Abstract
Background:
Little is known about the impact of socioeconomic status (SES) as a key element of social determinants of health on intensive care unit (ICU) outcomes for adults
Objective:
We assessed whether a validated individual SES index termed HOUSES (HOUsing-based socioeconomic Status index) derived from housing features was associated with short-term outcomes of critical illness including ICU mortality, ICU-free days, hospital-free days, and ICU readmission.
Methods:
We performed a population-based cohort study of adult patients living in Olmsted County, Minnesota admitted to 7 intensive care units at Mayo Clinic from 2011-2014. We compared outcomes between the lowest SES group (HOUSES quartile 1) and the higher SES group (HOUSES quartiles 2-4). We stratified the cohort based on age (<50 years old and ≥50 years old).
Results:
Among 4134 eligible patients, 3378 (82%) patients had SES successfully measured by the HOUSES index. Baseline characteristics, severity of illness and reason for ICU admission were similar among the different SES groups as measured by HOUSES except for larger number of intoxications and overdoses in younger patients from the lowest SES.
In all adult patients, there were no overall differences in mortality, ICU-free days, hospital-free days, or ICU readmissions in patients with higher SES compared to lower SES. Among older patients (>50 years), those with higher SES (HOUSES Q2-4) compared to those with lower SES (HOUSES Q1), had lower mortality rates (HR=0.72; 95% CI 0.56-0.93; adjusted p=0.01), increased ICU-free days (mean 1.08 days; 95% CI 0.34-1.84; adjusted p=0.004) and increased hospital-free days (mean 1.20 days; 95% CI 0.45, 1.96; adjusted p=0.002). There were no differences in ICU readmission rates. (OR=0.74; 95% CI 0.55-1.00; p=0.051)
Conclusion:
Individual-level SES may be an important determinant or predictor of critical care outcomes in older adults. HOUSES may be a useful tool for enhancing critical care research and practice.
INTRODUCTION
Although patients admitted to the ICU account for approximately one-quarter of hospitalized patients, they account for half of total hospital expenditures in the United States, with costs estimated at $110 to $260 billion per year or approximately 1% of the gross domestic product.1-3 The demand for ICU care varies geographically but overall continues to increase and costs also continue to accelerate.4-9 Furthermore as the population ages and presents with increased co-morbidities, the complexity of illness seen in the ICU increases.10
There are large number of patient-related factors influencing outcomes including acuity and severity of illness at presentation, as well as the presence of co-morbidities.11 Other important patient characteristics that influence outcomes and lead to differences in recovery and survival include age, and demographic factors such as sex, ethnicity, and race.10,12-15 However measured and unmeasured social determinants of health (SDH) may be among the most influential factors causing outcome differences and health disparities.
Socioeconomic status (SES) as a key element of SDH is a powerful determinant of disparities in health and healthcare outcomes yet there are many challenges in measuring it accurately, especially an individual-level SES.16-18 SES is defined by one’s ability to access desired resources including human, materialistic, and social capital.19 It is often represented and determined by education, income, and occupation or a combination of all three.20 Previous studies done to explore the effect of SES on critical care outcomes including mortality both here and in other countries have shown ambiguous results.21-23 These equivocal findings about the effect of SES on critical illness may be due to a variety of factors including measurement difficulties and the use of inappropriate proxies such as occupation and education, which may not reflect current SES, within-group heterogeneity, as well as SES data that does not measure individual SES in a granular manner.24
It is worth noting that the challenges to measuring SES include lack of standardized - reporting mechanisms in electronic medical records, ad hoc documentation originating from self-reported data that may be unreliable and has not been updated, as well as a paucity of SES measures in administrative data sets and some disease registries.25 A recent systematic review which included 10 studies examining the relationship between socioeconomic position and physical function, health-related quality of life and survival following critical illness recommended future studies based in the ICU should apply individual level SES assessments when exploring this issue.26 Our understanding of the relationship between age and SES during critical illness is limited. For all of the reasons mentioned above this is an important topic worthy of research scrutiny.
Recognizing the significance of improved SES measurement for health outcomes research, Juhn et al have developed and validated an individual housing based measure of SES, termed HOUSES index (HOUsing-based SocioEconomic Status index) (see the Method section for details).27 This index is derived from publically available property assessment data and geocodes address information in the medical record and directly links the address to real property data to determine an individual’s SES28. The main objective of this study was to identify whether low SES, assessed using the HOUSES index was associated with increased in-hospital mortality as a primary outcome. In addition, we wanted to examine the effect of SES using HOUSES on hospital –free days, ICU-free days, and ICU readmissions as secondary outcomes and whether age influenced this. We hypothesized that those with a low SES when compared to those with a higher SES would have worse ICU and hospital outcomes and age would be an influential factor.
METHODS
This study was approved as a minimal risk study by the Institutional review Board at Mayo Clinic.
Study setting and population
The study was conducted at Mayo Clinic, a quaternary care hospital that receives 15,000 ICU admissions per year. Olmsted County is the eighth most populated County in Minnesota with 144,248 residents (as reported in the 2010 census), and is an ideal setting to conduct population-based epidemiologic research. Approximately 98% of medical care received by county residents is delivered through Mayo Clinic, Olmsted Medical Center, and their affiliated health care facilities.29,30 Additionally, all Olmsted County ICU admissions come to Mayo Clinic.
Study design
The study was a population-based cohort study.
Study participants
Our study included all Olmsted County patients aged ≥ 18 years admitted to 7 ICUs at Mayo Clinic, Rochester, Minnesota between June 1, 2011 and May 31, 2014. If a patient had multiple ICU admissions, only the first admission was included for analysis of most outcomes, however we did analyze occurrence of re-admissions to the ICU. Olmsted county residents without research authorization or HOUSES information were excluded.
Data collection
Primary outcome:
The primary outcome of the study was in-hospital mortality.
