Abstract
Porcine somatic cell nuclear transfer (SCNT) is currently inefficient, as 1–3.95% of reconstructed embryos survive to term; inadequate or erroneous epigenetic reprogramming of the specialized donor somatic nucleus could be a primary reason. Therefore, a locus-specific analysis of DNA methylation dynamics in embryogenesis and the DNA methylation status of gametes and donor cells used for SCNT were conducted in the following developmentally important gene loci: POU5F1, NANOG, SOX2, H19, IGF2, IGF2R, XIST; and the retrotransposon LINE-1. There were significant epigenetic differences between the gametes and the somatic donor cells. Three gamete-specific differentially methylated regions (DMRs) in POU5F1, XIST, and LINE-1 were identified. A delayed demethylation process at POU5F1 and LINE-1 loci occurred after three successive cleavages, compared to the in vitro fertilized (IVF) embryos. Although cloned embryos could undergo de-methylation and re-methylation dynamics at the DMRs of imprinted genes (H19,IGF2R, and XIST), the re-methylation process was compromised, unlike in fertilized embryos. LINE-1 loci are widely dispersed across the whole genome, and LINE-1 DMR might be a potential porcine nuclear reprogramming epi-marker. Data from observations in our present and previous studies, and two published articles were pooled to produce a schematic diagram of locus-specific, DNA methylation dynamics of cloned and IVF embryos during porcine early embryogenesis. This also indicated aberrant DNA methylation reprogramming events, including inadequate DNA demethylation and insufficient re-methylation in cloned embryos. Further research should focus on mechanisms underlying demethylation during the early cleavage of embryos and de novo DNA methylation at the blastocyst stage.
Keywords: Cloned embryos, DNA methylation, Donor cells, In vitro fertilized embryos, Porcine
Thanks to groundbreaking studies, including the report of germline-competent induced pluripotent stem cells (iPSCs) in pigs [1], the completion of the Swine Genome Sequencing Project [2], and the large-scale utilization of somatic cell nuclear transfer (SCNT) in the pig industry [3, 4], pigs have become increasingly popular as biomedical models. They are strikingly similar to humans regarding their anatomy, physiology, genetics, and genome size (~2.7 Gb) [5]. SCNT technology is important for biomedical research and for the production of genetically modified pigs, due to the lack of germline-competent pig embryonic stem (ES) cells [6, 7]. Currently, the SCNT method is inefficient in pigs, with only 1–3.95% of transferred embryos surviving to term [3, 4, 8, 9]. The exact mechanisms underlying the extremely low efficiency of SCNT are still unknown. Inadequate or erroneous epigenetic reprogramming of specialized donor somatic nuclei, and consequent aberrant gene expression during development could be the main reasons [10,11,12].
Early studies using DNA 5-methylcytosine (5mC) immunological staining found that cloned embryos suffered from an incomplete demethylation process after activation of the embryos and had different methylation patterns in the inner cell mass (ICM) and the trophectoderm (TE), compared with their fertilized counterparts [10, 13]. Aberrant, locus-specific methylation patterns in cloned mammalian individuals (or during early embryogenesis) have been frequently reported, mainly concerning the imprinted loci (IGF2/H19, IGFR, and INS) [14, 15] and the XIST locus [16,17,18]. Genome-wide studies in mice and two recent studies in bovine animals found that epigenetic reprogramming in the germline had already started prior to fertilization [19,20,21,22], and that many gamete-specific, DNA differentially methylated regions (DMRs) presented DNA methylation reprogramming during early embryogenesis in control fertilized embryos [20,21,22,23]. It is therefore plausible that different epigenetic potentials exist between porcine somatic donor cells used for SCNT and mature gametes [24].
A comprehensive DNA methylation analysis, based on DNA 5-methylcytosine immunologic staining [13] and locus-specific DNA methylation reprogramming [25] has been performed in in vitro fertilized (IVF) porcine embryos. However, comprehensive comparisons of DNA methylation reprogramming dynamics throughout early embryogenesis between cloned and IVF embryos are limited.
We conducted a comprehensive, locus-specific, DNA methylation analysis of donor cells for SCNT, cloned embryos, gametes, and in vitro fertilized embryos at the following gene loci: POU5F1, NANOG, SOX2, H19, IGF2, IGF2R, XIST; and the retrotransposon LINE-1. Moreover, we present three newly identified, gamete-specific DMRs associated with POU5F1, XIST, and LINE-1. The results of a pooled analysis of data from the present study, from our previous studies, and from two other published articles were used to construct a schematic diagram of locus-specific, DNA methylation dynamics of cloned and IVF embryos during early porcine embryogenesis.
Materials and Methods
Unless otherwise stated, all chemicals were purchased from Sigma Chemical (St. Louis, MO, USA).
Ethics statement
This study was conducted in strict accordance with “The Instructive Notions with Respect to Caring for Laboratory Animals” issued by the Ministry of Science and Technology of China. The animal experimental protocol was approved by the Institutional Animal Care and Use Committee of Longyan University. All efforts were made to minimize animal suffering.
Porcine adult fibroblast isolation and culture
Adult ear fibroblasts were isolated from a 2-year-old, genetically proven superior Duroc boar (red coat), as previously described by Deng et al. [26]. Briefly, the ear tissue was sterilized with 75% ethanol for 5 min and washed thrice with phosphate-buffered saline (PBS) containing 1.5% penicillin-streptomycin. The ear tissue was then minced in PBS, and digested in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 15% fetal bovine serum (FBS), 1% penicillin-streptomycin, 0.32 mg/ml Collagenase IV, and 2,500 IU/ml DNase I for 6 h at 39°C. The cells were then centrifuged at 1,000 rpm for 5 min, and then re-suspended in DMEM medium supplemented with 15% FBS. The cells were cultured in 10-cm petri dishes for 12 h, and then frozen in liquid nitrogen until required.
Somatic cell nuclear transfer
Porcine SCNT embryos were produced using the method described by Shi et al. [27]. Briefly, ovaries of slaughtered prepubertal hybrid gilts (Duroc × Yorkshire × Landrace) were collected from the Guangzhou Tianhe abattoir located in the Tianhe district, Guangzhou City, China. Cumulus-oocyte complexes (COCs) were aspirated from the ovaries and were allowed to mature in vitro for 42–44 h, following the protocol described by Deng et al. [26].
For SCNT, matured COCs were freed from cumulus cells by repeatedly pipetted in 0.1% hyaluronidase, and oocytes with the first polar body were selected for enucleation. Fibroblasts were thawed and cultured up to passage three until confluence in DMEM (Gibco, Grand Island, NY, USA) supplemented with 10% (v/v) FBS at 39°C in a humidified atmosphere of 5% CO2 and 95% air. The matured oocyte was firmly sucked onto the holding pipette, and the first polar body and adjacent cytoplasm were aspirated into the enucleation pipette. A single fibroblast cell was microinjected into the perivitelline space of the oocyte. The oocyte–donor cell complexes were cultured in porcine zygote medium 3 (PZM3) at 39°C, 5% CO2, 5% O2, 90% N2, and 100% humidity for 1.5 h [28], and then activated to fuse in a medium described by Shi et al. [27]. After the post-activation treatment in PZM3 medium containing cytochalasin B (5 μg/ml) for 4 h, the reconstructed embryos were cultured in vitro in PZM3 medium at 39°C, 5% CO2, 7% O2, 88% N2, and 100% humidity.
