Abstract
Simple Summary
Tumor cell invasiveness and metastasis are key processes in cancer progression and are composed of many steps. All of them are regulated by multiple microRNAs that either promote or suppress tumor progression. Multiple studies demonstrated that microRNAs target the mRNAs of multiple genes involved in the regulation of cell motility, local invasion, and metastatic niche formation. Thus, microRNAs are promising biomarkers and therapeutic targets in oncology.
Abstract
Tumor cell invasiveness and metastasis are the main causes of mortality in cancer. Tumor progression is composed of many steps, including primary tumor growth, local invasion, intravasation, survival in the circulation, pre-metastatic niche formation, and metastasis. All these steps are strictly controlled by microRNAs (miRNAs), small non-coding RNA that regulate gene expression at the post-transcriptional level. miRNAs can act as oncomiRs that promote tumor cell invasion and metastasis or as tumor suppressor miRNAs that inhibit tumor progression. These miRNAs regulate the actin cytoskeleton, the expression of extracellular matrix (ECM) receptors including integrins and ECM-remodeling enzymes comprising matrix metalloproteinases (MMPs), and regulate epithelial–mesenchymal transition (EMT), hence modulating cell migration and invasiveness. Moreover, miRNAs regulate angiogenesis, the formation of a pre-metastatic niche, and metastasis. Thus, miRNAs are biomarkers of metastases as well as promising targets of therapy. In this review, we comprehensively describe the role of various miRNAs in tumor cell migration, invasion, and metastasis.
Keywords: miRNA, tumor invasiveness, metastasis, cell migration, epithelial–mesenchymal transition, tumor suppressor miR, oncomiR
1. Introduction
Tumor cell invasiveness is one of the hallmarks of cancer defined by Hanahan and Weinberg [1]. During oncogenesis, tumor cells acquire invasive potential, followed by the expansive growth and invasion of adjacent tissues and basement membrane. Tumor invasiveness is regarded as a heterogeneous and multistep process [2]. It is accompanied by angiogenesis, intravasation, and metastasis into the secondary site [3]. Initial steps in metastasis are completed very effectively, whereas the latest steps are very inefficient and are limiting for cancer progression [4]. Only 0.01% of cells that enter the circulation will successfully colonize distant sites [5]. Nonetheless, over 90% of the mortality due to cancer is attributable to metastases [6].
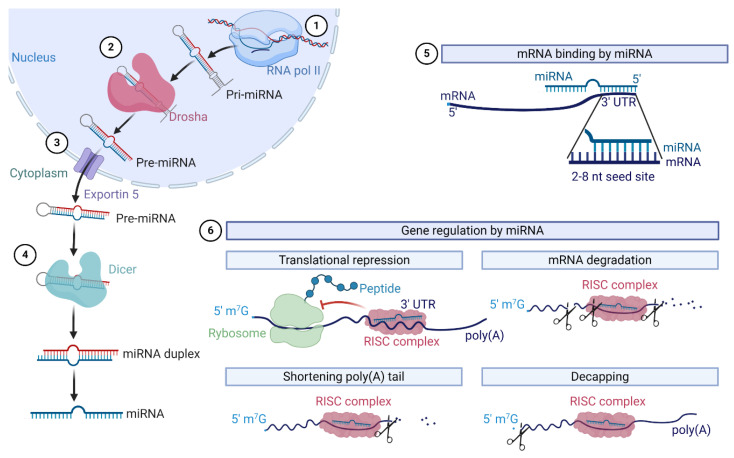
In 1993, Lee et al. described for the first time small RNA molecules encoded by the lin-4 gene regulating the expression of protein lin-14 in Caenorhabditis elegans [7]. Further studies revealed that microRNAs (miRNAs, miRs) are short single-stranded non-coding RNAs that regulate gene expression post-transcriptionally (Figure 1) [8]. Pri-miRNAs are transcribed in the nucleus by RNA polymerase II. Then, pri-miRNAs are cut by a protein complex consisting of Drosha and DGCR8. In the next step, pre-miRNA is exported to the cytoplasm and then cut by Dicer near the loop to form the miRNA duplex [9]. Cooperating with Argonaute proteins, miRNA creates an RNA-induced silencing complex (RISC) that targets mRNA and regulates genes the expression post-transcriptionally [10,11].
Figure 1.
MiRNAs biogenesis and the mechanism of mRNA regulation. The crucial steps in microRNAs biogenesis include (1) transcription by RNA polimerase II; (2) the processing of pri-miRNA by ribonuclease Drosha; (3) transport into the cytoplasm by Exportin 5; and (4) the maturation of miRNA. The mechanism of miRNA action includes binding to the seed site of mRNA (5) and gene regulation by the RNA-induced silencing complex (RISC) complex (6) by translational repression, mRNA degradation, shortening poly(A) tail and the removal of 5′ 7-methylguanylate cap.
The mechanism of this regulation involves the direct silencing of mRNA by the inhibition of the translation or destabilization of mRNA achieved by a shortening poly(A) tail, 5′-to-3′ exonucleolytic decay, and decapping [11]. MiRNAs bind to complementary sequences in the 3′ untranslated region (UTR) of target mRNA [9]. It has been identified that over 60% of human protein-coding genes harbor conserved miRNA target sites [12]. By targeting multiple mRNAs, miRNAs are involved in the regulation of a wide range of cellular processes including cell proliferation, differentiation, and apoptosis. Thus, the dysregulation of miRNAs is involved in the pathogenesis of many diseases, including cancer [13].
MiRNAs may play opposite roles in cancer after being either oncomiRs or tumor suppressor miRs (Table 1) [14]. The complexity of their effects makes them key regulators of all hallmarks of cancer [15]. MiRNAs may affect (promote or suppress) cancer cell proliferation, genomic instability [16], and apoptosis [17]. Moreover, miRNAs regulate tumor cell metabolism [18], angiogenesis [19], and cancer immune escape [20]. MiRNAs may either regulate gene expression in the cell or can be released outside the cell leading to the regulation of gene expression in adjacent cells. Therefore, miRNAs are not only key regulators of cancer cells but also of the complex regulatory network of the tumor microenvironment [21,22,23].
Table 1.
Role of miRNAs in cancer progression.
| miRNA | Cancer Type | Target | Role in Vitro | Role in Vivo | Ref. | |
|---|---|---|---|---|---|---|
| OncomiRs | miR-9 | Breast cancer | CDH1 | ↑ cell migration and invasiveness | ↑ tumor invasion and metastasis ↑ angiogenesis |
[24] |
| miR-10b | Breast cancer | HOXD10 | ↑ cell migration and invasiveness | ↑ tumor invasion and metastasis | [25] | |
| miR-17-5p | Colorectal cancer | PTEN, P130 | ↑ cell migration and invasiveness | ↑ tumor growth | [26,27] | |
| miR-19b | Breast cancer | TP53 | ↑ cell migration and invasiveness, cell cycle progression | ↑ tumor growth and metastasis | [28] | |
| miR-21 | Colorectal cancer | Pdcd4 | ↑ cell invasiveness | ↑ intravasation and metastasis | [29] | |
| miR-135b | Lung cancer | LZTS1, Hippo pathway | ↑ cell migration and invasiveness | ↑ tumor growth and metastasis | [30] | |
| miR-181a | Breast cancer | Bim | ↑ cell migration and invasiveness ↓ anoikis |
↑ tumor growth and metastasis | [31] | |
| miR-214 | Melanoma | TFAP2C, ITGA3 | ↑ cell migration and invasiveness | ↑ extravasation and metastasis | [32] | |
| miR-211 | Colorectal cancer | CHD5 | ↑ cells proliferation and migration | ↑ tumor growth | [33] | |
| miR-223 | Gastric cancer | EPB41L3 | ↑ cells motility and invasiveness | ↑ metastasis | [34] | |
| Tumor suppressor miRs | miR-34a | Neuroblastoma | MAP3K9 | ↑ induction of cell cycle arrest and apoptosis | ↓ tumor growth | [35] |
| miR-137 | Colorectal cancer | FMNL2 | ↓ cells proliferation and invasion | ↓ metastasis | [36] | |
| miR-192 | Colon cancer | Bcl-2, ZEB2 | ↑ apoptosis | ↓ metastasis | [37] | |
| miR-375 | Liver cancer | AEG-1 | ↓ cell growth and invasiveness | ↓ tumor growth | [38] | |
| miR-874 | Non-small cell lung cancer | MMP-2, uPA | ↓ cells invasiveness | ↓ tumor growth | [39] | |
| let-7 | Lung cancer | KRAS | ↓ cell proliferation | ↓ tumor growth | [40] | |
↑—increase, ↓—decrease.
MiRNAs expression is often aberrated in cancers and results in the dysregulation of gene expression. The pan-cancer analysis revealed a global downregulation of tumor suppressors by miRNAs in cancer cells [41]. Many oncogenes, including MYC, were reported to upregulate oncomiRs [9]. Moreover, oncogenes repress the expression of tumor suppressor miRNAs [42]. Mutations in proteins involved in miRNAs biogenesis and maturation, e.g., Drosha or Dicer, lead to the dysregulation of the expression of tumor suppressor miRs or oncomiRs resulting in cancer progression [9]. A study of Merritt et al. showed that the majority of ovarian tumor specimens had decreased Dicer or Drosha mRNA which affects miRNA expression [43]. Dicer is also downregulated by miRNAs, including miR-103/107 [44]. Moreover, epigenetic changes in miRNA promoters affect miRNA expression in cancer tissue [45].
MiRNAs have been reported to regulate every step of cancer progression. They promote or suppress primary tumor growth, local invasion, as well as metastasis (Figure 2). In this review, we aim to comprehensively review the role of miRNAs in the progression from a benign tumor to an invasive metastatic cancer.
Figure 2.
Regulation of cancer progression by miRNAs. Cancer progression involves several crucial steps, including (1) primary tumor growth, (2) migration and local invasion, (3) intravasation, (4) survival in the circulation, (5) extravasation, and (6) pre-metastatic niche formation (stromal cells, brown), recruitment of tumor-promoting immune cells (violet) and metastasis. Multiple miRNAs regulate each of these steps, and thus, act as either oncomiRs (promote cancer progression) or tumor suppressor miRs (suppress cancer progression).
2. Tumor Cell Migration and Local Invasion
Cell migration is necessary for the maintenance of homeostasis in the human body, enabling tissue repair, regeneration, and immune response. However, cell migration is also a crucial driver of cancer invasion and metastasis. It is known that these cells use multiple strategies for migration. Two main types of cells’ movement include single-cell migration and collective cell motility [6]. Single-cell migration is characterized by the lack of cell-to-cell interactions during migration and is divided into amoeboid-like, characteristic for leukemia or lymphoma cells, and mesenchymal migration, which occurs in stromal tumors or epithelial tumors after epithelial–mesenchymal transition (EMT) [2]. The multicellular migration includes collective cell migration [46] and collective cell invasion [47], which are characterized by the migration of a group of cells that retain cell-to-cell adhesions. In the collective cell migration model, the leader cell migrates according to the single-cell model and forms a proteolytic microtrack. It is excavated and expanded by the following multicellular group to form a larger path of migrating cells [2].
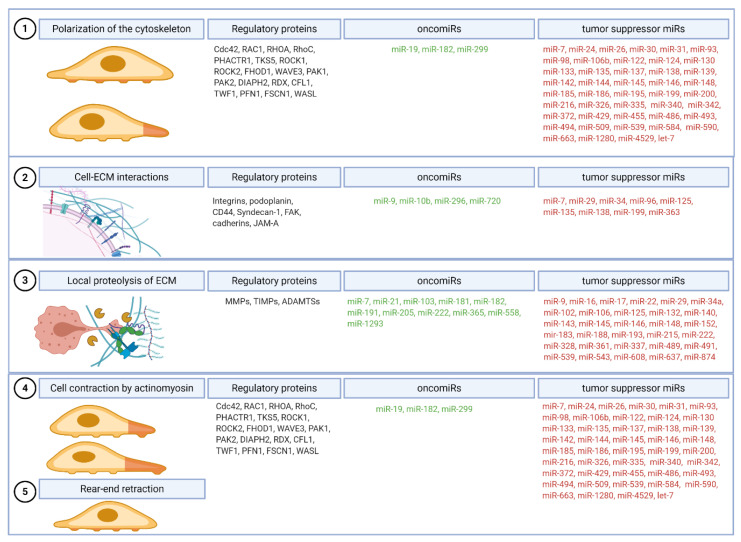
Cancer cell migration and local invasion are heterogeneous processes that include several steps regulated by diverse miRNAs. Multiple models have been established based on in vitro and in vivo studies [2]. In general, single-cell invasive migration consists of five molecular steps (1) the polarization of cytoskeleton by actin polymerization and the formation of pseudopod protrusion, (2) the recruitment and adhesion of cell surface receptors to extracellular matrix (ECM), (3) the focalized proteolysis of ECM, (4) the cell contraction by actomyosin, and (5) the rear-end retraction (Figure 3) [2].
Figure 3.
Stages of invasive cell migration. Five steps are required for successful tumor cell migration. The polarization of the cell cytoskeleton (1) begins the process of cell migration, followed by the formation of focalized clusters by the recruitment and adhesion of cell surface receptors to the extracellular matrix (ECM) (2) and the local proteolysis of ECM (3). Further steps include cell contraction by actomyosin (4) and the rotation of the adhesive bonds on the trailing edge (5). All steps are regulated by oncomiRs that promote each process and tumor suppressor miRs that inhibit cell migration and invasiveness by the regulation of mRNA of regulatory proteins.
2.1. Step 1: Polarization of the Cytoskeleton and Formation of the Leading Protrusion
Cell migration is initiated by the changes in the cell cytoskeleton that result in the formation of the protrusions of the cell membrane. These actin-based structures are termed filopodia, lamellipodia, invadopodia, and podosomes, based on their characters [48]. Despite being regulated mostly on posttranslational levels, multiple miRNAs regulate the reorganization and polarization of the cytoskeleton. The majority of miRNAs targeting the mRNA of cytoskeleton-regulating proteins are tumor suppressors and are downregulated in cancer. The overexpression of those miRNAs affects cytoskeleton remodeling and decreases cell migration and invasiveness.The first step of tumor cell invasion is the formation of the leading edge protrusion, which is controlled mainly by the members of the Rho family of small GTPases, Cdc42 and Rac [2]. Cdc42 is a direct target of miR-133 [49], miR-186 [50], miR-195 [51] and miR-330 [52]. Moreover, Cdc42 is targeted also by miR-137 which inhibits cell invasiveness [53]. Importantly, the expression of miR-137 gradually decreases during cancer progression due to epigenetic silencing at an early stage [54,55]. Cdc42 cooperates with Rac to promote cell invasiveness [56]. RAC1 is targeted by miR-142-3p and miR-145, leading to suppressed cell migration and invasiveness [57,58]. Moreover, miR-124 affects the subcellular localization of RAC1 [59]. MiRNAs regulate not only small GTPases but also their regulators, including guanine nucleotide exchange factors (RhoGEFs), GTPase-activating proteins (RhoGAPs), GDP dissociation inhibitors (RhoGDIs), and guanine nucleotide exchange modifiers (GEMs) [2,60].
Multiple miRNAs have been identified as crucial regulators of the actin cytoskeleton in cancer cells by direct targeting multiple cytoskeleton-associated proteins (Table 2). The reduced expression of miR-138, which targets RhoC and ROCK2, is associated with enhanced metastatic potential in oral squamous cell carcinomas [61]. The overexpression of miR-138 decreases tumor cell migration and invasiveness. Likewise, overexpression of miR-124 targeting ROCK1, a major downstream effector of RhoA and RhoC family members [62], results in the decreased length and number of actin fibers in cells as well as a reduction in long and thin protrusions on the cell surface [63]. Increased let-7b or miR-142-3p expression inhibits the formation of lamellipodia and filopodia which leads to the persistent stabilization of stress fibers [64,65]. MiR-145 promotes actin cytoskeleton rearrangements and cortical actin distribution, but it also reduces actin stress fiber and filopodia formation [66]. Thus, by targeting regulators of the actin cytoskeleton, miRNAs can potently affect cancer cell migration and invasiveness.
Table 2.
Direct regulation of actin cytoskeleton by miRNAs in cancer cells.
| Target | Role | miRNA | Role | Ref. |
|---|---|---|---|---|
| RhoC | Promotes reorganization of the actin cytoskeleton and regulates cell shape and motility. | miR-93, miR-106b, miR-138, miR-372, miR-493, miR-509 |
Decreases migration and invasiveness, reorganization of the stress fibers. | [67,68,69,70,71,72,73] |
| CDC42 | Transduces signals to the actin cytoskeleton, promotes the formation of filopodia. | miR-133, miR-137, miR-186, miR-195, miR-330 |
Decreases cell migration and invasiveness. | [49,50,51,52,53,74] |
| RAC1 | Regulates reorganization of the actin cytoskeleton, promotes the formation of lamellipodia. | miR-142-3p, miR-145 |
Decreases cell migration and invasiveness. | [57,58] |
| RHOA | Regulates cell adhesion and migration, provides contractile force by the formation of stress fibers. | miR-31, miR-122, miR-133a-3p, miR-146a, miR-200b, miR-340-5p |
Decreases cell migration. | [75,76,77,78,79,80] |
| PHACTR1 | Binds actin and regulates the reorganization of the actin cytoskeleton. | miR-584 | Decreases expression leads to the induction of migration. | [81] |
| TKS5 | Regulates actin cytoskeleton and invadopodia formation. | miR-200c | Decreases invasiveness. | [82] |
| MYLK | Regulates the phosphorylation of myosin light chains. | miR-155, miR-200c |
Decreases invasiveness and ability to form invadopodia. |
[82,83] |
| ROCK1 | Regulates actinomyosin cytoskeleton. | miR-124, miR-145, miR-148b, miR-199a, miR-335, miR-340, miR-584, miR-1280 |
Decreases cells migration and invasiveness. | [63,84,85,86,87,88,89] |
| ROCK2 | Regulates actinomyosin cytoskeleton. | miR-124, miR-130a, miR-135a, miR-138, miR-139, miR-144, miR-185, miR-4529-5p |
Decreases cells migration and invasiveness. | [90,91,92,93,94,95,96,97] |
| FHOD1 | Regulates actin cytoskeleton. | miR-200c | Decreases migration and invasiveness. | [98] |
| PPM1F | Regulates actin cytoskeleton. | miR-149, miR-200c, miR-490, miR-590 |
Decreases migration and invasiveness. | [98,99,100,101] |
| WAVE3 | Regulates actin cytoskeleton. | miR-31, miR-200 | Decreases invasiveness. | [102,103] |
| PAK1 | Regulates cell motility and cytoskeletal remodeling. | Let-7, miR-7, miR-26a, miR-26b, miR-98, miR-145, miR-485, miR-494, |
Decreases migration and invasiveness. | [64,103,104,105,106,107,108,109] |
| DIAPH2 | Regulates microtubule attachment to kinetochores. | Let-7, miR-10b |
Decreases migration and invasiveness. | [64,110] |
| RDX | Regulates of membrane domains through interaction with the cytoskeleton and transmembrane proteins. | Let-7, miR-31, mir-200b, miR-409 |
Decreases migration and invasiveness. | [64,111,112] |
| PAK2 | Regulates of cell motility and cytoskeletal remodeling. | miR-7, miR-23b, miR-137, miR-216a, miR-455, miR-4779, |
Decreases migration and invasiveness, cytoskelet remodeling. | [113,114,115,116,117] |
| CFL1 | Binds actin and regulates cell proliferation and migration. | miR-342 | Decreases invasion and migration. | [118] |
| TWF1 | Binds actin and promotes EMT. | miR-30c, miR-142, miR-486 |
Decreases stress fibers F-actin formation. | [119,120,121] |
| PFN1 | Binds actin and inhibits cells proliferation, migration, invasion and EMT. | miR-19a-3p, miR-182, miR-299-3p, miR-330-3p, |
Increases migration and invasiveness. | [122,123,124,125,126] |
| FSCN1 | Stabilizes actin filaments in invadopodia. | miR-24, miR-133a, miR-133b, miR-145, miR-200b, miR-326, miR-429, miR-539, miR-663 |
Decreases invasiveness. | [127,128,129,130,131,132,133,134,135] |
| WASL | Regulates actin polymerization. | miR-142-3p, miR-148a |
Decreases invasiveness, reduced number of membrane protrusions. | [65,136] |
2.2. Step 2: Formation of Focalized Clusters by Recruitment and Adhesion of Cell Surface Receptors to ECM
The second step in the tumor invasion is the activation of signaling pathways that control the tumor cells cytoskeleton as well as the cell-to-cell and cell-to-matrix interactions [2]. Extracellular matrix (ECM) is a key component of the tumor microenvironment [137]. ECM constituents serve as co-receptors, ligands, and signal presenters. Mechanocoupling between the cytoskeleton and ECM is crucial for the initiation of cell migration. For instance, miR-25-3p decreases adhesion to collagens I, II, and IV as to fibronectin, laminin, and tenascin [138]. Cancer cells sense ECM components and their mechanical properties by multiple adhesion and signal-transducing receptors, including integrins, syndecans, or CD44.
2.2.1. Integrins
The main mechanosensors and cell adhesion receptors for ECM are integrins, a family of 24 transmembrane proteins [139]. These heterodimeric αβ receptors bind either ECM proteins or membrane proteins on other cells. The activation of integrins by ligand binding leads to the formation of adhesome, which regulates multiple processes including cell proliferation, survival, differentiation, and migration [140]. Downstream integrin effectors include cytoskeletal adaptor proteins talin, paxillin, and kindlin as well as small GTPases Rac and Rho, that regulate cell protrusion and rear contraction [2]. Thus, the role of integrins goes far beyond only ECM–cell interaction. Moreover, the interaction between integrins and growth factor receptors regulates tumor growth and metastasis [141]. Importantly, the expression of a specific integrin determines the target organs for metastases [142]. Multiple studies reported disturbed integrins expression in cancer, thus, they are a promising target of therapy [143].
Integrin subunits are targeted by multiple miRNAs (Table 3). For instance, miR-31 directly targets integrins α2, α5, αV, and β3 leading to the inhibition of cell spreading in a ligand-dependent manner [144]. Moreover, miR-142-3p targeting integrin αV substantially decreases tumor cell invasiveness [65]. In addition to the direct targeting of integrin-coding mRNA, miRNAs may indirectly affect integrin levels. For example, miR-375 decreases HuD mRNA stability and translation and leads to a reduced expression of N-cadherin, RhoA, NCAM1, and integrin α1 [145].
Table 3.
The direct regulation of integrin subunits by miRNAs in cancer cells.
| Integrin | Protein Name | Synonyms [146] | miRNA | Ref. |
|---|---|---|---|---|
| ITGA1 | α1 | CD49a | miR-375 | [145] |
| ITGA2 | α2 | CD49b, α2 subunit of very late antigen 2 (VLA-2) | miR-31 | [144] |
| ITGA2B | αIIb | GTA, CD41, GP2B, HPA3, CD41b, GPIIb | miR-122 | [147] |
| ITGA3 | α3 | CD49c, α3 subunit of VLA-3 | miR-199 family | [148] |
| ITGA4 | α4 | CD49d, α4 subunit of VLA-4 | miR-30a | [149] |
| ITGA5 | α5 | CD49e, fibronectin receptor alpha | miR-25-3p, miR-26a, miR-31, miR-92, miR-142-3p, miR-148b, miR-149 |
[65,84,138,144,150] |
| ITGA6 | α6 | CD49f, ITGA6B | miR-25, miR-29s, miR-143-3p |
[151,152,153] |
| ITGA7 | α7 | nd | ||
| ITGA8 | α8 | nd | ||
| ITGA9 | α9 | miR-296-3p | [154] | |
| ITGA10 | α10 | miR-34a | [155] | |
| ITGA11 | α11 | miR-148a | [136] | |
| ITGAD | αD | nd | ||
| ITGAE | αE | CD103, human mucosal lymphocyte antigen 1α | nd | |
| ITGAL | αL | CD11a (p180), lymphocyte function-associated antigen 1 (LFA-1) α subunit | miR-126 | [156] |
| ITGAM | αM | Mac-1, CD11b, complement receptor 3 (CR3) subunit | miR-124 miR-223 |
[157,158] |
| ITGAV | αV | CD51, MSK8, vitronectin receptor α (VNRα) | miR-25, miR-31, miR-92, miR-142-3p |
[65,144,151,159] |
| ITGAX | αX | CD11c, CR4 subunit | miR-142 | [160] |
| ITGB1 | β1 | Fibronectin receptor β, CD29, MDF2, MSK12 | miR-29b, miR-29c miR-31, miR-124 miR-130b, miR-149 miR-183, miR-338-3p, miR-451 |
[144,161,162,163,164,165,166,167] |
| ITGB2 | β2 | Leukocyte cell adhesion molecule, CD18, CR3 subunit, CR4 subunit | miR-1, miR-133a | [168] |
| ITGB3 | β3 | CD61; GP3A; GPIIIa, platelet glycoprotein IIIa | miR-31, mir-150 | [144,169] |
| ITGB4 | β4 | CD104 | miR-29a, miR-184 | [170] |
| ITGB5 | β5 | miR-185 | [171] | |
| ITGB6 | β6 | miR-17/20a | [172] | |
| ITGB7 | β7 | nd | ||
| ITGB8 | β8 | let-7, miR-93 miR-145, miR-148a |
[64,136,173] |
nd—no data.
2.2.2. Podoplanin
Podoplanin is a transmembrane glycoprotein that mediates the degradation of ECM via controlling the stability of invadopodia [174]. Moreover, podoplanin binds the ERM (ezrin, radixin, moesin) protein family to enhance RhoA activity and cell invasiveness [175]. MiR-363 targets podoplanin, leading to the inhibition of cell migration and invasion, thus its level is downregulated in metastatic squamous cell carcinoma [176]. Similarly, podoplanin is also a target of miR-29b and miR-125a that are downregulated in glioblastoma [177].
2.2.3. CD44
CD44 is a cell-surface glycoprotein that mediates cell–cell interactions and cell adhesion. CD44 binds to hyaluronic acid and with low affinity to heparan sulfate, fibronectin, and collagen [178,179] It is overexpressed in several cell types, including cancer stem cells [180]. The level of CD44 is strongly regulated by miR-34a, which is a key negative regulator of prostate cancer stem cells [181]. MiR-34a, via targeting CD44, also suppresses angiogenesis [182]. Moreover, CD44 is targeted by miR-199a in ovarian cancer cells and so it suppresses the invasiveness, tumorigenicity, and multidrug resistance [183]. On the other side, the interaction of the CD44 with hyaluronan promotes miR-21 expression leading to the increased expression of anti-apoptotic proteins Bcl-2 and inhibitors of the apoptosis family of proteins (IAPs) [184] as well as increased cell migration and invasiveness [185]. Similarly, CD44–hyaluronan interaction induces the expression of miR-10b, which upregulates RhoA and RhoC resulting in the cytoskeleton activation and increased tumor cell invasiveness [186].
2.2.4. Syndecan-1
Another protein mediating cell–ECM adhesion is syndecan-1 (CD138). It is a heparan sulfate proteoglycan and one of the regulators of integrin function that is involved in the differentiation of tumor cells [187,188]. Syndecan-1 is targeted by miR-10b which promotes cancer cell motility and invasiveness [188].
2.2.5. Focal Adhesion Kinase (FAK)
FAK is a crucial component of the focal adhesion complex and functions as an integrator to control cell migration. FAK transduces extracellular stimuli into intracellular signaling, inducing the reorganization of the cytoskeleton. FAK has been identified as a target of tumor-suppressor miR-7, which inhibits EMT and metastasis [189,190]. Similarly, miR-138 and miR-135 target FAK and inhibit tumor cells invasiveness [191].
2.2.6. Production of ECM
MiRNAs are important modulators of major ECM components expression. MiR-200c targets fibronectin [192]. MiR-29c, which is downregulated in tumor cells, targets mRNA encoding ECM proteins, including collagens I, III, IV, and XV as well as laminin, and osteonectin [193]. Moreover, some miRNAs were reported to regulate collagen maturation, including miR-122 that targets prolyl 4-hydroxylase subunit alpha-1 (P4HA1) [194]. Two tumor suppressor miRNAs, miR-26 and miR-29 target lysyl oxidase-like 2 (LOXL-2), which is a collagen-modifying enzyme, crucial for tissue remodeling and metastasis [195].
2.2.7. Cadherins
In addition to cell–ECM interactions, tumor cells have dysregulated the expression of cell-to-cell adhesion proteins, including cadherins. Changes in the expression of cadherins promote or inhibit cell migration and invasiveness. MiR-9 targets E-cadherin resulting in the activation of β-catenin signaling [24]. Inhibiting miR-9 leads to the inhibition of metastasis formation [24]. Similarly, miR-720 targets E-cadherin and promotes metastasis [196]. Conversely, miR-96 upregulates E-cadherin expression by direct binding, which leads to the enhanced cell-to-cell adhesion [197].
2.2.8. JAM-A
Junctional adhesion molecule A (JAM-A) is a cell–cell adhesion protein and a key regulator of cell migration and invasion [198]. JAM-A has been identified as a direct target of miR-145, which finds its expression downregulated in cancer [66]. An increase in the miR-145 level leads to the inhibition of cell motility and invasiveness [66].
2.3. Step 3: Local Proteolysis of ECM
The proteolysis and remodeling of ECM are crucial for the invasiveness of the cancer cell. Moreover, ECM is a signal reservoir due to the binding of growth factors, sequestering them, and preventing their free diffusion [137]. The degradation of ECM releases growth factors, chemokines, and angiogenic factors, that promote tumor growth, invasiveness, and metastasis [199]. The main enzymes that generate paths for migrating cells are matrix metalloproteinases (MMPs) [200,201]. MMPs are a family of zinc-dependent endopeptidases that degrade all components of ECM. The formation of the invadopodia that promotes the degradation of ECM by the presentation of MMP-14 and secretion of MMP-2 and MMP-9 is a fundamental event during tumor cell invasion [202].
Multiple miRNAs have been identified as directly targeting MMP mRNA. These miRNAs are tumor suppressors and potently decrease tumor cell invasiveness. For instance, the overexpression of miR-874 targeting MMP-2 decreases tumor cells’ invasiveness in vitro as well as decreases tumor growth in vivo [39]. Moreover, downregulated in metastatic cancer miR-29c targets MMP-2 and integrin β1 [203]. The loss of miR-29c increases cell proliferation, adhesion to ECM, as well as migration, and invasiveness [203]. Importantly, miRNAs can also target components of transcription factors regulating MMP expression. Tumor suppressor miR-34a has been identified to target Fra-1 [204,205], a component of activator protein 1 (AP-1) necessary for MMP-1 expression [206]. On the other site, many oncomiRs target negative regulators of MMPs, increasing their expression and activity. A well described oncomiR-21 targets RECK, a membrane-anchored MMP inhibitor, and TIMP3, a tissue inhibitor of MMP activity [207]. The inhibition of miR-21 results in the downregulation of MMP activities and reduced the motility and invasiveness of tumor cells [207].
In addition to MMPs, disintegrin and metalloprotease domains with thrombospondins motifs (ADAMTSs) are important metalloproteases with a complex role in tumor biology [208]. Many of them were reported to be controlled by miRNAs. MiR-140, with a reduced expression in cancer, decreases ADAMTS5, and inhibits cell migration and invasiveness [209]. On the contrary, upregulated miR-365 promotes cell invasion by targeting ADAMTS1 [210]. MiR-221-3p targets ADAMTS6 [211]. Thus, miRNAs are crucial in the regulation of ECM proteolysis (Table 4).
Table 4.