Secondary Outcomes:
Secondary outcomes included hospital-free days, ICU-free days, and ICU readmission. Hospital-free days was defined as number of the days the patient was alive and outside of the hospital within 28 days of hospital admission. By definition, if a patient became deceased prior to hospital discharge, or had a hospital length of stay longer than 28 days, this outcome was defined as 0. This outcome was chosen over traditional length of stay to penalize early deaths within the ICU, as analyzing simple length of stay treats early death as a favorable outcome. The outcome ICU-free days was defined similarly. All variables were abstracted using electronic retrieval queries within the Unified Data platform (UDP). The demographic variables abstracted included age, sex, race, education level, and primary spoken language. We used the Charlson Comorbidity Index for assessment of medical complexity. Charlson Comorbidity Index quantifies medical complexities by incorporating the number and severity of 19 comorbid conditions as listed by the International Classification of Diseases, Ninth Revision codes.31 We calculated the Acute Physiology and Chronic Health Evaluation (APACHE) III.32 We defined the index date for most outcomes as the first admission to the ICU except for death when the index date was the final admission to the ICU.
Measurement of SES-HOUSES index
We used the HOUSES index, as an individual-level SES assessment tool. The HOUSES index uses address-linked real property data which is publically available from local Olmsted County governmental sources.27,28 Previous publications have reported in detail the development and validation of the HOUSES index.27 The four real property data points used to calculate the HOUSES index include estimated building value, housing unit size, number of bedrooms, and number of bathrooms. Variables are aggregated into an overall z-score such that a higher HOUSES score indicated higher SES. Subsequently our cohort was grouped into quartiles (Q1: lowest SES).
Juhn et al. have validated the HOUSES score within Olmsted County, Minnesota, Jackson County, Missouri, and Sioux Falls, South Dakota.27,33,34 Since HOUSES does not rely on self-reported data about SES (i.e., it is objective), can be updated with current address to reflect current SES (vs. educational attainment), and is publically available, it is very useful to better understand the role of SES for a broad range of important research questions, including ICU outcomes. Several health-related outcomes and risk factors have been evaluated using the HOUSES index in both adults and children.35,36 These include rheumatoid arthritis and mortality, vaccination uptake, rates of hospitalization, accidental falls, multiple chronic conditions as well as cardiovascular outcomes, and kidney transplant graft failure.34,37-43 The HOUSES index has also been used once previously in an ICU cohort to examine rates of advance care planning, healthcare utilization and discharge disposition.44 To focus on the main outcomes (eg, association of HOUSES with ICU outcomes) and avoid distraction, we did not report association of other SES measures with ICU outcomes, and Juhn et al demonstrated moderate to good correlation of HOUSES with other SES measures (eg, education, income, Hollingshead index, and Nakao-Treas index), which was not surprising given that each SES measure may reflect different aspects of SES. More importantly, HOUSES tended to show stronger association with health outcomes (eg, overweight, low birth weight) and behavioral risk factor (eg, smoking exposure) in a dose-response manner, compared to other SES measures.27
Reasons for ICU admission
To better understand mechanism between ICU outcomes and SES, we manually audited primary diagnosis of ICU admission and grouped them into four categories: medical diagnosis, surgical diagnosis, poisoning, and trauma.
Statistical analysis
Patient demographics and clinical characteristics were summarized using counts and percentages for categorical variables, and medians and interquartile ranges for continuous variables. Differences in proportions by quartile were analyzed using Pearson chi-square tests. Among patients differences in the distribution of the variables by HOUSES quartile were analyzed using chi-square/Fisher exact tests (where appropriate) for categorical variables and Kruskal-Wallis tests for continuous variables.
For the primary outcome of hospital mortality, we defined the duration of follow-up within the hospital as the date/time of ICU admission to the date/time of death or discharge alive from the hospital. Association between continuous HOUSES z-scores and the primary outcome of hospital mortality were examined using unadjusted and multivariable Cox proportional-hazards models adjusted for the effects of the a priori selected variables of age, sex, race, and APACHE. Interaction analyses were performed to determine if HOUSES modified any association between age, sex, race, and APACHE with mortality. The proportional hazards assumption was checked and met for all proportional hazards models. ICU mortality was analyzed using similar methods. The outcomes of ICU and hospital-free days were analyzed using linear regression, and ICU readmissions were analyzed using logistic regression models. As the lowest SES group (Q1) as determined by HOUSES would help identify a specific socially and medically underserved subgroup of our primary interest, HOUSES was stratified to allow for specific comparisons between Q2-4 versus Q1. Another rationale of using Q1 vs. Q2-4 was that demographic (eg, age, proportion of male and White race, English-speaking) and clinical characteristics (eg, APACHE score at admission to ICU) were similar among Q2-3, but not Q1. We have used this approach in previous HOUSES studies.43,44 All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary NC). All tests were 2-sided, and p < 0.05 was determined to be significant.
Interaction term
We stratified the cohort into those <50 years and those ≥50 years. This cut off has been used in previous studies of the effect of aging on health inequalities.45,46
RESULTS
Baseline Characteristics
Among the original cohort (n=4134), 250 were excluded as their address could not be retrieved or they no longer lived in Olmsted County, 468 were excluded as their address could not be successfully geocoded, they lived in a nursing home without sufficient prior residential home address information, or the address provided was a P.O. Box.44 A further 15 were excluded as they had retracted their research authorization since May 2014. The final cohort therefore included 3378 unique patients. Therefore 82% of the original cohort had their home address successfully formulated into a HOUSES score.
Baseline characteristics of the final cohort are summarized in Table 1. 1830/3378(54%) were male, and the median age and interquartile range (IQR) at the time of ICU admission was 67 (52, 59) years. The patients in the HOUSES Q1 group were more likely to be non-white, Muslim, and with limited English proficiency. Only 28.6% patients were found to have advance directive at the time of ICU admission among HOUSES Q1 compared to 41.2% among HOUSES Q2-Q4 (P=<0.001). There was no significant difference in the Charlson score among the quartile groups by HOUSES. Primary reasons for ICU admission were largely due to medical diagnoses, but differed by age group (ie, <50 years vs. ≥50 years) with most intoxications and overdoses occurring in those <50 years of age.
