Abstract
Ca2+ homeostasis is essential for multiple neuronal functions and thus, Ca2+ dyshomeostasis can lead to widespread impairment of cellular and synaptic signaling, subsequently contributing to dementia and Alzheimer’s disease (AD). While numerous studies implicate Ca2+ mishandling in AD, the cellular basis for loss of cognitive function remains under investigation. The process of synaptic degradation and degeneration in AD is slow, and constitutes a series of maladaptive processes each contributing to a further destabilization of the Ca2+ homeostatic machinery. Ca2+ homeostasis involves precise maintenance of cytosolic Ca2+ levels, despite extracellular influx via multiple synaptic Ca2+ channels, and intracellular release via organelles such as the endoplasmic reticulum (ER) via ryanodine receptor (RyRs) and IP3R, lysosomes via transient receptor potential mucolipin channel (TRPML) and two pore channel (TPC), and mitochondria via the permeability transition pore (PTP). Furthermore, functioning of these organelles relies upon regulated inter-organelle Ca2+ handling, with aberrant signaling resulting in synaptic dysfunction, protein mishandling, oxidative stress and defective bioenergetics, among other consequences consistent with AD. With few effective treatments currently available to mitigate AD, the past few years have seen a significant increase in the study of synaptic and cellular mechanisms as drivers of AD, including Ca2+ dyshomeostasis. Here, we detail some key findings and discuss implications for future AD treatments.
Keywords: calcium, synaptic, glutamate, nicotinic receptors, mitochondria, autophagy, lysosome
1. Ca2+ Dysregulation and Synaptic Defects in AD
The synapse, as the primary site of communication between neurons, plays a vital role in the transmission of neuronal impulses and information, and for encoding of learning and memory, all of which are affected in Alzheimer’s disease (AD). AD, as a progressive neurodegenerative disease, is characterized by Ca2+ dysregulation i.e., a “calciumopathy” [1] and synapse loss, i.e., a “synaptopathy” [2], with emerging evidence for a causal link between the two. Synaptic density is decreased in post-mortem brain tissue from AD patients [3,4], and while amyloid plaques have been implicated in AD related synaptic loss, synaptic deficits occur prior to and in the absence of amyloid plaques [5], and may also be due to Ca2+ dysregulation, an effect which is exhibited in presymptomatic AD mouse models [6,7,8,9]. Ca2+ dysregulation is characterized by exaggerated Ca2+ responses to synaptic and other stimuli, as well as abnormal Ca2+ homeostasis [10,11], both of which may result in elevated resting cytosolic Ca2+, an effect which is observed in AD and older non-AD rodent models [12,13,14,15,16].
1.1. Ca2+ Dysregulation Disrupts Synaptic Networks in AD
Synapses are unique Ca2+ entry points in the neuronal architecture, expressing both pre- and postsynaptic Ca2+ channels/receptors, including presynaptic RyRs, N/P/Q voltage gated Ca2+ channels (VGCCs), α7 nicotinic acetylcholine receptors (α7 nAChRs), and postsynaptic L-type VGCCs, RyRs and NMDA receptors (NMDARs). NMDARs in particular are one of the most well characterized postsynaptic glutamate receptors, with a high Ca2+ permeability and an established role in hippocampal synaptic plasticity [17]. At hyperpolarized potentials, NMDARs are blocked by Mg2+, but postsynaptic depolarization results in removal of Mg2+ block, and receptor disinhibition. Repeated NMDAR activation enhances postsynaptic Ca2+ entry, an effect which is facilitated by RyRs through Ca2+-induced-Ca2+ release (CICR), thus driving increased postsynaptic AMPA receptor (AMPAR) expression and subsequent synaptic long-term potentiation (LTP). This dual role of the NMDAR as coincidence detector and postsynaptic Ca2+ entry channel makes it uniquely positioned to mediate synaptic potentiation resulting from concurrent pre- and postsynaptic activation, thus forming a mechanistic basis for Hebbian plasticity and associative learning.
Paradoxically, NMDARS, as well as playing a role in synaptic plasticity, may also play a role in synaptic loss [18] and cell death [19]. The role of NMDARs in the deleterious effects of AD is further illustrated by the efficacy of the NMDAR antagonist memantine as a treatment for moderate to severe AD [20,21]. Interestingly, the clinical efficacy, or lack thereof, of specific Ca2+ channel antagonists could serve as a useful pointer for a role for those Ca2+ channels in the pathophysiology of AD, with the failure of large scale clinical trials for L-type VGCC antagonists in particular, contrasting with the positive effects of memantine [22]. AD is characterized by synaptic loss [2,4,23], including loss of synaptic terminals and dendritic spines [4], and similar dendritic spine loss is accompanied by impaired synaptic transmission and plasticity in animal models of AD [8,24,25,26,27,28,29]. Although the cause of synaptic loss in AD is not fully understood, it is thought to be associated with increased ER- Ca2+ release within spines [8,9,30,31], and at later disease states, toxic soluble Aβ species [32], resulting in hippocampal dendritic spine loss via NMDAR activation [33,34,35]. In contrast to Aβ, synaptic effects of abnormal tau expression have not been as extensively studied, however, a few recent studies have implicated effects of tau on VGCC function and synaptic signaling [36,37,38]. Specifically, tau accumulation may lead to synaptic loss and impairment of synaptic function via activation of calcineurin [39].
Hippocampal and cortical neurites in close proximity to amyloid plaques demonstrate Ca2+ hyperactivity in vivo, in presymptomatic AD mice, an effect which is blocked by AMPA receptor and NMDAR antagonists, suggesting that this hyperactivity is synaptically driven [6,7]. Although Ca2+ hyperactivity occurred mainly in the vicinity of insoluble dense core plaques, these plaques are also surrounded by soluble Aβ [40,41], which causes similar hyperactivity in WT mice [6]. Furthermore, plaque proximity has been reported to have no effect on evoked dendritic RyR and VGCC mediated Ca2+ signaling in AD mice [42], raising the possibility that some of the hyperactivity observed may be due to a presynaptic mechanism. Indeed, the presynaptic Ca2+ hyperactivity observed in an AD mouse model was inhibited by the sarcoplasmic endoplasmic Ca2+-ATPase (SERCA) pump inhibitor cyclopiazonic acid, indicating that this Ca2+ hyperactivity is driven by activation of presynaptic Ca2+ stores [43]. In contrast to reports of synaptically driven hyperexcitability in vivo, studies carried out using acute brain slices from AD mice demonstrate decreased basal hippocampal synaptic transmission, sometimes accompanied by increased paired-pulse ratio of evoked field potentials, indicative of decreased presynaptic glutamate release probability [29,44,45]. In addition, the membrane afterhyperpolarization mediated by activation of postsynaptic Ca2+ activated SK2 channels is increased in a 3xTg AD mouse model [46], leading to decreased postsynaptic membrane excitability and possible decreased synaptic transmission. It should also be noted that the decreased hippocampal synaptic transmission recently observed in a 5xTg AD mouse model was coupled with increased postsynaptic membrane excitability, due to an RyR2 mediated decrease in A-type K+ current (IA) [47], thus further illustrating the complexity of synaptic effects observed in AD mouse models. It is also noteworthy that the in vivo hyperexcitability studies mentioned above were conducted in animals anesthetized using the volatile inhalational anesthetic isoflurane. As isoflurane has been shown to result in increased cytosolic Ca2+ in hippocampal neurons [48], possibly due to IP3 receptor activation [49], effects which are exaggerated in AD mice [50], isoflurane anesthesia could be a potential mediator of the Ca2+ hyperexcitability observed in vivo in AD mice.
While the last two decades have seen a large increase in the number of studies using AD mouse models, a more recent development has been in the use of human induced neurons (HiNs), which are neurons derived from tissue samples taken from patients, to study synaptic transmission [51,52,53]. In a recent study, an increased frequency of spontaneous excitatory postsynaptic current (EPSC’s) was observed in AD derived HiNs, indicating an impulse-independent spontaneous increase in presynaptic glutamate release probability which is consistent with findings in human and animal studies [52]. Studies in patients with mild cognitive impairment have demonstrated hippocampal hyperactivity and decreased hippocampal volume [54,55], indicating a possible correlation between increased hippocampal activity and neurodegeneration.
More recently, proteomics has emerged as a method that allows for high throughput analysis of protein expression in small tissue samples [56], including post-mortem brain tissue from AD patients [57,58], and which has allowed for the study of changes in the interaction between presynaptic proteins [59], including SNAP25 and syntaxin [60]. Increased SNAP25 and syntaxin interaction results in reduced glutamatergic synaptic transmission [61,62] and decreased interaction between these proteins has been observed in the brains of AD patients, along with decreased levels of Complexin II [63], effects which would be expected to result in increased excitatory synaptic transmission [64,65]. SNAP25 has also been shown to negatively interact with presynaptic VGCCs to control presynaptic Ca2+ and affect neurotransmitter release [66,67], and the demonstrated therapeutic efficacy of putative AD medications such as levetiracetam, which targets presynaptic VGCCs [68,69], suggests that presynaptic Ca2+ channels could serve as a therapeutic target for AD.
1.2. Acetylcholine Signaling and α7nAChR Function in AD
nAChRs are essential for normal cognitive function [70,71], and this family of receptors includes the highly Ca2+ permeable homomeric α7nAChR isoform [72,73]. α7nAChRs are expressed throughout the septo-hippocampal circuit, both on medial septal nucleus/diagonal band cholinergic neurons [74], and also in the hippocampus. Cholinergic neurons in particular show significant degeneration in the course of AD [75,76], and this has resulted in development of medications to enhance cholinergic transmission, presumably through the activation of postsynaptic nAChRs. Hippocampal α7nAChRs are expressed presynaptically on mossy fiber terminals [77], and postsynaptically on CA1 interneurons [78,79], with activation of both resulting in Ca2+ influx [79,80,81,82,83]. Nicotine enhances hippocampal excitatory synaptic transmission via activation of α7nAChRs on mossy fiber terminals in the hippocampal CA3 region [80,81,84] and activation of CA1 α7nAChRs facilitates hippocampal LTP [85].
Aβ binds with high affinity to α7nAChRs [86,87], resulting in noncompetitive block of α7nAChR function, including at presynaptic α7nAChRs [88]. Cortical and hippocampal α7nAChR expression is reduced in AD patients [89,90] and AD mice [91] and Aβ binding to α7nAChRs results in the endocytosis of the Aβ α7nAChR complex with resulting accumulation within the lysosomal compartment [92]. Aβ binding to α7nAChRs results in Ca2+ influx, both in oocytes and presynaptic terminals in hippocampus [93,94]. Low micromolar concentrations of Aβ trigger glutamate release in the hippocampal dentate gyrus, CA3 and CA1 subfields via α7nAChRs [95] and picomolar concentrations of Aβ enhance hippocampal LTP via α7nAChRs [96]. In addition, α7nAChR activation rescues LTP deficits in hippocampal slices taken from Aβ infused rat brains, and Aβ treated hippocampal slices [97,98] and chronic treatment with an α7nAChR agonist restores cognition in AD mice [99]. Cells treated with the acetylcholinesterase inhibitor donepezil, used clinically in the treatment of AD, also showed reduced glutamate NMDAR mediated Ca2+ influx, an effect which was blocked by an α7nAChR antagonist [100]. Galantamine, also an acetylcholinesterase inhibitor, has been shown to positively modulate human α7nAChRs expressed in xenopus oocytes, thus allowing for a dual effect of increased synaptic acetylcholine and α7nAChR potentiation [101].
Although a role for α7nAChRs in the etiology of AD has not been established, many animal studies have demonstrated cognitive enhancing effects of compounds targeting α7nAChRs [102], including the α7nAChR partial agonist EVP-6124, which has also been shown to enhance cognition in patients with mild to moderate AD [103]. EVP-6124 and the α7nAChR positive allosteric modulator AVL-3288 have been shown to be well tolerated in patients [104,105], but some concerns exist about effects of potentiation of α7nAChR mediated Ca2+ effects in AD. In addition to α7nAChR mediated Ca2+ influx, activation of α7nAChRs triggers CICR via ryanodine sensitive Ca2+ stores [106], including at presynaptic α7nAChRs on hippocampal mossy fiber terminals [107], which are known to have strong RyR expression [108]. As RyR mediated CICR may be increased in AD, positive allosteric modulation of α7nAChRs may facilitate pre- and postsynaptic Ca2+ overload via already increased RyR function [109], possibly exacerbating AD related synaptic deficits. Based on these studies, the use of α7nAChR compounds in the treatment of cognitive impairment and AD looks promising, but caution should be exercised regarding the use of drugs which result in overt α7nAChR potentiation.
1.3. Potential Therapies for the Treatment of Synaptic Ca2+ Dysregulation in AD
As of now, there are only two FDA-approved classes of drugs used in the symptomatic treatment of AD: the noncompetitive NMDA antagonist memantine, and the acetylcholinesterase inhibitors, donepezil, galantamine and rivastigmine, with both classes of drugs having a synaptic site of action. Although both memantine and donepezil have been shown to be moderately effective in the treatment of AD symptoms, there is an urgent need for disease-modifying approaches, which currently requires the identification of novel compounds and receptor targets at the pre- or postsynaptic level. While a number of studies have identified promising small molecules targeting NMDARs [19,51], α7nAChRs [85,99], RyRs [110] and SERCA [111], few have made it past the preclinical stage of testing. In addition, the smoking cessation medication varenicline, which is an agonist at the α7nAChR [112], has been tested as a treatment for AD, but without any observed beneficial effects in patients [113]. Despite its failure, the clinical trial for varenicline illustrates the use of existing FDA approved medications as a strategy in the treatment of AD, bypassing many of the arduous and expensive aspects of drug development. The RyR modulator dantrolene (Ryanodex) is an FDA approved medication that has been shown to be effective in reversing many of the synaptic and cognitive effects seen in mouse models of AD [8,114,115,116], and has good CNS penetration when given orally or by nasal administration [117]. In addition, the clinically used L-type VGCC inhibitor isradipine has been shown to be neuroprotective in an AD mouse model [22], as has the beta-blocker carvedilol [47], however results from a recent large clinical study suggested no benefit of the VGCC antagonist nilvadipine as a treatment for AD [118]. Although the failure of large scale clinical trials for VGCC inhibitors as a treatment for AD has resulted in diminished enthusiasm for their use, the antiepileptic drug levetiracetam, which inhibits presynaptic VGCCs [68], has been shown to be beneficial in AD patients [69] and clinical trials for its use in the treatment of AD are ongoing [119]. The relative success of levetiracetam, along with the well documented failure of clinical trials targeting amyloid, strengthens the case for the use of synaptically targeted drugs in the treatment of AD and argues for the testing of FDA-approved medications as an important therapeutic strategy in the treatment of AD.
