Abstract
Environmental toxicants are chemicals that negatively affect human health. Although there are numerous ways to limit exposure, the ubiquitous nature of certain environmental toxicants makes it impossible to avoid them entirely. Consequently, scientists are continuously working toward developing strategies for combating their harmful effects. Using the nematode Caenorhabditis elegans, a model with many genetic and physiological similarities to humans, researchers in the Colaiácovo laboratory have identified several molecular mechanisms by which the toxic agent bisphenol A (BPA) interferes with reproduction. Here, we address their recent discovery that a widely available compound, Coenzyme Q10 (CoQ10), can rescue BPA-induced damage. This work is significant in that it poses a low-cost method for improving reproductive success in humans. The goal of this primer is to assist educators and students with navigating the paper entitled “Antioxidant CoQ10 Restores Fertility by Rescuing Bisphenol A-Induced Oxidative DNA Damage in the Caenorhabditis elegans Germline.” It is ideally suited for integration into an upper-level undergraduate course such as Genetics, Cell and Molecular Biology, Developmental Biology, or Toxicology. The primer provides background information on the history of BPA, the utility of the C. elegans germ line as a model for studying reproductive toxicity, and research methods including assessment of programmed cell death, fluorescent microscopy applications, and assays to quantify gene expression. Questions for deeper exploration in-class or online are provided.
Related article in GENETICS: Hornos Carneiro MF, Shin N, Karthikraj R, Barbosa F Jr, Kannan K, Colaiácovo MP. Antioxidant CoQ10 restores fertility by rescuing bisphenol A-induced oxidative DNA damage in the Caenorhabditis elegans Germline. Genetics 214:381–395.
Keywords: Bisphenol A, Coenzyme Q10, Caenorhabditis elegans germ line, oxidative stress
Summary
Certain chemicals in the environment are capable of altering hormone function, which can, in turn, compromise human health .One of the most widely studied examples is the plasticizer bisphenol A (BPA), which interferes with several aspects of reproduction (Siracusa et al. 2018). Although BPA can affect either sex, recent findings suggest that it disproportionately affects females, possibly by damaging the finite number of egg cells available in the ovary (Pivonello et al. 2020). While decreased use of BPA in commercial goods has reduced ingestion of BPA-tainted food and drink, BPA and compounds with a similar chemical structure are still widely used in many common consumer products. Thus, exposure has been reduced, but not eliminated, as evidenced by a >2.5-fold increase in blood serum levels of BPA detected in infertile women vs. fertile women of similar age living in the same metropolitan area (Pivonello et al. 2020). Since exposure to these ubiquitous compounds is impossible to avoid entirely, it is important to determine ways to circumvent damage at the cellular level. To do so, nonhuman models for studying how BPA causes toxicity are essential.
Background
Bisphenol A and toxicology: a short history
Toxicology is a multidisciplinary science in which the adverse effects of toxicants on living organisms are determined both qualitatively and quantitatively. It involves identifying, classifying, and characterizing physical, biological, and chemical substances and their effects on organisms. Toxicology includes determining how an organism could be exposed to an agent, and how the agent will enter the organism, (e.g., through dermal absorption, inhalation, or ingestion), as well as cataloging the adverse effects of exposure to various concentrations of the agent. Toxicologists determine whether effects are at the genetic, cellular, tissue, or organ level, and whether effects are seen in only specific subpopulations of organisms.
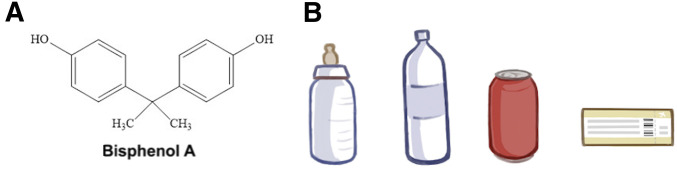
Bisphenol A (BPA) is a synthetically produced industrial chemical (formula C15H16O2; Figure 1A) that has been widely used commercially in epoxy resins and polycarbonate plastics since the 1950s. BPA has been used as a component of plastic storage containers of all types, plastic bottles, and food and beverage packaging including the lining of aluminum cans. In addition, BPA is found in thermal paper such as receipts and tickets (Geens et al. 2012 and Figure 1B). Consequently, there are multiple routes for BPA exposure in humans. The majority of exposures are via ingestion, while the next most likely exposure is by dermal absorption (Ma et al. 2019). The highest risk of exposure is through consumption of canned food, where BPA leaches from the can lining, thereby contaminating the interior product (Jalal et al. 2018).
Figure 1.
Bisphenol A (BPA) and items that contain it. (A) The chemical structure of BPA. (B) A selection of consumer products that contain BPA and/or its structural analogs. From left to right: a baby bottle, a plastic bottle, an aluminum can, and a thermal paper-based boarding pass.
BPA toxicology has been investigated due to its classification as an endocrine-disrupting chemical, as it interferes with normal hormone function. BPA is composed of two connected phenols (Figure 1A) and is structurally similar to diethylstilbestrol, a synthetic form of the steroid hormone estrogen that is known to cause birth defects and cancer (Reed and Fenton 2013). When naturally produced, estrogen promotes development of secondary sex characteristics in biological females, ensuring proper ovulation, and establishing and maintaining successful pregnancies. The mechanism by which BPA binds to human estrogen receptors has been identified, providing evidence for how BPA interferes with normal sexual function (Li et al. 2015).
Although BPA has been detected in ∼90% of individuals, in most cohort studies, BPA levels have been at or below the limits of 50 µg/kg body weight/day set by the US Environmental Protection Agency and the European Food Safety Authority (Jalal et al. 2018). Several sources of evidence, however, indicate that BPA can contribute to human disease, including cancers, neurobehavioral disorders, and infertility, even at very low doses (Vogel 2009). It is especially concerning that the cohort found to have the highest exposure to BPA is young children (Ma et al. 2019). For children, their increased BPA exposure is likely due to their higher food intake per pound of body mass and potential metabolic differences compared to adults (Braun and Hauser 2011). Additionally, high levels of BPA exposure have also been reported in pregnant women (Ma et al. 2019). Although this is presumed to result from increased dietary exposure to contaminated food (Gorecki et al. 2017; Pacyga et al. 2019), the link between diet and higher urinary BPA concentrations during pregnancy is still being investigated .
BPA’s ability to leach from polycarbonate sources and its effects were initially suggested in the early 1990s. The estrogenic effect of leached BPA was first suggested by Krishnan et al. (1993) when studying estrogen in yeast cells. They discovered that BPA had leached from an autoclaved flask into the culture media used for yeast. Once in the medium, BPA acted as an agonist on artificially introduced mammalian estrogen receptors in the yeast cells, suggesting BPA mimicked estrogen. By the mid-1990s, leaching of BPA into consumer goods intended for human consumption had been detected (Ben-Jonathan and Steinmetz 1998).
In 2003, two back-to-back publications showed that, on its own, BPA could function as a weak estrogen and disrupt normal reproductive processes in mammals. Howdeshell et al. (2003) reported that damage to mice cages and bottles made from polycarbonate and polysulfone led to the leaching of BPA, resulting in oral and dermal absorption of BPA by the caged mice. Corroborating the earlier findings in yeast, the leached BPA produced estrogenic activity in the exposed female mice. At the same time, Hunt et al. (2003) reported a similar scenario in their mouse populations. They uncovered a role for BPA in the disruption of meiosis, leading to infertility. Their initial observations were not from a planned experiment, rather they had observed loss of fertility in their mouse population for ∼5 years and were perplexed by this problem. Unexpectedly, the eggs of even their wild-type mice had a dramatic spike in chromosomal abnormalities, indicating severe defects in meiosis. To test their hypothesis that the meiosis abnormalities were due to caging material, Hunt and colleagues set up an experiment with three groups of cages and bottles: (1) new (i.e., nondamaged), (2) previously used/damaged, and (3) newly damaged. They found that mice using damaged cages and bottles showed an increased rate of defects in meiotic chromosome structure and number when compared to animals in nondamaged cages and bottles. In comparison, no defects were observed in animals given glass bottles.
