Abstract
Paraoxonase-1 (PON-1) activity is a new inflammatory and oxidative marker. Technical effects and biological factors could affect the accuracy of PON-1 activity measurement. We investigated the effects of storage at different temperatures, repeated freeze–thaw cycles, interferences from hemolytic, lipemic, and icteric samples, and seasonal effects on PON-1 activity in horses. We evaluated 2 substrates with an automated spectrophotometer. Ten equine serum samples were stored under different conditions. Although storage at room (21°C) or refrigeration (4°C) temperature induced a statistically significant decrease (p < 0.05) in PON-1 activity, this is not diagnostically relevant. PON-1 activity in frozen samples (−20°C) was stable for short-term storage; diagnostically significant (p < 0.01) fluctuations were observed after 1 mo. Four repeated freeze–thaw cycles were assessed, and all cycles affected PON-1 activity (p < 0.01); however, this was diagnostically significant only after the 4th cycle. Hemolysis induced an overestimation of PON-1 activity; lipemia and hyperbilirubinemia did not change PON-1 activity. Thirty-four horses were sampled monthly for 1 y, and PON-1 activity was higher in autumn (p < 0.05) and winter (p < 0.05) than in spring and summer.
Keywords: equine, interference, paraoxonase-1, repeated freeze–thaw cycles, seasonal variability, storage
Introduction
Preanalytical errors are defined by ISO 15189:2012 as “The processes that start, in chronological order, from the clinician’s request, and include the examination request, preparation and identification of the patient, collection of the primary sample(s), and transportation to and within the laboratory, and end when the analytical examination begins.”19 Preanalytical factors can be classified into 2 general categories: 1) technical effects as a result of the sampling technique and specimen management before analysis, and 2) biologic factors inherent with the animal sampled. The first category includes effects such as the choice of anticoagulant, the sampling technique, and the stability of the specimen during storage or shipping to a laboratory. The second category includes effects such as circadian rhythms, seasonal changes, fasting, stress, or exercise.3 Given that preanalytical errors are reported to be responsible for the majority of laboratory errors in veterinary medicine (range: 55–77%),18,38 published guidelines15 provide recommendations for control of preanalytical factors related to clinical chemistry in veterinary laboratories.
A new method to quantify paraoxonase-1 (PON-1) activity in horses has been validated.32 PON-1 is a negative acute-phase protein with both anti-inflammatory and anti-oxidative properties.1 It is a high-density lipoprotein (HDL)-associated enzyme synthesized by the liver that protects low-density lipoproteins (LDL) from peroxidation,37 and possesses anti-inflammatory properties blocking monocyte chemotaxis.22 PON-1 activity is closely associated with apolipoprotein A1 in HDL, and exerts its protective function through an arylesterase activity that hydrolyses oxidized lipids.36 During an acute-phase response, HDL molecules lose apolipoprotein A1, esterified cholesterol, and most of the HDL-associated enzymes, including PON-1, which is replaced mainly by serum amyloid A and ceruloplasmin. Changes in either composition or structure of the already synthesized PON-1 and decreased hepatic gene expression lead to decreased PON-1 serum activity during the acute-phase response.9,26 In humans, serum PON-1 activity is strongly determined by the enzyme genotype. Several polymorphisms in the promotor and the coding regions of the PON-1 gene have been described and are significantly associated with changes in enzyme activity.12,23 The physiologic substrate or substrates for PON-1 have not been identified. The available methods to measure serum PON-1 activity use a variety of synthetic substrates such as paraoxon, 4-nitrophenyl acetate (4-NPA), and coumarins, which take advantage of the catalytic versatility of this enzyme. From a practical point of view, PON-1 gene polymorphism could affect the rate of hydrolysis of PON-1 substrates differently. Therefore, it has been suggested that more than one activity should be used.4
Diagnostic utility of PON-1 activity in horses has been evaluated in few studies. PON-1 activity was lower in a study in horses infected with Theileria equi, supporting its role in oxidative stress.27 Another study investigating subclinical leptospirosis in horses explored PON-1 activity as an inflammatory protein, but no differences were found among control group, carriers, or subclinically infected group.34 It is unknown if preanalytical errors could contribute to conflicting study results.
Information about preanalytical variability of PON-1 activity in the horse has not been published, to our knowledge. Our aims were to: 1) perform the analytical validation of p-nitrophenyl acetate; and investigate 2) the correlation between PON-1 activity measured with 2 different substrates; 3) the effects of storage at different temperatures; 4) the effects of repeated freeze–thaw cycles; 5) the effects of interferences from hemolytic, lipemic, and icteric samples; and 6) the effects of seasonality on PON-1 activity.
Materials and methods
Study population and sample collection
Ten serum samples were collected as part of a teaching exercise from clinically normal Thoroughbred and Standardbred teaching herd horses at Murdoch University (Murdoch, Western Australia, Australia). Ethics approval was obtained from the Murdoch University Animal Ethics Committee (approval T3019/18). Blood was collected from the left or right jugular vein through a 20-ga, 3.8-cm Vacutainer needle into evacuated 4-mL plastic plain tubes with serum clot activator (Greiner Bio-One). After allowing 10 min for clot formation, samples were centrifuged in a refrigerated centrifuge at 4,000 × g for 5 min at 4°C. Serum obtained was aliquoted into 1.5-mL plastic vials (Eppendorf), and used for storage, interference, and the repeated freeze–thaw cycles studies described below.
