Abstract
Selenium (Se) is a widely distributed trace element with dual (beneficial or toxic) effects for humans, animals, and plants. The availability of Se in the soil is reliant on the structure of the parental material and the procedures succeeding to soil formation. Anthropogenic activities affect the content of Se in the environment. Although plants are the core source of Se in animal and human diet, the role of Se in plants is still debatable. A low concentration of Se can be beneficial for plant growth, development, and ecophysiology both under optimum and unfavorable environmental conditions. However, excess Se results in toxic effects, especially in Se sensitive plants, due to changing structure and function of proteins and induce oxidative/nitrosative stress, which disrupts several metabolic processes. Contrary, Se hyperaccumulators absorb and tolerate exceedingly large amounts of Se, could be potentially used to remediate, i.e., remove, transfer, stabilize, and/or detoxify Se-contaminants in the soil and groundwater. Thereby, Se-hyperaccumulators can play a dynamic role in overcoming global problem Se-inadequacy and toxicity. However, the knowledge of Se uptake and metabolism is essential for the effective phytoremediation to remove this element. Moreover, selecting the most efficient species accumulating Se is crucial for successful phytoremediation of a particular Se-contaminated area. This review emphasizes Se toxicity in plants and the environment with regards to Se biogeochemistry and phytoremediation aspects. This review follows a critical approach and stimulates thought for future research avenues.
Keywords: abiotic stress, environmental pollution, oxidative stress, phytoremediation, Se bioavailability, trace element
1. Introduction
Selenium (Se) is a trace element present in small amounts found in almost all organisms. It is recognized both as beneficial and toxic, wherein the boundary between these two dual effects is very narrow and differs among plant species. The term selenium is derived from the Greek word “Selene”, which means moon [1,2]. Selenium is a member of the oxygen family (group 16), also called the chalcogens. The physiochemical characteristics of Se and sulfur (S) are very close, which results in non-specific binding of Se rather than S. These substitutions can disrupt the cell metabolism and alter the protein structures, causing toxicity [2]. Selenium distribution varies greatly throughout the globe [3]. In nature, Se hardly exists in elemental form and is found only in a few minerals [1]. The physical, chemical and biological mechanisms are responsible for the speciation of Se, which is determined mainly by the pH and redox state of the environment [4,5]. The uptake and metabolism of Se are also greatly varied in different soil and plant systems due to the heterogeneity of physiological and biochemical nature [6,7,8]. Naturally, it exists in copper (Cu) crystals, sulfides of Cu, lead (Pb), and gold (Au). Selenium is also a derivation of metallurgical engineering and becomes one of the significant environmental pollutants [2,9,10].
The importance of Se was described by Schwarz and Foltz [11] as an essential trace element in animal nutrition. They found the inclusion of Se in fodder crops blocked degenerative muscular disorder and chronic liver damage in mouse [2,11]. Later Reeves and Hoffman [12] suggested that the deficiency of Se in the human diet is the main reason for growth retardation, impaired bone metabolism, and abnormalities in thyroid function. Notably, certain areas of the world (e.g., Italy, Egypt, Turkey, Nepal) are Se-inadequate, whereas some are Se-toxic because of natural and anthropogenic events [13,14]. Thus, both Se-inadequacy and Se-toxicity are harmful to humans and animals [10]. According to the World Health Organization (WHO), Se dose in the human diet should be 50–55 μg day−1 [15,16,17]. In humans, Se-deficiency arises when its dietary intake is lower than 40 μg day−1, and chronic poisonous when the consumption exceeds 400 μg day−1 [18]. In livestock, the necessity of Se is 0.05–0.10 mg kg−1 dry forage, whereas the toxic dose in animal feed is 2–5 mg kg−1 dry forage [17]. Crops are one of the primary sources of Se for most of the organisms; therefore, Se-abundant crops could prevent Se-inadequacy [19,20].
It has been reported that Se supplementation in a relatively low amount can alleviate the negative impact of a variety of abiotic stresses in plants by improving growth and development [21]. For example, Se application via plant roots restricted the uptake and translocation of heavy metals/metalloids [22,23,24]. On the other hand, Se toxicity, impeding plant growth, development, and disturbing plant ecophysiology, causing chlorosis and necrosis, restricted growth, and reduced protein biosynthesis [25,26,27]. Selenium toxicity or selenosis can occur in two ways, i.e., forming seleno-proteins and inducing oxidative stress. For instance, Se concentration ≥2 mg kg−1 dry weight (DW) in Arabidopsis, is toxic and cause a 10% reduction in the biomass without visible symptoms [28]. Selenium toxicity is reliant on the age of the plant and the chemical form of this element. The lowest concentration of Se, causing a significant decrease in the biomass of cucumber (Cucumis sativus) was 20 µM for SeO32– and 80 µM for SeO42– [29]. Similarly, SeO32– (50 or 100 μM) reduced vegetative growth and disrupted reproductive development [30]. Moreover, Se (SeO32–, 0.1 and 0.5 µM) decreased δ-aminolevulinic acid (ALA) content in maize (Zea mays) [31]. Selenium was also found to interact with other toxic metal/metalloid and accelerate the toxic effects [32].
Many plants efficiently take up Se and could be implemented to remove Se from the contaminated areas by several phytoremediation approaches such as phytoextraction, phytovolatilization, and rhizofiltration [33,34]. For instance, owing to the high level of accumulation, Brassica napus and B. juncea have been used for the Se phytoextraction [35]. For Se volatilization, Astragalus bisulcatus (Se hyperaccumulator) were used to volatilize the Se from the contaminated environment [36]. Notably, among numerous plant species, B. oleracea and A. bisulcatus volatilize higher Se, afterward Medicago sativa and Solanum lycopersicum [37]. For Se rhizofiltration, Typha angustifolia grown in wetland environments has been described in the elimination of supplemented Se as SeO32– (89%) and SeO42– (46%) [38], whereas muskgrass (Chara canescens Desv. and Lois) eliminate approximately 70–75% Se from the aqueous condition [39]. In recent decades, a great progress has been made using genetically engineered plants to remove metals [40]. Although there has been much interest in the dual role of Se in plants, the detail mechanism of Se toxicity and its remediation has not been summarized.
Therefore, in this review, we have discussed the recent advances in the Se toxicity in the plants and environment in the light of the recent research endeavors and experimental evidence. Moreover, Se biogeochemistry and phytoremediation possibilities have also been summarized, which would stimulate thought for future research avenues.
2. Selenium Biogeochemistry
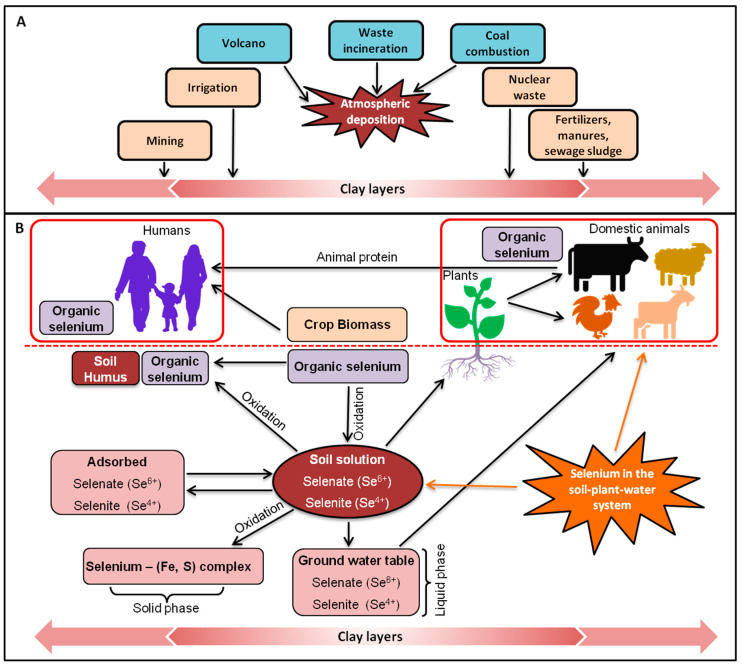
After the discovery in 1817 by Swedish chemist J.J. Berzelius, Se was considered a toxic element. Meanwhile, Se was found in all four compartments of the Earth, i.e., viz. atmosphere, hydrosphere, geosphere, and biosphere, ranked as the 67th abundant element on Earth (Figure 1) [41]. Moreover, it was placed 145th among the toxic and hazardous ingredients and 125th as the priority pollutant. Chemically Se resembles S and is coupled with the S depositions in the geosphere, such as coal [42]. The content of Se in coal ranged from 1–43 mg kg−1. The principal sources of terrestrial Se are Se rich minerals. Elemental Se or Se ore are very rare; nevertheless, Se can be combined with other elements, e.g., nickel (Ni), S, Cu, Ag, and Pb. Among the anthropogenic sources, industrial activities (pharmaceuticals production, ceramics factories, glass industry) mainly release Se [41].
Figure 1.
The schematic diagram for the mechanisms of Se-biogeochemistry in different Earth’s compartments. DMe-Se—dimethyl selenide; DMe-DSe—dimethyl diselenide; NOM—natural organic matter, OM—organic matter.
In Earth’s crust, sedimentary rocks contain a higher amount of Se than magmatic rocks. In contrast, organic-rich soils contain Se up to 600 mg kg−1 of soils and could adsorb more from water by silt and clay particles [43]. Naturally occurring 407 Se minerals are known, of which 259 are unique and divided into several classes (class I (elemental Se), class II (“sulfides”, includes 182 selenides), class IV (“oxides”, includes 49 selenites), and class VI (“sulfates”, includes four selenates)). Moreover, these Se containing minerals are classified according to their valences (−II, −I, 0, +IV, and +VI), for instance, metal selenide (Se–, Se2–), elemental Se (Se0), thioselenate (SSeO32–), selenite (SeO32–), selenate (SeO42–), respectively. Among the phases of Se, only one is recognized as organic, but yet not identified completely [44]. Hydrocarbons are also an important source of Se, but still, many remained undiscovered. In nature, physical (sorption effects of soils and sediments), chemical (pH, redox potential, organic matter content, and competitive ions), and biological (reduction, alkylation, dealkylation, and oxidation of Se by bacterial strains) mechanisms determined Se biogeochemistry (reactivity, mobility, and bioavailability) and are responsible for the speciation of this element [4,5].
2.1. Chemical Mechanisms Regulating Se Biogeochemistry
The availability of Se increases at higher pH. Environmental pH (soil or water) plays a vital role in the speciation of inorganic Se compounds. Selenium forms can remain oxidized or as diprotic acids (selenous and selenic acids) to maintain an acid-base equilibrium [44]. Under normal water pH (5.0–9.0), the dominant Se forms are HSeO3− and SeO32−, which remain in an equal fraction at pH 8.54 [45]. Further pH increases fully deprotonated, producing SeO42− (approximately 100%). The inorganic Se compounds are predominantly present in the anionic form (SeO42−, HSeO3−, and SeO32−), therefore, the bioavailability of Se will decrease with the decreasing pH [46]. In contrast, in strong acidic pH, Se-containing minerals dissolutes and releases Se; as a result, its mobility increases. Besides, adsorption by Fe-oxyhydroxides and co-precipitation with calcite abolishes the mobility of Se, thus abates Se-leaching under alkaline pH [47].
Redox potentially influences Se speciation too. Strong oxidizing conditions facilitate SeO42− formation, while mild oxidizing conditions enhance HSeO3− formation [46]. In contrast, elemental Se and volatile H2Se are predominant in strong reducing conditions. At strong reducing conditions and pH ≥ 4.0, HSe− shows dominancy while elemental Se remains insoluble and Se2− binds with metals [48].
Humic and fulvic acids of variable molecular weight contained by natural organic matter (NOM) bind Se to their functional groups containing nitrogen (N), S, and/or oxygen (O) [49]. Some reports suggest that Se concentration is positively correlated with NOM content. Interestingly, Se sorption by NOM is dependent on the NOM solubility, which is also ionic strength and pH-dependent [44]. Humic substances are also highly associated with Se, and upon adding SeO32− to the forest soil, Se was readily incorporated into the humic substances.
Competitive ions can reduce the availability of Se. Generally, an increase in pH was found to increase the sorption of metal cations on humic acid and immobilize SeO32−. Additionally, at low pH, the presence of Fe-oxyhydroxide immobilizes SeO32− [44]. Moreover, humic acids with redox potential from 0.4 to 0.8 V can reduce Cu(0), Cu(I), Sn(II), and U(IV) oxides, as well as Hg(II) and Fe(III), forming metal ion complexes and decrease the availability of Se [50].
2.2. Physical Mechanisms Regulating Se Biogeochemistry
Selenium bioavailability is affected by the mineral compositions of soils and sediments. Soil pH and the redox potential determine Se sorption on the aluminum (Al), iron (Fe), and manganese (Mn) oxyhydroxides. Only on the calcareous and the montmorillonite soils Se sorption was not affected at pH 2.0–9.0 along with 27 to 270 mg L−1 of SeO42−. Moreover, on the goethitic soils, SeO32− have weaker sorption, influenced by the pH and the SeO42− concentration. For example, a high SeO42− (300 mg L−1) concentration shows strong sorption at acidic pH, while lower near neutral. However, if the initial concentration of SeO42− is ≤30 mg L−1 no sorption was shown at pH 2.0–9.0. Notably, goethitic soils adsorbed SeO42− by a weak electrostatic attraction, which facilitates the plants to uptake Se easily. On the contrary, Fe and Al oxides of soils and sediments strongly adsorb SeO32−, where maximum sorption was observed at pH 3.0–4.0 and declined thereafter up to pH 8.0 [45,51].
Abiotic reactions could contribute to Se redox speciation, induced by sulfides, thiols, and ascorbic acid (AsA). For example, cysteine (Cys), can potentially reduce SeO32− to Se, which increases from pH 5.0–7.0, and decrease thereafter up to pH 9.0. On the contrary, cations (Mg2+ and Ca2+) can decrease the rate of Cys-induced SeO32− reduction by forming Cys-cations complexes [52]. Moreover, the bioavailability of sulfides influenced Se availability reversely. Similarly, the transformation of SeO32− to Se0 is influenced by the AsA level, which decreases from pH 2.0–5.5 affecting Se uptake by the plants. Moreover, temperature also affects the activation energy to reduce SeO32− to Se0 [52].
2.3. Biological Mechanisms Regulating Se Biogeochemistry
Microorganism-induced catalysis regulating Se speciation is the key biological mechanism for Se biogeochemistry, which affects the mobility and the bioavailability of Se [53,54]. Generally, assimilatory and disassimilatory reduction, alkylation, dealkylation, and oxidation are the primary transformation mechanisms for speciation of Se. Microbial reduction of SeO42− and SeO32− to Se is often used for bioremediation. Afterward, Se0 could further be reduced to Se−/Se2−, which is stable under reducing condition. However, these Se−/Se2− may react with metals (zinc, Zn and cadmium, Cd), forming highly insoluble metal-Se−/Se2− and reduce Se availability [55]. Moreover, alkylation leads to volatile dimethyl selenide (DMe-Se) and dimethyl diselenide (Dme–DSe) formation [56].
3. Selenium in the Environment
Selenium occurs in different environmental compartments in different forms (Figure 2). In nature, both organic (gaseous (DMe-Se, C2H6Se; DMe-DSe, C2H6Se2) and nongaseous (selenocysteine, Se-Cys; selenomethionine, Se-Met; Se-methylselenocysteine, SeMe-SeCys)) and inorganic (Se0, Se−, Se2−, SeO32−, and SeO42−) forms are found. On the other hand, the speciation of Se determines its accessibility and dispersal based on many features, like pH, NOM, microbial activity, redox potential, ionic elements, soil quality, temperature, and moisture [57,58].
Figure 2.
An overview of Se in the environment. (A) Sources of Se in the environment; (B) Selenium in the soil-plant-water consumer system. Plant roots take up Se (selenate or selenite) from the soil solution. The Se concentration in the soil solution depends on the solubility of the Se forms occurring and the biological transformations of organic forms. Modified from Hasanuzzaman et al. [59].
Naturally, Se exists in stratified rocks established during the Paleozoic era to the Cenozoic era [60]. The two anionic inorganic forms of Se (SeO32– and SeO42–) are readily soluble, moveable, bioavailable, and highly toxic. Organic Se originates mainly from the decaying of plants accumulating Se [60,61]. Globally, the mean Se level in soils varies from 0.1–0.7 mg kg−1, whereas clay soils contain 0.8–2 mg kg−1 and tropical soils 2–4.5 mg kg−1. The amount of Se in the soil depends on its texture, level of organic matter, and rainfall [62]. Notably, clay soils have higher Se content than coarse soils [63]. Volcanic soils and igneous rocks have a very low content of Se, found in mountainous countries like Finland, Scotland, and Sweden. Contrary sedimentary rocks are enriched with Se and incline to be mobile in rocks of the arid part climate, where it exerts harmful impacts on livestock [64,65].
