Abstract
Background:
Mucosal healing (MH) is the key aim of the treat-to-target strategy for patients with Crohn’s disease (CD). The efficacy of infliximab (IFX) on MH in different ileocolonic segments is unclear. The aim of this study was to investigate endoscopic MH in different ileocolonic segments in patients with CD who received IFX treatment.
Methods:
A retrospective, single-center study was performed in patients with active ileocolonic CD between January 2012 and December 2018. All patients underwent IFX treatment for at least 30 weeks. The MH of five ileocolonic segments was assessed by the Simple Endoscopic Score for CD (SES-CD) at baseline, 14/22 weeks and 30/38 weeks. The SES-CD values were analyzed by a mixed-effects model after the correction for confounding factors.
Results:
A total of 101 eligible patients were included. The baseline endoscopic severity was similar across segments. At 30/38 weeks, the greatest changes in the SES-CD ulcer size and ulcerated surface subscores were −94.29% and −94.32% both in the transverse colon (p < 0.0001), and the smallest changes were −67.88% and −69.67% both in the terminal ileum (p < 0.0001) compared with baseline. Stenosis mainly presented in the right colon (12/29, 41.38%). The change in the SES-CD stenosis subscore was −6.25% in the right colon at 30/38 weeks compared with −71.88% at 14/22 weeks (p = 0.0030). At 30/38 weeks, the transverse colon achieved the highest rate of complete MH (CMH) at 81.2%, and the lowest CMH rate occurred in the terminal ileum at 45.6%. Moreover, the degree of improvement in the rectum was negatively correlated with disease progression (p = 0.011).
Conclusions:
Ileocolonic segments in CD presented different degrees of endoscopic MH during IFX treatment. The transverse colon showed the highest CMH rate, whereas the right colon with stenosis showed the poorest improvement. The differing propensities of ileocolonic segments may provide an individualized IFX treatment strategy.
Keywords: Crohn’s disease, ileocolonic, infliximab, mucosal healing, SES-CD
Introduction
Crohn’s disease (CD) is a chronic relapsing inflammatory bowel disease (IBD) that frequently results in progressive complications of strictures, abscesses or fistulae. Medications for treating CD include corticosteroids, azathioprine (AZA), methotrexate, and biologics.1 With the advent of the first biologic, the antitumor necrosis factor (anti-TNF) monoclonal antibody infliximab (IFX), the “treat-to-target” strategy has become a major treatment strategy applied for IBD in recent years.2 The treatment goal has shifted from controlling clinical symptoms to achieving mucosal healing (MH) with considerably better long-term outcomes.3 MH usually refers to the absence of ulceration and erosions assessed by endoscopy. Nevertheless, there is currently no validated definition for MH in IBD. The standard and objective definition of MH is made through endoscopic activity indexes, including the Simple Endoscopic Score for Crohn’s Disease (SES-CD) and the Crohn’s Disease Endoscopic Index of Severity (CDEIS), which are widely used in clinical trials and in clinical practice.4,5
The ileocolonic segments show inconsistent or asynchronous responses under endoscopy to the same medication on clinical observation. The VERSIFY study demonstrated higher rates of MH in the transverse colon and right colon than in the left colon and ileum with vedolizumab therapy.6 Adalimumab has also shown varying efficacy in terms of MH.7 As the first biologic, IFX has been applied to treat CD for more than 20 years worldwide; however, less is known about its efficacy in terms of MH in different ileocolonic segments (i.e. the rectum, sigmoid/left colon, transverse colon, right colon and terminal ileum). Identifying different responses to IFX in the intestinal segments of patients with ileocolonic CD may be helpful to guide clinical treatment. In this study, we investigated MH assessed by the SES-CD in the terminal ileum and four colonic segments in patients who received IFX induction and maintenance treatment.
Methods
Study design and patients
This retrospective, single-center study was conducted by collecting medical data from patients diagnosed with CD who received IFX treatment from January 2012 to December 2018 at the Department of Gastroenterology of the Shanghai Tenth People’s Hospital affiliated with Tongji University (Shanghai, China). The diagnosis of CD was confirmed by reviewing patient medical, endoscopic, radiological and pathological records in accordance with the diagnostic criteria published by the European Crohn’s and Colitis Organization.8 The study complied with the Helsinki Declaration, and the study protocol and exemption of informed consent were approved by the Institutional Ethics Committee of the Tenth People’s Hospital of Tongji University, Shanghai, China (SHSY-IEC-4.1/20-40/01).