Twenty-six additional samples were used to assess the correlation between 2 substrates: paraoxon-ethyl and 4-NPA. These additional samples included samples from 9 other healthy horses of the teaching herd at Murdoch University plus 17 samples from horses with different pathologic conditions, collected as part of another study (approval R2887/16) to assess the correlation over a broader range of PON-1 activity. Samples used to identify seasonal differences came from a study investigating adrenocorticotropic hormone in horses in Australia.33 Sera were stored at −80°C in cryotubes (Cryo.s cryovials; Greiner Bio-One) and used as detailed below.
Measurement of serum PON-1 activity
Serum PON-1 activity was measured spectrophotometrically using an automated analyzer (Cobas Integra 400 plus; Roche Diagnostic). The paraoxon-ethyl method was adapted from a published study9: the reaction buffer was prepared using 0.05 mM glycine buffer (pH 10.5; MilliporeSigma) containing 1 mM paraoxon-ethyl as a substrate (purity >90%; MilliporeSigma) and 1 mM calcium chloride (Ajax Finechem). The enzymatic reaction was initiated with 6 μL of sample, 89 μL of distilled water, and 100 μL of reaction buffer, at 37°C. The rate of hydrolysis of paraoxon to p-nitrophenol was measured by monitoring the increase in absorbance at 504 nm using a molar extinction coefficient of 18,050 L/mol/cm. A PON-1 activity of 1 U/L is defined as 1 µmol of p-nitrophenol formed per minute under the assay conditions.
The other substrate, 4-NPA, has been validated in humans but not in horses; therefore, analytical validation was performed first (Suppl. material). The method was adapted from a published study16: the reaction buffer was prepared using 50 mM Tris-HCl (pH 8.0; Invitrogen UltraPure) and 1mM calcium chloride (Ajax Finechem). The starting reagent is prepared with 63 mg of 4-NPA (MilliporeSigma) dissolved in 10 mL of methanol (purity ≥99.9%; MilliporeSigma). One mL of this solution is added to 20 mL of distilled water. The enzymatic reaction is initiated by adding 14 μL of sample to 200 μL of buffer and then 50 μL of starting reagents. After 100 s, the reaction is monitored at 405 nm at 37°C for 210 s. The 4-NPA activity is expressed as units per liter of serum, in which 1 U equals 1 μmol of phenyl acetate hydrolyzed per minute. The molar extinction coefficient used to calculate the rate of hydrolysis is 14,000 M-1cm-1. Any measurement of PON-1 activity in our study was performed using both substrates.
Analytical validation of p-nitrophenyl acetate
Intra- and inter-assay precision of PON-1 activity using paraoxon-ethyl and p-nitrophenyl acetate as substrates was carried out by measuring the samples 20 times in succession (Suppl. Table 1). It was not possible to create a pool with high PON-1 activity for the inter-assay study, thus only 2 pools with medium and low activity were assessed. Accuracy was assessed using indirect methods, specifically the linearity under dilution test (LUD; Suppl. Table 2) and the spiking recovery test (SRT; Suppl. Table 3).
PON-1 storage stability
PON-1 activity was measured with both methods, immediately after collection (T0) on 10 samples. Then, simulating various sample delivery scenarios, serum aliquots were stored at room temperature (RT; 20–25°C) and analyzed after 24 (T24h) and 48 (T48h) h; refrigerated (4°C) and analyzed after 24 (T24h) and 48 (T48h) h; frozen (−20°C) and analyzed after 24 (T24h) and 48 (T48h) h, 1 wk (T1w), 1 mo (T1m), and 3 mo (T3m). Before analysis, refrigerated and frozen samples were brought to RT and measured in duplicate. Means of duplicate measurements were used to estimate the actual value of each sample.
Repeated freeze–thaw cycles
After the first analysis performed in 10 fresh sera, each sample was transferred into cryotubes (Greiner Bio-One) and stored at −20°C. After 24 h, all frozen sera were thawed at RT for ~1 h until completely thawed, and then mixed properly before analysis (freeze–thaw 1). Samples were immediately re-frozen at −20°C for the next study day. This cycle was repeated for 4 consecutive days (T1d, T2d, T3d, T4d). Means of duplicate measurements were used to estimate the actual value of each sample.
