Abstract
Metabolomics is improving the understanding of the mechanisms of the health effects of diet. Previous research has identified several metabolites associated with the Mediterranean Diet (MedDiet), but knowledge about longitudinal changes in metabolic biomarkers after a MedDiet intervention is scarce. A subsample of 48 firefighters from a cluster-randomized trial at Indianapolis fire stations was randomly selected for the metabolomics study at 12 months of follow up (time point 1), where Group 1 (n = 24) continued for another 6 months in a self-sustained MedDiet intervention, and Group 2 (n = 24), the control group at that time, started with an active MedDiet intervention for 6 months (time point 2). A total of 225 metabolites were assessed at the two time points by using a targeted NMR platform. The MedDiet score improved slightly but changes were non-significant (intervention: 24.2 vs. 26.0 points and control group: 26.1 vs. 26.5 points). The MedDiet intervention led to favorable changes in biomarkers related to lipid metabolism, including lower LDL-C, ApoB/ApoA1 ratio, remnant cholesterol, M-VLDL-CE; and higher HDL-C, and better lipoprotein composition. This MedDiet intervention induces only modest changes in adherence to the MedDiet and consequently in metabolic biomarkers. Further research should confirm these results based on larger study samples in workplace interventions with powerful study designs.
Keywords: Mediterranean Diet, metabolites, clinical trial, lipoprotein composition, biomarkers
1. Introduction
Currently, the understanding of diet-health relationships has gradually shifted from individual dietary components to overall dietary patterns that beneficially modulate metabolic physiology [1]. In this regard, several epidemiological and clinical studies have shown that the traditional Mediterranean-style eating pattern (characterized by high intake of fruits and vegetables, olive oil, legumes, whole grains, and fish and moderate consumption of white meat, dairy and wine during meals) has many health benefits [2,3], including beneficial changes in biomarkers of CVD risk [4,5] and lower risk of cardiovascular disease (CVD) [6,7,8].
However, the exact mechanisms of the benefits of the Mediterranean-style dietary pattern have yet to be understood. The application of metabolomics to measure changes in biological variables in response to dietary interventions has been proposed as a potential tool to discover biomarkers associated with healthier eating [9,10,11,12,13]. There is observational epidemiological evidence that acylcarnitines, Trimethylamine N-oxide (TMAO), some amino acids such as phenylalanine, glutamate as well as several lipid classes are associated with CVD risk [14]. While the effects of individual dietary ingredients on metabolome have been reported [15,16,17,18,19,20], only a few studies have focused on overall dietary patterns [21,22,23,24,25,26]. Some cross-sectional studies such as the Whitehall II study showed that a healthy diet was associated with specific fatty acids that reduced the risk of CVD [27]. In a British population, the association between the adherence to the MedDiet and cardiometabolic outcomes was mediated by baseline levels of acylcarnitines, sphingolipids, and phospholipids [24]. In the Supplementation en Vitamines et Mineraux Antioxydants (SU.VI.MAX) study, some metabolites were also cross-sectionally associated with dietary recommendations [28]. More recently, a metabolic signature of the Mediterranean Diet has been consistently identified in two large cohorts [26].
Importantly, studies analyzing changes in metabolites levels in response to MedDiet interventions are still scarce and inconclusive. In the Metabolic Syndrome Reduction in Navarra (RESMENA)study, after 2 months of an energy restricted MedDiet intervention, some significant changes in metabolites were shown but they were no longer observed after 6 months [22]. Additional evidence comes from the Prevention with Mediterranean Diet (PREDIMED) study, where several a priori-designed analyses found that the MedDiet may reduce the deleterious effect on cardiovascular or type-2 diabetes risk associated with 1 year changes in branched-chain amino acids [29,30], carnitines [31] and other metabolites. However, in most of these analyses, the 1 year metabolite changes were similar between the intervention and the control group, suggesting that the observed protective effect of the MedDiet could be due to other mechanisms.
Therefore, the objective of this study is to identify changes in plasma metabolic biomarkers associated with a MedDiet intervention within a subsample of a cluster-randomized controlled trial (Feeding America’s Bravest) among firefighters. We hypothesize that the MedDiet intervention induces changes in metabolites within clinically relevant pathways.
2. Materials and Methods
2.1. Study Design and Participants
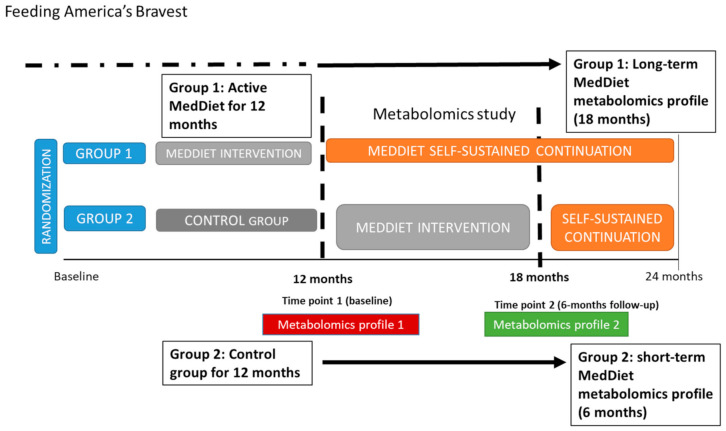
The overall study design, intervention strategies and primary outcomes of the Feeding America’s Bravest trial have been previously reported [32]. Briefly, Feeding America’s Bravest is a step-wedge cluster-randomized controlled trial within the 44 stations of Indianapolis Fire Department, which aims to compare a MedDiet Nutritional Intervention vs. an ad libitum Midwestern-style diet (control or no intervention) during 12 months. Group 1 (or the intervention group for 12 months) continued under a self-sustained continuation phase for another 12 months. Group 2 (initially controls) crossed over (at 12 months) to receive the active Mediterranean Diet Nutritional Intervention for 6 months followed by another 6 months of a self-sustained intervention phase. For this nested study, we randomly selected a sub-group of participants (n = 48) whose fire stations had been assigned to the MedDiet intervention (n = 24) or the control group (n = 24) for the previous 12 months. At that time (time point 1 for our study and 12 months follow up of the parent study), the intervention group (Group 1) continued under a self-sustained phase for another 6 months (time point 2 for our study or 18 months of the parent study) and the control group underwent the active MedDiet intervention for 6 months. Plasma metabolic biomarkers were analyzed at the two time points (Figure 1). The overarching Feeding America’s Bravest protocol was approved by the Harvard Institutional Review Board (IRB16-0170) and is registered at Clinical Trials (NCT02941757). All participants provided informed consent for participation.