Nearly 20 years later, it is now known that BPA interferes with reproduction by mechanisms that affect cellular and genomic integrity. For example, BPA-induced genotoxicity, the ability of chemical agents to damage DNA, is associated with the formation of multiple DNA lesions. These include adducts (chemical modification of DNA), and single- and double-strand DNA breaks (Jalal et al. 2018). At the molecular level, it is known that BPA acts as an endocrine disruptor via binding to at least five distinct nuclear receptors, including the estrogen receptor (Li et al. 2015). Strikingly, despite having a binding affinity ∼1000 times lower than that of estradiol, BPA is still able to produce estrogenic effects.
Finally, mounting evidence indicates that meiotic defects may also derive from BPA substitutes. Since the 2003 report by Hunt et al. that BPA disrupts chromosome, kinetochore and spindle alignment, Yang et al. (2020) found that chromosome and spindle-related defects occur in mouse oocytes treated with bisphenol F, a structural analog of BPA. Similarly, Chen et al. (2016) showed that exposure to bisphenol S alters germline function in C. elegans. At the cellular level, like BPA, bisphenols F and S cause meiotic defects in a dose-dependent manner.
How does BPA affect human fertility?
There have been many studies linking endocrine-disrupting chemicals to developmental and reproductive disorders in humans (Marques-Pinto and Carvalho 2013). In the years following the 2003 reports on BPA’s effects in mice (Howdeshell et al. 2003; Hunt et al. 2003), the potential for BPA to limit human reproduction became a topic of considerable attention. In one study, human oocytes derived from patients undergoing in vitro fertilization (IVF) were cultured in petri dishes, treated with BPA, and observed to exhibit a dose-dependent increase in meiotic arrest (Machtinger et al. 2013). At doses equivalent to the amount of BPA found in the fluid surrounding the ovum in the ovarian follicle, human oocytes exhibited defects in chromosome alignment and spindle formation in prophase and metaphase I of meiosis, respectively (Machtinger et al. 2013). Also, Ehrlich et al. (2012) found a positive association between the levels of BPA in urine and increased odds of implantation failure in women undergoing IVF treatments.
Findings from preclinical and clinical studies have now revealed ways in which prenatal, perinatal, and postnatal exposure to BPA affects human females (Pivonello et al. 2020). For example, BPA has been shown to accumulate in maternal blood, urine, amniotic fluid, placental tissue, and follicular fluid (Benachour and Aris 2009). Multiple lines of evidence have now uncovered potential mechanisms by which BPA accumulation interferes with fertilization and causes adverse pregnancy and birth outcomes (Cantonwine et al. 2013). For women undergoing reproductive therapies such as IVF, accumulation of BPA in follicular fluid was positively associated with a higher number of degenerated oocytes (Poormoosavi et al. 2019). Other studies have reported an association between BPA exposure and a decrease in the number of oocytes produced, as well as a reduction in the ability of oocytes to mature and be fertilized (Peretz et al. 2014). These effects are particularly concerning given that females are born with a finite number of eggs whose quality normally sharply declines at ages >35 years. (Nagaoka et al. 2012).
It is important to note that BPA-induced reproductive toxicity is not limited to females (Cariati et al. 2019). Thus far, fewer clinical studies have been conducted on the rate of male infertility due to BPA exposure. Nonetheless, studies have linked BPA exposure and decreased semen quality via effects on sperm concentration, total count, and vitality, which lessen the chance that sperm will successfully reach and fertilize a viable egg (Li et al. 2011; Rahman et al. 2015). Additionally, it is thought that BPA can inhibit fertilization by downregulating fertility-related proteins in the spermatozoa, such as the cytoskeletal component actin (Rahman et al. 2015), a protein required for sperm::egg interactions (Brener et al. 2003). Another study found that BPA exposure in adult rats leads to a decrease in follicle-stimulating hormone (Sadowski et al. 2014), a hormone that promotes the production of sperm and the growth and release of eggs.
Studying reproductive toxicology in animal models
Scientists studying toxicity work with compounds that have the potential to cause harm. Thus, experiments with human subjects presents significant ethical, as well as practical, limitations. To study the effects of these compounds, biomedical researchers have adopted the use of model organisms. Because of their similarities to human development and physiology, the predominant models for studying developmental and reproductive toxicity are rodents (Martin et al. 2009). Of the nonmammalian models, the nematode Caenorhabditis elegans is a popular choice as it is inexpensive, requires minimal upkeep, and shares multiple developmental and reproductive pathways with humans (Williams et al. 2017). Unlike rodent models, C. elegans has a life cycle of only 3–4 days from embryo to reproductive adult, and an enormous reproductive capacity, producing over 500 offspring in a life span when mated (Kimble and Ward 1988). Interestingly, most C. elegans are hermaphroditic. Thus, a single worm can produce both egg and sperm, and it can produce ∼300 viable offspring without mating.
When selecting a model organism to study reproductive toxicity, it is important that its genes and biochemical pathways are relatively well conserved so that findings may be applicable to humans. For example, Hornos Carneiro et al. (2020) showed that a compound called coenzyme Q10 (CoQ10) partially reversed DNA damage resulting from oxidative stress-induced repair defects caused by BPA exposure in C. elegans. C. elegans is useful for identifying drug-based therapies such as CoQ10 because the majority of the genetic pathways found in worms, such as those controlling DNA repair in meiosis, operate similarly in humans (Kaletta and Hengartner 2006). Remarkably, >83% of the >15,000 protein-coding sequences in C. elegans are also found in humans (Lai 2000). This subset accounts for an estimated 42% of genes linked to human diseases (Baumeister and Ge 2002). Because the genes and biochemical pathways controlling DNA repair are conserved between C. elegans and mammals, it is reasonable to hypothesize that in humans, CoQ10 may also reverse BPA reproductive toxicity caused by oxidative damage to DNA.
It is also advantageous for a model organism to be genetically tractable, that is, when it is easy to change, add, or delete a gene or multiple genes within it. For example, the strain of C. elegans tested by Hornos Carneiro et al. was col-121, which has a mutation in a collagen gene known to increase cuticle permeability and thus hypersensitivity to chemicals (Watanabe et al. 2005). Their use of the col-121 mutant allowed for lower, but relevant, doses of BPA to be tested.
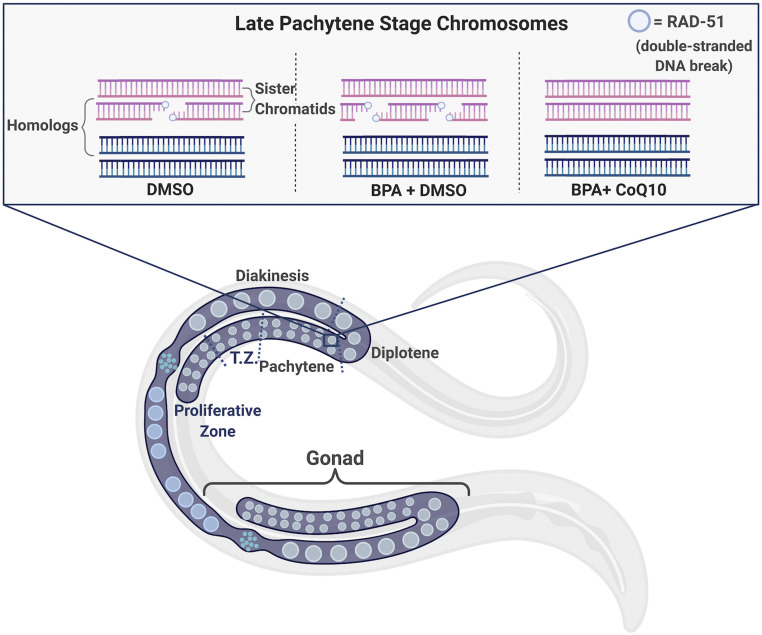
Lastly, for researchers studying fertility, the transparent cuticle of C. elegans allows scientists to study meiosis using a standard dissecting light microscope (Corsi et al. 2015). The gonads of an adult worm are quite large with respect the animal’s size. When dissected onto glass slides, researchers can easily examine the entire reproductive system of a single animal. Furthermore, the nuclei in the C. elegans gonad are ordered in a spatiotemporal gradient, meaning every stage of sperm or egg production can be visualized and quantified (Figure 2).
