Abstract
Aim:
Owing to the limited ability of current imaging modalities, several clinical T1 renal cell carcinomas (cT1 RCCa) can be pathologically upstaged to T3a (pT3a) after surgery. There have been some controversies regarding the oncological safety of partial nephrectomy (PNx) compared with radical nephrectomy (RNx) in these patients. We compared oncological outcomes of PNx and RNx in patients with upstaged pT3a RCCa.
Methods:
A systematic review was performed following the PRISMA guideline. PubMed, MEDLINE, Embase were searched. Oncological outcomes [recurrence-free survival (RFS), overall survival (OS) and cancer-specific survival (CSS)] between PNx and RNx were compared. The GRADE approach was used to rate the certainty of evidence.
Results:
A total of 7406 patients in 12 articles related to upstaged pT3a RCCa were included. In adjusted analysis, no difference was observed in RFS [hazard ratios (HR) 0.87; 95% confidence intervals (CI), 0.57–0.95; p = 0.88] and CSS (HR, 0.78; 95% CI, 0.59–1.04; p = 0.09) for PNx and RNx. Meanwhile, PNx was significantly associated with favorable OS compared with RNx (HR, 0.74; 95% CI, 0.57–0.95; p = 0.02).
Conclusions:
Our meta-analysis shows that patients treated with PNx have better or at least similar oncological outcomes compared with RNx in patients with upstaged pT3a RCCa from cT1. In particular, patients who had undergone PNx show a significantly improved OS. If PNx is available, we recommend performing PNx for all cT1 RCCa, even in patients with upstaging potential. However, due to the low level of evidence, large-scale randomized trials are required.
Keywords: partial nephrectomy, radical nephrectomy, renal cell carcinoma, T3a, upstaging
Introduction
Renal cell carcinoma (RCCa) is the most frequently diagnosed malignancy among renal tumors worldwide,1 and its incidence continues to steadily increase in most countries.2 With the increased use of modern imaging tools, including computed tomography and ultrasonography, the number of accidentally detected renal tumors is increasing,3 and most RCCa cases are detected as small renal masses in asymptomatic patients. According to the American Joint Committee on Cancer criteria, clinical stage T1 (cT1) are ⩽7 cm-sized tumor confined to the kidney.4 Patients with cT1 RCCa generally have a favorable prognosis. Current clinical guidelines recommend nephron-sparing surgery for the treatment of cT1 RCCa if technically feasible. Its advantages include offering renal function preservation and similar oncologic outcomes when compared with traditional radical nephrectomy (RNx).5,6 However, after surgery for these small renal masses, several tumors can be pathologically upstaged to T3a (pT3a). Regrettably, current imaging modalities still have limited ability to detect adverse pathological features that are associated with pT3a, such as renal sinus invasion and perinephric fat involvement.7
Previous studies have reported that patients with upstaged pT3 RCCa from cT1 have a poorer prognosis than those without.8–10 However, previous reports on the oncological consequences of partial nephrectomy (PNx) and RNx in these patients are still conflicting.11–22 In particular, when tumors are small but cT3 cannot be excluded from preoperative imaging studies, it is very troublesome to select PNx, although it is technically feasible. If clinicians obtain more convincing evidence on the oncological safety and efficacy of PNx in patients who are upstaged from cT1 to pT3a RCCa, it would be helpful in deciding a surgical strategy for cT1 renal tumors. In this study, we compared the oncological outcomes between PNx and RNx in patients with upstaged pT3a RCCa through a systematic review and meta-analysis including all recently published studies. In addition, the quality of evidence was assessed using complementary statistical methods for assessing the certainty of generated evidence.
Materials and methods
Search strategy
This systematic review is registered in PROSPERO, CRD42020176537. Literature search of all publications between January 2000 and April 2020 was conducted using the Embase, MEDLINE, and PubMed databases. In addition, a cross-reference search of eligible articles was performed to detect studies that were not found in the computerized search. We used combinations of the following MeSH terms and keywords, such as “kidney cancer,” “kidney carcinoma,” “renal cell cancer,” “renal cell carcinoma,” “partial nephrectomy,” “pT3,” “T3a,” and relevant variants. Two authors (DYC and JWK) independently reviewed the titles and abstracts based on the inclusion criteria and reviewed the identified articles. In case of disagreement regarding the inclusion of an article, it was discussed with a third author (KSC).
Inclusion criteria and study eligibility
The eligibility of a study was evaluated based on the participants, intervention, comparator, outcome, and study design approach, in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. We defined the participants and intervention as patients who were upstaged from cT1 renal tumor to pT3a RCCa after PNx, respectively. The comparator was defined as patients who underwent RNx for RCCa with the same characteristics. The first endpoint was recurrence-free survival (RFS), the second endpoint was overall survival (OS), and the third endpoint was cancer-specific survival (CSS). RFS was defined as the point at which radiographic or pathological evidence of local recurrence and/or distant metastases was found after surgery. CSS and OS were defined as the time from the date of surgery to the date of cancer-specific mortality or death from any cause, respectively. There was no restriction on research design, and both randomized controlled and non-randomized observational studies were included. The exclusion criteria were as follows: (1) non-human study, (2) not written in English, (3) conference and meeting abstracts, and (4) unable to extract outcome data. Conference and meeting abstracts were excluded even if they fit the inclusion criteria for reducing publication bias.
