Abstract
Background
Infants with heart failure remain at significant risk for wait list mortality, despite mechanical circulatory support (MCS). It is unclear if the outcomes are influenced by modality of support or underlying diagnosis. We sought to compare the outcomes of infants <10 kg, focusing on modality of support and underlying diagnosis.
Methods and Results
Using the Pediatric Heart Transplant Society database, we evaluated survival following first MCS device in children <10 kg who were listed for heart transplant between 2010 and 2018. There were 2049 children <10 kg, with the predominant diagnosis being congenital heart disease (CHD) (59.8% [n=1226]) and 28.1% (n=577) requiring MCS. Extracorporeal membrane oxygenation (ECMO) was the most common form of MCS at listing, with ventricular assist device (VAD) more common after listing. There was no difference in the use of ECMO at or after listing for cardiomyopathy versus CHD (8.9% versus 7.2%; P=0.2; 5.4% versus 6.4%; P=0.4). However, there was a significant difference in the use of VAD both at listing (8% versus 2.4%; P<0.001) and after (22.8% versus 5.1%; P<0.001) between the 2 groups. When comparing these groups, patients with CHD were smaller and younger and had a higher proportion with previous cardiac surgery. Survival at 3 months demonstrated better survival for VAD therapy compared with ECMO (74.3% versus 48.6%; P<0.001). In patients <5 kg, survival did not differ between ECMO and VAD (P=0.01) for the CHD or the cardiomyopathy group (P=0.38), but patients with cardiomyopathy demonstrated better survival on both forms of support.
Conclusions
Survival for patients <10 kg on ECMO is inferior compared with VAD. Patients with cardiomyopathy <5 kg had better survival with both modes of MCS compared with those with CHD. These findings support the need for small, durable devices for neonates and infants, with particular focus in patients with CHD.
Keywords: congenital heart disease, extracorporeal membrane oxygenation, mortality, pediatric, transplantation, ventricular assist device
Subject Categories: Transplantation, Congenital Heart Disease
Nonstandard Abbreviations and Acronyms
- HTx
heart transplant
- MCS
mechanical circulatory support
- PHTS
Pediatric Heart Transplant Society
Clinical Perspective
What Is New?
This article highlights the unique differences in outcomes for smaller children (<10 kg) requiring mechanical circulatory support with both the patients with cardiomyopathy/myocarditis and congenital heart disease who were supported on ventricular assist device having improved outcomes compared with those supported by extracorporeal membrane oxygenation.
Although a difference in outcome between ventricular assist device and extracorporeal membrane oxygenation was not demonstrated for children <5 kg, those <5 kg with dilated cardiomyopathy/myocarditis had improved outcomes compared with those with congenital heart disease.
What Are the Clinical Implications?
Smaller children requiring mechanical circulatory support are a heterogeneous patient population with variations in size, diagnosis, clinical condition, and timing of implantation.
Decisions about the need, timing, and type of mechanical circulatory support need to take all the above into consideration, and optimization of outcomes will require improving mechanical circulatory support options for these smaller children.
Mechanical circulatory support (MCS) is an important component in the management of end‐stage heart failure in children. 1 , 2 , 3 , 4 Although extracorporeal membrane oxygenation (ECMO) support has historically been the predominant form of support, advancements in the field of ventricular assist devices (VADs) have resulted in a transition away from ECMO as first‐line therapy.
This transition away from ECMO has likely been driven by the increase in availability and experience with VADs; however, the choice of device is dependent on the patient’s size, anatomical features, and clinical condition, with options being more limited in smaller patients as well as those with congenital heart disease (CHD). For children who require longer‐term support as a bridge to transplant, primary implantation of a durable device has become the preferred modality of support, with the possible exception of those with profound cardiogenic shock with multiorgan dysfunction. This shift in clinical practice is based on several studies examining the outcomes of children undergoing bridge to transplant with MCS. 1 , 2
However, it is unclear if survival with various forms of MCS is significantly different for infants requiring MCS as bridge to transplant, especially in the subset of patients who are small and those with CHD. Previous studies have shown that children <10 kg who are supported on a durable device achieved a successful outcome 57% of the time; however, CHD and liver dysfunction significantly increased the risk of death while awaiting heart transplant (HTx). 5 In addition, it has been suggested that within the group of patients who are <10 kg, the subgroup of those <5 kg is at higher risk for morbidity and mortality. 1 , 2 , 3 , 4 , 5
Currently, no studies have compared survival between ECMO and other forms of device therapy available for the smallest children (<10 kg). On the basis of data from a recent analysis of the United Network for Organ Sharing database, the frequency of VAD therapy as a bridge to transplant in infants <10 kg is similar to that of ECMO (10.3% versus 9.3%). 6 This observation differs from the trends seen in older children. 6 , 7 It is unclear if this practice variation is driven by outcomes. Therefore, further information is needed on the outcomes of device therapy in this unique and complex patient population. We sought to compare the outcomes of infants <10 kg with cardiomyopathy versus CHD requiring MCS pre‐HTX.
METHODS
Because of the nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to Pediatric Heart Transplant Society (PHTS) at phtsfexecutivedirector@gmail.com.
Patient Selection and Data Collection
The PHTS maintains a multicenter, prospective, event‐driven database that enrolls patients who are aged <18 years who have been listed for HTx. Data for this study were obtained from the PHTS database from January 1, 2010, to December 30, 2018, and included all patients from 55 participating institutions (Table S1).
Institutional Review Board approval was obtained at each institution. Patient consent to participate in the registry is left to the discretion of each institution as the registry serves as a quality improvement resource for centers. The Data Collection and Analysis Center is located at the University of Alabama at Birmingham. Information is collected on demographics and event data surrounding listing, transplantation, and death. Clinical information for any listing in PHTS is reported on the date of listing. The indications for listing and the decision for MCS and HTx were made at the discretion of the primary medical team on the basis of individual institutional clinical practice.
Study Cohort and Comparison Groups
The study included all children with weight <10 kg at time of listing who were diagnosed with CHD or cardiomyopathy/myocarditis (Figure 1). For patients with CHD, multiple secondary diagnosis details are collected. A small number of patients were excluded from the analysis because of having a different primary diagnosis (n=15).
Figure 1. Flowchart outlining the cohort of children <10 kg and divided by primary diagnosis, mechanical circulatory support (MCS) type, and time of initiation of support.
