Abstract
Altered circadian rhythms have negative consequences on health and behavior. Emerging evidence suggests genetics influences the physiological and behavioral responses to circadian disruption. We investigated the effects of a 21 h day (T = 21 cycle), with high-fat diet consumption, on locomotor activity, explorative behaviors, and health in male C57BL/6J and C57BL/6N mice. Mice were exposed to either a T = 24 or T = 21 cycle and given standard rodent chow (RC) or a 60% high-fat diet (HFD) followed by behavioral assays and physiological measures. We uncovered numerous strain differences within the behavioral and physiological assays, mainly that C57BL/6J mice exhibit reduced susceptibility to the obesogenic effects of (HFD) and anxiety-like behavior as well as increased circadian and novelty-induced locomotor activity compared to C57BL/6N mice. There were also substrain-specific differences in behavioral responses to the T = 21 cycle, including exploratory behaviors and circadian locomotor activity. Under the 21-h day, mice consuming RC displayed entrainment, while mice exposed to HFD exhibited a lengthening of activity rhythms. In the open-field and light-dark box, mice exposed to the T = 21 cycle had increased novelty-induced locomotor activity with no further effects of diet, suggesting daylength may affect mood-related behaviors. These results indicate that different circadian cycles impact metabolic and behavioral responses depending on genetic background, and despite circadian entrainment.
Keywords: Mouse, photoperiod, high-fat diet, locomotor activity, circadian, strain differences
Introduction
Numerous studies show that disrupted circadian rhythms can lead to abnormal behaviors. Individuals who are genetically predisposed to sleep and circadian rhythm disorders often also develop depression and anxiety symptoms (McClung 2007). Evidence suggests resetting the circadian clock may alleviate some of these symptoms (Katz et al. 2001), further highlighting the direct role for clocks in mood disorders. Environmental circadian disruptions, such as constant light or jet-lag, can also lead to changes in mood-related behaviors (Fonken and Nelson 2013b; Okuliarova et al. 2016; Tapia-Osorio et al. 2013). In addition, multiple studies have found that changing the photoperiod (i.e., hours of light per day) alters mood and motivated behaviors relative to standard light-dark (LD) cycles consisting of 12 of light and 12 h of darkness (12L:12D) (Ben-Hamo et al., 2016b; Bedrosian and Nelson 2017; Logan et al. 2018; Rosenwasser et al. 2015).
Circadian disruption can also adversely impact metabolism and overall health. Rotating shift-workers more commonly experience weight gain and obesity compared to regular work hour employees (Antunes et al. 2010). Similarly, rodents exposed to constant artificial light display altered levels of metabolic hormones, develop insulin resistance, and undergo marked weight gain (Coomans et al., 2013a; Maroni et al. 2018). This may be due to the timing of light exposure, as even dim light at night has consequences on metabolism (Fonken et al. 2010, 2013). These changes in metabolism are in part due to a combination of altered molecular rhythms in the suprachiasmatic nucleus (SCN; i.e., master circadian pacemaker in the brain) and peripheral tissues. For example, SCN ablation leads to progressive weight gain, likely because of an increase in glucose, insulin, and free-fatty acids, as well as fat deposits (Coomans et al., 2013b). Interestingly, genetic disruption to molecular rhythms results in physiological changes similar to type-2 diabetes and metabolic syndrome (Marcheva et al. 2010; Rudic et al. 2004; Sadacca et al. 2011; Shi et al. 2013; Turek et al. 2005). These relationships between clocks and metabolism are bidirectional. Poor eating habits and obesity alter circadian rhythms. For example, obesity in humans and animals is associated with sleep and circadian rhythms (Gómez-Santos et al. 2009; Laposky et al. 2008; Mistlberger et al. 1998). High-fat diet (HFD) alters free-running period (Kohsaka et al. 2007) and responses to light cues that help synchronize or entrain the circadian clock (Mendoza et al. 2008). Both circadian disruption and HFD consumption can lead to alterations in the timing of food consumption patterns, which can partially explain why these stimuli produce obesogenic and other adverse health effects (Coomans et al. 2013a; Fonken et al. 2010).
This study aims to uncover whether C57BL/6J (B6J) mice from Jackson Laboratories and C57BL/6N (B6N) mice from Charles River Laboratories differ in their response to altered environmental circadian disruption and high-fat diet. While these strains are highly genetically similar, B6J and B6N mice differ from each other regarding their free-running activity rhythm (Banks et al. 2015), and in their response to HFD consumption (B6N are more susceptible to obesity and type-2 diabetic symptoms) (Cardin et al. 2014; Heiker et al. 2014; Moreth et al. 2014; Podrini et al. 2013), and behavior (Sturm et al. 2015). More recently, studies have begun to explore the effects of non-24 h days on both metabolic physiology and mood-related behaviors (Fernandez et al., 2018; Karatsoreos et al. 2011; LeGates et al. 2012). In our studies, we subjected B6J and B6N male mice to 21 h days (T = 21), a shortened non-24 h day photoperiod, to compare to the standard 12L:12D cycle of 24 h days, to investigate the impact of photoperiod on metabolic phenotypes and exploratory behaviors related to mood.
Methods
This study had the approval of Bridgewater State University’s Institutional Animal Care and Use Committee. All experiments were conducted within the ethical guidelines set by Portaluppi et al. (2010).
Animals and circadian rhythm analysis
Thirty-six male B6J and twenty-eight male B6N mice were purchased from the aforementioned mouse laboratories at approximately 7 weeks of age. Upon arrival, mice were acclimated to a 12L:12D cycle for one week (from 06:00–18:00 h), consuming a regular chow diet (RC, 3.36 kcals per gram with kcal percentages 13.4% fat, 29.8% protein, and 56.8% carbohydrate, LabDiet 5001, St. Louis, MO, USA) and water ad libitum. The cages included continuous Infrared (IR) beam home-cage monitoring (StarrLife Sciences, Oakmont, PA, USA). Following acclimation, mice either remained in the 12L:12D cycle (06:00–18:00 h) or were introduced to a T = 21 cycle. Within each LD cycle, mice were either given the RC diet or a high-fat diet ad libitum (HFD, TestDiet 58Y1, 5.10 kcals per gram with kcal percentages 61.6% fat, 18.1% protein, and 20.3% carbohydrate, TestDiet 58Y1, St. Louis, MO, USA). Thus, there were four groups within each genotype of equal sample size for each specific genotype: 1) RC/24, 2) RC/21, 3) HFD/24, and 4) HFD/21. Weekly measurements of body mass and food intake were recorded.
Timing of behavioral and physiological assays
We conducted all of the behavioral and physiological assays 6 h after light onset for all animals in order to control for the amount of light exposure the animals had on the day of experimental procedures. As such, all assays described below were conducted at the same real time - on subsequent Fridays starting at the clock time of 12:00 h, which was 6 h after the lights turn on time for all groups and ending no later than 15:30h (Table 1). This time was selected to ensure that all groups of animals received the same light onset and amount of lighting for each behavioral and physiological assay on the day listed below regardless of photoperiod.
Table 1.
