Abstract
Background and Aims:
Glucosamine sulphate (GS) can be used as background therapy in people affected by knee osteoarthritis (OA). Knowledge regarding the efficacy and safety of GS is of importance since its use worldwide is increasing. Therefore, the present study aimed to map and grade the diverse health outcomes associated with GS using an umbrella review approach.
Methods:
Medline, Cinahl and Embase databases were searched until 1 April 2020. An umbrella review of systematic reviews and meta-analyses of randomized controlled trials (RCTs) was carried out. The evidence from the RCTs was graded using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) tool.
Results:
From 140 articles returned, 11 systematic reviews, for a total of 21 outcomes (37 RCTs; 3949 participants; almost all using 1500 mg/day), were included. No systematic reviews/meta-analyses of observational studies were included. Regarding the findings of the meta-analyses, 9/17 outcomes were statistically significant, indicating that GS is more effective than placebo. A high certainty of evidence, as assessed by GRADE, supported the use of GS (versus placebo) in improving the Lequesne Index, joint space width change, joint space width change after 3 years of follow up, joint space narrowing and OA progression. No difference in terms of adverse effects was found between GS and placebo. In systematic reviews, GS was associated with a better glucose profile and a better physical function performance than placebo.
Conclusion:
GS, when used as a prescription drug (i.e. crystalline glucosamine sulphate) at 1500 mg daily dosage, can positively affect the cartilage structure, reduce pain, improve function and glucose metabolism in people with knee OA, without having a greater incidence of adverse effects than placebo.
Keywords: glucosamine sulphate, osteoarthritis, umbrella review
Introduction
Osteoarthritis (OA) is among the most common diseases in older people, defined here as those greater than or equal to 65 years. This condition is traditionally characterized by joint pain and stiffness, with relevant consequences on functional decline/disability and finally loss in quality of life.1,2 In this regard, knee OA is the most common localisation within the symptomatic forms of OA, affecting more than approximately 250 million people worldwide, with symptomatic forms occurring in 10% of men and 13% of women aged 60 years or older.3,4
Currently, there is no definitive treatment for knee OA. The current therapeutic approach combines nonpharmacological and pharmacological strategies that aim to improve function, decrease pain and, if possible, improve structural aspects, with limited adverse events.5 In this regard, symptomatic slow-acting drugs for OA [such as chondroitin sulphate, glucosamine sulphate (GS), hyaluronic acid and diacerein]6 are an important background therapy for people affected by knee OA.7–9 In this class of medications, however, different drugs exist exhibiting different pharmacological profiles.
Glucosamine is a natural compound, present in different preparations. Briefly, glucosamine hydrochloride is used as nutraceutical or over-the-counter (OTC) products.10 In contrast, GS is obtained only by a proprietary semi-synthetic route and stabilisation process.10 GS is used only as a prescription drug product, prescription crystalline glucosamine sulphate (pCGS).10 However, multiple formulations of GS are available,11 both as prescription-grade and OTC products, with the latter having small/varying amounts of glucosamine.12 Moreover, GS is not available as a prescription-grade product in all countries. Importantly, there is extensive and increasing literature supporting the idea that only pCGS is able to deliver consistently high glucosamine bioavailability and plasma concentration in humans.13,14 In these experimental studies, the measurement of glucosamine concentration in patients affected by knee OA was also made as a plasma peak (7.17 µM) and as a site of action concentration (synovial fluid) equal to 4.34 µM.14 Plasma and synovial pCGS concentrations are highly correlated and both are in 10 µM range, a cut-off that seems important for some actions of pCGS, such as an anti-inflammatory effect15 that finally results in clinical efficacy.5,16–22
In addition to the use of GS for people affected with knee OA, GS may be an appropriate treatment for other conditions. For example, GS is used in hip OA23,24 or in other forms of OA.25–27 Moreover, the difference in efficacy and adverse effects incidence by prescription and OTC doses is still unclear.
The aim of the present work was to evaluate, through an umbrella review, the strength and credibility of the evidence derived from systematic reviews and meta-analyses of observational and/or intervention studies, that is, randomised controlled trials (RCTs), and obtain a general summary of their importance relative to health outcomes and adverse effects, in order to inform policies on the use of GS in humans.
Methods
This work followed a preplanned protocol (PROSPERO link: CRD42020179570). The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)28 recommendations and specific guidelines regarding how to conduct an umbrella review29 were followed for the reporting of this study.
Data sources and searches
An umbrella review was carried out,30 systematically searching the Medline, Cinahl and Embase databases from inception until 1 April 2020, using the terms ‘systematic reviews/meta-analyses’ and ‘glucosamine’, as free vocabulary words and/or controlled terms specific to each database, on a central platform hosted at Anglia Ruskin University. Reference lists of eligible articles and reviews in this field were also searched, including systematic reviews and meta-analyses under process.
Eligibility and selection criteria
We included systematic reviews, with or without formal meta-analysis of RCTs, in which at least one group used GS and one placebo reporting on health outcomes, both in terms of efficacy and safety. Only subjects taking GS only (not in combination with other medications) versus those not taking GS, independently from the length of treatment (i.e. no requirement that RCTs should be of a certain length was a priori set), were included. We included systematic reviews (with or without formal meta-analysis) that evaluated observational studies with longitudinal (prospective or retrospective) designs. We excluded studies comparing GS with another similar medication (e.g. chondroitin sulphate) or when GS was used together with another active medication (e.g. chondroitin sulphate).
Two reviewers (NV, JD) independently screened title/abstracts and full texts for eligibility, and when a consensus was not reached a third reviewer (SM) was consulted.
Data extraction
The following information was extracted: PMID/DOI, first author’s name, year of publication, study design (cohort, case control, RCT), number of included studies in each systematic review, the specific population under investigation (i.e. general population, subjects with OA and its location, etc.), the dosage of GS, the health outcome(s), the median follow-up period (in months), and for RCTs the risk of bias in included studies, according to the Cochrane review indications (high, unclear, low).31 If an article presented separate meta-analyses on more than one reported outcome, each one was assessed separately. Duplicated data from same papers were eliminated including only the largest sample size of the RCTs.