Interference study
The effects of hemolysis, hyperbilirubinemia, and hyperlipemia were assessed using published protocols.20,24,30 A pool from 8 sera with a medium PON-1 activity was prepared and divided into aliquots. Each aliquot was spiked with different concentrations of bilirubin (Bil; MilliporeSigma), commercial fat emulsion (Trigl; Intralipid 20% lipid injectable emulsion; Fresenius Kabi), or hemoglobin (Hb; Hemoglobin equine; MilliporeSigma), followed by duplicate measurements of PON-1 activity. Because Bil is water insoluble, dimethyl sulfoxide (MilliporeSigma) was used instead of distilled water. Interfering substances were added to each aliquot of the pooled sample to obtain final concentrations adequate to simulate the following levels of icterus, lipemia, and hemoglobinemia: slight (Bil = 1,540 µmol/L; Trigl = 35.0 mmol/L; Hb = 0.625 g/L), moderate (Bil = 3,080 µmol/L; Trigl = 70.6 mmol/L; Hb = 1.25 g/L), severe (Bil = 6,330 µmol/L; Trigl = 141 mmol/L; Hb = 2.50 g/L), very severe (Bil = 12,800 µmol/L; Trigl = 282 mmol/L; Hb = 5.00 g/L), and extreme (Bil = 25,700 µmol/L; Trigl = 565 mmol/L; Hb = 10.0 g/L). Interferograms were prepared according to reported protocols13,14 (Fig. 1). The original values were calculated by adding the same volume of distilled water or dimethyl sulfoxide as the volume of interfering solutions.
Figure 1.
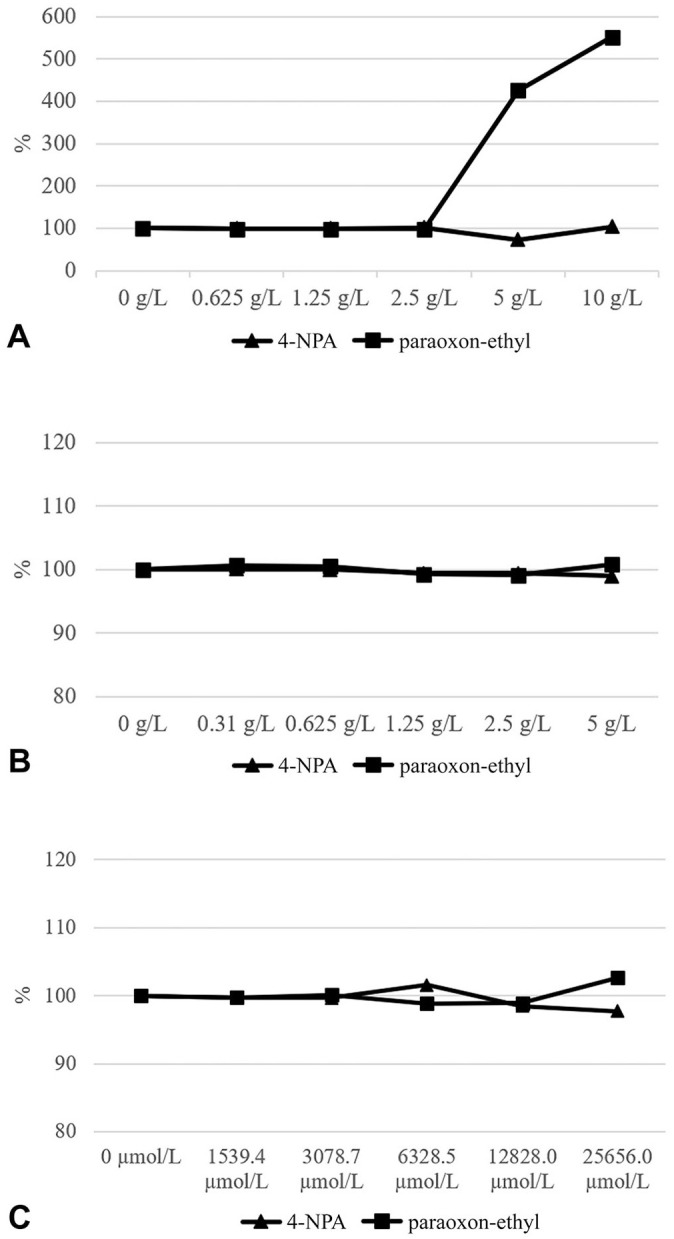
Interferograms for hemoglobin (A), lipid (B), and bilirubin (C) during measurement of PON-1 activity in a pool of equine serum obtained by mixing 8 different sera (using 2 different substrates: 4-nitrophenyl acetate [4-NPA] and paraoxon-ethyl). The concentrations on the x-axis refer to the final concentrations of interfering substances. The y-axes show the percentage change (final/original × 100%).
Seasonal study
Sera (n = 35) were collected monthly from April 2015 to February 2016 from 2 geographic locations within Australia: Perth in Western Australia and Townsville in North Queensland. All frozen sera were thawed at RT for 1 h until completely thawed, and then mixed properly before analysis. Means of duplicate measurements of PON-1 activity were used to estimate the actual value of each sample.