Selenium content in plant originated food varies due to soil Se level, which depends on the ability of plants to accumulate Se, as well as the geomorphological area [2,9]. Se accumulation level differs remarkably, from 0.006 to 3.06 μg g−1 DW in inadequate regions (few areas of New Zealand, Sweden, and Canada; [9]). Usually, fruits accumulate lower amounts of Se than vegetables. Similarly, cereal crops accumulate Se in their seeds, particularly in the form of Se-Met and Se content varies from 0.01–0.55 μg g−1. Besides, Grasses normally have a high amount of Se compared to legumes, and cow milk and other farm products contain 0.001–0.17 μg Se g−1 fresh weight (FW) [66,67]. Additionally, Brazil nuts, Brassica spp. (cabbage, broccoli, and mustard, etc.), garlic, and Astragalus spp. accumulate a great amount of Se and a good source of Se in the diet [68,69,70,71].
Selenium enters in groundwater from sediments, soil wastes and sub-soils, containing Se. Moreover, Se level in groundwater increases due to excessive use of Se-enriched fertilizers for instance; Western European countries like Belgium and France (0.12 μg L−1 and 2.4–40 μg L−1, respectively, and some Se-enriched west regions of Punjab, India (341 μg L−1) [9,18,72]. Therefore, Se concentration in drinking water should not exceed 10 μg L−1, together optimum human intake ought not to exceed from 55–200 μg day−1 for adults [73]. Elsewhere, Se level in seawater varies from 4000 to 12,000 μg L−1 [18].
Environmental pollution by human activities (burning of papers, tires, and fossil fuels etc.) and natural phenomena (wildfire and soil erosion) also introduces Se into the atmosphere [18]. In the atmosphere, Se usually exists as volatile organic compounds, such as diethylmaleate-selenide (DEMSe), DMe-Se, DMe-DSe, methaneselenol (CH3Se), and inorganic selenium dioxide (SeO2). Besides, SeO2 is volatile and transformed to selenious acid (H2SeO3). The level of the Se in the atmosphere varies between 1–10 ng m−3 and particularly lower than water and soil [9].
4. Selenium Abundance: A Global Distribution
The relationship between Se level in various soil/plant types and human health have been widely studied [58,62,74,75,76,77,78,79]. Regarding humans, it has been described that the Se uptake by an individual and the Se status in the population is closely related to Se level in the soil and the edible plants cultivated in that particular area.
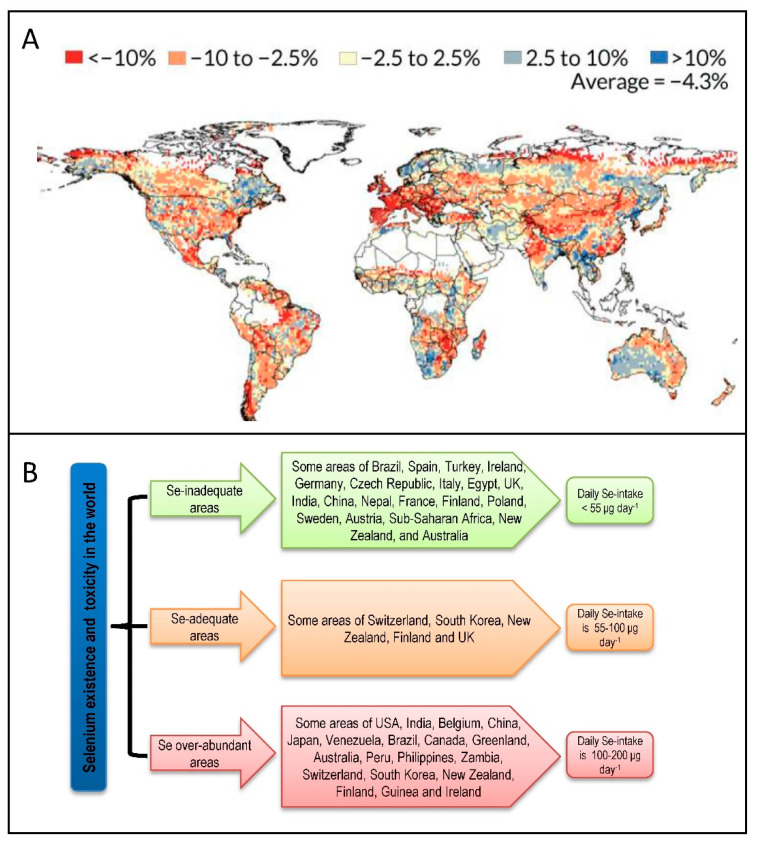
Selenium toxicity has been reported in several regions around the world, including west-central San Joaquin Valley of California, Colorado, Utah, Wyoming, and Idaho, US [80,81,82], Enshi district of Hubei province, China [83,84], Canada [85], Australia and New Zealand [73,86], West Bengal areas and northwestern Punjab regions, India [87,88]. On the other hand, in different regions, Se level is predicted to be decreased in the coming decades (Figure 3A).
Figure 3.
(A) Predicted percent changes in soil Se concentration from 1980–1999, 2080–2099. (B) Overview of Se occurrence and toxicity in various areas of the world based on human population and soil Se-availability. Source: LSA Honors Physics [89]; Hasanuzzaman et al. [21] (with permission from Elsevier).
Besides, many regions around the world are Se-inadequate. Countries such as France, Belgium, Brazil, Serbia, Slovenia, Spain, Portugal, Turkey, Poland, Germany, Denmark, United Kingdom, Slovakia, Austria, Ireland, Greece, Argentina, Italy, China, Nepal, Saudi Arabia, India, Czech Republic, Croatia, Egypt, Uruguay, Burundi, and New Guinea are accounted to have Se-inadequate areas (Figure 3B) [13,90,91,92,93]. Similarly, many regions are rich in Se, e.g., northwestern Punjab areas, India; Enshi district of Hubei, China; mountain and coastal communities, Japan; the Orinoco, Venezuela; western parts; Pakistan; western parts, Canada; Greenland; and Australia [72,83,86,94,95,96,97,98,99,100]. Almost 80% of the world’s total Se stocks are found in Canada, United States, Belgium, China, Peru, Chile, New Guinea, Zambia, Philippines, Zaire, and Australia [101].
Selenium malnutrition is a rising problem for humans globally, including Europe, Africa, Asia (mainly China), Australia, New Zealand, and southern US states. Almost forty countries documented having Se-inadequate regions, which is related to inadequate Se uptake (10 μg day−1) or even low in human, Clinical experimental reports suggested that good health is correlated with proper daily Se uptake, which can influence billions of people around the world. Selenium-deficiency can cause muscle weakness, pain/swelling or redness of joints and muscles, cancer susceptibility, erythrocytes fragility, unusual skin complexion, cardiomyopathy, Keshan, and Keshin-Beck diseases (KBD). For instance, 72% of the total area of China are Se-inadequate, where peoples are at risk of Keshan diseases, which is related to cardiomyopathy [102,103]. These problems can be solved by the development of Se-enriched foods (plants and products). On the other hand, animal health is also negatively influenced by Se-inadequacy; therefore, plant-based Se-supplementation may solve this problem for producing Se-enriched animal products [93]. However, the sufficient (0.05–0.10 mg kg−1) and toxic (4 to 5 mg kg−1) Se dose in the animal diet should be considered before administration [104]. For example, China has some highly Se-enriched soils, where they grow arable crops confirming sufficient regular Se intake by animals and humans [105,106].
On the other hand, Se toxicity has adverse effects in various regions around the globe. Meanwhile, Se toxicity involves kidney and liver failure, necrosis of heart and liver, nausea or vomiting, blood coagulation, loss of hair and nails, memory loss and tingling [28,107,108,109]. Therefore, it should be conscious regarding Se-concentration in food and drinking water.
5. Selenium Toxicity in Plants
5.1. Toxic Effects on Plant Growth and Development
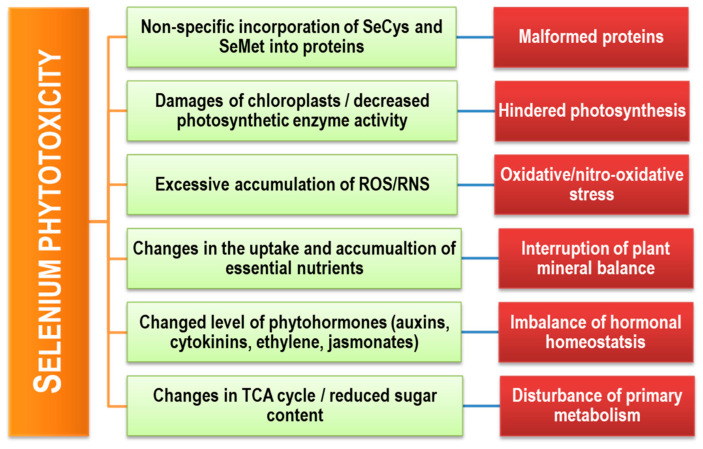
Selenium toxicity depends on the plant species, its age, and the availability of Se (Table 1; Figure 4) [30]. Young plants are much more sensitive to Se-toxicity than mature ones, and SeO32– is more phytotoxic than SeO42–. Selenate is as toxic as SeO32– only in the case of green algae Chlamydomonas reinhardtii (Figure 4) [110,111]. However, it has been suggested that Se is an essential element in this algal species because of the Se-dependent GSH peroxidase (GPX) component [112]. In the plant, higher toxicity of SeO32– than SeO42– is because of the faster incorporation of SeO32– into the Se-amino acids in roots after uptaken [113]. Previously, Hopper and Parker [114] reported that SeO32– was more toxic than SeO42– in perennial ryegrass and strawberry clover, and SeO32– preferentially inhibited the root growth, whereas SeO42– impeded shoot growth. In a recent study, Moreno et al. [115] reported that the maximum SeO32– concentration without growth inhibition for Raphanus sativus, Helianthus annuus, Medicago sativa, and Beta vulgaris to be 1, 10, 0.25, and 0.25 mg L−1, respectively. Notably, the uptake of SeO32– by roots and subsequent distribution within plants is slower than SeO42– [116].
Table 1.
Toxic effects of Se on the growth and physiological processes in plants.
| Plant Species | Form and Dose of Se | Negative Impact on Growth and Physiology | Reference |
|---|---|---|---|
| Arabidopsis thaliana | SeO32–; 50 or 100 μM | Se-induced secondary nitrooxidative stress. Decreased root growth and biomass (FW and DW). Reduced cell viability. Modified cell wall structure by modifying the pectin and callose. Decreased stomatal density and impaired stomatal regulations sensitive varieties were affected more than the tolerant. |
[26] |
|
Raphanus sativus, Helianthus annuus, Medicago sativa Beta vulgaris var. cicla |
SeO32–; 5 or 10 mg Se L–1 | Growth inhibition. | [115] |
| Pisum sativum cv. Petit Provençal | SeO32–; 50 or 100 μM | Altered vegetative and reproductive development. Shoot and root length and FW decreased. Chl a, chl b, chl a/b, total chl, total carotenoids content decreased. |
[30] |
| Cucumis sativus cv. Polan F1 | SeO42–; 80 µM SeO32–; 20 µM |
Decreased shoot root growth, biomass and leaf area. Impaired nutrient content. Reduced photosynthetic pigments accumulation and chl fluorescence. Increased lipid peroxidation. |
[117] |
| Oryza sativa | SeO32–; 100 g Se ha−1 | Increased Se content in root and shoot. Reduced photosynthesis and transpiration rate, and intercellular [CO2]. Impaired PSII quantum yield and diminished potential photosynthetic capacity. Reduced grain yield. |
[118] |
| Lactuca sativa var. capitata cv. Justyna | SeO42–; 20 µM SeO32–; 15 µM |
High accumulation of Se and S. Decreased biomass and leaf area. Reduced concentrations of photosynthetic pigments. Increased lipid peroxidation and H2O2 accumulation. |
[119] |
| Triticum aestivum | SeO42–; 100 μM | Reduction of PSII and PSI activities. | [120] |
| A. thaliana | SeO42–; 20 or 40 μM | Root growth inhibition. Loss of root apex cell viability and malformed root architecture. Reduction of primary root growth, an increase of lateral root growth. Decreased meristem cell activities. Hormonal imbalance. |
[121] |
| Spinacia oleracea cv. Missouri | SeO32–; 6 mg L−1 | Increased Se accumulation. Decreased growth parameters, e.g., shoot and root length, and FW and DW. Increased Na and Ca content, but decreased K content. |
[122] |
| Ulva sp. | SeO42–; 100 μM | Decreased level of chl and carotenoids. | [123] |
| Brassica juncea | SeO42–; 80 μM | Augmented Se and S concentration in different floral parts. Increased floral Se accumulation and impaired pollen germination. |
[124] |
| Lactuca sativa | SeO32– and SeO42–; 20 µM | Increased shoot Se concentration. Decreased P, S, Mg, Mn, and Fe concentrations. A slight reduction in shoot DW and yield. |
[125] |
| Hordeum vulgare | SeO42–; 2, 4, 8, or 16 ppm | Decreased plant height. Reduced chl concentrations. |
[126] |
| Stanleya albescens | SeO42–, 20 μM | Reduced growth. Chlorosis and impaired photosynthesis. Accumulation of the free amino acid selenocystathionine, a carbon-Se-carbon compounds (presumably selenocystathionine) together with some selenocysteine and selenate. |
[127] |
| Pteris vittata | SeO42–; 50 and 100 mg kg−1 in soil. | Suppressed uptake of Mg, K, P, Fe, Cu, and Zn. | [128] |
| Lactuca sativa var. capitata | SeO32–; 20 μM | Decreased productivity. Declined macronutrients accumulation in leaves. |
[129] |
| Zea mays | SeO32–; 50 and 100 µmol L−1 | Decreased DW accumulation. Root tolerance index severely decreased. |
[130] |
| Z. mays | SeO42– or selenomethionine (C5H11NO2Se); 100 µM |
High Se accumulation in root and shoot. Reduction in root and shoot FW. Altered anthocyanin level. Reduced chl level. |
[131] |
| Chlamydomonas reinhardtii | SeO32– and SeO42–; 4.5 ± 0.2 µM | Photosynthesis disorders. Ultrastructural damage. Inhibition and interruption of the photosynthetic electron transport chain. Growth inhibition. |
[111] |
Figure 4.
A schematic representation of the causes and consequences of Se toxicity in plants.
The toxicity thresholds for SeO32– and SeO42– in lettuce and cucumber were compared [29,119], where SeO32– was more toxic than SeO42– but both Se forms efficiently reduced roots biomass than shoots. In this context, a specific key role of the SULTR1;2 transporters regarding SeO42– sensitivity was found in Arabidopsis [132], which suggested that root growth, particularly root tip activity, might be a specific target of SeO42– toxicity in plants [132]. Furthermore, phytotoxic Se (both SeO32– and SeO42–) induced biomass reduction was accompanied by a corresponding decrease in leaf area [120]. Selenium supplemented irrigation water (10, 100, and 1000 µM SeO32–) did not affect the height of coffee plants, but the DW of roots and leaves, as well as the leaf area, was reduced, but the leaf thickness was increased at the maximum Se concentration [133]. Similarly, barley plants treated with different Se concentrations (2–16 ppm SeO42–) exhibited a reduction in plant height and chl content regardless of Se concentration, but the damaging effect was dose-dependent [126].
A positive correlation was found between the thickness, and specific root volume of lateral roots indicated the Se-inducted production of endogenous ethylene [121], which was suggested previously by Konze et al. [134]. They reported that two Se-amino acids, i.e., SeMet and selenoethionine, serve as ethylene precursors and enhanced ethylene production in auxin-treated Pisum sativum stem sections and senescing flower tissue of Ipomea tricolor. Moreover, Se toxicity induced cell viability inhibited root growth, and malformed root architecture, consequently reduced primary root elongation and facilitate lateral root growth [121]. In Brassica juncea, SeO42– and SeO32– (20 µM) treatment accumulated endogenous Se in floral parts and impaired pollen germination [124]. Moreover, Lehotai et al. [30] observed SeO32– (50 or 100 μM) reduced vegetative and inhibited reproductive development. In turn, SeO42– treatment (20 μM) altered hormonal balance, which alters morphological characteristics and modifies growth.
5.2. Toxic Effects on Physiological Processes
Selenium excess negatively affects several physiological and biochemical processes in plants. Among them, one of the foremost negative effects is the reduction of chl biosynthesis resulted in chlorosis. Saffaryazdi et al. [122] showed that SeO32– >1 mg L−1 in the nutrient solution decreased chl content in spinach, which might be related to lipoxygenase (LOX)-mediated lipid peroxidation and changes in the activity of antioxidant enzymes and/or negatively altered synthesis and activity of porphobilinogen synthetase (δ-aminolevulinate (ALA) dehydratase) required for chl biosynthesis [135]. Supporting this, Jain et al. [31] showed 0.1 and 0.5 µM SeO32– decreased ALA content in etiolated maize. Besides, Lehotai et al. [30] found an excess of Se diminished the chl a, chl b, chl a/b, total chl, and total carotenoid accumulation [30].
Selenite at concentrations >20 µM impaired the values of Fv/Fm, Fo, and Fm in hydroponically grown cucumber. However, SeO42– (2–80 µM) did not influence the chl fluorescence parameters [19]. A similar result was also postulated by Valkama et al. [136] using SeO42– (1 mg kg−1 soil) on. However, a high soil Se level increased sensitivity to enhanced UV-B radiation in strawberry. A high Se concentration increased quantum efficiency of PS II but decreased the number of leaves, biomass, and the starch/chloroplast area ratio, which was related to changes in the activity and/or biosynthesis of enzymes, rather than alteration of photosystem II (PSII [136]).