1.4. ER Ca2+ Channels in Synaptic Dysregulation in AD
Cytosolic Ca2+ levels are tightly regulated and maintained at low nM concentrations, despite a much higher extracellular Ca2+ concentration, and similarly elevated Ca2+ levels within intracellular organelles such as the ER. The ER is located throughout the cell, including pre- and postsynaptically, at synaptic terminals and dendritic spines respectively. RyRs, as well as having a role in gating ER Ca2+, are sensitive to changes in cytosolic Ca2+ through the process of CICR, with increases in postsynaptic Ca2+ resulting in RyR activation and release of ER Ca2+ into the cytosol. CICR has the effect of amplifying postsynaptic Ca2+ generated from influx via Ca2+ permeable receptors/ion channels such as NMDA receptors (NMDAR) and voltage gated Ca2+ channels (VGCCs), and RyR mediated CICR is upregulated in neurons in 3xTg AD mice [9]. Although RyR mediated amplification of postsynaptic Ca2+ allows for a large rapid increase in cytosolic Ca2+, this increased Ca2+ is usually rapidly removed from the cytosol, against a concentration gradient, by Ca2+ ATPases including the SERCA pump, which is also located on the ER membrane. The ER membrane also expresses IP3 receptors, although these are not thought to be synaptically expressed [120].
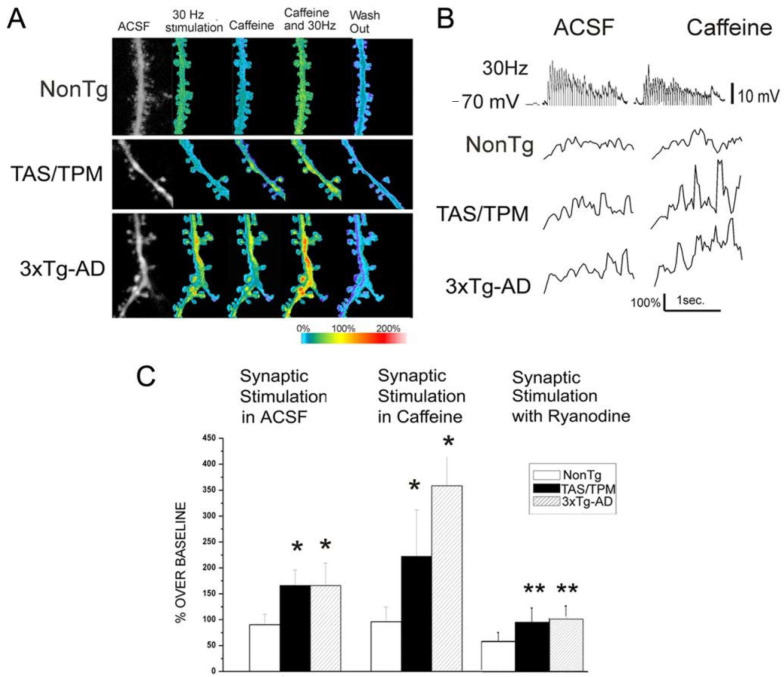
Mutations in the presenilin 1 (PS1) gene are linked to familial AD, an early onset form of the disease, and these mutations have specific functional implications for Ca2+ regulation. Although PS1 is a part of the γ-secretase complex which cleaves amyloid precursor protein (APP), it is also expressed on the ER membrane where it regulates RyR and IP3R channel properties [121,122,123,124], and may serve as a Ca2+ leak channel [125,126]. Mutations or altered expression of PS1 also affect the expression and sensitivity of neighboring RyRs [124]. RyRs play an important role in Ca2+ regulation, and RyR dysfunction is implicated in the Ca2+ dysregulation observed in AD [1]. RyR expression and RyR mediated Ca2+ responses are increased in the soma and dendritic spines of hippocampal and cortical pyramidal neurons of AD mice expressing PS1 mutations (Figure 1 and Figure 2) [9,30,127], effects which are normalized by acute or chronic treatment with dantrolene, a negative allosteric RyR modulator [50,114]. In particular, the RyR2 isoform, which is overexpressed in the hippocampus of AD mice [30,114], plays an important role in maintenance of synaptic function [128] and shortening of the RyR2 mean channel open time reverses the synaptic dysfunction and Ca2+ dyshomeostasis observed in an AD mouse model [47]. Human-induced neurons (HiN) derived from fibroblasts from AD patients expressing the PS1 mutation also display increased RyR expression and evoked RyR Ca2+ release [53], and RyR expression is also increased in post-mortem brains of AD patients, and patients with mild cognitive impairment [129,130]. Postsynaptic Ca2+ responses to high frequency stimulation (HFS) are increased in hippocampal and cortical neurons from several AD mouse models [8,9,131], effects which are mediated by RyR activation (Figure 1) [9]. Presynaptic RyR function is also increased in 3xTg AD mice and RyR activation by caffeine decreases the paired-pulse ratio of evoked CA1 field potentials to a greater extent in AD mice, as well as restoring normal frequency of spontaneously released vesicles, indicating increased facilitation of glutamate release by presynaptic RyRs [30]. Further indications of pathogenic synaptic effects resulting from altered RyR-Ca2+ signaling is the restoration of reduced presynaptic vesicle stores observed in AD mice back to normal levels upon treatment with Ryanodex [24].
Figure 1.
Synergistic Ca2+ interactions between RyR and glutamatergic synaptic transmission in AD mouse cortical neurons. (A) Pseudocolored images of relative Ca2+ changes in representative NonTg (top), TAS/TPM (a double transgenic AD mouse model) (middle), and 3xTg-AD (a triple transgenic AD mouse model) (bottom) neurons in the following conditions (from left to right): baseline 30 Hz synaptic stimulation (1.5 s), caffeine alone (10 mm), 30 Hz synaptic stimulation plus caffeine, and washout. (B) Representative Ca2+ response traces after 30 Hz synaptic stimulation (voltage trace shown in top) shown as percentage over baseline, in control aCSF (left panels) and in 10 mM caffeine (right panels) for NonTg (top), TAS/TPM (middle), and 3xTg-AD (bottom) neurons. (C) Bar graphs show averaged (mean ± SE) Ca2+ responses integrated over a 1.5 s time period of 30 Hz synaptic stimulation in control ACSF (left grouping), synaptic stimulation plus caffeine (center), and synaptic stimulation with ryanodine in the pipette (right grouping) for the NonTg, TAS/TPM, and 3xTg-AD neurons. Statistically significant differences are indicated by asterisks (one-way ANOVA, p < 0.05). * Significantly different from NonTg within treatment group; ** significantly different from synaptic stimulation in aCSF within transgenic strain (modified from [9]).
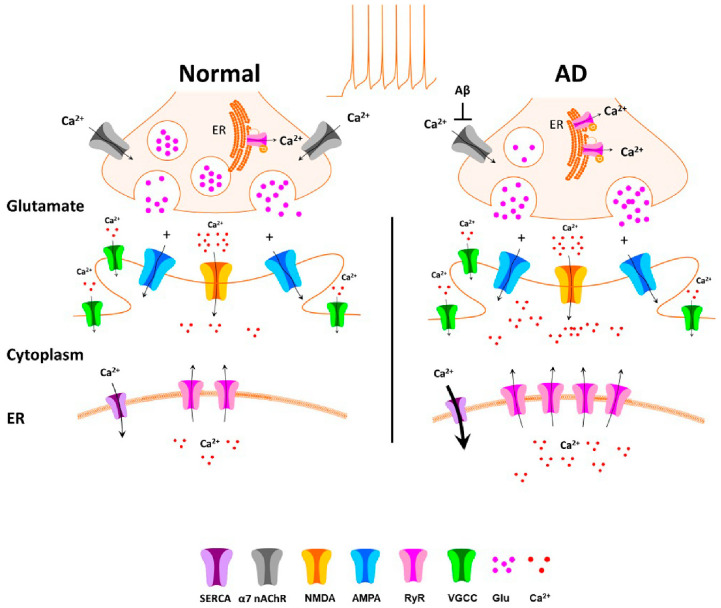
Figure 2.
Schematic of synaptic Ca2+ dysregulation in AD. Under normal circumstances (left), impulse mediated increases in presynaptic Ca2+ result in neurotransmitter release via a ready releasable vesicular pool. In addition, activation of presynaptic α7nAChRs triggers further Ca2+ influx, thus facilitating impulse mediated release, and presynaptic effects may be further increased via presynaptic RyR mediated Ca2+-induced-Ca2+ release (CICR). In mouse models of AD, although intrinsic cell excitability is not increased, presynaptic RyR mediated CICR may be increased, resulting in increased release probability of glutamate and depletion of vesicle stores. Aβ binding to presynaptic α7nAChRs may result in occlusion of the binding site, with decreased function and eventual decreased presynaptic α7nAChR expression due to endocytosis. In AD, increased ER Ca2+ stores, along with increased RyR expression results in increased postsynaptic CICR, which may facilitate stimulus-evoked postsynaptic Ca2+ increases.
In addition to effects on basal synaptic transmission, changes in RyR-Ca2+ signaling may also have implications for synaptic plasticity and LTP, which are impaired in AD mice [8,45]. High frequency stimulation (HFS) of hippocampal CA3-CA1 Schaffer collaterals, which generates LTP, initially results in a period of short-term, presynaptically mediated plasticity known as post tetanic potentiation (PTP), which results from an accumulation of presynaptic Ca2+ and is accompanied by an increased release probability of glutamate. This form of short term plasticity is necessary for the synaptic tagging processes involved in LTP [132], however in 3xTg AD mice, PTP is reduced, and this diminished short-term plasticity is followed by decreased LTP [8]. Chronic treatment with the RyR modulator dantrolene has been shown to restore PTP and LTP to control levels seen in non-AD mice, and this effect was accompanied by a restoration of presynaptic vesicles in the active zone, illustrating a role for aberrant presynaptic RyR-Ca2+ signaling in the impaired short and long-term synaptic plasticity observed in AD mice [8]. Presenilin deletion decreases Ca2+ effects of RyR activation, due to decreased RyR expression [121] and selective deletion of presynaptic presenilin decreases the release probability of glutamate, and LTP, an effect that is mimicked and occluded by RyR inhibition [133]. Thus, it would seem alterations in PS1 expression/function result in diminished LTP, either due to decreased or increased presynaptic RyR function, emphasizing the importance of RyR stabilization in maintenance of normal synaptic function.
Low resting cytosolic Ca2+ is maintained in part due to the actions of Ca2+-ATPases, which rapidly remove Ca2+ from the cell cytosol, against a concentration gradient. One of the major cellular Ca2+-ATPases is the SERCA pump, located on the ER membrane. SERCA function is facilitated by presenilin, and knockdown of the genes for presenilin 1 and presenilin 2 results in elevated cytosolic Ca2+, due to decreased SERCA mediated clearance of cytosolic Ca2+ [134]. Overexpression of the SERCA2b isoform typically found in neurons, and which physically interacts with the PS1 and PS2 Ca2+ channels on the ER membrane, results in increased Aβ [134]. Further evidence for presenilin’s role in SERCA function comes from a study showing that cells expressing a PS1 mutation show an exaggerated cytosolic Ca2+ response to SERCA inhibition by thapsigargin, indicating increased SERCA function [135]. SERCA inhibition also mimics the effects of selective deletion of presynaptic presenilin, on synaptic transmission and LTP [133], and inhibition of presynaptic SERCA function by cyclopiazonic acid diminished the Ca2+ hyperactivity observed in cortical neurons of AD mice in vivo [43]. In addition to SERCA, STIM Ca2+ sensors and Orai Ca2+ channels facilitate ER Ca2+ filling through the process of store-operated-Ca2+ entry (SOCE), a process which is deficient in PS1 mutant expressing neurons [136,137]. As ER Ca2+ release is elevated in AD neurons (Figure 2), in parallel with diminished SOCE activity, this opens up the possibility of an increased role for SERCA in this maladaptive pathology.
The characteristic features of AD, including maladaptive protein accumulation, increased free radicals and metabolic disruptions, are concurrent with aberrant intracellular Ca2+ signaling and contributes to the activation of the ER stress response in cells [138]. In an attempt to restore ER homeostasis, the ER triggers the unfolded protein response (UPR) by increasing the expression of transcription factors (ATP6c, XBPIs, and ATF4) which provides tolerance to cellular stress [139]. If the UPR is incompetent in decreasing stress, the ER triggers cell death by apoptosis [140,141] or autophagy [142]. Several animal [143,144,145] and human studies [138,146] report that AD mutations cause alterations in the UPR, thus in AD, aberrant ER-Ca2+ release disrupts the neuron’s compensatory mechanisms to restore cellular homeostasis and increases the vulnerability of neurons to stress and death.
2. Ca2+ Mishandling Impairs Cellular Organelle Functions in AD
In addition to synaptic signaling deficits, Ca2+ dyshomeostasis also has profound effects on the function of cell organelles, including the mitochondria and lysosomes, both of which play an important role in maintaining cellular and synaptic function. Like the ER, mitochondria and lysosomes act as intracellular Ca2+ stores, and dyshomeostasis of mitochondrial and lysosomal Ca2+ is emerging as a potential new source of cell dysfunction in AD, with profound implications for cellular and synaptic health.
2.1. Ca2+ Dysregulation Disrupts Mitochondrial Bioenergetics in AD
The mitochondria’s ability to buffer intracellular Ca2+ signaling is critical for neuronal signal transductions, ATP synthesis, and coordination with other organelles in physiological and pathological conditions. Mitochondrial dysfunction is a well-established characteristic of AD manifesting as increased free radical production and rate of oxidative damage, decreased ATP/ADP ratio and impaired bioenergetics [147,148,149,150]. Numerous differentially expressed mitochondria regulatory genes (133 in total) have been identified in the AD cohort and found that genes coding for mitochondrial oxidative phosphorylation were downregulated in both early and late AD brain specimens–specifically, NADH ubiquinone oxidoreductase subunits and complex I components which transfer electrons to the respiratory chain [151,152]. Proteomic and protein expression studies also confirmed dysregulated mitochondrial oxidative phosphorylation complexes [153] and defective enzymatic activity in the citric acid cycle and electron transport chain (ETC) [154,155].
The main function of the mitochondria is the production of ATP. Unavoidably, the by-products of electron transport in aerobic respiration are reactive oxygen species (ROS), due to electron leaks at complex I and III. Ca2+ overload, as is the case in AD, hinders glucose metabolism by disrupting components of the ETC such as, mitochondria complex I and II, tricarboxylic acid cycle (TCA), pyruvate dehydrogenase complex (PDHC), α-ketoglutarate dehydrogenase complex (KGDHC), malate dehydrogenase (MDH), and increasing ROS production while decreasing ATP production [156]. Redox proteomics studies identify increased oxidatively modified proteins, specifically antioxidant enzymes such as glutathione-S-transferase Mu, peroxiredoxin 6, multidrug-resistant protein 1 or 3, and GSH, in various brain regions of MCI and AD patients [157]. Additionally, enzymes involved in respiration were oxidized, specifically ATP synthase, aconitase, and creatine kinase [157]. This suggests that increased oxidative stress, as a consequence of mitochondrial Ca2+ overload, contributed to mitochondrial dysfunction and impaired energy metabolism in AD.