Statistical analysis
Statistical analysis was performed using SPSS statistics software (v.24; IBM). Data distribution was tested using the Shapiro–Wilk test; p ≤ 0.05 was considered statistically significant. Mean values were used for parametric data and median values for nonparametric data. The Spearman rank correlation test was used to determine the correlation between PON-1 activity estimated using 2 different substrates. Irrespective of statistical significance, preanalytical variability was also considered diagnostically relevant when the changes in PON-1 activity exceeded the total observed error (TEobs) calculated as 2 CV% + bias%, in which the CV was the average of the within- and between-run imprecision, and the bias the average inaccuracy of the method, as per ASVCP guidelines.17
For each storage condition (i.e., RT, 4°C, and −20°C), results obtained at different times were compared with a repeated-measurement ANOVA test, with a Greenhouse–Geisser correction if the assumption of sphericity was violated, followed by Bonferroni post hoc test to evaluate the differences between basal values (T0) and those recorded at every time point. Results obtained in different freeze–thaw cycles or in different months were compared with the nonparametric Friedman test followed by the Wilcoxon signed rank test. Results obtained from the pooled serum, with and without the different concentrations of interfering substances, were compared using a Friedman 2-way ANOVA for repeated measurement.
Results
Analytical validation and total observed error
Data for the analytical validation are reported with the imprecision and bias of the method used to calculate the TEobs (Suppl. Fig. 1, Tables 1–3). The least square linear regression on mean values of LUD and SRT demonstrated excellent correlation (r2 = 0.988, p = 0.000; r2 = 0.998, p = 0.000, respectively), and data fitted the linear model (Suppl. Fig. 1). The imprecision of the method was 2.92% and the bias was 8.12% with paraoxon-ethyl as substrate; TEobs was 14.0%. The imprecision of the method was 4.96% and the bias was 4.46% with 4-NPA as substrate; TEobs was 14.4%.
Correlation between the substrates
Based on the 54 serum samples included in this part of our study, there was a statistically significant, strong-positive correlation (rs = 0.723, p < 0.001) between PON-1 activity measured using paraoxon-ethyl and 4-NPA.
Storage stability
Ten samples were included in the storage study, and PON-1 activity was assessed with both substrates paraoxon-ethyl (PON-1) and 4-NPA. One PON-1 activity measured with paraoxon-ethyl was missing in a sample stored at room temperature for 48 h as a result of an analytical error.
In samples stored in different conditions, mean PON-1 activity were statistically significantly different between times with any storage condition irrespective of the substrate used (Table 1). Post hoc testing revealed that storage at RT induced a reduction in PON-1 activity, either using paraoxon-ethyl or 4-NPA as substrate, after 24 h and 48 h compared to basal values.
Table 1.
Results of PON-1 activity in equine sera (n = 10) stored under different temperatures and assessed using 2 different substrates.
| Storage condition/Time | PON-1 activity (U/L; mean ± SD) | |
|---|---|---|
| Paraoxon-ethyl | 4-nitrophenyl acetate | |
| Fresh | ||
| T0 | 67.6 ± 6.3 | 1,251 ± 85 |
| Room temperature, 21°C | ||
| T24h | 65.1 ± 6.1* | 1,191 ± 57* |
| T48h | 64.3 ± 6.1* | 1,179 ± 64* |
| Refrigerated at 4°C | ||
| T24h | 66.9 ± 5.9* | 1,200 ± 74** |
| T48h | 66.0 ± 6.1* | 1,179 ± 64 * |
| Frozen at −20°C | ||
| T24h | 66.9 ± 6.2 | 1,214 ± 85** |
| T48h | 63.2 ± 5.9* | 1,145 ± 69* |
| T1wk | 62.3 ± 6.0* | 1,203 ± 71* |
| T1mo | 62.0 ± 5.5* | 1,517 ± 82* |
| T3mo | 65.0 ± 5.8* | 1,299 ± 68* |
p < 0.01 versus T0.
p < 0.05 versus T0.
When samples were stored refrigerated, a similar pattern with decreasing mean PON-1 activity was observed with both substrates after 24 h and 48 h compared with the fresh samples. When 4-NPA was used as substrate, a more pronounced decrease was observed after 48 h of storage.
When samples were stored at −20°C and PON-1 activity was assessed with paraoxon-ethyl as substrate, a different pattern was observed. Except for short-term storage (24 h) that did not show any difference, there was a statistically significant decrease in PON-1 activity (p < 0.01) between T0 and any measured time up to 1 mo. Then, after 3 mo of storage, PON-1 activity increased again, but it was still significantly different from T0 (p < 0.01).
With 4-NPA, the pattern reflected the changes observed with paraoxon-ethyl as substrate, but the changes were even more pronounced, with PON-1 activity significantly lower after 24 and 48 h (p < 0.05 and p < 0.01, respectively) and 1 wk (p < 0.01). After 1 and 3 mo of storage, PON-1 activity was significantly higher (p < 0.01) than the basal value.
Despite numerous statistical differences observed with both substrates under different storage conditions, only samples frozen for 1 mo showed a variation greater than the TEobs calculated for PON-1 activity using 4-NPA; all other fluctuations were within diagnostically acceptable variation (Suppl. Fig. 2).
Repeated freeze–thaw cycles
Ten serum samples were included in this part of the study, and the PON-1 activity at each cycle was compared with the baseline value (Table 2). Using paraoxon-ethyl as substrate, the PON-1 activity showed numerous fluctuations, with a significant decrease (p < 0.01) after the first cycle followed by a significant increase (p < 0.01) in the following cycles. In contrast, when 4-NPA was used as substrate, PON-1 activity was significantly lower (p < 0.01) at the first freeze–thaw cycle compared with the baseline value, but then no significant difference was determined during the following cycles. When the results were compared with the TEobs, PON-1 activity was higher only in samples at the 4th freeze–thaw cycle, when assessed with paraoxon-ethyl as substrate (Suppl. Fig. 3).
