Abstract
Sustained imbalance in intracellular calcium (Ca2+) entry and clearance alters cellular integrity, ultimately leading to cellular homeostasis disequilibrium and cell death. Alzheimer’s disease (AD) is the most common cause of dementia. Beside the major pathological features associated with AD-linked toxic amyloid beta (Aβ) and hyperphosphorylated tau (p-tau), several studies suggested the contribution of altered Ca2+ handling in AD development. These studies documented physical or functional interactions of Aβ with several Ca2+ handling proteins located either at the plasma membrane or in intracellular organelles including the endoplasmic reticulum (ER), considered the major intracellular Ca2+ pool. In this review, we describe the cellular components of ER Ca2+ dysregulations likely responsible for AD. These include alterations of the inositol 1,4,5-trisphosphate receptors’ (IP3Rs) and ryanodine receptors’ (RyRs) expression and function, dysfunction of the sarco-endoplasmic reticulum Ca2+ ATPase (SERCA) activity and upregulation of its truncated isoform (S1T), as well as presenilin (PS1, PS2)-mediated ER Ca2+ leak/ER Ca2+ release potentiation. Finally, we highlight the functional consequences of alterations of these ER Ca2+ components in AD pathology and unravel the potential benefit of targeting ER Ca2+ homeostasis as a tool to alleviate AD pathogenesis.
Keywords: calcium, Alzheimer’s disease, endoplasmic reticulum, SERCA, IP3R, RyR, S1T, presenilin
1. Introduction
1.1. Ca2+ Signaling
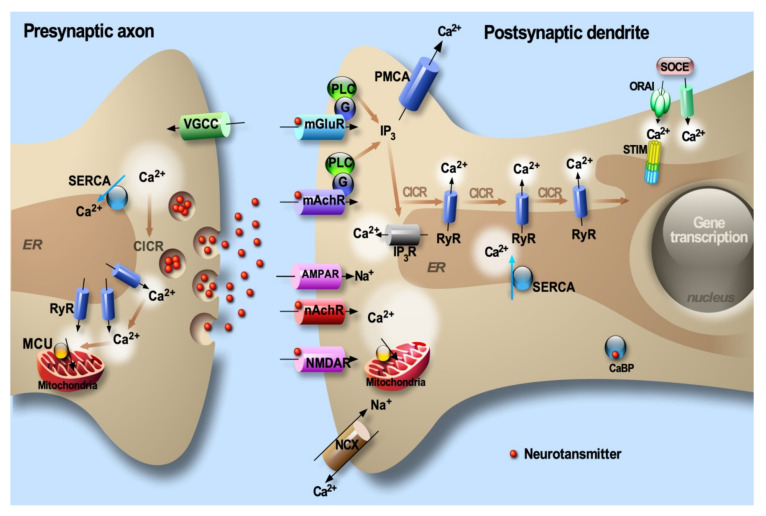
As a signal transduction molecule, calcium (Ca2+) regulates a large number of neuronal processes including growth and differentiation, neurotransmitter release and synaptic function, activity-dependent changes in gene expression and apoptosis [1]. Cytosolic Ca2+ ([Ca2+]cyt) signals are regulated in a spatiotemporal-dependent manner underlined by an intricate interplay between Ca2+ entry through the plasma membrane, storage in the internal stores (i.e., the endoplasmic reticulum (ER), considered the major dynamic Ca2+ intracellular pool), Ca2+ mobilization from the ER and its buffering by Ca2+-binding proteins (CaBP) (Figure 1). Ca2+ entry through the plasma membrane occurs through ligand-dependent Ca2+ receptors (i.e., N-methyl-d-aspartate receptor (NMDA) and Alpha7 nicotinic acetylcholine receptors (nAChRs)) and through voltage-gated Ca2+ channels (VGCC) (Figure 1). Ca2+ mobilization from the ER occurs through activation of the inositol 1,4,5-trisphosphate receptors (IP3R) downstream of metabotropic receptors (Figure 1), or through the activation of ryanodine receptors (RyRs) that are activated by a slight increase in [Ca2+]cyt, a mechanism known as Ca2+-induced Ca2+ release (CICR) (Figure 1). Elevations of cytosolic Ca2+ signals are “shut down” through the plasma membrane Na+/Ca2+ exchanger (NCX) and two Ca2+ ATPases which consume ATP to actively extrude Ca2+ out of the cells (i.e., the plasma membrane Ca2+ ATPase (PMCA)) or to actively sequester Ca2+ into the ER lumen (i.e., the sarco-endoplasmic reticulum Ca2+ ATPase (SERCA)) (Figure 1). Intriguingly, coupling between ER Ca2+ depletion and Ca2+ influx through the plasma membrane occurs through a canonical store-operated Ca2+ entry (SOCE) pathway [2] mainly consisting of a direct physical interaction between the Ca2+-sensing stromal interacting molecules (STIM1/2) oligomers within the ER membrane and the pore-forming ORAI proteins in the plasma membrane [3,4,5] (Figure 1). Several lines of evidence indicate that Ca2+ homeostasis could be disrupted upon cellular challenges as well as in neurodegenerative conditions.
Figure 1.
Elevations of intraneuronal [Ca2+] are the result of an influx across the plasma membrane and the release from the ER through various channels and receptors. The low intraneuronal Ca2+ level is then maintained by the activity of Ca2+-binding proteins (CaBP) and involves the sodium-Ca2+ exchanger (Na+/Ca2+) acting in concert with the ATP-dependent Ca2+ pumps located at the plasma membrane and the ER. Depletion of ER Ca2+ content activates the store-operated Ca2+ entry (SOCE) pathway.
1.2. Alzheimer’s Disease
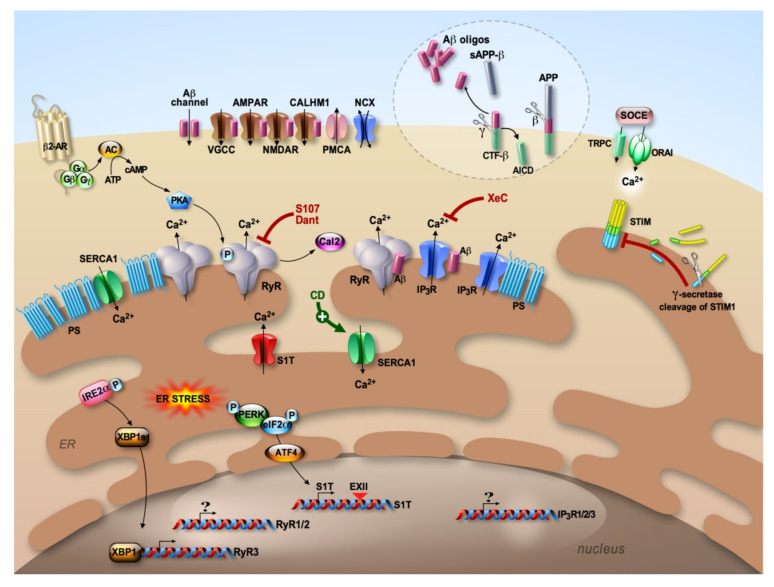
Alzheimer’s disease (AD) is an age-associated dementia disorder characterized by the accumulation of extracellular amyloid-beta (Aβ) peptides in the senile plaques and by the hyperphosphorylation of tau (pTau) protein, leading to intracellular protein aggregation into bundles or filaments that are deposited as neurofibrillary tangles [6,7,8]. Notably, Aβ peptide derives from the sequential processing of the β-amyloid precursor protein (βAPP referred to as APP hereafter) [9,10] by the β-seceretase (BACE1) and the γ–secretase complex (composed of presenilins (PSs: PS1 or PS2, the catalytic subunits of the enzyme), Nicastrin, anterior pharynx-defective-1 (APH-1) and presenilin enhancer-2 (PEN-2) [11,12])) (Figure 2). Importantly, a significant number of aggressive AD cases generally characterized by early onset are inherited in an autosomal-dominant manner (FAD: familial AD) and are caused by mutations on APP and on PS1 and PS2 [13,14] (Figure 2). These mutations either modify the nature of Aβ peptides and/or affect the levels of their production [15,16]. Besides the canonical disease-associated intracellular pTau and extracellular Aβ accumulations, recent studies unraveled additional processes that could contribute to AD progression, including: (i) the intracellular accumulation of Aβ [17,18] and other APP-derived fragments [18,19,20,21,22,23,24], and (ii) the spreading of both extracellular Tau and Aβ between neurons and between neurons and glial cells [25,26].
Figure 2.
Aβ peptides are derived from the processing of the βAPP (APP) through the amyloidogenic pathway. APP is first cleaved by β–secretase (β), generating APP C-terminal fragment β (CTF-β), which is then cleaved by γ–secretase complex (γ) to produce Aβ and APP intracellular domain (AICD). At the plasma membrane, Aβ form a cation channel and modulate several Ca2+ channels (VGCC, AMPAR, NMDAR and CALHM1). ER Ca2+ deregulation occurs through presenilin (PS)-associated Ca2+ leak and/or enhanced IP3R- and RyR-mediated Ca2+ release, dysfunctional Sarco-endoplasmic reticulum Ca2+ ATPase (SERCA) activity, enhanced expression of S1T driven by ER stress response and enhanced expression and dysfunctional IP3Rs and RyRs. SOCE is also deregulated in AD and implicates STIM, ORAI and TRPC. RyR2 macromolecular complex destabilization (PKA phosphorylation and calstabin2 (Cal2) dissociation) is linked to β2-adrenergic receptor activation. Pharmacological stabilization of ER Ca2+ content by S107, Dantrolene (Dant) blocking RyRs-mediated Ca2+ release/leak, Xestospongin C (XeC) blocking IP3R-mediated Ca2+ release and CD1163 (CD) activating SERCA provided beneficial effects in reversing several AD-related pathogenic paradigms in vitro and in vivo.
1.3. Physiology of ER Calcium Handling in Neurons
The ER forms a continuous and highly motile network distributed throughout the neuron. Within dendrites and dendritic spines, ER Ca2+ release is involved in modulating postsynaptic responses and synaptic plasticity [27]. In presynaptic nerve terminals, as well as in growth cones, ER is involved in vesicle fusion and neurotransmitter release [28,29]. In the soma, ER Ca2+ handling is coupled to the activation of Ca2+-sensitive kinases and phosphatases [30]. In the perinuclear space, ER Ca2+ handling triggers gene transcription [31]. Ca2+ mobilization from the ER has been shown to be involved in growth cone activity and in the formation of new connections and/or the strengthening of preexisting connections that occur during learning and memory in the adult brain [32].
1.4. Calcium Deregulation in AD
As stated above, the tight but subtle control of intracellular Ca2+ homeostasis is required for neuronal health, development and function [29,30,33,34]. Therefore, persistent imbalance in Ca2+ entry and clearance alters cellular integrity, leading to cellular homeostasis disequilibrium. These Ca2+ deregulations ultimately trigger excessive proliferation or cell death depending on the strength and the duration of the insult and in a cell-type-specific manner. Ca2+ signaling deregulation has a central role in AD pathophysiology [35]. The relevance of Ca2+ signaling in AD is supported by the fact that Ca2+ alterations were reported in both sporadic (SAD) and familial (FAD) forms of AD and that this can exacerbate Aβ formation and promote tau hyperphosphorylation [35,36,37]. As first evidence, in vitro studies have shown that Ca2+ may directly interact and enhance the proteolytic activity of BACE1 [38] and to stabilize γ–secretase and enhance its activity in reconstituted in vitro assay [39]. Moreover, tau hyperphosphorylation at disease-specific sites has been associated with abnormal intracellular Ca2+ signaling occurring upstream of Ca2+/calmodulin (CaM)-dependent protein kinase II (CaMKII) and CDK5 activation [37,40,41,42]. The bulk of data gathered these last 30 years allows us to draw up a scenario where Ca2+ deregulation is not only a consequence of the disease but also participates in a feedback loop to disease progression and amplification [35,36,43,44,45,46]. These studies reported a Ca2+-dependent enhancement of APP processing and the production of toxic APP-derived fragments, activation of signaling cascades through the modulation of kinases and phosphatases activities, thus affecting synaptic plasticity and cognitive function [34,35,36,47,48,49].
Several studies demonstrated a tight relationship between altered Ca2+ handling and the amyloidogenic cascade. These studies lead to identifying the physical or functional interaction of Aβ with several Ca2+ handling proteins in various AD models. At least four lines of evidence have emerged: (i) at the plasma membrane, Aβ has been shown to form a cation channel [50], or to act as a channel-modulator for the VGCCs, the nAChRs, the ionotropic glutamatereceptors NMDARs and AMPARs (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors), the Ca2+ homeostasis modulator 1 (CALHM1), and more recently the store-operated Ca2+channels (SOCE) (Figure 2) [51,52,53,54,55,56,57,58,59,60]; (ii) dysfunctional mitochondria were associated with Aβ-mediated Ca2+ toxicity [61,62] (discussed in this Special Issue [63]). Importantly, mitochondrial permeability transition pore, mitochondrial Ca2+ uniporter (MCU) dysfunctions and impaired mitochondrial Ca2+ efflux contribute to mitochondrial alteration in AD [63,64,65]; (iii) the autophagic failure in AD has been linked to lysosomal degradation defects [24,66] likely occurring upon lysosomal Ca2+ depletion [67,68]; (iv) a complex scenario of AD-associated ER Ca2+ dysregulation also emerged, where disturbances were linked to presenilin (PS1 and PS2)-associated ER Ca2+ leak and/or ER Ca2+ release potentiation functions [69,70,71,72,73], dysfunctional SERCA activity [74] and the upregulation of the recently described SERCA1 truncated isoform (S1T) [75], alterations of IP3Rs function [56,69,70,72,76,77,78,79,80,81] and dysfunctional RyRs [44,80,82,83,84,85,86,87,88,89,90,91,92,93,94] (Figure 2).
Besides APP-derived amyloidogenic products, previous studies described a physiological role of APP in regulating Ca2+ signaling. Knockdown of endogenous APP increases the frequency and reduces the amplitude of neuronal Ca2+ oscillations [95]. In addition, a recent study specifically reported that APP-deficient cells exhibited elevated resting Ca2+ levels in the ER and reduced ER Ca2+ leakage rates [96]. Pathogenic tau has also been associated with nuclear Ca2+ deregulation [97], with increasing the ionic current of artificial membranes [98], with inducing spontaneous Ca2+ oscillations in the neurons [99] and with the inhibition of mitochondrial Ca2+ efflux via the mitochondrial Na+/Ca2+ exchanger [99] (also discussed in this Special Issue [63]).
In this review, we will specifically present an update of the alterations of the molecular components controlling ER Ca2+ signaling in AD and discuss the potential benefit of targeting ER Ca2+ homeostasis as a tool to alleviate AD pathogenesis.
