Abstract
Nearly 100 years after the first report of tick-borne tularemia, questions remain about the tick vector(s) that pose the greatest risk for transmitting Francisella tularensis (Ft), the causative agent of tularemia. Additionally, few studies have identified genes/proteins required for Ft to infect, persist, and replicate in ticks. To answer questions about vector competence and Ft transmission by ticks, we infected Dermacentor variabilis (Dv), Amblyomma americanum (Aa), and Haemaphysalis longicornis (Hl; invasive species from Asia) ticks with Ft, finding that although Aa ticks initially become infected with 1 order of magnitude higher Ft, Ft replicated more robustly in Dv ticks, and did not persist in Hl ticks. In transmission studies, both Dv and Aa ticks efficiently transmitted Ft to naïve mice, causing disease in 57% and 46% of mice, respectively. Of four putative Ft chitinases, FTL1793 is the most conserved among Francisella sp. We generated a ΔFTL1793 mutant and found that ΔFTL1793 was deficient for infection, persistence, and replication in ticks. Recombinant FTL1793 exhibited chitinase activity in vitro, suggesting that FTL1793 may provide an alternative energy source for Ft in ticks. Taken together, Dv ticks appear to pose a greater risk for harboring and transmitting tularemia and FTL1793 plays a major role in promoting tick infections by Ft.
Keywords: Francisella tularensis, Dermacentor variabilis, Amblyomma americanum, Haemaphysalis longicornis, tick-borne disease
1. Introduction
Francisella tularensis (Ft) is a Gram-negative bacterium and the causative agent of the zoonotic disease tularemia. This facultative intracellular bacterium, lethal to over 300 species, is able to cause a range of flu-like symptoms in humans [1,2]. Due to high morbidity and mortality rates, ease of aerosolization, and low infectious dose, Ft has been classified as a Tier 1 Select Agent by the United States (U.S.) Centers for Disease Control and Prevention.
Ft is divided into three subspecies, including Ft subsp. tularensis (Type A), Ft subsp. holarctica (Type B), and Ft subsp. mediasiatica, although only Type A and Type B subsp. are virulent to humans. Type A strains are found exclusively in North America, have an LD50 of <10 organisms, cause up to 60% mortality if left untreated, and can be further divided into three subpopulations: Type A1a, A1b and A2, with Type A1b causing the most serious infections [3]. Although less virulent, Type B strains still cause disease in humans and are distributed throughout the Northern Hemisphere [4,5]. Through repeated subculturing of Ft subsp. holarctica, scientists in the former Soviet Union created a live attenuated strain in the 1930s, which has been named the live vaccine strain (LVS). However, LVS is not a licensed vaccine in the U.S. due to unknown mechanism(s) of attenuation, immunization side effects, and only partial protection against Type A aerosol infection [4,5]. Because of decreased biocontainment requirements, LVS has been used by many research groups to study pathogenesis in mice and other animal models [5]. Although interest in bioterrorism-related research has increased due to this classification, approximately half of U.S. tularemia cases are associated with tick bites [6].
Tick-borne disease cases, including Lyme disease, anaplasmosis, Rocky Mountain spotted fever, Powassan virus, and tularemia, have nearly doubled in the U.S. between 2004 and 2016 [7]. Increasing case numbers may be attributed to the geographic range expansion of tick vectors, presenting increased health risks to humans [8,9]. In 2015 alone, 314 cases of tularemia were reported—the highest recorded since 1964, with over 225 cases being reported per year since 2015 [10]. Ulceroglandular tularemia is the most common presentation in the U.S. and is generally attributed to the bite of an infected tick [11]. In the U.S., there are four tick species most commonly associated with tularemia transmission: Amblyomma americanum (Aa), Dermacentor andersoni, Dermacentor occidentalis, and Dermacentor variabilis (Dv) [12,13,14,15]. Although ticks have been speculated to play important roles in the environmental persistence and transmission of Ft [16], important questions remain about which tick vector(s) pose(s) the greatest risk for transmitting Ft and what Ft genes are important for infecting and persisting in ticks.
Between 2001 and 2010, three U.S. states (Arkansas, Missouri, Oklahoma) accounted for over 40% of all tularemia cases [17]. However, tularemia cases are widespread throughout the U.S., as highlighted by high numbers of cases from Colorado, Nebraska, South Dakota, and Wyoming in 2015 [10]. The expansive geographic range of Dv ticks, present in nearly every state east of the Rocky Mountains, have implicated Dv ticks as the main vector for tularemia in the U.S. [6,18]. Furthermore, Dv tick bites have been associated with two outbreaks occurring on Martha’s Vineyard, Massachusetts, both of which involved 15 cases of tularemia, and included one fatality [15]. Laboratory studies have demonstrated that Dv ticks can acquire Ft from infected mice [19] or through capillary tube feeding [20]. However, those studies also demonstrated major differences in the ability of Ft to infect, persist, and replicate in Dv ticks. Subsequent studies demonstrated that adult Dv ticks were able to transmit Ft infection to naïve mice, confirming their importance as vectors for Ft [18].
Whereas studies from the 1950s detected Ft in field-collected Aa ticks [21,22], there have not been any contemporary studies to examine Ft prevalence in Aa ticks. However, it is known that Aa ticks are the most abundant species in the south-central U.S., where higher numbers of tularemia cases occur, suggesting that Aa ticks also may be major vectors for tularemia [11,12]. Although performed in two separate studies, data from capillary tube-fed ticks indicated that Aa ticks may acquire higher Ft bacterial numbers than similarly-fed Dv ticks [12,20]. However, direct comparisons between tick species were not performed and the applicability of those studies to naturally infected (i.e., blood meal fed) Aa and Dv ticks is unknown. In addition, direct comparisons of Ft transmission from infected Aa and Dv ticks to naïve animals have not been performed, leaving major questions about which tick vector poses the greatest risk for Ft environmental persistence and/or transmission.
Haemaphysalis longicornis (Hl), a human-biting tick native to Asia, has been detected in at least nine U.S. states, including Arkansas, a state which regularly reports the highest number of tularemia cases in the U.S. [11,23]. Studies in Asia have shown that Hl experimentally acquires Anaplasma, Babesia, Borrelia, Ehrlichia, and Rickettsia infections, all of which circulate zoonotically in the U.S. [7,24]. However, the ability of Hl to acquire and transmit Ft has not been studied.
To provide new information about which tick vector(s) may pose the greatest risk for harboring and transmitting tularemia, this study directly compared the infection, persistence, and replication of Ft in Dv, Aa, and Hl ticks using a mouse–tick–Ft infection model. Dv and Aa ticks were selected for these studies because they are most commonly associated with U.S. tularemia cases and both of their geographic ranges overlap with the foci of tularemia infections in the south-central U.S. [6]. We also examined Ft infections of Hl ticks, given their invasion into the U.S. and questions about their ability to vector various diseases. Despite Aa ticks being initially colonized by significantly higher bacterial numbers, Ft persisted in and replicated more robustly in Dv ticks during our studies (up to 14 weeks). Interestingly, Ft was unable to persist in Hl ticks, indicating that it is unlikely to serve as a major vector for tularemia. In transmission studies, both Dv and Aa adult ticks efficiently transmitted Ft infections to naïve mice, with all mice succumbing to disease within seven days after tick infestation (two days after ticks completed their blood meal). Although not significant, Dv-infested mice had higher bacterial burdens in their blood, skin, and lungs, compared to Aa-infested mice. Chitin is a major component of the tick cuticle and, as such, tick-borne pathogens have been speculated to use chitinases as an alternative energy source. Although Ft has been reported to encode at least four putative chitinases [25], their roles in tick infections remain unknown. LVS gene locus FTL1793, chiD, contains a GH18 chitinase-like domain and is the most conserved putative chitinase among Francisella sp. To provide new information about Ft genes required for tick infections, we selected FTL1793 for further study. Recombinant FTL1793 was expressed and purified and in vitro assays with purified chitin confirmed that FTL1793 exhibits chitinase activity. An Ft gene deletion mutant, ΔFTL1793, was generated and used in mouse–tick–Ft infections, finding that FTL1793 was required for full infectivity and persistence of Ft in both Dv and Aa ticks, particularly during the time when nymphal ticks molt to adults (between weeks 4 and 6 after a blood meal). Taken together, these studies confirm that both Dv and Aa ticks serve as reservoirs and vectors for Ft. However, our results indicate that Dv ticks may pose a greater risk for tularemia transmission; Ft replicates more robustly in Dv ticks, and Dv ticks transmit higher Ft numbers to naïve animals. Finally, our results demonstrated that FTL1793 chitinase activity is important for Ft to survive and persist in ticks. Ft chitin degradation in ticks may provide an alternative energy source as this bacterium awaits transmission to a new mammalian host.
2. Results
2.1. Low Dose Ft Infects, Persists, and Replicates in Dv and Aa Ticks But Is Cleared from Hl Ticks
A previous study described a mouse–tick–Ft infection model to reproducibly infect nymphal Dv ticks by feeding ticks on Ft LVS-infected mice. When comparing infectious doses of 105 to 108 colony forming units (CFU)/mouse, that study found that infectious doses of 107 and 108 CFU/mouse were saturating for Dv ticks (no differences in CFU/tick between the two highest doses) but >105 CFU/mouse (2.2 × 102 CFU/tick) was needed for Ft LVS to infect, be maintained in, and replicate in nymphal Dv ticks [19]. Despite the importance of that study for understanding Ft infection and replication in Dv ticks, there still are major questions about whether different tick species (e.g., Dv and Aa) pose distinct risks as Ft reservoirs or if invasive tick species (e.g., Hl) can serve as new public health threats for transmitting tularemia. As such, the goal of this study was to compare and contrast Ft infections in three distinct tick species: Dv, Aa, and Hl. Many variables needed to be considered for this mouse–tick–Ft infection model, including the route of mouse infection that results in bacteremia, the time-to-death for bacteremic mice (approx. 36–48 h), and differences in tick blood meal feeding times (approx. 4–6 days). Given these considerations, we established a similar mouse–tick–Ft LVS infection model in our laboratory, where nymphal ticks (either Dv, Aa, or Hl) were placed onto mice three days before Ft infection (day 5; 12 ticks/mouse; ticks contained in a chamber on each mouse back), mice were i.v.-infected with Ft LVS (day 2), ticks continued taking a blood meal for approx. two more days (until day 0), and ticks were harvested when replete (five day total blood meal) or when mice were moribund.
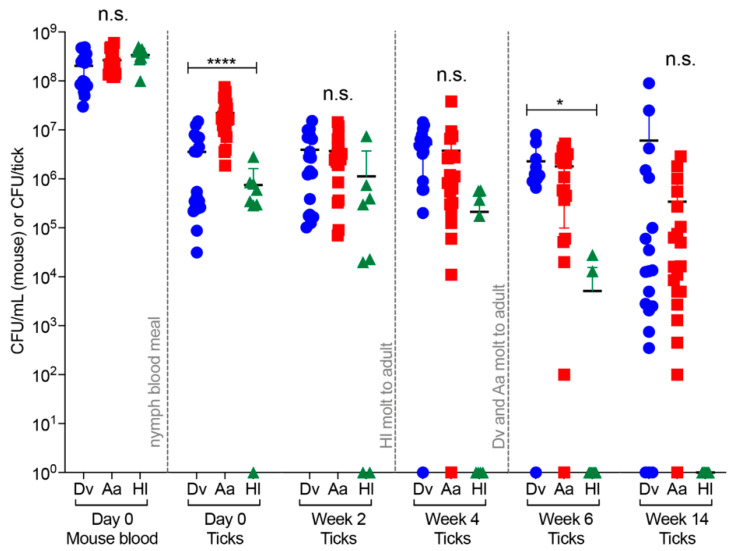
In the first series of studies, we infected mice with 105 CFU/mouse (1.4 × 105 CFU first experiment; 2.3 × 105 CFU second experiment), to directly compare our findings with the previous Dv–Ft infection study [19] and to assess whether threshold bacterial doses were needed to infect, be maintained in, and replicate in Aa and Hl ticks. On day 0, comparing groups of mice that had different tick species fed on them, there was no significant difference in Ft numbers in blood samples from infected mice (means: 3.1 × 105 CFU/mL for Dv mice, 3.9 × 105 CFU/mL for Aa mice, and 3.3 × 105 CFU/mL for Hl mice; Figure 1), highlighting the reproducibility of our model and demonstrating modest (approx. 0.2 orders of magnitude) Ft replication in mouse blood over two days. Subsets of replete ticks from all three tick species were homogenized on day 0, serially diluted, and plated to quantitate bacterial numbers/tick. On day 0, Aa ticks were found to contain significantly more (>1 order of magnitude) Ft than either Dv or Hl ticks (means: 5.6 × 104 CFU/Aa tick, 2.6 × 103 CFU/Dv tick, and 2.1 × 103 CFU/Hl tick; Figure 1). To account for the possibility that higher Ft numbers in individual ticks or tick species (e.g., Aa ticks) could be due to differences in tick blood meal volumes, we also analyzed CFU/mg tick weight, finding that Aa ticks still harbored significantly higher Ft numbers on day 0, compared to Dv or Hl ticks (Supplementary Figure S1). As such, 1 order of magnitude higher Ft numbers in Aa ticks on day 0 appear to be due to inherent differences in Aa ticks and not larger acquired blood volumes by this tick species.