Figure 1.
Study design and timeline of the metabolomics study within Feeding America´s Bravest parent study (step-wedge cluster-randomized control trial).
2.2. Mediterranean Diet Intervention
At the two time points of this study, data on sociodemographic characteristics, physical activity, sleep behaviors (a modified Pittsburgh Sleep Quality Index, where on a scale from 0 to 7 participants were asked to identify the statement which option best described their habitual level of physical activity over the past month [33]), food consumption based on a self- reported validated food frequency questionnaire [34] and a modified Mediterranean Diet scale [35], and anthropometric and clinical variables were assessed.
The MedDiet intervention has been described in detail elsewhere [32]. Briefly, it consisted of educational sessions and videos created by a certified nutritionist, leaflet and recommendations about the Mediterranean Dietand lifestyle, firefighter-tailored Mediterranean recipes (by modifying Firefighters´ favorite recipes according to the MedDiet principles by a chef and a nutritionist), in-site chef cooking demonstration, a firefighters’ food pyramid, Mediterranean food samples and discount coupons to a large supermarket chain for specific Mediterranean Diet-compatible foods [32].
Adherence to the MedDiet was assessed by the validated modified Mediterranean Diet Score (mMDS) [35,36] and the PREDIMED score. (36) The mMDS range from 0 (lowest) to 51 (highest conformity to the MedDiet) [36] and consists of 13 domains including consumption of fast food, fruits, vegetables, legumes, nuts, sweet desserts, fried foods, ocean fish, breads and starches (consumed at home and the fire station), the type and frequency of alcoholic beverages, non-alcoholic beverages (consumed at home and the fire station) and the type of cooking oil or fat (consumed at home and the fire station). Each component ranged from 0 (less adherence) to 4 points (better adherence) except the type of alcohol (wine; 0–2 points) and the type of cooking oil or fat (0–5 points). Weighted scores was considered for domains evaluated at both the homes and fire stations based on the percentage of meals consumed at each location [36].
The PREDIMED score [37] consists of 14 questions; 12 of them are about food consumption frequency (olive oil consumption, vegetables, fruits, red/processed meats, butter/margarine, soda drinks, wine, legumes, fish/seafood, nuts, commercial sweets, sofrito consumption), and another two about food intake habits considered characteristic of the Spanish Mediterranean Diet (preference of poultry consumption instead of red meats, use of olive oil as main culinary fat). Each question was scored 0 or 1, with a total possible range of 0 to 14; higher scores indicate greater adherence to the MedDiet.
2.3. Collection of Plasma Samples
In this nested study, 12 h fasting blood samples were collected at time point 1 (baseline for this metabolomics study and 12 months for the trial) and time point 2 (6 months follow up for this metabolomics study and 18 months for the trial) of follow up; samples were kept cold and immediately processed to separate the plasma with a refrigerated centrifuge. Next, the 200 µL cryovials were placed at −80 °C.
2.4. Plasma Biomarkers Measurements
Metabolites were quantified in plasma samples from 83 individuals that had optimal values using high-throughput proton Nuclear Magnetic Resonance (NMR) metabolomics (Nightingale Health Ltd., Helsinki, Finland). This method provides simultaneous quantification of routine lipids, lipoprotein subclass profiling with lipid concentrations within 14 subclasses, fatty acid composition, and various low-molecular metabolites including amino acids, ketone bodies and glycolysis-related metabolites. Details of the experimentation and applications of the NMR metabolomics platform have been described previously [38]. All measured metabolites fall in the range of detection; representative coefficients of variations (CVs) for the metabolic biomarkers were published previously [39,40].
2.5. Statistical Analysis
Differences in baseline characteristics between the two groups were examined by t-test ANOVA for continuous variables (means ± standard deviation [SD]) or Chi-square for categorical data (percentages). Statistical analyses of the metabolites were performed on log-transformed data that were scaled to SD units to facilitate comparisons across metabolites. The effect of the MedDiet intervention between point 1 and point 2 in this analysis (6 months) was assessed using a linear mixed-effects model adjusted for age and sex. As many of the metabolites are biologically correlated, applying a multiple testing correction using all the 225 biomarkers would be too strict. Thus, account for multiple testing, Bonferroni correction was applied with significance level defined as 0.05/21 = 0.002, with 21 being the number of principal components that explained 99% of the variation in the NMR data.
Cross-sectional linear associations between the adherence to the mMDS score and metabolite concentrations at both time points for both the MedDiet intervention and control groups were obtained using a linear regression model adjusted for age and sex. The analyses were performed using ggforestplot R package (version 0.0.2) and the linear mixed-effects model was fitted with the nlme R package (version 3.1.-144).
3. Results
3.1. Participant Characteristics
Characteristics of the 48 participants at time point 1, and changes after 6 months follow up (time point 2), are summarized in Table 1. Of the 48 participants at time point 1, 44 (92%) were followed up and provided dietary information and anthropometric measures at time point 2. Information for the plasma metabolites were available for n = 47 at baseline and n = 36 at the end of follow up (Supplementary Materials Figure S1). There were no statistically significant differences in the adherence to the mMDS or PREDIMED score at time 1 and after the 6 months follow up within and between groups. Similarly, no differences were seen for age or sex (Table 1).
Table 1.
