Abstract
Chemical compounds belonging to the class of coumarins have promising anti-inflammatory potential. Cinnamoyloxy-mammeisin (CNM) is a 4-phenylcoumarin that can be isolated from Brazilian geopropolis. To our knowledge, its anti-inflammatory activity has never been studied. Therefore, the present study investigated the anti-inflammatory activity of CNM and elucidated its mechanism of action on isolated macrophages. Pretreatment with CNM reduced neutrophil migration into the peritoneal and joint cavity of mice. Likewise, CNM reduced the in vitro and in vivo release of TNF-α and CXCL2/MIP-2. Regarding the possible molecular mechanism of action, CNM reduced the phosphorylation of proteins ERK 1/2, JNK, p38 MAPK, and AP-1 (subunit c-jun) in PG-stimulated macrophages. Pretreatment with CNM also reduced NF-κB activation in RAW 264.7 macrophages stably expressing the NF-κB-luciferase reporter gene. On the other hand, it did not alter IκBα degradation or nuclear translocation of p65. Thus, the results of this study demonstrate promising anti-inflammatory activity of CNM and provide an explanation of its mechanism of action in macrophages via inhibition of MAPK signaling, AP-1, and NF-κB.
Graphical Abstract

Natural products have been historically used in folk medicine for the treatment of various diseases and are considered a vital source for the discovery of novel drugs.1 A recent survey showed that 50% of the new drugs discovered in 2010 were natural products or their derivatives.2
Coumarins are a class of naturally occurring chemical compounds studied due to their numerous biological activities, such as antibacterial, anticoagulant, antitumor, and anti-inflammatory properties.3,4 Indeed, the coumarin isofraxidin reduces the number of neutrophils and TNF-α, IL-6, and PGE2 levels in bronchoalveolar lavage fluid in lipopolysaccharide (LPS)-induced acute lung injury.5 Notwithstanding, nodakenin, another coumarin, also reduced the inflammatory process in the airways of mice.6
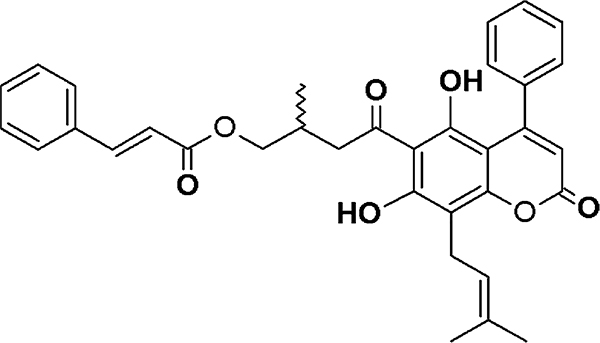
Geopropolis is a type of propolis-containing plant resin and wax and is characterized by the presence of soil. It can be collected by a native stingless bee species, such as Melipona scutellaris.7–9 Recent studies have found promising anti-inflammatory activity of geopropolis extract via inhibition of neutrophil migration.7 Moreover, another study isolated and identified seven coumarin-like compounds with antiproliferative activity against cancer cell lines in geopropolis of M. scutellaris.8 Among these compounds, cinnamoyloxy-mammeisin (CNM) (Figure 1), a 4-phenylcoumarin, was found to be the most abundant compound in geopropolis extract. Nevertheless, its activity on the inflammatory process has never been studied. Thus, the present study investigated the anti-inflammatory activity of CNM on peritonitis and arthritis models of inflammation and further elucidated its possible molecular mechanism of action.
Figure 1.
Chemical structure of cinnamoyloxy-mammeisin.