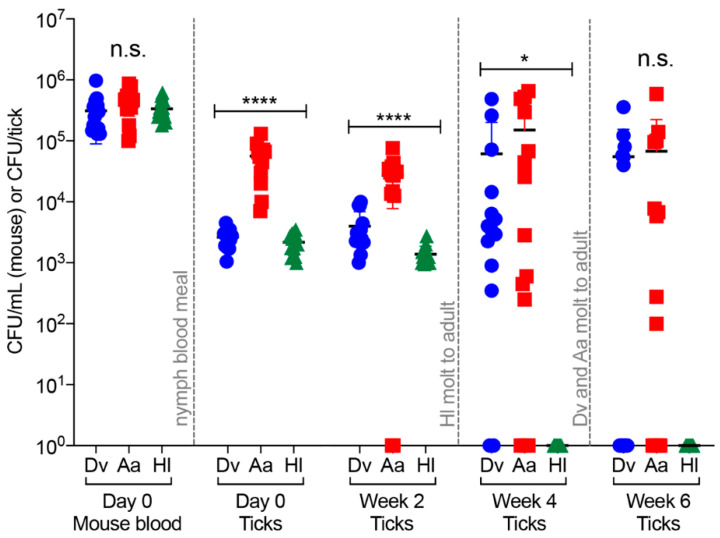
Figure 1.
Infection, persistence, and replication of Francisella tularensis (Ft) in ticks after feeding on mice infected with 105 CFU. Nymphal Dermacentor variabilis (Dv), Amblyomma americanum (Aa), and Haemaphysalis longicornis (Hl) ticks were placed onto non-infected C3H/HeN mice (day 5), the ticks fed for 3 days, mice were i.v. infected with 105 CFU of Ft LVS (day 2), and replete ticks were harvested 2–3 days later (day 0; 5–6 day total blood meal). Following tick harvest (day 0 ticks), blood was collected and plated from infected mice (day 0 mouse blood; n = 6–9/tick species/experiment) to enumerate bacterial numbers (CFU/mL blood). At the indicated time points, individual ticks (n = 6–9/tick species/experiment) were homogenized and plated to enumerate bacterial numbers (CFU/tick). Two independent experiments were performed to confirm reproducibility, with combined data shown. One-way ANOVA was used to compare groups of mice or ticks at each time point: n.s. indicates not significant; * indicates p < 0.05; **** indicates p < 0.0001.
Two weeks later (week 2), Ft numbers remained significantly higher in Aa ticks (mean 2.7 × 104 CFU/Aa tick), compared to Ft numbers in Dv (mean 3.9 × 103 CFU/Dv tick) and Hl ticks (mean 1.3 × 103 CFU/Hl tick) (Figure 1). As described above, when accounting for tick weight in week 2, Ft numbers remained significantly higher in Aa ticks (CFU/mg of tick), compared to either Dv or Hl ticks (Supplementary Figure S1) Interestingly, between day 0 and week 2, Ft replicated 1.5-fold in Dv ticks, while decreasing 4.8-fold in Aa ticks and 1.9-fold in Hl ticks (Figure 1).
At week 4, Ft numbers were not significantly different between Dv (mean 6.1 × 104 CFU/Dv tick) and Aa (mean 1.5 × 105 CFU/Aa tick) ticks (Figure 1). Between weeks 2 and 4, Ft replicated 11-fold in Dv ticks and 5.5-fold in Aa ticks (Figure 1). In addition, while Ft numbers were fairly consistent within each tick species in weeks 0 and 2, a wide range of bacterial numbers were detected in both Dv and Aa ticks in week 4 (range 0 to 6 × 105 CFU/tick) (Figure 1). Importantly, Ft was not detected in Hl ticks in week 4 (Figure 1). Between weeks 2 and 4, all Hl ticks molted from nymphs to adults, with all Hl ticks surviving the molt. Given that Ft was not detected in any Hl ticks in week 4, these results indicate that Ft may not be able to persist longer than four weeks in Hl ticks, Hl molting induces changes that result in either clearance or loss of Ft infection, or the initial (day 0) Ft infectious dose of 2.1 × 103 CFU/Hl tick is below the threshold for long-term Ft persistence in Hl ticks.
Between weeks 4 and 6, >99% of Dv and Aa ticks molted from nymphs to adults, with no adverse effects of Ft infection observed for either tick species (compared to non-infected Dv and Aa ticks; data not shown). Between weeks 4 and 6, average Ft numbers slightly declined in both Dv and Aa ticks (1.1-fold decrease in Dv ticks; 1.5-fold decrease in Aa ticks; week 6 means: 5.4 × 104 CFU/Dv tick and 6.7 × 104 CFU/Aa tick), with no significant difference between Ft numbers in Dv and Aa ticks. Although not significant, 46% of Dv ticks (6 of 13 Dv ticks) were infected with Ft in week 6, compared to 64% of Aa ticks (9 of 14 Aa ticks) that were infected with Ft in week 6 (Figure 1). Despite differences in infection rates, Dv ticks were more consistently infected with Ft in week 6 (range 4 × 104 to 3.5 × 105 CFU), compared to Aa ticks (range 1 × 102 to 5.8 × 105 CFU) (Figure 1). Finally, confirming the week 4 results, Ft was not detected in any Hl ticks in week 6. Compared to a previous mouse–Dv tick–Ft infection study [19], our mouse–tick–Ft infection model is much more efficient, delivering >1 order of magnitude more Ft/tick at the 105 CFU/mouse infectious dose. Our results demonstrated that Ft infections as low as 2.6 × 103 CFU/tick can persist and replicate in both Dv and Aa ticks for up to six weeks. Whereas Ft replicated 20-fold in Dv ticks between day 0 and week 6, the Ft replication rate in Aa ticks during the same time frame was only 1.1-fold (Figure 1), indicating that Dv ticks may be a better vector for Ft replication over six weeks. However, when considering percentages of infected ticks over time, higher percentages of Aa ticks (64%) harbored Ft, compared to Dv ticks (46%). Finally, the highest bacterial numbers in Dv and Aa ticks were observed in week 4 (4.8 × 105 CFU/Dv tick; 6.5 × 105 CFU/Aa tick; Figure 1), indicating that there may be a limit to Ft replication and bacterial numbers in both Dv and Aa ticks.
2.2. High Dose (107 CFU) Ft Infects, Persists, and Replicates in Dv and Aa Ticks But Is Cleared from Hl Ticks
Ft numbers in the blood of naturally infected animals, including mice and rabbits, have not been reported, therefore questions remain about biologically relevant bacteremia numbers for experimental tick infections. However, previous studies have noted Ft bacteremia of 106 to 1010 CFU/mL in the blood of experimentally infected mice [26,27]. A previous study noted that mouse infectious doses >107 CFU/mL blood did not necessarily result in increased Ft numbers in Dv ticks [19], suggesting that there may be a limit to the number of bacteria that ticks can support. To test whether higher infectious doses impacted the ability of Ft to infect, persist, and replicate in Dv, Aa, and Hl ticks, and to assess whether higher infectious doses could overcome Ft clearance/loss in Hl ticks, we performed a second series of mouse–tick–Ft infection studies using a higher infectious dose of 107 CFU/mouse of Ft LVS. Timing of tick placement, intravenous infection, tick harvest, and tick processing were identical to the low dose (105 CFU/mouse) experiments described above, with the exception of adding one additional time point (week 14) to assess longer-term Ft persistence in all three tick species, similar to an overwintering event. In these high-dose infection experiments, groups of mice were i.v. infected with 1.3 × 107 CFU/mouse (first experiment) and 2.4 × 107 CFU/mouse (second experiment) of Ft on day 2. On day 0, replete ticks (five-day total blood meal) were harvested, and blood was collected from all infected mice. No significant differences were calculated for Ft numbers in mouse blood on day 0 when comparing groups of mice that were infested by different tick species (means: 2.1 × 108 CFU/mL for Dv mice; 2.6 × 108 CFU/mL for Aa mice; 3.4 × 108 CFU/mL for Hl mice) (Figure 2). These results highlighted the reproducibility of our mouse infections and demonstrated that, at this higher infectious dose (compared to Figure 1), Ft replicated approx. 1 order of magnitude in mouse blood over two days (Figure 2). On day 0, Aa ticks were found to contain significantly more Ft (0.9 to 1.5 orders of magnitude) than either Dv or Hl ticks (means: 2.2 × 107 CFU/Aa tick, 3.5 × 106 CFU/Dv tick, 7.4 × 105 CFU/Hl tick; Figure 2). Given that Aa ticks harbored significantly higher Ft numbers on day 0 at both the lower infectious dose (105 CFU; Figure 1) and at this higher infectious dose (107 CFU; Figure 2), these data suggest that regardless of Ft numbers in the blood of infected animals (e.g., 105 or 108 CFU/mL), Aa ticks are infected by and harbor more Ft on day 0 than either Dv or Hl ticks. As described above, we calculated CFU/mg of tick to account for potential differences in Ft numbers due to inherent differences in tick weights (and/or blood volume), finding that Aa ticks still contained significantly higher Ft numbers on day 0, compared to Dv and Hl ticks (Supplementary Figure S2). In contrast to our 105 CFU infection study findings, at this higher infectious dose, Dv ticks were infected by 0.5 orders of magnitude higher Ft numbers than Hl ticks on day 0 (Figure 2).
Figure 2.
Infection, persistence, and replication of F. tularensis in ticks after feeding on mice infected with 107 CFU. Nymphal Dv, Aa, and Hl ticks were placed onto non-infected C3H/HeN mice (day 5), ticks fed for 3 days, mice were i.v. infected with 107 CFU of Ft live vaccine strain (LVS) (day 2), and replete ticks were harvested 2–3 days later (5–6 day total blood meal). Following tick harvest (day 0 ticks), blood was collected and plated from infected mice (day 0 mouse blood; n = 5–12/tick species/experiment) to enumerate bacterial numbers (CFU/mL blood). At the indicated time points, individual ticks (n = 7–13/tick species/experiment) were homogenized and plated to enumerate bacterial numbers (CFU/tick). Two independent experiments were performed to confirm reproducibility, with combined data shown. One-way ANOVA was used to compare groups of mice or ticks at each time point: n.s. indicates not significant; * indicates p < 0.05; **** indicates p < 0.0001.
Two weeks later (week 2), there were no significant differences in Ft numbers among the three tick species, with means of 3.9 × 106 CFU/Dv tick, 3.7 × 106 CFU/Aa tick, and 1.1 × 106 CFU/Hl tick (Figure 2). These results are in contrast to week 2 results from the 105 CFU infection studies, where Aa ticks contained significantly higher Ft numbers than either Dv or Hl ticks (Figure 1). At the 107 CFU infectious dose, Ft replicated 1.1-fold in Dv ticks and 1.4-fold in Hl ticks between day 0 and week 2, while decreasing 5.7-fold in Aa ticks (Figure 2). Changes in Ft numbers in Dv and Aa ticks at this higher infectious dose were similar to the 105 CFU infection studies, where Ft increased >1 order of magnitude in Dv ticks and decreased approx. 5-fold in Aa ticks between day 0 and week 2 (Figure 1). At this time, we are unable to completely explain the differences between week 2 results when comparing the 105 (Figure 1) and 107 CFU (Figure 2) infectious dose studies, but it remains possible that 105 CFU is near the lower threshold of infectious dose needed to infect Dv ticks and/or 107 CFU is near the upper threshold of infectious dose that Aa ticks can support. Indeed, the highest Ft numbers detected in Aa ticks throughout the study were observed on day 0 (Figure 1).
In week 4, no significant differences in Ft numbers were calculated among the three tick species, with means of 5.1 × 106 CFU/Dv tick, 3.7 × 106 CFU/Aa tick, and 2.1 × 105 CFU/Hl ticks (Figure 2). Between weeks 2 and 4, Ft replicated 1.3-fold in Dv ticks, while remaining constant in Aa ticks (Figure 2). All Hl ticks molted from nymphs to adults between weeks 2 and 4, and Ft numbers decreased 5.2-fold in Hl ticks during this time frame. Compared to the 105 CFU infection studies (Figure 1), Hl ticks did not clear Ft by week 4 (Figure 2), indicating that a higher infectious dose (7.4 × 105 CFU/Hl tick on day 0) can overcome the previously observed restriction/loss of Ft in Hl ticks (Figure 1). However, Ft only was detected in 50% (4 of 8) of Hl ticks in week 4 (compared to 94% infection rate for Dv ticks and 95% infection rate for Aa ticks), indicating that, even at higher infectious doses, Hl ticks are not ideal vectors for tularemia.