Lighting schematic for the 24-hour and 21-hour days showing onsets and offsets. The bold/italics/underline time on Friday (0600–1630 hours) at 1200 was the time in which all of the behavioral and physiological assays were conducted.
| Day | T = 24 Time | Light/Dark | T = 21 Time | Light/Dark |
|---|---|---|---|---|
| Sunday | 0000–0600 | Dark | 0000–1030 | Light |
| 0600–1800 | Light | 1030–2100 | Dark | |
| 1800–2400 | Dark | 2100–2400 | Light | |
| 0000–0600 | Dark | 0000–0730 | Light | |
| Monday | 0600–1800 | Light | 0730–1800 | Dark |
| 1800–2400 | Dark | 1800–2400 | Light | |
| 0000–0600 | Dark | 0000–0430 | Light | |
| Tuesday | 0600–1800 | Light | 0430–1500 | Dark |
| 1800–2400 | Dark | 1500–2400 | Light | |
| 0000–0600 | Dark | 0000–0130 | Light | |
| 0600–1800 | Light | 0130–1200 | Dark | |
| Wednesday | 1800–2400 | Dark | 1200–2230 | Light |
| 2230–2400 | Dark | |||
| 0000–0600 | Dark | 0000–0900 | Dark | |
| Thursday | 0600–1800 | Light | 0900–1930 | Light |
| 1800–2400 | Dark | 1930–2400 | Dark | |
| 0000–0600 | Dark | 0000–0600 | Dark | |
| Friday | 0600–1800 | Light | 0600–1630 | Light |
| 1800–2400 | Dark | 1630–2400 | Dark | |
| 0000–0600 | Dark | 0000–0300 | Dark | |
| Saturday | 0600–1800 | Light | 0300–1330 | Light |
| 1800–2400 | Dark | 1330–2400 | Dark |
Behavioral assays
After six weeks of photoperiod exposure, explorative and anxiety-like behaviors were assayed in an open field and light-dark box (i.e., L-D box) test using the SmartCage™software system as previously described (Hicks et al. 2016). The L-D box study was conducted one week after the open field study during the same subjective time as described above. Additionally, active time (time spent moving in the behavioral assay), the distance traveled, velocity, rears (raise itself upright on its hind legs), and time spent in the corners of the light and dark zones were measured for each zone of the L-D box.
Assessment of obesity and diabetic phenotype
After eight weeks of photoperiod exposure, a glucose tolerance test (GTT) was performed. For the GTT, food was removed from the cages and 12 h later, each mouse was given an intraperitoneal injection of 2 g/kg glucose after a baseline glucose measurement via tail prick (One-Touch Ultra-2 Glucose Monitors). Glucose levels were measured 30, 60, and 120 minutes post-injection and the Area Under the Curve (AUC) was calculated for each mouse.
After nine weeks of photoperiod exposure, 50 mg liver samples were obtained after CO2-induced euthanasia and immediately stored in −80°C. After storage, the liver samples were homogenized in 300 μL of 5% Triton-X100 (Sigma-Aldrich Merck, St. Louis, MO), centrifuged 4°C for 20 minutes at 2000 g, and tested in EnzyChrom™Triglyceride Assay Kits (Bioassay Systems, Hayward CA). Additionally, whole blood was collected and insulin levels were determined using Ultra Sensitive Mouse Insulin ELISA Kits (Crystal Chem, Downers Grove, IL, USA). Serum was obtained by allowing the collected whole blood (trunk blood post-euthanasia) to clot and centrifuging it at 4°C for 20 minutes.
BDNF
Brain-derived neurotrophic factor (BDNF) has been implicated in anxiety-like and exploration behaviors (Chen et al. 2006), and both circadian disruption (Ikeno et al. 2016) and HFD (Genzer et al. 2016) are known to reduce its levels. After euthanasia, whole hypothalamus sections (1 mm3) were removed and immediately stored in −80°C. After storage, tissue homogenates were created in a cocktail containing Pierce IP Lysis buffer (Thermo Scientific, Rockford, IL, USA) and protease inhibitor (Halt Protease Inhibitor Single-Use Cocktail EDTA-Free 100×; Thermo Scientific) at a ratio of 100 μl of protease inhibitor for each 10 ml of lysis buffer, and a ratio of 0.3 ml of protease/lysis cocktail was added to 20 mg of hypothalamus sample. After centrifugation, a low target concentration (working dilution 1:2) of sample and sample diluent buffer was created and then used in BDNF ELISA kits (Mouse BDNF PicoKine ELISA, Boster Biological Technology Co., Pleasanton, CA).
Statistical analyses
Circadian periods and overall activity during the initial 6-week T-cycle exposure were determined using ClockLab’s (Actimetrics, Wilmette IL, USA) and Lomb-scargal periodogram functions and bout analysis as previously described (Ahmad et al. 2013). In order to control for differences in the number of hours available for daily locomotor activity, the activity, alpha (the segment of a circadian cycle during which the organism is active, using Clocklab’s automated onset/offset detection), and number of bouts per day parameters for T = 21 mice were multiplied by 24/21 (8/7) to standardize to 24 h. Three-way ANOVAs were used to determine differences in all of the behavioral and physiological tests.
Results
Circadian locomotor activity
The means and SEM of all of the circadian locomotor activity parameters analyzed are summarized in Table 2 and representative actograms are provided in both Figure 1 and Figure 2. All mice, regardless of diet or genotype, were able to entrain to the 12 L:12D (T = 24) cycle. Additionally, all mice consuming RC, except for one B6N mouse (period of 23.25 h), entrained to the T = 21 cycle and exhibited periods of 21 h. Interestingly, only 2 of 9 mice for B6J and 1 of 7 for B6N consuming HFD were able to entrain to the 21 h day, while all of the others displayed periods of greater than 21 h. The average (without the entrained animals) for B6J was 24.31 ± 0.10 h, while the B6N average was 23.73 ± 0.33 h. As such, there was a diet and photoperiod interaction for circadian period (F1,51 = 30.87, p < .001). Mice consuming RC exhibited significantly shorter periods in the 21 h compared to the 24 h day schedule (p < .001), but this was not found for animals consuming HFD (p = .20).
Table 2.
Circadian locomotor activity profile and bout analysis (mean ± SEM) for all diet and lighting conditions for each genotype. Within the title of each column, the ‡ symbol refers to a diet difference, σ refers to a strain difference, and the X refers to an interaction. Different letters within each column indicate significant differences among each other at p < .05 (^ indicates at p = .061). For Bout Length and Counts per Bout, B6J > B6N and RC > HFD, also at p < .05.
| Genotype | Photoperiod | Diet | Average Daily Activity (X) | Bout Length (min) (σ, ‡) | Counts per Bout (σ, ‡) | No. Bouts per Day (X) | Circadian Period (h) (X) | Alpha (h) (X) | Rhythm Power (X) |
|---|---|---|---|---|---|---|---|---|---|
| B6J | T = 24 | RC | 25.65 ± 2.91a | 52.72 ± 3.49 | 334.67 ± 53.75 | 10.40 ± 0.40a | 24 ± 0a | 10.57 ± 0.44a | 2331.38 ± 252.03a |
| B6J | T = 24 | HF | 21.89 ± 1.75b^ | 47.18 ± 5.49 | 294.49 ± 49.31 | 10.37 ± 0.79a | 24 ± 0a | 10.21 ± 0.41a | 2449.09 ± 314.25a |
| B6J | T = 21 | RC | 26.38 ± 3.13a | 41.52 ± 3.60 | 311.01 ± 49.91 | 11.31 ± 0.41a | 21 ± 0b | 2.92 ± 0.35b | 872.08 ± 146.41b |
| B6J | T = 21 | HF | 20.96 ± 2.90b^ | 40.58 ± 2.21 | 264.76 ± 37.32 | 10.48 ± 0.30a | 23.57 ± 0.52a | 4.92 ± 0.83 c | 678.48 ± 61.17b |
| B6N | T = 24 | RC | 19.59 ± 1.89 c | 42.57 ± 2.51 | 221.79 ± 24.21 | 11.42 ± 0.42a | 24 ± 0a | 9.58 ± 1.48d | 1298.86 ± 74.52 c |
| B6N | T = 24 | HF | 9.75 ± 0.53d | 36.80 ± 1.36 | 114.76 ± 6.69 | 10.95 ± 0.65a | 24 ± 0a | 7.28 ± 0.48d | 965.86 ± 76.76 c |
| B6N | T = 21 | RC | 16.74 ± 2.58 c | 43.52 ± 4.25 | 196.39 ± 31.34 | 8.12 ± 0.49b | 21.36 ± 0.39b | 2.71 ± 0.72b | 454.05 ± 100.78d |
| B6N | T = 21 | HF | 9.12 ± 1.22d | 35.09 ± 3.21 | 136.09 ± 21.61 | 6.27 ± 0.32b | 23.28 ± 0.60a | 5.64 ± 1.03 c | 498.89 ± 111.06d |
Figure 1.

