Abstract
Background:
The prevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection among asymptomatic patients admitted to hospital has implications for personal protective equipment use, testing strategy and confidence in the safety of acute care services. Our aim was to estimate the positivity rate of reverse transcription polymerase chain reaction (RT-PCR) testing among people admitted to hospital without symptoms of coronavirus disease 2019 (COVID-19) in Alberta, Canada.
Methods:
Between Apr. 9 and May 24, 2020, we screened for COVID-19 symptoms and tested for SARS-CoV-2 infection in all consecutive adult patients (≥ 18 yr) admitted via emergency department to 3 Alberta hospitals. We summarized the parameters of the epidemic curve and assessed the performance of symptom screening versus RT-PCR results on nasopharyngeal or oropharyngeal swab samples.
Results:
The study period encompassed Alberta’s initial epidemic curve, with peak active cases per 100 000 of 71.4 (0.07%) on Apr. 30, 2020, and 14.7 and 14.6 at the beginning (Apr. 9, 2020) and end (May 24, 2020), respectively. Testing for SARS-CoV-2 infection (64.9% throat and 35.1% nasopharyngeal swabs) was done on 3375 adults (mean age 51, standard deviation 21, yr; 51.5% men). None of the asymptomatic patients (n = 1814) tested positive, and 71 of those with symptoms tested positive (n = 1561; 4.5%, 95% confidence interval [CI] 3.6%–5.7%). Sensitivity of symptom screening (v. RT-PCR) was 100% (95% CI 95%–100%), and specificity was 55% (95% CI 53%–57%). Posttest probabilities for prevalence of SARS-CoV-2 infection ranging from 1.5 to 14 times the peak prevalence of active cases during the study did not change when we assumed lower sensitivity (92%).
Interpretation:
In a region with low disease prevalence where protocolized symptom assessment was in place during the admission process, we did not identify people admitted to hospital without COVID-19 symptoms who were RT-PCR positive. There may not be additive benefit to universal testing of asymptomatic patients on hospital admission in a setting of low pretest probability and strong public health containment.
Compared with other highly pathogenic human coronaviruses (Middle East respiratory syndrome coronavirus and severe acute respiratory syndrome coronavirus), severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus responsible for coronavirus disease 2019 (COVID-19), has a lower case-fatality rate but spreads more efficiently.1 SARS-CoV-2 mostly spreads by respiratory droplets among people who are in close contact.2 Aerosol transmission can occur in some settings, especially in indoor, crowded and inadequately ventilated spaces where people stay for long periods.2 Studies are underway to examine the conditions under which aerosol transmission occurs outside of medical facilities where aerosol-generating procedures are conducted.2 Contact spread (direct or via contaminated articles or surfaces) can also occur.2
Transmission of SARS-CoV-2 is possible from people without classic respiratory symptoms (e.g., asymptomatic, presymptomatic and paucisymptomatic), although this has mostly been documented in close quarters (e.g., within households and cruise ships).3 Studies in various settings have shown that 15%–50% of people with positive results on reverse transcription polymerase chain reaction (RT-PCR) testing were asymptomatic at testing.4–6 Although presymptomatic spread has been described, the contribution of truly asymptomatic transmission remains unclear.6 If people who are unknowingly positive for SARS-CoV-2 infection are admitted to hospital, they can infect health care workers or other patients.3,7–9
The prevalence of asymptomatic carriers of SARS-CoV-2 has been shown to depend on how widespread SARS-CoV-2 infection is in a population, with estimates ranging from 0.34% in Iceland (where 0.8% of the population was positive for SARS-CoV-2) to 10% on the Diamond Princess cruise ship (where 20% of passengers were positive).3
Given the frequency of close human interaction in hospital settings, the risk of SARS-CoV-2 transmission from asymptomatic patients could be higher than in settings of community transmission, if appropriate standard precautions are neglected. According to a report during the first explosive outbreak in New York, 15% of currently asymptomatic women admitted for delivery had positive SARS-CoV-2 testing, further underscoring the importance of local epidemiology in guiding protective measures.10 Swab results alone without a 4-week symptom history, however, may overestimate the risk of asymptomatic transmission, as RT-PCR can remain positive after COVID-19 recovery owing to the detection of nonviable virus.11 According to a recent report from the United Kingdom, 40% of asymptomatic health care workers who tested positive had symptoms more than 1 week before testing.12
To inform the appropriate use of personal protective equipment (PPE) and other in-hospital precautions, including isolation requirements, room assignments and follow-up strategies for contact tracing, we screened for symptoms of COVID-19 and tested for SARS-CoV-2-infection in all patients admitted to 3 tertiary care hospitals via an emergency department during the peak of the epidemic curve in Alberta, Canada. Our aim was to estimate the positivity rate of RT-PCR testing among people admitted to hospital without COVID-19 symptoms.
