Abstract
Introduction: Cannabis sativa produces hundreds of bioactive compounds, including cannabinoids and terpenoids. It has been proposed that cannabinoids act in synergy with terpenoids to produce the entourage effect, a concept used to explain the therapeutic benefits of medicinal cannabis. One molecular explanation for the entourage effect is that the terpenoids augment the actions of cannabinoids at their molecular drug targets in cells. We recently reported that terpenoids commonly found in cannabis do not influence the functional effects of Δ9-tetrahydrocannabinol (Δ9-THC) on cannabinoid 1 and cannabinoid 2 receptors. The present study aimed to extend on this research by examining whether terpenoids influence the effects of phytocannabinoids and endocannabinoids on human transient receptor potential ankyrin 1 (hTRPA1) and human transient receptor potential vanilloid 1 (hTRPV1) channels heterologously expressed in mammalian cells.
Materials and Methods: The activity of terpenoids, phytocannabinoids, and endocannabinoids was assessed in inducible HEK Flp-In T-Rex cells transfected with hTRPA1 and hTRPV1 channels, respectively. Real-time changes in intracellular calcium ([Ca]i) were measured using the Calcium 5 dye and a FlexStation 3 plate reader.
Results: α-pinene, β-pinene, β-caryophyllene, linalool, limonene, β-myrcene or α-humulene did not affect [Ca]i in hTRPA1 and hTRPV1 overexpressing cells. Cinnamaldehyde (CA), Δ9-THC, and 2-arachidonoylglycerol (2-AG) activated TRPA1 receptors with high efficacy and similar potency (EC50s of ∼10 μM). Capsaicin and anandamide (AEA) activated TRPV1 receptors with an EC50 of 61 nM and 4.3 μM, respectively, but TRPV1 showed no response to Δ9-THC, cannabidiol, and other minor cannabinoids. Terpenoids did not significantly affect the responses of TRPA1 and TRPV1 receptors to submaximal and maximal concentrations of CA and Δ9-THC or the endocannabinoids AEA and 2-AG.
Discussion: We could not find any evidence that the terpenoids tested here activate TRPA1 and TRPV1 channels or modulate their activation by Δ9-THC and other agonists, including endocannabinoids.
Keywords: terpenoid, TRPA1, TRPV1, phytocannabinoid, endocannabinoid, entourage effect
Introduction
Cannabis plant matter contains hundreds of diverse bioactive molecules. The major components of cannabis include phytocannabinoids such as the main psychoactive constituent Δ9-tetrahydrocannabinol (Δ9-THC) and the nonpsychoactive cannabidiol (CBD).1 However, cannabis contains other phytochemicals, including various minor phytocannabinoids, as well as flavonoid and terpenoid molecules, which have their own biological activities.1,2 The burgeoning utilization of medicinal cannabis necessitates research that addresses the complex polypharmacology of cannabis. Relevant to such research is the “entourage effect” hypothesis, which posits that the effects of the whole of the cannabis plant is substantially greater than the sum of its individual components. The “entourage effect” is often quoted by the growing community of cannabis entrepreneurs and patient advocates to explain the therapeutic and safety benefits of medicinal cannabis.
While few scientific studies have directly addressed the “entourage effect” hypothesis, there is accumulating evidence for cannabis plant extracts having effects that cannot simply be attributed to individual cannabis constituents.3 One early study showed that Δ9-THC-rich cannabis extracts had more potent effects than Δ9-THC administered as a purified substance in humans and animals, implying that other components in the extract might enhance the effects of Δ9-THC.4 Consistent with this finding more recent studies showed that Δ9-THC-rich cannabis extracts had greater anticancer effects than purified Δ9-THC on human cancer cells in vitro and in vivo.5,6
One means through which “entourage effects” may occur is via the interaction of individual components of the cannabis plant. Interplay between different cannabinoids provides one possibility, and numerous studies have examined interactions between the phytocannabinoids CBD and Δ9-THC. These studies have revealed great complexity in the interaction, with CBD synergistically enhancing or inhibiting the effects of Δ9-THC dependent on the measured variable and the frequency of dosing.7–10 It has also been hypothesised that the varying effects of different cannabis chemovars might be due to variation in their terpene content, which can reach concentrations of 3.5% and 10% in flower and trichomes, respectively.11–13 However, few studies have addressed this hypothesis; thus, the present study sought to advance the evidence base on potential terpenoid–cannabinoid interactions.
One means to deconvolute the complex actions of whole plant cannabis formulations on human biology is to assess whether phytochemical synergy occurs at the level of drug targets heterologously expressed in mammalian cells. We recently used this approach by examining whether several terpenoids influenced the agonist activity of Δ9-THC at cannabinoid 1 (CB1) and 2 (CB2) receptors expressed in AtT20 cells.14 The terpenoids analyzed failed to affect the actions of Δ9-THC on these receptors; however, this doesn't preclude interactions at other cannabinoid drug targets. In the present study we aimed to extend on our prior research by examining whether the terpenoids modulate the effects of phytocannabinoids at transient receptor potential ankyrin 1 (TRPA1) and transient receptor potential vanilloid 1 (TRPV1) channels.
TRPA1 channels play an important role in pain, itch, allergic cough, and asthma,15 and TRPV1 channels are involved in epilepsy, pain, thermoregulation, and itch.15 Phytocannabinoids are known agonists of these receptors, with Δ9-THC activating TRPA1, as well as CBD and other minor phytocannabinoids being reported to activate TRPV1.16 Furthermore, endocannabinoids affect these receptors, and anandamide (AEA) and 2-arachidonoylglycerol (2-AG) are TRPV1 and TRPA1 agonists, respectively.17–19 Given that TRPA1 and TRPV1 receptors appear to have allosteric sites, it is possible that entourage effects are mediated by terpenoid allosteric modulation of cannabinoid actions at these receptors.20–22 In the present study we aimed to assess whether seven terpenoids most commonly found in cannabis have activity at TRPV1 and TRPA1 channels alone, as well as observing whether they modulate the effects of phytocannabinoids and endocannabinoids on these receptors.