The dynamic function of the mitochondria requires crosstalk between other major organelles, such as the ER (Figure 3). Mitochondria’s physical coupling to the ER is crucial for efficient Ca2+ transfer and cellular homeostasis. Mitochondrial Ca2+ uptake controls the rate of energy production, regulates intracellular Ca2+ signaling, and mediates cell death. Ca2+ transfer between these organelles is facilitated via the mitochondrial-associated membrane proteins (MAM). Numerous molecular proteins have been identified to support this physical interaction. Of interest, the glucose-regulated protein 75 (GRP75) is linked to IP3R and facilitates Ca2+ into the mitochondrial intermembrane space. From there, voltage-dependent anion-selective channel protein 1 (VDAC1) on the outer mitochondrial membrane, and the mitochondrial Ca2+ uniporter (MCU) on the inner mitochondrial membrane, transfer the Ca2+ to the mitochondrial matrix to stimulate the mitochondrial dehydrogenase and increase ETC activity and ATP synthesis. [158,159,160,161,162,163]. These MAMs play a crucial role in regulating mitochondrial Ca2+ uptake. In AD, many genes involved in mitochondrial Ca2+ transport are altered [164,165,166]. Of note, genes encoding mitochondrial Ca2+ influx, such as MCU, are downregulated whereas genes encoding mitochondrial Ca2+ efflux, such as NCLX, are upregulated, suggesting a compensatory mechanism to avoid excessive Ca2+ uptake. Additionally, studies report that, soluble Aβ aggregations increase cytosolic Ca2+, leading to mitochondrial Ca2+ overload via MCU. Excessive Ca2+ taken up by mitochondria leads to caspase activation and neuronal cell death [167].
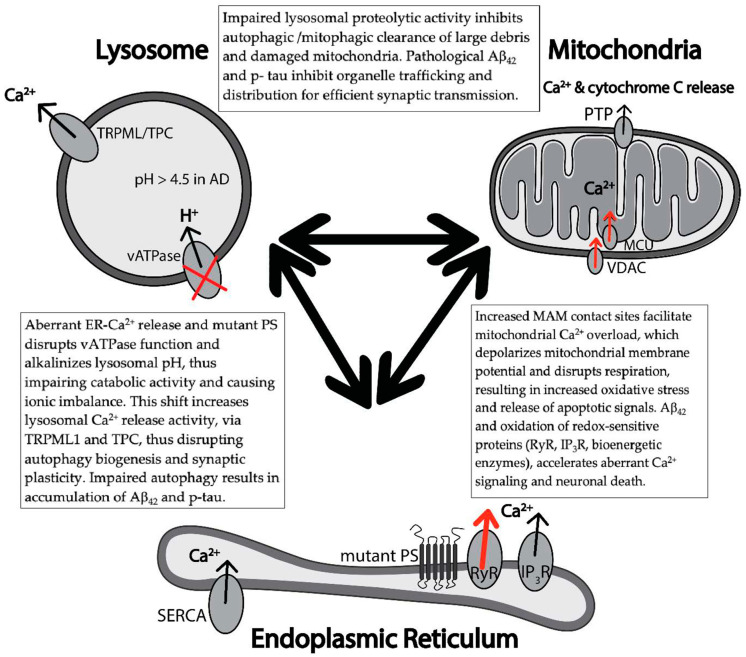
Figure 3.
Aberrant Ca2+ disrupts inter-organelle functional relationships in early AD pathology. Schematic of feed-forward cascades among various neuronal Ca2+ handling organelles: Endoplasmic reticulutm (ER), lysosome, and mitochondria, in early AD pathology. Excess ER Ca2+ release through RyR and IP3R cause mitochondrial Ca2+ overload that disrupts mitochondrial bioenergetics, resulting in increased oxidative stress and apoptosis. Additionally, ER Ca2+ disrupts lysosome-mediated clearance of maladaptive protein deposits and damaged organelle, such as Aβ, p-tau, and mitochondria, respectively. Aberrant intracellular Ca2+ signaling disrupts lysosomal Ca2+ release via TRPML and dysregulates autophagosome biosynthesis and impairs synaptic plasticity. Presenilin (PS) mutations disrupt vATPase trafficking resulting in alkaline lysosomes, thereby disrupting lysosomal ionic balance and lysosomal Ca2+ store. This alkaline environment impairs proteolysis and impairs autophagic clearance.
In addition to Ca2+, VDAC1 supports transport of superoxide anions [168] and since IP3R and RyRs have been shown to be redox-sensitive, ROS and Ca2+ may play a regulatory role in ER-mitochondria communication. During AD- associated Ca2+ overload, mitochondrial Ca2+ influx elevates oxidative stress and increases ROS production. In AD, accumulated ROS has profound effects on cellular functions by oxidizing several proteins, such as the redox-sensitive RyR and IP3R channels. Thus, Ca2+ induced ROS increase and ROS-mediated Ca2+ increase creates a self-amplifying loop that furthers neurotoxicity and cellular dyshomeostasis, and neuronal death [169,170,171,172]. In AD, MAM proteins are shown to be associated with presenilin 1 and 2, suggesting PSEN1/2 may alter mitochondrial Ca2+ transport. Additionally, increased contact sites between ER and mitochondria, via MAM proteins in AD result in elevation in ER-mitochondrial Ca2+ signaling and increased mitochondrial superoxide production [173,174,175,176,177,178].
Ca2+ is released from the mitochondria through the Na+/Ca2+ exchanger (NCLX) and the permeability transition pore [179]. The two functional states of the PTP regulates the amount of Ca2+ released; where the low conductance state amplifies Ca2+ waves and the high conductance state releases a surge of Ca2+ and apoptotic signals such as cytochrome C [180,181]. The biochemical signatures underlying apoptosis, rather than necrosis, indicates a choreographed and organized shutdown of the neuron. In AD, with continuous, prolonged increase in mitochondrial Ca2+ concentration, Ca2+ released from the mitochondria signals for apoptosis and increases AD pathology [172]. However the role of NCLX in AD needs further exploration as recent work suggests that impairment in glucose metabolism might reverse NCLX activity [182,183]. Additionally, impairment in NCLX accelerated memory declined and increased amyloidosis and tau pathology [184]. Mitochondrial calcium homeostasis may also rely on the activity of the plasma membrane NCLX, whose expression has been seen in differential patterns of mitochondrial expression dependent on cell type, and disruptions in expression may contribute to AD pathology [185,186,187].
Mitochondrial morphology and distribution are also crucial for neuronal homeostasis and synaptic function. Mitochondria undergo fusion and fission in the cytoplasm, which is a process to maintain a healthy pool of mitochondria with proper distribution. These mechanisms are controlled by DLP1 for fission and Mfn1, Mfn2, and OPA1 for fusion [188]. In AD, importantly, Aβ-induced and/or oxidative stress induced Ca2+ signaling led to increased DLP1 activation, resulting in excessive mitochondrial translocation and fission [189,190]. Recent studies reported that mitochondrial fragmentation, along with extensive oxidative stress and neuroinflammation, lead to neuronal loss in the cortex and hippocampus [191,192]. Excessive mitochondrial fragmentation as a result of improper Ca2+ handling and increased oxidative stress disrupts mitochondrial function, advancing AD pathology. Disrupted DLP1 and Mfn2 function is also responsible for reduced mitochondrial distribution. In AD, mitochondria are less abundant in neuronal processes of susceptible pyramidal neurons [193,194]. Increased tau phosphorylation negatively regulates mitochondrial movement in neurons. Tau phosphorylated at the AT8 sites inhibited mitochondrial movement in neurite processes of PC12 cells and mouse cortical neurons due to impaired microtubule spacing [195,196].
Damaged mitochondria are cleared through mitophagy, the selective degradation of mitochondria by autophagy following organelle damage or extreme cellular stress [197]. Mitophagy initiation involves the recruitment of PINK1 and PARKIN to the outer mitochondrial membrane, which tags the damaged mitochondria for degradation [198,199]. Additionally, mitophagy involves VDAC1 and MAM sensors, implicating the need for proper inter-organelle Ca2+ signaling and colocalization of the two organelles. Intracellular Ca2+ signaling relieves the inhibitory mammalian target of rapamycin (mTOR) block, thus activating mitophagy and initiating autophagosome biogenesis (ATG 32, 8, and 11) [200,201,202]. In AD patients, disruptions in mitophagy have been seen in the presence of Aβ, APP, and mutant PS1 expression. Aberrant inter-organelle Ca2+ signaling, as seen in AD, may disrupt degradation of damaged organelle via inactivation of lysosomal proteolysis and increase accumulation of cellular debris [198,199,203,204,205].
2.2. Ca2+ Dysregulation Impairs Lysosome-Autophagosome Mediated Protein Degradation
Lysosomal Ca2+ stores are responsible for regulating autophagy—a catabolic pathway utilizing the enzymatic activity of lysosomes to degrade and recycle large, bulky cellular debris, aggregated proteins, and damaged organelles. There are three types of autophagy: macroautophagy, microautophagy, and chaperone-mediated autophagy, however for simplicity, this review will focus on macroautophagy and be referred to as “autophagy”. More detailed descriptions of the aforementioned can be found in these reviews [163,206]. Autophagy is a systematic, dynamic degradation process regulated by the fusion of cargo vesicles, autophagosomes, to degradative compartments, lysosomes, with active hydrolases and proteases. While other cells rely on division to dilute cellular debris, neurons are specialized, post-mitotic cells that require efficient basal autophagy regulation to prevent accumulation of misfolded proteins and damaged organelles. Autophagy depends on the close proximity and communication between lysosomes and ER [207,208], therefore disruptions in inter-organelle Ca2+ signaling hinders clearance of pathological protein deposits.
Transcriptomic profiles from the collective ongoing studies known as Rush Memory and Aging Project (ROSMAP), reveal clusters of genes that are associated with pathological protein handling. Specifically, higher expression of SORL1 and ABCA7 transcripts are associated with tau tangle pathology, while elevated BIN1 transcripts are associated with beta amyloid in AD brains [209]. PLXNB1 abundance is associated with increased amyloid load and higher paired helical filaments (PHF) tau tangle density. Notably, BIN1, ABCA7, and SORL1 have functions in endocytic transport, APP metabolism and lysosome recycling, and thus are ideally positioned to serve a role in AD proteinopathy [210]. Altered expression of protein handling genes is linked to blunted endosomal trafficking, diminished degradative potential of lysosomes, and reduced autophagy-mediated clearance [210].
The key, critical feature of lysosomes is the acidic lumen (pH ~4.5) necessary for protein degradation and autophagosome digestion. The acidic pH is maintained by an active vacuolar-ATPase H+ pump (vATPase) driving the influx of H+ into the lysosome [211,212,213]. Genetic evidence linking endosomal H+ exchangers with AD suggest that proton leak pathways may regulate pathological Aβ generation and contribute to disease etiology [214]. In the ROSMAP AD population, there is downregulation of vATPase subunit (V1) genes, as well as the transcription factor “EB” (TFEB), a master regulator of lysosomal biogenesis that is associated with regulated autophagy [151]. Mutations in PS prevents the glycosylation, downstream maturation, and trafficking of the vATPase to the lysosome, resulting in an alkaline lysosomal lumen [215,216]. The alkaline environment inactivates protease activity, such as cathepsin B, which halts degradation of APP metabolites and dysregulates biogenesis of lysosomes and autophagosomes [217]. Additionally, cathepsins may also play a role in lysosomal trafficking along neuronal axons and dendrites, which is essential in mediating proper disposal of cellular debris. Studies showed that disrupting lysosomal proteolysis by inhibiting cathepsins or suppressing lysosomal acidification slowed axonal transport and caused selective accumulation within dystrophic neurites, a key feature of AD [218,219,220]. These are abnormally swollen regions of axons and dendrites filled mainly with autophagosomes and lysosomes, which implies improper transport of degradative organelles. Aberrant Ca2+ signaling, as seen in AD, can hinder lysosomal acidification and impair proteolytic enzymes in lysosomes, further AD proteinopathy and impair lysososmal trafficking, resulting in neuritic dystrophy.
Additionally, an increase in the lysosomal pH disrupts the homeostatic mechanisms to maintain the lysosomal membrane potential. The alkaline lumen depolarizes the lysosomal membrane via activation of lysosomal voltage-activated Na+ channels (lysoNaV). The Na+ efflux potentiates the influx of protons by the vATPase to restore lysosomal acidity. However, lysoNaV are also Ca2+ permeable, therefore aberrant Ca2+ increase and changes in the Ca2+ concentration gradient, as seen in early pathology of AD, can hinder lysosomal acidity by reducing the driving force of H+ influx to restore lysosomal function [213,221,222].
This shift to a more alkaline lysosomal lumen causes hyperactivity of the lysosomal Ca2+ efflux channels, lysosomal transient receptor potential Ca2+ channel mucolipin subfamily member (TRPML1) and two-pore channel (TPC) [205,215,216,223,224,225] (although debated [226,227,228]). Lysosomal Ca2+ efflux through TRPML1, activates a calcineurin-dependent pathway that, via TFEB, enhances the transcription of genes involved in autophagy and lysosomal expression, such as LC3-II, ATG9B, UVRAG, WIPI, SQSTM1, MAPLC3B, GLA, GNS, HEXA, MCOLN1, TMEM55B, and ATP6V1H [229,230,231].
This lysosomal-mediated Ca2+ release is also responsible for fusion of autophagosomes to lysosomes. In a manner similar to neuronal vesicular fusion, lysosomal Ca2+ efflux channels such as, P/Q type VGCC, facilitate fusion between lysosomal tethering proteins, such as synaptogamin 7, SNAP29, and SNARE VAMP 7/8, to autophagosomal tethering proteins such as syntaxin 17 and possible SNARE proteins [232,233,234,235]. Aberrant Ca2+ concentration, as in AD, can influence fusion of autophagosomes to malfunctioned lysosome, resulting in accumulation of cargo vesicles with maladaptive proteins, furthering neurotoxicity.
Recent studies have shown that lysosomal functions go beyond their primary role as the degradative compartment within a neuron. Lysosomal Ca2+ stores are also involved in maintaining synaptic transmission. When mGluR1 is activated, NAADP-evoked lysosomal Ca2+ release from lysosomal Ca2+ channels, presumably through TPC, is amplified into Ca2+ waves via RyR activation [236]. This signal inactivates SK channels and prevents local hyperpolarization, which allows for greater Ca2+ entry through GluN receptors and facilitates the induction of LTP [237]. When VGCC is activated, NAADP-evoked lysosomal Ca2+ release facilitates fusion to the plasma membrane and release of cathepsin B. Cathepsin B then regulates structural plasticity and dendritic spine formation [238,239], however in pathological conditions, protease activity is inhibited and therefore synaptic dysfunctions occur. Recent work has shown that blocking endogenous cathepsin inhibitors, such as cystatin B, decreases Aβ accumulation, autophagic-lysosomal pathology, and cognitive improvement in AD mice [240].
The systemic destruction of collective organelles leads to the neuron’s demise. The close proximity of the ER to the lysosome can enhance AD pathology (Figure 3) and PS mutations may alter the RyR-lysosomes trigger zone. In healthy neurons, RyR-mediated Ca2+ amplification suppresses autophagic flux [207] and induces LTP [237,238,239] however, increased Ca2+ signaling, as seen in neurodegenerative diseases, can alter this trigger zone and therefore disrupt autophagic clearance and synaptic plasticity.