Consecutive patients diagnosed with active CD were selected according to the following inclusion criteria: (a) 13−70 years of age; (b) ileocolonic location; (c) ileocolonoscopy performed at baseline (e.g. before IFX treatment), week 14 or 22 (recorded as 14/22 weeks, the second endoscopy examination), and week 30–38 (recorded as 30/38 weeks, the third endoscopy examination); (d) biologics-naïveté or previous use of IFX; (e) regular use of IFX for at least 30 weeks; (f) clinically active disease assessed as a Crohn’s Disease Activity Index (CDAI) score >150; and endoscopic activity defined as a SES-CD ⩾4 and the presence of definite ulcers (including aphthous ulcers). The endoscopic images were evaluated by two experienced physicians independently who were blinded to the clinical information based on the complete images (at least ⩾2 images) of each intestinal segment.
The exclusion criteria were as follows: extensive colonic resection; subtotal or total colectomy; existing ileostomy; induction therapy with corticosteroids or use of other biologics (e.g. adalimumab, vedolizumab or ustekinumab); no endoscopic follow-up; and primary nonresponse (PNR) or secondary loss of response (LOR) to IFX within 30 weeks. The current accepted clinical definition of a PNR is a lack of improvement in clinical signs and symptoms with induction therapy, which should not be assessed prior to 14 weeks following initial IFX treatment. A secondary LOR refers to patients who responded to induction therapy but subsequently lost the response during maintenance treatment within 30 weeks in this study.9,10
IFX was regularly administered intravenously at a dose of 5 mg/kg body weight at 0, 2, and 6 weeks and then every 8 weeks for at least 30 weeks. The clinical disease activity, biochemical tests, endoscopic activity and radiological images were assessed before and after IFX therapy. Figure 1 summarizes the flow of patients throughout the study.
Figure 1.
Patient flowchart of the study.
Five intestinal segments were assessed by ileocolonoscopy: the rectum, left colon (including the descending colon and sigmoid), transverse colon, right colon (including the ascending colon and cecum), and terminal ileum.
Study assessments
SES-CD
The endoscopic images were evaluated with the SES-CD, which is an easy-to-use and reproducible endoscopic scoring system for assessing endoscopic activity. The SES-CD is based on four endoscopic variables (presence and size of ulcers, proportion of surface covered by ulcers, proportion of affected surface, and presence and severity of stenosis) measured in the five ileocolonic segments (e.g. the terminal ileum, right colon, transverse colon, left colon and rectum).4
The total SES-CD ranges from 0 to 56 points, with higher values indicating more severe disease.4 The value for each variable ranges from 0 to 3, so that the score in each segment can range from 0 to 15: ulcer size: 0 = no ulcers, 1 = aphthous ulcers (>0.1–0.5 cm), 2 = large ulcers (>0.5–2 cm), and 3 = very large ulcers (>2 cm); ulcerated surface: 0 = none, 1 = <10%, 2 = 10–30%, and 3 = >30%; affected surface: 0 = none, 1 = <50%, 2 = 50–75%, and 3 = >75%; and stenosis, 0 = none, 1 = single and can be passed, 2 = multiple and can be passed, and 3 = cannot be passed. A total SES-CD ⩾ 16 was defined as severe, ⩾7–15 as moderate and ⩾3–6 as mild.11
Endoscopic efficacy and outcomes
The total SES-CD and subscore of each ileocolonic segment were assessed. The main outcome was the proportion of patients with endoscopic remission (defined as SES-CD = 0, i.e. complete mucosal healing, CMH) at 14/22 weeks and/or 30/38 weeks.12,13 Partial mucosal healing (PMH) was defined as a ⩾50% decrease in the SES-CD;6,14 no mucosal healing (NMH) was defined as an unchanged or worsened SES-CD.15
Clinical data collection
General demographic and clinical data were recorded in detail, including sex, age at disease onset, age at CD diagnosis, age at IFX treatment, CD duration, disease location, disease behavior, concomitant medications, and enteral nutrition. The long-term outcomes included clinical remission (CDAI <150), endoscopic remission, or disease progression 1 year after the last endoscopy examination. Disease progression refers to any new CD-related hospitalizations, surgeries, and complications (e.g. internal fistula/abscess, intestinal stricture or new perianal disease).