IVF
IVF and subsequent in vitro cultivation of pig embryos was performed as previously described [29]. Briefly, freshly ejaculated semen was obtained from the aforementioned Duroc boar and, after a short incubation at 39°C in a humidified atmosphere of 5% CO2 and 95% air, the semen was purified using the two-step Percoll gradient method. Semen was washed thrice with PBS supplemented with 0.1% (w/v) bovine serum albumin (BSA) using centrifugation at 250 × g for 5 min. The pellet was re-suspended and layered on top of a 45:90 discontinuous Percoll gradient and then centrifuged at 300 × g for 30 min. The pellet was then re-suspended in porcine gamete medium (PGM) [28] and washed twice at 150 × g for 5 min. The spermatozoa were diluted in PGM medium at a sperm concentration of 2 × 106 cells/ml and stored in a 5% CO2 incubator at 39°C, 5% CO2, 7% O2, 88% N2, and 100% humidity for 20 min. Matured oocytes were transferred into PGM medium and then incubated with capacitated spermatozoa at a final sperm concentration of 1 × 106 cells/ml for 6 h. Fertilized oocytes were washed three times with PZM3 medium and cultured in PZM3 medium at 39°C, 5% CO2, 7% O2, 88% N2, and 100% humidity.
Embryo transfer
Hybrid sows (Yorkshire × Landrace), in parity 2–5, showing natural standing estrus within the previous 30 h were used as embryo recipients. The recipient’s ovulation status was examined according to the criteria established by Koo et al. [30]. The anesthesia protocol for the recipient sow, and subsequent embryo transfer was performed following the method described by Shi et al. [27]. Briefly, after exposing the ovary and oviduct by making a 7-cm incision along the midline of the sow’s abdomen between the last two pairs of teats, cloned embryos and presumptive IVF zygotes cultured in PZM3 medium at 39°C for 20 h were loaded into a transparent transfer tube attached to a 1-ml syringe, and delivered in the site that was two-thirds of the distance down the oviduct towards the uterine horn. Subsequently, the transfer tube was examined under a microscope to ensure that all the embryos had been transferred.
Preparation of oocytes and embryos
Four replicates of experiments were conducted to prepare the matured oocytes and embryos at four developmental stages (two-cell, eight-cell, morula, and blastocyst stage). For each replicate, 50 in vitro, mature metaphase II (MII) oocytes (removed from the zona pellucida using an acidic Tyrode’s solution at 37°C) were collected for gamete DNA methylation analysis. Forty-five embryos (or presumptive zygotes) were added in each droplet to produce SCNT or IVF embryos to be collected at the four developmental stages. Embryos at the four developmental stages (two-cell, eight-cell, morula, and blastocyst stage) were collected at 24, 72, 120, and 168 h post-activation, respectively. Embryos obtained at each developmental stage from the four replicates were pooled, so that, at least 250 diploid cells were guaranteed in each pooled sample.
Oocytes and embryo DNA extraction
MII oocytes and embryos were removed from the zona pellucida using an acidic Tyrode’s solution at 37°C, and rapidly transferred into 100 ml of Buffer RLT (a lysis buffer solution), provided with the DNA/RNA Micro Kit (Qiagen, Hilden, Germany), supplemented with 10% 14.3 M β-mercaptoethanol. Embryos were briefly vortexed and stored at –80°C for up to one month before processing. It was guaranteed that the embryo transfer manipulations were carried out using minimal solution. DNA extractions were performed according to the appropriate protocols of the AllPrep DNA/RNA Micro Kit (Qiagen).
Sperm and donor cell genomic DNA extraction
Pretreatment of sperm, elimination of somatic cell contamination, and sperm genomic DNA extraction were performed as described by Zhao et al. [25]. Adult ear fibroblasts at passage three were cultured to full confluence and were then synchronized using contact inhibition. Donor cells were digested with 0.25% trypsin at 37°C and then they were washed with PBS three times. Approximately 1 × 106 cells were collected, and their genomic DNA was extracted according to the protocol of the E.Z.N.A.® Tissue DNA Kit (Omega Bio-Tek, Norcross, GA, USA).
Bisulfite-specific polymerase chain reaction (BS-PCR) and sequencing
Purified genomic DNA was pooled and treated with sodium bisulfite to convert all unmethylated cytosine to uracil by using the EZ DNA Methylation-Gold™ Kit (Zymo Research, Orange, CA, USA), according to the manufacturer’s recommendations. Primer information for BS-PCR products is listed in Supplementary Table 1 (online only). Nested PCRs were run using HotStarTaq plus DNA polymerase (Qiagen) using 30–35 cycles for the first amplification reaction and 40–45 cycles for the second amplification reaction. The amplified products were subjected to gel purification using the E.Z.N.A.® Gel Extraction Kit (Omega Bio-Tek), and then cloned into the TA cloning vector (pTZ57R/T) that was included in the InsTAclone™ PCR Cloning Kit (Fermentas, Plainville, MA, USA). The cloning reaction was subsequently transformed into Trans5α Chemically Competent cells (Beijing TransGen Biotech, Beijing, China), and then grown on Luria-Bertani-kanamycin (50 mg/ml) agar plates supplemented with ampicillin, X-Gal (5-Bromo-4-Chloro-3-Indolyl-beta-D-Galactoside; Fermentas), and IPTG (isopropyl-beta-D-thiogalactopyranoside, Fermentas) overnight. For each transformation, 10–20 white clones were randomly selected, and positive colonies were further confirmed using colony PCR. Positive clones were sent for plasmid DNA isolation and sequencing at Invitrogen Life Science Technologies (Guangzhou, China), until at least 14 qualified sequences were obtained. PCR amplifications and subsequent transformation were performed, at least, three times for each sample. To evaluate the bisulfite sequencing assay, the returned sequenced clones were filtered by setting 95% lower threshold conversion rates, according to the Bisulfite sequencing Data Presentation and Compilation (BDPC) DNA methylation analysis tool [31]. Combined bisulfite restriction analysis was also conducted to test the bisulfite conversion efficiency and the bisulfite-PCR bias during each round of BS-PCR. Adult porcine genomic DNA was used as a PCR template, which is known to be hemi-methylated at the H19-CTCF3 locus, and the qualified PCR products were meant to display equal amounts of image pixels in the cut bands (methylated bands) and in the uncut bands (unmethylated bands) when processed using Image J software. Finally, the qualified sequences were analyzed using BiQ Analyzer software, and the bead-diagram was plotted using the BIQ Analyzer platform. For LINE-1, sequencing saturation analysis was performed, and 20 sequenced clones tended to reach saturation (Supplementary Fig. 1: online only).
Calculation of DNA methylation levels of LINE-1 retrotransposon
Due to the high mutagenic rate in repetitive DNA elements, the mean methylation levels were calculated as suggested by Yang et al. [32], using the following equation:
Methylation Percentage (%) =
[Number of CpG/(Number of CpG + (Number of TpG – Number of TpA))] × 100 (Eq. 1)
Statistical analysis
Chi-square tests (χ2 test) were performed to analyze the ratio of developmental capacity and efficiency of the SCNT and IVF embryos data between the two groups. Differences were considered significant at a P < 0.05.