Both SeO32– and SeO42– (4.5 ± 0.2 µM) caused different toxic symptoms in Chlamydomonas reinhardtii, including ultrastructural damages of appressed domains of the chloroplast, subsequently interrupt photosynthetic electron chain, inhibit photosynthetic electron transport, and hindered photosynthesis [111]. Wheat exposed to 100 μM SeO42– showed decreased PSII and PSI system activities [120]. Moreover, accumulation of free amino acid selenocystathionine together with SeCys and SeO42– in SeO42– (20 μM) exposed Stanleya albescens, showed reduced growth, necrosis and chlorosis, and photosynthesis disorder [127]. In A. thaliana, Se-induced growth inhibition was attributed to decreased stomatal density and impaired stomatal regulation together with reduced cell viability [26].
Phytotoxic Se-induced growth reduction could be a consequence of disturbances in the balance of mineral nutrition. By modifying the uptake, accumulation, and transport of mineral nutrients, Se affects different biochemical reactions and physiological processes (growth, photosynthesis, respiration, gas exchange, water uptake, phloem unloading, and activation of protease inhibitor genes). In addition, Se could reduce or intensify the toxicity of essential or toxic elements by limiting or aggravating stresses induced by these elements. Kopsell et al. [137] observed decreased foliar concentrations of B, Fe, and P along with increased S and K in SeO42–-treated Brassica oleracea L. Similarly, Madagascar periwinkle, treated with both SeO32– and SeO42–, resulted in elevated Zn and Cu phytoaccumulation, and enhanced carbohydrate and alkaloids accumulation capacity, but this effect was more pronounced by SeO32– than SeO42– exposure [138].
Maize treated with SeO32– (5–100 µM) showed increased P and Ca but decreased K content [131]. However, Ca bioconcentration and an opposite P reduction was found in SeO42–-treated tall fescue and white clover [139]. Beyond, a synergic effect of Se and Fe was also found under Se exposure, where Fe concentration increased with the growing tissue Se concentration. The effect of Se was tested in a separate study using lettuce, which showed increased shoot Se concentrations, but decreased macronutrient accumulation, N, P, K, Ca, Mg, and S in leaves of lettuce together with growth reduction symptoms [129]. Besides, Se in the nutrient solution (10 and 20 mg L−1 SeO32) and to soil (50 and 100 mg kg−1 SeO32–) reduced bioaccumulation of Mg, K, P, Fe, Cu, and Zn in Pteris vittata [128].
Selenite-exposed maize treated or untreated with auxin (IAA) exhibited lower Mg content in the leaves and roots in comparison to mesocotyls. Moreover, Se supplementation increased the shoot Fe content [140]. In cucumber, 80 µM SeO42– is recognized as toxic, markedly reduced shoot K content but elevated amounts of Ca and S-SO4. In turn, SeO32– (>20 µM) caused a severe decrease in the P, K, Mg, Ca, and S-SO4 levels in shoots [117].
Selenium toxicity could be coupled with metal toxicity. In wheat and pea, Se (SeO32– and SeO42–) enhanced Cd and Cu uptake and toxicity and altered their distribution, especially in the SeO32–-treated peas. Besides, in wheat shoots, SeO42– elevated Cd bioaccumulation up to 50%, while Cd bioaccumulation increased up to 300% in pea roots by SeO32– supply [141]. In Brassica juncea, the use of SeO42– (50 mg Se L−1) and iodide (100 mg L−1) showed synergistic actions in reducing nitrate accumulation, enhancing flavonoid biosynthesis, increasing B and Al accumulation, and decreasing Sr and Cd bioconcentrations [142].
It was reported that the change in the transport abilities of some nutrient ions is one of the first observed symptoms of Se effects on plants [143]. It was found that SeO32– (2–10 µM) in the rooting medium inhibited root elongation in wheat, which is further enhanced by CaCl2, MgCl2, SrCl2 supplementation, along with pH reduction [144]. Moreover, these compounds raised the plasma membrane activity, enhancing Se uptake by roots. Yet, Bailey et al. [145] observed SO42–/SeO42– antagonism using SeO42– antagonist SO42– in wigeon grass. Similar SO42–/SeO42– antagonism has also been confirmed by White et al. [146] in Arabidopsis. The results of these studies show that increased levels of SO42– in the growing media increased FW and enhanced S accumulation, but reduced the Se content in shoots. On the contrary, the increase in the SeO42– concentration in the growth media increase both Se and S content in shoots but reduced shoot FW. However, the SO42–/SeO42– antagonism seems to be stronger than the PO42–/SeO32– antagonism [114]. Since SeO42– is transported across the cell membrane by high-affinity SO42– transporters, there is strong evidence that SeO42– directly competes with SO42– for uptake by plants, whereas PO42– transporters are involved in the SeO32– transport [147,148]. Therefore, the application of SeO32− increased the foliar concentration of S in lettuce shoots. Following the uptake, SeO32– is easily transformed into organic Se in roots, while SeO42– is quickly translocated and either metabolized or stored in plastids using the S metabolic pathway [19,91,148]. Both SeO42– and SeO32– are assimilated as their S analogs by the same pathway, leading to Se incorporation in almost all S metabolites. For that reason, Se non-accumulator plants contain higher amounts of Se in proteins than Se-accumulators [21].
The physicochemical differences between Se and S result in small but very significant changes in the biological properties of Se-substituted proteins. Although the Se-Se bond is longer and weaker but is more labile than S–S bonding and alters tertiary protein structure leading to catalytic malfunction function of enzymes. Due to its higher nucleophilicity, SeCys is more reactive than Cys, and replacement of Cys by SeCys induce Se-Se bridges preventing the formation of S-S bridges, thus altering redox potential and enzyme kinetics [149].
The Fe-S cluster proteins of the chloroplastic and mitochondrial electron transport chain are very much prone to SeCys replacement [150]. Due to its larger size, the Fe–Se cluster does not fit properly in proproteins. Hallenbeck et al. [151] found that the replacement of the Fe–S cluster with Fe–Se extremely reduced the activity of Klebsiella pneumoniae nitrogenase. Conversely, such substitution was beneficial for the activity of Citrus sinensis glutathione (GSH)-dependent peroxidase expressed in Escherichia coli [152]. Due to the crucial role of Cys residues in the protein structure, SeCys substitution is more detrimental than SeMet substitution [149]. Replacement of Met with SeMet disturbs protein synthesis via impairing the formation of the peptide bond [153]. However, SeCys having easy deprotonation capacity is more reactive than Cys and impaired protein function [154]. Coffee leaves supplied with toxic SeO32– (10 µM) showed an increased content of caffeine and soluble sugars as well as decrease photosynthetic pigments. Substantially lower nitrate reductase (NR) activity and disturbances in the free amino acid profile were found as well [133]. Moreover, Zhang et al. [155] demonstrated a high concentration of SeO32– inhibited the growth of chickpea sprouts and the biosynthesis of formononetin and biochanin an isoflavones.
5.3. Selenium-Induced Oxidative Stress in Plants
Being prooxidative, Se provokes oxidative stress. Different mechanisms have been described to explain Se-aggravated oxidative stress and its damaging effects in the plant cell (Figure 5; Table 2). Selenite restrains the ubiquitin-proteasome pathway and persuades the pentose phosphate pathway. Moreover, Se-induced inhibition of antioxidant defense provokes the overproduction of reactive oxygen species (ROS) [156]. Moreover, Se can directly react with several metabolites to generate ROS. The reaction of SeO32– with GSH causes O2•− generation and subsequent H2O2 overaccumulation. Therefore, excess Se causes depletion of GSH level and its activity, encouraging ROS production.
Figure 5.
Selenium-induced oxidative stress and consequent damage to the plant cell. The inhibition of the antioxidant defense system by Se excess provokes overproduction of reactive oxygen species (ROS). Excess of Se can directly react with several metabolites to generate ROS. Moreover, modified chloroplast and mitochondrial reactions under toxic Se concentrations cause ROS overproduction. Selenium causes nitric oxide-induced secondary nitrooxidative stress. Increasing lipoxygenase (LOX) activity under Se exposure generates peroxide radicals (LOO•) and inhibits the glyoxalase system causing methylglyoxal (MG) toxicity and subsequent oxidative stress.
Table 2.
Evidence for Se-induced oxidative stress in plants.
| Plant Species | Form and Concentration of Se | Indicators of Oxidative Stress and Changes in Antioxidant Enzymes Activities under Se Exposure | Reference |
|---|---|---|---|
| Arabidopsis thaliana | SeO32–; 50 or 100 μM | Distinct oxidative stress. Nitrosative modifications. Callose accumulation. Pectin accumulation. |
[26] |
| Pisum sativum | SeO32–; 50 or 100 μM | Increased H2O2 concentration in leaves and roots. Increased content of thiobarbituric acid reactive substances (TBARS). Altered GSH content, APX and CAT activities. Increased nitric oxide level in shoot and root. Nitric oxide-induced nitrooxidative stress by increasing peroxynitrite formation, as well as tyrosine nitration. |
[30] |
| Brassica rapa | SeO32–; 0.03–0.46 mM | Increased endogenous total ROS, O2•−, and enhanced lipid peroxidation. Loss of plasma membrane integrity in the roots. |
[157] |
| Triticum aestivum | SeO42–; 100 μM | Altered carbohydrates (soluble and starch) level. AsA and GSH contents were modified. Suppressed activities of SOD, APX, and GR. Higher generation of ROS. Augmented lipid peroxidation. Repressed PSII and PSI system activities. Modified redox status connected with Mn(II)/Mn(III), and semiquinone/quinone ratios. |
[120] |
| A. thaliana | SeO42–; 20 and 40 μM | Decreased NO content. Increased H2O2 content. Reduced cell viability. |
[121] |
| Vicia faba | SeO42–; 6 μM | Elevated lipid peroxidation and total -SH (T-SH) content. Increased GPX activity. Decreased guaiacol peroxidase (GPOX) activity. Increased O2•− production in the roots. Cell membrane injury and reduced cell viability. |
[158] |
| Stanleya pinnata | SeO42–; 40 and 80 μM | Oxidized proteins. Malformed or misfolded selenoproteins. |
[159] |
| Ulva sp. | SeO42–; 100 μM | Increased accumulation of H2O2. The activity of antioxidant enzymes such as SOD, CAT increased. Antioxidant metabolites including phenols, flavonoids, carotenoids, and gallic acid increased. |
[123] |
| A. thaliana | SeO42–; 20 μM | The cad2-1 mutant was recognized with a flawed GSH synthetic pathway that showed decreased root length, in contrast to the wild type. In the apr2-1 mutant, GSH depletion and ROS accretion were prominent. |
[160] |
| Hordeum vulgare | SeO42–; 4, 8 and 16 ppm | Increased membrane lipid peroxidation. Higher proline accumulation. Stimulated CAT, APX, GR, and glutathione-S-transferase (GST) activities. |
[126] |
| Stanleya albescens | SeO42–; 20 μM | Increased O2•− and H2O2 levels. Reduced AsA and GSH content. Declined radical-scavenging capacity. |
[127] |
| A. thaliana | SeO42–; 50 mM | Decreased GSH level. | [161] |
Selenium toxicity also induces reactive and malformed selenoproteins (SeCys/SeMet), thus alter redox potential, which distorts chloroplastic and mitochondrial enzyme kinetics [159]. Besides, Se toxicity disrupted chloroplast ultrastructure and function (photosystem and photoreactions) as well as upsetting mitochondrial functioning, causing ROS overgeneration. Selenite-induced overproduction of mitochondrial O2•− and consequently diminished aconitase activity activate the alternative oxidase pathway [150]. In turn, Se-induced peroxynitrite formation creates nitrosative stress. Therefore, nitrosative changes in protein (tyrosine nitration) occurs [30]. Yet, a Se-mediated increase in LOX activity generates lipid peroxide radicals (LOO•) [162]. The mechanisms accountable for Se-induced oxidative stress in plants are illustrated in Figure 5 and Table 2.
Both SeO42– and SeO32– induces ROS overaccumulation and oxidative stress in plant cells. In vitro investigations indicated that SeO32– reacts with GSH to produce O2•– [163]. Accordingly, Arabidopsis mutant vtc1 with defective AsA biosynthesis accumulated more O2•– and H2O2 under SeO32– exposure than wild-type plants, which indicated that the antioxidant properties of AsA reduce ROS accumulation in plants exposed to SeO32– [164]. Dimkovikj and Van Hoewyk [165] found SeO32–-induced quick formation of mitochondrial O2•− and consequent decrease in aconitase activity, which activated the alternative oxidase pathway. They also found an increased glucose concentration with higher respiratory rates and ATP levels. Selenite exposure also increases GSH concentrations along with elevated levels of γ-glutamyl cyclotransferase, which degrade Se metabolites conjugated to GSH.
At a high concentration, Se acts as a prooxidant. Hartikainen et al. [152] found an enhanced SOD activity and increased tocopherol content in ryegrass due to excess Se (>10 mg kg−1), which indicated the prooxidative activity of Se. They also observed excessive accumulation of toxic LOO•, which was scavenged by α-tocopherol to produce LOOH, and subsequently converted to less toxic LOH by enhanced GPX activity. Additionally, increased SOD activity prevents O2•− accumulation. Xue et al. [166] also observed similar results in leaves of both young and senescing lettuce plant, confirmed that excess Se diminished the antioxidative function, especially decreased activity of GPX and SOD. Moreover, Se bioaccumulation (both SeO32– and SeO42–) correlate positively with the GPX activity, where Se-dependent GPX activity was related particularly with the chemical form of Se rather than the Se bioconcentration [116].
Nowak et al. [167] demonstrated that higher Se concentrations (0.15 and 0.45 mM SeO32–) caused inhibition in the activity of antioxidant enzymes and provoked stress responses in wheat, where a slow Se concentration (0.05 mM) positively affected the antioxidant defense machinery, Contrary, excess Se-induced prooxidative effect may exert negative impact by reducing guaiacol peroxidase (GPOX) activity. In contrast, a low Se concentration stimulates GPOX activity, positively enhanced antioxidant defense [158,168]. In white clover and ryegrass, high Se concentrations promoted lipid peroxidation, increased shoot Se concentration as well as alters the activities of POD and APX [116,168]. Prooxidative high Se concentration (10 μM) exerted a toxic effect on cucumber also, by overgenerating ROS (O2•–, H2O2, HO•), resulted in enhanced lipid peroxidation and plasma membrane damage [156]. Oxidative stress caused by Se toxicity decreased respiration intensity, reduced chl content, decreased water balance, and accumulated free proline in common bean [169]. The authors also observed more severe lipid peroxidation compared to H2O2 damage accompanied by reduced activity of antioxidant enzymes (SOD, CAT, APX, and GR) and content of non-enzymatic antioxidants (AsA and GSH) [126,127,169].
Treatment with SeO32– (50 or 100 μM) was the source of nitrosative modifications in Arabidopsis. Distinctive oxidative stress, callose accumulation, pectin accumulation, etc. were well-recognized Se toxicity [26]. Similarly, SeO32– exposure (50 or 100 μM) modified the GSH content, APX, and CAT activities in Pisum sativum, where oxidative stress was the outcome of Se-induced nitric oxide-mediated secondary oxidative stress [30]. Selenium exerts nitro-oxidative stress by peroxynitrite overgeneration. Protein tyrosine nitration was also observed in Se affected plants [30]. On the other hand, SeO42– (100 μM) distorted carbohydrates metabolism in wheat along with declined AsA and GSH contents, as well as reduced activities of SOD, APX, and GR. Moreover, PSII and PSI activities were reduced together with distorted redox balance linked to Mn(II)/Mn(III), and semiquinone/quinone ratios under Se toxicity [120]. Mroczek-Zdyrska and Wójcik [158] also observed a similar result in Vicia faba plants exposed to SeO42– (6 μM) where severe oxidative stress was justified with higher O2•− production, lipid peroxidation, and cell membrane injury, while augmented GPX activity and diminished GPOX activity were evidenced in Se-stressed plants. Moreover, SeO42– treatment (40 and 80 μM) caused oxidation of proteins producing malformed or misfolded in selenoproteins of Stanleya pinnata [159]. The cad2-1 mutant of A. thaliana under SeO42– (20 μM) stress was characterized by a flawed GSH synthetic pathway, and the root length of these plants was reduced significantly, in contrast to the wild type. In other mutant apr2-1 GSH depletion and ROS accretion were prominent owing to Se toxicity [160]. In turn, Gomes-Junior et al. [170] detected ten SOD isoenzymes with two major Mn-SOD isoenzymes responding more efficiently to 0.05 than 0.5 mM of SeO32–. Moreover, an extra glutathione reductase (GR) isoenzyme, which has the potential for oxidative stress in coffee, was induced by SeO32–. However, the current challenges will probably be focused on discrimination between Se toxicity induced by oxidative/nitrooxidative stress and non-specific Se-proteins.
6. Phytoremediation of Selenium-Contaminated Environments
Phytoremediation, also known as green biotechnology, is an approach to eliminate toxic elements from the contaminated environment using various plant species. Further, toxic elements can effortlessly be removed through plant harvesting or volatilized into their less harmful volatile forms. Phytoremediation is recognized as eco-friendly and cheaper than other methods. It does not affect soil fertility, like some engineering interventions [13,19]. Almost all plants easily uptake Se, and this phenomenon could be implemented both for the removal of Se from the contaminated areas and for the biofortification of plants [33]. Table 3 shows the list of plant species (crops and non-crops) used for Se phytoremediation.