Figure 2.
Spatiotemporal organization of C. elegans germ line with respect to DNA double-stranded break (DSB) repair in the presence and absence of DMSO, BPA, and/or CoQ10. The graphic represents an adult C. elegans hermaphrodite containing one bilobed gonad (in purple), organized as a syncytium (i.e., nuclei sharing a common cytoplasm). Nuclei at the distal end (i.e., the proliferative zone) are undergoing mitosis and then enter meiotic prophase I. Other stages of prophase I are labeled in the top gonad, separated by blue dashed lines. In the transition zone (TZ, which corresponds to the leptotene/zygotene stages), DSBs start to form on all chromosomes. The peak of DSB formation happens in mid-pachytene, when homologous chromosomes recombine. By diplotene/diakinesis, all DSBs are repaired, and the paired, homologous chromosomes become condensed prior to being segregated. The inset is a graphical representation of bivalent chromosomes in late pachytene from worms exposed to DMSO only (left), BPA and DMSO (middle), and BPA and CoQ10 (right). During meiosis, the protein RAD-51, represented by dark blue circles, self-assembles on DNA following DSBs. For simplicity, the synaptonemal complex between paired homologs is not shown.
Experimental Rationale
Prior to starting their experiments, Hornos Carneiro et al. knew that BPA caused reproductive toxicity. A former postdoctoral researcher from the same laboratory, Dr. Patrick Allard, first discovered that BPA disrupted reproduction in C. elegans (Allard and Colaiacovo 2010). Hornos Carneiro et al. therefore sought to identify an agent that could limit BPA-induced toxicity, and thus could possibly improve fertility for humans. An important part of their experimental design relied upon BPA’s propensity to damage DNA, as well as prior knowledge of how DNA damage is handled by cells. For example, multiple laboratories confirmed that BPA causes cellular instability due to DNA damage resulting from oxidative stress (Gassman 2017), an underlying contributor to human infertility in both males and females (Agarwal et al. 2012).
Oxidative stress occurs at the cellular level in the form of free radicals and chemically unstable molecules of nitrogen or oxygen that can occur as byproducts of biochemical reactions. Their reactivity is due to the presence of unpaired electrons, which can damage macromolecules within the cell (Pham-Huy et al. 2008). To prevent oxidative stress-induced damage, cells have antioxidants, compounds which sequester free radicals to prevent oxidation of other compounds. Some antioxidants can be administered via the diet, such as CoQ10. In the eggs of aging female mice, CoQ10 has been shown to reverse the effects of oxidative stress (Ben-Meir et al. 2015). It has also shown promise for improving fertility in humans (Akarsu et al. 2017; Xu et al. 2018). Furthermore, exogenous CoQ10 supplementation has already been performed in C. elegans, and the authors discovered that it lowered oxidative stress levels (Ishii et al. 2004); although these experiments did not address reproduction directly, they did show that exogenous CoQ10 is biologically active in C. elegans.
Based on this information, the primary question asked by Hornos Carneiro et al. was whether addition of the supplement CoQ10 would suppress reproductive phenotypes of BPA-exposed worms. As a negative control group, they exposed worms only to dimethylsulfoxide (DMSO; a solvent for both BPA and CoQ10), and as a positive control, they examined worms treated with BPA dissolved in DMSO. Their experimental groups included worms exposed to CoQ10 alone, or CoQ10 with varying doses of BPA, which were precisely calculated to be equivalent to known exposures in humans. This was because a goal of their experimental design was to use approximate exposures occurring throughout the entire life span of an individual. If worms exposed to BPA with CoQ10 had fewer defects than those exposed to BPA alone, they could assume CoQ10 had rescued (suppressed) the BPA-induced phenotype. If their hypothesis was supported, this would substantiate using CoQ10 as an intervention prior to or during pregnancy in humans.
Tools, Techniques, and Results
Quantifying rescue of BPA-induced fertility defects
For the first of their experiments, Hornos Carneiro et al. allowed the adult worms (the P0 generation) to lay fertilized eggs (the F1 generation) on plates (the adult P0 worms were the ones exposed to either DMSO, DMSO and CoQ10, BPA, or BPA and CoQ10). After removing the parent (P0) worm, they counted the number of F1 animals that survived and classified these into three distinct categories: (1) fertilized eggs that arrested as embryos and never hatched (i.e., embryonic lethal); (2) embryos that hatched, but arrested as larvae, dying before adulthood (i.e., larval lethal); and (3) embryos that hatched and survived to adulthood without problems. By comparing the total number of surviving offspring, the researchers were able to assess reproductive as an outcome of BPA and/or CoQ10 exposure. As predicted, exposure to BPA resulted in fewer total embryos, and a significant portion of those that hatched died prior to reaching adulthood. However, statistical analysis revealed that addition of CoQ10 led to a significant decrease in lethality and an increase in total fertilized embryos produced by this population. Thus, CoQ10 rescued the BPA phenotype and restored fertility.
From prior studies performed in the Colaiácovo laboratory, Hornos Carneiro et al. knew that BPA inhibited successful reproduction in C. elegans by interfering with several steps of meiosis that involve DNA repair (Allard and Colaiacovo 2010). Somewhat paradoxically, successful meiosis requires that many DNA double-stranded breaks (DSBs) are purposely formed on all chromosomes. Although highly toxic in other contexts, DSB formation is what permits homologous chromosome pairs to exchange genetic material via homologous recombination during prophase I of meiosis. Likewise, DSBs are required for paired homologs to segregate properly during metaphase I. Since it was already known that BPA interfered with normal DSB repair in C. elegans (Allard and Colaiacovo 2010), Hornos Carneiro et al. tested whether adding CoQ10 would improve the ability of worms to repair DNA damage that occurs during meiosis.
To visualize detailed events during meiosis, the researchers used immunofluorescence, a technique that enables one to locate a protein of interest in fixed cells or tissue samples, usually relative to other labeled macromolecules. In these experiments, a fluorescently labeled antibody is produced or purchased that will bind very specifically to a protein of interest (i.e., an antigen). Soaking a tissue sample in one or more of these antibodies, then washing away any antibodies that did not bind to their target protein, allows researchers using epifluorescence microscopy to visualize where in the cell the fluorescent antibody is bound to target protein. For an extensive description of immunofluorescence and how it is used to study C. elegans meiosis, see Turcotte et al. (2016).