Representative actograms for animals in a T = 24 cycle. a) B6J/RC, b) B6J/HFD, c) B6N/RC, d) B6N/HFD.
Figure 2.

Representative actograms for animals in a T = 21 cycle. a) B6J/RC, b) B6J/HFD, c) B6N/RC, d) B6N/HFD.
Both diet and genotype differences were uncovered for average circadian locomotor activity (F1,51 = 13.11, p < .001, HFD < RC; F1,51 = 37.62, p < .001, B6N < B6J), but no interactions were present. The bout analysis revealed differences in diet (F1,51 = 4.29, p = .050, HFD < RC) and genotype (F1,51 = 4.29, p = .023, B6J > B6N) for length of an activity bout and the number of activity counts per bout, but no interactions. A photoperiod and genotype interaction was also found (F1,51 = 42.92, p < .001). While there are no differences in the number of bouts per day for B6J and B6N in LD (p = .66), in T = 21, B6J mice exhibited more bouts per day than B6N (p < .001).
Additionally, B6N in the T = 21 cycle had significantly fewer bouts per day than B6N in 12 L:12D (p < .001) and B6J in T = 21 (p < .001). HFD also reduced the number of bouts per day regardless of T-cycle or genotype (F1,51 = 5.04, p = .019).
Both photoperiod and genotype (F1,51 = 8.05, p = .007) and diet and photoperiod interactions (F1,51 = 18.43, p < .001) were observed for alpha. In LD, B6J mice had greater alpha than B6N mice (p = .012), but this was not found when these animals were in a 21 h day (p = .81). The T = 21 cycle reduced alpha for both B6N and B6J compared to respective controls (both p < .001). Additionally, HFD and RC consuming mice in 12:12 LD (regardless of genotype) exhibited similar alpha (p = .15), but in a T = 21 cycle HFD animals exhibited increased alpha compared to RC (p = .002). A photoperiod by genotype interaction was found for Rhythm Power (F1,51 = 12.83, p = .001). While rhythm power was reduced overall for mice in a T = 21 cycle (p = .015), in a standard LD cycle, B6N had reduced rhythm power compared to B6N (p < .001), but had no differences in a - 21 h day (p = .41).
Open field
The open field data are summarized in Table 3. A three-way interaction was uncovered for active time (F1,55 = 5.23, p = .026). While B6J mice exhibited no difference in active time when subjected to a 21 h day with either RC (p = .99) or HFD (p = .93), B6N mice consuming RC exhibited increased active time in the T = 21 cycle compared to 12 L:12D (p = .024), with no differences found when consuming HFD (p = .99). Baseline strain differences were found for active time (F1,55 = 9.92, p = .003, B6J > B6N), distance traveled (F1,55 = 23.52, p < .001, B6J > B6N), and number of rotations (F1,55 = 10.44, p = .002, B6J > B6N). Overall T-cycle differences were found for active time (F1,55 = 5.92, p = .018, T = 21 > T = 24), distance traveled (F1,55 = 10.25, p = .002, T = 21 > T = 24) and velocity (F1,55 = 10.25, p = .002, T = 21 > T = 24), regardless of strain. No differences were found for rears (F1,55 = 0.003, p = .99) or center zone time (F1,55 = 1.00, p = .32).
Table 3.
Open Field (mean ± SEM). a) Active time was increased in B6N/T = 21 mice compared to controls, but no other differences. b) Both strain (B6J > B6N) and photoperiod (T = 21 > T = 24) were present for Distance Traveled. c) Velocity was increased in mice under the 21-hour day compared to T = 24 mice. d) B6J exhibits increased Total Rotations compared to B6N. e) No significant differences for rearing or f) center zone Time. † indicates a T-cylce difference, σ indicates a baseline strain difference, and italics denotes significant pairwise comparison, at p < .05.
| Genotype | Diet | T-cycle | Active Time (min) (σ, †) | Distance (cm) (σ, †) | Velocity (cm/s) (†) | Rears | Total Rotations (σ) | Center Zone Time (min) |
|---|---|---|---|---|---|---|---|---|
| B6J | RC | T = 24 | 8.88 ± 0.33 | 1277.37 ± 65.98 | 2.99 ± 0.070 | 103.50 ± 6.94 | 16.06 ± 0.87 | 1.49 ± 0.17 |
| B6J | HF | T = 24 | 8.46 ± 0.13 | 1229.03 ± 56.79 | 2.79 ± 0.068 | 90.44 ± 6.83 | 18.57 ± 1.74 | 1.52 ± 0.18 |
| B6J | RC | T = 21 | 9.00 ± 0.24 | 1180.05 ± 47.99 | 3.10 ± 0.15 | 98.13 ± 9.27 | 18.97 ± 1.05 | 1.84 ± 0.21 |
| B6J | HF | T = 21 | 8.83 ± 0.21 | 1405.57 ± 59.85 | 3.07 ± 0.086 | 97.88 ± 9.51 | 16.22 ± 1.17 | 1.72 ± 0.18 |
| B6N | RC | T = 24 | 7.68 ± 0.27 | 999.01 ± 61.78 | 2.75 ± 0.11 | 91.85 ± 12.65 | 13.62 ± 1.84 | 1.49 ± 0.17 |
| B6N | HF | T = 24 | 8.26 ± 0.23 | 1092.26 ± 50.11 | 2.90 ± 0.12 | 95.85 ± 12.02 | 16.10 ± 1.80 | 1.30 ± 0.30 |
| B6N | RC | T = 21 | 8.91 ± 0.35 | 1415.40 ± 94.25 | 2.92 ± 0.087 | 69.28 ±21.92 | 14.81 ± 1.93 | 1.28 ± 0.28 |
| B6N | HF | T = 21 | 8.19 ± 0.19 | 1177.00 ± 75.73 | 2.95 ± 0.13 | 87.85 ±16.14 | 12.77 ± 1.02 | 1.64 ± 0.27 |
Light-dark box
B6N mice spent significantly more time in the dark zone of the L-D Box (F1,52 = 4.84, p = .032), but no other significant differences were uncovered (all p > .10) (Figure 3a). A strain by photoperiod interaction was revealed for the Number of Transitions between the two compartments (F1,52 = 4.60, p = .037). Whereas B6J mice in the 21 h day significantly increased their transitions compared to B6N mice in the same light cycle (p < .001), this was not the case in 12 L:12D (p = .15) (Figure 3b). Lastly, strain (F1,52 = 5.47, p = .023, B6J < B6N) and light cycle (F1,52 = 4.07, p = .049, T = 21 < 12 L:12D) differences were found in latency for the first dark zone entry, but no other differences were present (all p > .10) (Figure 3c). While no differences were found for the amount of time spent in the light zone corners (all p > .080) (Figure 3e), a genotype x cycle interaction was found for amount of time spent in the dark corners (F1,52 = 5.86, p = .019) (Figure 3d). B6N mice in the 21 h day spent more time in the dark corners than in the 24 h day (p < .001) and more than B6J mice in the 21 h day (p = .007).
Figure 3.

Light-Dark Box. a) B6N mice spend more time in the dark zone than B6J mice. b) Number of Transitions between the light and dark zones was increased for B6Js in the 21-hour day, but not for B6Ns. c) Strain (B6J < B6N) and light cycle (T = 21 < 12:12 LD) differences were found for Latency for the first dark zone entry. d) B6N under the 21-hour day spend more time in the Dark Corners than B6N in 12:12 LD and B6J in T = 21. e) No differences found for amount of time spent in the Light Corners. †: photoperiod difference, σ: strain difference, *: significantly different from each other, at p < .05.