Data extraction
Two authors (DYC and JWK) independently reviewed the included articles and extracted data at the trial level for each trial. Any discrepancy in extracted data was resolved through consensus. Extracted data included the details on study design, inclusion and exclusion criteria, whether participants were randomized or non-randomized, participant demographics and oncological characteristics, patient treatment characteristics (patients who were upstaged from cT1 renal tumor to pT3a RCCa after PNx or RNx), outcomes measured (RFS, OS, CSS), hazard ratios (HRs), 95% confidence intervals (CIs), and p-values.
Study quality assessments
After the final group of articles was agreed upon, the two authors (DYC and JWK) independently examined the quality. Quality evaluation of the non-randomized studies was performed according to the Newcastle–Ottawa Scale (NOS).23 The three major assessment categories of the NOS were selection, comparability, and exposure. A study can be given a rating of up to 9 stars, and a final score of 6 stars or more indicated high quality. In addition, we assessed the quality of the generated evidence using the Grading of Recommendations, Assessments, Developments, and Evaluation (GRADE) system.24 GRADE is used to systematically approach the evaluation and strength of the recommendations. It comprises domains for methodology evaluation, accuracy of results, consistency of results, immediacy, and risk of publication bias. Based on these five criteria, the quality of evidence was rated as belonging to one of four levels (high, moderate, low, and very low).
Statistical analysis
The effects of PNx compared with RNx were measured using HR values. Log HR values were obtained directly from the trials reporting HR point estimates and CIs, and the standard errors of log HR were calculated using the published CIs.25 Although some trials reported Kaplan–Meier estimates and log-rank p-values, they omitted HR, 95% CI, or both. In these cases, we estimated the HR and 95% CI using p-values, number of total events, and number of participants allocated to each arm.26 Estimates for the included studies were then combined using a random-effects model with inverse variance.27 Pooled HRs with 95% CIs indicated the effects of PNx or RNx on RFS, OS, and CSS. Chi-square heterogeneity tests were used to test for statistical heterogeneity between trials. The I2 statistic was calculated to measure the discrepancies between clinical trials. A Cochran Q statistic p-value < 0.05 or an I2 statistic > 50% was used to indicate the presence of statistically significant heterogeneity between clinical trials.28 Since <10 studies qualified for each analysis, funnel plots were not used to assess small study effects. Sensitivity analysis was performed by evaluating the stability of results by sequentially excluding each included study. We used the Review Manager v.5.3 (2008; Nordic Cochrane Center, Cochrane Collaboration, Copenhagen, Denmark) to perform the meta-analysis. All p-values were two-sided; and except for the test of discrepancy, a p-value < 0.05 was considered statistically significant.
Ethical approval
This meta-analysis is exempt from ethics approval, as we collected and synthesized data from previous clinical trials in which informed consent was already obtained by the trial investigators.
Results
Systematic review process and quality assessment
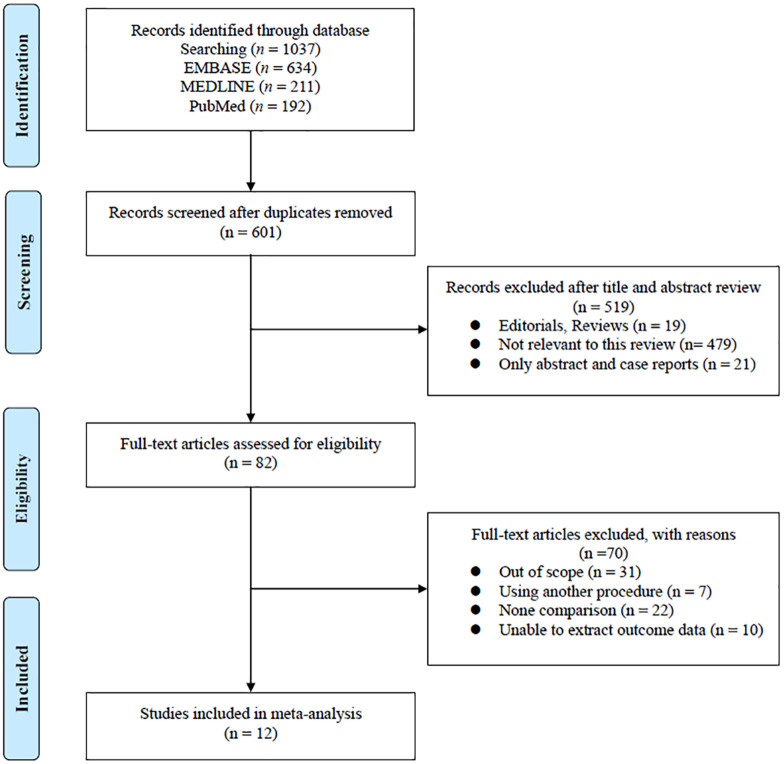
The results for the PRISMA flow diagram are presented in Figure 1. The initial database search found 1037 studies (634 in EMBASE, 211 in MEDLINE, and 192 in PubMed). Of these, 601 studies remained after duplicates were removed. After reviewing the titles and abstracts, 82 articles were excluded. Subsequently, the analysis of full-text articles was performed based on pre-established inclusion criteria. Finally, 12 studies11–22 with a total of 7406 patients were included. Information on the included studies is shown in Table 1. All studies were retrospective case-control studies. Among them, three studies used propensity score matching.11,15,18 There were five studies performed in the USA,11,15,19–21 three in Korea,12,14,17 two in China,13,18 and one each in Canada16 and Germany.22 All of the trials enrolled patients diagnosed with upstage pT3 RCCa from cT1 renal tumor, who had undergone either PNx or RNx. Meanwhile, patients upstaged from cT2 to pT3a were also included in several studies,17,20 but these patients were excluded from our study. Therefore, in this meta-analysis, we only analyzed the data of patients who were upstaged from cT1 to pT3a.