Next, the RCT-specific estimated estimates for any adverse effects or negative outcome for both systematic reviews and meta-analyses outcomes [risk ratio, odds ratio (OR), hazard ratio, incident risk ratio, standardized mean differences (SMDs), mean differences (MDs)], along with their 95% confidence intervals (CIs), were extracted.
Outcomes
Any efficacy/effectiveness outcome, adverse events or adverse effects potentially associated with GS use was included.
Risk of bias assessment
The methodological quality of each included systematic review was assessed using the Assessment of Multiple Systematic Reviews (AMSTAR) 2 tool (available at https://amstar.ca/Amstar-2.php), which is a recent update of AMSTAR,32 by two independent investigators (JD, NV). The AMSTAR 2 ranks the quality of a systematic review from critically low to high according to 16 predefined items.
Data synthesis and analysis
For each meta-analysis, we re-estimated the summary effect size and its 95% CIs under the assumption of a random-effects model. If the re-calculated effect size differed from the published effect size (e.g. in case of the use of a fixed-effects model instead of a random-effects model), we keep the re-calculated estimations. After the data extraction, we re-calculated the overall summary effect size, double checking with the original published ones. We also estimated the prediction intervals (PIs), which further accounts for between-study effects and estimates the certainty of the association if a new study addresses that same association.33–35 Between-study inconsistency was estimated with the I2 metric, with values >50% indicative of high heterogeneity and >75% very large heterogeneity.36 We calculated the evidence of small-study effects (i.e. whether small studies inflated effect sizes) using the regression asymmetry test37 with a p value < 0.10.38 We considered the effect size of the largest RCT included for each outcome, determining if it was statistically significant (p value < 0.05) or not.
All statistical analyses were conducted in Stata Statistical Software, version 14.0 (StataCorp., College Station, TX, USA).
Grading the evidence
Evidence from meta-analyses of RCTs was assessed in terms of the significance of the summary effect, using a p value < 0.05 as the threshold for statistical significance. When the p value for the random-effects model was < 0.05, we evaluated the evidence using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) assessment.39 Full details regarding the GRADE assessment are reported in Supplemental Table 1. We also reported 95% PIs (excluding the null or not), the presence of large heterogeneity (I2 >50%), small-study effects (p < 0.10) if the largest RCT in terms of participants, and excess significance (p > 0.10) as possible indicators of quality of the available evidence.
Sensitivity analyses
For outcomes of observational studies having a class I/II evidence, it was planned to conduct sensitivity analyses including only cohort studies. Moreover, for the outcomes of RCTs, it was planned to stratify analyses for risk of bias of the RCTs included using the original data if possible or evaluating the risk of bias using the Cochrane tool for risk of bias if not available in the original meta-analysis. Finally, it was planned to stratify the analyses of RCTs by prescription and not prescription doses. However, no observational studies were included and only prescription doses (i.e. >750 mg/day)40 were used. Only for one outcome (pain in OA) was it possible to run a sensitivity analysis, removing the RCTs at high risk of bias.
Results
Literature review
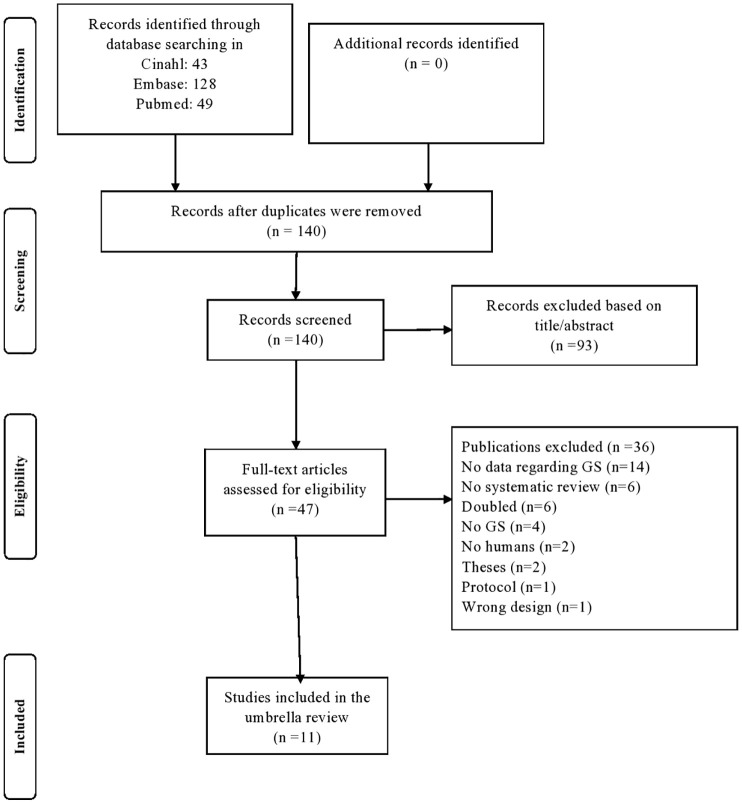
The initial search yielded 180 articles. After removing the duplicates, study selection commenced and 140 papers were evaluated, with 47 assessed as full text. As shown in the PRISMA flowchart (Figure 1), 11 articles were finally included,21,41–50 that is, 3 systematic reviews without meta-analysis, 1 network meta-analysis reporting narrative data on GS and 7 meta-analyses, for a total of 21 independent outcomes, as fully reported in Table 1. No systematic review or meta-analysis regarding observational studies was included, that is, no observational studies met the inclusion/exclusion criteria.
Figure 1.
PRISMA flowchart for study selection.
GS, glucosamine sulphate; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses.
Table 1.