Methods
Study design and setting
As part of the epidemic response, all patients admitted to hospital via emergency department who had symptoms according to a symptom assessment protocol were tested for SARS-CoV-2 infection. In this prospective quality-improvement study, we temporarily expanded this testing strategy (from Apr. 9 to May 24, 2020) to all consecutive people who were admitted through 3 Alberta Health Services emergency departments (1 each in Calgary [Peter Lougheed Centre], Central Zone [Red Deer Regional Hospital Centre], and Edmonton [Royal Alexandra Hospital]) and screened negative for COVID-19 symptoms. The first and third hospitals are tertiary teaching hospitals in large urban centres, and the second is a referral centre and the only hospital within a city of more than 100 000 people.
Participants
Study participants, who would not otherwise have been tested for SARS-CoV-2 infection, had nasopharyngeal or throat sampling for SARS-CoV-2 RT-PCR before transfer to an inpatient unit. We did not include in this study people who were discharged from the emergency department. We closely monitored all asymptomatic study participants for symptoms after the first test in the emergency department (Table 1). Asymptomatic patients were not routinely put on contact and droplet precautions, unless they had recently travelled or had been in contact with a probable or confirmed case (Table 2). We included adult patients (≥ 18 yr).
Table 1:
Alberta Health Services COVID-19 symptom screening questions*
| 1. Do you have the symptoms below? | Please circle | |
| • Fever (> 38°C) | Yes | No |
| • Cough | Yes | No |
| • Shortness of breath | Yes | No |
| • Difficulty breathing | Yes | No |
| • Sore throat | Yes | No |
Note: COVID-19 = coronavirus disease 2019.
Used to determine the need for testing (outside the context of this study) during the study period.
Table 2:
Alberta Health Services COVID-19 risk assessment screening questions*
| 1. Have you returned to Canada from outside the country (including the US) in the past 14 days? | Yes | No |
| 2. Do you live with or have had close contact† (within 2 m/6 ft) with a person with an influenza-like illness who has travelled outside of Canada within the last 14 days before their illness? | Yes | No |
| 3. Do you live with or have had close contact† (within 2 m/6 ft) with someone who is ill with fever and/or cough and influenza-like illness symptoms? | Yes | No |
| 4. Have you had close contact† (within 2 m/6 ft) with a confirmed or probable case of COVID-19? | Yes | No |
| If the answer is “No” to all of the above, the patient is considered low risk. |
Note: COVID-19 = coronavirus disease 2019, PPE = personal protective equipment.
Questionnaire used during the study period to determine the need for isolation, but not specifically to guide testing.
A close contact is an individual who provided care for the person who was ill, including health care workers, family members or other caregivers, or who had other similar close physical contact without consistent and appropriate use of PPE, or lived with or otherwise had close prolonged contact (within 2 m/6 ft) with a person while they were ill, or had direct contact with infectious bodily fluids of a person (e.g., was coughed or sneezed on) while not wearing recommended PPE.
Procedures
Emergency department staff screened all study participants for symptoms with the Alberta Health Services COVID-19 symptom screening questionnaire in use during the study period (Table 1) and collected nasopharyngeal or oropharyngeal specimens, depending on the provincial guidance at the time. The list was limited to core respiratory symptoms (e.g., fever, cough, sore throat and breathing difficulties); staff may also have considered exposure history and other symptoms (e.g., gastrointestinal symptoms or loss of taste or smell) when determining which patients to test as new evidence emerged during the study. ProvLab (Alberta Precision Laboratories) performed SARS-CoV-2 RT-PCR on each sample and provided data on age, sex, location of test order and test result. Provincial protocols were in place for notification and contact tracing of positive cases, including symptom assessment by Infection Prevention and Control.
Testing method
We used 2 swab kits for collecting samples: FLOQSwab and Universal Transport Medium for nasopharyngeal swabs, and APTIMA Unisex or Multi-test Swab Specimen Collection Kit for throat swabs. We used a validated laboratory-developed real-time RT-PCR assay.13
Outcome measure
We assessed the proportion of patients who tested positive for SARS-CoV-2 infection by presence versus absence of symptoms.