Materials and Methods
Cell culture
For this study, we used HEK293 cells as an expression system as they have been used extensively in previous studies assessing TRPV1 and TRPA1 channel signaling.23–25 Flp-In T-Rex HEK 293 cells (Life Technologies, Mulgrave, Victoria, Australia) were stably transfected with human TRPA1 (hTRPA1) or human TRPV1 (hTRPV1) cDNA (GenScript, Piscataway, NJ) and cultivated in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (FBS), 100 U mL−1 penicillin, and 100 μg mL−1 streptomycin. Medium for transfected cells was supplemented with hygromycin B 80 μg mL−1 and blasticidin S 10 μg mL−1. Medium for Flp-In T-Rex HEK293 cells was supplemented with zeocin 100 μg mL−1 and blasticidin S 15 μg mL−1.
Cells were incubated in 5% CO2 at 37°C in a humidified atmosphere. Cells were grown in flasks with a surface area of 75 mm2, once at optimum confluence (∼90%); cells were trypsinized and seeded into a poly-D-lysine coated black, clear-bottomed 96-well plate (Corning, Castle Hill, NSW, Australia) at a density of 110,000–130,000 cells per well in L15 medium supplemented with 1% FBS, 15 mM glucose, 100 U penicillin, and 100 μg mL−1 streptomycin. The cells were plated in a volume of 80 μL and were incubated overnight in a humidified chamber at 37°C and ambient CO2. Expression of the hTRPA1 and hTRPV1 channel was induced 4.5 h before experimentation by addition of 2 μg mL−1 tetracycline per well.
Calcium assay
Intracellular calcium [Ca]i was measured using the Calcium 5 Kit from Molecular Devices (Sunnyvale, CA) and a Flexstation 3 microplate reader (Molecular Devices). Eighty microliters of dye dissolved in HEPES-buffered saline solution (HBSS) containing (in mM): NaCl 140, KCl 5.33, CaCl2 1.3, MgCl2 0.5, HEPES 22, Na2HPO4 0.338, NaHCO3 4.17, KH2PO4 0.44, MgSO4 0.4, glucose 10 (pH to 7.4) containing probenecid was loaded into each well of the plate for 1–1.5 h before testing in the Flexstation at 37°C. Fluorescence was measured every 2 sec (λexcitation=485 nm, λemission=525 nm) for the duration of the experiment. Drugs were added after 2 min of baseline recording.
Drug dilutions
All terpenoids, cinnamaldehyde (CA), capsaicin (CAPS), and Δ9-THC were made up in dimethyl sulfoxide (DMSO) and stored at −25°C. Protease activated receptor 1 (PAR-1) peptide agonist stock solution was made up in water and stored at −25°C. AEA and 2-AG stock solution were made up in DMSO and stored at −80°C. Fresh aliquots were used each day, and drugs were diluted in HBSS containing 0.01% bovine serum albumin immediately before the assay. The final concentration of DMSO per well did not exceed 0.2%. In each column of the 96-well plate, one blank addition well was included, where HBSS plus solvent was added to the cells. The changes in fluorescence produced by this blank addition were subtracted from drug responses before determining maximum change in [Ca]i after each drug exposure.
Drugs and reagents
CA, bovine serum albumin, 2-AG, and AEA were from Sigma-Aldrich (Castle Hill, NSW, Australia). PAR-1 peptide agonist was from AusPep (Tullamarine, VIC, Australia). Δ9-THC was purchased from THC Pharm GmbH (Frankfurt, Germany). Terpenoids were purchased from Sigma-Aldrich; (+)-α-pinene, (+)-β-pinene, (−)-β-caryophyllene (BCP), (±)-linalool, (R)-(+)-limonene, β-myrcene, and α-humulene. The purity of terpenoids as stated by the supplier was as follows: 98.5% ((+)-α-pinene, (+)-β-pinene), 98% ((−)-BCP), 97% ((±)-linalool, (R)-(+)-limonene), 93% (β-myrcene), and 96% (α -humulene). All tissue culture reagents were from Sigma-Aldrich, Life Technologies (Mulgrave, Victoria, Australia) or InvivoGen (San Diego, CA).
Data analysis
Statistical analysis was performed using GraphPad Prism (Version 7.0b, San Diego, CA). The response to agonists was calculated as a percentage change of fluorescence over the baseline averaged for the 30 sec immediately before drug addition. All responses in HEK Flp-In T-Rex hTRPA1 cells were normalized to the response produced by a maximally effective concentration of CA (300 μM) included in each experiment. Responses in HEK Flp-In T-Rex hTRPV1 cells were normalized to the response produced by a maximally effective concentration of CAPS (10 μM) included in each experiment. Each determination was composed of two technical replicates, and the average of these replicates was used for analysis. Data for the response of hTRPA1 and hTRPV1 to terpenoids were extracted from experiments conducted to assess the effects of terpenoids on agonist-induced hTRPA1 and hTRPV1 activation, respectively, by analyzing the maximum change in [Ca]i before 2-AG, CAPS, CA, AEA, or Δ9-THC application.
Concentration–response data were fit to a four-parameter logistic Hill equation to derive the EC50 values. Data are expressed as mean with standard error of the mean (SEM) of at least five independent experiments. Data were scrutinized for outliers using the ROUT method (Q=1%).26 For experiments assessing activation of either wild-type (WT), hTRPA1, or hTRPV1 cells by terpenoids and phytocannabinoids, unpaired t-tests were used to compare means. For all other experiments, means were compared using two-way analysis of variance (ANOVA) with Bonferroni's post hoc analysis. Experiments assessing the effects of terpenoids on CA-induced hTRPA1 activation and on CAPS-induced hTRPV1 activation were analyzed using two-way ANOVA with Bonferroni's post hoc on raw data expressed as percentage change in fluorescence. The null hypothesis was rejected if the p-value was lower than 0.05 (p>0.05=not significant).
Results
Terpenoids do not induce nonspecific intracellular Ca2+ mobilization in WT cells
We first wished to ensure that the terpenoids did not have nonspecific effects on intracellular Ca2+ increase in nontransfected HEK Flp-In T-Rex WT cells. WT cells were challenged with 10 μM of α-pinene, β-pinene, BCP, linalool, limonene, β-myrcene, or α-humulene (Supplementary Fig. S1A). WT cells endogenously express PAR-1,27 and their activation by a ligand induces intracellular Ca2+ mobilization.28 Hence, the application of 10 μM of the peptide agonist of PAR-1 was used as a positive control for the responsiveness of WT cells (Supplementary Fig. S1B, open circles). Supplementary Figure S1B shows that terpenoids at 10 μM did not change [Ca]i in WT cells, ruling out confounding nonspecific effects of the terpenoids in experiments on hTRPA1- and hTRPV1-transfected cells.