In week 6, Ft numbers decreased in all three tick species, with means of 2.2 × 106 CFU/Dv tick (2.3-fold decrease), 1.8 × 106 CFU/Aa tick (two-fold decrease), and 5.1 × 103 CFU/Hl tick (42-fold decrease; significantly lower than either Dv or Aa ticks; p < 0.05) (Figure 2). In addition, only 25% (two out of eight) of Hl ticks were infected with Ft in week 6 (compared to 90% infection rate for Dv ticks and 95% infection rate for Aa ticks), providing further evidence that Hl ticks are not ideal vectors for tularemia. Between weeks 4 and 6, >99% of Dv and Aa ticks molted from nymphs to adults, with no adverse effects noted in Ft-infected ticks compared to non-infected ticks (data not shown).
Finally, a subset of ticks was processed in week 14 to assess the ability of Ft to persist in all three tick species long-term, similar to what would occur during an overwintering event in the south-central U.S. (approx. 3.5 months). In week 14, Ft numbers were more variable than week 6, however Ft replicated 2.7-fold in Dv ticks (compared to week 6; mean 6.1 × 106 CFU/Dv tick; 85% of Dv ticks infected with Ft), while Ft numbers declined 5.2-fold in Aa ticks (compared to week 6; mean 3.4 × 105 CFU/Aa tick; 95% of Aa ticks infected with Ft) (Figure 2). Importantly, Ft was not detected in any Hl ticks in week 14 (Figure 2) and indicated that, in agreement with our 105 CFU infection results where Ft did not persist in Hl ticks >4 weeks (Figure 1), Hl ticks are unlikely to serve as a major vector for tularemia, regardless of the infectious dose introduced into Hl ticks or a later time point examined (>4 weeks). In summary, at this higher infectious dose (106 CFU/Dv tick; 107 CFU/Aa tick), both Dv and Aa ticks supported the long-term (14 week) persistence of Ft. Despite Aa ticks being initially infected by 1 order of magnitude more Ft than Dv ticks (day 0; Figure 2), bacterial numbers decreased by nearly 2 orders of magnitude in Aa ticks over 14 weeks, compared to a modest replication (0.3 orders of magnitude) of Ft in Dv ticks over the same time period (Figure 2). In contrast, Hl ticks do not appear to be a major vector for tularemia; they initially were infected by 1 to 2 orders of magnitude less Ft than either Dv or Aa ticks (day 0) and, although Ft modestly (1.4-fold) replicated in Hl ticks between day 0 and week 2, Ft numbers rapidly declined in Hl ticks throughout the remainder of the experiment, with no Hl ticks containing Ft in week 14. Compared to the 105 CFU infection experiments, where Ft numbers increased 20-fold in Dv ticks and 1.1-fold in Aa ticks over six weeks, infections of Dv and Aa ticks at this higher infectious dose (107 CFU) revealed no Ft replication in Dv ticks and a reduction in Ft of approx. 1 order of magnitude in Aa ticks over six weeks (day 0 to week 6), suggesting that there may be a limit to Ft numbers that Dv and Aa ticks can support. Indeed, the highest number of bacteria detected in these 107 CFU infectious dose studies was 9 × 107 CFU/Dv tick (week 14) and 7.5 × 107 CFU/Aa tick (day 0) (Figure 2). Given that the highest Ft numbers were detected in Aa ticks at the beginning of the experiment (day 0) and the highest Ft numbers were detected in Dv ticks at the end of the experiment (week 14; Figure 2), together with replication rate data (20-fold replication in Dv ticks at 105 CFU infectious dose; 2.7-fold replication in Dv ticks between weeks 6 and 14 at 107 CFU infectious dose), our results indicate that of the three tick species examined here, Dv ticks pose the greatest risk for Ft persistence and replication.
2.3. Ft Is Efficiently Transmitted by Infected Dv and Aa Ticks to Naïve Mice
We next assessed the ability of infected Dv and Aa ticks to transmit Ft to naïve mice. Hl ticks were not included in transmission studies because of lower initial infection rates (compared to Dv and Aa ticks), clearance/loss of Ft in Hl ticks in low dose (105 CFU) infection studies by week 4 (Figure 1), and clearance/loss of Ft in Hl ticks in high dose (107 CFU) infection studies by week 14 (Figure 2). In these studies, nymphal Dv and Aa ticks were infected with Ft by feeding uninfected ticks on infected mice (107 CFU/mouse; as described above), ticks were collected when replete, maintained for 14 weeks (including through the nymph-to-adult molt), individually placed onto naïve mice, allowed to take a blood meal, replete ticks were collected, and then mice were monitored for 21 days (or until humanely euthanized because of severe signs of tularemia). In these transmission experiments, only infected adult female ticks were placed onto naïve mice. The rationale for using adult female ticks for infection studies included: (i) adult Dv ticks have been reported to more efficiently transmit infections than Dv nymphs [18,28]; (ii) adult male ticks only partially feed on hosts before detaching and mating with adult female ticks, likely limiting the ability of adult male ticks to efficiently transmit pathogens [29,30]; (iii) in parallel experiments, we found that female ticks harbored higher Ft numbers than male ticks (8.1 × 106 CFU/female Dv tick vs. 1.9 × 104 CFU/male Dv tick; 3.2 × 105 CFU/ female Aa tick vs. 1.8 × 105 CFU/male Aa tick; Supplementary Figure S3), although not significant. In transmission studies, we placed infected adult female ticks, along with uninfected adult male ticks, to promote attachment and efficient feeding by female ticks [29].
Infected adult female Dv and Aa ticks (week 14) were individually placed, together with a non-infected adult male tick of the same species, onto naïve mice to assess Ft transmission. Of the 15 adult Dv female ticks and 15 adult Aa female ticks placed onto mice, 14 Dv ticks (93% attachment) and 13 Aa ticks (66% attachment) attached and fed to repletion within 7–12 days on naïve mice. Following tick engorgement/detachment and collection (day 0), mice were bled to quantitate Ft numbers in individual mice, with 71% (10 of 14) of Dv-infested mice (mean 1.8 x 105 CFU/mL) and 61% (9 of 13) of Aa-infested mice (mean 7.8 × 104 CFU/mL) having detectable CFUs in the blood on day 0 (Figure 3). Although not significant, differences in Ft numbers in mouse blood on day 0 (Dv- vs. Aa-infested mice; Figure 3) appeared to correlate with differences in Ft numbers in infected female ticks (Dv- vs. Aa-infected ticks; Supplementary Figure S3), where Dv ticks harbored and transmitted higher numbers of Ft, compared to Aa ticks.
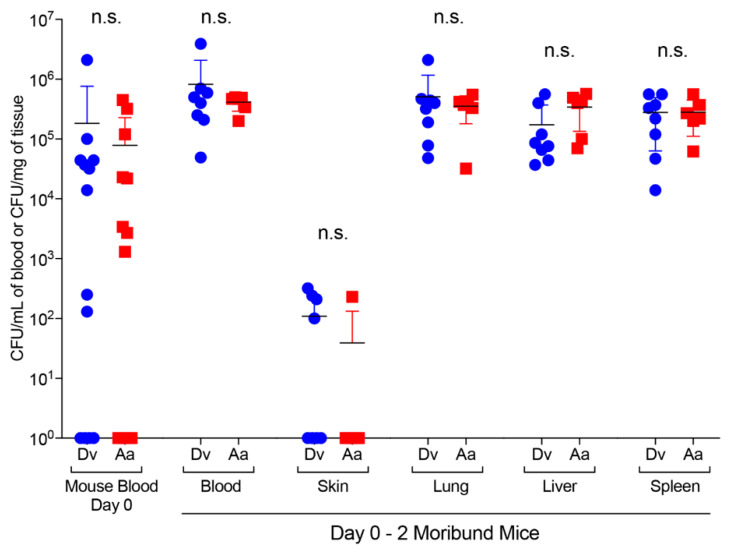
Figure 3.
Transmission of Ft from infected Dv and Aa ticks to naïve mice. Ft-infected Dv and Aa adult ticks were prepared as described for the 107 CFU infection studies (Figure 2). After 14 weeks, Ft-infected Dv and Aa adult ticks, together with an uninfected adult male tick of the same species, were individually placed onto naïve C3H/HeN mice and ticks were allowed to take a blood meal until replete (7–12 days). Upon repletion (day 0), ticks were harvested, and a small volume of mouse blood was collected from each mouse via retro-orbital bleeding to enumerate bacterial burdens in mice. Mice were monitored daily for signs of disease and humanely euthanized when moribund (within 2 days of tick repletion). Upon euthanasia, mouse blood and the indicated tissues (skin at attachment site, lungs, livers, and spleens) were collected and plated to enumerate bacterial numbers. Student’s t-test was used to compare bacterial numbers in blood samples or indicated samples: n.s. indicates not significant.
After tick harvest, mice (n = 14 for Dv-infested group; n = 13 for Aa-infested group) were monitored until they developed severe signs of tularemia (hunched, ruffled fur, conjunctivitis, unable to move when gently prodded), mice were humanely euthanized, blood, skin, lungs, livers, and spleens were harvested, and bacterial burdens were enumerated from each sample. From Dv-infested mice, 57% (8 of 14 mice) exhibited signs of tularemia within 2 days of tick detachment, with an average time to moribund status of 1.25 days. From Aa-infested mice, 46% (6 of 13 mice) exhibited signs of tularemia within 2 days after tick detachment, with an average time to moribund status of 1.20 days. Interestingly, two mice (1 Dv-infested; 1 Aa-infested) reached moribund status before their respective ticks completed feeding, demonstrating the ability of infected Dv and Aa ticks to cause rapid and lethal disease in animals. Between day 0 (tick repletion) and moribind status (within 2 days of tick repletion), Ft replicated approx. 0.6 orders of magnitude in the blood of both Dv- and Aa-infested mice and Ft was detected in all tissues collected from moribund mice (Figure 3). Comparing Ft numbers in Dv- and Aa-infested mouse tissues, Dv-infested mice had higher, although not significant, bacterial burdens in blood (mean 8.4 × 105 CFU/mL; Figure 3), lungs, livers, and spleens (means: 1.7 × 105–5.0 × 105 CFU/mg tissue; Figure 3) on days 0–2, compared to Aa-infested mice (blood mean 4.1 × 105 CFU/mL; lungs, livers, and spleens means: 2.8 × 105–3.5 × 105 CFU/mg tissue; Figure 3). When comparing Ft numbers at the tick attachment site, Dv-infested mice had higher bacterial numbers, although not signficiant, in mouse skin (1.0 × 102 CFU/mg), compared to Aa-infested mouse skin (3.9 × 101 CFU/mg). Additionally, classic tularemia skin ulcerations [31] were observed at the tick attachment site in 37% (3 of 8) of Dv-infested mice and 33% (2 of 6) of Aa-infested mice (Supplementary Figure S4). Although both Dv and Aa ticks transmitted rapid and lethal infections to naïve mice, higher Ft numbers in female Dv ticks in week 14 (Figure 2 and Supplementary Figure S3), higher rates of Dv-attachment to mice, and higher bacterial burdens in blood, skin, lungs, livers, and spleens of Dv-infested mice (Figure 3) indicate that Dv ticks may pose a greater health risk for transmitting and causing more severe tularemia infections.
2.4. FTL1793 Exhibits Chitinase Activity
Chitin, a polymer of N-acetylglucosamine (GlcNAc), is a major component of the tick cuticle and is remodeled during the tick molting process. As such, it has been speculated that chitin may be used as an energy source by tick-borne bacterial pathogens [32,33,34]. Chitin cleavage can be performed by chitinases, and bacterial chitinases have been shown to promote bacterial persistence in marine environments and mammals [35,36,37,38]. Using a conserved domain search, we searched the Ft LVS genome for putative chitinases and, confirming a previous report [25], found that gene locus FTL1793 (annotated as a hypothetical protein) contains a region encoding a putative glycosyl hydrolase (GH) 18 chitinase D-like region (Supplementary Figure S5A). Although some chitinase D-like proteins consist only of a catalytic domain [39,40], other chitinases are known to contain both a catalytic GH18 domain and a chitin-binding domain, linked by a fibronectin type III domain [41,42]. Different chitinase proteins are known to vary in chitin binding and chitin cleavage activity based on the presence/absence these domains [43]. FTL1793 was found to only contain a GH18 chitinase domain.