Table 1:
Baseline Characteristics HOUSES Q1 (lowest SES) versus Q2-4
| Characteristic | Houses Q1 N=838(%) |
Houses Q2-Q4 N=2540(%) |
Total N=3378(%) |
p-value | ||
|---|---|---|---|---|---|---|
|
Age at ICU admission Median(IQR) |
60.5(47.0, 75.0) | 69(54, 80) | 67(52, 79) | <0.001† | ||
| Sex | <0.001‡ | |||||
| Female | 435(51.9%) | 1113(43.8%) | 1548(45.8%) | |||
| Male | 403(48.1%) | 1427(56.2%) | 1830(54.2%) | |||
| Race | <0.001‡ | |||||
| White | 687(82%) | 2345(92.3%) | 3032(89.8%) | |||
| Non-White | 151(18%) | 195(7.7%) | 346(10.2%) | |||
| English | <0.001‡ | |||||
| No | 77(9.2%) | 96(3.8%) | 173(5.1%) | |||
| Yes | 761(90.8%) | 2444(96.2%) | 3205(94.9%) | |||
| Education | <0.001‡ | |||||
| Missing | 49 | 47 | 96 | |||
| Some high school or less | 148 (18.8%) | 278 (11.2%) | 426 (13.0%) | |||
| High School Graduate | 266 (33.7%) | 829 (33.3%) | 1095 (33.4%) | |||
| Any College | 254 (32.2%) | 696 (27.9%) | 950 (28.9%) | |||
| College Graduate | 121 (15.3%) | 690 (27.7%) | 811 (24.7%) | |||
| Religion | <0.001‡ | |||||
| Christian | 609(72.7%) | 2116(83.3%) | 2725(80.7%) | |||
| Muslim | 45(5.4%) | 30(1.2%) | 75(2.2%) | |||
| Jewish | 5(0.6%) | 13(0.5%) | 18(0.5%) | |||
| Other (Atheist, Hindu, Agnostic, Sikhism,) | 5(0.6%) | 12(0.5%) | 17(0.5%) | |||
| Chose not to disclose | 27(3.2%) | 64(2.5%) | 91(2.7%) | |||
| No affiliation | 147(17.5%) | 305(12%) | 452(13.4%) | |||
| Advance Directive at ICU admission | 240(28.6%) | 1046(41.2%) | 1286(38.1%) | <0.001‡ | ||
| APACHE | 33(23, 47) | 38(25, 51) | 37(24, 50) | <0.001† | ||
| Charlson Co-morbidity Score | 5(2, 8) | 5(3, 8) | 5(3, 8) | 0.06† | ||
| HOUSES (Z-score) | −4.0 (−4.4, −3.4) | 0.8 (−0.8, 2.4) | −0.3 (−2.4, 1.6) | <0.001† | ||
| Reason for admission to ICU | <50 | ≥50 | <50 | ≥ 50 | ||
| Medical diagnosis | 134(53) | 469(81) | 313(60) | 1638(82) | ||
| Poisoning | 83(33) | 26(5) | 113(22) | 17(1) | 0.001 | |
| Surgical diagnosis | 14(6) | 32(6) | 34(7) | 127(6) | ||
| Trauma | 23(9) | 53(9) | 62(12) | 220(11) | ||
| Missing | 3 | 9 | 7 | 24 | ||
Wilcoxon Rank-Sum
Chi-square
Numbers indicate N (%) unless otherwise noted
IQR=interquartile range
Reasons for ICU admission
We examined reasons for admissions within those <50 and those ≥50 to understand whether the reasons for admission may have influenced outcomes. We identified that almost one third of those <50 in the HOUSES Q1 were admitted for intoxications and overdoses 83/254(33%). This was significantly different to those in HOUSES Q2-Q4 in the same age range 113/522 (22%) (p=0.001).
Outcomes
Primary outcome: Hospital Mortality:
Almost 10% (321/3378) of the entire cohort died during hospital admission (Table 2, Figure 1). Patients in HOUSES Q2-Q4 did not show significantly different rates of in-hospital mortality compared to HOUSES Q1 in unadjusted (HR 0.88; 95% CI 0.69-1.12; p=0.31) and adjusted models (HR 0.81; 95% CI 0.63-1.04, p=0.10). There was a significant interaction between age and HOUSES (p=0.01). Relative to Q1, HOUSES Q2-Q4 was significantly associated with 28% decreased rates of hospital mortality (HR=0.72; 95% CI 0.56-0.93; p=0.01) in patients aged >=50 years, whereas there was no statistically significant difference in patients aged <50 (HR=2.70; p=0.07), maybe in part due to small sample size (Table 3).
Table 2.
Associations Between HOUSES Quartile and Outcomes Using Unadjusted and Multivariable Regression Models
| Outcome | Unadjusted Statistics |
Unadjusted Estimate |
Unadjusted p-value |
Adjusted Estimate† |
Adjusted p-value† |
|---|---|---|---|---|---|
| Hospital Mortality | N Deaths | Hazard Ratio | 0.31 | Hazard Ratio | 0.10 |
| HOUSES Q1, n=838 | 87 (10.4%) | 1.00 (ref) | 1.00 (ref) | ||
| HOUSES Q2-Q4, n=2540 | 234 (9.2%) | 0.88 (0.69, 1.12) | 0.81 (0.63, 1.04) | ||
| ICU-Free Days | Mean (SD) | Mean Estimate | 0.67 | Mean Estimate | 0.10 |
| HOUSES Q1, n=838 | 23.4 (8.2) | 0.00 (ref) | 0.00 (ref) | ||
| HOUSES Q2-Q4, n=2540 | 23.5 (7.9) | 0.14 (−0.48, 0.76) | 0.50 (−0.10, 1.09) | ||
| Hospital-Free Days | Mean (SD) | Mean Estimate | 0.57 | Mean Estimate | 0.03 |
| HOUSES Q1, n=838 | 19.7 (8.8) | 0.00 (ref) | 0.00 (ref) | ||
| HOUSES Q2-Q4, n=2540 | 19.9 (8.3) | 0.19 (−0.47, 0.85) | 0.70 (0.08, 1.32)* | ||
| ICU Readmission | N Readmissions | Odds Ratio | 0.31 | Odds Ratio | 0.051 |
| HOUSES Q1, n=838 | 69 (8.2%) | 1.00 (ref) | 1.00 (ref) | ||
| HOUSES Q2-Q4, n=2540 | 182 (7.2%) | 0.86 (0.64, 1.15) | 0.74 (0.55, 1.00) |
Unadjusted models consisted of just HOUSES as the predictor.
