Abstract
Background
Cardiovascular safety is an important consideration regarding the benefits versus risks of electronic cigarette use (EC) for public health. The single‐use cardiovascular effects of EC have been well studied but may not reflect effects of ad libitum use throughout the day. We aimed to compare the circadian hemodynamic effects as well as 24‐hour biomarkers of oxidative stress, and platelet aggregation and inflammation, with ad libitum cigarette smoking (CS) versus EC versus no tobacco product use.
Methods and Results
Thirty‐six healthy dual CS and EC users participated in a crossover study in a confined research setting. Circadian heart rate, blood pressure and plasma nicotine levels, 24‐hour urinary catecholamines, 8‐isoprostane and 11‐dehydro‐thromboxane B2, and plasma interleukin‐6 and interleukin‐8 were compared in CS, EC, and no nicotine conditions. Over 24 hours, and during daytime, heart rate and blood pressure were higher in CS and EC compared with no tobacco product conditions (P<0.01). Heart rate on average was higher with CS versus EC. Urinary catecholamines, 8‐isoprostane, and 11‐dehydro‐thromboxane B2 were not significantly different, but plasma IL‐6 and IL‐8 were higher with both CS and EC compared with no tobacco product (P<0.01).
Conclusions
CS and EC had similar 24‐hour patterns of hemodynamic effects compared with no tobacco product, with a higher average heart rate with CS versus EC, and similar effects on biomarkers of inflammation. EC may pose some cardiovascular risk, particularly to smokers with underlying cardiovascular disease, but may also provide a harm reduction opportunity for smokers willing to switch entirely to EC.
Registration
URL: https://www.clinicaltrials.gov; Unique Identifier: NCT02470754.
Keywords: biomarkers, electronic cigarettes, nicotine, tobacco
Subject Categories: Clinical Studies, Biomarkers, Hemodynamics, Inflammation
Nonstandard Abbreviations and Acronyms
- CS
cigarette smoking
- EC
electronic cigarette
Clinical Perspective
What Is New?
The 24‐hour cardiovascular effects of ad libitum electronic cigarettes (EC) versus tobacco cigarette use have not been described.
This study compares mechanistic biomarkers of cardiovascular disease for EC versus tobacco cigarette versus no nicotine use.
Results demonstrate ad libitum EC use is associated with similar circadian hemodynamic effects as cigarette smoking, reflecting persistent sympathetic neural stimulation.
What Are the Clinical Implications?
Both cigarette smoking and EC use are associated with increased inflammatory biomarker levels compared with no product use, which might contribute to cardiovascular risk.
The cardiovascular effects of ECs should be considered in counseling a patient with cardiovascular disease in the use of ECs to quit smoking, and in the duration of EC use once they have switched.
An important consideration in assessing the public health benefits versus risks of electronic cigarettes (ECs) is cardiovascular safety. Cardiovascular disease is a major source of morbidity and mortality in cigarette smokers. 1 , 2 Daily EC use can aid smoking cessation, and a recent clinical trial suggested that ECs are more effective in aiding quitting than nicotine replacement medication. 3 , 4 The question remains as to the potential cardiovascular harm of EC use, particularly in smokers who have underlying.
Mechanisms by which cigarette smoking causes cardiovascular disease include hemodynamic stress related to sympathetic neural stimulation, thrombogenesis, oxidant stress, inflammation, endothelial dysfunction, insulin resistance, production of an atherogenic lipid profile, and arrhythmogenesis. 2 , 5 Potentially cardiovascular‐toxic chemicals in cigarette smoke include oxidants, volatile organic compounds (such as acrolein, acetaldehyde, and formaldehyde), carbon monoxide, particulate matter, metals, and nicotine. In contrast, ECs generate an aerosol without combustion of organic materials. ECs deliver minimal levels or no carbon monoxide and usually much lower levels of oxidants, volatile organic compounds and most metals compared with cigarette smoke. 6 , 7 , 8 ECs can deliver levels of nicotine and particles (aerosols) similar to those of cigarettes, although the chemical composition of EC particles differs from that of cigarette smoke. 9 The number and size of particles also vary by the type of EC device and composition of the liquid. 9 To date, the toxicity of EC particles is unknown.
The main effect of nicotine with respect to the cardiovascular system is activation of the sympathetic nervous system. 10 The effects of nicotine that could contribute to acute cardiovascular events include increased heart rate, blood pressure, and myocardial contractility (resulting in increased myocardial work), and coronary vasoconstriction (resulting in reduced coronary blood flow reserve). 10 , 11 , 12 , 13 Other potentially adverse sympathetically mediated effects of nicotine may include arrhythmogenesis, insulin resistance, lipid abnormalities, and inflammation (the latter via activation of the splenocardiac axis). In addition, nicotine may produce oxidative stress and contribute to endothelial dysfunction. 10
A number of studies have examined acute effects of ECs in people, demonstrating the expected nicotine‐related increases in heart rate and blood pressure. Other reported acute effects of EC use include reduced heart rate variability, increased arterial stiffness, endothelial dysfunction, and platelet activation. 11 , 14 However, the cardiovascular effects of an acute vaping session may not reflect cardiovascular effects with more natural, ad libitum use. Studies of standardized use have generally tried to mimic cigarette smoking, with 10 to 20 puffs taken over 10 to 15 minutes. Most EC users do not puff the same way as smokers—rather they tend to take fewer puffs in clusters and puff more frequently than they puff cigarettes. 15 Furthermore, cardiovascular responses to acute exposure may not indicate effects with chronic exposure. For example, acute cigarette smoking and nicotine vaping impair endothelial function, as demonstrated by impaired flow‐mediated dilation, but in a study of cigarettes smokers who switched completely to vaping, endothelial function normalized, compared with those who continued smoking cigarettes. 16 With respect to actions of nicotine, substantial tolerance develops to cardiovascular effects of nicotine, including heart rate acceleration and epinephrine release, with repeated exposure. 17 , 18 The most relevant way to examine cardiovascular effects of vaping is by studying ad libitum use throughout the day. Circadian patterns are important because persistent sympathetic stimulation, particularly lasting overnight, may be associated with an increased risk of cardiovascular events. 19 , 20
The primary aim of this study was to compare the effects of ad libitum use of cigarettes and ECs on heart rate and blood pressure, during the day and overnight, as well as urine catecholamine excretion, which are indicators of sympathetic neural stimulation. Secondary aims compared the effects of cigarettes and ECs on biomarkers of other potential mechanisms of cardiovascular disease, including oxidative stress, platelet activation, and inflammation.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Participants
The participants were 36 healthy dual users (average age 35 years, 22% females), who used an EC at least 15 days and smoked at least 5 cigarettes/day over the past 30 days. They were recruited by advertisements via the Internet and flyers. To assure that daily nicotine intake was clinically significant, participants were required to have a salivary cotinine level of ≥50 ng/mL. Exclusion criteria included the following: use of EC liquids with <6 mg/mL nicotine concentration, <21 years of age, intent to quit ECs or cigarettes over the next 3 months, pregnancy, use of nicotine metabolism‐altering medications, use of cardiovascular medications such as α‐ and β‐blockers, chronic medical diseases, and active substance dependence or recent use of drugs of abuse other than marijuana. We excluded potential participants who vaped liquids containing <6 mg/mL nicotine to enhance the likelihood that they were vaping for pharmacologic effects of nicotine. Individuals who used other tobacco products >15 times per month or used EC devices with re‐buildable atomizers were excluded from the study. Re‐buildable atomizers were excluded because these devices need to be refilled often and because they tend to leak e‐liquid, making tracking the amount of e‐liquid consumed during the day challenging. Demographic data including baseline smoking and vaping behaviors, types of devices, and e‐liquid nicotine concentrations are shown in Table 1. The study was approved by the Institutional Review Board at the University of California San Francisco. Written, informed consent was obtained from each participant and all participants were financially compensated.