Since the amount of light exposure is linked to emotionality (see discussion below) and the T = 21 cycle produced increases in novelty-induced locomotor activity in the open field, we decided to analyze these parameters according to each zone of the L-D box (See Table 4 for summary). For the dark zone, a strain difference was uncovered for active time (F1,52 = 6.26, p = .015, B6J < B6N), velocity (F1,52 = 56.93, p < .001, B6J > B6N), but not distance (F1,52 = 0.28, p = .60) nor rears (F1,52 = 0.33, p = .86). In the Light Zone, strain differences were detected for active time (F1,52 = 7.73, p = .008, B6J > B6N), velocity (F1,52 = 11.30, p = .001, B6J > B6N), and rears (F1,52 = 23.61, p < .001, B6J > B6N). A three-way interaction was uncovered for distance traveled in the light lone (F1,52 = 4.12, p = .048), where B6J mice exhibited increased distance traveled compared to B6N mice in the 21 h day when consuming RC (p = .004), but this effect was not present in mice consuming HFD (p = .17).
Table 4.
Dark Zone and Light Zone Locomotor Activity in Light-Dark Box (mean ± SEM). Dark Zone (Top): B6N mice had increased active time and decreased velocity compared to B6J mice, but no differences for distance traveled or rearing were found. Light Zone (Bottom): B6J mice exhibited increased active time, velocity and rears compared to B6N mice. Additionally, B6J mice traveled more distance compared to B6N mice in the T = 21 cycle in mice consuming regular chow. σ indicates a baseline strain difference and italics denotes significant pairwise comparison, at p < .05.
| Genotype | Diet | T-cycle | Active Time (min) (σ) | Distance (cm) | Velocity (cm/s) (σ) | Rears |
|---|---|---|---|---|---|---|
| Dark Zone | ||||||
| B6J | RC | T = 24 | 3.71 ± 0.35 | 579.24 ± 52.72 | 3.06 ± 0.054 | 25.55 ± 6.16 |
| B6J | HF | T = 24 | 3.52 ± 0.36 | 522.37 ± 59.61 | 2.78 ± 0.072 | 28.00 ± 5.75 |
| B6J | RC | T = 21 | 3.88 ± 0.44 | 630.81 ± 86.27 | 3.07 ± 0.13 | 27.37 ± 7.12 |
| B6J | HF | T = 21 | 3.90 ± 0.30 | 619.02 ± 46.66 | 3.00 ± 0.076 | 29.22 ± 5.78 |
| B6N | RC | T = 24 | 3.96 ± 0.68 | 488.70 ± 65.61 | 2.47 ± 0.082 | 26.71 ± 6.38 |
| B6N | HF | T = 24 | 4.40 ± 0.45 | 554.65 ± 70.35 | 2.37 ± 0.085 | 25.66 ± 6.71 |
| B6N | RC | T = 21 | 5.35 ± 0.47 | 661.70 ± 64.28 | 2.50 ± 0.12 | 43.40 ± 6.22 |
| B6N | HF | T = 21 | 4.38 ± 0.66 | 555.21 ± 67.92 | 2.51 ± 0.19 | 26.14 ± 8.80 |
| Light Zone | ||||||
| B6J | RC | T = 24 | 4.91 ± 0.23 | 698.15 ± 31.76 | 2.97 ± 0.10 | 71.00 ± 5.86 |
| B6J | HF | T = 24 | 4.88 ± 0.32 | 706.64 ± 32.01 | 2.84 ± 0.094 | 56.44 ± 4.04 |
| B6J | RC | T = 21 | 4.91 ± 0.39 | 821.90 ± 18.81 | 3.19 ± 0.22 | 64.62 ± 10.01 |
| B6J | HF | T = 21 | 4.53 ± 0.30 | 728.77 ± 40.16 | 3.15 ± 0.11 | 60.66 ± 6.14 |
| B6N | RC | T = 24 | 4.79 ± 0.52 | 678.90 ± 99.26 | 2.77 ± 0.12 | 42.42 ± 10.62 |
| B6N | HF | T = 24 | 3.96 ± 0.47 | 506.60 ± 61.44 | 2.64 ± 0.20 | 42.80 ± 3.93 |
| B6N | RC | T = 21 | 3.59 ± 0.33 | 483.18 ± 58.32 | 2.74 ± 0.21 | 36.50 ± 7.28 |
| B6N | HF | T = 21 | 3.84 ± 0.69 | 529.84 ± 105.94 | 2.66 ± 0.15 | 31.00 ± 9.81 |
BDNF
HFD consumption produced significantly reduced BDNF levels in the hypothalamus compared to mice consuming RC (F1,55 = 8.16, p = .006), but there were no other differences present (all p > .080) (Figure 4).
Figure 4.

Hypothalamic BDNF. HFD consumption reduced BDNF in both strains but the 21 hour day had no effect. ‡: diet difference, at p < .05.
Physiological characteristics
As expected, HFD produced increased weight gain, but there was a diet and genotype interaction present (F1,56 = 7.07, p = .010). Whereas B6N and B6J mice consuming RC had similar weight gain (p = .33), B6J mice on HFD had less weight gain than B6N mice on HFD (p = .001). The T = 21 cycle had no effect on weight gain (F1,56 = 0.69, p = .54) (Figure 5a). Additionally, no differences were present for the average amount of kcals consumed per week (all p > .10) (Figure 5b). HFD produced increases in serum insulin (F1,53 = 16.68, p < .001) and there was an overall strain difference (F1,53 = 35.29, p < .001, B6J < B6N), but no other differences were found (all p > .10) (Figure 5c). HFD produced an increase in fasting glucose (F1,56 = 10.68, p = .002) and the AUC in response to the GTT (F1,56 = 26.06, p < .001) and there was a strain difference for fasting glucose (F1,56 = 8.20, p = .006, B6N > B6J), but no other differences were found (all p > .10) (Figure 5d,e). Strain differences (F1,56 = 14.21, p < .001, B6N > B6J) and diet differences (F1,56 = 5.53, p = .022, HFD > RC) were found for liver triglycerides (Figure 5f).
Figure 5.