Multivariable models were additionally adjusted for the effects of age, sex, race, and APACHE.
Missing data were handled using multiple imputation using 10 independent, imputed datasets.
Figure 1.
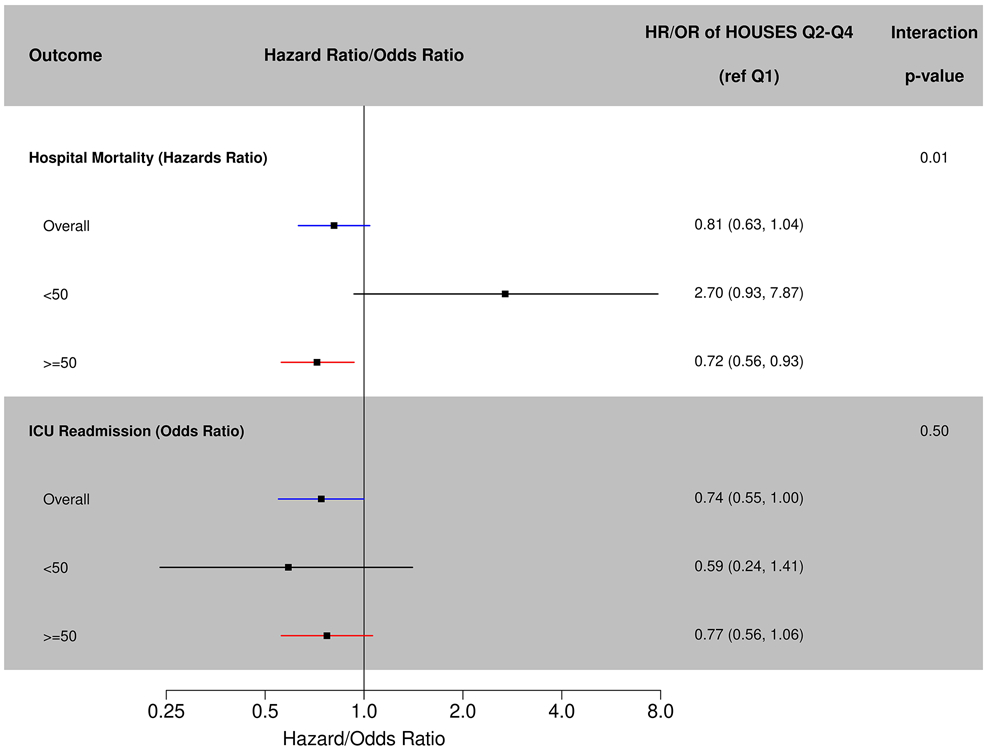
Hospital mortality and intensive care unit readmission.
Table 3.
Interactions Between Age and HOUSES and outcomes using multivariable Cox, linear, and logistic regression
| Age <50 N=740 |
Age ≥ 50 N=2683 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | N | Outcome | estimate † | p- value † |
N | Outcome | estimate † | p- value † |
Inter actio n p- valu e ‡ |
| Time to With-in Hospital Mortality | N | N Event (%) | Hazard Ratio | 0.07 | N | N Event (%) | Hazard Ratio | 0.01 | 0.01 |
| HOUSES Q1 | 243 | 4 (1.7%) | 1.00 (ref) | 595 | 83 (14.0%) | 1.00 (ref) | |||
| HOUSES Q2-Q4 | 497 | 24 (4.8%) | 2.70 (0.93, 7.87) | 2043 | 210 (10.3%) | 0.72 (0.56, 0.93) * | |||
| ICU Free Days | N | Mean (SD) | Mean Estimate | 0.005 | N | Mean (SD) | Mean Estimate | 0.004 | <.01 |
| HOUSES Q1 | 243 | 26.0 (4.1) | 0.00 (ref) | 595 | 22.3 (9.1) | 0.00 (ref) | |||
| HOUSES Q2-Q4 | 497 | 25.0 (6.1) | −1.16 (−1.96, −0.36) ** | 2043 | 23.1 (8.2) | 1.08 (0.34, 1.82) ** | |||
| Hospital Free Days | N | Mean (SD) | Mean Estimate | 0.13 | N | Mean (SD) | Mean Estimate | 0.002 | <.01 |
| HOUSES Q1 | 243 | 23.1 (6.1) | 0.00 (ref) | 595 | 18.4 (9.3) | 0.00 (ref) | |||
| HOUSES Q2-Q4 | 497 | 22.6 (6.9) | −0.73 (−1.68, 0.21) | 2043 | 19.2 (8.5) | 1.20 (0.45, 1.96) ** | |||
| ICU Readmission | N | N Event (%) | Odds Ratio | 0.23 | N | N Event (%) | Odds Ratio | 0.10 | 0.50 |
| HOUSES Q1 | 243 | 10 (4.1%) | 1.00 (ref) | 595 | 59 (9.9%) | 1.00 (ref) | |||
| HOUSES Q2-Q4 | 497 | 12 (2.4%) | 0.59 (0.24, 1.41) | 2043 | 170 (8.3%) | 0.77 (0.56, 1.06) | |||
P <.05
P <.01
Estimate and P-value are adjusted for all covariates listed in the table
P-value is the interaction p-value between HOUSES and AGE. If P<.05, it means the estimate for HOUSES is significantly modified by age <50 vs. >=50. P-values comes from a model on the full sepsis cohort, adjusted for the effects of HOUSES, age, sex, race, APACHE, and the HOUSES*age interaction.
Secondary outcomes
1). ICU-Free Days:
The mean number of ICU-free days in the cohort was 23. There was no significant difference in ICU- free days by HOUSES in unadjusted or adjusted models (Table 2, Figure 2). There was a significant interaction between age and HOUSES with ICU Free days (p<.01). Relative to Q1, Q2-Q4 patients had significantly increased ICU free days (mean 1.08; 95% CI 0.34-1.84; p=0.004) among patients aged >=50, whereas patients <50 had significantly fewer ICU free days (mean −1.16; 95% CI −1.96 to −0.36; p=0.005).
Figure 2.