Statistical analysis
We used frequencies, means and standard deviations (SDs) for sample description. We used the methods proposed by Wilson to estimate the 95% confidence intervals (CIs) of proportions,14 because standard methods for CI estimation that take a multiple of the standard error either side of the estimated quantity are sensitive to extreme values of these quantities. Accordingly, we did sample size estimation using a margin of error approach,15 assuming an α error of 0.05. We estimated that we needed to test at least 1500 participants to estimate a prevalence of 1% with a margin of error of 0.5%–1.5%. Of note, we designed this study before the epidemic peak (the highest prevalence of active cases during the outbreak in Alberta). To provide additional context, we summarized the incidence of daily cases in Alberta for the health zones, including the study sites (i.e., 3 zone-specific epidemic curves).
We also estimated the effective (time-dependent) reproductive number (Rt) using a Bayesian approach given the time series of daily incident (laboratory-confirmed) cases and the distribution of the serial interval (EpiEstim, R package version 2.2-1), to identify the point in time when it remained consistently below 1 (indicating the outbreak was under control). Rt is the average number of secondary cases that would be produced by a primary case infected at time t, if conditions remained constant after time t. Rt is useful to monitor the changes in the transmissibility of the epidemic over time in response to public health interventions, in contrast to the basic reproductive number (R0) used when all individuals of a population are susceptible to infection at the beginning of an outbreak. Since there are no Canadian data on serial interval (the time interval between the onset of symptoms in the primary case and the onset of symptoms in a secondary case), we assumed an uncertain distribution of the serial interval, with values drawn from a γ distribution, with the mean and variance sampled from truncated normal distributions. For these distributions, we used parameters estimated from existing studies.16–19
We estimated sensitivity, specificity, and positive and negative predictive values using epiR. We calculated posttest probabilities for a range of COVID-19 prevalence estimates, from 1.5 to 14 times the peak prevalence of active cases during the study, and assuming a lower sensitivity of the symptom screening to account for the fact that most of our RT-PCR tests were done using throat swabs.
Ethics approval
We obtained institutional review board approval for this study with waiver of patient consent (University of Calgary REB20-0689).
Results
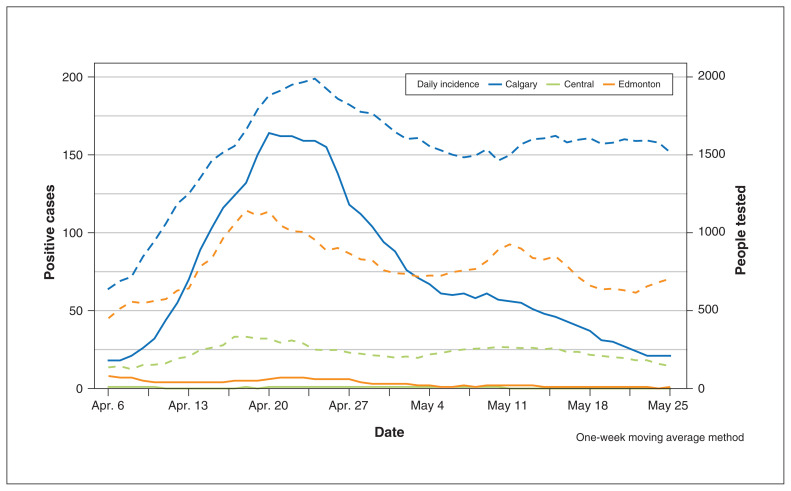
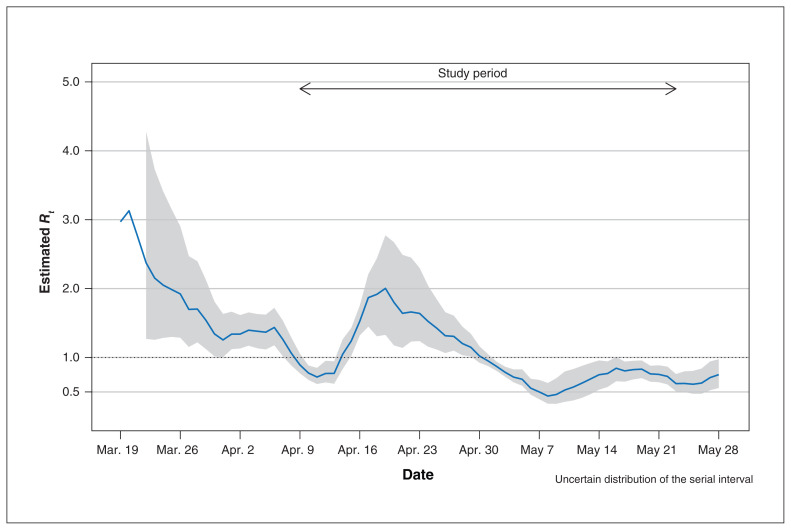
After the provincial declaration of the state of public health emergency (Mar. 17), about half of the hospital admissions at the 3 study sites occurred via emergency department access. Figure 1 shows the daily new cases of COVID-19 and the number of Albertans who underwent testing in these 3 zones (Calgary, Central Zone and Edmonton). The study period encompassed Alberta’s initial epidemic curve, with peak active cases per 100 000 of 71.4 (0.07%) (on Apr. 30, 2020), and 14.7 and 14.6 at the beginning (Apr. 9. 2020) and end of the study (May 24, 2020), respectively. The Calgary Zone accounted for most of the cases. The effective reproductive number remained below 1 after Apr. 30, 2020 (Figure 2).
