Abstract
Background
Failure rates after revascularization surgery remain high, both in vein grafts (VG) and arterial interventions. One promising approach to improve outcomes is endogenous upregulation of the gaseous transmitter‐molecule hydrogen sulfide, via short‐term dietary restriction. However, strict patient compliance stands as a potential translational barrier in the vascular surgery patient population. Here we present a new therapeutic approach, via a locally applicable gel containing the hydrogen sulfide releasing prodrug (GYY), to both mitigate graft failure and improve arterial remodeling.
Methods and Results
All experiments were performed on C57BL/6 (male, 12 weeks old) mice. VG surgery was performed by grafting a donor‐mouse cava vein into the right common carotid artery of a recipient via an end‐to‐end anastomosis. In separate experiments arterial intimal hyperplasia was assayed via a right common carotid artery focal stenosis model. All mice were harvested at postoperative day 28 and artery/graft was processed for histology. Efficacy of hydrogen sulfide was first tested via GYY supplementation of drinking water either 1 week before VG surgery (pre‐GYY) or starting immediately postoperatively (post‐GYY). Pre‐GYY mice had a 36.5% decrease in intimal/media+adventitia area ratio compared with controls. GYY in a 40% Pluronic gel (or vehicle) locally applied to the graft/artery had decreased intimal/media area ratios (right common carotid artery) and improved vessel diameters. GYY‐geltreated VG had larger diameters at both postoperative days 14 and 28, and a 56.7% reduction in intimal/media+adventitia area ratios. Intimal vascular smooth muscle cell migration was decreased 30.6% after GYY gel treatment, which was reproduced in vitro.
Conclusions
Local gel‐based treatment with the hydrogen sulfide‐donor GYY stands as a translatable therapy to improve VG durability and arterial remodeling after injury.
Keywords: hydrogen sulfide, vascular remodeling, vein graft disease
Subject Categories: Cardiovascular Surgery, Diet and Nutrition, Vascular Disease
Nonstandard Abbreviations and Acronyms
- DR
dietary restriction
- GYY
hydrogen sulfide releasing prodrug
- H2S
hydrogen sulfide
- I/M+A
intimal/media+adventitia
- NaHS
sodium hydrosulfide
- RCCA
right common carotid artery
- SF7‐AM
fluorescent probe binding free hydrogen sulfide
- VGD
vein graft disease
Clinical Perspective
What Is New?
This work evaluates a gel containing a slow‐releasing hydrogen sulfide prodrug that can be applied locally and directly onto the artery or graft during the surgical procedure.
One‐time periprocedural application of this hydrogen sulfide gel limits arterial intimal hyperplasia and attenuates vein graft disease.
What Are the Clinical Implications?
High failure rates after (cardio)vascular surgery interventions remain a significant clinical problem, with adverse vascular remodeling as a major contributor to high failure rates.
This one‐time, local, and periprocedural approach holds significant translational potential in improving vascular remodeling after (cardio)vascular surgery operations.
In lower extremity bypass surgery, autologous vein grafts remain the superior choice to achieve successful revascularization. 1 Although success rates are surpassing other conduits, 2 vein graft primary patency nevertheless falls toward 60% 1 year postintervention 3 with vein graft disease (VGD) as the main effector in prompting graft occlusion mid‐ to long‐term post implantation. 4 The intimal hyperplasia seen in VGD, that is, thickening of the intimal wall by vascular smooth muscle cell (VSMC) growth, 5 also drives open and endovascular arterial intervention failure. Taken together both these components render the vascular fibroproliferative response to injury a major unaddressed clinical challenge in cardiovascular surgery practice.
An emerging scientific field holding translational promise employs the concept of preconditioning of the patient, before surgery, against surgical stress/injury via short‐term dietary restriction (DR). The efficacy of DR, including the restriction of calories, proteins, or specific amino acids, is established in a wide range of preclinical surgical models, including surgical trauma, 6 , 7 renal 8 , 9 and hepatic 8 , 10 , 11 ischemia‐reperfusion injury, focal stroke in the brain, 12 poststenosis arterial hyperplasia, 13 and hindlimb ischemia, 14 without decelerating postinterventional wound healing processes. 15
One mechanism by which DR derives its benefit is via increased production of endogenous hydrogen sulfide (H2S), 16 a gaseous vasodilator and transmitter molecule 17 with anti‐inflammatory and cytoprotective potential. 18 In the vasculature, H2S is mainly enzymatically derived, with cystathionine y‐lyase being the most abundant H2S producing enzyme. 19 In the setting of vascular injury in rodent models, DR‐induced augmentation of endogenous H2S protects from ischemia‐reperfusion injury 20 and accelerates neovascularization after hindlimb ischemia. 14 Genetic (cardiac‐specific) overexpression of cystathionine y‐lyase protects from heart failure after transverse aortic constriction, 21 while knockdown of the same gene leads to increased neointima formation after carotid ligation, via increased VSMC migration. 22
In venous bypass surgery, DR‐induced increased endogenous H2S production (via cystathionine y‐lyase upregulation) attenuates inward remodeling and improves vein graft adaptation to its arterial environment, partly via limiting both VSMC migration and neutrophil transmigration toward the intimal layer. 23 Upregulation of endogenous H2S therefore possesses the capability to improve outcomes in various settings of (vascular) surgical injury. Nonetheless, translational barriers remain, not least of which includes the requirement for planning of the dietary intervention, as we found in a recent pilot study of DR in patients scheduled for vascular surgery. 24
Thus DR‐mimetic drugs, including direct H2S administration, are an attractive alternative approach. Although systemic treatment with various H2S donors shows therapeutic potential in a wide range of preclinical disease models, 25 including a single dose immediately before hepatic ischemia reperfusion injury, 16 its potential in vascular reconstructions has yet to be fully explored. Furthermore, the ability to use local delivery may address potential safety issues regarding systemic treatments, thereby improving clinical translatability. Here, we tested the potential of H2S to mitigate intimal hyperplasia and VGD, using a single, local periprocedural application.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Experimental Animals
All animal experiments were approved by the appropriate Harvard Medical Area or Brigham and Women’s Hospital Institutional Animal Care and Use Committee (04475 and A4752‐01 respectively) and in accordance with the NIH guidelines. All surgical experiments were performed on C57BL/6 mice (male, 10–12 weeks old, Stock No: 000664, Jackson Laboratory), fed a high‐fat diet (Research Diets D12492, 60% fat) during the experiment. Mice were housed 4 to 5 per cage and maintained on a 12‐hour light‐dark cycle at 22°C with 30% to 50% humidity. All in‐vivo experiments were conducted in male mice to limit intergroup variability while assessing intervention effectiveness, thereby lowering the number of experimental animals needed.
Vein Graft Surgery
Vein graft surgery was performed as described previously. 26 Briefly, mice were anesthetized with 5% isoflurane and maintained under 2% to 3% isoflurane via a nose cone for the duration of the procedure. The mouse was placed on a heating pad to maintain periprocedural body temperature. After removal of fur in the neck region a neckline incision was performed. After dissection of the right common carotid artery (RCCA) from its surrounding tissues, the artery was ligated with an 8‐0 nylon suture. Vascular clamps were placed at the proximal and distal arterial ends and the carotid wall was everted over a polyetheretherketone cuff. The thoracic inferior caval vein from a donor mouse was harvested just before the start of the vein graft procedure in the recipient and stored in ice cold sterile 0.9% NaCl supplemented with heparin (100 UI/mL). After everting the carotid artery of the recipient over the cuff, the donor caval vein was sleeved and then sutured over both ends of the cuff with an 8‐0 nylon suture, creating an end‐to‐end anastomosis. After grafting of the caval vein, vascular clamps were removed, and blood flow was restored. The incision was closed with 6‐0 Vicryl sutures. Postoperatively animals received warm lactate ringer solution (0.5 mL, subcutaneous) and buprenorphine (0.1 mg/kg, subcutaneous).
Focal Carotid Stenosis Procedure
A focal stenosis was created as described previously to generate a hemodynamically induced arterial intimal hyperplastic response in the setting of flowing blood. 27 Briefly, mice were anesthetized with 5% isoflurane and maintained under 2% to 3% isoflurane via nose cone for the duration of the procedure. After dissection of the RCCA from its surrounding tissues, a 35‐gauge blunt needle mandrel was placed longitudinally along the RCCA and tied with a 9‐0 nylon suture approximately 2 to 2.5 mm proximal to the bifurcation. The needle mandrel was then removed and skin closed with a 6‐0 Vicryl suture. Postoperatively mice received warm lactate ringer solution (0.5 mL, subcutaneous) and buprenorphine (0.1 mg/kg, subcutaneous).