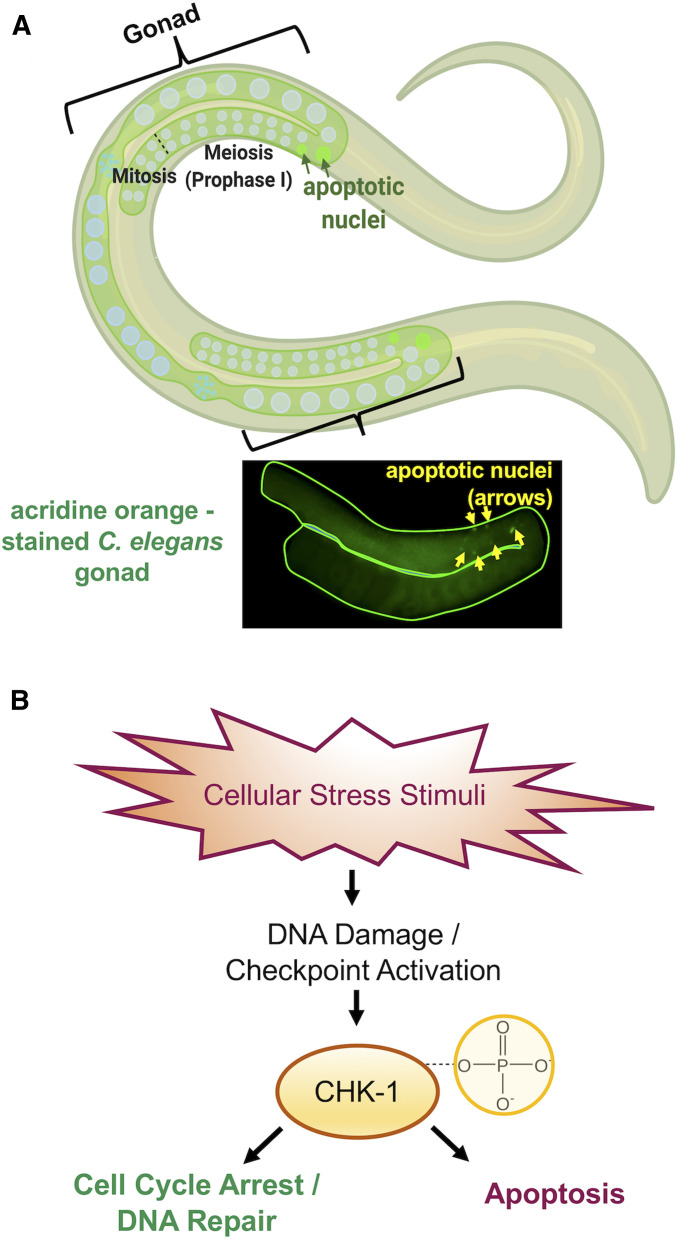
Hornos Carneiro et al. used immunofluorescence to visualize a protein involved in genetic recombination called RAD-51, a marker for DSBs (Sullivan and Bernstein 2018). They dissected gonads from worms in their control and experimental groups and quantified the number of RAD-51-stained foci (sites of DSBs) in each cell nucleus (Figure 2). As a complementary assay, they performed these experiments in rad-54 mutant worms that load the RAD-51 protein onto the ends of DSBs but cannot complete the subsequent steps of DSB repair (Ward et al. 2010). Thus, all RAD-51 foci become “trapped,” allowing researchers to quantify all DSBs formed during meiosis, rather than just those that happen to be in the middle of a DSB at the time the samples were fixed (Mets and Meyer 2009).Their next rescue experiments tested the ability of CoQ10 to suppress BPA-induced germ cell apoptosis. Apoptosis, or programmed cell death, is a tightly regulated process that leads to the removal of unnecessary or extensively damaged cells from a multicellular organism. In C. elegans, methods for studying apoptosis in meiotically dividing germ cells are well established. Because of its small size and transparent body, apoptotic nuclei are readily detected in live animals using basic microscopy. Hornos Carneiro et al. hypothesized that apoptosis might be a consequence of unrepaired DNA DSBs that could explain why BPA-treated worms had fewer offspring. They used a technique called acridine orange staining (Figure 3A) to assess apoptosis. Acridine orange is a cell-permeable dye that binds nucleic acids and emits green fluorescence in response to changes in pH, a hallmark of apoptotic cells (Lagadic-Gossmann et al. 2004).
Figure 3.
Causes and consequences of DNA damage in the C. elegans germ line. (A) Top: graphic showing apoptotic nuclei (depicted as fluorescent green circles) detected in the late pachytene stage of prophase I near the bend of the C. elegans gonads. Bottom: dissected C. elegans gonad stained with acridine orange; yellow arrowheads indicate apoptotic nuclei. (B) Cellular stress stimuli, such as BPA exposure, is a source of DNA damage that signals checkpoint proteins, such as the kinase CHK-1. This is activated by phosphorylation and leads to either DNA repair or the culling of damaged cells by apoptosis.
The upstream genetic pathways that respond to DNA damage, involving checkpoint proteins, are well conserved between human and C. elegans (Surova and Zhivotovsky 2013). When activated, checkpoint proteins transduce signals that culminate in either DNA repair or the removal of damaged cells (Gartner et al. 2008; Figure 3B). Using immunofluorescence, Hornos Carneiro et al. screened meiotic nuclei for the presence of the conserved checkpoint protein, CHK-1, in gonads dissected from animals treated with DMSO, BPA, and/or CoQ10. Since apoptosis does not eliminate all damaged germ cells, they also looked for abnormalities in chromosome structure in the oocytes, an indicator of incomplete DNA repair.
To complement their experiments examining oocytes, Hornos Carneiro et al. sorted C. elegans embryos to identify chromosomal abnormalities in the offspring. Environmental stressors, such as BPA, can cause chromosomal nondisjunction, leading to abnormal chromosome number (aneuploidy) in C. elegans (Shin et al. 2019). To assess aneuploidy in embryos, Hornos Carneiro et al. used a transgenic “reporter” gene engineered to produce an easily observable fluorescence signal whenever it is expressed. Hornos Carneiro used the jellyfish DNA sequence encoding green fluorescent protein (GFP) fused to the promoter sequence for a gene (xol-1) that only directs transcription of the attached gene in cells with one X chromosome (normally only in male worms) (Luz 2003). All cells of their worms contained this reporter gene. The reason for engineering this worm strain was that any cells containing only one X chromosome would synthesize GFP. This allowed the researchers to quantify the rate of X chromosome missegregation in worms treated with BPA. The rationale was that unmated, wild-type C. elegans are typically hermaphroditic, carrying two X chromosomes, and will only produce XX offspring unless mated with a male. In laboratory-maintained populations of wild-type worms, males are extremely rare and only occur as a result of a spontaneous nondisjunction event that causes a gamete with a missing X chromosome (Herman 2005). Therefore, xol-1 expression occurs in C. elegans offspring if there was a nondisjunction event during meiosis, producing a gamete missing a sex (X) chromosome. xol-1 expression can also be detected as result of X chromosome missegregation very early in embryogenesis.
To assess differences in xol-1 expression, Hornos Carneiro et al. used a COPAS Biosort, a “worm sorter” machine. The COPAS Biosort utilizes the principles of fluorescence-activated cell sorting, a technique originally developed for analyzing individual cells. A strength of the COPAS Biosort machine is its ability to separate thousands of small living organisms (such as C. elegans) based on physical and biological properties, including differences in GFP expression (Pulak 2006). Because xol-1::GFP is only detected in animals with one X chromosome, the COPAS Biosort could efficiently separate the GFP-expressing X-only males from their non-GFP (XX) counterparts.
Monitoring molecular changes: quantitative real-time PCR
Identifying molecular changes associated with infertility is an important strategy in identifying relevant therapeutic targets in humans (He et al. 2006). Prior to their current study, the Colaiácovo laboratory had shown that BPA exposure is correlated with increased transcription of known conserved regulators of DNA damage (Allard and Colaiacovo 2010). Hornos Carneiro et al. therefore began assessing the genetic mechanisms of BPA and CoQ10 effects on phenotype by reexamining messenger RNA (mRNA) expression levels of these previously identified gene targets as well as four conserved genes whose protein products respond to oxidative stress.