Main descriptive findings of the systematic reviews included.
| Author | Type of review | Mean GS dosage (mg) (number of studies) | Follow up in months (median) | Population | OA grade | Outcomes | Number of RCTs | GS | Placebo | Total sample size |
|---|---|---|---|---|---|---|---|---|---|---|
| Dostrovsky et al.43 | Systematic review | 1500 (n = 2) | 3 | General population | – | Glucose parameters | 2 | 18 | 16 | 34 |
| Eriksen et al.21 | Meta-analysis | 1500 (n = 13); lower dosages >750 (n = 8) | 3 | OA | K–L grade 2–3 | Pain (n = 9 WOMAC; n = 3 VAS; n = 1 numeric rating scale; n = 8 not reported) | 21 | 1334 | 1303 | 2637 |
| Gallagher et al.51 | Meta-analysis | 1500 (n = 2) | 36 | KOA | K–L grade 2–3 | OA progression, JSW | 2 | 207 | 207 | 414 |
| Gregori et al.46 | Network meta-analysis | 1500 (n = 2) | 36 | KOA | K–L grade 2–3 | Physical function | 1 | 207 | 207 | 414 |
| Honvo et al.50 | Meta-analysis | 1500 (n = 5) | 3 | OA | Not reported | Adverse events (total and specific) | 5 | 316 | 316 | 632 |
| Knapik et al.47 | Meta-analysis | 1500 (n = 2) | 36 | OA | Not reported | JSW | 2 | 207 | 207 | 414 |
| Lee et al.52 | Meta-analysis | 1500 (n = 2) | 36 | KOA | K–L grade 2–3 | JSW at 1 year and 3 years | 2 | 207 | 207 | 414 |
| Melo et al.48 | Systematic review | 1200 (n = 1) | 1.5 | TMJ OA | – | Pain (scale not reported) | 1 | 30 | 29 | 59 |
| Richy et al.41 | Meta-analysis | 1500 (n = 3) | 3 | OA | K–L grade 2–3 | JS narrowing, Lequesne Index, VAS pain, mobility, being a responder | 7 | 511 | 509 | 1020 |
| Simental-Mendìa et al.49 | Meta-analysis | 1500 (n = 5) | 3 | KOA | K–L grade 2–3 | Pain (VAS), WOMAC (total score), WOMAC physical function, WOMAC stiffness, WOMAC pain | 5 | 267 | 271 | 538 |
| Sodha et al.44 | Systematic review | 1500 (n = 1) | 6 | Spine OA | – | Pain (n = 1 numeric rating scale) | 1 | 125 | 125 | 250 |
GS, glucose sulphate; JS, joint space; JSW, joint space width; K–L, Kellgren and Lawrence; KOA, knee osteoarthritis; OA, osteoarthritis; RCT, randomised controlled trial; TMJ OA, temporomandibular joint osteoarthritis; VAS, visual analogue scale; WOMAC, Western Ontario and McMaster Universities Arthritis Index.
Descriptive findings of the articles included
Table 1 summarises key descriptive findings regarding the 11 articles included. Overall, 37 independent RCTs for a total of 3949 participants (1987 randomised to GS and 1962 to placebo), mainly affected by knee OA with a Kellgren and Lawrence grade of 2 (definite osteophytes and possible narrowing of joint space) or 3 (moderate multiple osteophytes, definite narrowing of joint space, some sclerosis and possible deformity of bony ends) were included. For each article included, the mean number of RCTs included was 4 (range: 2–21) for a median of 414 participants (range: 18–2228). The median follow-up period was 3 months (range: 1.5–36). Almost all RCTs (30/37), used the dosage of 1500 mg daily.
Main findings of the meta-analyses
Among the 17 outcomes included in the meta-analyses, 9 were statistically significant (p < 0.05). Overall, high heterogeneity (I2 >50%) was present in 8/17 outcomes, small-study effect was present in only 1 outcome (i.e. the use of GS on pain in people affected by OA). The largest RCT in terms of participants was statistically significant in 11/17 outcomes, as reported in Supplemental Table 2. Finally, no outcome included in the analysis had a 95% PI excluding the null value.
Table 2 shows the findings of the statistically significant outcomes using the GRADE approach, ranked by the level of evidence. A high certainty of evidence, as assessed by GRADE, supported the use of GS (versus placebo) in improving the Lequesne Index (3 RCTs; 454 participants; SMD = 0.363; 95% CI: 0.202–0.524), the joint space width change (2 RCTs; 414 participants; SMD = 0.250; 95% CI: 0.120–0.380), the joint space width change after 3 years of follow up (2 RCTs; 414 participants; SMD = 0.432; 95% CI: 0.235–0.628), joint space narrowing (2 RCTs; 414 participants; SMD = 0.410; 95% CI: 0.210–0.600), and, finally, OA progression (2 RCTs; 414 participants; OR = 0.382; 95% CI: 0.216–0.677) (Table 2). A moderate certainty of evidence supported the use of GS in improving the Western Ontario and McMaster Universities Arthritis Index (WOMAC) (total score) (6 RCTs; 621 participants; MD = –3.903; 95% CI: –7.418 to –0.658), whilst a very low certainty of evidence supported the use of GS in ameliorating pain (21 RCTs; 1772 participants; SMD = –0.646; 95% CI: –0.910 to –0.382) (including when the visual analogue scale was used; 5 RCTs; 538 participants; MD = –9.507; 95% CI: –17.128 to –1.797) and mobility (2 RCTs; 100 participants; SMD = 0.501; 95% CI: 0.09–0.912).
Table 2.
Summary of the findings according to the GRADE tool for randomized controlled trials.