Results
Developmental capacities of SCNT and IVF embryos
Thirteen healthy cloned piglets were derived from the donor cells in this study, and six healthy IVF-derived piglets were obtained using the sperm from the same donor boar. The IVF method seemed to be more successful than the SCNT regarding the ratio of clones that were born healthy (P = 0.072), and the efficiency of transferred embryos per piglet that was born healthy (P < 0.05) (Table 1).
Table 1. Developmental capacity of embryos derived from somatic cell nuclear transfer (SCNT) and in vitro fertilization (IVF).
| Sources of embryos | % Blast (the ratio of blastocysts to constructed embryos) | Total number of transferred constructed- embryos | Total number of recipients | Total number of farrowed recipients | Farrowing rate a) | Total number of piglets born | Total number of clones born healthy b) | Ratio of clones born healthy c) | Transferred embryos per piglets born healthy d) |
|---|---|---|---|---|---|---|---|---|---|
| SCNT | 21.41% (76/355) | 3,896 | 17 | 6 | 35.29% | 21 | 13 | 61.90% | 299.69 e) |
| IVF | 20.66% (56/271) | 362 | 3 | 1 | 33.33% | 6 | 6 | 100.00% | 60.33 f) |
a) Farrowing rate refers to the ratio of the farrowed recipients to the total number of recipients. b) Clones born healthy refers to piglets, excluding weak piglets, mummies, and stillborn piglets. c) Ratio of clones born healthy refers to the ratio of the number of clones born healthy to the total number of piglets born. d) Transferred embryos per piglet born healthy refers to the ratio of the number of transferred embryos to the number of clones born healthy. e) f) These values differ significantly from each other (P < 0.05).
Identification of three sex-specific DMRs of POU5F1, XIST, and LINE-1
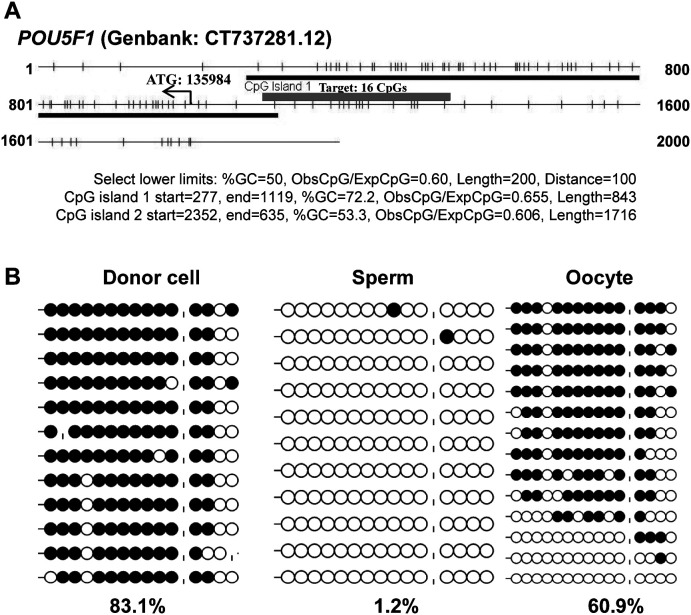
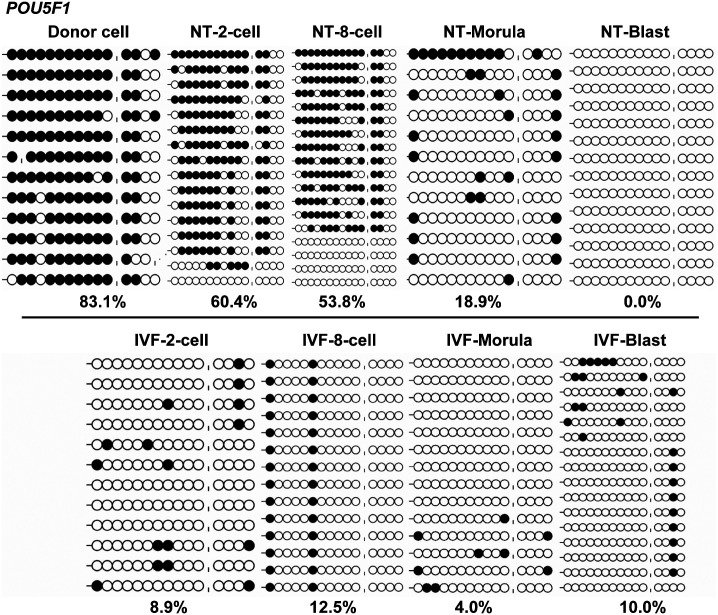
CpG island prediction results indicated that the 5' upstream region and the exons of POU5F1 (GenBank: CT737281.12) were in line with the typical characteristics of a CpG island (CGI) (Fig. 1A), and a certain region (–88 bp to –367 bp) was established as a gamete-specific DMR, with 1.2%, 60.9%, and 83.1% DNA methylation levels in sperm, oocytes, and donor cells, respectively (Fig. 1B). Considering that POU5F1 was previously reported as having a differentially methylated region (–1199 bp to –1379 bp) [33], the newly identified DMR was named as POU5F1-DMR2.
Fig. 1.
Gamete-specific DNA methylation patterns of the POU5F1 gene. The sequence of the POU5F1 gene (Genbank: CT737281.12) was analyzed for CpG islands (CGIs), using CpG Island Searcher (http://cpgislands.usc.edu/), and a typical island across the 5' upstream region, and the first exon was identified (blue bar in Fig. 1A). A CGI shore region (–88 bp to –367 bp, 280 bp, red bar in Fig. 1A) was subsequently identified as a gamete-specific differentially methylated region (DMR) (Fig. 1B). The use of “|” in Fig. 1B represents an absent CpG.
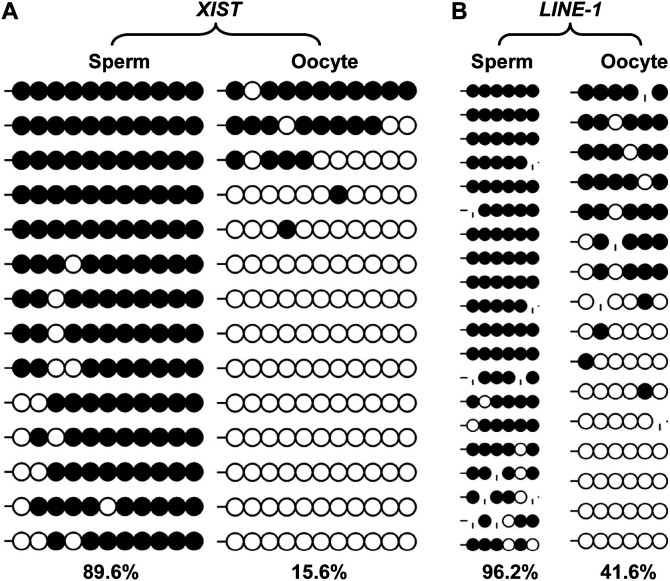
A CGI of the XIST gene (X chromosome in Sscrofa9.2: 58, 398, 546-58, 398, 756) at the 5' end upstream and the first exon were found to be differentially methylated in male and female somatic cells in our previous study [29], and herein were further identified as a gamete-specific DMR with 89.6% and 15.6% methylation levels in sperm and matured oocytes, respectively (Fig. 2A).
Fig. 2.