Table 1.
Demographics and Baseline Cigarette and EC Use Data
| N | 36 |
| Age, y | 35.4 ± 11.7 |
| Female, n | 8 (22) |
| Race | |
| Asian | 2 (6) |
| Black | 3 (8) |
| Latino | 4 (11) |
| White | 5 (14) |
| Mixed | 22 (61) |
| Device type | |
| Cig‐a‐like | 12 (33) |
| Fixed‐power tank | 15 (42) |
| Variable‐power tank | 6 (17) |
| Pod | 3 (8) |
| Cigarette dependence (FTCD) | 4.4 ± 2.0 |
| EC dependence (PS‐ECDI) | 5.9 ± 3.9 |
| Measured, liquid nicotine concentration, mg/g | |
| Whole sample | 17.0 ± 12.9 |
| Cig‐a‐like | 20.2 ± 13.4 |
| Fixed‐power tank | 12.2 ± 7.4 |
| Variable‐power tank | 9.4 ± 3.9 |
| Pod | 43.4 ± 4.8 |
| Screening salivary cotinine, ng/mL | 189.2 ± 92.8 |
| Baseline cigarettes/d | 12.9 ± 6.4 |
| Days of EC use in last 30 d | 22.6 ± 7.3 |
| Baseline EC sessions/d | 8.1 ± 7.2 |
Data are presented as n (%) or mean ± SD. EC indicates electronic cigarette; FTCD, Fagerstrom Test of Cigarette Dependence (Heatherton et al., Br J Addict 1991,86:1119–1127); and PS‐ECDI, Penn State E‐Cigarette Dependence Index (Foulds et al., Nicotine Tob Res 2015; 17: 186–192).
Experimental Protocol
Broadly, this within‐subject crossover study was designed to compare nicotine intake, subjective effects, biomarkers of exposure, and pharmacologic effects in dual users while smoking cigarettes only, vaping ECs only, and with no tobacco product use. The study was conducted between December 2015 and February 2018. The results of nicotine pharmacokinetics and titration, subjective effects, and toxicant exposure from this study have been published previously. 21 , 22 , 23
Participants were screened for eligibility, consent was obtained, questionnaires completed, and saliva samples collected for cotinine measurement. Participants were not asked to modify their smoking or EC use behavior before screening. After screening, participants completed two 1‐week study blocks: 1 cigarette only and 1 EC only block. The sequence of blocks was counterbalanced in sequential order of participant entry into the study. The first 4 days of each block consisted of at‐home use of ECs or cigarettes and served as a washout and stabilization period for the 5th to 7th days. On the 5th day, participants were admitted to a clinical research ward at Zuckerberg San Francisco General Hospital. On the first hospital day (5th study block day), fixed administration of the products was assessed and the 6th and 7th days (hereafter referred to as the “first” and “second” days of ad libitum use) consisted of ad libitum use of EC or cigarettes. An enforced tobacco product abstinence block of 2 days followed the last tobacco product block. While confined, the participants ate an unrestricted hospital diet with the exception that there was only 1 caffeinated beverage permitted per day.
On ad libitum days, participants were provided with an amount of their own brand cigarettes or EC supplies consistent with their normal consumption, with extra product provided considering that they normally dual use and the study would allow ad libitum use of only 1 product at a time. During the EC block, if participants were cig‐a‐like users, the study purchased their usual cig‐a‐like brand; if a fixed or variable power tank user, the study purchased their usual brand of e‐liquid; if a pod user, the study purchased the pods. Any remaining products were collected by study nurses each night at midnight. While on the hospital ward, participants were allowed access to television and Internet, and to go on supervised walks.
Starting at 8 am on the first day of each 2‐day in‐hospital block, following at least 8 hours of abstinence, participants were free to use their EC (at preferred power levels for variable‐power devices) or cigarettes ad libitum until midnight. Urine samples were collected over 24 hours each day. On the second day, blood samples were taken via indwelling intravenous catheter every 4 hours from 8 am to midnight, and at 8 am the following day.
Device Categorization
Device types and number of participants in each group were the following: (1) “Cig‐a‐likes”: small cylindrical devices resembling combustible cigarettes with nonrefillable liquid cartridges (N=12), (2) “Fixed‐power tanks”: devices with refillable liquid tanks and no user‐adjustable power parameters (N=15), (3) “Variable‐power tanks”: similar to fixed‐power devices but with the ability to change power parameters (eg, voltage or wattage) (N=6), and (4) “Pods”: devices utilizing disposable pods with liquid containing salt form nicotine (N=3).
Assessments
Nicotine and Cotinine Measures
Salivary cotinine concentrations at screening were determined by gas chromatography (GC), 24 modified for capillary GC, 25 limit of quantitation 10 ng/mL. Plasma nicotine and cotinine were determined by GC‐tandem mass spectrometry (GC‐MS/MS), using a published GC‐MS method 25 modified for MS/MS for improved sensitivity, nicotine limit of quantitation 0.2 ng/mL, and cotinine limit of quantitation 5 ng/mL.
Questionnaires
At screening, participants completed a basic demographic questionnaire including their age, sex, and race, and a nicotine use history questionnaire on cigarette and EC use in the past 30 days, EC type used most often, concentration of nicotine in e‐liquid used most frequently (in mg/mL), and how many mL of EC liquid were typically used per week (Table 1).
Ambulatory Heart Rate and Blood Pressure
Heart rate, systolic blood pressure and diastolic blood pressure of each participant were measured every 30 minutes for 24 consecutive hours in each of the 3 study arms with a 7100 series ambulatory blood pressure monitor (Welch Allyn, Skaneateles Falls, NY). Missing values or out‐of‐range values (<7%) were imputed using a “last‐observation‐carried‐forward” approach (excluding the first 8 am measurement). Out of range values included a heart rate <40 beats per minute, systolic blood pressure <70 or ≥180 mm Hg, or diastolic blood pressure <40 mm Hg.
Cardiovascular Effect Biomarkers
Urine epinephrine, norepinephrine, and dopamine concentrations were measured by quantitative high‐performance liquid chromatography–MS/MS by ARUP Laboratories, Salt Lake City, UT.