Direct regulation of matrix metalloproteinases (MMPs) by miRNAs in cancer cells.
| MMP | Role | Role in Cancer | miRNA | Ref. |
|---|---|---|---|---|
| Collagenases | ||||
| MMP-1 | Degradation of collagen types I, II, III, V, IX and fibrillary collagen | Initial invasion, metastasis | miR-222, miR-361-5p |
[212,213] |
| MMP-8 | Degradation of collagen types I, II, III, V, IX and fibrillary collagen | Inhibits invasion and metastasis | miR-539, miR-2682-3p | [214] |
| MMP-13 | Degradation of collagens types I, II, III, V, IX and fibrillary collagen | Tumor growth, invasion | miR-125b,miR-148a, miR-188-5p | [215,216] |
| Matrilysins | ||||
| MMP-7 | Proteolysis of fibronectin, collagen type IV, laminin, elastin, entactin, osteopontin, and cartilage proteoglycan aggregates | Invasive potential, proliferation, anti-apoptotic | miR-143, miR-489, miR-543 | [217,218] |
| MMP-26 | Degradation of collagen type IV, fibronectin, fibrinogen, casein, vitronectin, and others | Activates MMP-9 | nd | |
| Metalloelastase | ||||
| MMP-12 | Degradation of elastin | Protective inhibition of tumor growth | nd | |
| Stromelysins | ||||
| MMP-3 | Degradation of collagen types II, III, IV, IX, and X, proteoglycans, fibronectin, laminin, and elastin | Invasion, metastasis, EMT, angiogenesis | miR-17, miR-152 | [219,220] |
| MMP-10 | Degradation of proteoglycans and fibronectin | Invasion, migration, tumor growth | nd | |
| MMP-11 | Degradation of alpha-1 antitrypsin | Early tumor invasion | miR-125a-5p, miR-145, miR-192 | [221,222,223] |
| Gelatinases | ||||
| MMP-2 | Degradation of type IV collagen | Degradation of ECM | miR-29b, miR-29c, miR-106b, miR-874 | [39,203,224,225] |
| MMP-9 | Degradation of type IV collagen | Degradation of ECM | miR-29b, miR-183, miR-491-5p | [226,227,228] |
| Enamelysin | ||||
| MMP-20 | Tooth-specific MMP | nd | ||
| Membrane-Type | ||||
| MMP-14 | Degradation of fibronectin, collagen, and gelatin | Activation of other MMPs | miR-9, miR-22, miR-337-3p | [229,230,231] |
| MMP-15 | EMT, angiogenesis | miR-608 | [232] | |
| MMP-16 | Invasion, metastasis | miR-132, miR-146a, miR-146b, miR-215, miR-328-3p | [233,234,235,236,237] | |
| MMP-17 | Angiogenesis, metastasis | nd | ||
| MMP-24 | Migration, metastasis | nd | ||
| MMP-25 | Tumor growth | nd | ||
| Others | ||||
| MMP-19 | Tumor growth, adhesion, metastasis | miR-16, miR-193b-3p, miR-637 | [238,239,240] | |
| Inhibitors of metalloproteinase | ||||
| TIMP1 | Inhibition of MMP-14 -16, -19, -24 and ADAM10 | Inhibition of cancer growth and metastasis | miR-182, miR-1293 | [241,242] |
| TIMP2 | Inhibition of all MMPs and ADAM12 | Inhibition of cancer growth and metastasis | miR-205-5p | [243] |
| TIMP3 | Inhibition of all MMPs and ADAM10, 12, 17, 28 and 33; ADAMTS-1, -4, and -5, ADAMTS-2 | Inhibition of tumor growth, angiogenesis, and invasion | miR-21, miR-103, miR-181b, miR-191 | [244,245,246,247] |
| TIMP4 | Inhibition of most MMPs and ADAM17d, -28, and -33 | Inhibition of angiogenesis, and invasion, promotion of apoptosis | miR-558 | [248] |
| RECK | Inhibition of MMP-9 | Inhibition of metastasis | miR-7, miR-21, miR-222 | [207,249,250,251] |
nd—no data.
2.4. Step 4: Cell Contraction by Actomyosin, Myosin II Activation by the Small GTPase Rho and Step 5: Rotation of the Adhesive Bonds on the Trailing Edge
Actomyosin is the primary source of force in mammalian cells. Actin filaments are highly plastic and change dynamically in the cell. Actin polymerization and depolymerization are regulated mainly by the Ras homologue (Rho) superfamily of small GTPases [252], that are involved in the control of cell cytoskeleton organization, thus cell motility [253]. MiR-21 targets tropomyosin 1, an actin-binding protein and a putative tumor suppressor [254]. The administration of the miR-21 inhibitor substantially decreases tumor growth [255]. The regulation of the latest stages of invasive cell migration that includes cell contraction and rear-end retraction is similar to the regulation of the first step (Figure 3, Table 1).
Taken together, miRNAs create a complex network of interactions to regulate cell invasiveness (Table 5). The loss of miRNAs suppressing invasiveness is a crucial step during oncogenesis that allows local invasion and metastasis. On the other site, the upregulation of miRNAs promoting invasiveness accelerates cancer progression by increasing cell motility, invasion, and metastasis.
Table 5.
MiRNAs regulating cancer cell invasiveness and their direct targets.
| miRNAs Promoting Invasiveness | miRNAs Suppressing Invasiveness | ||||
|---|---|---|---|---|---|
| miRNA | Targets | Ref. | miRNA | Targets | Ref. |
| miR-9 | SOX7, CDH1, α-catenin | [24,256,257,258] | miR-10b | IGF-1R, HOXA-3, FGF13 | [259,260,261] |
| miR-10b | TP53, PAX6, NOTCH1, HOXD10, TIP30, KLF4, HOXB3 | [259,262,263,264,265] | miR-29c | CDK6, ITGB1, TIAM1, Collagens, Laminin γ1 | [193,266,267,268] |
| miR-21 | PDCD4, maspin, HBP1, LZTFL1, KLF5 | [185,269,270,271,272] | miR-34a | SATB2, BCL-2, HNF4α, Snail, MMP9, MMP14, Notch1 | [273,274,275,276,277,278] |
| miR-103 | DAPK, KLF4, OLFM4, LATS2, PTEN | [279,280,281,282] | miR-135a | ROCK1, Smo, ERRα | [283,284,285] |
| miR-107 | TPM1, DAPK, KLF4 | [279,286] | miR-145 | PAK4, ROCK1, MMP11, Rab27a, MUC1, MMP16, N-cadherin, ZEB2, Ets1, KLF4 | [88,287,288,289,290,291,292,293] |
| miR-135b | NR3C2, LZTS1, APC, FOXO1, ST6GALNAC2, RECK, EVI5 | [30,71,294,295,296,297] | miR-148b | WNT1, MTA2, ROCK1, Dock6 | [298,299,300,301] |
| miR-155 | DOCK-1, SDCBP, ANXA-2, CLDN-1, NDFIP1, SOCS1, TP53INP1, BCL6 | [302,303,304,305,306] | miR-200 | Foxf2, Flt1, BMP4, Onecut2, LIMK1, BMI-1, E2F3 | [307,308,309,310,311] |
| miR-223 | PAX6, hFBXW7, EPB41L3 | [34,312,313] | miR-214 | JAG1, ROCK1, CDC25B, ARL2, GALNT7, MKK3, JAK1 | [314,315,316,317,318,319,320] |
| miR-424 | CADM1, SMAD7 | [321,322] | miR-340 | NT5E, EphA3, SIRT7, NF-κB1, RhoA, ROCK1, JAK1 | [85,323,324,325,326,327,328] |
3. Epithelial–Mesenchymal Transition (EMT)
Epithelial–mesenchymal transition (EMT) is a process that is crucial for embryogenesis, wound healing but also malignant progression. EMT leads to the changes in cell–cell and cell–ECM interactions, that allow the migration of epithelial cells and confer them to the mesenchymal phenotype [329]. The process can be reversed and it is called a mesenchymal–epithelial transition (MET) and is associated with the colonization of distant organs and the formation of metastases [330]. The most important steps are changes in the cell polarity, cytoskeleton and adhesion to other cells and the basement membrane (Figure 4). This allows cells to invade local structures and migrate to distant localizations. Moreover, by undergoing an EMT, carcinoma cells can acquire stem-like cell capabilities such as unlimited self-renewal [331]. EMT is characterized by the downregulation of E-cadherin and the upregulation of N-cadherin and proteases including matrix metalloproteinases such as MMP2 and MMP9 [332]. The most important transcription factors initiating EMT are SNAIL, TWIST, and ZEB. EMT transcription factors regulate the expression of multiple miRNAs [333]. One of the targets of ZEB1 is miR-34a, which regulates multiple properties of tumor cells, including cell migration and invasiveness [333].
Figure 4.
Regulation of epithelial–mesenchymal transition (EMT) by miRNAs. EMT is a process of the acquisition of the mesenchymal features, including motility, invasiveness, and resistance to anoikis of epithelial cells. It is regulated by multiple signaling pathways and transcription factors. Several miRNAs have been identified as either inhibiting or promoting EMT.
All components of EMT signaling pathways are regulated by miRNAs post-transcriptionally [332] (Table 6). In general, EMT is promoted by oncomiRs and inhibited by tumor suppressor miRNAs (Figure 4). A group of oncromiRs called pro-EMT miRNAs promote EMT, tumor cell invasiveness, migration, and metastasis. For example, miR-9 and miR-92a bind to CDH1 which encodes E-cadherin [334]. MiR-10a promotes tumor cell migration and invasion by regulating EMT [335]. In cancer, the maturation of miR-10a is accelerated by XRN2, which leads to increased EMT and metastasis [336]. On the other side, the most important negative regulators of EMT are the miRNA-200 family members. MiR-200s target central regulators of EMT, ZEB1, and ZEB2 [337]. Similarly, miR-22 inhibits EMT via targeting EMT inducer SNAIL and ECM-remodeling MMP14, leading to the suppressed tumor growth, dissemination, and metastasis [231]. MiR-122 triggers a reverse process to EMT, mesenchymal–epithelial transition (MET), and causes cytoskeleton disruption, enhances cell adhesion, and inhibits the migration and invasiveness of cancer cells [80]. A similar effect was exerted by miR-200 family members that induced MET in cancer cell lines [338].The transformation of the growth factor-β (TGF-β) pathway is key signaling inducing EMT. Moreover, TGF-β controls other processes crucial for cancer progression including tumor cell proliferation and invasion. It was shown TGF-β regulates miRNAs expression but is also a target of miRNAs. Several miRNAs were described to be implicated in TGF-β-mediated EMT [339]. Among them, the miR-34 and miR-200 family seem to play the most important role as they form two negative feedback loops with transcription factors involved in EMT. MiR-34 participates with SNAIL1 with the first negative feedback loop and controls the initiation of the EMT process. TGF-β downregulates members of the miR-200 family by the methylation of their promoters and forms an autocrine TGF-β/miR-200b feedback loop. Thus, TGF- β induces EMT by miR-200/ZEB interaction [340]. TGF-β downregulates the expression of miR-584, a negative regulator of PHACTR1 (phosphatase and actin regulator 1), which in turn leads to actin rearrangement and cancer cell migration [81]. Moreover, TGF-β regulates miRNAs targeting adhesion genes [138].
Table 6.
Direct regulation of EMT signaling by miRNAs.
| Target | miRNAs | Ref. |
|---|---|---|
| TGF-β1 | miR-99a, miR-99b, miR-744 | [359,360] |
| TGFBR2 | miR-17 family, miR-21, miR-204, miR-211, miR-373, miR-520 | [361,362,363,364,365] |
| ZEB1 | miR-200 family, miR-205 | [366,367] |
| ZEB2 | miR-132, miR-138, miR-154, miR-200 family, miR-205, miR-215 | [367,368,369,370,371] |
| Twist1 | let-7d, miR-33a, miR-145a-5p, miR-151-5p, miR-214, miR-580 | [372,373,374,375] |
| Twist2 | miR-138, miR-215 | [376,377] |
| Notch | miR-23b, miR-30a, miR-34a, miR-206 | [131,378,379,380] |
| Snail1 | miR-22, miR-34a, miR-137, miR-182 | [231,276,381] |
| Snail2 | miR-30a, miR-124, miR-203, miR-204, miR-211 | [362,382,383,384] |
| EZH2 | miR-138 | [368] |
| Slug | miR-1, miR-30a, miR-124, miR-506, miR-630, miR-590-3p | [385,386,387,388,389,390] |
| N-cadherin | miR-145, miR-194 | [391,392] |
| Vimentin | miR-30c | [393] |
| Fibronectin | miR-1, miR-139, miR-200c, miR-432 | [394,395,396,397] |
| E-Cadherin | miR-10b, miR-22, miR-23b, miR-25, miR-92a, miR-221, miR-720, | [196,398,399,400,401,402,403] |
| ZO-1 | miR-103 | [404] |
| Claudins | miR-98 (claudin-1), miR-146-5p (claudin-12), miR-421 (claudin-11), miR-488 (claudin-2), miR-155 (claudin-1) | [405,406,407,408,409] |
Another crucial signaling pathway for tumor cell invasiveness and metastasis is Wnt/β-catenin. β-catenin-dependent canonical Wnt signaling regulates cell proliferation as well as the development and promotion of EMT, tumor cell invasiveness, and metastasis [341,342]. In the absence of Wnt, β-catenin forms a complex with the tumor suppressor adenomatous polyposis coli gene product (APC), glycogen synthase kinase 3 (GSK3), Axin, and casein kinase 1 (CK1) and is phosphorylated by CK1 and GSK3, which leads to the constant proteasomal degradation of β-catenin. After stimulation with the Wnt ligand, axin is recruited to the membrane complex of the Frizzled (Fz) receptor, low-density lipoprotein receptor-related protein 5 (LRP5) or LRP6 and the scaffolding protein Dishevelled (Dvl), which inhibits the phosphorylation of β-catenin, leading to its stabilization and accumulation in the nucleus [343]. Canonical Wnt signaling is regulated by multiple miRNAs. Wnt1 ligand is targeted by miR-122 [298], miR-148a [344], miR-148b [345], miR-152 [346], miR-329 [347]. Similarly, β-catenin is a target of multiple tumor suppressor miRNAs including miR-33a [348], miR-214 [349], miR-200c [350], miR-320a [351]. WWOX, a β-catenin inhibitor, is targeted by an oncogene, miR-153 [352]. The systemic administration of the miR-153 inhibitor suppressed the development of hepatocellular carcinoma in mice, while tumor cells with upregulated miR-153 expression exhibited increased growth [352]. The expression of miR-374a promotes cell migration and invasiveness by targeting crucial negative regulators of Wnt/β-catenin, including WIF1, PTEN, and WNT5A [353]. Moreover, other components of the Wnt signaling pathway are regulated by miRNAs, including Frizzled-7 by miR-27a [354], LRP6 by miR-202 [355] and miR-432 [356], axin 2 by miR-107 [357], and axin 2 and GSKβ by miR-1246 [358]. Altogether, miRNAs are important regulators of EMT and may either promote or suppress it by targeting different factors (Table 6).
4. Angiogenesis
4.1. Regulation of Angiogenesis by miRNA
Angiogenesis is a process of blood vessel creation. Tumor angiogenesis is classified as one of the hallmarks of cancer [410]. MiRNAs create a regulatory network that controls angiogenesis [411]. MiRNAs target multiple components of the angiogenesis regulatory pathway (Table 7). The tumor cell secretes miRNAs in exosomes to promote the angiogenesis of microvessel endothelial cells [412]. Moreover, multiple miRNAs either promote or inhibit endothelial cell proliferation, migration, and tube formation [408]. MiR-93 produced by glioblastoma cells promotes endothelial cell spreading, growth, migration, and tube formation, stimulating blood vessel formation and supporting tumor growth in vivo [173]. On the other hand, tumor suppressor miRNAs, like the miR-200 family suppress angiogenesis through multiple mechanisms, including targeting IL-8 and CXCL1. Moreover, tumor suppressor miRNAs inhibit crucial signaling pathways including PI3K/AKT, mTOR, and IGF1R pathways to inhibit angiogenesis. Targeting the miRNA-dependent regulation of angiogenesis seems to be a promising therapeutic approach [19]. Noteworthy, MMPs and the tissue inhibitors of metalloproteinases (TIMPs) are crucial for the ECM remodeling and angiogenesis [200], and their regulation by miRNAs was described above.
Table 7.
The direct regulation of the angiogenesis pathway by miRNA.
| Target | miRNA | Ref. |
|---|---|---|
| VEGF | miR-20b, miR-27b, miR-29b, miR-93, miR-126, miR-128, miR-140-5p, miR-195, miR-203, miR-205, miR-497, miR-503, miR-638, | [51,245,413,414,415,416,417,418,419,420,421,422,423,424] |
| VEGFR | miR-378a, miR-497 | [425] |
| NRP1 | miR-141, miR-338 | [426,427] |
| TSP-1 | miR-19a, miR-182, miR-467 | [428,429,430] |
| FGF | miR-503, miR-5582-5p | [421,431] |
| HDGF | miR-139, miR-195, miR-214, miR-497, miR-511, miR-873, miR-939 | [432,433,434,435,436,437,438] |
| Angiogenin | miR-204 | [363] |
| PDGF | miR-29a | [439] |
| HIF1a | miR-20b, miR-33a, miR-107, miR-135b, miR-519c | [440,441,442,443] |
| HIF2a | miR-145 | [58] |
| VHL | miR-21, miR-155, miR-222 | [444,445,446] |
| STAT3 | miR-125a, miR-411, miR-544, miR-874, miR-1299 | [447,448,449,450,451] |
| Bmi-1 | miR-16, miR-132, miR-183, miR-200c, miR-203, miR-218 | [452,453,454,455,456,457] |
| E2F3 | miR-194-5p, miR-200c, miR-217, miR-432, miR-449a | [311,458,459,460,461] |
| NF90 | miR-590-5p | [462] |
4.2. Vascular Endothelial Growth Factor-A (VEGF-A)
VEGF family members are growth factors that play a key role in angiogenesis. They bind to tyrosine kinase cell receptors, VEGFR-1, VEGFR-2, and VEGFR-3. Among them, VEGFR-2 is the most pro-angiogenic receptor [463]. Both, VEGF and VEGFR are regulated by miRNAs in cancer tissues. MiR-140-5p targets VEGF-A and suppresses angiogenesis and cell invasion [413]. Moreover, miR-205 and miR-497 bind directly to VEGF [414,415]. Expression of miR-140-5p, miR-205, and miR-497 targeting VEGF are substantially downregulated in cancer. VEGFR is targeted by miR-378a which acts as a suppressor of cell proliferation and invasion [425]. Furthermore, miR-497 via targeting VEGF suppresses cell proliferation and invasion and inhibits key signaling pathways as MEK/ERK and p38/MAPK [464].
4.3. Thrombospondin-1 (TSP-1)
TSP-1 is a known antioncogenic factor that controls many cellular processes. It influences cell proliferation, invasion, and migration. It functions as a ligand of CD47 [465]. MiR-467 binds to TSP-1 and promotes tumor angiogenesis and thus increases tumor growth. The expression of miR-467 is upregulated in tumor cells [428].
4.4. Platelet-Derived Growth Factor (PDGF)
PDGF, a member of the receptor tyrosine kinases family, is a growth factor involved in angiogenesis, cell proliferation, and migration. MiR-29a targets PDGFC and PDGFA and thus acts as a tumor suppressor [439]. Moreover, PDGF induces the expression of miRNAs, including miR-221, which regulates PDGF-induced EMT and cell migration [466].
4.5. Hypoxia-Inducible Factor 1 Alpha (HIF1a)
HIF-1α is a transcription factor that regulates angiogenesis, cell proliferation, and invasion. In normoxia, the proline residues of HIF-1α are hydroxylated, which is recognized by Hippel–Lindau tumor-suppressor protein (VHL) leading to the degradation in the proteasome [467]. HIF-1α accumulates in the cell under hypoxic conditions. Despite being regulated mainly by posttranslational modification, HIF-1α is also a target of several miRNAs, including miR-20b and miR-107 (Table 7).
VHL is described as a tumor suppressor gene and its inactivation may regulate cancer development and progression [467]. VHL is directly targeted by miR-21 in pancreatic cancer. The inhibition of miR-21 causes the suppression of tumor cell invasiveness via the HIF-1/VEGF pathway and the downregulating of MMP-2 and MMP-9 [445]. Moreover, miR-155 creates a signaling pathway with VHL/HIF/VEGF and regulates angiogenesis and the aggressive malignant phenotype of cancer cells [446].
5. Chemokines and Growth Factors
The mobilization of tumor cells from tissue-fixed state to migrating cells is regulated by multiple factors, including extracellular chemokines and growth factors. Several chemokines, CXCL12, CXCL10, CXCL11, CCL21, and CCL25 were identified as crucial in the induction of cell invasion [2,468]. CXCL12, which promotes invasiveness as well as the recruitment of monocytes to the tumor microenvironment, is a target of miR-342 [469]. The upregulation of miR-342 leads to the inhibition of tumor growth in vivo [469]. Moreover, the receptor for CXCL12, CXCR4, is targeted by miR-613, which suppresses cell invasiveness [470]. MiR-34a downregulates CXCL10 leading to a decrease in cell migration and invasiveness [471]. CCR7, a receptor for CCL21, promotes invasiveness and metastasis as well as regulates actin polymerization and pseudopodia formation is a target of Let-7a [472,473,474]. Moreover, CXCL11, which promotes cell migration, is a target of miR-144 [468,475,476]. Activation of the CXCL12/CXCR4 axis activates RhoA signaling, which regulates actin cytoskeleton and cell motility. This effect is mediated by the upregulation of lncRNA XIST, which acts as a sponge for miR-133a-3p, preventing RhoA downregulation, and promoting tumor cell invasion [76].
MiRNAs are important regulators of all crucial signaling pathways in cancer cells. They regulate the transduction of signaling from the growth factor receptors, including epidermal growth factor receptor (EGFR) [477], and regulate the MAPK signaling pathway [478,479], PI3K/Akt [480], p53 signaling [481], and JAK/STAT pathway [482]. For instance, SOCS2, a negative regulator of the JAK/STAT pathway, is targeted by multiple miRNAs including miR-196a, miR-196b, and miR-194 that promote cell migration, invasion, cell proliferation, and EMT [483,484].
6. Intravasation, Systemic Circulation, and Extravasation
After the local invasion, the tumor begins to grow. A fast increase in the cell number eventually leads to the dissemination of cancer cells to distant sites. Tumor dissemination occurs in early lesions as well as in mid- or late-stage tumors [485]. Cancer cells emigrate from the primary tumor to secondary sites via blood vessels, lymphatic vessels, interstitial fluid, and nerves [485,486,487]. Most miRNAs regulate multiple steps of metastasis, conferring tumor cells the ability to spread. For instance, miR-182, markedly overexpressed in metastatic cancer, targets four metastasis-suppressing genes [242]. The inhibition of miR-182 decreases cell migration and invasiveness as well as decreases the rate of tumor cells’ intravasation and metastasis to the lungs [242]. The first and critical step for metastasis is intravasation. To do this, tumor cells have to overcome the barrier of the basement membrane and the wall of the vessel.
6.1. Intravasation
Tumor cells secrete miR-105, which targets ZO-1, the tight junction protein-1 in endothelial cells. The exosome-mediated transfer of miR-105 from cancer cells destroys the integrity of endothelial monolayers, which enable intravasation [488]. Additionally, miR-181a disrupts the endothelial barrier by targeting Kruppel-like factor 6 (KL-F6), leading to the decreased expression of ZO-1, occluding, and claudin-5, which results in blood–tumor barrier permeability [489]. Tumor cells intravasation is also promoted by miR-21, which targets tumor suppressor Pdcd4 [29].
6.2. Systemic Circulation
Most of the tumor cells in the circulation are either killed by immune cells or die in the process called anoikis [490,491]. Natural killer (NK) cells are the main immune cells eliminating circulating tumor cells, thus suppressing metastasis. Circulating tumor cells use multiple mechanisms to escape from NK, including coating with platelets [492] and alterations in the expression of MHC molecules, NK cell ligand, and immune-checkpoints [490]. Importantly, miR-296-3p, which is overexpressed in metastatic cells, targets ICAM-1, rendering resistance to NK cells lysis in vasculature [491]. Moreover, Dicer-generated miR-222 and miR-339 suppress ICAM-1 on tumor cells, leading to the decreased susceptibility to cytotoxic T-cells cytolysis [490,491,493]. ICAM-1 is also a target of miR-296-3p, which enables invasiveness, intravasation, and enhances the survival of NK-resistant circulating tumor cells [491].
Anoikis is a form of programmed cell death induced by the loss of contact with the ECM or with other cells [494]. Anoikis depends on the activation of caspase and downstream pathways that includes the intrinsic and extrinsic apoptotic pathways [494]. Many miRNAs have been identified as crucial in the promotion or prevention of anoikis. MiR-141 enhances anoikis resistance and metastasis by targeting KLF12 [495]. Similarly, miR-214 promotes cell survival contributing to the enhanced metastasis of melanoma cells [32].
6.3. Extravasation
The extravasation of tumor cells determines their metastatic potential. Tumor cells were found to secrete extracellular vesicles (EVs) loaded with multiple miRNAs that are transferred to endothelial cells leading to changes in vascular permeability. Tumor-derived exosomes containing miR-181c are capable of destructing the blood–brain barrier by the dysregulation of the actin cytoskeleton via targeting PDPK1 [496]. Similarly, exosomal miR-25-3p promotes vascular permeability and angiogenesis by targeting KLF2 and KLF4, regulating tight junction proteins [497]. Moreover, miR-214 has been identified as crucial in the promotion of metastasis by an enhancement of extravasation [498]. On the other side, p38 activated by IL-1β promotes the expression of miR-31, which targets E-selectin [499]. This in turn leads to the decreased adhesion to the endothelium and inhibited transendothelial migration of tumor cells [499]. Similarly, tumor-suppressors miR-148b as well as miR-155 inhibit metastasis formation by affecting extravasation and survival [84,500].
7. Metastatic Colonization
The last stage of tumor invasion is the colonization of the secondary site. Metastasis to the sentinel lymph node is the most common and the most reliable factor for survival predicting in patients with different types of cancer [501]. Furthermore, tumor cells exit lymphatics, enabling systemic dissemination [502]. Tumor cells modulate premetastatic niches to enable the settlement and metastasis growth in tumor-draining lymph nodes or distant organs [5,142,501]. MiRNAs regulate this process either directly in tumor cells, promoting their migration, invasiveness, and survival or by affecting other cells in the premetastatic niches.
Tumor cells secrete multiple factors that reach distant sites via body fluids—blood, lymph, and interstitial fluid. The pro-metastatic secretome includes pro-angiogenic VEGF, PLGF, immunomodulating TGF-β, and S100 family proteins [142]. Moreover, tumors secrete extracellular vesicles that contain multiple proteins and miRNAs to prepare premetastatic niches [142,503]. It makes the tissue microenvironment supportive and receptive for the colonization by the metastatic tumor cell, according to the seed and soil hypothesis [504,505,506]. Premetastatic niche formation includes ECM remodeling, angiogenesis [507], and immune cell education towards a pro-metastatic phenotype [503]. All these processes are regulated by miRNAs in EVs. Secreted miRNAs, including miR-105-5p, miR-21-5p, miR-139-5p, regulate ECM remodeling by increasing the expression of MMPs in fibroblasts, as well as stimulate their proliferation creating a premetastatic niche [508]. Moreover, miR-122 in tumor-secreted extracellular vesicles reprograms the metabolism of stromal cells favoring a premetastatic microenvironment [509,510]. An exceptional pro-angiogenic and pro-metastatic role has been attributed to cancer stem cell (CSC)-released EVs containing miRNAs that regulate crucial biological processes [507,508].
Additionally, it seems that the secretion of tumor-suppressor miRNAs in the exosome is one of the mechanisms for decreasing their levels. Tumor cells secrete tumor-suppressors, including miR-23b, miR-224, and miR-921, which inhibit cell invasiveness, anoikis, angiogenesis, and metastasis [511]. Tumor suppressor miRNAs, miR-26, and miR-29, by targeting LOXL2, suppress tumor metastasis and the recruitment of myeloid cells to the metastatic site [195]. Moreover, miR-203 acts as a tumor suppressor miR and quells cancer cell proliferation and invasion [512]. However, miR-203 in exosome secreted by tumor cells promotes the polarization of monocytes into tumor-associated macrophages, thus supporting metastatic niche formation [512].
8. Tumor–Stroma Interactions
8.1. Cancer-Associated Fibroblasts (CAFs– Tumor Cells Interactions
Tumor cells and non-cancerous stromal cells interact with each other. Importantly, tumor invasiveness and metastasis greatly depend on stromal cells. Among the crucial stromal cells that induce ECM remodeling and enable cancer cell invasion are cancer-associated fibroblasts (CAFs) [513]. Cancer cells acquire migratory properties by the interaction between integrin α5β1 and fibronectin on the surface of CAFs, which enables migration through the ECM [514]. Tumor cells dysregulate miRNAs expression in resident fibroblasts favoring their polarization into tumor-promoting CAFs [515,516]. Many miRNAs have been identified as regulating CAF activation, including the miR-31, miR-214, miR-155 [515], and miR-200 family [517]. MiR-200s regulate collagen contraction by CAFs as well as trigger ECM remodeling, invasion, and tumor metastasis [517]. Similarly, miR-222 regulates CAFs’ reprogramming and its overexpression promotes fibroblast-induced cancer cell migration and invasiveness [518].
Moreover, also stromal cells secrete exosomes that regulate tumor cells. For instance, astrocytes secrete exosomes that contain miR-345 targeting KISS1, upregulate autophagy and promote brain invasion [519]. Additionally, astrocytes-derived exosomes contain miRNAs targeting PTEN, leading to its loss in brain metastasis [520].
Bones are a frequent location of solid tumors metastases. Tumor cells secrete factors that dysregulate miRNA expression in osteoclast, favoring bone metastasis and osteolysis [521]. Moreover, cancer-derived miRNA-218 decreases the production of type I collagen by directly targeting Col1a1 in preosteoblasts [522].
8.2. Immune Cells–Tumor Cells Interactions
MiRNAs are also important regulators of immune cells in the tumor microenvironment [22,523]. Tumor cells secrete miRNAs to directly suppress the antitumor response. The high expression of miR-424 in tumors decreases T-cell activation [524]. Similarly, a high level of miR-23a and miR-29a impairs the antitumor activity of cytotoxic T lymphocytes [525,526]. MiR-10b upregulated in tumor cells suppresses NK-mediated killing of tumor cells via targeting stress-induced cell surface molecule MICB [527].
In addition to directly suppressing antitumor immunity, miRNAs induce the polarization of immune cells [528]. Tumor cells as well as tumor-associated stromal cells secrete miRNAs that hijack immune cells to polarize them into immunosuppressive, tumor-promoting cells. MiRNAs may be secreted in extracellular vesicles, in the complexes with RNA-binding proteins including AGO2 and nucleophosmin, with lipoproteins, or by the direct exchange between cell via gap junctions [23,529]. In addition to directly suppressing antitumor immunity, miRNAs induce the polarization of immune cells [530]. Among others, cancer cells secrete miR-1246, which is delivered to macrophages and triggers the increased activity of TGF-β and an anti-inflammatory phenotype [530]. Similar effects are exerted by tumor-secreted miR-21 [531], miR-22-3p [532] and miR-203 [512]. Moreover, tumor-associated macrophages secrete miR-223 that promotes the invasiveness of tumor cells [533].
MiRNAs are also involved in the regulation of immune cell recruitment into the tumor microenvironment. MiR-155 enables the infiltration of innate immune cells and the suppression of antitumor immunity [534]. MiR-494 regulates the accumulation of myeloid-derived suppressor cells (MDSCs) and the inhibition of the miR-494 suppressed tumor growth and metastasis [535]. Moreover, miR-494 promotes arginase expression in MDSCs [535], which is crucial for the suppression of antitumor immunity [536]. Elevated TGF-β suppresses miR-34a which targets CCL22. Increased CCL22 production recruits regulatory T cells, which creates an immunosuppressive microenvironment and favors the colonization of tumor cells [537]. On the other hand, TGF-β promotes miR-155 and miR-21 expression in myeloid cells favoring polarization into immunosuppressive MDSCs [538].