RESULTS AND DISCUSSION
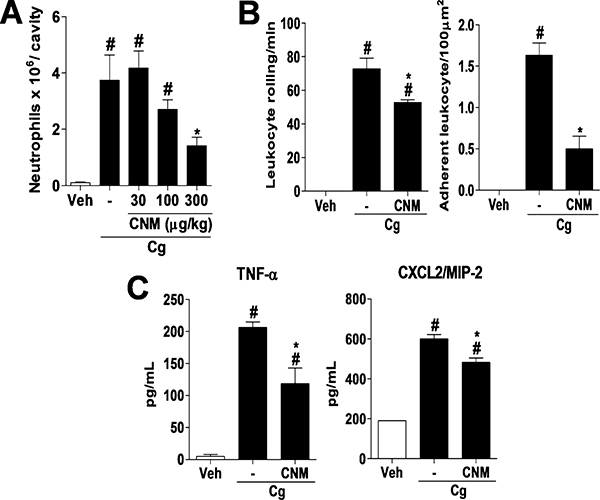
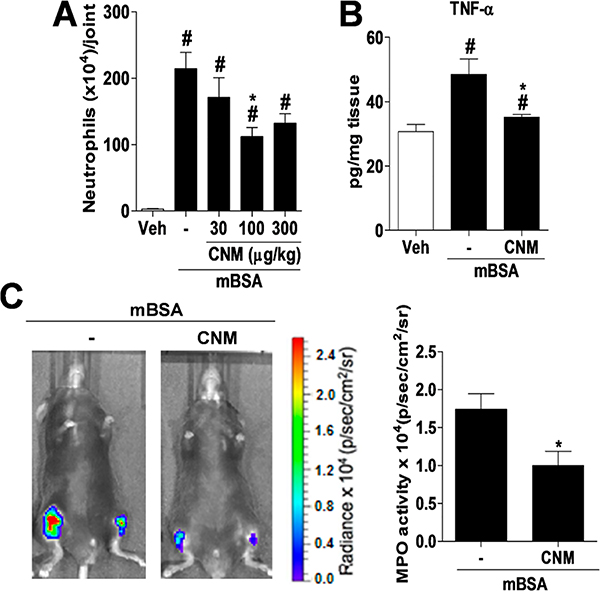
Studies have shown that the chemical composition of propolis may vary according to the collection site and type of vegetation in the area where bees collect plant resins.10,11 Due to this fact, many biologically active compounds have been identified and/or isolated from Brazilian propolis, such as caffeic acid phenethyl ester, artepelin C, apigenin, tt-farnesol, and pinocenbrin.12–15 Thus, several studies are carried out to isolate and identify new molecules from Brazilian propolis that could be applied as therapeutic agents.16 In the present study, we found that the subcutaneous (sc) administration of CNM (300 μg/kg) inhibited carrageenan-induced neutrophil migration into the peritoneal cavity of mice (Figure 2A). Further, the compound inhibited leukocyte rolling and adhesion to mesenteric venules (Figure 2B), which was associated with a decrease in the production of TNF-α and CXCL2/MIP-2 (Figure 2C). Likewise, in a model of antigen-induced arthritis, daily pretreatment with CNM (100 μg/kg, sc) (Figure 3A) for 3 days prior to challenge with methylated bovine serum albumin (mBSA) reduced neutrophil migration, release of TNF-α (Figure 3B), and the localized fluorescence representing the activity of myeloperoxidase (Figure 3C) in the joint cavity of mice.
Figure 2.
CNM reduces peritoneal inflammation induced by carrageenan. C57BL/6 mice were pretreated with CNM at doses of 30, 100, and 300 μg/kg sc or vehicle (veh), 30 min before ip administration of carrageenan (Cg) at 500 μg/cavity. (A) Migration of neutrophils into the peritoneal cavity 4 h after ip injection of Cg. (B) Leukocyte rolling and adhesion in the mesenteric microcirculation for 2 or 4 h, respectively, after ip injection of Cg in mice pretreated or not with CNM (300 μg/kg). (C) TNF-α and CXCL2/MIP-2 levels in the peritoneal cavity 1.5 or 3 h after ip injection of Cg, respectively, in mice pretreated or not with CNM (300 μg/kg). The data are expressed as the mean ± SEM, with n = 4 or 5 per group. Symbols indicate statistical difference (p < 0.05, Tukey’s post-test). #p < 0.05 compared to veh group; *p < 0.05 compared to Cg (−) group.
Figure 3.
CNM reduces joint inflammation induced by mBSA. C57BL/6 mice were pretreated with CNM at doses of 30, 100, and 300 μg/kg sc or vehicle (veh) 30 min prior to administration of mBSA (30 μg/joint). (A) Neutrophil migration in the joint cavity 6 h after intra-articular (ia) injection of mBSA. (B) TNF-α levels in the joint cavity after 1.5 h of ia injection of mBSA in mice pretreated or not with CNM (100 μg/kg). (C) Fluorescence intensity 6 h after ia injection of mBSA with the in vivo imaging system IVIS Spectrum representing the myeloperoxidase (MPO) in mice pretreated or not with CNM (100 μg/kg). The data are expressed as the mean ± SEM, with n = 5 or 6 per group. Symbols indicate statistical difference (p < 0.05, Tukey’s or Student’s t tests). #p < 0.05 compared to veh group; *p < 0.05 compared to mBSA (−) group.