Statistical analysis
Data analyses were conducted by SAS version 9.2 software (Cary, NC). Continuous variables are described as the mean ± standard deviation (mean ± SD) in cases of a normal distribution and as the median and interquartile range (IQR) in cases of a skewed distribution. Categorical variables are described as proportions. According to the last observation carried forward (LOCF), when the subscore of one variable for a certain segment was 0 at baseline or at 14/22 weeks due to absence of endoscopic results, these data were carried forward to the corresponding segmental variable at 14/22 weeks or 30/38 weeks accordingly. The endoscopic efficacy across different segments at 14/22 weeks and 30/38 weeks was analyzed by Pearson’s chi-squared test with Yates’ continuity correction. Kendall’s tau-b correlation was used to analyze the relationship between the degree of improvement of colonic segments and patient prognosis. Using the stepwise covariate testing approach, the baseline SES-CD, CD duration, stenosis and concomitant medications were identified as confounders. The mixed-effects model was used to evaluate the endoscopic efficacy after adjusting for these four confounders. A two-sided p-value < 0.05 was considered statistically significant.
Results
Patient characteristics
Among the 101 patients with ileocolonic CD, as shown in Table 1, the age of the patients was 23 (18–31) years, and 75 patients were male (74.3%). The median age at diagnosis was 22 (17–31) years, and that at initial IFX treatment was 23 (18–31) years. In total, 99 patients were biologically naïve. The median time of endoscopic examination was at 32 (24–39) weeks. The CDAI was 209.32 ± 103.35, and the C-reactive protein (CRP) level was 33.66 ± 33.25 before IFX treatment. The total SES-CD of patients at baseline was 12.22 ± 7.03. The number of patients treated with IFX concomitant with and without AZA was 76 and 25, respectively, and no significant difference was found in the MH (defined as a ⩾50% decrease in the total SES-CD score) rate between the two groups at 30/38 weeks (89.1% versus 75%, p = 0.217). The total SES-CD, CDAI and CRP level showed a significant decline over time before and after IFX use at 30/38 weeks (p < 0.001, Supplemental Figure 1). There were 61 and 30 patients without an endoscopy examination at 14/22 weeks and 30/38 weeks, respectively. The SES-CD subscores for each segment are shown in Table 2.
Table 1.
Demographic and clinical characteristics of the patients at baseline.
| Characteristic | Baseline (n = 101) |
|---|---|
| Male, n (%) | 75 (74.3) |
| Age at onset, years (median, IQR) | 21 (16–29) |
| Age at diagnosis, years (median, IQR) | 22 (17–31) |
| Age at IFX treatment, years (median, IQR) | 23 (18–31) |
| CD duration, years (median, IQR) | 2 (0.4–4) |
| CD location, n (%) | |
| Terminal ileum | 85 (84.2) |
| Right colon | 81 (80.2) |
| Transverse colon | 67 (66.3) |
| Left colon | 75 (74.3) |
| Rectum | 57 (56.4) |
| Upper GI | 15 (14.9) |
| Disease behavior, n (%) | |
| B1 | 67 (66.3) |
| B2 | 29 (28.7) |
| B3 | 5 (5.0) |
| Perianal disease | 49 (48.5) |
| Extraintestinal manifestations, n (%) | 27 (26.7) |
| Anti-TNF naïve, n (%) | 99 (98) |
| Concomitant Medication, n (%) | |
| AZA | 74 (73.3) |
| MTX | 4 (4.0) |
| GCS + AZA | 2 (2.0) |
| Concomitant enteral nutrition, n (%) | 68 (67.3) |
| CDAI score (mean ± SD) | 209.32 ± 103.35 |
| CRP (mean ± SD) | 33.66 ± 33.25 |
| Total SES-CD (mean ± SD) | 12.22 ± 7.03 |
AZA, azathioprine; B1, nonstricturing, nonpenetrating; B2, stricturing; B3, penetrating; CD, Crohn’s disease; CDAI, Crohn’s Disease Activity Index; CRP, C-reactive protein; GCS, glucocorticosteroid; GI, gastrointestinal tract; IFX, infliximab; IQR, interquartile range 25–75; MTX, methotrexate; SD, standard deviation; SES-CD, Simple Endoscopic Score for Crohn’s disease.
Table 2.