Table 2.
Results of PON-1 activity in equine sera (n = 10) repeatedly frozen and thawed, assessed using 2 different substrates.
| Cycle | PON-1 activity (U/L; mean ± SD) | |
|---|---|---|
| Paraoxon-ethyl | 4-nitrophenyl acetate | |
| 0 | 65.9 ± 8.2 | 1,256 ± 70 |
| 1 | 60.7 ± 7.1* | 1,178 ± 69* |
| 2 | 68.5 ± 8.4* | 1,254 ± 65 |
| 3 | 67.8 ± 9.4* | 1,301 ± 87 |
| 4 | 82.6 ± 9.4* | 1,282 ± 56 |
p < 0.01 versus cycle 0 (fresh samples).
Interference studies
Although icterus and lipemia did not interfere with PON-1 activity, severe hemolysis induced a significant (p < 0.001) overestimation with paraoxon-ethyl as substrate (Fig. 1). Specifically, when Hb is 5 g/L, PON-1 activity is overestimated by 425%, and overestimation increases further to 551% when Hb is 10 g/L; both results are above the TEobs.
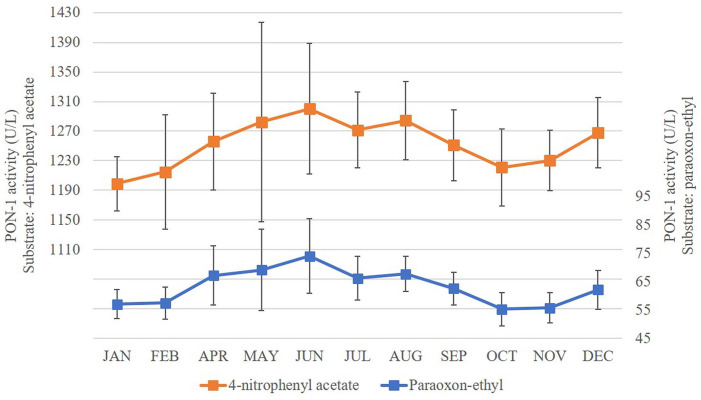
Seasonal study
Samples were available for only 11 mo (samples for March were not available). We analyzed 35 samples using paraoxon-ethyl as substrate and 34 samples using 4-NPA because 1 result was excluded as a result of an analytical error. PON-1 activity showed numerous significant differences across the year with both substrates (Suppl. Table 3, Fig. 2).
Figure 2.
Circannual serum PON-1 activity measured with 4-nitrophenyl acetate and paraoxon-ethyl in 35 horses. Error bars represent the standard deviation of the data.
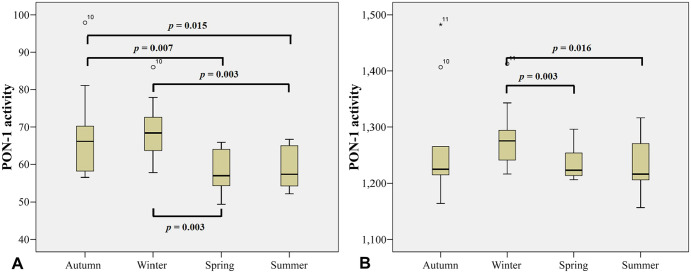
To better interpret these results, the data was grouped into the 4 seasons as follows: summer (December–February), autumn (April, May), winter (June–August), and spring (September–November). Changes in PON-1 activity, using paraoxon-ethyl as substrate, were significant across the seasons (Fig. 3a). In autumn, PON-1 activity was significantly higher than PON-1 activity during summer (p < 0.01) and spring (p < 0.05). PON-1 activity in winter is significantly higher than PON-1 activity in summer (p < 0.01) and spring (p < 0.01); PON-1 activity in spring and summer did not differ. Using 4-NPA as substrate, PON-1 activity showed a similar pattern across the seasons (Fig. 3b); PON-1 activity was significantly lower in summer than winter (p < 0.05) or spring (p < 0.01).
Figure 3.
Serum PON-1 activity in 35 horses sampled monthly and grouped into 4 seasons. PON-1 activity was measured using 2 different substrates: A. paraoxon-ethyl and B. 4-nitrophenyl acetate.
Discussion
The analytical validation of the substrate 4-NPA demonstrated good precision and accuracy, thus either paraoxon or 4-NPA could be used without affecting the reliability of the results. However, when compared with the analytical performances of paraoxon-ethyl, 4-NPA had higher intra- and inter-assay imprecision.32 A limitation of our analytical validation was the use of only 2 levels (medium and low) for the inter-assay precision. Unfortunately, the serum samples included in our study did not demonstrate enough variation in PON-1 activity. However, according to ASVCP guidelines,10 2 levels (normal and low) are sufficient if the analyte is medically significant when decreased. Three levels (low, normal, and high) are recommended if the analyte is medically significant when both decreased and increased.