Terpenoids do not have agonist activity at hTRPA1 and do not modulate the agonist effects of CA, Δ9-THC, or 2-AG at hTRPA1
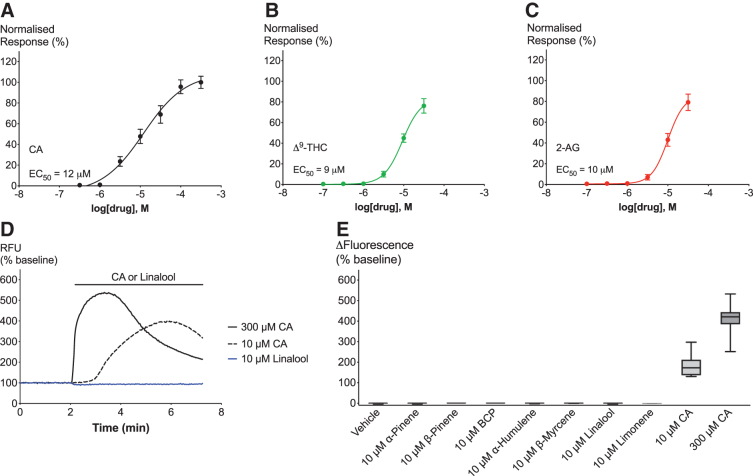
hTRPA1 activation was determined by measuring maximum change of [Ca]i in HEK Flp-In T-Rex cells expressing hTRPA1 channels. For the purpose of validating our hTRPA1 expressing cell system, we first analyzed the concentration–response relationship of an endogenous hTRPA1 agonist (2-AG), an exogenous agonist CA, and a phytocannabinoid agonist (Δ9-THC). Figure 1A–C shows the concentration response curves of all three ligands, which display an EC50 of 12 μM (CA), 9 μM (Δ9-THC), and 10 μM (2-AG). Calculation of the EC50 for Δ9-THC and 2-AG is restricted to changes in [Ca]i evoked by concentrations of up to 30 μM, due to limited drug solubility. Emax of 30 μM agonist reached 76% (Δ9-THC) and 79% (2-AG), respectively, when normalized to the response of 300 μM CA. Neither CA, Δ9-THC, nor 2-AG showed a response in WT cells, which confirms their specificity for TRPA1 (Supplementary Fig. S1C).
FIG. 1.
Effects of TRPA1 agonists and terpenoids on hTRPA1 cells. (A–C). Concentration–response curves of hTRPA1 agonists CA, Δ9-THC, and 2-AG. (D) Representative trace of effects of 10 μM linalool (blue), 10 μM CA (black broken), and 300 μM CA (black full) on hTRPA1. (E) Maximum change in fluorescence from baseline upon application of vehicle, 10 μM terpenoid, 10 μM CA, or 300 μM CA. Data derived from Δ9-THC, CA, and 2-AG experiments (see Figs. 2–4). N>5, mean±SEM. Drugs were added for the duration of the bar. Δ9-THC, Δ9-tetrahydrocannabinol; 2-AG, 2-arachidonoylglycerol; CA, cinnamaldehyde; BCP, β-caryophyllene; hTRPA1, human transient receptor potential ankyrin 1; SEM, standard error of the mean.
Following the establishment of hTRPA1 functional activation to known agonists in our cell system, we then sought to examine whether terpenoids commonly found in Cannabis activate hTRPA1 receptors. Considering the potency of known ligands at TRPA1, which show EC50 responses of around 10 μM (Fig. 1A–C), we decided to test 10 μM of α-pinene, β-pinene, BCP, linalool, limonene, β-myrcene, or α-humulene on hTRPA1 (Fig. 1D). We found no effect on [Ca]i by any of the terpenoids (Fig. 1E). As expected, 10 μM and 300 μM CA showed a substantial increase in [Ca]i (Fig. 1E).