Table 3.
List of selected plant species used for Se phytoremediation.
| Plant Species | Family | References |
|---|---|---|
| Brassica oleracea var. capitata, B. oleracea var. italica, B. oleracea var. botrytis, B. juncea, B. napus, Stanleya pinnata | Brassicaceae | [35,171,172,173,174] |
| Gaillardia aristata and Calendula officinalis | Asteraceae | [175,176,177] |
| Astragalus bisulcatus | Fabaceae | [171,178] |
| Arundo donax, Triticum aestivum, and Oryza sativa | Poaceae | [36,153,179] |
| Eichchornia crassipes | Pontederiaceae | [180] |
| Populus spp. | Salicaceae | [181] |
| Lemnoideae spp. | Lemnaceae | [182,183] |
| Hippuris vulgaris L. | Plantaginaceae | [184] |
| Typha latifolia | Typhaceae | [185] |
| Ipomoea purpurea | Convolvulaceae | [186] |
| Azolla caroliniana | Salviniaceae | [187] |
| Pteris vittata | Pteridaceae | [188] |
| Juncus xiphioides | Juncaceae | [189] |
| Bolboschoenus maritimus | Cyperaceae | [189] |
| Chara spp. | Characeae | [38,39] |
| Corchorus capsularis | Malvaceae | [190] |
| Eucalyptus globulus | Myrtaceae | [191] |
The choice of plant species for phytoremediation is crucial for the successful remediation of the Se-contaminated environment. Transgenic plants can also be used to enhance phytoremediation capacity. Different phytoremediation approaches, such as phytoextraction, phytovolatilization, and rhizofiltration are widely used for the remediation of Se-contaminated environments (Figure 6).
Figure 6.
Phytoremediation of Se-polluted environments. (A) plants types according to Se accumulation in biomass), (B) phytoremediation processes of Se polluted environments. Various phytotechnologies can be used to remediate Se contaminants by accumulating them at large amounts in various parts of plants, especially transgenic plants can provide safe and quick Se phytoremediation to avoid the adverse environmental impact and toxicity to consumers. The main bioavailable form of Se in soils is SeO42–. After uptaken up by plants, SeO42– can be accumulated in the root and easily translocated to the shoots. Inorganic SeO42– can be integrated into Se-Cys and other forms of organic Se. A few types of organic Se are volatile and can be released by the plants into the atmosphere as a harmless gas. Selenium accumulation and volatilization may be used to produce Se-biofortified crops and in phytoremediation, respectively.
6.1. Selenium Hyperaccumulation
Although Se is beneficial for many of the plants and animals, its essentiality for plants is not recognized yet. There are variations among plant species regarding uptake and accumulation of Se as well as producing volatile Se-compounds to avoid Se toxicity [19,192]. Therefore, according to the capacity to uptake, utilize, and accumulate Se, plants are categorized into three classes [Se hyperaccumulators (accumulate ≥ 1000 µg Se g−1 DW), secondary Se accumulators (accumulate 100–1000 μg Se kg−1 DW), non-accumulators (contain < 100 μg Se g−1 DW). The secondary Se accumulators can grow in seleniferous, and non-seleniferous soils and are termed as the Se-indicators, as their tissue Se content indicates the Se-phytoavailablity [19,148,193]. Nevertheless, there are variations among plants in terms of Se accumulation and tolerance within individual groups, genus, species, subspecies, ecotypes, or even cultivars [148,194,195].
In 1930, a group of researchers led by Orville Beath discovered Se hyperaccumulation and described indicator plant species grown on seleniferous soils. Thereafter, Se hyperaccumulators were identified in 6 families, 14 genera, and 45 taxa of the plant kingdom [148]. Among them, 25 Se hyperaccumulating taxa were reported in the family Fabaceae genus Astragalus. Others belong to the Brassicaceae family (species Stanleya pinnata and S. bipinnata) and the Asteraceae family (genera Oonopsis, Xylorhiza, and Symphyotrichum) [196]. Moreover, Neptunia amplexicaulis, a member of the Fabaceae family, is reported to be Se hyperaccumulator when growing on seleniferous soil. Although the Se content in a hyperaccumulator species could be up to 1.5% of their DW, there might be genetic variation among populations and within species [195]).
Previously it was stated that SeO42− is chemically similar to SO42−, and can occupy the SO42−-transporters (SULTR1;2 and SULTR1;1) to enter the root cells and move in the whole plant [132]. Many factors are responsible for uptake and transport of the SeO42−/SO42−, for instance, plant species, Se:S ratio in plant organs, and in growing media [19,195,197]. In hyperaccumulators, the expression of SO42− transporters is higher in comparison to secondary accumulators and non-accumulators, which is responsible for elevated Se content in their tissues. Moreover, the overexpression of SO42−-transporters in the hyperaccumulators bestow them not only to uptake but also to translocate Se to the aboveground plant organs [197].
Hyperaccumulators have some other traits that enable them to survive and grow successfully on Se rich soils. These species can efficiently convert the inorganic Se into non-protein organic Se and reduce the risk of oxidative stress [198]. They also uptake organic Se forms (Se-Cys, Se-Met, and MeSe-Cys) directly [199], but have the capability of preventing them from incorporation into proteins. Moreover, they can maintain the appropriate amount of Se in the tissue by the enzymatic transformation of MeSe-Cys to volatile dimethyldiselenide [193]. Additionally, they can transform Se-Cys to Se0 by the activity of selenocysteine lyase and preventing Se-Cys incorporation into the protein [200]. Furthermore, Se hyperaccumulators may sequestrate organic Se to tolerate Se toxicity. A considerable number of reports suggest that Se is involved in upregulating the antioxidant defense in the hyperaccumulator species, where enzymatic and non-enzymatic antioxidants and phytohormones, i.e., jasmonic acid (JA), salicylic acid (SA), and ethylene (ET), play key roles in Se tolerance [127,201,202].
Mechanisms of Se hyperaccumulation are of great interest to Se researchers. Hyperaccumulators may have evolved independently in different taxonomic families, genera, and species under similar ecological and physiological selection process [171,172,195,200]. Therefore, these traits might be beneficial both for Se phytoremediation and its biofortification.
6.2. Phytoextraction
Phytoextraction includes the harvesting of plants for the dismissal of metals/metalloids from contaminated soil; this approach is economical and ecofriendly but not very productive due to less phytoavailability of metals in soils and quite slow [171,203,204].
Various plants grown on Se-contaminated soils are Se-hyperaccumulators, but they show moderate growth, and low biomass production leads to inadequate removal of Se [205]. Due to the high potential of accumulation, some Brassica species, like rapeseed and mustard, were recognized for phytoextraction of Se from contaminated areas.
Chelating agents such as EDDS, DTPA, and ethylenediaminetetraacetic acid (EDTA) are considered as a potential weapon to increase the availability of metal/metalloids for increasing the efficiency of phytoextraction. However, the utility of various chelating agents depends on the plant species and the elements to be removed [174,206,207]. On the other hand, chelator-assisted phytoextraction may induce water contamination as they increase the mobilization of toxic ions, ultimate leaching [208,209]. In Brassica oleracea, Esringü and Turan [174] reported that the application of EDDS (7.5 mmol kg−1) and DTPA (1 mmol kg−1) increased Se removal by 12–20 fold from the contaminated soil.
The alteration in physiochemical properties of soils like pH, organic carbon, chelators, and Eh may affect the uptake and phytoaccumulation of Se [210]. However, Bañuelos et al. [171] showed that Brassica plants removed approx. 50% and barley approx. 20% of the Se from the soil. Besides, Johnsson [211] claims that 1.4 to 39% increased organic amendments in the plow layer decreased Se level from 1350 to 150 μg kg−1 in wheat seeds. Later, Dhillon et al. [212] reported that the addition of chicken manure and sugarcane press might reduce the uptake of Se by 44–97%. On the other hand, Yadav et al. [213] indicated onion as a Se remediator from contaminated soils in a cropping system. Hence, the attempts of phytoextraction for various Se-contaminated soils should be focused on the utilization of Se-enriched plant biomass, unescorted by the chelating agents.
6.3. Phytovolatilization
Plants can convert a toxic form of Se into less toxic compounds, e.g., volatile organic seleno-compounds. The process in which plants take up the pollutants from soil and release them in a volatile form is regarded as phytovolatilization [214]. The release of volatile organic elements from stems and leaves is known as direct phytovolatilization, while the increase in volatile contaminant flux from the contaminated soils by the root activity is known as indirect phytovolatilization [214]. The key benefit of this approach is, it can eliminate the contaminants without plant harvesting and/or biomass utilization.
Beath et al. [178] first reported Se volatilization by Se-hyperaccumulator (A. bisulcatus). Afterward, Evans et al. [215] found that the basic evaporative Se form released by Se-hyperactive plants was DMe-DSe. While non-accumulator B. oleracea released DMe-Se [216]. Dumont et al. [67] showed that these volatilized forms are nearly 600-fold less harmful compared to inorganic Se. Among various plant species, cabbage and A. bisulcatus volatilize a higher rate of Se, followed by alfalfa and tomato [37]. Later, Bañuelos et al. [217] and Terry and Zayed [218] reported that plants of the Brassicaceae family (cabbage and broccoli) have higher volatilization capability. The slow growth and low biomass production of Se-hyperaccumulators limit their potential for phytoremediation [172]. Hence, the combined effect of phytovolatilization and phytoextraction can increase the phytoremediation efficiency by two to three-fold.
Selenium volatilization efficiency is based upon a variety of factors, such as plant type, Se form, the configuration of the microbial group, type of macrophytes, temperature, the existence of other elements in the growing medium, microorganisms in the rhizosphere, and many other physiochemical parameters [38,218,219]. Selenium volatilization increases with temperature; higher temperature also improves the metabolic activities of plants [38,219]. Therefore, it is still required to explore the effects of these factors on phytovolatilization efficiency in field experiments.
6.4. Rhizofiltration
Rhizofiltration is a sub-technique of phytoremediation, which employs a plant root system to absorb contaminants, mainly toxic metals, from solution surrounding the rhizosphere, groundwater, surface water, and wastewater [220]. Suitability of various aquatic plants for Se rhizofiltration have been studied through short-term experiments in aqueous solutions including Myriophyllum brasiliense, Potamogeton crispus, Juncus xiphioides, Typha latifolia, Ruppia maritima, Scirpus robustus, and Hydrilla verticillate [220,221,222,223], and long-term experiments in constructed wetlands [224,225].
Cattail (Typha angustifolia) grown in wetland conditions has been reported effective in the removal of Se applied as SeO32– and SeO42– by 89% and 46%, respectively [38], while musk grass removed about 70–75% of supplemented Se from the aqueous environment [179,180]. Duckweed (Lemna minor), known for the natural capacity to accumulate Se, removed 55–99% of supplemented Se [222,226]. In turn, soft rush (Juncus effusus L.) can be used for Se rhizofiltration due to its availability in wetlands compared to cattail. Miranda et al. [222] demonstrated that the biomass of many aquatic plants possesses a considerable potential for the production of biofuel. Hence, the dual advantage of aquatic plants for polluted water management and the generation of renewable fuels and petrochemicals came up with an eco-friendly and cheap way for the remediation of Se-contaminated water.
6.5. Genetic Engineering for Se Phytoremediation
Genetic engineering is the modern tool used nowadays to enhance plant abiotic stress tolerance and in phytotechnologies (phytoremediation and biofortification). Recent advancements in omics approaches allowed altering plants at the molecular level resulted in efficient phytoremediation of Se [227]. The basics are to modify gene expression to target different routes and pathways for phytoremediation in non-accumulators, or secondary accumulators or to transfer the traits into a slow-growing hyperaccumulator (Table 4) [175]. Several researchers have successfully adopted the transgenics to enhance the Se-tolerance, Se-accumulation, as well as Se-volatilization using the traits from the Se-hyperaccumulators.
Table 4.
Transgenic plants and the candidate genes for the targeted Se-phytoremediation.
| Transgenic Species | Gene Transferred | Effects | Reference |
|---|---|---|---|
| Brassica juncea | Cystathionine-γ-synthase (CgS) | Increased Se volatilization | [228] |
| A. thaliana | Selenocysteine lyase (SL) | Enhanced Se accumulation | [229] |
| B. juncea | SL | Enhanced Se accumulation | [230] |
| A. thaliana | Selenocysteine methyltransferase (SMT) | Enhanced Se accumulation and volatilization | [231] |
| B. juncea | SMT | Enhanced Se accumulation and tolerance | [232] |
| B. juncea | APS | Three-fold increased Se accumulation in leaves | [233] |
| B. juncea | γ Glutamyl-cysteine synthetase (ECS) | Improved Se accumulation | [233] |
| B. juncea | APS×SMT | Increased Se accumulation under both SeO42− and SeO32− exposure | [217] |
| B. juncea | SL×SMT | Enhanced Se accumulation | [217] |
After entering in the root cell, SeO42− is first reduced to SeO32− with the ATP sulfurylase (APS), which is the initial step for the assimilation of SeO42− to organic Se. Therefore, an attempt was made to overexpress APS from A. thaliana in B. juncea. Consequently, the transgenic plants showed two to three-fold increased Se accumulation compared to the unaltered plants, but this transformation had no impact on the Se volatilization rate. In the Se metabolism pathway, the cystathionine-γ-synthase (CgS) enzyme is responsible for the conversion of Se-Cys to Se-Met, which is further converted to volatile DMe-Se. Therefore, A. thaliana CgS gene overexpression in B. juncea resulted in two to three-fold increased volatilization efficiency in comparison with untransformed plants [228].
As mentioned before, plants can take up Se-amino acids and incorporate them into the proteins leading to Se toxicity. To prevent Se-Cys incorporation to proteins, the enzyme selenocysteine methyltransferase (SMT) converts the Se-Cys to MeSe-Cys. Considering this, an attempt to overexpress the SMT gene from A. bisulcatus in both B. juncea and A. thaliana resulted in upregulated Se accumulation and Se tolerance, as well as increased Se-volatilization [231,232]. However, these plants were more efficient in Se volatilization when exposed to SeO32− than SeO42−. Therefore, an attempt to overexpress two enzymes (APS and SMT) showed approximately nine-fold higher Se-accumulation, where the majority of the Se was in MeSe-Cys form and 8-fold higher compared with wild plants [234]. Notably, Se tolerance, both single and double transgenics were the same. In turn, overexpression of Se-Cys lyase, an enzyme converting Se-Cys to Se0, in A. thaliana and B. juncea resulted in enhanced Se accumulation in comparison with the wild type [229,230].
7. Conclusions and Outlook
In this review, we discussed the causes of Se phytotoxicity, mechanisms of Se-induced cell damage, as well as Se biogeochemistry and phytoremediation features. A high amount of Se exerts several negative and harmful effects on the plant due to oxidative stress, altered and malformed protein structure, disrupted enzymatic function, interrupted biosynthesis and metabolism of carbohydrates, proteins, and other metabolites, distorted chloroplast, and mitochondrial ultrastructure and functioning. These negative effects considerably reduce plant growth, development, and overall production. Selenium has a very narrow gap between its adequacy and toxicity. Consequently, both Se-inadequacy and toxicity are widespread globally and overlap with soils that are low and rich in Se, respectively. Although some recent publications revealed both positive and negative effects of Se, there are still various aspects of Se biological action that required to be revealed, e.g., the essentiality of Se for plants and selection of plants show enhanced growth under Se exposure. Additionally, the effective concentrations of Se inducing a positive or negative effect on plant growth, development, and ecophysiology should be determined. Moreover, the actual mechanisms causing these effects should be revealed. It is also vital to explain the connection between Se and S biogeochemistry, which affects Se and S uptake in natural conditions. Moreover, plant tolerance to Se, their remediation potential, and Se detoxification mechanisms need to be enhanced for efficient phytoremediation of Se polluted areas.
Promising plant species for phytoremediation, phytoextraction, phytovolatilization, or rhizofiltration to reduce the Se concentration in polluted soils should be identified. Additionally, some exciting features of Se hyperaccumulators are still required to be revealed. For example, why and how these unique plant species vary in absorbing and accumulating Se, by which mechanisms plants trigger Se hyperaccumulation, the advantages and disadvantages of Se accumulation in plants, etc. Notably, the development of genetically engineered transgenic Brassica plants has a great potential to remove Se at a higher rate, which will probably help to remediate the Se from the polluted environment within a short period.
Therefore, to gain more insight into Se tolerance and toxicity, the results from several genomic, biochemical and genetic engineering experiments and the overexpression of the key Se and S accumulation pathways should be compared. Further, the state-of-the-art omics approaches, mainly transcriptomics, metabolomics, and proteomics, can help to identify the key genes, metabolites, proteins, and regulators encoding actual transporters of selenocompounds into and within hyperaccumulator plants; and the metabolic pathways responsible for the Se translocation. Afterward, the overexpression of such key genes would help to develop the Se hyperaccumulators with high biomass production for more effective remediation of Se polluted areas. Additionally, the engineered Se-associated metabolic pathways can provide novel ideas into the existing knowledge and aid to further discover the Se translocation mechanisms for future investigations.
Acknowledgments
Mirza Hasanuzzaman thanks Sher-e-Bangla Agricultural University Research System (SAURES) for funding his research on selenium. The authors thank Mahabub Alam for the critical readings and formatting of the manuscript.