Gamete-specific DNA methylation patterns of the XIST gene, and LINE-1 retrotransposon. The use of “|” represents an absent CpG due to mutation.
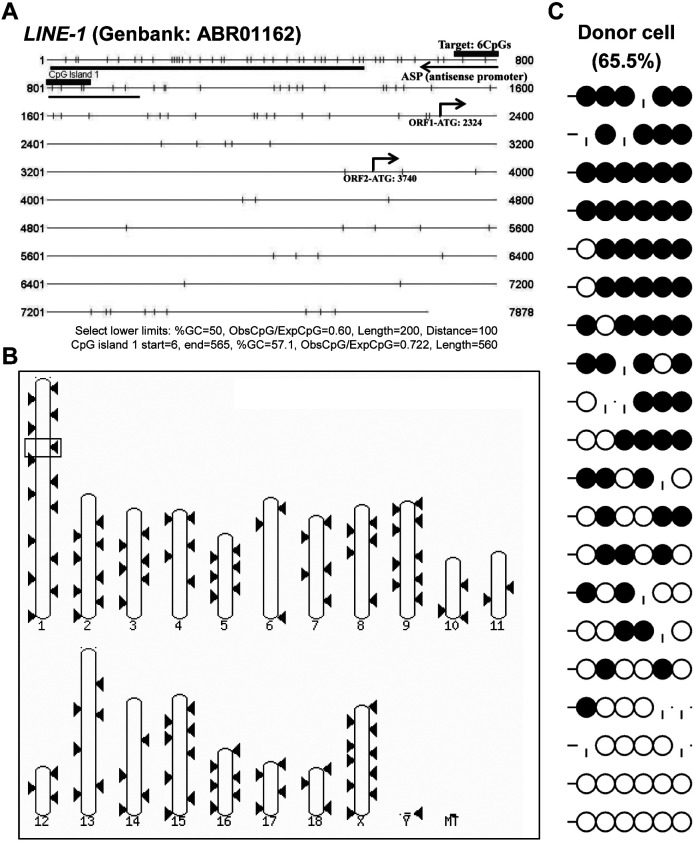
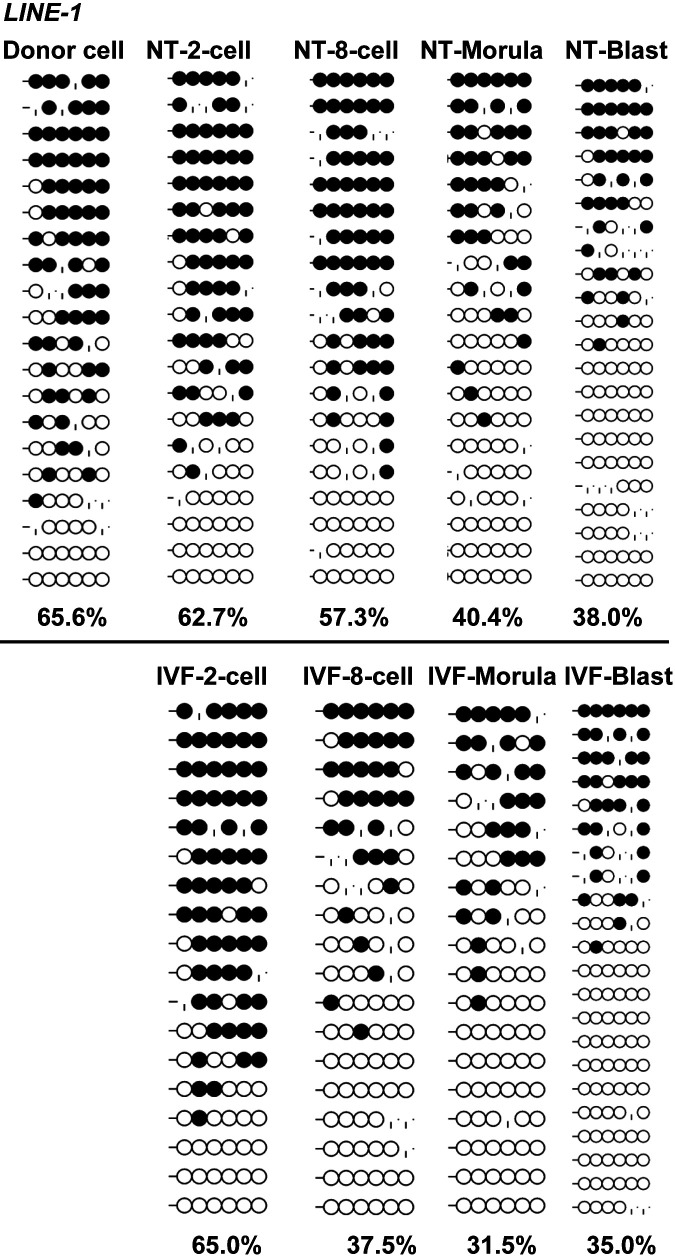
The antisense promoter (ASP) of the retrotransposon LINE-1 (GenBank: ABR01162) presented a gamete-specific, DNA methylation manner with 96.2%, 41.6%, and 65.6% DNA methylation levels in sperm, oocytes, and donor cells, respectively (Figs. 2B and 3C). The ASP of LINE-1 was BLASTed against the pig genome (Sscrofa10.2) on Ensemble, and 202 matched sequences with, at least, a 98.41% similarity and an E-value < 1e-1 were found, indicating that the region is widely distributed in the genome (Figs. 3A and B), and that it can represent the overall genomic methylation level, to some extent.
Fig. 3.
Sequence analysis for DNA methylation and genome-wide distribution of porcine LINE-1 retrotransposon. The CpG island prediction tool (http://cpgislands.usc.edu/) indicates a CpG island (blue bar) in the 5'-untranslated region (Fig. 3A). A CpG shore containing six CpGs (red bar) within the antisense promoter (ASP; based on GenBank: ABR01162 annotation) of the LINE-1 retrotransposon was defined as a gamete-specific DMR and was hypermethylated in donor somatic cells with 65.5% methylation levels (Fig. 3A). The LINE-1 DMR sequence was BLASTed against Pig Sscrofa11.1 (genomic sequence) and 202 matched sequences (red box, red triangle) with, at least, 98.41% of sequence similarity (E-value < 1e-1) were obtained (Fig. 3B).
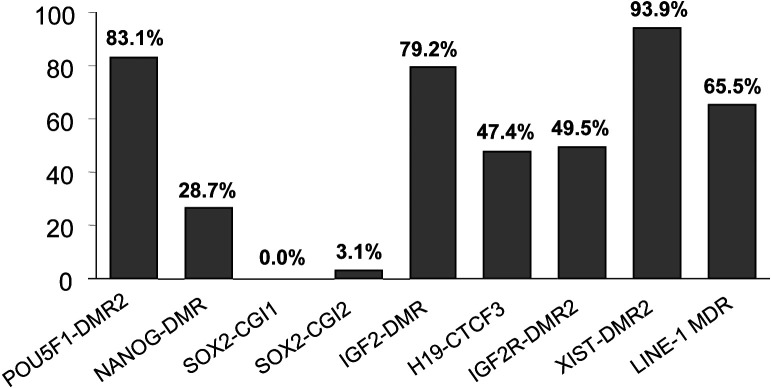
Examination of DNA methylation levels of donor cells for SCNT at nine DMRs
To screen the appropriate gene loci for the comparison of DNA dynamics during embryogenesis between SCNT and IVF embryos, nine loci (including four newly examined loci in the present study: POU5F1, SOX2-CGI1, SOX2-CGI2, and LINE-1), and five published DMRs (NANOG, IGF2, H19, IGF2R, and XIST [29, 34,35,36]), were analyzed and summarized for DNA methylation levels in male somatic donor cells (hereinafter referred to as the “donor cells”) (Fig. 4).