Oxidative stress was assessed using urine 8‐epi‐prostaglandin F2 α (8‐isoprostane), a lipid peroxidation product formed by degradation of arachidonic acid by free radicals. Platelet activation was assessed using urine 11‐dehydro‐thromboxane B2 (11‐dhTxB2), a metabolite of thromboxane A2 and a marker of in vivo platelet activation. These 2 biomarkers were measured using a high‐performance liquid chromatography–MS/MS technique at the Exposure Biology and Chemistry Lab of Duke University, described as follows. A 2 mL of urine aliquot was mixed with 2 mL of 0.1 mol/L acetate buffer (pH=5), 40 µL of internal standard mixture (250 µg/µL of d4‐8‐isoprostane and 250 µg/µL d4‐11‐dhTxB2 as internal standards), 20 μL of glucuronidase‐arylsulfatase, and 20 µL of ascorbic acid. The mixture was vortexed in a shaking bath at 37°C for 16 hours. Then 6 mL of ethyl‐acetate was added to the hydrolyzed mixture. Following shaking for 60 minutes, the mixture was centrifuged at 2360g for 30 minutes. The mixture from this last step was condensed by evaporation under nitrogen. Then we reconstituted the residue in 200 µL of H2O:MeOH (1:1) and centrifuged at 2360g for 30 minutes. This final solution was analyzed on a high‐performance liquid chromatography–MS/MS system (Thermo TSQ Quantum Access Max) equipped with a Luna 3 µm C18 reverse‐phase column (Phenomenex 50 × 2.1 mm). Mobile phases included Solvent A (water with 2.5 mol/L ammonium acetate) and Solvent B (100% methanol). The MS/MS parameters were 353.1/193.1 and 353.1/247.1 for 8‐isoprostane, 357.1/197.1 for d4‐8‐isoprostane, 367.0/161.0 and 367.0/305.0 for 11‐dhTxB2, and 371.0/309.0 for d4‐11‐dhTxB2. Calibration standards were purchased from ChemCruz. The limits of detection were 0.016 ng/mL for 8‐isoprostane and 0.065 ng/mL for 11‐dhTxB2. The recovery was 92% to 110% and 85% to 120%; and relative standard deviation from repeated injection was 11.27% and 10.02% for 8‐isoprostane and 11‐dhTxB2, respectively.
Inflammation was assessed using plasma interleukin‐6 (IL‐6), a pro‐inflammatory cytokine, and interleukin‐8 (IL‐8), a chemokine secreted as part of the inflammatory response, both measured by multiplex immunoassay (kits from R & D). These biomarkers are all known to be increased in cigarette smokers compared with nonsmokers, and all are associated with increased cardiovascular disease risk. 26
Statistical Analysis
Ambulatory heart rate and blood pressures are reported as mean and SD for 24‐hour (8 am to 8 am), daytime (8 am to 11 pm), and overnight periods (12 am to 8 am). The primary comparison between the 3 study arms was performed using nonlinear mixed models, where study treatment order was also included as a fixed effect. Post hoc comparisons are described using Tukey’s test.
Area under the plasma nicotine concentration–time curve values for plasma nicotine were calculated using the trapezoidal rule for 24‐hour plasma nicotine levels. For exposure biomarker analyses, concentrations with use of both EC and CS are reported relative to concentrations on the abstinence condition as geometric mean ratios with 95% CIs. Twenty‐four‐hour pooled urine biomarker concentrations were log‐transformed and plasma biomarkers rank‐transformed before further analysis. The effect of each exposure on these biomarkers was assessed using a mixed‐effect repeated measures model. We conducted post hoc pairwise comparisons between study arms applying a Bonferroni correction for multiple comparisons. Data from the EC‐only arm were used in an exploratory between‐subject analysis of device type effects describing urine and plasma biomarker values and reported as Wilcoxon rank‐sum tests. Because of the small number of pod users (N=3), the pod device users were not included in the between‐device analysis.
A priori, a sample size calculation determined that a sample size of 36 would give >80% power to detect corrected differences between cigarette and EC arms, on the primary outcome of mean heart rate, where values differed by at least 20% with coefficients of variation of 0.25. All analyses were conducted with SAS version 9.4 or R version 3.5, with 2‐tailed P‐values<0.05 considered significant.
RESULTS
Plasma Nicotine and Cotinine Levels
On average, participants smoked 15.6 (SD 22.2) cigarettes and vaped 1.95 g (SD 1.9) during ad libitum use days on the research unit. Mean steady‐state plasma nicotine concentrations over 24 hours while smoking cigarettes or using ECs, which have been described previously, 21 are shown in Figure 1. Overall, plasma nicotine levels were significantly higher during cigarette smoking, although in 25% of participants, nicotine levels during EC use exceeded levels during smoking. Mean (95% CI) plasma cotinine concentrations while smoking and using ECs were 246 ng/mL (229–264) and 182 ng/mL (158–207), respectively.
Figure 1. Plasma nicotine concentrations.
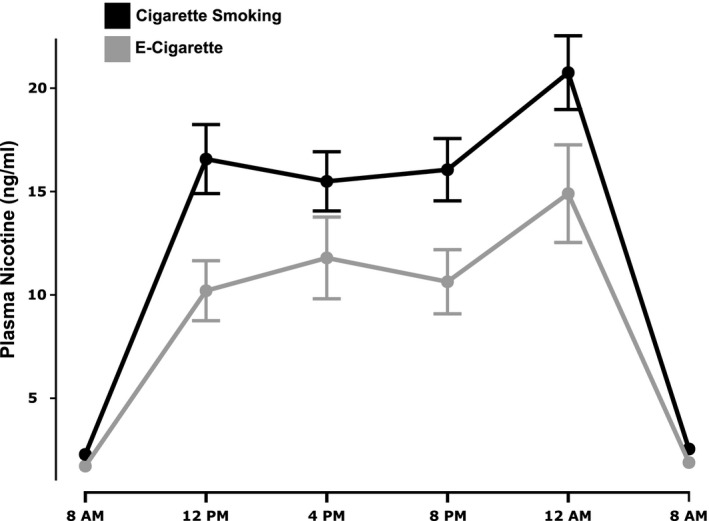
Twenty‐four‐hour plasma nicotine concentrations during ad libitum cigarette smoking or EC use, showing mean values and SE bars. EC indicates electronic cigarette.
Heart Rate and Blood Pressure
Mean heart rates and blood pressure while smoking, vaping, and using no nicotine product over 24 hours are shown in Figure 2 and mean heart rate and blood pressures over 24 hours and during daytime and overnight in Table 2. Over 24 hours and during daytime, heart rate was significantly higher with cigarette smoking than with EC use, and higher with EC use than with no product use. Overnight, heart rate was higher in the smoking condition than other conditions, while heart rate in the EC and no product conditions were similar. Systolic and diastolic blood pressure were similar with smoking and vaping during daytime and overnight, with both conditions higher than with no product use.
Figure 2. Mean ambulatory heart rate and blood pressure measurements.