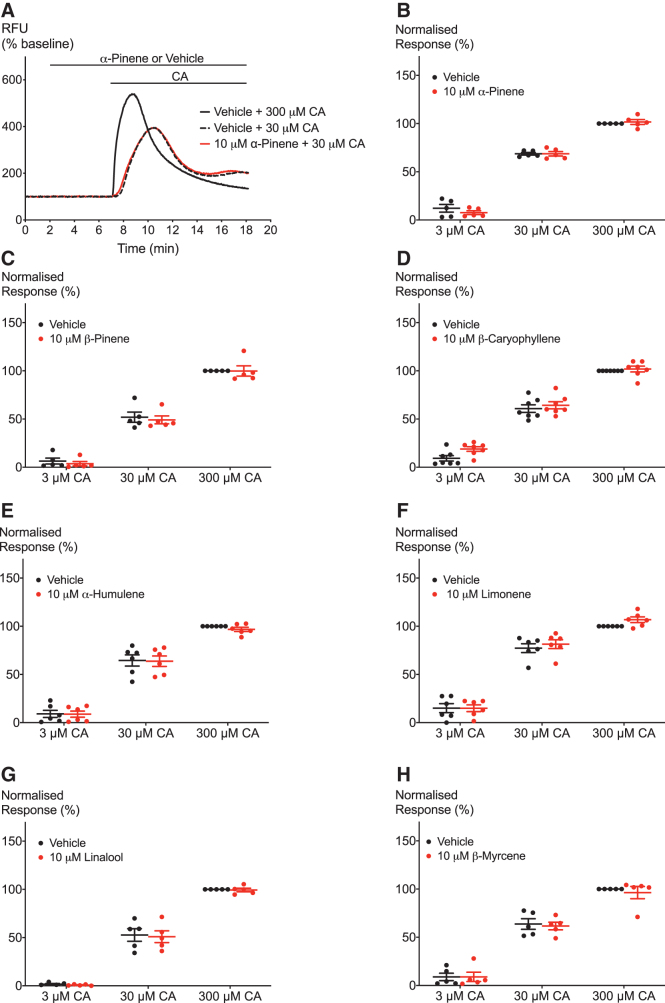
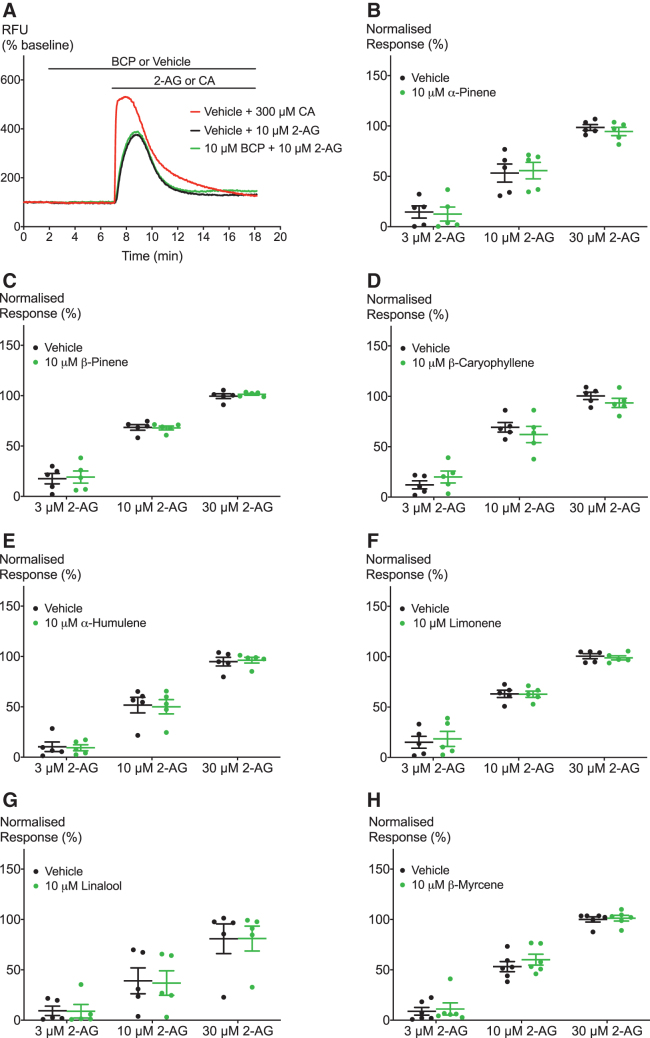
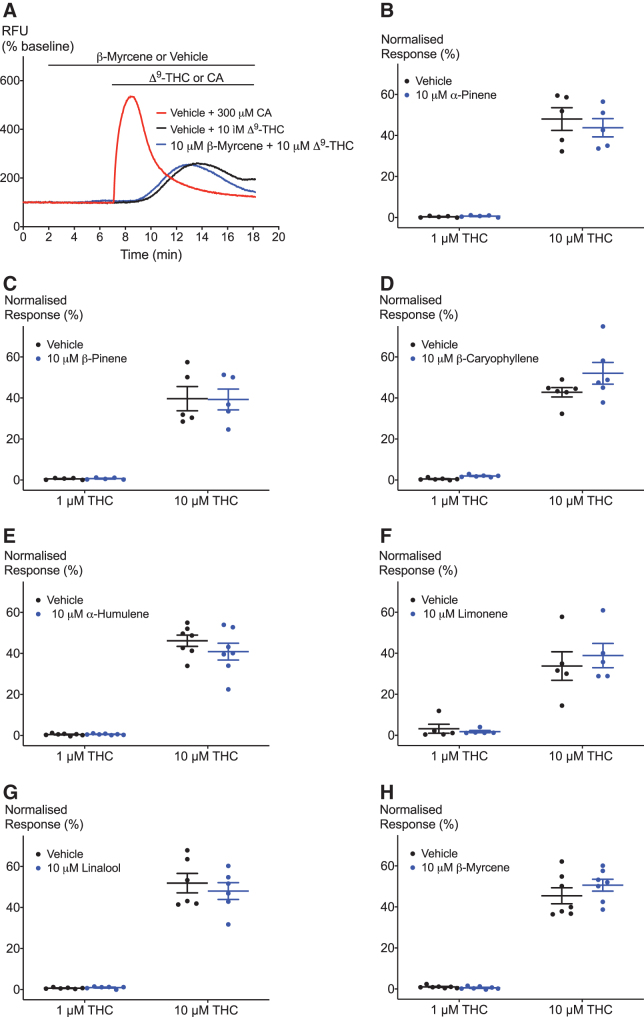
The second aim was to investigate whether terpenoids have a modulatory effect on agonist-induced TRPA1 activation. To address this question, the response of hTRPA1 to a range of concentrations of the agonists CA, Δ9-THC, and 2-AG was measured after 5 min of application of 10 μM of terpenoid (Figs. 2–4A). Figures 2–4 show that none of the seven terpenoids tested significantly altered activation of hTRPA1 by any of the agonists. One limitation of working with the FLIPR Calcium assay is the fact that compounds cannot be removed from the solution. This might lead to continued activation of the channels and therewith a fluctuation in fluorescence over a prolonged period of time as seen in Figures 2A and 3A. Moreover, prolonged exposure to agonists can also lead to cytotoxicity as described by Stueber et al.29
FIG. 2.
Effect of terpenoids on CA-induced hTRPA1 activation. (A) Representative trace showing the effect of preincubation with 10 μM α-pinene on activation of hTRPA1 by CA. (B–H) Terpenoids do not affect the activation of hTRPA1 by one maximal and two submaximal concentrations of CA. Two-way ANOVA with Bonferroni's post hoc analysis performed on raw data expressed as % change fluorescence, N=5–7, mean±SEM. Data presented as % of maximum CA (300 μM) response. Drugs were added for the duration of the bar. ANOVA, analysis of variance.
FIG. 4.
Effects of terpenoids on 2-AG-induced hTRPA1 activation. (A) Representative trace of effects of BCP on hTRPA1 activation by 10 μM 2-AG. (B–H) Terpenoids do not significantly modulate 2-AG-induced hTRPA1 signaling. Two-way ANOVA with Bonferroni's post hoc analysis, N=5–6, mean±SEM. Data presented as % of maximum CA (300 μM) response. Drugs were added for the duration of the bar.
FIG. 3.
Effect of terpenoids on Δ9-THC-induced hTRPA1 activation. (A) Representative trace of effects of β-myrcene on hTRPA1 activation by 10 μM Δ9-THC. (B–H) Terpenoids do not affect the response of hTRPA1 to two submaximal Δ9-THC concentrations. Two-way ANOVA with Bonferroni's post hoc analysis, N=5–7, mean±SEM. Data presented as % of maximum CA (300 μM) response. Drugs were added for the duration of the bar.