Systemic Treatment with GYY4137
For systemic treatment, mice were randomized in pre‐GYY, post‐GYY, and control groups. Based on our previous work, 16 in the pre‐GYY treatment group, 250 µmol/L GYY4137 (Sigma, cat# SML0100) (GYY) was supplied in the drinking water for 1 week preceding vein graft surgery and replaced fresh after 3 days. Immediately post surgery, drinking water was replaced with regular (without GYY). In the post‐GYY treatment group, 250 µmol/L GYY was supplemented in the drinking water immediately postoperative until harvest at postoperative day 28 and replaced weekly. 28
Periprocedural Local Treatment With GYY
For periprocedural local treatment with GYY, a 40% Pluronic gel (Sigma, cat# P2443) was created the day before surgery (40% w/v, 4‐g Pluronic gel, 10 mL sterile 0.9% NaCl) and kept at 4°C overnight under continuous stirring. Mice were randomized into GYY treatment or vehicle groups. Ten minutes before application of the gel onto the vessel/graft, GYY was dissolved into a stock solution (0.9% NaCl) and resuspended in 100 µL of 40% Pluronic gel to a final concentration of 250 µmol/L. During the focal stenosis procedure, 100 µL gel containing GYY or vehicle only was applied immediately after stenosis creation. During bypass surgery, the gel (GYY or vehicle) was applied onto the caval vein after it was grafted into the RCCA but before opening the proximal and distal vascular clamps. Immediately after enveloping the graft with the gel, vascular clamps were opened, and blood flow was restored.
Vein Graft/Focal Carotid Stenosis Harvest
Animals were anesthetized (induction under 5% isoflurane, 1–2% continued isoflurane to maintain anesthesia), anesthetic depth was confirmed by toe pinch. A percutaneous cardiac puncture was performed to obtain whole blood, followed by a thoracotomy. Mice were then euthanized via exsanguination by cutting and removing the caval vein. Perfusion was performed with lactate Ringers solution via the left ventricle for 3 minutes, then switched to 3 minutes of perfusion‐fixation with 10% formalin. To harvest the graft/RCCA, a midline neck incision was made, the graft/RCCA was excised en‐block and fixed in 10% formalin for 24 hours. After 24 hours the tissue was transferred to a 70% ethanol solution and then processed for paraffin embedding.
Graft and Artery Processing for Histology
After paraffin embedding, both vein grafts and RCCA were cut with a microtome in 5 µm sections and mounted on slides. Grafts were sectioned at regular intervals of 200 µm, starting from the proximal cuff until 1000 µm post proximal cuff. Arteries were cut at regular intervals of 200 µm, starting at 200 µm proximal from the focal stenosis, until 2800 µm proximal from the stenosis. After section cutting, a Masson‐trichome histology staining was performed. In short, slides were deparaffinized to 95% ethanol, 3 minutes in 5% picric acid (in 95% ethanol), tap water wash, 3 minute stain in working Harris Hematoxylin Solution (Fisher Scientific, cat# 245–678), tap water wash, stained with 1% Biebrich Scarlet in 1% acetic acid (Fisher Scientific, cat# A38S‐500) for 3 minutes, quick rinse in distilled water, 1 minute stain in 5% Phosphomolybdic/Phosphotungstic acid solution then stained with 2.5% light green SF yellowish in 2.5% acetic acid (Fisher Scientific, cat# A38S‐500) for 4 minutes, rinsed in distilled water and rinsed in 1% acetic acid solution (Fisher Scientific, cat# A38S‐500) for 2 minutes. After dehydration with Xylene slides were covered with a cover glass employing Permount (Electron Microscopy Science, cat# 17986‐05).
Immunohistochemistry
For fluorescent immunohistochemistry, slides were first preheated to 60°C in a vacuum oven for 30 minutes. Afterwards, slides were immediately deparaffinized (Xylene 3×5 minutes, 100% ethanol 3×5 minutes, 70% ethanol 5 minutes, 50% ethanol 5 minutes, deionized water 5 minutes). After deparaffinization, antigen retrieval was performed for 30 minutes at 97°C in citrate buffer (pH 6.0 in PBS, Abcam, ab93678) and slides were cooled to room temperature (RT) afterwards. Next, slides were incubated with 10% goat serum (Life Technologies, cat# 50062Z), in PBS with 0.3 mol/L glycine (Aijnomoto, cat# R015N0080039), for 1 hour at RT. Consecutively, slides were incubated with primary antibodies for smooth muscle cell‐α (SMC‐α; mouse anti‐mouse, Abcam, ab7817, 1:800 dilution) and Ki‐67 (Ki‐67 (rabbit anti‐mouse, Abcam, ab16667, 1:100 dilution) o/n at 4°C for SMC‐α – Ki‐67 double staining. The next day, slides were washed 3 times in PBS+0.05% tween and incubated in secondary antibody for 2 hours at RT. For SMC‐α+Ki‐67 double staining, slides were incubated with Alexa Fluor 647 (goat anti‐mouse, A‐32728, 1:600 dilution) and Alexa Flour 568 (goat anti‐rabbit, A‐11011, 1:600 dilution). After secondary antibody incubation, slides were washed in PBS+0.05% tween 3×5 minutes and mounted with DAPI (Vector, CB‐1000) and imaged by confocal microscopy.
Histology and Immunohistochemistry Analyses
All histology and immunohistochemistry analysis were done by a blinded observer. Vein grafts were excluded from analysis in case of complete occlusion of the graft/RCCA at postoperative day (POD) 28. Based on our previous vein graft experiments with endogenous H2S treatment, 23 our initial systemic GYY experiments were conducted with n=10/group. After systemic GYY experiments (n=10/group), 2 control, 2 pre‐GYY, and 2 post‐GYY vein grafts were occluded at POD 28. Observed differences between experimental groups after systemic GYY treatment led us to increase group size from n=10 to n=18 for local (one‐time) treatment with GYY. For local GYY experiments (n=18/group), 0 vehicle and 2 local GYY vein grafts were occluded at POD 28. For focal stenosis experiments (n=10/group), 1 vehicle and 0 local‐GYY RCCA were occluded at POD 28. Failure rates after both vein graft and focal stenosis experiments were comparable to previously conducted experiments by microsurgeon (~10%). Brightfield images of vein graft and carotid artery cross‐sections were taken with a Zeiss Axio A1 microscope (Carl Zeiss). For vein grafts, histomorphometric analysis was performed on 5 cross‐sections per vein graft (200–400–600–800–1000 µm) using Image J 1.51p (Java 1.8.0_66). Luminal, intimal (I), medial, and adventitial (M+A) areas and circumferences were measured for each cross‐section with 3 cross‐sections per slide, then averaged per slide. Next, I/M+A area, intimal thickness, M+A thickness, I/M+A thickness ratios, and corrected luminal area were calculated as described previously, 29 to account for morphometric changes induced by vein graft processing. For collagen measurements, Masson‐trichome stained slides were processed via the color deconvolution tool in Image J and percentage of total vein graft layer occupied by the deconvoluted green channel was then calculated. For VSMC+Ki‐67 analysis, images were processed in Image J 1.51p by measuring intimal and M+A total area based on DAPI positive cells, followed by color thresholding of the SMC‐α positive cells. Area occupied by SMC‐α was then calculated as a percentage of total area in that respective vein graft layer. Proliferating VSMCs were defined was SMC‐α/Ki‐67 double‐positive cells, counted per vein graft layer and normalized to mm2. For histomorphometric analysis of carotid artery cross‐sections, luminal, I, and M circumferences and area were measured, followed by calculation of I/M area, M thickness, I/M thickness ratio, and corrected luminal diameter.
Duplex Ultrasound Biomicroscopy
A Vevo 2100 imaging system with 18 to 70 MhZ linear array transducers (VisualSonics Inc., Toronto, ON, Canada) was employed for high resolution in vivo ultrasonography of vein grafts and carotid arteries poststenosis creation. At POD 14 and 28 mice were anesthetized via 5% isoflurane inhalation and maintained under 2% to 3% via nose cone inhalation, body temperature was controlled via heating pad. To measure vessel cross‐dimensional sections, M‐mode was employed. Three luminal axial images were taken (at proximal, mid, and distal graft/poststenosis) and mean vessel diameters were calculated.