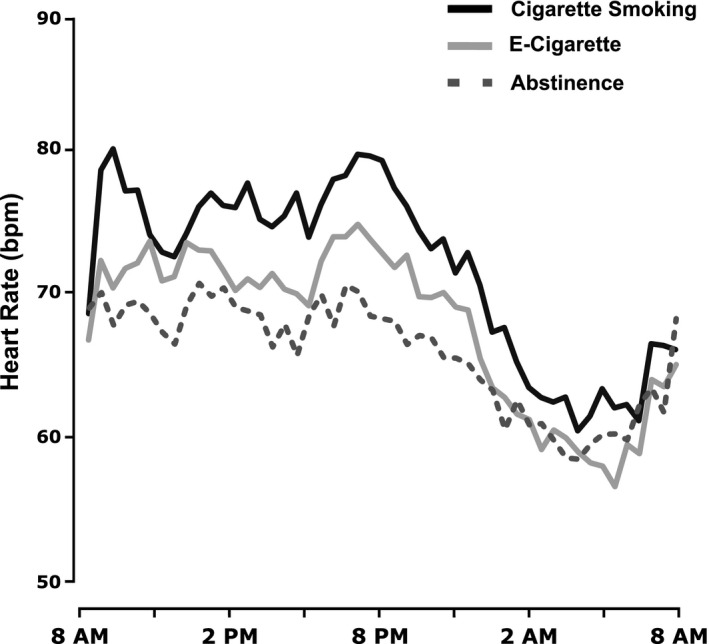
Twenty‐four‐hour mean heart rate during ad libitum cigarette smoking or EC use compared with no nicotine product use. EC indicates electronic cigarette.
Table 2.
Mean Heart Rate and Blood Pressure in Each Condition
| Mean (SD) | Heart Rate (bpm)*†‡ | Systolic (mm Hg)*‡ | Diastolic (mm Hg)*‡ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CS | EC | Abs | CS | EC | Abs | CS | EC | Abs | |
| 24‐h | 72.5 (12.6) | 68.7 (11.8) | 66.8 (11.2) | 119.9 (14.7) | 120.2 (15.3) | 116.8 (15.6) | 76.8 (12.4) | 76.7 (12.5) | 73.2 (11.9) |
| Daytime (8 am–11 pm) | 76.4 (11.7) | 72.3 (10.8) | 69.2 (11.1) | 123.2 (13.9) | 124.2 (13.0) | 119.5 (15.0) | 80.0 (11.4) | 80.0 (10.8) | 75.7 (11.1) |
| Overnight (12 am–8 am) | 65.3 (11.0) | 62.2 (10.6) | 62.6 (10.1) | 113.8 (14.3) | 113.1 (16.4) | 111.7 (15.4) | 71.0 (12.3) | 70.6 (13.2) | 68.6 (12.0) |
Means in each study period compared with nonlinear mixed models, which also included random effects for repeated measures within subject and fixed effects for treatment order. Study arm effects noted in 24‐h measurements: *Cigarette smoking>Abstinence, †Cigarette smoking>EC, ‡EC>Abstinence (all 2‐sided P values≤0.01). Abs indicates abstinence; CS, cigarette smoking; and EC, electronic cigarette.
Urine Catecholamines and Cardiovascular Effect Biomarkers
Urine epinephrine, norepinephrine, and dopamine excretion was not significantly different across conditions (Table 3). Urine 8‐isoprostane and 11‐dhTxB2 were also not significantly different, although for the latter there was a trend for higher levels with EC use and smoking compared with no product use (Figure 3). Plasma IL‐6 and IL‐8 were similar while smoking and vaping, while both were significantly higher compared with no product use (Figure 4).
Table 3.
Relative Biomarker Concentrations
| Geometric Mean of Ratio (95% CI) | |||
|---|---|---|---|
| EC/Abstinence | Smoking/Abstinence | Smoking/EC | |
| Urine biomarkers | |||
| Epinephrine (µg/24 h) | 1.15 (0.94–1.40) | 1.15 (0.97–1.36) | 1.00 (0.87–1.15) |
| Epinephrine (ng/mL) | 1.12 (0.96–1.31) | 1.04 (0.90–1.20) | 0.92 (0.80–1.07) |
| Epinephrine (ng/mg Cr) | 1.18 (0.98–1.40) | 1.22 (1.03–1.45)* | 1.04 (0.90–1.20) |
| Norepinephrine (µg/24 h) | 0.95 (0.85–1.06) | 0.97 (0.88–1.07) | 1.02 (0.90–1.15) |
| Norepinephrine (ng/mL) | 0.94 (0.81–1.08) | 0.87 (0.75–1.01) | 0.93 (0.81–1.06) |
| Norepinephrine (ng/mg Cr) | 1.00 (0.92–1.08) | 1.03 (0.93–1.14) | 1.03 (0.93–1.13) |
| Dopamine (µg/24 h) | 0.95 (0.87–1.04) | 0.95 (0.86–1.04) | 1.00 (0.90–1.11) |
| Dopamine (ng/mL) | 0.94 (0.81–1.08) | 0.86 (0.74–0.99) | 0.91 (0.80–1.04) |
| Dopamine (ng/mg Cr) | 1.00 (0.93–1.07) | 1.01 (0.93–1.09) | 1.01 (0.93–1.10) |
| 8‐Isoprostane (ng/24 h) | 1.09 (0.81–1.48) | 1.23 (0.94–1.63) | 1.13 (0.88–1.45) |
| 8‐Isoprostane (ng/mL) | 1.18 (0.88–1.57) | 1.19 (0.89–1.61) | 1.02 (0.83–1.24) |
| 8‐Isoprostane (ng/mg cr) | 1.23 (0.92–1.64) | 1.42 (1.07–1.90)* | 1.16 (0.94–1.42) |
| 11‐dhTxB2 (ng/24 h) | 1.25 (0.68–2.30) | 1.49 (0.86–2.61) | 1.19 (0.92–1.54) |
| 11‐dhTxB2 (ng/mL) | 1.35 (0.76–2.39) | 1.45 (0.85–2.45) | 1.07 (0.86–1.34) |
| 11‐dhTxB2 (ng/mg Cr) | 1.40 (0.77–2.59) | 1.72 (0.98–3.03) | 1.22 (0.97–1.54) |
| Plasma biomarkers | |||
| Interleukin‐6 (pg/mL) | 1.84 (1.20–2.82)† | 2.26 (1.37–3.74)† | 0.89 (0.51–1.53) |
| Interleukin‐8 (pg/mL) | 1.36 (1.12–1.63)† | 1.43 (1.21–1.69)† | 1.06 (0.86–1.30) |
Geometric means of relative biomarker concentrations reflect pairwise‐differences following mixed model analysis with random effects for repeated measures within subject and fixed effects for treatment order. Study arm effects: * P<0.05, † P<0.01. 11‐dhTxB2 indicates 11‐dehydro‐thromboxane B2; Cr, creatinine; and EC, electronic cigarette.
Figure 3. Within‐participant relative 24‐hour urine biomarkers concentrations.
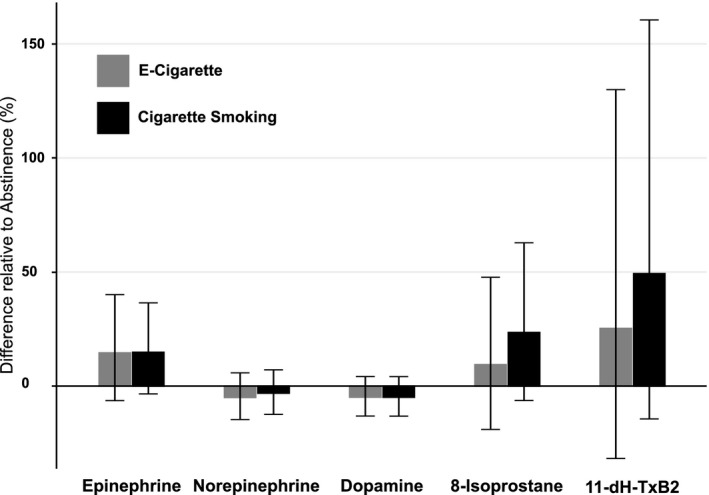
Estimated mean difference (%) in urinary biomarker concentrations from total 24‐h urine collection in 2 exposure periods (ad libitum cigarette smoking and EC use) relative to the nicotine abstinence period. Error bars indicate 95% CIs. 11‐dhTxB2 indicates 11‐dehydro‐thromboxane B2; and EC indicates electronic cigarette.
