Abstract
Heart failure (HF) and diabetes mellitus (DM) frequently coexist, with a prevalence of DM of 35–40% in patients with HF, independent of the level of impairment of the ejection fraction (EF). Furthermore, DM is considered a strong independent risk factor for the progression of HF with either preserved or reduced EF and is associated with poor prognosis. The ability of neprilysin inhibitors to elevate levels of biologically active natriuretic peptides has made them a potential therapeutic approach in HF. In the Prospective comparison of ARNi with ACEi to Determine Impact on Global Mortality and morbidity in Heart Failure (PARADIGM-HF) trial, a dual-acting angiotensin-receptor–neprilysin inhibitor, sacubitril/valsartan was superior to enalapril in reducing the risks of death and HF hospitalization in patients with HF with reduced EF. In addition, in a post-hoc analysis of this trial, among patients with DM, treatment with sacubitril/valsartan resulted in improved glycemic control compared with enalapril. Also, there are additional studies suggesting beneficial metabolic effects of this class of drugs. In this review we discuss potential mechanisms of sacubitril/valsartan effect on glycemic control. Sacubitril/valsartan concomitantly blocks the renin–angiotensin system and inhibits neprilysin, a ubiquitous enzyme responsible for the breakdown of more than 50 vasoactive peptides, including the biologically active natriuretic peptides, bradykinin, angiotensin I and II, endothelin 1, glucagon, glucagon-like peptide-1, insulin-B chain, and others. There are a number of potential mechanisms by which inhibition of neprilysin may lead to improvement in glycemic control, with most evidence suggesting modulation of neprilysin circulating substrates. Although there is some evidence suggesting the improvement of glucose metabolism by renin–angiotensin system inhibition, this effect is most likely modest. As these mechanisms are not fully understood, detailed mechanistic studies, as well as large randomized clinical trials in patients with DM, are needed to further clarify beneficial metabolic properties of sacubitril/valsartan.
Keywords: heart failure, diabetes mellitus, sacubitril/valsartan
Introduction
Heart failure (HF) and diabetes mellitus (DM) are frequent co-morbidities, with a prevalence of DM as high as 35–40% in patients with HF, independent of ejection fraction (EF) impairment.1 Moreover, DM is considered to be a strong independent risk factor for the progression of HF with either preserved or reduced EF,2 while presence of both is associated with a poor subsequent prognosis, as shown in both observational studies and clinical trials.3–5
Despite the fact that angiotensin-converting enzyme (ACE) inhibitors, angiotensin AT1 receptor blockers (ARBs), beta-blockers, and mineralocorticoid receptor antagonists (MRAs) were beneficial in HF (including DM), mortality in these patients still remains high.6 Also, they are usually combined with diuretics and ivabradine.
ACE inhibitors improved symptoms and reduced morbidity and mortality in patients with HF and type 2 DM, and are recommended in this group of patients. In the Studies Of Left Ventricular Dysfunction (SOLVD) trial, there was a significant mortality reduction in DM patients with HF randomized to enalapril.7 In the Assessment of Treatment with Lisinopril and Survival in heart failure (ATLAS) trial high-dose lisinopril treatment resulted in mortality and HF hospitalization risk reduction in patients with and without type 2 DM.8 Meta-analysis including six studies (2398 DM and 10,188 non-DM patients) found that HF treatment with ACE inhibitors resulted in reduced mortality in both groups.9 However, hypoglycemia has been reported with ACE inhibitors treatment in type 2 DM on glucose-lowering treatment, so glucose monitoring is necessary.10 ARBs also have beneficial effects in patients with HF and DM.11 In the CHARM-Alternative trial, candesartan reduced cardiovascular (CV) mortality and morbidity in HF patients intolerant to ACE inhibitors.12 However, as DM patients treated with candesartan had a 2-fold risk of hyperkalemia, kidney function and potassium monitoring is necessary when ARBs are used in this group of patients.12,13 Beta-blockers reduced mortality and hospital admission, and improved symptoms in both DM and non-DM patients.14 In a meta-analysis of six trials, beta-blockers reduced all-cause mortality in patients with and without type 2 DM.15 Also, in a meta-analysis of CIBIS-2, MERIT-HF and COPERNICUS, patients with type 2 DM (n = 1883) had reduced mortality when treated with beta-blockers.9 Hypoglycemia has been described with non-cardio-selective beta-blockade (propranolol), but not with beta-1-selective agents or with carvedilol.16,17 Treatment with MRAs was associated with improved outcomes in HF patients with and without DM.18 Spironolactone and eplerenone reduced mortality in both patients with HF, regardless of the presence or absence of type 2 DM.19 Meta-analysis of four studies found that MRA treatment was associated with improved clinical outcomes in patients with DM and HF. Also, eplerenone seems to have a neutral metabolic profile.20 The most serious adverse effect of MRAs is hyperkalemia, so surveillance of kidney function and potassium is mandatory. Loop diuretics are recommended to reduce HF symptoms, while thiazides have been shown to promote hyperglycemia.21 Also, ivabradine demonstrated a significant reduction in CV death or HF hospitalization, regardless of the presence or absence of type 2 DM.22
On the other hand, sodium–glucose co-transporter type 2 inhibitors are novel glucose-lowering agents shown to reduce HF hospitalizations in CV outcome trials.23,24 Furthermore, two agents from this class have been shown to reduce the combined risk of CV death or HF hospitalization in patients with HF with reduced EF (HFrEF), regardless of the presence or absence of DM, and seem to have become game changers in the treatment of HFrEF.25,26 Also, there is currently an ongoing study investigating the potential benefit of empagliflozin in patients with HF with preserved EF (HFpEF) (EMPagliflozin outcomE tRial in Patients With chrOnic heaRt Failure With Preserved Ejection Fraction-EMPEROR-Preserved).