Primary VSMC Isolation
Primary VSMCs were harvested from murine aortas as described before. 30 Briefly, C57BL/6 (4–6 weeks old) mice were anesthetized (induction under 5% isoflurane, 1–2% continues isoflurane to maintain anesthesia) and a thoracoabdominal incision was performed after which the thoracic aorta was exposed. Primary euthanasia occurred via removal of the cava vein followed by exsanguination. The left cardiac ventricle was perfused with sterile saline and the perivascular adipose tissue surrounding the aorta was carefully removed with forceps and scissors. The thoracic aorta was removed and placed in Hanks Balanced Salt Solution (Thermo Fisher Scientific, cat# 14025076) with 1% penicillin/streptomycin (Corning, cat# 30‐002‐CL) on ice. After a brief wash in PBS, aortas where then placed in 6‐well plates (n=2 per well) in enzyme digestion solution consisting of 1 mg/mL Collagenase type 2 (Gibco, cat# 17101015), 0.24 mg/mL Elastase (Worthington Biochemical, LS002279), 1 mg/mL Soybean Trypsin Inhibitor (Worthington Biochemical, LS0033570), 1% penicillin/streptomycin in Hanks Balanced Salt Solution at 37°C/5% CO2 for 10 minutes. After the first digestion the aortas were transferred to another 6‐well well containing Dulbecco’s Modified Eagle Medium (DMEM) (Thermo Fisher Scientific, cat#10566016), 20% CCS, 1% pen/strep and washed briefly. Next, employing forceps, the adventitia layer of the aorta was carefully everted over the intimal and medial layer of the aorta and removed. After removal of the adventitial layer, the aorta was cut longitudinally, and the endothelium carefully scraped off with angled forceps. The artery was then placed in enzyme digestion solution and incubated for 60 minutes at 37°C/5% CO2. After the second digestion, the resulting single cell suspension was washed with DMEM (20% CCS, 1% pen/strep), centrifuged for 5 minutes at 1200 rpm/4°C, resuspended in complete DMEM and plated in 24‐well plates (Falcon, cat#353047). Cells were passaged at 90% confluency and used for subsequent assays at passage 3‐8.
Human Umbilical Endothelial Cell Isolation and Culture
Cords were collected from full‐term pregnancies and stored in sterile PBS at 4°C and subsequently used within 7 days. A cannula was inserted in the vein and flushed with sterile PBS. The vein was infused with 0.075% collagenase type II (Worthington, Lakewood, NJ, USA) and incubated for 20 minutes at 37°C. The collagenase solution was collected, and the vein was flushed with PBS. The cell suspension was centrifuged at 1200 rpm for 5 minutes and the pellet was resuspended in complete culture medium, EGMTM‐2 Endothelial Cell Growth Medium‐2 (BulletKit, Lonza). The cells were cultured in plates coated with fibronectin from bovine plasma (Sigma).
Cells were maintained at 37°C in a humidified 5% CO2 environment. Culture medium was refreshed every 2 to 3 days. Cells were grown until cobblestone morphology was reached. Cells were passed 1:3 using trypsin‐EDTA (Sigma) and human umbilical endothelial cell (HUVECs) were used up to passage three.
MTT (3‐(4,5‐Dimethylthiazol‐2‐yl)‐2,5‐Diphenyltetrazolium Bromide) Assay
HUVEC and murine VSMCs, both p3‐4, were seeded in gelatin coated 96‐well plates (0.1% gelatin, Sigma, G9391) in complete medium and left to adhere overnight. Then, 125 to 250 to 500 to 1000 µmol/L solutions of GYY4137 were prepared fresh in complete medium and supplemented in 6 replicate wells per cell type. After 2, 6, and 12 hours of incubation with GYY, 10 µL of MTT (5 mg/mL) was added per well and left to incubate for another 4 hours. After 4 hours of MTT incubation, supernatant was removed and replaced with 75 µL of isopropanol/hydrochloride and incubated for 1.5 hours. Using a plate reader, 96‐well plates were then read at an optical density of 590 nm.
Live‐Cell Imaging and Analysis
HUVEC and murine VSMCs, both p3‐4, were seeded in gelatin‐coated, tissue‐cell treated 96‐well plates in complete medium and left to adhere overnight. Then, 125 to 250 to 500 to 1000 µmol/L solutions of GYY4137 were prepared fresh in complete medium. For both VSMCs and HUVECs, propidium iodide (PI) (1:200) was added to the GYY containing medium and supplemented in duplicate wells. Live imaging was performed using a fluorescence microscope with an automated stage and temperature and CO2 control (Leica AF6000) taking pictures every 15 minutes at 2 locations/well. The number of cells positive for PI was then counted per image and totaled per well.
In‐Vitro Transmigration Assay
VSMCs were serum starved overnight (0.5% CCS) and seeded in the apical chamber of 6.5 mm Transwell inserts with 8 µm pore polyester membranes (Corning, cat#3464). Before seeding 5×104 cells in DMEM per apical chamber, apical membranes were coated with collagen type 1 (Thermo Fisher Scientific, A1048301). VSMCs were seeded with or in absence of 250 µmol/L GYY. In a separate experiment, VSMCs were preincubated with 250 µmol/L GYY for 6 hours before seeding. The bottom chamber contained serum‐free DMEM with/without 10 ng/mL platelet‐derived growth factor‐BB (PDGF‐BB, R&D Systems, cat# 520‐BB‐050). After 6 hours incubation, apical and bottom chambers were washed with PBS twice and fixed in 4% paraformaldehyde (PFA, Chemcruz, cat# sc‐281692) for 10 minutes at RT. Next, inserts were washed twice in PBS and incubated with DAPI for 10 minutes at RT in the dark. After washing with PBS, membranes were cut out from inserts and mounted on slides. Per insert, 8 images were taken at 20× magnification, number of migrated VSMCs were then counted with ImageJ and normalized per mm2.
Immunocytochemistry
VSMCs were first grown on gelatin‐coated slides (0.1% gelatin, Sigma, G9391) till near‐confluency then fixed with 4% PFA, washed twice in PBS and permeabilized with 0.01% Triton and incubated with 0.1% bovine serum albumin in PBS for 45 minutes. After removal of the blocking reagent, slides where incubated with SMC‐α primary antibody (mouse anti‐mouse, Abcam, ab7817, 1:400 dilution in 0.1% BSA/PBS) o/n at 4°C. After 3 washes with PBS, slides were then stained with Alexa Fluor 647 (goat anti‐mouse, A‐32728, 1:600 dilution in BSA/PBS) for 2 hours at RT. After 2 washes with PBS, slides were incubated with DAPI mounting medium and covered with coverslips. Slides were imaged by confocal microscope.
Ex‐Vivo Measurement of H2S
To establish the release profile of GYY/NaHS ex vivo, GYY (250 µmol/L, 1 mmol/L) and NaHS (1 mmol/L) were dissolved in 3 mL PBS and incubated at 37°C. At various time points, solutions were sampled and incubated with 0.25 µmol/L SF7‐AM (Tocris, cat# 4943) for 30 minutes at 37°C in the dark in a glass bottom 96‐well plate (Cellvis, cat# P96‐0‐N). After incubation, plate was imaged with a GE Typhoon FLA 9500 Laser Scanner (GE Healthcare, cat# 15342) and mean fluorescent intensity of each well was measured with ImageJ.
H2S Release by GYY in a 40% Pluronic Gel
To assess the release profile of GYY while dissolved in a 40% Pluronic gel. PBS, GYY (1 mmol/L, 250 µmol/L) and NaHS (1 mmol/L, Sigma, cat# 161527) were dissolved in 3 mL 40% Pluronic gel in separate wells of a 6‐well plate, and incubated at 37°C. After gel was solidified, each gel was overlaid with 3 mL of sterile PBS. At various time points, supernatant was sampled and incubated with 0.25 µmol/L SF7‐AM for 30 minutes at 37°C in the dark in a glass 96‐well plate and imaged with a GE Typhoon laser scanner. Starting at 12 hours after first overlaying the Pluronic gel with PBS, supernatant was removed every 24 hours and renewed with fresh 3 mL PBS.
H2S Release After Local GYY Treatment
C57BL/6 mice (n=4/group) were anesthetized via 5% isoflurane induction then switched to 2% to 3% isoflurane. Midline neck incision was performed and the right and left CCA were exposed via surgical manipulation. Both arteries were then enveloped with the same Pluronic gel with or without 250 µmol/L GYY. After 30 seconds incubation, midline incision was closed with 6/0 Vicryl suture and postoperatively mice received warm lactate ringer solution (0.5 mL, subcutaneous) and buprenorphine (0.1 mg/kg, subcutaneous). Twenty‐four hours after surgery, mice were anesthetized, and the wound bed was reopened. THen, 100 µL of gel remnant visible in the wound bed was collected and incubated with 0.25 µmol/L SF7‐AM for 30 minutes in a 96‐well plate. Mice were then euthanized via cervical dislocation. After incubation, plates were imaged with the GE Typhoon laser scanner, wells were then analyzed with ImageJ and corrected for background signal (PBS+0.25 µmol/L SF7‐AM).
Statistical Analysis
Data are expressed as Mean±SD. Normality testing was performed with a Shapiro‐Wilk normality test. Student’s t test, 1‐way or 2‐way analysis of variance was used to analyze normally distributed data. Nonnormally distributed data were analyzed by Mann–Whitney test or Kruskal–Wallis test. All statistical analyses were performed with Graphpad (8.12).