Fig. 4.
DNA methylation levels at nine loci of porcine donor cells. DNA methylation patterns of male somatic donor cells at nine loci in eight genes, POU5F1, NANOG, SOX2, IGF2, H19, IGF2R, XIST, and LINE-1.
For the three pluripotency genes, NANOG was hypomethylated in donor cells (26.7%), and could be completely demethylated after the first cleavage of the cloned embryos (Fig. 4) (Supplementary Fig. 2: online only). Similarly, the SOX2 gene (GenBank: CU914271.8) was resistant to DNA methylation modification in donor cells, although the entire gene region met the typical CpG island definition (Fig. 4) (Supplementary Fig. 3: online only). POU5F1 was hypermethylated at the CGI shore region in donor cells (Fig. 1).
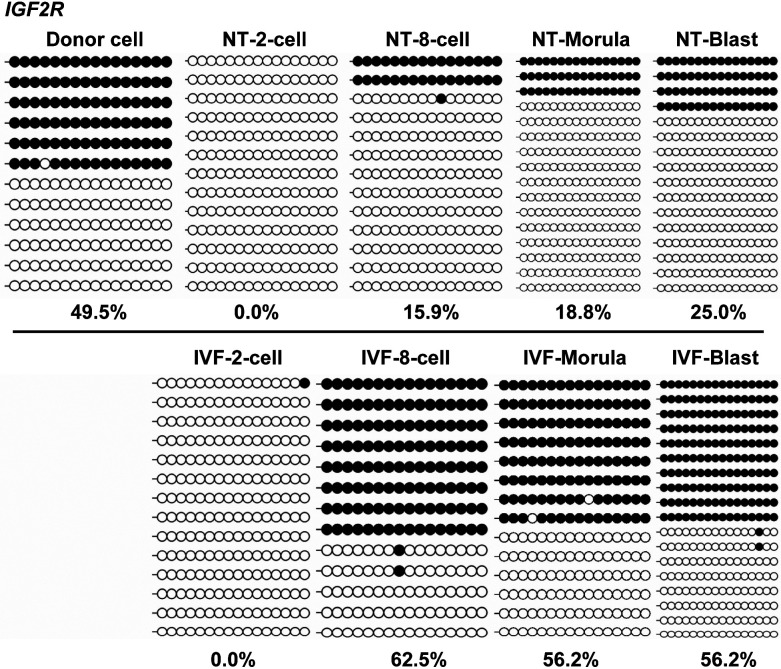
For three imprinted genes in mice, the H19-CTCF3 locus (Fig. 4) (Supplementary Fig. 3), and IGF2R-DMR2 (Fig. 4) (Supplementary Fig. 5: online only) were confirmed to have imprinted DNA methylation patterns in porcine donor cells, whereas this was not the case for IGF2-DMR2 (Fig. 4) (Supplementary Fig. 4: online only).
The ASP of LINE-1 was hypermethylated in donor cells (Fig. 3C). XIST-DMR2 was found to be hypermethylated in male somatic donor cells, and hemi-methylated in female donor cells in our previous study [29].
DNA methylation reprogramming dynamics of SCNT and IVF embryos before implantation
Because NANOG and SOX2 were hypomethylated, and IGF2-DMR2 presented a non-typical, imprinted DNA methylation pattern in donor cells, the DNA methylation reprogramming dynamics of three loci (POU5F1-DMR2, IGF2R-DMR2, and LINE-1-DMR) were selectively compared in cloned and IVF embryos.
The results showed that POU5F1 underwent a delayed demethylation process from two-cell to the blastocyst-stage in cloned embryos, compared to the IVF counterparts, which rapidly demethylated after the first cleavage (60.4% in cloned embryos vs. 8.9% in IVF embryos; Fig. 5). Similarly, LINE-1 also underwent a delayed demethylation process after three cleavages (8-cell embryos) in cloned embryos (Fig. 6A), compared to the IVF counterparts, which had lower methylation levels (57.3% in cloned embryos vs. 37.5% in IVF embryos; Fig. 6B). For IGF2R, cloned and IVF embryos were completely demethylated after the first cleavage (Fig. 7), but differed in the re-methylation process when the cloned embryos could not establish the imprinted hemi-methylation patterns, compared with the IVF counterparts which reached hemi-methylation levels at the blastocyst stage (25.0% in cloned embryos vs. 56.2% in IVF embryos; Fig. 7).
Fig. 5.
DNA Methylation reprogramming dynamics of POU5F1 during early embryogenesis. Cloned embryos undergo a delayed demethylation process from two-cell to the blastocyst-stage, compared with their in vitro fertilization (IVF) counterparts, which rapidly demethylate after the first cleavage.
Fig. 6.
DNA Methylation reprogramming dynamics of LINE-1 during early embryogenesis. Cloned embryos undergo a delayed demethylation process after three cleavages, compared with their in vitro fertilization (IVF) counterparts, which reach a lower methylation level (57.5% of cloned embryos vs. 37.5% of IVF embryos).
Fig. 7.
DNA Methylation reprogramming dynamics of IGF2R during early embryogenesis. Cloned and in vitro fertilization (IVF) embryos completely demethylate after the first cleavage but differ in the re-methylation process when the cloned embryos cannot establish imprinted hemi-methylation patterns, compared to the IVF embryos, that reach semi-methylation at the blastocyst stage.
To observe the effects of sex on DNA methylation patterns in cloned embryos, DNA methylation levels of embryos at the blastocyst stage at four typical loci were compared between the male embryos and the mixed-sex embryos. Results indicated no evident differences between the two types of embryos, except for the XIST gene (Supplementary Fig. 6: online only), which might imply that the sex has little influence on DNA methylation at the autosomal gene loci.