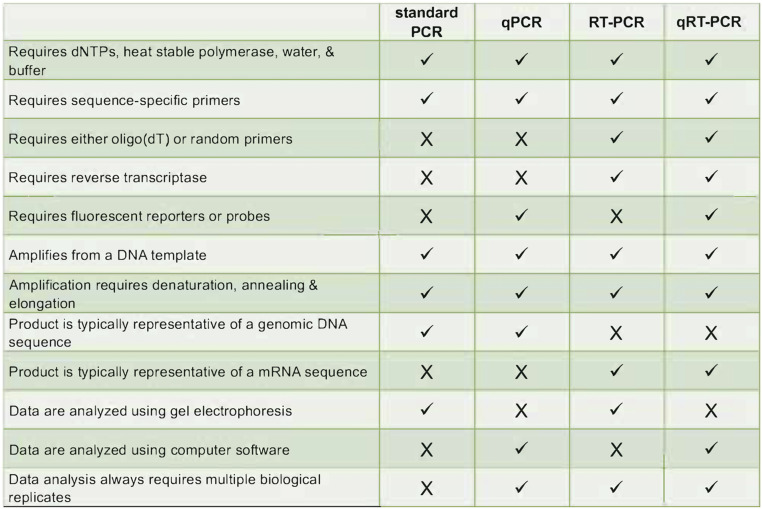
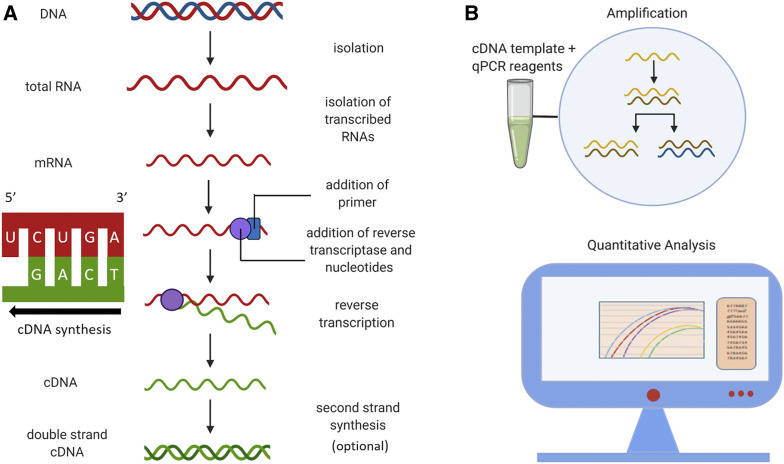
To study how CoQ10 affects changes in gene expression caused by BPA, Hornos Carneiro et al. used quantitative reverse transcriptase polymerase chain reaction (qRT-PCR). Of the many techniques available to assess gene expression, qRT-PCR is widely accepted as the most robust means for comparing mRNA abundance in different environmental conditions, mutant backgrounds, or developmental time points. The terminology for the various types of PCR are confusing and are often incorrectly used. Standard PCR amplifies a DNA template and the products are separated by size in a gel matrix for observation. This method is not quantitative as measurement of product abundance is done at the end point where signal saturation may occur. Quantitative PCR (qPCR) circumvents this problem because transcript abundance is measured at the end of each cycle, in real time, hence name for this method. Since it is a quantitative method, qPCR is more practical for diagnostics wherein small differences in input DNA, such as copy number variation, may be used for molecular diagnostics (Zonta et al. 2015). Both standard PCR and qPCR can be used to measure mRNA transcript abundance once the RNA template is converted to a complementary DNA (cDNA) copy using the enzyme reverse transcriptase (Pfaffl 2010). cDNA is subsequently amplified using standard PCR (RT-PCR) or quantitative, real-time methods (qRT-PCR). These methods allow researchers to compare differences in gene expression by identifying which genes are actively being transcribed under certain conditions (refer to Figure 5 for a comparison of all four methods).
Figure 5.
Comparison of techniques and requirements for different types of polymerase chain reaction (PCR). dNTPs, deoxynucleotide triphosphates; qPCR, quantitative PCR; qRT-PCR, quantitative reverse transcriptase PCR; RT-PCR, reverse transcriptase PCR.
Hornos Carneiro et al. used qRT-PCR to measure differences in expression levels of genes known to be affected by BPA. To perform this type of experiment, the first step is always to isolate RNA (Figure 4A) in the presence of RNase inhibitors. Purified RNA samples are converted to cDNA using reverse transcriptase. cDNA is more stable than mRNA, and unlike genomic DNA, which includes both coding and noncoding sequences, cDNAs consist only of exons and flanking untranslated regions as they are derived from mature mRNAs. Reverse transcription of mRNA to cDNA requires standard PCR reagents (e.g., dNTPs, water, and buffer) along with either oligo(dT) primers that bind to the poly-A tail or random base sequences (random hexamer primers). The advantage of primers binding the poly-A tail is that more full-length transcripts are isolated, whereas random hexamers primers have the ability to recognize degraded RNAs. Random primers are also useful for amplifying nonpolyadenylated RNAs, such as those in bacteria. cDNA can be amplified immediately following reverse transcription or used as a template for an additional “second-strand synthesis,” which generates a double-stranded cDNA molecule. qRT-PCR involves quantification of target cDNAs at every cycle of amplification and uses a specialized thermocycler, and fluorescent dye binds double-stranded DNA to allow detection of the amount cDNA produced after each cycle of amplification (Figure 4B). Following all qRT-PCR runs/fluorescence analysis, target DNA molecules may be quantified using absolute quantification by generating a standard curve which compares the test samples with samples of known concentrations (Taylor et al. 2019). The second means of quantification is relative quantification, where an internal reference gene, one that is ubiquitously and stably expressed in all samples, is used as a baseline to compare fold differences among samples.
Figure 4.
Workflow for cDNA isolation and quantitative real-time PCR (qRT-PCR). (A) Total RNA is isolated from samples and is reverse transcribed in a 5′ to 3′ direction to form complementary DNA (cDNA). This step requires a reverse transcriptase (RT) enzyme which uses mRNA as a template. Depending on the type of RT used and whether RNase is added to the RT reaction, the initial product is either a single-stranded cDNA or a DNA::RNA hybrid. Second strand synthesis is an optional step to produce double-stranded cDNA which may be useful for other applications, such as molecular cloning or generating cDNA libraries. (B) The cDNA and qRT-PCR reagents are added to a microwell plate (a single tube is shown for simplicity). Negative controls (not shown) include the following: (1) a no-template control, (2) a no-reverse-transcriptase control, and (3) a no-amplification control, meaning no DNA polymerase. During qRT-PCR, amplification and quantification of cDNA occur simultaneously. Experimental and positive control reactions are depicted by colored curves on data analysis display, and horizontal black line corresponds to negative control.
Hornos Carneiro et al. measured mRNA concentrations using the reference gene tba-1. In C. elegans, the tba-1 gene encodes tubulin, a subunit of the cytoskeletal microtubules, expressed ubiquitously in the adult germ line and embryos (Hurd 2018). This means that tubulin is expected to be present in similar amounts in all of their samples, hence its selection as a reference. Relative (fold) differences in expression were established using the DMSO-only (control) samples as a baseline. Expression levels of the 19 target genes could then be compared among the three experimental groups (see their Figure 4).
Measuring oxidative stress
Molecular mechanisms dealing with oxidative stress are largely conserved; therefore, findings in model organisms such as C. elegans can often be applied to humans (Miranda-Vizuete and Veal 2017). There are multiple tools for studying oxidative stress and metabolic function in C. elegans. To understand how CoQ10 suppresses BPA-induced oxidative stress, Hornos Carneiro et al. used another reporter strain. This time, the GFP DNA sequence was fused to the promoter region required for transcribing the gst-4 gene. In their qRT-PCR experiments, gst-4 was one of the four genes tested for responding to oxidative stress. gst-4 encodes a conserved protein involved in the metabolism of glutathione (Leiers et al. 2003), critical for regulating multiple physiological processes in worms and humans, including antioxidant activity (Ferguson and Bridge 2019). Since transcription of the gst-4 gene increases in response to oxidative stress (Leiers et al. 2003), the use of a gst-4 reporter can identify conditions where oxidative stress is increased (Link and Johnson 2002). Reactive oxygen species can form as a byproduct of the mitochondrial electron transport chain during oxidative phosphorylation, as well as through chemical interactions between reactive xenobiotics, including BPA, and cell organelles such as proteins, membranes, and DNA. Therefore, as a complementary assay, the researchers used a dye called MitoTrackerRed as an indicator of normal mitochondrial function.