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Risk with placebo | Risk with GS | ||||
| Lequesne Index | − | SMD 0.363 SD higher (0.202 higher to 0.524 higher) | − | 454 (3 RCTs) | ⨁⨁⨁⨁ HIGH |
| JSW (at 3 years) | − | SMD 0.432 SD higher (0.235 higher to 0.628 higher) | − | 414 (2 RCTs) | ⨁⨁⨁⨁ HIGH |
| JS narrowing | − | SMD 0.41 SD higher (0.21 higher to 0.6 higher) | − | 414 (2 RCTs) | ⨁⨁⨁⨁ HIGH |
| OA progression | − | − | OR 0.382 (0.216–0.677) | 414 (2 RCTs) | ⨁⨁⨁⨁ HIGH |
| JSW | − | SMD 0.25 SD higher (0.12 higher to 0.38 higher) | − | 414 (2 RCTs) | ⨁⨁⨁⨁ HIGH |
| WOMAC (total score) | − | MD 3.903 lower (7.418 lower to 0.658 lower) | − | 621 (6 RCTs) | ⨁⨁⨁◯ MODERATEb |
| Pain VAS | − | MD 9.507 lower (17.128 lower to 1.797 lower) | − | 538 (5 RCTs) | ⨁◯◯◯ VERY LOWa,b |
| Pain | − | SMD 0.646 SD lower (0.91 lower to 0.382 lower) | − | 1772 (21 RCTs) | ⨁◯◯◯ VERY LOWa,d,e |
| Mobility | − | SMD 0.501 SD higher (0.091 higher to 0.912 higher) | − | 100 (2 RCTs) | ⨁◯◯◯ VERY LOWa,c |
>30% of the RCTs included reporting high risk of bias.
I2 between 50% and 75%.
Sample size, in each arm, fewer than 100 participants.
I2 >75%.
Egger’s test (p value) < 0.05.
CI, confidence interval; GRADE, Grading of Recommendations, Assessment, Development and Evaluation; GS, glucose sulphate; JS, joint space; JSW, joint space width; MD, mean difference; OA, osteoarthritis; OR, odds ratio; RCT, randomised controlled trial; SD, standard deviation; SMD, standardised mean difference; VAS, visual analogue scale; WOMAC, Western Ontario and McMaster Universities Arthritis Index.
A sensitivity analysis was carried out for the outcome pain. A total of 10 RCTs at high risk of bias were removed, the re-calculated SMD was –0.298 (11 RCTs; n = 1493; 95% CI: –0.546; –0.05); however, no differences in terms of heterogeneity (I2 = 87%) or prediction intervals (–0.84 to 0.28) was observed.
Of importance, as shown in Supplemental Table 2, no significant differences in terms of adverse events between GS and placebo were observed (5 RCTs; 632 participants; OR = 1.236; 95% CI: 0.623–2.454; p = 0.54).
Findings from the narrative systematic reviews
As shown in Table 3, of the four outcomes included in the systematic reviews without meta-analysis, GS was associated with a better glucose profile43 and a better physical function performance compared with placebo.46 On the contrary, when people suffering from spine or temporomandibular joint OA were included no significant effect of GS on physical function or pain was observed.44,48
Table 3.
Main findings of the systematic reviews, without meta-analysis.
| Author | Type of review | Population | Outcome | Number of RCTs | GS | Placebo | Total sample size | Main findings |
|---|---|---|---|---|---|---|---|---|
| Dostrovsky et al.43 | Systematic review | General population | Glucose parameters | 2 | 16 | 18 | 34 | Better glucose parameters in both RCTs in GS compared with placebo |
| Gregori et al.46 | Network meta-analysis | KOA | Physical function | 1 | 106 | 106 | 212 | Slight improvement in intervention group compared with placebo |
| Melo et al.48 | Systematic review | TMJ OA | Pain | 1 | 30 | 29 | 59 | No significant difference between placebo and GS |
| Sodha et al.44 | Systematic review | Spine OA | Pain | 1 | 125 | 125 | 250 | No significant difference between placebo and GS for pain and physical function |
GS, glucosamine sulphate; KOA, knee osteoarthritis; OA, osteoarthritis; RCT, randomised controlled trial; TMJ OA, temporomandibular joint osteoarthritis.
Quality assessment
The assessment of the risk of bias in the meta-analyses included is fully reported in Supplemental Table 3. Four systematic reviews/meta-analyses were adjudicated as having high (i.e. one noncritical weakness) confidence of the results found, whilst, for the others, one was low (i.e. one critical flaw with or without noncritical weaknesses) and six were critically low (i.e. having more than one critical flaw with or without noncritical weaknesses).
Discussion
In this umbrella review, including 11 systematic reviews comprising 37 RCTs, the current research regarding GS and health outcomes in humans is reported. Overall, the findings suggest that GS is a safe product and, when used as prescription drug at 1500 mg/daily dosage, is able to positively modify the cartilage structure, reduce pain and improve function in people with knee OA, without having a greater incidence of adverse effects than placebo. The efficacy of GS was supported by different degrees of certainty of evidence, according to the GRADE evaluation, similar to that made in the 2019 European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) updated algorithm, which supports the use of prescription GS as background therapy for knee OA.53 Altogether, our findings suggest that GS might provide clinical benefits at 1500 mg/daily; we should differentiate the formulation of GS that is essential for maximising the clinical benefit, patient adherence and satisfaction with treatment.18
GS is widely used, particularly in older people, for the treatment of knee OA and its global use is increasing overall.54,55 The present umbrella review supports the assumption that, when compared with placebo, GS is able to delay the joint space narrowing, finally resulting in a minor OA progression. This analysis showed that in two RCTs,16,17 those randomised to GS had a 62% reduction in OA progression compared with those randomised to placebo. This evidence is supported by a high certainty of evidence, meaning that the role of potential biases is limited. These effects may be explained through several mechanisms. First, GS is able to reduce inflammatory parameters, in particular IL-1.56 In this regard, GS, if it reaches appropriate doses in blood and cartilage cells, can positively interfere with the IL-1 intracellular signalling pathway and gene expression.56 However, the dose-dependent effect of GS on IL-1β-induced gene expression of stromelysin-1 (MMP-3) and aggrecanase-2 (ADAMTS5) in human chondrocytes is optimised at clinically relevant concentrations (~10 μM) that can be obtained only at pharmacological doses of GS.57 Through this mechanism, GS is able to reduce the degradation of cartilage, therefore improving the cartilage structure of the knee.