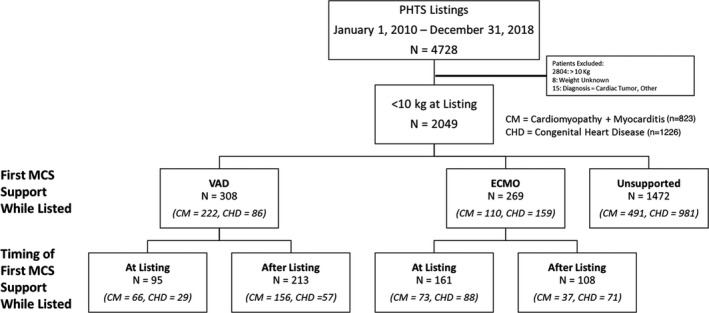
CHD indicates congenital heart disease; CM, cardiomyopathy; ECMO, extracorporeal membrane oxygenation; PHTS, Pediatric Heart Transplant Society; and VAD, ventricular assist device.
Patient characteristics were compared by diagnosis group and by the presence and timing of initial MCS support. Patients were supported by either ECMO or VAD at the time of listing or were unsupported at the time of listing. In addition, the patients who were unsupported at the time of listing could have received MCS after listing.
Both temporary and durable devices were included in the study. The term temporary device was used for devices that traditionally have been used for short‐term support, including: Abbott PediMag and CentriMag, Maquet Rotaflow, Sorin Revolution, and TandemHeart. This definition was solely based on device type and not duration of support. All other devices were considered durable devices.
Statistical Analysis
Differences in demographic and clinical characteristics between the groups were determined by independent t tests for continuous variables and reported as means±SD or median and interquartile range. For categorical variables, χ2 testing was performed and reported as frequency and percentage.
To accurately compare the risk of death on the wait list after MCS initiation (ECMO or VAD), patient time was segmented as before MCS initiation or without MCS initiation or after MCS initiation. For patients on support at listing, the start time of MCS was the day of listing and their entire follow‐up was categorized on the basis of support at listing. For patients unsupported at listing and initiating MCS after listing, follow‐up time was segmented to before MCS initiation (with censoring at initiation of MCS) and then transition to after MCS on the basis of the implant date until reaching a registry end point. This patient‐level outcome after VAD initiation was selected because most patients received no or only one device. This same approach was also used for patients with ECMO. Using this patient time segmentation approach, Kaplan‐Meier analysis was used to evaluate survival on the wait list and compare survival without MCS with survival after MCS initiation with either ECMO or VAD. Additional comparisons were made for patients on the basis of cause and size. Survival on the wait list after ECMO as bridge to VAD was compared with survival on the wait list after initiating MCS directly with VAD and with survival on the wait list after ECMO support before or without a bridge to VAD.
Multiphase parametric hazard modeling 8 was used to evaluate the risk of death on the wait list in the following patient groups: (1) patients undergoing ECMO, (2) patients receiving VAD, (3) patients with CHD, and (4) patients with cardiomyopathy/myocarditis. Patient time was segmented by support initiation as described above. Numerous factors were evaluated as covariates (Table S2). Final models were determined using forward stepwise selection with entry α of 0.1 and an exit α of 0.05. The final models were used to depict predicted mortality curves for different patient scenarios.
Competing outcome analysis was used to evaluate the time‐related probabilities of the mutually exclusive device‐related outcomes for MCS initiation with VAD (HTx from VAD, death on VAD, switch to ECMO, and explant) and for MCS initiation with ECMO (HTx from ECMO, death on ECMO [death on ECMO or within 1 week of decannulation], switch to VAD, and decanulation). In the competing outcome depictions, at any given point in time, the sum of the percentages for each mutually exclusive event equals 100%.
The statistical analysis was performed using SAS package 9.4 (SAS Institute, Cary, NC).
RESULTS
Demographics and Clinical Characteristics of All Infants
Between, 2010 and 2018, 4728 patients were listed for primary HTx in PHTS, of which the study group consisted of the 2049 patients having a weight of <10 kg at the time of listing. Within the study group, 40.2% (n=823) had a diagnosis of cardiomyopathy and 59.8% (n=1226) had CHD. In terms of MCS, 269 (13.1%) (n=110 cardiomyopathy and n=159 CHD) required ECMO support and 308 (15%) (n=222 cardiomyopathy and n=86 CHD) required VAD support at or after listing. There were 1472 patients who remained and did not require MCS support during their entire time on the wait list (Figure 1).
Table 1 highlights the demographic characteristics and differences between the CHD and cardiomyopathy cohorts. Notably, the patients with CHD were younger, smaller, and less likely to be on inotropes at listing. These patients also had a tendency toward a higher listing creatinine but similar levels of total bilirubin as patients with cardiomyopathy.
Table 1.
Demographics at Listing
| Demographics at Listing | Cardiomyopathy (n=823) | Congenital Heart Disease (n=1226) | P Value |
|---|---|---|---|
| Male sex | 368 (44.7) | 701 (57.2) | <0.001 |
| White race | 523 (63.5) | 844 (68.8) | 0.01 |
| Status 1A | 498 (75.1) | 968 (87.8) | <0.001 |
| Status 1B | 123 (18.6) | 82 (7.4) | <0.001 |
| PRA >10 | 96 (18.9) | 176 (24.4) | 0.02 |
| Ventilator at listing | 314 (40.1) | 509 (43.2) | 0.2 |
| Inotropes at listing | 611 (74.6) | 831 (68.0) | 0.001 |
| ECMO at listing | 73 (8.9) | 88 (7.2) | 0.2 |
| ECMO after listing | 37 (5.4) | 71 (6.4) | 0.4 |
| VAD at listing | 66 (8.0) | 29 (2.4) | <0.001 |
| VAD after listing | 156 (22.8) | 57 (5.1) | <0.001 |
| History of surgery at listing | 67 (8.2) | 930 (75.9) | <0.001 |
| History of renal insufficiency | 21 (2.7) | 64 (5.4) | 0.004 |
| Age at listing, mo | 7.0±6.5 | 5.2±6.4 | <0.001 |
| Weight at listing, kg | 6.0±2.1 | 5.1±2.0 | <0.001 |
| Patients with weight <5 kg | 305 (37.1) | 697 (56.9) | <0.001 |
| Serum albumin, g/dL | 3.5±1.6 | 3.3±1.6 | 0.004 |
| Bilirubin at listing, mg/dL | 1.1±2.0 | 1.8±3.0 | <0.001 |
| Creatinine at listing, mg/dL | 0.4±0.2 | 0.4±0.5 | 0.01 |
| Transplant year | 2014.1±2.5 | 2014.4±2.5 | 0.05 |
Data are given as number (percentage) or mean±SD. ECMO indicates extracorporeal membrane oxygenation; PRA, panel reactive antibody; and VAD, ventricular assist device.