9. miRNAs as Biomarkers in Cancer
Due to the dysregulated pattern of miRNA expression in cancer, miRNAs arose as promising biomarkers [539,540]. MiRNA profiling is feasible because of the stability of miRNAs and their presence in different body fluids, fresh frozen tissues, and even routinely collected formalin-fixed paraffin-embedded (FFPE) tumor tissue [541]. Multiple miRNAs have been identified as diagnostic or prognostic biomarkers [542,543]. Moreover, several miRNAs have been reported as an important prognostic marker of lymph node metastasis and distant organ metastasis (Table 8). MiR-21 which inhibits apoptosis [184] and potently promotes invasiveness (Table 5), correlates with the lymph node metastasis in many types of cancer [544], including breast cancer [545] and pancreatic ductal adenocarcinoma [546]. Similarly, miR-10b increases tumor cell migration, and invasiveness is a biomarker of distant metastasis in colorectal cancer [547]. Furthermore, as miRNAs regulate tumor cells’ response to the therapy [548], there are promising tools to monitor anticancer treatment.
Table 8.
MiRNAs as biomarkers of metastasis.
| miRNA | Cancer | Type of Tissue | Mirna Level | Lymph Node Metastasis | Distant Metastasis | Ref. |
|---|---|---|---|---|---|---|
| miR-203 | Colorectal | Serum | High | OR 2.9; 95%CI 1.4–6.1; p = 0.0035 | OR 5.3; 95%CI 2.4–11.5; p < 0.0001 | [549] |
| miR-885-5p | Colorectal | Serum | High | OR 3.0; 95%CI 1.3–7.2; p = 0.0116 | OR 3.1; 95%CI 1.0–10.0; p = 0.0456 | [547] |
| miR-19a | Various carcinomas | Serum and tissue | High | OR 0.564; 95%CI 0.346–0.921 | nd | [550] |
| miR-20a | Cervical | Serum | High | OR 1.552; 95%CI 1.137–2.118 | nd | [551] |
| miR-21 | Breast | Serum and tissue | High | OR 2.36; 95%CI 1.04–4.78; p = 0.03 | nd | [545] |
| miR-21 | Pancreatic ductal adenocarcinoma | Serum and tissue | High | OR 1.45; 95%CI 1.02–2.06; p = 0.038 | [546] | |
| miR-122-5p | Colorectal | Serum | High | OR 1.621; 95%CI 1.255–2.092; p = 0.0002 |
nd | [552] |
| miR-146b-5p | Colorectal | Serum | High | OR 2.096; 95%CI 1.594–2.756; p < 0.0001 |
nd | [552] |
| miR-186-5 | Colorectal | Serum | High | OR 2.910; 95%CI 1.810–4.678; p < 0.0001 |
nd | [552] |
| miR-193a-5p | Colorectal | Serum | High | OR 0.656; 95%CI 0.577–0.774; p < 0.0001 |
nd | [552] |
| let-7i | Colorectal | Tissue | Low | nd | OR 5.5; 95%CI 1.1–26.8; p = 0.0334 |
[547] |
| miR-10b | Colorectal | Tissue | High | nd | OR 4.9; 95%CI 1.2–19.7; p = 0.0248 | [547] |
| miR-29a | Colorectal | Serum | High | nd | OR 3.500; 95%CI 1.274–9.617; p < 0.05 | [553] |
OR—odds ratio, 95%CI—95% confidence interval, nd—no data.
10. Challenges for the Use of miRNAs in Clinical Oncology
MiRNAs seem to have the potential for therapeutic use [554,555,556]. However, the first clinical trials did not live up to expectations. The first trial tested the miR-34-based compound—MRX34—in several types of cancer. X34 is a liposomal miR-34a-mimic that entered the phase I study. MiR-34a is a tumor suppressor miRNA which targets several genes from the different oncogenic pathway. The results confirmed antitumor actions and showed acceptable safety when used twice a week in patients with different solid tumors in the advanced stadium [557]. However, further studies were terminated due to serious adverse events (NCT01829971, NCT02862145) [558]. Other compounds tested in clinical trials involved TargomiRs, targeted minicells containing miR-16 family (NCT02369198, NCT03713320) [559,560], and cobomarsen, an oligonucleotide inhibitor of miR-155 (NCT03837457, NCT02580552) [561].
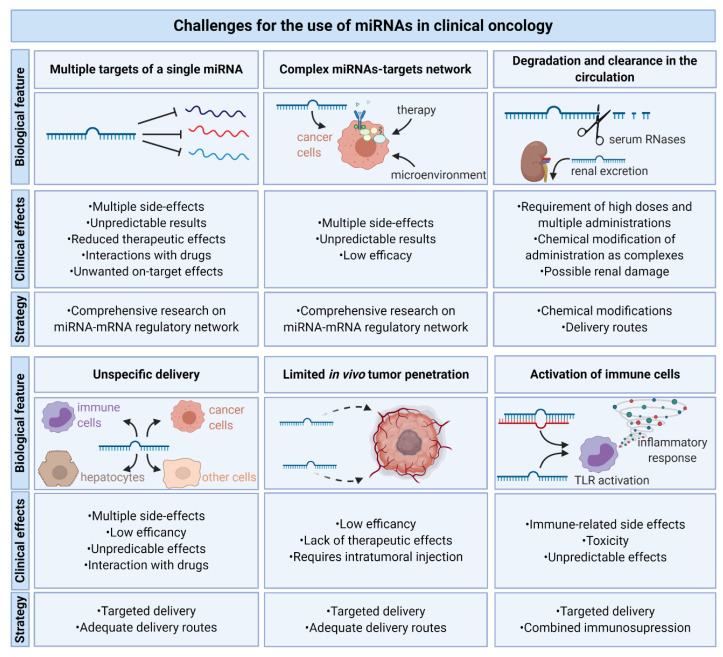
Despite great expectations, only a few miRNA-based therapies were tested in clinical trials and did not achieve satisfactory effects. There are several crucial challenges for the use of miRNAs in oncology that limit their efficacy (Figure 5). The most important biological feature of miRNAs that leads to unpredictable results as well as putative multiple side-effects is the complexity of miRNAs–targets network. That is, numerous pathways are affected, hence any miRNA-based therapy will always have diverse effects depending on the initial expression of their targets. Moreover, miRNA off-targets on mismatched targets must be taken into account [562].
Figure 5.
Challenges for the use of miRNAs in clinical oncology. Biological features of miRNAs include multiple targets, the complexity of the miRNAs–mRNAs network, the degradation by RNases and clearance in the circulation via renal excretion, unspecific delivery—not only to their destination, but also to healthy unaffected tissue including hepatocytes, limited in vivo tumor penetration and the activation of immune cells. This leads to a lack of therapy efficiency and there is a need for further comprehensive research on the miRNA–mRNA network and miRNAs’ delivery methods to overcome them.
MiRNAs act as modulators of the levels of multiple rather than only strong post transcriptional repressors [563]. However, many targets of miRNAs remain unknown as most studies focus on simple miRNA–target axes. Importantly, the upregulation of a single miRNA affects the global regulation of gene expression by endogenous miRNAs [564]. Therefore, comprehensive studies on miRNA–mRNA interactions with the use of high-throughput methods are required.
Recent advances provided reliable models to investigate the complex role of miRNAs in cancer [565], since the role of miRNA depends on the context and may be modulated by the tumor microenvironment or therapy. Thus, miRNAs must be tested in in vivo preclinical studies in different models, since single miRNA may act as either oncomiR or tumor-suppressor miR, even in similar tumor types. Xenograft models of human tumor-derived cells in immune deficient mice are the most reliable to evaluate the in vivo potential of miRNAs as well as their therapeutic potential [565].
Importantly, regardless of their relatively high stability, unmodified miRNAs administered to the circulation are degraded quickly by serum RNases [566]. Thus, chemical modifications are required to increase miRNA stability, providing their longer half-life time and higher therapeutic efficacy [567,568]. Another major limitation of miRNAs therapy, common to almost all types of gene therapy, is the targeted delivery of oligonucleotides to cancer cells. Unmodified miRNAs poorly penetrate the cell membrane. Therefore, delivery vehicles are required. Currently, different types of vectors are being tested for miRNA delivery, including inorganic materials, lipid-based nanocarriers, cell-derived membrane vesicles and viral vectors [568].
miRNA delivery is limited by several factors including limited tumor penetration and unavoidable yet undesired delivery to healthy tissues, including immune cells and hepatocytes. Over 60% of lipid-conjugated miRNAs are accumulated in the liver [569]. This leads to multiple side effects, unpredictable results, interactions with other drugs, low therapy efficiency or a lack of therapeutic effects. Intratumor injections, targeted delivery, or adequate delivery routes may overcome these obstacles. The more targeted delivery of miRNAs to the tumor increases the amount of miRNA that reach destination cells [570]. A tumor targeting antibody-guided nanoparticles with miR-34a effectively reached the metastasis of melanoma, which increased the amount of absorbed miRNA in tumor cells [571,572]. A similar approach was tested in patient-derived xenografts of pancreatic ductal adenocarcinoma. Tumor-penetrating nanocomplexes targeting cell surface proteins carrying antimiR oligonucleotides inhibiting identified oncomiRs, which potently suppressed tumor growth [573].
The administration of high doses of miRNAs also increases a high risk of immune cell activation. Exogenous single-stranded RNAs and double-stranded duplexes are recognized by Toll-like receptors (TLRs), triggering the expression of pro-inflammatory cytokines, including interferons (IFNs) [574,575]. Indeed, the first-in-human clinical trial of miRNA therapy was closed early due to serious immune-related adverse effects that resulted in patients deaths [558].
Considering all the aforementioned limitations, despite a huge progress in our understanding of miRNA engagement in cancer, before successfully entering clinical medicine, more comprehensive studies are required. Not only on the mechanisms of miRNAs action, but also on the safety and specificity of miRNAs delivery.
11. Conclusions
In recent years, significant progress has been made in understanding the role of miRNAs in orchestrating cancer progression. Molecular studies enabled the identification of tumor suppressor genes and oncogenes as direct targets of miRNAs. Multiple reports described the role of miRNAs in promoting or suppressing tumor cell proliferation, migration, invasiveness, and metastasis. Moreover, miRNAs are important players in chemoresistance and tumor immune evasion. Importantly, the function of miRNAs is tissue specific as well as context dependent. Single miRNA may act as oncomiR that promotes tumor cell invasiveness and metastasis in one type of cancer but in another type of tumor it can act as a suppressor miR [14]. Some oncomiRs are specific biomarkers and their inhibition seems to be a promising therapeutic approach. However, due to fact that a single miRNA can target multiple mRNAs, further research and careful data analysis are necessary.
Acknowledgments
Figures were generated using Biorender.com.
Author Contributions
Conceptualization, T.M.G.; writing—original draft preparation, T.M.G., K.K.; writing—review and editing, T.M.G., K.K., P.K.W. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by Medical University of Warsaw.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hanahan D., Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Friedl P., Alexander S. Cancer invasion and the microenvironment: Plasticity and reciprocity. Cell. 2011;147:992–1009. doi: 10.1016/j.cell.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 3.Jiang W.G., Sanders A.J., Katoh M., Ungefroren H., Gieseler F., Prince M., Thompson S.K., Zollo M., Spano D., Dhawan P., et al. Tissue invasion and metastasis: Molecular, biological and clinical perspectives. Semin. Cancer Biol. 2015;35:S244–S275. doi: 10.1016/j.semcancer.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Chambers A.F., Groom A.C., MacDonald I.C. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 5.Doglioni G., Parik S., Fendt S.-M. Interactions in the (Pre)metastatic Niche Support Metastasis Formation. Front. Oncol. 2019;9:219. doi: 10.3389/fonc.2019.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valastyan S., Weinberg R.A. Tumor metastasis: Molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee R.C., Feinbaum R.L., Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-Y. [DOI] [PubMed] [Google Scholar]
- 8.Gebert L.F.R., MacRae I.J. Regulation of microRNA function in animals. Nat. Rev. Mol. Cell Biol. 2019;20:21–37. doi: 10.1038/s41580-018-0045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin S., Gregory R.I. MicroRNA biogenesis pathways in cancer. Nat. Rev. Cancer. 2015;15:321–333. doi: 10.1038/nrc3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ha M., Kim V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 11.Bartel D.P. Metazoan MicroRNAs. Cell. 2018;173:20–51. doi: 10.1016/j.cell.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedman R.C., Farh K.K.-H., Burge C.B., Bartel D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Condrat C.E., Thompson D.C., Barbu M.G., Bugnar O.L., Boboc A., Cretoiu D., Suciu N., Cretoiu S.M., Voinea S.C. miRNAs as Biomarkers in Disease: Latest Findings Regarding Their Role in Diagnosis and Prognosis. Cells. 2020;9:276. doi: 10.3390/cells9020276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grzywa T.M., Klicka K., Rak B., Mehlich D., Garbicz F., Zieliński G., Maksymowicz M., Sajjad E., Włodarski P.K. Lineage-dependent role of miR-410-3p as oncomiR in gonadotroph and corticotroph pituitary adenomas or tumor suppressor miR in somatotroph adenomas via MAPK, PTEN/AKT, and STAT3 signaling pathways. Endocrine. 2019;65:646–655. doi: 10.1007/s12020-019-01960-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalmay T., Edwards D.R. MicroRNAs and the hallmarks of cancer. Oncogene. 2006;25:6170–6175. doi: 10.1038/sj.onc.1209911. [DOI] [PubMed] [Google Scholar]
- 16.Vincent K., Pichler M., Lee G.-W., Ling H. MicroRNAs, genomic instability and cancer. Int J. Mol. Sci. 2014;15:14475–14491. doi: 10.3390/ijms150814475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shirjang S., Mansoori B., Asghari S., Duijf P.H.G., Mohammadi A., Gjerstorff M., Baradaran B. MicroRNAs in cancer cell death pathways: Apoptosis and necroptosis. Free Radic. Biol. Med. 2019;139:1–15. doi: 10.1016/j.freeradbiomed.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 18.Pedroza-Torres A., Romero-Córdoba S.L., Justo-Garrido M., Salido-Guadarrama I., Rodríguez-Bautista R., Montaño S., Muñiz-Mendoza R., Arriaga-Canon C., Fragoso-Ontiveros V., Álvarez-Gómez R.M., et al. MicroRNAs in Tumor Cell Metabolism: Roles and Therapeutic Opportunities. Front. Oncol. 2019;9:1404. doi: 10.3389/fonc.2019.01404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y., Wang L., Chen C., Chu X. New insights into the regulatory role of microRNA in tumor angiogenesis and clinical implications. Mol. Cancer. 2018;17:22. doi: 10.1186/s12943-018-0766-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yi M., Xu L., Jiao Y., Luo S., Li A., Wu K. The role of cancer-derived microRNAs in cancer immune escape. J. Hematol. Oncol. 2020;13:25. doi: 10.1186/s13045-020-00848-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bayraktar R., Van Roosbroeck K., Calin G.A. Cell-to-cell communication: MicroRNAs as hormones. Mol. Oncol. 2017;11:1673–1686. doi: 10.1002/1878-0261.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paladini L., Fabris L., Bottai G., Raschioni C., Calin G.A., Santarpia L. Targeting microRNAs as key modulators of tumor immune response. J. Exp. Clin. Cancer Res. 2016;35:103. doi: 10.1186/s13046-016-0375-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Syed S.N., Frank A.-C., Raue R., Brüne B. MicroRNA-A Tumor Trojan Horse for Tumor-Associated Macrophages. Cells. 2019;8:1482. doi: 10.3390/cells8121482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma L., Young J., Prabhala H., Pan E., Mestdagh P., Muth D., Teruya-Feldstein J., Reinhardt F., Onder T.T., Valastyan S., et al. miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat. Cell Biol. 2010;12:247–256. doi: 10.1038/ncb2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma L., Teruya-Feldstein J., Weinberg R.A. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449:682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 26.Fang L., Li H., Wang L., Hu J., Jin T., Wang J., Yang B.B. MicroRNA-17-5p promotes chemotherapeutic drug resistance and tumour metastasis of colorectal cancer by repressing PTEN expression. Oncotarget. 2014;5:2974–2987. doi: 10.18632/oncotarget.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma Y., Zhang P., Wang F., Zhang H., Yang Y., Shi C., Xia Y., Peng J., Liu W., Yang Z., et al. Elevated oncofoetal miR-17-5p expression regulates colorectal cancer progression by repressing its target gene P130. Nat. Commun. 2012;3:1291. doi: 10.1038/ncomms2276. [DOI] [PubMed] [Google Scholar]
- 28.Fan Y., Yin S., Hao Y., Yang J., Zhang H., Sun C., Ma M., Chang Q., Xi J.J. miR-19b promotes tumor growth and metastasis via targeting TP53. RNA. 2014;20:765–772. doi: 10.1261/rna.043026.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asangani I.A., Rasheed S.A., Nikolova D.A., Leupold J.H., Colburn N.H., Post S., Allgayer H. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27:2128–2136. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- 30.Lin C.-W., Chang Y.-L., Chang Y.-C., Lin J.-C., Chen C.-C., Pan S.-H., Wu C.-T., Chen H.-Y., Yang S.-C., Hong T.-M., et al. MicroRNA-135b promotes lung cancer metastasis by regulating multiple targets in the Hippo pathway and LZTS1. Nat. Commun. 2013;4:1877. doi: 10.1038/ncomms2876. [DOI] [PubMed] [Google Scholar]
- 31.Taylor M.A., Sossey-Alaoui K., Thompson C.L., Danielpour D., Schiemann W.P. TGF-β upregulates miR-181a expression to promote breast cancer metastasis. J. Clin. Investig. 2013;123:150–163. doi: 10.1172/JCI64946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Penna E., Orso F., Cimino D., Tenaglia E., Lembo A., Quaglino E., Poliseno L., Haimovic A., Osella-Abate S., De Pittà C., et al. microRNA-214 contributes to melanoma tumour progression through suppression of TFAP2C. EMBO J. 2011;30:1990–2007. doi: 10.1038/emboj.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cai C., Ashktorab H., Pang X., Zhao Y., Sha W., Liu Y., Gu X. MicroRNA-211 expression promotes colorectal cancer cell growth in vitro and in vivo by targeting tumor suppressor CHD5. PLoS ONE. 2012;7:e29750. doi: 10.1371/journal.pone.0029750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X., Zhang Y., Zhang H., Liu X., Gong T., Li M., Sun L., Ji G., Shi Y., Han Z., et al. miRNA-223 promotes gastric cancer invasion and metastasis by targeting tumor suppressor EPB41L3. Mol. Cancer Res. 2011;9:824–833. doi: 10.1158/1541-7786.MCR-10-0529. [DOI] [PubMed] [Google Scholar]
- 35.Tivnan A., Tracey L., Buckley P.G., Alcock L.C., Davidoff A.M., Stallings R.L. MicroRNA-34a is a potent tumor suppressor molecule in vivo in neuroblastoma. BMC Cancer. 2011;11:33. doi: 10.1186/1471-2407-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang L., Li X., Zhang X., Lv Z., He G., Zhao W., Ren X., Li Y., Bian X., Liao W., et al. MicroRNA-137, an HMGA1 target, suppresses colorectal cancer cell invasion and metastasis in mice by directly targeting FMNL2. Gastroenterology. 2013;144:624–635.e4. doi: 10.1053/j.gastro.2012.11.033. [DOI] [PubMed] [Google Scholar]
- 37.Geng L., Chaudhuri A., Talmon G., Wisecarver J.L., Are C., Brattain M., Wang J. MicroRNA-192 suppresses liver metastasis of colon cancer. Oncogene. 2014;33:5332–5340. doi: 10.1038/onc.2013.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He X.X., Chang Y., Meng F.Y., Wang M.Y., Xie Q.H., Tang F., Li P.Y., Song Y.H., Lin J.S. MicroRNA-375 targets AEG-1 in hepatocellular carcinoma and suppresses liver cancer cell growth in vitro and in vivo. Oncogene. 2012;31:3357–3369. doi: 10.1038/onc.2011.500. [DOI] [PubMed] [Google Scholar]
- 39.Kesanakurti D., Maddirela D.R., Chittivelu S., Rao J.S., Chetty C. Suppression of tumor cell invasiveness and in vivo tumor growth by microRNA-874 in non-small cell lung cancer. Biochem. Biophys. Res. Commun. 2013;434:627–633. doi: 10.1016/j.bbrc.2013.03.132. [DOI] [PubMed] [Google Scholar]
- 40.Esquela-Kerscher A., Trang P., Wiggins J.F., Patrawala L., Cheng A., Ford L., Weidhaas J.B., Brown D., Bader A.G., Slack F.J. The let-7 microRNA reduces tumor growth in mouse models of lung cancer. Cell Cycle. 2008;7:759–764. doi: 10.4161/cc.7.6.5834. [DOI] [PubMed] [Google Scholar]
- 41.Dhawan A., Scott J.G., Harris A.L., Buffa F.M. Pan-cancer characterisation of microRNA across cancer hallmarks reveals microRNA-mediated downregulation of tumour suppressors. Nat. Commun. 2018;9:5228. doi: 10.1038/s41467-018-07657-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang T.C., Yu D., Lee Y.S., Wentzel E.A., Arking D.E., West K.M., Dang C.V., Thomas-Tikhonenko A., Mendell J.T. Widespread microRNA repression by Myc contributes to tumorigenesis. Nat. Genet. 2008;40:43–50. doi: 10.1038/ng.2007.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Merritt W.M., Lin Y.G., Han L.Y., Kamat A.A., Spannuth W.A., Schmandt R., Urbauer D., Pennacchio L.A., Cheng J.F., Nick A.M., et al. Dicer, Drosha, and outcomes in patients with ovarian cancer. N. Engl. J. Med. 2008;359:2641–2650. doi: 10.1056/NEJMoa0803785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martello G., Rosato A., Ferrari F., Manfrin A., Cordenonsi M., Dupont S., Enzo E., Guzzardo V., Rondina M., Spruce T., et al. A MicroRNA Targeting Dicer for Metastasis Control. Cell. 2010;141:1195–1207. doi: 10.1016/j.cell.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 45.Acunzo M., Romano G., Wernicke D., Croce C.M. MicroRNA and cancer—A brief overview. Adv. Biol. Regul. 2015;57:1–9. doi: 10.1016/j.jbior.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 46.Theveneau E., Mayor R. Cadherins in collective cell migration of mesenchymal cells. Curr. Opin. Cell Biol. 2012;24:677–684. doi: 10.1016/j.ceb.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Friedl P., Locker J., Sahai E., Segall J.E. Classifying collective cancer cell invasion. Nat. Cell Biol. 2012;14:777–783. doi: 10.1038/ncb2548. [DOI] [PubMed] [Google Scholar]
- 48.Caswell P.T., Zech T. Actin-Based Cell Protrusion in a 3D Matrix. Trends Cell Biol. 2018;28:823–834. doi: 10.1016/j.tcb.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheng Z., Liu F., Wang G., Li Y., Zhang H., Li F. miR-133 is a key negative regulator of CDC42-PAK pathway in gastric cancer. Cell. Signal. 2014;26:2667–2673. doi: 10.1016/j.cellsig.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 50.Dong Y., Jin X., Sun Z., Zhao Y., Song X. MiR-186 Inhibited Migration of NSCLC via Targeting cdc42 and Effecting EMT Process. Mol. Cells. 2017;40:195–201. doi: 10.14348/molcells.2017.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang R., Zhao N., Li S., Fang J.H., Chen M.X., Yang J., Jia W.H., Yuan Y., Zhuang S.M. MicroRNA-195 suppresses angiogenesis and metastasis of hepatocellular carcinoma by inhibiting the expression of VEGF, VAV2, and CDC42. Hepatology. 2013;58:642–653. doi: 10.1002/hep.26373. [DOI] [PubMed] [Google Scholar]
- 52.Li Y., Zhu X., Xu W., Wang D., Yan J. miR-330 regulates the proliferation of colorectal cancer cells by targeting Cdc42. Biochem. Biophys. Res. Commun. 2013;431:560–565. doi: 10.1016/j.bbrc.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 53.Liu M., Lang N., Qiu M., Xu F., Li Q., Tang Q., Chen J., Chen X., Zhang S., Liu Z., et al. miR-137 targets Cdc42 expression, induces cell cycle G1 arrest and inhibits invasion in colorectal cancer cells. Int. J. Cancer. 2011;128:1269–1279. doi: 10.1002/ijc.25452. [DOI] [PubMed] [Google Scholar]
- 54.Huang Y.C., Lee C.T., Lee J.C., Liu Y.W., Chen Y.J., Tseng J.T., Kang J.W., Sheu B.S., Lin B.W., Hung L.Y. Epigenetic silencing of miR-137 contributes to early colorectal carcinogenesis by impaired Aurora-A inhibition. Oncotarget. 2016;7:76852–76866. doi: 10.18632/oncotarget.12719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Balaguer F., Link A., Lozano J.J., Cuatrecasas M., Nagasaka T., Boland C.R., Goel A. Epigenetic silencing of miR-137 is an early event in colorectal carcinogenesis. Cancer Res. 2010;70:6609–6618. doi: 10.1158/0008-5472.CAN-10-0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilkinson S., Paterson H.F., Marshall C.J. Cdc42-MRCK and Rho-ROCK signalling cooperate in myosin phosphorylation and cell invasion. Nat. Cell Biol. 2005;7:255–261. doi: 10.1038/ncb1230. [DOI] [PubMed] [Google Scholar]
- 57.Wu L., Cai C., Wang X., Liu M., Li X., Tang H. MicroRNA-142-3p, a new regulator of RAC1, suppresses the migration and invasion of hepatocellular carcinoma cells. FEBS Lett. 2011;585:1322–1330. doi: 10.1016/j.febslet.2011.03.067. [DOI] [PubMed] [Google Scholar]
- 58.Zhang H., Pu J., Qi T., Qi M., Yang C., Li S., Huang K., Zheng L., Tong Q. MicroRNA-145 inhibits the growth, invasion, metastasis and angiogenesis of neuroblastoma cells through targeting hypoxia-inducible factor 2 alpha. Oncogene. 2014;33:387–397. doi: 10.1038/onc.2012.574. [DOI] [PubMed] [Google Scholar]
- 59.Yu J.-Y., Chung K.-H., Deo M., Thompson R.C., Turner D.L. MicroRNA miR-124 regulates neurite outgrowth during neuronal differentiation. Exp. Cell Res. 2008;314:2618–2633. doi: 10.1016/j.yexcr.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Humphries B.A., Wang Z., Yang C. MicroRNA Regulation of the Small Rho GTPase Regulators-Complexities and Opportunities in Targeting Cancer Metastasis. Cancers. 2020;12:1092. doi: 10.3390/cancers12051092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu X., Jiang L., Wang A., Yu J., Shi F., Zhou X. MicroRNA-138 suppresses invasion and promotes apoptosis in head and neck squamous cell carcinoma cell lines. Cancer Lett. 2009;286:217–222. doi: 10.1016/j.canlet.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rath N., Olson M.F. Rho-associated kinases in tumorigenesis: Re-considering ROCK inhibition for cancer therapy. EMBO Rep. 2012;13:900–908. doi: 10.1038/embor.2012.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.An L., Liu Y., Wu A., Guan Y. microRNA-124 inhibits migration and invasion by down-regulating ROCK1 in glioma. PLoS ONE. 2013;8:e69478. doi: 10.1371/journal.pone.0069478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hu X., Guo J., Zheng L., Li C., Zheng T.M., Tanyi J.L., Liang S., Benedetto C., Mitidieri M., Katsaros D., et al. The heterochronic microRNA let-7 inhibits cell motility by regulating the genes in the actin cytoskeleton pathway in breast cancer. Mol. Cancer Res. 2013;11:240–250. doi: 10.1158/1541-7786.MCR-12-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schwickert A., Weghake E., Brüggemann K., Engbers A., Brinkmann B.F., Kemper B., Seggewiß J., Stock C., Ebnet K., Kiesel L., et al. microRNA miR-142-3p Inhibits Breast Cancer Cell Invasiveness by Synchronous Targeting of WASL, Integrin Alpha V, and Additional Cytoskeletal Elements. PLoS ONE. 2015;10:e0143993. doi: 10.1371/journal.pone.0143993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Götte M., Mohr C., Koo C.Y., Stock C., Vaske A.K., Viola M., Ibrahim S.A., Peddibhotla S., Teng Y.H.F., Low J.Y., et al. miR-145-dependent targeting of Junctional Adhesion Molecule A and modulation of fascin expression are associated with reduced breast cancer cell motility and invasiveness. Oncogene. 2010;29:6569–6580. doi: 10.1038/onc.2010.386. [DOI] [PubMed] [Google Scholar]
- 67.Wang Q., Tang H., Yin S., Dong C. Downregulation of microRNA-138 enhances the proliferation, migration and invasion of cholangiocarcinoma cells through the upregulation of RhoC/p-ERK/MMP-2/MMP-9. Oncol. Rep. 2013;29:2046–2052. doi: 10.3892/or.2013.2304. [DOI] [PubMed] [Google Scholar]
- 68.Jiang L., Liu X., Kolokythas A., Yu J., Wang A., Heidbreder C.E., Shi F., Zhou X. Downregulation of the Rho GTPase signaling pathway is involved in the microRNA-138-mediated inhibition of cell migration and invasion in tongue squamous cell carcinoma. Int. J. Cancer. 2010;127:505–512. doi: 10.1002/ijc.25320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen X., Chen S., Xiu Y.L., Sun K.X., Zong Z.H., Zhao Y. RhoC is a major target of microRNA-93-5P in epithelial ovarian carcinoma tumorigenesis and progression. Mol. Cancer. 2015;14:31. doi: 10.1186/s12943-015-0304-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu B.L., Sun K.X., Zong Z.H., Chen S., Zhao Y. MicroRNA-372 inhibits endometrial carcinoma development by targeting the expression of the Ras homolog gene family member C (RhoC) Oncotarget. 2016;7:6649–6664. doi: 10.18632/oncotarget.6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pei H., Jin Z., Chen S., Sun X., Yu J., Guo W. MiR-135b promotes proliferation and invasion of osteosarcoma cells via targeting FOXO1. Mol. Cell Biochem. 2015;400:245–252. doi: 10.1007/s11010-014-2281-2. [DOI] [PubMed] [Google Scholar]