To confirm putative chitinase activity of FTL1793, we cloned, expressed, and purified recombinant FTL1793 protein. Recombinant FTL1793 was estimated to be approx. 90% pure, based on visualization of purified protein and immunoblot analysis (Supplementary Figure S5B,C). The ability of recombinant FTL1793 to cleave purified chitin was tested in chitin azure assays [44], where chitin has been covalently linked to remazol brilliant violet 5R (RBV) dye and chitin cleavage (dye release) can be monitored at 570 nm. Chitinase activity was measured for FTL1793, along with a positive control (chitinase from Streptomyces griseus), a negative control (Ft outer membrane protein FopA; [45,46]), and chitin azure assay buffer alone. An increase in absorbance was observed within 48 h in samples containing either FTL1793 or S. griseus chitinase, confirming the predicted chitinase activity of FTL1793 (Figure 4A). Given the lower relative chitinase activity of FTL1793 during the first 24 h, compared to S. griseus chitinase (Figure 4A), and the likely need for sustained Ft chitinase activity in ticks during an overwintering, separate chitin azure assays were set up and monitored over 30 days to assess long-term FTL1793 activity. In these long-term chitin azure assays, FTL1793 and S. griseus chitinase both demonstrated sustained chitinase activity during the 30 days incubation (Figure 4B). Negative control protein FopA and buffer alone did not display chitinase activity in either the 48 h or 30 days chitin azure assays (Figure 4A,B). These results confirmed that FTL1793 exhibits chitinase activity and suggested that Ft may use this chitinase to degrade chitin in ticks. Furthermore, sustained Ft chitinase activity (Figure 4B) may be needed for long-term persistence in ticks (e.g., overwintering event).
Figure 4.
FTL1793 exhibits chitinase activity. Recombinant FTL1793 protein was expressed and purified from Escherichia coli. Chitin azure assays were used to measure chitinase activity. In these assays, 94 μg of each of the following proteins were added per reaction: recombinant FTL1793, chitinase from Streptomyces griseus (positive control), or recombinant Ft FopA protein (negative control). Chitin azure in assay buffer alone (buffer) was also tested. Reactions were incubated on an end-over-end carousel for either: (A) 48 h at 37 °C; or (B) 30 d at 37 °C. Absorbance at 570 nm was measured to determine chitin cleavage. Samples were prepared in triplicate and measurements recorded every 24 h.
2.5. Ft FTL1793, a Putative Chitinase, Is Required for Ft Persistence in Ticks
Although previous studies speculated that chitinases may play important roles in Ft environmental persistence or in Ft tick infections [25,47], only one study has examined if chitinases are required for or contribute to Ft infections of ticks. However, that study co-infected D. andersoni ticks with a combination of three independent Francisella novicida chitinase mutants (chiA, chiB, chiAB), finding that all three mutants were present in ticks four days later, suggesting that F. novicida chitinases may not be required for tick infections [48]. Given the lack of studies testing Ft and putative Ft chitinases in either Dv or Aa ticks, the true role of Ft chitinases in tick infections remains unclear. A separate study examined an Ft purMCD mutant (purine auxotroph), which is avirulent in mice, in Dv ticks, finding that the purMCD mutant did not persist in ticks through the molt to the adult stage [19]. However, given the high degree of attenuation of that mutant in mice and low initial infectivity of purMCD in Dv ticks, it is unlikely that purine biosynthesis, alone, plays a major role in Ft persistence in ticks. To examine the role of FTL1793 and associated chitinase activity in tick infections, an isogenic Ft LVS mutant was generated using homologous recombination [49], referred to hereafter as ΔFTL1793. To confirm that ΔFTL1793 did not possess an inherent growth defect, the growth of WT Ft LVS and ΔFTL1793 were compared in liquid growth medium, demonstrating no substantial differences in bacterial growth over 32 h (Supplementary Figure S6A).
Before testing the infectivity of the ΔFTL1793 mutant in ticks, we first assessed the virulence of ΔFTL1793 in mice. These initial mouse virulence testing studies were important because our mouse–tick–Ft infection model relies on Ft virulence, including bacteremia in mice, to reproducibly infect ticks (Figure 1 and Figure 2). Although our mouse–tick–Ft infection model infects mice i.v. with Ft two days before tick repletion, it remained possible that this two-day i.v. infection window was too narrow to assess potential virulence defects of ΔFTL1793. As such, we performed mouse pulmonary infections (intranasal delivery) to assess ΔFTL1793 virulence. We, and others, have previously reported on the virulence of Ft LVS in mice via the intranasal route, which typically results in morbidity/mortality within 5–8 days [50,51,52]. Groups of mice were intranasally infected with either 104 CFU of wild-type (WT) Ft LVS, 104 CFU of ΔFTL1793, 107 CFU of ΔFTL1793, or 109 CFU of ΔFTL1793, and monitored for 21 days after infection (or euthanized when moribund). Confirming previous intranasal infection studies in our laboratory, all mice intranasally infected with 104 CFU of WT Ft LVS died within seven days (Supplementary Figure S6B). At 104 CFU, ΔFTL1793 was partially attenuated, with 60% (three of five) of mice surviving until day 21 (Supplementary Figure S6B). In contrast, only 25% (one of four) of mice infected with either 107 CFU or 109 CFU of ΔFTL1793 survived infection (Supplementary Figure S6B). Given that there was no difference in percentage survival of mice infected with either 107 or 109 CFU of ΔFTL1793, we selected the 107 CFU infectious dose of ΔFTL1793 for subsequent mouse–tick–Ft infection studies and to correlate these findings with our previous 107 CFU infectious doses studies (Figure 2).
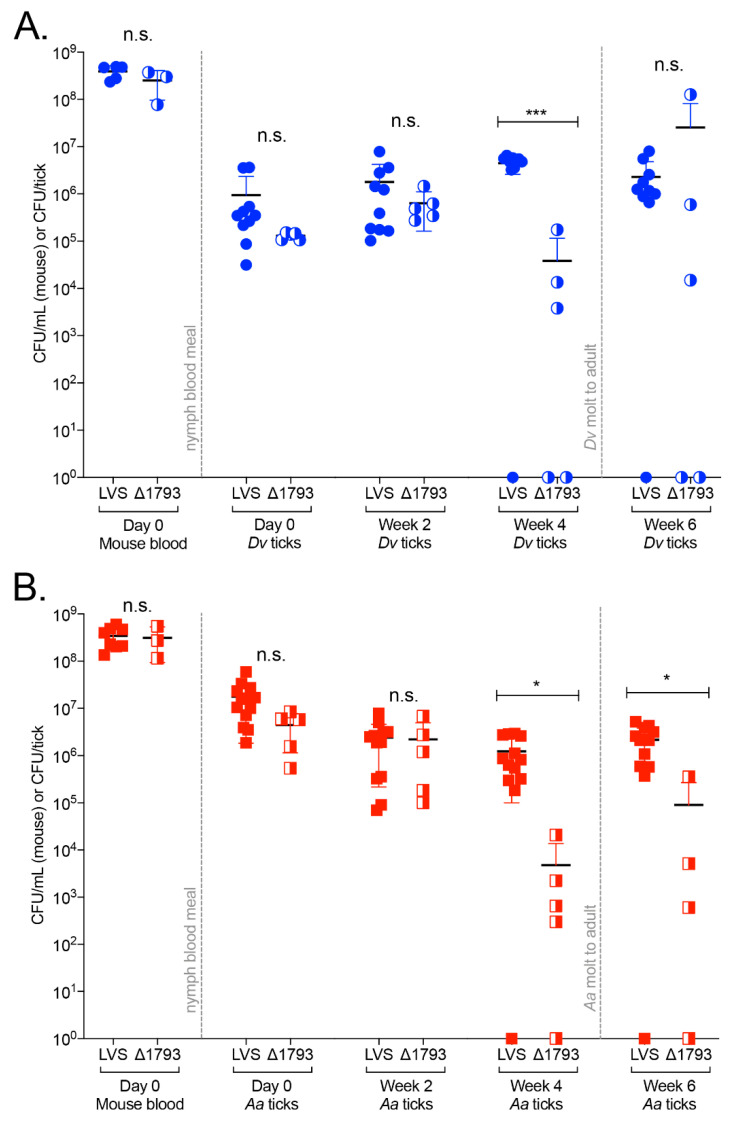
To assess the role of FTL1793 in promoting Ft infections of ticks, Dv and Aa ticks were infected with either WT Ft LVS or ΔFTL1793, identical to what is described above for the high dose (107 CFU) tick infection studies (Figure 2). In these studies, uninfected Dv and Aa ticks were placed onto naïve mice (day 5), the ticks fed for three days, mice were i.v. infected (day 2) with either 2.4 × 107 CFU of WT Ft LVS or 3.8 × 107 CFU of ΔFTL1793, and replete ticks and mouse blood were collected two days later (day 0). Despite partial attenuation of ΔFTL1793 via the intranasal route (Supplementary Figure S6B), ΔFTL1793 was not attenuated via the i.v. route, because no significant differences were found between WT Ft LVS and ΔFTL1793 numbers in the blood of infected mice (either Dv-infested or Aa-infested) on day 0 (Figure 5A,B). In addition, both WT Ft LVS and ΔFTL1793 were found to have replicated approx. 1 order of magnitude in mouse blood over two days (Figure 5A,B).
Figure 5.
Comparison of Ft LVS and ΔFTL1793 in Dv and Aa ticks. Nymphal Dv (panel (A); top; blue circles) or Aa (panel (B); bottom; red squares) ticks were placed onto non-infected C3H/HeN mice (day 5), the ticks fed for 3 days, mice were i.v. infected with 107 CFU of Ft LVS or the ΔFTL1793 mutant (day 2), and replete ticks were harvested approx. 2 days later (day 0; 5-day total blood meal). Following tick harvest (day 0 ticks), blood was collected and plated from infected mice (day 0 mouse blood; n = 3–8/group) to enumerate bacterial numbers (CFU/mL blood). At the indicated time points, individual ticks (n = 4–13/tick species) were homogenized and plated to enumerate bacterial numbers (CFU/tick). Student’s t test was used to calculate differences between LVS and ΔFTL1793 at each time point: n.s. indicates not significant; * indicates p < 0.05; *** indicates p < 0.001.
On day 0, replete Dv ticks were found to be infected by nearly 1 order of magnitude more WT Ft LVS (mean 9.4 × 105 CFU/Dv tick) than ΔFTL1793 (mean 1.3 × 105 CFU/Dv tick), although not significantly (Figure 5A). Similarly, in week 2, nearly 0.5 orders of magnitude more WT Ft LVS (mean 1.7 × 106 CFU/Dv tick) was present in Dv ticks, compared to ΔFTL1793 (mean 6.3 × 105 CFU/Dv tick), although not significantly (Figure 5A). In week 4, WT Ft LVS was 2 orders of magnitude higher (mean 4.4 × 106 CFU/Dv tick) than ΔFTL1793 (mean 3.4 × 104 CFU/Dv tick) in Dv ticks (p = 0.0002; Figure 5A). Between weeks 2 and 4, differences in WT and ΔFTL1793 bacterial numbers in Dv ticks were further highlighted by a 2.5-fold replication of WT Ft LVS compared to a 16-fold decrease in ΔFTL1793 in Dv ticks during the same time period (Figure 5A). Between weeks 4 and 6, nymphal Dv ticks molted to adults, with WT Ft LVS decreasing two-fold (mean 2.2 × 106 CFU/Dv tick in week 6), while ΔFTL1793 increased 650-fold (mean 2.5 × 107 CFU/Dv tick in week 6) (Figure 5A). However, differences between WT Ft LVS and ΔFTL1793 were not signficantly different in week 6.
In Aa ticks, approx. 0.7 orders of magnitude more WT Ft LVS (mean 1.7 × 107 CFU/Aa tick) was present on day 0, compared to ΔFTL1793 (mean 4.4 × 106 CFU/Aa tick), although not significantly (Figure 5B). In week 2, WT Ft LVS (mean 2.4 × 106 CFU/Aa tick) and ΔFTL1793 (mean 2.2 × 106 CFU/Aa tick) numbers were similar, with both bacterial strains having decreased in Aa ticks from day 0 to week 2 (Figure 5B). In week 4, Ft LVS numbers (mean 1.2 × 106 CFU/Aa tick) were 2.6 orders of magnitude higher (p = 0.0332) than ΔFTL1793 (mean 4.7 × 103 CFU/Aa tick) (Figure 5B). Differences between WT Ft LVS and ΔFTL1793 numbers in week 4 were highlighted by differences in replication rates between weeks 2 and 4, with WT Ft LVS decreasing two-fold in Aa ticks, while ΔFTL1793 decreased over 460-fold (Figure 5B). In week 6, WT Ft LVS was >1 order of magnitude higher (mean 2.1 × 106 CFU/Aa tick) than ΔFTL1793 (mean 9.0 × 104 CFU/Aa tick; p < 0.05), with WT Ft LVS having replicated 1.7-fold between weeks 4 to 6, compared with 18-fold replication of ΔFTL1793 during the same time period (Figure 5B). Comparing ΔFTL1793 and WT Ft LVS in both Dv and Aa ticks, ΔFTL1793 was less efficient at infecting (day 0), replicating in, and persisting in nymphal ticks until week 4 (Figure 5A,B). However, after the molt to adult (week 6), ΔFTL1793 was found in higher numbers in Dv ticks and replicated faster than WT Ft LVS in both Dv and Aa ticks. Taken together, these data indicate that FTL1793 and its associated chitinase activity contribute to the infection and persistence of Ft in nymphal ticks, prior to the molt to the adult life stage (e.g., first four weeks). Increases in ΔFTL1793 numbers after the molt indicate that additional chitinases or other genes are required for Ft to persist and replicate in ticks long-term.