Figure 4. Within‐participant relative plasma biomarkers concentrations.
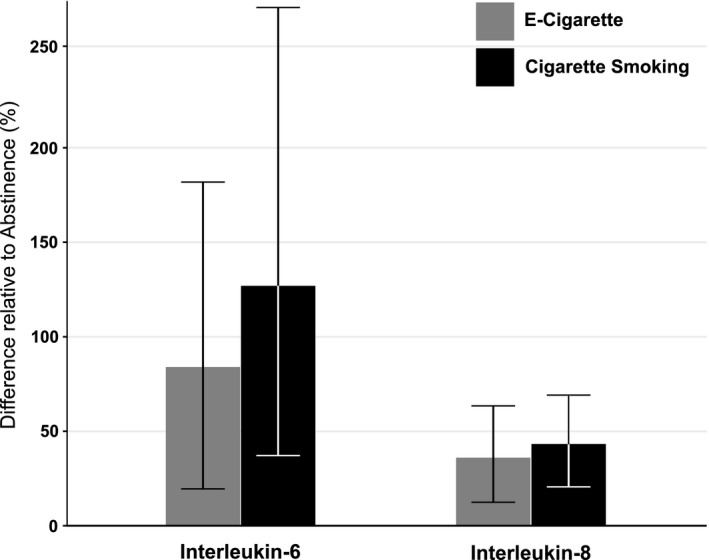
Estimated mean difference (%) in plasma biomarker concentrations in ad libitum exposure periods (cigarette smoking and EC use) relative to the nicotine abstinence period. Error bars indicate 95% CIs. and EC indicates electronic cigarette.
Comparison of Effects of Different Electronic Cigarette Devices
Plasma nicotine levels were on average higher in users of variable voltage devices compared with users of fixed‐voltage devices (P=0.019), the latter of which was similar to the cig‐a‐like condition. Mean (95% CI) plasma area under the plasma nicotine concentration–time curve over 24 hours (ng/mL × h) was 209 (154–249), 190 (146–231), and 413 (322–504) for users of cig‐alike, fixed and variable‐voltage devices, respectively. Correspondingly, heart rate was significantly greater with the variable‐voltage device use compared with fixed voltage, which was higher than in the cig‐a‐like condition (Table 4). Systolic and diastolic blood pressures were significantly higher with use of fixed voltage than cig‐a‐like, which were higher than variable voltage. No significant differences were observed in urinary biomarkers or IL‐8 by device type. Plasma IL‐6 was higher with variable voltage versus cig‐a‐like (P=0.03). Across subjects, there were no significant correlations between nicotine plasma area under the plasma nicotine concentration–time curve with EC use and various cardiovascular biomarkers.
Table 4.
Mean Heart Rate and Blood Pressures by EC Device Type
| Mean (SD) | Heart rate (bpm)*† | Systolic (mm Hg)† | Diastolic (mm Hg)† | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cig‐A‐Like | Fixed | Variable | Cig‐A‐Like | Fixed | Variable | Cig‐A‐Like | Fixed | Variable | |
| EC (24 h) | 66.3 (11.4) | 69.8 (11.7) | 72.3 (12.3) | 118.9 (14.7) | 123.0 (15.8) | 116.6 (14.0) | 76.1 (11.3) | 79.0 (13.1) | 72.8 (11.1) |
| EC daytime (8 am–11 pm) | 69.7 (11.0) | 72.7 (10.7) | 77.1 (10.0) | 123.4 (13.1) | 127.1 (12.9) | 118.4 (10.4) | 80.1 (10.0) | 82.0 (11.3) | 74.8 (8.3) |
| EC Overnight (12 am–8 am) | 60.0 (9.2) | 64.5 (11.7 | 63.1 (10.7) | 110.8 (13.7) | 115.7 (17.9) | 113.0 (18.3) | 68.8 (10.0) | 73.5 (14.5) | 68.9 (14.3) |
| Abstinence (24 h) | 65.4 (10.7) | 68.3 (11.7) | 67.5 (11.2) | 113.3 (12.6) | 120.8 (17.6) | 114.0 (13.9) | 72.6 (10.2) | 76.1 (13.2) | 68.7 (9.9) |
Device effects compared in 24‐h measurements with nonlinear mixed models, which also included random effects for repeated measures within subject and fixed effects for treatment order. Two‐sided P values≤0.01: *Variable Tank>Fixed Tank>Cig‐a‐ Like †Fixed>Cig‐a‐Like>Variable. EC indicates electronic cigarette.
Discussion
This study was performed to investigate and compare the 24‐hour cardiovascular effects of EC use compared with cigarette smoking and no product use in people who regularly used both electronic and conventional cigarettes. ECs and conventional cigarettes deliver nicotine rapidly by inhalation, but the pattern of nicotine self‐administration differs. Smokers generally take 8 to 12 puffs over 5 to 8 minutes from a cigarette, then smoke another cigarette at a later time, while vapers tend to inhale intermittently and in a more continuous manner. 15 Cigarettes deliver high levels of many combustion products, including oxidants, carbon monoxide, carbonyls, and particles, while ECs deliver much lower levels of oxidants, minimal levels or no carbon monoxide, lesser amounts of carbonyl compounds, and particles of a different physical composition than cigarette smoke and with unclear toxicity. 6 , 7 , 8 , 9
We observed that in general, nicotine exposure was greater with smoking than with EC use, and correspondingly average daily heart rate was higher with smoking than with EC use, which was in turn higher than with no product use. The heart rate elevation while smoking persisted over the 24‐hour period, while the heart rate effect of EC use was observed during daytime only. A small but significant increase in blood pressure was observed in both cigarette and EC conditions during daytime.
We also observed, as might be expected, that variable‐voltage EC use, which delivered higher doses of nicotine, was associated with higher heart rate. Despite this observation, we did not observe across participants a significant correlation between nicotine exposure (24‐hour plasma area under the plasma nicotine concentration–time curve) and hemodynamic effects. The finding of no significant correlation could be a statistical power issue or it could be that variable‐voltage devices, which generate greater on average amounts of aerosol and particles, have effects on heart rate independent of nicotine delivery.
While we observed the expected evidence of sympathetic neural activation based on heart and blood pressure, we did not observe the expected increase in urine epinephrine excretion, which we have observed in prior studies of smoking versus no nicotine use. 27 The reason for this negative outcome is unclear, but may have been related to individual differences in physical activity, which can also affect catecholamine excretion. This warrants future studies with a larger sample size and detailed physical activity assessment.