2. The Ryanodine Receptors: RyRs
RyRs are a family of three mammalian isoforms, RyR1, RyR2 and RyR3, mainly expressed in the skeletal muscle, heart and brain. All RyRs isoforms are expressed in the brain, with an abundance range of order as follows, RyR2 > RyR1 >> RyR3 [100,101]. RyRs activity is influenced on the one hand by Ca2+, Mg2+ and ATP [102,103,104,105] and, on the other hand, by the integrated effects of co-proteins forming RyR1 and RyR2 homotetramer macromolecular complexes [106,107,108]. These include calmodulin (CaM) [109,110], FKBP12 (12.0 kDa) and FKBP12.6 (12.6 kDa), known as Calstabin1 (Cal1) and Calstabin2 (Cal2), respectively [111]; PKA anchored to RyR1 and RyR2 via a kinase anchoring protein (mAKAP) [112,113], and Ca2+/calmodulin-dependent protein kinase II (CaMKII) [114]. Other regulatory proteins were also described to interact with RYR1, thus controlling the channel gating activity [109]. RyR1/2 macromolecular complexes contain also the requisite molecular machinery allowing channel dephosphorylation (i.e., PP1 and PP2A) [113,115,116].
Enhanced RyR-mediated Ca2+ release was reported in primary cultured neurons derived from 3xTg-AD mice (knock in (KI) for the mutated PS1M146V and overexpressing mutated APP and microtubule-associated tau protein (PS1M146V/APPswe/tauP301L)) [85,87]. This was further confirmed in cellular models expressing wild-type or mutated APP, PS1 or PS2 [44,80,82,83,84,86,87,88,89,90,91,92,93,94,117]. Exacerbated IP3R-evoked Ca2+ signals in AD mice (PS1KI and 3xTg-AD)-derived neurons were shown to be linked to RYR-associated CICR [85]. These findings were further supported by using the RyR blocker dantrolene (Dant), shown to reduce enhanced [Ca2+]cyt level [92,93,118]. While some studies reported that RyR dysfunction in AD-related study models occurs independently of PS mutation or overexpression, namely in models expressing APP and overproducing Aβ [86,92,119,120,121,122], in many cases, PS mutation-mediated Ca2+ deregulation was associated with the alteration of the activity of RyRs (discussed beyond in PSs chapter). In addition, it was also reported that exogenous Aβ oligomers may directly stimulate RyR-mediated Ca2+ release [123] and that the application of soluble Aβ caused a marked increase in channel open probability [124].
RyR isoform expression is modified throughout AD progression and between different brain regions [125]. Exogenous application of Aβ peptide was also shown to specifically increase RyR3 isoform expression [86,123]. RyRs mRNAs increase throughout the lifetime of PS1-M146V transgenic mice and 3xTg-AD mice [84,85,87] as well as in cellular and mice AD models overexpressing wild-type or mutated APP (bearing the Swedish mutation APPswe) [92]. Conversely, neuronal conditional PS1/2 knockout (KO) (PScDKO) is associated with a downregulation of RyR2 expression, demonstrating that PS may regulate Ca2+ homeostasis and synaptic function via RyRs [126]. It has been proposed that the modulation of RyR expression may act as a disease promoter or a compensatory beneficial mechanism. In fact, while on the one hand, enhanced [Ca2+]cyt response is associated with the increased expression of RyRs [127], the activation of the ER stress response factor X-box binding protein 1 spliced isoform (XBP1s) may occur upon Aβ oligomer treatment [128,129], triggering a reduction of [Ca2+]cyt linked to the down-expression of the RyR3 isoform [130]. Accordingly, a dual role for endogenous RyR3 has been suggested in an AD mouse model. Thus, the deletion of RyR3 in young (≤ 3 mo) APPPS1 mice increased hippocampal neuronal network excitability and accelerated AD pathology, leading to mushroom spine loss and increased Aβ accumulation. Meanwhile, deletion of RyR3 in older APPPS1 mice (≥6 mo) rescued network excitability and mushroom spine loss, reduced Aβ load and reduced spontaneous seizure occurrence [131] (Figure 2).
RyRs mutations are liked to various pathologies affecting muscle and heart [132,133]. The development of transgenic mouse models (i.e., KO of RyR1, RyR2 or RyR3, or expressing RyR harboring disease mutations, or lacking exon sequence) [134] strengthens the fact that RyRs play a key role in physiology and pathophysiology. The viability of RyR3 KO mouse, in contrast to the RyR1 and RyR2 KO mice [135,136], led to the demonstration that RyR3-deficient mice exhibit decreased social behavior [137], greater locomotor activity [136,138], altered memory [138,139] associated with impaired maintenance of long-term potentiation (LTP) [140]. To date, no mutations have been reported in RYRs linked to brain disorders. Nevertheless, the role of leaky RyR2 in the pathogenesis of epilepsy has been described in the RyR2-R2474S mice model [101]. Interestingly, three single nuclear polymorphisms were significantly associated with risk for hypertension, diabetes and AD [141]. A meta-analysis based on four genome-wide association study (GWAS) also identified RYR3 association with AD risk [142]. Another study observed a significant interaction between RYR3 and CACNA1C (gene encoding for the Ca2+ voltage-gated channel subunit Alpha1 C) in three independent datasets of AD Neuroimaging Initiative cohorts [143].
RyRs post-translational modifications (PTMs) shift the channel from a finely regulated state to a non-regulated Ca2+ leak channel. RyR PTMs were associated with different pathologies affecting skeletal muscle, heart and, recently, brain [133] [113,144,145,146,147,148,149,150]. Experimental transgenic mice expressing RyR harboring PKA-non-phosphorylated sites or phosphomimetic RyR mutants demonstrated the role of the PKA phosphorylation site in RyR macromolecular complex remodeling, Calstabin dissociation and ER Ca2+ leak [133]. In addition to phosphorylation sites, RyRs also contain a large number of amino acid residues that are potential targets for reactive oxygen species (ROS) and for reactive nitrogen species (RNS) [108,151,152]. Recently, we described a new molecular mechanism and signaling cascade underlying altered RyR-mediated intracellular Ca2+ release in AD [116,150,153]. We reported that the RyR2 channel undergoes PKA phosphorylation, oxidation/nitrosylation and depletion of the channel stabilizing subunit Calstabin2 in SH-SY5Y neuroblastoma cells expressing APP harboring the familial Swedish mutations (APPswe), in APP/PS1 (APPswe, PS1-M146V), as well as in 3xTg-AD, transgenic mice models and, most importantly, in human SAD brains [150,153]. We further reported that RyR2 macromolecular complex remodeling occurs through synergistic mitochondrial reactive oxygen species (ROS) production and β-adrenergic stimulation [150,153]. Notably, oxidative stress is considered a major contributor to AD pathogenesis [154,155], and β2-adrenergic receptors (β2-ARs) have also been implicated in the development of AD [51,156,157,158,159,160,161]. However, targeting β-adrenergic signaling is questionable, since both beneficial versus defective effects were described in AD mice [162,163,164]. In our study, we specifically targeted the downstream PKA-mediated RyR2 phosphorylation and macromolecular complex destabilization (Figure 2). We showed that pharmacological stabilization of calstabin2 on the RyR2 macromolecular complex by S107 (a benzothiazepine derivative molecule [101]) reduces elevated Ca2+ signals in AD cells [153], prevents ER Ca2+ leakage and reduces single channel open probabilities in AD mice brains [150]. Most importantly, S107 treatment reduces APP processing and Aβ production both in vitro and in vivo [150,153]. S107 administration also inhibited calpain activity and AMPK-dependent tau phosphorylation in an APP/PS1 mouse model [150]. These data agree well with previously reported studies demonstrating the beneficial effects of the pharmacological targeting of RyR with dantrolene [88,92,93,165]. In support of these findings, RyR macromolecular complex stabilization improved the hippocampal synaptic plasticity (LTP and LTD) and cognitive function of APP/PS1 and 3xTg-AD mice [150]. Importantly, we further showed that crossing APP/PS1 mice with RyR2-S2808A KI mice, harboring RyR2 channels that cannot be PKA-phosphorylated, resulted in improved cognitive function and decreased neuropathology. In contrast, phosphormimetic RyR2-S2808D KI mice exhibit early altered hippocampal synaptic plasticity (LTP and LTD) and cognitive dysfunction [150]. Overall, these results emphasize the broad implication of RyRs in ER Ca2+ signaling deregulation in AD occurring through the regulation of RYRs expression, CICR-dependent activity, macromolecular complex stability-linked to β2-AR signaling cascade, Aβ- and PS-mediated RyRs channel opening and likely RYR3 gene polymorphism.
3. The Inositol 1,4,5-Trisphosphate Receptors: IP3Rs
Among the three IP3Rs isoforms, the predominant one in neurons is IP3R1 [166,167,168,169]. In addition to Ca2+ and IP3, there are other allosteric IP3R modulators, including ATP [170]. The activity of IP3R can also be regulated by its phosphorylation by different kinases [166,170]. Among them are PKA, protein kinase C (PKC), cGMP-dependent protein kinase (PKG), CaMKII and different protein tyrosine kinases. Moreover, similarly to RyRs, the IP3Rs can also be regulated by the redox status and by several interacting proteins (i.e., CaM-related Ca2+-binding proteins (CaBPs), Bcl2 family members, proteases (Caspase-3 and calpain) and ER lumen-specific protein (ERp44) [170]).
IP3Rs activity controls spine morphology, synaptic plasticity and memory consolidation [171,172,173]. Notably, alterations of IP3Rs expression and function were reported to be implicated in Ca2+ signaling deregulation in several AD models [174]. Ca2+ imaging experiments demonstrated that orthologous expression of FAD PS1 mutants potentiates IP3-mediated Ca2+ release [175]. These data were confirmed in cortical neurons isolated from PS1-M146V KI mice [79] and in cells expressing FAD PS1-DeltaE9 mutant [176]. PS1-DeltaE9 mutant cells harbored enhanced basal phosphoinositide hydrolysis and cyt[Ca2+], which were both reversed by the PLC inhibitor neomycin. PS1-DeltaE9 mutant cells also showed high basal [Ca2+] and agonist-evoked Ca2+ signals that were reversed by xestospongin C (XeC, a reversible IP3R antagonist) [176]. The molecular mechanisms underlying enhanced IP3R-mediated Ca2+ release have been described to be PS-dependent and/or PS-independent [73,177] (also discussed in PSs chapter below). The computational modeling of single IP3R activity was used to analyze and quantify the pathological enhancement of IP3R function by FAD-causing mutant PS [178]. This study revealed that the gain-of-function enhancement of IP3R was sensitive to both IP3 and Ca2+, thus triggering a higher frequency of local Ca2+ signals, while enhancing the activity of the channel at extremely low ligand concentrations will lead to spontaneous Ca2+ signals in cells expressing FAD-causing mutant PS [178]. It has been consequently observed that the gain-of-function enhancement of IP3R channels in cells expressing PS1-M146L leads to the opening of mitochondrial permeability transition pore (PTP) in high-conductance state, triggering a reduction in the inner mitochondrial membrane potential and in NADH and ATP levels [179]. Conversely, genetic reduction of IP3R1 normalizes disturbed Ca2+ signaling in PS1-M146V KI mice and most importantly alleviates AD pathogenesis (i.e., rescues aberrant hippocampal long-term potentiation (LTP), attenuates Aβ accumulation and tau hyperphosphorylation and memory deficits) in both PS1-M146V KI and 3xTg-AD mice [72]. Accordingly, in vitro experiments showed that XeC effectively ameliorated Aβ42-induced apoptosis and intracellular Ca2+ overload in the primary hippocampal neurons [180]. Notably, intracerebroventricular injection of XeC reduced the number of Aβ plaques, alleviated ER stress response and significantly improved the cognitive behavior of APP/PS1 mice [180]. Exacerbated IP3R-mediated Ca2+ release is also linked to Aβ, independently of PS overexpression/mutation. Jensen L.E., et al. reported that Aβ42 induced elevation of cytosolic Ca2+ in an IP3R-dependent and -independent manner [91]. In addition, it was also shown that the treatment with Aβ42 significantly increased mRNA levels of IP3R1/2 and mGluR5 [181]. Enhanced IP3R1 expression and ER Ca2+ release were also reported in astrocytes derived from the entorhinal cortex and from the hippocampus from WT mice and mice treated with Aβ42 oligomers [182]. Finally, as stated above, IP3R function is regulated by several binding proteins. Thus, it is also conceivable that any alteration of the expression, localization, activity and binding affinity of these proteins may affect IP3R structural/functional state, thus impacting AD development.
4. Presenilins 1 and 2: PS1/2
PS1 and PS2 are multispanning transmembrane (TM) proteins located in intracellular membranous organelles such as the ER, nuclear envelope and Golgi apparatus but also in multiple secretory and endocytic organelles as well as the plasma membrane [183]. PS1 was first cloned as a causative gene of FAD [184]. Its homologue PS2 gene was then identified, sharing an approximately 60% sequence homology as a whole and approximately 90% within the TM domains [185,186]. Accordingly, PS1 and PS2 were also shown to share similar predicted topology [187,188,189].