3. Discussion
Although tick-borne tularemia was described as early as 1924, major questions remain about which arthropod vectors pose the greatest risk for harboring and transmitting Ft to humans. Data from the CDC indicate that D. andersoni, Dv, and Aa ticks, as well as deer flies (Chrysops sp.), can transmit tularemia. In addition, modeling has predicted that climate change, specifically milder winters and increased precipitation at higher latitudes, will drive major increases in tick numbers and expansion of ticks into new geographic areas in upcoming years [8,9,53,54]. Finally, the appearance of invasive tick species, including Hl from Asia, in the U.S. has raised concerns about the introduction of exotic tick-borne diseases into the U.S., the transmission of existing U.S. tick-borne diseases by invasive tick species, and co-transmission of two or more diseases by ticks to humans [55]. Although Hl ticks were shown not to vector Borrelia burgdorferi [56], the causative agent of Lyme disease, Hl ticks were shown to harbor and transmit Rickettsia rickettsii [57], the agent of Rocky Mountain spotted fever, leaving unanswered questions about what other tick-borne diseases can be harbored and transmitted by Hl ticks. Those findings are not unique to Hl ticks; it was previously reported that B. burgdorferi was unable to persist in Dv ticks, likely due to the presence of Dv-specific antimicrobial peptides that are lytic to the spirochete [58].
Despite a number of previous studies examining Ft infection or transmission by Dv ticks [15,16,18,19,28,59,60], much less information is available about Ft infections of Aa ticks [20,21]. Importantly, none of those previous studies directly compared Ft infection of and transmission by Dv and Aa ticks. Furthermore, given concerns about the invasion of Hl ticks into the U.S., no studies have examined whether Ft can infect Hl ticks. In this study, we directly compared Ft infections of Dv, Aa, and Hl nymphal ticks, at two different infectious doses, and monitored Ft persistence, replication, and/or clearance in these ticks over the course of 6–14 weeks. This is the first study to report that, although Ft initially infects Hl ticks, Ft is unable to persist or is cleared by Hl ticks, at both 105 and 107 CFU infectious doses. Due to the lack of Ft in Hl ticks in week 14, we were unable to perform transmission studies. However, given that a low dose (105 CFU) of Ft was not detected in Hl ticks after two weeks and a high dose (107 CFU) of Ft was not detected in Hl ticks after six weeks, our results indicate that Hl ticks are unlikely to serve as a major vector for tularemia. By directly comparing Ft infection and persistence in both Dv and Aa ticks, the two major U.S. tick vectors for tularemia, we found that Aa ticks initially were infected by approx. 1 order of magnitude more Ft (at both 105 and 107 CFU infectious doses), compared to Dv ticks. Higher Ft numbers in Aa ticks were not due to larger blood meal volumes by Aa ticks (when relative CFU/tick were adjusted for weights of engorged ticks), suggesting either that there are inherent differences in how Ft infects Dv and Aa ticks, or there are differences in how Dv and Aa ticks respond to Ft infections. Another possibility is that Aa ticks concentrate their blood meals more efficiently than Dv ticks. One previous study examined the blood volumes imbibed by various life stages of Aa ticks (larvae, nymph, adult), finding that Aa ticks remove excess ions and water to concentrate the blood meals [61]. Other studies have used isotopes to accurately measure the amount of red blood cells and plasma imbibed by other tick species [62]. However, it is not known if Aa ticks concentrate their blood meal more efficiently than Dv ticks, which could help explain differences in Ft numbers in engorged ticks. Separately, other studies have shown that erythrocyte invasion by Ft may enhance its ability to colonize ticks following a blood meal [63]. Although it is unclear how Ft invades erythrocytes, given that these cells do not phagocytose or endocytose, it is possible that Aa ticks imbibe an increased number of erythrocytes via blood meal concentration, compared to Dv ticks, which also may help to explain higher Ft numbers in engorged Aa ticks [63].
Alternatively, differences in Dv and Aa endosymbionts or tick midgut microbiota [64,65,66,67] may impact the ability of Ft to initially colonize and persist in ticks by competing with Ft for nutrients, providing essential nutrients to Ft, or modulating tick immune responses that either promote or restrict Ft infection. Although some previous studies have speculated that tick endosymbionts may provide nutrients to other tick-borne pathogens [64,68], nothing has been reported about how Dv and Aa endosymbionts influence the Ft persistence in ticks. Our studies demonstrated that Ft persisted in both Dv and Aa ticks for up to 14 weeks (3.5 months) following engorgement. After the tick processes its blood meal, nutrients become extremely limited [69]. For Ft, the lack of nutrients in ticks during an overwintering event is likely to be further confounded by missing and/or incomplete amino acid biosynthesis pathways [70], suggesting that Ft may need to acquire nutrients exogenously. Although it remains possible that tick endosymbionts could provide essential nutrients to Ft and that differences in Dv and Aa endosymbionts could explain differences in Ft numbers and Ft replication rates in either tick, detailed studies on Dv and Aa endosymbionts, in the context of Ft infections, are needed. Such studies could provide important information about tick–microbiome–pathogen interactions.
Despite higher initial Ft numbers in Aa ticks, we found that Ft replicated more robustly in Dv ticks, increasing 20-fold in Dv ticks over six weeks at the low infectious dose (compared to a 1.1-fold replication in Aa ticks during the same time period) and increasing 1.7-fold in Dv ticks over 14 weeks at the high infectious dose (compared to a 64-fold decrease in Aa ticks over the same time period). Although higher Ft replication rates in Dv ticks did not correlate with a significantly higher Ft transmission to naïve mice, Ft numbers in Dv-infested mice were generally higher in all tissues examined, compared to Ft numbers in Aa-infested mice. Interestingly, at the low infectious dose, Ft replicated robustly in both Dv and Aa ticks between weeks 2 and 4. Other tick researchers have speculated that the breakdown of the peritrophic matrix, a semipermeable membrane that surrounds the tick blood meal, contributes to the availability of nutrients for residing pathogens [32,33]. Because the tick peritrophic matrix is composed of chitin [71], bacteria able to degrade this N-acetyl-glucosamine polymer may be able to generate carbon as an energy source [35,72]. Given that Dv and Aa ticks molted between weeks 4 and 6, chitin may have been available preceding the molt (between weeks 2 and 4), which may explain the 11-fold Ft replication in Dv ticks and 5-fold Ft replication in Aa ticks between weeks 2 and 4. In contrast, Ft replication between weeks 2 and 4 in the high infectious dose study was modest in both Dv and Aa ticks. One possibility is that the amount of free chitin in ticks before the molt is limited and higher Ft numbers consumed all available chitin. Indeed, in these higher dose infections, bacterial burdens did not exceed 107 CFU/tick, suggesting that there is a limit to Ft numbers in ticks.
Other pathogenic bacteria, including Vibrio cholerae, have been shown to utilize chitinases to promote environmental persistence and form biofilms on chitin in the marine environment [36,73]. Similarly, F. novicida was reported to form biofilms on chitin surfaces and use chitin as a sole energy source. In that study, two F. novicida chitinase mutants, ΔchiA (F. novicida gene locus FTN_0627) and ΔchiB (F. novicida gene locus FTN_1744), were unable to colonize or form biofilms on chitin [47]. However, given the many differences between Ft and F. novicida [74,75], and previously-noted differences among Ft Type A, Ft Type B, and F. novicida putative chitinases [25], the application of those findings to Ft strains remains unknown. Considering those studies, and that chitin is a major component of ticks (including the peritrophic matrix), we hypothesized that Ft chitinases may play important roles in infection and persistence in ticks. Of the four putative Ft chitinases, ChiA (gene loci FTT_0715 in Type A strain SchuS4, FTL_1521 in LVS, and FTN_0627 in F. novicida), ChiB (gene loci FTT_1768 in SchuS4, FTL_0093 in LVS, and FTN_1744 in F. novicida), and ChiC (gene loci FTT_1592c and FTT_1593c in SchuS4, FTL_1635 in LVS, and FTN_0627 in F. novicida) were reported to vary substantially among Francisella sp. in amino acid identity, predicted open reading frame lengths, and predicted domains within each gene [25]. Here, we focused on ChiD (gene loci FTT_0066 in SchuS4, FTL_1793 in LVS, and FTN_1644 in F. novicida) given that chiD sequences were highly conserved (98.6% identity) among Ft Type A, Ft Type B, and F. novicida. Our ΔFTL1793 mutant, lacking a GH18 chitinase, was detected at significantly lower numbers (2 orders of magnitude less in Dv ticks; 2.5 orders of magnitude less in Aa ticks) in ticks in week 4 (immediately before the tick molt). In addition, our in vitro chitinase activity assays confirmed that recombinant FTL1793 continuously cleaved chitin (throughout 30-day incubation). Taken together, these results suggest that FTL1793 degrades tick chitin, likely liberated immediately prior to tick molting, to promote Ft replication in both Dv and Aa ticks. Despite significantly lower ΔFTL1793 numbers in week 4, ΔFTL1793 numbers subsequently increased in both ticks between weeks 4 and 6, suggesting that other proteins, including other chitinases, may provide nutrients to Ft to promote bacterial replication. As noted above, a previous study noted that Ft contains at least four chitinases (ChiA, ChiB, ChiC, ChiD) and speculated that different chitinases may function at different stages throughout Ft tick infection [25,47]. Interestingly, in that previous study, none of the ChiD orthologs (including gene loci FTT_0066 in SchuS4, FTL_1793 in LVS, and FTN_1644 in F. novicida) were found to possess chitinase activity. However, those authors tested chitinase activity using different chitin substrates, including chitin analogs, and incubation times and conditions also varied from our studies. Regardless, given our findings that FTL1793 exhibits chitinase activity and FTL1793 contributes to persistence and replication of Ft in both Dv and Aa ticks, future studies are needed to fully elucidate the function of all Ft chitin-related genes in ticks.
Finally, it remains possible that FTL1793, and other Ft chitinases, may perform functions in addition to degrading arthropod/tick chitin. In Pseudomonas aeruginosa, a pulmonary pathogen, endochitinase activity correlates with antifungal activity, which may be important for microbial competition in the lung [76]. In Legionalla pneumophila, another pulmonary pathogen, the ChiA chitinase is required to cleave lung mucin and promote bacterial penetration through the alveolar mucosa [38]. Other bacterial chitinases have been shown to play similar roles in bacterial virulence, including cleavage of mammalian glycolipids, glycoproteins, and extracellular matrix components [77]. Here, our initial virulence testing of the ΔFTL1793 mutant in a mouse pulmonary infection model revealed that ΔFTL1793 was partially attenuated. However, ΔFTL1793 bacteremia numbers (i.e., intravenous infection) were equivalent to WT Ft LVS after two days of infection, indicating that FTL1793 may be required for Ft virulence in lungs but not in the bloodstream. Given these findings, future studies should examine the potential multi-functional role(s) of FTL1793, and other Ft chitinases, in both ticks and mammalian hosts, and consider different infection routes for these studies.
4. Materials and Methods
4.1. Bacterial Stains and Culture Conditions
Francisella tularensis Type B strain LVS was obtained from BEI Resources and cultured as previously described [50,51]. Routine F. tularensis cultures were grown overnight on supplemented Mueller-Hinton agar (sMHA) at 37 °C with 5% CO2. sMHA plates were prepared with the following: Mueller-Hinton broth powder (Becton Dickinson) was mixed with 1.6% (wt/vol) Bacto Agar (Becton Dickinson), autoclaved, and further supplemented with 2.5% (vol/vol) bovine calf serum (Hyclone), 2% (vol/vol) IsoVitaleX (Becton Dickinson), 0.1% (wt/vol) glucose, and 0.025% (wt/vol) iron pyrophosphate. For mouse infections, F. tularensis strains were first grown for 24 h on sMHA then transferred to Brain heart infusion agar (BHI; Becton Dickinson) and grown overnight at 37 °C with 5% CO2.