The ability of neprilysin (NEP) inhibitors to elevate levels of biologically active natriuretic peptides has made them a potential therapeutic approach in HF. First NEP inhibitors ecadotril and candoxatril were initially tested in HF, but lack of efficacy and side effects led to discontinuation of their development.27 Although the dual ACE/NEP inhibitor omapatrilat was found to be protective against HF,28 it was not approved for clinical use due to increased frequency of angioedema29,30
Sacubitril/valsartan (formerly known as LCZ696) is a dual-acting angiotensin-receptor–NEP inhibitor, that contains equimolar amounts of valsartan, a type 1 angiotensin II receptor (AT1) blocker (ARB), and sacubitril, a prodrug that is hydrolyzed into the active NEP inhibitor LBQ657.30,31 The combined ARB/NEP inhibitor was developed to address two pathophysiological mechanisms underlying HF—activation of the renin–angiotensin aldosterone system (RAS) and decreased sensitivity to natriuretic peptides (NPs). In the Prospective comparison of ARNi with ACEi to Determine Impact on Global Mortality and morbidity in Heart Failure (PARADIGM-HF trial), sacubitril/valsartan was superior to ACE inhibitor enalapril in reducing the risks of death and HF hospitalization in patients with HFrEF, which led to its approval by U.S. Food and Drug Administration for the reduction of HF hospitalization.32
The effect of earlier NEP inhibitors on glycemic control has been investigated. Omapatrilat improved whole-body insulin-mediated glucose disposal, induced profound insulin sensitization and increased myocardial glucose uptake in obese insulin-resistant Zucker rats.33 In addition, both acute and chronic dual ACE/NEP inhibition with mixanpril improved whole-body insulin-mediated glucose disposal and insulin sensitivity in obese Zucker rats.34,35 Because of these prior studies, the reduction of new-onset DM was a pre-specified outcome in PARADIGM-HF. However, sacubitril/valsartan did not reduce the new-onset DM compared with enalapril, probably due to a very small number of new-onset diabetes cases (n = 84) during the course of the trial. In addition, this endpoint might have been less sensitive to the effect of sacubitril/valsartan on glucose control as the study might not have been sufficiently powered, and may also have been to insensitive clinical diagnosis of new-onset DM.
However, analysis investigating the relationship between glycemic status and clinical outcomes in this trial found that sacubitril/valsartan was beneficial compared with enalapril, irrespective of glycemic status.36 Furthermore, in a post-hoc analysis of PARADIGM-HF trial among 3778 patients with DM and HFrEF, treatment with sacubitril/valsartan resulted in a greater HbA1c reduction compared with enalapril. Moreover, during the 3-year course of the study, fewer subjects in the sacubitril/valsartan arm required initiation of insulin therapy for glucose control.37 In addition, an 8-week study among 92 obese hypertensive patients found that sacubitril/valsartan improved peripheral insulin sensitivity compared with amlodipine, using the hyperinsulinemic–euglycemic clamp.38
Taking into account both experimental and clinical data previously mentioned, the aim of this review is to present a detailed overview of potential mechanisms of sacubitril/valsartan on glycemic impairment and discuss its potential favorable metabolic effects.
Sacubitril/valsartan
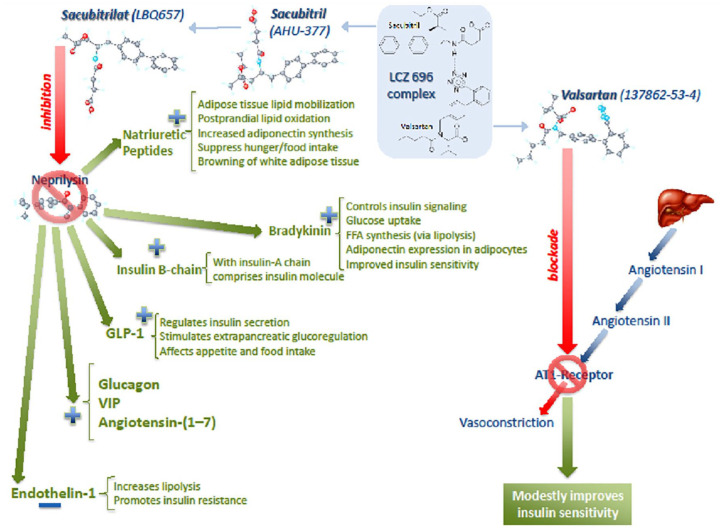
Sacubitril/valsartan concomitantly inhibits NEP and blocks RAS. NEP is an enzyme widely distributed in various tissues, including endothelial, epithelial and smooth muscle cells, cardiac myocytes, adipocytes, and pancreatic islets. It is responsible for the breakdown of more than 50 vasoactive peptides, including the biologically active NPs, bradykinin, angiotensin-(1–7), endothelin-1, glucagon, glucagon-like peptide-1, insulin-B chain, vasoactive intestinal peptide (VIP), and many others.39 There are a number of potential mechanisms by which inhibition of NEP may lead to improvement in glycemic control (Figure 1). Beneficial metabolic effect previously shown is most likely due to the synergistic effect of multiple circulating NEP substrates.