Figure 1.
Flowchart for systematic review process and data acquisition.
Table 1.
Characteristics of the eligible studies.
| Author(s) | Country | Study design | Study summary | Surgical method | No. of patients | Tumor size (cm, IQR) | Median follow-up (months, IQR) | Tumor characteristics between both groups (Tumor size, Fuhrman grade, histology, and positive surgical margin, etc) |
|---|---|---|---|---|---|---|---|---|
| Weight et al.21 | USA | Retrospective, multi-institutional | Comparisons of oncologic outcomes between partial nephrectomy and radical nephrectomy in upstaged pT2/pT3 or high-grade renal tumors. *This meta-analysis only analyzed upgraded pT3a data, excluding other data. |
RNx | 80 | 6 (5.0–6.6) | 50 (34–74) | There is a statistically different tumor size between both groups. |
| PNx | 66 | 3.5 (5.0–6.6) | 61 (39–83) | |||||
| Hansen et al.11 | USA | Retrospective, multi-institutional, propensity score matched study | Comparisons of oncologic outcomes between partial nephrectomy and radical nephrectomy in >7 cm renal tumor or pT3a or Fuhrman grade III–IV renal tumors. *This meta-analysis only analyzed upgraded pT3a data, excluding other data. |
RNx | 477 (after matching) | 3.9 (2.5–4.8) | NR | There is no statistically significant difference in tumor characteristics between both groups after propensity score matching. |
| PNx | 477 (after matching) | 3.9 (2.4–4.5) | NR | |||||
| Oh et al.17 | Korea | Retrospective, multi-institutional | Comparisons of oncologic outcomes between partial nephrectomy and radical nephrectomy in pT3a and additional analysis using only cT1 renal tumor. *This meta-analysis only analyzed upgraded pT3a from clinical T1a renal tumor, excluding other data. |
RNx | 33 | 3.37 (1.80–4.00) | 35.0 ± 33.2 | There is a statistically different tumor size between both groups. |
| PNx | 30 | 2.75 (0.80–4.00) | 30.5 ± 35.3 | |||||
| Jeong et al.12 | Korea | Retrospective, single-institutional | Comparisons of oncologic outcomes between partial nephrectomy and radical nephrectomy in upstaged pT3a from cT1 renal tumor. | RNx | 54 | NR | 50.8 ± 32.4 | NR |
| PNx | 37 | NR | ||||||
| Maurice et al.15 | USA | Retrospective, single-institutional, propensity score matched study | Comparisons of oncologic outcomes between partial nephrectomy and radical nephrectomy in high-grade renal cell carcinoma included pT3a from cT1 renal tumor. *This meta-analysis only analyzed upgraded pT3a data, excluding other data. |
RNx | 138 (after matching) | 3.0 (2.1–4.5) | 68 (47–82) | There is no statistically significant difference in tumor characteristics between both groups after propensity score matching. |
| PNx | 138 (after matching) | 3.0 (2.1–4.5) | 63 (41–83) | |||||
| Nayak et al.16 | Canada | Prospective, multi-institutional | Comparisons of oncologic outcomes between partial nephrectomy and radical nephrectomy in upstaged pT3a from cT1 renal tumor. | RNx | 68 | 4.7 (3.5–5.7) | 22.3 (6.4–33.2) | NR |
| PNx | 66 | |||||||
| Peng et al.18 | China | Retrospective, single-institutional, propensity score matched study | Comparisons of oncologic outcomes between partial nephrectomy and radical nephrectomy in upstaged pT3a from cT1 renal tumor. | RNx | 18 (after matching) | 5.03 ± 1.42 | 35.5 (10–86) | There is no statistically significant difference in tumor characteristics between both groups after propensity score matching. |
| PNx | 18 (after matching) | 5.27 ± 1.50 | ||||||
| Shah et al.19 | USA | Retrospective, single-institutional | Comparisons of oncologic outcomes between partial nephrectomy and radical nephrectomy in upstaged pT3a from cT1 renal tumor. | RNx | 91 | 5.0 ± 1.6 | 38 | There is a statistically different tumor size and histology type between both groups. |
| PNx | 49 | 3.2 ± 1.4 | ||||||
| Lee et al.14 | Korea | Retrospective, single-institutional | Comparisons of oncologic outcomes between partial nephrectomy and radical nephrectomy in upstaged pT3a from cT1 renal tumor. | RNx | 57 | 5.0 (3.7–6.2) | 39.0 (15.0–69.0). | NR |
| PNx | 158 | |||||||
| Srivastava et al.20 | USA | Retrospective, multi-institutional | Comparisons of oncologic outcomes between partial nephrectomy and radical nephrectomy in upstaged pT3a. *This meta-analysis only analyzed upgraded pT3a data from cT1 excluding other data. |
RNx | 4158 (total) (cT1a: 1432, cT1b: 2726) | 6.8 (4.9–9.0) | 37 (16–76) | NR |
| PNx | 1083 (total) (cT1a: 762, cT1b: 321) | 3.5 (2.5–5.0) | 36 (16–69) | |||||
| Ziegelmueller et al.22 | Germany | Retrospective, single-institutional | Comparisons of oncologic outcomes between partial nephrectomy and radical nephrectomy in upstaged pT3a from cT1 renal tumor. | RNx | 17 | 4 (0.8–6.9) | 80 | No statistical difference in tumor characteristics between the two groups was reported. |
| PNx | 38 | 3.9 (1.0–6.5) | ||||||
| Lai et al.13 | China | Retrospective, single-institutional | Comparisons of oncologic outcomes between partial nephrectomy and radical nephrectomy in upstaged pT3a from cT1 renal tumor. | RNx | 7 | 5 (3.5–6.5) | 44.5 (24–70) | NR |
| PNx | 48 |
NR, not reported; PNx, partial nephrectomy; RNx, radical nephrectomy.