There was strong and positive correlation between the 2 substrates, as demonstrated in humans21 and animals.5,7 The correlation between the 2 substrates further supports that the changes we observed are secondary to preanalytical factors, more than to genetic polymorphisms. In human patients, polymorphism has greater influence on paraoxon-ethyl than 4-NPA,2 and further studies are warranted to investigate the genetic polymorphism in horses and, if present, its influence on different substrates.
Because both substrates provided reliable results, the selection of the substrate could be based on preanalytical factors. Our study was focused on the investigation of preanalytical conditions that could affect the accuracy of PON-1 activity measurement. Information concerning optimal specimen requirements, such as handling and delivery or shipping temperature for the assays performed in the laboratory, should be investigated in case of delays in shipping samples, power cut, or voltage fluctuations of the freezers.6,15
Our results demonstrated fast degradation of paraoxonase in serum samples when stored at RT or refrigerated, and the degradation is time-related considering that the PON-1 activity was significantly lower after 24 h and even lower after 48 h. Nevertheless, it seems that the differences were not diagnostically relevant and still within the intrinsic analytical error of the methods. Given the consistent trend of degradation of the enzymatic activity in the samples stored at RT and in the refrigerator, we suggest that serum samples that cannot be processed immediately (e.g., travelling from remote collection points) are frozen and tested later with the paraoxon-ethyl substrate. In frozen samples, we observed several fluctuations of PON-1 activity, with initial reduction in PON-1 activity followed by almost normal activity after 3 mo of storage. Because the variation of PON-1 activity observed during storage was within the total imprecision of the method, the fluctuations of PON-1 activity in frozen samples are attributable to the analytical imprecision rather than real degradation of the enzyme. A similar fluctuation has been reported in another study31 in dog serum for PON-1 activity quantified using 4-NPA with any storage condition (RT, refrigeration, and freezing at −20°C and −80°C). It is important to highlight that even in dog serum, as in horse samples included in our study, the magnitude of the fluctuations was never higher than the minimum significant change calculated based on imprecision of the method, despite statistically significant differences compared with T0. In contrast with our results, another study on canine serum32 reported clinically significant variation in PON-1 activity measured with paraoxon-ethyl as substrate during storage at RT and refrigeration, but not in frozen samples for up to 3 mo. Therefore, it seems that PON-1 activity is more stable in frozen samples from horses than in canine species.
In practice, it is possible to reanalyze stored samples to confirm previous results or to perform additional analysis; however, the stability of the analytes after repeated freeze–thaw cycles must be assured before giving results, or before establishing new investigations. When we repeatedly froze and thawed our samples, there were differences between the 2 substrates; paraoxon-ethyl displayed more fluctuation than 4-NPA. After the 4th freeze–thaw cycle, PON-1 activity was higher than in fresh samples, and the difference was higher than the imprecision of the method. Therefore, when sample handling requires repeated freeze–thaw cycles (e.g., a single aliquot is available, but multiple tests are required), the substrate should be 4-NPA. A similar study performed in dogs31 showed the same fluctuations after 2 and 3 cycles, both greater than the imprecision of the method using 4-NPA as substrate. This further supports the premise that PON-1 activity is more stable in horse serum than in dog serum.
High levels of select blood contents (i.e., lipids, bilirubin, and hemoglobin) may interfere with the laboratory test.24 PON-1 activity in strongly hemolytic samples should be assessed using 4-nitrophenyl acetate as substrate, whereas there were no interferences from lipids and bilirubin. This is in contrast with a study32 in which lipids induced a significant decrease in PON-1 activity; however, there were no interferences from bilirubin and an overestimation of PON-1 activity with hemoglobin (despite starting the interference in our study with 5 g/L and in the other study32 with 10 g/L). The interference from lipids has also been reported in dogs,29,35 and although interference with the hemolysate of canine blood has been reported, this has not been demonstrated with purified hemoglobin.29 Considering the differences between equine and canine species, it could be speculated that the interference from hemoglobin is not purely the result of spectrophotometric tampering, but perhaps to the result of molecules present in the hemoglobin powder (e.g., calcium released during hemoglobin purification).
Given the strong link between PON-1 activity and lipids, preanalytical factors could affect PON-1 activity measurements. Specifically, lipid concentration and composition are affected by seasons; serum triglyceride concentration is lower in colder months than in warmer months in Morgan and Thoroughbred horses.28 Therefore, seasonal changes in PON-1 activity could be hypothesized. PON-1 activity demonstrated seasonal variation and both substrates showed a similar pattern with an increase from April, a peak in June, and then a progressive decrease until October. When data are grouped into seasons, PON-1 activity is higher in autumn and winter than in spring or summer. Because PON-1 is bound to HDL, the seasonal differences could be related to the changes in lipid profile across the seasons. Studies investigating the seasonal lipid profile in Thoroughbred horses8,11 have been undertaken in the northern hemisphere, where seasonal variations of lipids have not been detected. It is well known that proopiomelanocortin peptides produced by the equine pituitary pars intermedia demonstrate circannual rhythm,33 and it is plausible that dopaminergic tone, which affects metabolism, appetite, and obesity, could potentially influence PON-1 activity.25 Further investigations of seasonal variation of PON-1 activity are required to determine if seasonal reference intervals are required.