SES-CD subscores by ileocolonic segment at baseline, 14/22 weeks and 30/38 weeks.
| SES-CD (mean ± SD) | Baseline | 14/22 weeks | 30/38 weeks |
|---|---|---|---|
| Terminal ileum, n | 86 | 33 | 61 |
| Total score, mean ± D | 4.00 ± 1.71 | 1.36 ± 1.82 | 1.66 ± 1.69 |
| Ulcer size | 1.65 ± 0.78 | 0.54 ± 0.84 | 0.53 ± 0.77 |
| Ulcerated surface | 1.22 ± 0.68 | 0.35 ± 0.54 | 0.37 ± 0.49 |
| Affected surface | 1.02 ± 0.55 | 0.28 ± 0.46 | 0.36 ± 0.48 |
| Stenosis | 0.12 ± 0.52 | 0.01 ± 0.11 | 0.02 ± 0.21 |
| Right colon, n | 94 | 38 | 71 |
| Total score, mean ± SD | 3.97 ± 2.40 | 1.66 ± 1.83 | 1.89 ± 2.25 |
| Ulcer size | 1.45 ± 0.82 | 0.41 ± 0.72 | 0.41 ± 0.74 |
| Ulcerated surface | 1.12 ± 0.80 | 0.28 ± 0.46 | 0.30 ± 0.53 |
| Affected surface | 1.10 ± 0.82 | 0.49 ± 0.51 | 0.48 ± 0.67 |
| Stenosis | 0.32 ± 0.85 | 0.09 ± 0.48 | 0.30 ± 0.84 |
| Transverse colon, n | 99 | 38 | 71 |
| Total score, mean ± SD | 2.91 ± 2.54 | 0.68 ± 1.60 | 0.44 ± 1.04 |
| Ulcer size | 1.05 ± 0.93 | 0.13 ± 0.46 | 0.06 ± 0.28 |
| Ulcerated surface | 0.88 ± 0.86 | 0.10 ± 0.35 | 0.05 ± 0.22 |
| Affected surface | 0.83 ± 0.86 | 0.19 ± 0.54 | 0.20 ± 0.54 |
| Stenosis | 0.16 ± 0.63 | 0.00 ± 0.00 | 0.01 ± 0.10 |
| Left colon, n | 101 | 40 | 71 |
| Total score, mean ± SD | 3.08 ± 2.46 | 1.55 ± 2.60 | 0.77 ± 1.32 |
| Ulcer size | 1.11 ± 0.89 | 0.33 ± 0.73 | 0.18 ± 0.48 |
| Ulcerated surface | 0.96 ± 0.82 | 0.26 ± 0.58 | 0.15 ± 0.39 |
| Affected surface | 0.89 ± 0.84 | 0.39 ± 0.73 | 0.26 ± 0.51 |
| Stenosis | 0.17 ± 0.65 | 0.06 ± 0.43 | 0.01 ± 0.10 |
| Rectum, n | 101 | 40 | 71 |
| Total score, mean ± SD | 1.95 ± 2.26 | 1.10 ± 2.22 | 0.55 ± 1.16 |
| Ulcer size | 0.70 ± 0.88 | 0.16 ± 0.49 | 0.12 ± 0.38 |
| Ulcerated surface | 0.54 ± 0.74 | 0.17 ± 0.53 | 0.11 ± 0.34 |
| Affected surface | 0.60 ± 0.74 | 0.21 ± 0.61 | 0.18 ± 0.46 |
| Stenosis | 0.09 ± 0.47 | 0.03 ± 0.30 | 0.01 ± 0.10 |
SES-CD, Simple Endoscopic Score for Crohn’s disease.