Figure 1:
Daily incidence of testing (dashed lines) and positive cases (solid lines) across Alberta by zone using moving average methodology (with a width of 7 days for the rolling window).
Figure 2:
Estimates of the effective reproductive number (Rt, solid line) and 95% credible interval (grey area) during the coronavirus disease 2019 outbreak in Alberta.
Cohort description
During the study period, 3375 people (mean age 51 [SD 21] yr; 51.5% men) were admitted to hospital through the emergency departments in the 3 study sites, screened for symptoms and tested (64.9% via throat swab, 35.1% via nasopharyngeal swab). Of these (Table 3), 1814 (53.7%) people were asymptomatic (mean age 55 [SD 22] yr; 51.7% men) and 1561 had symptoms (mean age 47 [SD 19] yr; 51.4% men).
Table 3:
Characteristics of the study participants
| Study participants | Asymptomatic | Symptomatic | |
|---|---|---|---|
| No. (%) | 3375 (100) | 1814 (53.7) | 1561 (46.3) |
| Age, yr, mean ± standard deviation | 51 ± 21 | 55 ± 22 | 47 ± 19 |
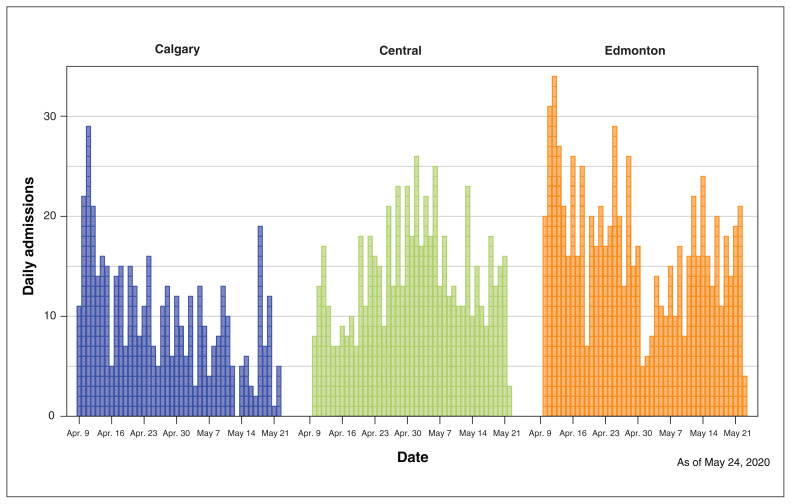
Figure 3 shows the distribution of study participants who were admitted daily by site over the study period: 755 in the Edmonton Zone (41.6%), 614 in the Central Zone (33.8%) and 445 (24.5%) in the Calgary Zone. A mean of 40 study participants were admitted each day (SD 12; median 39, range 4–73). Results were available within the same day of testing (16.2%), within 1 day (75.4%) or within 2 days of testing (98.1%); 2.3% were available on days 3–7.
Figure 3:
Distribution of daily hospital admissions via emergency department by hospital during the study period in each study site (Calgary, Peter Lougheed Centre; Central Zone, Red Deer Regional Hospital Centre; and Edmonton, Royal Alexandra Hospital).
Outcomes
None of the patients without symptoms of COVID-19 tested positive for SARS-CoV-2 at emergency department screening. During their time in hospital, 26 asymptomatic patients developed symptoms possibly suggestive of COVID-19 and were tested a second time; 7 were tested a third time and 1 patient was tested 5 times. All tests were negative.
Among those who were admitted via emergency departments during the study period and had symptoms, 71 were positive (4.5%, 95% CI 3.6%–5.7%). Of these, 68 were from the Calgary Zone hospital (prevalence in the Calgary Zone 7.5%, 95% CI 6.0%–9.5%), 3 were from Edmonton and none were from the Central Zone.