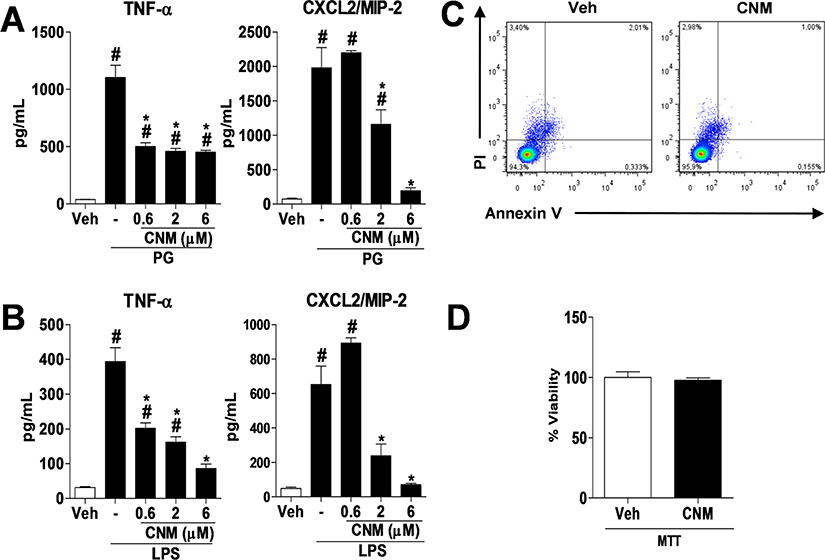
Macrophages are immune cells that play key roles in homeostasis, tissue repair, and immunity. Among the functions attributed to macrophages in the immune response are the ability to phagocytize and destroy infectious agents and release inflammatory mediators that contribute to activation of the immune system.17,18 Besides contributing to the host protective response, macrophages are also involved in tissue damage and loss of function in multiple pathologies in cases where the inflammatory response is not adequately controlled. Thus, the excessive release of reactive oxygen species and nitrogen by macrophages triggers tissue damage and often contributes to disease progression.17 Thus, after determining the activity of CNM on the in vivo release of TNF-α and CXCL2/MIP-2, we next investigated its mechanism of action in RAW 264.7 macrophages. As seen in the results, pretreatment with CNM at concentrations of 0.6, 2, or 6 μM reduced the release of TNF-α and CXCL2/MIP-2 in macrophages stimulated with peptidoglycan (PG) or LPS, as shown in Figure 4A and B, respectively. Furthermore, the highest CNM concentration used in in vitro (6 μM) experiments showed no cytotoxic effect in RAW macrophages as compared to the vehicle group (Figure 4C and D, annexin V and PI or MTT assays).
Figure 4.
CNM reduces the release of cytokines from macrophages. RAW 264.7 macrophages were pretreated with CNM at concentrations of 0.6, 2, and 6 μM or vehicle (veh), 30 min prior to stimulation with PG (5 μg/mL) or LPS (10 ng/mL). (A, B) TNF-α and CXCL2/MIP-2 levels in the supernatant of RAW 264.7 macrophages stimulated with PG or LPS for 4 h. (C) Cell viability using flow cytometry (annexin V and PI) of RAW 264.7 macrophages incubated with CNM 6 μM for 4 h. (D) Cell viability by MTT assay of RAW 264.7 macrophages incubated with 6 μM CNM for 4 h. The data are expressed as the mean ± SEM, with n = 3−5 per group. Symbols indicate statistical difference (p < 0.05, Tukey’s or Student’s t tests). #p < 0.05 compared to veh group; *p < 0.05 compared to PG (−) or LPS (−) group.
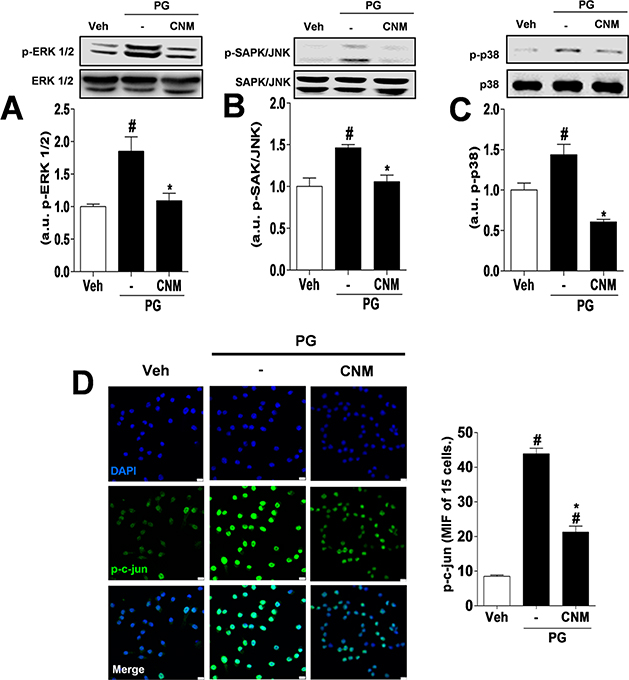
Given the above, the signaling pathways involved in the inflammatory activity of macrophages have been investigated as potential targets for the development of new drugs.19 MAPK plays an important role in the activation of multiple inflammatory genes. The presence of receptors expressed on the cell membrane of macrophages or in their cytoplasm when activated in response to pathogenic infections or tissue damage triggers a chain reaction in which MAPK is also activated.20 In the present study the activity of CNM on MAPK ERK 1/2, JNK, and p38 MAPK was investigated. ERK 1/2 is an important MAPK that participates in the regulation of cytokine production in macrophages. Its intracellular signaling may occur via post-transcriptional mechanisms via Tpl2/ERK or activation of the AP-1 transcription factor.20–22 Likewise, JNK and p38 MAPK participate in the regulation of the production of inflammatory mediators in macrophages by activation of AP-1. In the case of JNK, part of its modulatory mechanism is through induction of c-Jun phosphorylation, a subunit of AP-1.22,23 This study demonstrated that the modulatory effect of CNM (6 μM) on the release of TNF-α and CXCL2/MIP-2 in RAW 264.7 macrophages is associated with the reduced phosphorylation of ERK 1/2 (Figure 5A), JNK (Figure 5B), and p38 MAPK (Figure 5C). Furthermore, phosphorylated c-Jun was also reduced by the treatment with CNM (Figure 5D), therefore proving the modulation of AP-1 activity.