Terpenoids do not affect [Ca]i through hTRPV1 and do not modulate hTRPV1 activation
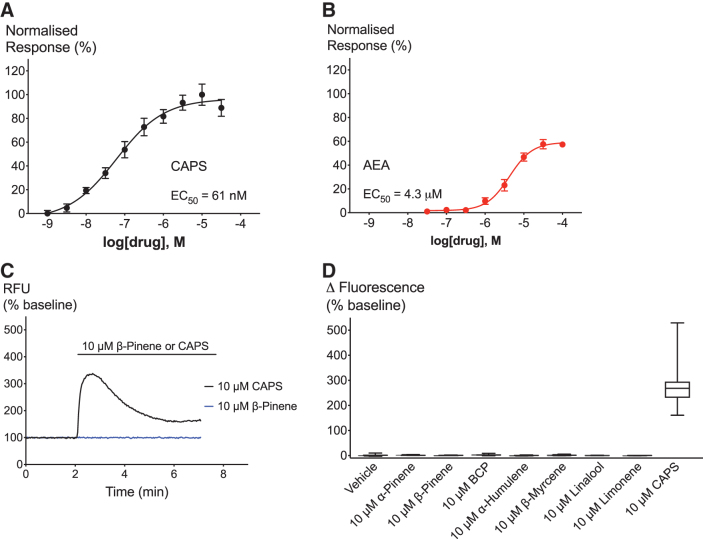
For the experimental design and execution on HEK FlpIn T-Rex cells expressing hTRPV1 channels, we adopted a similar rational to the experiments performed on hTRPA1 cells. We first sought to validate our hTRPV1 channel-expressing cell system (hTRPV1) by determining the concentration–response curves of known agonists. Both the exogenous agonist CAPS and the endogenous agonist AEA activated hTRPV1 in a concentration-dependent manner with an EC50 of 61 nM for CAPS and 4.3 μM for AEA (Fig. 5A, B). The Emax of 100 μM AEA reached 57% when normalized to the response of 10 μM CAPS. Neither CAPS nor AEA showed a response in WT cells, which confirms their specificity for TRPV1 (Supplementary Fig. S1C). Surprisingly, we also saw no specific response to any of the common phytocannabinoids present in Cannabis sativa in hTRPV1 cells (Supplementary Fig. S2). Responses to 30 μM of cannabichromene, CBD, cannabidiolic acid, cannabigerol, cannabigerolic acid, cannabigerovarin, Δ9-THC, or tetrahydrocannabinolic acid were not significantly different between hTRPV1 (Supplementary Fig. S2A) and WT cells (Supplementary Fig. S2B).
FIG. 5.
Effects of TRPV1 agonists and terpenoids on hTRPV1 cells. (A, B) Concentration–response curves of hTRPV1 agonists CAPS and AEA. (C) Representative trace showing the application of 10 μM β-pinene (blue) and 10 μM CAPS on hTRPV1 (black). (D) Maximum change in fluorescence upon application of vehicle, 10 μM terpenoid or 10 μM CAPS. N>5, mean±SEM, unpaired t-test. Drugs were added for the duration of the bar. AEA, anandamide; CAPS, capsaicin; hTRPV1, human transient receptor potential vanilloid 1.
We then sought out to determine the effects of terpenoids on hTRPV1 (Fig. 5C). hTRPV1 cells displayed significant response to 10 μM CAPS, but we saw no significant difference of the response to any of the seven terpenoids compared to vehicle (Fig. 5D).
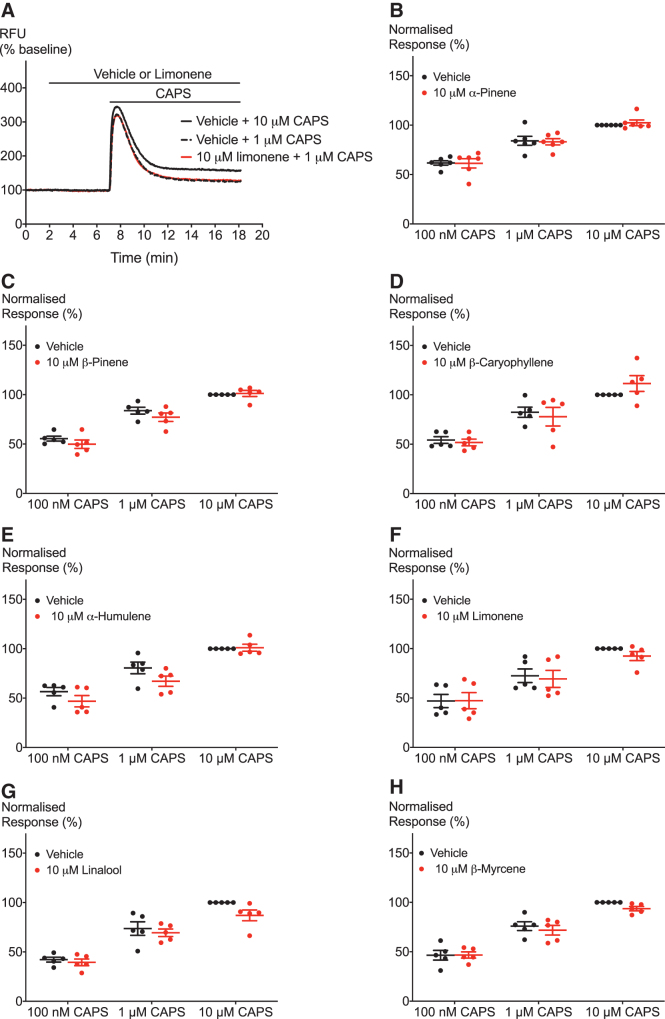
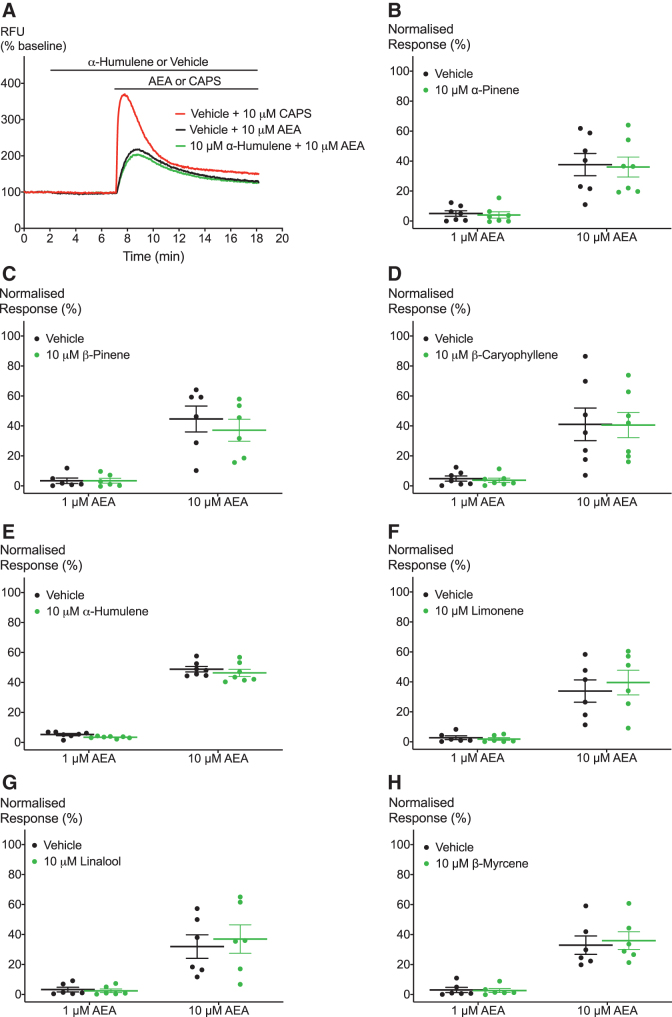
Finally, we aimed to examine the effects of terpenoids on hTRPV1 activation by known agonists. Again, we tested the effects of 10 μM of the terpenoids on activation of hTRPV1 by an exogenous agonist CAPS and an endogenous agonist AEA. We applied 10 μM of the terpenoids for 5 min followed by addition of a range of concentrations of the agonist (Figs. 6A and 7A). Figures 6 and 7 show that none of the seven terpenoids significantly shifted the response of hTRPV1 to CAPS and AEA, respectively.