Looking Ahead
Multiple lines of evidence have now uncovered potential mechanisms by which BPA interferes with fertilization and causes adverse pregnancy and birth outcomes (Cantonwine et al. 2013). The possibility that BPA accelerates reproductive senescence is a major concern, given the number of females who are now delaying pregnancy until the later portion of their reproductive years when egg quality is already declining. As it is not feasible for humans to avoid BPA entirely, developing measures to counter BPA toxicity are an important step to increase the potential for reproductive success. Future studies are needed to further investigate the molecular mechanisms by which CoQ10, and possibly other antioxidants, attenuate oxidative stress and suppress toxicity caused by BPA and its structural analogs.
Questions for review
What did Hornos Carneiro et al. find when assessing levels of RAD-51 foci (figure 1, E and F of Hornos Carneiro et al.)? Be sure you can explain what the figure is showing. What conclusion could be drawn from those results and the ones presented in Figure 2, B and C?
What is the difference between antibody staining and acridine orange staining? Why were both used by Hornos Carneiro et al. (figures 1 and 2, respectively)? Briefly compare and contrast the techniques. Also, explain why it was necessary for the authors to stain germ line with DAPI in addition to the RAD-51 antibody.
There are multiple types of PCR used today. Why was qRT-PCR used by Hornos Carneiro et al. vs. other PCR techniques? Explain the differences between the qRT-PCR results depicted in figure 4A vs. figure 4B of Hornos Carneiro et al.; what was the purpose of using two different temperatures?
Hornos Carneiro et al. used several controls to validate their results regarding oxidative stress. Briefly explain the purpose of using paraquat (figure 5, B and C of Hornos Carneiro et al.), as well as the rationale for using the sek-1 mutant (figure 5, G and H).
Given your knowledge of reporter strains and the experiments conducted by Hornos Carneiro et al., what was the rationale for using the reporter in figure 5? How is this strain different from the one used in figure 6, D and E of Hornos Carneiro et al.? In your own words, explain how reporter strains are generated using genetic engineering.
Discuss the rationale for using the COPAS Biosort to quantify nondisjunction in figure 6, C and E. How do the results in figure 3 and figure 6, A and B of Hornos Carneiro et al. support their rationale for conducting the experiments using the Biosort?
Many journal articles now include graphical abstracts, schematic images that visually represent the authors’ primary findings. These abstracts allow the reader to easily identify the article’s main message. Using Hornos Carneiro et al.’s findings, construct a graphical abstract depicting how CoQ10 repairs BPA-induced oxidative damage.
Questions for further discussion
8. Why is BPA still a problem and why is it important to still study it? Provide two reasons. Now that BPA’s harmful effects are becoming known, what actions can individuals take to decrease their exposure to BPA?
9. BPA exposure was shown to produce several harmful phenotypes that CoQ10 exposure rescued. What are other endogenous (internal) forms of cell stress? Would CoQ10 possibly be able to relieve this stress too? Why or why not?
10. Hornos Carneiro et al. found that CoQ10 rescued BPA-induced oxidative DNA damage in the C. elegans germ line suggesting it could restore fertility. Regarding function, what remains unknown about CoQ10 in C. elegans? What further knowledge is needed to inform the use of CoQ10 in humans? What is one experiment you can think of to answer this question?
11. There has been talk about formally including CoQ10 in human fertility trials for many years. Currently, some clinicians recommend that patients purchase it over the counter. What limitations currently exist with using CoQ10 as an over-the-counter adjunctive therapy? Based on the findings from Hornos Carneiro et al. and other researchers, what considerations should clinicians take in account when recommending this to patients?
Acknowledgments
We thank Julia Rigothi, Nicolas Andrews, and Elizabeth De Stasio for proofreading the manuscript and for helpful suggestions. We also thank the Genetics Society of America for their continued support of undergraduate education by permitting a team of student researchers to write this article during an unprecedented time of laboratory shutdown. Some graphics were created with BioRender.com. P.M.C. is supported by National Institutes of Health grant 1R15GM117479-01. B.R.B. is supported by an National Institute of Environmental Health Sciences training grant (T32 ES007324).
Footnotes
Communicating editor: E. De Stasio
Literature Cited
- Agarwal A., Aponte-Mellado A., Premkumar B. J., Shaman A., and Gupta S., 2012. The effects of oxidative stress on female reproduction: a review. Reprod. Biol. Endocrinol. 10: 49 10.1186/1477-7827-10-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akarsu S., Gode F., Isik A. Z., Dikmen Z. G., and Tekindal M. A., 2017. The association between coenzyme Q10 concentrations in follicular fluid with embryo morphokinetics and pregnancy rate in assisted reproductive techniques. J. Assist. Reprod. Genet. 34: 599–605. 10.1007/s10815-017-0882-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard P., and Colaiacovo M. P., 2010. Bisphenol A impairs the double-strand break repair machinery in the germline and causes chromosome abnormalities. Proc. Natl. Acad. Sci. USA 107: 20405–20410. 10.1073/pnas.1010386107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asencio C., Navas P., Cabello J., Schnabel R., Cypser J. R. et al. , 2009. Coenzyme Q supports distinct developmental processes in Caenorhabditis elegans. Mech. Ageing Dev. 130: 145–153. 10.1016/j.mad.2008.10.004 [DOI] [PubMed] [Google Scholar]
- Baumeister R., and Ge L., 2002. The worm in us – Caenorhabditis elegans as a model of human disease. Trends Biotechnol. 20: 147–148. 10.1016/S0167-7799(01)01925-4 [DOI] [PubMed] [Google Scholar]
- Benachour N., and Aris A., 2009. Toxic effects of low doses of bisphenol-A on human placental cells. Toxicol. Appl. Pharmacol. 241: 322–328. 10.1016/j.taap.2009.09.005 [DOI] [PubMed] [Google Scholar]
- Ben-Jonathan N., and Steinmetz R., 1998. Xenoestrogens: the emerging story of bisphenol A. Trends Endocrinol. Metab. 9: 124–128. 10.1016/S1043-2760(98)00029-0 [DOI] [PubMed] [Google Scholar]
- Ben-Meir A., Burstein E., Borrego-Alvarez A., Chong J., Wong E. et al. , 2015. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell 14: 887–895. 10.1111/acel.12368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisht S., Faiq M., Tolahunase M., and Dada R., 2017. Oxidative stress and male infertility. Nat. Rev. Urol. 14: 470–485. 10.1038/nrurol.2017.69 [DOI] [PubMed] [Google Scholar]
- Braun J. M., and Hauser R., 2011. Bisphenol A and children’s health. Curr. Opin. Pediatr. 23: 233–239. 10.1097/MOP.0b013e3283445675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brener E., Rubinstein S., Cohen G., Shternall K., Rivlin J. et al. , 2003. Remodeling of the actin cytoskeleton during mammalian sperm capacitation and acrosome Reaction1. Biol. Reprod. 68: 837–845. 10.1095/biolreprod.102.009233 [DOI] [PubMed] [Google Scholar]