We did not see significant effects of smoking or vaping compared with no product use on biomarkers of oxidative stress or platelet activation. Both 8‐isoprostane and 11‐dhTx B2 levels are on average 50% higher in regular smokers compared with nonsmokers. 26 The fact that we did not see a significant change during 2 days of product abstinence may have been because the duration of our study and no product use were too short. Urine excretion of 8‐isoprostane is reported to decrease 3 to 7 days after smoking cessation. 28 In a prior study we observed a significant decrease in urine 11‐dhTxB2 3 to 4 days after stopping smoking or switching from cigarettes to nicotine patches, indicating a rapid change in thrombogenic risk. 29 In the present study, we did see a trend with higher average levels of 8‐isoprostane and 11‐dhTxB2 during smoking compared with vaping, but there was much interindividual variability and differences were not statistically significant.
While in the current study there were no significant differences between EC use and abstinence for 8‐isoprostane, 2 cross‐sectional studies of cardiovascular biomarkers in EC users compared with non–tobacco users have reported effects of ECs on oxidative stress. Singh et al found in a small group of adult self‐reported vapers who reported never using tobacco products that urinary excretion of 8‐isoprostane and the oxidative DNA damage biomarker 8‐oxo‐dG were significantly higher in vapers compared with never EC users. 30 Sakamaki‐Ching confirmed in another small group that urine 8‐isoprostane and 8‐oxo‐dG were significantly higher in vapers than in non–tobacco users. 31 Unfortunately, in neither study was cannabis smoking, a source of exposure to toxic combustion products and which is very common in EC users, biochemically assessed. The lack of a significant difference in 8‐isoprostane in the current study may be because the abstinence period was too short for significant changes to take place in this biomarker.
Both cigarette smoking and EC use were associated with higher plasma levels of the inflammatory cytokines IL‐6 and IL‐8 compared with no product use in our study. Similarly, higher levels of IL‐6 and IL‐8 as well as other inflammatory biomarkers in vapers compared with never tobacco users were reported by Singh et al. 30 A pro‐inflammatory effect of EC use could be mediated by exposure to reactive oxygen species, and/or particulates in EC aerosol. Carbonyls such as acrolein have been considered as possible sources of oxidant stress with EC use, but in the present study and in other studies there is little evidence of increased urine excretion of acrolein metabolites with EC use, while excretion is high with cigarette smoking. 22 , 32
The role of nicotine in promoting inflammation is unclear. The direct effect of nicotine acting via activation of the cholinergic nervous system is anti‐inflammatory. 33 However, sympathetic nervous system activation of the splenocardiac axis can have pro‐inflammatory effects. 12 , 34 The effect of nicotine on inflammation is informed by studies of users of smokeless tobacco, which exposes users to as much nicotine as smoking but without combustion products, and is associated with a similar circadian profile of plasma nicotine concentrations and cardiovascular effects as smoking. 35 Moist snuff use is associated with relatively few increases in inflammatory biomarkers compared with non–tobacco users, while smokers have much higher levels of a number of inflammatory biomarkers compared with moist snuff users. 36 Furthermore, inflammatory gene expression in peripheral blood mononuclear cells in snuff users is similar to that of non–tobacco users, and much different from that of smokers. 37 Such studies suggest that nicotine does not play a major role in promoting inflammation.
Strengths of our study include the fact that participants were confined to a research unit so that product use, nicotine exposure, and various cardiovascular effects could be carefully monitored. Ours is also the first study to examine cardiovascular effects of ad libitum EC use over 24 hours. Outpatient studies are often confounded by noncompliance, with study conditions and factors such as other drug use, exercise, stress, or heat that can affect cardiovascular measurements. On the other hand, we recognize that studying ad libitum tobacco use on a clinical research unit reduces external validity. Many of the usual social and environmental cues for tobacco product use are not present in a research unit environment. However, we do not believe that this limitation affects the validity of our cardiovascular measurements.
The main limitation of our study is the relatively brief duration of experimental conditions, particularly product abstinence, making our findings related to biomarkers of harm limited to those that change in a short period of time. For example, the normalization of urine 11‐dhTxB2 and 8‐isoprostane, and plasma IL‐6 and IL‐8 takes weeks to months after a smoker stops smoking. 26 The rate of change in the first few days of stopping smoking has not been fully characterized, so we may have missed the true extent of differences between smoking and EC conditions compared with abstinence. Furthermore, we studied users of a variety of EC devices, but these devices continue to evolve. Newer lower‐wattage devices (such as pod devices, of which we had only 3 users) deliver lower levels of oxidants and volatile organic compounds and may have different cardiovascular effects than higher‐wattage devices. 38 , 39 , 40 An alternative study design would have been to investigate only 1 EC device, but that would have even less generalizability to EC users in general. Future studies of cardiovascular risk of EC should compare specific devices and EC liquids. Another limitation with respect to understanding how EC use might affect vapers with underlying cardiovascular disease is that we studied healthy volunteers, none of whom were taking cardiovascular medications.
The implications of our study to assessing cardiovascular risk are as follows. We find circadian changes in heart rate reflecting persistent sympathetic neural activation produced by nicotine with ad libitum smoking and EC use. The magnitude of sympathetic neural stimulation was generally less with ECs than smoking, and smoking had persistent effects overnight, while ECs did not. Persistent sympathetic neural activation and heart rate acceleration may be a risk factor for future cardiovascular events. Increased resting heart rate and/or decreased heart rate variability (a marker of sympathetic predominance) has been associated with increased risk of future cardiovascular events, greater myocardial ischemia in the presence of coronary heart disease, increased vascular shear stress and endothelial dysfunction, and accelerated atherogenesis. 19 Increased sympathetic neural activity is associated with poorer outcome in people with chronic heart failure, and smoking cessation is associated with considerable clinical benefit in such individuals. 19 , 41 Sympathetic stimulation may play a role in atrial and ventricular arrhythmogenesis and sudden death. We found a small but significant increase in blood pressure during the day with EC use compared with no product use, which could convey cardiovascular risk. Of note, however, is that 2 studies have found that blood pressure declined in smokers who were able to switch to ECs, suggesting reduced risk of EC use compared with CS. 42 , 43
While there is a concern about adverse effects of nicotine‐induced sympathetic neural stimulation and persistent heart rate acceleration on cardiovascular health, it is important to consider epidemiological data from lifelong users of smokeless tobacco (also known as snus) in Sweden. Swedish snus users are exposed to nicotine at levels similar to cigarette smokers, but without exposure to products of combustion. Ad libitum use of smokeless tobacco throughout the day is associated with the same circadian pattern of heart rate acceleration as we see with cigarettes and smokeless tobacco. 35 Myocardial infarction and stroke risk is no higher in snus users compared with non–tobacco users, but when they do occur are more likely to be fatal in snus users. 44 In Swedish snus users who have had a myocardial infarction, mortality is reported to be higher in those who continue to use smokeless tobacco compared with those who quit. 45 An increased risk of cardiac death in snus users with coronary heart disease could be related to sympathetic neural‐stimulating effects of nicotine aggravating ischemia and/or producing fatal arrhythmogenesis.
Our data and the data of Singh et al and Sakamaki‐Ching et al suggest that EC use is associated with a chronic inflammatory response, most likely related to oxidant delivery. 30 , 31 Chronic inflammation could pose long‐term cardiovascular risk, contributing both to accelerated atherogenesis and, by inducing plaque instability, increasing the risk of acute cardiovascular events. A pro‐inflammatory state produced by EC use may also have important implications beyond cardiovascular disease, given the wide range of associations between biomarkers of inflammation and a variety of acute and chronic inflammatory conditions. It should be noted that insofar as the pro‐inflammatory effects of ECs is related to oxidant stress, lower‐power devices may present much less of an oxidant effect. 38 , 39 This could be a place where regulation of EC devices could reduce cardiovascular and other disease risk.