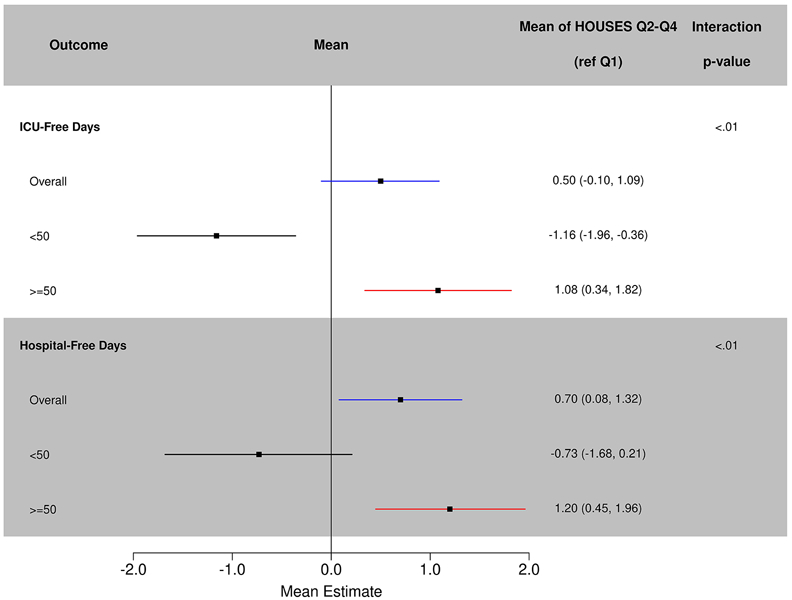
Adjusted mean estimate of HOUsing-based SocioEconomic status with intensive care unit and hospital-free days.
2). Hospital-Free Days:
The mean number of hospital-free days was 20 in the cohort. After adjustment for age, sex, race, and APACHE, HOUSES Q2-Q4 had significantly fewer hospital- free days than those in Q1 (mean=0.70, 95% CI 0.08-1.32; p=0.03) (Table 2, Figure 2). Similar to ICU free days, there was a significant interaction between age and HOUSES (p<0.01). Relative to Q1, HOUSES Q2-Q4 had significantly increased hospital-free days in patients aged >=50, (mean=1.20, 95% CI 0.45-1.96; p=0.002) whereas for patients aged <50 there were no significant differences. The interaction between age and HOUSES was significant at p<0.01.
3). ICU Readmission:
About 7.5% (251/3378) of the entire cohort was readmitted to the ICU. There were no statistically significant differences for rates of ICU readmission for HOUSES Q1 versus Q2-Q4 (p-value =0.31) in unadjusted analysis, odds ratio=0.86 (95% CI: 0.64-1.15). After adjustment for age, sex, race, and APACHE, Q2-Q4 had decreased odds of ICU readmission (OR=0.74; 95% CI 0.55-1.00; p=0.051), but this comparison was insignificant (Table 2, Figure 1). There was no significant interaction between HOUSES and age for ICU readmissions (Table 3, Figure 1).
Education as a predictor variable instead of HOUSES
Education was not associated with hospital mortality, ICU mortality, or ICU readmissions, but college graduates had 1.28 more mean hospital free days (95% CI 0.35-2.21, p=0.006) and 1.10 more mean ICU free days (95% CI 1.03-1.33, p=0.01) relative to some high school or less patients.
DISCUSSION
Socioeconomic status, as measured by the HOUSES index, was associated with differences in ICU and hospital outcomes in older adults>50 years. Age was an important effect modifier for SES on ICU and hospital outcomes including hospital mortality, ICU-free days and hospital-free days. There was no significant effect modification between SES and age for ICU readmission.
The use of the HOUSES index allows us to establish an accurate and individual measure of SES for the ICU patients in this study cohort. Census tract and area-level SES have become popular in recent years for measuring SES and examining disparities in a variety of domains.47 While helpful these tools may introduce misclassification bias up to one third of the time, (the so-called ecological fallacy-when group data is used to make inferences about individuals), leading to conflicting research findings.48,49 Furthermore, the extent of and actual effects of area level SES versus individual level SES on clinical outcomes differ.50 The HOUSES index provides a unique opportunity to study the effect of individual-level SES on a variety of healthcare outcomes but has only been used once previously in the ICU setting.44
This paper is unique as it examines the interaction or effect modification between age and HOUSES index on critical illness outcomes. We used a cut-off age of 50 years and demonstrated that some outcomes showed significant differences associated with the interaction between age and SES status. An interaction is a statistical term that describes the effect of one variable on the outcome of interest when modified by the value of another variable.51,52 Interactions between variables are present when their joint effect is larger or smaller than their summative effect on the outcome. In this study few overall differences were noted between those in the lowest SES group and those in the higher SES group. However when we examined the groups by age(<50 and >50 years) we saw significant differences that suggest that the modifying effects of increasing age on SES lead to associations with poorer ICU and hospital outcomes such as hospital mortality, ICU-free days and hospital-free days. Although there were no significant differences in hospital mortality between HOUSES 1 versus HOUSES 2-4 overall, older ICU adult patients from higher socioeconomic groups had hospital mortality rates that were almost 30% lower when compared to older patients from lower socioeconomic groups (HOUSES 1). We also found that older patients with high SES had a higher number of days outside ICU (shorter ICU stays) than older patients with lower SES. When we explored overall hospital stay (hospital-free days) we found that older patients with higher SES were more likely to be discharged quickly than those of the same age group from the lower SES group. There is evidence to support the concept that older patients with lower SES face multiple challenges and do worse after discharge for a number of reasons.53 These may include lack of the necessary financial and social supports after hospital discharge leading to longer hospital stays. Our previous study demonstrated that low SES was associated with higher rates of discharge to nursing home instead of home, however we did not examine the interaction between age and SES in that study.44 All of the above findings suggests that as aging occurs SES becomes a more prominent predictor of outcomes in critical illness and associated with worse outcomes.