Schematic diagram of locus-specific DNA methylation dynamics of cloned and IVF embryos during early embryogenesis
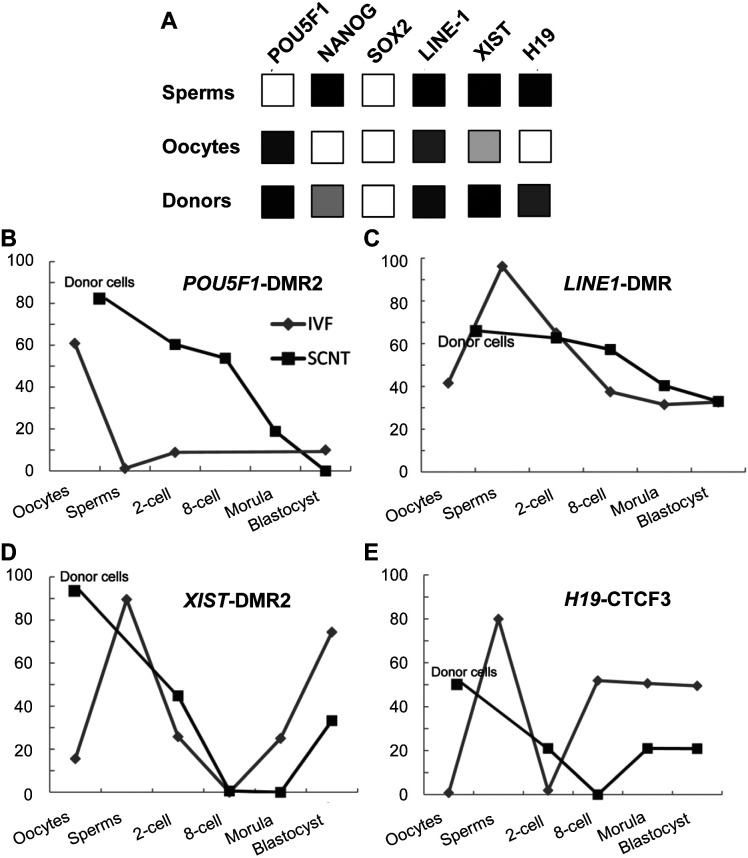
Genome-wide DNA methylation sequencing data is limited, and to this end, data were pooled from related articles [25, 35], previous studies [29], and the current study, to construct a schematic diagram of locus-specific DNA methylation dynamics of cloned and IVF embryos during porcine early embryogenesis (Fig. 8). From the diagram, significant epigenetic differences between porcine gametes and somatic donor cells can be inferred. Aberrant DNA methylation reprogramming, including an inadequate DNA demethylation (POU5F1 from 2-cell to 8-cell stage, and LINE-1 at 8-cell stage), and insufficient re-methylation (XIST from morula stage to blastocyst stage, and H19 from the 8-cell stage to the blastocyst stage) occurred in cloned embryos, compared with in vitro fertilized embryos.
Fig. 8.
Summary of locus-specific DNA methylation dynamics of cloned and in vitro fertilization (IVF) embryos during early porcine embryogenesis. (A) Locus-specific DNA methylation patterns of six gene loci at the level of sperms, oocytes, and somatic donor cells (B–E). DNA methylation reprogramming dynamics of four gene loci during early embryogenesis of cloned embryos (dark red lines) and IVF embryos (blue lines).
Discussion
In this study, the IVF method was more successful than SCNT regarding the number of healthy offspring generated and the number of transferred embryos per healthy piglets (Table 1). Similarly, a high developmental efficiency of IVF embryos was found by another research group that obtained 21 live piglets from 5-day IVF-derived blastocysts; of these, four recipient sows became pregnant and farrowed healthy piglets [28]. In a previous RNA-Seq transcriptome analysis study, cloned and in vitro fertilized porcine blastocysts yielded 628 differentially expressed transcripts [37]. These findings imply that there is a profound distinction at the level of gene expression and/or epigenetic regulation during embryogenesis and development between SCNT- and IVF-derived embryos.
DNA demethylation of pluripotency genes is essential for the epigenetic reprogramming of somatic cell nuclei [38] and sperm [19]. In the current study, the POU5F1 5'-UTR region (–88 bp to –367 bp) was found to be hypermethylated in porcine somatic donor cell nuclei, and to undergo a delayed DNA demethylation process during early embryogenesis of cloned embryos, compared with the IVF embryos. This phenomenon could be explained either by the different reproductive patterns (1.2% and 60.9% DNA methylation levels in sperm and oocytes, respectively), or because cloned embryos were not able to rapidly demethylate. The NANOG promoter was found to be hypomethylated in porcine somatic donor cell nuclei and seemed to be easily demethylated during the early embryogenesis of cloned embryos, whereas NANOG was not found to be expressed in somatic donor cells and two-cell embryos, which implies that demethylation is not crucial for activating NANOG [34]. Similar to the previous observation that SOX2 was demethylated in sperm, oocytes, and early embryos [25], SOX2 was practically demethylated in donor cells, although the entire gene region met the typical CpG island criteria.
In the current study, there was a dynamic process of de-methylation and re-methylation before implantation at DMR2 of IGF2R, and in the CTCF3 region of H19 in cloned porcine embryos and IVF embryos (in our previous work) [29], which contradicts the knowledge that germ-line imprinted methylation could escape DNA methylation reprogramming in early embryonic development [39, 40]. However, our results are in agreement with a previous observation in porcine embryos, in which H19-CTCF3 was hemi-methylated in in vitro fertilized zygotes, fully methylated in parthenogenetic zygotes, and demethylated in androgenetic zygotes. The hemi-methylated pattern (as seen in IVF zygotes) was observed up to the four-cell embryo stage; embryos were exclusively demethylated at the eight-cell stage, and then restored at the morula stage [41]. This dynamic adjustment of DNA methylation during early embryogenesis might be a part of imprinting mechanisms [42]. The results of the current study also showed that cloned embryos displayed insufficient ability to re-establish a hemi-methylated status, whereas their IVF counterparts succeeded in acquiring the normal hemi-methylated status at the eight-cell stage. This phenomenon also occurred in the XIST gene, which might indicate that cloned embryos lack the capacity to restore DNA imprints before implantation.
Retrotransposable elements (TEs) comprise approximately 50% of the mammalian genome, and they might represent a threat to the integrity of the genome when they escape from the restraint of epigenetic control because of their de novo insertions nature. They can also be convenient, since they can be used by mammalian genomes for a repertoire of regulatory sequences, especially during early embryogenesis and germline development [43]. Of the TEs, the expression of LINE-1 was reported to be essential for murine preimplantation development [44]. Recent genome-wide DNA methylation studies in mice reported that the LINE-1 (unlike the short interspersed elements – SINEs) was refractory to SCNT reprogramming, whereas its repressive methylation coating could be largely removed in natural reprogramming [12, 20, 23]. In the porcine, LINE-1 and glutamic acid transfer RNA (tRNAGlu)-derived SINEs constitute the two main repetitive element groups [2]. Similar to observations in mice, SINEs and micro-satellites could be effectively demethylated in cloned porcine embryos, and in in vitro and in vivo fertilized embryos [45]. In our previous analysis of the RNA-Seq transcriptome of cloned and in vitro fertilized porcine blastocysts, eight LINE-1 (GenBank: ABR01162) BLAST-matched differentially expressed transcripts were screened [37]. The above-mentioned studies suggested that LINE-1 might present a large obstacle hindering porcine SCNT reprogramming. The ASP of LINE-1 was found to be more refractory to SCNT reprogramming than IVF-derived reprogramming (57.3% of DNA methylation in 8-cell embryos in SCNT, and 37.5% in IVF, respectively [37]), which was similar to observations in mice [12, 20, 23].
Different methylation patterns arise in gametes. This study compared several locus-specific DNA methylation reprogramming dynamics during embryogenesis in cloned and in vitro fertilized embryos. Global DNA methylomes based on genome-scale sequencing of porcine gametes and donor cells for cloning and embryos will be imperative, going forward. Additionally, further research efforts should focus on the underlying mechanisms of demethylation during the early cleavage of embryos and the establishment of de novo DNA methylation at the blastocyst stage.
Supplementary
Acknowledgments
This work was supported by the Natural Science Foundation of Fujian Province, China (Grant No. 2015J01620 and 2018J01457), and the Fund for Local Science and Technology Development Guided by the Chinese Government (Grant No. 2018L3011). We would like to thank Professor Zhenfang Wu’s group for their selfless help during the investigation of this project.