Author Contributions
Conceptualization, M.H. and M.F.; writing—original draft preparation, M.H., M.H.M.B.B., A.R., B.H.-N., R.M.-G., K.N.; writing—review and editing, M.H., M.H.M.B.B., M.F.; visualization, M.H., M.H.M.B.B., K.N.; supervision, M.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Boyd R. Selenium stories. Nat. Chem. 2011;3:570. doi: 10.1038/nchem.1076. [DOI] [PubMed] [Google Scholar]
- 2.Bodnar M., Konieczka P., Namiesnik J. The properties, functions, and use of selenium compounds in living organisms. J. Environ. Sci. Health Part C. 2012;30:225–252. doi: 10.1080/10590501.2012.705164. [DOI] [PubMed] [Google Scholar]
- 3.Floor G.H., Román-Ross G. Selenium in volcanic environments: A review. Appl. Geochem. 2012;27:517–531. doi: 10.1016/j.apgeochem.2011.11.010. [DOI] [Google Scholar]
- 4.Peng Q., Wang M., Cui Z., Huang J., Chen C., Guo L., Liang D. Assessment of bioavailability of selenium in different plant-soil systems by diffusive gradients in thin-films (DGT) Environ. Pollut. 2017;225:637–643. doi: 10.1016/j.envpol.2017.03.036. [DOI] [PubMed] [Google Scholar]
- 5.Chauhan R., Awasthi S., Srivastava S., Dwivedi S., Pilon-Smits E.A., Dhankher O.P., Tripathi R.D. Understanding selenium metabolism in plants and its role as a beneficial element. Crit. Rev. Environ. Sci. Technol. 2019;49:1937–1958. doi: 10.1080/10643389.2019.1598240. [DOI] [Google Scholar]
- 6.Sobolev O.I., Gutyj B.V., Sobolieva S.V., Borshch O.O., Nedashkivsky V.M., Kachan L.M., Karkach P.M., Nedashkivska N.V., Poroshinska O.A., Stovbetska L.S., et al. Selenium in natural environment and food chains. A Review. Ukrainian J. Ecol. 2020;4:148–158. [Google Scholar]
- 7.Trippe R.C., 3rd, Pilon-Smits E.A.H. Selenium transport and metabolism in plants: Phytoremediation and biofortification implications. J. Hazard. Mater. 2020;404:124178. doi: 10.1016/j.jhazmat.2020.124178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wrobel K., Esperanza M.G., Barrientos E.Y., Escobosa A.R.C., Wrobel K. Different approaches in metabolomic analysis of plants exposed to selenium: A comprehensive review. Acta Physiol. Plant. 2020;42:1–20. doi: 10.1007/s11738-020-03113-0. [DOI] [Google Scholar]
- 9.Mehdi Y., Hornick J.-L., Istasse L., Dufrasne I. Selenium in the environment, metabolism and involvement in body functions. Molecules. 2013;18:3292–3311. doi: 10.3390/molecules18033292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar A., Prasad K.S. Role of nano-selenium in health and environment. J. Biotechnol. 2020 doi: 10.1016/j.jbiotec.2020.11.004. in press. [DOI] [PubMed] [Google Scholar]
- 11.Schwarz K., Foltz C.M. Selenium as an integral part of factor 3 against dietary necrotic liver degeneration. J. Am. Chem. Soc. 1957;79:3292–3293. doi: 10.1021/ja01569a087. [DOI] [PubMed] [Google Scholar]
- 12.Reeves M., Hoffmann P. The human selenoproteome: Recent insights into functions and regulation. Cell. Mol. Life Sci. 2009;66:2457–2478. doi: 10.1007/s00018-009-0032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu Y.-G., Pilon-Smits E.A., Zhao F.-J., Williams P.N., Meharg A.A. Selenium in higher plants: Understanding mechanisms for biofortification and phytoremediation. Trends Plant Sci. 2009;14:436–442. doi: 10.1016/j.tplants.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Etteieb S., Magdouli S., Zolfaghari M., Brar S. Monitoring and analysis of selenium as an emerging contaminant in mining industry: A critical review. Sci. Total Environ. 2020;698:134339. doi: 10.1016/j.scitotenv.2019.134339. [DOI] [PubMed] [Google Scholar]
- 15.WHO Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. [(accessed on 5 March 2014)];2009 Available online: http://www.who.int/healthinfo/global_burden_disease/GlobalHealth2009:Risks_report_annex.pdf.
- 16.Malagoli M., Schiavon M., Pilon-Smits E.A. Effects of selenium biofortification on crop nutritional quality. Front. Plant Sci. 2015;6:280. doi: 10.3389/fpls.2015.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu Z., Bañuelos G.S., Lin Z.-Q., Liu Y., Yuan L., Yin X., Li M. Biofortification and phytoremediation of selenium in China. Front. Plant Sci. 2015;6:136. doi: 10.3389/fpls.2015.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winkel L.H., Johnson C.A., Lenz M., Grundl T., Leupin O.X., Amini M., Charlet L. Environmental selenium research: From microscopic processes to global understanding. Environ. Sci. Technol. 2012;46:571–579. doi: 10.1021/es203434d. [DOI] [PubMed] [Google Scholar]
- 19.Schiavon M., Pilon-Smits E.A. Selenium biofortification and phytoremediation phytotechnologies: A review. J. Environ. Qual. 2017;46:10–19. doi: 10.2134/jeq2016.09.0342. [DOI] [PubMed] [Google Scholar]
- 20.Ye Y., Qu J., Pu Y., Rao S., Xu F., Wu C. Selenium Biofortification of Crop Food by Beneficial Microorganisms. J. Fungi. 2020;6:59. doi: 10.3390/jof6020059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasanuzzaman M., Bhuyan M.B., Raza A., Hawrylak-Nowak B., Matraszek-Gawron R., Al Mahmud J., Nahar K., Fujita M. Selenium in plants: Boon or bane? Environ. Exp. Bot. 2020;178:104170. doi: 10.1016/j.envexpbot.2020.104170. [DOI] [Google Scholar]
- 22.Rizwan M., Ali S., Rehman M.Z., Rinklebe J., Tsang D.C.W., Tack F.M.G., Abbasi G.H., Hussain A., Igalavithana A.D., Lee B.C., et al. Effects of selenium on the uptake of toxic trace elements by crop plants: A review. Crit. Rev. Environ. Sci. Technol. 2020 doi: 10.1080/10643389.2020.1796566. [DOI] [Google Scholar]
- 23.Zwolak I. The Role of Selenium in Arsenic and Cadmium Toxicity: An Updated Review of Scientific Literature. Biol. Trace Elem. Res. 2020;193:44–63. doi: 10.1007/s12011-019-01691-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng R., Wang L., Yang J., Zhao P., Zhu Y., Li Y., Yu Y., Liu H., Rensing C., Wu Z., et al. Underlying mechanisms responsible for restriction of uptake and translocation of heavy metals (metalloids) by selenium via root application in plants. J. Hazard. Mater. 2020;402:123570. doi: 10.1016/j.jhazmat.2020.123570. [DOI] [PubMed] [Google Scholar]
- 25.Mroczek-Zdyrska M., Strubińska J., Hanaka A. Selenium improves physiological parameters and alleviates oxidative stress in shoots of lead-exposed Vicia faba L. minor plants grown under phosphorus-deficient conditions. J. Plant Growth Regul. 2017;36:186–199. doi: 10.1007/s00344-016-9629-7. [DOI] [Google Scholar]
- 26.Molnár Á., Kolbert Z., Kéri K., Feigl G., Ördög A., Szőllősi R., Erdei L. Selenite-induced nitro-oxidative stress processes in Arabidopsis thaliana and Brassica juncea. Ecotoxicol. Environ. Saf. 2018;148:664–674. doi: 10.1016/j.ecoenv.2017.11.035. [DOI] [PubMed] [Google Scholar]
- 27.Pyrzynska K., Sentkowska A. Selenium in plant foods: Speciation analysis, bioavailability, and factors affecting composition. Crit. Rev. Food Sci. Nutr. 2020 doi: 10.1080/10408398.2020.1758027. [DOI] [PubMed] [Google Scholar]
- 28.Kabata-Pendias A. Trace Elements in Soils and Plants. CRC Press/Taylor & Francis Group ICC; Boca Raton, FL, USA: 2010. [Google Scholar]
- 29.Hawrylak-Nowak B. Beneficial effects of exogenous selenium in cucumber seedlings subjected to salt stress. Biol. Trace Elem. Res. 2009;132:259–269. doi: 10.1007/s12011-009-8402-1. [DOI] [PubMed] [Google Scholar]
- 30.Lehotai N., Lyubenova L., Schröder P., Feigl G., Ördög A., Szilágyi K., Erdei L., Kolbert Z. Nitro-oxidative stress contributes to selenite toxicity in pea (Pisum sativum L) Plant Soil. 2016;400:107–122. doi: 10.1007/s11104-015-2716-x. [DOI] [Google Scholar]
- 31.Jain M., Panwar M., Gadre R. Influence of selenium supplementation on δ-aminolevulinic acid formation in greening maize leaf segments. Res. J. Phytochem. 2017;11:111–117. doi: 10.3923/rjphyto.2017.111.117. [DOI] [Google Scholar]
- 32.Ali W., Zhang H., Junaid M., Mao K., Xu N., Chang C., Rasool A., Aslam M.W., Ali J., Yang Z. Insights into the mechanisms of arsenic-selenium interactions and the associated toxicity in plants, animals, and humans: A critical review. Crit. Rev. Environ. Sci. Technol. 2020 doi: 10.1080/10643389.2020.1740042. [DOI] [Google Scholar]
- 33.Feng R., Wei C., Tu S. The roles of selenium in protecting plants against abiotic stresses. Environ. Exp. Bot. 2013;87:58–68. doi: 10.1016/j.envexpbot.2012.09.002. [DOI] [Google Scholar]
- 34.Ponton D.E., Graves S.D., Fortin C., Janz D., Amyot M., Schiavon M. Selenium interactions with algae: Chemical processes at biological uptake sites, bioaccumulation, and intracellular metabolism. Plants. 2020;9:528. doi: 10.3390/plants9040528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Banuelos G., Ajwa H., Mackey B., Wu L., Cook C., Akohoue S., Zambruzuski S. Evaluation of different plant species used for phytoremediation of high soil selenium. J. Environ. Qual. 1997;26:639–646. doi: 10.2134/jeq1997.00472425002600030008x. [DOI] [Google Scholar]
- 36.Yasin M., El-Mehdawi A.F., Anwar A., Pilon-Smits E.A., Faisal M. Microbial-enhanced selenium and iron biofortification of wheat (Triticum aestivum L.)-applications in phytoremediation and biofortification. Int. J. Phytoremed. 2015;17:341–347. doi: 10.1080/15226514.2014.922920. [DOI] [PubMed] [Google Scholar]
- 37.Duckart E., Waldron L., Donner H. Selenium uptake and volatilization from plants growing in soil. Soil Sci. 1992;153:94–99. doi: 10.1097/00010694-199202000-00002. [DOI] [Google Scholar]
- 38.Salhani N., Boulyga S., Stengel E. Phytoremediation of selenium by two helophyte species in subsurface flow constructed wetland. Chemosphere. 2003;50:967–973. doi: 10.1016/s0045-6535(02)00607-0. [DOI] [PubMed] [Google Scholar]
- 39.Lin Z.Q., De Souza M., Pickering I., Terry N. Evaluation of the macroalga, muskgrass, for the phytoremediation of selenium-contaminated agricultural drainage water by microcosms. J. Environ. Qual. 2002;31:2104–2110. doi: 10.2134/jeq2002.2104. [DOI] [PubMed] [Google Scholar]
- 40.Ozyigit I.I., Can H., Dogan I. Phytoremediation using genetically engineered plants to remove metals: A review. Environ. Chem. Lett. 2020 doi: 10.1007/s10311-020-01095-6. [DOI] [Google Scholar]
- 41.Charya L.S. Selenium pollution in the marine environment and marine bacteria in selenium bioremediation. In: Naik M., Dubey S., editors. Marine Pollution and Microbial Remediation. Springer; Berlin/Heidelberg, Germany: 2017. pp. 223–237. [Google Scholar]
- 42.Wang Z., Becker H. Ratios of S, Se and Te in the silicate Earth require a volatile-rich late veneer. Nature. 2013;499:328–331. doi: 10.1038/nature12285. [DOI] [PubMed] [Google Scholar]
- 43.Fernández-Martínez A., Charlet L. Selenium environmental cycling and bioavailability: A structural chemist point of view. Rev. Environ. Sci. Biotechnol. 2009;8:81–110. doi: 10.1007/s11157-009-9145-3. [DOI] [Google Scholar]
- 44.Sharma V.K., McDonald T.J., Sohn M., Anquandah G.A., Pettine M., Zboril R. Biogeochemistry of selenium. A review. Environ. Chem. Lett. 2015;13:49–58. doi: 10.1007/s10311-014-0487-x. [DOI] [Google Scholar]
- 45.Torres J., Pintos V., Domínguez S., Kremer C., Kremer E. Selenite and selenate speciation in natural waters: Interaction with divalent metal ions. J. Sol. Chem. 2010;39:1–10. doi: 10.1007/s10953-009-9491-3. [DOI] [Google Scholar]
- 46.Stroud J.L., McGrath S.P., Zhao F.-J. Selenium speciation in soil extracts using LC-ICP-MS. Int. J. Environ. Anal. Chem. 2012;92:222–236. doi: 10.1080/03067310903111661. [DOI] [Google Scholar]
- 47.Cui Y., Chen J., Zhang Y., Peng D., Huang T., Sun C. pH-Dependent Leaching Characteristics of Major and Toxic Elements from Red Mud. Int. J. Environ. Res. Public Health. 2019;16:2046. doi: 10.3390/ijerph16112046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoon I.-H., Bang S., Kim K.-W., Kim M.G., Park S.Y., Choi W.-K. Selenate removal by zero-valent iron in oxic condition: The role of Fe (II) and selenate removal mechanism. Environ. Sci. Pollut. Res. 2016;23:1081–1090. doi: 10.1007/s11356-015-4578-4. [DOI] [PubMed] [Google Scholar]
- 49.Wallschläger D., Feldmann J. Formation, occurrence, significance, and analysis of organoselenium and organotellurium compounds in the environment. Metal Ions Life Sci. 2010;7:319–364. doi: 10.1039/BK9781847551771-00319. [DOI] [PubMed] [Google Scholar]
- 50.Maurer F., Christl I., Kretzschmar R. Reduction and reoxidation of humic acid: Influence on spectroscopic properties and proton binding. Environ. Sci. Technol. 2010;44:5787–5792. doi: 10.1021/es100594t. [DOI] [PubMed] [Google Scholar]
- 51.Nakamaru Y.M., Altansuvd J. Speciation and bioavailability of selenium and antimony in non-flooded and wetland soils: A review. Chemosphere. 2014;111:366–371. doi: 10.1016/j.chemosphere.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 52.Gennari F., Sharma V.K., Pettine M., Campanella L., Millero F.J. Reduction of selenite by cysteine in ionic media. Geochim. Cosmochim. Acta. 2014;124:98–108. doi: 10.1016/j.gca.2013.09.019. [DOI] [Google Scholar]
- 53.Vriens B., Lenz M., Charlet L., Berg M., Winkel L.H. Natural wetland emissions of methylated trace elements. Nat. Commun. 2014;5:3035. doi: 10.1038/ncomms4035. [DOI] [PubMed] [Google Scholar]
- 54.Nancharaiah Y.V., Lens P. Ecology and biotechnology of selenium-respiring bacteria. Microbiol. Mol. Biol. Rev. 2015;79:61–80. doi: 10.1128/MMBR.00037-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mal J., Nancharaiah Y., Van Hullebusch E., Lens P. Effect of heavy metal co-contaminants on selenite bioreduction by anaerobic granular sludge. Bioresour. Technol. 2016;206:1–8. doi: 10.1016/j.biortech.2016.01.064. [DOI] [PubMed] [Google Scholar]
- 56.Kagami T., Narita T., Kuroda M., Notaguchi E., Yamashita M., Sei K., Soda S., Ike M. Effective selenium volatilization under aerobic conditions and recovery from the aqueous phase by Pseudomonas stutzeri NT-I. Water Res. 2013;47:1361–1368. doi: 10.1016/j.watres.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 57.Sager M. Selenium in agriculture, food, and nutrition. Pure Appl. Chem. 2006;78:111–133. doi: 10.1351/pac200678010111. [DOI] [Google Scholar]
- 58.El-Ramady H., Abdalla N., Taha H.S., Alshaal T., El-Henawy A., Salah E.-D.F., Shams M.S., Youssef S.M., Shalaby T., Bayoumi Y. Selenium and nano-selenium in plant nutrition. Environ. Chem. Lett. 2016;14:123–147. doi: 10.1007/s10311-015-0535-1. [DOI] [Google Scholar]
- 59.Hasanuzzaman M., Hossain M.A., Fujita M. Selenium in higher plants: Physiological role, antioxidant metabolism and abiotic stress tolerance. J. Plant Sci. 2010;5:354–375. [Google Scholar]
- 60.Rosenfeld I., Beath O.A. Selenium: Geobotany, Biochemistry, Toxicity, and Nutrition. Academic Press; New York, NY, USA: 2013. [Google Scholar]
- 61.Martens D.A., Suarez D.L. Selenium speciation of soil/sediment determined with sequential extractions and hydride generation atomic absorption spectrophotometry. Environ. Sci. Technol. 1996;31:133–139. doi: 10.1021/es960214+. [DOI] [Google Scholar]