- Cantonwine D. E., Hauser R., and Meeker J. D., 2013. Bisphenol A and human reproductive health. Expert Rev. Obstet. Gynecol. 8: 329–335. 10.1586/17474108.2013.811939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cariati F., D’Uonno N., Borrillo F., Iervolino S., Galdiero G. et al. , 2019. Bisphenol A: an emerging threat to male fertility. Reprod. Biol. Endocrinol. 17: 6 10.1186/s12958-018-0447-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Shu L., Qiu Z., Lee D. Y., Settle S. J. et al. , 2016. Exposure to the BPA-substitute bisphenol S causes unique alterations of germline function. PLOS Genet. 12: e1006223 10.1371/journal.pgen.1006223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi A. K., Wightman B., and Chalfie M. A transparent window into biology: a primer on Caenorhabditis elegans (June 18, 2015), WormBook, ed. The C. elegans Research Community WormBook, doi/10.1895/wormbook.1.177.1, 10.1895/wormbook.1.177.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich S., Williams P. L., Missmer S. A., Flaws J. A., Berry K. F. et al. , 2012. Urinary bisphenol A concentrations and implantation failure among women undergoing in vitro fertilization. Environ. Health Perspect. 120: 978–983. 10.1289/ehp.1104307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson G. D., and Bridge W. J., 2019. The glutathione system and the related thiol network in Caenorhabditis elegans. Redox Biol. 24: 101171 10.1016/j.redox.2019.101171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner A., Boag P. R., and Blackwell K. T., 2008. Germline survival and apoptosis. WormBook. 10.1895/wormbook.1.145.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassman N. R., 2017. Induction of oxidative stress by bisphenol A and its pleiotropic effects. Environ. Mol. Mutagen. 58: 60–71. 10.1002/em.22072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geens T., Aerts D., Berthot C., Bourguignon J.-P., Goeyens L. et al. , 2012. A review of dietary and non-dietary exposure to bisphenol-A. Food Chem. Toxicol. 50: 3725–3740. 10.1016/j.fct.2012.07.059 [DOI] [PubMed] [Google Scholar]
- Gorecki S., Bemrah N., Roudot A.-C., Marchioni E., Le Bizec B., et al. , 2017. Human health risks related to the consumption of foodstuffs of animal origin contaminated by bisphenol A. Food Chem. Toxicol. 110: 333–339. 10.1016/j.fct.2017.10.045 [DOI] [PubMed] [Google Scholar]
- He Z., Chan W.-Y., and Dym M., 2006. Microarray technology offers a novel tool for the diagnosis and identification of therapeutic targets for male infertility. Reproduction 132: 11–19. 10.1530/rep.1.01070 [DOI] [PubMed] [Google Scholar]
- Herman, R. K., Introduction to sex determiniation (December 24, 2005), WormBook, ed. The C. elegans Research 1.71.1 [Google Scholar]
- Hornos Carneiro M. F., Shin N., Karthikraj R., Barbosa F., Kannan K., et al. , 2020. Antioxidant CoQ10 restores fertility by rescuing bisphenol A-induced oxidative DNA damage in the Caenorhabditis elegans germline. Genetics 214: 381–395. 10.1534/genetics.119.302939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howdeshell K. L., Peterman P. H., Judy B. M., Taylor J. A., Orazio C. E. et al. , 2003. Bisphenol A is released from used polycarbonate animal cages into water at room temperature. Environ. Health Perspect. 111: 1180–1187. 10.1289/ehp.5993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard, E.J.A., and Greenstein, D. Introduction to the germ line (September 1, 2005), WormBook, ed. The C. elegans Research Community WormBook, doi/10.1895/wormbook.1.18.1, http://www.wormbook.org. 10.1895/wormbook.1.18.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt P. A., Koehler K. E., Susiarjo M., Hodges C. A., Ilagan A. et al. , 2003. Bisphenol A exposure causes meiotic aneuploidy in the female mouse. Curr. Biol. 13: 546–553. 10.1016/S0960-9822(03)00189-1 [DOI] [PubMed] [Google Scholar]
- Hurd, D. D., Tubulins in C. elegans (August 4, 2018), WormBook, ed. The C. elegans Research Community WormBook, doi/10.1895/wormbook.1.182.1 [Google Scholar]
- Ishii N., Senoo-Matsuda N., Miyake K., Yasuda K., Ishii T. et al. , 2004. Coenzyme Q10 can prolong C. elegans lifespan by lowering oxidative stress. Mech. Ageing Dev. 125: 41–46. 10.1016/j.mad.2003.10.002 [DOI] [PubMed] [Google Scholar]
- Jalal N., Surendranath A. R., Pathak J. L., Yu S., and Chung C. Y., 2018. Bisphenol A (BPA) the mighty and the mutagenic. Toxicol. Rep. 5: 76–84. 10.1016/j.toxrep.2017.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaletta T., and Hengartner M. O., 2006. Finding function in novel targets: C. elegans as a model organism. Nat. Rev. Drug Discov. 5: 387–399. 10.1038/nrd2031 [DOI] [PubMed] [Google Scholar]
- Kimble J., and Ward S., 1988. Germ-line development and fertilization, pp. 191–214 in The nematode Caenorhabditis elegans, edited by Wood W. B. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Krishnan A. V., Stathis P., Permuth S. F., Tokes L., and Feldman D., 1993. Bisphenol-A: an estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology 132: 2279–2286. 10.1210/endo.132.6.8504731 [DOI] [PubMed] [Google Scholar]
- Lagadic-Gossmann D., Huc L., and Lecureur V., 2004. Alterations of intracellular pH homeostasis in apoptosis: origins and roles. Cell Death Differ. 11: 953–961. 10.1038/sj.cdd.4401466 [DOI] [PubMed] [Google Scholar]
- Lai C.-H., 2000. Identification of novel human genes evolutionarily conserved in Caenorhabditis elegans by comparative proteomics. Genome Res. 10: 703–713. 10.1101/gr.10.5.703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiers B., Kampkötter A., Grevelding C. G., Link C. D., Johnson T. E. et al. , 2003. A stress-responsive glutathione S-transferase confers resistance to oxidative stress in Caenorhabditis elegans. Free Radic. Biol. Med. 34: 1405–1415. 10.1016/S0891-5849(03)00102-3 [DOI] [PubMed] [Google Scholar]
- Li D.-K., Zhou Z., Miao M., He Y., Wang J. et al. , 2011. Urine bisphenol-A (BPA) level in relation to semen quality. Fertil. Steril. 95: 625–630.e1–e4. 10.1016/j.fertnstert.2010.09.026 [DOI] [PubMed] [Google Scholar]
- Li L., Wang Q., Zhang Y., Niu Y., Yao X. et al. , 2015. The molecular mechanism of bisphenol A (BPA) as an endocrine disruptor by interacting with nuclear receptors: Insights from molecular dynamics (MD) simulations. PLOS One 10: e0120330 10.1371/journal.pone.0120330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link C. D., and Johnson C. J., 2002. Reporter transgenes for study of oxidant stress in Caenorhabditis elegans. Methods Enzymol. 353: 497–505. 10.1016/s0076-6879(02)53072-x [DOI] [PubMed] [Google Scholar]
- Luz J. G., 2003. XOL-1, primary determinant of sexual fate in C. elegans, is a GHMP kinase family member and a structural prototype for a class of developmental regulators. Genes Dev. 17: 977–990. 10.1101/gad.1082303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Liu H., Wu J., Yuan L., Wang Y. et al. , 2019. The adverse health effects of bisphenol A and related toxicity mechanisms. Environ. Res. 176: 108575 10.1016/j.envres.2019.108575 [DOI] [PubMed] [Google Scholar]