Figure 1.
Potential mechanisms of sacubitril/valsartan effect on glycemic control.
Natriuretic peptides
Experimental, clinical and epidemiological studies suggest that DM, obesity, and metabolic syndrome are characterized by NP deficiency, known as NP handicap. This concept is based on numerous reports showing that lower plasma NP concentrations in subjects without overt CV disorders are associated with insulin resistance and DM, whereas higher concentrations appear to be protective.40 NP handicap leads to drop of plasma NP level and/or tissue response in adipose tissue and skeletal muscle, and therefore increase in circulating NP level and/or tissue signaling may help in treatment of metabolic disturbances. Thus, chronic NEP inhibition, which degrades NP, could raise circulating NP levels and preserve the beneficial actions of NP signaling in metabolic tissues.41
Several studies have suggested that both atrial (ANP) and brain natriuretic peptide (BNP) levels may relate to development of DM. During a median follow-up of 12 years in 7822 patients included in the Atherosclerosis Risk in Communities (ARIC) study, higher levels of NT-proBNP were associated with a significantly decreased risk of DM, even after adjustment for traditional risk factors and fasting glucose.42 Similarly, individuals with one copy of an allele previously shown to be associated with elevated mid-regional ANP levels in plasma had a lower likelihood of incident DM at a follow-up of 14 years.43 Also, three out of four tested variants in NPPA-NPPB (rs632793, rs198389) associated with increased NT-proBNP concentrations and reduced risk of type 2 DM in the Women’s Health Study.44 One such variant, rs198389 in the NPPB promoter region, was associated with a reduced risk of type 2 DM, independently of established risk factors.45 Using data from the Malmo Diet and Cancer study, during 16-year follow-up, levels of circulating mid-regional ANP (MRANP) were significantly inversely associated with new-onset DM [odds ratio (OR) 0.85; 95% confidence interval (CI) = 0.73–0.99; p = 0.034] but not BNP (OR 0.92; 95% CI = 0.80–1.06; p = 0.262). This was also true for the degree of fasting glucose progression over time, independently of other risk factors. In this study, lowest quartile of MRANP was associated with an approximately 60% increased risk of developing DM when compared with the top MRANP quartile and with a 40% increased risk compared with all other subjects (the three upper MRANP quartiles). A further large prospective study was conducted and found that the carriers of at least one copy of G allele of rs5068, a genetic loci that was previously shown to be associated with MR-ANP levels in plasma,46 had lower likelihood of incident DM at a follow-up of 14 years, confirming a causal role of ANP metabolism in DM development.
In addition, studies have investigated the effect of NPs on adipose tissue metabolism, suggesting potential mechanisms and benefits of NEP inhibition. Systemic ANP infusion led to a concentration-dependent increased lipolysis and secondary increase in hepatic gluconeogenesis, most likely through direct stimulation of NP receptors. However, additional studies are needed to address the exact mechanisms by which ANP changes carbohydrate metabolism in humans.47 Also, intravenous ANP infusion has been shown to increase postprandial lipid oxidation [circulating free fatty acids (FFA)], driving an increase in postprandial energy expenditure.27 In addition, both ANP and BNP increased adiponectin (an adipokine with insulin sensitizing properties) synthesis by adipocytes, improving both glucose metabolism and insulin resistance via the AMPK signaling pathway.48,49 Intravenous administration of BNP may also contribute to a beneficial metabolic profile by reducing circulating ghrelin concentrations, decreasing hunger and increasing satiety in healthy people.50 In addition, ANP was shown to inhibit the secretion of pro-inflammatory cytokines by a direct and indirect effect on adipose tissue macrophages.51 Thus, increasing adiponectin and reducing interleukin- 6 and tumor necrosis factor-α secretion from adipose tissue could enhance systemic insulin sensitivity. Both ANP and BNP infusions have been shown to lead to a functional switch of white adipocytes to behave like brown adipocytes with an increased capacity for thermogenic energy expenditure.52 Transplantation of brown fat in mice has been shown to increase insulin sensitivity, improve glucose tolerance, and reduce body weight.53
Glucagon-like peptide 1
Recently published experimental and clinical studies support the notion that augmentation of glucagon-like-peptide 1 (GLP-1), a neuropeptide of the incretin family and potent antihyperglycemic hormone, by NEP inhibition might be one of several mechanisms by which treatment with sacubitril/valsartan could improve glucose control. In high-fat-fed NEP-deficient mice, improved glycemic status was associated with elevated active GLP-1 levels, reduced plasma DPP-4 activity and improved beta cell function, suggesting beneficial metabolic effects of NEP inhibition.54,55 Initial human data supporting beneficial effect of NEP inhibition via GLP-1 increase were recently published. Three-month sacubitril/valsartan treatment in 27 patients with HFrEF (five with DM) resulted in a median 57% (interquartile range 46–65) plasma GLP-1 increase, irrespective of clinical characteristics or antihyperglycemic treatment.56