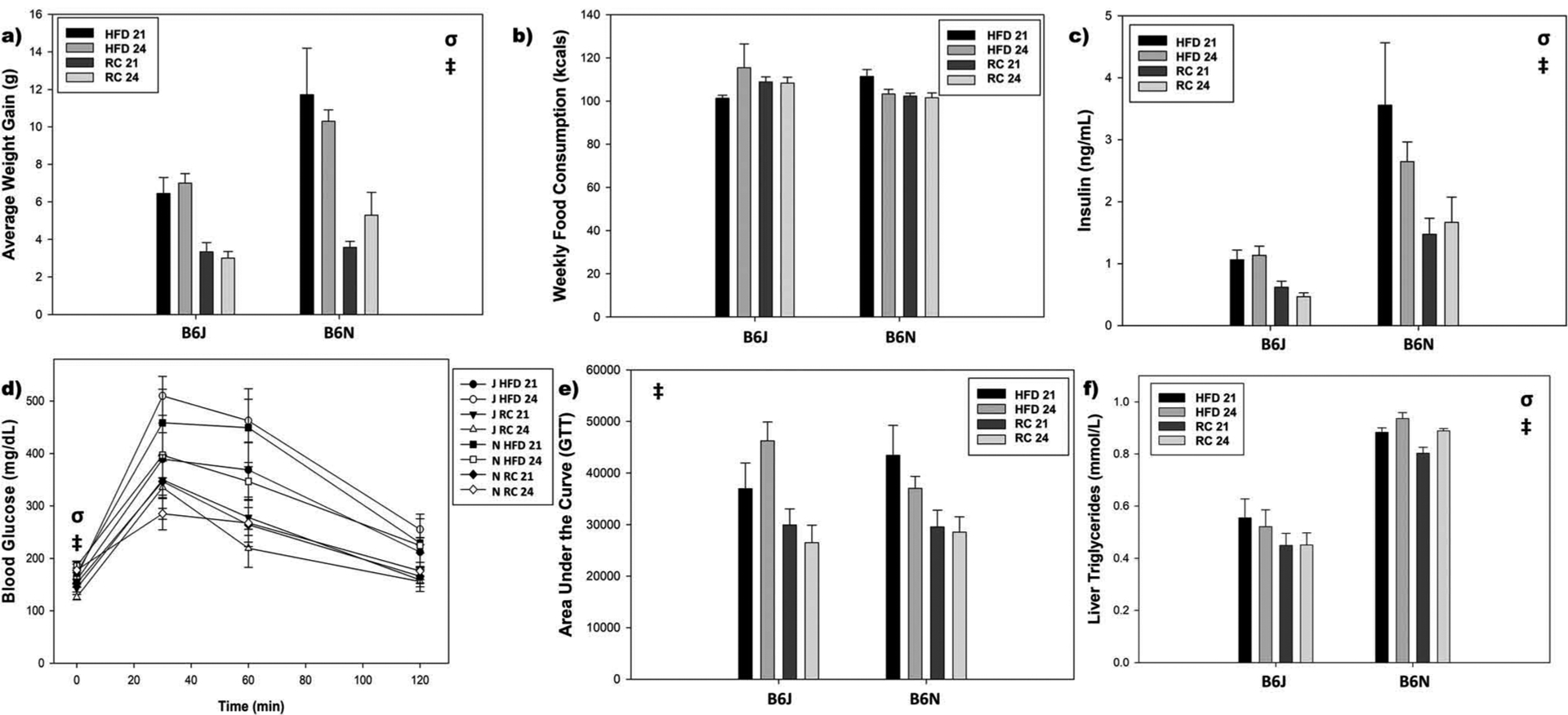
Obesogenic and diabetic phenotypes. a) HFD produced greater weight gain than RC and this difference was more pronounced in B6Ns over B6Js. b) No differences in weekly food consumption. c) Again, HFD produced greater insulin levels than RC and this difference was more pronounced in B6Ns over B6Js. d) HFD produced increases to fasting glucose and B6N mice had higher fasting glucose than B6J mice. e) However, only HFD produced worsened Glucose Tolerance and no strain or photoperiod differences were present. f) Both HFD consuming mice and B6N mice exhibited increased Liver Triglycerides compared to RC and B6J mice. σ: strain difference, ‡: diet difference, at p < .05.
Discussion
In this study, we report several baseline behavioral and physiological differences between the B6J and B6N substrains. Overall, the B6J strain exhibits increased amplitude of home-cage circadian locomotor activity and novelty-induced locomotor activity compared to B6N mice, which is manifested in increasedday and nighttime activity. While the ability to entrain to the photoperiod (on regular chow) was similar between the two strains, B6J mice exhibited increased rhythm power and alpha, as well as longer and more sustainable home-cage locomotor activity bouts compared to B6N mice. Aprevious study showed that these substrains also differ in their circadian periods with B6J mice exhibit longer free-running rhythms in constant darkness compared to B6N, suggesting these differences are not solely due to baseline differences in locomotor activity (Banks etal. 2015). In addition, B6J mice compared to B6N mice exhibited increased exploratory activity in the open field and L-Dbox assessment, evident by increased distance and active time, and increased transitions and decreased dark zone time, respectively.
A recent study from our laboratory has also shown these two substrains differ in their behavioral and physiological responses to constant light and continuous wheel-running access, with B6J mice compared to B6N mice exhibiting longer periods in constant light and more locomotor activity (Capri et al. 2019). The variation in both circadian period and locomotor activity seen between these two substrains may be due to several key genetic differences which may regulate circadian clock function and locomotor activity, including Adcy5 and Pmch (Simon et al. 2013). B6J mice and B6N mice also differ in their stress response and corticosterone function (Sturm et al. 2015), which explains some of the baseline differences seen in this study and others comparing these two substrains. Additionally, we also corroborated that the B6N mouse compared to the B6J mouse is more susceptible to diet-induced obesity and type-2 diabetic-like symptoms when given HFD. While HFD feeding in both genotypes increased insulin levels, liver triglycerides, fasting glucose, and body weight, the B6N mouse compared to the B6J mouse had larger increases in those metabolic characteristics. This disparity may be due to differences in the regulation and expression of Aplp2 and Snap29 between the substrains, genes that may regulate glucose homeostasis and fat development (Heiker et al. 2014). Overall, it would seem that the differing phenotypes seen between the two substrains in both the T = 21 and T = 24 cycles may be due to their underlying genetic differences and that genetic background can modulate both behavioral and physiological responses to biological clock dysregulation.
We uncovered circadian differences between HFD-consuming and RC-consuming animals in their response to a T = 21 cycle, regardless of substrain. We also showed that consumption of HFD lead to a reduction in overall home-cage locomotor activity as seen previously (Gelineau et al. 2017; Hatzidis et al. 2017; Kohsaka et al. 2007). Over 80% of the HFD animals (for both strains) exposed to the T = 21 cycle was not able to entrain to the novel photoperiod and exhibited circadian periods greater than 21 h (B6J mice exhibiting a statistically non-significant longer period in these conditions compared to B6N mice). This dissimilarity is most likely due to HFD exposure producing period lengthening effects, as seen in HFD-consuming animals held in DD (Kohsaka et al. 2007). Both obesity and HFD alter responses to photic phase shifting and simulated jet-lag, with declines in c-fos induction within the SCN after those stimuli (Mendoza et al. 2008; Miranda-Anaya et al. 2017). Additionally, HFD exposure leads to variations in core clock gene transcription in peripheral tissues, but not within the SCN (Kohsaka et al. 2007; Pendergast et al. 2013). Nevertheless, in the current study, and ones previously listed, both HFD-consuming and obese animals exhibited no problems entraining their locomotor activity to a standard 24 h day. These results imply that circadian period and phase changes consequent to HFD exposure are due to alterations in circadian rhythmicity in organs and tissues downstream of the SCN as well as a lack of phase alignment between the master clock and peripheral clocks.
Interestingly, all mice of both substrains, except for one in the current study consuming RC, were able to entrain to the 21 h day. A previous study by Karatsoreos et al. (2011) reported that mice exposed to a 10L:10D cycle could not entrain; shorter days (<10 L:10D) also lead to a lack of entrainment (Fernandez et al. 2018; LeGates et al. 2012). In those studies, the mice experienced worsened physiological characteristics, including poorer glucose tolerance and weight gain. Synchronization to the LD cycle might prevent many of the negative metabolic consequences associated with circadian dysregulation. This idea is corroborated by the finding mice in the T = 21 cycle groups did not have any increased obesity or diabetic characteristics. The lack of entrainment is well known to produce disruptions in metabolic processes. Whether it is dim-light-at-night (Fonken et al. 2013a, 2010), constant room-level lighting (Coomans et al. 2013a; Maroni et al. 2018), or acute (Nascimento et al., 2016a; b) and chronic simulated jet-lag (Antunes et al. 2010; Casiraghi et al. 2016; Gale et al. 2011; Varcoe et al. 2011), all of these animal studies describe similar impairments of health, including obesity, glucose processing, and metabolic hormone concentrations in animals not entrained to the LD cycle. It would appear the inability to synchronize to a photoperiod is associated with poorer metabolic outcomes. Removal of the SCN also produces metabolic syndrome and type-2 diabetic symptoms (Coomans et al. 2013b). Additionally, core clock gene mutants, which by default are circadian dysregulated, are obese and diabetic (Marcheva et al. 2010; Turek et al. 2005). Mice in the T = 21 did not exhibit altered physiological characteristics, including B6N, the substrain which is more susceptible to obesity. This is in contrast to Karatsoreos et al. (2011), who found that a 10 L:10D cycle (T = 20) produced negative metabolic consequences in B6N mice; however, the mice in this study were unable to entrain to the T = 20 cycle, which may be a reason for the differences between the two studies regarding metabolic outcomes. Interestingly, food consumption during the active time reduces the negative health problems associated with both HFD and circadian disruption and increases the robustness of the circadian clock as well (Hatori et al. 2012; Oike et al. 2015). These results imply that the desynchronization between the master circadian clock and peripheral tissues is a driver of the poor metabolic outcomes caused by either clock gene mutations or environmental circadian disruption (shift-work, jet-lag, etc.).