Both PS1 and PS2 are expressed in neurons [190] and are essential for embryonic development since PS1 KO mice die at birth [191] and PS1/PS2 double KO mice (PSDKO) mice die before embryonic day 9.5 [192]. Importantly, PSs were also shown to play key roles in neuronal function and survival. Therefore, conditional PSDKO mice show impaired spatial and associative memory, deficits in short- and long-term plasticity [193,194] and develop synaptic, dendritic and neuronal degeneration in an age-dependent manner [193]. Importantly, being the catalytic component of γ–secretase complex cleaving the APP [195], most of the PS mutations associated with early-onset FAD affect APP processing and, more particularly, the ratio Aβ40/42 by increasing the aggregation-prone Aβ42 species [196,197,198]. Several studies proposed the contribution of PSs to ER Ca2+ signaling deregulation in AD. It has been proposed that PSs act as ER Ca2+ leak channels, and FAD mutations in PSs disrupt this function, leading to ER Ca2+ overload [69,70,76]. Tu et al. also proposed that the full-length PSs function as ER Ca2+ leak channels independently of other γ–secretase components [69]. Cysteine point mutants combined with NMR studies revealed that TM7 and TM9, but not TM6, could play an important role in forming the conductance pore of mouse PS1 [77]. A recent study investigated the interaction of Ca2+ with both PS1 and PS2 using all-atom molecular dynamics (MD) simulations in realistic membrane models [199]. Although the Ca2+ leak event linked to PS1 or PS2 has been challenged in this study, the obtained data demonstrated the presence of four Ca2+ sites in membrane-bound PS1 and PS2 [199]. The authors speculated that Ca2+ may prevent PS maturation (i.e., “presenilinase” endoproteolysis generating PS N-and PS C-terminal derivatives [200]) by triggering conformational changes, thus preserving the immature Ca2+ regulation function. Meanwhile, conversely, PS maturation yielding a biologically active PS would abolish this Ca2+-regulatory function [199]. Nevertheless, the PS-associated Ca2+ leak function was discussed in other studies proposing that FAD PSs directly potentiate the gating of IP3R [81]. Exaggerated IP3R-mediated Ca2+ responses were also reported in cells and neurons derived from transgenic mice expressing FAD-linked mutant PS1 or PS2 [84]. These findings agree well with data obtained in PS1-M146V KI mice neurons using whole-cell patch-clamp recording, flash photolysis and two-photon imaging [79]. Accordingly, genetic reduction of IP3R 1 normalizes disturbed Ca2+ signaling in FAD PS1 mice and alleviates AD pathogenesis in PS1-M146V KI mice [72]. Other studies point to PS-linked disruptions in RyR signaling as an important ER molecular component associated with enhanced ER Ca2+ signals in both 3xTg-AD and PS1-M146V (KI) neurons [80]. PS1/PS2 were also shown to harbor a physical interaction with RyRs in the ER [83,117,201]. Specifically, PS2 interacts with RyR and with sorcin (a RyR regulator) in a Ca2+-dependent manner in both cellular models and in the brain [201,202], thus increasing both mean currents and open probability of single brain RyR channels [203,204]. Discrepancies regarding the role of PSs in ER Ca2+ handling alterations were further highlighted in a recent study [205] showing that FAD PS2 mutants, but not FAD PS1, are able to partially block SERCA activity, thereby reducing ER Ca2+ content in either SH-SY5Y cells or FAD patient-derived fibroblasts [205]. Despite this incongruity concerning the exact molecular mechanism underlying PS-mediated ER Ca2+ deregulation, FAD PSs undoubtedly directly or indirectly contribute to the Ca2+ hypothesis in AD. However, whether PS-mediated ER Ca2+ deregulation is dependent on or independent of its endoproteolysis generating PS N-and PS C-terminal derivatives still remains an open question.
5. The Sarco-Endoplasmic Reticulum (SR/ER) Ca2+-ATPase and Its Truncated Isoform: SERCA and S1T
SERCAs are integral ER proteins preserving low [Ca2+]cyt by pumping free Ca2+ ions into the ER lumen, utilizing ATP hydrolysis. The SERCA pumps are encoded by three distinct genes (SERCA1-3), resulting in 12 known protein isoforms, with tissue-specific expression patterns. SERCA2b is the most expressed isoform in neurons [206]. Despite the well-established structure and function of the SERCA pumps, their role in the central nervous system and whether it could be affected in brain diseases remain to be definitely established. Interestingly, SERCA-mediated Ca2+ dyshomeostasis has been associated with neuropathological conditions, such as bipolar disorder, schizophrenia, Parkinson’s disease but also AD [207]. An initial study showed that SERCA activity is reduced in fibroblasts isolated from PSDKO. Immunoprecipitation analyses suggested a physical interaction between SERCA and PS1 and PS2 [74] and that modulation of SERCA expression regulates Aβ levels [74]. The interaction of PS1 holoprotein was further demonstrated in cells overexpressing PS1 and subjected to tunicamycin treatment [208]. It has also been shown that overexpressed wild-type or mutated PS2 triggered ER-passive leakage through IP3R and RyR but also potently reduced ER Ca2+ uptake, an effect that has been counteracted by the overexpression of SERCA2b [71]. A recent study reported that the pharmacological SERCA activation by a quinoline derivative (CD1163), discovered via high-throughput screening of small molecules library, provides some beneficial effects in APP/PS1 mice [209] (Figure 2). Overall, these studies pinpointed the potential contribution of SERCA to ER Ca2+ dyshomeostsis in AD cellular study models. However, dedicated studies in mice AD models and in human-derived samples are still needed to further support the beneficial versus pathogenic role of the modulation of SERCA expression and activity in AD development.
Accumulation of unfolded proteins into the ER as well as alteration of ER Ca2+ homeostasis induce ER stress, eliciting an unfolded protein response (UPR) [210,211]. Several studies have reported that UPR occurs in human AD brains [212,213] and in several AD study systems [214,215,216]. We previously demonstrated that the human SERCA1 truncated isoform (S1T) [217] is induced under pharmacological and physiopathological ER stress through the activation of the PERK-eIF2α-ATF4-CHOP pathway [218]. In turn, S1T expression induction triggered an amplification of ER stress and mitochondrial apoptosis [218]. UPR activation has been proposed to be linked to intracellular Aβ accumulation [219]. In a recent study, we revealed that S1T is upregulated in SH-SY5Y cells expressing APPswe [75]. Importantly, biochemical data indicate that enhanced human S1T expression correlates with Aβ load in human AD-affected brains and that S1T high neuronal immunostaining is selectively observed in human AD cases harboring focal Aβ. We further demonstrated that S1T expression is induced by exogenous application of Aβ oligomers in cells [75]. Interestingly, S1T overexpression in return enhances APP processing and the production of APP-derived toxic fragments (APP C-terminal fragments and Aβ) in cells and in 3xTgAD mice. Mechanistically, we find that S1T-mediated elevation of APP proteolysis occurs through the upregulation of BACE1 expression and enhanced activity [75]. In agreement with these findings, several lines of evidence indicated that enhanced phosphorylation of PERK and eIF2α in the AD brain is associated with increased amyloidogenic APP processing [214,215,216] through increased BACE1 expression [220,221]. We have also to consider that BACE1 upregulation occurring downstream of [Ca2+]cyt elevation acts in a positive feedback loop with AD progression [222]. In addition, the induction of ER stress and the activation of UPR trigger neuroinflammation [223]. Accordingly, we demonstrated that S1T overexpression, as well as tunicamycin treatment, induce the expression of proinflammatory cytokines and increase the proliferation of active microglia [75]. Altogether, our data strengthen the molecular link between ER Ca2+ leak, ER stress and APP processing contributing to AD setting and/or progression.
6. The Molecular Bridge between ER Ca2+ Depletion and Plasma Membrane Ca2+ Entry: STIM/ORAI
The store-operated Ca2+ entry (SOCE) is an essential route for Ca2+ uptake to replenish intracellular Ca2+ stores [224]. The stromal interaction molecules STIM1 and STIM2 have been identified as essential components of SOCE and major sensors of the Ca2+ concentration located in the ER membrane (reviewed in [225]). Both STIM homologues are ubiquitously expressed in different cell types, with a higher STIM1 level in most tissues and a predominant expression of STIM2 in the brain [226]. A decrease in ER luminal Ca2+ concentration results in dissociation of Ca2+ from the STIM EF-hand domain, which, in turn, triggers oligomerization and activation of STIM1, a process that is reversed when luminal [Ca2+] returns to resting level [3,4]. Active STIM oligomers translocate to ER plasma membrane junctions and recruit and interact with ORAI channels located on the plasma membrane [227]. There are three ORAI isoforms displaying tissue-specific expression and activation patterns [225]. In addition, transient receptor potential channels (TRPC) can also be recruited by the ORAI/STIM complex, constituting an additional route for Ca2+ entry through the plasma membrane upon ER Ca2+ depletion [228] (Figure 1). Several TRPC isoforms were also identified and were described to harbor tissue/cell-specific expression and activation patterns [229].
Several studies pinpointed a role for STIM/ORAI in neuronal Ca2+ signaling-associated synaptic function [230,231]. Thus, the maturation of dendritic spines and the formation of functional synapses in immature hippocampal neurons is facilitated by the influx of Ca2+ through ORAI1 [232]. STIM also interact with/and or control the activity of several Ca2+ channels on the plasma membrane (L-type Ca2+ channels (CaV1.2), L-type VGCCs and mGluR [232]). Several studies suggested that the disruption of neuronal SOCE underlies AD pathogenesis. A direct connection between Aβ-induced synaptic mushroom spine loss and the neuronal SOCE pathway was reported in two studies. Popugaeva et al. reported that the application of exogenous Aβ42 oligomers to hippocampal cultures or injection of Aβ42 oligomers directly into the hippocampal region resulted in the reduction of mushroom spines and activity of synaptic CaMKII, which were rescued by STIM2 overexpression [224]. Accordingly, similar findings were reported in APPKI hippocampal neurons accumulating extracellular Aβ42. Thus, it was shown that Aβ triggers mGluR5 receptor overactivation, leading to elevated ER Ca2+ levels, compensatory downregulation of STIM2 expression, impairment of synaptic SOCE and reduced CaMKII activity [58]. Inversely, overexpression of the constitutively active STIM1-D76A mutant and ORAI1 significantly reduced Aβ secretion [55]. A link between STIM1/2 and PS1 was also reported. The STIM2–SOCE–CaMKII pathway was downregulated in a PS1-M146V KI mouse model of AD, associated with loss of hippocampal mushroom spines [233], and conversely, STIM2 overexpression rescued synaptic SOCE and mushroom spine deficit in hippocampal neurons from PS1-M146V KI mice [233]. Intriguingly, even if STIM1 expression is not altered in the AD models cited above, it has been identified as a target of PS1-containing γ–secretase activity. In particular, FAD-linked PS1 mutations enhanced γ–secretase cleavage of STIM1, reducing the activation of ORAI1 and attenuating SOCE [234]. As a consequence, the inhibition of SOCE in hippocampal neurons triggered an alteration of the dendritic spine architecture [234]. A recent study showed that the hyperactivation of SOCE channels in neurons expressing PS1-DeltaE9 mutant is mediated by the STIM1 sensor and can be attenuated by pharmacological inhibition and genetic KO STIM1 [235]. Interestingly, SOCE in PS1-DeltaE9 mutant-expressing cells is not contributed by STIM2 but involves TRPC and ORAI subunits. Importantly, transgenic Drosophila flies expressing PS1-DeltaE9 in the cholinergic neuron system showed short-term memory loss, which was reversed upon pharmacological inhibition of STIM1 [235]. Accordingly, a recent study further supports the link between FAD PSs and altered SOCE, through the demonstration of reduced STIM1 expression in SH-SY5Y cells and in patient-derived fibroblasts expressing different FAD-PS mutations [205].
TRPC may also play a role in SOCE deregulation in AD. TRPC expression is not altered in mice and human AD brains [233]. However, reduced TRPC1 expression was observed in astrocytes derived from APP KO mice [181,236]. In addition, TRPC6 was shown to specifically interact with APP, thereby blocking its cleavage by γ–secretase and reducing Aβ production independently from its ion channel activity [237]. Conversely, PS2 mutations abolish agonist-induced TRPC6 activation [238]. Importantly, activation of TRPC6 stimulates the activity of the neuronal SOCE pathway in the spines and rescues mushroom spine loss and long-term potentiation impairment in APP KI mice [58]. A review by Prikhodko, V. et al. in this Special Issue addresses the potential use of TRPC modulators as drugs to treat AD [239].
7. Conclusions
Studies demonstrating the implication of ER Ca2+ deregulation in AD highlight a complex picture integrating several molecular ER Ca2+ components. This includes enhanced ER Ca2+ release through IP3R and RyR, dysfunctional ER Ca2+ uptake by SERCA and upregulation of the S1T truncated isoform, gain- or loss-of-function of PS components of the γ–secretase complex. Disease-associated remodeling of this Ca2+ machinery toolkit is also coupled to specific cellular signaling cascades modulating the activity (i.e., post-translational modifications, interactions with regulatory proteins) and/or the expression of these Ca2+ channels and pump (i.e., linked to ER stress). Several studies also pinpointed the direct interaction of Aβ peptide with several members of the ER Ca2+ machinery, thus contributing to ER Ca2+ dyshomeostasis. In addition, recent studies demonstrated that the failure of the SOCE molecular bridge between the ER and the plasma membrane has to be seriously considered as a major molecular mechanism controlling ER Ca2+ content and consequently ER-mediated Ca2+ release. Finally, it becomes now evident that ER Ca2+ dyshomeostasis is significantly associated with AD development and/or progression. The treatment options for AD remain supportive and symptomatic, without attenuation of the ultimate prognosis; thus efforts have still to be made in defining therapeutic approaches targeting ER Ca2+ machinery to cure AD (Figure 2). The described ER Ca2+ toolkits are enriched in ER–mitochondria contact sites known as mitochondria-associated membranes (MAMs) [240]. Importantly, besides Ca2+ tunneling from ER to mitochondria, MAMs impact various cellular housekeeping functions such as phospholipid, glucose, cholesterol and fatty acid metabolism, which are all altered in AD [240,241]. This may further highlight the potential relevance of targeting ER Ca2+ handling proteins as an attempt to alleviate both ER and mitochondria dysfunctions associated with AD.
Acknowledgments
We thank Franck Aguila for figure drawing.