4.2. Mouse–Tick–F. tularensis Infection and Transmission
Dermacentor variabilis, Amblyomma americanum, and Haemaphysalis longicornis nymphal and adult ticks were obtained from either the Centers for Disease Control and Prevention through the Biodefense and Emerging Infectious Diseases (BEI) Resources Repository or from the Tick Rearing Facility, National Tick Research and Education Resource, Oklahoma State University, Stillwater, OK, U.S.A. Ticks were housed in 3-dram plastic vials in a glass desiccator, with 12 h light–dark cycles, at ambient temperature over saturated potassium nitrate (KNO3), which generated a humidified atmosphere of >90% at 20 °C. All studies with mice and ticks were approved by the University of Toledo Institutional Animal Care and Use Committee (IACUC; protocol 108672 approved May 2019 and valid until May 2022) and Institutional Biosafety Committee (IBC; protocol 108665 approved March 2016 and valid until March 2021). C3H/HeN mice, female, 8–10 weeks old, were purchased from Charles River Laboratories. One day prior to tick placement (day 6), mice were anesthetized with an i.p. injection of ketamine-xylazine, an area approximately 2.5 cm in diameter between the shoulder blades was shaved with surgical clippers, and plastic chambers (top portion of 15 mL conical tubes) were adhered to shaved skin using Kamar adhesive. Mice were individually housed to prevent chamber removal by cage mates and mice were maintained in disposable, plastic cages with sealable lids and high-efficiency particulate air (HEPA) filters (Innovive). The next day (day 5), mice were anesthetized, 12 nymphal D. variabilis, A. americanum or H. longicornis ticks were placed in each chamber, chambers were closed with a fine-mesh polyester fabric, and fabric was secured to each chamber using a rubber band. Double-sided tape was adhered to the inside upper rim of each cage bottom, cage lids were secured onto each cage, and cages were placed onto tack mats to prevent the loss of any escaped ticks. All tick studies were performed in designated BSL2/A-BSL2 rooms with double-sided tape placed around the entry/exit door and tack mats placed in front of entry/exit doors to prevent the loss of any escaped ticks. For mouse infections, overnight LVS growth was scraped from BHI agar plates, suspended in sterile PBS, and diluted to either 105 or 107 CFU/100 µL, based on previous OD600 measurements and bacterial enumeration studies. Three days after tick placement (day 2), mice were anesthetized by an i.p. infection of ketamine-xylazine, and intravenously infected (retro-orbital) with F. tularensis LVS. Bacterial inocula were serially diluted and plated in quadruplets onto sMHA to confirm CFUs. Approximately 48 h after infection (day 0), mice were anesthetized by an i.p. injection of ketamine-xylazine, replete ticks were collected, and blood was harvested from infected mice by cardiac puncture for serial dilution in PBS and plated onto sMHA. Individual mice and groups of replete ticks from each mouse were sequentially numbered so that bacterial numbers could be correlated between mouse blood and associated ticks. Prior to tick homogenization, ticks were individually weighed, and weights recorded for subsequent analysis. Ticks were processed on day 0 (replete/engorgement), week 2, week 4, week 6, and week 14 after harvesting from mice. Prior to homogenization, ticks were surface-sterilized by being placed into 30% H2O2 for five seconds, 70% ethanol for five seconds, rinsed with molecular biology grade water (Corning) for 5 s, then homogenized in RNase-free disposable pellet pestle tubes (Fisher) containing 200 µL of sterile PBS. Tick homogenates were serially diluted in PBS and plated onto sMHA containing 100 mg/L cycloheximide, 80,000 U/L polymyxin B, and 2.5 mg/L amphotericin B. Following 72 h of incubation, colonies were counted, and CFU/mL (mouse blood), CFU/tick, or CFU/mg tick were calculated. Transmission studies (from infected ticks to naïve mice) were performed essentially as described above, with the following modifications: LVS-infected adult female ticks (14 weeks after their nymphal blood meal) were individually placed into chambers and allowed to feed to repletion on naïve mice. A single non-infected adult male tick of the same species was added to each chamber to promote female tick attachment and efficient feeding. Replete adult ticks were collected 7–12 days after tick attachment. To quantitate bacterial burdens in mouse blood during transmission studies, mouse blood was collected after ticks completed their blood meal (7–12 days after tick placement) via retro-orbital bleeding. Following tick detachment, mice were monitored at least three times daily for signs of tularemia and were humanely euthanized when moribund (within 2 days of tick detachment). For euthanasia, mice were anesthetized by an i.p. infection of ketamine-xylazine, blood was collected by cardiac puncture, mice were cervically dislocated, skin from the tick attachment site was harvested using an 8 mm biopsy punch (Accuderm, Fort Lauderdale, FL, USA), and lungs, livers, and spleens were aseptically harvested and transferred to sterile Whirlpack (Madison, WI, USA) bags. Skin, lungs, livers, and spleens were homogenized, 25 µL of PBS/mg of tissue was added to each tissue, serially diluted, and dilutions were plated onto sMHA. After 72 h of incubation at 37 °C, bacterial numbers were enumerated from all samples. Fold changes in CFU/tick between time points were calculated by the formula: final value/initial value (Y/X).
4.3. Bioinformatic Predictions
A putative chitinase domain in gene locus FTL1793 was identified using the National Center for Biotechnology Information (NCBI) Conserved Domain Search: https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi).
4.4. Generation of F. tularensis Gene Deletion Mutant
Isogenic deletion mutants in F. tularensis were generated by homologous recombination as previously described [49]. F. tularensis LVS genomic DNA was extracted using phenol-chloroform (Fisher Bioreagents, Chicago, IL, USA). Approx. 700 bp regions immediately upstream and downstream from gene locus FTL1793 were PCR-amplified from F. tularensis Type B strain LVS genomic DNA using the following primers, respectively: FTL1793_A (5′-GGTAAGGGGCCCGCTTTTAACTGACTTGAAGCC-3′) and FTL1793_B (5′-AACTTCCGCCGGCGTAGTATCGCCAGATTCATTCATTTCC-3′); FTL1793_C (5′-AACTTCCGCCGGCGTAGTAAACCCTTGTTGTTGAACCTG-3′) and FTL1793_D (5′-GGTAAGGGGCCCAGCTGATATGGTGAGTCTGC-3′). Separately, a flippase (FLP) recombination target (FRT)-flanked Pfn-kanamycin resistance cassette (kan) was PCR-amplified from pLG66a, as previously described [49]. Splicing overlap extension (SOE) PCR was performed to join the upstream and downstream flanking regions with the FRT-Pfn-kan-FRT amplicon, which replaced FTL1793. The resulting upstream-FRT-Pfn-kan-FRT-downstream insert was digested with ApaI (New England Biolabs, Ipswich, MA, USA) and ligated into similarly digested pTP163 (suicide plasmid) using T4 DNA ligase (New England Biolabs, Ipswich, MA, USA). The resulting gene deletion construct was transformed into NEB 10-β Escherichia coli cells (New England Biolabs, Ipswich, MA, USA) and DNA sequencing was performed to verify the integrity of the insert. Positive constructs were transformed into E. coli S17-1 cells and conjugation was performed with F. tularensis LVS on chocolate agar (Mueller-Hinton medium supplemented with 1% (wt/vol) tryptone, 0.5% (wt/vol) NaCl, 1.6% (wt/vol) agar, 1% (wt/vol) bovine hemoglobin powder (Neogen, Lansing, MI, USA), 0.1% (wt/vol) glucose, and 2% (vol/vol) IsoVitaleX (Becton Dickinson, Franklin Lakes, NJ, USA) overnight at 30 °C with 5% CO2. The next day, conjugants were transferred onto chocolate agar supplemented with 200 mg/L hygromycin and 100 mg/L polymyxin B, incubated for 3 to 4 days, individual colonies were transferred to sMHA supplemented with 10 mg/L kanamycin (sMHA-kan10), and kanamycin-resistant colonies were transferred to sMHA supplemented with 10 mg/L kanamycin and 8% (wt/vol) sucrose. Final replica plating of individual clones was performed by transferring colonies onto sMHA containing either 200 mg/L hygromycin (sMHA-hyg200) or sMHA-kan10. Hyg-sensitive and kan-resistant colonies, indicating loss of the suicide plasmid and replacement of FTL1793 with kan, were sequence verified. The resulting gene deletion strain was designated ΔFTL1793.
4.5. Intranasal Mouse Infections
Both F. tularensis LVS and ΔFTL1793 were grown on sMHA overnight, then transferred to BHI for 20–24 h. Bacteria were scraped, resuspended in sterile PBS, and diluted to the desired concentration (104 to 109 CFU/20 μL) based on previous OD600 measurements and bacterial enumeration studies. To confirm infectious doses, bacterial inocula were serially diluted and plated in quadruplets on sMHA. Groups of female C3H/HeN mice (6–8 weeks old; Charles River Laboratories, Wilmington, MA, USA) were anesthetized by an i.p. infection of ketamine-xylazine and intranasally (i.n.) infected with 20 μL of the indicated bacterial resuspension. Mice were monitored daily for signs of disease and the individual health status of all mice was recorded using 5-point health status scale (‘1′ indicated healthy mice, and ‘5′ indicated mice found dead). Moribund mice (health status ‘4′) were humanely euthanized to minimize suffering.
4.6. Expression and Purification of Recombinant FTL1793 Protein
Gene locus FTL1793, without the amino-terminal signal sequence (amino acid residues 1–32), was PCR-amplified using LVS genomic DNA, AccuPrime DNA Polymerase (ThermoFisher, Chicago, IL, USA), and primers 5′FTL1793_SalI (5′-GCGCGTCGACAGGAGGAAACGGATGAAGTCACTACTACCGAATAGAACAATTG-3′) and 3′FTL1793_BamHI (5′-GCGCGGATCCTTAGTGGTGATGGTGATGATGTTTACTATCTATTTTTGTCCAAGCATCTG-3′). Primer 3′FTL1793_BamHI encoded a 6 × histidine fusion tag at the 3′ end, which was added to the C-terminal end of recombinant FTL1793 to aid in affinity purification. The resulting amplicon was double-digested with SalI and BamHI, ligated into similarly digested pBad18 using T4 DNA ligase, and transformed into NEB 10-β E. coli cells. Following overnight selection on Luria-Bertani (LB) agar plates containing 100 µg/mL ampicillin, individual colonies were selected, plasmids purified using Qiagen (Germantown, MD, USA) QIAprep Spin Miniprep kits, and diagnostic PCR was performed to confirm insert presence and correct size. DNA sequencing was performed for positive clones to confirm the integrity of the insert and verified expression plasmids were transformed into Rosetta DE3 E. coli cells (Millipore, Burlington, MA, USA) for recombinant protein expression.
To express recombinant FTL1793 protein, bacteria were grown to an OD600 of 0.5 in LB medium supplemented with 100 µg/mL ampicillin, and protein expression was induced by the addition of arabinose to a final concentration of 0.4%. After 4 h of protein induction, bacteria were pelleted by centrifugation at 8000× g for 30 min at 4 °C, supernatant was removed, and pellets were stored at −80 °C until processing. Bacterial pellets were thawed, suspended in soluble extraction buffer (10 mM Tris, 500 mM NaCl, 10 mM imidazole, 1 mM PMSF, pH 8.0), sonicated on ice for 5 min with 30 sec intervals at 50% power, insoluble material was removed by centrifugation at 8000× g at 15 °C for 30 min, and supernatants were collected. Supernatants were applied to pre-equilibrated nickel-nitrilotriacetic acid (Ni-NTA) agarose (Qiagen, Germantown, MD, USA) columns, columns were washed with >10-fold excess volume of soluble extraction buffer (4 °C), and purified protein was eluted in 1 mL fractions with elution buffer (10 mM Tris, 500 mM NaCl, 200 mM imidazole, 1 mM PMSF, pH 8.0, 4 °C). Individual elution fractions were assessed by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie staining for recombinant protein concentration and purity, with four elution fractions selected for concentration and buffer exchange (10 mM Tris, 500 mM NaCl, pH 8.0) using Amicon Ultra-4 centrifugal filter units with a 50-kDa cutoff (Millipore, Burlington, MA, USA). The final recombinant protein concentration was determined using the detergent compatible (DC) protein assay (BioRad, Hercules, CA, USA) and purity was subsequently assessed by SDS-PAGE Coomassie staining and immunoblot analysis.
4.7. Immunoblotting
Immunoblot analysis was performed as previously described [45]. Briefly, recombinant FTL1793 was diluted in SDS-PAGE loading buffer, boiled for 10 min, and loaded onto 12.5% SDS-PAGE gels, together with a molecular mass standard (Precision Plus protein all blue prestained protein; BioRad Laboratories, Hercules, CA, USA). Proteins were separated, transferred to nitrocellulose, blots were incubated overnight in blot block (0.1% [vol/vol] Tween 20 and 2% [wt/vol] bovine serum albumin in PBS) at 4 °C, and immunoblotting was performed using a 1:10,000 dilution (in blot block) of Penta-His HRP conjugate antibody (Qiagen, Germantown, MD, USA). Immunoblots were developed with SuperSignal West Pico chemiluminescent detection reagent (Thermo Fisher, Rockford, IL, USA).
4.8. Enzymatic Assays for FTL1793 Activity
Independent chitin azure assays were prepared by adding 10 mg of chitin azure (Sigma, St. Louis, MO, USA) to 750 μL of 200 mM sodium phosphate buffer, pH 7.0, followed by one of the following: 94 μg of recombinant FTL1793; 94 µg of recombinant FopA (negative control Ft outer membrane protein; [45]); 200 U of purified chitinase from Streptomyces griseus (Sigma, St. Louis, MO, USA); or buffer alone (buffer control). Recombinant FopA was prepared as previously described [45]. Enzyme assays were prepared in triplicates and incubated end-over-end at 37 °C. Enzyme activity was assessed every 24 h for 30 d, with samples being centrifuged at 7000× g for 10 min, and the supernatant absorbance at 570 nm being measured. After absorbance measurements were recorded, samples were resuspended and returned to the incubated carousel for further analysis.