There were no significant differences in hospital mortality or hospital-free days for younger patients regardless of their socioeconomic status. Younger patients with lower SES had a higher number of days outside the ICU (ICU-free days). In order to understand this phenomenon better we examined the reasons for admission. We identified that there was a significantly higher proportion of patients in the low SES group (one third of cohort versus one fifth) admitted with poisoning, a diagnosis that usually requires a brief ICU admission. It is likely that those from higher SES required longer ICU stays as their admissions were less likely to be related to poisoning (ie, acute medical conditions) and needed more complex ICU management and longer stays We could not do further analysis on this as our database lacked the granular detail necessary therefore our interpretation of these findings is speculative and deserves more rigorous exploration.
We did not find any significant differences between patients with lower SES and patients with higher SES when we measured readmission rates to Additionally there was no significant interaction between SES measured by HOUSES index and age for ICU readmissions. It may be that to mitigate risks of readmission patients of lower SES are kept longer in hospital as shown by our hospital-free days data.
Several studies have shown that among the large number of patient-related factors affecting outcomes of critical illness, age over 65 is usually a significant independent risk for worse outcomes.54-56 However, other research has shown that emergent surgeries, severity of illness and chronic co-morbidities were all more important predictors of critical illness outcomes than age itself.57-59 Ho Kwok et al reported that SES adjusted with age was significantly associated with hospital mortality, but this association disappeared once other important confounders were included in the analysis.21 Depending on resources, older age may guide treatment intensity, with less aggressive measures being deployed in the very elderly and consequently outcomes influenced by management choices secondary to age.60,61
The cut-off of 50 years was chosen for a number of reasons. We considered it a more relevant (biological) age cut-off for this study as age-related immune function (immune senescence) is likely to be influential in the outcomes we were concerned with.62 For example, herpes zoster is considered to be a disease that occurs due to age-related immune senescence and the incidence of herpes zoster increases dramatically after 50 years of age.63,64 As a result of this age-related immune senescence, zoster vaccine has been licensed for those ages 50 years or older. Furthermore the live influenza vaccine has not been approved in individuals older than 50 years in the United States for similar reasons.63 We also considered the prevalence of multiple chronic conditions within our population in Olmsted when we considered our age cut-off and its importance for the study outcomes. Our previous work in Olmsted County, using the HOUSES index demonstrated that in older adults (median age of 57 years) 90% already had one or more chronic conditions (multiple chronic conditions).65 Our study findings highlight that even at the relatively young age of 50 years, when adults are actively engaged in the workforce and should be major contributors to the economy, significant health inequalities in critical care outcomes exist.
Our study has some limitations. It is a single-center cohort study based in a quaternary-care academic medical center in in the Midwest. However, Mayo Clinic, Rochester, is the only hospital in Olmsted County providing ICU care and is therefore a suitable environment for a population-based study. We included residents from Olmsted County, which has a predominantly white population. Although increasingly validated across counties in the Midwest, the HOUSES index may not be available in all counties and its application to study the effects of SES on healthcare outcomes may thus be limited at present. We were only able to complete a HOUSES score for 82% of our study cohort however this compares favorably to abstraction of other proxies of SES including education.44 While it is critical to assess and understand why patterns of association of HOUSES with ICU outcomes differ by age, given heterogeneity of reasons of admission to ICU and small number of the study cohort, a larger sample-sized study may be warranted to address this issue, especially for younger age < 50 years.
Strengths of this study include the use of an objective and granular individual-level measure of SES that can mitigate the negative implications of misclassification bias and subsequent misleading research findings. Furthermore it is the first time the HOUSES index has been applied in this population through the lens of aging as a modifier of critical illness outcomes. The HOUSES index is a composite index derived from housing attributes and can be calculated by linking address in EMR to housing assessment data and geographic location. An important advantage of the HOUSES index is that it can follow changes in patient’s SES over time with change of address but also serves as an objective measure of SES at a specific time point. Based on the strengths of the HOUSES index outlined above and its use in many different settings and among several different patient populations, we believe the HOUSES index addresses Jones’ call to action for improved SES measures to understand health outcomes following critical illness.26,43 We have demonstrated that the HOUSES index is a useful tool and can provide clarity about SES and critical illness outcomes that other measures fail to do. It is important to better understand the effect of SES as a key element on critical care outcomes as the role of social determinants of health become increasingly apparent in other healthcare domains. The accuracy of the HOUSES index is an important feature as the HOUSES index may provide a more robust reflection of a patient’s “social” risk factors as well as one’s ability to access to desirable resources.19,66
Acknowledgments
Funding: This study was supported by CTSA Grant Number TL1 TR002380 from the National Center for Advancing Translational Science (NCATS) and by R01 HL126667 from NHLBI. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The study was also supported by an internal grant awarded by the Critical Care Research Committee at Mayo Clinic.
Footnotes
This work was performed at the Mayo Clinic, Rochester Minnesota.
The authors have not disclosed any potential conflicts of interest.