A limitation of our study is the inclusion of serum samples stored for almost 24 mo at −80°C for the seasonal study. Long-term storage could induce variation in PON-1 activity, thus affecting the interpretation of results. However, a long-term storage study performed in dogs demonstrated good stability of PON-1 activity in canine serum samples for up to 1 y.31 Although there are no reports in which samples were stored at −80°C beyond 1 y, it could be speculated that, at ultra-low temperatures, enzyme stability is high. Data in our study did not show a pattern with significant or linear increase or decrease during storage at −80°C when comparing PON-1 activity in the samples collected at the beginning of the study (April 2015) and the following samples up to February 2016. The absence of a pattern further supports our hypothesis that the significant differences observed were seasonal-related and that constant degradation or activation of the enzymatic activity was not induced by prolonged storage. Given that samples went through only one freeze–thaw cycle, it is unlikely this cycle would significantly affect the PON-1 activity from a clinical perspective. In the long-term storage study in dogs,31 repeated freeze–thaw cycles of samples stored at −80°C did not affect PON-1 activity in canine serum. Studies on long-term storage at ultra-low temperatures are warranted to confirm that the seasonal variation observed was not affected by storage.
Supplemental Material
Supplemental material, sj-pdf-1-vdi-10.1177_1040638720974745 for Preanalytical variables affecting the measurement of serum paraoxonase-1 activity in horses by Gabriele Rossi, Amy Richardson, Hali Jamaludin and Cristy Secombe in Journal of Veterinary Diagnostic Investigation
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Gabriele Rossi  https://orcid.org/0000-0003-4879-9504
https://orcid.org/0000-0003-4879-9504
Supplementary material: Supplementary material for this article is available online.
References
- 1. Aviram M, et al. Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions. A possible peroxidative role for paraoxonase. J Clin Invest 1998;101:1581–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Billecke S, et al. Human serum paraoxonase (PON1) isozymes Q and R hydrolyze lactones and cyclic carbonate esters. Drug Metab Dispos 2000;28:1335–1342. [PubMed] [Google Scholar]
- 3. Braun JP, et al. The preanalytic phase in veterinary clinical pathology. Vet Clin Pathol 2015;44:8–25. [DOI] [PubMed] [Google Scholar]
- 4. Camps J, et al. The paraoxonases: role in human diseases and methodological difficulties in measurement. Crit Rev Clin Lab Sci 2009;46:83–106. [DOI] [PubMed] [Google Scholar]
- 5. Ceron JJ, et al. Serum paraoxonase 1 (PON1) measurement: an update. BMC Vet Res 2014;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cuhadar S, et al. The effect of storage time and freeze-thaw cycles on the stability of serum samples. Biochem Med (Zagreb) 2013;23:70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Farid AS, et al. Serum paraoxonase-1 as biomarker for improved diagnosis of fatty liver in dairy cows. BMC Vet Res 2013;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fazio E, et al. Seasonal thyroid and lipid profiles in Thoroughbred pregnant and nonpregnant mares (Equus caballus). Theriogenology 2016;85:1582–1589. [DOI] [PubMed] [Google Scholar]
- 9. Feingold KR, et al. Paraoxonase activity in the serum and hepatic mRNA levels decrease during the acute phase response. Atherosclerosis 1998;139:307–315. [DOI] [PubMed] [Google Scholar]
- 10. Flatland B, et al. ASVCP quality assurance guidelines: control of general analytical factors in veterinary laboratories. Vet Clin Pathol 2010;39:264–277. [DOI] [PubMed] [Google Scholar]
- 11. Frank N, et al. Association of season and pasture grazing with blood hormone and metabolite concentrations in horses with presumed pituitary pars intermedia dysfunction. J Vet Intern Med 2010;24:1167–1175. [DOI] [PubMed] [Google Scholar]
- 12. Furlong CE. Genetic variability in the cytochrome P450-paraoxonase 1 (PON1) pathway for detoxication of organophosphorus compounds. J Biochem Mol Toxicol 2007;21:197–205. [DOI] [PubMed] [Google Scholar]
- 13. Glick MR, et al. Interferographs: A User’s Guide to Interferences in Clinical Chemistry Instruments. 2nd ed. Science Enterprises, 1991. [Google Scholar]
- 14. Glick MR, et al. Graphical comparisons of interferences in clinical chemistry instrumentation. Clin Chem 1986;32:470. [PubMed] [Google Scholar]
- 15. Gunn-Christie RG, et al. ASVCP quality assurance guidelines: control of preanalytical, analytical, and postanalytical factors for urinalysis, cytology, and clinical chemistry in veterinary laboratories. Vet Clin Pathol 2012;41:18–26. [DOI] [PubMed] [Google Scholar]