The symptom screening tool had 100% sensitivity and 55% specificity in identifying cases of SARS-CoV-2 infection among people admitted to hospital via the emergency department. Posttest probabilities for prevalence of SARS-CoV-2 infection ranging from 1.5 to 14 times the peak prevalence of active cases during the study did not change when we assumed lower sensitivity (Tables 4, 5 and 6).
Table 4:
Performance of the symptom screening tool
| SARS-CoV-2 positive | SARS-CoV-2 negative | Total | |
|---|---|---|---|
| Screening positive | 71 | 1490 | 1561 |
| Screening negative | 0 | 1814 | 1814 |
| Total | 71 | 3304 | 3375 |
| Point estimates (95% CI) | |||
| Apparent prevalence | 0.46 (0.45–0.48) | ||
| True prevalence | 0.02 (0.02–0.03) | ||
| Accuracy | 0.56 (0.54–0.58) | ||
| Sensitivity | 1.00 (0.95–1.00) | ||
| Specificity | 0.55 (0.53–0.57) | ||
| Positive predictive value | 0.04 (0.04–0.06) | ||
| Negative predictive value | 1.00 (0.998–1.00) | ||
| Positive likelihood ratio* | 2.19 (2.09–2.29) | ||
| Negative likelihood ratio* | 0.02 (0.004–0.18) | ||
| Odds ratio* | 87.65 (12.16–631.50) |
Note: CI = confidence interval, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
To estimate positive likelihood ratio, negative likelihood ratio and diagnostic odds ratio, we added 1 to each cell.
Table 5:
Performance of the symptom screening tool (sensitivity analysis)*
| SARS-CoV-2 positive | SARS-CoV-2 negative | Total | |
|---|---|---|---|
| Screening positive | 71 | 1490 | 1561 |
| Screening negative | 6 | 1808 | 1814 |
| Total | 77 | 3298 | 3375 |
| Point estimates (95% CI) | |||
| Apparent prevalence | 0.46 (0.45–0.48) | ||
| True prevalence | 0.02 (0.02–0.03) | ||
| Accuracy | 0.56 (0.54–0.57) | ||
| Sensitivity | 0.92 (0.84–0.97) | ||
| Specificity | 0.55 (0.53–0.56) | ||
| Positive predictive value | 0.04 (0.04–0.06) | ||
| Negative predictive value | 0.997 (0.993–0.998) | ||
| Positive likelihood ratio | 2.04 (1.89–2.20) | ||
| Negative likelihood ratio | 0.14 (0.07–0.31) | ||
| Odds ratio | 14.36 (6.22–33.13) |
Note: CI = confidence interval, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
Assuming asymptomatic prevalence = 0.0035 (6 people with SARS-CoV-2 infection among 1814 without symptoms).
Table 6:
Probabilities of SARS-CoV-2 infection before and after symptom screening*
| Pretest probability | Posttest probability | T+ | Posttest probability | T− | |
|---|---|---|---|
| SE = 0.986 SP = 0.549 (Table 4) |
0.001 | 0.0022 | 0 |
| 0.005 | 0.01 | 0.0001 | |
| 0.010 | 0.02 | 0.0003 | |
| SE = 0.922 SP = 0.548 (Table 5) |
0.001 | 0.002 | 0.0001 |
| 0.005 | 0.01 | 0.0007 | |
| 0.010 | 0.02 | 0.0014 |
Note: SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2, SE = sensitivity, SP = specificity.
Pretest probability indicates the prevalence of SARS-CoV-2 infection in the population; posttest probability | T+ indicates the probability of having SARS-CoV-2 infection given a positive symptom screening; posttest probability | T− indicates the probability of having SARS-CoV-2 infection given a negative symptom screening. Test performance measures are from main results (Table 4) and from sensitivity analysis (Table 5). Of note, a pretest probability of 0.005 is about 7 times the prevalence of active cases in Alberta during the study period (0.07%).