Acknowledgments
The authors thank Nikki Barrington for editorial assistance in the preparation of this review.
Funding
This work was supported by NIH RF1 AG065628, R56 AG055497, and RF1 AG047237 (GES).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stutzmann G.E. The pathogenesis of Alzheimers disease is it a lifelong “calciumopathy”? Neuroscientist. 2007;13:546–559. doi: 10.1177/1073858407299730. [DOI] [PubMed] [Google Scholar]
- 2.Selkoe D.J. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 3.DeKosky S.T., Scheff S.W. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: Correlation with cognitive severity. Ann. Neurol. 1990;27:457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- 4.Terry R.D., Masliah E., Salmon D.P., Butters N., DeTeresa R., Hill R., Hansen L.A., Katzman R. Physical basis of cognitive alterations in Alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 5.Hsia A.Y., Masliah E., McConlogue L., Yu G.Q., Tatsuno G., Hu K., Kholodenko D., Malenka R.C., Nicoll R.A., Mucke L. Plaque-independent disruption of neural circuits in Alzheimer’s disease mouse models. Proc. Natl. Acad. Sci. USA. 1999;96:3228–3233. doi: 10.1073/pnas.96.6.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Busche M.A., Chen X., Henning H.A., Reichwald J., Staufenbiel M., Sakmann B., Konnerth A. Critical role of soluble amyloid-β for early hippocampal hyperactivity in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2012;109:8740–8745. doi: 10.1073/pnas.1206171109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Busche M.A., Eichhoff G., Adelsberger H., Abramowski D., Wiederhold K.-H., Haass C., Staufenbiel M., Konnerth A., Garaschuk O. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer’s disease. Science. 2008;321:1686–1689. doi: 10.1126/science.1162844. [DOI] [PubMed] [Google Scholar]
- 8.Chakroborty S., Hill E.S., Christian D.T., Helfrich R., Riley S., Schneider C., Kapecki N., Mustaly-Kalimi S., Seiler F.A., Peterson D.A., et al. Reduced presynaptic vesicle stores mediate cellular and network plasticity defects in an early-stage mouse model of Alzheimer’s disease. Mol. Neurodegener. 2019;14:7. doi: 10.1186/s13024-019-0307-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goussakov I., Miller M.B., Stutzmann G.E. NMDA-mediated Ca2+ influx drives aberrant ryanodine receptor activation in dendrites of young Alzheimer’s disease mice. J. Neurosci. 2010;30:12128–12137. doi: 10.1523/JNEUROSCI.2474-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murchison D., McDermott A.N., LaSarge C.L., Peebles K.A., Bizon J.L., Griffith W.H. Enhanced Calcium Buffering in F344 Rat Cholinergic Basal Forebrain Neurons Is Associated with Age-Related Cognitive Impairment. J. Neurophysiol. 2009;102:2194–2207. doi: 10.1152/jn.00301.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moyer J.R., Furtak S.C., McGann J.P., Brown T.H. Aging-related changes in calcium binding proteins in rat perirhinal cortex. Neurobiol. Aging. 2011;32:1693–1706. doi: 10.1016/j.neurobiolaging.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oh M.M., Oliveira F.A., Waters J., Disterhoft J.F. Altered Calcium Metabolism in Aging CA1 Hippocampal Pyramidal Neurons. J. Neurosci. 2013;33:7905–7911. doi: 10.1523/JNEUROSCI.5457-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuchibhotla K.V., Goldman S.T., Lattarulo C.R., Wu H.-Y., Hyman B.T., Bacskai B.J. Abeta plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron. 2008;59:214–225. doi: 10.1016/j.neuron.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez J.R., Lyckman A., Oddo S., Laferla F.M., Querfurth H.W., Shtifman A. Increased intraneuronal resting [Ca2+] in adult Alzheimer’s disease mice. J. Neurochem. 2008;105:262–271. doi: 10.1111/j.1471-4159.2007.05135.x. [DOI] [PubMed] [Google Scholar]
- 15.Uryash A., Flores V., Adams J.A., Allen P.D., Lopez J.R. Memory and Learning Deficits Are Associated With Ca2+ Dyshomeostasis in Normal Aging. Front. Aging Neurosci. 2020;12:224. doi: 10.3389/fnagi.2020.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thibault O., Hadley R., Landfield P.W. Elevated postsynaptic [Ca2+]i and L-type calcium channel activity in aged hippocampal neurons: Relationship to impaired synaptic plasticity. J. Neurosci. 2001;21:9744–9756. doi: 10.1523/JNEUROSCI.21-24-09744.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bliss T.V., Collingridge G.L. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 18.Talantova M., Sanz-Blasco S., Zhang X., Xia P., Akhtar M.W., Okamoto S., Dziewczapolski G., Nakamura T., Cao G., Pratt A.E., et al. Aβ induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss. Proc. Natl. Acad. Sci. USA. 2013;110:E2518–E2527. doi: 10.1073/pnas.1306832110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan J., Bengtson C.P., Buchthal B., Hagenston A.M., Bading H. Coupling of NMDA receptors and TRPM4 guides discovery of unconventional neuroprotectants. Science. 2020;370 doi: 10.1126/science.aay3302. [DOI] [PubMed] [Google Scholar]
- 20.Winblad B., Jones R.W., Wirth Y., Stöffler A., Möbius H.J. Memantine in moderate to severe Alzheimer’s disease: A meta-analysis of randomised clinical trials. Dement. Geriatr. Cogn. Disord. 2007;24:20–27. doi: 10.1159/000102568. [DOI] [PubMed] [Google Scholar]
- 21.Gauthier S., Loft H., Cummings J. Improvement in behavioural symptoms in patients with moderate to severe Alzheimer’s disease by memantine: A pooled data analysis. Int. J. Geriatr. Psychiatry. 2008;23:537–545. doi: 10.1002/gps.1949. [DOI] [PubMed] [Google Scholar]
- 22.Copenhaver P.F., Anekonda T.S., Musashe D., Robinson K.M., Ramaker J.M., Swanson T.L., Wadsworth T.L., Kretzschmar D., Woltjer R.L., Quinn J.F. A translational continuum of model systems for evaluating treatment strategies in Alzheimer’s disease: Isradipine as a candidate drug. Dis. Model. Mech. 2011;4:634–648. doi: 10.1242/dmm.006841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scheff S.W., Price D.A., Schmitt F.A., Mufson E.J. Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol. Aging. 2006;27:1372–1384. doi: 10.1016/j.neurobiolaging.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Jacobsen J.S., Wu C.-C., Redwine J.M., Comery T.A., Arias R., Bowlby M., Martone R., Morrison J.H., Pangalos M.N., Reinhart P.H., et al. Early-onset behavioral and synaptic deficits in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2006;103:5161–5166. doi: 10.1073/pnas.0600948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spires T.L., Meyer-Luehmann M., Stern E.A., McLean P.J., Skoch J., Nguyen P.T., Bacskai B.J., Hyman B.T. Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. J. Neurosci. 2005;25:7278–7287. doi: 10.1523/JNEUROSCI.1879-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lanz T.A., Carter D.B., Merchant K.M. Dendritic spine loss in the hippocampus of young PDAPP and Tg2576 mice and its prevention by the ApoE2 genotype. Neurobiol. Dis. 2003;13:246–253. doi: 10.1016/S0969-9961(03)00079-2. [DOI] [PubMed] [Google Scholar]
- 27.Fitzjohn S.M., Morton R.A., Kuenzi F., Rosahl T.W., Shearman M., Lewis H., Smith D., Reynolds D.S., Davies C.H., Collingridge G.L., et al. Age-related impairment of synaptic transmission but normal long-term potentiation in transgenic mice that overexpress the human APP695SWE mutant form of amyloid precursor protein. J. Neurosci. 2001;21:4691–4698. doi: 10.1523/JNEUROSCI.21-13-04691.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chapman P.F., White G.L., Jones M.W., Cooper-Blacketer D., Marshall V.J., Irizarry M., Younkin L., Good M.A., Bliss T.V., Hyman B.T., et al. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat. Neurosci. 1999;2:271–276. doi: 10.1038/6374. [DOI] [PubMed] [Google Scholar]
- 29.Larson J., Lynch G., Games D., Seubert P. Alterations in synaptic transmission and long-term potentiation in hippocampal slices from young and aged PDAPP mice. Brain Res. 1999;840:23–35. doi: 10.1016/S0006-8993(99)01698-4. [DOI] [PubMed] [Google Scholar]
- 30.Chakroborty S., Goussakov I., Miller M.B., Stutzmann G.E. Deviant ryanodine receptor-mediated calcium release resets synaptic homeostasis in presymptomatic 3xTg-AD mice. J. Neurosci. 2009;29:9458–9470. doi: 10.1523/JNEUROSCI.2047-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang H., Wu L., Pchitskaya E., Zakharova O., Saito T., Saido T., Bezprozvanny I. Neuronal Store-Operated Calcium Entry and Mushroom Spine Loss in Amyloid Precursor Protein Knock-In Mouse Model of Alzheimer’s Disease. J. Neurosci. 2015;35:13275–13286. doi: 10.1523/JNEUROSCI.1034-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lue L.F., Kuo Y.M., Roher A.E., Brachova L., Shen Y., Sue L., Beach T., Kurth J.H., Rydel R.E., Rogers J. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer’s disease. Am. J. Pathol. 1999;155:853–862. doi: 10.1016/S0002-9440(10)65184-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arbel-Ornath M., Hudry E., Boivin J.R., Hashimoto T., Takeda S., Kuchibhotla K.V., Hou S., Lattarulo C.R., Belcher A.M., Shakerdge N., et al. Soluble oligomeric amyloid-β induces calcium dyshomeostasis that precedes synapse loss in the living mouse brain. Mol. Neurodegener. 2017;12:27. doi: 10.1186/s13024-017-0169-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calabrese B., Shaked G.M., Tabarean I.V., Braga J., Koo E.H., Halpain S. Rapid, concurrent alterations in pre- and postsynaptic structure induced by naturally-secreted amyloid-β protein. Mol. Cell. Neurosci. 2007;35:183–193. doi: 10.1016/j.mcn.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shankar G.M., Bloodgood B.L., Townsend M., Walsh D.M., Selkoe D.J., Sabatini B.L. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J. Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Esteras N., Kundel F., Amodeo G.F., Pavlov E.V., Klenerman D., Abramov A.Y. Insoluble tau aggregates induce neuronal death through modification of membrane ion conductance, activation of voltage-gated calcium channels and NADPH oxidase. FEBS J. 2020 doi: 10.1111/febs.15340. [DOI] [PubMed] [Google Scholar]
- 37.Zhou L., McInnes J., Wierda K., Holt M., Herrmann A.G., Jackson R.J., Wang Y.-C., Swerts J., Beyens J., Miskiewicz K., et al. Tau association with synaptic vesicles causes presynaptic dysfunction. Nat. Commun. 2017;8:15295. doi: 10.1038/ncomms15295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gómez-Ramos A., Díaz-Hernández M., Cuadros R., Hernández F., Avila J. Extracellular tau is toxic to neuronal cells. FEBS Lett. 2006;580:4842–4850. doi: 10.1016/j.febslet.2006.07.078. [DOI] [PubMed] [Google Scholar]
- 39.Yin Y., Gao D., Wang Y., Wang Z.-H., Wang X., Ye J., Wu D., Fang L., Pi G., Yang Y., et al. Tau accumulation induces synaptic impairment and memory deficit by calcineurin-mediated inactivation of nuclear CaMKIV/CREB signaling. Proc. Natl. Acad. Sci. USA. 2016;113:E3773–E3781. doi: 10.1073/pnas.1604519113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keskin A.D., Kekuš M., Adelsberger H., Neumann U., Shimshek D.R., Song B., Zott B., Peng T., Förstl H., Staufenbiel M., et al. BACE inhibition-dependent repair of Alzheimer’s pathophysiology. Proc. Natl. Acad. Sci. USA. 2017;114:8631–8636. doi: 10.1073/pnas.1708106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koffie R.M., Meyer-Luehmann M., Hashimoto T., Adams K.W., Mielke M.L., Garcia-Alloza M., Micheva K.D., Smith S.J., Kim M.L., Lee V.M., et al. Oligomeric amyloid beta associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc. Natl. Acad. Sci. USA. 2009;106:4012–4017. doi: 10.1073/pnas.0811698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Briggs C.A., Schneider C., Richardson J.C., Stutzmann G.E. β amyloid peptide plaques fail to alter evoked neuronal calcium signals in APP/PS1 Alzheimer’s disease mice. Neurobiol. Aging. 2013;34:1632–1643. doi: 10.1016/j.neurobiolaging.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lerdkrai C., Asavapanumas N., Brawek B., Kovalchuk Y., Mojtahedi N., del Moral M.O., Garaschuk O. Intracellular Ca2+ stores control in vivo neuronal hyperactivity in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2018;115:E1279–E1288. doi: 10.1073/pnas.1714409115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He Y., Wei M., Wu Y., Qin H., Li W., Ma X., Cheng J., Ren J., Shen Y., Chen Z., et al. Amyloid β oligomers suppress excitatory transmitter release via presynaptic depletion of phosphatidylinositol-4,5-bisphosphate. Nat. Commun. 2019;10:1193. doi: 10.1038/s41467-019-09114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oddo S., Caccamo A., Shepherd J.D., Murphy M.P., Golde T.E., Kayed R., Metherate R., Mattson M.P., Akbari Y., LaFerla F.M. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: Intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/S0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 46.Chakroborty S., Kim J., Schneider C., Jacobson C., Molgó J., Stutzmann G.E. Early presynaptic and postsynaptic calcium signaling abnormalities mask underlying synaptic depression in presymptomatic Alzheimer’s disease mice. J. Neurosci. 2012;32:8341–8353. doi: 10.1523/JNEUROSCI.0936-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yao J., Sun B., Institoris A., Zhan X., Guo W., Song Z., Liu Y., Hiess F., Boyce A.K.J., Ni M., et al. Limiting RyR2 Open Time Prevents Alzheimer’s Disease-Related Neuronal Hyperactivity and Memory Loss but Not β-Amyloid Accumulation. Cell Rep. 2020;32:108169. doi: 10.1016/j.celrep.2020.108169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kindler C.H., Eilers H., Donohoe P., Ozer S., Bickler P.E. Volatile anesthetics increase intracellular calcium in cerebrocortical and hippocampal neurons. Anesthesiology. 1999;90:1137–1145. doi: 10.1097/00000542-199904000-00029. [DOI] [PubMed] [Google Scholar]