Potential effects of other NEP substrates
Augmentation of other NEP substrates by NEP inhibition may also play a role in glycemic control. Bradykinin, a NEP substrate, was shown to have numerous effects that would contribute to metabolic homeostasis. For example, bradykinin significantly enhances insulin-stimulated glucose transport in adipocytes via a nitric oxide (NO)-dependent pathway that acts by modulating the feedback inhibition of insulin signaling at the level of insulin receptor signal 1.57 Also, the bradykinin–NO system plays an important role in glucose uptake in skeletal muscle independent of insulin.58 In addition, bradykinin enhances synthesis of FFA, via lipolysis.59
Insulin-B chain, which, together with the insulin-A chain, comprises the insulin molecule, has been recognized as a NEP substrate and might have beneficial antihyperglycemic effects.16 Long-term incubation of human adipocytes with endothelin-1, which is reduced by NEP inhibition, results in a significant increase in lipolysis. Moreover, endothelin-1 attenuates the inhibiting effect of insulin on lipolysis in visceral fat cells, thereby contributing to the development of insulin resistance in obesity.60 In vitro and in vivo studies have shown that the NEP inhibitor, candoxatril, reduces glucagon degradation which is involved in elevating glucose by promoting gluconeogenesis and glycogenolysis, as well as in regulating lipolysis.61 Another NEP substrate, VIP62 increases glycogenolysis playing an important role in glucose control. Angiotensin-(1–7) is also considered a NEP substrate and involved in glucose homeostasis improvement.63,64 NEP more efficiently hydrolyzes angiotensin I to angiotensin-(1–7) compared with ACE2.65 It has been shown that local RAS in pancreatic islet regulates local blood flow, insulin synthesis and secretion, and beta cell survival.66–68 In addition, recent studies suggest that NEP is expressed in islets and that both NEP and ACE2 are required for angiotensin-(1–7) to enhance insulin secretion in vitro.69,70 The findings suggest that concurrent use of angiotensin-(1–7) and NEP inhibitors as antihyperglycemic agents requires further investigation.
Renin–angiotensin system
Inhibition of the RAS has also been shown to improve glycemic control, although the potential mechanism is not clear.71,72 Angiotensin II promotes insulin resistance, while AT1-receptor blockade modestly improves insulin sensitivity.73 Also, it has been shown that ACE inhibitors and ARBs improved metabolic syndrome in patients with DM.74,75 The possible mechanisms involve improvement of insulin sensitivity, enhancement of adipocyte differentiation, and ameliorated inflammation. RAS blockade was found to reduce the incidence of new onset of type 2 DM in clinical trials such as Heart Outcomes Prevention Evaluation (HOPE),76 while in the Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication (DREAM) study, ramipril did not reduce the incidence of DM, although plasma glucose levels measured 2 h after an oral glucose load were significantly lower in the ramipril group.77 Also, in the large population of patients with impaired glucose tolerance and CV disease or risk factors, the use of valsartan for 5 years, along with lifestyle modification, led to a 14% relative reduction in the incidence of DM.78 Although there is some evidence suggesting the improvement of glucose metabolism by RAS inhibition, this effect is most likely to be modest.
Finally, experimental animal studies and clinical trials suggested benefits of angiotensin-receptor–neprilysin inhibitors in DM complications. A combination of irbesartan and thiorphan was more effective in preventing diabetic nephropathy and retinopathy compared with ARB monotherapy.79,80 Furthermore, in HFpEF patients, sacubitril/valsartan improved serum creatinine and estimated glomerular filtration rate, lowered the incidence of worsening renal function, while urinary albumin to creatinine ratio was higher compared with the valsartan treated group.81 However, a clinical trial enrolling patients with DM is needed in order to evaluate safety and efficacy of sacubitril/valsartan in DM.
Conclusion
There is evidence suggesting that treatment with a dual-acting angiotensin-receptor–neprilysin inhibitor (sacubitril/valsartan) resulted in improved glycemic control. This beneficial metabolic effect is most likely secondary to NEP inhibition and consequent modulation of its circulating substrates. However, these mechanisms of action are still elusive. Thus, in future, detailed mechanistic studies and large randomized clinical trials in patients with type 2 DM are needed to look into the beneficial metabolic properties of drugs of this class.