- 72.Xing F., Sharma S., Liu Y., Mo Y.Y., Wu K., Zhang Y.Y., Pochampally R., Martinez L.A., Lo H.W., Watabe K. miR-509 suppresses brain metastasis of breast cancer cells by modulating RhoC and TNF-α. Oncogene. 2015;34:4890–4900. doi: 10.1038/onc.2014.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ueno K., Hirata H., Majid S., Yamamura S., Shahryari V., Tabatabai Z.L., Hinoda Y., Dahiya R. Tumor suppressor microRNA-493 decreases cell motility and migration ability in human bladder cancer cells by downregulating RhoC and FZD4. Mol. Cancer Ther. 2012;11:244–253. doi: 10.1158/1535-7163.MCT-11-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu X., Li Y., Shen H., Li H., Long L., Hui L., Xu W. miR-137 inhibits the proliferation of lung cancer cells by targeting Cdc42 and Cdk6. FEBS Lett. 2013;587:73–81. doi: 10.1016/j.febslet.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 75.Algaber A., Al-Haidari A., Madhi R., Rahman M., Syk I., Thorlacius H. MicroRNA-340-5p inhibits colon cancer cell migration via targeting of RhoA. Sci. Rep. 2020;10:16934. doi: 10.1038/s41598-020-73792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yu X., Wang D., Wang X., Sun S., Zhang Y., Wang S., Miao R., Xu X., Qu X. CXCL12/CXCR4 promotes inflammation-driven colorectal cancer progression through activation of RhoA signaling by sponging miR-133a-3p. J. Exp. Clin. Cancer Res. 2019;38:32. doi: 10.1186/s13046-018-1014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ge F., Wang C., Wang W., Liu W., Wu B. MicroRNA-31 inhibits tumor invasion and metastasis by targeting RhoA in human gastric cancer. Oncol. Rep. 2017;38:1133–1139. doi: 10.3892/or.2017.5758. [DOI] [PubMed] [Google Scholar]
- 78.Liu Q., Wang W., Yang X., Zhao D., Li F., Wang H. MicroRNA-146a inhibits cell migration and invasion by targeting RhoA in breast cancer. Oncol. Rep. 2016;36:189–196. doi: 10.3892/or.2016.4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang B.G., Li J.S., Liu Y.F., Xu Q. MicroRNA-200b suppresses the invasion and migration of hepatocellular carcinoma by downregulating RhoA and circRNA_000839. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2017;39:1010428317719577. doi: 10.1177/1010428317719577. [DOI] [PubMed] [Google Scholar]
- 80.Wang S.C., Lin X.L., Li J., Zhang T.T., Wang H.Y., Shi J.W., Yang S., Zhao W.T., Xie R.Y., Wei F., et al. MicroRNA-122 triggers mesenchymal-epithelial transition and suppresses hepatocellular carcinoma cell motility and invasion by targeting RhoA. PLoS ONE. 2014;9:e101330. doi: 10.1371/journal.pone.0101330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fils-Aimé N., Dai M., Guo J., El-Mousawi M., Kahramangil B., Neel J.C., Lebrun J.J. MicroRNA-584 and the protein phosphatase and actin regulator 1 (PHACTR1), a new signaling route through which transforming growth factor-β Mediates the migration and actin dynamics of breast cancer cells. J. Biol. Chem. 2013;288:11807–11823. doi: 10.1074/jbc.M112.430934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sundararajan V., Gengenbacher N., Stemmler M.P., Kleemann J.A., Brabletz T., Brabletz S. The ZEB1/miR-200c feedback loop regulates invasion via actin interacting proteins MYLK and TKS5. Oncotarget. 2015;6:27083–27096. doi: 10.18632/oncotarget.4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Weber M., Kim S., Patterson N., Rooney K., Searles C.D. MiRNA-155 targets myosin light chain kinase and modulates actin cytoskeleton organization in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2014;306:H1192–H1203. doi: 10.1152/ajpheart.00521.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cimino D., De Pittà C., Orso F., Zampini M., Casara S., Penna E., Quaglino E., Forni M., Damasco C., Pinatel E., et al. miR148b is a major coordinator of breast cancer progression in a relapse-associated microRNA signature by targeting ITGA5, ROCK1, PIK3CA, NRAS, and CSF1. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2013;27:1223–1235. doi: 10.1096/fj.12-214692. [DOI] [PubMed] [Google Scholar]
- 85.Maskey N., Li D., Xu H., Song H., Wu C., Hua K., Song J., Fang L. MicroRNA-340 inhibits invasion and metastasis by downregulating ROCK1 in breast cancer cells. Oncol. Lett. 2017;14:2261–2267. doi: 10.3892/ol.2017.6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Du W., Tang H., Lei Z., Zhu J., Zeng Y., Liu Z., Huang J.A. miR-335-5p inhibits TGF-β1-induced epithelial-mesenchymal transition in non-small cell lung cancer via ROCK1. Respir. Res. 2019;20:225. doi: 10.1186/s12931-019-1184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhu Q.D., Zhou Q.Q., Dong L., Huang Z., Wu F., Deng X. MiR-199a-5p Inhibits the Growth and Metastasis of Colorectal Cancer Cells by Targeting ROCK1. Technol. Cancer Res. Treat. 2018;17:1533034618775509. doi: 10.1177/1533034618775509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zheng M., Sun X., Li Y., Zuo W. MicroRNA-145 inhibits growth and migration of breast cancer cells through targeting oncoprotein ROCK1. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2016;37:8189–8196. doi: 10.1007/s13277-015-4722-2. [DOI] [PubMed] [Google Scholar]
- 89.Majid S., Dar A.A., Saini S., Shahryari V., Arora S., Zaman M.S., Chang I., Yamamura S., Chiyomaru T., Fukuhara S., et al. MicroRNA-1280 inhibits invasion and metastasis by targeting ROCK1 in bladder cancer. PLoS ONE. 2012;7:e46743. doi: 10.1371/journal.pone.0046743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wong C.C., Wong C.M., Tung E.K., Au S.L., Lee J.M., Poon R.T., Man K., Ng I.O. The microRNA miR-139 suppresses metastasis and progression of hepatocellular carcinoma by down-regulating Rho-kinase 2. Gastroenterology. 2011;140:322–331. doi: 10.1053/j.gastro.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 91.Sun X., Zhang X., Chen S., Fan M., Ma S., Zhai H. Myosin Heavy Chain-Associated RNA Transcripts Promotes Gastric Cancer Progression Through the miR-4529-5p/ROCK2 Axis. Dig. Dis. Sci. 2019;64:3539–3548. doi: 10.1007/s10620-019-05708-1. [DOI] [PubMed] [Google Scholar]
- 92.Niu Y., Tang G. miR-185-5p targets ROCK2 and inhibits cell migration and invasion of hepatocellular carcinoma. Oncol. Lett. 2019;17:5087–5093. doi: 10.3892/ol.2019.10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang W., Zhou X., Wei M. MicroRNA-144 suppresses osteosarcoma growth and metastasis by targeting ROCK1 and ROCK2. Oncotarget. 2015;6:10297–10308. doi: 10.18632/oncotarget.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kroiss A., Vincent S., Decaussin-Petrucci M., Meugnier E., Viallet J., Ruffion A., Chalmel F., Samarut J., Allioli N. Androgen-regulated microRNA-135a decreases prostate cancer cell migration and invasion through downregulating ROCK1 and ROCK2. Oncogene. 2015;34:2846–2855. doi: 10.1038/onc.2014.222. [DOI] [PubMed] [Google Scholar]
- 95.Wang Y., Li J., Xu C., Zhang X. MicroRNA-139-5p Inhibits Cell Proliferation and Invasion by Targeting RHO-Associated Coiled-Coil-Containing Protein Kinase 2 in Ovarian Cancer. Oncol. Res. 2018;26:411–420. doi: 10.3727/096504017X14974343584989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zheng F., Liao Y.J., Cai M.Y., Liu Y.H., Liu T.H., Chen S.P., Bian X.W., Guan X.Y., Lin M.C., Zeng Y.X., et al. The putative tumour suppressor microRNA-124 modulates hepatocellular carcinoma cell aggressiveness by repressing ROCK2 and EZH2. Gut. 2012;61:278–289. doi: 10.1136/gut.2011.239145. [DOI] [PubMed] [Google Scholar]
- 97.Zheng Y., Xiang L., Chen M., Xiang C. MicroRNA‑130a inhibits the proliferation, migration and invasive ability of hepatocellular carcinoma cells by downregulating Rho‑kinase 2. Mol. Med. Rep. 2018;18:3077–3084. doi: 10.3892/mmr.2018.9283. [DOI] [PubMed] [Google Scholar]
- 98.Jurmeister S., Baumann M., Balwierz A., Keklikoglou I., Ward A., Uhlmann S., Zhang J.D., Wiemann S., Sahin Ö. MicroRNA-200c represses migration and invasion of breast cancer cells by targeting actin-regulatory proteins FHOD1 and PPM1F. Mol. Cell. Biol. 2012;32:633–651. doi: 10.1128/MCB.06212-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang J., Jin M., Chen X., Zhang R., Huang Y., Liu H., Zhu J. Loss of PPM1F expression predicts tumour recurrence and is negatively regulated by miR-590-3p in gastric cancer. Cell Prolif. 2018;51:e12444. doi: 10.1111/cpr.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang H., Chen W., Jin M., Hou L., Chen X., Zhang R., Zhang J., Zhu J. CircSLC3A2 functions as an oncogenic factor in hepatocellular carcinoma by sponging miR-490-3p and regulating PPM1F expression. Mol. Cancer. 2018;17:165. doi: 10.1186/s12943-018-0909-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Luo G., Chao Y.L., Tang B., Li B.S., Xiao Y.F., Xie R., Wang S.M., Wu Y.Y., Dong H., Liu X.D., et al. miR-149 represses metastasis of hepatocellular carcinoma by targeting actin-regulatory proteins PPM1F. Oncotarget. 2015;6:37808–37823. doi: 10.18632/oncotarget.5676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sossey-Alaoui K., Downs-Kelly E., Das M., Izem L., Tubbs R., Plow E.F. WAVE3, an actin remodeling protein, is regulated by the metastasis suppressor microRNA, miR-31, during the invasion-metastasis cascade. Int. J. Cancer. 2011;129:1331–1343. doi: 10.1002/ijc.25793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sossey-Alaoui K., Bialkowska K., Plow E.F. The miR200 family of microRNAs regulates WAVE3-dependent cancer cell invasion. J. Biol. Chem. 2009;284:33019–33029. doi: 10.1074/jbc.M109.034553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhan M.N., Yu X.T., Tang J., Zhou C.X., Wang C.L., Yin Q.Q., Gong X.F., He M., He J.R., Chen G.Q., et al. MicroRNA-494 inhibits breast cancer progression by directly targeting PAK1. Cell Death Dis. 2017;8:e2529. doi: 10.1038/cddis.2016.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yue K., Wang X., Wu Y., Zhou X., He Q., Duan Y. microRNA-7 regulates cell growth, migration and invasion via direct targeting of PAK1 in thyroid cancer. Mol. Med. Rep. 2016;14:2127–2134. doi: 10.3892/mmr.2016.5477. [DOI] [PubMed] [Google Scholar]
- 106.Kou B., Gao Y., Du C., Shi Q., Xu S., Wang C.Q., Wang X., He D., Guo P. miR-145 inhibits invasion of bladder cancer cells by targeting PAK1. Urol. Oncol. 2014;32:846–854. doi: 10.1016/j.urolonc.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 107.Yang G., Zhang X., Shi J. MiR-98 inhibits cell proliferation and invasion of non-small cell carcinoma lung cancer by targeting PAK1. Int. J. Clin. Exp. Med. 2015;8:20135–20145. [PMC free article] [PubMed] [Google Scholar]
- 108.Lin X.J., He C.L., Sun T., Duan X.J., Sun Y., Xiong S.J. hsa-miR-485-5p reverses epithelial to mesenchymal transition and promotes cisplatin-induced cell death by targeting PAK1 in oral tongue squamous cell carcinoma. Int. J. Mol. Med. 2017;40:83–89. doi: 10.3892/ijmm.2017.2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wei Z., Chang K., Fan C., Zhang Y. MiR-26a/miR-26b represses tongue squamous cell carcinoma progression by targeting PAK1. Cancer Cell Int. 2020;20:82. doi: 10.1186/s12935-020-1166-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wimmer M., Zauner R., Ablinger M., Piñón-Hofbauer J., Guttmann-Gruber C., Reisenberger M., Lettner T., Niklas N., Proell J., Sajinovic M., et al. A cancer stem cell-like phenotype is associated with miR-10b expression in aggressive squamous cell carcinomas. Cell Commun. Signal. 2020;18:61. doi: 10.1186/s12964-020-00550-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yuan J., Xiao C., Lu H., Yu H., Hong H., Guo C., Wu Z. miR-200b regulates breast cancer cell proliferation and invasion by targeting radixin. Exp. Ther. Med. 2020;19:2741–2750. doi: 10.3892/etm.2020.8516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hua D., Ding D., Han X., Zhang W., Zhao N., Foltz G., Lan Q., Huang Q., Lin B. Human miR-31 targets radixin and inhibits migration and invasion of glioma cells. Oncol. Rep. 2012;27:700–706. doi: 10.3892/or.2011.1555. [DOI] [PubMed] [Google Scholar]
- 113.Pellegrino L., Stebbing J., Braga V.M., Frampton A.E., Jacob J., Buluwela L., Jiao L.R., Periyasamy M., Madsen C.D., Caley M.P., et al. miR-23b regulates cytoskeletal remodeling, motility and metastasis by directly targeting multiple transcripts. Nucleic Acids Res. 2013;41:5400–5412. doi: 10.1093/nar/gkt245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li Q., Wu X., Guo L., Shi J., Li J. MicroRNA-7-5p induces cell growth inhibition, cell cycle arrest and apoptosis by targeting PAK2 in non-small cell lung cancer. FEBS Open Bio. 2019;9:1983–1993. doi: 10.1002/2211-5463.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Koo K.H., Kwon H. MicroRNA miR-4779 suppresses tumor growth by inducing apoptosis and cell cycle arrest through direct targeting of PAK2 and CCND3. Cell Death Dis. 2018;9:77. doi: 10.1038/s41419-017-0100-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hao S., Luo C., Abukiwan A., Wang G., He J., Huang L., Weber C.E., Lv N., Xiao X., Eichmüller S.B., et al. miR-137 inhibits proliferation of melanoma cells by targeting PAK2. Exp. Dermatol. 2015;24:947–952. doi: 10.1111/exd.12812. [DOI] [PubMed] [Google Scholar]
- 117.Ni X., Ding Y., Yuan H., Shao J., Yan Y., Guo R., Luan W., Xu M. Long non-coding RNA ZEB1-AS1 promotes colon adenocarcinoma malignant progression via miR-455-3p/PAK2 axis. Cell Prolif. 2020;53:e12723. doi: 10.1111/cpr.12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu C., Xing H., Luo X., Wang Y. MicroRNA-342 targets Cofilin 1 to suppress the growth, migration and invasion of human breast cancer cells. Arch. Biochem. Biophys. 2020;687:108385. doi: 10.1016/j.abb.2020.108385. [DOI] [PubMed] [Google Scholar]
- 119.Bockhorn J., Dalton R., Nwachukwu C., Huang S., Prat A., Yee K., Chang Y.-F., Huo D., Wen Y., Swanson K.E., et al. MicroRNA-30c inhibits human breast tumour chemotherapy resistance by regulating TWF1 and IL-11. Nat. Commun. 2013;4:1393. doi: 10.1038/ncomms2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hua Y.Q., Zhu Y.D., Xie G.Q., Zhang K., Sheng J., Zhu Z.F., Ning Z.Y., Chen H., Chen Z., Meng Z.Q., et al. Long non-coding SBF2-AS1 acting as a competing endogenous RNA to sponge microRNA-142-3p to participate in gemcitabine resistance in pancreatic cancer via upregulating TWF1. Aging. 2019;11:8860–8878. doi: 10.18632/aging.102307. [DOI] [PubMed] [Google Scholar]
- 121.Jin X., Pang W., Zhang Q., Huang H. MicroRNA-486-5p improves nonsmall-cell lung cancer chemotherapy sensitivity and inhibits epithelial-mesenchymal transition by targeting twinfilin actin binding protein 1. J. Int. Med. Res. 2019;47:3745–3756. doi: 10.1177/0300060519850739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang Z., Shi Z., Zhang L., Zhang H., Zhang Y. Profilin 1, negatively regulated by microRNA-19a-3p, serves as a tumor suppressor in human hepatocellular carcinoma. Pathol. Res. Pract. 2019;215:499–505. doi: 10.1016/j.prp.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 123.Jiang X.-M., Yu X.-N., Liu T.-T., Zhu H.-R., Shi X., Bilegsaikhan E., Guo H.-Y., Song G.-Q., Weng S.-Q., Huang X.-X., et al. microRNA-19a-3p promotes tumor metastasis and chemoresistance through the PTEN/Akt pathway in hepatocellular carcinoma. Biomed. Pharmacother. 2018;105:1147–1154. doi: 10.1016/j.biopha.2018.06.097. [DOI] [PubMed] [Google Scholar]
- 124.Liu H., Wang Y., Li X., Zhang Y.J., Li J., Zheng Y.Q., Liu M., Song X., Li X.R. Expression and regulatory function of miRNA-182 in triple-negative breast cancer cells through its targeting of profilin 1. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2013;34:1713–1722. doi: 10.1007/s13277-013-0708-0. [DOI] [PubMed] [Google Scholar]
- 125.Bai N., Ma Y., Zhao J., Li B. Knockdown of lncRNA HCP5 Suppresses the Progression of Colorectal Cancer by miR-299-3p/PFN1/AKT Axis. Cancer Manag. Res. 2020;12:4747–4758. doi: 10.2147/CMAR.S255866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Huang Y., Sun H., Ma X., Zeng Y., Pan Y., Yu D., Liu Z., Xiang Y. HLA-F-AS1/miR-330-3p/PFN1 axis promotes colorectal cancer progression. Life Sci. 2020;254:117180. doi: 10.1016/j.lfs.2019.117180. [DOI] [PubMed] [Google Scholar]
- 127.Akanuma N., Hoshino I., Akutsu Y., Murakami K., Isozaki Y., Maruyama T., Yusup G., Qin W., Toyozumi T., Takahashi M., et al. MicroRNA-133a regulates the mRNAs of two invadopodia-related proteins, FSCN1 and MMP14, in esophageal cancer. Br. J. Cancer. 2014;110:189–198. doi: 10.1038/bjc.2013.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chiyomaru T., Enokida H., Tatarano S., Kawahara K., Uchida Y., Nishiyama K., Fujimura L., Kikkawa N., Seki N., Nakagawa M. miR-145 and miR-133a function as tumour suppressors and directly regulate FSCN1 expression in bladder cancer. Br. J. Cancer. 2010;102:883–891. doi: 10.1038/sj.bjc.6605570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kano M., Seki N., Kikkawa N., Fujimura L., Hoshino I., Akutsu Y., Chiyomaru T., Enokida H., Nakagawa M., Matsubara H. miR-145, miR-133a and miR-133b: Tumor-suppressive miRNAs target FSCN1 in esophageal squamous cell carcinoma. Int. J. Cancer. 2010;127:2804–2814. doi: 10.1002/ijc.25284. [DOI] [PubMed] [Google Scholar]
- 130.Liu Y., Hong W., Zhou C., Jiang Z., Wang G., Wei G., Li X. miR-539 inhibits FSCN1 expression and suppresses hepatocellular carcinoma migration and invasion. Oncol. Rep. 2017;37:2593–2602. doi: 10.3892/or.2017.5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ortega M., Bhatnagar H., Lin A.P., Wang L., Aster J.C., Sill H., Aguiar R.C.T. A microRNA-mediated regulatory loop modulates NOTCH and MYC oncogenic signals in B- and T-cell malignancies. Leukemia. 2015;29:968–976. doi: 10.1038/leu.2014.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yu S., Xie H., Zhang J., Wang D., Song Y., Zhang S., Zheng S., Wang J. MicroRNA‑663 suppresses the proliferation and invasion of colorectal cancer cells by directly targeting FSCN1. Mol. Med. Rep. 2017;16:9707–9714. doi: 10.3892/mmr.2017.7794. [DOI] [PubMed] [Google Scholar]
- 133.Li Y., Gao Y., Xu Y., Ma H., Yang M. Down-regulation of miR-326 is associated with poor prognosis and promotes growth and metastasis by targeting FSCN1 in gastric cancer. Growth Factors. 2015;33:267–274. doi: 10.3109/08977194.2015.1076406. [DOI] [PubMed] [Google Scholar]
- 134.Xiao P., Liu W., Zhou H. miR-200b inhibits migration and invasion in non-small cell lung cancer cells via targeting FSCN1. Mol. Med. Rep. 2016;14:1835–1840. doi: 10.3892/mmr.2016.5421. [DOI] [PubMed] [Google Scholar]
- 135.Zhang M., Dong B.B., Lu M., Zheng M.J., Chen H., Ding J.Z., Xu A.M., Xu Y.H. miR-429 functions as a tumor suppressor by targeting FSCN1 in gastric cancer cells. OncoTargets Ther. 2016;9:1123–1133. doi: 10.2147/OTT.S91879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li H.P., Huang H.Y., Lai Y.R., Huang J.X., Chang K.P., Hsueh C., Chang Y.S. Silencing of miRNA-148a by hypermethylation activates the integrin-mediated signaling pathway in nasopharyngeal carcinoma. Oncotarget. 2014;5:7610–7624. doi: 10.18632/oncotarget.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Walker C., Mojares E., Del Río Hernández A. Role of Extracellular Matrix in Development and Cancer Progression. Int. J. Mol. Sci. 2018;19:3028. doi: 10.3390/ijms19103028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bogusławska J., Rodzik K., Popławski P., Kędzierska H., Rybicka B., Sokół E., Tański Z., Piekiełko-Witkowska A. TGF-β1 targets a microRNA network that regulates cellular adhesion and migration in renal cancer. Cancer Lett. 2018;412:155–169. doi: 10.1016/j.canlet.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 139.Hamidi H., Ivaska J. Every step of the way: Integrins in cancer progression and metastasis. Nat. Rev. Cancer. 2018;18:533–548. doi: 10.1038/s41568-018-0038-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Winograd-Katz S.E., Fässler R., Geiger B., Legate K.R. The integrin adhesome: From genes and proteins to human disease. Nat. Rev. Mol. Cell Biol. 2014;15:273–288. doi: 10.1038/nrm3769. [DOI] [PubMed] [Google Scholar]
- 141.Ivanova I.A., Vermeulen J.F., Ercan C., Houthuijzen J.M., Saig F.A., Vlug E.J., van der Wall E., van Diest P.J., Vooijs M., Derksen P.W.B. FER kinase promotes breast cancer metastasis by regulating α6- and β1-integrin-dependent cell adhesion and anoikis resistance. Oncogene. 2013;32:5582–5592. doi: 10.1038/onc.2013.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Peinado H., Zhang H., Matei I.R., Costa-Silva B., Hoshino A., Rodrigues G., Psaila B., Kaplan R.N., Bromberg J.F., Kang Y., et al. Pre-metastatic niches: Organ-specific homes for metastases. Nat. Rev. Cancer. 2017;17:302–317. doi: 10.1038/nrc.2017.6. [DOI] [PubMed] [Google Scholar]
- 143.Raab-Westphal S., Marshall J.F., Goodman S.L. Integrins as Therapeutic Targets: Successes and Cancers. Cancers. 2017;9:110. doi: 10.3390/cancers9090110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Augoff K., Das M., Bialkowska K., McCue B., Plow E.F., Sossey-Alaoui K. miR-31 is a broad regulator of β1-integrin expression and function in cancer cells. Mol. Cancer Res. 2011;9:1500–1508. doi: 10.1158/1541-7786.MCR-11-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Abdelmohsen K., Hutchison E.R., Lee E.K., Kuwano Y., Kim M.M., Masuda K., Srikantan S., Subaran S.S., Marasa B.S., Mattson M.P., et al. miR-375 inhibits differentiation of neurites by lowering HuD levels. Mol. Cell. Biol. 2010;30:4197–4210. doi: 10.1128/MCB.00316-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Takada Y., Ye X., Simon S. The integrins. Genome Biol. 2007;8:215. doi: 10.1186/gb-2007-8-5-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Liu da Z., Jickling G.C., Ander B.P., Hull H., Zhan X., Cox C., Shroff N., Dykstra-Aiello C., Stamova B., Sharp F.R. Elevating microRNA-122 in blood improves outcomes after temporary middle cerebral artery occlusion in rats. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2016;36:1374–1383. doi: 10.1177/0271678X15610786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sakaguchi T., Yoshino H., Yonemori M., Miyamoto K., Sugita S., Matsushita R., Itesako T., Tatarano S., Nakagawa M., Enokida H. Regulation of ITGA3 by the dual-stranded microRNA-199 family as a potential prognostic marker in bladder cancer. Br. J. Cancer. 2017;116:1077–1087. doi: 10.1038/bjc.2017.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Darzi L., Boshtam M., Shariati L., Kouhpayeh S., Gheibi A., Mirian M., Rahimmanesh I., Khanahmad H., Tabatabaiefar M.A. The silencing effect of miR-30a on ITGA4 gene expression in vitro: An approach for gene therapy. Res. Pharm. Sci. 2017;12:456–464. doi: 10.4103/1735-5362.217426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang X., Cheng S.-L., Bian K., Wang L., Zhang X., Yan B., Jia L.-T., Zhao J., Gammoh N., Yang A.-G., et al. MicroRNA-26a promotes anoikis in human hepatocellular carcinoma cells by targeting alpha5 integrin. Oncotarget. 2015;6:2277–2289. doi: 10.18632/oncotarget.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zoni E., van der Horst G., van de Merbel A.F., Chen L., Rane J.K., Pelger R.C., Collins A.T., Visakorpi T., Snaar-Jagalska B.E., Maitland N.J., et al. miR-25 Modulates Invasiveness and Dissemination of Human Prostate Cancer Cells via Regulation of αv- and α6-Integrin Expression. Cancer Res. 2015;75:2326–2336. doi: 10.1158/0008-5472.CAN-14-2155. [DOI] [PubMed] [Google Scholar]
- 152.Kinoshita T., Nohata N., Hanazawa T., Kikkawa N., Yamamoto N., Yoshino H., Itesako T., Enokida H., Nakagawa M., Okamoto Y., et al. Tumour-suppressive microRNA-29s inhibit cancer cell migration and invasion by targeting laminin-integrin signalling in head and neck squamous cell carcinoma. Br. J. Cancer. 2013;109:2636–2645. doi: 10.1038/bjc.2013.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jin Y.P., Hu Y.P., Wu X.S., Wu Y.S., Ye Y.Y., Li H.F., Liu Y.C., Jiang L., Liu F.T., Zhang Y.J., et al. miR-143-3p targeting of ITGA6 suppresses tumour growth and angiogenesis by downregulating PLGF expression via the PI3K/AKT pathway in gallbladder carcinoma. Cell Death Dis. 2018;9:182. doi: 10.1038/s41419-017-0258-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fan J., Kang X., Zhao L., Zheng Y., Yang J., Li D. Long Noncoding RNA CCAT1 Functions as a Competing Endogenous RNA to Upregulate ITGA9 by Sponging MiR-296-3p in Melanoma. Cancer Manag. Res. 2020;12:4699–4714. doi: 10.2147/CMAR.S252635. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 155.Ngalame N.N., Tokar E.J., Person R.J., Xu Y., Waalkes M.P. Aberrant microRNA expression likely controls RAS oncogene activation during malignant transformation of human prostate epithelial and stem cells by arsenic. Toxicol. Sci. Off. J. Soc. Toxicol. 2014;138:268–277. doi: 10.1093/toxsci/kfu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhao S., Wang Y., Liang Y., Zhao M., Long H., Ding S., Yin H., Lu Q. MicroRNA-126 regulates DNA methylation in CD4+ T cells and contributes to systemic lupus erythematosus by targeting DNA methyltransferase 1. Arthritis Rheum. 2011;63:1376–1386. doi: 10.1002/art.30196. [DOI] [PubMed] [Google Scholar]
- 157.Conrad A.T., Dittel B.N. Taming of macrophage and microglial cell activation by microRNA-124. Cell Res. 2011;21:213–216. doi: 10.1038/cr.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Liu Q., Zhang M., Jiang X., Zhang Z., Dai L., Min S., Wu X., He Q., Liu J., Zhang Y., et al. miR-223 suppresses differentiation of tumor-induced CD11b+ Gr1+ myeloid-derived suppressor cells from bone marrow cells. Int. J. Cancer. 2011;129:2662–2673. doi: 10.1002/ijc.25921. [DOI] [PubMed] [Google Scholar]
- 159.Berrien-Elliott M.M., Sun Y., Neal C., Ireland A., Trissal M.C., Sullivan R.P., Wagner J.A., Leong J.W., Wong P., Mah-Som A.Y., et al. MicroRNA-142 Is Critical for the Homeostasis and Function of Type 1 Innate Lymphoid Cells. Immunity. 2019;51:479–490.e6. doi: 10.1016/j.immuni.2019.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sun Y., Varambally S., Maher C.A., Cao Q., Chockley P., Toubai T., Malter C., Nieves E., Tawara I., Wang Y., et al. Targeting of microRNA-142-3p in dendritic cells regulates endotoxin-induced mortality. Blood. 2011;117:6172–6183. doi: 10.1182/blood-2010-12-325647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sekiya Y., Ogawa T., Yoshizato K., Ikeda K., Kawada N. Suppression of hepatic stellate cell activation by microRNA-29b. Biochem. Biophys. Res. Commun. 2011;412:74–79. doi: 10.1016/j.bbrc.2011.07.041. [DOI] [PubMed] [Google Scholar]
- 162.Fowler A., Thomson D., Giles K., Maleki S., Mreich E., Wheeler H., Leedman P., Biggs M., Cook R., Little N., et al. miR-124a is frequently down-regulated in glioblastoma and is involved in migration and invasion. Eur. J. Cancer. 2011;47:953–963. doi: 10.1016/j.ejca.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 163.Cao X., Pfaff S.L., Gage F.H. A functional study of miR-124 in the developing neural tube. Genes Dev. 2007;21:531–536. doi: 10.1101/gad.1519207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Li G., Luna C., Qiu J., Epstein D.L., Gonzalez P. Targeting of integrin beta1 and kinesin 2alpha by microRNA 183. J. Biol. Chem. 2010;285:5461–5471. doi: 10.1074/jbc.M109.037127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tsuchiya S., Oku M., Imanaka Y., Kunimoto R., Okuno Y., Terasawa K., Sato F., Tsujimoto G., Shimizu K. MicroRNA-338-3p and microRNA-451 contribute to the formation of basolateral polarity in epithelial cells. Nucleic Acids Res. 2009;37:3821–3827. doi: 10.1093/nar/gkp255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chan S.H., Huang W.C., Chang J.W., Chang K.J., Kuo W.H., Wang M.Y., Lin K.Y., Uen Y.H., Hou M.F., Lin C.M., et al. MicroRNA-149 targets GIT1 to suppress integrin signaling and breast cancer metastasis. Oncogene. 2014;33:4496–4507. doi: 10.1038/onc.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhao Y., Miao G., Li Y., Isaji T., Gu J., Li J., Qi R. MicroRNA- 130b suppresses migration and invasion of colorectal cancer cells through downregulation of integrin β1 [corrected] PLoS ONE. 2014;9:e87938. doi: 10.1371/journal.pone.0087938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wang H., Zhu Y., Zhao M., Wu C., Zhang P., Tang L., Zhang H., Chen X., Yang Y., Liu G. miRNA-29c suppresses lung cancer cell adhesion to extracellular matrix and metastasis by targeting integrin β1 and matrix metalloproteinase2 (MMP2) PLoS ONE. 2013;8:e70192. doi: 10.1371/journal.pone.0070192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Slezak S., Jin P., Caruccio L., Ren J., Bennett M., Zia N., Adams S., Wang E., Ascensao J., Schechter G., et al. Gene and microRNA analysis of neutrophils from patients with polycythemia vera and essential thrombocytosis: Down-regulation of micro RNA-1 and -133a. J. Transl. Med. 2009;7:39. doi: 10.1186/1479-5876-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lu J., Guo S., Ebert B.L., Zhang H., Peng X., Bosco J., Pretz J., Schlanger R., Wang J.Y., Mak R.H., et al. MicroRNA-mediated control of cell fate in megakaryocyte-erythrocyte progenitors. Dev. Cell. 2008;14:843–853. doi: 10.1016/j.devcel.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hughes A.E., Bradley D.T., Campbell M., Lechner J., Dash D.P., Simpson D.A., Willoughby C.E. Mutation altering the miR-184 seed region causes familial keratoconus with cataract. Am. J. Hum. Genet. 2011;89:628–633. doi: 10.1016/j.ajhg.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lin Z., He R., Luo H., Lu C., Ning Z., Wu Y., Han C., Tan G., Wang Z. Integrin-β5, a miR-185-targeted gene, promotes hepatocellular carcinoma tumorigenesis by regulating β-catenin stability. J. Exp. Clin. Cancer Res. 2018;37:17. doi: 10.1186/s13046-018-0691-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 173.Jing C., Ma G., Li X., Wu X., Huang F., Liu K., Liu Z. MicroRNA-17/20a impedes migration and invasion via TGF-β/ITGB6 pathway in esophageal squamous cell carcinoma. Am. J. Cancer Res. 2016;6:1549–1562. [PMC free article] [PubMed] [Google Scholar]