Endoscopic outcomes
SES-CD ulcer size subscore by ileocolonic segment
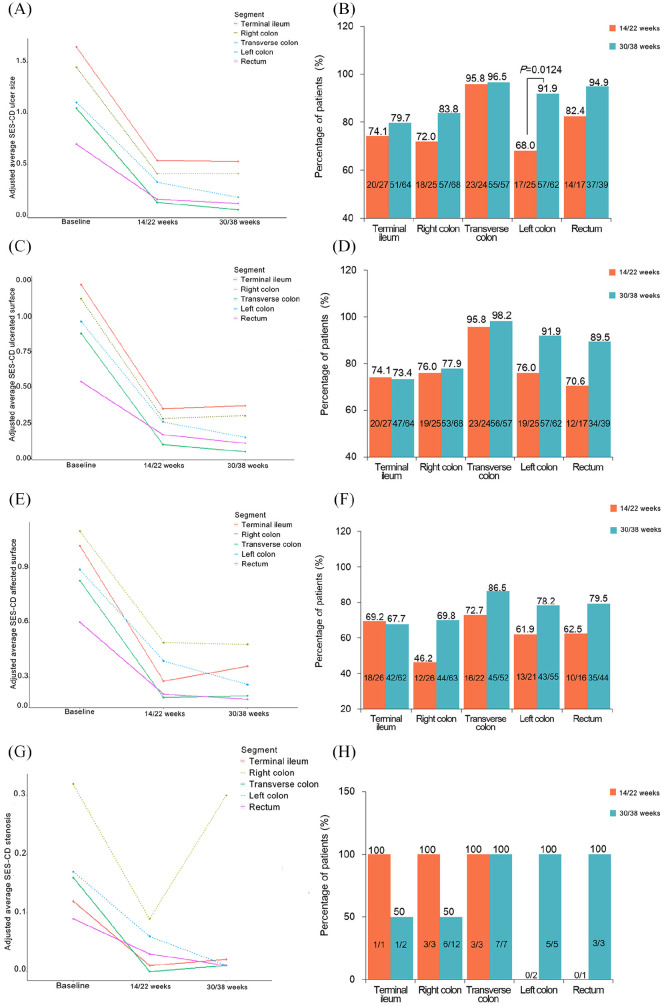
From baseline to 14/22 weeks, the greatest improvement in the SES-CD ulcer size subscore was in the transverse colon (–87.62%, p < 0.0001), followed by the rectum (–77.14%, p < 0.0001), the right colon (–71.72%, p < 0.0001), and the left colon (–70.27%, p < 0.0001). The least improvement was seen in the terminal ileum (–67.27%, p < 0.0001) (Figure 2A).
Figure 2.
Changes and improvements from baseline to weeks 14/22 and weeks 30/38 in SES-CD subscores by ileocolonic segment in patients with baseline subscore ⩾ 1. (A, B) Ulcer size; (C, D) ulcerated surface; (E, F) affected surface; and (G, H) stenosis.
At 30/38 weeks, the SES-CD ulcer size subscore showed the most improvement in the transverse colon (–94.29%, p < 0.0001), the left colon (–83.78%, p < 0.0001), the rectum (–82.86%, p < 0.0001), the right colon (–71.72%, p < 0.0001), and the terminal ileum (–67.88%, p < 0.0001) compared with that at baseline (Figure 2A). All four colonic segments showed significant changes in the SES-CD ulcer size subscore compared with that at 14/22 weeks (p < 0.05, Figure 2A). A significant improvement in the size of ulcers in patients was only seen in the left colon (68–91.9% of patients, p = 0.0124, Figure 2B) from 14/22 weeks to 30/38 weeks.
SES-CD ulcerated surface subscore by ileocolonic segment
The greatest shift from baseline to 14/22 weeks in the SES-CD ulcerated surface subscore was also in the transverse colon (–88.64%, p < 0.0001). The terminal ileum (–71.31%, p < 0.0001) and the rectum (–68.52%, p = 0.0004) showed the least change (Figure 2C).
At 30/38 weeks, the greatest improvement in this subsocre was also seen in the transverse colon (–94.32%, p < 0.0001). The terminal ileum showed the worst effect after IFX treatment (–69.67%, p < 0.0001, Figure 2C).
SES-CD affected surface subscore by ileocolonic segment
The improvement in the SES-CD affected surface subscore in each segment after IFX treatment showed a similar trend as that of the ulcer size and ulcerated surface subscores (p < 0.05, Figure 2E).
At 30/38 weeks, the four colonic segments all showed significant improvement compared with 14/22 weeks (p < 0.05). No significant changes were observed in the terminal ileum (p > 0.05, Figure 2E).
SES-CD stenosis subscore by ileocolonic segment
The same pattern was also observed in the SES-CD stenosis subscores from baseline to 14/22 weeks. The transverse colon showed the greatest improvement (–100.00%, p = 0.0198, Figure 2G). A significant decline in the SES-CD subscores from baseline to 30/38 weeks was observed in the left colon (–94.12%, p = 0.0063) and transverse colon (–93.75%, p = 0.0006, Figure 2G). The SES-CD stenosis subscore of the right colon increased significantly due to recurrence (0.09 ± 0.48 versus 0.30 ± 0.84, p = 0.0030) at 30/38 weeks.
From 14/22 weeks to 30/38 weeks, no significant improvements in the SES-CD ulcerated surface, affected surface, and stenosis subscores were found (p > 0.05, Figure 2D, F, H).