Interpretation
Asymptomatic transmission of SARS-CoV-2 could reduce the effectiveness of control measures informed by symptoms, with potentially serious consequences in hospital settings where the frequency of close human interaction is high. In this study, we found that none of the 1814 consecutive people who were admitted to hospital via 1 of the 3 emergency departments in Alberta without symptoms consistent with SARS-CoV-2 infection had a positive RT-PCR swab. Conversely, 4.5% of people with symptoms in the same emergency department settings tested positive (7.5% at the hospital within the Calgary Zone). These findings suggest that symptom assessment during admission processes may effectively identify patients who do not require RT-PCR testing or isolation in geographic regions where the prevalence of active cases (pretest probability) is low, as it was in most regions of Canada during the first wave of COVID-19.20
The government of Alberta implemented a rapid response to the COVID-19 outbreak, instituting aggressive public health measures of containment within 3 weeks of detecting the first positive case in Alberta (Mar. 5, 2020). In addition, all patients presenting to hospital during this period were screened for core respiratory symptoms. We conducted our study during a time frame when localized outbreaks were identified in many long-term care and designated supportive living facilities, and others in workplaces, shelters and the community, including the largest outbreak in a meat packing plant with more than 1000 cases within the Calgary Zone. During the first 3 weeks of the study the estimated effective reproductive number was still above 1, with a prevalence of active cases peaking at 71.4 per 100 000 during this time (0.07%). While estimates of prevalent active cases varied across Alberta as they did in regions within other Canadian provinces, the provincial average of prevalent active cases in Alberta was higher than in most Canadian provinces during the first wave of COVID-19, including Ontario (0.04%), though lower than in Quebec (0.35%).20 As a result, our findings would appear relevant to most jurisdictions who are presently experiencing similar or lower rates of active cases of COVID-19 in the community.
Our study raises the possibility that continuous droplet and contact PPE for health care workers versus continuous masking alone may not be necessary when caring for low-risk patients in areas of relatively low community transmission. However, further data from outbreak investigations and research into modes of transmission in health care settings are accruing and will further inform the evolution of health care worker PPE recommendations.
Our data suggest that when there is low community prevalence, routine testing for SARS-CoV-2 infection on admission appears to have no additional benefit when symptom screening is negative; this may result in unnecessary extension of isolation protocols, increase potential harms associated with false positive tests, and offer low value in terms of health care resources. Even assuming a pretest probability as high as 1%, the posttest probability of a positive test in an asymptomatic person is very low, at 0.03%.
The prevalence of active cases has been used as a measure of community transmission to inform other policy decisions for the management of COVID-19. For instance, Alberta uses a cut-off of 50 active cases per 100 000 to consider reimplementing public health measures at a regional level,21 consistent with the criteria Germany adopted. Similarly, jurisdictions may choose a threshold for prevalent active cases to recommend testing for COVID-19 among asymptomatic patients who are admitted to hospital. However, further studies are needed to assess the benefit of testing in this group when the prevalence of active cases is higher than we observed.
The true prevalence of SARS-CoV-2 infection in asymptomatic people and the risk of SARS-CoV-2 transmission from an asymptomatic person are crucial questions in the pandemic response. The prevalence of asymptomatic people varies across studies, which reflects different population risks (highest rates in closed populations such as cruise ships and long-term care facilities)22,23 and failure to rule out postsymptomatic positive tests (which requires both current and previous symptom history). Prolonged RT-PCR positivity, while noninfectious, has been well described and influences interpretation of previous studies. During the first outbreak in New York, 15% of currently asymptomatic women admitted for delivery had positive SARS-CoV-2 testing.10 Not unexpectedly, a recent meta-analysis of similar studies found a comparable prevalence of asymptomatic people with positive RT-PCR tests.24 However, positive swab results in currently asymptomatic people with unknown 4-week symptom history may overestimate both the prevalence of asymptomatic infection with SARS-CoV-2 and the risk of asymptomatic transmission.11 Although the degree of symptoms varies considerably, current data suggest the period of transmission risk is from 2 days before symptoms through the first week after onset, although PCR positivity can continue for several weeks, without transmission risk.
Consistent with our findings, negative testing of all asymptomatic people included in a point-prevalence study across 4 hospitals in a low COVID-19 prevalence area of Ontario further suggests low utility of testing patients without COVID-19 symptoms in low-prevalence settings,25 which is an important consideration in prioritization of testing to optimize turnaround times. Finally, existing estimates of the risk of transmission from an asymptomatic person with SARS-CoV-2 infection ranging from 0.03% to 0.79% indicate that SARS-CoV-2 spreads less efficiently from an asymptomatic person,24 and this risk would be expected to be ameliorated by standard COVID-19 precautions in community and health care settings.
Limitations
Our study has limitations. Results from the province of Alberta, where aggressive health policy measures were implemented rapidly, may not be generalizable to other contexts and settings. For example, the proportion of people admitted via emergency department who were determined to be symptomatic was high (almost 50% of all admissions), suggesting that exposure history and nonrespiratory symptoms may have also been considered as new evidence emerged during the pandemic.