Use of Mechanical Support of All Infants <10 kg
Figure 1 outlines the patients supported with MCS, including timing of first MCS support. For those listed on MCS, time in days on ECMO before listing was a median of 4 days (interquartile range, 2–7 days); and for VAD, 5 days (interquartile range, 1–13 days). For those not on device therapy at listing, but who eventually required MCS initiation, the time from listing to ECMO initiation was a median of 20.5 days (interquartile range, 9–47 days); and for VAD, a median of 13 days (interquartile range, 5–35 days). The demographics varied across device strategy at listing with a higher proportion of children <5 kg undergoing ECMO support at the time of listing. In addition, as outlined on Table 2, the weights, ages, history of surgery, bilirubin, and creatinine differed across the support strategies at listing. For all infants on VAD therapy, most underwent isolated left VAD implantation (n=238, 77.3%), 37 patients (17.7%) required biventricular assist device support, and 14 received an isolated right VAD (4.5%).
Table 2.
Characteristics at Listing for Support Group
| Characteristics at Listing | VAD (n=95) | ECMO (n=161) | Unsupported (n=1793) | P Value | VAD or ECMO at Listing (n=256) | VAD or ECMO After Listing (n=321) | P Value |
|---|---|---|---|---|---|---|---|
| Male sex | 49 (51.6) | 87 (54.0) | 933 (52.0) | 0.9 | 136 (53.1) | 164 (51.1) | 0.6 |
| Primary diagnosis | <0.0001 | <0.001 | |||||
| Cardiomyopathy/myocarditis | 59 (62.1) | 58 (36.0) | 653 (36.4) | 139 (54.3) | 193 (60.2) | ||
| Congenital heart disease | 29 (30.5) | 88 (54.7) | 1109 (61.9) | 117 (45.7) | 128 (39.9) | ||
| White race | 51 (53.7) | 104 (64.6) | 1212 (67.6) | 0.02 | 155 (60.5) | 202 (62.9) | 0.6 |
| Status 1A | 63 (95.5) | 141 (97.2) | 1262 (81.2) | <0.0001 | 204 (96.7) | 229 (85.8) | <0.001 |
| Status 1B | 0 (0.0) | 1 (0.7) | 204 (13.1) | <0.0001 | 1 (0.5) | 34 (12.7) | <0.001 |
| PRA >10 | 21 (25.3) | 24 (16.9) | 452 (27.6) | 0.02 | 21 (17.8) | 38 (21.0) | 0.5 |
| Ventilator at listing | 58 (61.7) | 138 (85.7) | 627 (36.7) | <0.0001 | 196 (76.9) | 145 (46.5) | <0.001 |
| Inotropes at listing | 57 (62.0) | 123 (76.9) | 1262 (70.5) | 0.04 | 180 (71.4) | 252 (78.5) | 0.05 |
| History of surgery at listing | 32 (33.7) | 83 (51.9) | 882 (49.2) | 0.009 | 115 (45.1) | 107 (33.4) | 0.004 |
| History of renal insufficiency | 5 (5.5) | 18 (11.5) | 62 (3.6) | <0.0001 | 23 (9.3) | 7 (2.4) | <0.001 |
| Age at listing, mo | 8.6±6.3 | 3.8±4.8 | 6.0±6.6 | <0.0001 | 5.6±5.8 | 6.6±6.5 | 0.06 |
| Weight at listing, kg | 6.5±2.0 | 5.0±2.2 | 5.5±2.1 | <0.0001 | 5.6±2.3 | 5.8±2.1 | 0.3 |
| Patients with weight <5 kg | 27 (28.4) | 101 (62.7) | 874 (48.7) | <0.0001 | 128 (50.0) | 136 (42.4) | 0.07 |
| Serum albumin, g/dL | 3.3±0.7 | 3.1±0.8 | 3.4±1.6 | 0.03 | 3.2±0.8 | 3.3±0.7 | 0.02 |
| Bilirubin at listing, mg/dL | 0.9±1.2 | 2.5±4.2 | 1.4±2.5 | <0.0001 | 1.9±3.5 | 1.2±2.0 | 0.006 |
| Creatinine at listing, mg/dL | 0.4±0.2 | 0.5±0.4 | 0.4±0.4 | <0.0001 | 0.5±0.3 | 0.4±0.2 | <0.001 |
| Transplant year | 2014.2±2.4 | 2014.3±2.8 | 2014.2±2.5 | 1.0 | 2014.3±2.6 | 2014.5±2.6 | 0.5 |
Data are given as number (percentage) or mean±SD. ECMO indicates extracorporeal membrane oxygenation; PRA, panel reactive antibody; and VAD, ventricular assist device.
When further examined, only 9.5% (n=117) of patients with CHD were on some form of MCS at listing (VAD, n=29; and ECMO, n=88), compared with 139 (16.9%) patients with cardiomyopathy (VAD, n=66; and ECMO, n=73) (P<0.001). Following listing, an additional 57 patients with CHD underwent VAD implant and 71 were placed on ECMO, compared with 156 unique patients with cardiomyopathy who underwent VAD implant and 37 who were placed on ECMO after listing (Figure 1). Among patients with CHD receiving MCS, the median time between listing and MCS was 0.6 months (interquartile range, 0.3–1.3 months) and was similar to those with cardiomyopathy (0.59 months [interquartile range, 0.25–1.3 months]).
MCS in Infants With CHD
Most patients in the CHD cohort were male (n=701, 57.2%) with 75.9% having a history of cardiac surgery before listing (Table 1). Within this patient group, there were differences in the weight and age distribution of patients with CHD based on initial MCS strategy at listing, with those on ECMO at listing being younger and smaller than those who underwent VAD placement or who did not require MCS at listing (Table 3). History of cardiac surgery differed between the 3 groups, as most infants in the ECMO group at listing underwent previous heart surgical interventions. There were significantly more infants with single ventricles in the ECMO and unsupported groups, compared with single‐ventricle patients on VAD support. Also, end‐organ function differed at listing between the 3 groups, with the total median bilirubin level in the ECMO and unsupported cohort being higher than the VAD group. There was a higher percentage of patients in the ECMO group who had a history of renal insufficiency but at the time listing there was no difference in the mean creatinine level. The diagnosis for all patients with CHD <10 kg included in this cohort is outlined in Table 4.
Table 3.