- 49.Wei H., Liang G., Yang H., Wang Q., Hawkins B., Madesh M., Wang S., Eckenhoff R.G. The Common Inhalational Anesthetic Isoflurane Induces Apoptosis via Activation of Inositol 1,4,5-Trisphosphate Receptors. Anesthesiol. J. Am. Soc. Anesthesiol. 2008;108:251–260. doi: 10.1097/01.anes.0000299435.59242.0e. [DOI] [PubMed] [Google Scholar]
- 50.Stutzmann G.E., Smith I., Caccamo A., Oddo S., Laferla F.M., Parker I. Enhanced ryanodine receptor recruitment contributes to Ca2+ disruptions in young, adult, and aged Alzheimer’s disease mice. J. Neurosci. 2006;26:5180–5189. doi: 10.1523/JNEUROSCI.0739-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghatak S., Dolatabadi N., Gao R., Wu Y., Scott H., Trudler D., Sultan A., Ambasudhan R., Nakamura T., Masliah E., et al. NitroSynapsin ameliorates hypersynchronous neural network activity in Alzheimer hiPSC models. Mol. Psychiatry. 2020 doi: 10.1038/s41380-020-0776-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghatak S., Dolatabadi N., Trudler D., Zhang X., Wu Y., Mohata M., Ambasudhan R., Talantova M., Lipton S.A. Mechanisms of hyperexcitability in Alzheimer’s disease hiPSC-derived neurons and cerebral organoids vs. isogenic controls. eLife. 2019;8 doi: 10.7554/eLife.50333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schrank S., McDaid J., Briggs C.A., Mustaly-Kalimi S., Brinks D., Houcek A., Singer O., Bottero V., Marr R.A., Stutzmann G.E. Human-Induced Neurons from Presenilin 1 Mutant Patients Model Aspects of Alzheimer’s Disease Pathology. Int. J. Mol. Sci. 2020;21:1030. doi: 10.3390/ijms21031030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huijbers W., Mormino E.C., Schultz A.P., Wigman S., Ward A.M., Larvie M., Amariglio R.E., Marshall G.A., Rentz D.M., Johnson K.A., et al. Amyloid-β deposition in mild cognitive impairment is associated with increased hippocampal activity, atrophy and clinical progression. Brain J. Neurol. 2015;138:1023–1035. doi: 10.1093/brain/awv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hämäläinen A., Pihlajamäki M., Tanila H., Hänninen T., Niskanen E., Tervo S., Karjalainen P.A., Vanninen R.L., Soininen H. Increased fMRI responses during encoding in mild cognitive impairment. Neurobiol. Aging. 2007;28:1889–1903. doi: 10.1016/j.neurobiolaging.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 56.Drummond E., Wisniewski T. Using Proteomics to Understand Alzheimer’s Disease Pathogenesis. In: Wisniewski T., editor. Alzheimer’s Disease. Codon Publications; Brisbane, Australia: 2019. [PubMed] [Google Scholar]
- 57.Honer W.G., Ramos-Miguel A., Alamri J., Sawada K., Barr A.M., Schneider J.A., Bennett D.A. The synaptic pathology of cognitive life. Dialogues Clin. Neurosci. 2019;21:271–279. doi: 10.31887/DCNS.2019.21.3/whoner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bennett D.A., Buchman A.S., Boyle P.A., Barnes L.L., Wilson R.S., Schneider J.A. Religious Orders Study and Rush Memory and Aging Project. J. Alzheimers Dis. JAD. 2018;64:S161–S189. doi: 10.3233/JAD-179939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dieterich D.C., Kreutz M.R. Proteomics of the Synapse—A Quantitative Approach to Neuronal Plasticity. Mol. Cell. Proteomics MCP. 2016;15:368–381. doi: 10.1074/mcp.R115.051482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Honer W.G., Barr A.M., Sawada K., Thornton A.E., Morris M.C., Leurgans S.E., Schneider J.A., Bennett D.A. Cognitive reserve, presynaptic proteins and dementia in the elderly. Transl. Psychiatry. 2012;2:e114. doi: 10.1038/tp.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jeans A.F., Oliver P.L., Johnson R., Capogna M., Vikman J., Molnár Z., Babbs A., Partridge C.J., Salehi A., Bengtsson M., et al. A dominant mutation in Snap25 causes impaired vesicle trafficking, sensorimotor gating, and ataxia in the blind-drunk mouse. Proc. Natl. Acad. Sci. USA. 2007;104:2431–2436. doi: 10.1073/pnas.0610222104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Toft-Bertelsen T.L., Ziomkiewicz I., Houy S., Pinheiro P.S., Sørensen J.B. Regulation of Ca2+ channels by SNAP-25 via recruitment of syntaxin-1 from plasma membrane clusters. Mol. Biol. Cell. 2016;27:3329–3341. doi: 10.1091/mbc.E16-03-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ramos-Miguel A., Sawada K., Jones A.A., Thornton A.E., Barr A.M., Leurgans S.E., Schneider J.A., Bennett D.A., Honer W.G. Presynaptic proteins complexin-I and complexin-II differentially influence cognitive function in early and late stages of Alzheimer’s disease. Acta Neuropathol. 2017;133:395–407. doi: 10.1007/s00401-016-1647-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Verderio C., Pozzi D., Pravettoni E., Inverardi F., Schenk U., Coco S., Proux-Gillardeaux V., Galli T., Rossetto O., Frassoni C., et al. SNAP-25 Modulation of Calcium Dynamics Underlies Differences in GABAergic and Glutamatergic Responsiveness to Depolarization. Neuron. 2004;41:599–610. doi: 10.1016/S0896-6273(04)00077-7. [DOI] [PubMed] [Google Scholar]
- 65.Malsam J., Bärfuss S., Trimbuch T., Zarebidaki F., Sonnen A.F.-P., Wild K., Scheutzow A., Rohland L., Mayer M.P., Sinning I., et al. Complexin Suppresses Spontaneous Exocytosis by Capturing the Membrane-Proximal Regions of VAMP2 and SNAP25. Cell Rep. 2020;32:107926. doi: 10.1016/j.celrep.2020.107926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rettig J., Sheng Z.H., Kim D.K., Hodson C.D., Snutch T.P., Catterall W.A. Isoform-specific interaction of the alpha1A subunits of brain Ca2+ channels with the presynaptic proteins syntaxin and SNAP-25. Proc. Natl. Acad. Sci. USA. 1996;93:7363–7368. doi: 10.1073/pnas.93.14.7363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Antonucci F., Corradini I., Morini R., Fossati G., Menna E., Pozzi D., Pacioni S., Verderio C., Bacci A., Matteoli M. Reduced SNAP-25 alters short-term plasticity at developing glutamatergic synapses. EMBO Rep. 2013;14:645–651. doi: 10.1038/embor.2013.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vogl C., Mochida S., Wolff C., Whalley B.J., Stephens G.J. The synaptic vesicle glycoprotein 2A ligand levetiracetam inhibits presynaptic Ca2+ channels through an intracellular pathway. Mol. Pharmacol. 2012;82:199–208. doi: 10.1124/mol.111.076687. [DOI] [PubMed] [Google Scholar]
- 69.Cumbo E., Ligori L.D. Levetiracetam, lamotrigine, and phenobarbital in patients with epileptic seizures and Alzheimer’s disease. Epilepsy Behav. EB. 2010;17:461–466. doi: 10.1016/j.yebeh.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 70.Newhouse P.A., Potter A., Corwin J., Lenox R. Acute nicotinic blockade produces cognitive impairment in normal humans. Psychopharmacology. 1992;108:480–484. doi: 10.1007/BF02247425. [DOI] [PubMed] [Google Scholar]
- 71.Kadir A., Almkvist O., Wall A., Långström B., Nordberg A. PET imaging of cortical 11C-nicotine binding correlates with the cognitive function of attention in Alzheimer’s disease. Psychopharmacology. 2006;188:509–520. doi: 10.1007/s00213-006-0447-7. [DOI] [PubMed] [Google Scholar]
- 72.Fucile S. Ca2+ permeability of nicotinic acetylcholine receptors. Cell Calcium. 2004;35:1–8. doi: 10.1016/j.ceca.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 73.Séguéla P., Wadiche J., Dineley-Miller K., Dani J.A., Patrick J.W. Molecular cloning, functional properties, and distribution of rat brain alpha 7: A nicotinic cation channel highly permeable to calcium. J. Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thinschmidt J.S., Frazier C.J., King M.A., Meyer E.M., Papke R.L. Medial septal/diagonal band cells express multiple functional nicotinic receptor subtypes that are correlated with firing frequency. Neurosci. Lett. 2005;389:163–168. doi: 10.1016/j.neulet.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 75.Davies P., Maloney A.J. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet Lond. Engl. 1976;2:1403. doi: 10.1016/S0140-6736(76)91936-X. [DOI] [PubMed] [Google Scholar]
- 76.Mufson E.J., Counts S.E., Perez S.E., Ginsberg S.D. Cholinergic system during the progression of Alzheimer’s disease: Therapeutic implications. Expert Rev. Neurother. 2008;8:1703–1718. doi: 10.1586/14737175.8.11.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grybko M., Sharma G., Vijayaraghavan S. Functional distribution of nicotinic receptors in CA3 region of the hippocampus. J. Mol. Neurosci. MN. 2010;40:114–120. doi: 10.1007/s12031-009-9266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Frazier C.J., Rollins Y.D., Breese C.R., Leonard S., Freedman R., Dunwiddie T.V. Acetylcholine activates an alpha-bungarotoxin-sensitive nicotinic current in rat hippocampal interneurons, but not pyramidal cells. J. Neurosci. 1998;18:1187–1195. doi: 10.1523/JNEUROSCI.18-04-01187.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Khiroug L., Giniatullin R., Klein R.C., Fayuk D., Yakel J.L. Functional mapping and Ca2+ regulation of nicotinic acetylcholine receptor channels in rat hippocampal CA1 neurons. J. Neurosci. 2003;23:9024–9031. doi: 10.1523/JNEUROSCI.23-27-09024.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gray R., Rajan A.S., Radcliffe K.A., Yakehiro M., Dani J.A. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996;383:713–716. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- 81.Radcliffe K.A., Dani J.A. Nicotinic stimulation produces multiple forms of increased glutamatergic synaptic transmission. J. Neurosci. 1998;18:7075–7083. doi: 10.1523/JNEUROSCI.18-18-07075.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fayuk D., Yakel J.L. Ca2+ permeability of nicotinic acetylcholine receptors in rat hippocampal CA1 interneurones. J. Physiol. 2005;566:759–768. doi: 10.1113/jphysiol.2005.089789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cheng Q., Yakel J.L. Presynaptic α7 nicotinic acetylcholine receptors enhance hippocampal mossy fiber glutamatergic transmission via PKA activation. J. Neurosci. 2014;34:124–133. doi: 10.1523/JNEUROSCI.2973-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sharma G., Grybko M., Vijayaraghavan S. Action potential-independent and nicotinic receptor-mediated concerted release of multiple quanta at hippocampal CA3-mossy fiber synapses. J. Neurosci. 2008;28:2563–2575. doi: 10.1523/JNEUROSCI.5407-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Townsend M., Whyment A., Walczak J.-S., Jeggo R., van den Top M., Flood D.G., Leventhal L., Patzke H., Koenig G. α7-nAChR agonist enhances neural plasticity in the hippocampus via a GABAergic circuit. J. Neurophysiol. 2016;116:2663–2675. doi: 10.1152/jn.00243.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang H.Y., Lee D.H., D’Andrea M.R., Peterson P.A., Shank R.P., Reitz A.B. beta-Amyloid(1-42) binds to alpha7 nicotinic acetylcholine receptor with high affinity. Implications for Alzheimer’s disease pathology. J. Biol. Chem. 2000;275:5626–5632. doi: 10.1074/jbc.275.8.5626. [DOI] [PubMed] [Google Scholar]
- 87.Wang H.Y., Lee D.H., Davis C.B., Shank R.P. Amyloid peptide Abeta(1-42) binds selectively and with picomolar affinity to alpha7 nicotinic acetylcholine receptors. J. Neurochem. 2000;75:1155–1161. doi: 10.1046/j.1471-4159.2000.0751155.x. [DOI] [PubMed] [Google Scholar]
- 88.Liu Q., Kawai H., Berg D.K. beta -Amyloid peptide blocks the response of alpha 7-containing nicotinic receptors on hippocampal neurons. Proc. Natl. Acad. Sci. USA. 2001;98:4734–4739. doi: 10.1073/pnas.081553598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Burghaus L., Schütz U., Krempel U., de Vos R.A., Jansen Steur E.N., Wevers A., Lindstrom J., Schröder H. Quantitative assessment of nicotinic acetylcholine receptor proteins in the cerebral cortex of Alzheimer patients. Brain Res. Mol. Brain Res. 2000;76:385–388. doi: 10.1016/S0169-328X(00)00031-0. [DOI] [PubMed] [Google Scholar]
- 90.Guan Z.-Z., Zhang X., Ravid R., Nordberg A. Decreased Protein Levels of Nicotinic Receptor Subunits in the Hippocampus and Temporal Cortex of Patients with Alzheimer’s Disease. J. Neurochem. 2000;74:237–243. doi: 10.1046/j.1471-4159.2000.0740237.x. [DOI] [PubMed] [Google Scholar]
- 91.Oddo S., Caccamo A., Green K.N., Liang K., Tran L., Chen Y., Leslie F.M., LaFerla F.M. Chronic nicotine administration exacerbates tau pathology in a transgenic model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2005;102:3046–3051. doi: 10.1073/pnas.0408500102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.D’Andrea M.R., Nagele R.G. Targeting the alpha 7 nicotinic acetylcholine receptor to reduce amyloid accumulation in Alzheimer’s disease pyramidal neurons. Curr. Pharm. Des. 2006;12:677–684. doi: 10.2174/138161206775474224. [DOI] [PubMed] [Google Scholar]
- 93.Dougherty J.J., Wu J., Nichols R.A. Beta-amyloid regulation of presynaptic nicotinic receptors in rat hippocampus and neocortex. J. Neurosci. 2003;23:6740–6747. doi: 10.1523/JNEUROSCI.23-17-06740.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dineley K.T., Bell K.A., Bui D., Sweatt J.D. beta-Amyloid peptide activates alpha 7 nicotinic acetylcholine receptors expressed in Xenopus oocytes. J. Biol. Chem. 2002;277:25056–25061. doi: 10.1074/jbc.M200066200. [DOI] [PubMed] [Google Scholar]
- 95.Hascup K.N., Hascup E.R. Soluble Amyloid-β42 Stimulates Glutamate Release through Activation of the α7 Nicotinic Acetylcholine Receptor. J. Alzheimers Dis. JAD. 2016;53:337–347. doi: 10.3233/JAD-160041. [DOI] [PubMed] [Google Scholar]