However, GS is able, according to this umbrella review, to improve clinical outcomes commonly affected in knee OA. In particular, it was found that GS is able to improve the Lequesne Index,58 a tool that evaluates several aspects compromised in OA, including activities of daily living, pain and physical function. In this sense, this umbrella review indicates that GS is able to reduce pain and disability, however, this evidence is supported by a lower certainty of evidence according to GRADE. Traditionally, Cohen defined an effect size of 0.20 as small, 0.50 as moderate and 0.80 or greater as large.59 Given this, the effect of GS, compared with placebo, is ranked between small and moderate. However, as already discussed in other relevant papers,7,11 these effects are almost doubled those of paracetamol,60 a common medication used for knee OA pain-relief treatment. Moreover, other medications commonly used for the treatment of knee OA have a similar effect as observed for GS, as indicated in the recent network meta-analysis of Gregori et al. in which GS and celecoxib were the only long-term OA treatments associated with pain reduction [effect size (ES) = 0.29 and ES = 0.18, respectively].46
Another important aspect of this umbrella review is the potential association between GS and favourable glucose profile. Glucosamine, in fact, is an amino sugar, therefore, one might claim that this medication can lead to hyperglycaemia, insulin resistance and finally to diabetes by overactivating the hexosamine pathway.61 In this sense, however, a large RCT comprising 407 overweight and obese women, followed up for 2.5 years, reported that there was no significant effect of a 2.5-year GS intervention on mean glycosylated haemoglobin level.62 Present data, although limited by the narrative nature of the review, confirmed that GS is safe from a metabolic point of view, being in agreement with a large longitudinal study using the UK Biobank showing that the use of GS in OA is associated with a lower incidence of diabetes, over 8 years of follow up.63 In the same database, it is reported that GS can lead to a reduction in cardiovascular disease64 and all-cause and specific-cause mortality.65 Further studies are encouraged in order to confirm these promising findings, since in the UK Biobank the data are reported for different glucosamine preparations and not specifically for GS. Regarding the mechanisms of action that can justify these epidemiological findings, we can argue that GS may interfere with some pro-inflammatory pathways such as nuclear factor kappa-B, mitogen-activated protein kinase and phosphatidyl-inositol-3-kinase-dependent pathways66,67 that are usually involved in the pathogenesis of diabetes.68
Finally, our umbrella review is, in our opinion, important since GS use was associated with a similar incidence in adverse effects, compared with placebo, suggesting that its use is safe. One pivotal meta-analysis regarding this topic and included in this umbrella review, in fact, reports that the use of pCGS is not associated with a higher incidence of total and specific gastrointestinal, skin and subcutaneous tissue, renal and urinary adverse events when compared with placebo.50 The topic of safety for medications is clinically relevant, particularly in older people, in which knee OA is widely diffused. It is known that the median age of knee OA detection is 55 years and typically people with this condition live about 30 years with the disease.69 Therefore, to have medications with a good safety profile is of importance, but still debated in geriatric medicine since older people often use a high number of medications (polypharmacy) that may have unwanted interactions.
Findings from the present review should be interpreted in light of its limitations. First, the use of already established tools for quality assessment of evidence, which indirectly rely on the data reported in the selected articles, can cumulatively bring some biases. In order to overcome these potential biases we used low heterogeneity in the GRADE assessment as one of the criteria for high certainty of level. However, I2 estimates can also carry substantial uncertainty in terms of clinical parameters. Second, meta-analyses might have important limitations70 and their results depend on the choice of estimate from each primary study and its representation in the meta-analysis. In this regard, an umbrella review is totally dependent on the quality of the systematic reviews/meta-analyses performed and mainly the ‘exhaustive’ character of these systematic reviews/meta-analyses; for example, if the search strategies were not exhaustive, some important studies may have been missed. Moreover, it is also possible that some recent RCTs have not been included in this work. Third, the meta-analyses included in this umbrella review reported data on a median of four RCTs, independently from the follow-up duration. This work should encourage further intervention research on GS, particularly in forms of OA other than knee OA. At the same time, this work has some important strengths, including the fact that, differently from previously published literature, only GS (and not other forms of glucosamine) was included and the safety profile of GS in humans is confirmed. Finally, we were not able to analyse the economic aspects of GS, since no systematic review has been published, which is indeed of great clinical importance.9,71,72
In conclusion, the present umbrella review suggests that prescription GS, when used at 1500 mg/daily dosage, can positively affect the cartilage structure and improve the pain and function in people with knee OA, without having a greater incidence of adverse effects than placebo, indicating a possible role in older people. Moreover, some promising results indicated that GS is associated with a better glucose profile than placebo. Overall, these findings encourage further research regarding GS and other forms of OA not affecting the knee.
Supplemental Material
Supplemental material, sj-pdf-1-tab-10.1177_1759720X20975927 for Glucosamine sulphate: an umbrella review of health outcomes by Nicola Veronese, Jacopo Demurtas, Lee Smith, Jean-Yves Reginster, Olivier Bruyère, Charlotte Beaudart, Germain Honvo and Stefania Maggi in Therapeutic Advances in Musculoskeletal Disease
Footnotes
Author contributions: NV: conceptualization; data curation; formal analysis; funding acquisition; methodology; writing original draft. JD: data curation; writing original draft. LS: data curation. J-YR: writing – review and editing. OB: writing – review and editing. CB: formal analysis; methodology. GH: formal analysis; methodology. SM: writing – review and editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by an unrestricted grant from Mylan Company.
Conflict of interest statement: J-YR reports grants and personal fees from IBSA-Genevrier, Mylan, Radius Health and CNIEL, personal fees from Laboratoires Pierre Fabre, Faes Pharma, Rejuvenate Biomed, Samumed, Teva, Theramex, Pfizer, Mithra Pharmaceuticals, Dairy Research Council, Nutricia, Danone and AgNovos, and grants from TRB. GH reports lecture fees and travel support from IBSA. SM reports grants from Sanofi Pasteur, MSD, GlaxoSmithKline, Pfizer and Takeda as organiser of meetings/congresses and as principal investigator of epidemiological studies, for taking part on advisory boards and in expert meetings. OB reports grants from Biophytis, IBSA, Meda, Servier Laboratories and SMB, and personal fees from Amgen, Aptissen, Biophytis, IBSA, Meda, Novartis, Sanofi, Servier Laboratories, SMB and UCB. NV reports personal fees from Mylan, IBSA and Fidia.