- Machtinger R., Combelles C. M. H., Missmer S. A., Correia K. F., Williams P. et al. , 2013. Bisphenol-A and human oocyte maturation in vitro. Hum. Reprod. 28: 2735–2745. 10.1093/humrep/det312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques-Pinto A., and Carvalho D., 2013. Human infertility: are endocrine disruptors to blame? Endocr. Connect. 2: R15–R29. 10.1530/EC-13-0036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. L., Breslin W., Rocca M., Wright D., and Cavagnaro J., 2009. Considerations in assessing the developmental and reproductive toxicity potential of biopharmaceuticals. Birth Defects Res. B Dev. Reprod. Toxicol. 86: 176–203. 10.1002/bdrb.20197 [DOI] [PubMed] [Google Scholar]
- Mets D. G., and Meyer B. J., 2009. Condensins regulate meiotic DNA break distribution, thus crossover frequency, by controlling chromosome structure. Cell 139: 73–86. 10.1016/j.cell.2009.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda-Vizuete A., and Veal E. A., 2017. Caenorhabditis elegans as a model for understanding ROS function in physiology and disease. Redox Biol. 11: 708–714. 10.1016/j.redox.2016.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka S. I., Hassold T. J., and Hunt P. A., 2012. Human aneuploidy: mechanisms and new insights into an age-old problem. Nat. Rev. Genet. 13: 493–504. 10.1038/nrg3245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacyga D. C., Sathyanarayana S., and Strakovsky R. S., 2019. Dietary predictors of phthalate and bisphenol exposures in pregnant women. Adv. Nutr. Bethesda Md 10: 803–815. 10.1093/advances/nmz029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz J., Vrooman L., Ricke W. A., Hunt P. A., Ehrlich S., et al. , 2014. Bisphenol A and reproductive Health: update of experimental and human evidence, 2007–2013. Environ. Health Perspect. 122: 775–786. 10.1289/ehp.1307728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl M. W., 2010. The ongoing evolution of qPCR. Methods 50: 215–216. 10.1016/j.ymeth.2010.02.005 [DOI] [PubMed] [Google Scholar]
- Pham-Huy L. A., He H., and Pham-Huy C., 2008. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 4: 89–96. [PMC free article] [PubMed] [Google Scholar]
- Pivonello C., Muscogiuri G., Nardone A., Garifalos F., Provvisiero D. P. et al. , 2020. Bisphenol A: an emerging threat to female fertility. Reprod. Biol. Endocrinol. 18: 22 10.1186/s12958-019-0558-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poormoosavi S. M., Behmanesh M. A., Janati S., and Najafzadehvarzi H., 2019. Level of bisphenol A in follicular fluid and serum and oocyte morphology in patients undergoing IVF treatment. J. Family Reprod. Health 13: 154–159. [PMC free article] [PubMed] [Google Scholar]
- Pulak R., 2006. Techniques for analysis, sorting, and dispensing of C. elegans on the COPAS flow-sorting system. Methods Mol. Biol. 351: 275–286. 10.1385/1-59745-151-7:275 [DOI] [PubMed] [Google Scholar]
- Rahman M. S., Kwon W.-S., Lee J.-S., Yoon S.-J., Ryu B.-Y. et al. , 2015. Bisphenol-A affects male fertility via fertility-related proteins in spermatozoa. Sci. Rep. 5: 9169 10.1038/srep09169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed C. E., and Fenton S. E., 2013. Exposure to diethylstilbestrol during sensitive life stages: a legacy of heritable health effects: diethylstilbestrol during sensitive life stages. Birth Defects Res. Part C Embryo Today Rev. 99: 134–146. 10.1002/bdrc.21035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski R. N., Park P., Neese S. L., Ferguson D. C., Schantz S. L. et al. , 2014. Effects of perinatal bisphenol A exposure during early development on radial arm maze behavior in adult male and female rats. Neurotoxicol. Teratol. 42: 17–24. 10.1016/j.ntt.2014.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber M., and Chandel N. S., 2014. ROS function in redox signaling and oxidative stress. Curr. Biol. 24: R453–R462. 10.1016/j.cub.2014.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin N., Cuenca L., Karthikraj R., Kannan K., and Colaiácovo M. P., 2019. Assessing effects of germline exposure to environmental toxicants by high-throughput screening in C. elegans. PLOS Genet. 15: e1007975 10.1371/journal.pgen.1007975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siracusa J. S., L. Yin, E. Measel, S. Liang, and X. Yu, 2018 Effects of bisphenol A and its analogs on reproductive health: a mini review. Reprod. Toxicol. 79: 96–123. 10.1016/j.reprotox.2018.06.005 10.1016/j.reprotox.2018.06.005 [DOI] [PMC free article] [PubMed]
- Stergiou L., and Hengartner M. O., 2004. Death and more: DNA damage response pathways in the nematode C. elegans. Cell Death Differ. 11: 21–28. 10.1038/sj.cdd.4401340 [DOI] [PubMed] [Google Scholar]
- Sullivan M. R., and Bernstein K. A., 2018. RAD-ical new insights into RAD51 regulation. Genes (Basel) 9: 629 10.3390/genes9120629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surova O., and Zhivotovsky B., 2013. Various modes of cell death induced by DNA damage. Oncogene 32: 3789–3797. 10.1038/onc.2012.556 [DOI] [PubMed] [Google Scholar]
- Taylor S. C., Nadeau K., Abbasi M., Lachance C., Nguyen M. et al. , 2019. The ultimate qPCR experiment: producing publication quality, reproducible data the first time. Trends Biotechnol. 37: 761–774. 10.1016/j.tibtech.2018.12.002 [DOI] [PubMed] [Google Scholar]
- Turcotte C. A., Andrews N. P., Sloat S. A., and Checchi P. M., 2016. CRISPR technology reveals RAD(51)-ical mechanisms of repair in roundworms: an educational primer for use with “promotion of homologous recombination by SWS-1 in complex with RAD-51 paralogs in Caenorhabditis elegans.”. Genetics 204: 883–891. 10.1534/genetics.116.195479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S. A., 2009. The politics of plastics: the making and unmaking of bisphenol A “safety.” Am. J. Public Health 99: S559–S566. 10.2105/AJPH.2008.159228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. D., Muzzini D. M., Petalcorin M. I. R., Martinez-Perez E., Martin J. S. et al. , 2010. Overlapping mechanisms promote postsynaptic RAD-51 filament disassembly during meiotic double-strand break repair. Mol. Cell 37: 259–272. 10.1016/j.molcel.2009.12.026 [DOI] [PubMed] [Google Scholar]
- Watanabe M., Mitani N., Ishii N., and Miki K., 2005. A mutation in a cuticle collagen causes hypersensitivity to the endocrine disrupting chemical, bisphenol A, in Caenorhabditis elegans. Mutat. Res. Mol. Mech. Mutagen. 570: 71–80. 10.1016/j.mrfmmm.2004.10.005 [DOI] [PubMed] [Google Scholar]
- Williams, D. C., D. C. Bailey, and V. A. Fitsanakis, 2017 Caenorhabditis elegans as a model to assess reproductive and developmental toxicity, pp. 303–314 in Reproductive and Developmental Toxicology, edited by Gupta, R. C. Academic Press, Cambridge, MA. [Google Scholar]
- Xu Y., Nisenblat V., Lu C., Li R., Qiao J. et al. , 2018. Pretreatment with coenzyme Q10 improves ovarian response and embryo quality in low-prognosis young women with decreased ovarian reserve: a randomized controlled trial. Reprod. Biol. Endocrinol. RBE 16: 29 10.1186/s12958-018-0343-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Baumann C., De La Fuente R., and Viveiros M. M., 2020. Mechanisms underlying disruption of oocyte spindle stability by bisphenol compounds. Reproduction 159: 383–396. 10.1530/REP-19-0494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonta E., Nizard P., and Taly V., 2015. Assessment of DNA integrity, applications for cancer research. Adv. Clin. Chem. 70: 197–246. 10.1016/bs.acc.2015.03.002 [DOI] [PubMed] [Google Scholar]