Funding
This work has been developed and supported through the LABEX (excellence laboratory, program investment for the future) DISTALZ (Development of Innovative Strategies for a Transdisciplinary approach to Alzheimer’s disease) to FC and by Fondation Vaincre Alzheimer to MC.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Berridge M.J., Bootman M.D., Roderick H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 2.Bootman M.D., Collins T.J., Peppiatt C.M., Prothero L.S., MacKenzie L., De Smet P., Travers M., Tovey S.C., Seo J.T., Berridge M.J., et al. Calcium signalling—An overview. Semin. Cell Dev. Biol. 2001;12:3–10. doi: 10.1006/scdb.2000.0211. [DOI] [PubMed] [Google Scholar]
- 3.Cahalan M.D. STIMulating store-operated Ca(2+) entry. Nat. Cell Biol. 2009;11:669–677. doi: 10.1038/ncb0609-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Putney J.W., Jr. New molecular players in capacitative Ca2+ entry. J. Cell Sci. 2007;120:1959–1965. doi: 10.1242/jcs.03462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smyth J.T., Hwang S.Y., Tomita T., DeHaven W.I., Mercer J.C., Putney J.W. Activation and regulation of store-operated calcium entry. J. Cell Mol. Med. 2010;14:2337–2349. doi: 10.1111/j.1582-4934.2010.01168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gulisano W., Maugeri D., Baltrons M.A., Fa M., Amato A., Palmeri A., D’Adamio L., Grassi C., Devanand D.P., Honig L.S., et al. Role of Amyloid-beta and Tau Proteins in Alzheimer’s Disease: Confuting the Amyloid Cascade. J. Alzheimer’s Dis. 2018;64:S611–S631. doi: 10.3233/JAD-179935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selkoe D.J., Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016;8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones D.T., Graff-Radford J., Lowe V.J., Wiste H.J., Gunter J.L., Senjem M.L., Botha H., Kantarci K., Boeve B.F., Knopman D.S., et al. Tau, amyloid, and cascading network failure across the Alzheimer’s disease spectrum. Cortex. 2017;97:143–159. doi: 10.1016/j.cortex.2017.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Checler F. Processing of the beta-amyloid precursor protein and its regulation in Alzheimer’s disease. J. Neurochem. 1995;65:1431–1444. doi: 10.1046/j.1471-4159.1995.65041431.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y.W., Thompson R., Zhang H., Xu H. APP processing in Alzheimer’s disease. Mol. Brain. 2011;4:3. doi: 10.1186/1756-6606-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edbauer D., Winkler E., Regula J.T., Pesold B., Steiner H., Haass C. Reconstitution of gamma-secretase activity. Nat. Cell Biol. 2003;5:486–488. doi: 10.1038/ncb960. [DOI] [PubMed] [Google Scholar]
- 12.Kimberly W.T., LaVoie M.J., Ostaszewski B.L., Ye W., Wolfe M.S., Selkoe D.J. Gamma-secretase is a membrane protein complex comprised of presenilin, nicastrin, Aph-1, and Pen-2. Proc. Natl. Acad. Sci. USA. 2003;100:6382–6387. doi: 10.1073/pnas.1037392100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanzi R.E., Bertram L. Twenty years of the Alzheimer’s disease amyloid hypothesis: A genetic perspective. Cell. 2005;120:545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Van Cauwenberghe C., Van Broeckhoven C., Sleegers K. The genetic landscape of Alzheimer disease: Clinical implications and perspectives. Genet. Med. 2015;18:421–430. doi: 10.1038/gim.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golde T.E., Cai X.D., Shoji M., Younkin S.G. Production of amyloid beta protein from normal amyloid beta-protein precursor (beta APP) and the mutated beta APPS linked to familial Alzheimer’s disease. Ann. N.Y. Acad. Sci. 1993;695:103–108. doi: 10.1111/j.1749-6632.1993.tb23036.x. [DOI] [PubMed] [Google Scholar]
- 16.Wolfe M.S. When loss is gain: Reduced presenilin proteolytic function leads to increased Abeta42/Abeta40. Talking Point on the role of presenilin mutations in Alzheimer disease. EMBO Rep. 2007;8:136–140. doi: 10.1038/sj.embor.7400896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Billings L.M., Oddo S., Green K.N., McGaugh J.L., LaFerla F.M. Intraneuronal Abeta causes the onset of early Alzheimer’s disease-related cognitive deficits in transgenic mice. Neuron. 2005;45:675–688. doi: 10.1016/j.neuron.2005.01.040. [DOI] [PubMed] [Google Scholar]
- 18.Del Prete D., Suski J.M., Oules B., Debayle D., Gay A.S., Lacas-Gervais S., Bussiere R., Bauer C., Pinton P., Paterlini-Brechot P., et al. Localization and Processing of the Amyloid-beta Protein Precursor in Mitochondria-Associated Membranes. J. Alzheimer’s Dis. 2017;55:1549–1570. doi: 10.3233/JAD-160953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pardossi-Piquard R., Petit A., Kawarai T., Sunyach C., Alves da Costa C., Vincent B., Ring S., D’Adamio L., Shen J., Muller U., et al. Presenilin-dependent transcriptional control of the Abeta-degrading enzyme neprilysin by intracellular domains of betaAPP and APLP. Neuron. 2005;46:541–554. doi: 10.1016/j.neuron.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Nhan H.S., Chiang K., Koo E.H. The multifaceted nature of amyloid precursor protein and its proteolytic fragments: Friends and foes. Acta Neuropathol. 2015;129:1–19. doi: 10.1007/s00401-014-1347-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baranger K., Marchalant Y., Bonnet A.E., Crouzin N., Carrete A., Paumier J.M., Py N.A., Bernard A., Bauer C., Charrat E., et al. MT5-MMP is a new pro-amyloidogenic proteinase that promotes amyloid pathology and cognitive decline in a transgenic mouse model of Alzheimer’s disease. Cell Mol. Life Sci. 2016;73:217–236. doi: 10.1007/s00018-015-1992-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willem M., Tahirovic S., Busche M.A., Ovsepian S.V., Chafai M., Kootar S., Hornburg D., Evans L.D., Moore S., Daria A., et al. eta-Secretase processing of APP inhibits neuronal activity in the hippocampus. Nature. 2015;526:443–447. doi: 10.1038/nature14864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lauritzen I., Pardossi-Piquard R., Bauer C., Brigham E., Abraham J.D., Ranaldi S., Fraser P., St-George-Hyslop P., Le Thuc O., Espin V., et al. The beta-Secretase-Derived C-Terminal Fragment of betaAPP, C99, But Not Abeta, Is a Key Contributor to Early Intraneuronal Lesions in Triple-Transgenic Mouse Hippocampus. J. Neurosci. 2012;32:16243–16255. doi: 10.1523/JNEUROSCI.2775-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lauritzen I., Pardossi-Piquard R., Bourgeois A., Pagnotta S., Biferi M.G., Barkats M., Lacor P., Klein W., Bauer C., Checler F. Intraneuronal aggregation of the beta-CTF fragment of APP (C99) induces Abeta-independent lysosomal-autophagic pathology. Acta Neuropathol. 2016;132:257–276. doi: 10.1007/s00401-016-1577-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vergara C., Houben S., Suain V., Yilmaz Z., De Decker R., Vanden Dries V., Boom A., Mansour S., Leroy K., Ando K., et al. Amyloid-beta pathology enhances pathological fibrillary tau seeding induced by Alzheimer PHF in vivo. Acta Neuropathol. 2019;137:397–412. doi: 10.1007/s00401-018-1953-5. [DOI] [PubMed] [Google Scholar]
- 26.He Z., Guo J.L., McBride J.D., Narasimhan S., Kim H., Changolkar L., Zhang B., Gathagan R.J., Yue C., Dengler C., et al. Amyloid-beta plaques enhance Alzheimer’s brain tau-seeded pathologies by facilitating neuritic plaque tau aggregation. Nat. Med. 2018;24:29–38. doi: 10.1038/nm.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holbro N., Grunditz A., Oertner T.G. Differential distribution of endoplasmic reticulum controls metabotropic signaling and plasticity at hippocampal synapses. Proc. Natl. Acad. Sci. USA. 2009;106:15055–15060. doi: 10.1073/pnas.0905110106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dailey M.E., Bridgman P.C. Dynamics of the endoplasmic reticulum and other membranous organelles in growth cones of cultured neurons. J. Neurosci. 1989;9:1897–1909. doi: 10.1523/JNEUROSCI.09-06-01897.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emptage N.J., Reid C.A., Fine A. Calcium stores in hippocampal synaptic boutons mediate short-term plasticity, store-operated Ca2+ entry, and spontaneous transmitter release. Neuron. 2001;29:197–208. doi: 10.1016/S0896-6273(01)00190-8. [DOI] [PubMed] [Google Scholar]
- 30.Berridge M.J., Bootman M.D., Lipp P. Calcium--a life and death signal. Nature. 1998;395:645–648. doi: 10.1038/27094. [DOI] [PubMed] [Google Scholar]
- 31.Li W., Llopis J., Whitney M., Zlokarnik G., Tsien R.Y. Cell-permeant caged InsP3 ester shows that Ca2+ spike frequency can optimize gene expression. Nature. 1998;392:936–941. doi: 10.1038/31965. [DOI] [PubMed] [Google Scholar]
- 32.Bandtlow C.E., Schmidt M.F., Hassinger T.D., Schwab M.E., Kater S.B. Role of intracellular calcium in NI-35-evoked collapse of neuronal growth cones. Science. 1993;259:80–83. doi: 10.1126/science.8418499. [DOI] [PubMed] [Google Scholar]
- 33.Popugaeva E., Bezprozvanny I. Can the calcium hypothesis explain synaptic loss in Alzheimer’s disease? Neurodegener. Dis. 2014;13:139–141. doi: 10.1159/000354778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bojarski L., Herms J., Kuznicki J. Calcium dysregulation in Alzheimer’s disease. Neurochem. Int. 2008;52:621–633. doi: 10.1016/j.neuint.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Tong B.C., Wu A.J., Li M., Cheung K.H. Calcium signaling in Alzheimer’s disease & therapies. Biochim. Biophys. Acta Mol. Cell Res. 2018;1865:1745–1760. doi: 10.1016/j.bbamcr.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 36.Del Prete D., Checler F., Chami M. Ryanodine receptors: Physiological function and deregulation in Alzheimer disease. Mol. Neurodegener. 2014;9:21. doi: 10.1186/1750-1326-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao L.L., Guan P.P., Liang Y.Y., Huang X.S., Wang P. Calcium Ions Stimulate the Hyperphosphorylation of Tau by Activating Microsomal Prostaglandin E Synthase 1. Front. Aging Neurosci. 2019;11:108. doi: 10.3389/fnagi.2019.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayley M., Perspicace S., Schulthess T., Seelig J. Calcium enhances the proteolytic activity of BACE1: An in vitro biophysical and biochemical characterization of the BACE1-calcium interaction. Biochim. Biophys. Acta. 2009;1788:1933–1938. doi: 10.1016/j.bbamem.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 39.Ho M., Hoke D.E., Chua Y.J., Li Q.X., Culvenor J.G., Masters C., White A.R., Evin G. Effect of Metal Chelators on gamma-Secretase Indicates That Calcium and Magnesium Ions Facilitate Cleavage of Alzheimer Amyloid Precursor Substrate. Int. J. Alzheimer’s Dis. 2010;2011:950932. doi: 10.4061/2011/950932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller S.G., Kennedy M.B. Regulation of brain type II Ca2+/calmodulin-dependent protein kinase by autophosphorylation: A Ca2+-triggered molecular switch. Cell. 1986;44:861–870. doi: 10.1016/0092-8674(86)90008-5. [DOI] [PubMed] [Google Scholar]
- 41.Oka M., Fujisaki N., Maruko-Otake A., Ohtake Y., Shimizu S., Saito T., Hisanaga S.I., Iijima K.M., Ando K. Ca2+/calmodulin-dependent protein kinase II promotes neurodegeneration caused by tau phosphorylated at Ser262/356 in a transgenic Drosophila model of tauopathy. J. Biochem. 2017;162:335–342. doi: 10.1093/jb/mvx038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Litersky J.M., Johnson G.V., Jakes R., Goedert M., Lee M., Seubert P. Tau protein is phosphorylated by cyclic AMP-dependent protein kinase and calcium/calmodulin-dependent protein kinase II within its microtubule-binding domains at Ser-262 and Ser-356. Biochem. J. 1996;316 (Pt 2):655–660. doi: 10.1042/bj3160655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chakroborty S., Stutzmann G.E. Early calcium dysregulation in Alzheimer’s disease: Setting the stage for synaptic dysfunction. Sci. China Life Sci. 2011;54:752–762. doi: 10.1007/s11427-011-4205-7. [DOI] [PubMed] [Google Scholar]
- 44.Mattson M.P. ER calcium and Alzheimer’s disease: In a state of flux. Sci. Signal. 2010;3:pe10. doi: 10.1126/scisignal.3114pe10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.LaFerla F.M. Calcium dyshomeostasis and intracellular signalling in Alzheimer’s disease. Nat. Rev. Neurosci. 2002;3:862–872. doi: 10.1038/nrn960. [DOI] [PubMed] [Google Scholar]
- 46.Supnet C., Bezprozvanny I. The dysregulation of intracellular calcium in Alzheimer disease. Cell Calcium. 2010;47:183–189. doi: 10.1016/j.ceca.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bezprozvanny I., Mattson M.P. Neuronal calcium mishandling and the pathogenesis of Alzheimer’s disease. Trends Neurosci. 2008;31:454–463. doi: 10.1016/j.tins.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berridge M.J. Calcium signalling and Alzheimer’s disease. Neurochem. Res. 2011;36:1149–1156. doi: 10.1007/s11064-010-0371-4. [DOI] [PubMed] [Google Scholar]