- 16. Haagen L, Brock A. A new automated method for phenotyping arylesterase (EC 3.1.1.2) based upon inhibition of enzymatic hydrolysis of 4-nitrophenyl acetate by phenyl acetate. Eur J Clin Chem Clin Biochem 1992;30:391–395. [DOI] [PubMed] [Google Scholar]
- 17. Harr KE, et al. ASVCP guidelines: allowable total error guidelines for biochemistry. Vet Clin Pathol 2013;42:424–436. [DOI] [PubMed] [Google Scholar]
- 18. Hooijberg E, et al. An error management system in a veterinary clinical laboratory. J Vet Diagn Invest 2012;24:458–468. [DOI] [PubMed] [Google Scholar]
- 19. International Organization for Standardization. ISO 15189: 2012. Medical Laboratories—Requirements for Quality and Competence. ISO, 2012. [DOI] [PubMed] [Google Scholar]
- 20. Jacobs RM, et al. Effects of bilirubinemia, hemolysis, and lipemia on clinical chemistry analytes in bovine, canine, equine, and feline sera. Can Vet J 1992;33:605–608. [PMC free article] [PubMed] [Google Scholar]
- 21. Kotani K, et al. Changes on the physiological lactonase activity of serum paraoxonase 1 by a diet intervention for weight loss in healthy overweight and obese women. J Clin Biochem Nutrition 2009;45:329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mackness B, et al. Paraoxonase-1 inhibits oxidised LDL-induced MCP-1 production by endothelial cells. Biochem Biophys Res Commun 2004;318:680–683. [DOI] [PubMed] [Google Scholar]
- 23. Marsillach J, et al. The measurement of the lactonase activity of paraoxonase-1 in the clinical evaluation of patients with chronic liver impairment. Clin Biochem 2009;42:91–98. [DOI] [PubMed] [Google Scholar]
- 24. Martínez-Subiela S, Cerón JJ. Effects of hemolysis, lipemia, hyperbilirrubinemia, and anticoagulants in canine C-reactive protein, serum amyloid A, and ceruloplasmin assays. Can Vet J 2005;46:625–629. [PMC free article] [PubMed] [Google Scholar]
- 25. McFarlane D. Diagnostic testing for equine endocrine diseases: confirmation versus confusion. Vet Clin North Am Equine Pract 2019;35:327–338. [DOI] [PubMed] [Google Scholar]
- 26. Novak F, et al. Decreased paraoxonase activity in critically ill patients with sepsis. Clin Exp Med 2010;10:21–25. [DOI] [PubMed] [Google Scholar]
- 27. O’Neill SL, Feldman BF. Hemolysis as a factor in clinical chemistry and hematology of the dog. Vet Clin Pathol 1989;18:58–68. [DOI] [PubMed] [Google Scholar]
- 28. Radakovic M, et al. Oxidative stress and DNA damage in horses naturally infected with Theileria equi. Vet J 2016;217:112–118. [DOI] [PubMed] [Google Scholar]
- 29. Robie SM, et al. Equine serum lipids: serum lipids and glucose in Morgan and Thoroughbred horses and Shetland ponies. Am J Vet Res 1975;36:1705–1708. [PubMed] [Google Scholar]
- 30. Rossi G, et al. Serum paraoxonase 1 activity in dogs: preanalytical and analytical factors and correlation with C-reactive protein and alpha-2-globulin. Vet Clin Pathol 2013;42:329–341. [DOI] [PubMed] [Google Scholar]
- 31. Rossi S, et al. Homocysteine measurement by an enzymatic method and potential role of homocysteine as a biomarker in dogs. J Vet Diagn Invest 2008;20:644–649. [DOI] [PubMed] [Google Scholar]
- 32. Rubio CP, et al. Stability of biomarkers of oxidative stress in canine serum. Res Vet Sci 2018;121:85–93. [DOI] [PubMed] [Google Scholar]
- 33. Ruggerone B, et al. Validation of a paraoxon-based method for measurement of paraoxonase (PON-1) activity and establishment of RIs in horses. Vet Clin Pathol 2018;47:69–77. [DOI] [PubMed] [Google Scholar]
- 34. Secombe CJ, et al. The effect of geographic location on circannual adrenocorticotropic hormone plasma concentrations in horses in Australia. J Vet Intern Med 2017;31:1533–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Turk R, et al. Serum platelet-activating factor acetylhydrolase and paraoxonase-1 activity in horses infected with Leptospira spp. Acta Trop 2011;118:97–100. [DOI] [PubMed] [Google Scholar]
- 36. Tvarijonaviciute A, et al. Validation of spectrophotometric assays for serum paraoxonase type-1 measurement in dogs. Am J Vet Res 2012;73:34–41. [DOI] [PubMed] [Google Scholar]
- 37. Watson AD, et al. Protective effect of high density lipoprotein associated paraoxonase. Inhibition of the biological activity of minimally oxidized low density lipoprotein. J Clin Invest 1995;96:2882–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. West J, et al. Preanalytical errors in medical laboratories: a review of the available methodologies of data collection and analysis. Ann Clin Biochem 2017;54:14–19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-vdi-10.1177_1040638720974745 for Preanalytical variables affecting the measurement of serum paraoxonase-1 activity in horses by Gabriele Rossi, Amy Richardson, Hali Jamaludin and Cristy Secombe in Journal of Veterinary Diagnostic Investigation