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: Dr. Solomon has received research grants from Actelion, Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, BMS, Celladon, Cytokinetics, Eidos, Gilead, GSK, Ionis, Lilly, Lone Star Heart, Mesoblast, MyoKardia, NIH/NHLBI, Neurotronik, Novartis, NovoNordisk, Respicardia, Sanofi Pasteur, Theracos, and has consulted for Abbott, Action Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, Boeringer-Ingelheim, BMS, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi-Sankyo, Gilead, GSK, Ironwood, Lilly, Merck, Myokardia, Novartis, Roche, Takeda, Theracos, Quantum Genetics, Cardurion, AoBiome, Janssen, Cardiac Dimensions, Tenaya, Sanofi-Pasteur, Dinaqor, Tremeau, CellProThera, Moderna, American Regent. Dr. Seferovic is a full time employee of Cyclerion Therapeutics. Dr. Seely reports no conflicts of interest.
ORCID Id: Jelena P. Seferovic  https://orcid.org/0000-0002-3267-6828
https://orcid.org/0000-0002-3267-6828
Contributor Information
Jelena P. Seferovic, Cardiovascular Division, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115, USA.
Scott D. Solomon, Cardiovascular Division, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA
Ellen W. Seely, Endocrinology, Diabetes, and Hypertension Division, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA
References
- 1. McMurray JJ, Gerstein HC, Holman RR, et al. Heart failure: a cardiovascular outcome in diabetes that can no longer be ignored. Lancet Diabetes Endocrinol 2014; 2: 843–851. [DOI] [PubMed] [Google Scholar]
- 2. Pocock SJ, Wang D, Pfeffer MA, et al. Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J 2006; 27: 65–75. [DOI] [PubMed] [Google Scholar]
- 3. Badar AA, Perez-Moreno AC, Hawkins NM, et al. Clinical characteristics and outcomes of patients with angina and heart failure in the CHARM (Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity) programme. Eur J Heart Fail 2015; 17: 196–204. [DOI] [PubMed] [Google Scholar]
- 4. Velazquez EJ, Pfeffer MA, McMurray JV, et al. ; VALIANT Investigators. VALsartan In Acute Myocardial iNfarcTion (VALIANT) trial: baseline characteristics in context. Eur J Heart Fail 2003; 5: 537–544. [DOI] [PubMed] [Google Scholar]
- 5. McMurray JJ, Packer M, Desai AS, et al. ; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 6. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics-2015 update: a report from the American Heart Association. Circulation 2015; 131: e29–e322. [DOI] [PubMed] [Google Scholar]
- 7. Vermes E, Ducharme A, Bourassa MG, et al. Enalapril reduces the incidence of diabetes in patients with chronic heart failure: insight from the Studies Of Left Ventricular Dysfunction (SOLVD). Circulation 2003; 107: 1291–1296. [DOI] [PubMed] [Google Scholar]
- 8. Ryden L, Armstrong PW, Cleland JG, et al. Efficacy and safety of high-dose lisinopril in chronic heart failure patients at high cardiovascular risk, including those with diabetes mellitus. Results from the ATLAS trial. Eur Heart J 2000; 21: 1967–1978. [DOI] [PubMed] [Google Scholar]
- 9. Shekelle PG, Rich MW, Morton SC, et al. Efficacy of angiotensin-converting enzyme inhibitors and beta-blockers in the management of left ventricular systolic dysfunction according to race, gender and diabetic status: a meta-analysis of major clinical trials. J Am Coll Cardiol 2003; 41: 1529–1538. [DOI] [PubMed] [Google Scholar]
- 10. Morris AD, Boyle DI, McMahon AD, et al. ACE inhibitor use is associated with hospitalization for severe hypoglycemia in patients with diabetes. DARTS/MEMO Collaboration. Diabetes audit and research in Tayside, Scotland. Medicines Monitoring Unit. Diabetes Care 1997; 20: 1363–1367. [DOI] [PubMed] [Google Scholar]
- 11. Dickstein K, Kjekshus J. Effects of losartan and captopril on mortality and morbidity in high-risk patients after acute myocardial infarction: the OPTIMAAL randomised trial. Optimal trial in myocardial infarction with angiotensin II antagonist losartan. Lancet 2002; 360: 752–760. [DOI] [PubMed] [Google Scholar]
- 12. Granger CB, McMurray JJ, Yusuf S, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin converting-enzyme inhibitors: the CHARM-alternative trial. Lancet 2003; 362: 772–776. [DOI] [PubMed] [Google Scholar]
- 13. Cohn JN, Tognoni G. Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001; 345: 1667–1675. [DOI] [PubMed] [Google Scholar]
- 14. Deedwania PC, Giles TD, Klibaner M, et al. Efficacy, safety and tolerability of metoprolol CR/XL in patients with diabetes and chronic heart failure: experiences from MERIT-HF. Am Heart J 2005; 149: 159–167. [DOI] [PubMed] [Google Scholar]