- 96.Puzzo D., Privitera L., Leznik E., Fà M., Staniszewski A., Palmeri A., Arancio O. Picomolar Amyloid-β Positively Modulates Synaptic Plasticity and Memory in Hippocampus. J. Neurosci. 2008;28:14537–14545. doi: 10.1523/JNEUROSCI.2692-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kroker K.S., Moreth J., Kussmaul L., Rast G., Rosenbrock H. Restoring long-term potentiation impaired by amyloid-beta oligomers: Comparison of an acetylcholinesterase inhibitior and selective neuronal nicotinic receptor agonists. Brain Res. Bull. 2013;96:28–38. doi: 10.1016/j.brainresbull.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 98.Ondrejcak T., Wang Q., Kew J.N.C., Virley D.J., Upton N., Anwyl R., Rowan M.J. Activation of α7 nicotinic acetylcholine receptors persistently enhances hippocampal synaptic transmission and prevents Aß-mediated inhibition of LTP in the rat hippocampus. Eur. J. Pharmacol. 2012;677:63–70. doi: 10.1016/j.ejphar.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 99.Medeiros R., Castello N.A., Cheng D., Kitazawa M., Baglietto-Vargas D., Green K.N., Esbenshade T.A., Bitner R.S., Decker M.W., LaFerla F.M. α7 Nicotinic receptor agonist enhances cognition in aged 3xTg-AD mice with robust plaques and tangles. Am. J. Pathol. 2014;184:520–529. doi: 10.1016/j.ajpath.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 100.Shen H., Kihara T., Hongo H., Wu X., Kem W.R., Shimohama S., Akaike A., Niidome T., Sugimoto H. Neuroprotection by donepezil against glutamate excitotoxicity involves stimulation of alpha7 nicotinic receptors and internalization of NMDA receptors. Br. J. Pharmacol. 2010;161:127–139. doi: 10.1111/j.1476-5381.2010.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Texidó L., Ros E., Martín-Satué M., López S., Aleu J., Marsal J., Solsona C. Effect of galantamine on the human alpha7 neuronal nicotinic acetylcholine receptor, the Torpedo nicotinic acetylcholine receptor and spontaneous cholinergic synaptic activity. Br. J. Pharmacol. 2005;145:672–678. doi: 10.1038/sj.bjp.0706221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang T., Xiao T., Sun Q., Wang K. The current agonists and positive allosteric modulators of α 7 nAChR for CNS indications in clinical trials. Acta Pharm. Sin. B. 2017;7:611–622. doi: 10.1016/j.apsb.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Deardorff W.J., Shobassy A., Grossberg G.T. Safety and clinical effects of EVP-6124 in subjects with Alzheimer’s disease currently or previously receiving an acetylcholinesterase inhibitor medication. Expert Rev. Neurother. 2015;15:7–17. doi: 10.1586/14737175.2015.995639. [DOI] [PubMed] [Google Scholar]
- 104.Barbier A.J., Hilhorst M., Van Vliet A., Snyder P., Palfreyman M.G., Gawryl M., Dgetluck N., Massaro M., Tiessen R., Timmerman W., et al. Pharmacodynamics, pharmacokinetics, safety, and tolerability of encenicline, a selective α7 nicotinic receptor partial agonist, in single ascending-dose and bioavailability studies. Clin. Ther. 2015;37:311–324. doi: 10.1016/j.clinthera.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 105.Gee K.W., Olincy A., Kanner R., Johnson L., Hogenkamp D., Harris J., Tran M., Edmonds S.A., Sauer W., Yoshimura R., et al. First in human trial of a type I positive allosteric modulator of alpha7-nicotinic acetylcholine receptors: Pharmacokinetics, safety, and evidence for neurocognitive effect of AVL-3288. J. Psychopharmacol. Oxf. Engl. 2017;31:434–441. doi: 10.1177/0269881117691590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dajas-Bailador F.A., Mogg A.J., Wonnacott S. Intracellular Ca2+ signals evoked by stimulation of nicotinic acetylcholine receptors in SH-SY5Y cells: Contribution of voltage-operated Ca2+ channels and Ca2+ stores. J. Neurochem. 2002;81:606–614. doi: 10.1046/j.1471-4159.2002.00846.x. [DOI] [PubMed] [Google Scholar]
- 107.Sharma G., Vijayaraghavan S. Modulation of presynaptic store calcium induces release of glutamate and postsynaptic firing. Neuron. 2003;38:929–939. doi: 10.1016/S0896-6273(03)00322-2. [DOI] [PubMed] [Google Scholar]
- 108.Padua R.A., Nagy J.I., Geiger J.D. Subcellular localization of ryanodine receptors in rat brain. Eur. J. Pharmacol. 1996;298:185–189. doi: 10.1016/0014-2999(95)00797-0. [DOI] [PubMed] [Google Scholar]
- 109.Guerra-Álvarez M., Moreno-Ortega A.J., Navarro E., Fernández-Morales J.C., Egea J., López M.G., Cano-Abad M.F. Positive allosteric modulation of alpha-7 nicotinic receptors promotes cell death by inducing Ca2+ release from the endoplasmic reticulum. J. Neurochem. 2015;133:309–319. doi: 10.1111/jnc.13049. [DOI] [PubMed] [Google Scholar]
- 110.Lacampagne A., Liu X., Reiken S., Bussiere R., Meli A.C., Lauritzen I., Teich A.F., Zalk R., Saint N., Arancio O., et al. Post-translational remodeling of ryanodine receptor induces calcium leak leading to Alzheimer’s disease-like pathologies and cognitive deficits. Acta Neuropathol. 2017;134:749–767. doi: 10.1007/s00401-017-1733-7. [DOI] [PubMed] [Google Scholar]
- 111.Krajnak K., Dahl R. A new target for Alzheimer’s disease: A small molecule SERCA activator is neuroprotective in vitro and improves memory and cognition in APP/PS1 mice. Bioorg. Med. Chem. Lett. 2018;28:1591–1594. doi: 10.1016/j.bmcl.2018.03.052. [DOI] [PubMed] [Google Scholar]
- 112.Mihalak K.B., Carroll F.I., Luetje C.W. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol. Pharmacol. 2006;70:801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- 113.Kim S.Y., Choi S.H., Rollema H., Schwam E.M., McRae T., Dubrava S., Jacobsen J. Phase II crossover trial of varenicline in mild-to-moderate Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2014;37:232–245. doi: 10.1159/000355373. [DOI] [PubMed] [Google Scholar]
- 114.Chakroborty S., Briggs C., Miller M.B., Goussakov I., Schneider C., Kim J., Wicks J., Richardson J.C., Conklin V., Cameransi B.G., et al. Stabilizing ER Ca2+ Channel Function as an Early Preventative Strategy for Alzheimer’s Disease. PLoS ONE. 2012;7:e52056. doi: 10.1371/journal.pone.0052056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Oulès B., Del Prete D., Greco B., Zhang X., Lauritzen I., Sevalle J., Moreno S., Paterlini-Bréchot P., Trebak M., Checler F., et al. Ryanodine Receptor Blockade Reduces Amyloid-β Load and Memory Impairments in Tg2576 Mouse Model of Alzheimer Disease. J. Neurosci. 2012;32:11820–11834. doi: 10.1523/JNEUROSCI.0875-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Peng J., Liang G., Inan S., Wu Z., Joseph D.J., Meng Q., Peng Y., Eckenhoff M.F., Wei H. Dantrolene ameliorates cognitive decline and neuropathology in Alzheimer triple transgenic mice. Neurosci. Lett. 2012;516:274–279. doi: 10.1016/j.neulet.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang J., Shi Y., Yu S., Wang Y., Meng Q., Liang G., Eckenhoff M.F., Wei H. Intranasal administration of dantrolene increased brain concentration and duration. PLoS ONE. 2020;15:e0229156. doi: 10.1371/journal.pone.0229156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lawlor B., Segurado R., Kennelly S., Olde Rikkert M.G.M., Howard R., Pasquier F., Börjesson-Hanson A., Tsolaki M., Lucca U., Molloy D.W., et al. Nilvadipine in mild to moderate Alzheimer disease: A randomised controlled trial. PLoS Med. 2018;15:e1002660. doi: 10.1371/journal.pmed.1002660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Study of AGB101 in Mild Cognitive Impairment Due to Alzheimer’s Disease—Full Text View—ClinicalTrials.gov. [(accessed on 15 August 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03486938.
- 120.Sharp A.H., McPherson P.S., Dawson T.M., Aoki C., Campbell K.P., Snyder S.H. Differential immunohistochemical localization of inositol 1,4,5-trisphosphate- and ryanodine-sensitive Ca2+ release channels in rat brain. J. Neurosci. 1993;13:3051–3063. doi: 10.1523/JNEUROSCI.13-07-03051.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu B., Yamaguchi H., Lai F.A., Shen J. Presenilins regulate calcium homeostasis and presynaptic function via ryanodine receptors in hippocampal neurons. Proc. Natl. Acad. Sci. USA. 2013;110:15091–15096. doi: 10.1073/pnas.1304171110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Payne A.J., Gerdes B.C., Naumchuk Y., McCalley A.E., Kaja S., Koulen P. Presenilins regulate the cellular activity of ryanodine receptors differentially through isotype-specific N-terminal cysteines. Exp. Neurol. 2013;250:143–150. doi: 10.1016/j.expneurol.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cheung K.-H., Shineman D., Müller M., Cárdenas C., Mei L., Yang J., Tomita T., Iwatsubo T., Lee V.M.-Y., Foskett J.K. Mechanism of Ca2+ disruption in Alzheimer’s disease by presenilin regulation of InsP3 receptor channel gating. Neuron. 2008;58:871–883. doi: 10.1016/j.neuron.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chan S.L., Mayne M., Holden C.P., Geiger J.D., Mattson M.P. Presenilin-1 mutations increase levels of ryanodine receptors and calcium release in PC12 cells and cortical neurons. J. Biol. Chem. 2000;275:18195–18200. doi: 10.1074/jbc.M000040200. [DOI] [PubMed] [Google Scholar]
- 125.Tu H., Nelson O., Bezprozvanny A., Wang Z., Lee S.-F., Hao Y.-H., Serneels L., De Strooper B., Yu G., Bezprozvanny I. Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer’s disease-linked mutations. Cell. 2006;126:981–993. doi: 10.1016/j.cell.2006.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nelson O., Tu H., Lei T., Bentahir M., de Strooper B., Bezprozvanny I. Familial Alzheimer disease-linked mutations specifically disrupt Ca2+ leak function of presenilin 1. J. Clin. Investig. 2007;117:1230–1239. doi: 10.1172/JCI30447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Aloni E., Oni-Biton E., Tsoory M., Moallem D.H., Segal M. Synaptopodin Deficiency Ameliorates Symptoms in the 3xTg Mouse Model of Alzheimer’s Disease. J. Neurosci. 2019;39:3983–3992. doi: 10.1523/JNEUROSCI.2920-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bertan F., Wischhof L., Sosulina L., Mittag M., Dalügge D., Fornarelli A., Gardoni F., Marcello E., Di Luca M., Fuhrmann M., et al. Loss of Ryanodine Receptor 2 impairs neuronal activity-dependent remodeling of dendritic spines and triggers compensatory neuronal hyperexcitability. Cell Death Differ. 2020:1–20. doi: 10.1038/s41418-020-0584-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kelliher M., Fastbom J., Cowburn R.F., Bonkale W., Ohm T.G., Ravid R., Sorrentino V., O’Neill C. Alterations in the ryanodine receptor calcium release channel correlate with Alzheimer’s disease neurofibrillary and beta-amyloid pathologies. Neuroscience. 1999;92:499–513. doi: 10.1016/S0306-4522(99)00042-1. [DOI] [PubMed] [Google Scholar]
- 130.Bruno A., Huang J., Bennett D.A., Marr R., Hastings M.L., Stutzmann G.E. Altered Ryanodine Receptor Expression in Mild Cognitive Impairment and Alzheimer’s Disease. Neurobiol. Aging. 2012;33:1001.e1–1001.e6. doi: 10.1016/j.neurobiolaging.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chakroborty S., Kim J., Schneider C., West A.R., Stutzmann G.E. Nitric Oxide Signaling Is Recruited as a Compensatory Mechanism for Sustaining Synaptic Plasticity in Alzheimer’s Disease Mice. J. Neurosci. 2015;35:6893–6902. doi: 10.1523/JNEUROSCI.4002-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Redondo R.L., Morris R.G.M. Making memories last: The synaptic tagging and capture hypothesis. Nat. Rev. Neurosci. 2011;12:17–30. doi: 10.1038/nrn2963. [DOI] [PubMed] [Google Scholar]
- 133.Zhang C., Wu B., Beglopoulos V., Wines-Samuelson M., Zhang D., Dragatsis I., Südhof T.C., Shen J. Presenilins are essential for regulating neurotransmitter release. Nature. 2009;460:632–636. doi: 10.1038/nature08177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Green K.N., Demuro A., Akbari Y., Hitt B.D., Smith I.F., Parker I., LaFerla F.M. SERCA pump activity is physiologically regulated by presenilin and regulates amyloid beta production. J. Cell Biol. 2008;181:1107–1116. doi: 10.1083/jcb.200706171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Guo Q., Sopher B.L., Furukawa K., Pham D.G., Robinson N., Martin G.M., Mattson M.P. Alzheimer’s presenilin mutation sensitizes neural cells to apoptosis induced by trophic factor withdrawal and amyloid beta-peptide: Involvement of calcium and oxyradicals. J. Neurosci. 1997;17:4212–4222. doi: 10.1523/JNEUROSCI.17-11-04212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Leissring M.A., Akbari Y., Fanger C.M., Cahalan M.D., Mattson M.P., LaFerla F.M. Capacitative Calcium Entry Deficits and Elevated Luminal Calcium Content in Mutant Presenilin-1 Knockin Mice. J. Cell Biol. 2000;149:793–798. doi: 10.1083/jcb.149.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tong B.C.-K., Lee C.S.-K., Cheng W.-H., Lai K.-O., Foskett J.K., Cheung K.-H. Familial Alzheimer’s disease–associated presenilin 1 mutants promote γ-secretase cleavage of STIM1 to impair store-operated Ca2+ entry. Sci. Signal. 2016;9:ra89. doi: 10.1126/scisignal.aaf1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hoozemans J.J.M., van Haastert E.S., Nijholt D.A.T., Rozemuller A.J.M., Eikelenboom P., Scheper W. The Unfolded Protein Response Is Activated in Pretangle Neurons in Alzheimer’s Disease Hippocampus. Am. J. Pathol. 2009;174:1241–1251. doi: 10.2353/ajpath.2009.080814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Uddin M.S., Tewari D., Sharma G., Kabir M.T., Barreto G.E., Bin-Jumah M.N., Perveen A., Abdel-Daim M.M., Ashraf G.M. Molecular Mechanisms of ER Stress and UPR in the Pathogenesis of Alzheimer’s Disease. Mol. Neurobiol. 2020;57:2902–2919. doi: 10.1007/s12035-020-01929-y. [DOI] [PubMed] [Google Scholar]