Results
Systemic Therapy With the H2S Donor GYY Limits Vein Graft Disease
To test the effectiveness of exogenous H2S in vein graft surgery, we first supplemented 250 µmol/L GYY to the drinking water of C57BL/6 mice, either throughout the 7 days preceding vein graft surgery (pre‐GYY) or starting immediately after surgery and continuing until harvest at POD 28 (post‐GYY). Figure 1A exemplifies the experimental design and Figure 1B outlines the vein graft procedure, which encompasses the transplantation of a donor caval vein in a recipient RCCA via an end‐to‐end anastomosis. At POD 28, all mice were anesthetized and then euthanized via exsanguination, vein grafts were harvested and processed for histology. Figure 1C shows representative histology images of vein graft cross‐sections from control, pre‐GYY, and post‐GYY groups after Masson‐trichome staining at POD 28.
Figure 1. Systemic therapy with the H2S donor GYY limits vein graft disease.
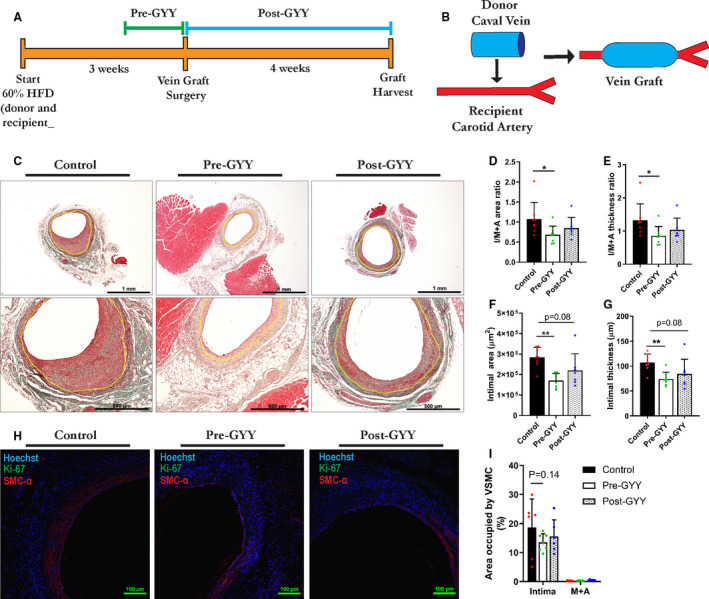
A, Experimental design: 60% high‐fat diet (HFD), 1 week of preconditioning (pre‐GYY) or 4 weeks of postconditioning (post‐GYY) vs control. B, Schematic depiction of the vein graft procedure: end‐to‐end anastomosis of a donor mouse caval vein into a recipient mouse right common carotid artery. C, Representative images of control, pre‐GYY, and post‐GYY vein grafts harvested at POD 28 after Masson‐trichome staining, with yellow lining highlighting the intimal‐M+A border. Scale bars=1 mm or 500 µm as indicated. D through G, Morphometric analysis of vein grafts at POD 28 in control, pre‐GYY, and post‐GYY treated mice as indicated; n=7 to 8/group. Multiple comparisons are via Kruskal‐Wallis test with Dunn’s multiple comparisons test unless indicated otherwise. D, I/M+A area ratios. E, I/M+A thickness. F, Intimal area. G, Intimal thickness. F through G, One‐way analysis of variance with Dunnett’s multiple comparisons test. H, Vein grafts after VSMC+Ki‐67 IHC. Scale bars=100 µm as indicated. I, Percentage of intimal and M+A layers occupied by VSMCs; n=6–7/group; via 2‐way analysis of variance with Tukey’s multiple comparisons test. Data represented as Mean±SD. *P<0.05, **P<0.01. GYY indicates hydrogen sulfide prodrug; I/M+A, intimal/media+adventitia; IHC, immunohistochemistry; POD, postoperative day; and VSMC, vascular smooth muscle cell.
Pretreatment with GYY decreased the I/M+A area and thickness ratios with 36.5% and 35.8% respectively (Figure 1D through E) compared with control mice, whereas there was no difference in control versus post‐GYY area and thickness ratios (Figure 1D through E). In concurrence with a decrease in area and thickness ratios, intimal area (Figure 1F), and thickness (Figure 1G) were diminished in the pre‐GYY group. Although there was a trend toward decreased intimal area and thickness in the post‐GYY group (Figure 1F through G). In both treatment groups, there was no difference in lumen area (Figure S1A), M+A area (Figure S1B) and thickness (Figure S1C). Further analysis of the VSMC content of pre‐ and postconditioned vein grafts (Figure 1H) hinted toward decreased intimal VSMC infiltration in the pre‐GYY group (Figure 1H through I). Interestingly, in the post‐GYY group there was increased M+A collagen content (Figure S1D) and a trend toward increased overall collagen content, signifying an outward remodeling response.
Taken together these data suggest a significant beneficial effect for systemic GYY treatment in attenuating VGD, with preconditioning yielding an additional beneficial response compared with postconditioning.
Development of a Locally Applicable Pluronic Gel that Ensures Extended H2S Release Both Ex Vivo and In Vivo
Because of potential translational barriers associated with preconditioning for extended time periods before surgery, we next explored the potential local use of GYY at the time of surgery to prevent potentially dangerous side effects associated with excess H2S. To understand the kinetics of local delivery of different sulfide donors we first tested the slow and extended release qualities of different sulfide donors ex vivo by dissolving the well‐known sulfide donor sodium hydrosulfide (NaHS) at 1 mmol/L in PBS as a positive control, and GYY at 1 mmol/L and 250 µmol/L respectively. 28
To first compare the presence of free H2S at various time points after dissolving slow‐ and fast‐releasing H2S‐donors, we dissolved GYY and NaHS in PBS and took a 100µL sample at regular intervals. This sample was placed in a 96‐well plate and free H2S was then measured by adding the H2S‐binding molecular probe SF7‐AM. 31 As expected, PBS‐dissolved GYY proved capable of extended release of H2S molecules up until 1 day after dissolvement, whereas NaHS‐derived H2S release diminished after 6 hours (Figure 2A). After the 24‐hour time point there was no GYY/PBS present due to evaporation. In the same experiment, we compared the sulfide release rate of both donors, by a repeat measurement of the T=0 sample, after SF7‐AM was added. We found that GYY maintained a steady and continuous release of H2S molecules, as shown by a slow increase in intensity of the SF7‐AM signal, whereas the H2S molecules released by NaHS rapidly saturated the SF7‐AM signal (Figure 2B).
Figure 2. Development of a locally applicable Pluronic gel that ensures extended H2S release both ex‐vivo and in‐vivo.
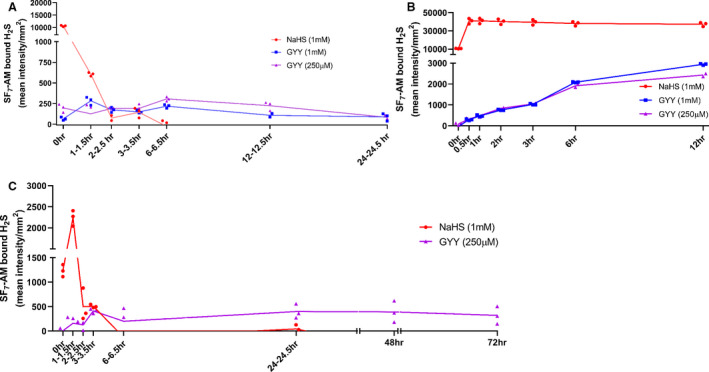
A through C, All samples were incubated with 0.25 µmol/L SF7‐AM in a 96‐well plate in triplicates for 30 minutes at 37°C and then imaged. Resulting fluorescent intensity was corrected for background signal with a PBS+SF7‐AM control. A, NaHS (1 mmol/L), GYY (1 mmol/L, 250 µmol/L), and PBS dissolved in equal volume PBS, H2S release was measured at regular intervals. B, Repeated measurement of same wells in 96‐wells plate to assess release rate of respective H2S releasing compounds. C, H2S‐drugs dissolved in equal volume of 40% Pluronic gel and overlaid with equal volume PBS. At regular intervals H2S release in PBS supernatant was measured. GYY indicates hydrogen sulfide prodrug; H2S, hydrogen sulfide; NaHS, sodium hydrosulfide; and SF7‐AM, fluorescent probe binding free hydrogen sulfide.
Next, to enable periprocedural local sustained and targeted release, we tested whether dissolving GYY in a 40% Pluronic gel would extend the release profile of GYY. We dissolved PBS, GYY, or NaHS in 40% Pluronic gel at 37°C, then overlaid the gel with PBS. At regular intervals, the supernatant was tested for free H2S by incubation with the SF7‐AM probe. Measurements were continued until the Pluronic gel was dissolved in supernatant PBS and consecutively evaporated. Interestingly, we were able to extend the release profile of GYY from 24 to 72 hours by utilizing Pluronic gel as a vehicle, while NaHS was fully released from the gel at 6 hours postdissolvement (Figure 2C).