References
- 1.West FD, Uhl EW, Liu Y, Stowe H, Lu Y, Yu P, Gallegos-Cardenas A, Pratt SL, Stice SL. Brief report: chimeric pigs produced from induced pluripotent stem cells demonstrate germline transmission and no evidence of tumor formation in young pigs. Stem Cells 2011; 29: 1640–1643. [DOI] [PubMed] [Google Scholar]
- 2.Groenen MA, Archibald AL, Uenishi H, Tuggle CK, Takeuchi Y, Rothschild MF, Rogel-Gaillard C, Park C, Milan D, Megens HJ, Li S, Larkin DM, Kim H, Frantz LA, Caccamo M, Ahn H, Aken BL, Anselmo A, Anthon C, Auvil L, Badaoui B, Beattie CW, Bendixen C, Berman D, Blecha F, Blomberg J, Bolund L, Bosse M, Botti S, Bujie Z, Bystrom M, Capitanu B, Carvalho-Silva D, Chardon P, Chen C, Cheng R, Choi SH, Chow W, Clark RC, Clee C, Crooijmans RP, Dawson HD, Dehais P, De Sapio F, Dibbits B, Drou N, Du ZQ, Eversole K, Fadista J, Fairley S, Faraut T, Faulkner GJ, Fowler KE, Fredholm M, Fritz E, Gilbert JG, Giuffra E, Gorodkin J, Griffin DK, Harrow JL, Hayward A, Howe K, Hu ZL, Humphray SJ, Hunt T, Hornshøj H, Jeon JT, Jern P, Jones M, Jurka J, Kanamori H, Kapetanovic R, Kim J, Kim JH, Kim KW, Kim TH, Larson G, Lee K, Lee KT, Leggett R, Lewin HA, Li Y, Liu W, Loveland JE, Lu Y, Lunney JK, Ma J, Madsen O, Mann K, Matthews L, McLaren S, Morozumi T, Murtaugh MP, Narayan J, Nguyen DT, Ni P, Oh SJ, Onteru S, Panitz F, Park EW, Park HS, Pascal G, Paudel Y, Perez-Enciso M, Ramirez-Gonzalez R, Reecy JM, Rodriguez-Zas S, Rohrer GA, Rund L, Sang Y, Schachtschneider K, Schraiber JG, Schwartz J, Scobie L, Scott C, Searle S, Servin B, Southey BR, Sperber G, Stadler P, Sweedler JV, Tafer H, Thomsen B, Wali R, Wang J, Wang J, White S, Xu X, Yerle M, Zhang G, Zhang J, Zhang J, Zhao S, Rogers J, Churcher C, Schook LB. Analyses of pig genomes provide insight into porcine demography and evolution. Nature 2012; 491: 393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurome M, Geistlinger L, Kessler B, Zakhartchenko V, Klymiuk N, Wuensch A, Richter A, Baehr A, Kraehe K, Burkhardt K, Flisikowski K, Flisikowska T, Merkl C, Landmann M, Durkovic M, Tschukes A, Kraner S, Schindelhauer D, Petri T, Kind A, Nagashima H, Schnieke A, Zimmer R, Wolf E. Factors influencing the efficiency of generating genetically engineered pigs by nuclear transfer: multi-factorial analysis of a large data set. BMC Biotechnol 2013; 13: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Z, Shi J, Liu D, Zhou R, Zeng H, Zhou X, Mai R, Zeng S, Luo L, Yu W, Zhang S, Wu Z. Effects of donor fibroblast cell type and transferred cloned embryo number on the efficiency of pig cloning. Cell Reprogram 2013; 15: 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bendixen E, Danielsen M, Larsen K, Bendixen C. Advances in porcine genomics and proteomics—a toolbox for developing the pig as a model organism for molecular biomedical research. Brief Funct Genomics 2010; 9: 208–219. [DOI] [PubMed] [Google Scholar]
- 6.Koh S, Piedrahita JA. From “ES-like” cells to induced pluripotent stem cells: a historical perspective in domestic animals. Theriogenology 2014; 81: 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu K, Mao J, Song L, Fan A, Zhang S, Wang J, Fan N, Liu N, Ye X, Fu H, Zhou Z, Wang Y, Wei H, Liu Z, Li Z, Lai L, Wang X, Liu L. DNA repair and replication links to pluripotency and differentiation capacity of pig iPS cells. PLoS One 2017; 12: e0173047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao J, Ross JW, Hao Y, Spate LD, Walters EM, Samuel MS, Rieke A, Murphy CN, Prather RS. Significant improvement in cloning efficiency of an inbred miniature pig by histone deacetylase inhibitor treatment after somatic cell nuclear transfer. Biol Reprod 2009; 81: 525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitworth KM, Prather RS. Somatic cell nuclear transfer efficiency: how can it be improved through nuclear remodeling and reprogramming? Mol Reprod Dev 2010; 77: 1001–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang X, Smith SL, Tian XC, Lewin HA, Renard JP, Wakayama T. Nuclear reprogramming of cloned embryos and its implications for therapeutic cloning. Nat Genet 2007; 39: 295–302. [DOI] [PubMed] [Google Scholar]
- 11.Zhao J, Whyte J, Prather RS. Effect of epigenetic regulation during swine embryogenesis and on cloning by nuclear transfer. Cell Tissue Res 2010; 341: 13–21. [DOI] [PubMed] [Google Scholar]
- 12.Peat JR, Reik W. Incomplete methylation reprogramming in SCNT embryos. Nat Genet 2012; 44: 965–966. [DOI] [PubMed] [Google Scholar]
- 13.Deshmukh RS, Østrup O, Østrup E, Vejlsted M, Niemann H, Lucas-Hahn A, Petersen B, Li J, Callesen H, Hyttel P. DNA methylation in porcine preimplantation embryos developed in vivo and produced by in vitro fertilization, parthenogenetic activation and somatic cell nuclear transfer. Epigenetics 2011; 6: 177–187. [DOI] [PubMed] [Google Scholar]
- 14.Wei Y, Zhu J, Huan Y, Liu Z, Yang C, Zhang X, Mu Y, Xia P, Liu Z. Aberrant expression and methylation status of putatively imprinted genes in placenta of cloned piglets. Cell Reprogram 2010; 12: 213–222. [DOI] [PubMed] [Google Scholar]
- 15.Shen CJ, Cheng WT, Wu SC, Chen HL, Tsai TC, Yang SH, Chen CM. Differential differences in methylation status of putative imprinted genes among cloned swine genomes. PLoS One 2012; 7: e32812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu JH, Yin S, Xiong B, Hou Y, Chen DY, Sun QY. Aberrant DNA methylation imprints in aborted bovine clones. Mol Reprod Dev 2008; 75: 598–607. [DOI] [PubMed] [Google Scholar]