References:
- 1.Barrett ML, Smith MW, Elixhauser A, Honigman LS, Pines JM. STATISTICAL BRIEF# 185. 2014. [Google Scholar]
- 2.Coopersmith CM, Wunsch H, Fink MP, et al. A comparison of critical care research funding and the financial burden of critical illness in the United States. Critical care medicine. 2012;40(4):1072–1079. [DOI] [PubMed] [Google Scholar]
- 3.Halpern NA, Pastores SM. Critical care medicine beds, use, occupancy and costs in the United States: a methodological review. Critical care medicine. 2015;43(11):2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halpern NA. Can the costs of critical care be controlled? Current opinion in critical care. 2009;15(6):591–596. [DOI] [PubMed] [Google Scholar]
- 5.Mullins PM, Goyal M, Pines JM. National growth in intensive care unit admissions from emergency departments in the United States from 2002 to 2009. Academic Emergency Medicine. 2013;20(5):479–486. [DOI] [PubMed] [Google Scholar]
- 6.Cooke CR. Risk of death influences regional variation in intensive care unit admission rates among the elderly in the United States. PloS one. 2016;11(11):e0166933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallace DJ, Angus DC, Seymour CW, Barnato AE, Kahn JM. Critical care bed growth in the United States. A comparison of regional and national trends. American journal of respiratory and critical care medicine. 2015;191(4):410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallace DJ, Seymour CW, Kahn JM. Hospital-level changes in adult ICU bed supply in the United States. Critical care medicine. 2017;45(1):e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ward NS, Chong DH. Critical care beds and resource utilization: current trends and controversies. Paper presented at: Seminars in respiratory and critical care medicine2015. [DOI] [PubMed] [Google Scholar]
- 10.Fuchs L, Chronaki CE, Park S, et al. ICU admission characteristics and mortality rates among elderly and very elderly patients. Intensive Care Medicine. 2012;38(10):1654–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horner R, Lawler FH, Hainer BL. Relationship between patient race and survival following admission to intensive care among patients of primary care physicians. Health services research. 1991;26(4):531. [PMC free article] [PubMed] [Google Scholar]
- 12.Foreman MG, Willsie SK. Health care disparities in critical illness. Clinics in chest medicine. 2006;27(3):473–486. [DOI] [PubMed] [Google Scholar]
- 13.Soto GJ, Martin GS, Gong MN. Healthcare disparities in critical illness. Critical care medicine. 2013;41(12):2784–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Degenholtz HB, Thomas SB, Miller MJ. Race and the intensive care unit: disparities and preferences for end-of-life care. Critical care medicine. 2003;31(5):S373–S378. [DOI] [PubMed] [Google Scholar]
- 15.Muni S, Engelberg RA, Treece PD, Dotolo D, Curtis JR. The influence of race/ethnicity and socioeconomic status on end-of-life care in the ICU. Chest Journal. 2011;139(5):1025–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calderon VY. Socioeconomic Determinants of Health: The Facts Are In. AMA J Ethics. 2006;8(11):744–747. [DOI] [PubMed] [Google Scholar]
- 17.Braveman P, Gottlieb L. The social determinants of health: it's time to consider the causes of the causes. Public health reports. 2014;129(1_suppl2):19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galobardes B, Shaw M, Lawlor DA, Lynch JW, Davey Smith G. Indicators of socioeconomic position (part 1). Journal of Epidemiology and Community Health. 2006;60(1):7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oakes JM, Rossi PH. The measurement of SES in health research: current practice and steps toward a new approach. Soc Sci Med. 2003;56(4):769–784. [DOI] [PubMed] [Google Scholar]
- 20.Winkleby MA, Jatulis DE, Frank E, Fortmann SP. Socioeconomic status and health: how education, income, and occupation contribute to risk factors for cardiovascular disease. American journal of public health. 1992;82(6):816–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho KM, Dobb GJ, Knuiman M, Finn J, Webb SA. The effect of socioeconomic status on outcomes for seriously ill patients: a linked data cohort study. Medical Journal of Australia. 2008;189(1):26–30. [DOI] [PubMed] [Google Scholar]
- 22.Welch CA, Harrison DA, Hutchings A, Rowan K. The association between deprivation and hospital mortality for admissions to critical care units in England. Journal of critical care. 2010;25(3):382–390. [DOI] [PubMed] [Google Scholar]
- 23.Hutchings A, Raine R, Brady A, Wildman M, Rowan K. Socioeconomic status and outcome from intensive care in England and Wales. Medical care. 2004:943–951. [DOI] [PubMed] [Google Scholar]
- 24.Geronimus AT. Invited commentary: using area-based socioeconomic measures—think conceptually, act cautiously. American Journal of Epidemiology. 2006;164(9):835–840. [DOI] [PubMed] [Google Scholar]
- 25.Krieger N Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. American journal of public health. 1992;82(5):703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones JR, Berney S, Connolly B, et al. Socioeconomic Position and Health Outcomes Following Critical Illness: A Systematic Review. Critical care medicine. 2019;47(6):e512–e521. [DOI] [PubMed] [Google Scholar]
- 27.Juhn YJ, Beebe TJ, Finnie DM, et al. Development and initial testing of a new socioeconomic status measure based on housing data. Journal of Urban Health. 2011;88(5):933–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Juhn Y, Krusemark E, Rand-Weaver J, et al. A novel measure of socioeconomic status using individual housing data in health disparities research for asthma in adults. Allergy: European Journal of Allergy and Clinical Immunology. 2014;69:327. [Google Scholar]
- 29.Melton LJ III. History of the Rochester epidemiology project. Paper presented at: Mayo Clinic Proceedings 1996. [DOI] [PubMed] [Google Scholar]
- 30.Rocca WA, Yawn BP, Sauver JLS, Grossardt BR, Melton LJ. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Paper presented at: Mayo Clinic proceedings 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of clinical epidemiology. 1992;45(6):613–619. [DOI] [PubMed] [Google Scholar]
- 32.Knaus WA, Wagner DP, Draper EA, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100(6):1619–1636. [DOI] [PubMed] [Google Scholar]
- 33.Butterfield MC, Williams AR, Beebe T, et al. A two-county comparison of the HOUSES index on predicting self-rated health. Journal of Epidemiology and Community Health. 2010:jech.2008.084723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris MN, Lundien MC, Finnie DM, et al. Application of a novel socioeconomic measure using individual housing data in asthma research: an exploratory study. NPJ primary care respiratory medicine. 2014;24:14018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bang DW, Manemann SM, Gerber Y, et al. A novel socioeconomic measure using individual housing data in cardiovascular outcome research. International journal of environmental research and public health. 2014;11(11):11597–11615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghawi H, Crowson CS, Rand-Weaver J, Krusemark E, Gabriel SE, Juhn YJ. A novel measure of socioeconomic status using individual housing data to assess the association of SES with rheumatoid arthritis and its mortality: a population-based case–control study. BMJ open. 2015;5(4):e006469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wi C-I, Gauger J, Bachman M, et al. Role of individual-housing–based socioeconomic status measure in relation to smoking status among late adolescents with asthma. Annals of epidemiology. 2016;26(7):455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ryu E, Juhn YJ, Wheeler PH, et al. Individual housing-based socioeconomic status predicts risk of accidental falls among adults. Ann Epidemiol. 2017;27(7):415–420.e412. [DOI] [PubMed] [Google Scholar]