4.9. Statistics
GraphPad (San Diego, CA, USA) Prism 8 was used to compile data into graphs and was used for the following statistical analyses: differences in bacterial burdens in ticks and mouse blood (unpaired t-test; one-way ANOVA); differences in tick weight (unpaired t-test; one-way ANOVA); and percent survival of intranasal F. tularensis mouse infection (log-rank Mantel-Cox test).
Acknowledgments
The authors thank Michael L. Levin, Medical Entomology Laboratory Director, Rickettsial Zoonoses Branch, Centers for Disease Control and Prevention (through BEI Resources) for providing Dv and Aa ticks for the majority of these experiments. The authors also thank the Tick Rearing Facility, National Tick Research and Education Resource at Oklahoma State University for providing Aa ticks for a portion of these studies. The authors also wish to thank Dara Frank and Jennifer Coburn at the Medical College of Wisconsin and Joao Pedra at the University of Maryland Baltimore School of Medicine for helpful discussions about tick infection studies.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-0817/9/12/1037/s1. Figure S1: Bacterial numbers (CFU) per mg of tick weight to assess infection, persistence, and replication of Ft in ticks after feeding on mice infected with 105 CFU; Figure S2: Bacterial numbers (CFU) per mg of tick weight to assess infection, persistence, and replication of Ft in ticks after feeding on mice infected with 107 CFU; Figure S3: Adult female ticks harbor higher Ft numbers than adult male ticks; Figure S4: Ft-infected ticks induce skin ulcerations on naive mice; Figure S5: Expression and purification of recombinant FTL1793; Figure S6: ΔFTL1793 is partially attenuated in an intranasal mouse infection model.
Author Contributions
Conceptualization, J.F.H.; methodology, B.G.T. and J.F.H.; validation, B.G.T. and J.F.H.; formal analysis, B.G.T. and J.F.H.; investigation, B.G.T. and J.F.H.; resources, J.F.H.; data curation, B.G.T. and J.F.H.; writing—original draft preparation, B.G.T.; writing—review and editing, B.G.T. and J.F.H.; visualization, J.F.H.; supervision, J.F.H.; project administration, J.F.H.; funding acquisition, J.F.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grant R01 AI093351 from the National Institute of Allergy and Infectious Disease of the National Institutes of Health (NIAID-NIH) to J.F.H. and by bridge funding from the University of Toledo College of Medicine and Life Sciences to J.F.H.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dennis D.T., Inglesby T.V., Henderson D.A., Bartlett J.G., Ascher M.S., Eitzen E., Fine A.D., Friedlander A.M., Hauer J., Layton M., et al. Tularemia as a biological weapon: Medical and public health management. JAMA. 2001;285:2763–2773. doi: 10.1001/jama.285.21.2763. [DOI] [PubMed] [Google Scholar]
- 2.Keim P., Johansson A., Wagner D.M. Molecular epidemiology, evolution, and ecology of Francisella. Ann. N. Y. Acad. Sci. 2007;1105:30–66. doi: 10.1196/annals.1409.011. [DOI] [PubMed] [Google Scholar]
- 3.Kugeler K.J., Mead P.S., Janusz A.M., Staples J.E., Kubota K.A., Chalcraft L.G., Petersen J.M. Molecular Epidemiology of Francisella tularensis in the United States. Clin. Infect. Dis. 2009;48:863–870. doi: 10.1086/597261. [DOI] [PubMed] [Google Scholar]
- 4.Oyston P.C., Quarry J.E. Tularemia vaccine: Past, present and future. Antonie Van Leeuwenhoek. 2005;87:277–281. doi: 10.1007/s10482-004-6251-7. [DOI] [PubMed] [Google Scholar]
- 5.Ellis J., Oyston P.C., Green M., Titball R.W. Tularemia. Clin. Microbiol. Rev. 2002;15:631–646. doi: 10.1128/CMR.15.4.631-646.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zellner B., Huntley J.F. Ticks and Tularemia: Do We Know What We Don’t Know? Front. Cell Infect. Microbiol. 2019;9:146. doi: 10.3389/fcimb.2019.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberg R., Lindsey N.P., Fischer M., Gregory C.J., Hinckley A.F., Mead P.S., Paz-Bailey G., Waterman S.H., Drexler N.A., Kersh G.J., et al. Vital Signs: Trends in Reported Vectorborne Disease Cases—United States and Territories, 2004–2016. MMWR Morb. Mortal. Wkly. Rep. 2018;67:496–501. doi: 10.15585/mmwr.mm6717e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molaei G., Little E.A.H., Williams S.C., Stafford K.C. Bracing for the Worst—Range Expansion of the Lone Star Tick in the Northeastern United States. N. Engl. J. Med. 2019;381:2189–2192. doi: 10.1056/NEJMp1911661. [DOI] [PubMed] [Google Scholar]
- 9.Sagurova I., Ludwig A., Ogden N.H., Pelcat Y., Dueymes G., Gachon P. Predicted Northward Expansion of the Geographic Range of the Tick Vector Amblyomma americanum in North America under Future Climate Conditions. Environ. Health Perspect. 2019;127:107014. doi: 10.1289/EHP5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pedati C., House J., Hancock-Allen J., Colton L., Bryan K., Ortbahn D., Kightlinger L., Kugeler K., Petersen J., Mead P., et al. Notes from the Field: Increase in Human Cases of Tularemia--Colorado, Nebraska, South Dakota, and Wyoming, January-September 2015. MMWR Morb. Mortal. Wkly. Rep. 2015;64:1317–1318. doi: 10.15585/mmwr.mm6447a4. [DOI] [PubMed] [Google Scholar]
- 11.Eisen L. A call for renewed research on tick-borne Francisella tularensis in the Arkansas-Missouri primary national focus of tularemia in humans. J. Med. Entomol. 2007;44:389–397. doi: 10.1093/jmedent/44.3.389. [DOI] [PubMed] [Google Scholar]
- 12.Mani R.J., Metcalf J.A., Clinkenbeard K.D. Amblyomma americanum as a Bridging Vector for Human Infection with Francisella tularensis. PLoS ONE. 2015;10:e0130513. doi: 10.1371/journal.pone.0130513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parker R.R., Spencer R.R., Francis E. Tularæmia: XI. Tularæmia Infection in Ticks of the Species Dermacentor Andersoni Stiles in the Bitterroot Valley, Mont. Public Health Rep. 1924;39 doi: 10.2307/4577151. [DOI] [Google Scholar]
- 14.Parker R.R., Brooks C.S., Marsh H. The Occurrence of Bacterium tularense in the Wood Tick, Dermacentor occidentalis, in California. Public Health Rep. 1929;44 doi: 10.2307/4579265. [DOI] [Google Scholar]
- 15.Goethert H.K., Shani I., Telford S.R., 3rd Genotypic diversity of Francisella tularensis infecting Dermacentor variabilis ticks on Martha’s Vineyard, Massachusetts. J. Clin. Microbiol. 2004;42:4968–4973. doi: 10.1128/JCM.42.11.4968-4973.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goethert H.K., Telford S.R., 3rd Quantum of infection of Francisella tularensis tularensis in host-seeking Dermacentor variabilis. Ticks Tick-Borne Dis. 2010;1:66–68. doi: 10.1016/j.ttbdis.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention Tularemia—United States, 2001–2010. MMWR Morb. Mortal. Wkly. Rep. 2013;62:963–966. [PMC free article] [PubMed] [Google Scholar]
- 18.Reese S.M., Petersen J.M., Sheldon S.W., Dolan M.C., Dietrich G., Piesman J., Eisen R.J. Transmission efficiency of Francisella tularensis by adult american dog ticks (Acari: Ixodidae) J. Med. Entomol. 2011;48:884–890. doi: 10.1603/ME11005. [DOI] [PubMed] [Google Scholar]
- 19.Coburn J., Maier T., Casey M., Padmore L., Sato H., Frank D.W. Reproducible and quantitative model of infection of Dermacentor variabilis with the live vaccine strain of Francisella tularensis. Appl. Environ. Microbiol. 2015;81:386–395. doi: 10.1128/AEM.02917-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mani R.J., Reichard M.V., Morton R.J., Kocan K.M., Clinkenbeard K.D. Biology of Francisella tularensis subspecies holarctica live vaccine strain in the tick vector Dermacentor variabilis. PLoS ONE. 2012;7:e35441. doi: 10.1371/journal.pone.0035441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calhoun E.L. Natural occurrence of tularemia in the lone star tick, Amblyomma americanus (Linn.), and in dogs in Arkansas. Am. J. Trop. Med. Hyg. 1954;3:360–366. doi: 10.4269/ajtmh.1954.3.360. [DOI] [PubMed] [Google Scholar]
- 22.Hopla C.E. Experimental studies on tick transmission of tularemia organisms. Am. J. Hyg. 1953;58:101–118. doi: 10.1093/oxfordjournals.aje.a119585. [DOI] [PubMed] [Google Scholar]
- 23.Beard C.B., Occi J., Bonilla D.L., Egizi A.M., Fonseca D.M., Mertins J.W., Backenson B.P., Bajwa W.I., Barbarin A.M., Bertone M.A., et al. Multistate Infestation with the Exotic Disease-Vector Tick Haemaphysalis longicornis—United States, August 2017–September 2018. MMWR Morb. Mortal. Wkly. Rep. 2018;67:1310–1313. doi: 10.15585/mmwr.mm6747a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang J.G., Ko S., Smith W.B., Kim H.C., Lee I.Y., Chae J.S. Prevalence of Anaplasma, Bartonella and Borrelia Species in Haemaphysalis longicornis collected from goats in North Korea. J. Vet. Sci. 2016;17:207–216. doi: 10.4142/jvs.2016.17.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chandler J.C., Molins C.R., Petersen J.M., Belisle J.T. Differential chitinase activity and production within Francisella species, subspecies, and subpopulations. J. Bacteriol. 2011;193:3265–3275. doi: 10.1128/JB.00093-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forestal C.A., Malik M., Catlett S.V., Savitt A.G., Benach J.L., Sellati T.J., Furie M.B. Francisella tularensis has a significant extracellular phase in infected mice. J. Infect. Dis. 2007;196:134–137. doi: 10.1086/518611. [DOI] [PubMed] [Google Scholar]
- 27.Molins C.R., Delorey M.J., Yockey B.M., Young J.W., Sheldon S.W., Reese S.M., Schriefer M.E., Petersen J.M. Virulence differences among Francisella tularensis subsp. tularensis clades in mice. PLoS ONE. 2010;5:e10205. doi: 10.1371/journal.pone.0010205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reese S.M., Dietrich G., Dolan M.C., Sheldon S.W., Piesman J., Petersen J.M., Eisen R.J. Transmission dynamics of Francisella tularensis subspecies and clades by nymphal Dermacentor variabilis (Acari: Ixodidae) Am. J. Trop. Med. Hyg. 2010;83:645–652. doi: 10.4269/ajtmh.2010.10-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sonenshine D.E., Roe R.M., editors. Biology of Ticks. 2nd ed. Volume 1. Oxford University Press; Oxford, UK: New York, NY, USA: 1991. p. 3. [Google Scholar]
- 30.Tahir D., Meyer L., Fourie J., Jongejan F., Mather T., Choumet V., Blagburn B., Straubinger R.K., Varloud M. Interrupted Blood Feeding in Ticks: Causes and Consequences. Microorganisms. 2020;8:910. doi: 10.3390/microorganisms8060910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oyston P.C., Sjostedt A., Titball R.W. Tularaemia: Bioterrorism defence renews interest in Francisella tularensis. Nat. Rev. Microbiol. 2004;2:967–978. doi: 10.1038/nrmicro1045. [DOI] [PubMed] [Google Scholar]
- 32.Yang X., Koci J., Smith A.A., Zhuang X., Sharma K., Dutta S., Rana V.S., Kitsou C., Yas O.B., Mongodin E.F., et al. A novel tick protein supports integrity of gut peritrophic matrix impacting existence of gut microbiome and Lyme disease pathogens. Cell Microbiol. 2020:e13275. doi: 10.1111/cmi.13275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tilly K., Elias A.F., Errett J., Fischer E., Iyer R., Schwartz I., Bono J.L., Rosa P. Genetics and regulation of chitobiose utilization in Borrelia burgdorferi. J. Bacteriol. 2001;183:5544–5553. doi: 10.1128/JB.183.19.5544-5553.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tully B.G., Huntley J.F. Mechanisms Affecting the Acquisition, Persistence and Transmission of Francisella tularensis in Ticks. Microorganisms. 2020;8:1639. doi: 10.3390/microorganisms8111639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Markov E.Y., Kulikalova E.S., Urbanovich L.Y., Vishnyakov V.S., Balakhonov S.V. Chitin and Products of Its Hydrolysis in Vibrio cholerae Ecology. Biochemistry. 2015;80:1109–1116. doi: 10.1134/S0006297915090023. [DOI] [PubMed] [Google Scholar]