- 174.Fang L., Deng Z., Shatseva T., Yang J., Peng C., Du W.W., Yee A.J., Ang L.C., He C., Shan S.W., et al. MicroRNA miR-93 promotes tumor growth and angiogenesis by targeting integrin-β8. Oncogene. 2011;30:806–821. doi: 10.1038/onc.2010.465. [DOI] [PubMed] [Google Scholar]
- 175.Martín-Villar E., Borda-d’Agua B., Carrasco-Ramirez P., Renart J., Parsons M., Quintanilla M., Jones G.E. Podoplanin mediates ECM degradation by squamous carcinoma cells through control of invadopodia stability. Oncogene. 2015;34:4531–4544. doi: 10.1038/onc.2014.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Martín-Villar E., Megías D., Castel S., Yurrita M.M., Vilaró S., Quintanilla M. Podoplanin binds ERM proteins to activate RhoA and promote epithelial-mesenchymal transition. J. Cell Sci. 2006;119 Pt 21:4541–4553. doi: 10.1242/jcs.03218. [DOI] [PubMed] [Google Scholar]
- 177.Sun Q., Zhang J., Cao W., Wang X., Xu Q., Yan M., Wu X., Chen W. Dysregulated miR-363 affects head and neck cancer invasion and metastasis by targeting podoplanin. Int. J. Biochem. Cell Biol. 2013;45:513–520. doi: 10.1016/j.biocel.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 178.Cortez M.A., Nicoloso M.S., Shimizu M., Rossi S., Gopisetty G., Molina J.R., Carlotti C., Jr., Tirapelli D., Neder L., Brassesco M.S., et al. miR-29b and miR-125a regulate podoplanin and suppress invasion in glioblastoma. Genes Chromosomes Cancer. 2010;49:981–990. doi: 10.1002/gcc.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Senbanjo L.T., Chellaiah M.A. CD44: A Multifunctional Cell Surface Adhesion Receptor Is a Regulator of Progression and Metastasis of Cancer Cells. Front. Cell Dev. Biol. 2017;5:18. doi: 10.3389/fcell.2017.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zöller M. CD44: Can a cancer-initiating cell profit from an abundantly expressed molecule? Nat. Rev. Cancer. 2011;11:254–267. doi: 10.1038/nrc3023. [DOI] [PubMed] [Google Scholar]
- 181.Chen C., Zhao S., Karnad A., Freeman J.W. The biology and role of CD44 in cancer progression: Therapeutic implications. J. Hematol. Oncol. 2018;11:64. doi: 10.1186/s13045-018-0605-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Liu C., Kelnar K., Liu B., Chen X., Calhoun-Davis T., Li H., Patrawala L., Yan H., Jeter C., Honorio S., et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat. Med. 2011;17:211. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yu G., Yao W., Xiao W., Li H., Xu H., Lang B. MicroRNA-34a functions as an anti-metastatic microRNA and suppresses angiogenesis in bladder cancer by directly targeting CD44. J. Exp. Clin. Cancer Res. 2014;33:779. doi: 10.1186/s13046-014-0115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cheng W., Liu T., Wan X., Gao Y., Wang H. MicroRNA-199a targets CD44 to suppress the tumorigenicity and multidrug resistance of ovarian cancer-initiating cells. FEBS J. 2012;279:2047–2059. doi: 10.1111/j.1742-4658.2012.08589.x. [DOI] [PubMed] [Google Scholar]
- 185.Chen L., Bourguignon L.Y.W. Hyaluronan-CD44 interaction promotes c-Jun signaling and miRNA21 expression leading to Bcl-2 expression and chemoresistance in breast cancer cells. Mol. Cancer. 2014;13:52. doi: 10.1186/1476-4598-13-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wang H., Tan Z., Hu H., Liu H., Wu T., Zheng C., Wang X., Luo Z., Wang J., Liu S., et al. microRNA-21 promotes breast cancer proliferation and metastasis by targeting LZTFL1. BMC Cancer. 2019;19:738. doi: 10.1186/s12885-019-5951-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bourguignon L.Y., Wong G., Earle C., Krueger K., Spevak C.C. Hyaluronan-CD44 interaction promotes c-Src-mediated twist signaling, microRNA-10b expression, and RhoA/RhoC up-regulation, leading to Rho-kinase-associated cytoskeleton activation and breast tumor cell invasion. J. Biol. Chem. 2010;285:36721–36735. doi: 10.1074/jbc.M110.162305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Szatmári T., Ötvös R., Hjerpe A., Dobra K. Syndecan-1 in Cancer: Implications for Cell Signaling, Differentiation, and Prognostication. Dis. Markers. 2015;2015:796052. doi: 10.1155/2015/796052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ibrahim S.A., Yip G.W., Stock C., Pan J.-W., Neubauer C., Poeter M., Pupjalis D., Koo C.Y., Kelsch R., Schüle R., et al. Targeting of syndecan-1 by microRNA miR-10b promotes breast cancer cell motility and invasiveness via a Rho-GTPase- and E-cadherin-dependent mechanism. Int. J. Cancer. 2012;131:E884–E896. doi: 10.1002/ijc.27629. [DOI] [PubMed] [Google Scholar]
- 190.Kong X., Li G., Yuan Y., He Y., Wu X., Zhang W., Wu Z., Chen T., Wu W., Lobie P.E., et al. MicroRNA-7 inhibits epithelial-to-mesenchymal transition and metastasis of breast cancer cells via targeting FAK expression. PLoS ONE. 2012;7:e41523. doi: 10.1371/journal.pone.0041523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zeng C.Y., Zhan Y.S., Huang J., Chen Y.X. MicroRNA‑7 suppresses human colon cancer invasion and proliferation by targeting the expression of focal adhesion kinase. Mol. Med. Rep. 2016;13:1297–1303. doi: 10.3892/mmr.2015.4643. [DOI] [PubMed] [Google Scholar]
- 192.Golubovskaya V.M., Sumbler B., Ho B., Yemma M., Cance W.G. MiR-138 and MiR-135 directly target focal adhesion kinase, inhibit cell invasion, and increase sensitivity to chemotherapy in cancer cells. Anti-cancer Agents Med. Chem. 2014;14:18–28. doi: 10.2174/187152061401140108113435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Howe E.N., Cochrane D.R., Richer J.K. Targets of miR-200c mediate suppression of cell motility and anoikis resistance. Breast Cancer Res. 2011;13:R45. doi: 10.1186/bcr2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sengupta S., den Boon J.A., Chen I.H., Newton M.A., Stanhope S.A., Cheng Y.J., Chen C.J., Hildesheim A., Sugden B., Ahlquist P. MicroRNA 29c is down-regulated in nasopharyngeal carcinomas, up-regulating mRNAs encoding extracellular matrix proteins. Proc. Natl. Acad. Sci. USA. 2008;105:5874–5878. doi: 10.1073/pnas.0801130105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Li J., Ghazwani M., Zhang Y., Lu J., Li J., Fan J., Gandhi C.R., Li S. miR-122 regulates collagen production via targeting hepatic stellate cells and suppressing P4HA1 expression. J. Hepatol. 2013;58:522–528. doi: 10.1016/j.jhep.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wong C.C.-L., Tse A.P.-W., Huang Y.-P., Zhu Y.-T., Chiu D.K.-C., Lai R.K.-H., Au S.L.-K., Kai A.K.-L., Lee J.M.-F., Wei L.L., et al. Lysyl oxidase-like 2 is critical to tumor microenvironment and metastatic niche formation in hepatocellular carcinoma. Hepatology. 2014;60:1645–1658. doi: 10.1002/hep.27320. [DOI] [PubMed] [Google Scholar]
- 197.Bhat N.S., Colden M., Dar A.A., Saini S., Arora P., Shahryari V., Yamamura S., Tanaka Y., Kato T., Majid S., et al. MicroRNA-720 Regulates E-cadherin–αE-catenin Complex and Promotes Renal Cell Carcinoma. Mol. Cancer Ther. 2017;16:2840–2848. doi: 10.1158/1535-7163.MCT-17-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Voss G., Haflidadóttir B.S., Järemo H., Persson M., Catela Ivkovic T., Wikström P., Ceder Y. Regulation of cell–cell adhesion in prostate cancer cells by microRNA-96 through upregulation of E-Cadherin and EpCAM. Carcinogenesis. 2019;41:865–874. doi: 10.1093/carcin/bgz191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Naik M.U., Naik T.U., Suckow A.T., Duncan M.K., Naik U.P. Attenuation of Junctional Adhesion Molecule-A Is a Contributing Factor for Breast Cancer Cell Invasion. Cancer Res. 2008;68:2194–2203. doi: 10.1158/0008-5472.CAN-07-3057. [DOI] [PubMed] [Google Scholar]
- 200.Barash U., Cohen-Kaplan V., Dowek I., Sanderson R.D., Ilan N., Vlodavsky I. Proteoglycans in health and disease: New concepts for heparanase function in tumor progression and metastasis. FEBS J. 2010;277:3890–3903. doi: 10.1111/j.1742-4658.2010.07799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Quintero-Fabián S., Arreola R., Becerril-Villanueva E., Torres-Romero J.C., Arana-Argáez V., Lara-Riegos J., Ramírez-Camacho M.A., Alvarez-Sánchez M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019;9:1370. doi: 10.3389/fonc.2019.01370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Cathcart J., Pulkoski-Gross A., Cao J. Targeting matrix metalloproteinases in cancer: Bringing new life to old ideas. Genes Dis. 2015;2:26–34. doi: 10.1016/j.gendis.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Jacob A., Prekeris R. The regulation of MMP targeting to invadopodia during cancer metastasis. Front. Cell Dev. Biol. 2015;3:4. doi: 10.3389/fcell.2015.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yang S., Li Y., Gao J., Zhang T., Li S., Luo A., Chen H., Ding F., Wang X., Liu Z. MicroRNA-34 suppresses breast cancer invasion and metastasis by directly targeting Fra-1. Oncogene. 2013;32:4294–4303. doi: 10.1038/onc.2012.432. [DOI] [PubMed] [Google Scholar]
- 205.Wu J., Wu G., Lv L., Ren Y.F., Zhang X.J., Xue Y.F., Li G., Lu X., Sun Z., Tang K.F. MicroRNA-34a inhibits migration and invasion of colon cancer cells via targeting to Fra-1. Carcinogenesis. 2012;33:519–528. doi: 10.1093/carcin/bgr304. [DOI] [PubMed] [Google Scholar]
- 206.Kimura R., Ishikawa C., Rokkaku T., Janknecht R., Mori N. Phosphorylated c-Jun and Fra-1 induce matrix metalloproteinase-1 and thereby regulate invasion activity of 143B osteosarcoma cells. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2011;1813:1543–1553. doi: 10.1016/j.bbamcr.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 207.Gabriely G., Wurdinger T., Kesari S., Esau C.C., Burchard J., Linsley P.S., Krichevsky A.M. MicroRNA 21 promotes glioma invasion by targeting matrix metalloproteinase regulators. Mol. Cell. Biol. 2008;28:5369–5380. doi: 10.1128/MCB.00479-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Cal S., López-Otín C. ADAMTS proteases and cancer. Matrix Biol. 2015;44–46:77–85. doi: 10.1016/j.matbio.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 209.Yu L., Lu Y., Han X., Zhao W., Li J., Mao J., Wang B., Shen J., Fan S., Wang L., et al. microRNA -140-5p inhibits colorectal cancer invasion and metastasis by targeting ADAMTS5 and IGFBP5. Stem Cell Res. Ther. 2016;7:180. doi: 10.1186/s13287-016-0438-5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 210.Li M., Liu L., Zang W., Wang Y., Du Y., Chen X., Li P., Li J., Zhao G. miR‑365 overexpression promotes cell proliferation and invasion by targeting ADAMTS-1 in breast cancer. Int. J. Oncol. 2015;47:296–302. doi: 10.3892/ijo.2015.3015. [DOI] [PubMed] [Google Scholar]
- 211.Xie Y., Gou Q., Xie K., Wang Z., Wang Y., Zheng H. ADAMTS6 suppresses tumor progression via the ERK signaling pathway and serves as a prognostic marker in human breast cancer. Oncotarget. 2016;7:61273. doi: 10.18632/oncotarget.11341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Liu X., Yu J., Jiang L., Wang A., Shi F., Ye H., Zhou X. MicroRNA-222 regulates cell invasion by targeting matrix metalloproteinase 1 (MMP1) and manganese superoxide dismutase 2 (SOD2) in tongue squamous cell carcinoma cell lines. Cancer Genom. Proteom. 2009;6:131–139. [PMC free article] [PubMed] [Google Scholar]
- 213.Ma F., Zhang L., Ma L., Zhang Y., Zhang J., Guo B. MiR-361-5p inhibits glycolytic metabolism, proliferation and invasion of breast cancer by targeting FGFR1 and MMP-1. J. Exp. Clin. Cancer Res. 2017;36:158. doi: 10.1186/s13046-017-0630-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Jin H., Wang W. MicroRNA-539 suppresses osteosarcoma cell invasion and migration in vitro and targeting Matrix metallopeptidase-8. Int. J. Clin. Exp. Pathol. 2015;8:8075–8082. [PMC free article] [PubMed] [Google Scholar]
- 215.Hou H., Gao F., Liang H., Lv Y., Li M., Yao L., Zhang J., Dou G., Wang Y. MicroRNA-188-5p regulates contribution of bone marrow-derived cells to choroidal neovascularization development by targeting MMP-2/13. Exp. Eye Res. 2018;175:115–123. doi: 10.1016/j.exer.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 216.Xu N., Zhang L., Meisgen F., Harada M., Heilborn J., Homey B., Grandér D., Ståhle M., Sonkoly E., Pivarcsi A. MicroRNA-125b down-regulates matrix metallopeptidase 13 and inhibits cutaneous squamous cell carcinoma cell proliferation, migration, and invasion. J. Biol. Chem. 2012;287:29899–29908. doi: 10.1074/jbc.M112.391243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Yu B., Liu X., Chang H. MicroRNA-143 inhibits colorectal cancer cell proliferation by targeting MMP7. Minerva Med. 2017;108:13–19. doi: 10.23736/S0026-4806.16.04651-6. [DOI] [PubMed] [Google Scholar]
- 218.Song N., Liu H., Ma X., Zhang S. Placental growth factor promotes metastases of ovarian cancer through MiR-543-regulated MMP7. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2015;37:1104–1112. doi: 10.1159/000430235. [DOI] [PubMed] [Google Scholar]
- 219.Lin Y.H., Liao C.J., Huang Y.H., Wu M.H., Chi H.C., Wu S.M., Chen C.Y., Tseng Y.H., Tsai C.Y., Chung I.H., et al. Thyroid hormone receptor represses miR-17 expression to enhance tumor metastasis in human hepatoma cells. Oncogene. 2013;32:4509–4518. doi: 10.1038/onc.2013.309. [DOI] [PubMed] [Google Scholar]
- 220.Zheng X., Chopp M., Lu Y., Buller B., Jiang F. MiR-15b and miR-152 reduce glioma cell invasion and angiogenesis via NRP-2 and MMP-3. Cancer Lett. 2013;329:146–154. doi: 10.1016/j.canlet.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Shang G., Mi Y., Mei Y., Wang G., Wang Y., Li X., Wang Y., Li Y., Zhao G. MicroRNA-192 inhibits the proliferation, migration and invasion of osteosarcoma cells and promotes apoptosis by targeting matrix metalloproteinase-11. Oncol. Lett. 2018;15:7265–7272. doi: 10.3892/ol.2018.8239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Waresijiang N., Sun J., Abuduaini R., Jiang T., Zhou W., Yuan H. The downregulation of miR‑125a‑5p functions as a tumor suppressor by directly targeting MMP‑11 in osteosarcoma. Mol. Med. Rep. 2016;13:4859–4864. doi: 10.3892/mmr.2016.5141. [DOI] [PubMed] [Google Scholar]
- 223.Wu D., Li M., Wang L., Zhou Y., Zhou J., Pan H., Qu P. microRNA‑145 inhibits cell proliferation, migration and invasion by targeting matrix metallopeptidase-11 in renal cell carcinoma. Mol. Med. Rep. 2014;10:393–398. doi: 10.3892/mmr.2014.2149. [DOI] [PubMed] [Google Scholar]
- 224.Ni X., Xia T., Zhao Y., Zhou W., Wu N., Liu X., Ding Q., Zha X., Sha J., Wang S. Downregulation of miR-106b induced breast cancer cell invasion and motility in association with overexpression of matrix metalloproteinase 2. Cancer Sci. 2014;105:18–25. doi: 10.1111/cas.12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Steele R., Mott J.L., Ray R.B. MBP-1 upregulates miR-29b that represses Mcl-1, collagens, and matrix-metalloproteinase-2 in prostate cancer cells. Genes Cancer. 2010;1:381–387. doi: 10.1177/1947601910371978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Luo D.J., Li L.J., Huo H.F., Liu X.Q., Cui H.W., Jiang D.M. MicroRNA-29b sensitizes osteosarcoma cells to doxorubicin by targeting matrix metalloproteinase 9 (MMP-9) in osteosarcoma. Eur. Rev. Med. Pharmacol. Sci. 2019;23:1434–1442. doi: 10.26355/eurrev_201902_17100. [DOI] [PubMed] [Google Scholar]
- 227.Fan D., Wang Y., Qi P., Chen Y., Xu P., Yang X., Jin X., Tian X. MicroRNA-183 functions as the tumor suppressor via inhibiting cellular invasion and metastasis by targeting MMP-9 in cervical cancer. Gynecol. Oncol. 2016;141:166–174. doi: 10.1016/j.ygyno.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 228.Yan W., Zhang W., Sun L., Liu Y., You G., Wang Y., Kang C., You Y., Jiang T. Identification of MMP-9 specific microRNA expression profile as potential targets of anti-invasion therapy in glioblastoma multiforme. Brain Res. 2011;1411:108–115. doi: 10.1016/j.brainres.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 229.Zhang H., Qi M., Li S., Qi T., Mei H., Huang K., Zheng L., Tong Q. microRNA-9 targets matrix metalloproteinase 14 to inhibit invasion, metastasis, and angiogenesis of neuroblastoma cells. Mol. Cancer Ther. 2012;11:1454–1466. doi: 10.1158/1535-7163.MCT-12-0001. [DOI] [PubMed] [Google Scholar]
- 230.Xiang X., Mei H., Zhao X., Pu J., Li D., Qu H., Jiao W., Zhao J., Huang K., Zheng L., et al. miRNA-337-3p suppresses neuroblastoma progression by repressing the transcription of matrix metalloproteinase 14. Oncotarget. 2015;6:22452–22466. doi: 10.18632/oncotarget.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Zuo Q.F., Cao L.Y., Yu T., Gong L., Wang L.N., Zhao Y.L., Xiao B., Zou Q.M. MicroRNA-22 inhibits tumor growth and metastasis in gastric cancer by directly targeting MMP14 and Snail. Cell Death Dis. 2015;6:e2000. doi: 10.1038/cddis.2015.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Zuo W., Zhou K., Deng M., Lin Q., Yin Q., Zhang C., Zhou J., Song Y. LINC00963 facilitates acute myeloid leukemia development by modulating miR-608/MMP-15. Aging. 2020;12:18970–18981. doi: 10.18632/aging.103252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Wang H., Li X.T., Wu C., Wu Z.W., Li Y.Y., Yang T.Q., Chen G.L., Xie X.S., Huang Y.L., Du Z.W., et al. miR-132 can inhibit glioma cells invasion and migration by target MMP16 in vitro. OncoTargets Ther. 2015;8:3211–3218. doi: 10.2147/OTT.S79282. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 234.Yao Y., Shen H., Zhou Y., Yang Z., Hu T. MicroRNA-215 suppresses the proliferation, migration and invasion of non-small cell lung carcinoma cells through the downregulation of matrix metalloproteinase-16 expression. Exp. Ther. Med. 2018;15:3239–3246. doi: 10.3892/etm.2018.5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Shi J., An G., Guan Y., Wei T., Peng Z., Liang M., Wang Y. miR-328-3p mediates the anti-tumor effect in osteosarcoma via directly targeting MMP-16. Cancer Cell Int. 2019;19:104. doi: 10.1186/s12935-019-0829-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Astarci E., Erson-Bensan A.E., Banerjee S. Matrix metalloprotease 16 expression is downregulated by microRNA-146a in spontaneously differentiating Caco-2 cells. Dev. Growth Differ. 2012;54:216–226. doi: 10.1111/j.1440-169X.2011.01324.x. [DOI] [PubMed] [Google Scholar]
- 237.Xia H., Qi Y., Ng S.S., Chen X., Li D., Chen S., Ge R., Jiang S., Li G., Chen Y., et al. microRNA-146b inhibits glioma cell migration and invasion by targeting MMPs. Brain Res. 2009;1269:158–165. doi: 10.1016/j.brainres.2009.02.037. [DOI] [PubMed] [Google Scholar]
- 238.Chang Z.K., Meng F.G., Zhang Z.Q., Mao G.P., Huang Z.Y., Liao W.M., He A.S. MicroRNA-193b-3p regulates matrix metalloproteinase 19 expression in interleukin-1β-induced human chondrocytes. J. Cell. Biochem. 2018;119:4775–4782. doi: 10.1002/jcb.26669. [DOI] [PubMed] [Google Scholar]
- 239.Tian G., Wang S.W., Song M., Hu Y.F., Cao X.N., Ge J.W. MicroRNA-16 inhibits the proliferation, migration and invasion of non-small cell lung carcinoma cells by down-regulating matrix metalloproteinase-19 expression. Eur. Rev. Med. Pharmacol. Sci. 2019;23:5260–5269. doi: 10.26355/eurrev_201906_18192. [DOI] [PubMed] [Google Scholar]
- 240.Li X., He M., Guo J., Cao T. Upregulation of circular RNA circ-ERBB2 predicts unfavorable prognosis and facilitates the progression of gastric cancer via miR-503/CACUL1 and miR-637/MMP-19 signaling. Biochem. Biophys. Res. Commun. 2019;511:926–930. doi: 10.1016/j.bbrc.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 241.Li P., Ma Y., Wang Y., Chen T., Wang H., Chu H., Zhao G., Zhang G. Identification of miR-1293 potential target gene: TIMP-1. Mol. Cell. Biochem. 2013;384:1–6. doi: 10.1007/s11010-013-1775-7. [DOI] [PubMed] [Google Scholar]
- 242.Sachdeva M., Mito J.K., Lee C.-L., Zhang M., Li Z., Dodd R.D., Cason D., Luo L., Ma Y., Van Mater D., et al. MicroRNA-182 drives metastasis of primary sarcomas by targeting multiple genes. J. Clin. Investig. 2014;124:4305–4319. doi: 10.1172/JCI77116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Nagai H., Hasegawa S., Uchida F., Terabe T., Ishibashi Kanno N., Kato K., Yamagata K., Sakai S., Kawashiri S., Sato H., et al. MicroRNA-205-5p suppresses the invasiveness of oral squamous cell carcinoma by inhibiting TIMP‑2 expression. Int. J. Oncol. 2018;52:841–850. doi: 10.3892/ijo.2018.4260. [DOI] [PubMed] [Google Scholar]
- 244.Zhao Y., Gu X., Wang Y. MicroRNA-103 promotes nasopharyngeal carcinoma through targeting TIMP-3 and the Wnt/β-catenin pathway. Laryngoscope. 2020;130:E75–E82. doi: 10.1002/lary.28045. [DOI] [PubMed] [Google Scholar]
- 245.Qin S., Zhu Y., Ai F., Li Y., Bai B., Yao W., Dong L. MicroRNA-191 correlates with poor prognosis of colorectal carcinoma and plays multiple roles by targeting tissue inhibitor of metalloprotease 3. Neoplasma. 2014;61:27–34. doi: 10.4149/neo_2014_005. [DOI] [PubMed] [Google Scholar]
- 246.Bo P., Julang L. MicroRNA-21 up-regulates metalloprotease by down-regulating TIMP3 during cumulus cell-oocyte complex in vitro maturation. Mol. Cell. Endocrinol. 2018;477:29–38. doi: 10.1016/j.mce.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 247.Wang B., Hsu S.H., Majumder S., Kutay H., Huang W., Jacob S.T., Ghoshal K. TGFbeta-mediated upregulation of hepatic miR-181b promotes hepatocarcinogenesis by targeting TIMP3. Oncogene. 2010;29:1787–1797. doi: 10.1038/onc.2009.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Cheng D., Jiang S., Chen J., Li J., Ao L., Zhang Y. Upregulated long noncoding RNA Linc00261 in pre-eclampsia and its effect on trophoblast invasion and migration via regulating miR-558/TIMP4 signaling pathway. J. Cell. Biochem. 2019;120:13243–13253. doi: 10.1002/jcb.28598. [DOI] [PubMed] [Google Scholar]
- 249.Jung H.M., Phillips B.L., Patel R.S., Cohen D.M., Jakymiw A., Kong W.W., Cheng J.Q., Chan E.K. Keratinization-associated miR-7 and miR-21 regulate tumor suppressor reversion-inducing cysteine-rich protein with kazal motifs (RECK) in oral cancer. J. Biol. Chem. 2012;287:29261–29272. doi: 10.1074/jbc.M112.366518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Li N., Tang B., Zhu E.D., Li B.S., Zhuang Y., Yu S., Lu D.S., Zou Q.M., Xiao B., Mao X.H. Increased miR-222 in H. pylori-associated gastric cancer correlated with tumor progression by promoting cancer cell proliferation and targeting RECK. FEBS Lett. 2012;586:722–728. doi: 10.1016/j.febslet.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 251.Zhang Z., Li Z., Gao C., Chen P., Chen J., Liu W., Xiao S., Lu H. miR-21 plays a pivotal role in gastric cancer pathogenesis and progression. Lab. Investig. J. Tech. Methods Pathol. 2008;88:1358–1366. doi: 10.1038/labinvest.2008.94. [DOI] [PubMed] [Google Scholar]
- 252.Jung H., Yoon S.R., Lim J., Cho H.J., Lee H.G. Dysregulation of Rho GTPases in Human Cancers. Cancers. 2020;12:1179. doi: 10.3390/cancers12051179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Parri M., Chiarugi P. Rac and Rho GTPases in cancer cell motility control. Cell Commun. Signal. 2010;8:23. doi: 10.1186/1478-811X-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Zhu S., Si M.L., Wu H., Mo Y.Y. MicroRNA-21 targets the tumor suppressor gene tropomyosin 1 (TPM1) J. Biol. Chem. 2007;282:14328–14336. doi: 10.1074/jbc.M611393200. [DOI] [PubMed] [Google Scholar]
- 255.Si M.L., Zhu S., Wu H., Lu Z., Wu F., Mo Y.Y. miR-21-mediated tumor growth. Oncogene. 2007;26:2799–2803. doi: 10.1038/sj.onc.1210083. [DOI] [PubMed] [Google Scholar]
- 256.Han L., Wang W., Ding W., Zhang L. MiR-9 is involved in TGF-β1-induced lung cancer cell invasion and adhesion by targeting SOX7. J. Cell. Mol. Med. 2017;21:2000–2008. doi: 10.1111/jcmm.13120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Xu X.Z., Li X.A., Luo Y., Liu J.F., Wu H.W., Huang G. MiR-9 promotes synovial sarcoma cell migration and invasion by directly targeting CDH1. Int. J. Biochem. Cell Biol. 2019;112:61–71. doi: 10.1016/j.biocel.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 258.White R.A., Neiman J.M., Reddi A., Han G., Birlea S., Mitra D., Dionne L., Fernandez P., Murao K., Bian L., et al. Epithelial stem cell mutations that promote squamous cell carcinoma metastasis. J. Clin. Investig. 2013;123:4390–4404. doi: 10.1172/JCI65856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Hou R., Wang D., Lu J. MicroRNA-10b inhibits proliferation, migration and invasion in cervical cancer cells via direct targeting of insulin-like growth factor-1 receptor. Oncol. Lett. 2017;13:5009–5015. doi: 10.3892/ol.2017.6033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.He C., Chen Z.Y., Li Y., Yang Z.Q., Zeng F., Cui Y., He Y., Chen J.B., Chen H.Q. miR-10b suppresses cell invasion and metastasis through targeting HOXA3 regulated by FAK/YAP signaling pathway in clear-cell renal cell carcinoma. BMC Nephrol. 2019;20:127. doi: 10.1186/s12882-019-1322-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Song J.J., Li W. MiR-10b suppresses the growth and metastasis of colorectal cancer cell by targeting FGF13. Eur. Rev. Med. Pharmacol. Sci. 2019;23:576–587. doi: 10.26355/eurrev_201901_16870. [DOI] [PubMed] [Google Scholar]
- 262.Ouyang H., Gore J., Deitz S., Korc M. microRNA-10b enhances pancreatic cancer cell invasion by suppressing TIP30 expression and promoting EGF and TGF-β actions. Oncogene. 2014;33:4664–4674. doi: 10.1038/onc.2013.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Liu Z., Zhu J., Cao H., Ren H., Fang X. miR-10b promotes cell invasion through RhoC-AKT signaling pathway by targeting HOXD10 in gastric cancer. Int. J. Oncol. 2012;40:1553–1560. doi: 10.3892/ijo.2012.1342. [DOI] [PubMed] [Google Scholar]
- 264.Wang J., Wang B., Chen L.Q., Yang J., Gong Z.Q., Zhao X.L., Zhang C.Q., Du K.L. miR-10b promotes invasion by targeting KLF4 in osteosarcoma cells. Biomed. Pharmacother. Biomed. Pharmacother. 2016;84:947–953. doi: 10.1016/j.biopha.2016.09.108. [DOI] [PubMed] [Google Scholar]
- 265.Chen H., Fan Y., Xu W., Chen J., Xu C., Wei X., Fang D., Feng Y. miR-10b Inhibits Apoptosis and Promotes Proliferation and Invasion of Endometrial Cancer Cells via Targeting HOXB3. Cancer Biother. Radiopharm. 2016;31:225–231. doi: 10.1089/cbr.2016.1998. [DOI] [PubMed] [Google Scholar]
- 266.Jiang H., Liu Z.N., Cheng X.H., Zhang Y.F., Dai X., Bao G.M., Zhou L.B. MiR-29c suppresses cell invasion and migration by directly targeting CDK6 in gastric carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2019;23:7920–7928. doi: 10.26355/eurrev_201909_19006. [DOI] [PubMed] [Google Scholar]
- 267.Lu Y., Hu J., Sun W., Li S., Deng S., Li M. MiR-29c inhibits cell growth, invasion, and migration of pancreatic cancer by targeting ITGB1. OncoTargets Ther. 2016;9:99–109. doi: 10.2147/OTT.S92758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Liu N., Tang L.L., Sun Y., Cui R.X., Wang H.Y., Huang B.J., He Q.M., Jiang W., Ma J. MiR-29c suppresses invasion and metastasis by targeting TIAM1 in nasopharyngeal carcinoma. Cancer Lett. 2013;329:181–188. doi: 10.1016/j.canlet.2012.10.032. [DOI] [PubMed] [Google Scholar]