Finally, we evaluated the capability of this GYY gel to release H2S in vivo by enveloping the left and right common carotid arteries (LCCA/RCCA) in the 40% Pluronic gel with/without 250 µmol/L GYY. At 24 hours postsurgery, we tested for H2S release from the gel remnants surrounding the LCCA/RCCA. Although sampling was hampered by high background signal because of the presence of blood, there was a trend for an increase in fluorescent intensity after incubation with SF7‐AM in the local‐GYY group (1±0.37 versus 1.791±0.72, n=4/group, P=0.1) supporting extended H2S release in vivo.
Local Application of the H2S Donor GYY Attenuates the Arterial Fibroproliferative Response to Injury
To determine the effectiveness of local therapy with GYY during a vascular procedure, we first tested our GYY gel in a model of arterial hyperplasia and vascular remodeling. Figure 3A illustrates the employed surgical model of focal stenosis of the RCCA in order to create proximal arterial hyperplasia and remodeling, and Figure 3B outlines the experimental design.
Figure 3. Local application of the H2S donor GYY mitigates injury‐induced arterial intimal hyperplasia.
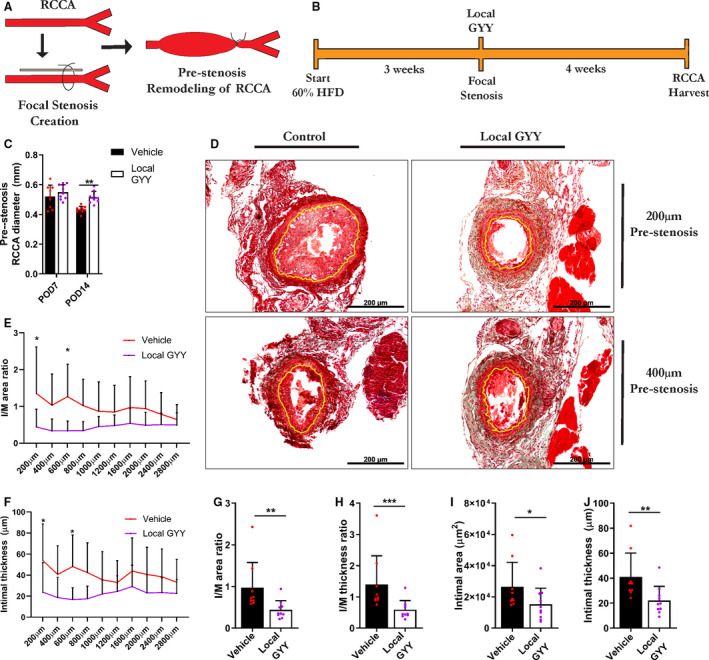
A, Schematic depiction of surgical procedure, with partial ligation of the RCCA and resulting remodeling proximal from the stenosis. B, Experimental outline. C, Prestenosis diameter of RCCA at POD 7 and POD 14 in vehicle and local GYY treated animals, via 2‐way analysis of variance, n=9 to 10/group. D, Masson‐Trichome staining of RCCA cross‐sections at POD 28 after focal stenosis, with yellow lining indicating the intima‐media border. Scale bars are 200 µm as indicated. E through F, Measurement of I/M area and thickness ratio respectively at regular intervals proximal of stenosis at POD 28. G through H, Morphometric analysis of prestenosis RCCA, via Mann–Whitney test, n=9 to 10/group. G, I/M area. H, I/M thickness ratio. I, Intimal area. J, Intimal thickness. Data represented as Mean±SD. *P<0.05, **P<0.01, ***P<0.001. GYY indicates hydrogen sulfide prodrug; HFD, high‐fat diet; H2S, hydrogen sulfide; I/M, intimal/media; POD, postoperative day; and RCCA, right common carotid artery.
Interestingly, at POD 14 there was an increase in RCCA diameter proximal from the stenosis in the GYY group, as measured by ultrasound (Figure 3C). After harvesting the RCCA at POD 28, a Masson‐trichome staining was performed with representative images in Figure 3D at 200 µm and 400 µm proximal from the stenosis. Figure 3E and 3F visualize the remodeling response at regular distance intervals prestenosis, per I/M area ratio and intimal area respectively. When averaged, the group treated with local GYY showed decreased I/M area and thickness (Figure 3G through H) ratios and decreased intimal thickness (Figure 3J), and there was a trend for decreased intimal area (Figure 3I). Local treatment with GYY had no significant effect on lumen area (Figure S2A), medial area (Figure S2B), or medial thickness (Figure S2C).
Thus, local therapy was effective in mitigating the remodeling response to vascular surgical injury.
Periprocedural H2S Therapy Protects from Vein Graft Disease and Attenuates Intimal VSMC Migration
Consequently, we set out to test whether this intervention could be advantageous in attenuating VGD and intimal hyperplasia. As outlined in Figure 4A, we next applied our GYY gel locally during the bypass procedure. During the procedure, the vein graft was enveloped with Pluronic gel (vehicle or GYY), just before the vascular clamps were opened. Interestingly, at POD 14 and POD 28, vein grafts treated with GYY showed a sustained increase in vein graft diameters in vivo compared with vehicle‐treated grafts, as measured by ultrasound (Figure 4B).
Figure 4. Periprocedural local H2S therapy protects from vein graft disease by attenuating intimal VSMC migration.
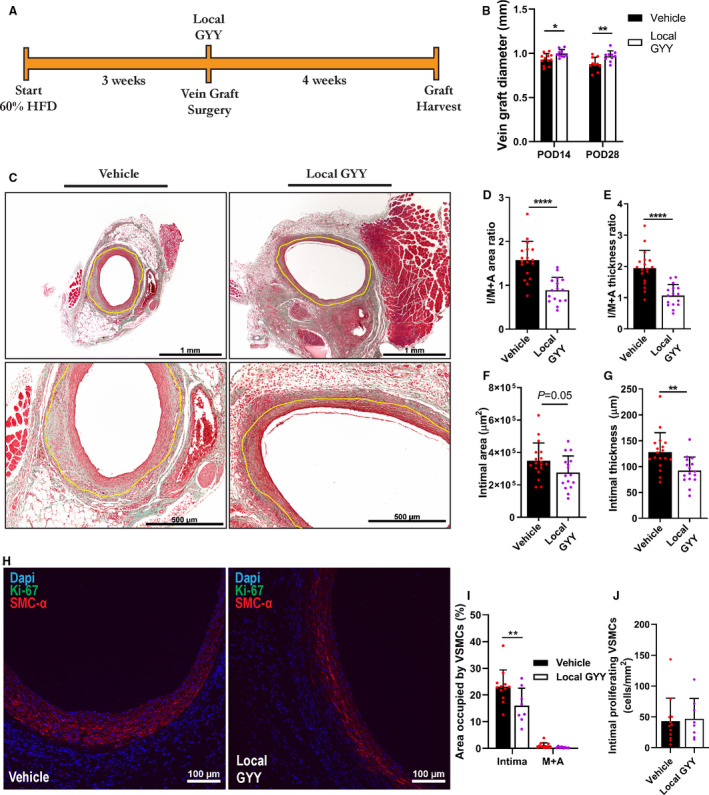
A, Experimental outline with local GYY application during vein graft surgery. B, Ultrasound measurements of vein graft diameters at POD 14 and POD 28 by 2‐way analysis of variance with Sidak’s multiple comparisons test; n=10 to 12/group. C, Representative images of locally treated vein grafts at POD 28 after Masson‐trichome staining. Yellow lining indicating the intima‐media+adventitia border. Scale bars 1 mm/500 µm as indicated. D through G, Morphometric analysis of vein grafts at POD 28, n=16 to18/group, Student’s t test was performed unless indicated otherwise. D, I/M+A area ratios. E, I/M+A thickness ratios F, Intimal area. G, Intimal thickness. H, IHC for SMC‐α+Ki‐67. Scale bars are 100 µm as indicated. I, Percentage of area occupied by SMC‐α positive cells in intimal and M+A layers, via 2‐way analysis of variance with Sidak’s multiple comparisons test; n=8 to 12/group. J, Intimal colocalization of VSMC and Ki‐67. Data represented as Mean±SD. *P<0.05, **P<0.01, ****P<0.0001. GYY indicates hydrogen sulfide prodrug; HFD, high‐fat diet; H2S, hydrogen sulfide; I/M+A, intimal/media+adventitia; IHC, immunohistochemistry; POD, postoperative day; SMC‐α, smooth muscle cell‐α; and VSMC, vascular smooth muscle cell.
After graft harvest at POD 28 and consecutive histology, the local GYY group had increased luminal graft diameters (Figure 4C, representative images), confirmed by an increase in corrected luminal area (Figure S3A), which correlated with the in‐vivo ultrasound measurements on an individual mouse basis (Figure S3B). After quantification of the vein graft wall, we found that local GYY treatment decreased I/M+A area ratios with 56.7% (Figure 4D) and resulted in a 44.8% reduction in I/M+A thickness ratios (Figure 4E), signifying a strong attenuation of VGD after periprocedural treatment with GYY. Intimal area (Figure 4F) and thickness (Figure 4G) were also decreased combined with an increase in M+A area (Figure S3C) and thickness (Figure S3D). Overall, this pointed toward improved and beneficial adaption of the vein graft to its new arterial environment, as was shown by a limited inward remodeling response (Figure 4B through G) and increased outward remodeling (Figure S3C through Figure S3D) after local GYY treatment.