The results of quality assessment using NOS for the included studies are shown in Table 2. All studies received a score of 6 points. Overall, the quality scores within the subscales represented relatively high quality. However, there was some selection bias in the control group cases.
Table 2.
Results of the quality assessment using the Newcastle–Ottawa Scale.
| Author(s) | Selection (4) | Comparability (2) | Exposure (3) | Total score | |||||
|---|---|---|---|---|---|---|---|---|---|
| Adequate definition of cases | Representativeness of cases | Selection of controls | Definition of controls | Control for important factor or additional factor | Ascertainment of exposure | Same method of ascertainment for cases and controls | Non-Response rate | ||
| Weight et al.21 | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 0 | 6 |
| Hansen et al.11 | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 0 | 6 |
| Oh et al.17 | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 0 | 6 |
| Jeong et al.12 | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 0 | 6 |
| Maurice et al.15 | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 0 | 6 |
| Nayak et al.16 | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 0 | 6 |
| Peng et al.18 | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 0 | 6 |
| Shah et al.19 | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 0 | 6 |
| Lee et al.14 | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 0 | 6 |
| Srivastava et al.20 | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 0 | 6 |
| Ziegelmueller et al.22 | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 0 | 6 |
| Lai et al.13 | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 0 | 6 |
Recurrence-free survival
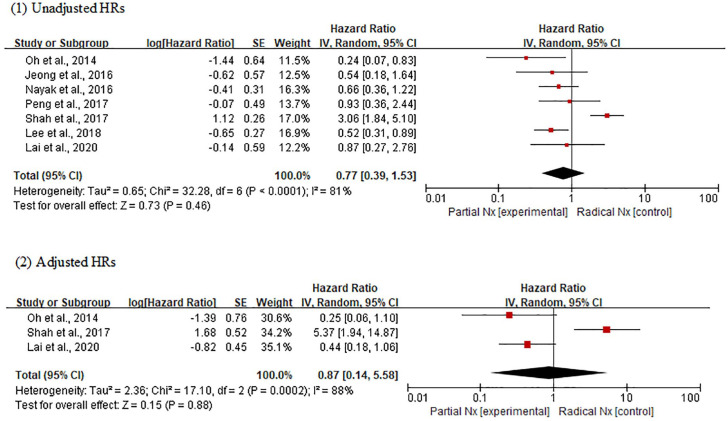
In the analysis using unadjusted HRs, meta-analysis revealed an overall HR of 0.77 for RFS in patients receiving PNx (95% CI, 0.39–1.53; p = 0.46) (Figure 2). Heterogeneity was found across studies (Cochran Q statistic, p < 0.0001; I2 statistic, 81%). In the analysis using adjusted HRs, an overall HR was 0.87 for RFS in patients receiving PNx (95% CI, 0.14–5.58; p = 0.88). Heterogeneity was found across studies (Cochran Q statistic, p = 0.0002; I2 statistic, 88%). In both analyses, there was no significant difference in RFS between PNx and RNx groups, but the certainty of each comparison was very low using the GRADE approach (Table 3).
Figure 2.
Forest plots of recurrence-free survival according to surgical methods.
Table 3.