- 49.Chakroborty S., Stutzmann G.E. Calcium channelopathies and Alzheimer’s disease: Insight into therapeutic success and failures. Eur. J. Pharmacol. 2014;739:83–95. doi: 10.1016/j.ejphar.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 50.Arispe N., Rojas E., Pollard H.B. Alzheimer disease amyloid beta protein forms calcium channels in bilayer membranes: Blockade by tromethamine and aluminum. Proc. Natl. Acad. Sci. USA. 1993;90:567–571. doi: 10.1073/pnas.90.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alberdi E., Sanchez-Gomez M.V., Cavaliere F., Perez-Samartin A., Zugaza J.L., Trullas R., Domercq M., Matute C. Amyloid beta oligomers induce Ca2+ dysregulation and neuronal death through activation of ionotropic glutamate receptors. Cell Calcium. 2010;47:264–272. doi: 10.1016/j.ceca.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 52.Texido L., Martin-Satue M., Alberdi E., Solsona C., Matute C. Amyloid beta peptide oligomers directly activate NMDA receptors. Cell Calcium. 2011;49:184–190. doi: 10.1016/j.ceca.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 53.Thibault O., Pancani T., Landfield P.W., Norris C.M. Reduction in neuronal L-type calcium channel activity in a double knock-in mouse model of Alzheimer’s disease. Biochim. Biophys. Acta. 2012;1822:546–549. doi: 10.1016/j.bbadis.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma Z., Siebert A.P., Cheung K.H., Lee R.J., Johnson B., Cohen A.S., Vingtdeux V., Marambaud P., Foskett J.K. Calcium homeostasis modulator 1 (CALHM1) is the pore-forming subunit of an ion channel that mediates extracellular Ca2+ regulation of neuronal excitability. Proc. Natl. Acad. Sci. USA. 2012;109:E1963–E1971. doi: 10.1073/pnas.1204023109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zeiger W., Vetrivel K.S., Buggia-Prevot V., Nguyen P.D., Wagner S.L., Villereal M.L., Thinakaran G. Ca2+ influx through store-operated Ca2+ channels reduces Alzheimer disease beta-amyloid peptide secretion. J. Biol. Chem. 2013;288:26955–26966. doi: 10.1074/jbc.M113.473355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoo A.S., Cheng I., Chung S., Grenfell T.Z., Lee H., Pack-Chung E., Handler M., Shen J., Xia W., Tesco G., et al. Presenilin-mediated modulation of capacitative calcium entry. Neuron. 2000;27:561–572. doi: 10.1016/S0896-6273(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 57.Chernyuk D., Zernov N., Kabirova M., Bezprozvanny I., Popugaeva E. Antagonist of neuronal store-operated calcium entry exerts beneficial effects in neurons expressing PSEN1DeltaE9 mutant linked to familial Alzheimer disease. Neuroscience. 2019;410:118–127. doi: 10.1016/j.neuroscience.2019.04.043. [DOI] [PubMed] [Google Scholar]
- 58.Zhang H., Sun S., Wu L., Pchitskaya E., Zakharova O., Fon Tacer K., Bezprozvanny I. Store-Operated Calcium Channel Complex in Postsynaptic Spines: A New Therapeutic Target for Alzheimer’s Disease Treatment. J. Neurosci. 2016;36:11837–11850. doi: 10.1523/JNEUROSCI.1188-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang H., Wu L., Pchitskaya E., Zakharova O., Saito T., Saido T., Bezprozvanny I. Neuronal Store-Operated Calcium Entry and Mushroom Spine Loss in Amyloid Precursor Protein Knock-In Mouse Model of Alzheimer’s Disease. J. Neurosci. 2015;35:13275–13286. doi: 10.1523/JNEUROSCI.1034-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Popugaeva E., Pchitskaya E., Speshilova A., Alexandrov S., Zhang H., Vlasova O., Bezprozvanny I. STIM2 protects hippocampal mushroom spines from amyloid synaptotoxicity. Mol. Neurodegener. 2015;10:37. doi: 10.1186/s13024-015-0034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tang J., Oliveros A., Jang M.H. Dysfunctional Mitochondrial Bioenergetics and Synaptic Degeneration in Alzheimer Disease. Int. Neurourol. J. 2019;23:S5–S10. doi: 10.5213/inj.1938036.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Garcia-Escudero V., Martin-Maestro P., Perry G., Avila J. Deconstructing mitochondrial dysfunction in Alzheimer disease. Oxid. Med. Cell Longev. 2013;2013:162152. doi: 10.1155/2013/162152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Esteras N., Abramov A.Y. Mitochondrial Calcium Deregulation in the Mechanism of Beta-Amyloid and Tau Pathology. Cells. 2020;9:2135. doi: 10.3390/cells9092135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Perez M.J., Ponce D.P., Aranguiz A., Behrens M.I., Quintanilla R.A. Mitochondrial permeability transition pore contributes to mitochondrial dysfunction in fibroblasts of patients with sporadic Alzheimer’s disease. Redox Biol. 2018;19:290–300. doi: 10.1016/j.redox.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jadiya P., Kolmetzky D.W., Tomar D., Di Meco A., Lombardi A.A., Lambert J.P., Luongo T.S., Ludtmann M.H., Pratico D., Elrod J.W. Impaired mitochondrial calcium efflux contributes to disease progression in models of Alzheimer’s disease. Nat. Commun. 2019;10:3885. doi: 10.1038/s41467-019-11813-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wolfe D.M., Lee J.H., Kumar A., Lee S., Orenstein S.J., Nixon R.A. Autophagy failure in Alzheimer’s disease and the role of defective lysosomal acidification. Eur. J. Neurosci. 2013;37:1949–1961. doi: 10.1111/ejn.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee J.H., Yu W.H., Kumar A., Lee S., Mohan P.S., Peterhoff C.M., Wolfe D.M., Martinez-Vicente M., Massey A.C., Sovak G., et al. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell. 2010;141:1146–1158. doi: 10.1016/j.cell.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee J.H., McBrayer M.K., Wolfe D.M., Haslett L.J., Kumar A., Sato Y., Lie P.P., Mohan P., Coffey E.E., Kompella U., et al. Presenilin 1 Maintains Lysosomal Ca(2+) Homeostasis via TRPML1 by Regulating vATPase-Mediated Lysosome Acidification. Cell Rep. 2015;12:1430–1444. doi: 10.1016/j.celrep.2015.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tu H., Nelson O., Bezprozvanny A., Wang Z., Lee S.F., Hao Y.H., Serneels L., De Strooper B., Yu G., Bezprozvanny I. Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer’s disease-linked mutations. Cell. 2006;126:981–993. doi: 10.1016/j.cell.2006.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nelson O., Tu H., Lei T., Bentahir M., de Strooper B., Bezprozvanny I. Familial Alzheimer disease-linked mutations specifically disrupt Ca2+ leak function of presenilin 1. J. Clin. Invest. 2007;117:1230–1239. doi: 10.1172/JCI30447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brunello L., Zampese E., Florean C., Pozzan T., Pizzo P., Fasolato C. Presenilin-2 dampens intracellular Ca2+ stores by increasing Ca2+ leakage and reducing Ca2+ uptake. J. Cell Mol. Med. 2009;13:3358–3369. doi: 10.1111/j.1582-4934.2009.00755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shilling D., Muller M., Takano H., Mak D.O., Abel T., Coulter D.A., Foskett J.K. Suppression of InsP3 receptor-mediated Ca2+ signaling alleviates mutant presenilin-linked familial Alzheimer’s disease pathogenesis. J. Neurosci. 2014;34:6910–6923. doi: 10.1523/JNEUROSCI.5441-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shilling D., Mak D.O., Kang D.E., Foskett J.K. Lack of evidence for presenilins as endoplasmic reticulum Ca2+ leak channels. J. Biol. Chem. 2012;287:10933–10944. doi: 10.1074/jbc.M111.300491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Green K.N., Demuro A., Akbari Y., Hitt B.D., Smith I.F., Parker I., LaFerla F.M. SERCA pump activity is physiologically regulated by presenilin and regulates amyloid beta production. J. Cell Biol. 2008;181:1107–1116. doi: 10.1083/jcb.200706171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bussiere R., Oules B., Mary A., Vaillant-Beuchot L., Martin C., El Manaa W., Vallee D., Duplan E., Paterlini-Brechot P., Alves Da Costa C., et al. Upregulation of the Sarco-Endoplasmic Reticulum Calcium ATPase 1 Truncated Isoform Plays a Pathogenic Role in Alzheimer’s Disease. Cells. 2019;8:1539. doi: 10.3390/cells8121539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nelson O., Supnet C., Liu H., Bezprozvanny I. Familial Alzheimer’s disease mutations in presenilins: Effects on endoplasmic reticulum calcium homeostasis and correlation with clinical phenotypes. J. Alzheimer’s Dis. 2010;21:781–793. doi: 10.3233/JAD-2010-100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nelson O., Supnet C., Tolia A., Horre K., De Strooper B., Bezprozvanny I. Mutagenesis mapping of the presenilin 1 calcium leak conductance pore. J. Biol. Chem. 2011;286:22339–22347. doi: 10.1074/jbc.M111.243063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zampese E., Fasolato C., Kipanyula M.J., Bortolozzi M., Pozzan T., Pizzo P. Presenilin 2 modulates endoplasmic reticulum (ER)-mitochondria interactions and Ca2+ cross-talk. Proc. Natl. Acad. Sci. USA. 2011;108:2777–2782. doi: 10.1073/pnas.1100735108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stutzmann G.E., Caccamo A., LaFerla F.M., Parker I. Dysregulated IP3 signaling in cortical neurons of knock-in mice expressing an Alzheimer’s-linked mutation in presenilin1 results in exaggerated Ca2+ signals and altered membrane excitability. J. Neurosci. 2004;24:508–513. doi: 10.1523/JNEUROSCI.4386-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stutzmann G.E., Smith I., Caccamo A., Oddo S., Parker I., Laferla F. Enhanced ryanodine-mediated calcium release in mutant PS1-expressing Alzheimer’s mouse models. Ann. N.Y. Acad. Sci. 2007;1097:265–277. doi: 10.1196/annals.1379.025. [DOI] [PubMed] [Google Scholar]
- 81.Cheung K.H., Shineman D., Muller M., Cardenas C., Mei L., Yang J., Tomita T., Iwatsubo T., Lee V.M., Foskett J.K. Mechanism of Ca2+ disruption in Alzheimer’s disease by presenilin regulation of InsP(3) receptor channel gating. Neuron. 2008;58:871–883. doi: 10.1016/j.neuron.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Querfurth H.W., Jiang J., Geiger J.D., Selkoe D.J. Caffeine stimulates amyloid beta-peptide release from beta-amyloid precursor protein-transfected HEK293 cells. J. Neurochem. 1997;69:1580–1591. doi: 10.1046/j.1471-4159.1997.69041580.x. [DOI] [PubMed] [Google Scholar]
- 83.Chan S.L., Mayne M., Holden C.P., Geiger J.D., Mattson M.P. Presenilin-1 mutations increase levels of ryanodine receptors and calcium release in PC12 cells and cortical neurons. J. Biol. Chem. 2000;275:18195–18200. doi: 10.1074/jbc.M000040200. [DOI] [PubMed] [Google Scholar]
- 84.Smith I.F., Hitt B., Green K.N., Oddo S., LaFerla F.M. Enhanced caffeine-induced Ca2+ release in the 3xTg-AD mouse model of Alzheimer’s disease. J. Neurochem. 2005;94:1711–1718. doi: 10.1111/j.1471-4159.2005.03332.x. [DOI] [PubMed] [Google Scholar]
- 85.Stutzmann G.E., Smith I., Caccamo A., Oddo S., Laferla F.M., Parker I. Enhanced ryanodine receptor recruitment contributes to Ca2+ disruptions in young, adult, and aged Alzheimer’s disease mice. J. Neurosci. 2006;26:5180–5189. doi: 10.1523/JNEUROSCI.0739-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Supnet C., Grant J., Kong H., Westaway D., Mayne M. Amyloid-beta-(1-42) increases ryanodine receptor-3 expression and function in neurons of TgCRND8 mice. J. Biol. Chem. 2006;281:38440–38447. doi: 10.1074/jbc.M606736200. [DOI] [PubMed] [Google Scholar]
- 87.Chakroborty S., Goussakov I., Miller M.B., Stutzmann G.E. Deviant ryanodine receptor-mediated calcium release resets synaptic homeostasis in presymptomatic 3xTg-AD mice. J. Neurosci. 2009;29:9458–9470. doi: 10.1523/JNEUROSCI.2047-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Paula-Lima A.C., Adasme T., SanMartin C., Sebollela A., Hetz C., Carrasco M.A., Ferreira S.T., Hidalgo C. Amyloid beta-peptide oligomers stimulate RyR-mediated Ca2+ release inducing mitochondrial fragmentation in hippocampal neurons and prevent RyR-mediated dendritic spine remodeling produced by BDNF. Antioxid. Redox Signal. 2011;14:1209–1223. doi: 10.1089/ars.2010.3287. [DOI] [PubMed] [Google Scholar]
- 89.Ito E., Oka K., Etcheberrigaray R., Nelson T.J., McPhie D.L., Tofel-Grehl B., Gibson G.E., Alkon D.L. Internal Ca2+ mobilization is altered in fibroblasts from patients with Alzheimer disease. Proc. Natl. Acad. Sci. USA. 1994;91:534–538. doi: 10.1073/pnas.91.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Goussakov I., Miller M.B., Stutzmann G.E. NMDA-mediated Ca(2+) influx drives aberrant ryanodine receptor activation in dendrites of young Alzheimer’s disease mice. J. Neurosci. 2010;30:12128–12137. doi: 10.1523/JNEUROSCI.2474-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jensen L.E., Bultynck G., Luyten T., Amijee H., Bootman M.D., Roderick H.L. Alzheimer’s disease-associated peptide Abeta42 mobilizes ER Ca(2+) via InsP3R-dependent and -independent mechanisms. Front. Mol. Neurosci. 2013;6:36. doi: 10.3389/fnmol.2013.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Oules B., Del Prete D., Greco B., Zhang X., Lauritzen I., Sevalle J., Moreno S., Paterlini-Brechot P., Trebak M., Checler F., et al. Ryanodine receptor blockade reduces amyloid-beta load and memory impairments in Tg2576 mouse model of Alzheimer disease. J. Neurosci. 2012;32:11820–11834. doi: 10.1523/JNEUROSCI.0875-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chakroborty S., Briggs C., Miller M.B., Goussakov I., Schneider C., Kim J., Wicks J., Richardson J.C., Conklin V., Cameransi B.G., et al. Stabilizing ER Ca2+ channel function as an early preventative strategy for Alzheimer’s disease. PLoS ONE. 2012;7:e52056. doi: 10.1371/journal.pone.0052056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chakroborty S., Kim J., Schneider C., Jacobson C., Molgo J., Stutzmann G.E. Early presynaptic and postsynaptic calcium signaling abnormalities mask underlying synaptic depression in presymptomatic Alzheimer’s disease mice. J. Neurosci. 2012;32:8341–8353. doi: 10.1523/JNEUROSCI.0936-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Santos S.F., Pierrot N., Morel N., Gailly P., Sindic C., Octave J.N. Expression of human amyloid precursor protein in rat cortical neurons inhibits calcium oscillations. J. Neurosci. 2009;29:4708–4718. doi: 10.1523/JNEUROSCI.4917-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gazda K., Kuznicki J., Wegierski T. Knockdown of amyloid precursor protein increases calcium levels in the endoplasmic reticulum. Sci. Rep. 2017;7:14512. doi: 10.1038/s41598-017-15166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mahoney R., Ochoa Thomas E., Ramirez P., Miller H.E., Beckmann A., Zuniga G., Dobrowolski R., Frost B. Pathogenic Tau Causes a Toxic Depletion of Nuclear Calcium. Cell Rep. 2020;32:107900. doi: 10.1016/j.celrep.2020.107900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Esteras N., Kundel F., Amodeo G.F., Pavlov E.V., Klenerman D., Abramov A.Y. Insoluble tau aggregates induce neuronal death through modification of membrane ion conductance, activation of voltage-gated calcium channels and NADPH oxidase. FEBS J. 2020 doi: 10.1111/febs.15340. [DOI] [PubMed] [Google Scholar]