- 269.Yao Q., Cao S., Li C., Mengesha A., Kong B., Wei M. Micro-RNA-21 regulates TGF-β-induced myofibroblast differentiation by targeting PDCD4 in tumor-stroma interaction. Int. J. Cancer. 2011;128:1783–1792. doi: 10.1002/ijc.25506. [DOI] [PubMed] [Google Scholar]
- 270.Zhu S., Wu H., Wu F., Nie D., Sheng S., Mo Y.-Y. MicroRNA-21 targets tumor suppressor genes in invasion and metastasis. Cell Res. 2008;18:350–359. doi: 10.1038/cr.2008.24. [DOI] [PubMed] [Google Scholar]
- 271.Su C., Cheng X., Li Y., Han Y., Song X., Yu D., Cao X., Liu Z. MiR-21 improves invasion and migration of drug-resistant lung adenocarcinoma cancer cell and transformation of EMT through targeting HBP1. Cancer Med. 2018;7:2485–2503. doi: 10.1002/cam4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Wang J., Chu Y., Xu M., Zhang X., Zhou Y., Xu M. miR-21 promotes cell migration and invasion of hepatocellular carcinoma by targeting KLF5. Oncol. Lett. 2019;17:2221–2227. doi: 10.3892/ol.2018.9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Ge X., Gao J., Sun Q.W., Wang C.X., Deng W., Mao G.Y., Li H.Q., Guo S.S., Cheng J., Wu Y.N., et al. MiR-34a inhibits the proliferation, migration, and invasion of oral squamous cell carcinoma by directly targeting SATB2. J. Cell. Physiol. 2020;235:4856–4864. doi: 10.1002/jcp.29363. [DOI] [PubMed] [Google Scholar]
- 274.Zhao Y., Wang X. miR-34a targets BCL-2 to suppress the migration and invasion of sinonasal squamous cell carcinoma. Oncol. Lett. 2018;16:6566–6572. doi: 10.3892/ol.2018.9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Li Z., Chen H. miR-34a inhibits proliferation, migration and invasion of paediatric neuroblastoma cells via targeting HNF4α. Artif. Cells Nanomed. Biotechnol. 2019;47:3072–3078. doi: 10.1080/21691401.2019.1637886. [DOI] [PubMed] [Google Scholar]
- 276.Dong P., Xiong Y., Watari H., Hanley S.J., Konno Y., Ihira K., Yamada T., Kudo M., Yue J., Sakuragi N. MiR-137 and miR-34a directly target Snail and inhibit EMT, invasion and sphere-forming ability of ovarian cancer cells. J. Exp. Clin. Cancer Res. 2016;35:132. doi: 10.1186/s13046-016-0415-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Jia L.F., Wei S.B., Mitchelson K., Gao Y., Zheng Y.F., Meng Z., Gan Y.H., Yu G.Y. miR-34a inhibits migration and invasion of tongue squamous cell carcinoma via targeting MMP9 and MMP14. PLoS ONE. 2014;9:e108435. doi: 10.1371/journal.pone.0108435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Rui X., Zhao H., Xiao X., Wang L., Mo L., Yao Y. MicroRNA-34a suppresses breast cancer cell proliferation and invasion by targeting Notch1. Exp. Ther. Med. 2018;16:4387–4392. doi: 10.3892/etm.2018.6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Chen H.Y., Lin Y.M., Chung H.C., Lang Y.D., Lin C.J., Huang J., Wang W.C., Lin F.M., Chen Z., Huang H.D., et al. miR-103/107 promote metastasis of colorectal cancer by targeting the metastasis suppressors DAPK and KLF4. Cancer Res. 2012;72:3631–3641. doi: 10.1158/0008-5472.CAN-12-0667. [DOI] [PubMed] [Google Scholar]
- 280.Xiong B., Lei X., Zhang L., Fu J. miR-103 regulates triple negative breast cancer cells migration and invasion through targeting olfactomedin 4. Biomed. Pharmacother. Biomed. Pharmacother. 2017;89:1401–1408. doi: 10.1016/j.biopha.2017.02.028. [DOI] [PubMed] [Google Scholar]
- 281.Han L.L., Yin X.R., Zhang S.Q. miR-103 promotes the metastasis and EMT of hepatocellular carcinoma by directly inhibiting LATS2. Int. J. Oncol. 2018;53:2433–2444. doi: 10.3892/ijo.2018.4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Tan Y., Zhao L. miR-103 promotes hepatocellular carcinoma cell proliferation and migration in the simulation transition zone of RFA through PI3K/Akt signaling pathway by targeting PTEN. Int. J. Clin. Exp. Pathol. 2020;13:473–479. [PMC free article] [PubMed] [Google Scholar]
- 283.Zhao Y., Sun X., Zhu K., Cheng M. miR-135a inhibits malignant proliferation and diffusion of non-small cell lung cancer cells by down-regulating ROCK1 protein. Biosci. Rep. 2020;40:BSR20201276. doi: 10.1042/BSR20201276. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 284.Yang C., Zheng X., Ye K., Sun Y., Lu Y., Fan Q., Ge H. miR-135a Inhibits the Invasion and Migration of Esophageal Cancer Stem Cells through the Hedgehog Signaling Pathway by Targeting Smo. Mol. Ther. Nucleic Acids. 2020;19:841–852. doi: 10.1016/j.omtn.2019.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 285.Tribollet V., Barenton B., Kroiss A., Vincent S., Zhang L., Forcet C., Cerutti C., Périan S., Allioli N., Samarut J., et al. miR-135a Inhibits the Invasion of Cancer Cells via Suppression of ERRα. PLoS ONE. 2016;11:e0156445. doi: 10.1371/journal.pone.0156445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Jiang R., Zhang C., Liu G., Gu R., Wu H. MicroRNA-107 Promotes Proliferation, Migration, and Invasion of Osteosarcoma Cells by Targeting Tropomyosin 1. Oncol. Res. 2017;25:1409–1419. doi: 10.3727/096504017X14882829077237. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 287.Sheng N., Tan G., You W., Chen H., Gong J., Chen D., Zhang H., Wang Z. MiR-145 inhibits human colorectal cancer cell migration and invasion via PAK4-dependent pathway. Cancer Med. 2017;6:1331–1340. doi: 10.1002/cam4.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Tang L., Wei D., Yan F. MicroRNA-145 functions as a tumor suppressor by targeting matrix metalloproteinase 11 and Rab GTPase family 27a in triple-negative breast cancer. Cancer Gene Ther. 2016;23:258–265. doi: 10.1038/cgt.2016.27. [DOI] [PubMed] [Google Scholar]
- 289.Sachdeva M., Mo Y.Y. MicroRNA-145 suppresses cell invasion and metastasis by directly targeting mucin 1. Cancer Res. 2010;70:378–387. doi: 10.1158/0008-5472.CAN-09-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Chen B., Huang Z., Zhang Y., Chen Y., Li Z. MicroRNA-145 Suppresses Osteosarcoma Metastasis via Targeting MMP16. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2015;37:2183–2193. doi: 10.1159/000438575. [DOI] [PubMed] [Google Scholar]
- 291.Jiang S.B., He X.J., Xia Y.J., Hu W.J., Luo J.G., Zhang J., Tao H.Q. MicroRNA-145-5p inhibits gastric cancer invasiveness through targeting N-cadherin and ZEB2 to suppress epithelial-mesenchymal transition. OncoTargets Ther. 2016;9:2305–2315. doi: 10.2147/OTT.S101853. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 292.Zheng L., Pu J., Qi T., Qi M., Li D., Xiang X., Huang K., Tong Q. miRNA-145 targets v-ets erythroblastosis virus E26 oncogene homolog 1 to suppress the invasion, metastasis, and angiogenesis of gastric cancer cells. Mol. Cancer Res. 2013;11:182–193. doi: 10.1158/1541-7786.MCR-12-0534. [DOI] [PubMed] [Google Scholar]
- 293.Minami K., Taniguchi K., Sugito N., Kuranaga Y., Inamoto T., Takahara K., Takai T., Yoshikawa Y., Kiyama S., Akao Y., et al. MiR-145 negatively regulates Warburg effect by silencing KLF4 and PTBP1 in bladder cancer cells. Oncotarget. 2017;8:33064–33077. doi: 10.18632/oncotarget.16524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Zhang Z., Che X., Yang N., Bai Z., Wu Y., Zhao L., Pei H. miR-135b-5p Promotes migration, invasion and EMT of pancreatic cancer cells by targeting NR3C2. Biomed. Pharmacother. Biomed. Pharmacother. 2017;96:1341–1348. doi: 10.1016/j.biopha.2017.11.074. [DOI] [PubMed] [Google Scholar]
- 295.Lv Z.D., Xin H.N., Yang Z.C., Wang W.J., Dong J.J., Jin L.Y., Li F.N. miR-135b promotes proliferation and metastasis by targeting APC in triple-negative breast cancer. J. Cell. Physiol. 2019;234:10819–10826. doi: 10.1002/jcp.27906. [DOI] [PubMed] [Google Scholar]
- 296.Jia L., Luo S., Ren X., Li Y., Hu J., Liu B., Zhao L., Shan Y., Zhou H. miR-182 and miR-135b Mediate the Tumorigenesis and Invasiveness of Colorectal Cancer Cells via Targeting ST6GALNAC2 and PI3K/AKT Pathway. Dig. Dis. Sci. 2017;62:3447–3459. doi: 10.1007/s10620-017-4755-z. [DOI] [PubMed] [Google Scholar]
- 297.Li Y., Xu D., Bao C., Zhang Y., Chen D., Zhao F., Ding J., Liang L., Wang Q., Liu L., et al. MicroRNA-135b, a HSF1 target, promotes tumor invasion and metastasis by regulating RECK and EVI5 in hepatocellular carcinoma. Oncotarget. 2015;6:2421–2433. doi: 10.18632/oncotarget.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Zhang J.G., Shi Y., Hong D.F., Song M., Huang D., Wang C.Y., Zhao G. MiR-148b suppresses cell proliferation and invasion in hepatocellular carcinoma by targeting WNT1/β-catenin pathway. Sci. Rep. 2015;5:8087. doi: 10.1038/srep08087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Wu M., Ye X., Wang S., Li Q., Lai Y., Yi Y. MicroRNA-148b suppresses proliferation, migration, and invasion of nasopharyngeal carcinoma cells by targeting metastasis-associated gene 2. OncoTargets Ther. 2017;10:2815–2822. doi: 10.2147/OTT.S135664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Chen X., Bo L., Lu W., Zhou G., Chen Q. MicroRNA-148b targets Rho-associated protein kinase 1 to inhibit cell proliferation, migration and invasion in hepatocellular carcinoma. Mol. Med. Rep. 2016;13:477–482. doi: 10.3892/mmr.2015.4500. [DOI] [PubMed] [Google Scholar]
- 301.Li X., Jiang M., Chen D., Xu B., Wang R., Chu Y., Wang W., Zhou L., Lei Z., Nie Y., et al. miR-148b-3p inhibits gastric cancer metastasis by inhibiting the Dock6/Rac1/Cdc42 axis. J. Exp. Clin. Cancer Res. 2018;37:71. doi: 10.1186/s13046-018-0729-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Lopez-Ramirez M.A., Wu D., Pryce G., Simpson J.E., Reijerkerk A., King-Robson J., Kay O., de Vries H.E., Hirst M.C., Sharrack B., et al. MicroRNA-155 negatively affects blood-brain barrier function during neuroinflammation. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2014;28:2551–2565. doi: 10.1096/fj.13-248880. [DOI] [PubMed] [Google Scholar]
- 303.Peng J., Liu H., Liu C. MiR-155 Promotes Uveal Melanoma Cell Proliferation and Invasion by Regulating NDFIP1 Expression. Technol. Cancer Res. Treat. 2017;16:1160–1167. doi: 10.1177/1533034617737923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Zhang W., Ji W., Zhao X. MiR-155 promotes anaplastic thyroid cancer progression by directly targeting SOCS1. BMC Cancer. 2019;19:1093. doi: 10.1186/s12885-019-6319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Li N., Cui T., Guo W., Wang D., Mao L. MiR-155-5p accelerates the metastasis of cervical cancer cell via targeting TP53INP1. OncoTargets Ther. 2019;12:3181–3196. doi: 10.2147/OTT.S193097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Zeng Q., Tao X., Huang F., Wu T., Wang J., Jiang X., Kuang Z., Cheng B. Overexpression of miR-155 promotes the proliferation and invasion of oral squamous carcinoma cells by regulating BCL6/cyclin D2. Int. J. Mol. Med. 2016;37:1274–1280. doi: 10.3892/ijmm.2016.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Kundu S.T., Byers L.A., Peng D.H., Roybal J.D., Diao L., Wang J., Tong P., Creighton C.J., Gibbons D.L. The miR-200 family and the miR-183~96~182 cluster target Foxf2 to inhibit invasion and metastasis in lung cancers. Oncogene. 2016;35:173–186. doi: 10.1038/onc.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Roybal J.D., Zang Y., Ahn Y.H., Yang Y., Gibbons D.L., Baird B.N., Alvarez C., Thilaganathan N., Liu D.D., Saintigny P., et al. miR-200 Inhibits lung adenocarcinoma cell invasion and metastasis by targeting Flt1/VEGFR1. Mol. Cancer Res. 2011;9:25–35. doi: 10.1158/1541-7786.MCR-10-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Kim J.S., Kurie J.M., Ahn Y.H. BMP4 depletion by miR-200 inhibits tumorigenesis and metastasis of lung adenocarcinoma cells. Mol. Cancer. 2015;14:173. doi: 10.1186/s12943-015-0441-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Sun Y., Shen S., Liu X., Tang H., Wang Z., Yu Z., Li X., Wu M. MiR-429 inhibits cells growth and invasion and regulates EMT-related marker genes by targeting Onecut2 in colorectal carcinoma. Mol. Cell Biochem. 2014;390:19–30. doi: 10.1007/s11010-013-1950-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Liu L., Qiu M., Tan G., Liang Z., Qin Y., Chen L., Chen H., Liu J. miR-200c inhibits invasion, migration and proliferation of bladder cancer cells through down-regulation of BMI-1 and E2F3. J. Transl. Med. 2014;12:305. doi: 10.1186/s12967-014-0305-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Huang B.S., Luo Q.Z., Han Y., Li X.B., Cao L.J., Wu L.X. microRNA-223 promotes the growth and invasion of glioblastoma cells by targeting tumor suppressor PAX6. Oncol. Rep. 2013;30:2263–2269. doi: 10.3892/or.2013.2683. [DOI] [PubMed] [Google Scholar]
- 313.Li J., Guo Y., Liang X., Sun M., Wang G., De W., Wu W. MicroRNA-223 functions as an oncogene in human gastric cancer by targeting FBXW7/hCdc4. J. Cancer Res. Clin. Oncol. 2012;138:763–774. doi: 10.1007/s00432-012-1154-x. [DOI] [PubMed] [Google Scholar]
- 314.Cao T.H., Ling X., Chen C., Tang W., Hu D.M., Yin G.J. Role of miR-214-5p in the migration and invasion of pancreatic cancer cells. Eur. Rev. Med. Pharmacol. Sci. 2018;22:7214–7221. doi: 10.26355/eurrev_201811_16255. [DOI] [PubMed] [Google Scholar]
- 315.Zhang M., Wang D., Zhu T., Yin R. miR-214-5p Targets ROCK1 and Suppresses Proliferation and Invasion of Human Osteosarcoma Cells. Oncol. Res. 2017;25:75–81. doi: 10.3727/096504016X14719078133401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Wang M., Wang L., Zhang M., Li X., Zhu Z., Wang H. MiR-214 inhibits the proliferation and invasion of esophageal squamous cell carcinoma cells by targeting CDC25B. Biomed. Pharmacother. Biomed. Pharmacother. 2017;95:1678–1683. doi: 10.1016/j.biopha.2017.09.048. [DOI] [PubMed] [Google Scholar]
- 317.Peng R., Men J., Ma R., Wang Q., Wang Y., Sun Y., Ren J. miR-214 down-regulates ARL2 and suppresses growth and invasion of cervical cancer cells. Biochem. Biophys. Res. Commun. 2017;484:623–630. doi: 10.1016/j.bbrc.2017.01.152. [DOI] [PubMed] [Google Scholar]
- 318.Lu Q., Xu L., Li C., Yuan Y., Huang S., Chen H. miR-214 inhibits invasion and migration via downregulating GALNT7 in esophageal squamous cell cancer. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2016;37:14605–14614. doi: 10.1007/s13277-016-5320-7. [DOI] [PubMed] [Google Scholar]
- 319.Peng R., Cheng X., Zhang Y., Lu X., Hu Z. miR-214 down-regulates MKK3 and suppresses malignant phenotypes of cervical cancer cells. Gene. 2020;724:144146. doi: 10.1016/j.gene.2019.144146. [DOI] [PubMed] [Google Scholar]
- 320.Chen X., Du J., Jiang R., Li L. MicroRNA-214 inhibits the proliferation and invasion of lung carcinoma cells by targeting JAK1. Am. J. Transl. Res. 2018;10:1164–1171. [PMC free article] [PubMed] [Google Scholar]
- 321.Li Y., Liu J., Hu W., Zhang Y., Sang J., Li H., Ma T., Bo Y., Bai T., Guo H., et al. miR-424-5p Promotes Proliferation, Migration and Invasion of Laryngeal Squamous Cell Carcinoma. OncoTargets Ther. 2019;12:10441–10453. doi: 10.2147/OTT.S224325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Wang F., Wang J., Yang X., Chen D., Wang L. MiR-424-5p participates in esophageal squamous cell carcinoma invasion and metastasis via SMAD7 pathway mediated EMT. Diagn. Pathol. 2016;11:88. doi: 10.1186/s13000-016-0536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Wang N., Xiang X., Chen K., Liu P., Zhu A. Targeting of NT5E by miR-30b and miR-340 attenuates proliferation, invasion and migration of gallbladder carcinoma. Biochimie. 2018;146:56–67. doi: 10.1016/j.biochi.2017.10.027. [DOI] [PubMed] [Google Scholar]
- 324.Xiao H., Yu L., Li F., Wang H., Li W., He X. MiR-340 suppresses the metastasis by targeting EphA3 in cervical cancer. Cell Biol. Int. 2018;42:1115–1123. doi: 10.1002/cbin.10974. [DOI] [PubMed] [Google Scholar]
- 325.Wang X., Song Y. MicroRNA-340 inhibits the growth and invasion of angiosarcoma cells by targeting SIRT7. Biomed. Pharmacother. Biomed. Pharmacother. 2018;103:1061–1068. doi: 10.1016/j.biopha.2018.04.148. [DOI] [PubMed] [Google Scholar]
- 326.Chen Q., Zhang Y., Xu L. microRNA-340 influences cell proliferation, apoptosis and invasion by targeting NF-κB1 in gastric cancer. Int. J. Clin. Exp. Pathol. 2018;11:3812–3824. [PMC free article] [PubMed] [Google Scholar]
- 327.Wang H., Guo W., Jian Q., Xue K., Huang M., Chi S., Li C., Li C. MicroRNA-340 inhibits squamous cell carcinoma cell proliferation, migration and invasion by downregulating RhoA. J. Dermatol. Sci. 2018;92:197–206. doi: 10.1016/j.jdermsci.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 328.Yuan J., Ji H., Xiao F., Lin Z., Zhao X., Wang Z., Zhao J., Lu J. MicroRNA-340 inhibits the proliferation and invasion of hepatocellular carcinoma cells by targeting JAK1. Biochem. Biophys. Res. Commun. 2017;483:578–584. doi: 10.1016/j.bbrc.2016.12.102. [DOI] [PubMed] [Google Scholar]
- 329.Dongre A., Weinberg R.A. New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019;20:69–84. doi: 10.1038/s41580-018-0080-4. [DOI] [PubMed] [Google Scholar]
- 330.Kalluri R., Weinberg R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Mani S.A., Guo W., Liao M.-J., Eaton E.N., Ayyanan A., Zhou A.Y., Brooks M., Reinhard F., Zhang C.C., Shipitsin M., et al. The Epithelial-Mesenchymal Transition Generates Cells with Properties of Stem Cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Lamouille S., Xu J., Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Ahn Y.-H., Gibbons D.L., Chakravarti D., Creighton C.J., Rizvi Z.H., Adams H.P., Pertsemlidis A., Gregory P.A., Wright J.A., Goodall G.J., et al. ZEB1 drives prometastatic actin cytoskeletal remodeling by downregulating miR-34a expression. J. Clin. Investig. 2012;122:3170–3183. doi: 10.1172/JCI63608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Ding X.-M. MicroRNAs: Regulators of cancer metastasis and epithelial-mesenchymal transition (EMT) Chin. J. Cancer. 2014;33:140–147. doi: 10.5732/cjc.013.10094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Yan Y., Wang Q., Yan X.-L., Zhang Y., Li W., Tang F., Li X., Yang P. miR-10a controls glioma migration and invasion through regulating epithelial–mesenchymal transition via EphA8. FEBS Lett. 2015;589:756–765. doi: 10.1016/j.febslet.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 336.Zhang H., Lu Y., Chen E., Li X., Lv B., Vikis H.G., Liu P. XRN2 promotes EMT and metastasis through regulating maturation of miR-10a. Oncogene. 2017;36:3925–3933. doi: 10.1038/onc.2017.39. [DOI] [PubMed] [Google Scholar]
- 337.Choi P.-W., Ng S.-W. The Functions of MicroRNA-200 Family in Ovarian Cancer: Beyond Epithelial-Mesenchymal Transition. Int. J. Mol. Sci. 2017;18:1207. doi: 10.3390/ijms18061207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Behbahani G.D., Ghahhari N.M., Javidi M.A., Molan A.F., Feizi N., Babashah S. MicroRNA-Mediated Post-Transcriptional Regulation of Epithelial to Mesenchymal Transition in Cancer. Pathol. Oncol. Res. 2017;23:1–12. doi: 10.1007/s12253-016-0101-6. [DOI] [PubMed] [Google Scholar]
- 339.Suzuki H.I. MicroRNA Control of TGF-β Signaling. Int. J. Mol. Sci. 2018;19:1901. doi: 10.3390/ijms19071901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Zhang J., Tian X.J., Zhang H., Teng Y., Li R., Bai F., Elankumaran S., Xing J. TGF-β-induced epithelial-to-mesenchymal transition proceeds through stepwise activation of multiple feedback loops. Science Signal. 2014;7:ra91. doi: 10.1126/scisignal.2005304. [DOI] [PubMed] [Google Scholar]
- 341.Jung Y.-S., Park J.-I. Wnt signaling in cancer: Therapeutic targeting of Wnt signaling beyond β-catenin and the destruction complex. Exp. Mol. Med. 2020;52:183–191. doi: 10.1038/s12276-020-0380-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Sedgwick A.E., D’Souza-Schorey C. Wnt Signaling in Cell Motility and Invasion: Drawing Parallels between Development and Cancer. Cancers. 2016;8:80. doi: 10.3390/cancers8090080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Zhan T., Rindtorff N., Boutros M. Wnt signaling in cancer. Oncogene. 2017;36:1461–1473. doi: 10.1038/onc.2016.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Ahsani Z., Mohammadi-Yeganeh S., Kia V., Karimkhanloo H., Zarghami N., Paryan M. WNT1 Gene from WNT Signaling Pathway Is a Direct Target of miR-122 in Hepatocellular Carcinoma. Appl. Biochem. Biotechnol. 2017;181:884–897. doi: 10.1007/s12010-016-2256-8. [DOI] [PubMed] [Google Scholar]
- 345.Yan H., Dong X., Zhong X., Ye J., Zhou Y., Yang X., Shen J., Zhang J. Inhibitions of epithelial to mesenchymal transition and cancer stem cells-like properties are involved in miR-148a-mediated anti-metastasis of hepatocellular carcinoma. Mol. Carcinog. 2014;53:960–969. doi: 10.1002/mc.22064. [DOI] [PubMed] [Google Scholar]
- 346.Huang S., Xie Y., Yang P., Chen P., Zhang L. HCV core protein-induced down-regulation of microRNA-152 promoted aberrant proliferation by regulating Wnt1 in HepG2 cells. PLoS ONE. 2014;9:e81730. doi: 10.1371/journal.pone.0081730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Wu L., Pei F., Men X., Wang K., Ma D. miR‑329 inhibits papillary thyroid cancer progression via direct targeting WNT1. Oncol. Lett. 2018;16:3561–3568. doi: 10.3892/ol.2018.9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Fang Y., Feng Y., Wu T., Srinivas S., Yang W., Fan J., Yang C., Wang S. Aflatoxin B1 negatively regulates Wnt/β-catenin signaling pathway through activating miR-33a. PLoS ONE. 2013;8:e73004. doi: 10.1371/journal.pone.0073004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Wang X., Chen J., Li F., Lin Y., Zhang X., Lv Z., Jiang J. MiR-214 inhibits cell growth in hepatocellular carcinoma through suppression of β-catenin. Biochem. Biophys. Res. Commun. 2012;428:525–531. doi: 10.1016/j.bbrc.2012.10.039. [DOI] [PubMed] [Google Scholar]
- 350.Liu J., Ruan B., You N., Huang Q., Liu W., Dang Z., Xu W., Zhou T., Ji R., Cao Y., et al. Downregulation of miR-200a induces EMT phenotypes and CSC-like signatures through targeting the β-catenin pathway in hepatic oval cells. PLoS ONE. 2013;8:e79409. doi: 10.1371/journal.pone.0079409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Lu C., Liao Z., Cai M., Zhang G. MicroRNA-320a downregulation mediates human liver cancer cell proliferation through the Wnt/β-catenin signaling pathway. Oncol. Lett. 2017;13:573–578. doi: 10.3892/ol.2016.5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Hua H.W., Jiang F., Huang Q., Liao Z., Ding G. MicroRNA-153 promotes Wnt/β-catenin activation in hepatocellular carcinoma through suppression of WWOX. Oncotarget. 2015;6:3840–3847. doi: 10.18632/oncotarget.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Cai J., Guan H., Fang L., Yang Y., Zhu X., Yuan J., Wu J., Li M. MicroRNA-374a activates Wnt/β-catenin signaling to promote breast cancer metastasis. J. Clin. Investig. 2013;123:566–579. doi: 10.1172/JCI65871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Chen Z., Ma T., Huang C., Zhang L., Lv X., Xu T., Hu T., Li J. MiR-27a modulates the MDR1/P-glycoprotein expression by inhibiting FZD7/β-catenin pathway in hepatocellular carcinoma cells. Cell. Signal. 2013;25:2693–2701. doi: 10.1016/j.cellsig.2013.08.032. [DOI] [PubMed] [Google Scholar]
- 355.Zhang Y., Zheng D., Xiong Y., Xue C., Chen G., Yan B., Ye Q. miR-202 suppresses cell proliferation in human hepatocellular carcinoma by downregulating LRP6 post-transcriptionally. FEBS Lett. 2014;588:1913–1920. doi: 10.1016/j.febslet.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 356.Jiang N., Chen W.-J., Zhang J.-W., Xu C., Zeng X.-C., Zhang T., Li Y., Wang G.-Y. Downregulation of miR-432 activates Wnt/β-catenin signaling and promotes human hepatocellular carcinoma proliferation. Oncotarget. 2015;6:7866–7879. doi: 10.18632/oncotarget.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Zhang J.J., Wang C.Y., Hua L., Yao K.H., Chen J.T., Hu J.H. miR-107 promotes hepatocellular carcinoma cell proliferation by targeting Axin2. Int. J. Clin. Exp. Pathol. 2015;8:5168–5174. [PMC free article] [PubMed] [Google Scholar]
- 358.Chai S., Ng K.Y., Tong M., Lau E.Y., Lee T.K., Chan K.W., Yuan Y.F., Cheung T.T., Cheung S.T., Wang X.Q., et al. Octamer 4/microRNA-1246 signaling axis drives Wnt/β-catenin activation in liver cancer stem cells. Hepatology. 2016;64:2062–2076. doi: 10.1002/hep.28821. [DOI] [PubMed] [Google Scholar]
- 359.Martin J., Jenkins R.H., Bennagi R., Krupa A., Phillips A.O., Bowen T., Fraser D.J. Post-transcriptional regulation of Transforming Growth Factor Beta-1 by microRNA-744. PLoS ONE. 2011;6:e25044. doi: 10.1371/journal.pone.0025044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Turcatel G., Rubin N., El-Hashash A., Warburton D. MIR-99a and MIR-99b modulate TGF-β induced epithelial to mesenchymal plasticity in normal murine mammary gland cells. PLoS ONE. 2012;7:e31032. doi: 10.1371/journal.pone.0031032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Mishra S., Deng J.J., Gowda P.S., Rao M.K., Lin C.L., Chen C.L., Huang T., Sun L.Z. Androgen receptor and microRNA-21 axis downregulates transforming growth factor beta receptor II (TGFBR2) expression in prostate cancer. Oncogene. 2014;33:4097–4106. doi: 10.1038/onc.2013.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Wang F.E., Zhang C., Maminishkis A., Dong L., Zhi C., Li R., Zhao J., Majerciak V., Gaur A.B., Chen S., et al. MicroRNA-204/211 alters epithelial physiology. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2010;24:1552–1571. doi: 10.1096/fj.08-125856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Flores-Pérez A., Marchat L.A., Rodríguez-Cuevas S., Bautista-Piña V., Hidalgo-Miranda A., Ocampo E.A., Martínez M.S., Palma-Flores C., Fonseca-Sánchez M.A., Astudillo-de la Vega H., et al. Dual targeting of ANGPT1 and TGFBR2 genes by miR-204 controls angiogenesis in breast cancer. Sci. Rep. 2016;6:34504. doi: 10.1038/srep34504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Jiang Z., Yin J., Fu W., Mo Y., Pan Y., Dai L., Huang H., Li S., Zhao J. MiRNA 17 family regulates cisplatin-resistant and metastasis by targeting TGFbetaR2 in NSCLC. PLoS ONE. 2014;9:e94639. doi: 10.1371/journal.pone.0094639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Keklikoglou I., Koerner C., Schmidt C., Zhang J.D., Heckmann D., Shavinskaya A., Allgayer H., Gückel B., Fehm T., Schneeweiss A., et al. MicroRNA-520/373 family functions as a tumor suppressor in estrogen receptor negative breast cancer by targeting NF-κB and TGF-β signaling pathways. Oncogene. 2012;31:4150–4163. doi: 10.1038/onc.2011.571. [DOI] [PubMed] [Google Scholar]
- 366.Gregory P.A., Bert A.G., Paterson E.L., Barry S.C., Tsykin A., Farshid G., Vadas M.A., Khew-Goodall Y., Goodall G.J. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat. Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 367.Korpal M., Lee E.S., Hu G., Kang Y. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J. Biol. Chem. 2008;283:14910–14914. doi: 10.1074/jbc.C800074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Liu X., Wang C., Chen Z., Jin Y., Wang Y., Kolokythas A., Dai Y., Zhou X. MicroRNA-138 suppresses epithelial-mesenchymal transition in squamous cell carcinoma cell lines. Biochem. J. 2011;440:23–31. doi: 10.1042/BJ20111006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.White N.M., Khella H.W., Grigull J., Adzovic S., Youssef Y.M., Honey R.J., Stewart R., Pace K.T., Bjarnason G.A., Jewett M.A., et al. miRNA profiling in metastatic renal cell carcinoma reveals a tumour-suppressor effect for miR-215. Br. J. Cancer. 2011;105:1741–1749. doi: 10.1038/bjc.2011.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Lin X., Yang Z., Zhang P., Liu Y., Shao G. miR-154 inhibits migration and invasion of human non-small cell lung cancer by targeting ZEB2. Oncol. Lett. 2016;12:301–306. doi: 10.3892/ol.2016.4577. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 371.You J., Li Y., Fang N., Liu B., Zu L., Chang R., Li X., Zhou Q. MiR-132 suppresses the migration and invasion of lung cancer cells via targeting the EMT regulator ZEB2. PLoS ONE. 2014;9:e91827. doi: 10.1371/journal.pone.0091827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Chang C.J., Hsu C.C., Chang C.H., Tsai L.L., Chang Y.C., Lu S.W., Yu C.H., Huang H.S., Wang J.J., Tsai C.H., et al. Let-7d functions as novel regulator of epithelial-mesenchymal transition and chemoresistant property in oral cancer. Oncol. Rep. 2011;26:1003–1010. doi: 10.3892/or.2011.1360. [DOI] [PubMed] [Google Scholar]