Given the relatively low degree of community transmission in Alberta with a peak of prevalent active cases at 0.071% of the population, our study may have been underpowered to detect asymptomatic cases. However, the true population prevalence of active cases may have been higher considering that during this study asymptomatic people as well as many symptomatic people were not tested in Alberta. Recent population-based screening data from Iceland indicate a prevalence of active cases that was more than 10-fold higher at 0.8%, nearly half of which were asymptomatic.26
We focused on the prevalence of asymptomatic people admitted to hospital through emergency departments. People seeking care in emergency departments during a pandemic may not represent the general population, other types of health care or congregate settings, or patients admitted directly to hospital. We did not have information on the reason for the emergency department visit leading to hospital admission. The context of the environment and population need to be considered before extrapolating these results to other settings and regions. Although the presence of comorbidities appears to influence the likelihood of documented or severe COVID-19, it is unknown whether comorbidities influence the likelihood of detection of asymptomatic infection; these and other demographic and clinical data were not available within this laboratory-based study.
The sensitivity of the RT-PCR nasopharyngeal swabs, especially for asymptomatic screening, is not well described.27,28 Considering that nearly two-thirds of our study participants received oropharyngeal swabs, which may have lower sensitivity than nasopharyngeal swabs (and sputum) for SARS-CoV-2 detection,29 the possibility of higher rates of false negative testing cannot be excluded. We explored the implications of this through extensive additional analyses (Tables 5 and 6).
Finally, the use of an expanded COVID-19 symptom screening list may be more sensitive in defining low-risk patients for whom isolation is not required, and since this study, a comprehensive screening tool that includes nonrespiratory symptoms (e.g., chills, loss of smell and taste, gastrointestinal symptoms) and a comprehensive risk exposure assessment has been implemented across Alberta.30
Conclusion
In this 6-week study involving 3375 consecutive patients admitted to 3 emergency departments in a region with low disease prevalence, no cases of SARS-CoV-2 infection were detected on RT-PCR screening among patients with no defined COVID-19 symptoms (54% of the study cohort) at hospital admission, though we note that the RT-PCR testing was done using throat swabs in 65% of patients. In a setting with low community transmission, there may not be additive benefit to universal testing of asymptomatic patients on hospital admission.
Supplementary Material
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: Eddy Lang, Braden Manns, Laura McDougall, Lynora Saxinger and Uma Chandran contributed to the study concept, and design and analysis plan. Kevin Fonseca, Stephanie Murphy, Braden Manns and Pietro Ravani contributed to the data collection. Stephanie Murphy and Pietro Ravani contributed to the analysis. All authors contributed to drafting the manuscript and revising it for important intellectual content. Pietro Ravani and Braden Manns contributed to the study supervision. All authors affirm that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Data sharing: The authors are not able to make the data set available to other researchers owing to their contractual arrangements with the provincial health ministry (Alberta Health), which is the data custodian. Researchers may make requests to obtain a similar data set at https://absporu.ca.
Disclaimer: The interpretation and conclusions contained herein are those of the researchers and do not represent the views of the Government of Alberta, Alberta Health, Alberta Health Services, the Cumming School of Medicine or the University of Calgary.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/8/4/E887/suppl/DC1.
References
- 1.Gordon DE, Jang GM, Bouhaddou M, et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–68. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coronavirus disease (COVID-19): How is it transmitted? Geneva: World Health Organization; 2020. [accessed 2020 Dec. 4]. Available: www.who.int/news-room/q-a-detail/coronavirus-disease-covid-19-how-is-it-transmitted. [Google Scholar]
