappan M, Crawley C, et al. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open‐label phase 2a proof‐of‐concept study. Lancet Neurol. 2012;11:150‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Park SR, Kim JW, Jun HS, Roh JY, Lee HY, Hong IS. Stem cell secretome and its effect on cellular mechanisms relevant to wound healing. Mol Ther. 2018;26:606‐617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen H, Zhang H, Zheng Y, et al. Prolyl hydroxylase 2 silencing enhances the paracrine effects of mesenchymal stem cells on necrotizing enterocolitis in an NF‐kappaB‐dependent mechanism. Cell Death Dis. 2020;11:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yanagisawa H, Schluterman MK, Brekken RA. Fibulin‐5, an integrin‐binding matricellular protein: its function in development and disease. J Cell Commun Signal. 2009;3:337‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Colognato H, Tzvetanova ID. Glia unglued: how signals from the extracellular matrix regulate the development of myelinating glia. Dev Neurobiol. 2011;71:924‐955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Belin S, Zuloaga KL, Poitelon Y. Influence of mechanical stimuli on Schwann cell biology. Front Cell Neurosci. 2017;11:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Auer‐Grumbach M, Weger M, Fink‐Puches R, et al. Fibulin‐5 mutations link inherited neuropathies, age‐related macular degeneration and hyperelastic skin. Brain. 2011;134:1839‐1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cheng S, Lv H, Zhang W, et al. Adult‐onset demyelinating neuropathy associated with FBLN5 gene mutation. Clin Neuropathol. 2017;36:171‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Toda K, Small JA, Goda S, Quarles RH. Biochemical and cellular properties of three immortalized Schwann cell lines expressing different levels of the myelin‐associated glycoprotein. J Neurochem. 1994;63:1646‐1657. [DOI] [PubMed] [Google Scholar]
- 29. Hai M, Muja N, DeVries GH, Quarles RH, Patel PI. Comparative analysis of Schwann cell lines as model systems for myelin gene transcription studies. J Neurosci Res. 2002;69:497‐508. [DOI] [PubMed] [Google Scholar]
- 30. Sroka IC, Chopra H, Das L, Gard JM, Nagle RB, Cress AE. Schwann cells increase prostate and pancreatic tumor cell invasion using laminin binding A6 integrin. J Cell Biochem. 2016;117:491‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fogarty EA, Kitzman JO, Antonellis A. SOX10‐regulated promoter use defines isoform‐specific gene expression in Schwann cells. BMC Genomics. 2020;21:549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sonnenberg‐Riethmacher E, Miehe M, Riethmacher D. Promotion of periostin expression contributes to the migration of Schwann cells. J Cell Sci. 2015;128:3345‐3355. [DOI] [PubMed] [Google Scholar]
- 33. Shamash S, Reichert F, Rotshenker S. The cytokine network of Wallerian degeneration: tumor necrosis factor‐alpha, interleukin‐1alpha, and interleukin‐1beta. J Neurosci. 2002;22:3052‐3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen G, Luo X, Wang W, Wang Y, Zhu F, Wang W. Interleukin‐1beta promotes Schwann cells de‐differentiation in Wallerian degeneration via the c‐JUN/AP‐1 pathway. Front Cell Neurosci. 2019;13:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Safka Brozkova D, Lassuthova P, Neupauerova J, et al. Czech family confirms the link between FBLN5 and Charcot‐Marie‐Tooth type 1 neuropathy. Brain. 2013;136:e232. [DOI] [PubMed] [Google Scholar]
- 36. Yamagishi Y, Samukawa M, Kuwahara M, et al. Charcot‐Marie‐Tooth disease with a mutation in FBLN5 accompanying with the small vasculitis and widespread onion‐bulb formations. J Neurol Sci. 2020;410:116623. [DOI] [PubMed] [Google Scholar]
- 37. Tsuda T. Extracellular interactions between Fibulins and transforming growth factor (TGF)‐beta in physiological and pathological conditions. Int J Mol Sci. 2018;19:2787–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Munzel EJ, Schaefer K, Obirei B, et al. Claudin k is specifically expressed in cells that form myelin during development of the nervous system and regeneration of the optic nerve in adult zebrafish. Glia. 2012;60:253‐270. [DOI] [PubMed] [Google Scholar]