- 99.Britti E., Ros J., Esteras N., Abramov A.Y. Tau inhibits mitochondrial calcium efflux and makes neurons vulnerable to calcium-induced cell death. Cell Calcium. 2020;86:102150. doi: 10.1016/j.ceca.2019.102150. [DOI] [PubMed] [Google Scholar]
- 100.Furuichi T., Furutama D., Hakamata Y., Nakai J., Takeshima H., Mikoshiba K. Multiple types of ryanodine receptor/Ca2+ release channels are differentially expressed in rabbit brain. J. Neurosci. 1994;14:4794–4805. doi: 10.1523/JNEUROSCI.14-08-04794.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lehnart S.E., Mongillo M., Bellinger A., Lindegger N., Chen B.X., Hsueh W., Reiken S., Wronska A., Drew L.J., Ward C.W., et al. Leaky Ca2+ release channel/ryanodine receptor 2 causes seizures and sudden cardiac death in mice. J. Clin. Investig. 2008;118:2230–2245. doi: 10.1172/JCI35346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Meissner G., Darling E., Eveleth J. Kinetics of rapid Ca2+ release by sarcoplasmic reticulum. Effects of Ca2+, Mg2+, and adenine nucleotides. Biochemistry. 1986;25:236–244. doi: 10.1021/bi00349a033. [DOI] [PubMed] [Google Scholar]
- 103.Meissner G. The structural basis of ryanodine receptor ion channel function. J. Gen. Physiol. 2017;149:1065–1089. doi: 10.1085/jgp.201711878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Laver D.R., Honen B.N. Luminal Mg2+, a key factor controlling RYR2-mediated Ca2+ release: Cytoplasmic and luminal regulation modeled in a tetrameric channel. J. Gen. Physiol. 2008;132:429–446. doi: 10.1085/jgp.200810001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tencerova B., Zahradnikova A., Gaburjakova J., Gaburjakova M. Luminal Ca2+ controls activation of the cardiac ryanodine receptor by ATP. J. Gen. Physiol. 2012;140:93–108. doi: 10.1085/jgp.201110708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yan Z., Bai X., Yan C., Wu J., Li Z., Xie T., Peng W., Yin C., Li X., Scheres S.H.W., et al. Structure of the rabbit ryanodine receptor RyR1 at near-atomic resolution. Nature. 2015;517:50–55. doi: 10.1038/nature14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Peng W., Shen H., Wu J., Guo W., Pan X., Wang R., Chen S.R., Yan N. Structural basis for the gating mechanism of the type 2 ryanodine receptor RyR2. Science. 2016;354 doi: 10.1126/science.aah5324. [DOI] [PubMed] [Google Scholar]
- 108.Denniss A., Dulhunty A.F., Beard N.A. Ryanodine receptor Ca(2+) release channel post-translational modification: Central player in cardiac and skeletal muscle disease. Int. J. Biochem. Cell Biol. 2018;101:49–53. doi: 10.1016/j.biocel.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 109.Lanner J.T., Georgiou D.K., Joshi A.D., Hamilton S.L. Ryanodine receptors: Structure, expression, molecular details, and function in calcium release. Cold Spring Harb. Perspect. Biol. 2010;2:a003996. doi: 10.1101/cshperspect.a003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Balshaw D.M., Yamaguchi N., Meissner G. Modulation of intracellular calcium-release channels by calmodulin. J. Membr. Biol. 2002;185:1–8. doi: 10.1007/s00232-001-0111-4. [DOI] [PubMed] [Google Scholar]
- 111.MacMillan D. FK506 binding proteins: Cellular regulators of intracellular Ca2+ signalling. Eur. J. Pharmacol. 2013;700:181–193. doi: 10.1016/j.ejphar.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 112.Zalk R., Lehnart S.E., Marks A.R. Modulation of the ryanodine receptor and intracellular calcium. Annu. Rev. Biochem. 2007;76:367–385. doi: 10.1146/annurev.biochem.76.053105.094237. [DOI] [PubMed] [Google Scholar]
- 113.Marx S.O., Reiken S., Hisamatsu Y., Jayaraman T., Burkhoff D., Rosemblit N., Marks A.R. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): Defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/S0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 114.Currie S. Cardiac ryanodine receptor phosphorylation by CaM Kinase II: Keeping the balance right. Front. Biosci. 2009;14:5134–5156. doi: 10.2741/3591. [DOI] [PubMed] [Google Scholar]
- 115.Allen P.B., Ouimet C.C., Greengard P. Spinophilin, a novel protein phosphatase 1 binding protein localized to dendritic spines. Proc. Natl. Acad. Sci. USA. 1997;94:9956–9961. doi: 10.1073/pnas.94.18.9956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chami M., Checler F. Targeting Post-Translational Remodeling of Ryanodine Receptor: A New Track for Alzheimer’s Disease Therapy? Curr. Alzheimer Res. 2020;17:313–323. doi: 10.2174/1567205017666200225102941. [DOI] [PubMed] [Google Scholar]
- 117.Lee S.Y., Hwang D.Y., Kim Y.K., Lee J.W., Shin I.C., Oh K.W., Lee M.K., Lim J.S., Yoon D.Y., Hwang S.J., et al. PS2 mutation increases neuronal cell vulnerability to neurotoxicants through activation of caspase-3 by enhancing of ryanodine receptor-mediated calcium release. FASEB J. 2006;20:151–153. doi: 10.1096/fj.05-4017fje;1. [DOI] [PubMed] [Google Scholar]
- 118.Guo Q., Sopher B.L., Furukawa K., Pham D.G., Robinson N., Martin G.M., Mattson M.P. Alzheimer’s presenilin mutation sensitizes neural cells to apoptosis induced by trophic factor withdrawal and amyloid beta-peptide: Involvement of calcium and oxyradicals. J. Neurosci. 1997;17:4212–4222. doi: 10.1523/JNEUROSCI.17-11-04212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Leissring M.A., Murphy M.P., Mead T.R., Akbari Y., Sugarman M.C., Jannatipour M., Anliker B., Muller U., Saftig P., De Strooper B., et al. A physiologic signaling role for the gamma -secretase-derived intracellular fragment of APP. Proc. Natl. Acad. Sci. USA. 2002;99:4697–4702. doi: 10.1073/pnas.072033799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lopez J.R., Lyckman A., Oddo S., Laferla F.M., Querfurth H.W., Shtifman A. Increased intraneuronal resting [Ca(2+)] in adult Alzheimer’s disease mice. J. Neurochem. 2008;105:262–271. doi: 10.1111/j.1471-4159.2007.05135.x. [DOI] [PubMed] [Google Scholar]
- 121.Rojas G., Cardenas A.M., Fernandez-Olivares P., Shimahara T., Segura-Aguilar J., Caviedes R., Caviedes P. Effect of the knockdown of amyloid precursor protein on intracellular calcium increases in a neuronal cell line derived from the cerebral cortex of a trisomy 16 mouse. Exp. Neurol. 2008;209:234–242. doi: 10.1016/j.expneurol.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 122.Niu Y., Su Z., Zhao C., Song B., Zhang X., Zhao N., Shen X., Gong Y. Effect of amyloid beta on capacitive calcium entry in neural 2a cells. Brain Res. Bull. 2009;78:152–157. doi: 10.1016/j.brainresbull.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 123.Paula-Lima A.C., Hidalgo C. Amyloid beta-peptide oligomers, ryanodine receptor-mediated Ca(2+) release, and Wnt-5a/Ca(2+) signaling: Opposing roles in neuronal mitochondrial dynamics? Front. Cell Neurosci. 2013;7:120. doi: 10.3389/fncel.2013.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shtifman A., Ward C.W., Laver D.R., Bannister M.L., Lopez J.R., Kitazawa M., LaFerla F.M., Ikemoto N., Querfurth H.W. Amyloid-beta protein impairs Ca2+ release and contractility in skeletal muscle. Neurobiol. Aging. 2010;31:2080–2090. doi: 10.1016/j.neurobiolaging.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kelliher M., Fastbom J., Cowburn R.F., Bonkale W., Ohm T.G., Ravid R., Sorrentino V., O’Neill C. Alterations in the ryanodine receptor calcium release channel correlate with Alzheimer’s disease neurofibrillary and beta-amyloid pathologies. Neuroscience. 1999;92:499–513. doi: 10.1016/S0306-4522(99)00042-1. [DOI] [PubMed] [Google Scholar]
- 126.Wu B., Yamaguchi H., Lai F.A., Shen J. Presenilins regulate calcium homeostasis and presynaptic function via ryanodine receptors in hippocampal neurons. Proc. Natl. Acad. Sci. USA. 2013;110:15091–15096. doi: 10.1073/pnas.1304171110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kipanyula M.J., Contreras L., Zampese E., Lazzari C., Wong A.K., Pizzo P., Fasolato C., Pozzan T. Ca2+ dysregulation in neurons from transgenic mice expressing mutant presenilin 2. Aging Cell. 2012;11:885–893. doi: 10.1111/j.1474-9726.2012.00858.x. [DOI] [PubMed] [Google Scholar]
- 128.Cisse M., Duplan E., Lorivel T., Dunys J., Bauer C., Meckler X., Gerakis Y., Lauritzen I., Checler F. The transcription factor XBP1s restores hippocampal synaptic plasticity and memory by control of the Kalirin-7 pathway in Alzheimer model. Mol. Psychiatry. 2017;22:1562–1575. doi: 10.1038/mp.2016.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gerakis Y., Dunys J., Bauer C., Checler F. Abeta42 oligomers modulate beta-secretase through an XBP-1s-dependent pathway involving HRD1. Sci. Rep. 2016;6:37436. doi: 10.1038/srep37436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Casas-Tinto S., Zhang Y., Sanchez-Garcia J., Gomez-Velazquez M., Rincon-Limas D.E., Fernandez-Funez P. The ER stress factor XBP1s prevents amyloid-beta neurotoxicity. Hum. Mol. Genet. 2011;20:2144–2160. doi: 10.1093/hmg/ddr100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liu J., Supnet C., Sun S., Zhang H., Good L., Popugaeva E., Bezprozvanny I. The role of ryanodine receptor type 3 in a mouse model of Alzheimer disease. Channels. 2014;8:230–242. doi: 10.4161/chan.27471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mickelson J.R., Gallant E.M., Litterer L.A., Johnson K.M., Rempel W.E., Louis C.F. Abnormal sarcoplasmic reticulum ryanodine receptor in malignant hyperthermia. J. Biol. Chem. 1988;263:9310–9315. [PubMed] [Google Scholar]
- 133.Kushnir A., Wajsberg B., Marks A.R. Ryanodine receptor dysfunction in human disorders. Biochim. Biophys. Acta Mol. Cell Res. 2018;1865:1687–1697. doi: 10.1016/j.bbamcr.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 134.Kushnir A., Betzenhauser M.J., Marks A.R. Ryanodine receptor studies using genetically engineered mice. FEBS Lett. 2010;584:1956–1965. doi: 10.1016/j.febslet.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Takeshima H., Iino M., Takekura H., Nishi M., Kuno J., Minowa O., Takano H., Noda T. Excitation-contraction uncoupling and muscular degeneration in mice lacking functional skeletal muscle ryanodine-receptor gene. Nature. 1994;369:556–559. doi: 10.1038/369556a0. [DOI] [PubMed] [Google Scholar]
- 136.Takeshima H., Ikemoto T., Nishi M., Nishiyama N., Shimuta M., Sugitani Y., Kuno J., Saito I., Saito H., Endo M., et al. Generation and characterization of mutant mice lacking ryanodine receptor type 3. J. Biol. Chem. 1996;271:19649–19652. doi: 10.1074/jbc.271.33.19649. [DOI] [PubMed] [Google Scholar]
- 137.Matsuo N., Tanda K., Nakanishi K., Yamasaki N., Toyama K., Takao K., Takeshima H., Miyakawa T. Comprehensive behavioral phenotyping of ryanodine receptor type 3 (RyR3) knockout mice: Decreased social contact duration in two social interaction tests. Front. Behav. Neurosci. 2009;3:3. doi: 10.3389/neuro.08.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Balschun D., Wolfer D.P., Bertocchini F., Barone V., Conti A., Zuschratter W., Missiaen L., Lipp H.P., Frey J.U., Sorrentino V. Deletion of the ryanodine receptor type 3 (RyR3) impairs forms of synaptic plasticity and spatial learning. EMBO J. 1999;18:5264–5273. doi: 10.1093/emboj/18.19.5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Futatsugi A., Kato K., Ogura H., Li S.T., Nagata E., Kuwajima G., Tanaka K., Itohara S., Mikoshiba K. Facilitation of NMDAR-independent LTP and spatial learning in mutant mice lacking ryanodine receptor type 3. Neuron. 1999;24:701–713. doi: 10.1016/S0896-6273(00)81123-X. [DOI] [PubMed] [Google Scholar]
- 140.Shimuta M., Yoshikawa M., Fukaya M., Watanabe M., Takeshima H., Manabe T. Postsynaptic modulation of AMPA receptor-mediated synaptic responses and LTP by the type 3 ryanodine receptor. Mol. Cell Neurosci. 2001;17:921–930. doi: 10.1006/mcne.2001.0981. [DOI] [PubMed] [Google Scholar]
- 141.Gong S., Su B.B., Tovar H., Mao C., Gonzalez V., Liu Y., Lu Y., Wang K.S., Xu C. Polymorphisms Within RYR3 Gene Are Associated With Risk and Age at Onset of Hypertension, Diabetes, and Alzheimer’s Disease. Am. J. Hypertens. 2018;31:818–826. doi: 10.1093/ajh/hpy046. [DOI] [PubMed] [Google Scholar]
- 142.Sun J., Song F., Wang J., Han G., Bai Z., Xie B., Feng X., Jia J., Duan Y., Lei H. Hidden risk genes with high-order intragenic epistasis in Alzheimer’s disease. J. Alzheimer’s Dis. 2014;41:1039–1056. doi: 10.3233/JAD-140054. [DOI] [PubMed] [Google Scholar]
- 143.Koran M.E., Hohman T.J., Thornton-Wells T.A. Genetic interactions found between calcium channel genes modulate amyloid load measured by positron emission tomography. Hum. Genet. 2014;133:85–93. doi: 10.1007/s00439-013-1354-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wehrens X.H., Lehnart S.E., Reiken S.R., Deng S.X., Vest J.A., Cervantes D., Coromilas J., Landry D.W., Marks A.R. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science. 2004;304:292–296. doi: 10.1126/science.1094301. [DOI] [PubMed] [Google Scholar]