Figure 5.
CNM reduces MAPK and c-Jun phosphorylation in stimulated macrophages. RAW 264.7 macrophages were pretreated with CNM (6 μM) or vehicle (veh), 30 min prior to stimulation with PG (5 μg/mL). (A, B, C) MAPK phosphorylation (ERK 1/2, SAPK/JNK, and p38 MAPK) was evaluated in RAW 264.7 macrophages stimulated with PG for 15 min. (D) p-c-Jun was analyzed in RAW 264.7 macrophages after 30 min of stimulation with PG. The data are expressed as the mean ± SEM, with n = 3 or 4 per group. Symbols indicate statistical difference (p < 0.05, Tukey’s post-test). #p < 0.05 compared to veh group; *p < 0.05 compared to PG (−) group.
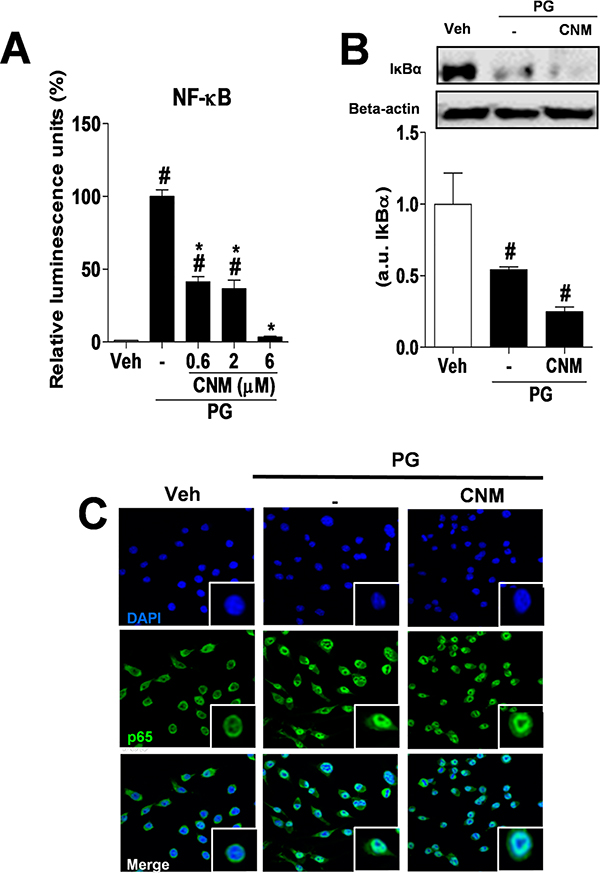
The transcription factor NF-κB has also been the target of novel anti-inflammatory drugs as part of the innate and adaptive immune response. In addition, NF-κB activity is elevated in various inflammatory diseases, including rheumatoid arthritis.24 Its activation via toll-like receptors involves the cascade reaction by Myd88/IRAK/TRAF6/TAK-1/IKK signaling, followed by IκBα degradation, NF-κB activation, and release of inflammatory cytokines and chemokines.24 Glucocorticoid drugs exert their inhibitory activity on NF-κB through the induction of IκBα.25 At 0.6, 2 or 6 μM, CNM reduced NF-κB activation compared to the PG group, characterized by reduced luminescence emission (Figure 6A). On the other hand, CNM at 6 μM did not alter IκB-α degradation (Figure 6B) or nuclear translocation of p65 (Figure 6C). Studies have shown that ERK 1/2 and p38 MAPK play a key role in NF-κB activation and are essential for NF-κB-DNA binding.26,27 The current study suggests that inhibition of ERK 1/2 and p38 MAPK by CNM may cause reduction of NF-κB activation.
Figure 6.
CNM reduces NF-κB activity but not IκBα degradation or p65 translocation to the nucleus. RAW 264.7 macrophages were pretreated with CNM at concentrations of 0.6, 2, and 6 μM or vehicle (veh), 30 min prior to stimulation with PG (5 μg/mL). (A) NF-κB activity was quantified using RAW 264.7 macrophages stably expressing the NF-κB pLUC gene, after stimulation with PG for 4 h. (B) IκBα levels were evaluated in RAW 264.7 macrophages stimulated with PG for 15 min, pretreated or not with 6 μM CNM. (C) RAW 264.7 macrophages pretreated or not with 6 μM CNM were fixed 30 min after PG stimulation (5 μg/mL), and then p65 (green) and the nucleus (blue) were stained. The data are expressed as the mean ± SEM, with n = 3−5 per group. Symbols indicate statistical difference (p < 0.05, Tukey’s post-test). #p < 0.05 compared to veh group; *p < 0.05 compared to PG (−) group.