Characteristics of the firefighters participating in the Feeding America’s Bravest intervention study.
| Time Point 1 (n = 48) | Time Point 2 (n = 44 *) | |||||||
|---|---|---|---|---|---|---|---|---|
| 12 Months MedDiet Intervention (n = 24) | Control Group (n = 24) | p-Value | 18 Months MedDiet Intervention (n = 22) | Control Group After a 6 Months of Active MedDiet Intervention (n = 22) | p-Value | p-Value (Follow Up-Baseline) Intervention Group | p-Value (Follow Up-Baseline) Control Group | |
| Sex, male (%) | 91.7 | 95.8 | 0.55 | 84.6 | 94.1 | 0.39 | N/A | N/A |
| Age (years) | 47.5 (6.7) | 47.6 (8.6) | 0.95 | 45.9 (6.7) | 49.9 (8.4) | 0.17 | N/A | N/A |
| PREDIMED score (0–14 points) | 6.1 (2.1) | 6.6 (2.1) | 0.31 | 6.4 (1.9) | 6.7 (1.9) | 0.64 | 0.48 | 0.64 |
| mMDs score (0–51 points) | 24.2 (6.5) | 26.1 (4.9) | 0.27 | 26.0 (6.5) | 26.5 (5.6) | 0.81 | 0.23 | 0.52 |
| Fast food consumption (0–4 points) | 2.8 (0.94) | 3 (0.61) | 0.47 | 2.76 (0.83) | 2.69 (0.63) | 0.79 | 0.06 | 0.13 |
| Fruit (0–4 points) | 1.56 (0.61) | 1.65 (0.79) | 0.70 | 1.62 (0.56) | 1.71 (0.69) | 0.693 | 0.62 | 0.71 |
| Vegetable (0–4 points) | 1.8 (0.93) | 2.11 (0.69) | 0.314 | 1.70 (0.85) | 2.06 (0.56) | 0.16 | 0.07 | 0.11 |
| Sweet desserts (0–4 points) | 1 (0.69) | 1.24 (0.75) | 0.340 | 0.85 (0.69) | 1.35 (0.61) | 0.04 | 0.05 | 0.84 |
| Cooking oil or fat use at home (0–5 points) | 3.0 (2.20) | 2.88 (1.99) | 0.57 | 3.85 (1.77) | 3.59 (1.87) | 0.70 | 0.07 | 0.56 |
| Fried food consumption (0–4 points) | 0.12 (0.47) | 0.35 (0.78) | 0.27 | 0.46 (0.88) | 0.71 (0.98) | 0.49 | 0.33 | 0.33 |
| Breads and starches at home (0–4 points) | 2.70 (1.82) | 2.39 (1.97) | 0.63 | 1.15 (1.80) | 1.41 (1.62) | 0.92 | 0.07 | 0.21 |
| Ocean fish (0–4 points) | 0.78 (0.88) | 0.53 (0.72) | 0.37 | 0.39 (0.87) | 0.65 (0.86) | 0.42 | 0.09 | 0.33 |
| Non-alcoholic beverage at home | 2.61 (1.72) | 3.06 (1.39) | 0.41 | 2.85 (1.41) | 3.11 (1.45) | 0.61 | 0.12 | 0.38 |
| Alcoholic beverages(0–4 points) | 1.06 (1.26) | 0.94 (1.14) | 0.78 | 1.31 (1.43) | 1.12 (1.22) | 0.61 | 1.0 | 0.58 |
| Wine (0–2 points) | 1.58 (0.51) | 1.89 (0.31) | 0.03 | 1.61 (0.51) | 1.82 (0.39) | 0.22 | 0.35 | 0.33 |
| Legumes (0–4 points) | 3.05 (0.87) | 3.06 (0.87) | 0.99 | 3.15 (1.28) | 2.53 (1.12) | 0.17 | 0.04 | 0.04 |
| Nuts (0–4 points) | 2.40 (1.09) | 2.6 (0.9) | 0.57 | 2.69 (1.11) | 2.47 (0.71) | 0.61 | 0.09 | 0.29 |
Pairwise comparisons over time include only those participants with complete information at baseline and at the end of follow up (n = 23). * At the end of follow up, 44 participants provided information about diet, n = 36 plasma serum and diet information, and 30 participants were assessed for anthropometric and other cardiovascular risk factors (Figure S1). Thus, mMDS and PREDIMED included n = 44 for the analysis at the end of follow up and for the comparisons.
3.2. The 6 Month Effect of the MedDiet Intervention
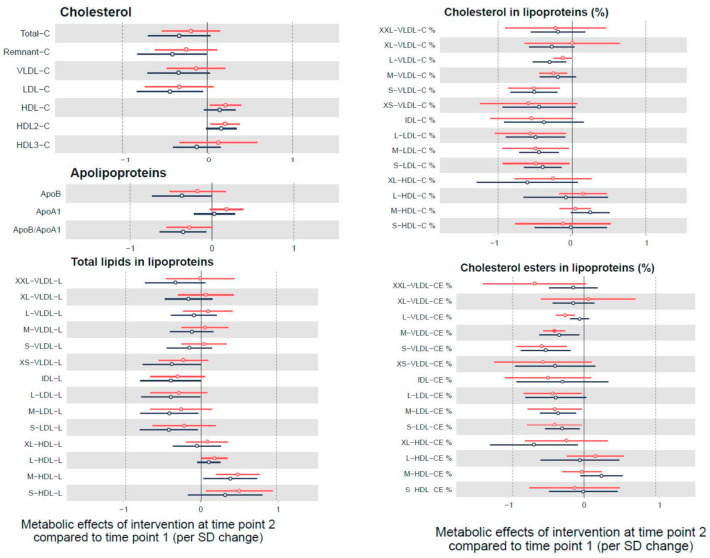
Figure 2 shows the 6 months effects of the intervention in the most relevant metabolites pathways. Data on the effect of the intervention in all the metabolites studied by group are presented in Table S1. The main subgroups affected by the intervention were the lipids and lipoproteins. Specifically, we observed a reduction in LDL-C, ApoB/ApoA1 ratio, remnant cholesterol, and higher HDL-C, and other subfractions such as lower cholesterol in L-VLDL-C, S-VLDL-C, L-LDL-C, M-LDL-C, S-LDL-C lipoproteins, and the composition of the lipoproteins after 6 months of intervention. Of note, these associations did not reach statistical significance after correcting the p-values for multiple testing (except for a decrease in M-VLDL-CE and an increase in lactate).
Figure 2.
Metabolic effects of the intervention after 6 months follow up (time point 2) compared to baseline (time point 1) (linear mixed models adjusted by age and sex). Results show changes by SD in each metabolite per unit change in mMDS score and are displayed by hollow points. Only those significant results (after correction of multiple testing) are indicated by filled points along with their 95% confidence intervals. In black is shown Group 1 (the intervention group for 12 months) that continued under a self-sustained continuation phase for another 6 months. In red is shown Group 2 (control) that received the active Mediterranean Diet Nutritional Intervention for 6 months.