- 140.Szegezdi E., Logue S.E., Gorman A.M., Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880–885. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gorman A.M., Healy S.J.M., Jäger R., Samali A. Stress management at the ER: Regulators of ER stress-induced apoptosis. Pharmacol. Ther. 2012;134:306–316. doi: 10.1016/j.pharmthera.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 142.Ogata M., Hino S., Saito A., Morikawa K., Kondo S., Kanemoto S., Murakami T., Taniguchi M., Tanii I., Yoshinaga K., et al. Autophagy Is Activated for Cell Survival after Endoplasmic ReticulumStress. Mol. Cell. Biol. 2006;26:9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cissé M., Duplan E., Lorivel T., Dunys J., Bauer C., Meckler X., Gerakis Y., Lauritzen I., Checler F. The transcription factor XBP1s restores hippocampal synaptic plasticity and memory by control of the Kalirin-7 pathway in Alzheimer model. Mol. Psychiatry. 2017;22:1562–1575. doi: 10.1038/mp.2016.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ma T., Trinh M.A., Wexler A.J., Bourbon C., Gatti E., Pierre P., Cavener D.R., Klann E. Suppression of eIF2α kinases alleviates Alzheimer’s disease–related plasticity and memory deficits. Nat. Neurosci. 2013;16:1299–1305. doi: 10.1038/nn.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Soejima N., Ohyagi Y., Nakamura N., Himeno E., Iinuma K.M., Sakae N., Yamasaki R., Tabira T., Murakami K., Irie K., et al. Intracellular accumulation of toxic turn amyloid-β is associated with endoplasmic reticulum stress in Alzheimer’s disease. Curr. Alzheimer Res. 2013;10:11–20. [PubMed] [Google Scholar]
- 146.Katayama T., Imaizumi K., Sato N., Miyoshi K., Kudo T., Hitomi J., Morihara T., Yoneda T., Gomi F., Mori Y., et al. Presenilin-1 mutations downregulate the signalling pathway of the unfolded-protein response. Nat. Cell Biol. 1999;1:479–485. doi: 10.1038/70265. [DOI] [PubMed] [Google Scholar]
- 147.Hidalgo C., Arias-Cavieres A. Calcium, Reactive Oxygen Species, and Synaptic Plasticity. Physiology. 2016;31:201–215. doi: 10.1152/physiol.00038.2015. [DOI] [PubMed] [Google Scholar]
- 148.Mostafavi S., Gaiteri C., Sullivan S.E., White C.C., Tasaki S., Xu J., Taga M., Klein H.-U., Patrick E., Komashko V., et al. A molecular network of the aging human brain provides insights into the pathology and cognitive decline of Alzheimer’s disease. Nat. Neurosci. 2018;21:811–819. doi: 10.1038/s41593-018-0154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tramutola A., Lanzillotta C., Perluigi M., Butterfield D.A. Oxidative stress, protein modification and Alzheimer disease. Brain Res. Bull. 2017;133:88–96. doi: 10.1016/j.brainresbull.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 150.Zhang H., Menzies K.J., Auwerx J. The role of mitochondria in stem cell fate and aging. Development. 2018;145 doi: 10.1242/dev.143420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Canchi S., Raao B., Masliah D., Rosenthal S.B., Sasik R., Fisch K.M., De Jager P.L., Bennett D.A., Rissman R.A. Integrating Gene and Protein Expression Reveals Perturbed Functional Networks in Alzheimer’s Disease. Cell Rep. 2019;28:1103–1116.e4. doi: 10.1016/j.celrep.2019.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Manczak M., Park B.S., Jung Y., Reddy P.H. Differential expression of oxidative phosphorylation genes in patients with Alzheimer’s disease. NeuroMolecular Med. 2004;5:147–162. doi: 10.1385/NMM:5:2:147. [DOI] [PubMed] [Google Scholar]
- 153.Adav S.S., Park J.E., Sze S.K. Quantitative profiling brain proteomes revealed mitochondrial dysfunction in Alzheimer’s disease. Mol. Brain. 2019;12 doi: 10.1186/s13041-019-0430-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bubber P., Haroutunian V., Fisch G., Blass J.P., Gibson G.E. Mitochondrial abnormalities in Alzheimer brain: Mechanistic implications. Ann. Neurol. 2005;57:695–703. doi: 10.1002/ana.20474. [DOI] [PubMed] [Google Scholar]
- 155.Parker W.D., Parks J., Filley C.M., Kleinschmidt-DeMasters B.K. Electron transport chain defects in Alzheimer’s disease brain. Neurology. 1994;44:1090–1096. doi: 10.1212/WNL.44.6.1090. [DOI] [PubMed] [Google Scholar]
- 156.Gibson G.E., Thakkar A. Interactions of Mitochondria/Metabolism and Calcium Regulation in Alzheimer’s Disease: A Calcinist Point of View. Neurochem. Res. 2017;42:1636–1648. doi: 10.1007/s11064-017-2182-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Swomley A.M., Butterfield D.A. Oxidative stress in Alzheimer disease and mild cognitive impairment: Evidence from human data provided by redox proteomics. Arch. Toxicol. 2015;89:1669–1680. doi: 10.1007/s00204-015-1556-z. [DOI] [PubMed] [Google Scholar]
- 158.Duchen M.R. Contributions of mitochondria to animal physiology: From homeostatic sensor to calcium signalling and cell death. J. Physiol. 1999;516:1–17. doi: 10.1111/j.1469-7793.1999.001aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hajnóczky G., Hager R., Thomas A.P. Mitochondria suppress local feedback activation of inositol 1,4, 5-trisphosphate receptors by Ca2+ J. Biol. Chem. 1999;274:14157–14162. doi: 10.1074/jbc.274.20.14157. [DOI] [PubMed] [Google Scholar]
- 160.Robb-Gaspers L.D., Burnett P., Rutter G.A., Denton R.M., Rizzuto R., Thomas A.P. Integrating cytosolic calcium signals into mitochondrial metabolic responses. EMBO J. 1998;17:4987–5000. doi: 10.1093/emboj/17.17.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rowland A.A., Voeltz G.K. Endoplasmic reticulum-mitochondria contacts: Function of the junction. Nat. Rev. Mol. Cell Biol. 2012;13:607–615. doi: 10.1038/nrm3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Schon E.A., Area-Gomez E. Mitochondria-associated ER membranes in Alzheimer disease. Mol. Cell. Neurosci. 2013;55:26–36. doi: 10.1016/j.mcn.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 163.Szabadkai G., Bianchi K., Várnai P., De Stefani D., Wieckowski M.R., Cavagna D., Nagy A.I., Balla T., Rizzuto R. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J. Cell Biol. 2006;175:901–911. doi: 10.1083/jcb.200608073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wang M., Beckmann N.D., Roussos P., Wang E., Zhou X., Wang Q., Ming C., Neff R., Ma W., Fullard J.F., et al. The Mount Sinai cohort of large-scale genomic, transcriptomic and proteomic data in Alzheimer’s disease. Sci. Data. 2018;5:180185. doi: 10.1038/sdata.2018.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.De Jager P.L., Ma Y., McCabe C., Xu J., Vardarajan B.N., Felsky D., Klein H.-U., White C.C., Peters M.A., Lodgson B., et al. A multi-omic atlas of the human frontal cortex for aging and Alzheimer’s disease research. Sci. Data. 2018;5:180142. doi: 10.1038/sdata.2018.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hodes R.J., Buckholtz N. Accelerating Medicines Partnership: Alzheimer’s Disease (AMP-AD) Knowledge Portal Aids Alzheimer’s Drug Discovery through Open Data Sharing. Expert Opin. Ther. Targets. 2016;20:389–391. doi: 10.1517/14728222.2016.1135132. [DOI] [PubMed] [Google Scholar]
- 167.Calvo-Rodriguez M., Hou S.S., Snyder A.C., Kharitonova E.K., Russ A.N., Das S., Fan Z., Muzikansky A., Garcia-Alloza M., Serrano-Pozo A., et al. Increased mitochondrial calcium levels associated with neuronal death in a mouse model of Alzheimer’s disease. Nat. Commun. 2020;11:2146. doi: 10.1038/s41467-020-16074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Han D., Antunes F., Canali R., Rettori D., Cadenas E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J. Biol. Chem. 2003;278:5557–5563. doi: 10.1074/jbc.M210269200. [DOI] [PubMed] [Google Scholar]
- 169.Paula-Lima A.C., Adasme T., SanMartín C., Sebollela A., Hetz C., Carrasco M.A., Ferreira S.T., Hidalgo C. Amyloid β-Peptide Oligomers Stimulate RyR-Mediated Ca2+ Release Inducing Mitochondrial Fragmentation in Hippocampal Neurons and Prevent RyR-Mediated Dendritic Spine Remodeling Produced by BDNF. Antioxid. Redox Signal. 2010;14:1209–1223. doi: 10.1089/ars.2010.3287. [DOI] [PubMed] [Google Scholar]
- 170.SanMartín C.D., Veloso P., Adasme T., Lobos P., Bruna B., Galaz J., García A., Hartel S., Hidalgo C., Paula-Lima A.C. RyR2-Mediated Ca2+ Release and Mitochondrial ROS Generation Partake in the Synaptic Dysfunction Caused by Amyloid β Peptide Oligomers. Front. Mol. Neurosci. 2017;10 doi: 10.3389/fnmol.2017.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Smaili S.S., Russell J.T. Permeability transition pore regulates both mitochondrial membrane potential and agonist-evoked Ca2+ signals in oligodendrocyte progenitors. Cell Calcium. 1999;26:121–130. doi: 10.1054/ceca.1999.0061. [DOI] [PubMed] [Google Scholar]
- 172.Wang W., Zhao F., Ma X., Perry G., Zhu X. Mitochondria dysfunction in the pathogenesis of Alzheimer’s disease: Recent advances. Mol. Neurodegener. 2020;15:30. doi: 10.1186/s13024-020-00376-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Area-Gomez E., del Carmen Lara Castillo M., Tambini M.D., Guardia-Laguarta C., de Groof A.J., Madra M., Ikenouchi J., Umeda M., Bird T.D., Sturley S.L., et al. Upregulated function of mitochondria-associated ER membranes in Alzheimer disease. EMBO J. 2012;31:4106–4123. doi: 10.1038/emboj.2012.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Area-Gomez E., de Groof A., Bonilla E., Montesinos J., Tanji K., Boldogh I., Pon L., Schon E.A. A key role for MAM in mediating mitochondrial dysfunction in Alzheimer disease. Cell Death Dis. 2018;9:1–10. doi: 10.1038/s41419-017-0215-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Del Prete D., Suski J.M., Oulès B., Debayle D., Gay A.S., Lacas-Gervais S., Bussiere R., Bauer C., Pinton P., Paterlini-Bréchot P., et al. Localization and Processing of the Amyloid-β Protein Precursor in Mitochondria-Associated Membranes. J. Alzheimers Dis. JAD. 2017;55:1549–1570. doi: 10.3233/JAD-160953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hedskog L., Pinho C.M., Filadi R., Rönnbäck A., Hertwig L., Wiehager B., Larssen P., Gellhaar S., Sandebring A., Westerlund M., et al. Modulation of the endoplasmic reticulum-mitochondria interface in Alzheimer’s disease and related models. Proc. Natl. Acad. Sci. USA. 2013;110:7916–7921. doi: 10.1073/pnas.1300677110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sarasija S., Laboy J.T., Ashkavand Z., Bonner J., Tang Y., Norman K.R. Presenilin mutations deregulate mitochondrial Ca2+ homeostasis and metabolic activity causing neurodegeneration in Caenorhabditis elegans. eLife. 2018;7:e33052. doi: 10.7554/eLife.33052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Völgyi K., Badics K., Sialana F.J., Gulyássy P., Udvari E.B., Kis V., Drahos L., Lubec G., Kékesi K.A., Juhász G. Early Presymptomatic Changes in the Proteome of Mitochondria-Associated Membrane in the APP/PS1 Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2018;55:7839–7857. doi: 10.1007/s12035-018-0955-6. [DOI] [PubMed] [Google Scholar]
- 179.Luongo T.S., Lambert J.P., Gross P., Nwokedi M., Lombardi A.A., Shanmughapriya S., Carpenter A.C., Kolmetzky D., Gao E., van Berlo J.H., et al. The Mitochondrial Na+/Ca2+ Exchanger Is Essential for Ca2+ Homeostasis and Viability. [(accessed on 26 October 2020)]; doi: 10.1038/nature22082. Available online: https://pubmed.ncbi.nlm.nih.gov/28445457/ [DOI] [PMC free article] [PubMed]
- 180.Kroemer G., Galluzzi L., Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 181.Smaili S.S., Pereira G.J.S., Costa M.M., Rocha K.K., Rodrigues L., do Carmo L.G., Hirata H., Hsu Y.-T. The Role of Calcium Stores in Apoptosis and Autophagy. Curr. Mol. Med. 2013;13:252–265. doi: 10.2174/156652413804810772. [DOI] [PubMed] [Google Scholar]
- 182.Magi S., Piccirillo S., Maiolino M., Lariccia V., Amoroso S. NCX1 and EAAC1 transporters are involved in the protective action of glutamate in an in vitro Alzheimer’s disease-like model. Cell Calcium. 2020;91:102268. doi: 10.1016/j.ceca.2020.102268. [DOI] [PubMed] [Google Scholar]
- 183.Zilberter Y., Zilberter M. The vicious circle of hypometabolism in neurodegenerative diseases: Ways and mechanisms of metabolic correction. J. Neurosci. Res. 2017;95:2217–2235. doi: 10.1002/jnr.24064. [DOI] [PubMed] [Google Scholar]
- 184.Jadiya P., Kolmetzky D.W., Tomar D., Di Meco A., Lombardi A.A., Lambert J.P., Luongo T.S., Ludtmann M.H., Praticò D., Elrod J.W. Impaired mitochondrial calcium efflux contributes to disease progression in models of Alzheimer’s disease. Nat. Commun. 2019;10:3885. doi: 10.1038/s41467-019-11813-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Piccirillo S., Magi S., Castaldo P., Preziuso A., Lariccia V., Amoroso S. NCX and EAAT transporters in ischemia: At the crossroad between glutamate metabolism and cell survival. Cell Calcium. 2020;86:102160. doi: 10.1016/j.ceca.2020.102160. [DOI] [PubMed] [Google Scholar]
- 186.Magi S., Piccirillo S., Preziuso A., Amoroso S., Lariccia V. Mitochondrial localization of NCXs: Balancing calcium and energy homeostasis. Cell Calcium. 2020;86:102162. doi: 10.1016/j.ceca.2020.102162. [DOI] [PubMed] [Google Scholar]