Characteristics at Listing for Support Group for Patients With CHD
| Characteristics at Listing | VAD (n=29) | ECMO (n=88) | Unsupported (n=1109) | P Value | VAD or ECMO at Listing (n=117) | VAD or ECMO After Listing (n=128) | P Value |
|---|---|---|---|---|---|---|---|
| Male sex | 16 (55.2) | 50 (56.8) | 635 (57.3) | 1.0 | 66 (56.4) | 79 (61.7) | 0.4 |
| White race | 22 (75.9) | 56 (63.6) | 766 (69.1) | 0.4 | 78 (66.7) | 87 (68.0) | 0.8 |
| Single ventricle | 7 (24.1) | 40 (45.5) | 674 (60.8) | <0.0001 | 47 (40.2) | 77 (60.2) | 0.002 |
| Status 1A | 19 (95.0) | 78 (98.7) | 871 (86.8) | 0.005 | 97 (98.0) | 108 (93.1) | 0.09 |
| Status 1B | 0 (0.0) | 0 (0.0) | 82 (8.2) | 0.01 | 0 (0.0) | 6 (5.2) | 0.02 |
| PRA >10 | 6 (24.0) | 9 (11.7) | 297 (29.2) | 0.004 | 15 (14.7) | 29 (25.0) | 0.1 |
| Ventilator at listing | 20 (71.4) | 78 (88.6) | 411 (38.7) | <0.0001 | 98 (84.5) | 60 (48.4) | <0.001 |
| Inotropes at listing | 16 (59.3) | 66 (75.9) | 749 (67.6) | 0.2 | 82 (71.9) | 94 (73.4) | 0.8 |
| History of surgery at listing | 25 (86.2) | 80 (90.9) | 825 (74.5) | 0.001 | 105 (89.7) | 93 (72.7) | <0.001 |
| History of renal insufficiency | 2 (7.1) | 10 (11.6) | 52 (4.8) | 0.02 | 12 (10.5) | 4 (3.3) | 0.03 |
| Age at listing, mo | 9.0±6.4 | 3.1±4.3 | 5.3±6.5 | <0.0001 | 4.5±5.5 | 5.1±5.7 | 0.5 |
| Weight at listing, kg | 6.0±2.1 | 4.4±1.9 | 5.2±2.0 | 0.0001 | 4.8±2.0 | 5.2±2.0 | 0.1 |
| Patients with weight <5 kg | 12 (41.4) | 65 (73.9) | 620 (55.9) | 0.001 | 77 (65.8) | 71 (55.5) | 0.1 |
| Serum albumin, g/dL | 3.3±0.5 | 3.0±0.7 | 3.4±1.6 | 0.1 | 3.1±0.7 | 3.2±0.7 | 0.3 |
| Bilirubin at listing, mg/dL | 0.8±0.8 | 3.0±5.0 | 1.7±2.7 | 0.0002 | 2.5±4.5 | 1.8±2.5 | 0.1 |
| Creatinine at listing, mg/dL | 0.4±0.2 | 0.5±0.3 | 0.4±0.5 | 0.1 | 0.5±0.3 | 0.4±0.2 | <0.001 |
| Transplant year | 2015.3±2.3 | 2014.9±2.6 | 2014.3±2.5 | 0.1 | 2015.0±2.5 | 2015.0±2.6 | 1.0 |
Data are given as number (percentage) or mean±SD. CHD indicates congenital heart disease; ECMO, extracorporeal membrane oxygenation; PRA, panel reactive antibody; and VAD, ventricular assist device.
Table 4.
Type of CHD
| Type of CHD | Yes, n | Yes, % |
|---|---|---|
| Arch hypoplasia/interruption/hypoplasia | 34 | 0.12 |
| Atrial septal defect/ventricular septal defect | 117 | 0.1 |
| Atrioventricular discordance | 0 | 0 |
| Bilateral SVC | 4 | 0.02 |
| Complete atrioventricular septal defect/atrioventricular canal | 97 | 0.08 |
| Congenitally corrected transposition | 17 | 0.01 |
| Coronary anomaly | 34 | 0.05 |
| Dextrocardia | 7 | 0.03 |
| Double‐inlet left ventricle | 17 | 0.02 |
| Ebstein anomaly | 24 | 0.02 |
| Heterotaxy | 32 | 0.05 |
| Hypoplastic left heart | 578 | 0.47 |
| Hypoplastic right ventricle not otherwise specified | 40 | 0.05 |
| Interrupted inferior vena cava | 1 | 0 |
| Left SVC (no right SVC) | 0 | 0 |
| Left ventricular outflow tract obstruction/ aortic stenosis | 74 | 0.08 |
| Mitral stenosis | 21 | 0.08 |
| Right aortic arch | 0 | 0 |
| PDA | 1 | 0 |
| Pulmonary atresia (with complex heart disease, not intact septum or TOF) | 10 | 0.04 |
| Pulmonary atresia with intact ventricular septum | 197 | 0.16 |
| Situs inversus | 0 | 0 |
| Total anomalous pulmonary venous return | 18 | 0.02 |
| Partial anomalous pulmonary venous return | 1 | 0 |
| TOF/TOF variant/double‐outlet right ventricle/right ventricular outflow tract obstruction | 90 | 0.07 |
| Transposition of the treated arteries | 66 | 0.05 |
| Tricuspid atresia | 31 | 0.04 |
| Truncus arteriosus | 12 | 0.01 |
| Unknown | 5 | 0.11 |
| Other | 44 | 0.04 |
CHD indicates congenital heart disease; PDA, patent ductus arteriosus; SVC, superior vena cava; and TOF, tetralogy of Fallot.
The listing characteristics of patients with CHD with MCS at listing compared with initiation after listing are also outlined in Table 3. Those patients who were on support at listing were more likely to be ventilated, have a history of cardiac surgery and history of renal insufficiency, and a slightly higher creatinine at the time of listing compared with those patients in whom MCS was initiated after listing.
MCS in Infants With Cardiomyopathy
Most patients with cardiomyopathy were female, with >70% on inotropes and 40% requiring a ventilator at listing (Table 1). In general, the weight and age of the patients with cardiomyopathy at listing was higher than those with CHD. Within the cardiomyopathy group, those patients with ECMO at listing (Table 5) were more likely to be ventilated, have a history of renal insufficiency, have higher bilirubin and creatinine at listing, and were smaller than those on VAD or unsupported. Patients with cardiomyopathy on MCS at listing had a higher proportion of patients on ventilators with history of renal insufficiency compared with those in whom MCS was initiated after listing. In addition, the average listing bilirubin and creatinine were higher (Table 5).
Table 5.