- 17.Jiang L, Lai L, Samuel M, Prather RS, Yang X, Tian XC. Expression of X-linked genes in deceased neonates and surviving cloned female piglets. Mol Reprod Dev 2008; 75: 265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matoba S, Inoue K, Kohda T, Sugimoto M, Mizutani E, Ogonuki N, Nakamura T, Abe K, Nakano T, Ishino F, Ogura A. RNAi-mediated knockdown of Xist can rescue the impaired postimplantation development of cloned mouse embryos. Proc Natl Acad Sci USA 2011; 108: 20621–20626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farthing CR, Ficz G, Ng RK, Chan CF, Andrews S, Dean W, Hemberger M, Reik W. Global mapping of DNA methylation in mouse promoters reveals epigenetic reprogramming of pluripotency genes. PLoS Genet 2008; 4: e1000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith ZD, Chan MM, Mikkelsen TS, Gu H, Gnirke A, Regev A, Meissner A. A unique regulatory phase of DNA methylation in the early mammalian embryo. Nature 2012; 484: 339–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang Z, Lin J, Dong H, Zheng X, Marjani SL, Duan J, Ouyang Z, Chen J, Tian XC. DNA methylomes of bovine gametes and in vivo produced preimplantation embryos. Biol Reprod 2018; 99: 949–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duan JE, Jiang ZC, Alqahtani F, Mandoiu I, Dong H, Zheng X, Marjani SL, Chen J, Tian XC. Methylome dynamics of bovine gametes and in vivo early embryos. Front Genet 2019; 10: 512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan MM, Smith ZD, Egli D, Regev A, Meissner A. Mouse ooplasm confers context-specific reprogramming capacity. Nat Genet 2012; 44: 978–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castillo J, Amaral A, Oliva R. Sperm nuclear proteome and its epigenetic potential. Andrology 2014; 2: 326–338. [DOI] [PubMed] [Google Scholar]
- 25.Zhao MT, Rivera RM, Prather RS. Locus-specific DNA methylation reprogramming during early porcine embryogenesis. Biol Reprod 2013; 88: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deng W, Yang D, Zhao B, Ouyang Z, Song J, Fan N, Liu Z, Zhao Y, Wu Q, Nashun B, Tang J, Wu Z, Gu W, Lai L. Use of the 2A peptide for generation of multi-transgenic pigs through a single round of nuclear transfer. PLoS One 2011; 6: e19986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi J, Zhou R, Luo L, Mai R, Zeng H, He X, Liu D, Zeng F, Cai G, Ji H, Tang F, Wang Q, Wu Z, Li Z. Influence of embryo handling and transfer method on pig cloning efficiency. Anim Reprod Sci 2015; 154: 121–127. [DOI] [PubMed] [Google Scholar]
- 28.Yoshioka K, Suzuki C, Itoh S, Kikuchi K, Iwamura S, Rodriguez-Martinez H. Production of piglets derived from in vitro-produced blastocysts fertilized and cultured in chemically defined media: effects of theophylline, adenosine, and cysteine during in vitro fertilization. Biol Reprod 2003; 69: 2092–2099. [DOI] [PubMed] [Google Scholar]
- 29.Xu W, Li Z, Yu B, He X, Shi J, Zhou R, Liu D, Wu Z. Effects of DNMT1 and HDAC inhibitors on gene-specific methylation reprogramming during porcine somatic cell nuclear transfer. PLoS One 2013; 8: e64705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koo OJ, Kang JT, Kwon DK, Park HJ, Lee BC. Influence of ovulation status, seasonality and embryo transfer method on development of cloned porcine embryos. Reprod Domest Anim 2010; 45: 773–778. [DOI] [PubMed] [Google Scholar]
- 31.Rohde C, Zhang Y, Jurkowski TP, Stamerjohanns H, Reinhardt R, Jeltsch A. Bisulfite sequencing Data Presentation and Compilation (BDPC) web server—a useful tool for DNA methylation analysis. Nucleic Acids Res 2008; 36: e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang AS, Estécio MR, Doshi K, Kondo Y, Tajara EH, Issa JP. A simple method for estimating global DNA methylation using bisulfite PCR of repetitive DNA elements. Nucleic Acids Res 2004; 32: e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Z, Chen J, Ren J, Bao L, Liao J, Cui C, Rao L, Li H, Gu Y, Dai H, Zhu H, Teng X, Cheng L, Xiao L. Generation of pig induced pluripotent stem cells with a drug-inducible system. J Mol Cell Biol 2009; 1: 46–54. [DOI] [PubMed] [Google Scholar]
- 34.Miyamoto K, Tsukiyama T, Yang Y, Li N, Minami N, Yamada M, Imai H. Cell-free extracts from mammalian oocytes partially induce nuclear reprogramming in somatic cells. Biol Reprod 2009; 80: 935–943. [DOI] [PubMed] [Google Scholar]
- 35.Han DW, Im YB, Do JT, Gupta MK, Uhm SJ, Kim JH, Schöler HR, Lee HT. Methylation status of putative differentially methylated regions of porcine IGF2 and H19. Mol Reprod Dev 2008; 75: 777–784. [DOI] [PubMed] [Google Scholar]
- 36.Hyldig SM, Croxall N, Contreras DA, Thomsen PD, Alberio R. Epigenetic reprogramming in the porcine germ line. BMC Dev Biol 2011; 11: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu WH, Li ZC, Ouyang ZP, Yu B, Shi JS, Liu DW, Wu ZF. RNA-Seq transcriptome analysis of porcine cloned and in vitro fertilized blastocysts. J Integr Agric 2015; 14: 926–938. [Google Scholar]
- 38.Simonsson S, Gurdon J. DNA demethylation is necessary for the epigenetic reprogramming of somatic cell nuclei. Nat Cell Biol 2004; 6: 984–990. [DOI] [PubMed] [Google Scholar]
- 39.Stöger R, Kubicka P, Liu CG, Kafri T, Razin A, Cedar H, Barlow DP. Maternal-specific methylation of the imprinted mouse Igf2r locus identifies the expressed locus as carrying the imprinting signal. Cell 1993; 73: 61–71. [DOI] [PubMed] [Google Scholar]
- 40.Tremblay KD, Saam JR, Ingram RS, Tilghman SM, Bartolomei MS. A paternal-specific methylation imprint marks the alleles of the mouse H19 gene. Nat Genet 1995; 9: 407–413. [DOI] [PubMed] [Google Scholar]
- 41.Park CH, Kim HS, Lee SG, Lee CK. Methylation status of differentially methylated regions at Igf2/H19 locus in porcine gametes and preimplantation embryos. Genomics 2009; 93: 179–186. [DOI] [PubMed] [Google Scholar]
- 42.Shemer R, Birger Y, Dean WL, Reik W, Riggs AD, Razin A. Dynamic methylation adjustment and counting as part of imprinting mechanisms. Proc Natl Acad Sci USA 1996; 93: 6371–6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zamudio N, Bourc’his D. Transposable elements in the mammalian germline: a comfortable niche or a deadly trap? Heredity (Edinb) 2010; 105: 92–104. [DOI] [PubMed] [Google Scholar]
- 44.Beraldi R, Pittoggi C, Sciamanna I, Mattei E, Spadafora C. Expression of LINE-1 retroposons is essential for murine preimplantation development. Mol Reprod Dev 2006; 73: 279–287. [DOI] [PubMed] [Google Scholar]
- 45.Kang YK, Koo DB, Park JS, Choi YH, Kim HN, Chang WK, Lee KK, Han YM. Typical demethylation events in cloned pig embryos. Clues on species-specific differences in epigenetic reprogramming of a cloned donor genome. J Biol Chem 2001; 276: 39980–39984. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.