A major hallmark of VGD and consecutive graft failure, is the proliferation and migration of VSMCs from medial and adventitial layers of the vein conduit into the intimal layer. 5 Interestingly, at POD 28 there was a 30.6% decrease in intimal VSMCs (Figure 4H) in the local GYY group (Figure 4I) whereas there was no difference in proliferating VSMCs at this post‐operative time point (Figure 4J), nor was there a difference in collagen deposition (Figure S3E). This pointed toward attenuation of VSMC migration by local GYY treatment as an underlying mechanism for its therapeutic effect; therefore, we examined the effects of GYY on VSMCs in vitro.
Periprocedural H2S Therapy Limits VSMC Migration and Proliferation In Vitro
To further investigate this phenotype, we isolated VSMCs from murine aortas and confirmed their origin by immunocytochemistry with alpha‐SMC (Figure 5A). Intracellular H2S levels were measured with SF7‐AM upon 6‐hour GYY stimulation (Figure 5B). Next, we tested whether GYY treatment would impair VSMC migration in vitro by either preconditioning VSMCs with GYY (6 hours, 250 µmol/L GYY) or stimulate them with GYY for the duration of the Boyden chamber assay (6 hours). Interestingly, VSMC transmigration was limited both by preconditioning (Figure 5, 6, 6 hours preconditioning) and costimulating (Figure 5C and 5D peri‐GYY) PDGF‐BB stimulated VSMCs.
Figure 5. Periprocedural H2S therapy limits VSMC migration and proliferation in vitro.
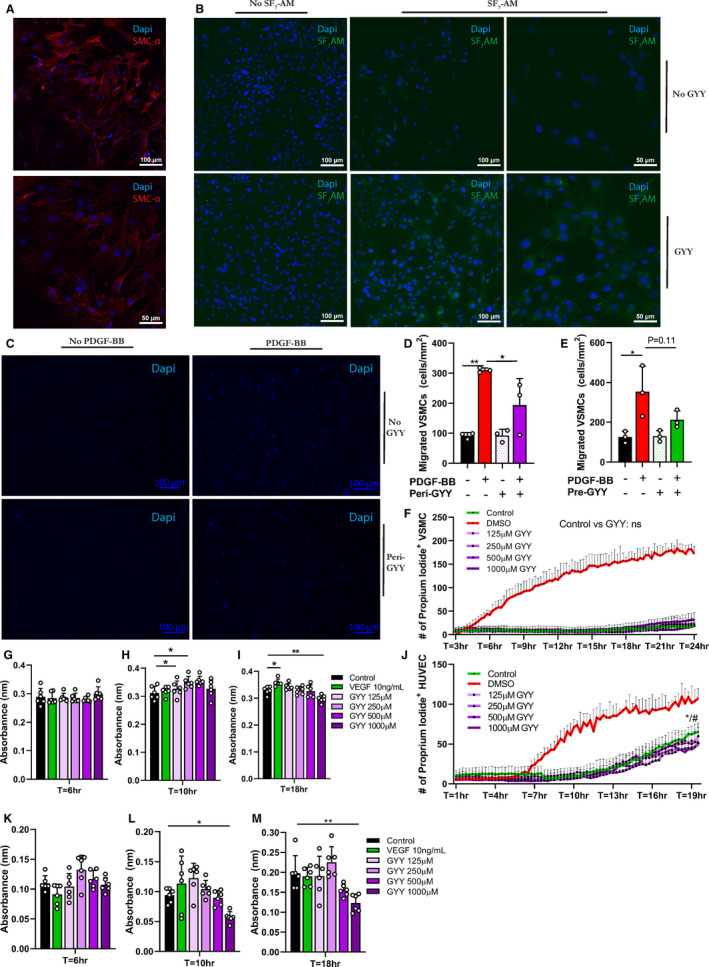
A, Representative immunocytochemistry image (20× & 40× magnification) of primary VSMC SMC‐α staining. Scale bars 100 and 50 µm as indicated. B, VSMCs after 6 hours treatment with 250 µmol/L GYY, with/without SF7‐AM incubation (20× & 40× magnification). Scale bars 100 and 50 µm as indicated. C, VSMCs after Transwell migration assay (6 hours in presence or absence of PDGF‐BB and/or 250 µm GYY). D, Quantification of Transwell migration assay. Number of migrated VSMCs normalized to per mm2 after 6 hours PDGF‐BB and/or 250 µmol/L GYY incubation in triplicates via 1‐way analysis of variance with Sidak’s multiple comparisons test. E, Quantification of Transwell migration assay after 6 hours of PDGF‐BB incubation and/or 6 hours of 250 µmol/L GYY pretreatment. F, Live imaging of VSMC positive for PI during incubation with 0 to 1000 µmol/L GYY or DMSO. Number of PI+ VSMC in GYY versus control via 2‐way analysis of variance with Dunnett’s multiple comparison test. G through I, MTT assay of VSMC incubated with 0 to 1000 µmol/L GYY or VEGF (10 ng/mL), 6 replicates per condition. One‐way analysis of variance with Dunnett’s multiple comparison test. J, Live imaging of HUVECs positive for PI during incubation with 0 to 1000 µmol/L GYY or DMSO. Number of PI‐positive HUVEC in GYY versus control via 2‐way analysis of variance with Dunnett’s multiple comparison test. */#: significant decrease in PI+ HUVECs supplemented with 250 µmol/L and 1000 µmol/L GYY respectively compared with control at T=18 to 19 hour. K through M, MTT assay of HUVECs incubated with 0 to 1000 µmol/L GYY or VEGF (10 ng/mL), 6 replicates per condition. One‐way analysis of variance with Dunnett’s multiple comparison test. Data represented as Mean±SD. *P<0.05, **P<0.01. DMSO indicates dimethyl sulfoxide; GYY, hydrogen sulfide prodrug; H2S, hydrogen sulfide; HUVEC, human umbilical endothelial cells; MTT, 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide; PDGF‐BB, platelet‐derived growth factor; PI, proprium iodide; SMC‐α, smooth muscle cell‐α; VEGF, vascular endothelial growth factor; and VSMC, vascular smooth muscle cell.
Figure 6. Periprocedural local hydrogen sulfide therapy limits vein graft disease.
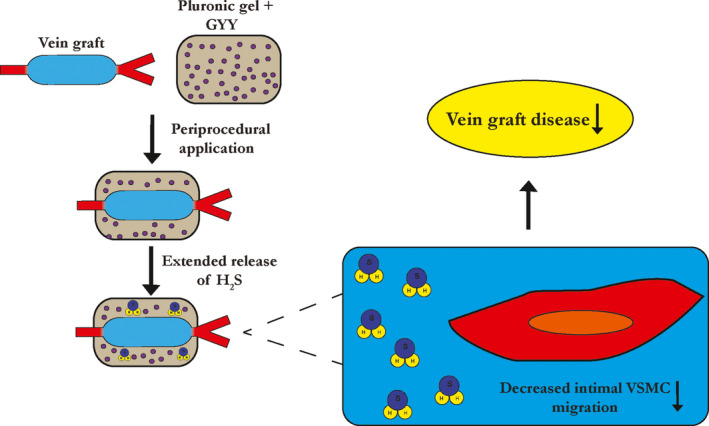
GYY indicates hydrogen sulfide prodrug; H2S, hydrogen sulfide; VSMC, vascular smooth muscle cell.
To rule out cell death as an explanation for limited VSMC migration during/after GYY stimulation and to test the toxicity of our compound, we next performed live imaging on VSMC stained with PI during GYY supplementation in various concentrations. Most important, 250 µmol/L did not increase cell death, but of note was that even 1000 µmol/L of GYY did not prove toxic for VSMCs (Figure 5F). Because GYY did not have any detectable toxic effects on VSMC we next assessed whether it influenced metabolic activity/proliferation via an MTT assay. Proliferation was not different after short incubations with GYY (Figure 5G), and medium length incubation slightly amplified VSMC proliferation (Figure 5H). At the 18‐hour time point, however, GYY limited VSMC proliferation, especially at higher concentrations (Figure 5I).
Lastly, because endothelial cells, next to VSMC, play an important role in both early and late vascular remodeling, 32 we repeated our analysis of the toxicity and metabolic effects of GYY but then on HUVECs. Interestingly, live imaging with PI revealed a slight increase in cell survival compared with control conditions in HUVECs supplemented with 250 µmol/L or 1000 µmol/L GYY at T=18 to 19 hours (Figure 5J), indicative of a potential cytoprotective effect of GYY on endothelial cells. Further analysis of HUVEC proliferation via MTT assays (Figure 5K) and 5M) showed reduced metabolic activity/proliferation after longer periods of incubation (T=18 hour) and high (1000 µmol/L) GYY concentration (Figure 5M).