ORCID iD: Nicola Veronese  https://orcid.org/0000-0002-9328-289X
https://orcid.org/0000-0002-9328-289X
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Nicola Veronese, Geriatric Unit, Department of Internal Medicine and Geriatrics, University of Palermo, Viale Scaduto, Palermo, 90100, Italy.
Jacopo Demurtas, Clinical and Experimental Medicine PhD Program, University of Modena and Reggio Emilia, Modena, Italy; Primary Care Department, USL Toscana Sud Est-Grosseto, Grosseto, Italy.
Lee Smith, The Cambridge Centre for Sport and Exercise Sciences, Anglia Ruskin University, Cambridge, UK.
Jean-Yves Reginster, Division of Public Health, Epidemiology and Health Economics, University of Liège, Liège, Belgium; WHO Collaborating Centre for Public Heath Aspects of Musculoskeletal Health and Aging, Liège, Belgium; Biochemistry Department, College of Science, King Saud University, Riyadh, Kingdom of Saudi Arabia.
Olivier Bruyère, Division of Public Health, Epidemiology and Health Economics, University of Liège, Liège, Belgium; WHO Collaborating Centre for Public Heath Aspects of Musculoskeletal Health and Aging, Liège, Belgium.
Charlotte Beaudart, Division of Public Health, Epidemiology and Health Economics, University of Liège, Liège, Belgium; WHO Collaborating Centre for Public Heath Aspects of Musculoskeletal Health and Aging, Liège, Belgium.
Germain Honvo, Division of Public Health, Epidemiology and Health Economics, University of Liège, Liège, Belgium; WHO Collaborating Centre for Public Heath Aspects of Musculoskeletal Health and Aging, Liège, Belgium.
Stefania Maggi, National Research Council, Neuroscience Institute, Padua, Italy.
References
- 1. Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ 2003; 81: 646–656. [PMC free article] [PubMed] [Google Scholar]
- 2. Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the global burden of disease study 2010. Lancet 2012; 380: 2197–2223. [DOI] [PubMed] [Google Scholar]
- 3. Corti MC, Rigon C. Epidemiology of osteoarthritis: prevalence, risk factors and functional impact. Aging Clin Exp Res 2003; 15: 359–363. [DOI] [PubMed] [Google Scholar]
- 4. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet 2012; 380: 2163–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bruyère O, Altman RD, Reginster J-Y. Efficacy and safety of glucosamine sulfate in the management of osteoarthritis: evidence from real-life setting trials and surveys. Semin Arthritis Rheum 2016; 45: S12–S17. [DOI] [PubMed] [Google Scholar]
- 6. Reginster J-Y, Veronese N. Highly purified chondroitin sulfate: a literature review on clinical efficacy and pharmacoeconomic aspects in osteoarthritis treatment. Aging Clin Exp Res. Epub ahead of print 7 July 2020. DOI: 10.1007/s40520-020-01643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bruyère O, Cooper C, Pelletier J-P, et al. An algorithm recommendation for the management of knee osteoarthritis in Europe and internationally: a report from a task force of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Semin Arthritis Rheum 2014; 44: 253–263. [DOI] [PubMed] [Google Scholar]
- 8. Bruyère O, Cooper C, Pelletier J-P, et al. A consensus statement on the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) algorithm for the management of knee osteoarthritis – from evidence-based medicine to the real-life setting. Semin Arthritis Rheum 2016; 45: S3–S11. [DOI] [PubMed] [Google Scholar]
- 9. Bruyère O, Honvo G, Veronese N, et al. An updated algorithm recommendation for the management of knee osteoarthritis from the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Semin Arthritis Rheum 2019; 49: 337–350. [DOI] [PubMed] [Google Scholar]
- 10. De Wan M, Volpi G. Method of preparing mixed glucosamine salts. Google Patents US5847107A, USA, 1998. [Google Scholar]
- 11. Bruyère O, Cooper C, Al-Daghri NM, et al. Inappropriate claims from non-equivalent medications in osteoarthritis: a position paper endorsed by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Aging Clin Exp Res 2018; 30: 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Russell AS, Aghazadeh-Habashi A, Jamali F. Active ingredient consistency of commercially available glucosamine sulfate products. J Rheumatol 2002; 29: 2407–2409. [PubMed] [Google Scholar]
- 13. Persiani S, Roda E, Rovati L, et al. Glucosamine oral bioavailability and plasma pharmacokinetics after increasing doses of crystalline glucosamine sulfate in man. Osteoarthritis Cartilage 2005; 13: 1041–1049. [DOI] [PubMed] [Google Scholar]
- 14. Persiani S, Rotini R, Trisolino G, et al. Synovial and plasma glucosamine concentrations in osteoarthritic patients following oral crystalline glucosamine sulphate at therapeutic dose. Osteoarthritis Cartilage 2007; 15: 764–772. [DOI] [PubMed] [Google Scholar]
- 15. Chiusaroli R, Piepoli T, Zanelli T, et al. Experimental pharmacology of glucosamine sulfate. Int J Rheumatol 2011; 2011: 939265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reginster JY, Deroisy R, Rovati LC, et al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet 2001; 357: 251–256. [DOI] [PubMed] [Google Scholar]
- 17. Pavelká K, Gatterová J, Olejarová M, et al. Glucosamine sulfate use and delay of progression of knee osteoarthritis: a 3-year, randomized, placebo-controlled, double-blind study. Arch Intern Med 2002; 162: 2113–2123. [DOI] [PubMed] [Google Scholar]
- 18. Herrero-Beaumont G, Ivorra JA, Del Carmen Trabado M, et al. Glucosamine sulfate in the treatment of knee osteoarthritis symptoms: a randomized, double-blind, placebo-controlled study using acetaminophen as a side comparator. Arthritis Rheum 2007; 56: 555–567. [DOI] [PubMed] [Google Scholar]