- 3.Lee S, Meyler P, Mozel M, et al. Asymptomatic carriage and transmission of SARS-CoV-2: What do we know? Can J Anaesth. 2020;67:1424–30. doi: 10.1007/s12630-020-01729-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mizumoto K, Kagaya K, Zarebski A, et al. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25:2000180. doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishiura H, Kobayashi T, Miyama T, et al. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19) Int J Infect Dis. 2020;94:154–5. doi: 10.1016/j.ijid.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei WE, Li Z, Chiew CJ, et al. Presymptomatic transmission of SARS-CoV-2 — Singapore, January 23–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:411–5. doi: 10.15585/mmwr.mm6914e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chau NVV, Lam VT, Thanh Dung NT, et al. The natural history and transmission potential of asymptomatic SARS-CoV-2 infection. Clin Infect Dis. 2020 Jun 4; doi: 10.1093/cid/ciaa711. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Shamsi HO, Coomes EA, Alrawi S. Screening for COVID-19 in asymptomatic patients with cancer in a hospital in the United Arab Emirates. JAMA Oncol. 2020;6:1627–8. doi: 10.1001/jamaoncol.2020.2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albalate M, Arribas P, Torres E, et al. High prevalence of asymptomatic COVID-19 in haemodialysis: learning day by day in the first month of the COVID-19 pandemic. Nefrologia. 2020;40:279–86. doi: 10.1016/j.nefro.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sutton D, Fuchs K, D’Alton M, et al. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med. 2020;382:2163–4. doi: 10.1056/NEJMc2009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asymptomatic transmission of SARS-CoV-2 infection. Edmonton: Alberta Health Services; 2020. [accessed 2020 Apr. 29]. Available: www.albertahealthservices.ca/assets/info/ppih/if-ppih-covid-19-rapid-response-asymptomatic-transmission.pdf. [Google Scholar]
- 12.Rivett L, Sridhar S, Sparkes D, et al. Screening of healthcare workers for SARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission. eLife. 2020;9:e58728. doi: 10.7554/eLife.58728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alberta Precision Laboratories. COVID-19 test performance. Edmonton: Alberta Health Services; 2020. [accessed 2020 July 14]. Available: www.albertahealthservices.ca/assets/wf/lab/wf-lab-bulletin-COVID-19-test-performance.pdf. [Google Scholar]
- 14.Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc. 1927;22:209–12. doi: 10.2307/2276774. [DOI] [Google Scholar]
- 15.Gerstman B. 19: Sample size, precision, and power. San Jose (CA): San Jose State University; 2003. [accessed 2020 June 2]. Available: www.sjsu.edu/faculty/gerstman/StatPrimer/sampsize.PDF. [Google Scholar]
- 16.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linton NM, Kobayashi T, Yang Y, et al. Incubation period and other epidemiological characteristics of 2019 novel coronavirus infections with right truncation: a statistical analysis of publicly available case data. J Clin Med. 2020;9:538. doi: 10.3390/jcm9020538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishiura H, Linton NM, Akhmetzhanov AR. Serial interval of novel coronavirus (COVID-19) infections. Int J Infect Dis. 2020;93:284–6. doi: 10.1016/j.ijid.2020.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berry I, Soucy J-PR, Tuite A, et al. Open access epidemiologic data and an interactive dashboard to monitor the COVID-19 outbreak in Canada. CMAJ. 2020;192:E420. doi: 10.1503/cmaj.75262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.COVID-19 relaunch status map. Edmonton: Government of Alberta; [accessed 2020 July 13]. Available: www.alberta.ca/maps/covid-19-status-map.htm. [Google Scholar]
- 22.Expert Taskforce for the COVID-19 Cruise Ship Outbreak. Epidemiology of COVID-19 outbreak on cruise ship quarantined at Yokohama, Japan, February 2020. [accessed 2020 Dec. 9];EID Journal. 2020 26(11) doi: 10.3201/eid2611.201165. Available: wwwnc.cdc.gov/eid/article/26/11/20-1165_article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gmehlin CG, Munoz-Price LS. Coronavirus disease 2019 (COVID-19) in long-term care facilities: a review of epidemiology, clinical presentations, and containment interventions. Infect Control Hosp Epidemiol. 2020 Oct 26;:1–6. doi: 10.1017/ice.2020.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Byambasuren O, Magnolia C, Bell K, et al. Estimating the extent of true asymptomatic COVID-19 and its potential for community transmission: systematic review and meta-analysis. Lancet. 2020. Jun 4, [accessed 2020 July 15]. Available: https://ssrn.com/abstract=3586675. [DOI] [PMC free article] [PubMed]
- 25.Bai AD, Li XX, Alsalem M, et al. Utility of asymptomatic inpatient testing for COVID-19 in a low-prevalence setting: a multicenter point-prevalence study. Infect Control Hosp Epidemiol. 2020;41:1233–5. doi: 10.1017/ice.2020.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gudbjartsson DF, Helgason A, Jonsson H, et al. Spread of SARS-CoV-2 in the Icelandic population. N Engl J Med. 2020;382:2302–15. doi: 10.1056/NEJMoa2006100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vinh DB, Zhao X, Kiong KL, et al. Overview of COVID-19 testing and implications for otolaryngologists. Head Neck. 2020;42:1629–33. doi: 10.1002/hed.26213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zitek T. The appropriate use of testing for COVID-19. West J Emerg Med. 2020;21:470–2. doi: 10.5811/westjem.2020.4.47370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohammadi A, Esmaeilzadeh E, Li Y, et al. SARS-CoV-2 detection in different respiratory sites: a systematic review and meta-analysis. EBioMedicine. 2020;59:102903. doi: 10.1016/j.ebiom.2020.102903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Symptoms and testing. Edmonton: Government of Alberta; [accessed 2020 Apr. 29]. Available: www.alberta.ca/covid-19-testing-in-alberta.aspx#symptoms. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.