Characteristics at Listing for Support Group for Patients With Cardiomyopathy/Myocarditis
| Characteristics at Listing | VAD (n=66) | ECMO (n=73) | Unsupported (n=684) | P Value | VAD or ECMO at Listing (n=117) | VAD or ECMO After Listing (n=128) | P Value |
|---|---|---|---|---|---|---|---|
| Male sex | 33 (50.0) | 37 (50.7) | 298 (43.6) | 0.3 | 70 (50.4) | 85 (44.0) | 0.3 |
| White race | 29 (43.9) | 48 (65.8) | 446 (65.2) | 0.003 | 77 (55.4) | 115 (59.6) | 0.4 |
| Status 1A | 44 (95.7) | 63 (95.5) | 391 (71.0) | <0.0001 | 107 (95.5) | 121 (80.1) | <0.001 |
| Status 1B | 0 (0.0) | 1 (1.5) | 122 (22.1) | <0.0001 | 1 (0.9) | 28 (18.5) | <0.001 |
| PRA >10 | 15 (25.9) | 15 (23.1) | 155 (25.0) | 0.9 | 30 (24.4) | 43 (25.7) | 0.8 |
| Ventilator at listing | 38 (57.6) | 60 (82.2) | 216 (33.5) | <0.0001 | 98 (70.5) | 85 (45.2) | <0.001 |
| Inotropes at listing | 41 (63.1) | 57 (78.1) | 513 (75.3) | 0.07 | 98 (71.0) | 158 (81.9) | 0.02 |
| History of surgery at listing | 7 (10.6) | 3 (4.2) | 57 (8.3) | 0.4 | 10 (7.2) | 14 (7.3) | 1.0 |
| History of renal insufficiency | 3 (4.8) | 8 (11.3) | 10 (1.5) | <0.0001 | 11 (8.2) | 3 (1.7) | 0.007 |
| Age at listing, mo | 8.5±6.3 | 4.7±5.1 | 7.1±6.6 | 0.002 | 6.5±6.0 | 7.6±6.9 | 0.1 |
| Weight at listing, kg | 6.8±1.9 | 5.7±2.4 | 6.0±2.1 | 0.008 | 6.2±2.2 | 6.2±2.1 | 0.8 |
| Patients with weight <5 kg | 15 (22.7) | 36 (49.3) | 254 (37.1) | 0.005 | 51 (36.7) | 65 (33.7) | 0.6 |
| Serum albumin, g/dL | 3.4±0.8 | 3.2±0.8 | 3.6±1.7 | 0.1 | 3.3±0.8 | 3.5±0.7 | 0.07 |
| Bilirubin at listing, mg/dL | 0.9±1.3 | 1.9±2.7 | 1.0±2.0 | 0.002 | 1.4±2.2 | 0.9±1.3 | 0.006 |
| Creatinine at listing, mg/dL | 0.4±0.2 | 0.5±0.4 | 0.3±0.2 | <0.0001 | 0.5±0.4 | 0.4±0.2 | <0.001 |
| Transplant year | 2013.9±2.4 | 2013.8±2.8 | 2014.1±2.5 | 0.6 | 2013.9±2.6 | 2014.3±2.6 | 0.2 |
Data are given as number (percentage) or mean±SD. ECMO indicates extracorporeal membrane oxygenation; PRA, panel reactive antibody; and VAD, ventricular assist device.
Survival for All Patients <10 kg
In the overall group (cardiomyopathy+CHD), wait list survival was significantly different between those who required ECMO or VAD support at any time, compared with those who did not (Figure 2). Furthermore, comparison of survival estimates at the 3‐month time point (3 months after device placement for those who required ECMO or VAD) demonstrates a better survival for VAD compared with patients supported with ECMO (74.3% versus 48.6%; P<0.001) (Figure 2). This survival advantage was also observed for patients who were able to transition from ECMO to VAD (Figures 3, 5A and 5B).
Figure 2. Wait list survival, stratified by absence of support, ventricular assist device (VAD) while listed, and extracorporeal membrane oxygenation (ECMO) while listed.
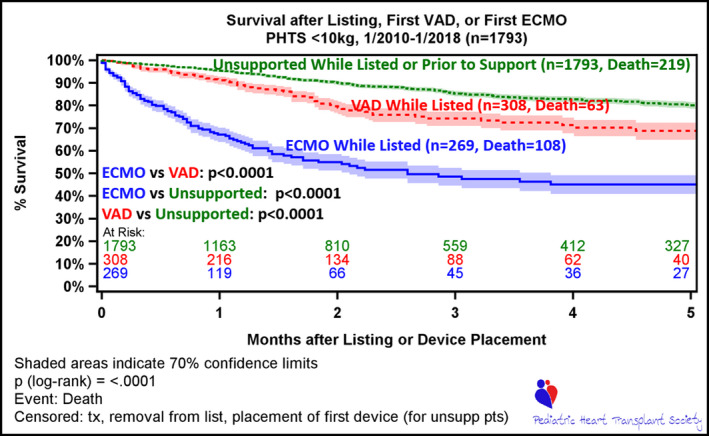
Time 0 is time of listing for unsupported patients (unsupp pts), who are followed up to the event of death or censored at transplant, removal from list, or at time of first device (ECMO or VAD). The lower 2 curves depict survival while on first device, either ECMO or VAD. Time 0 for patients on device (ECMO or VAD) is time of listing (if patient on device at listing) or time implant of first device following listing. Patients in this cohort are censored at transplant or removal from list. PHTS indicates Pediatric Heart Transplant Society; and tx, transplant.
Figure 3. Survival after ventricular assist device (VAD) implant on wait list, stratified by VAD as initial device vs VAD following extracorporeal membrane oxygenation (ECMO).
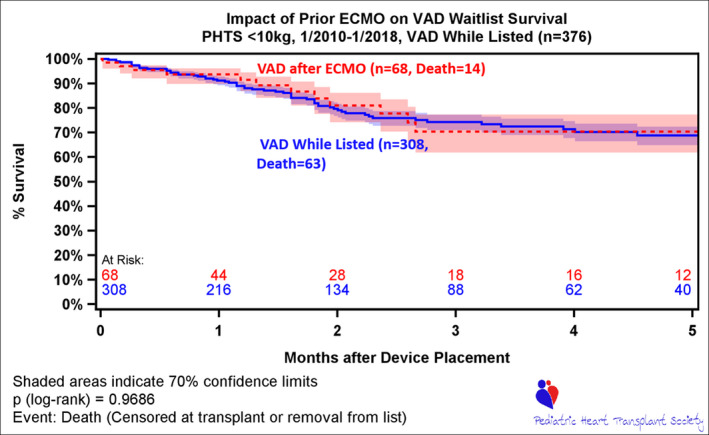
Time 0 is device placement or listing (if device placed before listing). PHTS indicates Pediatric Heart Transplant Society.
Figure 5. Survival after ventricular assist device (VAD) implant on wait list, stratified by VAD as initial device vs VAD following extracorporeal membrane oxygenation (ECMO).