Results of the GRADE quality assessment of direct evidence of each comparison.
| No. of studies | Study design | Risk of bios | Inconsistency | Indirectness | Imprecision | Other consideration | No. of patients | Effect | Overall quality of evidence | |
|---|---|---|---|---|---|---|---|---|---|---|
| Radical nephrectomy | Partial nephrectomy | |||||||||
| 1. Recurrence-free survival | ||||||||||
| 7 Unadjusted | observational studies | not serious | not serious | not serious | serious* | none | 328 | 406 | HR 0.77 (0.39–1.53) | •○○○ VERY LOW |
| 3 Adjusted | observational studies | not serious | not serious | not serious | serious** | none | 128 | 127 | HR 0.87 (0.14–5.58) | •○○○ VERY LOW |
| 2. Overall survival | ||||||||||
| 5 Unadjusted | observational studies | not serious | not serious | not serious | serious* | none | 4367 | 1416 | HR 0.61 (0.57–0.95) | •○○○ VERY LOW |
| 3 Adjusted | observational studies | not serious | not serious | not serious | not serious | none | 4293 | 1220 | HR 0.74 (0.57–0.95) | ••○○ LOW |
| 3. Cancer-specific survival | ||||||||||
| 6 Unadjusted | observational studies | not serious | not serious | not serious | serious* | none | 4787 | 1801 | HR 0.74 (0.59–0.93) | •○○○ VERY LOW |
| 3 Adjusted | observational studies | not serious | not serious | not serious | not serious | none | 4632 | 1559 | HR 0.78 (0.59–1.04) | ••○○LOW |
Unadjusted values are applied.
Total number of participants is small.
Overall survival
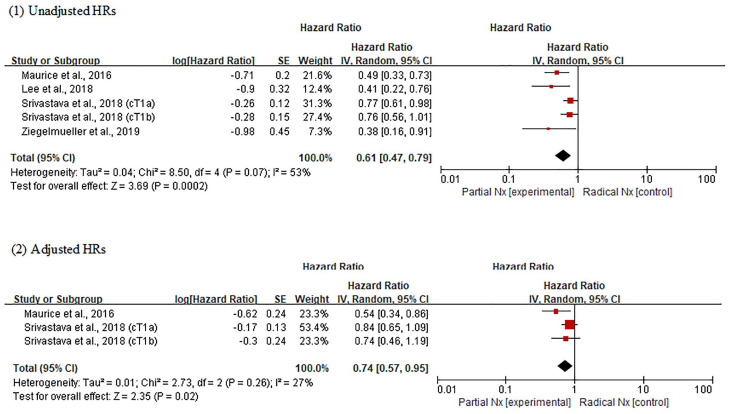
In the analysis using unadjusted HRs, the OS outcome in PNx group was superior to that in RNx group (overall HR, 0.61; 95% CI, 0.47–0.79; p = 0.0002) (Figure 3). Heterogeneity was found across studies (Cochran Q statistic, p = 0.07; I2 statistic, 53%). In the analysis using adjusted HRs, meta-analysis also revealed that PNx was significantly associated with favorable OS compared with RNx (overall HR, 0.74; 95% CI, 0.57–0.95; p = 0.02). No heterogeneity was found across studies (Cochran Q statistic, p = 0.26; I2 statistic, 27%). The certainty of comparisons was low for adjusted analysis, but very low for unadjusted analysis (Table 3).
Figure 3.
Forest plots of overall survival according to surgical methods.
Cancer-specific survival
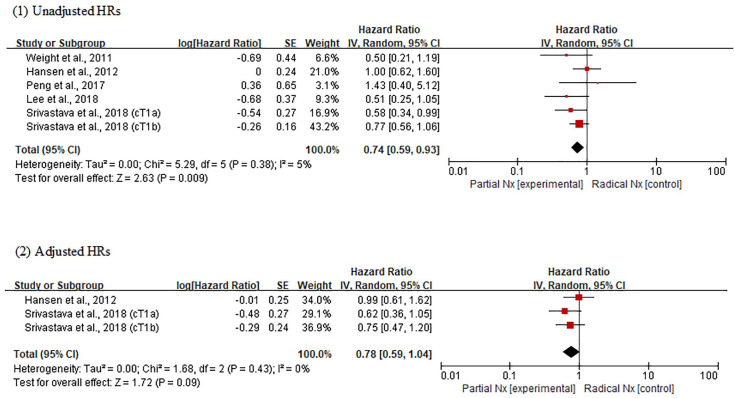
Meta-analysis using unadjusted HRs revealed an overall HR of 0.74 for CSS in patients receiving PNx (95% CI, 0.59–0.93; p = 0.009) (Figure 4). No heterogeneity was found across studies (Cochran Q statistic, p = 0.38; I2 statistic, 5%). However, in the analysis using adjusted HRs, there was no difference in CSS between PNx and RNx groups (overall HR, 0.78; 95% CI, 0.59–1.04; p = 0.09). No heterogeneity was found across studies (Cochran Q statistic, p = 0.43; I2 statistic, 0%). The certainty of comparisons was low for adjusted analysis, but very low for unadjusted analysis (Table 3).
Figure 4.
Forest plots of cancer-specific survival according to surgical methods.
Discussion
In our meta-analysis, patients treated with PNx showed better or at least similar oncological outcomes compared with RNx in patients with upstaged T3aRCCa from a cT1 tumor. Patients who had undergone PNx showed a significantly improved OS compared with those who had undergone RNx, but there was no difference in RFS and CSS between PNx and RNx groups. These findings suggest that (1) PNx has no negative effect on cancer control in patients with upstaged T3a RCCa from cT1 tumor, (2) the nephron-sparing strategy may be beneficial in improving OS, and (3) PNx should be offered in cT1 renal tumor if technically feasible. Our study is the first meta-analysis to show that PNx has an advantage in improving OS in upstaged T3a RCCa, but clinicians should be aware that the level of evidence was low.