- 373.Li B., Han Q., Zhu Y., Yu Y., Wang J., Jiang X. Down-regulation of miR-214 contributes to intrahepatic cholangiocarcinoma metastasis by targeting Twist. FEBS J. 2012;279:2393–2398. doi: 10.1111/j.1742-4658.2012.08618.x. [DOI] [PubMed] [Google Scholar]
- 374.Nairismägi M.L., Vislovukh A., Meng Q., Kratassiouk G., Beldiman C., Petretich M., Groisman R., Füchtbauer E.M., Harel-Bellan A., Groisman I. Translational control of TWIST1 expression in MCF-10A cell lines recapitulating breast cancer progression. Oncogene. 2012;31:4960–4966. doi: 10.1038/onc.2011.650. [DOI] [PubMed] [Google Scholar]
- 375.Nairismägi M.L., Füchtbauer A., Labouriau R., Bramsen J.B., Füchtbauer E.M. The proto-oncogene TWIST1 is regulated by microRNAs. PLoS ONE. 2013;8:e66070. doi: 10.1371/journal.pone.0066070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Long L., Huang G., Zhu H., Guo Y., Liu Y., Huo J. Down-regulation of miR-138 promotes colorectal cancer metastasis via directly targeting TWIST2. J. Transl. Med. 2013;11:275. doi: 10.1186/1479-5876-11-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Zhang J., Wang Q., Quan Z. Long non-coding RNA CASC9 enhances breast cancer progression by promoting metastasis through the meditation of miR-215/TWIST2 signaling associated with TGF-β expression. Biochem. Biophys. Res. Commun. 2019;515:644–650. doi: 10.1016/j.bbrc.2019.05.080. [DOI] [PubMed] [Google Scholar]
- 378.Huang T.T., Ping Y.H., Wang A.M., Ke C.C., Fang W.L., Huang K.H., Lee H.C., Chi C.W., Yeh T.S. The reciprocal regulation loop of Notch2 pathway and miR-23b in controlling gastric carcinogenesis. Oncotarget. 2015;6:18012–18026. doi: 10.18632/oncotarget.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Song G., Zhang Y., Wang L. MicroRNA-206 targets notch3, activates apoptosis, and inhibits tumor cell migration and focus formation. J. Biol. Chem. 2009;284:31921–31927. doi: 10.1074/jbc.M109.046862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Zhang X., Ai F., Li X., Tian L., Wang X., Shen S., Liu F. MicroRNA-34a suppresses colorectal cancer metastasis by regulating Notch signaling. Oncol. Lett. 2017;14:2325–2333. doi: 10.3892/ol.2017.6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Zhan Y., Li X., Liang X., Li L., Cao B., Wang B., Ma J., Ding F., Wang X., Pang D., et al. MicroRNA-182 drives colonization and macroscopic metastasis via targeting its suppressor SNAI1 in breast cancer. Oncotarget. 2017;8:4629–4641. doi: 10.18632/oncotarget.13542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Xia H., Cheung W.K., Ng S.S., Jiang X., Jiang S., Sze J., Leung G.K., Lu G., Chan D.T., Bian X.W., et al. Loss of brain-enriched miR-124 microRNA enhances stem-like traits and invasiveness of glioma cells. J. Biol. Chem. 2012;287:9962–9971. doi: 10.1074/jbc.M111.332627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Zhang Z., Zhang B., Li W., Fu L., Fu L., Zhu Z., Dong J.T. Epigenetic Silencing of miR-203 Upregulates SNAI2 and Contributes to the Invasiveness of Malignant Breast Cancer Cells. Genes Cancer. 2011;2:782–791. doi: 10.1177/1947601911429743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Kumarswamy R., Mudduluru G., Ceppi P., Muppala S., Kozlowski M., Niklinski J., Papotti M., Allgayer H. MicroRNA-30a inhibits epithelial-to-mesenchymal transition by targeting Snai1 and is downregulated in non-small cell lung cancer. Int. J. Cancer. 2012;130:2044–2053. doi: 10.1002/ijc.26218. [DOI] [PubMed] [Google Scholar]
- 385.Cui Z., Hu Y. MicroRNA-124 suppresses Slug-mediated lung cancer metastasis. Eur. Rev. Med. Pharmacol. Sci. 2016;20:3802–3811. [PubMed] [Google Scholar]
- 386.Tominaga E., Yuasa K., Shimazaki S., Hijikata T. MicroRNA-1 targets Slug and endows lung cancer A549 cells with epithelial and anti-tumorigenic properties. Exp. Cell Res. 2013;319:77–88. doi: 10.1016/j.yexcr.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 387.Chang C.W., Yu J.C., Hsieh Y.H., Yao C.C., Chao J.I., Chen P.M., Hsieh H.Y., Hsiung C.N., Chu H.W., Shen C.Y., et al. MicroRNA-30a increases tight junction protein expression to suppress the epithelial-mesenchymal transition and metastasis by targeting Slug in breast cancer. Oncotarget. 2016;7:16462–16478. doi: 10.18632/oncotarget.7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Chen W.X., Zhang Z.G., Ding Z.Y., Liang H.F., Song J., Tan X.L., Wu J.J., Li G.Z., Zeng Z., Zhang B.X., et al. MicroRNA-630 suppresses tumor metastasis through the TGF-β- miR-630-Slug signaling pathway and correlates inversely with poor prognosis in hepatocellular carcinoma. Oncotarget. 2016;7:22674–22686. doi: 10.18632/oncotarget.8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Yan M., Ye L., Feng X., Shi R., Sun Z., Li Z., Liu T. MicroRNA-590-3p inhibits invasion and metastasis in triple-negative breast cancer by targeting Slug. Am. J. Cancer Res. 2020;10:965–974. [PMC free article] [PubMed] [Google Scholar]
- 390.Ma H.B., Yao Y.N., Yu J.J., Chen X.X., Li H.F. Extensive profiling of circular RNAs and the potential regulatory role of circRNA-000284 in cell proliferation and invasion of cervical cancer via sponging miR-506. Am. J. Transl. Res. 2018;10:592–604. [PMC free article] [PubMed] [Google Scholar]
- 391.Gao P., Xing A.Y., Zhou G.Y., Zhang T.G., Zhang J.P., Gao C., Li H., Shi D.B. The molecular mechanism of microRNA-145 to suppress invasion-metastasis cascade in gastric cancer. Oncogene. 2013;32:491–501. doi: 10.1038/onc.2012.61. [DOI] [PubMed] [Google Scholar]
- 392.Miao J., Wang W., Wu S., Zang X., Li Y., Wang J., Zhan R., Gao M., Hu M., Li J., et al. miR-194 Suppresses Proliferation and Migration and Promotes Apoptosis of Osteosarcoma Cells by Targeting CDH2. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018;45:1966–1974. doi: 10.1159/000487973. [DOI] [PubMed] [Google Scholar]
- 393.Bockhorn J., Yee K., Chang Y.F., Prat A., Huo D., Nwachukwu C., Dalton R., Huang S., Swanson K.E., Perou C.M., et al. MicroRNA-30c targets cytoskeleton genes involved in breast cancer cell invasion. Breast Cancer Res. Treat. 2013;137:373–382. doi: 10.1007/s10549-012-2346-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Yang C.H., Wang Y., Sims M., Cai C., Pfeffer L.M. MicroRNA-1 suppresses glioblastoma in preclinical models by targeting fibronectin. Cancer Lett. 2019;465:59–67. doi: 10.1016/j.canlet.2019.08.021. [DOI] [PubMed] [Google Scholar]
- 395.Wang S., Gao B., Yang H., Liu X., Wu X., Wang W. MicroRNA-432 is downregulated in cervical cancer and directly targets FN1 to inhibit cell proliferation and invasion. Oncol. Lett. 2019;18:1475–1482. doi: 10.3892/ol.2019.10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Ye Y., Zhuang J., Wang G., He S., Ni J., Xia W. MicroRNA-139 targets fibronectin 1 to inhibit papillary thyroid carcinoma progression. Oncol. Lett. 2017;14:7799–7806. doi: 10.3892/ol.2017.7201. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 397.Zhang H., Sun Z., Li Y., Fan D., Jiang H. MicroRNA-200c binding to FN1 suppresses the proliferation, migration and invasion of gastric cancer cells. Biomed. Pharmacother. Biomed. Pharmacother. 2017;88:285–292. doi: 10.1016/j.biopha.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 398.Dhar S., Kumar A., Gomez C.R., Akhtar I., Hancock J.C., Lage J.M., Pound C.R., Levenson A.S. MTA1-activated Epi-microRNA-22 regulates E-cadherin and prostate cancer invasiveness. FEBS Lett. 2017;591:924–933. doi: 10.1002/1873-3468.12603. [DOI] [PubMed] [Google Scholar]
- 399.Liu Y., Zhao J., Zhang P.Y., Zhang Y., Sun S.Y., Yu S.Y., Xi Q.S. MicroRNA-10b targets E-cadherin and modulates breast cancer metastasis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2012;18:Br299–Br308. doi: 10.12659/MSM.883262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Pan Y., Li J., Zhang Y., Wang N., Liang H., Liu Y., Zhang C.Y., Zen K., Gu H. Slug-upregulated miR-221 promotes breast cancer progression through suppressing E-cadherin expression. Sci. Rep. 2016;6:25798. doi: 10.1038/srep25798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Wang J.Y., Li X.F., Li P.Z., Zhang X., Xu Y., Jin X. MicroRNA-23b regulates nasopharyngeal carcinoma cell proliferation and metastasis by targeting E-cadherin. Mol. Med. Rep. 2016;14:537–543. doi: 10.3892/mmr.2016.5206. [DOI] [PubMed] [Google Scholar]
- 402.Xu X., Chen Z., Zhao X., Wang J., Ding D., Wang Z., Tan F., Tan X., Zhou F., Sun J., et al. MicroRNA-25 promotes cell migration and invasion in esophageal squamous cell carcinoma. Biochem. Biophys. Res. Commun. 2012;421:640–645. doi: 10.1016/j.bbrc.2012.03.048. [DOI] [PubMed] [Google Scholar]
- 403.Chen Z.L., Zhao X.H., Wang J.W., Li B.Z., Wang Z., Sun J., Tan F.W., Ding D.P., Xu X.H., Zhou F., et al. microRNA-92a promotes lymph node metastasis of human esophageal squamous cell carcinoma via E-cadherin. J. Biol. Chem. 2011;286:10725–10734. doi: 10.1074/jbc.M110.165654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Du J., Zhang F., Zhang L., Jia Y., Chen H. MicroRNA-103 regulates the progression in endometrial carcinoma through ZO-1. Int. J. Immunopathol. Pharmacol. 2019;33:2058738419872621. doi: 10.1177/2058738419872621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Sun X., Cui S., Fu X., Liu C., Wang Z., Liu Y. MicroRNA-146-5p promotes proliferation, migration and invasion in lung cancer cells by targeting claudin-12. Cancer Biomark. Sect. A Dis. Markers. 2019;25:89–99. doi: 10.3233/CBM-182374. [DOI] [PubMed] [Google Scholar]
- 406.Yang P., Zhang M., Liu X., Pu H. MicroRNA-421 promotes the proliferation and metastasis of gastric cancer cells by targeting claudin-11. Exp. Ther. Med. 2017;14:2625–2632. doi: 10.3892/etm.2017.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Wang Y.B., Shi Q., Li G., Zheng J.H., Lin J., Qiu W. MicroRNA-488 inhibits progression of colorectal cancer via inhibition of the mitogen-activated protein kinase pathway by targeting claudin-2. Am. J. Physiol. Cell Physiol. 2019;316:C33–C47. doi: 10.1152/ajpcell.00047.2018. [DOI] [PubMed] [Google Scholar]
- 408.Zheng Y.F., Luo J., Gan G.L., Li W. Overexpression of microRNA-98 inhibits cell proliferation and promotes cell apoptosis via claudin-1 in human colorectal carcinoma. J. Cell. Biochem. 2019;120:6090–6105. doi: 10.1002/jcb.27895. [DOI] [PubMed] [Google Scholar]
- 409.Zhang G.-J., Xiao H.-X., Tian H.-P., Liu Z.-L., Xia S.-S., Zhou T. Upregulation of microRNA-155 promotes the migration and invasion of colorectal cancer cells through the regulation of claudin-1 expression. Int. J. Mol. Med. 2013;31:1375–1380. doi: 10.3892/ijmm.2013.1348. [DOI] [PubMed] [Google Scholar]
- 410.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 411.Rosano S., Corà D., Parab S., Zaffuto S., Isella C., Porporato R., Hoza R.M., Calogero R.A., Riganti C., Bussolino F., et al. A regulatory microRNA network controls endothelial cell phenotypic switch during sprouting angiogenesis. eLife. 2020;9:e48095. doi: 10.7554/eLife.48095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Wang Z.-F., Liao F., Wu H., Dai J. Glioma stem cells-derived exosomal miR-26a promotes angiogenesis of microvessel endothelial cells in glioma. J. Exp. Clin. Cancer Res. 2019;38:201. doi: 10.1186/s13046-019-1181-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Lu Y., Qin T., Li J., Wang L., Zhang Q., Jiang Z., Mao J. MicroRNA-140-5p inhibits invasion and angiogenesis through targeting VEGF-A in breast cancer. Cancer Gene Ther. 2017;24:386–392. doi: 10.1038/cgt.2017.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Yue X., Wang P., Xu J., Zhu Y., Sun G., Pang Q., Tao R. MicroRNA-205 functions as a tumor suppressor in human glioblastoma cells by targeting VEGF-A. Oncol. Rep. 2012;27:1200–1206. doi: 10.3892/or.2011.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Gu A., Lu J., Wang W., Shi C., Han B., Yao M. Role of miR-497 in VEGF-A-mediated cancer cell growth and invasion in non-small cell lung cancer. Int. J. Biochem. Cell Biol. 2016;70:118–125. doi: 10.1016/j.biocel.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 416.Lei Z., Li B., Yang Z., Fang H., Zhang G.M., Feng Z.H., Huang B. Regulation of HIF-1alpha and VEGF by miR-20b tunes tumor cells to adapt to the alteration of oxygen concentration. PLoS ONE. 2009;4:e7629. doi: 10.1371/journal.pone.0007629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Chou J., Lin J.H., Brenot A., Kim J.W., Provot S., Werb Z. GATA3 suppresses metastasis and modulates the tumour microenvironment by regulating microRNA-29b expression. Nat. Cell Biol. 2013;15:201–213. doi: 10.1038/ncb2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Yang I.P., Tsai H.L., Hou M.F., Chen K.C., Tsai P.C., Huang S.W., Chou W.W., Wang J.Y., Juo S.H. MicroRNA-93 inhibits tumor growth and early relapse of human colorectal cancer by affecting genes involved in the cell cycle. Carcinogenesis. 2012;33:1522–1530. doi: 10.1093/carcin/bgs166. [DOI] [PubMed] [Google Scholar]
- 419.Liu B., Peng X.C., Zheng X.L., Wang J., Qin Y.W. MiR-126 restoration down-regulate VEGF and inhibit the growth of lung cancer cell lines in vitro and in vivo. Lung Cancer. 2009;66:169–175. doi: 10.1016/j.lungcan.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 420.Zhu X., Er K., Mao C., Yan Q., Xu H., Zhang Y., Zhu J., Cui F., Zhao W., Shi H. miR-203 suppresses tumor growth and angiogenesis by targeting VEGFA in cervical cancer. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2013;32:64–73. doi: 10.1159/000350125. [DOI] [PubMed] [Google Scholar]
- 421.Zhou B., Ma R., Si W., Li S., Xu Y., Tu X., Wang Q. MicroRNA-503 targets FGF2 and VEGFA and inhibits tumor angiogenesis and growth. Cancer Lett. 2013;333:159–169. doi: 10.1016/j.canlet.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 422.Cheng J., Chen Y., Zhao P., Liu X., Dong J., Li J., Huang C., Wu R., Lv Y. Downregulation of miRNA-638 promotes angiogenesis and growth of hepatocellular carcinoma by targeting VEGF. Oncotarget. 2016;7:30702–30711. doi: 10.18632/oncotarget.8930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Ye J., Wu X., Wu D., Wu P., Ni C., Zhang Z., Chen Z., Qiu F., Xu J., Huang J. miRNA-27b targets vascular endothelial growth factor C to inhibit tumor progression and angiogenesis in colorectal cancer. PLoS ONE. 2013;8:e60687. doi: 10.1371/journal.pone.0060687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Hu J., Cheng Y., Li Y., Jin Z., Pan Y., Liu G., Fu S., Zhang Y., Feng K., Feng Y. microRNA-128 plays a critical role in human non-small cell lung cancer tumourigenesis, angiogenesis and lymphangiogenesis by directly targeting vascular endothelial growth factor-C. Eur. J. Cancer. 2014;50:2336–2350. doi: 10.1016/j.ejca.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 425.Fu H., Zhang J., Pan T., Ai S., Tang L., Wang F. miR‑378a enhances the sensitivity of liver cancer to sorafenib by targeting VEGFR, PDGFRβ and c‑Raf. Mol. Med. Rep. 2018;17:4581–4588. doi: 10.3892/mmr.2018.8390. [DOI] [PubMed] [Google Scholar]
- 426.Peng Y., Liu Y.-M., Li L.-C., Wang L.-L., Wu X.-L. MicroRNA-338 Inhibits Growth, Invasion and Metastasis of Gastric Cancer by Targeting NRP1 Expression. PLoS ONE. 2014;9:e94422. doi: 10.1371/journal.pone.0094422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Ma L., Zhai B., Zhu H., Li W., Jiang W., Lei L., Zhang S., Qiao H., Jiang X., Sun X. The miR-141/neuropilin-1 axis is associated with the clinicopathology and contributes to the growth and metastasis of pancreatic cancer. Cancer Cell Int. 2019;19:248. doi: 10.1186/s12935-019-0963-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Bhattacharyya S., Sul K., Krukovets I., Nestor C., Li J., Adognravi O.S. Novel tissue-specific mechanism of regulation of angiogenesis and cancer growth in response to hyperglycemia. J. Am. Heart Assoc. 2012;1:e005967. doi: 10.1161/JAHA.112.005967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Amodeo V., Bazan V., Fanale D., Insalaco L., Caruso S., Cicero G., Bronte G., Rolfo C., Santini D., Russo A. Effects of anti-miR-182 on TSP-1 expression in human colon cancer cells: There is a sense in antisense? Expert Opin. Ther. Targets. 2013;17:1249–1261. doi: 10.1517/14728222.2013.832206. [DOI] [PubMed] [Google Scholar]
- 430.Yin Q., Wang P.P., Peng R., Zhou H. MiR-19a enhances cell proliferation, migration, and invasiveness through enhancing lymphangiogenesis by targeting thrombospondin-1 in colorectal cancer. Biochem. Cell Biol. Biochim. Biol. Cell. 2019;97:731–739. doi: 10.1139/bcb-2018-0302. [DOI] [PubMed] [Google Scholar]
- 431.Han T., Mei Y., Wang Y., Feng Z. miR-5582-5p inhibits cell proliferation of non-small cell lung cancer through targeting FGF-10. Int. J. Clin. Exp. Pathol. 2019;12:1087–1094. [PMC free article] [PubMed] [Google Scholar]
- 432.Zhao W.Y., Wang Y., An Z.J., Shi C.G., Zhu G.A., Wang B., Lu M.Y., Pan C.K., Chen P. Downregulation of miR-497 promotes tumor growth and angiogenesis by targeting HDGF in non-small cell lung cancer. Biochem. Biophys. Res. Commun. 2013;435:466–471. doi: 10.1016/j.bbrc.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 433.Shih T.C., Tien Y.J., Wen C.J., Yeh T.S., Yu M.C., Huang C.H., Lee Y.S., Yen T.C., Hsieh S.Y. MicroRNA-214 downregulation contributes to tumor angiogenesis by inducing secretion of the hepatoma-derived growth factor in human hepatoma. J. Hepatol. 2012;57:584–591. doi: 10.1016/j.jhep.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 434.Song R., Cong L., Ni G., Chen M., Sun H., Sun Y., Chen M. MicroRNA-195 inhibits the behavior of cervical cancer tumors by directly targeting HDGF. Oncol. Lett. 2017;14:767–775. doi: 10.3892/ol.2017.6210. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 435.Situ J., Zhang H., Jin Z., Li K., Mao Y., Huang W. MicroRNA-939 Directly Targets HDGF to Inhibit the Aggressiveness of Prostate Cancer via Deactivation of the WNT/β-Catenin Pathway. OncoTargets Ther. 2020;13:4257–4270. doi: 10.2147/OTT.S250101. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 436.Wang Q., Zhu W. MicroRNA-873 inhibits the proliferation and invasion of endometrial cancer cells by directly targeting hepatoma-derived growth factor. Exp. Ther. Med. 2019;18:1291–1298. doi: 10.3892/etm.2019.7713. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 437.He S., Wang G., Ni J., Zhuang J., Zhuang S., Wang G., Ye Y., Xia W. MicroRNA-511 Inhibits Cellular Proliferation and Invasion in Colorectal Cancer by Directly Targeting Hepatoma-Derived Growth Factor. Oncol. Res. 2018;26:1355–1363. doi: 10.3727/096504018X15154094331876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Zhang Z., Li W., Jiang D., Liu C., Lai Z. MicroRNA-139-5p inhibits cell viability, migration and invasion and suppresses tumor growth by targeting HDGF in non-small cell lung cancer. Oncol. Lett. 2020;19:1806–1814. doi: 10.3892/ol.2020.11296. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 439.Yang Y., Dodbele S., Park T., Glass R., Bhat K., Sulman E.P., Zhang Y., Abounader R. MicroRNA-29a inhibits glioblastoma stem cells and tumor growth by regulating the PDGF pathway. J. Neuro-Oncol. 2019;145:23–34. doi: 10.1007/s11060-019-03275-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Zhou J., Xu D., Xie H., Tang J., Liu R., Li J., Wang S., Chen X., Su J., Zhou X., et al. miR-33a functions as a tumor suppressor in melanoma by targeting HIF-1α. Cancer Biol. Ther. 2015;16:846–855. doi: 10.1080/15384047.2015.1030545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Cha S.T., Chen P.S., Johansson G., Chu C.Y., Wang M.Y., Jeng Y.M., Yu S.L., Chen J.S., Chang K.J., Jee S.H., et al. MicroRNA-519c suppresses hypoxia-inducible factor-1alpha expression and tumor angiogenesis. Cancer Res. 2010;70:2675–2685. doi: 10.1158/0008-5472.CAN-09-2448. [DOI] [PubMed] [Google Scholar]
- 442.Cascio S., D’Andrea A., Ferla R., Surmacz E., Gulotta E., Amodeo V., Bazan V., Gebbia N., Russo A. miR-20b modulates VEGF expression by targeting HIF-1 alpha and STAT3 in MCF-7 breast cancer cells. J. Cell. Physiol. 2010;224:242–249. doi: 10.1002/jcp.22126. [DOI] [PubMed] [Google Scholar]
- 443.Umezu T., Tadokoro H., Azuma K., Yoshizawa S., Ohyashiki K., Ohyashiki J.H. Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood. 2014;124:3748–3757. doi: 10.1182/blood-2014-05-576116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Li C., Zhao J., Sun W. microRNA-222-Mediated VHL Downregulation Facilitates Retinoblastoma Chemoresistance by Increasing HIF1α Expression. Investig. Ophthalmol. Vis. Sci. 2020;61:9. doi: 10.1167/iovs.61.10.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Sun J., Jiang Z., Li Y., Wang K., Chen X., Liu G. Downregulation of miR-21 inhibits the malignant phenotype of pancreatic cancer cells by targeting VHL. OncoTargets Ther. 2019;12:7215–7226. doi: 10.2147/OTT.S211535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Kong W., He L., Richards E.J., Challa S., Xu C.X., Permuth-Wey J., Lancaster J.M., Coppola D., Sellers T.A., Djeu J.Y., et al. Upregulation of miRNA-155 promotes tumour angiogenesis by targeting VHL and is associated with poor prognosis and triple-negative breast cancer. Oncogene. 2014;33:679–689. doi: 10.1038/onc.2012.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Zhang X., Tang J., Zhi X., Xie K., Wang W., Li Z., Zhu Y., Yang L., Xu H., Xu Z. miR-874 functions as a tumor suppressor by inhibiting angiogenesis through STAT3/VEGF-A pathway in gastric cancer. Oncotarget. 2015;6:1605–1617. doi: 10.18632/oncotarget.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Wang Y., Lu Z., Wang N., Zhang M., Zeng X., Zhao W. MicroRNA-1299 is a negative regulator of STAT3 in colon cancer. Oncol. Rep. 2017;37:3227–3234. doi: 10.3892/or.2017.5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Shan D., Shang Y., Hu T. MicroRNA-411 Inhibits Cervical Cancer Progression by Directly Targeting STAT3. Oncol. Res. 2019;27:349–358. doi: 10.3727/096504018X15247361080118. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 450.Fan Z., Cui H., Xu X., Lin Z., Zhang X., Kang L., Han B., Meng J., Yan Z., Yan X., et al. MiR-125a suppresses tumor growth, invasion and metastasis in cervical cancer by targeting STAT3. Oncotarget. 2015;6:25266–25280. doi: 10.18632/oncotarget.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Zhu Z., Wang S., Zhu J., Yang Q., Dong H., Huang J. MicroRNA-544 down-regulates both Bcl6 and Stat3 to inhibit tumor growth of human triple negative breast cancer. Biol. Chem. 2016;397:1087–1095. doi: 10.1515/hsz-2016-0104. [DOI] [PubMed] [Google Scholar]
- 452.Kim J.S., Choi D.W., Kim C.S., Yu S.K., Kim H.J., Go D.S., Lee S.A., Moon S.M., Kim S.G., Chun H.S., et al. MicroRNA-203 Induces Apoptosis by Targeting Bmi-1 in YD-38 Oral Cancer Cells. Anticancer Res. 2018;38:3477–3485. doi: 10.21873/anticanres.12618. [DOI] [PubMed] [Google Scholar]
- 453.Zhang X.L., Sun B.L., Tian S.X., Li L., Zhao Y.C., Shi P.P. MicroRNA-132 reverses cisplatin resistance and metastasis in ovarian cancer by the targeted regulation on Bmi-1. Eur. Rev. Med. Pharmacol. Sci. 2019;23:3635–3644. doi: 10.26355/eurrev_201905_17787. [DOI] [PubMed] [Google Scholar]
- 454.Xu L., Li Y., Yan D., He J., Liu D. MicroRNA-183 inhibits gastric cancer proliferation and invasion via directly targeting Bmi-1. Oncol. Lett. 2014;8:2345–2351. doi: 10.3892/ol.2014.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Qiu M., Liang Z., Chen L., Tan G., Liu L., Wang K., Chen H., Liu J. MicroRNA-200c suppresses cell growth and metastasis by targeting Bmi-1 and E2F3 in renal cancer cells. Exp. Ther. Med. 2017;13:1329–1336. doi: 10.3892/etm.2017.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Cheng Y., Yang X., Deng X., Zhang X., Li P., Tao J., Lu Q. MicroRNA-218 inhibits bladder cancer cell proliferation, migration, and invasion by targeting BMI-1. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2015;36:8015–8023. doi: 10.1007/s13277-015-3532-x. [DOI] [PubMed] [Google Scholar]
- 457.Chen F., Chen L., He H., Huang W., Zhang R., Li P., Meng Y., Jiang X. Up-regulation of microRNA-16 in Glioblastoma Inhibits the Function of Endothelial Cells and Tumor Angiogenesis by Targeting Bmi-1. Anti-Cancer Agents Med. Chem. 2016;16:609–620. doi: 10.2174/1871520615666150916092251. [DOI] [PubMed] [Google Scholar]
- 458.Wang T., Du M., Zhang W., Bai H., Yin L., Chen W., He X., Chen Q. MicroRNA-432 Suppresses Invasion and Migration via E2F3 in Nasopharyngeal Carcinoma. OncoTargets Ther. 2019;12:11271–11280. doi: 10.2147/OTT.S233435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Yang J., Zhang H.F., Qin C.F. MicroRNA-217 functions as a prognosis predictor and inhibits pancreatic cancer cell proliferation and invasion via targeting E2F3. Eur. Rev. Med. Pharmacol. Sci. 2017;21:4050–4057. [PubMed] [Google Scholar]
- 460.Li X., Li H., Zhang R., Liu J., Liu J. MicroRNA-449a inhibits proliferation and induces apoptosis by directly repressing E2F3 in gastric cancer. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2015;35:2033–2042. doi: 10.1159/000374010. [DOI] [PubMed] [Google Scholar]
- 461.Wang Y., Sun G., Wang C., Guo W., Tang Q., Wang M. MiR-194-5p inhibits cell migration and invasion in bladder cancer by targeting E2F3. J. BUON. 2018;23:1492–1499. [PubMed] [Google Scholar]
- 462.Zhou Q., Zhu Y., Wei X., Zhou J., Chang L., Sui H., Han Y., Piao D., Sha R., Bai Y. MiR-590-5p inhibits colorectal cancer angiogenesis and metastasis by regulating nuclear factor 90/vascular endothelial growth factor A axis. Cell Death Dis. 2016;7:e2413. doi: 10.1038/cddis.2016.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Melincovici C.S., Boşca A.B., Şuşman S., Mărginean M., Mihu C., Istrate M., Moldovan I.M., Roman A.L., Mihu C.M. Vascular endothelial growth factor (VEGF)—Key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol. Rev. Roum. Morphol. Embryol. 2018;59:455–467. [PubMed] [Google Scholar]
- 464.Pengcheng S., Ziqi W., Luyao Y., Xiangwei Z., Liang L., Yuwei L., Lechen L., Wanhai X. MicroRNA-497 suppresses renal cell carcinoma by targeting VEGFR-2 in ACHN cells. Biosci. Rep. 2017;37:BSR20170270. doi: 10.1042/BSR20170270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Kamijo H., Miyagaki T., Takahashi-Shishido N., Nakajima R., Oka T., Suga H., Sugaya M., Sato S. Thrombospondin-1 promotes tumor progression in cutaneous T-cell lymphoma via CD47. Leukemia. 2020;34:845–856. doi: 10.1038/s41375-019-0622-6. [DOI] [PubMed] [Google Scholar]
- 466.Su A., He S., Tian B., Hu W., Zhang Z. MicroRNA-221 Mediates the Effects of PDGF-BB on Migration, Proliferation, and the Epithelial-Mesenchymal Transition in Pancreatic Cancer Cells. PLoS ONE. 2013;8:e71309. doi: 10.1371/journal.pone.0071309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Kaelin W.G., Jr. The VHL Tumor Suppressor Gene: Insights into Oxygen Sensing and Cancer. Trans. Am. Clin. Climatol. Assoc. 2017;128:298–307. [PMC free article] [PubMed] [Google Scholar]
- 468.Puchert M., Obst J., Koch C., Zieger K., Engele J. CXCL11 promotes tumor progression by the biased use of the chemokine receptors CXCR3 and CXCR7. Cytokine. 2020;125:154809. doi: 10.1016/j.cyto.2019.154809. [DOI] [PubMed] [Google Scholar]
- 469.Tian Y., Matsui S., Touma M., Wu Q., Sugimoto K. MicroRNA-342 inhibits tumor growth via targeting chemokine CXCL12 involved in macrophages recruitment/activation. Genes Cells. 2018;23:1009–1022. doi: 10.1111/gtc.12650. [DOI] [PubMed] [Google Scholar]
- 470.Zhu Y., Tang L., Zhao S., Sun B., Cheng L., Tang Y., Luo Z., Lin Z., Zhu J., Zhu W., et al. CXCR4-mediated osteosarcoma growth and pulmonary metastasis is suppressed by MicroRNA-613. Cancer Sci. 2018;109:2412–2422. doi: 10.1111/cas.13653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Xu M., Li D., Yang C., Ji J.S. MicroRNA-34a Inhibition of the TLR Signaling Pathway Via CXCL10 Suppresses Breast Cancer Cell Invasion and Migration. Cell. Physiol. Biochem. 2018;46:1286–1304. doi: 10.1159/000489111. [DOI] [PubMed] [Google Scholar]