- 19. Reginster JY. The efficacy of glucosamine sulfate in osteoarthritis: financial and nonfinancial conflict of interest. Arthritis Rheum 2007; 56: 2105–2110. [DOI] [PubMed] [Google Scholar]
- 20. Towheed TE, Maxwell L, Anastassiades TP, et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev 2009; CD002946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eriksen P, Bartels EM, Altman RD, et al. Risk of bias and brand explain the observed inconsistency in trials on glucosamine for symptomatic relief of osteoarthritis: a meta-analysis of placebo-controlled trials. Arthritis Care Res (Hoboken) 2014; 66: 1844–1855. [DOI] [PubMed] [Google Scholar]
- 22. Kucharz EJ, Kovalenko V, Szanto S, et al. A review of glucosamine for knee osteoarthritis: why patented crystalline glucosamine sulfate should be differentiated from other glucosamines to maximize clinical outcomes. Curr Med Res Opin 2016; 32: 997–1004. [DOI] [PubMed] [Google Scholar]
- 23. Bruyère O, Reginster J-Y. Glucosamine and chondroitin sulfate as therapeutic agents for knee and hip osteoarthritis. Drugs Aging 2007; 24: 573–580. [DOI] [PubMed] [Google Scholar]
- 24. Rozendaal RM, Uitterlinden EJ, van Osch GJ, et al. Effect of glucosamine sulphate on joint space narrowing, pain and function in patients with hip osteoarthritis; subgroup analyses of a randomized controlled trial. Osteoarthritis Cartilage 2009; 17: 427–432. [DOI] [PubMed] [Google Scholar]
- 25. Towheed T. Glucosamine sulphate in osteoarthritis: a systematic review. Arthritis Rheum 1998; 41: S198. [Google Scholar]
- 26. Towheed TE, Maxwell L, Anastassiades TP, et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev 2005; CD002946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tenti S, Giordano N, Mondanelli N, et al. A retrospective observational study of glucosamine sulfate in addition to conventional therapy in hand osteoarthritis patients compared to conventional treatment alone. Aging Clin Exp Res 2020; 32: 1161–1172. [DOI] [PubMed] [Google Scholar]
- 28. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aromataris E, Fernandez R, Godfrey CM, et al. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc 2015; 13: 132–140. [DOI] [PubMed] [Google Scholar]
- 30. Ioannidis JP. Integration of evidence from multiple meta-analyses: a primer on umbrella reviews, treatment networks and multiple treatments meta-analyses. CMAJ 2009; 181: 488–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Higgins JP, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions. Chichester: John Wiley & Sons, 2019. [Google Scholar]
- 32. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017; 358: j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Higgins JP, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc 2009; 172: 137–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. IntHout J, Ioannidis JPA, Rovers MM, et al. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open 2016; 6: e010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Serghiou S, Goodman SN. Random-effects meta-analysis: summarizing evidence with caveats. JAMA 2019; 321: 301–302. [DOI] [PubMed] [Google Scholar]
- 36. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 37. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Carvalho AF, Kohler CA, Brunoni AR, et al. Bias in peripheral depression biomarkers. Psychother Psychosom 2016; 85: 81–90. [DOI] [PubMed] [Google Scholar]
- 39. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336: 924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hsu C-H, Hsu N-C, Shih C-L, et al. Medication-taking habit and outcome of glucosamine sulfate for osteoarthritis patients influenced by national health insurance regulations in Taiwan. J Clin Med 2019; 8: 1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Richy F, Bruyère O, Ethgen O, et al. Structural and symptomatic efficacy of glucosamine and chondroitin in knee osteoarthritis: a comprehensive meta-analysis. Arch Intern Med 2003; 163: 1514–1522. [DOI] [PubMed] [Google Scholar]
- 42. Lee YH, Woo JH, Choi SJ, et al. Effect of glucosamine or chondroitin sulfate on the osteoarthritis progression: a meta-analysis. Rheumatol Int 2010; 30: 357–363. [DOI] [PubMed] [Google Scholar]
- 43. Dostrovsky NR, Towheed TE, Hudson RW, et al. The effect of glucosamine on glucose metabolism in humans: a systematic review of the literature. Osteoarthritis Cartilage 2011; 19: 375–380. [DOI] [PubMed] [Google Scholar]
- 44. Sodha R, Sivanadarajah N, Alam M. The use of glucosamine for chronic low back pain: a systematic review of randomised control trials. BMJ Open 2013; 3: e001167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gallagher B, Tjoumakaris FP, Harwood MI, et al. Chondroprotection and the prevention of osteoarthritis progression of the knee: a systematic review of treatment agents. Am J Sports Med 2015; 43: 734–744. [DOI] [PubMed] [Google Scholar]
- 46. Gregori D, Giacovelli G, Minto C, et al. Association of pharmacological treatments with long-term pain control in patients with knee osteoarthritis: a systematic review and meta-analysis. JAMA 2018; 320: 2564–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Knapik JJ, Pope R, Hoedebecke SS, et al. Effects of oral glucosamine sulfate on osteoarthritis-related pain and joint-space changes: systematic review and meta-analysis. J Spec Oper Med 2018; 18: 139–147. [DOI] [PubMed] [Google Scholar]
- 48. Melo G, Casett E, Stuginski-Barbosa J, et al. Effects of glucosamine supplements on painful temporomandibular joint osteoarthritis: a systematic review. J Oral Rehabil 2018; 45: 414–422. [DOI] [PubMed] [Google Scholar]