- 36.Wucher B.R., Bartlett T.M., Hoyos M., Papenfort K., Persat A., Nadell C.D. Vibrio cholerae filamentation promotes chitin surface attachment at the expense of competition in biofilms. Proc. Natl. Acad. Sci. USA. 2019;116:14216–14221. doi: 10.1073/pnas.1819016116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DebRoy S., Dao J., Soderberg M., Rossier O., Cianciotto N.P. Legionella pneumophila type II secretome reveals unique exoproteins and a chitinase that promotes bacterial persistence in the lung. Proc. Natl. Acad. Sci. USA. 2006;103:19146–19151. doi: 10.1073/pnas.0608279103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rehman S., Grigoryeva L.S., Richardson K.H., Corsini P., White R.C., Shaw R., Portlock T.J., Dorgan B., Zanjani Z.S., Fornili A., et al. Structure and functional analysis of the Legionella pneumophila chitinase ChiA reveals a novel mechanism of metal-dependent mucin degradation. PLoS Pathog. 2020;16:e1008342. doi: 10.1371/journal.ppat.1008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tuveng T.R., Hagen L.H., Mekasha S., Frank J., Arntzen M.O., Vaaje-Kolstad G., Eijsink V.G.H. Genomic, proteomic and biochemical analysis of the chitinolytic machinery of Serratia marcescens BJL200. Biochim. Biophys. Acta Proteins Proteom. 2017;1865:414–421. doi: 10.1016/j.bbapap.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 40.Madhuprakash J., Singh A., Kumar S., Sinha M., Kaur P., Sharma S., Podile A.R., Singh T.P. Structure of chitinase D from Serratia proteamaculans reveals the structural basis of its dual action of hydrolysis and transglycosylation. Int. J. Biochem. Mol. Biol. 2013;4:166–178. [PMC free article] [PubMed] [Google Scholar]
- 41.Kezuka Y., Bando K., Kobayashi H., Yonou Y., Sato T., Watanabe T., Nonaka T. Crystallization and preliminary X-ray analysis of the catalytic domain of chitinase D from Bacillus circulans WL-12. Protein Pept. Lett. 2006;13:951–954. doi: 10.2174/092986606778256108. [DOI] [PubMed] [Google Scholar]
- 42.Wang S., Moyne A., Thottappilly G., Wu S., Locy R.D., Singh N.K. Purification and characterization of a Bacillus cereus exochitinase. Enzym. Microb. Technol. 2001;28:492–498. doi: 10.1016/S0141-0229(00)00362-8. [DOI] [PubMed] [Google Scholar]
- 43.Manjeet K., Purushotham P., Neeraja C., Podile A.R. Bacterial chitin binding proteins show differential substrate binding and synergy with chitinases. Microbiol. Res. 2013;168:461–468. doi: 10.1016/j.micres.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 44.Thompson S.E., Smith M., Wilkinson M.C., Peek K. Identification and characterization of a chitinase antigen from Pseudomonas aeruginosa strain 385. Appl. Environ. Microbiol. 2001;67:4001–4008. doi: 10.1128/AEM.67.9.4001-4008.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huntley J.F., Conley P.G., Hagman K.E., Norgard M.V. Characterization of Francisella tularensis outer membrane proteins. J. Bacteriol. 2007;189:561–574. doi: 10.1128/JB.01505-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chong A., Child R., Wehrly T.D., Rockx-Brouwer D., Qin A., Mann B.J., Celli J. Structure-Function Analysis of DipA, a Francisella tularensis Virulence Factor Required for Intracellular Replication. PLoS ONE. 2013;8:e67965. doi: 10.1371/journal.pone.0067965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Margolis J.J., El-Etr S., Joubert L.M., Moore E., Robison R., Rasley A., Spormann A.M., Monack D.M. Contributions of Francisella tularensis subsp. novicida chitinases and Sec secretion system to biofilm formation on chitin. Appl. Environ. Microbiol. 2010;76:596–608. doi: 10.1128/AEM.02037-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reif K.E., Palmer G.H., Ueti M.W., Scoles G.A., Margolis J.J., Monack D.M., Noh S.M. Dermacentor andersoni transmission of Francisella tularensis subsp. novicida reflects bacterial colonization, dissemination, and replication coordinated with tick feeding. Infect. Immun. 2011;79:4941–4946. doi: 10.1128/IAI.05676-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu X., Ren G., Huntley J.F. Generating Isogenic Deletions (Knockouts) in Francisella tularensis, a Highly-infectious and Fastidious Gram-negative Bacterium. Bio. Protoc. 2015;5:e1500. doi: 10.21769/BioProtoc.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu X., Ren G., Gunning W.T., 3rd, Weaver D.A., Kalinoski A.L., Khuder S.A., Huntley J.F. FmvB: A Francisella tularensis Magnesium-Responsive Outer Membrane Protein that Plays a Role in Virulence. PLoS ONE. 2016;11:e0160977. doi: 10.1371/journal.pone.0160977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ren G., Champion M.M., Huntley J.F. Identification of disulfide bond isomerase substrates reveals bacterial virulence factors. Mol. Microbiol. 2014;94:926–944. doi: 10.1111/mmi.12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kurtz S.L., Bosio C.M., De Pascalis R., Elkins K.L. GM-CSF has disparate roles during intranasal and intradermal Francisella tularensis infection. Microbes Infect. 2016;18:758–767. doi: 10.1016/j.micinf.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 53.Sonenshine D.E. Range Expansion of Tick Disease Vectors in North America: Implications for Spread of Tick-Borne Disease. Int. J. Environ. Res. Public Health. 2018;15:478. doi: 10.3390/ijerph15030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma Y., Bring A., Kalantari Z., Destouni G. Potential for Hydroclimatically Driven Shifts in Infectious Disease Outbreaks: The Case of Tularemia in High-Latitude Regions. Int. J. Environ. Res. Public Health. 2019;16:3717. doi: 10.3390/ijerph16193717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wormser G.P., McKenna D., Piedmonte N., Vinci V., Egizi A.M., Backenson B., Falco R.C. First Recognized Human Bite in the United States by the Asian Longhorned Tick, Haemaphysalis longicornis. Clin. Infect. Dis. 2020;70:314–316. doi: 10.1093/cid/ciz449. [DOI] [PubMed] [Google Scholar]
- 56.Breuner N.E., Ford S.L., Hojgaard A., Osikowicz L.M., Parise C.M., Rosales Rizzo M.F., Bai Y., Levin M.L., Eisen R.J., Eisen L. Failure of the Asian longhorned tick, Haemaphysalis longicornis, to serve as an experimental vector of the Lyme disease spirochete, Borrelia burgdorferi sensu stricto. Ticks Tick-Borne Dis. 2020;11:101311. doi: 10.1016/j.ttbdis.2019.101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stanley H.M., Ford S.L., Snellgrove A.N., Hartzer K., Smith E.B., Krapiunaya I., Levin M.L. The Ability of the Invasive Asian Longhorned Tick Haemaphysalis longicornis (Acari: Ixodidae) to Acquire and Transmit Rickettsia rickettsii (Rickettsiales: Rickettsiaceae), the Agent of Rocky Mountain Spotted Fever, Under Laboratory Conditions. J. Med. Entomol. 2020;57:1635–1639. doi: 10.1093/jme/tjaa076. [DOI] [PubMed] [Google Scholar]
- 58.Johns R., Sonenshine D.E., Hynes W.L. Identification of a defensin from the hemolymph of the American dog tick, Dermacentor variabilis. Insect. Biochem. Mol. Biol. 2001;31:857–865. doi: 10.1016/S0965-1748(01)00031-5. [DOI] [PubMed] [Google Scholar]
- 59.Goethert H.K., Telford S.R., 3rd A new Francisella (Beggiatiales: Francisellaceae) inquiline within Dermacentor variabilis say (Acari: Ixodidae) J. Med. Entomol. 2005;42:502–505. doi: 10.1093/jmedent/42.3.502. [DOI] [PubMed] [Google Scholar]
- 60.Goethert H.K., Telford S.R., 3rd Nonrandom distribution of vector ticks (Dermacentor variabilis) infected by Francisella tularensis. PLoS Pathog. 2009;5:e1000319. doi: 10.1371/journal.ppat.1000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sauer J.R., Hair J.A. The Quantity of Blood Ingested by the Lone Star Tick (Acarina: Ixodidae) Ann. Entomol. Soc. Am. 1972;65:15. [Google Scholar]
- 62.Rechav Y., Strydom W.J., Clarke F.C., Burger L.B., Mackie A.J., Fielden L.J. Isotopes as Host Blood Markers To Measure Blood Intake by Feeding Ticks (Acari: Ixodidae) J. Med Entomol. 1994;31:5. doi: 10.1093/jmedent/31.4.511. [DOI] [PubMed] [Google Scholar]
- 63.Schmitt D.M., Barnes R., Rogerson T., Haught A., Mazzella L.K., Ford M., Gilson T., Birch J.W., Sjostedt A., Reed D.S., et al. The Role and Mechanism of Erythrocyte Invasion by Francisella tularensis. Front. Cell Infect. Microbiol. 2017;7:173. doi: 10.3389/fcimb.2017.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gall C.A., Reif K.E., Scoles G.A., Mason K.L., Mousel M., Noh S.M., Brayton K.A. The bacterial microbiome of Dermacentor andersoni ticks influences pathogen susceptibility. ISME J. 2016;10:1846–1855. doi: 10.1038/ismej.2015.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Duron O., Binetruy F., Noel V., Cremaschi J., McCoy K.D., Arnathau C., Plantard O., Goolsby J., Perez de Leon A.A., Heylen D.J.A., et al. Evolutionary changes in symbiont community structure in ticks. Mol. Ecol. 2017;26:2905–2921. doi: 10.1111/mec.14094. [DOI] [PubMed] [Google Scholar]
- 66.Rynkiewicz E.C., Hemmerich C., Rusch D.B., Fuqua C., Clay K. Concordance of bacterial communities of two tick species and blood of their shared rodent host. Mol. Ecol. 2015;24:2566–2579. doi: 10.1111/mec.13187. [DOI] [PubMed] [Google Scholar]
- 67.Clay K., Klyachko O., Grindle N., Civitello D., Oleske D., Fuqua C. Microbial communities and interactions in the lone star tick, Amblyomma americanum. Mol. Ecol. 2008;17:4371–4381. doi: 10.1111/j.1365-294x.2008.03914.x. [DOI] [PubMed] [Google Scholar]
- 68.Gerhart J.G., Moses A.S., Raghavan R. A Francisella-like endosymbiont in the Gulf Coast tick evolved from a mammalian pathogen. Sci. Rep. 2016;6:33670. doi: 10.1038/srep33670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sojka D., Franta Z., Horn M., Caffrey C.R., Mares M., Kopacek P. New insights into the machinery of blood digestion by ticks. Trends Parasitol. 2013;29:276–285. doi: 10.1016/j.pt.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 70.Meibom K.L., Charbit A. Francisella tularensis metabolism and its relation to virulence. Front. Microbiol. 2010;1:140. doi: 10.3389/fmicb.2010.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schorderet S., Pearson R.D., Vuocolo T., Eisemann C., Riding G.A., Tellam R.L. cDNA and deduced amino acid sequences of a peritrophic membrane glycoprotein, ‘peritrophin-48′, from the larvae of Lucilia cuprina. Insect. Biochem. Mol. Biol. 1998;28:99–111. doi: 10.1016/s0965-1748(97)00103-3. [DOI] [PubMed] [Google Scholar]
- 72.Hayes C.A., Dalia T.N., Dalia A.B. Systematic genetic dissection of chitin degradation and uptake in Vibrio cholerae. Environ. Microbiol. 2017;19:4154–4163. doi: 10.1111/1462-2920.13866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mueller R.S., McDougald D., Cusumano D., Sodhi N., Kjelleberg S., Azam F., Bartlett D.H. Vibrio cholerae strains possess multiple strategies for abiotic and biotic surface colonization. J. Bacteriol. 2007;189:5348–5360. doi: 10.1128/JB.01867-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sarva S.T., Waldo R.H., Belland R.J., Klose K.E. Comparative Transcriptional Analyses of Francisella tularensis and Francisella novicida. PLoS ONE. 2016;11:e0158631. doi: 10.1371/journal.pone.0158631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kingry L.C., Petersen J.M. Comparative review of Francisella tularensis and Francisella novicida. Front. Cell Infect. Microbiol. 2014;4:35. doi: 10.3389/fcimb.2014.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Folders J., Algra J., Roelofs M.S., van Loon L.C., Tommassen J., Bitter W. Characterization of Pseudomonas aeruginosa chitinase, a gradually secreted protein. J. Bacteriol. 2001;183:7044–7052. doi: 10.1128/JB.183.24.7044-7052.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Frederiksen R.F., Paspaliari D.K., Larsen T., Storgaard B.G., Larsen M.H., Ingmer H., Palcic M.M., Leisner J.J. Bacterial chitinases and chitin-binding proteins as virulence factors. Microbiology. 2013;159:833–847. doi: 10.1099/mic.0.051839-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.