- 62.El-Ramady H.R., Domokos-Szabolcsy É., Shalaby T.A., Prokisch J., Fári M. Selenium in agriculture: Water, air, soil, plants, food, animals and nanoselenium. In: Eric L., Jan S., Didier R., editors. CO2 Sequestration, Biofuels and Depollution. Springer; Cham, Switzerland: 2015. pp. 153–232. [Google Scholar]
- 63.Hartikainen H. Biogeochemistry of selenium and its impact on food chain quality and human health. J. Trace Elem. Med. Biol. 2005;18:309–318. doi: 10.1016/j.jtemb.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 64.McNeal J.M., Balistrieri L.S. Geochemistry and occurrence of selenium: An overview. In: Jacobs L.W., editor. Selenium in Agriculture and the Environment. ACSESS; Madison, WI, USA: 1989. pp. 1–13. [Google Scholar]
- 65.Gupta U.C., Gupta S.C. Selenium in soils and crops, its deficiencies in livestock and humans: Implications for management. Commun. Soil Sci. Plant Anal. 2000;31:1791–1807. doi: 10.1080/00103620009370538. [DOI] [Google Scholar]
- 66.Beale A., Fasulo D., Craigmill A. Effects of oral and parenteral selenium supplements on residues in meat, milk and eggs. In: George W.W., editor. Reviews of Environmental Contamination and Toxicology. Springer; New York, NY, USA: 1990. pp. 125–150. [DOI] [PubMed] [Google Scholar]
- 67.Dumont E., Vanhaecke F., Cornelis R. Selenium speciation from food source to metabolites: A critical review. Anal. Bioanal. Chem. 2006;385:1304–1323. doi: 10.1007/s00216-006-0529-8. [DOI] [PubMed] [Google Scholar]
- 68.Thomson C.D., Chisholm A., McLachlan S.K., Campbell J.M. Brazil nuts: An effective way to improve selenium status. Am. J. Clin. Nutr. 2008;87:379–384. doi: 10.1093/ajcn/87.2.379. [DOI] [PubMed] [Google Scholar]
- 69.Sors T.G., Martin C.P., Salt D.E. Characterization of selenocysteine methyltransferases from Astragalus species with contrasting selenium accumulation capacity. Plant J. 2009;59:110–122. doi: 10.1111/j.1365-313X.2009.03855.x. [DOI] [PubMed] [Google Scholar]
- 70.Sun H.W., Ha J., Liang S.X., Kang W.J. Protective role of selenium on garlic growth under cadmium stress. Commun. Soil Sci. Plant Anal. 2010;41:1195–1204. doi: 10.1080/00103621003721395. [DOI] [Google Scholar]
- 71.Prins C.N., Hantzis L.J., Quinn C.F., Pilon-Smits E.A. Effects of selenium accumulation on reproductive functions in Brassica juncea and Stanleya pinnata. J. Exp. Bot. 2011;62:5633–5640. doi: 10.1093/jxb/err247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bajaj M., Eiche E., Neumann T., Winter J., Gallert C. Hazardous concentrations of selenium in soil and groundwater in North-West India. J. Hazard. Mater. 2011;189:640–646. doi: 10.1016/j.jhazmat.2011.01.086. [DOI] [PubMed] [Google Scholar]
- 73.Thomson C. Assessment of requirements for selenium and adequacy of selenium status: A review. Eur. J. Clin. Nutr. 2004;58:391–402. doi: 10.1038/sj.ejcn.1601800. [DOI] [PubMed] [Google Scholar]
- 74.Winkel L.H., Vriens B., Jones G.D., Schneider L.S., Pilon-Smits E., Bañuelos G.S. Selenium cycling across soil-plant-atmosphere interfaces: A critical review. Nutrients. 2015;7:4199–4239. doi: 10.3390/nu7064199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qingyun W., Zhang J., Bingzi Z., Xiuli X., Xihai D., Zhang H. Influence of long-term fertilization on selenium accumulation in soil and uptake by crops. Pedosphere. 2016;26:120–129. [Google Scholar]
- 76.Dos Reis A.R., El-Ramady H., Santos E.F., Gratão P.L., Schomburg L. Overview of selenium deficiency and toxicity worldwide: Affected areas, selenium-related health issues, and case studies. In: Pilon-Smits E.A.H., Lenny H.E.W., Lin Z.-Q., editors. Selenium in Plants. Springer; Cham, Switzerland: 2017. pp. 209–230. [Google Scholar]
- 77.White P.J. The genetics of selenium accumulation by plants. In: Elizabeth A.H., Lenny P.-S., Winkel Z.-Q., editors. Selenium in Plants. Springer; Cham, Switzerland: 2017. pp. 143–163. [Google Scholar]
- 78.Dinh Q.T., Cui Z., Huang J., Tran T.A.T., Wang D., Yang W., Zhou F., Wang M., Yu D., Liang D. Selenium distribution in the Chinese environment and its relationship with human health: A review. Environ. Int. 2018;112:294–309. doi: 10.1016/j.envint.2017.12.035. [DOI] [PubMed] [Google Scholar]
- 79.Shahid M., Niazi N.K., Khalid S., Murtaza B., Bibi I., Rashid M.I. A critical review of selenium biogeochemical behavior in soil-plant system with an inference to human health. Environ. Pollut. 2018;234:915–934. doi: 10.1016/j.envpol.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 80.Presser T.S. The geologic origin and pathways of mobility of selenium from the California Coast Ranges to the west-central San Joaquin valley. In: Frankenberger W.T., Benson S., editors. Publication of an Organization Other than the U.S. Geological Survey. Marcel Dekker; New York, NY, USA: 1994. pp. 139–156. [Google Scholar]
- 81.Fessler A.J., Moller G., Talcott P., Exon J. Selenium toxicity in sheep grazing reclaimed phosphate mining sites. Veter. Hum. Toxicol. 2003;45:294. [PubMed] [Google Scholar]
- 82.Bullock L.A., Parnell J. Selenium and molybdenum enrichment in uranium roll-front deposits of Wyoming and Colorado, USA. J. Geochem. Exp. 2017;180:101–112. doi: 10.1016/j.gexplo.2017.06.013. [DOI] [Google Scholar]
- 83.Huang Y., Wang Q., Gao J., Lin Z., Banuelos G.S., Yuan L., Yin X. Daily dietary selenium intake in a high selenium area of Enshi, China. Nutrients. 2013;5:700–710. doi: 10.3390/nu5030700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yuan L., Zhu Y., Lin Z.-Q., Banuelos G., Li W., Yin X. A novel selenocystine-accumulating plant in selenium-mine drainage area in Enshi, China. PLoS ONE. 2013;8:e65615. doi: 10.1371/journal.pone.0065615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jenkins K., Hidiroglou M. A review of selenium/vitamin E responsive problems in livestock: A case for selenium as a feed additive in Canada. Can. J. Animal Sci. 1972;52:591–620. doi: 10.4141/cjas72-072. [DOI] [Google Scholar]
- 86.Tinggi U. Essentiality and toxicity of selenium and its status in Australia: A review. Toxicol. Lett. 2003;137:103–110. doi: 10.1016/S0378-4274(02)00384-3. [DOI] [PubMed] [Google Scholar]
- 87.Ghosh A., Sarkar S., Pramanik A., Chowdhury S., Ghosh S. Selenium toxicosis in grazing buffaloes and its relationship with soil and plant of West Bengal. Ind. J. Animal Sci. 1993;63:557–560. [Google Scholar]
- 88.Wadgaonkar S.L., Ferraro A., Race M., Nancharaiah Y.V., Dhillon K.S., Fabbricino M., Esposito G., Lens P.N. Optimization of soil washing to reduce the selenium levels of seleniferous soil from Punjab, Northwestern India. J. Environ. Qual. 2018;47:1530–1537. doi: 10.2134/jeq2018.05.0187. [DOI] [PubMed] [Google Scholar]
- 89.LSA Honors Physics The Nutrition of Food Could Worsen with Climate Change. [(accessed on 14 August 2020)];2017 Available online: http://lasallehonorsphysics1617.blogspot.com/2017/03/the-nutrition-of-food-could-worsen-with.html.
- 90.Yu D., Liang D., Lei L., Zhang R., Sun X., Lin Z. Selenium geochemical distribution in the environment and predicted human daily dietary intake in northeastern Qinghai, China. Environ. Sci. Pollut. Res. 2015;22:11224–11235. doi: 10.1007/s11356-015-4310-4. [DOI] [PubMed] [Google Scholar]
- 91.Gupta M., Gupta S. An overview of selenium uptake, metabolism, and toxicity in plants. Front. Plant Sci. 2017;7:2074. doi: 10.3389/fpls.2016.02074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rodriguez A.M., Schild C.O., Cantón G.J., Riet-Correa F., Armendano J.I., Caffarena R.D., Brambilla E.C., García J.A., Morrell E.L., Poppenga R. White muscle disease in three selenium deficient beef and dairy calves in Argentina and Uruguay. Ciência Rural. 2018;48:e20170733. doi: 10.1590/0103-8478cr20170733. [DOI] [Google Scholar]
- 93.Pilarczyk B., Tomza-Marciniak A., Pilarczyk R., Marciniak A., Bąkowska M., Nowakowska E. In: Selenium, Se. Mammals and Birds as Bioindicators of Trace Element Contaminations in Terrestrial Environments. Elżbieta K., editor. Springer; Cham, Switzerland: 2019. pp. 301–362. [Google Scholar]
- 94.Yee H., Measures C., Edmond J. Selenium in the tributaries of the Orinoco in Venezuela. Nature. 1987;326:686–689. doi: 10.1038/326686a0. [DOI] [Google Scholar]
- 95.Hansen J.C., Deutch B., Pedersen H.S. Selenium status in Greenland Inuit. Sci. Total Environ. 2004;331:207–214. doi: 10.1016/j.scitotenv.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 96.Miyazaki Y., Koyama H., Sasada Y., Satoh H., Nojiri M., Suzuki S. Dietary habits and selenium intake of residents in mountain and coastal communities in Japan. J. Nutr. Sci. Vitaminol. 2004;50:309–319. doi: 10.3177/jnsv.50.309. [DOI] [PubMed] [Google Scholar]
- 97.Orr P.L., Guiguer K.R., Russel C.K. Food chain transfer of selenium in lentic and lotic habitats of a western Canadian watershed. Ecotoxicol. Environ. Saf. 2006;63:175–188. doi: 10.1016/j.ecoenv.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 98.Khan S.D., Mahmood K., Sultan M.I., Khan A.S., Xiong Y., Sagintayev Z. Trace element geochemistry of groundwater from Quetta Valley, western Pakistan. Environ. Earth Sci. 2010;60:573–582. doi: 10.1007/s12665-009-0197-z. [DOI] [Google Scholar]
- 99.Kagawa M., Ishizaka Y., Ohta K. Sources of sulfate in winter aerosols over the Sea of Japan, as inferred from selenium composition. Atmos. Environ. 2003;37:1593–1600. doi: 10.1016/S1352-2310(03)00006-2. [DOI] [Google Scholar]
- 100.Junior E.S., Wadt L., Silva K., Lima R., Batista K., Guedes M., Carvalho G., Carvalho T., Reis A., Lopes G. Natural variation of selenium in Brazil nuts and soils from the Amazon region. Chemosphere. 2017;188:650–658. doi: 10.1016/j.chemosphere.2017.08.158. [DOI] [PubMed] [Google Scholar]
- 101.Liu Y., Li F., Yin X., Lin Z. Plant-based biofortification: From phytoremediation to Se-enriched agriculture products. In: Sharma S.K., Mudhoo A., editors. Green Chemistry for Environmental Sustainability. CRC Press; Boca Raton, FL, USA: 2011. pp. 341–356. [Google Scholar]
- 102.Li Y., Peng T., Yang Y., Niu C., Archard L., Zhang H. High prevalence of enteroviral genomic sequences in myocardium from cases of endemic cardiomyopathy (Keshan disease) in China. Heart. 2000;83:696–701. doi: 10.1136/heart.83.6.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xia Y., Hill K.E., Byrne D.W., Xu J., Burk R.F. Effectiveness of selenium supplements in a low-selenium area of China. Am. J. Clin. Nutr. 2005;81:829–834. doi: 10.1093/ajcn/81.4.829. [DOI] [PubMed] [Google Scholar]
- 104.Zanetti M., Correa L., Netto A.S., Cunha J., Santana R., Cozzolino S. Influence of canola oil, vitamin E and selenium on cattle meat quality and its effects on nutrition and health of humans. In: Banuelos G.S., Lin Z.-Q., Moraes M.F., Guilherme L.R.G., dos Reis A.R., editors. Proceedings of the Global Advances in Selenium Research from Theory to Application: Proceedings of the 4th International Conference on Selenium in the Environment and Human Health. CRS Press; London, UK: 2015. pp. 97–98. [Google Scholar]
- 105.Gao J., Liu Y., Huang Y., Lin Z.-q., Bañuelos G.S., Lam M.H.-W., Yin X. Daily selenium intake in a moderate selenium deficiency area of Suzhou, China. Food Chem. 2011;126:1088–1093. doi: 10.1016/j.foodchem.2010.11.137. [DOI] [Google Scholar]
- 106.Han J., Liang H., Yi J., Tan W., He S., Wu X., Shi X., Ma J., Guo X. Selenium deficiency induced damages and altered expressions of metalloproteinases and their inhibitors (MMP1/3, TIMP1/3) in the kidneys of growing rats. J. Trace Elem. Med. Biol. 2016;34:1–9. doi: 10.1016/j.jtemb.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 107.Tan J.A., Zhu W., Wang W., Li R., Hou S., Wang D., Yang L. Selenium in soil and endemic diseases in China. Sci. Total Environ. 2002;284:227–235. doi: 10.1016/S0048-9697(01)00889-0. [DOI] [PubMed] [Google Scholar]
- 108.Fairweather-Tait S.J., Bao Y., Broadley M.R., Collings R., Ford D., Hesketh J.E., Hurst R. Selenium in human health and disease. Antioxid. Redox Signal. 2011;14:1337–1383. doi: 10.1089/ars.2010.3275. [DOI] [PubMed] [Google Scholar]
- 109.MacFarquhar J.K., Broussard D.L., Melstrom P., Hutchinson R., Wolkin A., Martin C., Burk R.F., Dunn J.R., Green A.L., Hammond R. Acute selenium toxicity associated with a dietary supplement. Arch. Intern. Med. 2010;170:256–261. doi: 10.1001/archinternmed.2009.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Morlon H., Fortin C., Floriani M., Adam C., Garnier-Laplace J., Boudou A. Toxicity of selenite in the unicellular green alga Chlamydomonas reinhardtii: Comparison between effects at the population and sub-cellular level. Aquat. Toxicol. 2005;73:65–78. doi: 10.1016/j.aquatox.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 111.Geoffroy L., Gilbin R., Simon O., Floriani M., Adam C., Pradines C., Cournac L., Garnier-Laplace J. Effect of selenate on growth and photosynthesis of Chlamydomonas reinhardtii. Aquat. Toxicol. 2007;83:149–158. doi: 10.1016/j.aquatox.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 112.Fu L.H., Wang X.F., Eyal Y., She Y.M., Donald L.J., Standing K.G., Ben-Hayyim G. A selenoprotein in the plant kingdom mass spectrometry confirms that an opal codon (UGA) encodes selenocysteine in Chlamydomonas reinhardtii glutathione peroxidase. J. Biol. Chem. 2002;277:25983–25991. doi: 10.1074/jbc.M202912200. [DOI] [PubMed] [Google Scholar]
- 113.Lyons G.H., Stangoulis J.C., Graham R.D. Tolerance of wheat (Triticum aestivum L.) to high soil and solution selenium levels. Plant Soil. 2005;270:179–188. doi: 10.1007/s11104-004-1390-1. [DOI] [Google Scholar]
- 114.Hopper J.L., Parker D.R. Plant availability of selenite and selenate as influenced by the competing ions phosphate and sulfate. Plant Soil. 1999;210:199–207. doi: 10.1023/A:1004639906245. [DOI] [Google Scholar]
- 115.Moreno O.D., Acevedo Aguilar F.J., Yanez Barrientos E. Selenium uptake and biotransformation and effect of selenium exposure on the essential and trace elements status: Comparative evaluation of four edible plants. J. Mex. Chem. Soc. 2018;62:247–258. [Google Scholar]
- 116.Cartes P., Gianfreda L., Mora M. Uptake of selenium and its antioxidant activity in ryegrass when applied as selenate and selenite forms. Plant Soil. 2005;276:359–367. doi: 10.1007/s11104-005-5691-9. [DOI] [Google Scholar]
- 117.Hawrylak-Nowak B., Matraszek R., Pogorzelec M. The dual effects of two inorganic selenium forms on the growth, selected physiological parameters and macronutrients accumulation in cucumber plants. Acta Physiol. Plant. 2015;37:41. doi: 10.1007/s11738-015-1788-9. [DOI] [Google Scholar]
- 118.Zhang M., Tang S., Huang X., Zhang F., Pang Y., Huang Q., Yi Q. Selenium uptake, dynamic changes in selenium content and its influence on photosynthesis and chlorophyll fluorescence in rice (Oryza sativa L.) Environ. Exp. Bot. 2014;107:39–45. doi: 10.1016/j.envexpbot.2014.05.005. [DOI] [Google Scholar]