FIG. 6.
Effect of terpenoids on CAPS-induced hTRPV1 signaling. (A) Representative trace of effects of 10 μM limonene on TRPV1 activation by 1 μM CAPS. (B–H) Terpenoids do not significantly change hTRPV1 response to one maximal and two submaximal concentrations of CAPS. Two-way ANOVA with Bonferroni's post hoc analysis performed on raw data expressed as % change fluorescence, N=5, mean±SEM. Data presented as % of maximum CAPS (10 μM) response. Drugs were added for the duration of the bar.
FIG. 7.
Effects of terpenoids on hTRPV1 activation by AEA. (A) Representative trace of effects of 10 μM α-humulene on hTRPV1 activation by 10 μM AEA. (B–H) Application of terpenoids does not lead to significant change in hTRPV1 activation by AEA at two submaximal concentrations. Two-way ANOVA with Bonferroni's post hoc analysis. N=5–7, mean±SEM. Data presented as % of maximum CAPS (10 μM) response. Drugs were added for the duration of the bar.
Since we did not see a response of hTRPV1 to any of the common phytocannabinoids (Supplementary Fig. S2), we were unable to study the effects of terpenoids on the activation of hTRPV1 by other phytocannabinoids.
Discussion
Our work was inspired by the idea that terpenoids might influence the actions of endocannabinoids and phytocannabinoids at their receptor targets.11,30 Terpenoid-cannabinoid synergy at cannabinoid drug targets may provide one mechanism for the “entourage effect:” the notion that the pharmacological effects of the cannabis plant, and its mixture of 500 bioactive molecules, are greater than the sum of its individual components. We have addressed this hypothesis recently by examining the interaction between terpenoids and Δ9-THC at CB1 and CB2 receptors but could not find any evidence of terpenoid modulation of the actions of Δ9-THC at these receptors.14 In this study we further explored the entourage hypothesis by examining potential interactions at TRPV1 and TRPA1 channels. The principal findings of our work are that seven terpenoids commonly found in Cannabis, namely α-pinene, β-pinene, BCP, linalool, limonene, β-myrcene, and α-humulene, did not elicit a change in [Ca]i in either hTRPA1 or hTRPV1 expressing cells and did not affect activation of either receptor by endocannabinoids and the phytocannabinoid Δ9-THC.
The validation of our cell system makes us confident that they provide reliable tools to study hTRPA1 and hTRPV1 functional activation. Our data on the potency of CA (EC50=12 μM) and Δ9-THC (EC50=9 μM) to activate TRPA1 channels are consistent with the literature.19,24,31 We report here for the first time that 2-AG is a TRPA1 receptor agonist. Interestingly, 2-AG had an almost identical concentration–response curve to Δ9-THC with a similar EC50 (10 μM) and Emax. This finding is consistent with research showing that endocannabinoids activate TRP channels.32 We also showed that our TRPV1 cell system is sensitive to the effects of known agonists. CAPS and AEA activated hTRPV1 with comparable potency to prior reports (EC50=61 nM and 4.3 μM, respectively).17,19,33 None of the agonists changed [Ca]i in our WT cells confirming the specificity of these agonists.
To date, only two studies have demonstrated an effect of terpenoids from Cannabis on TRPA1 and TRPV1.34,35 Riera et al. showed that linalool activated human TRPA1 but only at very high concentrations (EC50 of 117 μM).34 Consistent with our results here, Riera et al. showed that linalool had no effect on TRPV1.34 Our results confirmed the findings of Jansen and coworkers who showed that BCP, limonene, linalool, β-pinene, and α-humulene do not activate rat TRPV1.35 Yet, unlike the null finding reported here with myrcene against human TRPV1, they showed that 10 μM β-myrcene activated rat TRPV1 when using the Fluo-4 calcium assay to assess changes in [Ca]i. We did not see a significant or specific response of hTRPV1 to any of the phytocannabinoids commonly found in Cannabis and hence did not address the potential of terpenoids to modulate CBD-induced TRPV1 activation.