- 15. Haas SJ, Vos T, Gilbert RE, et al. Are beta-blockers as efficacious in patients with diabetes mellitus as in patients without diabetes mellitus who have chronic heart failure? A meta-analysis of large-scale clinical trials. Am Heart J 2003; 146: 848–853. [DOI] [PubMed] [Google Scholar]
- 16. Giugliano D, Acampora R, Marfella R, et al. Metabolic and cardiovascular effects of carvedilol and atenolol in non-insulin-dependent diabetes mellitus and hypertension. A randomized, controlled trial. Ann Intern Med 1997; 126: 955–959. [DOI] [PubMed] [Google Scholar]
- 17. Shorr RI, Ray WA, Daugherty JR, et al. Antihypertensives and the risk of serious hypoglycemia in older persons using insulin or sulfonylureas. JAMA 1997; 278: 40–43. [PubMed] [Google Scholar]
- 18. Gilbert RE, Krum H. Heart failure in diabetes: effects of anti-hyperglycaemic drug therapy. Lancet 2015; 385: 2107–2117. [DOI] [PubMed] [Google Scholar]
- 19. Fernandez HM, Leipzig RM. Spironolactone in patients with heart failure. N Engl J Med 2000; 342: 132. [DOI] [PubMed] [Google Scholar]
- 20. Dei Cas A, Khan SS, Butler J, et al. Impact of diabetes on epidemiology, treatment, and outcomes of patients with heart failure. JACC Heart Fail 2015; 3: 136–145. [DOI] [PubMed] [Google Scholar]
- 21. MacDonald MR, Petrie MC, Hawkins NM, et al. Diabetes, left ventricular systolic dysfunction, and chronic heart failure. Eur Heart J 2008; 29: 1224–1240. [DOI] [PubMed] [Google Scholar]
- 22. Swedberg K, Komajda M, Bohm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 2010; 376: 875–885. [DOI] [PubMed] [Google Scholar]
- 23. Zinman B, Wanner C, Lachin JM, et al. ; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 24. Neal B, Perkovic V, de Zeeuw D, et al. ; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657. [DOI] [PubMed] [Google Scholar]
- 25. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 26. Packer M, Anker SD, Butler J, et al. ; EMPEROR-Reduced Trial Investigators. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020; 383: 1413–1424. [DOI] [PubMed] [Google Scholar]
- 27. Vardeny O, Miller R, Solomon SD. Combined neprilysin and renin-angiotensin system inhibition for the treatment of heart failure. JACC Heart Fail 2014; 2: 663–670. [DOI] [PubMed] [Google Scholar]
- 28. Weber MA. Vasopeptidase inhibitors. Lancet 2001; 358: 1525–1532. [DOI] [PubMed] [Google Scholar]
- 29. Pickering TG. Effects of stress and behavioral interventions in hypertension: the rise and fall of omapatrilat. J Clin Hypertens 2002; 4: 371–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gu J, Noe A, Chandra P, et al. Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (ARNi). J Clin Pharmacol 2010; 50: 401–414. [DOI] [PubMed] [Google Scholar]
- 31. Kobalava Z, Kotovskaya Y, Averkov O, et al. Pharmacodynamic and pharmacokinetic profiles of sacubitril/valsartan (LCZ696) in patients with heart failure and reduced ejection fraction. Cardiovasc Ther 2016; 34: 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. U.S. Food & Drug Administration. http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2015/207620Orig1s000ltr.pdf (2015).
- 33. Wang CH, Leung N, Lapointe N, et al. Vasopeptidase inhibitor omapatrilat induces profound insulin sensitization and increases myocardial glucose uptake in Zucker fatty rats: studies comparing a vasopeptidase inhibitor, angiotensin-converting enzyme inhibitor, and angiotensin II type I receptor blocker. Circulation 2003; 107: 1923–1929. [DOI] [PubMed] [Google Scholar]
- 34. Arbin V, Claperon N, Fournié-Zaluski MC, et al. Acute effect of the dual angiotensin-converting enzyme and neutral endopeptidase 24-11 inhibitor mixanpril on insulin sensitivity in obese Zucker rat. Br J Pharmacol 2001; 133: 495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Arbin V, Claperon N, Fournie-Zaluski MC, et al. Effects of dual angiotensin-converting enzyme and neutral endopeptidase 24-11 chronic inhibition by mixanpril on insulin sensitivity in lean and obese Zucker rats. J Cardiovasc Pharmacol 2003; 41: 254–264. [DOI] [PubMed] [Google Scholar]
- 36. Kristensen SL, Preiss D, Jhund PS, et al. ; PARADIGM-HF Investigators and Committees. Risk related to pre-diabetes mellitus and diabetes mellitus in heart failure with reduced ejection fraction: insights from prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial. Circ Heart Fail 2016; 9: e002560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Seferovic JP, Claggett B, Seidelmann SB, et al. Effect of sacubitril/valsartan versus enalapril on glycaemic control in patients with heart failure and diabetes: a post-hoc analysis from the PARADIGM-HF trial. Lancet Diabetes Endocrinol 2017; 5: 333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jordan J, Stinkens R, Jax T, et al. Improved insulin sensitivity with angiotensin receptor neprilysin inhibition in individuals with obesity and hypertension. Clin Pharmacol Ther 2017; 101: 254–263. [DOI] [PubMed] [Google Scholar]