- 49. Simental-Mendia M, Sanchez-Garcia A, Vilchez-Cavazos F, et al. Effect of glucosamine and chondroitin sulfate in symptomatic knee osteoarthritis: a systematic review and meta-analysis of randomized placebo-controlled trials. Rheumatol Int 2018; 38: 1413–1428. [DOI] [PubMed] [Google Scholar]
- 50. Honvo G, Reginster JY, Rabenda V, et al. Safety of symptomatic slow-acting drugs for osteoarthritis: outcomes of a systematic review and meta-analysis. Drugs Aging 2019; 36: 65–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gallagher B, Tjoumakaris FP, Harwood MI, et al. Chondroprotection and the prevention of osteoarthritis progression of the knee: a systematic review of treatment agents. Am J Sports Med. 2015; 43: 734–744. [DOI] [PubMed] [Google Scholar]
- 52. Lee YH, Woo JH, Choi SJ, Ji JD, et al. Effect of glucosamine or chondroitin sulfate on the osteoarthritis progression: a meta-analysis. Rheumatol Int. 2010; 30:357–363. [DOI] [PubMed] [Google Scholar]
- 53. Bruyère O, Reginster J-Y, Honvo G, et al. Cost-effectiveness evaluation of glucosamine for osteoarthritis based on simulation of individual patient data obtained from aggregated data in published studies. Aging Clin Exp Res 2019; 31: 881–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hopman W, Towheed T, Gao Y, et al. Prevalence of and factors associated with glucosamine use in Canada. Osteoarthritis Cartilage 2006; 14: 1288–1293. [DOI] [PubMed] [Google Scholar]
- 55. Galvin R, Cousins G, Boland F, et al. Prescribing patterns of glucosamine in an older population: a national cohort study. BMC Complement Altern Med 2013; 13: 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dechant JE, Baxter GM, Frisbie DD, et al. Effects of glucosamine hydrochloride and chondroitin sulphate, alone and in combination, on normal and interleukin-1 conditioned equine articular cartilage explant metabolism. Equine Vet J 2005; 37: 227–231. [DOI] [PubMed] [Google Scholar]
- 57. Chiusaroli R, Visentini M, Galimberti C, et al. Targeting of ADAMTS5’s ancillary domain with the recombinant mAb CRB0017 ameliorates disease progression in a spontaneous murine model of osteoarthritis. Osteoarthritis Cartilage 2013; 21: 1807–1810. [DOI] [PubMed] [Google Scholar]
- 58. Lequesne MG, Mery C, Samson M, et al. Indexes of severity for osteoarthritis of the hip and knee. Validation–value in comparison with other assessment tests. Scand J Rheumatol Suppl 1987; 65: 85–89. [DOI] [PubMed] [Google Scholar]
- 59. Cohen J. Statistical power analysis for the behavioral sciences. New York, NY: Academic Press, 2013. [Google Scholar]
- 60. Towheed TE, Maxwell L, Judd MG, et al. Acetaminophen for osteoarthritis. Cochrane Database Syst Rev 2006; CD004257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Veronese N, Cooper C, Reginster J-Y, et al. Type 2 diabetes mellitus and osteoarthritis. Semin Arthritis Rheum 2019; 49: 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gommans YMM, Runhaar J, Jacobs ML, et al. The effect of prolonged glucosamine usage on HbA1c levels and new-onset diabetes mellitus in overweight and obese middle-aged women. Am J Med 2017; 130: 731–737.e736. [DOI] [PubMed] [Google Scholar]
- 63. Ma H, Li X, Zhou T, et al. Glucosamine use, inflammation, and genetic susceptibility, and incidence of type 2 diabetes: a prospective study in UK Biobank. Diabetes Care 2020; 43: 719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ma H, Li X, Sun D, et al. Association of habitual glucosamine use with risk of cardiovascular disease: prospective study in UK Biobank. BMJ 2019; 365: l1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Li Z-H, Gao X, Chung VC, et al. Associations of regular glucosamine use with all-cause and cause-specific mortality: a large prospective cohort study. Ann Rheum Dis 2020; 79: 829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Largo R, Alvarez-Soria MA, Díez-Ortego I, et al. Glucosamine inhibits IL-1β-induced NFκB activation in human osteoarthritic chondrocytes. Osteoarthritis Cartilage 2003; 11: 290–298. [DOI] [PubMed] [Google Scholar]
- 67. Herrero-Beaumont G, Largo R. Glucosamine and O-GlcNAcylation: a novel immunometabolic therapeutic target for OA and chronic, low-grade systemic inflammation? London: BMJ Publishing Group Ltd, 2020. [DOI] [PubMed] [Google Scholar]
- 68. Tsalamandris S, Antonopoulos AS, Oikonomou E, et al. The role of inflammation in diabetes: current concepts and future perspectives. Eur Cardiol 2019; 14: 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Charlesworth J, Fitzpatrick J, Perera NKP, et al. Osteoarthritis – a systematic review of long-term safety implications for osteoarthritis of the knee. BMC Musculoskelet Disord 2019; 20: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ioannidis JP. The mass production of redundant, misleading, and conflicted systematic reviews and meta-analyses. Milbank Q 2016; 94: 485–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bruyère O, Pavelka K, Rovati LC, et al. Total joint replacement after glucosamine sulphate treatment in knee osteoarthritis: results of a mean 8-year observation of patients from two previous 3-year, randomised, placebo-controlled trials. Osteoarthritis Cartilage 2008; 16: 254–260. [DOI] [PubMed] [Google Scholar]
- 72. Scholtissen S, Bruyère O, Neuprez A, et al. Glucosamine sulphate in the treatment of knee osteoarthritis: cost-effectiveness comparison with paracetamol. Int J Clin Pract 2010; 64: 756–762. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tab-10.1177_1759720X20975927 for Glucosamine sulphate: an umbrella review of health outcomes by Nicola Veronese, Jacopo Demurtas, Lee Smith, Jean-Yves Reginster, Olivier Bruyère, Charlotte Beaudart, Germain Honvo and Stefania Maggi in Therapeutic Advances in Musculoskeletal Disease