3.3. Cross-Sectional Association between Mediterranean Diet Adherence and Biomarkers
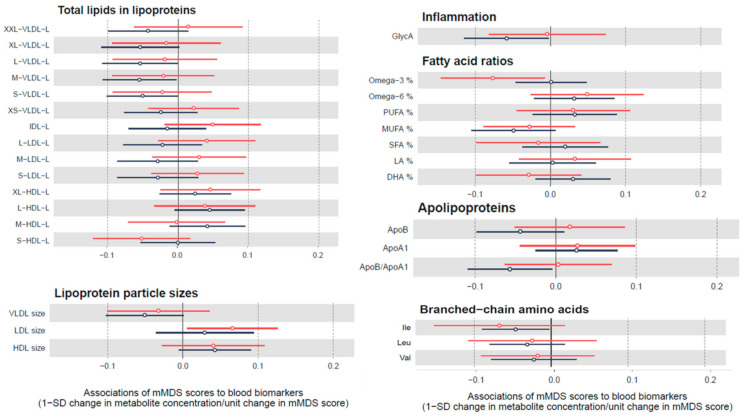
We also examined the cross-sectional linear association between biomarkers and adherence to the MedDiet at point 1 and 2 of this study, regardless of the participant’s group at baseline and follow up. Results were similar at both time points, although somewhat higher effect sizes where observed at time 2 where all participants had received at least 6 months of the MedDiet intervention (Figure 3). A 1 unit difference in mMDS score was associated with lower total lipids in lipoproteins of different sizes (VLDL, LDL) and ApoB/ApoA1 ratio, lower concentrations of a marker of inflammation (Glyc A), lower concentrations of branched chain amino acids and higher polyunsaturated fatty acids (PUFAs) and Docosahexaenoic acid (DHA) (Figure 3). We further included all the participants grouped together and analyzed the association between unit changes in the MedDiet score and SD changes in metabolic markers. We found a similar pattern in the results, but not significant, with a tendency to higher lipoprotein particle size with higher MedDiet scores (Figure S2).
Figure 3.
Cross-sectional association between biomarkers and adherence to the MedDiet for all participants at time point 1 and time point 2. Red lines show the results for the participants at baseline and black lines for the participants at follow up. Results show changes by SD in each biomarker per unit change in mMDS score and are displayed by hollow points along with their 95% confidence intervals.
Similar results were found when we used the PREDIMED score instead of the mMDS (Figure S3).
4. Discussion
In this sub-study of firefighters in Indianapolis participating in a cluster-randomized MedDiet intervention trial, we found that the MedDiet intervention was associated with favorable changes in markers of cardiovascular risk, specifically those related to the lipid metabolism (cholesterol, lipid composition, or cholesterol esters in the VLDL, IDL, and LDL lipoprotein subclasses, and ApoB/ApoA ratio) that were non-significant after correcting for multiple testing (except for a decrease in M-VLDL-CE). When the adherence to the MedDiet was measured with a self-reported scale (mMDS), the direction of the association with metabolites was similar at both time points (baseline and 6 months after the follow up).
Our results highlighting the changes in plasma metabolites related to lipid metabolism are in line with other studies [20,26]. A recent investigation that identified a metabolic signature of adherence to the MedDiet showed that out of 67 metabolites, 45 were lipids followed by 19 amino acids, 2 vitamins and 1 xenobiotic [26]. Although we used a different methodology and a different set of biomarkers, and thus we could not replicate this metabolic signature, our results support that the MedDiet may induce changes in relevant lipid species and subclasses related to atherogenic risk. In fact, the MedDiet is high in healthy fats (>35–40% of the total energy) mostly from monounsaturated fatty acids (MUFAs) (olive oil mostly) and PUFAs (from nuts and fish), and therefore the results are not surprising. In the firefighter population, we previously reported good correlation between nutrient intake from the food frequency questionnaire and the corresponding plasma biomarkers (omega-3, Eicosapentaenoic acid (EPA), and DHA) [36]. In line with these results, we found that changes in the MedDiet scores showed some tendency to be associated with fatty acids (an increase in PUFA% specifically DHA% in the expense of MUFA%). Although olive oil is a main component of the MedDiet, previous research found that higher consumption of this oil was linked to changes in omega 3, but not MUFA concentrations [36,41,42]. In addition, the average olive oil consumption in the firefighters is only approximately 0.5 tbsp/day, which is similar to other US cohorts [43] but much lower than in a Spanish cohort (4 tbsp/day) [44]. Nonetheless, it looks like changes in omega 3 to fatty acid ratio, PUFA to FA ratio and DHA to FA ratio increase with changes in the adherence to the MedDiet. This is in line with other studies [20,36], and suggests that those biomarkers could serve as indicators of adherence to the MedDiet.
We found that the MedDiet intervention induced a decrease in total cholesterol, remnant-C, VLDL-C and LDL-C and an increase in HDL-C and HDL2-C. Many studies have already demonstrated the effect of the MedDiet on total lipid metabolism, especially reducing total cholesterol and increasing HDL-C [45]. For example, the PREDIMED study, a randomized control trial, found that those in the MedDiet intervention (with olive oil or nuts) over 6 months had an increase in HDL-C but not a reduction in LDL-C [45]. Other studies support that replacing dietary saturated fatty acids (SFAs) with PUFA reduces the plasma LDL-C and subsequently the risk of cardiovascular disease [46,47,48].
In our study, we also observed a decrease in large, medium and small LDL fractions such as total lipids, cholesterol, particle concentration or cholesterol esters. Similarly, the MedDiet intervention decreased total cholesterol and cholesterol esters in the large, medium and small VLDL. Literature shows that VLDL concentrations are related directly or indirectly in the development of atherosclerosis [49]; for example the fatty acid composition of VLDL is critical for the activity of lipoprotein lipase and the formation of proatherogenic LDL and VLDL remnants [50]. In the FINRISK cohort, increased risk of cardiovascular disease was associated with all VLDL, IDL, and LDL subclasses, while the L- and M-HDL subclasses were associated with lower risk [51]. Despite the evidence of the role of these metabolites in CVD development, few studies have studied the effect of a MedDiet intervention in different lipids composition of lipoproteins or its subfractions. Interestingly, our results on lipid subfractions agree with a recent publication using the same metabolomic approach, where 47 participants were randomized to a SFA-rich diet, a MUFA-rich diet or a MED diet for 8 weeks. Additionally, in another study, compared to the control group, those participants that replaced SFAs with PUFAs reduced the lipoprotein particle concentration [52]. Finally, olive oil consumption modifies the lipid composition of VLDL [53] as well as the lipoprotein subfractions [54].