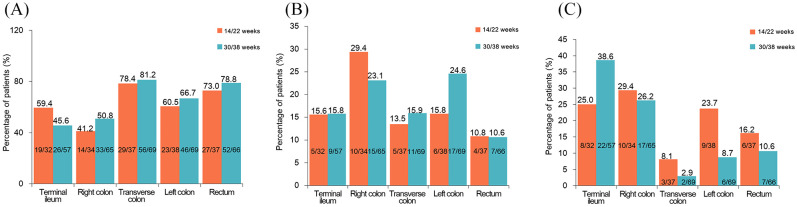
Endoscopic mucosal healing by ileocolonic segment
After IFX treatment, at 14/22 weeks, 78.4% of the transverse colon and 41.2% of the right colon segments achieved CMH (p = 0.0031). The patients showed an increase in the CMH rate in the four colonic segments from 14/22 weeks to 30/38 weeks. The transverse colon showed the highest CMH rate (81.2%) at 30/38 weeks, which was significantly higher than that in the terminal ileum (45.6%, p < 0.0001, Figure 3A). A marked difference in PMH across segments was only observed at 30/38 weeks (p < 0.0001, Figure 3B). The transverse colon showed a lower NMH rate than the right colon at 14/22 weeks (29.4% versus 8.1%, p = 0.0443, Figure 3C).
Figure 3.
Mucosal healing by segments at weeks 14/22 and weeks 30/38. (A) Complete mucosal healing; (B) partial mucosal healing; and (C) no mucosal healing.
Relationship between colonic mucosal healing and prognosis
Through Kendall’s tau-b correlation, among the four colonic segments, only the degree of improvement in the rectum was negatively correlated with disease progression (p = 0.011, Supplemental Table 1). No significant correlation was found between the degree of endoscopic improvement in the other colon segments and clinical remission, endoscopic remission, or disease progression (Supplemental Table 1).
Discussion
In patients with CD who receive IFX treatment, the pattern of MH in ileocolonic segments varies in clinical practice. Recently, small bowel ulcerations treated with anti-TNF in CD patients have been proven to be more difficult to heal than colon ulcerations.16 However, no research has been done to evaluate the improvements with IFX treatment in different colonic segments. Therefore, identifying the pattern of MH following IFX treatment can provide useful predictions of the treatment response in different involved segments.
The patients in our study mostly had endoscopically moderate-to-severe disease activity, and the severity of disease before IFX treatment was similar across segments. We examined the four SES-CD subscores and MH in each ileocolonic segment of patients with active CD who were treated with IFX. The results indicated that each subscore (ulcer size, ulcerated surface, affected surface and stenosis) of the SES-CD improved following IFX induction and maintenance treatment. However, the MH in different ileocolonic regions was not uniform. The greatest improvements occurred in the transverse colon in terms of the SES-CD ulcer size, ulcerated surface, and affected surface subscores at 14/22 weeks and 30/38 weeks. Stenosis was only improved at 14/22 weeks. Furthermore, the CMH rate in the transverse colon (81.2%) was significantly higher than that in the terminal ileum (45.6%). The MH rates in the left colon and the rectum were better than those in the right colon and terminal ileum. Only the degree of improvement in the rectum was negatively correlated with disease progression. These results suggest that MH in different ileocolonic regions was not uniform after IFX treatment. Some CD patients needed long-term IFX treatment to achieve MH in some intestinal segments (e.g. the right colon and terminal ileum).
Although the patient population differed, a study on vedolizumab treatment in patients with moderate-to-severe active CD for 52 weeks reported results similar to our findings in all active CD patients. In the vedolizumab study, the proportion of CMH (defined as the absence of any ulcers, including aphthous ulcers) was much higher in the transverse colon, right colon and rectum than in the left colon; however, it was the lowest in the ileum.6 The MH of the different segments in patients with moderate-to-severe ileocolonic CD treated with adalimumab was assessed using the CDEIS, SES-CD subscores, and histological findings. Favorable changes in the ulcer size and ulcerated surface according to the SES-CD were more pronounced in the rectum, sigmoid/left colon, and transverse colon than in the right colon and ileum at weeks 52.7 These inconsistent results may be due to the difference in the disease severity (moderate-to-severe versus all active disease) and the difference in the primary endpoint (52 weeks versus 38 weeks) between the two studies.