Meanwhile, the physiological differences in this study were mainly caused by HFD consumption and not the T = 21 cycle. HFD consumption leads to desynchrony between the master clock within the suprachiasmatic nucleus (SCN) and peripheral oscillators - molecular rhythms within the SCN are normal, but the molecular clock of digestive system tissues (liver and adipose) and metabolic hormones are altered in a standard LD cycle (Kohsaka et al. 2007). Interestingly, other studies investigating the combined effects of circadian dysregulation (rotating shift-schedules and light-at-night) and HFD consumption found increased obesity and diabetic symptoms in HFD-circadian disrupted animals compared to entrained HFD animals (Fonken et al. 2013a; Kim et al. 2018).Furthermore, HFD consuming mice that did not entrain to the T = 21 cycle did not exhibit poorer metabolic phenotypes, which may indicate differences in physiological responses depending upon the type of circadian disruption. Still it is worth noting that when considering each condition individually (HFD or circadian disruption), consumption of HFD had a much greater effect on metabolic characteristics than circadian disruption, alone, as evident in this study and another recent study (Appiakannan et al. 2019), which only show a moderate increase in obesity and type-2 diabetes symptoms under circadian disruption. For example, HFD reduced BDNF levels within the hypothalamus, the area of the brain that controls both the circadian clock and food regulation, regardless of strain or photoperiod, similar to what was found in other studies using mice and rats (Liu et al. 2014; Molteni et al. 2002).
The T = 21 cycle produced alterations in exploratory locomotor activity within the open-field and L-D box. Overall, the 21 h day produced increases in active time, distance traveled, and velocity, which may be signs of increased impulsivity. Nevertheless, there were some substrain differences in the response to the T = 21 cycle as B6N mice increased their active time in the open field and spent more time in the dark corners of the L-D box, while B6J mice increased their transitions and locomotor activity in the L-D box. Studies of animals held in non-24 h day cycles report abnormal behaviors, including anxiety, exploration, and learning and memory tasks (Fernandez et al. 2018; Karatsoreos et al. 2011; LeGates et al. 2012). Other studies found that genetic disruption of the molecular clock loop within the SCN is sufficient to produce anxiety-like and depressive-like behaviors in rodent models under free-running conditions (Ben-Hamo et al., 2016a; Landgraf et al. 2016). This result implies that environmentally induced circadian disruptions, including abnormal day lengths such as used in these experiments, can affect SCN outputs that lead to altered rhythms downstream of the master clock that control mood regulation. Additionally, reduced rhythm power can also lead to abnormal neurobehavioral outcomes (McGowan and Coogan 2013). Unlike what was found for physiological phenotypes, it would appear that non-24 h day circadian cycles, even if they can be synchronized, produce altered behavioral outcomes. Of note, it would appear that decreased alpha (the time it takes to go from activity onset to offset each day) and rhythm power is correlated with increased in impulsivity and novelty-induced activity in rodent models, which may partially explain the behavioral differences seen here and elsewhere (McGowan and Coogan 2018). Additional studies that directly investigate the role of rhythm power and alpha in modulating behavioral outcomes would be of particular interest.
In this study, the mice were subjected to a shortened T-cycle (T = 21), which means they were exposed to a shortened photoperiod as well (10.5 h of light exposure during the 21 h day). As such, it may be difficult to distinguish whether the differences in the behavioral effects seen here between the two types of ‘day’ cycles are due to a shorter duration of light exposure per day or the desynchronization of the internal clock caused by the non-24 h day. Based upon previous research, the amount of light exposure may be a main modulator of behavior seen here and in previous studies. Previous studies have shown that mice exposed to a 7 L:7D (T = 14) or 10 L:10D (T = 20) cycle do not entrain and exhibit alterations of emotionality and cognitive functions (Fernandez et al. 2018; Karatsoreos et al. 2011; LeGates et al. 2012). Remarkably, we report similar behavioral changes in mice that can entrain to the T = 21 cycle, while the mice in the aforementioned studies did not synchronize to those T-cycles. These results indicate that behavioral changes can occur in mice exposed to circadian cycles shorter than T = 24, whether or not the animal can synchronize to the light cycle. In addition, mice lacking melanopsin (the photopigment that relays light information from the retina to the SCN) are unaffected by the mood-altering effects of the T = 14 cycle, indicating that light exposure to the circadian clock plays a large role in regulating emotionality (Fernandez et al. 2018). It is worth noting, however, that these effects may be relegated to mood regulation, as cognitive deficits occur similarly between melanopsin KO and control mice exposed to a T = 14 cycle (Fernandez et al. 2018). It would appear that the amount of light exposure to the SCN affects mood regulation more so, and these behavioral changes may be less dependent upon dysregulation of the internal circadian clock, per se. Future experiments that investigate the role of photic inputs to the circadian clock would be beneficial to understanding how light exposure, entrainment, and circadian misalignment may differently or similarly affect behavioral outcomes in both humans and animal models.
In conclusion, we report numerous differences between the B6J and B6N strains of male mice for both behavior and physiology, alone, as well as in response to altered circadian cycles and a high-fat diet. B6J mice compared to B6N mice exhibit increased novelty-induced and circadian locomotor activity, even under normally housed conditions without any experimental design; additionally, B6N mice spend more time in the dark zone of the L-D box. B6N mice are more susceptible to the negative metabolic consequences of HFD consumption than B6J. Lastly, both strains of mice exhibited difficulty in entraining to the T = 21 cycle when consuming HFD. Still, some similarities still exist as mice exposed to a 21 h day exhibit altered exploratory behaviors compared to 24 h entrained mice, regardless of genotype. Overall, B6N mice compared to B6Js mice are more susceptible to the negative physiological effects of circadian dysregulation and poor diet consumption with higher baseline anxiety-like behaviors, while B6Js mice compared to B6N mice are more susceptible to behavioral changes in response to circadian disruptions. As recent studies are determining genetic links between metabolic and mood disorders (Penninx and Lange 2018; Zuccoli et al. 2017), the behavioral and physiological differences seen between the two strains under both T-cycles may be connected.
Footnotes
Competing financial interests
The authors declare no competing financial interests.