Taken together, these data suggest that a single local periprocedural administration of the H2S donor GYY is able to attenuate VGD and that this is at least partly through limiting VSMC migration and proliferation. Benefits from local GYY therapy in vascular remodeling could, parallel to limited VSMC migration/proliferation, possibly extend to increased cell survival and cytoprotection in endothelial cells.
Discussion
The prospect of translating short‐term dietary preconditioning strategies into everyday surgical practice appears appealing as such approaches have been tested in certain patient populations. For example, in healthy kidney donors scheduled for transplant surgery, a 2‐week protein‐calorie restriction diet was proven feasible in terms of compliance. 33 Furthermore, just 1 week of preoperative caloric restriction diet was able to reduce intraoperative blood loss in patients undergoing liver resection. 34 , 35 In the vascular surgery patient population, however, with a predisposition toward unhealthy lifestyle choices and increased metabolic disease even a short‐term change in dietary patterns may prove challenging from a DR‐compliance perspective. In our recent pilot study only about 10% of appropriate patients were able to undergo a defined 3‐day dietary intervention before elective vascular surgery. 24
Here we established a promising new approach to mitigate graft failure after bypass surgery by local delivery of the H2S donor GYY during the vein graft procedure. This local periprocedural therapy would circumvent the need for preconditioning via DR or pharmacological therapy (with H2S donors) and increase the potential for clinical translation. A 1‐time periprocedural application of this H2S gel onto the graft proved sufficient to limit inward graft remodeling, while simultaneously improving vein graft outward adaptations. Furthermore, even when compared with systemic pre‐ or postconditioning with GYY, local application had increased efficacy in attenuating VGD. Protection from VGD and vein intimal hyperplasia notwithstanding, our H2S gel was additionally able to reduce arterial hyperplasia and improve arterial remodeling in a mouse model of arterial focal stenosis, thereby bolstering the potential of this intervention beyond vein graft surgery, to extend toward both open and endovascular arterial procedures.
Previous research found that systemic treatment with H2S donors, via IP injection, attenuated arterial remodeling after balloon angioplasty. 36 In a follow‐up study the same group showed decreased VSMC proliferation and migration in vitro as a result of exogenous H2S therapy, due to downregulation of matrix‐metalloproteinase‐2. 22 And recently, a slow‐releasing H2S‐peptide gel was developed that limited VSMC migration in vitro and ex vivo in transplanted human vein graft segments. 37 Here we show that local (exogenous) H2S therapy limits intimal VSMC migration in vivo, which in turn contributes to protection from graft failure. (Figure 6) This supports our previous work on the role of H2S in vein grafts, where we found that endogenous upregulation of H2S (via DR) protected from VGD, also partly via inhibition of the fibroproliferative response. 23 Whether exogenous H2S therapy (and endogenous) also mitigates dedifferentiated VSMC migration or is involved in VSMC phenotype switching, both important hallmarks in the intimal hyperplastic response, 38 remains to be determined in future studies. However, H2S is also known for its anti‐inflammatory properties. 18 For example, systemic H2S therapy decreased circulating tissue necrosis factor‐α after transient aortic occlusion, implying a decrease in the inflammatory response after vascular injury. 39 It is likely that increased locally available H2S not only directly inhibits VSMC migration but also indirectly, by blocking the influx and activation of immune cells.
A possible concern in exogenous H2S supplementation is its narrow therapeutic window and the toxicity of H2S in high concentrations. 40 We therefore opted for a slow‐releasing H2S donor (GYY4137) as opposed to the more conventional exogenous H2S treatment with the fast‐releasing H2S donor NaHS. Because GYY continuously releases free H2S molecules in low concentrations, we circumvented any potential issues of toxicity via oral administration. Furthermore, GYY being a prodrug, its parent molecule ZYJ112 has not shown any biological (or adverse) effects in vitro or in vivo, 41 which further reinforces the potential of GYY as a safe candidate for (oral) exogenous H2S treatment.
Despite the translational promise this work holds, there are several limitations to be acknowledged. First, this intervention was tested only on vein grafts and arteries at 28 days postsurgery, because in this model long‐term protection cannot be established. Second, although we established protection from VGD at POD 28, the effects of GYY during early vein graft remodeling are unknown. We hope that this work will incite future research on the effects of local H2S therapy on the different cell types (VSMC, endothelial cells, leukocytes) that play a major role in facilitating or accelerating this remodeling response. Third, only a single concentration of GYY was tested, therefore the most efficacious and optimal dose of GYY in VGD/arterial remodeling is unknown. Lastly, although a high‐fat diet was employed to mimic the vascular surgery patient population, other factors were not accounted for (age, sex, underlying comorbidities).
In short, we developed and tested a H2S releasing gel that can be applied locally during the procedure and that is capable of attenuating both vein graft failure and arterial remodeling. Future directions should focus on exploring its potential in vascular access surgery and endovascular interventions.
Sources of Funding
This work was supported by an American Heart Association Post‐Doctoral Grant [#19POST34400059] and grants from Foundation ‘De Drie Lichten’, Prins Bernhard Cultural Foundation and Michael‐van Vloten Foundation to P.K.; the Harvard‐Longwood Research Training in Vascular Surgery NIH T32 Grant 5T32HL007734‐22 to KT; American Heart Association Grant‐in‐Aid 16GRNT27090006; National Institutes of Health, 1R01HL133500 to C.K.O.; and NIH (AG036712, DK090629) and Charoen Pokphand Group to J.R.M.
Disclosures
None.
Supporting information
Figures S1–S3
Acknowledgments
We thank M.L. van der Bent for her expertise and support with live imaging of primary cells. Author Contributions: P.K. provided funding, conceived of experimental designs, performed experiments and wrote the article. M.T. performed surgeries, processed vein grafts and analyzed histology. M.R.V. conceived of experimental designs, performed surgeries and advised with P.H.Q. on analysis of vein grafts. K.T., M.R.M. and S.J.M. assisted with animal care and in‐vivo studies. C.M., J.W.J., H.A.B, M.L.B and T.S. assisted with ex‐vivo and in‐vitro experiments. S.P. assisted with histomorphometric analysis of vein grafts. J.R.M. and C.K.O. provided funding, conceived of experimental designs and supervised the project.
(J. Am. Heart Assoc. 2020;9:e016391 DOI: 10.1161/JAHA.120.016391.)
For Sources of Funding and Disclosures, see page 15.