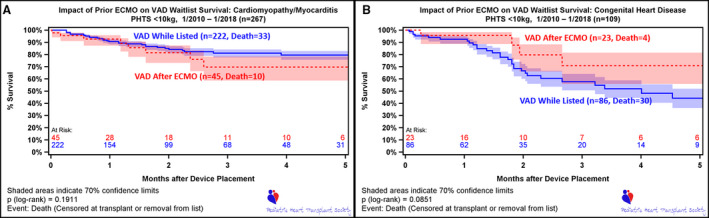
Time 0 is device placement or listing (if device placed before listing). A, Information for patients with cardiomyopathy/myocarditis. B, Information for patients with congenital heart disease. PHTS indicates Pediatric Heart Transplant Society.
This difference in outcomes between ECMO and VAD was observed in both the CHD and cardiomyopathy populations, with a greater negative impact on the CHD cohort (Figure 4). By 3 months after implant, the survival after support in the cardiomyopathy group was 62.2% with ECMO compared with 81.2% on VAD support (Figure 4). For the CHD group, however, survival dropped to 38.9% for the ECMO group versus 57.7% for the VAD support cohort at 3 months.
Figure 4. Wait list survival, stratified by absence of support, ventricular assist device (VAD) while listed, and extracorporeal membrane oxygenation (ECMO) while listed.
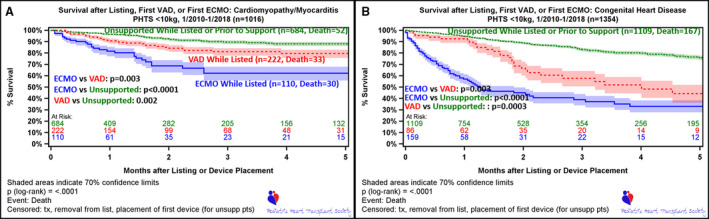
A, Includes patients with cardiomyopathy/myocarditis. B, Includes patients with congenital heart disease. Time 0 is time of listing for unsupported patients (unsupp pts), who are followed up to the event of death or censored at transplant, removal from list, or at time of first device (ECMO or VAD). The lower 2 curves depict survival while on first device, either ECMO or VAD. Time 0 for patients on device (ECMO or VAD) is time of listing (if patient on device at listing) or time implant of first device following listing. Patients in this cohort are censored at transplant or removal from list. PHTS indicates Pediatric Heart Transplant Society; and tx, transplant.
As mentioned above, within the CHD population requiring MCS, outcomes on device while waiting for HTx are not equivalent between MCS. However, they were not statistically different between single‐ventricle and biventricular hearts (Figure S1). There was a significant survival advantage to support with a VAD compared with ECMO, and this was demonstrated when patients with CHD were able to transition from ECMO to VAD support during the waiting period (Figure 5B).
Competing Outcomes
The competing outcome curves for VADs in children with CHD show that at 1‐month after implant 67.2% were alive on VAD therapy, 15.2% were transplanted, 5.8% had died, 5.9% had been switched to ECMO, and 5.8% were explanted (Figure 6A). Curves for ECMO therapy in patients with CHD at 30 days show 10.7% were alive on ECMO, 35.2% of the patients had died, 27.7% had been decannulated, 13.2% were switched to a VAD, and 13.2% had undergone HTx (Figure 6C).
Figure 6. Competing outcome depictions for mutually exclusive outcomes after first mechanical circulatory support.
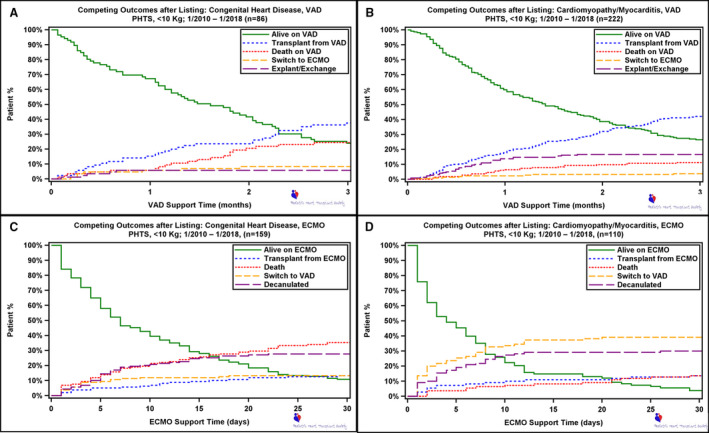
A, Ventricular assist device (VAD) support in patients with congenital heart disease. B, Extracorporeal membrane oxygenation (ECMO) support in patients with congenital heart disease. C, VAD support in patients with cardiomyopathy/myocarditis. D, ECMO support in patients with cardiomyopathy/myocarditis. PHTS indicates Pediatric Heart Transplant Society.
When examining device‐related outcomes in the patients with cardiomyopathy at 1‐month after VAD implant, 60.0% were alive on VAD therapy, 17.8% were transplanted, 6.4% had died, 2.3% had been switched to ECMO, and 13.7% were explanted (Figure 6B). ECMO therapy in patients with cardiomyopathy revealed that at 30 days, 4.6% were alive on ECMO, 13.6% of the patients had died, 30.0% had been decannulated, 39.1% were switched to a VAD, and 13.6% had undergone transplant (Figure 6D).
Risk Models
Multivariate analysis was performed both for survival after first ECMO or VAD implantation while listed (Table S2). The models all revealed a single early decreasing hazard for death. This analysis confirmed that CHD was a risk factor for death on MCS when in infants <10 kg. For ECMO, the hazard of death was in the early phase (hazard ratio [HR], 2.70 [1.75–4.16]; P<0.001). For VAD therapy, the hazard for death also occurred in the early phase (HR, 2.19 [1.31–3.66]; P=0.003). In addition to diagnosis, weight was also associated with mortality in the VAD model but not ECMO (HR, 0.32 for each unit change in the log scale [0.17–0.61]; P<0.001), with a higher weight being protective. For example, the HR for mortality of a 6‐kg child compared with a 5‐kg child is 0.81 (Table 6).
Table 6.
Adjusted Risk of Mortality on the Wait List by Diagnosis and MSC Use
| Variable | Hazard Ratio | 95% CI | P Value |
|---|---|---|---|
| Parametric hazard modeling results for CHD | |||
| Race (White) | 0.76 | 0.60–1.0 | 0.04 |
| List year (since 2010) | 0.92 | 0.88–0.96 | 0.0004 |
| ECMO | 4.40 | 3.30–5.85 | <0.0001 |
| VAD | 2.46 | 1.65–3.66 | <0.0001 |
| Weight at listing, kg | 0.94 | 0.88–1.00 | 0.05 |
| Status 1A at listing | 2.04 | 1.27–3.28 | 0.003 |
| Ventilator at listing | 1.54 | 1.18–2.01 | 0.001 |
| Parametric hazard modeling results for cardiomyopathy | |||
| ECMO | 3.48 | 2.16–5.60 | <0.0001 |
| VAD | 1.83 | 1.18–2.86 | 0.008 |
| Ventilator at listing | 1.79 | 1.20–2.68 | 0.005 |
| Parametric hazard modeling results for ECMO | |||
| List year (since 2010) | 0.89 | 0.82–0.96 | 0.002 |
| CHD | 2.7 | 1.75–4.16 | <0.0001 |
| Parametric hazard modeling results for VAD | |||
| Weight at listing (logarithmic), kg | 0.324 | 0.17–0.62 | 0.0006 |
| CHD | 2.19 | 1.31–3.66 | 0.003 |
Patients are in >1 cohort. CHD indicates congenital heart disease; ECMO, extracorporeal membrane oxygenation; MSC, mechanical circulatory support; and VAD, ventricular assist device.