The data obtained herein corroborate those of other studies investigating the activity of coumarins and derivatives on the MAPK and NF-κB pathway. A recent study showed that angelicin reduced TNF-α and IL-6 levels in vivo and in vitro.28 This compound reduced NF-κB (p65) signaling, as well as p38 MAPK and JNK phosphorylation.28 In another study, imperatorin reduced the inflammatory cytokines TNF-α, IL-6, and IL-1β produced by RAW 264.7 macrophages and the phosphorylated proteins p38 MAPK and JNK.29 However, unlike our findings, imperatorin reduced NF-κB translocation to the nucleus by blocking the phosphorylation and degradation of IκBα.29
It is worth noting that this is a pioneer study regarding the actions and pharmacological effects of CNM and that the stingless bees M. scutellaris that collect the geopropolis are native, primitive, and vulnerable to extinction in Brazil. Considering the important role of bees in the ecosystem and food production chain, the findings in this study add scientific value to bees and underscore the need for their preservation.
In summary, we conclude that CNM reduced neutrophil migration in the inflammatory process by inhibiting the release of TNF-α and CXCL2/MIP-2. Furthermore, we found that the effect of CNM in macrophages is associated with inhibition of ERK 1/2, JNK, and p38 MAPK phosphorylation, AP-1, and NF-κB. These results suggest that CNM could be a promising agent to combat inflammatory diseases.
EXPERIMENTAL SECTION
General Experimental Procedures.
Hexane, 2-propanol, dichloromethane, ethyl acetate, acetone, and methanol were purchased from Merck (Sao Paulo, SP, Brazil). RPMI, penicillin, L-glutamine, carrageenan, mBSA, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), dimethyl sulfoxide (DMSO), LPS, PG, and Triton X-100 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Anti-ERK 1/2, anti-p-ERK 1/2, anti-SAPK/JNK, anti-p-SAPK/JNK, anti-p38 MAPK, anti-p-p38 MAPK, anti-IκBα, and Beta-actin antibodies were purchased from Cell Signaling (Danvers, MA, USA). The Apo Screen annexin V-FITC kit was purchased from SouthernBiotech (Birmingham, AL, USA). Fetal bovine serum was from Gibco (Grand Island, NY, USA). LPS, PG, carrageenan, and mBSA dissolution was performed in sterilized saline solution, while CNM was dissolved in 1% DMSO in 1× phosphate-buffered saline (PBS).
Experimental Biological Material.
Samples of M. scutellaris geopropolis were collected between June and July 2012 in the city of Entre Rios (12°22′ S, 37°54′ W) in the state of Bahia, Brazil. Samples of M. scutellaris bees were deposited in the Bioscience Institute of Sao Paulo University, in the “Nogueira Neto” Entomological Collection under the voucher number CEPANN 42.863. The present research received authorization for access to genetic heritage components granted by the National Council for Technological and Scientific Development (CNPq), no. 010666/2014–1.
Extraction and Isolation.
The isolation and identification of the CNM were performed as previously described.8 The purity of CNM (≥95%) was determined by reverse-phase high-performance liquid chromatography (RP-HPLC).
Animals.
C57BL/6 SPF (specific pathogen-free) male mice weighing between 20 and 22 g were provided by CEMIB/UNICAMP (Multidisciplinary Center for Biological Research, SP, Brazil). The animals were kept at temperatures of 22−25 °C with food (standard pellet diet, Presence) and water ad libitum, with a controlled light/dark cycle and a humidity of 40−60%. The use of animals in this study was previously approved by the ethics committee for animal use (CEUA/UNICAMP, process no. 2793–1).
Cell Culture.
RAW 264.7 macrophages, or RAW 264.7 stably bearing the luciferase reporter gene controlled by an NF-κB-sensitive promoter (NF-κB-pLUC) were cultured in RPMI supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 2 mM L-glutamine at 37 °C in a 5% CO2/95% atmosphere.
Pharmacological Protocol in Vivo and in Vitro.
Mice were pretreated with CNM at 30, 100, or 300 μg/kg by sc injection. After 30 min they received a challenge consisting of intraperitoneal (ip) injection of carrageenan (500 μg/cavity) or intra-articular (ia) injection of mBSA (30 μg/joint). In the in vitro test, RAW 264.7 macrophages were pretreated with CNM at concentrations of 0.6, 2, or 6 μM 30 min before stimulation with PG (5 μg/mL) or LPS (10 ng/mL). In the in vivo and in vitro assays, the negative control group received the vehicle used to dissolve CNM, as previously described.
Migration of Neutrophils into the Peritoneal Cavity.
Neutrophil infiltration was determined in the peritoneum 4 h after ip injection of carrageenan (500 μg/cavity). Washing of the peritoneal cavity was performed with 3 mL of PBS containing EDTA. The fluid was collected (approximately 95% of the injected volume), and total leukocyte counting was carried out in a Neubauer chamber. A differential smear was prepared using a cytospin (Cytospin 4, Thermo Fisher Scientific, Waltham, MA, USA) and stained with Panotic Solution Kit (Laborclin, Pinhais, PR, Brazil). A total of 100 cells were counted using an optical microscope (100×). Results are presented as the number of neutrophils per cavity.30
Leukocyte Rolling and Adhesion in the Mesenteric Microcirculation.