- 145.Andersson D.C., Betzenhauser M.J., Reiken S., Meli A.C., Umanskaya A., Xie W., Shiomi T., Zalk R., Lacampagne A., Marks A.R. Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab. 2011;14:196–207. doi: 10.1016/j.cmet.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bellinger A.M., Reiken S., Carlson C., Mongillo M., Liu X., Rothman L., Matecki S., Lacampagne A., Marks A.R. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat. Med. 2009;15:325–330. doi: 10.1038/nm.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bellinger A.M., Reiken S., Dura M., Murphy P.W., Deng S.X., Landry D.W., Nieman D., Lehnart S.E., Samaru M., LaCampagne A., et al. Remodeling of ryanodine receptor complex causes “leaky” channels: A molecular mechanism for decreased exercise capacity. Proc. Natl. Acad. Sci. USA. 2008;105:2198–2202. doi: 10.1073/pnas.0711074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fauconnier J., Meli A.C., Thireau J., Roberge S., Shan J., Sassi Y., Reiken S.R., Rauzier J.M., Marchand A., Chauvier D., et al. Ryanodine receptor leak mediated by caspase-8 activation leads to left ventricular injury after myocardial ischemia-reperfusion. Proc. Natl. Acad. Sci. USA. 2011;108:13258–13263. doi: 10.1073/pnas.1100286108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liu X., Betzenhauser M.J., Reiken S., Meli A.C., Xie W., Chen B.X., Arancio O., Marks A.R. Role of leaky neuronal ryanodine receptors in stress-induced cognitive dysfunction. Cell. 2012;150:1055–1067. doi: 10.1016/j.cell.2012.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lacampagne A., Liu X., Reiken S., Bussiere R., Meli A.C., Lauritzen I., Teich A.F., Zalk R., Saint N., Arancio O., et al. Post-translational remodeling of ryanodine receptor induces calcium leak leading to Alzheimer’s disease-like pathologies and cognitive deficits. Acta Neuropathol. 2017;134:749–767. doi: 10.1007/s00401-017-1733-7. [DOI] [PubMed] [Google Scholar]
- 151.Petrotchenko E.V., Yamaguchi N., Pasek D.A., Borchers C.H., Meissner G. Mass spectrometric analysis and mutagenesis predict involvement of multiple cysteines in redox regulation of the skeletal muscle ryanodine receptor ion channel complex. Res. Rep. Biol. 2011;2011:13–21. doi: 10.2147/RRB.S15776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sun J., Yamaguchi N., Xu L., Eu J.P., Stamler J.S., Meissner G. Regulation of the cardiac muscle ryanodine receptor by O(2) tension and S-nitrosoglutathione. Biochemistry. 2008;47:13985–13990. doi: 10.1021/bi8012627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bussiere R., Lacampagne A., Reiken S., Liu X., Scheuerman V., Zalk R., Martin C., Checler F., Marks A.R., Chami M. Amyloid beta production is regulated by beta2-adrenergic signaling-mediated post-translational modifications of the ryanodine receptor. J. Biol. Chem. 2017;292:10153–10168. doi: 10.1074/jbc.M116.743070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hidalgo C., Carrasco M.A. Redox control of brain calcium in health and disease. Antioxid. Redox Signal. 2011;14:1203–1207. doi: 10.1089/ars.2010.3711. [DOI] [PubMed] [Google Scholar]
- 155.Von Bernhardi R., Eugenin J. Alzheimer’s disease: Redox dysregulation as a common denominator for diverse pathogenic mechanisms. Antioxid. Redox Signal. 2012;16:974–1031. doi: 10.1089/ars.2011.4082. [DOI] [PubMed] [Google Scholar]
- 156.Echeverria V., Ducatenzeiler A., Chen C.H., Cuello A.C. Endogenous beta-amyloid peptide synthesis modulates cAMP response element-regulated gene expression in PC12 cells. Neuroscience. 2005;135:1193–1202. doi: 10.1016/j.neuroscience.2005.06.057. [DOI] [PubMed] [Google Scholar]
- 157.Igbavboa U., Johnson-Anuna L.N., Rossello X., Butterick T.A., Sun G.Y., Wood W.G. Amyloid beta-protein1-42 increases cAMP and apolipoprotein E levels which are inhibited by beta1 and beta2-adrenergic receptor antagonists in mouse primary astrocytes. Neuroscience. 2006;142:655–660. doi: 10.1016/j.neuroscience.2006.06.056. [DOI] [PubMed] [Google Scholar]
- 158.Prapong T., Uemura E., Hsu W.H. G protein and cAMP-dependent protein kinase mediate amyloid beta-peptide inhibition of neuronal glucose uptake. Exp. Neurol. 2001;167:59–64. doi: 10.1006/exnr.2000.7519. [DOI] [PubMed] [Google Scholar]
- 159.Palavicini J.P., Wang H., Bianchi E., Xu S., Rao J.S., Kang D.E., Lakshmana M.K. RanBP9 aggravates synaptic damage in the mouse brain and is inversely correlated to spinophilin levels in Alzheimer’s brain synaptosomes. Cell Death Dis. 2013;4:e667. doi: 10.1038/cddis.2013.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Marambaud P., Ancolio K., Alves da Costa C., Checler F. Effect of protein kinase A inhibitors on the production of Abeta40 and Abeta42 by human cells expressing normal and Alzheimer’s disease-linked mutated betaAPP and presenilin 1. Br. J. Pharmacol. 1999;126:1186–1190. doi: 10.1038/sj.bjp.0702406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bekris L.M., Yu C.E., Bird T.D., Tsuang D.W. Genetics of Alzheimer disease. J. Geriatr. Psychiatry Neurol. 2010;23:213–227. doi: 10.1177/0891988710383571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Branca C., Wisely E.V., Hartman L.K., Caccamo A., Oddo S. Administration of a selective beta2 adrenergic receptor antagonist exacerbates neuropathology and cognitive deficits in a mouse model of Alzheimer’s disease. Neurobiol. Aging. 2014;35:2726–2735. doi: 10.1016/j.neurobiolaging.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dobarro M., Gerenu G., Ramirez M.J. Propranolol reduces cognitive deficits, amyloid and tau pathology in Alzheimer’s transgenic mice. Int. J. Neuropsychopharmacol. 2013;16:2245–2257. doi: 10.1017/S1461145713000631. [DOI] [PubMed] [Google Scholar]
- 164.Dang V., Medina B., Das D., Moghadam S., Martin K.J., Lin B., Naik P., Patel D., Nosheny R., Wesson Ashford J., et al. Formoterol, a long-acting beta2 adrenergic agonist, improves cognitive function and promotes dendritic complexity in a mouse model of Down syndrome. Biol. Psychiatry. 2014;75:179–188. doi: 10.1016/j.biopsych.2013.05.024. [DOI] [PubMed] [Google Scholar]
- 165.Peng J., Liang G., Inan S., Wu Z., Joseph D.J., Meng Q., Peng Y., Eckenhoff M.F., Wei H. Dantrolene ameliorates cognitive decline and neuropathology in Alzheimer triple transgenic mice. Neurosci. Lett. 2012;516:274–279. doi: 10.1016/j.neulet.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mikoshiba K. IP3 receptor/Ca2+ channel: From discovery to new signaling concepts. J. Neurochem. 2007;102:1426–1446. doi: 10.1111/j.1471-4159.2007.04825.x. [DOI] [PubMed] [Google Scholar]
- 167.Ross C.A., Meldolesi J., Milner T.A., Satoh T., Supattapone S., Snyder S.H. Inositol 1,4,5-trisphosphate receptor localized to endoplasmic reticulum in cerebellar Purkinje neurons. Nature. 1989;339:468–470. doi: 10.1038/339468a0. [DOI] [PubMed] [Google Scholar]
- 168.Furuichi T., Yoshikawa S., Miyawaki A., Wada K., Maeda N., Mikoshiba K. Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400. Nature. 1989;342:32–38. doi: 10.1038/342032a0. [DOI] [PubMed] [Google Scholar]
- 169.Mignery G.A., Sudhof T.C., Takei K., De Camilli P. Putative receptor for inositol 1,4,5-trisphosphate similar to ryanodine receptor. Nature. 1989;342:192–195. doi: 10.1038/342192a0. [DOI] [PubMed] [Google Scholar]
- 170.Foskett J.K., White C., Cheung K.H., Mak D.O. Inositol trisphosphate receptor Ca2+ release channels. Physiol. Rev. 2007;87:593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Inoue T., Kato K., Kohda K., Mikoshiba K. Type 1 inositol 1,4,5-trisphosphate receptor is required for induction of long-term depression in cerebellar Purkinje neurons. J. Neurosci. 1998;18:5366–5373. doi: 10.1523/JNEUROSCI.18-14-05366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Baker K.D., Edwards T.M., Rickard N.S. The role of intracellular calcium stores in synaptic plasticity and memory consolidation. Neurosci. Biobehav. Rev. 2013;37:1211–1239. doi: 10.1016/j.neubiorev.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 173.Sugawara T., Hisatsune C., Le T.D., Hashikawa T., Hirono M., Hattori M., Nagao S., Mikoshiba K. Type 1 inositol trisphosphate receptor regulates cerebellar circuits by maintaining the spine morphology of purkinje cells in adult mice. J. Neurosci. 2013;33:12186–12196. doi: 10.1523/JNEUROSCI.0545-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Egorova P.A., Bezprozvanny I.B. Inositol 1,4,5-trisphosphate receptors and neurodegenerative disorders. FEBS J. 2018;285:3547–3565. doi: 10.1111/febs.14366. [DOI] [PubMed] [Google Scholar]
- 175.Leissring M.A., Paul B.A., Parker I., Cotman C.W., LaFerla F.M. Alzheimer’s presenilin-1 mutation potentiates inositol 1,4,5-trisphosphate-mediated calcium signaling in Xenopus oocytes. J. Neurochem. 1999;72:1061–1068. doi: 10.1046/j.1471-4159.1999.0721061.x. [DOI] [PubMed] [Google Scholar]
- 176.Cedazo-Minguez A., Popescu B.O., Ankarcrona M., Nishimura T., Cowburn R.F. The presenilin 1 deltaE9 mutation gives enhanced basal phospholipase C activity and a resultant increase in intracellular calcium concentrations. J. Biol. Chem. 2002;277:36646–36655. doi: 10.1074/jbc.M112117200. [DOI] [PubMed] [Google Scholar]
- 177.Bezprozvanny I., Supnet C., Sun S., Zhang H., De Strooper B. Response to Shilling et al. (10.1074/jbc.M111.300491) J. Biol. Chem. 2012;287:20469. doi: 10.1074/jbc.L112.356790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mak D.O., Cheung K.H., Toglia P., Foskett J.K., Ullah G. Analyzing and Quantifying the Gain-of-Function Enhancement of IP3 Receptor Gating by Familial Alzheimer’s Disease-Causing Mutants in Presenilins. PLoS Comput. Biol. 2015;11:e1004529. doi: 10.1371/journal.pcbi.1004529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Toglia P., Ullah G. The gain-of-function enhancement of IP3-receptor channel gating by familial Alzheimer’s disease-linked presenilin mutants increases the open probability of mitochondrial permeability transition pore. Cell Calcium. 2016;60:13–24. doi: 10.1016/j.ceca.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 180.Wang Z.J., Zhao F., Wang C.F., Zhang X.M., Xiao Y., Zhou F., Wu M.N., Zhang J., Qi J.S., Yang W. Xestospongin C, a Reversible IP3 Receptor Antagonist, Alleviates the Cognitive and Pathological Impairments in APP/PS1 Mice of Alzheimer’s Disease. J. Alzheimer’s Dis. 2019;72:1217–1231. doi: 10.3233/JAD-190796. [DOI] [PubMed] [Google Scholar]
- 181.Ronco V., Grolla A.A., Glasnov T.N., Canonico P.L., Verkhratsky A., Genazzani A.A., Lim D. Differential deregulation of astrocytic calcium signalling by amyloid-beta, TNFalpha, IL-1beta and LPS. Cell Calcium. 2014;55:219–229. doi: 10.1016/j.ceca.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 182.Grolla A.A., Sim J.A., Lim D., Rodriguez J.J., Genazzani A.A., Verkhratsky A. Amyloid-beta and Alzheimer’s disease type pathology differentially affects the calcium signalling toolkit in astrocytes from different brain regions. Cell Death Dis. 2013;4:e623. doi: 10.1038/cddis.2013.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.De Strooper B., Beullens M., Contreras B., Levesque L., Craessaerts K., Cordell B., Moechars D., Bollen M., Fraser P., George-Hyslop P.S., et al. Phosphorylation, subcellular localization, and membrane orientation of the Alzheimer’s disease-associated presenilins. J. Biol. Chem. 1997;272:3590–3598. doi: 10.1074/jbc.272.6.3590. [DOI] [PubMed] [Google Scholar]
- 184.Sherrington R., Rogaev E.I., Liang Y., Rogaeva E.A., Levesque G., Ikeda M., Chi H., Lin C., Li G., Holman K., et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature. 1995;375:754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- 185.Rogaev E.I., Sherrington R., Rogaeva E.A., Levesque G., Ikeda M., Liang Y., Chi H., Lin C., Holman K., Tsuda T., et al. Familial Alzheimer’s disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer’s disease type 3 gene. Nature. 1995;376:775–778. doi: 10.1038/376775a0. [DOI] [PubMed] [Google Scholar]
- 186.Levy-Lahad E., Wasco W., Poorkaj P., Romano D.M., Oshima J., Pettingell W.H., Yu C.E., Jondro P.D., Schmidt S.D., Wang K., et al. Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science. 1995;269:973–977. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- 187.Lu P., Bai X.C., Ma D., Xie T., Yan C., Sun L., Yang G., Zhao Y., Zhou R., Scheres S.H.W., et al. Three-dimensional structure of human gamma-secretase. Nature. 2014;512:166–170. doi: 10.1038/nature13567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bai X.C., Yan C., Yang G., Lu P., Ma D., Sun L., Zhou R., Scheres S.H.W., Shi Y. An atomic structure of human gamma-secretase. Nature. 2015;525:212–217. doi: 10.1038/nature14892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yang G., Zhou R., Shi Y. Cryo-EM structures of human gamma-secretase. Curr. Opin. Struct. Biol. 2017;46:55–64. doi: 10.1016/j.sbi.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 190.Lee M.K., Slunt H.H., Martin L.J., Thinakaran G., Kim G., Gandy S.E., Seeger M., Koo E., Price D.L., Sisodia S.S. Expression of presenilin 1 and 2 (PS1 and PS2) in human and murine tissues. J. Neurosci. 1996;16:7513–7525. doi: 10.1523/JNEUROSCI.16-23-07513.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wong P.C., Zheng H., Chen H., Becher M.W., Sirinathsinghji D.J., Trumbauer M.E., Chen H.Y., Price D.L., Van der Ploeg L.H., Sisodia S.S. Presenilin 1 is required for Notch1 and DII1 expression in the paraxial mesoderm. Nature. 1997;387:288–292. doi: 10.1038/387288a0. [DOI] [PubMed] [Google Scholar]
- 192.Donoviel D.B., Hadjantonakis A.K., Ikeda M., Zheng H., Hyslop P.S., Bernstein A. Mice lacking both presenilin genes exhibit early embryonic patterning defects. Genes Dev. 1999;13:2801–2810. doi: 10.1101/gad.13.21.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Beglopoulos V., Sun X., Saura C.A., Lemere C.A., Kim R.D., Shen J. Reduced beta-amyloid production and increased inflammatory responses in presenilin conditional knock-out mice. J. Biol. Chem. 2004;279:46907–46914. doi: 10.1074/jbc.M409544200. [DOI] [PubMed] [Google Scholar]