The role of nephron-sparing surgery is becoming more significant in the treatment of RCCa. McKiernan et al. reported that when controlling for preoperative risk factors for renal insufficiency, patients undergoing RNx are at a greater risk of chronic renal insufficiency compared with a similar cohort of patients undergoing PNx.29 Furthermore, Huang et al. reported that RNx have significant statistically increased cardiovascular events and mortality than PNx when adjusting for preoperative demographic and comorbid variables.30 Therefore, the importance of nephron-sparing was further recognized. In 2009, the AUA guideline recommended PNx as a standard treatment for cT1a. Since then, in cT1a renal tumors, PNx has been performed more frequently than RNx.31 In addition, the development of surgical techniques, such as robotic surgery, is not limited to cT1 in localized RCCa, and PNx is being performed even in cancers over cT2. Several studies32,33 have shown that the oncologic outcome for this is not negative. A meta-analysis by Mir et al. reported that34 PNx is a viable treatment option for larger renal tumors, as it offers equivalent cancer control and better preservation of renal function with potential for better long-term survival. However, they also reported that more careful selection is required in patients with a renal tumor sized >7 cm.
Therefore, recently, most surgeries for cT1 renal tumor are being performed as nephron-sparing surgery. However, it is inevitable that some upstaged T3a RCCa has been reported among them. In contemporary literature, the incidence of pathological upstaging showed an incidence between 5% and 14%.9,12,16,19 Some researchers have predicted these upstaged T3a preoperatively, but the predictability is still limited. Teishima et al.7 showed that upstaged T3a was related to the irregular shape of the tumor margin through enhanced computed tomography. Other studies have reported that it was associated with age, tumor size, and the serum AST/ALT ratio.16,35,36 Several studies have reported the prognosis in upstaged patients. Gorin et al.9 reported that upstaged T3a patients underwent robotic PNx. The 24-month RFS estimates for pT1-2 and pT3a tumors were 99.2% and 91.8%, respectively. Through an institutional study, Russell et al.10 reported that patients with pT3a upstaging experience a significantly reduced RFS and CSS compared with patients with pT1 disease. Chen et al.8 performed meta-analysis about this, which strongly indicated that postoperative pT3a upstaging is significantly associated with poor RFS, OS, and CSS in patients with cT1 RCC. Meanwhile, the result of the oncologic outcomes of PNx and RNx in this upstaged T3a RCCa remains controversial. Most studies did not show differences in oncologic outcomes between the two surgical procedures. However, the study by Shah et al. showed that RFS is better with RNx in upstaged T3a.19 Ziegelmueller et al. reported that PNx in OS showed better results compared with RNx.22 To resolve these conflicting issues, we performed this meta-analysis. In the present study, we found that PNx, as treatment for upstaged T3a, showed better or at least similar oncological outcomes, especially in terms of OS, compared with RNx. Therefore, upstaged T3a can be an adverse prognostic feature in cT1 RCCa, but PNx does not worsen the prognosis.
Recently, a meta-analysis on the present issue was published by Deng et al.,37 but they did not include recently published studies between 2019 and 2020. Also, there were significant differences in the characteristics of renal tumor according to the two surgical methods, as they included cT2 patients as well as cT1 patients. However, we included the studies that only analyzed cT1 patients; therefore, the studies included in our meta-analysis showed few significant differences in renal tumor character between the two groups. In addition, the previous studies used a mixture of unadjusted and adjusted values for their meta-analysis, while we collected unadjusted and adjusted results separately in this study. The unadjusted result reflects the bivariate relationship between an independent and dependent variable that does not control for covariates or confounders. Unadjusted findings are sometimes presented in cohort studies, but are generally perceived as likely to be biased due to confounding factors. Therefore, for these types of studies, adjusted findings are usually preferred for meta-analysis.38 We obtained different results from previous meta-analysis through these additional statistical methods. As in the previous study, there was no difference in RFS between the two surgical methods in the upstaged pT3a, and there was no difference in CSS in the adjusted analysis as well. However, unlike the previous study, our study statistically proved that when PNx is performed in upstaged pT3a patients, they may have superior OS than when RNx is performed. These results show that the oncological results of PNx are similar to those of RNx in the upstaged pT3a patients, and PNx reduces the incidence of renal dysfunction with a lower rate of non-cancer-specific deaths.39
Our study had some limitations. First, the included studies were retrospective in design, and inevitably come with limitations such as selection bias. In most studies, a specific schedule has not been established for the examination to evaluate recurrence in patients. Second, the small number of studies and sample sizes could affect the overall data quality. Finally, some studies did not provide accurate HR and 95% CI for RFS, OS, and CSS. Therefore, the estimates obtained using the Kaplan–Meier curve may have some errors. In addition, more than half of the studies did not provide adjusted HR for RFS, CSS, and OS through multivariate analysis; therefore, the number of studies would be limited when the adjusted results are pooled for survival analysis. To overcome these limitations, well-designed randomized trials must be performed in the future. Despite these limitations, our study has several strengths. Compared with previous meta-analysis, we updated the included studies with recently published literatures, and extracted data from studies that were more strictly selected. Therefore, this study provides clinical information on the usefulness and safety of PNx in upstaged T3a RCCa. Our findings may explain that in patients with upstaged T3a after PNx, pT3a may have a poor prognosis rather than pT1, but no additional treatment is required. Moreover, the improvement in OS can be explained by the nephron sparing. Therefore, we think that our results would offer great help in treating patients with upstaged T3a RCCa.