Compared with the highest CMH rate in the transverse colon (81.2%), the terminal ileum showed the poorest response (45.6%) to IFX at 30/38 weeks in our study. Takenaka et al. showed that the rate of endoscopic MH in patients with CD who were treated with IFX for 1 year was 79% in the colon in comparison with 36% in the small bowel.16 These results may indicate that anti-TNF agents have different efficacies in terms of MH in different disease locations in patients with CD, and that colonic segments may heal more easily than ileal segments. The exact mechanism of such a discrepancy is not clear. Recently, single-cell technologies were applied to study cytokine blockade in ileal CD to address whether cellular heterogeneity contributes to treatment resistance. They found a unique cellular expression pattern in the inflamed tissues of a subset of patients, the presence of which at diagnosis correlated with failure to achieve durable corticosteroid-free remission upon anti-TNF therapy.17 These results suggest that patients with CD might have different unique cellular expression patterns among inflamed segments, correlating to the response to anti-TNF therapy or treatment with other biologics. In addition, the mucosal concentration of anti-TNF agents was associated with MH.18 Investigating the cellular expression pattern and measuring the mucosal concentration of biologics may be helpful to explain the different responses to therapy in each segment.
This was the first study to analyze the endoscopic efficacy of IFX treatment in different ileocolonic segments in patients with active CD. The SES-CD of ulcer size, ulcerated surface, affected surface subscores and endoscopic MH were analyzed across different segments separately. A mixed-effects model was applied to analyze the data more accurately and objectively. The differing propensities and MH rates among the ileocolonic segments may enable the individualized treatment of patients with active ileocolonic CD receiving IFX therapy.
There were some limitations to this study. This was a single-center and retrospective study. We included patients who were PNR or secondary LOR to IFX, thus easily producing information bias and selection bias. Only one endoscopic subscore of the SES-CD system was used to evaluate MH. Not all cases of stenosis were examined by CD/MRI at the same time. The severity of the lesions was not assessed in our study. Through 1 year after the last endoscopy, some patients were lost for follow-up. Thus, the correlation between the degree of improvement in each colonic segment and prognosis was poor. Thus, further multicenter, prospective studies are needed in the future to investigate all of the above in detail.
In conclusion, our results indicate that different bowel segments in patients with ileocolonic CD had different MH rates under endoscopy in response to IFX treatment. In general, better efficacy was observed in the transverse colon and left colon. However, the poorest therapeutic efficacy was observed in the terminal ileum and the right colon with stenosis. These results provide potential guidance for the personalized use of IFX treatment strategies for better MH and an improved long-term prognosis in CD.
Supplemental Material
Supplemental material, sj-pdf-1-tag-10.1177_1756284820976923 for Efficacy of infliximab treatment on the mucosal healing of different intestinal segments in patients with ileocolonic Crohn’s disease by Yaling Wu, Lei Zhang, Jingli Cao, Haichao Wang, Chen Ye, Deji Zhuoma, Pengyu Yang and Xiaolei Wang in Therapeutic Advances in Gastroenterology
Acknowledgments
The authors would like to thank the Department staff for their generous assistance.
Footnotes
Disclaimers: The authors statements that the views expressed in the submitted article are our own and not any official position of the institution or funder.
Authorship: XL-W and YL-W contributed to the design of the study; YL-W, JL-C, HC-W, C-Y, DJ-ZM and PY-Y performed the acquisition of data, the statistical analysis and interpretation of data; YL-W and L-Z drafted the manuscript; and XL-W critically revised the manuscript for important intellectual content. All authors reviewed and approved the final version of the manuscript.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this work was supported by National Natural Science Foundation of China (grant number 81670476).
Declaration of conflicting interests: The authors declare that there is no conflict of interest.
ORCID iD: Xiaolei Wang  https://orcid.org/0000-0001-8922-1368
https://orcid.org/0000-0001-8922-1368
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Yaling Wu, Department of Gastroenterology, Shanghai Tenth People’s Hospital, Tongji University, Shanghai, China.
Lei Zhang, Department of Gastroenterology, Shanghai Tenth People’s Hospital, Tongji University, Shanghai, China.
Jingli Cao, Department of Gastroenterology, Shanghai First People’s Hospital Branch Hospital, Shanghai, China.
Haichao Wang, Department of Gastroenterology, Shanghai Tenth People’s Hospital, Tongji University, Shanghai, China.
Chen Ye, Department of Gastroenterology, Shanghai Tenth People’s Hospital, Soochow University, Jiangsu, China.
Deji Zhuoma, Department of Gastroenterology, Shanghai Tenth People’s Hospital, Tongji University, Shanghai, China.
Pengyu Yang, Department of Gastroenterology, Shanghai Tenth People’s Hospital, Tongji University, Shanghai, China.
Xiaolei Wang, Department of Gastroenterology, Shanghai Tenth People’s Hospital, Tongji University, Shanghai, 200072, China.