- 472.Kim S.J., Shin J.Y., Lee K.D., Bae Y.K., Sung K.W., Nam S.J., Chun K.H. MicroRNA let-7a suppresses breast cancer cell migration and invasion through downregulation of C-C chemokine receptor type 7. Breast Cancer Res. 2012;14:R14. doi: 10.1186/bcr3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Rizeq B., Malki M.I. The Role of CCL21/CCR7 Chemokine Axis in Breast Cancer Progression. Cancers. 2020;12:1036. doi: 10.3390/cancers12041036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Tang G., Du R., Tang Z., Kuang Y. MiRNALet-7a mediates prostate cancer PC-3 cell invasion, migration by inducing epithelial-mesenchymal transition through CCR7/MAPK pathway. J. Cell. Biochem. 2018;119:3725–3731. doi: 10.1002/jcb.26595. [DOI] [PubMed] [Google Scholar]
- 475.Han B., Feng D., Yu X., Liu Y., Yang M., Luo F., Zhou L., Liu F. MicroRNA-144 mediates chronic inflammation and tumorigenesis in colorectal cancer progression via regulating C-X-C motif chemokine ligand 11. Exp. Ther. Med. 2018;16:1935–1943. doi: 10.3892/etm.2018.6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Ji X., Liu Y., Kao X., Chen X., Zhao Y., Zhang S., Chen L., Yu M., Wei J., Yang Z., et al. miR-144 suppresses cell proliferation and migration in colorectal cancer by targeting NRAS. J. Cell. Biochem. 2020;121:3871–3881. doi: 10.1002/jcb.29543. [DOI] [PubMed] [Google Scholar]
- 477.Kedmi M., Sas-Chen A., Yarden Y. MicroRNAs and Growth Factors: An Alliance Propelling Tumor Progression. J. Clin. Med. 2015;4:1578–1599. doi: 10.3390/jcm4081578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Masliah-Planchon J., Garinet S., Pasmant E. RAS-MAPK pathway epigenetic activation in cancer: miRNAs in action. Oncotarget. 2016;7:38892–38907. doi: 10.18632/oncotarget.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Roncarati R., Lupini L., Shankaraiah R.C., Negrini M. The Importance of microRNAs in RAS Oncogenic Activation in Human Cancer. Front. Oncol. 2019;9:988. doi: 10.3389/fonc.2019.00988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Rahmani F., Ferns G.A., Talebian S., Nourbakhsh M., Avan A., Shahidsales S. Role of regulatory miRNAs of the PI3K/AKT signaling pathway in the pathogenesis of breast cancer. Gene. 2020;737:144459. doi: 10.1016/j.gene.2020.144459. [DOI] [PubMed] [Google Scholar]
- 481.Liu J., Zhang C., Zhao Y., Feng Z. MicroRNA Control of p53. J. Cell. Biochem. 2017;118:7–14. doi: 10.1002/jcb.25609. [DOI] [PubMed] [Google Scholar]
- 482.Zhang L., Li J., Wang Q., Meng G., Lv X., Zhou H., Li W., Zhang J. The relationship between microRNAs and the STAT3-related signaling pathway in cancer. Tumor Biol. 2017;39:1010428317719869. doi: 10.1177/1010428317719869. [DOI] [PubMed] [Google Scholar]
- 483.Ren W., Wu S., Wu Y., Liu T., Zhao X., Li Y. MicroRNA-196a/-196b regulate the progression of hepatocellular carcinoma through modulating the JAK/STAT pathway via targeting SOCS2. Cell Death Dis. 2019;10:333. doi: 10.1038/s41419-019-1530-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Das R., Gregory P.A., Fernandes R.C., Denis I., Wang Q., Townley S.L., Zhao S.G., Hanson A.R., Pickering M.A., Armstrong H.K., et al. MicroRNA-194 Promotes Prostate Cancer Metastasis by Inhibiting SOCS2. Cancer Res. 2017;77:1021–1034. doi: 10.1158/0008-5472.CAN-16-2529. [DOI] [PubMed] [Google Scholar]
- 485.Follain G., Herrmann D., Harlepp S., Hyenne V., Osmani N., Warren S.C., Timpson P., Goetz J.G. Fluids and their mechanics in tumour transit: Shaping metastasis. Nat. Rev. Cancer. 2020;20:107–124. doi: 10.1038/s41568-019-0221-x. [DOI] [PubMed] [Google Scholar]
- 486.Amit M., Na’ara S., Gil Z. Mechanisms of cancer dissemination along nerves. Nat. Rev. Cancer. 2016;16:399–408. doi: 10.1038/nrc.2016.38. [DOI] [PubMed] [Google Scholar]
- 487.Karaman S., Detmar M. Mechanisms of lymphatic metastasis. J. Clin. Investig. 2014;124:922–928. doi: 10.1172/JCI71606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Zhou W., Fong M.Y., Min Y., Somlo G., Liu L., Palomares M.R., Yu Y., Chow A., O’Connor S.T.F., Chin A.R., et al. Cancer-Secreted miR-105 Destroys Vascular Endothelial Barriers to Promote Metastasis. Cancer Cell. 2014;25:501–515. doi: 10.1016/j.ccr.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Ma J., Yao Y., Wang P., Liu Y., Zhao L., Li Z., Li Z., Xue Y. MiR-181a regulates blood-tumor barrier permeability by targeting Krüppel-like factor 6. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2014;34:1826–1836. doi: 10.1038/jcbfm.2014.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Mohme M., Riethdorf S., Pantel K. Circulating and disseminated tumour cells—Mechanisms of immune surveillance and escape. Nat. Rev. Clin. Oncol. 2017;14:155–167. doi: 10.1038/nrclinonc.2016.144. [DOI] [PubMed] [Google Scholar]
- 491.Liu X., Chen Q., Yan J., Wang Y., Zhu C., Chen C., Zhao X., Xu M., Sun Q., Deng R., et al. MiRNA-296-3p-ICAM-1 axis promotes metastasis of prostate cancer by possible enhancing survival of natural killer cell-resistant circulating tumour cells. Cell Death Dis. 2013;4:e928. doi: 10.1038/cddis.2013.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Schlesinger M. Role of platelets and platelet receptors in cancer metastasis. J. Hematol. Oncol. 2018;11:125. doi: 10.1186/s13045-018-0669-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Ueda R., Kohanbash G., Sasaki K., Fujita M., Zhu X., Kastenhuber E.R., McDonald H.A., Potter D.M., Hamilton R.L., Lotze M.T., et al. Dicer-regulated microRNAs 222 and 339 promote resistance of cancer cells to cytotoxic T-lymphocytes by down-regulation of ICAM-1. Proc. Natl. Acad. Sci. USA. 2009;106:10746–10751. doi: 10.1073/pnas.0811817106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Paoli P., Giannoni E., Chiarugi P. Anoikis molecular pathways and its role in cancer progression. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2013;1833:3481–3498. doi: 10.1016/j.bbamcr.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 495.Mak C.S.L., Yung M.M.H., Hui L.M.N., Leung L.L., Liang R., Chen K., Liu S.S., Qin Y., Leung T.H.Y., Lee K.-F., et al. MicroRNA-141 enhances anoikis resistance in metastatic progression of ovarian cancer through targeting KLF12/Sp1/survivin axis. Mol. Cancer. 2017;16:11. doi: 10.1186/s12943-017-0582-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Tominaga N., Kosaka N., Ono M., Katsuda T., Yoshioka Y., Tamura K., Lötvall J., Nakagama H., Ochiya T. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nat. Commun. 2015;6:6716. doi: 10.1038/ncomms7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Zeng Z., Li Y., Pan Y., Lan X., Song F., Sun J., Zhou K., Liu X., Ren X., Wang F., et al. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat. Commun. 2018;9:5395. doi: 10.1038/s41467-018-07810-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Penna E., Orso F., Cimino D., Vercellino I., Grassi E., Quaglino E., Turco E., Taverna D. miR-214 Coordinates Melanoma Progression by Upregulating ALCAM through TFAP2 and miR-148b Downmodulation. Cancer Res. 2013;73:4098–4111. doi: 10.1158/0008-5472.CAN-12-3686. [DOI] [PubMed] [Google Scholar]
- 499.Zhong L., Huot J., Simard M.J. p38 activation induces production of miR-146a and miR-31 to repress E-selectin expression and inhibit transendothelial migration of colon cancer cells. Sci. Rep. 2018;8:2334. doi: 10.1038/s41598-018-20837-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Thomsen K.G., Terp M.G., Lund R.R., Søkilde R., Elias D., Bak M., Litman T., Beck H.C., Lyng M.B., Ditzel H.J. miR-155, identified as anti-metastatic by global miRNA profiling of a metastasis model, inhibits cancer cell extravasation and colonization in vivo and causes significant signaling alterations. Oncotarget. 2015;6:29224–29239. doi: 10.18632/oncotarget.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Jones D., Pereira E.R., Padera T.P. Growth and Immune Evasion of Lymph Node Metastasis. Front. Oncol. 2018;8:36. doi: 10.3389/fonc.2018.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Brown M., Assen F.P., Leithner A., Abe J., Schachner H., Asfour G., Bago-Horvath Z., Stein J.V., Uhrin P., Sixt M., et al. Lymph node blood vessels provide exit routes for metastatic tumor cell dissemination in mice. Science. 2018;359:1408–1411. doi: 10.1126/science.aal3662. [DOI] [PubMed] [Google Scholar]
- 503.Peinado H., Alečković M., Lavotshkin S., Matei I., Costa-Silva B., Moreno-Bueno G., Hergueta-Redondo M., Williams C., García-Santos G., Ghajar C., et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Chin A.R., Wang S.E. Cancer Tills the Premetastatic Field: Mechanistic Basis and Clinical Implications. Clin. Cancer Res. 2016;22:3725–3733. doi: 10.1158/1078-0432.CCR-16-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Langley R.R., Fidler I.J. The seed and soil hypothesis revisited--the role of tumor-stroma interactions in metastasis to different organs. Int. J. Cancer. 2011;128:2527–2535. doi: 10.1002/ijc.26031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.de Groot A.E., Roy S., Brown J.S., Pienta K.J., Amend S.R. Revisiting Seed and Soil: Examining the Primary Tumor and Cancer Cell Foraging in Metastasis. Mol. Cancer Res. 2017;15:361–370. doi: 10.1158/1541-7786.MCR-16-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Grange C., Tapparo M., Collino F., Vitillo L., Damasco C., Deregibus M.C., Tetta C., Bussolati B., Camussi G. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011;71:5346–5356. doi: 10.1158/0008-5472.CAN-11-0241. [DOI] [PubMed] [Google Scholar]
- 508.Sánchez C.A., Andahur E.I., Valenzuela R., Castellón E.A., Fullá J.A., Ramos C.G., Triviño J.C. Exosomes from bulk and stem cells from human prostate cancer have a differential microRNA content that contributes cooperatively over local and pre-metastatic niche. Oncotarget. 2016;7:3993–4008. doi: 10.18632/oncotarget.6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Fong M.Y., Zhou W., Liu L., Alontaga A.Y., Chandra M., Ashby J., Chow A., O’Connor S.T.F., Li S., Chin A.R., et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat. Cell Biol. 2015;17:183–194. doi: 10.1038/ncb3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Shu S.L., Yang Y., Allen C.L., Maguire O., Minderman H., Sen A., Ciesielski M.J., Collins K.A., Bush P.J., Singh P., et al. Metabolic reprogramming of stromal fibroblasts by melanoma exosome microRNA favours a pre-metastatic microenvironment. Sci. Rep. 2018;8:12905. doi: 10.1038/s41598-018-31323-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Ostenfeld M.S., Jeppesen D.K., Laurberg J.R., Boysen A.T., Bramsen J.B., Primdal-Bengtson B., Hendrix A., Lamy P., Dagnaes-Hansen F., Rasmussen M.H., et al. Cellular disposal of miR23b by RAB27-dependent exosome release is linked to acquisition of metastatic properties. Cancer Res. 2014;74:5758–5771. doi: 10.1158/0008-5472.CAN-13-3512. [DOI] [PubMed] [Google Scholar]
- 512.Takano Y., Masuda T., Iinuma H., Yamaguchi R., Sato K., Tobo T., Hirata H., Kuroda Y., Nambara S., Hayashi N., et al. Circulating exosomal microRNA-203 is associated with metastasis possibly via inducing tumor-associated macrophages in colorectal cancer. Oncotarget. 2017;8:78598–78613. doi: 10.18632/oncotarget.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Attieh Y., Clark A.G., Grass C., Richon S., Pocard M., Mariani P., Elkhatib N., Betz T., Gurchenkov B., Vignjevic D.M. Cancer-associated fibroblasts lead tumor invasion through integrin-β3-dependent fibronectin assembly. J. Cell Biol. 2017;216:3509–3520. doi: 10.1083/jcb.201702033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Miyazaki K., Oyanagi J., Hoshino D., Togo S., Kumagai H., Miyagi Y. Cancer cell migration on elongate protrusions of fibroblasts in collagen matrix. Sci. Rep. 2019;9:292. doi: 10.1038/s41598-018-36646-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Mitra A.K., Zillhardt M., Hua Y., Tiwari P., Murmann A.E., Peter M.E., Lengyel E. MicroRNAs reprogram normal fibroblasts into cancer-associated fibroblasts in ovarian cancer. Cancer Discov. 2012;2:1100–1108. doi: 10.1158/2159-8290.CD-12-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Yang F., Ning Z., Ma L., Liu W., Shao C., Shu Y., Shen H. Exosomal miRNAs and miRNA dysregulation in cancer-associated fibroblasts. Mol. Cancer. 2017;16:148. doi: 10.1186/s12943-017-0718-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Tang X., Hou Y., Yang G., Wang X., Tang S., Du Y.E., Yang L., Yu T., Zhang H., Zhou M., et al. Stromal miR-200s contribute to breast cancer cell invasion through CAF activation and ECM remodeling. Cell Death Differ. 2016;23:132–145. doi: 10.1038/cdd.2015.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Chatterjee A., Jana S., Chatterjee S., Wastall L.M., Mandal G., Nargis N., Roy H., Hughes T.A., Bhattacharyya A. MicroRNA-222 reprogrammed cancer-associated fibroblasts enhance growth and metastasis of breast cancer. Br. J. Cancer. 2019;121:679–689. doi: 10.1038/s41416-019-0566-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Kaverina N., Borovjagin A.V., Kadagidze Z., Baryshnikov A., Baryshnikova M., Malin D., Ghosh D., Shah N., Welch D.R., Gabikian P., et al. Astrocytes promote progression of breast cancer metastases to the brain via a KISS1-mediated autophagy. Autophagy. 2017;13:1905–1923. doi: 10.1080/15548627.2017.1360466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Zhang L., Zhang S., Yao J., Lowery F.J., Zhang Q., Huang W.C., Li P., Li M., Wang X., Zhang C., et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527:100–104. doi: 10.1038/nature15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Ell B., Mercatali L., Ibrahim T., Campbell N., Schwarzenbach H., Pantel K., Amadori D., Kang Y. Tumor-induced osteoclast miRNA changes as regulators and biomarkers of osteolytic bone metastasis. Cancer Cell. 2013;24:542–556. doi: 10.1016/j.ccr.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Liu X., Cao M., Palomares M., Wu X., Li A., Yan W., Fong M.Y., Chan W.C., Wang S.E. Metastatic breast cancer cells overexpress and secrete miR-218 to regulate type I collagen deposition by osteoblasts. Breast Cancer Res. 2018;20:127. doi: 10.1186/s13058-018-1059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Omar H.A., El-Serafi A.T., Hersi F., Arafa E.-S.A., Zaher D.M., Madkour M., Arab H.H., Tolba M.F. Immunomodulatory MicroRNAs in cancer: Targeting immune checkpoints and the tumor microenvironment. FEBS J. 2019;286:3540–3557. doi: 10.1111/febs.15000. [DOI] [PubMed] [Google Scholar]
- 524.Xu S., Tao Z., Hai B., Liang H., Shi Y., Wang T., Song W., Chen Y., OuYang J., Chen J., et al. miR-424(322) reverses chemoresistance via T-cell immune response activation by blocking the PD-L1 immune checkpoint. Nat. Commun. 2016;7:11406. doi: 10.1038/ncomms11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Lin R., Chen L., Chen G., Hu C., Jiang S., Sevilla J., Wan Y., Sampson J.H., Zhu B., Li Q.J. Targeting miR-23a in CD8+ cytotoxic T lymphocytes prevents tumor-dependent immunosuppression. J. Clin. Investig. 2014;124:5352–5367. doi: 10.1172/JCI76561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Xu H., Cheung I.Y., Guo H.F., Cheung N.K. MicroRNA miR-29 modulates expression of immunoinhibitory molecule B7-H3: Potential implications for immune based therapy of human solid tumors. Cancer Res. 2009;69:6275–6281. doi: 10.1158/0008-5472.CAN-08-4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Tsukerman P., Stern-Ginossar N., Gur C., Glasner A., Nachmani D., Bauman Y., Yamin R., Vitenshtein A., Stanietsky N., Bar-Mag T., et al. MiR-10b downregulates the stress-induced cell surface molecule MICB, a critical ligand for cancer cell recognition by natural killer cells. Cancer Res. 2012;72:5463–5472. doi: 10.1158/0008-5472.CAN-11-2671. [DOI] [PubMed] [Google Scholar]
- 528.Daveri E., Vergani E., Shahaj E., Bergamaschi L., La Magra S., Dosi M., Castelli C., Rodolfo M., Rivoltini L., Vallacchi V., et al. microRNAs Shape Myeloid Cell-Mediated Resistance to Cancer Immunotherapy. Front. Immunol. 2020;11:1214. doi: 10.3389/fimmu.2020.01214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.O’Brien K., Breyne K., Ughetto S., Laurent L.C., Breakefield X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020;21:585–606. doi: 10.1038/s41580-020-0251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Cooks T., Pateras I.S., Jenkins L.M., Patel K.M., Robles A.I., Morris J., Forshew T., Appella E., Gorgoulis V.G., Harris C.C. Mutant p53 cancers reprogram macrophages to tumor supporting macrophages via exosomal miR-1246. Nat. Commun. 2018;9:771. doi: 10.1038/s41467-018-03224-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Hsieh C.H., Tai S.K., Yang M.H. Snail-overexpressing Cancer Cells Promote M2-Like Polarization of Tumor-Associated Macrophages by Delivering MiR-21-Abundant Exosomes. Neoplasia. 2018;20:775–788. doi: 10.1016/j.neo.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Ying X., Wu Q., Wu X., Zhu Q., Wang X., Jiang L., Chen X., Wang X. Epithelial ovarian cancer-secreted exosomal miR-222-3p induces polarization of tumor-associated macrophages. Oncotarget. 2016;7:43076–43087. doi: 10.18632/oncotarget.9246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Yang M., Chen J., Su F., Yu B., Su F., Lin L., Liu Y., Huang J.D., Song E. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol. Cancer. 2011;10:117. doi: 10.1186/1476-4598-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Zonari E., Pucci F., Saini M., Mazzieri R., Politi L.S., Gentner B., Naldini L. A role for miR-155 in enabling tumor-infiltrating innate immune cells to mount effective antitumor responses in mice. Blood. 2013;122:243–252. doi: 10.1182/blood-2012-08-449306. [DOI] [PubMed] [Google Scholar]
- 535.Liu Y., Lai L., Chen Q., Song Y., Xu S., Ma F., Wang X., Wang J., Yu H., Cao X., et al. MicroRNA-494 is required for the accumulation and functions of tumor-expanded myeloid-derived suppressor cells via targeting of PTEN. J. Immunol. 2012;188:5500–5510. doi: 10.4049/jimmunol.1103505. [DOI] [PubMed] [Google Scholar]
- 536.Grzywa T.M., Sosnowska A., Matryba P., Rydzynska Z., Jasinski M., Nowis D., Golab J. Myeloid Cell-Derived Arginase in Cancer Immune Response. Front. Immunol. 2020;11:938. doi: 10.3389/fimmu.2020.00938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Yang P., Li Q.J., Feng Y., Zhang Y., Markowitz G.J., Ning S., Deng Y., Zhao J., Jiang S., Yuan Y., et al. TGF-β-miR-34a-CCL22 signaling-induced Treg cell recruitment promotes venous metastases of HBV-positive hepatocellular carcinoma. Cancer Cell. 2012;22:291–303. doi: 10.1016/j.ccr.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Li L., Zhang J., Diao W., Wang D., Wei Y., Zhang C.-Y., Zen K. MicroRNA-155 and MicroRNA-21 Promote the Expansion of Functional Myeloid-Derived Suppressor Cells. J. Immunol. 2014;192:1034–1043. doi: 10.4049/jimmunol.1301309. [DOI] [PubMed] [Google Scholar]
- 539.Lan H., Lu H., Wang X., Jin H. MicroRNAs as Potential Biomarkers in Cancer: Opportunities and Challenges. BioMed Res. Int. 2015;2015:125094. doi: 10.1155/2015/125094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540.Cortez M.A., Bueso-Ramos C., Ferdin J., Lopez-Berestein G., Sood A.K., Calin G.A. MicroRNAs in body fluids—the mix of hormones and biomarkers. Nat. Rev. Clin. Oncol. 2011;8:467–477. doi: 10.1038/nrclinonc.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Iorio M.V., Croce C.M. MicroRNA dysregulation in cancer: Diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Mol. Med. 2012;4:143–159. doi: 10.1002/emmm.201100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Filipów S., Łaczmański Ł. Blood Circulating miRNAs as Cancer Biomarkers for Diagnosis and Surgical Treatment Response. Front. Genet. 2019;10:169. doi: 10.3389/fgene.2019.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Cui M., Wang H., Yao X., Zhang D., Xie Y., Cui R., Zhang X. Circulating MicroRNAs in Cancer: Potential and Challenge. Front. Gen. 2019;10:626. doi: 10.3389/fgene.2019.00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Bouyssou J.M., Manier S., Huynh D., Issa S., Roccaro A.M., Ghobrial I.M. Regulation of microRNAs in cancer metastasis. Biochim. Biophys. Acta. 2014;1845:255–265. doi: 10.1016/j.bbcan.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Jinling W., Sijing S., Jie Z., Guinian W. Prognostic value of circulating microRNA-21 for breast cancer: A systematic review and meta-analysis. Artif. Cells Nanomed. Biotechnol. 2017;45:1–6. doi: 10.1080/21691401.2016.1216856. [DOI] [PubMed] [Google Scholar]
- 546.Hu G.Y., Tao F., Wang W., Ji K.W. Prognostic value of microRNA-21 in pancreatic ductal adenocarcinoma: A meta-analysis. World J. Surg. Oncol. 2016;14:82. doi: 10.1186/s12957-016-0842-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Hur K., Toiyama Y., Schetter A.J., Okugawa Y., Harris C.C., Boland C.R., Goel A. Identification of a metastasis-specific MicroRNA signature in human colorectal cancer. J. Natl. Cancer Inst. 2015;107:dju492. doi: 10.1093/jnci/dju492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Grzywa T.M., Klicka K., Paskal W., Dudkiewicz J., Wejman J., Pyzlak M., Włodarski P.K. miR-410-3p is induced by vemurafenib via ER stress and contributes to resistance to BRAF inhibitor in melanoma. PLoS ONE. 2020;15:e0234707. doi: 10.1371/journal.pone.0234707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Hur K., Toiyama Y., Okugawa Y., Ide S., Imaoka H., Boland C.R., Goel A. Circulating microRNA-203 predicts prognosis and metastasis in human colorectal cancer. Gut. 2017;66:654–665. doi: 10.1136/gutjnl-2014-308737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Peng Y., Huang D., Ma K., Deng X., Shao Z. MiR-19a as a prognostic indicator for cancer patients: A meta-analysis. Biosci. Rep. 2019;39:BSR20182370. doi: 10.1042/BSR20182370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Zhao S., Yao D., Chen J., Ding N. Circulating miRNA-20a and miRNA-203 for screening lymph node metastasis in early stage cervical cancer. Genet. Test. Mol. Biomark. 2013;17:631–636. doi: 10.1089/gtmb.2013.0085. [DOI] [PubMed] [Google Scholar]
- 552.Qu A., Yang Y., Zhang X., Wang W., Liu Y., Zheng G., Du L., Wang C. Development of a preoperative prediction nomogram for lymph node metastasis in colorectal cancer based on a novel serum miRNA signature and CT scans. EBioMedicine. 2018;37:125–133. doi: 10.1016/j.ebiom.2018.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Wang L.G., Gu J. Serum microRNA-29a is a promising novel marker for early detection of colorectal liver metastasis. Cancer Epidemiol. 2012;36:e61–e67. doi: 10.1016/j.canep.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 554.Ling H., Fabbri M., Calin G.A. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat. Rev. Drug Discov. 2013;12:847–865. doi: 10.1038/nrd4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Forterre A., Komuro H., Aminova S., Harada M. A Comprehensive Review of Cancer MicroRNA Therapeutic Delivery Strategies. Cancers. 2020;12:1852. doi: 10.3390/cancers12071852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Setten R.L., Rossi J.J., Han S.P. The current state and future directions of RNAi-based therapeutics. Nat. Rev. Drug Discov. 2019;18:421–446. doi: 10.1038/s41573-019-0017-4. [DOI] [PubMed] [Google Scholar]
- 557.Beg M.S., Brenner A.J., Sachdev J., Borad M., Kang Y.-K., Stoudemire J., Smith S., Bader A.G., Kim S., Hong D.S. Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Invest. New Drugs. 2017;35:180–188. doi: 10.1007/s10637-016-0407-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Hong D.S., Kang Y.K., Borad M., Sachdev J., Ejadi S., Lim H.Y., Brenner A.J., Park K., Lee J.L., Kim T.Y., et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br. J. Cancer. 2020;122:1630–1637. doi: 10.1038/s41416-020-0802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.van Zandwijk N., Pavlakis N., Kao S.C., Linton A., Boyer M.J., Clarke S., Huynh Y., Chrzanowska A., Fulham M.J., Bailey D.L., et al. Safety and activity of microRNA-loaded minicells in patients with recurrent malignant pleural mesothelioma: A first-in-man, phase 1, open-label, dose-escalation study. Lancet Oncol. 2017;18:1386–1396. doi: 10.1016/S1470-2045(17)30621-6. [DOI] [PubMed] [Google Scholar]
- 560.Reid G., Pel M.E., Kirschner M.B., Cheng Y.Y., Mugridge N., Weiss J., Williams M., Wright C., Edelman J.J., Vallely M.P., et al. Restoring expression of miR-16: A novel approach to therapy for malignant pleural mesothelioma. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2013;24:3128–3135. doi: 10.1093/annonc/mdt412. [DOI] [PubMed] [Google Scholar]
- 561.Seto A.G., Beatty X., Lynch J.M., Hermreck M., Tetzlaff M., Duvic M., Jackson A.L. Cobomarsen, an oligonucleotide inhibitor of miR-155, co-ordinately regulates multiple survival pathways to reduce cellular proliferation and survival in cutaneous T-cell lymphoma. Br. J. Haematol. 2018;183:428–444. doi: 10.1111/bjh.15547. [DOI] [PubMed] [Google Scholar]
- 562.Singh S., Narang A.S., Mahato R.I. Subcellular Fate and Off-Target Effects of siRNA, shRNA, and miRNA. Pharm. Res. 2011;28:2996–3015. doi: 10.1007/s11095-011-0608-1. [DOI] [PubMed] [Google Scholar]
- 563.Bartoszewski R., Sikorski A.F. Editorial focus: Understanding off-target effects as the key to successful RNAi therapy. Cell Mol. Biol. Lett. 2019;24:69. doi: 10.1186/s11658-019-0196-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Khan A.A., Betel D., Miller M.L., Sander C., Leslie C.S., Marks D.S. Transfection of small RNAs globally perturbs gene regulation by endogenous microRNAs. Nat. Biotechnol. 2009;27:549–555. doi: 10.1038/nbt.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Pal A.S., Kasinski A.L. Animal Models to Study MicroRNA Function. Adv. Cancer Res. 2017;135:53–118. doi: 10.1016/bs.acr.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Segal M., Biscans A., Gilles M.E., Anastasiadou E., De Luca R., Lim J., Khvorova A., Slack F.J. Hydrophobically Modified let-7b miRNA Enhances Biodistribution to NSCLC and Downregulates HMGA2 In Vivo. Mol. Ther. Nucleic Acids. 2020;19:267–277. doi: 10.1016/j.omtn.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567.Lennox K.A., Behlke M.A. Chemical modification and design of anti-miRNA oligonucleotides. Gene Ther. 2011;18:1111–1120. doi: 10.1038/gt.2011.100. [DOI] [PubMed] [Google Scholar]
- 568.Fu Y., Chen J., Huang Z. Recent progress in microRNA-based delivery systems for the treatment of human disease. ExRNA. 2019;1:24. doi: 10.1186/s41544-019-0024-y. [DOI] [Google Scholar]
- 569.Segal M., Slack F.J. Challenges identifying efficacious miRNA therapeutics for cancer. Expert Opin. Drug Discov. 2020;15:987–991. doi: 10.1080/17460441.2020.1765770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Varshney A., Panda J.J., Singh A.K., Yadav N., Bihari C., Biswas S., Sarin S.K., Chauhan V.S. Targeted delivery of microRNA-199a-3p using self-assembled dipeptide nanoparticles efficiently reduces hepatocellular carcinoma in mice. Hepatology. 2018;67:1392–1407. doi: 10.1002/hep.29643. [DOI] [PubMed] [Google Scholar]
- 571.Zhang Y., Wang Z., Gemeinhart R.A. Progress in microRNA delivery. J. Control. Release. 2013;172:962–974. doi: 10.1016/j.jconrel.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Chen Y., Zhu X., Zhang X., Liu B., Huang L. Nanoparticles modified with tumor-targeting scFv deliver siRNA and miRNA for cancer therapy. Mol. Ther. J. Am. Soc. Gene Ther. 2010;18:1650–1656. doi: 10.1038/mt.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Gilles M.E., Hao L., Huang L., Rupaimoole R., Lopez-Casas P.P., Pulver E., Jeong J.C., Muthuswamy S.K., Hidalgo M., Bhatia S.N., et al. Personalized RNA Medicine for Pancreatic Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018;24:1734–1747. doi: 10.1158/1078-0432.CCR-17-2733. [DOI] [PubMed] [Google Scholar]
- 574.Bayraktar R., Bertilaccio M.T.S., Calin G.A. The Interaction Between Two Worlds: MicroRNAs and Toll-Like Receptors. Front. Immunol. 2019;10:1053. doi: 10.3389/fimmu.2019.01053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Fabbri M., Paone A., Calore F., Galli R., Croce C.M. A new role for microRNAs, as ligands of Toll-like receptors. RNA Biol. 2013;10:169–174. doi: 10.4161/rna.23144. [DOI] [PMC free article] [PubMed] [Google Scholar]