References
- 1. Almasri J, Adusumalli J, Asi N, Lakis S, Alsawas M, Prokop LJ, Bradbury A, Kolh P, Conte MS, Murad MH. A systematic review and meta‐analysis of revascularization outcomes of infrainguinal chronic limb‐threatening ischemia. J Vasc Surg. 2018;624–633. [DOI] [PubMed] [Google Scholar]
- 2. Klinkert P, Schepers A, Burger DHC, Bockel JHV, Breslau PJ. Vein versus polytetrafluoroethylene in above‐knee femoropopliteal bypass grafting: five‐year results of a randomized controlled trial. J Vasc Surg. 2003;149–155. [DOI] [PubMed] [Google Scholar]
- 3. Conte MS, Bandyk DF, Clowes AW, Moneta GL, Seely L, Lorenz TJ, Namini H, Hamdan AD, Roddy SP, Belkin M, et al. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg. 2006;742–751.e1. [DOI] [PubMed] [Google Scholar]
- 4. de Vries MR, Simons KH, Jukema JW, Braun J, Quax PHA. Vein graft failure: from pathophysiology to clinical outcomes. Nat Rev Cardiol. 2016;451–470. [DOI] [PubMed] [Google Scholar]
- 5. de Vries MR, Quax PHA. Inflammation in Vein Graft disease. Front Cardiovasc Med. 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nguyen B, Tao M, Yu P, Mauro C, Seidman MA, Wang YE, Mitchell J, Ozaki CK. Pre‐operative diet impacts the adipose tissue response to surgical trauma. Surgery. 2013;584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peng W, Robertson L, Gallinetti J, Mejia P, Vose S, Charlip A, Chu T, Mitchell JR. Surgical stress resistance induced by single amino acid deprivation requires Gcn2 in mice. Sci Transl Med. 2012;118ra11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mitchell JR, Verweij M, Brand K, van de Ven M, Goemaere N, van den Engel S, Chu T, Forrer F, Muller C, de Jong M, et al. Short‐term dietary restriction and fasting precondition against ischemia reperfusion injury in mice. Aging Cell. 2010;40–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Robertson LT, Treviño‐Villarreal JH, Mejia P, Grondin Y, Harputlugil E, Hine C, Vargas D, Zheng H, Ozaki CK, Kristal BS, et al. Protein and calorie restriction contribute additively to protection from renal ischemia reperfusion injury partly via leptin reduction in male mice. J Nutr. 2015;1717–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Ginhoven TM, Dik WA, Mitchell JR, Smits‐te Nijenhuis MA, van Holten‐Neelen C, Hooijkaas H, Hoeijmakers JH, de Bruin RW, IJzermans JNM. Dietary restriction modifies certain aspects of the postoperative acute phase response. J Surg Res. 2011;582–589. [DOI] [PubMed] [Google Scholar]
- 11. Verweij M, van Ginhoven TM, Mitchell JR, Sluiter W, van den Engel S, Roest HP, Torabi E, Ijzermans JN, Hoeijmakers JH, de Bruin RW. Preoperative fasting protects mice against hepatic ischemia/reperfusion injury: mechanisms and effects on liver regeneration. Liver Transplant. 2011;695–704. [DOI] [PubMed] [Google Scholar]
- 12. Varendi K, Airavaara M, Anttila J, Vose S, Planken A, Saarma M, Mitchell JR, Andressoo JO. Short‐term preoperative dietary restriction is neuroprotective in a rat focal stroke model. PLoS One. 2014;e93911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mauro CR, Tao M, Yu P, Treviño‐Villarreal H, Longchamp A, Kristal BS, Ozaki CK, Mitchell JR. Pre‐operative dietary restriction reduces intimal hyperplasia and protects from ischemia reperfusion injury. J Vasc Surg. 2016;500–509.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Longchamp A, Mirabella T, Arduini A, MacArthur MR, Das A, Treviño‐Villarreal JH, Hine C, Ben‐Sahra I, Knudsen NH, Brace LE, et al. Amino acid restriction triggers angiogenesis via GCN2/ATF4 regulation of VEGF and H2S production. Cell. 2018;117–129.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Trocha K, Kip P, MacArthur MR, Mitchell SJ, Longchamp A, Treviño‐Villarreal JH, Tao M, Bredella MA, De Amorim BK, Mitchell JR, et al. Preoperative protein or methionine restriction preserves wound healing and reduces hyperglycemia. J Surg Res. 2019;216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hine C, Harputlugil E, Zhang Y, Ruckenstuhl C, Lee BC, Brace L, Longchamp A, Trevino‐Villarreal JH, Mejia P, Ozaki CK, et al. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell. 2015;132–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev. 2012;791–896. [DOI] [PubMed] [Google Scholar]
- 18. Kanagy NL, Szabo C, Papapetropoulos A. Vascular biology of hydrogen sulfide. Am J Physiol ‐ Cell Physiol. 2017;C537–C549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bibli SI, Hu J, Sigala F, Wittig I, Heidler J, Zukunft S, Tsilimigras DI, Randriamboavonjy V, Wittig J, Kojonazarov B, et al. Cystathionine gamma Lyase sulfhydrates the RNA binding protein human antigen r to preserve endothelial cell function and delay atherogenesis. Circulation. 2019;101–114. [DOI] [PubMed] [Google Scholar]
- 20. Hine C, Harputlugil E, Zhang Y, Ruckenstuhl C, Lee BC, Brace L, Longchamp A, Trevino‐Villarreal JH, Mejia P, Ozaki CK, et al. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell. 2015;132–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kondo K, Bhushan S, King AL, Prabhu SD, Hamid T, Koenig S, Murohara T, Predmore BL, Gojon G, Wang R, et al. H(2)S Protects against pressure overload induced heart failure via upregulation of endothelial nitric oxide synthase (eNOS). Circulation. 2013;1116–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang G, Li H, Tang G, Wu L, Zhao K, Cao Q, Xu C, Wang R. Increased neointimal formation in cystathionine gamma‐lyase deficient mice: role of hydrogen sulfide in alpha5beta1‐integrin and matrix metalloproteinase‐2 expression in smooth muscle cells. J Mol Cell Cardiol. 2012;677–688. [DOI] [PubMed] [Google Scholar]
- 23. Trocha KM, Kip P, Tao M, MacArthur MR, Trevino‐Villarreal JH, Longchamp A, Toussaint W, Lambrecht BN, de Vries MR, Quax PHA, et al. Short‐term preoperative protein restriction attenuates vein graft disease via induction of cystathionine gamma‐lyase. Cardiovasc Res. 2020;416–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kip P, Trocha KM, Tao M, O'Leary JJ, Ruske J, Giulietti JM, Trevino‐Villareal JH, MacArthur MR, Bolze A, Burak MF, et al. Insights from a short‐term protein‐calorie restriction exploratory trial in elective carotid endarterectomy patients. Vasc Endovasc Surg. 2019;470–476. DOI: 10.1177/1538574419856453 [DOI] [PubMed] [Google Scholar]
- 25. Wallace JL, Wang R. Hydrogen sulfide‐based therapeutics: exploiting a unique but ubiquitous gasotransmitter. Nat Rev Drug Discov. 2015;329–345. [DOI] [PubMed] [Google Scholar]
- 26. Yu P, Nguyen BT, Tao M, Campagna C, Ozaki CK. Rationale and practical techniques for mouse models of early vein graft adaptations. J Vasc Surg. 2010;444–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tao M, Mauro CR, Yu P, Favreau JT, Nguyen B, Gaudette GR, Ozaki CK. A simplified murine intimal hyperplasia model founded on a focal carotid stenosis. Am J Pathol. 2013;277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, Lee SW, Zhao Y, Baskar R, Tan CH, Moore PK. Characterization of a novel, water‐soluble hydrogen sulfide‐releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation. 2008;2351–2360. [DOI] [PubMed] [Google Scholar]
- 29. Yu P, Nguyen BT, Tao M, Bai Y, Ozaki CK. Mouse Vein Graft hemodynamic manipulations to enhance experimental utility. Am J Pathol. 2011;2910–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Patel JJ, Srivastava S, Siow RC. Isolation, culture, and characterization of vascular smooth muscle cells. Methods Mol Biol. 2016;91–105. [DOI] [PubMed] [Google Scholar]
- 31. Lin VS, Lippert AR, Chang CJ. Cell‐trappable fluorescent probes for endogenous hydrogen sulfide signaling and imaging H2O2‐dependent H2S production. Proc Natl Acad Sci USA. 2013;7131–7135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. de Vries MR, Quax PH. Plaque angiogenesis and its relation to inflammation and atherosclerotic plaque destabilization. Curr Opin Lipidol. 2016;499–506. [DOI] [PubMed] [Google Scholar]
- 33. Jongbloed F, de Bruin RWF, Klaassen RA, Beekhof P, van Steeg H, Dor FJMF, van der Harst E, Dollé MET, Ijzermans JNM. Short‐term preoperative calorie and protein restriction is feasible in healthy kidney donors and morbidly obese patients scheduled for surgery. Nutrients. 2016;306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Reeves JG, Suriawinata AA, Ng DP, Holubar SD, Mills JB, Barth RJ Jr. Short‐term preoperative diet modification reduces steatosis and blood loss in patients undergoing liver resection. Surgery. 2013;1031–1037. [DOI] [PubMed] [Google Scholar]
- 35. Barth RJ Jr, Mills JB, Suriawinata AA, Putra J, Tosteson TD, Axelrod D, Freeman R, Whalen GF, LaFemina J, Tarczewski SM, et al. Short‐term preoperative diet decreases bleeding after partial hepatectomy: results from a multi‐institutional randomized controlled trial. Ann Surg. 2019;48–52. [DOI] [PubMed] [Google Scholar]
- 36. Meng QH, Yang G, Yang W, Jiang B, Wu L, Wang R. Protective effect of hydrogen sulfide on balloon injury‐induced neointima hyperplasia in rat carotid arteries. Am J Pathol. 2007;1406–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Longchamp A, Kaur K, Macabrey D, Dubuis C, Corpataux JM, Deglise S, Matson JB, Allagnat F. Hydrogen sulfide‐releasing peptide hydrogel limits the development of intimal hyperplasia in human vein segments. Acta Biomater. 2019;374–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rzucidlo EM, Martin KA, Powell RJ. Regulation of vascular smooth muscle cell differentiation. J Vasc Surg. 2007;A25–A32. [DOI] [PubMed] [Google Scholar]
- 39. Hunter JP, Hosgood SA, Patel M, Furness P, Sayers RD, Nicholson ML. Hydrogen sulfide reduces inflammation following abdominal aortic occlusion in rats. Ann Vasc Surg. 2015;353–360. [DOI] [PubMed] [Google Scholar]
- 40. Zheng Y, Yu B, De La Cruz LK, Roy Choudhury M, Anifowose A, Wang B. Toward hydrogen sulfide based therapeutics: critical drug delivery and developability issues. Med Res Rev. 2018;57–100. [DOI] [PubMed] [Google Scholar]
- 41. Lee ZW, Zhou J, Chen CS, Zhao Y, Tan CH, Li L, Moore PK, Deng LW. The slow‐releasing hydrogen sulfide donor, GYY4137, exhibits novel anti‐cancer effects in vitro and in vivo. PLoS One. 2011;e21077. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1–S3