References
- 1. Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology Consensus Guidelines on the management of inflammatory bowel disease in adults. Gut 2019; 68: 1–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol 2015; 110: 1324–1338. [DOI] [PubMed] [Google Scholar]
- 3. Rutgeerts P, Feagan BG, Lichtenstein GR, et al. Comparison of scheduled and episodic treatment strategies of infliximab in Crohn’s disease. Gastroenterology 2004; 126: 402–413. [DOI] [PubMed] [Google Scholar]
- 4. Daperno M, D’Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc 2004; 60: 505–512. [DOI] [PubMed] [Google Scholar]
- 5. Mary JY, Modigliani R. Development and validation of an endoscopic index of the severity for Crohn’s disease: a prospective multicentre study. Groupe d’Etudes Thérapeutiques des Affections Inflammatoires du Tube Digestif (GETAID). Gut 1989; 30: 983–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Danese S, Sandborn WJ, Colombel JF, et al. Endoscopic, radiologic, and histologic healing with vedolizumab in patients with active Crohn’s disease. Gastroenterology 2019; 157: 1007–1018. [DOI] [PubMed] [Google Scholar]
- 7. Reinisch W, Colombel JF, D’Haens G, et al. Characterisation of mucosal healing with adalimumab treatment in patients with moderately to severely active Crohn’s disease: results from the extend trial. J Crohns Colitis 2017; 11: 425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gomollón F, Dignass A, Annese V, et al. III European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohns Colitis 2017; 11: 3–25. [DOI] [PubMed] [Google Scholar]
- 9. Sprakes MB, Ford AC, Warren L, et al. Efficacy, tolerability, and predictors of response to infliximab therapy for Crohn’s disease: a large single centre experience. J Crohns Colitis 2012; 6: 143–153. [DOI] [PubMed] [Google Scholar]
- 10. Ben-Hori S, Kopylov U, Chowers Y. Optimizing anti-TNF treatments in inflammatory bowel disease. Autoimmun Rev 2014; 13: 24–30. [DOI] [PubMed] [Google Scholar]
- 11. Narula N, Wong ECL, Aruljothy A, et al. Ileal and rectal ulcer size affects the ability to achieve endoscopic remission: a post hoc analysis of the SONIC trial. Am J Gastroenterol 2020; 115: 1236–1245. [DOI] [PubMed] [Google Scholar]
- 12. Baert F, Moortgat L, Van Assche G, et al. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn’s disease. Gastroenterology 2010; 138: 463–468. [DOI] [PubMed] [Google Scholar]
- 13. Kang B, Choi SY, Choi YO, et al. Subtherapeutic infliximab trough levels and complete mucosal healing are associated with sustained clinical remission after infliximab cessation in paediatric-onset Crohn’s disease patients treated with combined immunosuppressive therapy. J Crohns Colitis 2018; 12: 644–652. [DOI] [PubMed] [Google Scholar]
- 14. Nuti F, Civitelli F, Bloise S, et al. Prospective evaluation of the achievement of mucosal healing with anti-TNF-α therapy in a paediatric Crohn’s disease cohort. J Crohns Colitis 2016; 10: 5–12. [DOI] [PubMed] [Google Scholar]
- 15. Schnitzler F, Fidder H, Ferrante M, et al. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn’s disease. Inflamm Bowel Dis 2009; 15: 1295–1301. [DOI] [PubMed] [Google Scholar]
- 16. Takenaka K, Fujii T, Suzuki K, et al. Small bowel healing detected by endoscopy in patients with Crohn’s disease after treatment with antibodies against tumor necrosis factor. Clin Gastroenterol Hepatol 2020; 18: 1545–1552. [DOI] [PubMed] [Google Scholar]
- 17. Martin JC, Chang C, Boschetti G, et al. Single-cell analysis of Crohn’s disease lesions identifies a pathogenic cellular module associated with resistance to anti-TNF therapy. Cell 2019; 178: 1493–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Papamichael K, Van Stappen T, Vande Casteele N, et al. Infliximab concentration thresholds during induction therapy are associated with short-term mucosal healing in patients with ulcerative colitis. Clin Gastroenterol Hepatol 2016; 14: 543–549. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tag-10.1177_1756284820976923 for Efficacy of infliximab treatment on the mucosal healing of different intestinal segments in patients with ileocolonic Crohn’s disease by Yaling Wu, Lei Zhang, Jingli Cao, Haichao Wang, Chen Ye, Deji Zhuoma, Pengyu Yang and Xiaolei Wang in Therapeutic Advances in Gastroenterology