In our study, the effect was consistently shown in both groups, usually being stronger in the group undergoing a longer MedDiet intervention/exposure (MedDiet intervention + a self-sustained continuation phase), suggesting that the MedDiet induces favorable changes in metabolites related to CVD disease while the adherence to the MedDiet is sustained. For example, in our study, the ApoB/ApoA1 ratio was decreased in both groups after the intervention and also by adherence to the mMDS, which agrees with other short-term randomized trial with the MedDiet supplemented with olive oil, suggesting that these ratios may predict CVD beyond conventional lipid measures [55]. Finally, we found a significant association with lactate, a metabolite that was previously shown to increase the diabetes risk in the PREDIMED study [56]. However, we found that it occurs in the opposite direction.
Study Limitations
This was a pilot study with a small sample size. Possibly because of this, most associations lost statistical significance after correcting for multiple testing. However, results were in line with previous research, suggesting that a larger sample size would have retrieved significant results. In addition, the fact that we did not find differences in the mMDS adherence between groups may reflect selection bias since this is a sub-study within 400 firefighters participating in the Feeding America’s Bravest trial, and participants willing to participate could potentially be healthier and more health conscious. Moreover, the control group had higher scores of mMDS at baseline and their scores were slightly improved during the intervention; however, the results were consistent in the cross-sectional analysis. We only adjusted for age and sex, since it was a randomized study with no significant differences between this sub-study and the parent study for the rest of the variables which suggests the need to perform a study that includes larger metabolites. In addition, we did not analyze other metabolites included in other studies nor at baseline for the parent study. In this pilot trial, Group 2 could be considered as the intervention group but Group 1 was not a pure control group because they already finished an active MedDiet intervention and began their self-sustained MedDiet phase. The changes in biomarkers in Group 1 (between time point 1 and 2) are more likely to reflect both residual effects of the MedDiet and continued effects from self-sustained diet intervention. Additionally, most of our participants were male and thus generalizability should be explored. In any case, the results in this study should be corroborated in larger clinical studies with longer follow up due to the pilot study nature and with a powerful study design.
5. Conclusions
This MedDiet intervention induces only modest changes in adherence to the MedDiet and consequently in metabolic biomarkers related to lipid metabolism. Further research should confirm these results based on larger study samples in workplace interventions with powerful study designs.
Acknowledgments
We appreciate the participation of the Indianapolis Fire Departments as well as the firefighters and their spouses. We also thank Noora Kanerva and Heli Julkunen for their help with data analysis, as well as the Indiana Clinical and Translational Science Institute for helping with sample processing, the Kroger Company (coupons and customer loyalty discounts), Barilla America (Barilla Plus Products), Arianna Trading Company, Innoliva and Molino de Zafra, Spain (extra virgin olive oil samples and discounts) and the Almond Board of California (free samples of roasted unsalted almonds) for study support. The sponsors had no involvement in the overall study design; collection, analysis and interpretation of data; writing of the report; or the decision to submit the report for publication.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6643/12/12/3610/s1, Figure S1: Flowchart of participants at time point 1 and 2 of the Feeding America’s Bravest trial, Figure S2: Associations of unit changes in mMDS score by SD changes in plasma biomarkers between month 6 (time point 2) and baseline (time point 1) in all participants grouped together, Figure S3: Association between biomarkers and the PREDIMED score adherence (cross-sectional analysis), Table S1: The 6 months effect of the Mediterranean Diet intervention on metabolic biomarkers (linear mixed model analysis with p-values corrected for multiple testing).
Author Contributions
All authors have participated sufficiently and meaningfully to the research study and the preparation of this manuscript. M.S.-P. formulated the study question and design, interpreted the results, and drafted the manuscript. M.S.-P. and M.R.-C. were involved in statistical modeling. M.S.-P., S.N.K., Y.S. and S.M. contributed to the conception and design of the study and acquisition of the data. All authors contributed to the interpretation of data and critical revision of the manuscript and approved the final version. M.S.P., M.R.-C. and S.N.K. share primary responsibility for the final content. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by EMW-2014-FP-00612, US Department of Homeland Security, a Ohio University OURC grant and a CHSP Research Innovation grant, and the 2018 Southeast Center for Integrated Metabolomics Pilot & Feasibility Project. M.S.-P. holds a Ramón y Cajal contract (RYC-2018-025069-I) from the Ministry of Science, Innovation and Universities and FEDER/FSE and FIS grant PI20/00896. The funding agencies had no role in study design, data collection and analysis, interpretation of results, manuscript preparation or in the decision to submit this manuscript for publication