- 194.Zhang C., Wu B., Beglopoulos V., Wines-Samuelson M., Zhang D., Dragatsis I., Sudhof T.C., Shen J. Presenilins are essential for regulating neurotransmitter release. Nature. 2009;460:632–636. doi: 10.1038/nature08177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wolfe M.S., Xia W., Ostaszewski B.L., Diehl T.S., Kimberly W.T., Selkoe D.J. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and gamma-secretase activity. Nature. 1999;398:513–517. doi: 10.1038/19077. [DOI] [PubMed] [Google Scholar]
- 196.Borchelt D.R., Thinakaran G., Eckman C.B., Lee M.K., Davenport F., Ratovitsky T., Prada C.M., Kim G., Seekins S., Yager D., et al. Familial Alzheimer’s disease-linked presenilin 1 variants elevate Abeta1-42/1-40 ratio in vitro and in vivo. Neuron. 1996;17:1005–1013. doi: 10.1016/S0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- 197.Tomita T., Maruyama K., Saido T.C., Kume H., Shinozaki K., Tokuhiro S., Capell A., Walter J., Grunberg J., Haass C., et al. The presenilin 2 mutation (N141I) linked to familial Alzheimer disease (Volga German families) increases the secretion of amyloid beta protein ending at the 42nd (or 43rd) residue. Proc. Natl. Acad. Sci. USA. 1997;94:2025–2030. doi: 10.1073/pnas.94.5.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Scheuner D., Eckman C., Jensen M., Song X., Citron M., Suzuki N., Bird T.D., Hardy J., Hutton M., Kukull W., et al. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat. Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 199.Mehra R., Kepp K.P. Identification of Structural Calcium Binding Sites in Membrane-Bound Presenilin 1 and 2. J. Phys. Chem. B. 2020;124:4697–4711. doi: 10.1021/acs.jpcb.0c01712. [DOI] [PubMed] [Google Scholar]
- 200.Thinakaran G., Borchelt D.R., Lee M.K., Slunt H.H., Spitzer L., Kim G., Ratovitsky T., Davenport F., Nordstedt C., Seeger M., et al. Endoproteolysis of presenilin 1 and accumulation of processed derivatives in vivo. Neuron. 1996;17:181–190. doi: 10.1016/S0896-6273(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 201.Takeda T., Asahi M., Yamaguchi O., Hikoso S., Nakayama H., Kusakari Y., Kawai M., Hongo K., Higuchi Y., Kashiwase K., et al. Presenilin 2 regulates the systolic function of heart by modulating Ca2+ signaling. FASEB J. 2005;19:2069–2071. doi: 10.1096/fj.05-3744fje. [DOI] [PubMed] [Google Scholar]
- 202.Pack-Chung E., Meyers M.B., Pettingell W.P., Moir R.D., Brownawell A.M., Cheng I., Tanzi R.E., Kim T.W. Presenilin 2 interacts with sorcin, a modulator of the ryanodine receptor. J. Biol. Chem. 2000;275:14440–14445. doi: 10.1074/jbc.M909882199. [DOI] [PubMed] [Google Scholar]
- 203.Hayrapetyan V., Rybalchenko V., Rybalchenko N., Koulen P. The N-terminus of presenilin-2 increases single channel activity of brain ryanodine receptors through direct protein-protein interaction. Cell Calcium. 2008;44:507–518. doi: 10.1016/j.ceca.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 204.Rybalchenko V., Hwang S.Y., Rybalchenko N., Koulen P. The cytosolic N-terminus of presenilin-1 potentiates mouse ryanodine receptor single channel activity. Int. J. Biochem. Cell Biol. 2008;40:84–97. doi: 10.1016/j.biocel.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 205.Greotti E., Capitanio P., Wong A., Pozzan T., Pizzo P., Pendin D. Familial Alzheimer’s disease-linked presenilin mutants and intracellular Ca(2+) handling: A single-organelle, FRET-based analysis. Cell Calcium. 2019;79:44–56. doi: 10.1016/j.ceca.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 206.Baba-Aissa F., Raeymaekers L., Wuytack F., Dode L., Casteels R. Distribution and isoform diversity of the organellar Ca2+ pumps in the brain. Mol. Chem. Neuropathol. 1998;33:199–208. doi: 10.1007/BF02815182. [DOI] [PubMed] [Google Scholar]
- 207.Britzolaki A., Saurine J., Klocke B., Pitychoutis P.M. A Role for SERCA Pumps in the Neurobiology of Neuropsychiatric and Neurodegenerative Disorders. Adv. Exp. Med. Biol. 2020;1131:131–161. doi: 10.1007/978-3-030-12457-1_6. [DOI] [PubMed] [Google Scholar]
- 208.Jin H., Sanjo N., Uchihara T., Watabe K., St George-Hyslop P., Fraser P.E., Mizusawa H. Presenilin-1 holoprotein is an interacting partner of sarco endoplasmic reticulum calcium-ATPase and confers resistance to endoplasmic reticulum stress. J. Alzheimer’s Dis. 2010;20:261–273. doi: 10.3233/JAD-2010-1360. [DOI] [PubMed] [Google Scholar]
- 209.Krajnak K., Dahl R. A new target for Alzheimer’s disease: A small molecule SERCA activator is neuroprotective in vitro and improves memory and cognition in APP/PS1 mice. Bioorg. Med. Chem. Lett. 2018;28:1591–1594. doi: 10.1016/j.bmcl.2018.03.052. [DOI] [PubMed] [Google Scholar]
- 210.Zhao L., Ackerman S.L. Endoplasmic reticulum stress in health and disease. Curr. Opin. Cell Biol. 2006;18:444–452. doi: 10.1016/j.ceb.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 211.Hetz C., Papa F.R. The Unfolded Protein Response and Cell Fate Control. Mol. Cell. 2018;69:169–181. doi: 10.1016/j.molcel.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 212.Hoozemans J.J., Veerhuis R., Van Haastert E.S., Rozemuller J.M., Baas F., Eikelenboom P., Scheper W. The unfolded protein response is activated in Alzheimer’s disease. Acta Neuropathol. 2005;110:165–172. doi: 10.1007/s00401-005-1038-0. [DOI] [PubMed] [Google Scholar]
- 213.Hoozemans J.J., van Haastert E.S., Nijholt D.A., Rozemuller A.J., Eikelenboom P., Scheper W. The unfolded protein response is activated in pretangle neurons in Alzheimer’s disease hippocampus. Am. J. Pathol. 2009;174:1241–1251. doi: 10.2353/ajpath.2009.080814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Chang R.C., Suen K.C., Ma C.H., Elyaman W., Ng H.K., Hugon J. Involvement of double-stranded RNA-dependent protein kinase and phosphorylation of eukaryotic initiation factor-2alpha in neuronal degeneration. J. Neurochem. 2002;83:1215–1225. doi: 10.1046/j.1471-4159.2002.01237.x. [DOI] [PubMed] [Google Scholar]
- 215.Page G., Rioux Bilan A., Ingrand S., Lafay-Chebassier C., Pain S., Perault Pochat M.C., Bouras C., Bayer T., Hugon J. Activated double-stranded RNA-dependent protein kinase and neuronal death in models of Alzheimer’s disease. Neuroscience. 2006;139:1343–1354. doi: 10.1016/j.neuroscience.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 216.Kim H.S., Choi Y., Shin K.Y., Joo Y., Lee Y.K., Jung S.Y., Suh Y.H., Kim J.H. Swedish amyloid precursor protein mutation increases phosphorylation of eIF2alpha in vitro and in vivo. J. Neurosci. Res. 2007;85:1528–1537. doi: 10.1002/jnr.21267. [DOI] [PubMed] [Google Scholar]
- 217.Chami M., Gozuacik D., Lagorce D., Brini M., Falson P., Peaucellier G., Pinton P., Lecoeur H., Gougeon M.L., le Maire M., et al. SERCA1 truncated proteins unable to pump calcium reduce the endoplasmic reticulum calcium concentration and induce apoptosis. J. Cell Biol. 2001;153:1301–1314. doi: 10.1083/jcb.153.6.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Chami M., Oules B., Szabadkai G., Tacine R., Rizzuto R., Paterlini-Brechot P. Role of SERCA1 truncated isoform in the proapoptotic calcium transfer from ER to mitochondria during ER stress. Mol. Cell. 2008;32:641–651. doi: 10.1016/j.molcel.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Nishitsuji K., Tomiyama T., Ishibashi K., Ito K., Teraoka R., Lambert M.P., Klein W.L., Mori H. The E693Delta mutation in amyloid precursor protein increases intracellular accumulation of amyloid beta oligomers and causes endoplasmic reticulum stress-induced apoptosis in cultured cells. Am. J. Pathol. 2009;174:957–969. doi: 10.2353/ajpath.2009.080480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.O’Connor T., Sadleir K.R., Maus E., Velliquette R.A., Zhao J., Cole S.L., Eimer W.A., Hitt B., Bembinster L.A., Lammich S., et al. Phosphorylation of the translation initiation factor eIF2alpha increases BACE1 levels and promotes amyloidogenesis. Neuron. 2008;60:988–1009. doi: 10.1016/j.neuron.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Devi L., Ohno M. PERK mediates eIF2alpha phosphorylation responsible for BACE1 elevation, CREB dysfunction and neurodegeneration in a mouse model of Alzheimer’s disease. Neurobiol. Aging. 2014;35:2272–2281. doi: 10.1016/j.neurobiolaging.2014.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Chami L., Checler F. BACE1 is at the crossroad of a toxic vicious cycle involving cellular stress and beta-amyloid production in Alzheimer’s disease. Mol. Neurodegener. 2012;7:52. doi: 10.1186/1750-1326-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Salminen A., Kauppinen A., Suuronen T., Kaarniranta K., Ojala J. ER stress in Alzheimer’s disease: A novel neuronal trigger for inflammation and Alzheimer’s pathology. J. Neuroinflamm. 2009;6:41. doi: 10.1186/1742-2094-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Popugaeva E., Bezprozvanny I. STIM proteins as regulators of neuronal store-operated calcium influx. Neurodegener. Dis. Manag. 2018;8:5–7. doi: 10.2217/nmt-2017-0053. [DOI] [PubMed] [Google Scholar]
- 225.Secondo A., Bagetta G., Amantea D. On the Role of Store-Operated Calcium Entry in Acute and Chronic Neurodegenerative Diseases. Front. Mol. Neurosci. 2018;11:87. doi: 10.3389/fnmol.2018.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kraft R. STIM and ORAI proteins in the nervous system. Channels. 2015;9:245–252. doi: 10.1080/19336950.2015.1071747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Luik R.M., Wu M.M., Buchanan J., Lewis R.S. The elementary unit of store-operated Ca2+ entry: Local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J. Cell Biol. 2006;174:815–825. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ambudkar I.S., Ong H.L., Liu X., Bandyopadhyay B.C., Cheng K.T. TRPC1: The link between functionally distinct store-operated calcium channels. Cell Calcium. 2007;42:213–223. doi: 10.1016/j.ceca.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 229.Chen X., Sooch G., Demaree I.S., White F.A., Obukhov A.G. Transient Receptor Potential Canonical (TRPC) Channels: Then and Now. Cells. 2020;9:1983. doi: 10.3390/cells9091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Moccia F., Zuccolo E., Soda T., Tanzi F., Guerra G., Mapelli L., Lodola F., D’Angelo E. Stim and Orai proteins in neuronal Ca(2+) signaling and excitability. Front. Cell Neurosci. 2015;9:153. doi: 10.3389/fncel.2015.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wegierski T., Kuznicki J. Neuronal calcium signaling via store-operated channels in health and disease. Cell Calcium. 2018;74:102–111. doi: 10.1016/j.ceca.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 232.Heine M., Heck J., Ciuraszkiewicz A., Bikbaev A. Dynamic compartmentalization of calcium channel signalling in neurons. Neuropharmacology. 2020;169:107556. doi: 10.1016/j.neuropharm.2019.02.038. [DOI] [PubMed] [Google Scholar]
- 233.Sun S., Zhang H., Liu J., Popugaeva E., Xu N.J., Feske S., White C.L., 3rd, Bezprozvanny I. Reduced synaptic STIM2 expression and impaired store-operated calcium entry cause destabilization of mature spines in mutant presenilin mice. Neuron. 2014;82:79–93. doi: 10.1016/j.neuron.2014.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Tong B.C., Lee C.S., Cheng W.H., Lai K.O., Foskett J.K., Cheung K.H. Familial Alzheimer’s disease-associated presenilin 1 mutants promote gamma-secretase cleavage of STIM1 to impair store-operated Ca2+ entry. Sci. Signal. 2016;9:ra89. doi: 10.1126/scisignal.aaf1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ryazantseva M., Goncharova A., Skobeleva K., Erokhin M., Methner A., Georgiev P., Kaznacheyeva E. Presenilin-1 Delta E9 Mutant Induces STIM1-Driven Store-Operated Calcium Channel Hyperactivation in Hippocampal Neurons. Mol. Neurobiol. 2018;55:4667–4680. doi: 10.1007/s12035-017-0674-4. [DOI] [PubMed] [Google Scholar]
- 236.Linde C.I., Baryshnikov S.G., Mazzocco-Spezzia A., Golovina V.A. Dysregulation of Ca2+ signaling in astrocytes from mice lacking amyloid precursor protein. Am. J. Physiol. Cell Physiol. 2011;300:C1502–C1512. doi: 10.1152/ajpcell.00379.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Wang J., Lu R., Yang J., Li H., He Z., Jing N., Wang X., Wang Y. TRPC6 specifically interacts with APP to inhibit its cleavage by gamma-secretase and reduce Abeta production. Nat. Commun. 2015;6:8876. doi: 10.1038/ncomms9876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Lessard C.B., Lussier M.P., Cayouette S., Bourque G., Boulay G. The overexpression of presenilin2 and Alzheimer’s-disease-linked presenilin2 variants influences TRPC6-enhanced Ca2+ entry into HEK293 cells. Cell Signal. 2005;17:437–445. doi: 10.1016/j.cellsig.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 239.Prikhodko V., Chernyuk D., Sysoev Y., Zernov N., Okovityi S., Popugaeva E. Potential Drug Candidates to Treat TRPC6 Channel Deficiencies in the Pathophysiology of Alzheimer’s Disease and Brain Ischemia. Cells. 2020;9:2351. doi: 10.3390/cells9112351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Giorgi C., Missiroli S., Patergnani S., Duszynski J., Wieckowski M.R., Pinton P. Mitochondria-associated membranes: Composition, molecular mechanisms, and physiopathological implications. Antioxid. Redox Signal. 2015;22:995–1019. doi: 10.1089/ars.2014.6223. [DOI] [PubMed] [Google Scholar]
- 241.Area-Gomez E., de Groof A., Bonilla E., Montesinos J., Tanji K., Boldogh I., Pon L., Schon E.A. A key role for MAM in mediating mitochondrial dysfunction in Alzheimer disease. Cell Death Dis. 2018;9:335. doi: 10.1038/s41419-017-0215-0. [DOI] [PMC free article] [PubMed] [Google Scholar]