Conclusion
Our meta-analysis shows that patients treated with PNx have better or at least similar oncological outcomes compared with RNx in patients with upstaged T3a RCCa from cT1 tumor. In particular, patients who had undergone PNx show significantly improved OS compared with patients who had received RNx. Therefore, we recommend that PNx may be performed for all cT1 renal tumors, including the ones with upstaged potential, if nephron-saving surgical approaches are available. However, due to the low level of evidence, this result must be interpreted with caution. Therefore, large-scale randomized trials and a long-term follow-up study are needed to verify this result.
Footnotes
Author Contributions: Conceptualization, D.Y.C. and K.S.C.; methodology, D.Y.C., D.H.K. and K.S.C.; validation, J.Y.L., K.S.C.; formal analysis, D.Y.C. and D.K.K.; data curation, D.Y.C., J.W.K.; writing—original draft preparation, D.Y.C.; writing—review and editing, K.S.C.; visualization, D.Y.C. and D.H.K.; supervision, K.S.C.; project administration, K.S.C.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a research grant from Inha University Hospital.
ORCID iD: Kang Su Cho  https://orcid.org/0000-0002-3500-8833
https://orcid.org/0000-0002-3500-8833
Contributor Information
Doo Yong Chung, Department of Urology, Inha University School of Medicine, Incheon, Korea.
Dong Hyuk Kang, Department of Urology, Inha University School of Medicine, Incheon, Korea.
Jong Won Kim, Department of Urology, Gangnam Severance Hospital, Yonsei University College of Medicine, Gangnam-gu, Seoul, Korea.
Do Kyung Kim, Department of Urology, Soonchunhyang University Medical College, Soonchunhyang University Seoul Hospital, Seoul, Korea.
Joo Yong Lee, Department of Urology, Urological Science Institute, Yonsei University College of Medicine, Seoul, Korea.
Kang Su Cho, Department of Urology, Gangnam Severance Hospital, Yonsei University College of Medicine, 211 Eonju-ro, Gangnam-gu, Seoul 06273, Korea.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019; 69: 7–34. [DOI] [PubMed] [Google Scholar]
- 2. Znaor A, Lortet-Tieulent J, Laversanne M, et al. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol 2015; 67: 519–530. [DOI] [PubMed] [Google Scholar]
- 3. Turner RM, II, Morgan TM, Jacobs BL. Epidemiology of the small renal mass and the treatment disconnect phenomenon. Urol Clin North Am 2017; 44: 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Swami U, Nussenzveig RH, Haaland B, et al. Revisiting AJCC TNM staging for renal cell carcinoma: quest for improvement. Ann Transl Med 2019; 7: S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Campbell SC, Novick AC, Belldegrun A, et al. Guideline for management of the clinical T1 renal mass. J Urol 2009; 182: 1271–1279. [DOI] [PubMed] [Google Scholar]
- 6. Gill IS, Aron M, Gervais DA, et al. Clinical practice. Small renal mass. N Engl J Med 2010; 362: 624–634. [DOI] [PubMed] [Google Scholar]
- 7. Teishima J, Hayashi T, Kitano H, et al. Impact of radiological morphology of clinical T1 renal cell carcinoma on the prediction of upstaging to pathological T3. Jpn J Clin Oncol 2020; 50: 473–478. [DOI] [PubMed] [Google Scholar]
- 8. Chen L, Deng W, Liu X, et al. Impact of pathological T3a upstaging on oncological outcomes of clinical T1 renal cell carcinoma: a meta-analysis. J Cancer 2019; 10: 4998–5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gorin MA, Ball MW, Pierorazio PM, et al. Outcomes and predictors of clinical T1 to pathological T3a tumor up-staging after robotic partial nephrectomy: a multi-institutional analysis. J Urol 2013; 190: 1907–1911. [DOI] [PubMed] [Google Scholar]
- 10. Russell CM, Lebastchi AH, Chipollini J, et al. Multi-institutional survival analysis of incidental pathologic T3a upstaging in clinical T1 renal cell carcinoma following partial nephrectomy. Urology 2018; 117: 95–100. [DOI] [PubMed] [Google Scholar]
- 11. Hansen J, Sun M, Bianchi M, et al. Assessment of cancer control outcomes in patients with high-risk renal cell carcinoma treated with partial nephrectomy. Urology 2012; 80: 347–353. [DOI] [PubMed] [Google Scholar]
- 12. Jeong SH, Kim JK, Park J, et al. Pathological T3a upstaging of clinical T1 renal cell carcinoma: outcomes according to surgical technique and predictors of upstaging. PLoS One 2016; 11: e0166183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lai GS, Li JR, Wang SS, et al. Survival analysis of pathological T3a upstaging in clinical T1 renal cell carcinoma. In Vivo 2020; 34: 799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee H, Lee M, Lee SE, et al. Outcomes of pathologic stage T3a renal cell carcinoma up-staged from small renal tumor: emphasis on partial nephrectomy. BMC Cancer 2018; 18: 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maurice MJ, Zhu H, Kim S, et al. Survival after partial and radical nephrectomy for high-risk disease: a propensity-matched comparison. Can Urol Assoc J 2016; 10: E282–E289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nayak JG, Patel P, Saarela O, et al. Pathological upstaging of clinical T1 to pathological T3a renal cell carcinoma: a multi-institutional analysis of short-term outcomes. Urology 2016; 94: 154–160. [DOI] [PubMed] [Google Scholar]