References
- Ahmad ST, Steinmetz SB, Bussey HM, Possidente B, Seggio JA. 2013. Larval ethanol exposure alters free-running circadian rhythm and per Locus transcription in adult D. melanogaster period mutants. Behav Brain Res. 241:50–55.doi: 10.1016/j.bbr.2012.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes LC, Levandovski R, Dantas G, Caumo W, Hidalgo MP. 2010. Obesity and shift work: chronobiological aspects. Nutr Res Rev. 231:155–168. doi: 10.1017/S0954422410000016 [DOI] [PubMed] [Google Scholar]
- Appiakannan HS, Kestyus DR, Weber ET. 2019. Effects of high fat diet and chronic circadian challenge on glucocorticoid regulation in C57BL/6J mice. Physiol Behav. 204:100–105. doi: 10.1016/j.physbeh.2019.01.014 [DOI] [PubMed] [Google Scholar]
- Banks G, Heise I, Starbuck B, Osborne T, Wisby L, Potter P, … Nolan PM. 2015. Genetic background influences age-related decline in visual and nonvisual retinal responses, circadian rhythms, and sleep. Neurobiol Aging. 361:380–393. doi: 10.1016/j.neurobiolaging.2014.07.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedrosian TA, Nelson RJ. 2017. Timing of light exposure affects mood and brain circuits. Transl Psychiatry. 71: e1017. doi: 10.1038/tp.2016.262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Hamo M, Larson TA, Duge LS, Sikkema C, Wilkinson CW, de la Iglesia HO, González MM. 2016a. Circadian forced desynchrony of the master clock leads to phenotypic manifestation of depression in rats. eNeuro. 36. doi: 10.1523/ENEURO.0237-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Hamo M, Tal K, Paz-Cohen R, Kronfeld-Schor N, Einat H. 2016b. Differential effects of photoperiod length on depression- and anxiety-like behavior in female and male diurnal spiny mice. Physiol Behav. 165:1–6. doi: 10.1016/j.physbeh.2016.06.030 [DOI] [PubMed] [Google Scholar]
- Capri KM, Maroni MJ, Deane HV, Concepcion HA, DeCourcey H, Logan RW, Seggio JA. 2019. Male C57BL/6N and C57BL/6J mice respond differently to constant light and running-wheel access. Front Behav Neurosci. 13:268. doi: 10.3389/fnbeh.2019.00268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin S, Scott-Boyer MP, Praktiknjo S, Jeidane S, Picard S, Reudelhuber TL, Deschepper CF. 2014. Differences in cell-type-specific responses to angiotensin II explain cardiac remodeling differences in C57BL/6 mouse substrains. Hypertension. 645:1040–1046. doi: 10.1161/HYPERTENSIONAHA.114.04067 [DOI] [PubMed] [Google Scholar]
- Casiraghi LP, Alzamendi A, Giovambattista A, Chiesa JJ, Golombek DA. 2016. Effects of chronic forced circadian desynchronization on body weight and metabolism in male mice. Physiol Rep. 48. doi: 10.14814/phy2.12743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, … Lee FS. 2006. Genetic variant BDNF Val66Met polymorphism alters anxiety-related behavior. Science. 3145796:140–143. doi: 10.1126/science.1129663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coomans CP, van den Berg SA, Houben T, van Klinken JB, van den Berg R, Pronk AC, … Meijer JH. 2013a. Detrimental effects of constant light exposure and high-fat diet on circadian energy metabolism and insulin sensitivity. Faseb J. 274:1721–1732. doi: 10.1096/fj.12-210898 [DOI] [PubMed] [Google Scholar]
- Coomans CP, van den Berg SA, Lucassen EA, Houben T, Pronk AC, van der Spek RD, … Meijer JH. 2013b. The suprachiasmatic nucleus controls circadian energy metabolism and hepatic insulin sensitivity. Diabetes. 624:1102–1108. doi: 10.2337/db12-0507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez DC, Fogerson PM, Lazzerini Ospri L, Thomsen MB, Layne RM, Severin D, … Hattar S. 2018. Light affects mood and learning through distinct retina-brain pathways. Cell. 1751:71–84.e18. doi: 10.1016/j.cell.2018.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Lieberman RA, Weil ZM, Nelson RJ. 2013a. Dim light at night exaggerates weight gain and inflammation associated with a high-fat diet in male mice. Endocrinology. 15410:3817–3825. doi: 10.1210/en.2013-1121 [DOI] [PubMed] [Google Scholar]
- Fonken LK, Nelson RJ. 2013b. Dim light at night increases depressive-like responses in male C3H/HeNHsd mice. Behav Brain Res. 243:74–78. doi: 10.1016/j.bbr.2012.12.046 [DOI] [PubMed] [Google Scholar]
- Fonken LK, Workman JL, Walton JC, Weil ZM, Morris JS, Haim A, Nelson RJ. 2010. Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci U S A. 10743:18664–18669. doi: 10.1073/pnas.1008734107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale JE, Cox HI, Qian J, Block GD, Colwell CS, Matveyenko AV. 2011. Disruption of circadian rhythms accelerates development of diabetes through pancreatic beta-cell loss and dysfunction. J Biol Rhythms. 265:423–433. doi: 10.1177/0748730411416341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelineau RR, Arruda NL, Hicks JA, Monteiro De Pina I, Hatzidis A, Seggio JA. 2017. The behavioral and physiological effects of high-fat diet and alcohol consumption: sex differences in C57BL/6J mice. Brain Behav. 76:e00708. doi: 10.1002/brb3.708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genzer Y, Dadon M, Burg C, Chapnik N, Froy O. 2016. Effect of dietary fat and the circadian clock on the expression of brain-derived neurotrophic factor BDNF. Mol Cell Endocrinol. 430:49–55. doi: 10.1016/j.mce.2016.04.015 [DOI] [PubMed] [Google Scholar]
- Gómez-Santos C, Gómez-Abellán P, Madrid JA, Hernández-Morante JJ, Lujan JA, Ordovas JM, Garaulet M. 2009. Circadian rhythm of clock genes in human adipose explants. Obesity Silver Spring. 178:1481–1485. doi: 10.1038/oby.2009.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, … Panda S. 2012. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 156:848–860. doi: 10.1016/j.cmet.2012.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzidis A, Hicks JA, Gelineau RR, Arruda NL, Monteiro De Pina I, O’Connell KE, Seggio JA. 2017. Removal of a high-fat diet, but not voluntary exercise, reverses obesity and diabetic-like symptoms in male C57BL/6J mice. Hormones Athens. 161:62–74. doi: 10.14310/horm.2002.1720 [DOI] [PubMed] [Google Scholar]
- Heiker JT, Kunath A, Kosacka J, Flehmig G, Knigge A, Kern M, … Klöting N. 2014. Identification of genetic loci associated with different responses to high-fat diet-induced obesity in C57BL/6N and C57BL/6J substrains. Physiol Genomics. 4611:377–384. doi: 10.1152/physiolgenomics.00014.2014 [DOI] [PubMed] [Google Scholar]
- Hicks JA, Hatzidis A, Arruda NL, Gelineau RR, De Pina IM, Adams KW, Seggio JA. 2016. Voluntary wheel-running attenuates insulin and weight gain and affects anxiety-like behaviors in C57BL/6J mice exposed to a high-fat diet. Behav Brain Res. 310:1–10. doi: 10.1016/j.bbr.2016.04.051 [DOI] [PubMed] [Google Scholar]
- Ikeno T, Deats SP, Soler J, Lonstein JS, Yan L. 2016. Decreased daytime illumination leads to anxiety-like behaviors and HPA axis dysregulation in the diurnal grass rat Arvicanthis niloticus. Behav Brain Res. 300:77–84. doi: 10.1016/j.bbr.2015.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH, McEwen BS. 2011. Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc Natl Acad Sci U S A. 1084:1657–1662. doi: 10.1073/pnas.1018375108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz G, Durst R, Zislin Y, Barel Y, Knobler HY. 2001. Psychiatric aspects of jet lag: review and hypothesis. Med Hypotheses. 561:20–23. doi: 10.1054/mehy.2000.1094 [DOI] [PubMed] [Google Scholar]
- Kim SM, Neuendorff N, Alaniz RC, Sun Y, Chapkin RS, Earnest DJ. 2018. Shift work cycle-induced alterations of circadian rhythms potentiate the effects of high-fat diet on inflammation and metabolism. Faseb J. 326:3085–3095. doi: 10.1096/fj.201700784R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, … Bass J. 2007. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 65:414–421. doi: 10.1016/j.cmet.2007.09.006 [DOI] [PubMed] [Google Scholar]