In conclusion, we report similar 24‐hour patterns of hemodynamic effects of CS and ECs, with lesser average effects of ECs, particularly overnight. We also find similar effects of smoking and ECs on biomarkers of inflammation, confirming findings of other recent cross‐sectional studies. Our findings raise concerns about the potential of EC use to cause cardiovascular harm, particularly in people with underlying coronary heart or cerebrovascular disease. However, given that exposure to toxic combustion products from cigarette smoke is known to be markedly reduced in EC users, and our observations of lower heart rate and trends towards reduction in biomarkers of oxidative stress and platelet aggregation with EC compared with CS, we believe that EC, while not harmless, may provide an important harm‐reduction opportunity for smokers who are able to switch completely from CS to EC. Data such as ours may be useful for regulatory purposes in the short term, but epidemiological studies of long‐term EC users, including those with preexisting cardiovascular disease, are needed to fully understand the impact of EC use on cardiovascular health.
Sources of Funding
This study was supported by grants R01DA039264, P30DA012393, and R25DA035163 from the National Institute on Drug Abuse, 1 U54HL147127 and T32CA113710 from the National Cancer Institute, and was carried out in part at the Clinical Research Center at Zuckerberg San Francisco General Hospital (NIH/NCRR UCSF‐CTSI UL1 RR024131). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH) or the US Food and Drug Administration (FDA).
Disclosures
Dr. Benowitz is a consultant to Pfizer and Achieve Life Sciences, companies that market or are developing smoking‐cessation medications, and has served as a paid expert witness in litigation against tobacco companies. The remaining authors have no disclosures to report.
Acknowledgments
We thank Marian Shahid, Annalise Davis, and Jennifer Ko for clinical research coordination; Kristina Bello, Trisha Mao, Polly Cheung, and Lisa Yu for performing analytical chemistry; and Zuckerberg San Francisco General Hospital Clinical Research Center nurses and staff for research participant care and study procedures.
(J Am Heart Assoc. 2020;9:e017317 DOI: 10.1161/JAHA.120.017317.)
For Sources of Funding and Disclosures, see page 10.
References
- 1. Barua RS, Rigotti NA, Benowitz NL, Cummings KM, Jazayeri M‐A, Morris PB, Ratchford EV, Sarna L, Stecker EC, Wiggins BS. 2018 ACC expert consensus decision pathway on tobacco cessation treatment: a report of the American college of cardiology task force on clinical expert consensus documents. J Am Coll Cardiol. 2018;72:3332–3365. [DOI] [PubMed] [Google Scholar]
- 2. Morris PB, Ference BA, Jahangir E, Feldman DN, Ryan JJ, Bahrami H, El‐Chami MF, Bhakta S, Winchester DE, Al‐Mallah MH, et al. Cardiovascular effects of exposure to cigarette smoke and electronic cigarettes: clinical perspectives from the prevention of cardiovascular disease section leadership council and early career councils of the American College of Cardiology. J Am Coll Cardiol. 2015;66:1378–1391. [DOI] [PubMed] [Google Scholar]
- 3. Hajek P, Phillips‐Waller A, Przulj D, Pesola F, Myers Smith K, Bisal N, Li J, Parrott S, Sasieni P, Dawkins L, et al. A randomized trial of e‐cigarettes versus nicotine‐replacement therapy. N Engl J Med. 2019;380:629–637. [DOI] [PubMed] [Google Scholar]
- 4. Zhu S‐H, Zhuang Y‐L, Wong S, Cummins SE, Tedeschi GJ. E‐cigarette use and associated changes in population smoking cessation: evidence from US current population surveys. BMJ. 2017;358:j3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. U.S. Department of Health and Human Services . How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking‐Attributable Disease: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010. [Google Scholar]
- 6. Belushkin M, Tafin Djoko D, Esposito M, Korneliou A, Jeannet C, Lazzerini M, Jaccard G. Selected harmful and potentially harmful constituents levels in commercial e‐cigarettes. Chem Res Toxicol. 2020;33:657–668. [DOI] [PubMed] [Google Scholar]
- 7. Bitzer ZT, Goel R, Reilly SM, Bhangu G, Trushin N, Foulds J, Muscat J, Richie JP. Emissions of free radicals, carbonyls, and nicotine from the NIDA standardized research electronic cigarette and comparison to similar commercial devices. Chem Res Toxicol. 2019;32:130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gray N, Halstead M, Gonzalez‐Jimenez N, Valentin‐Blasini L, Watson C, Pappas RS. Analysis of toxic metals in liquid from electronic cigarettes. Int J Environ Res Public Health. 2019;16:4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mulder HA, Patterson JL, Halquist MS, Kosmider L, Turner JBM, Poklis JL, Poklis A, Peace MR. The effect of electronic cigarette user modifications and e‐liquid adulteration on the particle size profile of an aerosolized product. Sci Rep. 2019;9:10221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Benowitz NL, Burbank AD. Cardiovascular toxicity of nicotine: implications for electronic cigarette use. Trends Cardiovasc Med. 2016;26:515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Benowitz NL, Fraiman JB. Cardiovascular effects of electronic cigarettes. Nat Rev Cardiol. 2017;14:447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boas Z, Gupta P, Moheimani RS, Bhetraratana M, Yin F, Peters KM, Gornbein J, Araujo JA, Czernin J, Middlekauff HR. Activation of the "splenocardiac axis" by electronic and tobacco cigarettes in otherwise healthy young adults. Physiol Rep. 2017;5:e13393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moheimani RS, Bhetraratana M, Yin F, Peters KM, Gornbein J, Araujo JA, Middlekauff HR. Increased cardiac sympathetic activity and oxidative stress in habitual electronic cigarette users: implications for cardiovascular risk. JAMA Cardiol. 2017;2:278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nocella C, Biondi‐Zoccai G, Sciarretta S, Peruzzi M, Pagano F, Loffredo L, Pignatelli P, Bullen C, Frati G, Carnevale R. Impact of tobacco versus electronic cigarette smoking on platelet function. Am J Cardiol. 2018;122:1477–1481. [DOI] [PubMed] [Google Scholar]
- 15. St Helen G, Ross KC, Dempsey DA, Havel CM, Jacob P, Benowitz NL. Nicotine delivery and vaping behavior during ad libitum e‐cigarette access. Tob Regul Sci. 2016;2:363–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. George J, Hussain M, Vadiveloo T, Ireland S, Hopkinson P, Struthers AD, Donnan PT, Khan F, Lang CC. Cardiovascular effects of switching from tobacco cigarettes to electronic cigarettes. J Am Coll Cardiol. 2019;74:3112–3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fattinger K, Verotta D, Benowitz NL. Pharmacodynamics of acute tolerance to multiple nicotinic effects in humans. J Pharmacol Exp Ther. 1997;281:1238–1246. [PubMed] [Google Scholar]