- 17. Oh JJ, Byun SS, Lee SE, et al. Partial nephrectomy versus radical nephrectomy for non-metastatic pathological T3a renal cell carcinoma: a multi-institutional comparative analysis. Int J Urol 2014; 21: 352–357. [DOI] [PubMed] [Google Scholar]
- 18. Peng D, He ZS, Li XS, et al. Partial nephrectomy for T3aN0M0 renal cell carcinoma: shall we step forward? Int Braz J Urol 2017; 43: 849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shah PH, Moreira DM, Patel VR, et al. Partial nephrectomy is associated with higher risk of relapse compared with radical nephrectomy for clinical stage T1 renal cell carcinoma pathologically up staged to T3a. J Urol 2017; 198: 289–296. [DOI] [PubMed] [Google Scholar]
- 20. Srivastava A, Patel HD, Joice GA, et al. Incidence of T3a up-staging and survival after partial nephrectomy: size-stratified rates and implications for prognosis. Urol Oncol 2018; 36: 12 e17–12 e13. [DOI] [PubMed] [Google Scholar]
- 21. Weight CJ, Lythgoe C, Unnikrishnan R, et al. Partial nephrectomy does not compromise survival in patients with pathologic upstaging to pT2/pT3 or high-grade renal tumors compared with radical nephrectomy. Urology 2011; 77: 1142–1146. [DOI] [PubMed] [Google Scholar]
- 22. Ziegelmueller BK, Spek A, Szabados B, et al. Partial nephrectomy in pT3a tumors less than 7 cm in diameter has a superior overall survival compared to radical nephrectomy. Cureus 2019; 11: e5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25: 603–605. [DOI] [PubMed] [Google Scholar]
- 24. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336: 924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998; 17: 2815–2834. [DOI] [PubMed] [Google Scholar]
- 26. Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007; 8: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Berkey CS, Hoaglin DC, Mosteller F, et al. A random-effects regression model for meta-analysis. Stat Med 1995; 14: 395–411. [DOI] [PubMed] [Google Scholar]
- 28. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McKiernan J, Simmons R, Katz J, et al. Natural history of chronic renal insufficiency after partial and radical nephrectomy. Urology 2002; 59: 816–820. [DOI] [PubMed] [Google Scholar]
- 30. Huang WC, Elkin EB, Levey AS, et al. Partial nephrectomy versus radical nephrectomy in patients with small renal tumors-is there a difference in mortality and cardiovascular outcomes? J Urol 2009; 181: 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. May A, Guduru A, Syed J, et al. Current trends and disparities in use of partial nephrectomy for T1a renal masses. J Urol 2019; 201: E522. [Google Scholar]
- 32. Kopp RP, Mehrazin R, Palazzi KL, et al. Survival outcomes after radical and partial nephrectomy for clinical T2 renal tumours categorised by R.E.N.A.L. nephrometry score. BJU Int 2014; 114: 708–718. [DOI] [PubMed] [Google Scholar]
- 33. Hansen J, Sun M, Bianchi M, et al. Assessment of cancer control outcomes in patients with high-risk renal cell carcinoma treated with partial nephrectomy. Urology 2012; 80: 347–353. [DOI] [PubMed] [Google Scholar]
- 34. Mir MC, Derweesh I, Porpiglia F, et al. Partial nephrectomy versus radical nephrectomy for clinical T1b and T2 renal tumors: a systematic review and meta-analysis of comparative studies. Eur Urol 2017; 71: 606–617. [DOI] [PubMed] [Google Scholar]
- 35. Fukui S, Miyake M, Iida K, et al. The preoperative predictive factors for pathological T3a upstaging of clinical T1 renal cell carcinoma. Diagnostics (Basel) 2019; 9: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ramaswamy K, Kheterpal E, Pham H, et al. Significance of pathologic T3a upstaging in clinical T1 renal masses undergoing nephrectomy. Clin Genitourin Cancer 2015; 13: 344–349. [DOI] [PubMed] [Google Scholar]
- 37. Deng H, Fan Y, Yuan F, et al. Partial nephrectomy provides equivalent oncologic outcomes and better renal function preservation than radical nephrectomy for pathological T3a renal cell carcinoma: a meta-analysis. Int Braz J Urol. Epub ahead of print 10 April 2020. DOI: 10.1590/S1677-5538.IBJU.2020.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Voils CI, Crandell JL, Chang Y, et al. Combining adjusted and unadjusted findings in mixed research synthesis. J Eval Clin Pract 2011; 17: 429–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim SP, Thompson RH, Boorjian SA, et al. Comparative effectiveness for survival and renal function of partial and radical nephrectomy for localized renal tumors: a systematic review and meta-analysis. J Urol 2012; 188: 51–57. [DOI] [PubMed] [Google Scholar]