The evaluation of leukocyte rolling in the mesenteric microcirculation was determined 2 h after ip stimulation with carrageenan (500 μg/cavity), while adhesion was verified 4 h after stimulation. For visualization of leukocytes, the animals were anesthetized with 90 mg/kg ketamine and 10 mg/kg xylazine, and their mesentery was exteriorized for observation of the microcirculation (intravital microscope DM 6000, Leica Microsystems, Wetzlar, Germany). The rolling of leukocytes was analyzed in three different fields per animal over a period of 10 min. For adhesion, the number of leukocytes adhering to the endothelium was evaluated over a venular area of 100 μm2 (10 μm in the tissue corresponds to 3.4 cm on the monitor screen).31,32
Migration of Neutrophils in the Joint Cavity.
Mice were immunized sc with an emulsion containing 100 μL of Complete Freud’s Adjuvant, 100 μL of 1× PBS, and 500 μg of mBSA. The reinforcement (7 and 14 days after the first injection) was administered with Incomplete Freud’s Adjuvant emulsion and mBSA. On day 21, the challenge was administered ia with mBSA (30 μg/joint). Six hours later the animals were euthanized, their articular cavity was washed with PBS containing EDTA, and total and differential leukocyte counts were performed as previously described.33
Myeloperoxidase Activity in the Joint Tissue.
The myeloperoxidase activity in vivo was assessed 6 h after stimulation with mBSA (30 μg/joint). Mice were anesthetized and received ip administration of the solution (100 mg/kg) used to determine the activity of myeloperoxidase by bioluminescence (XenoLight Rediject Inflammation Probe, PerkinElmer, Inc., Waltham, MA, USA). Image acquisition was performed using the IVIS Spectrum System device (PerkinElmer, Inc., Waltham, MA, USA). The results were expressed as bioluminescence intensity (radiance p/sec/cm2/sr).34
RAW 264.7 Culture and Collection of the Supernatant for Quantification of TNF-α and CXCL2/MIP-2.
RAW 264.7 macrophages were cultured in 96-well plates (2 × 105 cell/well) at 37 °C, 5% CO2 overnight. Four hours after stimulation with PG (5 μg/mL) or LPS (10 ng/mL) the supernatant was collected and stored at −70 °C.
Enzyme Linked Immunosorbent Assay (ELISA).
The levels of cytokines and chemokines in the peritoneal cavity of the animals were determined 1.5 h (TNF-α) and 3 h (CXCL2/MIP-2) after ip stimulation with carrageenan at 500 μg/cavity. The levels of TNF-α (1.5 h) were determined after challenge with mBSA at 30 μg/joint. In the supernatant of RAW 264.7 macrophages, the levels of TNF-α or CXCL2/MIP-2 were determined 4 h after stimulation with PG (5 μg/mL) or LPS (10 ng/mL). ELISA was performed using protocols supplied by the manufacturers (R&D Systems, Minneapolis, MN, USA). Results are expressed as pg/mL or pg/mg tissue.
Viability Assay by Flow Cytometry (Annexin V and Propidium Iodide (PI)).
RAW 264.7 macrophages were grown in six-well plates (2 × 106 cells/well) at 37 °C, 5% CO2 overnight. After 4 h of incubation with CNM at 6 μM, the cells were washed and resuspended in annexin buffer. Anti-annexin V-FITC antibody (1:50) was added and incubated at 4 °C for 20 min. Then the anti-PI antibody (1:100) was added, and the analysis carried out in a FACSVerse (BD Biosciences, San Diego, CA, USA). Data were analyzed with FlowJo software (Tree Star, Ashland, OR, USA).35 Three to four separate experiments were performed for analysis (annexin and PI), and approximately 10 000 gated events were collected in each analysis.
Cell Viability Assay by MTT.
RAW 264.7 macrophages were cultured in 96-well plates (2 × 105 cells/well) at 37 °C, 5% CO2 overnight. After 4 h of incubation with CNM at 6 μM, the supernatant was removed, RPMI with MTT (1 mg/mL) was added to the plate, and the plate was incubated for 2 h. Then the supernatant was removed again, and the cells were resuspended in 200 μL of absolute ethanol. The absorbance was measured at 540 nm using a microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Western Blotting.