- 18. Porchet HC, Benowitz NL, Sheiner LB. Pharmacodynamic model of tolerance: application to nicotine. J Pharmacol Exp Ther. 1988;244:231–236. [PubMed] [Google Scholar]
- 19. Caetano J, Delgado AJ. Heart rate and cardiovascular protection. Eur J Intern Med. 2015;26:217–222. [DOI] [PubMed] [Google Scholar]
- 20. Stone PH. Triggers of transient myocardial ischemia: circadian variation and relation to plaque rupture and coronary thrombosis in stable coronary artery disease. Am J Cardiol. 1990;66:32G–36G. [DOI] [PubMed] [Google Scholar]
- 21. Harvanko AM, St Helen G, Nardone N, Addo N, Benowitz NL. Twenty‐four‐hour subjective and pharmacological effects of ad‐libitum electronic and combustible cigarette use among dual users. Addiction. 2020;115:1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. St Helen G, Liakoni E, Nardone N, Addo N, Jacob P III, Benowitz NL. Comparison of systemic exposure to toxic and/or carcinogenic volatile organic compounds (VOC) during vaping, smoking, and abstention. Cancer Prev Res. 2020;13:153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. St Helen G, Nardone N, Addo N, Dempsey D, Havel C, Jacob P III, Benowitz NL. Differences in nicotine intake and effects from electronic and combustible cigarettes among dual users. Addiction. 2020;115:757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jacob P III, Wilson M, Benowitz NL. Improved gas chromatographic method for the determination of nicotine and cotinine in biologic fluids. J Chromatogr. 1981;222:61–70. [DOI] [PubMed] [Google Scholar]
- 25. Jacob P III, Yu L, Wilson M, Benowitz NL. Selected ion monitoring method for determination of nicotine, cotinine and deuterium‐labeled analogs: absence of an isotope effect in the clearance of (s)‐nicotine‐3',3'‐d2 in humans. Biol Mass Spectrom. 1991;20:247–252. [DOI] [PubMed] [Google Scholar]
- 26. Scherer G. Suitability of biomarkers of biological effects (BOBEs) for assessing the likelihood of reducing the tobacco related disease risk by new and innovative tobacco products: a literature review. Regul Toxicol Pharmacol. 2018;94:203–233. [DOI] [PubMed] [Google Scholar]
- 27. Zevin S, Jacob P III, Benowitz NL. Dose‐related cardiovascular and endocrine effects of transdermal nicotine. Clin Pharmacol Ther. 1998;64:87–95. [DOI] [PubMed] [Google Scholar]
- 28. Pilz H, Oguogho A, Chehne F, Lupattelli G, Palumbo B, Sinzinger H. Quitting cigarette smoking results in a fast improvement of in vivo oxidation injury (determined via plasma, serum and urinary isoprostane). Thromb Res. 2000;99:209–221. [DOI] [PubMed] [Google Scholar]
- 29. Benowitz NL, Fitzgerald GA, Wilson M, Zhang Q. Nicotine effects on eicosanoid formation and hemostatic function: comparison of transdermal nicotine and cigarette smoking. J Am Coll Cardiol. 1993;22:1159–1167. [DOI] [PubMed] [Google Scholar]
- 30. Singh KP, Lawyer G, Muthumalage T, Maremanda KP, Khan NA, McDonough SR, Ye D, McIntosh S, Rahman I. Systemic biomarkers in electronic cigarette users: implications for noninvasive assessment of vaping‐associated pulmonary injuries. ERJ Open Res. 2019;5:00182‐2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sakamaki‐Ching S, Williams M, Hua M, Li J, Bates SM, Robinson AN, Lyons TW, Goniewicz ML, Talbot P. Correlation between biomarkers of exposure, effect and potential harm in the urine of electronic cigarette users. BMJ Open Respir Res. 2020;7:e000452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shahab L, Goniewicz ML, Blount BC, Brown J, McNeill A, Alwis KU, Feng J, Wang L, West R. Nicotine, carcinogen, and toxin exposure in long‐term e‐cigarette and nicotine replacement therapy users: a cross‐sectional study. Ann Intern Med. 2017;166:390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Filippini P, Cesario A, Fini M, Locatelli F, Rutella S. The yin and yang of non‐neuronal alpha7‐nicotinic receptors in inflammation and autoimmunity. Curr Drug Targets. 2012;13:644–655. [DOI] [PubMed] [Google Scholar]
- 34. Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, et al. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487:325–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Benowitz NL, Jacob P III, Yu L. Daily use of smokeless tobacco: systemic effects. Ann Intern Med. 1989;111:112–116. [DOI] [PubMed] [Google Scholar]
- 36. Sgambato JA, Jones BA, Caraway JW, Prasad GL. Inflammatory profile analysis reveals differences in cytokine expression between smokers, moist snuff users, and dual users compared to non‐tobacco consumers. Cytokine. 2018;107:43–51. [DOI] [PubMed] [Google Scholar]
- 37. Arimilli S, Madahian B, Chen P, Marano K, Prasad GL. Gene expression profiles associated with cigarette smoking and moist snuff consumption. BMC Genom. 2017;18:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bitzer ZT, Goel R, Reilly SM, Foulds J, Muscat J, Elias RJ, Richie JP Jr. Effects of solvent and temperature on free radical formation in electronic cigarette aerosols. Chem Res Toxicol. 2018;31:4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reilly SM, Bitzer ZT, Goel R, Trushin N, Richie JP. Free radical, carbonyl, and nicotine levels produced by Juul electronic cigarettes. Nicotine Tob Res. 2019;21:1274–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sleiman M, Logue JM, Montesinos VN, Russell ML, Litter MI, Gundel LA, Destaillats H. Emissions from electronic cigarettes: key parameters affecting the release of harmful chemicals. Environ Sci Technol. 2016;50:9644–9651. [DOI] [PubMed] [Google Scholar]
- 41. Suskin N, Sheth T, Negassa A, Yusuf S. Relationship of current and past smoking to mortality and morbidity in patients with left ventricular dysfunction. J Am Coll Cardiol. 2001;37:1677–1682. [DOI] [PubMed] [Google Scholar]
- 42. Farsalinos K, Cibella F, Caponnetto P, Campagna D, Morjaria JB, Battaglia E, Caruso M, Russo C, Polosa R. Effect of continuous smoking reduction and abstinence on blood pressure and heart rate in smokers switching to electronic cigarettes. Intern Emerg Med. 2016;11:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Polosa R, Morjaria JB, Caponnetto P, Battaglia E, Russo C, Ciampi C, Adams G, Bruno CM. Blood pressure control in smokers with arterial hypertension who switched to electronic cigarettes. Int J Environ Res Public Health. 2016;13:1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hansson J, Galanti MR, Hergens M‐P, Fredlund P, Ahlbom A, Alfredsson L, Bellocco R, Eriksson M, Hallqvist J, Hedblad B, et al. Use of snus and acute myocardial infarction: pooled analysis of eight prospective observational studies. Eur J Epidemiol. 2012;27:771–779. [DOI] [PubMed] [Google Scholar]
- 45. Arefalk G, Hambraeus K, Lind L, Michaelsson K, Lindahl B, Sundstrom J. Discontinuation of smokeless tobacco and mortality risk after myocardial infarction. Circulation. 2014;130:325–332. [DOI] [PubMed] [Google Scholar]