Besides MCS‐specific hazard models, models were created for patients with cardiomyopathy and CHD (Table 6). For patients with cardiomyopathy, the use of ECMO (HR, 3.48 [2.16–5.6]; P<0.001) and VAD (HR, 1.83 [1.17–2.86]; P=0.008) or a ventilator at listing (HR, 1.79 [1.2–2.68]; P=0.005) was associated with mortality in the early phase of the model. For patients with CHD, there were several factors associated with early‐phase mortality in the multivariate (MV) analysis, with White race, list year since 2010, and higher weight being protective, whereas ECMO, VAD, status 1A at listing, and ventilator support at listing were associated with mortality. The impact of size and difference between the cardiomyopathy and CHD group on outcomes can clearly be seen in the Kaplan‐Meier survival analysis (Figure 7), especially early after implant. In the smallest of patients, those <5 kg, overall survival did not differ between ECMO versus VAD (P=0.10) for the CHD group or the cardiomyopathy group (P=0.38). However, survival was better on both forms of support for those <5 kg with a diagnosis of cardiomyopathy (Figure 7).
Figure 7. Wait list survival after first mechanical circulatory support, stratified by ventricular assist device (VAD) vs extracorporeal membrane oxygenation (ECMO) in patients weighing <5 kg.
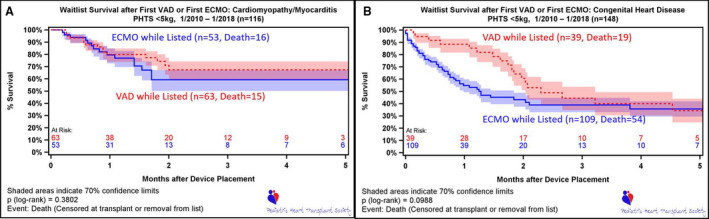
Time 0 is device placement or listing (if device placed before listing). A, Patients with cardiomyopathy/myocarditis. B, Patients with congenital heart disease. PHTS indicates Pediatric Heart Transplant Society.
DISCUSSION
This study explored the characteristics and outcomes of infants <10 kg who required MCS while awaiting HTx with attention to mode of support and diagnosis. This particular group was chosen because of previously reported inferior outcomes while supported on MCS in this subset of patients. 5 , 9 , 10 , 11 A previous analysis through the PHTS examined all patients, regardless of age, supported on ECMO and found that just over half of the patients with CHD listed and transplanted were on ECMO 10 and that smaller children had the highest risk of mortality. Our analysis adds further to this previous analysis by examining further detail in those smaller patients requiring MCS, and highlights the difference in outcomes for patients with cardiomyopathy compared with CHD. 12
This analysis, like others, found that ECMO was associated with higher mortality in both patients with CHD and cardiomyopathy but the difference was more striking in those with CHD. In patients with CHD, death on ECMO at 1 month was 35%, with only 15% of patients achieving HTx. This differed from patients with CHD treated with VAD support, in whom death on VAD at 1 month was 5.8%. Interestingly, a similar proportion of patients with CHD underwent HTx (15.8%) in the competing outcomes curve (COC) analysis. We were able to show in this study that the ECMO survival disadvantage was reversed in patients who were able to switch to VAD support in both the overall cohort and children with CHD. Although the above results incorporated all patients <10 kg, the story for those <5 kg was less clear. Similar to the overall cohort, those <5 kg with cardiomyopathy had better outcomes compared with patients with CHD on support. However, from the Kaplan‐Meier survival analysis, there was no significant survival difference between ECMO and VAD within each group. These findings highlight the importance of finding alternative treatment options in these small children.
Although ECMO has traditionally been used as a means of MCS, VAD therapy provides an alternative strategy of MCS for children awaiting HTx. Although most pediatric patients supported with VADs have a favorable outcome, the combination of CHD and small size has been suggested to have a significant impact on survival in this patient cohort. 5 , 9 Currently, VAD support is the predominant means of support in most children; however, as highlighted in this study, ECMO continues to play an important role in young infants, with 33.1% of patients with cardiomyopathy and 64.9% of patients with CHD using ECMO as the first device. The predominance of ECMO use in children with CHD may be driven by the known challenges of supporting these patients with VAD, the predominance of children <5 kg in this group, the need for an oxygenator, and the use of ECMO as a first‐line strategy following congenital surgery.
Although there are many factors that could be responsible for these differences in MCS outcomes between patients with cardiomyopathy and CHD, the role of cardiac surgery cannot be dismissed. A recent article by Morales et al showed that survival outcomes for infants on a pulsatile VAD who had preimplant congenital heart surgery plus ECMO during the same admission had poor outcomes, with only 8% survival. In contrast, patients who did not have congenital surgery plus ECMO had a 61% survival. The authors cautioned that if patients had undergone preimplant surgery plus ECMO, VAD support might not provide a survival benefit. Although we know the number of patients in our study who underwent previous congenital heart surgery, we do not know what proportion had surgery during the same admission as the VAD implantation. Nevertheless, because of the high percentage of children with CHD <10 kg, we suspect that using VAD as a rescue therapy following surgery may have played a significant role in our results. 13
Our findings showing a difference in outcomes between infants with cardiomyopathy and CHD are supported by previous literature, including a report by Conway et al, who examined the outcomes of 97 patients <10 kg supported with the Berlin Heart EXCOR, and found 57% of patients were able to achieve good outcomes (weaned or transplanted), with this number decreasing to 27% in patients weighing <5 kg. 5 When the results from this study are examined more closely, it is clear that the patients <10 kg were not homogeneous, with 26 (26.8%) patients having a diagnosis of CHD. The authors observed that for patients with no CHD and <10 kg, no preimplant ECMO, and a normal bilirubin, 87.5% were successfully supported to HTx. However, this rate dropped to 30.8% in those with CHD, and even further for patients with CHD on ECMO and/or who had an elevated bilirubin. These reported survival patterns remained true for those <5 kg, with a 66.7% success rate in children without CHD, who did not require pre‐ECMO and had normal bilirubin; whereas only 1 of 12 infants with CHD <5 kg (n=13) survived to transplant. 5 This is similar to our results in patients <5 kg, where most had single‐ventricle physiological features and a survival rate of 44% at 3 months after VAD implant. These results are in contrast to children <10 kg with cardiomyopathy or myocarditis, in whom survival to HTx on VAD therapy has been reported to be as high as 91%. 11 This is consistent with our observation of excellent survival on device therapy for small patients with cardiomyopathy.