- 39. Campbell DJ. Long-term neprilysin inhibition - implications for ARNIs. Nat Rev Cardiol 2017; 14: 171–186. [DOI] [PubMed] [Google Scholar]
- 40. Coué M, Moro C. Natriuretic peptide control of energy balance and glucose homeostasis Biochimie 2016; 124: 84–91. [DOI] [PubMed] [Google Scholar]
- 41. Birkenfeld AL, Adams F, Schroeder C, et al. Metabolic actions could confound advantageous effects of combined angiotensin II receptor and neprilysin inhibition. Hypertension 2011; 57: e4–e5. [DOI] [PubMed] [Google Scholar]
- 42. Lazo M, Young JH, Brancati FL, et al. NH2-terminal pro-brain natriuretic peptide and risk of diabetes. Diabetes 2013; 62: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jujić A, Nilsson PM, Engström G, et al. Atrial natriuretic peptide and type 2 diabetes development-biomarker and genotype association study. PLoS One 2014; 9: e89201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Everett BM, Cook NR, Chasman DI, et al. Prospective evaluation of B-type natriuretic peptide concentrations and the risk of type 2 diabetes in women. Clin Chem 2013; 59: 557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pfister R, Sharp S, Luben R, et al. Mendelian randomization study of B-type natriuretic peptide and type 2 diabetes: evidence of causal association from population studies. PLoS Med 2011; 8: e1001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Magnusson M, Jujic A, Hedblad B, et al. Low plasma level of atrial natriuretic peptide predicts development of diabetes: the prospective Malmo diet and cancer study. J Clin Endocrinol Metab 2012; 97: 638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Birkenfeld AL, Boschmann M, Moro C, et al. Lipid mobilization with physiological atrial natriuretic peptide concentrations in humans. J Clin Endocrinol Metab 2005; 90: 3622–3628. [DOI] [PubMed] [Google Scholar]
- 48. Birkenfeld AL, Boschmann M, Engeli S, et al. Atrial natriuretic peptide and adiponectin interactions in man. PLoS One 2012; 7: e43238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tsukamoto O, Fujita M, Kato M, et al. Natriuretic peptides enhance the production of adiponectin in human adipocytes and in patients with chronic heart failure. J Am Coll Cardiol 2009; 53: 2070–2077. [DOI] [PubMed] [Google Scholar]
- 50. Vila G, Grimm G, Resl M, et al. B-type natriuretic peptide modulates ghrelin, hunger, and satiety in healthy men. Diabetes 2012; 61: 2592–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Moro C, Klimcakova E, Lolmede K, et al. Atrial natriuretic peptide inhibits the production of adipokines and cytokines linked to inflammation and insulin resistance in human subcutaneous adipose tissue. Diabetologia 2007; 50: 1038–1047. [DOI] [PubMed] [Google Scholar]
- 52. Bordicchia M, Liu D, Amri EZ, et al. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest 2012; 122: 1022–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stanford KI, Middelbeek RJ, Townsend KL, et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest 2013; 123: 215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Plamboeck A, Holst JJ, Carr RD, et al. Neutral endopeptidase 24.11 and dipeptidyl peptidase IV are both mediators of the degradation of glucagon-like peptide 1 in the anaesthetised pig. Diabetologia 2005; 48: 1882–1890. [DOI] [PubMed] [Google Scholar]
- 55. Willard JR, Barrow BM, Zraika S. Improved glycaemia in high-fat-fed neprilysin-deficient mice is associated with reduced DPP-4 activity and increased active GLP-1 levels. Diabetologia 2017; 60: 701–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Vodovar N, Nougué H, Launay JM, et al. Sacubitril/valsartan in PARADIGM-HF. Lancet Diabetes Endocrinol 2017; 5: 495–496. [DOI] [PubMed] [Google Scholar]
- 57. Beard K, Lu H, Ho K, et al. Bradykinin augments insulin-stimulated glucose transport in rat adipocytes via endothelial nitric oxide synthase-mediated inhibition of Jun NH2-terminal kinase. Diabetes 2006; 55: 2678–2687. [DOI] [PubMed] [Google Scholar]
- 58. Shiuchi T, Cui TX, Wu L, et al. ACE inhibitor improves insulin resistance in diabetic mouse via bradykinin and NO. Hypertension 2002; 40: 329–334. [DOI] [PubMed] [Google Scholar]
- 59. Mori MA, Sales VM, Motta FL, et al. Kinin B1 receptor in adipocytes regulates glucose tolerance and predisposition to obesity. PLoS One 2012; 7: e44782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Eriksson AK, van Harmelen V, Stenson BM, et al. Endothelin-1 stimulates human adipocyte lipolysis through the ET A receptor. Int J Obes (Lond) 2009; 33: 67–74. [DOI] [PubMed] [Google Scholar]