- 119.Hawrylak-Nowak B. Comparative effects of selenite and selenate on growth and selenium accumulation in lettuce plants under hydroponic conditions. Plant Growth Regul. 2013;70:149–157. doi: 10.1007/s10725-013-9788-5. [DOI] [Google Scholar]
- 120.Łabanowska M., Filek M., Kościelniak J., Kurdziel M., Kuliś E., Hartikainen H. The effects of short-term selenium stress on Polish and Finnish wheat seedlings—EPR, enzymatic and fluorescence studies. J. Plant Physiol. 2012;169:275–284. doi: 10.1016/j.jplph.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 121.Lehotai N., Kolbert Z., Pető A., Feigl G., Ördög A., Kumar D., Tari I., Erdei L. Selenite-induced hormonal and signalling mechanisms during root growth of Arabidopsis thaliana L. J. Exp. Bot. 2012;63:5677–5687. doi: 10.1093/jxb/ers222. [DOI] [PubMed] [Google Scholar]
- 122.Saffaryazdi A., Lahouti M., Ganjeali A., Bayat H. Impact of selenium supplementation on growth and selenium accumulation on spinach (Spinacia oleracea L.) plants. Not. Sci. Biol. 2012;4:95–100. doi: 10.15835/nsb448029. [DOI] [Google Scholar]
- 123.Schiavon M., Moro I., Pilon-Smits E.A., Matozzo V., Malagoli M., Dalla Vecchia F. Accumulation of selenium in Ulva sp. and effects on morphology, ultrastructure and antioxidant enzymes and metabolites. Aquat. Toxicol. 2012;122:222–231. doi: 10.1016/j.aquatox.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 124.Quinn C.F., Prins C.N., Freeman J.L., Gross A.M., Hantzis L.J., Reynolds R.J., in Yang S., Covey P.A., Bañuelos G.S., Pickering I.J. Selenium accumulation in flowers and its effects on pollination. New Phytol. 2011;192:727–737. doi: 10.1111/j.1469-8137.2011.03832.x. [DOI] [PubMed] [Google Scholar]
- 125.Ramos S.J., Faquin V., Almeida H.J.d., Avila F.W., Guilherme L.R.G., Bastos C.E.A., Avila P.A. Selenate and selenite on yield, mineral nutrition and biofortification with selenium in lettuce cultivars. Revista Brasileira de Ciência do Solo. 2011;35:1347–1355. doi: 10.1590/S0100-06832011000400029. [DOI] [Google Scholar]
- 126.Akbulut M., Çakır S. The effects of Se phytotoxicity on the antioxidant systems of leaf tissues in barley (Hordeum vulgare L.) seedlings. Plant Physiol. Biochem. 2010;48:160–166. doi: 10.1016/j.plaphy.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 127.Freeman J.L., Tamaoki M., Stushnoff C., Quinn C.F., Cappa J.J., Devonshire J., Fakra S.C., Marcus M.A., McGrath S.P., Van Hoewyk D. Molecular mechanisms of selenium tolerance and hyperaccumulation in Stanleya pinnata. Plant Physiol. 2010;153:1630–1652. doi: 10.1104/pp.110.156570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Feng R., Wei C., Tu S., Wu F. Effects of Se on the uptake of essential elements in Pteris vittata L. Plant Soil. 2009;325:123–132. doi: 10.1007/s11104-009-9961-9. [DOI] [Google Scholar]
- 129.Matraszek R., Hawrylak-Nowak B. Macronutrients accumulation in useable parts of lettuce as affected by nickel and selenium concentrations in nutrient solution. Fres. Environ. Bullet. 2009;18:1059–1065. [Google Scholar]
- 130.Hawrylak-Nowak B. Changes in anthocyanin content as indicator of maize sensitivity to selenium. J. Plant Nutr. 2008;31:1232–1242. doi: 10.1080/01904160802134962. [DOI] [Google Scholar]
- 131.Hawrylak-Nowak B. Effect of selenium on selected macronutrients in maize plants. J. Elementol. 2008;13:513–519. [Google Scholar]
- 132.El Kassis E., Cathala N., Rouached H., Fourcroy P., Berthomieu P., Terry N., Davidian J.-C. Characterization of a selenate-resistant Arabidopsis mutant. Root growth as a potential target for selenate toxicity. Plant Physiol. 2007;143:1231–1241. doi: 10.1104/pp.106.091462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mazzafera P. Growth and biochemical alterations in coffee due to selenite toxicity. Plant Soil. 1998;201:189–196. doi: 10.1023/A:1004328717851. [DOI] [Google Scholar]
- 134.Konze J.R., Schilling N., Kende H. Enhancement of ethylene formation by selenoamino acids. Plant Physiol. 1978;62:397–401. doi: 10.1104/pp.62.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Padmaja K., Somasekharaiah B., Prasad A. Inhibition of chlorophyll synthesis by selenium: Involvement of lipoxygenase mediated lipid peroxidation and antioxidant enzymes. Photosynthetica. 1995;31:1–7. [Google Scholar]
- 136.Valkama E., Kivimäenpää M., Hartikainen H., Wulff A. The combined effects of enhanced UV-B radiation and selenium on growth, chlorophyll fluorescence and ultrastructure in strawberry (Fragaria× ananassa) and barley (Hordeum vulgare) treated in the field. Agric. Forest Meteorol. 2003;120:267–278. doi: 10.1016/j.agrformet.2003.08.021. [DOI] [Google Scholar]
- 137.Kopsell D.A., Randle W.M., Mills H.A. Nutrient accumulation in leaf tissue of rapid-cycling Brassica oleracea responds to increasing sodium selenate concentrations. J. Plant Nutr. 2000;23:927–935. doi: 10.1080/01904160009382071. [DOI] [Google Scholar]
- 138.Arvy M., Thiersault M., Doireau P. Relationships between selenium, micronutrients, carbohydrates, and alkaloid accumulation in Catharanthus roseus cells. J. Plant Nutr. 1995;18:1535–1546. doi: 10.1080/01904169509365002. [DOI] [Google Scholar]
- 139.Wu L., Huang Z.-Z. Selenium assimilation and nutrient element uptake in white clover and tall fescue under the influence of sulphate concentration and selenium tolerance of the plants. J. Exp. Bot. 1992;43:549–555. doi: 10.1093/jxb/43.4.549. [DOI] [Google Scholar]
- 140.Pazurkiewicz-Kocot K., Kita A., Pietruszka M. Effect of selenium on magnesium, iron, manganese, copper, and zinc accumulation in corn treated by indole-3-acetic acid. Commun. Soil Sci. Plant Anal. 2008;39:2303–2318. doi: 10.1080/00103620802292343. [DOI] [Google Scholar]
- 141.Landberg T., Greger M. Influence of selenium on uptake and toxicity of copper and cadmium in pea (Pisum sativum) and wheat (Triticum aestivum) Physiol. Plant. 1994;90:637–644. doi: 10.1111/j.1399-3054.1994.tb02518.x. [DOI] [Google Scholar]
- 142.Golubkina N., Kekina H., Caruso G. Yield, quality and antioxidant properties of Indian mustard (Brassica juncea L.) in response to foliar biofortification with selenium and iodine. Plants. 2018;7:80. doi: 10.3390/plants7040080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nawaz F., Ahmad R., Ashraf M., Waraich E., Khan S. Effect of selenium foliar spray on physiological and biochemical processes and chemical constituents of wheat under drought stress. Ecotoxicol. Environ. Saf. 2015;113:191–200. doi: 10.1016/j.ecoenv.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 144.Kinraide T.B. The controlling influence of cell-surface electrical potential on the uptake and toxicity of selenate (SeO42–) Physiol. Plant. 2003;117:64–71. doi: 10.1046/j.0031-9317.2002.00002.x. [DOI] [Google Scholar]
- 145.Bailey F., Knight A., Ogle R., Klaine S. Effect of sulfate level on selenium uptake by Ruppia maritima. Chemosphere. 1995;30:579–591. doi: 10.1016/0045-6535(94)00419-U. [DOI] [Google Scholar]
- 146.White P.J., Bowen H.C., Parmaguru P., Fritz M., Spracklen W., Spiby R., Meacham M., Mead A., Harriman M., Trueman L. Interactions between selenium and sulphur nutrition in Arabidopsis thaliana. J. Exp. Bot. 2004;55:1927–1937. doi: 10.1093/jxb/erh192. [DOI] [PubMed] [Google Scholar]
- 147.Li H.F., McGrath S.P., Zhao F.J. Selenium uptake, translocation and speciation in wheat supplied with selenate or selenite. New Phytol. 2008;178:92–102. doi: 10.1111/j.1469-8137.2007.02343.x. [DOI] [PubMed] [Google Scholar]
- 148.White P.J. Selenium accumulation by plants. Annal. Bot. 2016;117:217–235. doi: 10.1093/aob/mcv180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hondal R.J., Marino S.M., Gladyshev V.N. Selenocysteine in thiol/disulfide-like exchange reactions. Antioxid. Redox Signal. 2013;18:1675–1689. doi: 10.1089/ars.2012.5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Balk J., Pilon M. Ancient and essential: The assembly of iron–sulfur clusters in plants. Trends Plant Sci. 2011;16:218–226. doi: 10.1016/j.tplants.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 151.Hallenbeck P.C., George G.N., Prince R.C., Thorneley R.N. Characterization of a modified nitrogenase Fe protein from Klebsiella pneumoniae in which the 4Fe4S cluster has been replaced by a 4Fe4Se cluster. JBIC J. Biol. Inorg. Chem. 2009;14:673–682. doi: 10.1007/s00775-009-0480-1. [DOI] [PubMed] [Google Scholar]
- 152.Hartikainen H., Xue T., Piironen V. Selenium as an anti-oxidant and pro-oxidant in ryegrass. Plant Soil. 2000;225:193–200. doi: 10.1023/A:1026512921026. [DOI] [Google Scholar]
- 153.Terry N., Zayed A., De Souza M., Tarun A. Selenium in higher plants. Annu. Rev. Plant Biol. 2000;51:401–432. doi: 10.1146/annurev.arplant.51.1.401. [DOI] [PubMed] [Google Scholar]
- 154.Châtelain E., Satour P., Laugier E., Vu B.L., Payet N., Rey P., Montrichard F. Evidence for participation of the methionine sulfoxide reductase repair system in plant seed longevity. Proc. Natl. Acad. Sci. USA. 2013;110:3633–3638. doi: 10.1073/pnas.1220589110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang L., Li Q., Yang X., Xia Z. Effects of sodium selenite and germination on the sprouting of chickpeas (Cicer arietinum L.) and its content of selenium, formononetin and biochanin A in the sprouts. Biol. Trace Elem. Res. 2012;146:376–380. doi: 10.1007/s12011-011-9261-0. [DOI] [PubMed] [Google Scholar]
- 156.Jóźwiak W., Politycka B. Effect of selenium on alleviating oxidative stress caused by a water deficit in cucumber roots. Plants. 2019;8:217. doi: 10.3390/plants8070217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chen Y., Mo H.-Z., Hu L.-B., Li Y.-Q., Chen J., Yang L.-F. The endogenous nitric oxide mediates selenium-induced phytotoxicity by promoting ROS generation in Brassica rapa. PLoS ONE. 2014;9:e110901. doi: 10.1371/journal.pone.0110901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mroczek-Zdyrska M., Wójcik M. The influence of selenium on root growth and oxidative stress induced by lead in Vicia faba L. minor plants. Biol. Trace Elem. Res. 2012;147:320–328. doi: 10.1007/s12011-011-9292-6. [DOI] [PubMed] [Google Scholar]
- 159.Sabbagh M., Van Hoewyk D. Malformed selenoproteins are removed by the ubiquitin–proteasome pathway in Stanleya pinnata. Plant Cell Physiol. 2012;53:555–564. doi: 10.1093/pcp/pcs015. [DOI] [PubMed] [Google Scholar]
- 160.Grant K., Carey N.M., Mendoza M., Schulze J., Pilon M., Pilon-Smits E.A., Van Hoewyk D. Adenosine 5′-phosphosulfate reductase (APR2) mutation in Arabidopsis implicates glutathione deficiency in selenate toxicity. Biochem. J. 2011;438:325–335. doi: 10.1042/BJ20110025. [DOI] [PubMed] [Google Scholar]
- 161.Hugouvieux V., Dutilleul C., Jourdain A., Reynaud F., Lopez V., Bourguignon J. Arabidopsis putative selenium-binding protein1 expression is tightly linked to cellular sulfur demand and can reduce sensitivity to stresses requiring glutathione for tolerance. Plant Physiol. 2009;151:768–781. doi: 10.1104/pp.109.144808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ríos J., Blasco B., Cervilla L., Rosales M., Sanchez-Rodriguez E., Romero L., Ruiz J. Production and detoxification of H2O2 in lettuce plants exposed to selenium. Annal. Appl. Biol. 2009;154:107–116. doi: 10.1111/j.1744-7348.2008.00276.x. [DOI] [Google Scholar]
- 163.Spallholz J.E. On the nature of selenium toxicity and carcinostatic activity. Free Radic. Biol. Med. 1994;17:45–64. doi: 10.1016/0891-5849(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 164.Tamaoki M., Freeman J.L., Pilon-Smits E.A. Cooperative ethylene and jasmonic acid signaling regulates selenite resistance in Arabidopsis. Plant Physiol. 2008;146:1219–1230. doi: 10.1104/pp.107.110742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dimkovikj A., Van Hoewyk D. Selenite activates the alternative oxidase pathway and alters primary metabolism in Brassica napus roots: Evidence of a mitochondrial stress response. BMC Plant Biol. 2014;14:1–15. doi: 10.1186/s12870-014-0259-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Xue T., Hartikainen H., Piironen V. Antioxidative and growth-promoting effect of selenium on senescing lettuce. Plant Soil. 2001;237:55–61. doi: 10.1023/A:1013369804867. [DOI] [Google Scholar]
- 167.Nowak J., Kaklewski K., Ligocki M. Influence of selenium on oxidoreductive enzymes activity in soil and in plants. Soil Biol. Biochem. 2004;36:1553–1558. doi: 10.1016/j.soilbio.2004.07.002. [DOI] [Google Scholar]
- 168.de la Luz Mora M., Pinilla L., Rosas A., Cartes P. Selenium uptake and its influence on the antioxidative system of white clover as affected by lime and phosphorus fertilization. Plant Soil. 2008;303:139–149. doi: 10.1007/s11104-007-9494-z. [DOI] [Google Scholar]
- 169.Aggarwal M., Sharma S., Kaur N., Pathania D., Bhandhari K., Kaushal N., Kaur R., Singh K., Srivastava A., Nayyar H. Exogenous proline application reduces phytotoxic effects of selenium by minimising oxidative stress and improves growth in bean (Phaseolus vulgaris L.) seedlings. Biol. Trace Elem. Res. 2011;140:354–367. doi: 10.1007/s12011-010-8699-9. [DOI] [PubMed] [Google Scholar]
- 170.Gomes-Junior R.A., Gratão P.L., Gaziola S.A., Mazzafera P., Lea P.J., Azevedo R.A. Selenium-induced oxidative stress in coffee cell suspension cultures. Funct. Plant Biol. 2007;34:449–456. doi: 10.1071/FP07010. [DOI] [PubMed] [Google Scholar]
- 171.Bañuelos G., Zambrzuski S., Mackey B. Phytoextraction of selenium from soils irrigated with selenium-laden effluent. Plant Soil. 2000;224:251–258. doi: 10.1023/A:1004881803469. [DOI] [Google Scholar]
- 172.Bañuelos G.S., Arroyo I., Pickering I.J., Yang S.I., Freeman J.L. Selenium biofortification of broccoli and carrots grown in soil amended with Se-enriched hyperaccumulator Stanleya pinnata. Food Chem. 2015;166:603–608. doi: 10.1016/j.foodchem.2014.06.071. [DOI] [PubMed] [Google Scholar]
- 173.Parker D.R., Feist L.J., Varvel T.W., Thomason D.N., Zhang Y. Selenium phytoremediation potential of Stanleya pinnata. Plant Soil. 2003;249:157–165. doi: 10.1023/A:1022545629940. [DOI] [Google Scholar]
- 174.Esringü A., Turan M. The roles of diethylenetriamine pentaacetate (DTPA) and ethylenediamine disuccinate (EDDS) in remediation of selenium from contaminated soil by Brussels sprouts (Brassica oleracea var. gemmifera) Water Air Soil Pollut. 2012;223:351–362. doi: 10.1007/s11270-011-0863-0. [DOI] [Google Scholar]
- 175.Barceló J., Poschenrieder C. Hyperaccumulation of trace elements: From uptake and tolerance mechanisms to litter decomposition; selenium as an example. Plant Soil. 2011;341:31–35. doi: 10.1007/s11104-010-0469-0. [DOI] [Google Scholar]
- 176.Dhillon K.S., Bañuelos G.S. Overview and prospects of selenium phytoremediation approaches. In: Pilon-Smits E.A.H., Lenny H.E.W., Lin Z.-Q., editors. Selenium in Plants. Springer; Cham, Switzerland: 2017. pp. 277–321. [Google Scholar]