- 187.Gobbi P., Castaldo P., Minelli A., Salucci S., Magi S., Corcione E., Amoroso S. Mitochondrial localization of Na+/Ca2+ exchangers NCX1-3 in neurons and astrocytes of adult rat brain in situ. Pharmacol. Res. 2007;56:556–565. doi: 10.1016/j.phrs.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 188.Mishra P., Chan D.C. Mitochondrial dynamics and inheritance during cell division, development and disease. Nat. Rev. Mol. Cell Biol. 2014;15:634–646. doi: 10.1038/nrm3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kim B., Park J., Chang K.-T., Lee D.-S. Peroxiredoxin 5 prevents amyloid-beta oligomer-induced neuronal cell death by inhibiting ERK-Drp1-mediated mitochondrial fragmentation. Free Radic. Biol. Med. 2016;90:184–194. doi: 10.1016/j.freeradbiomed.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 190.Kim D.I., Lee K.H., Gabr A.A., Choi G.E., Kim J.S., Ko S.H., Han H.J. Aβ-Induced Drp1 phosphorylation through Akt activation promotes excessive mitochondrial fission leading to neuronal apoptosis. Biochim. Biophys. Acta. 2016;1863:2820–2834. doi: 10.1016/j.bbamcr.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 191.Han S., Nandy P., Austria Q., Siedlak S.L., Torres S., Fujioka H., Wang W., Zhu X. Mfn2 Ablation in the Adult Mouse Hippocampus and Cortex Causes Neuronal Death. Cells. 2020;9:116. doi: 10.3390/cells9010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Jiang S., Nandy P., Wang W., Ma X., Hsia J., Wang C., Wang Z., Niu M., Siedlak S.L., Torres S., et al. Mfn2 ablation causes an oxidative stress response and eventual neuronal death in the hippocampus and cortex. Mol. Neurodegener. 2018;13:5. doi: 10.1186/s13024-018-0238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Pickett E.K., Rose J., McCrory C., McKenzie C.-A., King D., Smith C., Gillingwater T.H., Henstridge C.M., Spires-Jones T.L. Region-specific depletion of synaptic mitochondria in the brains of patients with Alzheimer’s disease. Acta Neuropathol. (Berl.) 2018;136:747–757. doi: 10.1007/s00401-018-1903-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sheng Z.-H., Cai Q. Mitochondrial transport in neurons: Impact on synaptic homeostasis and neurodegeneration. Nat. Rev. Neurosci. 2012;13:77–93. doi: 10.1038/nrn3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ebneth A., Godemann R., Stamer K., Illenberger S., Trinczek B., Mandelkow E. Overexpression of tau protein inhibits kinesin-dependent trafficking of vesicles, mitochondria, and endoplasmic reticulum: Implications for Alzheimer’s disease. J. Cell Biol. 1998;143:777–794. doi: 10.1083/jcb.143.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Shahpasand K., Uemura I., Saito T., Asano T., Hata K., Shibata K., Toyoshima Y., Hasegawa M., Hisanaga S. Regulation of Mitochondrial Transport and Inter-Microtubule Spacing by Tau Phosphorylation at the Sites Hyperphosphorylated in Alzheimer’s Disease. J. Neurosci. 2012;32:2430–2441. doi: 10.1523/JNEUROSCI.5927-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lemasters J.J. Selective Mitochondrial Autophagy, or Mitophagy, as a Targeted Defense Against Oxidative Stress, Mitochondrial Dysfunction, and Aging. Rejuvenation Res. 2005;8:3–5. doi: 10.1089/rej.2005.8.3. [DOI] [PubMed] [Google Scholar]
- 198.Kerr J.S., Adriaanse B.A., Greig N.H., Mattson M.P., Cader M.Z., Bohr V.A., Fang E.F. Mitophagy and Alzheimer’s Disease: Cellular and Molecular Mechanisms. Trends Neurosci. 2017;40:151–166. doi: 10.1016/j.tins.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Martín-Maestro P., Gargini R., Perry G., Avila J., García-Escudero V. PARK2 enhancement is able to compensate mitophagy alterations found in sporadic Alzheimer’s disease. Hum. Mol. Genet. 2016;25:792–806. doi: 10.1093/hmg/ddv616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Cárdenas C., Foskett J.K. Mitochondrial Ca2+ signals in autophagy. Cell Calcium. 2012;52:44–51. doi: 10.1016/j.ceca.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ding W.X., Yin X.M. Mitophagy: Mechanisms, pathophysiological roles, and analysis. Biol. Chem. 2012;393:547–564. doi: 10.1515/hsz-2012-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Okamoto K., Kondo-Okamoto N., Ohsumi Y. Mitochondria-anchored receptor Atg32 mediates degradation of mitochondria via selective autophagy. Dev. Cell. 2009;17:87–97. doi: 10.1016/j.devcel.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 203.Martín-Maestro P., Gargini R., A Sproul A., García E., Antón L.C., Noggle S., Arancio O., Avila J., García-Escudero V. Mitophagy Failure in Fibroblasts and iPSC-Derived Neurons of Alzheimer’s Disease-Associated Presenilin 1 Mutation. Front. Mol. Neurosci. 2017;10:291. doi: 10.3389/fnmol.2017.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Nguyen T.N., Padman B.S., Lazarou M. Deciphering the Molecular Signals of PINK1/Parkin Mitophagy. Trends Cell Biol. 2016;26:733–744. doi: 10.1016/j.tcb.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 205.Mustaly S., Littlefield A., Stutzmann G.E. Calcium Signaling Deficits in Glia and Autophagic Pathways Contributing to Neurodegenerative Disease. Antioxid. Redox Signal. 2018;29:1158–1175. doi: 10.1089/ars.2017.7266. [DOI] [PubMed] [Google Scholar]
- 206.Li W., Li J., Bao J. Microautophagy: Lesser-known self-eating. Cell. Mol. Life Sci. 2012;69:1125–1136. doi: 10.1007/s00018-011-0865-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Vervliet T., Pintelon I., Welkenhuyzen K., Bootman M.D., Bannai H., Mikoshiba K., Martinet W., Nadif Kasri N., Parys J.B., Bultynck G. Basal ryanodine receptor activity suppresses autophagic flux. Biochem. Pharmacol. 2017;132:133–142. doi: 10.1016/j.bcp.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 208.Wu Y., Whiteus C., Xu C.S., Hayworth K.J., Weinberg R.J., Hess H.F., De Camilli P. Contacts between the endoplasmic reticulum and other membranes in neurons. Proc. Natl. Acad. Sci. USA. 2017;114:E4859–E4867. doi: 10.1073/pnas.1701078114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Yu L., Chibnik L.B., Srivastava G.P., Pochet N., Yang J., Xu J., Kozubek J., Obholzer N., Leurgans S.E., Schneider J.A., et al. Association of Brain DNA methylation in SORL1, ABCA7, HLA-DRB5, SLC24A4, and BIN1 with pathological diagnosis of Alzheimer disease. JAMA Neurol. 2015;72:15–24. doi: 10.1001/jamaneurol.2014.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Van Acker Z.P., Bretou M., Annaert W. Endo-lysosomal dysregulations and late-onset Alzheimer’s disease: Impact of genetic risk factors. Mol. Neurodegener. 2019;14:20. doi: 10.1186/s13024-019-0323-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.De Duve C., Wattiaux R. Functions of Lysosomes. Annu. Rev. Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- 212.Nakamura N., Matsuura A., Wada Y., Ohsumi Y. Acidification of vacuoles is required for autophagic degradation in the yeast, Saccharomyces cerevisiae. J. Biochem. 1997;121:338–344. doi: 10.1093/oxfordjournals.jbchem.a021592. [DOI] [PubMed] [Google Scholar]
- 213.Xu H., Ren D. Lysosomal Physiology. Annu. Rev. Physiol. 2015;77:57–80. doi: 10.1146/annurev-physiol-021014-071649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Prasad H., Rao R. The Na+/H+ Exchanger NHE6 Modulates Endosomal pH to Control Processing of Amyloid Precursor Protein in a Cell Culture Model of Alzheimer Disease. J. Biol. Chem. 2015;290:5311–5327. doi: 10.1074/jbc.M114.602219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lee J.H., Yu W.H., Kumar A., Lee S., Mohan P.S., Peterhoff C.M., Wolfe D.M., Martinez-Vicente M., Massey A.C., Sovak G., et al. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell. 2010;141:1146–1158. doi: 10.1016/j.cell.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Lee J.H., McBrayer M.K., Wolfe D.M., Haslett L.J., Kumar A., Sato Y., Lie P.P.Y., Mohan P., Coffey E.E., Kompella U., et al. Presenilin 1 Maintains Lysosomal Ca2+ Homeostasis via TRPML1 by Regulating vATPase-Mediated Lysosome Acidification. Cell Rep. 2015;12:1430–1444. doi: 10.1016/j.celrep.2015.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Man S.M., Kanneganti T.-D. Regulation of lysosomal dynamics and autophagy by CTSB/cathepsin B. Autophagy. 2016;12:2504–2505. doi: 10.1080/15548627.2016.1239679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Lee S., Sato Y., Nixon R.A. Lysosomal Proteolysis Inhibition Selectively Disrupts Axonal Transport of Degradative Organelles and Causes an Alzheimer’s-Like Axonal Dystrophy. J. Neurosci. 2011;31:7817–7830. doi: 10.1523/JNEUROSCI.6412-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Lee S., Sato Y., Nixon R.A. Primary lysosomal dysfunction causes cargo-specific deficits of axonal transport leading to Alzheimer-like neuritic dystrophy. Autophagy. 2011;7:1562–1563. doi: 10.4161/auto.7.12.17956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Torres M., Jimenez S., Sanchez-Varo R., Navarro V., Trujillo-Estrada L., Sanchez-Mejias E., Carmona I., Davila J.C., Vizuete M., Gutierrez A., et al. Defective lysosomal proteolysis and axonal transport are early pathogenic events that worsen with age leading to increased APP metabolism and synaptic Abeta in transgenic APP/PS1 hippocampus. Mol. Neurodegener. 2012;7:59. doi: 10.1186/1750-1326-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Calcraft P.J., Ruas M., Pan Z., Cheng X., Arredouani A., Hao X., Tang J., Rietdorf K., Teboul L., Chuang K.-T., et al. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 2009;459:596–600. doi: 10.1038/nature08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Cang C., Bekele B., Ren D. The voltage-gated sodium channel TPC1 confers endolysosomal excitability. Nat. Chem. Biol. 2014;10:463–469. doi: 10.1038/nchembio.1522. [DOI] [PubMed] [Google Scholar]
- 223.Carmona-Gutierrez D., Hughes A.L., Madeo F., Ruckenstuhl C. The crucial impact of lysosomes in aging and longevity. Ageing Res. Rev. 2016;32:2–12. doi: 10.1016/j.arr.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Colacurcio D.J., Nixon R.A. Disorders of lysosomal acidificstion-The emerging role of v-ATPase in aging and neurodegenerative disease. Ageing Res. Rev. 2016;32:75–88. doi: 10.1016/j.arr.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Neely Kayala K.M., Dickinson G.D., Minassian A., Walls K.C., Green K.N., Laferla F.M. Presenilin-null cells have altered two-pore calcium channel expression and lysosomal calcium: Implications for lysosomal function. Brain Res. 2012;1489:8–16. doi: 10.1016/j.brainres.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Coen K., Flannagan R.S., Baron S., Carraro-Lacroix L.R., Wang D., Vermeire W., Michiels C., Munck S., Baert V., Sugita S., et al. Lysosomal calcium homeostasis defects, not proton pump defects, cause endo-lysosomal dysfunction in PSEN-deficient cells. J. Cell Biol. 2012;198:23–35. doi: 10.1083/jcb.201201076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Mauvezin C., Nagy P., Juhász G., Neufeld T.P. Autophagosome–lysosome fusion is independent of V-ATPase-mediated acidification. Nat. Commun. 2015;6:7007. doi: 10.1038/ncomms8007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Zhang X., Garbett K., Veeraraghavalu K., Wilburn B., Gilmore R., Mirnics K., Sisodia S.S. A Role for Presenilins in Autophagy Revisited: Normal Acidification of Lysosomes in Cells Lacking PSEN1 and PSEN2. J. Neurosci. 2012;32:8633–8648. doi: 10.1523/JNEUROSCI.0556-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Medina D.L., Di Paola S., Peluso I., Armani A., De Stefani D., Venditti R., Montefusco S., Scotto-Rosato A., Prezioso C., Forrester A., et al. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat. Cell Biol. 2015;17:288–299. doi: 10.1038/ncb3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Sardiello M., Palmieri M., di Ronza A., Medina D.L., Valenza M., Gennarino V.A., Malta C.D., Donaudy F., Embrione V., Polishchuk R.S., et al. A Gene Network Regulating Lysosomal Biogenesis and Function. Science. 2009;325:473–477. doi: 10.1126/science.1174447. [DOI] [PubMed] [Google Scholar]
- 231.Settembre C., Zoncu R., Medina D.L., Vetrini F., Erdin S., Erdin S., Huynh T., Ferron M., Karsenty G., Vellard M.C., et al. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J. 2012;31:1095–1108. doi: 10.1038/emboj.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Glavan G., Schliebs R., Živin M. Synaptotagmins in neurodegeneration. Anat. Rec. 2009;292:1849–1862. doi: 10.1002/ar.21026. [DOI] [PubMed] [Google Scholar]
- 233.Jun K., Piedras-Rentería E.S., Smith S.M., Wheeler D.B., Lee S.B., Lee T.G., Chin H., Adams M.E., Scheller R.H., Tsien R.W., et al. Ablation of P/Q-type Ca2+ channel currents, altered synaptic transmission, and progressive ataxia in mice lacking the α1A-subunit. Proc. Natl. Acad. Sci. USA. 1999;96:15245–15250. doi: 10.1073/pnas.96.26.15245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Saftig P., Klumperman J. Lysosome biogenesis and lysosomal membrane proteins: Trafficking meets function. Nat. Rev. Mol. Cell Biol. 2009;10:623–635. doi: 10.1038/nrm2745. [DOI] [PubMed] [Google Scholar]
- 235.Tian X., Gala U., Zhang Y., Shang W., Jaiswal S.N., di Ronza A., Jaiswal M., Yamamoto S., Sandoval H., Duraine L., et al. A voltage-gated calcium channel regulates lysosomal fusion with endosomes and autophagosomes and is required for neuronal homeostasis. PLoS Biol. 2015;13 doi: 10.1371/journal.pbio.1002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Kinnear N.P., Wyatt C.N., Clark J.H., Calcraft P.J., Fleischer S., Jeyakumar L.H., Nixon G.F., Evans A.M. Lysosomes co-localize with ryanodine receptor subtype 3 to form a trigger zone for calcium signalling by NAADP in rat pulmonary arterial smooth muscle. Cell Calcium. 2008;44:190–201. doi: 10.1016/j.ceca.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Foster W.J., Taylor H.B.C., Padamsey Z., Jeans A.F., Galione A., Emptage N.J. Hippocampal mGluR1-dependent long-term potentiation requires NAADP-mediated acidic store Ca2+ signaling. Sci. Signal. 2018;11 doi: 10.1126/scisignal.aat9093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Padamsey Z., McGuinness L., Bardo S.J., Reinhart M., Tong R., Hedegaard A., Hart M.L., Emptage N.J. Activity-Dependent Exocytosis of Lysosomes Regulates the Structural Plasticity of Dendritic Spines. Neuron. 2017;93:132–146. doi: 10.1016/j.neuron.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Padamsey Z., McGuinness L., Emptage N.J. Inhibition of lysosomal Ca2+ signalling disrupts dendritic spine structure and impairs wound healing in neurons. Commun. Integr. Biol. 2017;10:e1344802. doi: 10.1080/19420889.2017.1344802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Yang D.S., Stavrides P., Mohan P.S., Kaushik S., Kumar A., Ohno M., Schmidt S.D., Wesson D., Bandyopadhyay U., Jiang Y., et al. Reversal of autophagy dysfunction in the TgCRND8 mouse model of Alzheimer’s disease ameliorates amyloid pathologies and memory deficits. Brain. 2011;134:258–277. doi: 10.1093/brain/awq341. [DOI] [PMC free article] [PubMed] [Google Scholar]