The pharmacological validation of our cell systems presented herein demonstrates clearly that our cells are sensitive to known agonists of TRPA1 and TRPV1. However, that the phytocannabinoids failed to activate hTRPV1 here is contrary to the existing literature.16,25,35,36 For example, CBD has been shown to be a TRPV1 channel agonist with potencies between 1 and 30 μM, but in our study CBD did not activate hTRPV1 cells at a 30 μM concentration.16,25 There are numerous differences in assay conditions that may help explain the discrepant findings, including differences in expression levels of TRPV1, which may render other systems more sensitive to the effects of cannabinoids.16,25,35,36 Differences in the method utilized to measure changes in intracellular calcium, the temperature at which the experiments were performed, and the concentration of solvents might also contribute to the discordant findings.37–39
The present study is limited to assessing terpenoid and cannabinoid interactions in a HEK cell tetracycline-regulated expression system. Future studies could use whole cell patch clamp electrophysiology in brain slices to assess effects on TRPA1 and TRPV1 channels in a native system that better mimics in vivo complexity.40 Moreover, the translational potential of our results might be improved by utilizing neurons derived from human inducible pluripotent stem cells, which have been developed to study TRP channel responses.41 Another limitation of the current study is that we have only studied the seven terpenoids that are most commonly reported to be present in Cannabis; however, there are likely to be hundreds of these molecules in the plant.2 With increasing knowledge of the terpenoid content of cannabis, novel terpenoid molecules will need to be incorporated in studies assessing terpenoid–cannabinoid interactions.
Collectively our studies show that there is currently no evidence for terpenoid–cannabinoid interactions at CB1, CB2, TRPV1, or TRPA1 receptors, but given the promiscuity of the cannabinoids, the search should continue by exploring interactions at additional molecular targets.14 For example, terpenoid modulation of Δ9-THC effects at TRPV2, TRPV3, GPR18, GPR55, glycine, and PPARγ receptors could be examined.42 Moreover, in vivo research is required as synergy might be attained through effects on distinct but intersecting biochemical systems.43 Pharmacokinetic entourage might play a role where the terpenoids modulate the absorption, distribution, metabolism, and excretion of the cannabinoids. The complexity of both human biochemistry and cannabis phytochemistry demands that mechanisms explaining possible entourage effects will be multifaceted. Increased global utilization of medicinal cannabis necessitates research on the entourage effect to continue in earnest.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge Barry and Joy Lambert for their continued support of the Lambert Initiative for Cannabinoid Therapeutics.
Abbreviations Used
- Δ9-THC
Δ9-tetrahydrocannabinol
- 2-AG
2-arachidonoylglycerol
- AEA
anandamide
- ANOVA
analysis of variance
- BCP
β-caryophyllene
- CA
cinnamaldehyde
- CAPS
capsaicin
- CBD
cannabidiol
- DMSO
dimethyl sulfoxide
- FBS
fetal bovine serum
- HBSS
HEPES-buffered saline solution
- hTRPA1
human transient receptor potential ankyrin 1
- hTRPV1
human transient receptor potential vanilloid 1
- PAR-1
protease activated receptor 1
- SEM
standard error of the mean
- WT
wild-type
Author Disclosure Statement
J.C.A. is Deputy Academic Director of the Lambert Initiative. He has served as an expert witness in various medicolegal cases involving cannabis and cannabinoids and served as a temporary advisor to the World Health Organization (WHO) on their review of cannabis and the cannabinoids. He receives funding from the Australian National Health and Medical Research Council (NHMRC). I.S.M. is Academic Director of the Lambert Initiative and a NHMRC Principal Research Fellow and receives research funding from the ARC and NHMRC. He is involved in an NHMRC-funded clinical trial using the cannabis extract, Nabiximols (Sativex). He has served as an expert witness in various medicolegal cases involving cannabis and cannabinoids. I.S.M. and J.C.A. are inventors on several patents involving cannabinoid therapeutics. J.S. was supported by an International Research Excellence Scholarship from Macquarie University. The remaining authors have no conflicts of interest.
Funding Information
This study was supported by the Lambert Initiative for Cannabinoid Therapeutics, a philanthropically-funded center for medicinal cannabis research at the University of Sydney.
Supplementary Material
Cite this article as: Heblinski M, Santiago M, Fletcher C, Stuart J, Connor M, McGregor IS, Arnold JC (2020) Terpenoids commonly found in Cannabis sativa do not modulate the actions of phytocannabinoids or endocannabinoids on TRPA1 and TRPV1 channels, Cannabis and Cannabinoid Research 5:4, 305–317, DOI: 10.1089/can.2019.0099.
References
- 1. Hanus LO, Meyer SM, Munoz E, et al. Phytocannabinoids: a unified critical inventory. Nat Prod Rep. 2016;33:1357–1392 [DOI] [PubMed] [Google Scholar]
- 2. Booth JK, Bohlmann J. Terpenes in Cannabis sativa – From plant genome to humans. Plant Sci. 2019;284:67–72 [DOI] [PubMed] [Google Scholar]
- 3. Russo EB. The case for the entourage effect and conventional breeding of clinical cannabis: no “Strain,” no gain. Front Plant Science. 2018;9:1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carlini EA, Karniol IG, Renault PF, et al. Effects of marihuana in laboratory animals and in man. Br J Pharmacol. 1974;50:299–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baram L, Peled E, Berman P, et al. The heterogeneity and complexity of Cannabis extracts as antitumor agents. Oncotarget. 2019;10:4091–4106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blasco-Benito S, Seijo-Vila M, Caro-Villalobos M, et al. Appraising the “entourage effect”: antitumor action of a pure cannabinoid versus a botanical drug preparation in preclinical models of breast cancer. Biochem Pharmacol. 2018;157:285–293 [DOI] [PubMed] [Google Scholar]
- 7. Casey SL, Atwal N, Vaughan CW. Cannabis constituent synergy in a mouse neuropathic pain model. Pain. 2017;158:2452–2460 [DOI] [PubMed] [Google Scholar]
- 8. Todd SM, Zhou C, Clarke DJ, et al. Interactions between cannabidiol and Delta(9)-THC following acute and repeated dosing: rebound hyperactivity, sensorimotor gating and epigenetic and neuroadaptive changes in the mesolimbic pathway. Eur Neuropsychopharmacol. 2017;27:132–145 [DOI] [PubMed] [Google Scholar]