- 177.Liu J., Xin X., Zhou Q. Phytoremediation of contaminated soils using ornamental plants. Environ. Rev. 2018;26:43–54. doi: 10.1139/er-2017-0022. [DOI] [Google Scholar]
- 178.Beath O.A., Gilbert C.S., Eppson H. The use of indicator plants in locating seleniferous areas in Western United States. I. General. Am. J. Bot. 1939;26:257–269. doi: 10.1002/j.1537-2197.1939.tb12900.x. [DOI] [Google Scholar]
- 179.Carey A.-M., Scheckel K.G., Lombi E., Newville M., Choi Y., Norton G.J., Price A.H., Meharg A.A. Grain accumulation of selenium species in rice (Oryza sativa L.) Environ. Sci. Technol. 2012;46:5557–5564. doi: 10.1021/es203871j. [DOI] [PubMed] [Google Scholar]
- 180.Pal R., Rai J. The phytoextraction potential of water hyacinth (Eichchornia crassipes): Removal of selenium and copper. Chem. Ecol. 2010;26:163–172. doi: 10.1080/02757541003785833. [DOI] [Google Scholar]
- 181.Bañuelos G., Shannon M., Ajwa H., Draper J., Jordahl J., Licht J. Phytoextraction and accumulation of boron and selenium by poplar (Populus) hybrid clones. Int. J. Phytoremed. 1999;1:81–96. doi: 10.1080/15226519908500006. [DOI] [Google Scholar]
- 182.Zayed A., Gowthaman S., Terry N. Phytoaccumulation of trace elements by wetland plants: I. Duckweed. J. Environ. Qual. 1998;27:715–721. doi: 10.2134/jeq1998.00472425002700030032x. [DOI] [Google Scholar]
- 183.Landesman L., Fedler C., Duan R. Plant nutrient phytoremediation using duckweed. In: Ansari A.A., Gill S.S., Lanza G.R., Rast W., editors. Eutrophication: Causes, Consequences and Control. Springer; Dordrecht, The Netherlands: 2010. pp. 341–354. [Google Scholar]
- 184.Carvalho K.M., Gallardo M.T., McGettigan M.J., Martin D.F. Remediation of selenium contamination by plants and microbes: An annotated bibliography. Fla. Sci. 2000;63:133–141. [Google Scholar]
- 185.Jeke N.N., Zvomuya F., Cicek N., Ross L., Badiou P. Biomass, nutrient, and trace element accumulation and partitioning in cattail (Typha latifolia L.) during wetland phytoremediation of municipal biosolids. J. Environ. Qual. 2015;44:1541–1549. doi: 10.2134/jeq2015.02.0064. [DOI] [PubMed] [Google Scholar]
- 186.Sabogal A., Borkowski D. Estado actual de la investigación sobre Ipomoea carnea: Toxicidad en ganado caprino. Revista Química. 2007;21:29–35. [Google Scholar]
- 187.Pilon-Smits E., De Souza M., Hong G., Amini A., Bravo R., Payabyab S., Terry N. Selenium volatilization and accumulation by twenty aquatic plant species. J. Environ. Qual. 1999;28:1011–1018. doi: 10.2134/jeq1999.00472425002800030035x. [DOI] [Google Scholar]
- 188.Feng R., Wei C. Antioxidative mechanisms on selenium accumulation in Pteris vittata L., a potential selenium phytoremediation plant. Plant Soil Environ. 2012;58:105–110. doi: 10.17221/162/2011-PSE. [DOI] [Google Scholar]
- 189.Pilon-Smits E.A., Hwang S., Lytle C.M., Zhu Y., Tai J.C., Bravo R.C., Chen Y., Leustek T., Terry N. Overexpression of ATP sulfurylase in Indian mustard leads to increased selenate uptake, reduction, and tolerance. Plant Physiol. 1999;119:123–132. doi: 10.1104/pp.119.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Dhillon S., Dhillon K. Phytoremediation of selenium-contaminated soils: The efficiency of different cropping systems. Soil Use Manag. 2009;25:441–453. doi: 10.1111/j.1475-2743.2009.00217.x. [DOI] [Google Scholar]
- 191.Dhillon K., Dhillon S., Thind H. Evaluation of different agroforestry tree species for their suitability in the phytoremediation of seleniferous soils. Soil Use Manag. 2008;24:208–216. doi: 10.1111/j.1475-2743.2008.00143.x. [DOI] [Google Scholar]
- 192.White P.J., Bowen H.C., Marshall B., Broadley M.R. Extraordinarily high leaf selenium to sulfur ratios define ‘Se-accumulator’plants. Annal. Bot. 2007;100:111–118. doi: 10.1093/aob/mcm084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Pilon-Smits E.A., LeDuc D.L. Phytoremediation of selenium using transgenic plants. Curr. Opin. Biotechnol. 2009;20:207–212. doi: 10.1016/j.copbio.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 194.Cappa J.J., Cappa P.J., El Mehdawi A.F., McAleer J.M., Simmons M.P., Pilon-Smits E.A. Characterization of selenium and sulfur accumulation across the genus Stanleya (Brassicaceae): A field survey and common-garden experiment. Am. J. Bot. 2014;101:830–839. doi: 10.3732/ajb.1400041. [DOI] [PubMed] [Google Scholar]
- 195.El Mehdawi A.F., Paschke M.W., Pilon-Smits E.A. Symphyotrichum ericoides populations from seleniferous and nonseleniferous soil display striking variation in selenium accumulation. New Phytol. 2015;206:231–242. doi: 10.1111/nph.13164. [DOI] [PubMed] [Google Scholar]
- 196.El Mehdawi A.F., Reynolds R.J.B., Prins C.N., Lindblom S.D., Cappa J.J., Fakra S.C., Pilon-Smits E.A. Analysis of selenium accumulation, speciation and tolerance of potential selenium hyperaccumulator Symphyotrichum ericoides. Physiol. Plant. 2014;152:70–83. doi: 10.1111/ppl.12149. [DOI] [PubMed] [Google Scholar]
- 197.Cabannes E., Buchner P., Broadley M.R., Hawkesford M.J. A comparison of sulfate and selenium accumulation in relation to the expression of sulfate transporter genes in Astragalus species. Plant Physiol. 2011;157:2227–2239. doi: 10.1104/pp.111.183897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Van Hoewyk D. A tale of two toxicities: Malformed selenoproteins and oxidative stress both contribute to selenium stress in plants. Annal. Bot. 2013;112:965–972. doi: 10.1093/aob/mct163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kikkert J., Berkelaar E. Plant uptake and translocation of inorganic and organic forms of selenium. Arch. Environ. Contam. Toxicol. 2013;65:458–465. doi: 10.1007/s00244-013-9926-0. [DOI] [PubMed] [Google Scholar]
- 200.Van Hoewyk D., Garifullina G.F., Ackley A.R., Abdel-Ghany S.E., Marcus M.A., Fakra S., Ishiyama K., Inoue E., Pilon M., Takahashi H. Overexpression of AtCpNifS enhances selenium tolerance and accumulation in Arabidopsis. Plant Physiol. 2005;139:1518–1528. doi: 10.1104/pp.105.068684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hasanuzzaman M., Hossain M.A., Fujita M. Exogenous selenium pretreatment protects rapeseed seedlings from cadmium-induced oxidative stress by upregulating antioxidant defense and methylglyoxal detoxification systems. Biol. Trace Elem. Res. 2012;149:248–261. doi: 10.1007/s12011-012-9419-4. [DOI] [PubMed] [Google Scholar]
- 202.Malik J.A., Goel S., Kaur N., Sharma S., Singh I., Nayyar H. Selenium antagonises the toxic effects of arsenic on mungbean (Phaseolus aureus Roxb.) plants by restricting its uptake and enhancing the antioxidative and detoxification mechanisms. Environ. Exp. Bot. 2012;77:242–248. doi: 10.1016/j.envexpbot.2011.12.001. [DOI] [Google Scholar]
- 203.Bañuelos G.S. Phytoremediation of selenium contaminated soil, and water produces biofortified products and new agricultural byproducts. In: Bañuelos G.S., Lin Z.Q., editors. Development and Uses of Biofortified Agricultural Products. CRC Press; Boca Raton, FL, USA: 2009. pp. 57–70. [Google Scholar]
- 204.Bhargava A., Carmona F.F., Bhargava M., Srivastava S. Approaches for enhanced phytoextraction of heavy metals. J. Environ. Manag. 2012;105:103–120. doi: 10.1016/j.jenvman.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 205.Chaney R. Plant uptake of inorganic waste. In: Parr J.E., Marsh P.B., Kla J.M., editors. Land Treatment of Hazardous Wastes. Noyes Data Corp.; Park Ridge, IL, USA: 1983. pp. 50–67. [Google Scholar]
- 206.Schmidt U. Enhancing phytoextraction: The effect of chemical soil manipulation on mobility, plant accumulation, and leaching of heavy metals. J. Environ. Qual. 2003;32:1939–1954. doi: 10.2134/jeq2003.1939. [DOI] [PubMed] [Google Scholar]
- 207.Shahid M., Austruy A., Echevarria G., Arshad M., Sanaullah M., Aslam M., Nadeem M., Nasim W., Dumat C. EDTA-enhanced phytoremediation of heavy metals: A review. Soil Sediment Contam. 2014;23:389–416. doi: 10.1080/15320383.2014.831029. [DOI] [Google Scholar]
- 208.Robinson B., Fernández J.-E., Madejón P., Marañón T., Murillo J.M., Green S., Clothier B. Phytoextraction: An assessment of biogeochemical and economic viability. Plant Soil. 2003;249:117–125. doi: 10.1023/A:1022586524971. [DOI] [Google Scholar]
- 209.Wu Q., Wei Z., Ouyang Y. Phytoextraction of metal-contaminated soil by Sedum alfredii H: Effects of chelator and co-planting. Water Air Soil Pollut. 2007;180:131–139. doi: 10.1007/s11270-006-9256-1. [DOI] [Google Scholar]
- 210.Vamerali T., Bandiera M., Lucchini P., Dickinson N.M., Mosca G. Long-term phytomanagement of metal-contaminated land with field crops: Integrated remediation and biofortification. Eur. J. Agron. 2014;53:56–66. doi: 10.1016/j.eja.2013.11.008. [DOI] [Google Scholar]
- 211.Johnsson L. Selenium uptake by plants as a function of soil type, organic matter content and pH. Plant Soil. 1991;133:57–64. doi: 10.1007/BF00011899. [DOI] [Google Scholar]
- 212.Dhillon K., Dhillon S., Dogra R. Selenium accumulation by forage and grain crops and volatilization from seleniferous soils amended with different organic materials. Chemosphere. 2010;78:548–556. doi: 10.1016/j.chemosphere.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 213.Yadav S., Gupta S., Prakash R., Spallholz J., Prakash N. Selenium uptake by Allium cepa grown in Se-spiked soils. Am.-Eur. J. Agric. Environ. Sci. 2007;2:80–84. [Google Scholar]
- 214.Limmer M., Burken J. Phytovolatilization of organic contaminants. Environ. Sci. Technol. 2016;50:6632–6643. doi: 10.1021/acs.est.5b04113. [DOI] [PubMed] [Google Scholar]
- 215.Evans C.S., Asher C., Johnson C. Isolation of dimethyl diselenide and other volatile selenium compounds from Astragalus racemosus (Pursh.) Aust. J. Biol. Sci. 1968;21:13–20. doi: 10.1071/BI9680013. [DOI] [Google Scholar]
- 216.Lewis B., Johnson C., Broyer T. Cleavage of Se-ethylselenomethionine selenonium salt by a cabbage leaf enzyme fraction. Biochim. Biophy. Acta. 1971;237:603–605. doi: 10.1016/0304-4165(71)90281-9. [DOI] [PubMed] [Google Scholar]
- 217.Bañuelos G., Leduc D.L., Pilon-Smits E.A., Terry N. Transgenic Indian mustard overexpressing selenocysteine lyase or selenocysteine methyltransferase exhibit enhanced potential for selenium phytoremediation under field conditions. Environ. Sci. Technol. 2007;41:599–605. doi: 10.1021/es061152i. [DOI] [PubMed] [Google Scholar]
- 218.Terry N., Zayed A.M. Selenium Volatilization by Plants. Volume 343 Marcel Dekker; New York, NY, USA: 1994. [Google Scholar]
- 219.Lin Z.-Q., Terry N., Gao S., Mohamed S., Ye Z. Vegetation changes and partitioning of selenium in 4-year-old constructed wetlands treating agricultural drainage. Int. J. Phytoremed. 2010;12:255–267. doi: 10.1080/15226510903563868. [DOI] [PubMed] [Google Scholar]
- 220.Krishna R., Fulekar M., Patakg B. Rhizofiltration: A green technology for remediation of heavy metals. Int. J. Innov. Bio-Sci. 2012;2:193–199. [Google Scholar]
- 221.Ornes W., Sajwan K., Dosskey M., Adriano D. Bioaccumulation of selenium by floating aquatic plants. Water Air Soil Pollut. 1991;57:53–57. doi: 10.1007/BF00282868. [DOI] [Google Scholar]
- 222.Miranda A., Muradov N., Gujar A., Stevenson T., Nugegoda D., Ball A., Mouradov A. Application of aquatic plants for the treatment of selenium-rich mining wastewater and production of renewable fuels and petrochemicals. J. Sustain. Bioenergy Syst. 2014;4:97–112. doi: 10.4236/jsbs.2014.41010. [DOI] [Google Scholar]
- 223.Nattrass M., McGrew N.R., Morrison J.I., Baldwin B.S. Phytoremediation of selenium-impacted water by aquatic macrophytes1. J. Amer. Soc. Min. Reclam. 2019;8:69–79. doi: 10.21000/JASMR19010069. [DOI] [Google Scholar]
- 224.Lin Z.-Q., Terry N. Selenium removal by constructed wetlands: Quantitative importance of biological volatilization in the treatment of selenium-laden agricultural drainage water. Environ. Sci. Technol. 2003;37:606–615. doi: 10.1021/es0260216. [DOI] [PubMed] [Google Scholar]
- 225.Ohlbaum M., Wadgaonkar S.L., van Bruggen J.J., Nancharaiah Y.V., Lens P.N. Phytoremediation of seleniferous soil leachate using the aquatic plants Lemna minor and Egeria densa. Ecol. Eng. 2018;120:321–328. doi: 10.1016/j.ecoleng.2018.06.013. [DOI] [Google Scholar]
- 226.Carvalho K.M., Martin D.F. Removal of aqueous selenium by four aquatic plants. J. Aquat. Plant Manag. 2001;39:33–36. [Google Scholar]
- 227.Visioli G., D’Egidio S., Sanangelantoni A.M. The bacterial rhizobiome of hyperaccumulators: Future perspectives based on omics analysis and advanced microscopy. Front. Plant Sci. 2015;5:752. doi: 10.3389/fpls.2014.00752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Huysen T., Terry N., Pilon-Smits E. Exploring the selenium phytoremediation potential of transgenic Indian mustard overexpressing ATP sulfurylase or cystathionine-γ-synthase. Int. J. Phytoremed. 2004;6:111–118. doi: 10.1080/16226510490454786. [DOI] [PubMed] [Google Scholar]
- 229.Pilon M., Owen J.D., Garifullina G.F., Kurihara T., Mihara H., Esaki N., Pilon-Smits E.A. Enhanced selenium tolerance and accumulation in transgenic Arabidopsis expressing a mouse selenocysteine lyase. Plant Physiol. 2003;131:1250–1257. doi: 10.1104/pp.102.014639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Garifullina G.F., Owen J.D., Lindblom S.D., Tufan H., Pilon M., Pilon-Smits E.A. Expression of a mouse selenocysteine lyase in Brassica juncea chloroplasts affects selenium tolerance and accumulation. Physiol. Plant. 2003;118:538–544. doi: 10.1034/j.1399-3054.2003.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.LeDuc D.L., Tarun A.S., Montes-Bayon M., Meija J., Malit M.F., Wu C.P., AbdelSamie M., Chiang C.-Y., Tagmount A., de Souza M. Overexpression of selenocysteine methyltransferase in Arabidopsis and Indian mustard increases selenium tolerance and accumulation. Plant Physiol. 2004;135:377–383. doi: 10.1104/pp.103.026989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Ellis D.R., Sors T.G., Brunk D.G., Albrecht C., Orser C., Lahner B., Wood K.V., Harris H.H., Pickering I.J., Salt D.E. Production of Se-methylselenocysteine in transgenic plants expressing selenocysteine methyltransferase. BMC Plant Biol. 2004;4:1. doi: 10.1186/1471-2229-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Bañuelos G., Terry N., LeDuc D.L., Pilon-Smits E.A., Mackey B. Field trial of transgenic Indian mustard plants shows enhanced phytoremediation of selenium-contaminated sediment. Environ. Sci. Technol. 2005;39:1771–1777. doi: 10.1021/es049035f. [DOI] [PubMed] [Google Scholar]
- 234.LeDuc D.L., AbdelSamie M., Móntes-Bayon M., Wu C.P., Reisinger S.J., Terry N. Overexpressing both ATP sulfurylase and selenocysteine methyltransferase enhances selenium phytoremediation traits in Indian mustard. Environ. Pollut. 2006;144:70–76. doi: 10.1016/j.envpol.2006.01.008. [DOI] [PubMed] [Google Scholar]