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cespedes E.M., Hu F.B. Dietary patterns: From nutritional epidemiologic analysis to national guidelines. Am. J. Clin. Nutr. 2015;101:899–900. doi: 10.3945/ajcn.115.110213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martínez-González M.A., Gea A., Ruiz-Canela M. The Mediterranean Diet and Cardiovascular Health. Circ. Res. 2019;124:779–798. doi: 10.1161/CIRCRESAHA.118.313348. [DOI] [PubMed] [Google Scholar]
- 3.Dinu M., Pagliai G., Casini A., Sofi F. Mediterranean diet and multiple health outcomes: An umbrella review of meta-analyses of observational studies and randomised trials. Eur. J. Clin. Nutr. 2017 doi: 10.1016/j.numecd.2016.11.055. [DOI] [PubMed] [Google Scholar]
- 4.Godos J., Zappala G., Bernardini S., Giambini I., Bes-Rastrollo M., Martinez-Gonzalez M. Adherence to the Mediterranean diet is inversely associated with metabolic syndrome occurrence: A meta-analysis of observational studies. Int. J. Food Sci. Nutr. 2017;68:138–148. doi: 10.1080/09637486.2016.1221900. [DOI] [PubMed] [Google Scholar]
- 5.Mattei J., Sotos-Prieto M., Bigornia S.J., Noel S.E., Tucker K.L. The Mediterranean Diet Score Is More Strongly Associated with Favorable Cardiometabolic Risk Factors over 2 Years Than Other Diet Quality Indexes in Puerto Rican Adults. J. Nutr. 2017;147:661–669. doi: 10.3945/jn.116.245431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosato V., Temple N.J., La Vecchia C., Castellan G., Tavani A., Guercio V. Mediterranean diet and cardiovascular disease: A systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2017 doi: 10.1007/s00394-017-1582-0. [DOI] [PubMed] [Google Scholar]
- 7.Sotos-Prieto M., Bhupathiraju S.N., Mattei J., Fung T.T., Li Y., Pan A., Willett W.C., Rimm E.B., Hu F.B. Changes in Diet Quality Scores and Risk of Cardiovascular Disease Among US Men and Women. Circulation. 2015;132:2212–2219. doi: 10.1161/CIRCULATIONAHA.115.017158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sotos-Prieto M., Bhupathiraju S.N., Mattei J., Fung T.T., Li Y., Pan A., Willett W.C., Rimm E.B., Hu F.B. Association of Changes in Diet Quality with Total and Cause-Specific Mortality. N. Engl. J. Med. 2017;377:143–153. doi: 10.1056/NEJMoa1613502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guasch-Ferré M., Bhupathiraju S.N., Hu F.B. Use of Metabolomics in Improving Assessment of Dietary Intake. Clin. Chem. 2018;64:82–98. doi: 10.1373/clinchem.2017.272344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brennan L., Hu F.B. Metabolomics Based Dietary Biomarkers in Nutritional Epidemiology-Current Status and Future Opportunities. Mol. Nutr. Food Res. 2018:1701064. doi: 10.1002/mnfr.201701064. [DOI] [PubMed] [Google Scholar]
- 11.Hardin D.S. Validating dietary intake with biochemical markers. J. Am. Diet. Assoc. 2009;109:1698–1699. doi: 10.1016/j.jada.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Perez I., Posma J.M., Gibson R., Chambers E.S., Hansen T.H., Vestergaard H., Hansen T., Beckmann M., Pedersen O., Elliott P., et al. Objective assessment of dietary patterns by use of metabolic phenotyping: A randomised, controlled, crossover trial. Lancet Diabetes Endocrinol. 2017;5:184–195. doi: 10.1016/S2213-8587(16)30419-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scalbert A., Brennan L., Manach C., Andres-Lacueva C., Dragsted L.O., Draper J., Rappaport S.M., van der Hooft J.J., Wishart D.S. The food metabolome: A window over dietary exposure. Am. J. Clin. Nutr. 2014;99:1286–1308. doi: 10.3945/ajcn.113.076133. [DOI] [PubMed] [Google Scholar]
- 14.Ruiz-Canela M., Hruby A., Clish C.B., Liang L., Martinez-Gonzalez M.A., Hu F.B. Comprehensive Metabolomic Profiling and Incident Cardiovascular Disease: A Systematic Review. J. Am. Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.005705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheung W., Keski-Rahkonen P., Assi N., Ferrari P., Freisling H., Rinaldi S., Slimani N., Zamora-Ros R., Rundle M., Frost G., et al. A metabolomic study of biomarkers of meat and fish intake. Am. J. Clin. Nutr. 2017;105:600–608. doi: 10.3945/ajcn.116.146639. [DOI] [PubMed] [Google Scholar]
- 16.Papandreou C., Hernández-Alonso P., Bulló M., Ruiz-Canela M., Yu E., Guasch-Ferré M., Toledo E., Dennis C., Deik A., Clish C., et al. Plasma Metabolites Associated with Coffee Consumption: A Metabolomic Approach within the PREDIMED Study. Nutrients. 2019;11:32. doi: 10.3390/nu11051032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernández-Alonso P., Papandreou C., Bulló M., Ruiz-Canela M., Dennis C., Deik A., Wang D.D., Guasch-Ferré M., Yu E., Toledo E., et al. Plasma Metabolites Associated with Frequent Red Wine Consumption: A Metabolomics Approach within the PREDIMED Study. Mol. Nutr. Food Res. 2019;63:e1900140. doi: 10.1002/mnfr.201900140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt J.A., Rinaldi S., Ferrari P., Carayol M., Achaintre D., Scalbert A., Cross A.J., Gunter M.J., Fensom G.K., Appleby P.N., et al. Metabolic profiles of male meat eaters, fish eaters, vegetarians, and vegans from the EPIC-Oxford cohort. Am. J. Clin. Nutr. 2015;102:1518–1526. doi: 10.3945/ajcn.115.111989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edmands W.M.B., Beckonert O.P., Stella C., Campbell A., Lake B.G., Lindon J.C., Holmes E., Gooderham N.J. Identification of human urinary biomarkers of cruciferous vegetable consumption by metabonomic profiling. J. Proteome Res. 2011;10:4513–4521. doi: 10.1021/pr200326k. [DOI] [PubMed] [Google Scholar]
- 20.Michielsen C.C.J.R., Hangelbroek R.W.J., Feskens E.J.M., Afman L.A. Disentangling the Effects of Monounsaturated Fatty Acids from Other Components of a Mediterranean Diet on Serum Metabolite Profiles: A Randomized Fully Controlled Dietary Intervention in Healthy Subjects at Risk of the Metabolic Syndrome. Mol. Nutr. Food Res. 2019;63:e1801095. doi: 10.1002/mnfr.201801095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin Q., Black A., Kales S.N., Vattem D., Ruiz-Canela M., Sotos-Prieto M. Metabolomics and Microbiomes as Potential Tools to Evaluate the Effects of the Mediterranean Diet. Nutrients. 2019;11:207. doi: 10.3390/nu11010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bondia-Pons I., Martinez J.A., de la Iglesia R., Lopez-Legarrea P., Poutanen K., Hanhineva K., Zulet M.d.l.Á. Effects of short- and long-term Mediterranean-based dietary treatment on plasma LC-QTOF/MS metabolic profiling of subjects with metabolic syndrome features: The Metabolic Syndrome Reduction in Navarra (RESMENA) randomized controlled trial. Mol. Nutr. Food Res. 2015;59:711–728. doi: 10.1002/mnfr.201400309. [DOI] [PubMed] [Google Scholar]