- 9. Todd SM, Arnold JC. Neural correlates of interactions between cannabidiol and Delta(9) -tetrahydrocannabinol in mice: implications for medical cannabis. Br J Pharmacol. 2016;173:53–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Silveira MM, Arnold JC, Laviolette SR, et al. Seeing through the smoke: human and animal studies of cannabis use and endocannabinoid signalling in corticolimbic networks. Neurosci Biobehav Rev. 2017;76(Pt B):380–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol. 2011;163:1344–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fischedick JT, Hazekamp A, Erkelens T, et al. Metabolic fingerprinting of Cannabis sativa L., cannabinoids and terpenoids for chemotaxonomic and drug standardization purposes. Phytochemistry. 2010;71:2058–2073 [DOI] [PubMed] [Google Scholar]
- 13. Potter D. The propagation, characterisation and optimisation of Cannabis sativa L. as a phytopharmaceutical [Doctoral Thesis]. King's College: London; 2009 [Google Scholar]
- 14. Santiago M, Sachdev S, Arnold JC, et al. Absence of entourage: terpenoids commonly found in Cannabis sativa do not modulate the functional activity of delta(9)-THC at human CB1 and CB2 receptors. Cannabis Cannabinoid Res. 2019;4:165–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Muller C, Morales P, Reggio PH. Cannabinoid ligands targeting TRP channels. Front Mol Neurosci. 2018;11:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. De Petrocellis L, Ligresti A, Moriello AS, et al. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharmacol. 2011;163:1479–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smart D, Gunthorpe MJ, Jerman JC, et al. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1). Br J Pharmacol. 2000;129:227–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. De Petrocellis L, Di Marzo V. Non-CB1, non-CB2 receptors for endocannabinoids, plant cannabinoids, and synthetic cannabimimetics: focus on G-protein-coupled receptors and transient receptor potential channels. J Neuroimmune Pharmacol. 2010;5:103–121 [DOI] [PubMed] [Google Scholar]
- 19. Stuart JM. The pharmacology of novel illicit synthetic cannabinoids [Empirical thesis]. Faculty of Human Sciences, Macquarie University; Sydney, Australia; 2015 [Google Scholar]
- 20. Diaz-Franulic I, Caceres-Molina J, Sepulveda RV, et al. Structure-driven pharmacology of transient receptor potential channel vanilloid 1. Mol Pharmacol. 2016;90:300–308 [DOI] [PubMed] [Google Scholar]
- 21. Brewster MS, Gaudet R. How the TRPA1 receptor transmits painful stimuli: inner workings revealed by electron cryomicroscopy. Bioessays. 2015;37:1184–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lin King JV, Emrick JJ, Kelly MJS, et al. A cell-penetrating scorpion toxin enables mode-specific modulation of TRPA1 and pain. Cell. 2019;178:1362–1374 e1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. De Petrocellis L, Vellani V, Schiano-Moriello A, et al. Plant-derived cannabinoids modulate the activity of transient receptor potential channels of ankyrin type-1 and melastatin type-8. J Pharmacol Exp Ther. 2008;325:1007–1015 [DOI] [PubMed] [Google Scholar]
- 24. Redmond WJ, Gu L, Camo M, et al. Ligand determinants of fatty acid activation of the pronociceptive ion channel TRPA1. PeerJ. 2014;2:e248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Starkus J, Jansen C, Shimoda LMN, et al. Diverse TRPV1 responses to cannabinoids. Channels (Austin, Tex). 2019;13:172–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Motulsky HJ, Brown RE. Detecting outliers when fitting data with nonlinear regression - a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinformatics. 2006;7:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Atwood BK, Lopez J, Wager-Miller J, et al. Expression of G protein-coupled receptors and related proteins in HEK293, AtT20, BV2, and N18 cell lines as revealed by microarray analysis. BMC Genomics. 2011;12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boire A, Covic L, Agarwal A, et al. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell. 2005;120:303–313 [DOI] [PubMed] [Google Scholar]
- 29. Stueber T, Eberhardt MJ, Caspi Y, et al. Differential cytotoxicity and intracellular calcium-signalling following activation of the calcium-permeable ion channels TRPV1 and TRPA1. Cell Calcium. 2017;68:34–44 [DOI] [PubMed] [Google Scholar]
- 30. Russo EB, Marcu J. Cannabis pharmacology: the usual suspects and a few promising leads. Adv Pharmacol. 2017;80:67–134 [DOI] [PubMed] [Google Scholar]
- 31. Jordt SE, Bautista DM, Chuang HH, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265 [DOI] [PubMed] [Google Scholar]
- 32. Storozhuk MV, Zholos AV. TRP channels as novel targets for endogenous ligands: focus on endocannabinoids and nociceptive signalling. Curr Neuropharmacol. 2018;16:137–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Islam MS. Transient receptor Potential Channels. Springer: Dordrecht, 2011 [Google Scholar]
- 34. Riera CE, Menozzi-Smarrito C, Affolter M, et al. Compounds from Sichuan and Melegueta peppers activate, covalently and non-covalently, TRPA1 and TRPV1 channels. Br J Pharmacol. 2009;157:1398–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jansen C, Shimoda LMN, Kawakami JK, et al. Myrcene and terpene regulation of TRPV1. Channels (Austin). 2019;13:344–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Iannotti FA, Hill CL, Leo A, et al. Nonpsychotropic plant cannabinoids, cannabidivarin (CBDV) and cannabidiol (CBD), activate and desensitize transient receptor potential vanilloid 1 (TRPV1) channels in vitro: potential for the treatment of neuronal hyperexcitability. ACS Chem Neurosci. 2014;5:1131–1141 [DOI] [PubMed] [Google Scholar]
- 37. Lam PM, McDonald J, Lambert DG. Characterization and comparison of recombinant human and rat TRPV1 receptors: effects of exo- and endocannabinoids. Br J Anaesth. 2005;94:649–656 [DOI] [PubMed] [Google Scholar]
- 38. Cortright DN, Szallasi A. Biochemical pharmacology of the vanilloid receptor TRPV1. An update. Eur J Biochem. 2004;271:1814–1819 [DOI] [PubMed] [Google Scholar]
- 39. Vetter I, Wyse BD, Roberts-Thomson SJ, et al. Mechanisms involved in potentiation of transient receptor potential vanilloid 1 responses by ethanol. Eur J Pain. 2008;12:441–454 [DOI] [PubMed] [Google Scholar]
- 40. Evans MS, Cheng X, Jeffry JA, et al. Sumatriptan inhibits TRPV1 channels in trigeminal neurons. Headache. 2012;52:773–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vangeel L. TRP channel function in iPSC-derived sensory neurons. Biophy J. 2017;112 [Google Scholar]
- 42. Banister SD, Arnold JC, Connor M, et al. Dark classics in chemical neuroscience: delta(9)-tetrahydrocannabinol. ACS Chem Neurosci. 2019;10:2160–2175 [DOI] [PubMed] [Google Scholar]
- 43. Piomelli D. Waiting for the entourage. Cannabis Cannabinoid Res. 2019;4:137–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.