This study had several limitations inherent to a retrospective analysis, especially with small patient numbers. Another major limitation identified during the study was the difference in patient cohorts supported by the 2 modes of MCS. ECMO was predominantly used as first‐line therapy in younger and smaller patients, with a higher percentage of patients having single‐ventricle physiological features. In addition, children <5 kg were left on ECMO for longer periods of time, likely secondary to the lack of available support options, increasing the risk of mortality as time on device increased. Moreover, as we did not know the reason for ECMO initiation, we could not account for the impact of the primary indication for ECMO, which may have influenced the difference in outcomes between the forms of MCS. Last, as time on VAD support before HTx is limited, conclusions cannot be drawn about long‐term outcomes while waiting. Therefore, when examining the results of previous studies and when designing analyses moving forward, it would be important to take these observations into consideration.
Although this study confirms previous reports of the negative impact of ECMO on outcomes in small children listed for HTx, it has also begun to tease out key information in understanding the patients <10 kg who require MCS. Characterizing this patient population is essential for moving forward to aid in determining which treatment options will have the most impact for a particular diagnosis. The observations throughout this study speak to the tremendous need for development of device options for these small patients, especially those with CHD. Supporting these children remains a challenge, and there is ongoing need for research and development of smaller pumps that are designed for the unique features of the pediatric population.
Sources of Funding
None.
Disclosures
Dr Kirklin is the Principle Investigator of Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) and is partially supported through the INTERMACS contract. Dr Conway reports an educational grant from Heartware INC. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S2
Figure S1
(J Am Heart Assoc. 2020;9:e011890 DOI: 10.1161/JAHA.118.011890.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.118.011890
For Sources of Funding and Disclosures, see page 12.
References
- 1. Almond CS, Morales DL, Blackstone EH, Turrentine MW, Imamura M, Massicotte MP, Jordan LC, Devaney EJ, Ravishankar C, Kante KK, et al. Berlin Heart EXCOR pediatric ventricular assist device for bridge to heart transplantation in US children. Circulation. 2013;1702–1711. [DOI] [PubMed] [Google Scholar]
- 2. Fraser CD Jr, Jaquiss RDB, Rosenthal DN, Humpl T, Canter CE, Blackstone EH, Naftel D, Ichord R, Bomgaars L, Twedell JS, et al. Prospective trial of a pediatric ventricular assist device. N Engl J Med. 2012;532–541. [DOI] [PubMed] [Google Scholar]
- 3. Davies RR, Russo MJ, Hong KN, O'Byrne ML, Cork DP, Moskowitz AJ, Gelijns AC, Mital S, Mosca RS, Chen JM, et al. The use of mechanical circulatory support as a bridge to transplantation in pediatric patients: an analysis of the United Network for Organ Sharing database. J Thorac Cardiovasc Surg. 2008;421–431. [DOI] [PubMed] [Google Scholar]
- 4. Rossano JW, Lorts A, VanderPluym CJ, Jeewa A, Guleserian KJ, Bleiweis MS, Reinhartz O, Blume ED, Rosenthal DN, Naftel DC, et al. Utilization and outcomes of continuous‐flow ventricular assist devices in pediatric patients: a report from the Pediatric Interagency Registry for Mechanical Circulatory Support (PediMACS). J Heart Lung Transplant. 2015;S102. [DOI] [PubMed] [Google Scholar]
- 5. Conway J, St Louis J, Morales DLS, Law S, Tjossem C, Humpl T. Delineating survival outcomes in children <10 kg bridged to transplant or recovery with the Berlin Heart EXCOR ventricular assist device. JACC Heart Fail. 2015;70–77. [DOI] [PubMed] [Google Scholar]
- 6. Davies RR, Haldeman S, McCulloch MA, Pizarro C. Ventricular assist devices as a bridge‐to-transplant improve early post‐transplant outcomes in children. J Heart Lung Transplant. 2014;704–712. [DOI] [PubMed] [Google Scholar]
- 7. Dipchand AI, Rossano JW, Edwards LB, Kucheryavaya AY, Benden C, Goldfarb S, Levvey BJ, Lund LH, Meiser B, Yusen RD, et al. The Registry of the International Society for Heart and Lung Transplantation: eighteenth official pediatric heart transplantation report‐2015. J Heart Transplant. 2015;1233–1243. [DOI] [PubMed] [Google Scholar]
- 8. Blackstone EH, Naftel DC, Turner ME Jr. The decomposition of time‐varying hazard into phases, each incorporating a separate stream of concomitant information. J Am Stat Assoc. 1986;615–624. [Google Scholar]
- 9. Weinstein S, Bello R, Pizarro C, Fynn‐Thompson F, Kirklin JK, Guleserian KJ, Woods RK, Tjossem C, Kroslowitz R, Friedmann P. The use of the Berlin Heart EXCOR in patients with functional single ventricle. J Thorac Cardiovasc Surg. 2014;697–705. [DOI] [PubMed] [Google Scholar]
- 10. Dipchand AI, Mahle WT, Tresler M, Naftel DC, Almond C, Kirklin JK, Pruitt E, Webber SA. Extracorporeal membrane oxygenation as a bridge to pediatric heart transplantation effect on post‐listing and post‐transplantation outcomes. Circ Heart Fail. 2015;960–969. [DOI] [PubMed] [Google Scholar]
- 11. Karimova A, van Doorn C, Brown K, Giardini A, Kostolny M, Mathias M, Hoskote A, Burch M. Mechanical bridging to orthotopic heart transplantationin children weighing less than 10 kg: feasibility and limitations. Eur J Cardiothorac Surg. 2011;304–309. [DOI] [PubMed] [Google Scholar]
- 12. Almond CSD, Thiagarajan RR, Piercey GE, Gauvreau K, Blume ED, Bastardi HJ, Fynn‐Thompson F, Singh TP. Waiting list mortality among children listed for heart transplantation in the United States. Circulation. 2009;717–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morales DLS, Zafar F, Almond CS, Canter C, Fynn‐Thompson F, Conway J, Adachi I, Lorts A. Berlin Heart EXCOR use in patients with congenital heart disease. J Heart Lung Transplant. 2017;1209–1216. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2
Figure S1