- 23.Playdon M.C., Moore S.C., Derkach A., Reedy J., Subar A.F., Sampson J.N., Albanes D., Gu F., Kontto J., Lassale C., et al. Identifying biomarkers of dietary patterns by using metabolomics. Am. J. Clin. Nutr. 2017;105:450–465. doi: 10.3945/ajcn.116.144501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tong T.Y.N., Koulman A., Griffin J.L., Wareham N.J., Forouhi N.G., Imamura F. A Combination of Metabolites Predicts Adherence to the Mediterranean Diet Pattern and Its Associations with Insulin Sensitivity and Lipid Homeostasis in the General Population: The Fenland Study, United Kingdom. J. Nutr. 2020;150:568–578. doi: 10.1093/jn/nxz263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rebholz C.M., Lichtenstein A.H., Zheng Z., Appel L.J., Coresh J. Serum untargeted metabolomic profile of the Dietary Approaches to Stop Hypertension (DASH) dietary pattern. Am. J. Clin. Nutr. 2018;108:243–255. doi: 10.1093/ajcn/nqy099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J., Guasch-Ferré M., Chung W., Ruiz-Canela M., Toledo E., Corella D., Bhupathiraju S.N., Tobias D.K., Tabung F.K., Hu J., et al. The Mediterranean diet, plasma metabolome, and cardiovascular disease risk. Eur. Heart J. 2020 doi: 10.1093/eurheartj/ehaa209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akbaraly T., Würtz P., Singh-Manoux A., Shipley M.J., Haapakoski R., Lehto M., Desrumaux C., Kähönen M., Lehtimäki T., Mikkilä V., et al. Association of circulating metabolites with healthy diet and risk of cardiovascular disease: Analysis of two cohort studies. Sci. Rep. 2018;8:8620. doi: 10.1038/s41598-018-26441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lécuyer L., Dalle C., Micheau P., Pétéra M., Centeno D., Lyan B., Lagree M., Galan P., Hercberg S., Rossary A., et al. Untargeted plasma metabolomic profiles associated with overall diet in women from the SU.VI.MAX cohort. Eur. J. Nutr. 2020 doi: 10.1007/s00394-020-02177-5. [DOI] [PubMed] [Google Scholar]
- 29.Ruiz-Canela M., Guasch-Ferré M., Toledo E., Clish C.B., Razquin C., Liang L., Wang D.D., Corella D., Estruch R., Hernáez Á., et al. Plasma branched chain/aromatic amino acids, enriched Mediterranean diet and risk of type 2 diabetes: Case-cohort study within the PREDIMED Trial. Diabetologia. 2018;61:1560–1571. doi: 10.1007/s00125-018-4611-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruiz-Canela M., Toledo E., Clish C.B., Hruby A., Liang L., Salas-Salvadó J., Razquin C., Corella D., Estruch R., Ros E., et al. Plasma Branched-Chain Amino Acids and Incident Cardiovascular Disease in the PREDIMED Trial. Clin. Chem. 2016;62:582–592. doi: 10.1373/clinchem.2015.251710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guasch-Ferré M., Ruiz-Canela M., Li J., Zheng Y., Bulló M., Wang D.D., Toledo E., Clish C., Corella D., Estruch R., et al. Plasma Acylcarnitines and Risk of Type 2 Diabetes in a Mediterranean Population at High Cardiovascular Risk. J. Clin. Endocrinol. Metab. 2019;104:1508–1519. doi: 10.1210/jc.2018-01000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sotos-Prieto M., Cash S.B., Christophi C.A., Folta S., Moffatt S., Muegge C., Korre M., Mozaffarian D., Kales S.N. Rationale and design of feeding America’s bravest: Mediterranean diet-based intervention to change firefighters’ eating habits and improve cardiovascular risk profiles. Contemp. Clin. Trials. 2017;61:101–107. doi: 10.1016/j.cct.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 33.Jackson A.S., Blair S.N., Mahar M.T., Wier L.T., Ross R.M., Stuteville J.E. Prediction of functional aerobic capacity without exercise testing. Med. Sci. Sport. Exerc. 1990;22:863–870. doi: 10.1249/00005768-199012000-00021. [DOI] [PubMed] [Google Scholar]
- 34.Salvini S., Hunter D.J., Sampson L., Stampfer M.J., Colditz G.A., Rosner B., Willett W.C. Food-based validation of a dietary questionnaire: The effects of week-to-week variation in food consumption. Int. J. Epidemiol. 1989;18:858–867. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 35.Gong Y., Yang J., Farioli A., Korre M., Kales S.N. Modified Mediterranean Diet Score and Cardiovascular Risk in a North American Working Population. PLoS ONE. 2014;9:e87539. doi: 10.1371/journal.pone.0087539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sotos-Prieto M., Christophi C., Black A., Furtado J.D., Song Y., Magiatis P., Papakonstantinou A., Melliou E., Moffatt S., Kales S.N. Assessing Validity of Self-Reported Dietary Intake within a Mediterranean Diet Cluster Randomized Controlled Trial among US Firefighters. Nutrients. 2019;11:2250. doi: 10.3390/nu11092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schroder H., Fito M., Estruch R., Martinez-Gonzalez M.A., Corella D., Salas-Salvado J., Lamuela-Raventos R., Ros E., Salaverria I., Fiol M., et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J. Nutr. 2011;141:1140–1145. doi: 10.3945/jn.110.135566. [DOI] [PubMed] [Google Scholar]
- 38.Soininen P., Kangas A.J., Würtz P., Suna T., Ala-Korpela M. Quantitative serum nuclear magnetic resonance metabolomics in cardiovascular epidemiology and genetics. Circ. Cardiovasc. Genet. 2015;8:192–206. doi: 10.1161/CIRCGENETICS.114.000216. [DOI] [PubMed] [Google Scholar]