RAW 264.7 macrophages were grown in six-well plates (2 × 106 cell/well) at 37 °C, 5% CO2 and incubated overnight. After 15 min of PG stimulation (previously standardized, as shown in Figures S1 and S2), the cells were lysed with RIPA-containing protease inhibitor (1:25) and phosphatase (1:50). The protein quantification was performed using Bradford reagent (Sigma-Aldrich). Mini-PROTEAN Tetra Cell (Bio-Rad, Hercules, CA, USA) and gel with 10% polyacrylamide were used for running. Then the gels were transferred to a nitrocellulose membrane, and anti-ERK 1/2 primary antibody (1:1000), anti-p-ERK 1/2 (1:1000), anti-SAPK/JNK (1:500), anti-p-SAPK/JNK (1:500), anti-p38 MAPK (1:500), anti-p-p38 MAPK (1:500), anti-IκBα (1:500), and Beta-actin (1:5000) were added. After overnight incubation at 4 °C, anti-mouse or anti-rabbit secondary antibodies (1:10 000) were added and incubated for 1 h. The membranes were washed, and the substrate Luminata (Millipore, Billerica, MA, USA) was added for chemiluminescence revelation using a ChemiDoc XRS apparatus (Bio-Rad) with the software Image Lab 3.0.
Immunofluorescence.
RAW 264.7 macrophages were cultivated on circular coverslips in 24-well plates (2 × 105 cell/well) at 37 °C, 5% CO2 and incubated overnight. After 30 min of PG stimulation, the cells were fixed with 4% paraformaldehyde for 20 min and washed with 1× PBS containing 10 mM glycine. The permeabilization was carried out with Triton X-100 for 30 min, and the blocking with 1% bovine serum albumin (BSA) for 1 h. Anti-p65 primary antibody 1:100 (Santa Cruz Biotechnology, Dallas, TX, USA) or anti-p-c-Jun 1:100 (Cell Signaling) was incubated overnight. The secondary antibody Alexa Fluor 488-conjugated anti-mouse IgG 1:400 (Molecular Probes, Eugene, OR, USA) was incubated for 1 h. Hoechst 33342 (Sigma-Aldrich) at 1 μg/mL was used as a nucleus marker. The coverslips were fixed on slides with Fluormount (SouthernBiotech), and the images were acquired in a TCS SP5 Leica microscope (Leica Microsystems, Wetzlar, Germany).
NF-κB Activation Assay.
RAW 264.7 macrophages stably transfected with the NF-κB-pLUC gene to express luciferase by the transcription factor NF-κB were cultured in 24-well plates (3 × 105 cells/well) and incubated overnight. The evaluation of NF-κB activation was performed 4 h after challenge with PG (5 μg/mL). The cells were lysed with 50 μL of Tris-NaCl-Tween buffer, and an aliquot of the suspension was added along with 25 μL of the Luciferase Assay Reagent containing luciferin (Promega Corporation, Madison, WI, USA). A microplate reader (FlexStation 3 Multi-Mode microplate reader, Molecular Devices) was used to quantify the luminescence.36,37
Statistical Analysis.
Statistical analysis was done using GraphPad Prism software version 5.03 (San Diego, CA, USA). Data were expressed as mean ± SEM. The statistical comparison between groups was performed using analysis of variance (ANOVA) followed by Tukey’s post-test. Comparison only between two groups was performed using Student’s t test. Significance was indicated when p < 0.05.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by the São Paulo Research Foundation (FAPESP) (2012/01365-0, 2012/22378-2, and 2013/08216-2/Center for Research in Inflammatory Disease-CRID). The authors are grateful to Mr. J. E. Borges de Souza for providing the geopropolis samples.
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jnatprod.6b00263.
Additional information (PDF)
REFERENCES
- (1).Newman DJ; Cragg GM; Snader KM J. Nat. Prod. 2003, 66, 1022–1037. [DOI] [PubMed] [Google Scholar]
- (2).Newman DJ; Cragg GM J. Nat. Prod. 2012, 75, 311–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Musa MA; Cooperwood JS; Khan MO Curr. Med. Chem. 2008, 15, 2664–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Ishita IJ; Nurul Islam M; Kim YS; Choi RJ; Sohn HS; Jung HA; Choi JS Arch. Pharmacal Res. 2016, 39, 115–126. [DOI] [PubMed] [Google Scholar]
- (5).Niu X; Wang Y; Li W; Mu Q; Li H; Yao H; et al. Int. Immunopharmacol. 2015, 24, 432–439. [DOI] [PubMed] [Google Scholar]
- (6).Xiong Y; Wang J; Yu H; Zhang X; Miao C; Ma S Immunopharmacol. Immunotoxicol. 2014, 36, 341–348. [DOI] [PubMed] [Google Scholar]