- 61. Trebbien R, Klarskov L, Olesen M, et al. Neutral endopeptidase 24.11 is important for the degradation of both endogenous and exogenous glucagon in anesthetized pigs. Am J Physiol Endocrino Metab 2004; 287: E431–E438. [DOI] [PubMed] [Google Scholar]
- 62. Goetzl EJ, Sreedharan SP, Turck CW, et al. Preferential cleavage of amino- and carboxyl-terminal oligopeptides from vasoactive intestinal polypeptide by human recombinant enkephalinase (neutral endopeptidase, EC 3.4.24.11). Biochem Biophys Res Commun 1989; 158: 850–854. [DOI] [PubMed] [Google Scholar]
- 63. Gafford JT, Skidgel RA, Erdös EG, et al. Human kidney “enkephalinase”, a neutral metalloendopeptidase that cleaves active peptides. Biochemistry 1983; 22: 3265–3271. [DOI] [PubMed] [Google Scholar]
- 64. Yamamoto K, Chappell MC, Brosnihan KB, et al. In vivo metabolism of angiotensin I by neutral endopeptidase (EC 3.4.24.11) in spontaneously hypertensive rats. Hypertension 1992; 19: 692–696. [DOI] [PubMed] [Google Scholar]
- 65. Rice GI, Thomas DA, Grant PJ, et al. Evaluation of angiotensin converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem J 2004; 383: 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Leung PS. The physiology of a local renin-angiotensin system in the pancreas. J Physiol 2007; 580: 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Carlsson PO, Berne C, Jansson L. Angiotensin II and the endocrine pancreas: effects on islet blood flow and insulin secretion in rats. Diabetologia 1998; 41: 127–133. [DOI] [PubMed] [Google Scholar]
- 68. Tikellis C, Wookey PJ, Candido R, et al. Improved islet morphology after blockade of the renin-angiotensin system in the ZDF rat. Diabetes 2004; 53: 989–997. [DOI] [PubMed] [Google Scholar]
- 69. Zraika S, Hull RL, Udayasankar J, et al. Identification of the amyloid-degrading enzyme neprilysin in mouse islets and potential role in islet amyloidogenesis. Diabetes 2007; 56: 304–310. [DOI] [PubMed] [Google Scholar]
- 70. Brar GS, Barrow BM, Watson M, et al. Neprilysin is required for angiotensin-(1-7)’s ability to enhance insulin secretion via its proteolytic activity to generate angiotensin-(1-2). Diabetes 2017; 66: 2201–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jing F, Mogi M, Horiuchi M. Role of renin-angiotensin-aldosterone system in adipose tissue dysfunction. Mol Cell Endocrinol 2013; 378: 23–28. [DOI] [PubMed] [Google Scholar]
- 72. Messerli FH, Weber MA, Brunner HR. Angiotensin II receptor inhibition: a new therapeutic principle. Arch Intern Med 1996; 156: 1957–1965. [PubMed] [Google Scholar]
- 73. van der Zijl NJ, Moors CC, Goossens GH, et al. Valsartan improves {beta}-cell function and insulin sensitivity in subjects with impaired glucose metabolism: a randomized controlled trial. Diabetes Care 2011; 34: 845–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Elliott WJ, Meyer PM. Incident diabetes in clinical trials of antihypertensive drugs: a network meta-analysis. Lancet 2007; 369: 201–207. [DOI] [PubMed] [Google Scholar]
- 75. Gillespie EL, White CM, Kardas M, et al. The impact of ACE inhibitors or angiotensin II type 1 receptor blockers on the development of new-onset type 2 diabetes. Diabetes Care 2005; 28: 2261–2266. [DOI] [PubMed] [Google Scholar]
- 76. Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The heart outcomes prevention evaluation study investigators. N Engl J Med 2000; 342: 145–153. [DOI] [PubMed] [Google Scholar]
- 77. Bosch J, Yusuf S, Gerstein HC, et al. DREAM Trial Investigators. Effect of ramipril on the incidence of diabetes. N Engl J Med 2006; 355: 1551–1562. [DOI] [PubMed] [Google Scholar]
- 78. McMurray JJ, Holman RR, Haffner SM, et al. NAVIGATOR Study Group. Effect of valsartan on the incidence of diabetes and cardiovascular events. N Engl J Med 2010; 362: 1477–1490. [DOI] [PubMed] [Google Scholar]
- 79. Roksnoer LC, van Veghel R, Clahsen-van Groningen MC, et al. Blood pressure-independent renoprotection in diabetic rats treated with AT1 receptor-neprilysin inhibition versus AT1 receptor blockade alone. Clin Sci (Lond) 2016; 30: 1209–1220. [DOI] [PubMed] [Google Scholar]
- 80. Prasad T, Roksnoer LC, Zhu P, et al. Beneficial effects of combined AT1 Receptor/Neprilysin inhibition (ARNI) versus AT1 receptor blockade alone in the diabetic eye ARNI protects against diabetic retinopathy. Invest Ophthalmol Vis Sci 2016; 57: 6722–6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Voors AA, Gori M, Liu LC, et al. Renal effects of the angiotensin receptor neprilysin inhibitor LCZ696 in patients with heart failure and preserved ejection fraction. Eur J Heart Fail 2015; 17: 510–517. [DOI] [PubMed] [Google Scholar]