- 39.Kettunen J., Demirkan A., Würtz P., Draisma H.H.M., Haller T., Rawal R., Vaarhorst A., Kangas A.J., Lyytikäinen L.-P., Pirinen M., et al. Genome-wide study for circulating metabolites identifies 62 loci and reveals novel systemic effects of LPA. Nat. Commun. 2016;7:11122. doi: 10.1038/ncomms11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Würtz P., Kangas A.J., Soininen P., Lawlor D.A., Davey Smith G., Ala-Korpela M. Quantitative Serum Nuclear Magnetic Resonance Metabolomics in Large-Scale Epidemiology: A Primer on -Omic Technologies. Am. J. Epidemiol. 2017;186:1084–1096. doi: 10.1093/aje/kwx016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hebestreit K., Yahiaoui-Doktor M., Engel C., Vetter W., Siniatchkin M., Erickson N., Halle M., Kiechle M., Bischoff S.C. Validation of the German version of the Mediterranean Diet Adherence Screener (MEDAS) questionnaire. BMC Cancer. 2017;17:341. doi: 10.1186/s12885-017-3337-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barceló F., Perona J.S., Prades J., Funari S.S., Gomez-Gracia E., Conde M., Estruch R., Ruiz-Gutiérrez V. Mediterranean-style diet effect on the structural properties of the erythrocyte cell membrane of hypertensive patients: The Prevencion con Dieta Mediterranea Study. Hypertension. 2009;54:1143–1150. doi: 10.1161/HYPERTENSIONAHA.109.137471. [DOI] [PubMed] [Google Scholar]
- 43.Guasch-Ferré M., Hu F.B., Martínez-González M.A., Fitó M., Bulló M., Estruch R., Ros E., Corella D., Recondo J., Gómez-Gracia E., et al. Olive oil intake and risk of cardiovascular disease and mortality in the PREDIMED Study. BMC Med. 2014;12:78. doi: 10.1186/1741-7015-12-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guasch-Ferré M., Liu G., Li Y., Sampson L., Manson J.E., Salas-Salvadó J., Martínez-González M.A., Stampfer M.J., Willett W.C., Sun Q., et al. Olive Oil Consumption and Cardiovascular Risk in U.S. Adults. J. Am. Coll. Cardiol. 2020;75:1729–1739. doi: 10.1016/j.jacc.2020.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Estruch R., Martínez-González M.A., Corella D., Salas-Salvadó J., Ruiz-Gutiérrez V., Covas M.I., Fiol M., Gómez-Gracia E., López-Sabater M.C., Vinyoles E., et al. Effects of a Mediterranean-style diet on cardiovascular risk factors: A randomized trial. Ann. Intern. Med. 2006;145:1–11. doi: 10.7326/0003-4819-145-1-200607040-00004. [DOI] [PubMed] [Google Scholar]
- 46.Mensink R.P., Zock P.L., Kester A.D.M., Katan M.B. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: A meta-analysis of 60 controlled trials. Am. J. Clin. Nutr. 2003;77:1146–1155. doi: 10.1093/ajcn/77.5.1146. [DOI] [PubMed] [Google Scholar]
- 47.Sacks F.M., Lichtenstein A.H., Wu J.H.Y., Appel L.J., Creager M.A., Kris-Etherton P.M., Miller M., Rimm E.B., Rudel L.L., Robinson J.G., et al. Dietary Fats and Cardiovascular Disease: A Presidential Advisory From the American Heart Association. Circulation. 2017;136:e1–e23. doi: 10.1161/CIR.0000000000000510. [DOI] [PubMed] [Google Scholar]
- 48.Stone N.J., Robinson J.G., Lichtenstein A.H., Bairey Merz C.N., Blum C.B., Eckel R.H., Goldberg A.C., Gordon D., Levy D., Lloyd-Jones D.M., et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014;63:2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 49.Wilhelm M.G., Cooper A.D. Induction of atherosclerosis by human chylomicron remnants: A hypothesis. J. Atheroscler. Thromb. 2003;10:132–139. doi: 10.5551/jat.10.132. [DOI] [PubMed] [Google Scholar]
- 50.Liu J., Sempos C.T., Donahue R.P., Dorn J., Trevisan M., Grundy S.M. Non-high-density lipoprotein and very-low-density lipoprotein cholesterol and their risk predictive values in coronary heart disease. Am. J. Cardiol. 2006;98:1363–1368. doi: 10.1016/j.amjcard.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 51.Metabolite Profiling and Cardiovascular Event Risk: A Prospective Study of 3 Population-Based Cohorts—PubMed. [(accessed on 3 June 2020)]; doi: 10.1161/CIRCULATIONAHA.114.013116. Available online: https://pubmed.ncbi.nlm.nih.gov/25573147/?from_single_result=Metabolite+profiling+and+cardiovascular+event+risk%3A+a+prospective+study+of+3+population-based+cohorts. [DOI] [PMC free article] [PubMed]
- 52.Ulven S.M., Christensen J.J., Nygård O., Svardal A., Leder L., Ottestad I., Lysne V., Laupsa-Borge J., Ueland P.M., Midttun Ø., et al. Using metabolic profiling and gene expression analyses to explore molecular effects of replacing saturated fat with polyunsaturated fat-a randomized controlled dietary intervention study. Am. J. Clin. Nutr. 2019;109:1239–1250. doi: 10.1093/ajcn/nqy356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perona J.S., Cañizares J., Montero E., Sánchez-Domínguez J.M., Pacheco Y.M., Ruiz-Gutierrez V. Dietary virgin olive oil triacylglycerols as an independent determinant of very low-density lipoprotein composition. Nutrition. 2004;20:509–514. doi: 10.1016/j.nut.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 54.Damasceno N.R.T., Sala-Vila A., Cofán M., Pérez-Heras A.M., Fitó M., Ruiz-Gutiérrez V., Martínez-González M.-Á., Corella D., Arós F., Estruch R., et al. Mediterranean diet supplemented with nuts reduces waist circumference and shifts lipoprotein subfractions to a less atherogenic pattern in subjects at high cardiovascular risk. Atherosclerosis. 2013;230:347–353. doi: 10.1016/j.atherosclerosis.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 55.Solá R., Fitó M., Estruch R., Salas-Salvadó J., Corella D., de La Torre R., Muñoz M.A., López-Sabater M.d.C., Martínez-González M.-A., Arós F., et al. Effect of a traditional Mediterranean diet on apolipoproteins B, A-I, and their ratio: A randomized, controlled trial. Atherosclerosis. 2011;218:174–180. doi: 10.1016/j.atherosclerosis.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 56.Guasch-Ferré M., Santos J.L., Martínez-González M.A., Clish C.B., Razquin C., Wang D., Liang L., Li J., Dennis C., Corella D., et al. Glycolysis/gluconeogenesis- and tricarboxylic acid cycle-related metabolites, Mediterranean diet, and type 2 diabetes. Am. J. Clin. Nutr. 2020;111:835–844. doi: 10.1093/ajcn/nqaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.