- (7).Franchin M; Da Cunha MG; Denny C; Napimoga MH; Cunha TM; Bueno-Silva B; Alencar SM; Ikegaki M; Rosalen PL Evid. Based. Complement. Alternat. Med. 2013, 2013, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Da Cunha MG; Rosalen PL; Franchin M; de Alencar SM; Ikegaki M; Ransom T; Beutler JA Planta Med. 2016, 82, 190–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Ribeiro-Junior JA; Franchin M; Cavallini ME; Denny C; Alencar SM; Ikegaki M; Rosalen PL Evid. Based. Complement. Alternat. Med. 2015, 2015, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Park YK; Alencar SM; Aguiar CL J. Agric. Food Chem. 2002, 50, 2502–2506. [DOI] [PubMed] [Google Scholar]
- (11).Silva BB; Rosalen PL; Cury JA; Ikegaki M; Souza VC; Esteves A; Alencar SM Evid. Based. Complement. Alternat. Med. 2008, 5, 313–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Natarajan K; Singh S; Burke TR Jr.; Grunberger D; Aggarwal BB Proc. Natl. Acad. Sci. U. S. A. 1996, 93, 9090–9095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Paulino N; Abreu SR; Uto Y; Koyama D; Nagasawa H; Hori H; Dirsch VM; Vollmar AM; Scremin A; Bretz WA Eur. J. Pharmacol. 2008, 587, 296–301. [DOI] [PubMed] [Google Scholar]
- (14).Koo H; Hayacibara MF; Schobel BD; Cury JA; Rosalen PL; Park YK; Vacca-Smith AM; Bowen WH J. Antimicrob. Chemother. 2003, 52, 782–789. [DOI] [PubMed] [Google Scholar]
- (15).Koo H; Rosalen PL; Cury JA; Park YK; Ikegaki M; Sattler A Caries Res. 1999, 33, 393–400. [DOI] [PubMed] [Google Scholar]
- (16).Sforcin JM; Bankova V J. Ethnopharmacol. 2011, 133, 253–260. [DOI] [PubMed] [Google Scholar]
- (17).Wynn TA; Chawla A; Pollard JW Nature 2013, 496, 445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Davies LC; Jenkins SJ; Allen JE; Taylor PR Nat. Immunol. 2013, 14, 986–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Kim EK; Choi EJ Biochim. Biophys. Acta, Mol. Basis Dis. 2010, 1802, 396–405. [Google Scholar]
- (20).Arthur JS; Ley SC Nat. Rev. Immunol. 2013, 13, 679–692. [DOI] [PubMed] [Google Scholar]
- (21).Dumitru CD; Ceci JD; Tsatsanis C; Kontoyiannis D; Stamatakis K; Lin JH; Patriotis C; Jenkins NA; Copeland NG; Kollias G; Tsichlis PN Cell 2000, 103, 1071–1083. [DOI] [PubMed] [Google Scholar]
- (22).Karin M J. Biol. Chem. 1995, 270, 16483–16486. [DOI] [PubMed] [Google Scholar]
- (23).Zarubin T; Han J Cell Res. 2005, 15, 11–18. [DOI] [PubMed] [Google Scholar]
- (24).Li Q; Verma IM Nat. Rev. Immunol. 2002, 2, 725–734. [DOI] [PubMed] [Google Scholar]
- (25).Auphan N; DiDonato JA; Rosette C; Helmberg A; Karin M Science 1995, 270, 286–290. [DOI] [PubMed] [Google Scholar]
- (26).Olson CM; Hedrick MN; Izadi H; Bates TC; Oliveira ER; Anguita J Infect. Immun. 2007, 75, 270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Reber L; Vermeulen L; Haegeman G; Frossard N PLoS One 2009, 4, e4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Liu F; Sun GQ; Gao HY; Li RS; Soromou LW; Chen N; Deng YH; Feng HH J. Surg. Res. 2013, 185, 300–309. [DOI] [PubMed] [Google Scholar]
- (29).Guo W; Sun J; Jiang L; Duan L; Huo M; Chen N; Zhong W; Wassy L; Yang Z; Feng H Inflammation 2012, 35, 1764–1772. [DOI] [PubMed] [Google Scholar]
- (30).Dal Secco D; Moreira AP; Freitas A; Silva JS; Rossi MA; Ferreira SH; Cunha FQ Nitric Oxide 2006, 15, 77–86. [DOI] [PubMed] [Google Scholar]
- (31).Baez S Circ. Res. 1969, 25, 315–329. [DOI] [PubMed] [Google Scholar]
- (32).Fortes ZB; Farsky SP; Oliveira MA; Garcia-Leme J Diabetes 1991, 40, 1267–1273. [DOI] [PubMed] [Google Scholar]
- (33).Vieira SM; Lemos HP; Grespan R; Napimoga MH; Dal-Secco D; Freitas A; Cunha TM; Verri WA Jr.; Souza-Junior DA; Jamur MC; Fernandes KS; Oliver C; Silva JS; Teixeira MM; Cunha FQ Br. J. Pharmacol. 2009, 158, 779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Veras FP; Peres RS; Saraiva AL; Pinto LG; Louzada-Junior P; Cunha TM; Paschoal JA; Cunha FQ; Alves-Filho JC Sci. Rep. 2015, 5, 15171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Vermes I; Haanen C; Steffens-Nakken H; Reutellingsperger C J. Immunol. Methods 1995, 184, 39–51. [DOI] [PubMed] [Google Scholar]
- (36).Cooper ZA; Ghosh A; Gupta A; Maity T; Benjamin IJ; Vogel SN; Hasday JD; Singh IS Am. J. Physiol. Cell. Physiol. 2010, 298, C171–C181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Silva RL; Lopes AH; França RO; Vieira SM; Silva EC; Amorim RC; Cunha FQ; Pohlit AM; Cunha TM J. Nat. Prod. 2015, 78, 241–249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.