- 39.Hammer R, Capili C, Wi C-I, Ryu E, Rand-Weaver J, Juhn YJ. A new socioeconomic status measure for vaccine research in children using individual housing data: a population-based case-control study. BMC public health. 2016;16(1):1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wi C-I, Sauver JLS, Jacobson DJ, et al. Ethnicity, Socioeconomic Status, and Health Disparities in a Mixed Rural-Urban US Community—Olmsted County, Minnesota. Paper presented at: Mayo Clinic Proceedings 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson M, Urm S, Jung J, et al. Housing data-based socioeconomic index and risk of invasive pneumococcal disease: an exploratory study. Epidemiology and infection. 2013;141(04):880–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ryu E, Wi C-I, Crow SS, et al. Assessing health disparities in children using a modified housing-related socioeconomic status measure: a cross-sectional study. BMJ open. 2016;6(7):e011564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stevens MA, Beebe TJ, Wi C-I, Taler SJ, Sauver JLS, Juhn YJ. HOUSES index as an innovative socioeconomic measure predicts graft failure among kidney transplant recipients. Transplantation. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barwise A, Juhn YJ, Wi C-I, et al. An Individual Housing-Based Socioeconomic Status Measure Predicts Advance Care Planning and Nursing Home Utilization. American Journal of Hospice and Palliative Medicine®. 2018:1049909118812431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shaw BA, McGeever K, Vasquez E, Agahi N, Fors S. Socioeconomic inequalities in health after age 50: are health risk behaviors to blame? Soc Sci Med. 2014;101:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McMunn A, Nazroo J, Breeze E. Inequalities in health at older ages: a longitudinal investigation of the onset of illness and survival effects in England. Age and Ageing. 2008;38(2):181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steenland K, Henley J, Calle E, Thun M. Individual-and area-level socioeconomic status variables as predictors of mortality in a cohort of 179,383 persons. American journal of epidemiology. 2004;159(11):1047–1056. [DOI] [PubMed] [Google Scholar]
- 48.Pardo-Crespo MR, Narla NP, Williams AR, et al. Comparison of individual-level versus area-level socioeconomic measures in assessing health outcomes of children in Olmsted County, Minnesota. J Epidemiol Community Health. 2013:jech-2012-201742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Narla NP, Pardo-Crespo MR, Beebe TJ, et al. Concordance between individual vs. Area-level socioeconomic measures in an Urban setting. Journal of health care for the poor and underserved. 2015;26(4):1157–1172. [DOI] [PubMed] [Google Scholar]
- 50.Stafford M, Marmot M. Neighbourhood deprivation and health: does it affect us all equally? International journal of epidemiology. 2003;32(3):357–366. [DOI] [PubMed] [Google Scholar]
- 51.Fitzmaurice G The meaning and interpretation of interaction. Nutrition. 2000;16(4):313–314. [DOI] [PubMed] [Google Scholar]
- 52.Fitzmaurice G How to explain an interaction. Nutrition (Burbank, Los Angeles County, Calif). 2001;17(2):170–171. [DOI] [PubMed] [Google Scholar]
- 53.Kangovi S, Barg FK, Carter T, et al. Challenges Faced by Patients with Low Socioeconomic Status During the Post-Hospital Transition. Journal of General Internal Medicine. 2014;29(2):283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosenthal GE, Kaboli PJ, Barnett MJ, Sirio CA. Age and the risk of in-hospital death: insights from a multihospital study of intensive care patients. Journal of the American Geriatrics Society. 2002;50(7):1205–1212. [DOI] [PubMed] [Google Scholar]
- 55.Kaarlola A, Tallgren M, Pettilä V. Long-term survival, quality of life, and quality-adjusted life-years among critically ill elderly patients. Critical care medicine. 2006;34(8):2120–2126. [DOI] [PubMed] [Google Scholar]
- 56.Somme D, Maillet J-M, Gisselbrecht M, Novara A, Ract C, Fagon J-Y. Critically ill old and the oldest-old patients in intensive care: short-and long-term outcomes. Intensive care medicine. 2003;29(12):2137–2143. [DOI] [PubMed] [Google Scholar]
- 57.Tang E, Hsu L, Lam K, Pang W. Critically ill elderly who require mechanical ventilation: the effects of age on survival outcomes and resource utilisation in the medical intensive care unit of a general hospital. ANNALS-ACADEMY OF MEDICINE SINGAPORE. 2003;32(5):691–696. [PubMed] [Google Scholar]
- 58.Chelluri L, Pinsky MR, Donahoe MP, Grenvik A. Long-term Outcome of Critically III Elderly Patients Requiring Intensive Care. Jama. 1993;269(24):3119–3123. [PubMed] [Google Scholar]
- 59.de Rooij SE, Govers A, Korevaar J, Abu-Hanna A, Levi M, De Jonge E. Short-term and long-term mortality in very elderly patients admitted to an intensive care unit. Intensive care medicine. 2006;32(7):1039–1044. [DOI] [PubMed] [Google Scholar]
- 60.de Rooij SE, Abu-Hanna A, Levi M, de Jonge E. Factors that predict outcome of intensive care treatment in very elderly patients: a review. Critical Care. 2005;9(4):R307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boumendil A, Aegerter P, Guidet B, Network CR. Treatment intensity and outcome of patients aged 80 and older in intensive care units: a multicenter matched-cohort study. Journal of the American Geriatrics Society. 2005;53(1):88–93. [DOI] [PubMed] [Google Scholar]
- 62.Goronzy JJ, Weyand CM. Understanding immunosenescence to improve responses to vaccines. Nature immunology. 2013;14(5):428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Levin MJ. Immune senescence and vaccines to prevent herpes zoster in older persons. Current opinion in immunology. 2012;24(4):494–500. [DOI] [PubMed] [Google Scholar]
- 64.Yawn BP, Saddier P, Wollan PC, Sauver JLS, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Paper presented at: Mayo Clinic Proceedings 2007. [DOI] [PubMed] [Google Scholar]
- 65.Takahashi PY, Ryu E, Hathcock MA, et al. A novel housing-based socioeconomic measure predicts hospitalisation and multiple chronic conditions in a community population. J Epidemiol Community Health. 2016;70(3):286–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin SS, Kelsey JL. Use of race and ethnicity in epidemiologic research: concepts, methodological issues, and suggestions for research. Epidemiologic reviews. 2000;22(2):187–202. [DOI] [PubMed] [Google Scholar]