- Landgraf D, Long JE, Proulx CD, Barandas R, Malinow R, Welsh DK. 2016. Genetic disruption of circadian rhythms in the suprachiasmatic nucleus causes helplessness, behavioral despair, and anxiety-like behavior in mice. Biol Psychiatry. 8011:827–835. doi: 10.1016/j.biopsych.2016.03.1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laposky AD, Bradley MA, Williams DL, Bass J, Turek FW. 2008. Sleep-wake regulation is altered in leptin-resistant db/db genetically obese and diabetic mice. Am J Physiol Regul Integr Comp Physiol. 2956:R2059–2066. doi: 10.1152/ajpregu.00026.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeGates TA, Altimus CM, Wang H, Lee HK, Yang S, Zhao H, … Hattar S. 2012. Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature. 4917425:594–598. doi: 10.1038/nature11673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhu Z, Kalyani M, Janik JM, Shi H. 2014. Effects of energy status and diet on Bdnf expression in the ventro-medial hypothalamus of male and female rats. Physiol Behav. 130:99–107. doi: 10.1016/j.physbeh.2014.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan RW, Hasler BP, Forbes EE, Franzen PL, Torregrossa MM, Huang YH, … McClung CA. 2018. Impact of sleep and circadian rhythms on addiction vulnerability in adolescents. Biol Psychiatry. 8312:987–996. doi: 10.1016/j.biopsych.2017.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, … Bass J. 2010. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 4667306:627–631. doi: 10.1038/nature09253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroni MJ, Capri KM, Cushman AV, Monteiro De Pina IK, Chasse MH, Seggio JA. 2018. Constant light alters serum hormone levels related to thyroid function in male CD-1 mice. Chronobiol Int. 3510:1456–1463. doi: 10.1080/07420528.2018.1488259 [DOI] [PubMed] [Google Scholar]
- McClung CA. 2007. Circadian genes, rhythms and the biology of mood disorders. Pharmacol Ther. 1142:222–232. doi: 10.1016/j.pharmthera.2007.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan NM, Coogan AN. 2013. Circadian and behavioural responses to shift work-like schedules of light/dark in the mouse. J Mol Psychiatry. 11:7. doi: 10.1186/2049-9256-1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan NM, Coogan AN. 2018. Sleep and circadian rhythm function and trait impulsivity: an actigraphy study. Psychiatry Res. 268:251–256. doi: 10.1016/j.psychres.2018.07.030 [DOI] [PubMed] [Google Scholar]
- Mendoza J, Pévet P, Challet E. 2008. High-fat feeding alters the clock synchronization to light. J Physiol. 58624:5901–5910. doi: 10.1113/jphysiol.2008.159566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda-Anaya M, Luna-Moreno D, Carmona-Castro A, Díaz-Muñoz M. 2017. Differences in photic entrainment of circadian locomotor activity between lean and obese volcano mice. J Circadian Rhythms. 15:1. doi: 10.5334/jcr.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistlberger RE, Lukman H, Nadeau BG. 1998. Circadian rhythms in the Zucker obese rat: assessment and intervention. Appetite. 303:255–267. doi: 10.1006/appe.1997.0134 [DOI] [PubMed] [Google Scholar]
- Molteni R, Barnard RJ, Ying Z, Roberts CK, Gómez-Pinilla F. 2002. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience. 1124:803–814. doi: 10.1016/s0306-4522(02)00123-9 [DOI] [PubMed] [Google Scholar]
- Moreth K, Fischer R, Fuchs H, Gailus-Durner V, Wurst W, Katus HA, … Hrabě de Angelis M. 2014. High-throughput phenotypic assessment of cardiac physiology in four commonly used inbred mouse strains. J Comp Physiol B. 1846:763–775. doi: 10.1007/s00360-014-0830-3 [DOI] [PubMed] [Google Scholar]
- Nascimento NF, Hicks JA, Carlson KN, Hatzidis A, Amaral DN, Logan RW, Seggio JA. 2016a. Long-term wheel-running and acute 6-h advances alter glucose tolerance and insulin levels in TALLYHO/JngJ mice. Chronobiol Int. 331:108–116. doi: 10.3109/07420528.2015.1108330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento NF, Hicks JA, Carlson KN, Hatzidis A, Amaral DN, Seggio JA. 2016b. 6-h advances alter circadian activity patterns, fasting glucose, and insulin levels in C57BL/6J mice. Biol Rhythm Res. 471:133–143. doi: 10.1080/09291016.2015.1088188 [DOI] [Google Scholar]
- Oike H, Sakurai M, Ippoushi K, Kobori M. 2015. Time-fixed feeding prevents obesity induced by chronic advances of light/dark cycles in mouse models of jet-lag/shift work. Biochem Biophys Res Commun. 4653:556–561. doi: 10.1016/j.bbrc.2015.08.059 [DOI] [PubMed] [Google Scholar]
- Okuliarova M, Molcan L, Zeman M. 2016. Decreased emotional reactivity of rats exposed to repeated phase shifts of light-dark cycle. Physiol Behav. 156:16–23. doi: 10.1016/j.physbeh.2016.01.003 [DOI] [PubMed] [Google Scholar]
- Pendergast JS, Branecky KL, Yang W, Ellacott KL, Niswender KD, Yamazaki S. 2013. High-fat diet acutely affects circadian organisation and eating behavior. Eur J Neurosci. 378:1350–1356. doi: 10.1111/ejn.12133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninx BWJH Lange SMM. 2018. Metabolic syndrome in psychiatric patients: overview, mechanisms, and implications. Dialogues Clin Neurosci. 20(1):63–73. PMCID: PMC6016046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podrini C, Cambridge EL, Lelliott CJ, Carragher DM, Estabel J, Gerdin AK, … Project SMG. 2013. High-fat feeding rapidly induces obesity and lipid derangements in C57BL/6N mice. Mamm Genome. 245–6:240–251. doi: 10.1007/s00335-013-9456-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portaluppi F, Smolensky MH, Touitou Y. 2010. Ethics and methods for biological rhythm research on animals and human beings. Chronobiol Int. 27:1911–1929. doi: 10.3109/07420528.2010.516381 [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM, Fixaris MC, McCulley WD. 2015. Photoperiodic modulation of voluntary ethanol intake in C57BL/6 mice. Physiol Behav. 147:342–347. doi: 10.1016/j.physbeh.2015.05.011 [DOI] [PubMed] [Google Scholar]
- Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB, Fitzgerald GA. 2004. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 211:e377. doi: 10.1371/journal.pbio.0020377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadacca LA, Lamia KA, deLemos AS, Blum B, Weitz CJ. 2011. An intrinsic circadian clock of the pancreas is required for normal insulin release and glucose homeostasis in mice. Diabetologia. 541:120–124. doi: 10.1007/s00125-010-1920-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi SQ, Ansari TS, McGuinness OP, Wasserman DH, Johnson CH. 2013. Circadian disruption leads to insulin resistance and obesity. Curr Biol. 235:372–381. doi: 10.1016/j.cub.2013.01.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon MM, Greenaway S, White JK, Fuchs H, Gailus-Durner V, Wells S, … Brown SD. 2013. A comparative phenotypic and genomic analysis of C57BL/6J and C57BL/6N mouse strains. Genome Biol. 147:R82. doi: 10.1186/gb-2013-14-7-r82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm M, Becker A, Schroeder A, Bilkei-Gorzo A, Zimmer A. 2015. Effect of chronic corticosterone application on depression-like behavior in C57BL/6N and C57BL/6J mice. Genes Brain Behav. 143:292–300. doi: 10.1111/gbb.12208 [DOI] [PubMed] [Google Scholar]
- Tapia-Osorio A, Salgado-Delgado R, Angeles-Castellanos M, Escobar C. 2013. Disruption of circadian rhythms due to chronic constant light leads to depressive and anxiety-like behaviors in the rat. Behav Brain Res. 252:1–9. doi: 10.1016/j.bbr.2013.05.028 [DOI] [PubMed] [Google Scholar]
- Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, … Bass J. 2005. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 3085724:1043–1045. doi: 10.1126/science.1108750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varcoe TJ, Wight N, Voultsios A, Salkeld MD, Kennaway DJ. 2011. Chronic phase shifts of the photoperiod throughout pregnancy programs glucose intolerance and insulin resistance in the rat. PLoS One. 64:e18504. doi: 10.1371/journal.pone.0018504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccoli GS, Saia-Cereda VM, Nascimento JM, Martins-de-Souza D. 2017. The energy metabolism dysfunction in psychiatric disorders postmortem brains: focus on proteomic evidence. Front Neurosci. 11:493. doi: 10.3389/fnins.2017.00493 [DOI] [PMC free article] [PubMed] [Google Scholar]