- 39. Kim S, Lee YI, Chang KY, et al. Promotion of remyelination by sulfasalazine in a transgenic zebrafish model of demyelination. Mol Cells. 2015;38:1013‐1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee YH, Albig AR, Regner M, Schiemann BJ, Schiemann WP. Fibulin‐5 initiates epithelial‐mesenchymal transition (EMT) and enhances EMT induced by TGF‐beta in mammary epithelial cells via a MMP‐dependent mechanism. Carcinogenesis. 2008;29:2243‐2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang M, Topalovski M, Toombs JE, et al. Fibulin‐5 blocks microenvironmental ROS in pancreatic cancer. Cancer Res. 2015;75:5058‐5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Finzsch M, Schreiner S, Kichko T, et al. Sox10 is required for Schwann cell identity and progression beyond the immature Schwann cell stage. J Cell Biol. 2010;189:701‐712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rehberg S, Lischka P, Glaser G, Stamminger T, Wegner M, Rosorius O. Sox10 is an active nucleocytoplasmic shuttle protein, and shuttling is crucial for Sox10‐mediated transactivation. Mol Cell Biol. 2002;22:5826‐5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. She ZY, Yang WX. SOX family transcription factors involved in diverse cellular events during development. Eur J Cell Biol. 2015;94:547‐563. [DOI] [PubMed] [Google Scholar]
- 45. Singh A, Karnoub AE, Palmby TR, Lengyel E, Sondek J, Der CJ. Rac1b, a tumor associated, constitutively active Rac1 splice variant, promotes cellular transformation. Oncogene. 2004;23:9369‐9380. [DOI] [PubMed] [Google Scholar]
- 46. Zhang ZG, Lambert CA, Servotte S, et al. Effects of constitutively active GTPases on fibroblast behavior. Cell Mol Life Sci. 2006;63:82‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nakaya M, Kitano M, Matsuda M, Nagata S. Spatiotemporal activation of Rac1 for engulfment of apoptotic cells. Proc Natl Acad Sci U S A. 2008;105:9198‐9203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lupski JR, de Oca‐Luna RM, Slaugenhaupt S, et al. DNA duplication associated with Charcot‐Marie‐Tooth disease type 1A. Cell. 1991;66:219‐232. [DOI] [PubMed] [Google Scholar]
- 49. Birouk N, Gouider R, Le Guern E, et al. Charcot‐Marie‐Tooth disease type 1A with 17p11.2 duplication. Clinical and electrophysiological phenotype study and factors influencing disease severity in 119 cases. Brain. 1997;120(pt 5):813‐823. [DOI] [PubMed] [Google Scholar]
- 50. Schiemann WP, Blobe GC, Kalume DE, Pandey A, Lodish HF. Context‐specific effects of fibulin‐5 (DANCE/EVEC) on cell proliferation, motility, and invasion. Fibulin‐5 is induced by transforming growth factor‐beta and affects protein kinase cascades. J Biol Chem. 2002;277:27367‐27377. [DOI] [PubMed] [Google Scholar]
- 51. Okuyama T, Shirakawa J, Yanagisawa H, et al. Identification of the matricellular protein Fibulin‐5 as a target molecule of glucokinase‐mediated calcineurin/NFAT signaling in pancreatic islets. Sci Rep. 2017;7:2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rodriguez‐Molina JF, Lopez‐Anido C, Ma KH, et al. Dual specificity phosphatase 15 regulates Erk activation in Schwann cells. J Neurochem. 2017;140:368‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Newbern JM, Li X, Shoemaker SE, et al. Specific functions for ERK/MAPK signaling during PNS development. Neuron. 2011;69:91‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cervellini I, Galino J, Zhu N, Allen S, Birchmeier C, Bennett DL. Sustained MAPK/ERK activation in adult Schwann cells impairs nerve repair. J Neurosci. 2018;38:679‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Napoli I, Noon LA, Ribeiro S, et al. A central role for the ERK‐signaling pathway in controlling Schwann cell plasticity and peripheral nerve regeneration in vivo. Neuron. 2012;73:729‐742. [DOI] [PubMed] [Google Scholar]
- 56. Li NW, Yin YX, Li JY, Zhang CH, Guo YG. Passivation of lithium metal anode via hybrid ionic liquid electrolyte toward stable Li plating/stripping. Adv Sci (Weinh). 2017;4:1600400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chernousov MA, Yu WM, Chen ZL, Carey DJ, Strickland S. Regulation of Schwann cell function by the extracellular matrix. Glia. 2008;56:1498‐1507. [DOI] [PubMed] [Google Scholar]
- 58. Joronen K, Konu A, Rankin HS, Astedt‐Kurki P. An evaluation of a drama program to enhance social relationships and anti‐bullying at elementary school: a controlled study. Health Promot Int. 2012;27:5‐14. [DOI] [PubMed] [Google Scholar]
- 59. Chicri RO, Sasaki RT, Carvalho AS, Nouer PR, Lima‐Arsati YB. Effect of enamel pretreatment on shear bond strength of brackets bonded with resin‐modified glass‐ionomer cement. World J Orthod. 2010;11:11‐15. [PubMed] [Google Scholar]
- 60. Guo L, Moon C, Niehaus K, Zheng Y, Ratner N. Rac1 controls Schwann cell myelination through cAMP and NF2/merlin. J Neurosci. 2012;32:17251‐17261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ness JK, Snyder KM, Tapinos N. Lck tyrosine kinase mediates beta1‐integrin signalling to regulate Schwann cell migration and myelination. Nat Commun. 2013;4:1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Feltri ML, Suter U, Relvas JB. The function of RhoGTPases in axon ensheathment and myelination. Glia. 2008;56:1508‐1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bacallao K, Monje PV. Requirement of cAMP signaling for Schwann cell differentiation restricts the onset of myelination. PLoS One. 2015;10:e0116948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Grove M, Kim H, Santerre M, et al. YAP/TAZ initiate and maintain Schwann cell myelination. Elife. 2017;6:e20982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fu J, Zheng M, Zhang X, et al. Fibulin‐5 promotes airway smooth muscle cell proliferation and migration via modulating Hippo‐YAP/TAZ pathway. Biochem Biophys Res Commun. 2017;493:985‐991. [DOI] [PubMed] [Google Scholar]
- 66. Montani L, Buerki‐Thurnherr T, de Faria JP, et al. Profilin 1 is required for peripheral nervous system myelination. Development. 2014;141:1553‐1561. [DOI] [PubMed] [Google Scholar]
- 67. Shen YA, Chen Y, Dao DQ, et al. Phosphorylation of LKB1/Par‐4 establishes Schwann cell polarity to initiate and control myelin extent. Nat Commun. 2014;5:4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gerber D, Ghidinelli M, Tinelli E, et al. Schwann cells, but not oligodendrocytes, depend strictly on dynamin 2 function. Elife. 2019;8:e42404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Leitman EM, Tewari A, Horn M, et al. MLCK regulates Schwann cell cytoskeletal organization, differentiation and myelination. J Cell Sci. 2011;124:3784‐3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Samanta J, Salzer JL. Myelination: actin disassembly leads the way. Dev Cell. 2015;34:129‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sparrow N, Manetti ME, Bott M, et al. The actin‐severing protein cofilin is downstream of neuregulin signaling and is essential for Schwann cell myelination. J Neurosci. 2012;32:5284‐5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zuchero JB, Fu MM, Sloan SA, et al. CNS myelin wrapping is driven by actin disassembly. Dev Cell. 2015;34:152‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Nawaz S, Sanchez P, Schmitt S, et al. Actin filament turnover drives leading edge growth during myelin sheath formation in the central nervous system. Dev Cell. 2015;34:139‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Vargas ME, Watanabe J, Singh SJ, Robinson WH, Barres BA. Endogenous antibodies promote rapid myelin clearance and effective axon regeneration after nerve injury. Proc Natl Acad Sci U S A. 2010;107:11993‐11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kopper TJ, Gensel JC. Myelin as an inflammatory mediator: myelin interactions with complement, macrophages, and microglia in spinal cord injury. J Neurosci Res. 2018;96:969‐977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Vaquie A, Sauvain A, Duman M, et al. Injured axons instruct Schwann cells to build constricting actin spheres to accelerate axonal disintegration. Cell Rep. 2019;27:3152‐3166. e3157. [DOI] [PubMed] [Google Scholar]
- 77. Nocera G, Jacob C. Mechanisms of Schwann cell plasticity involved in peripheral nerve repair after injury. Cell Mol Life Sci. 2020;77:3977–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Park HT, Feltri ML. Rac1 GTPase controls myelination and demyelination. Bioarchitecture. 2011;1:110‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Tricaud N, Park HT. Wallerian demyelination: chronicle of a cellular cataclysm. Cell Mol Life Sci. 2017;74:4049‐4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Liu JA, Cheung M. Neural crest stem cells and their potential therapeutic applications. Dev Biol. 2016;419:199‐216. [DOI] [PubMed] [Google Scholar]
- 81. Osorio MJ, Rowitch DH, Tesar P, Wernig M, Windrem MS, Goldman SA. Concise review: stem cell‐based treatment of Pelizaeus‐Merzbacher disease. Stem Cells. 2017;35:311‐315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wang Y, Pan J, Wang D, Liu J. The use of stem cells in neural regeneration: a review of current opinion. Curr Stem Cell Res Ther. 2018;13:608‐617. [DOI] [PubMed] [Google Scholar]
- 83. Pittenger MF, Discher DE, Peault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kwon S, Ki SM, Park SE, et al. Anti‐apoptotic effects of human Wharton's jelly‐derived mesenchymal stem cells on skeletal muscle cells mediated via secretion of XCL1. Mol Ther. 2016;24:1550‐1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315‐317. [DOI] [PubMed] [Google Scholar]
- 86. Lee J, Kwon SJ, Kim JH, et al. Cerebrospinal fluid from Alzheimer's disease patients as an optimal formulation for therapeutic application of mesenchymal stem cells in Alzheimer's disease. Sci Rep. 2019;9:564. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Figure S2
Figure S3
Figure S4
Figure S5
Figure S6
Figure S7
Figure S8
Appendix S1. Tables
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


