Abstract
Phospholipases D (PLDs) catalyze hydrolysis of the diester bond of phospholipids to generate phosphatidic acid and the free lipid headgroup. In mammals, PLD enzymes comprise the intracellular enzymes PLD1 and PLD2 and possibly the proteins encoded by related genes, as well as a class of cell-surface and secreted enzymes with structural homology to ecto nucleotide phosphatases/phosphodiesterases as typified by autotaxin (ENPP2) that have lysoPLD activities. Genetic and pharmacological loss-of-function approaches implicate these enzymes in intra- and inter- cellular signaling mediated by the lipid products phosphatidic acid, lysophosphatidic acid, and their metabolites, while the possibility that the water-soluble product of their reactions is biologically relevant has received far less attention. PLD1 and PLD2 are highly selective for phosphatidylcholine (PC), whereas autotaxin has broader substrate specificity for lysophospholipids but by virtue of the high abundance of lysphosphatidylcholine (LPC) in extracellular fluids predominantly hydrolyses this substrate. In all cases, the water-soluble product of these PLD activities is choline. Although choline can be formed de novo by methylation of phosphatidylethanolamine, this activity is absent in most tissues, so mammals are effectively auxotrophic for choline. Dietary consumption of choline in both free and esterified forms is substantial. Choline is necessary for synthesis of the neurotransmitter acetylcholine and of the choline-containing phospholipids PC and sphingomyelin (SM) and also plays a recently-appreciated important role as a methyl donor in the pathways of “one-carbon (1C)” metabolism. This review discusses emerging evidence that some of the biological functions of these intra and extracellular PLD enzymes involves generation of choline.
Overview of Choline Metabolism
Figure 1 summarizes the pathways of choline metabolism that are discussed in detail in the following sections. Choline is a ubiquitous metabolite present in tissues, plasma and other biological fluids. Humans can synthesize small amounts of choline by sequential methylation of phosphatidylethanolamine (PE) to phosphatidylcholine (PC) in the liver1. Hence, choline is considered an essential nutrient that must be obtained from the diet to supplement this smaller endogenously/de novo synthesized pool2. Plasma choline is derived from three major sources (1) dietary choline - as free base or as a constituent of phospholipids present within many foods (2) endogenous synthesis – principally in the liver (3) liberation from choline-containing phospholipids, which are major constituents of all cell membranes. The most abundant sources of esterified choline, phosphatidylcholine and sphingomyelin, are essential for both the structural integrity of cellular membranes and for lipid-dependent signaling pathways, both of which are required for cancer cell growth3. Free choline is released from esterified choline lipid sources by hydrolysis catalyzed by phospholipase D enzymes. Dietary choline is absorbed by enterocytes in the lumen of the small intestines mediated by choline transporter proteins3. Free choline enters the portal circulation of the liver, whereas PC enters the circulation via lymph as a phospholipid constituent of triglyceride-rich chylomicrons. Choline can be phosphorylated to phosphocholine or oxidized to form betaine by the gut microbiota, or in some cell types such as hepatocytes, to feed into the choline biosynthetic pathway and one-carbon metabolism4. It is also a precursor to the neurotransmitter acetylcholine. Although choline plays essential roles, it is also involved, either directly or indirectly via its metabolites, in chronic non-communicable diseases, cardiovascular diseases, and cancers.
Figure 1. Role of Diet and Phospholipase D enzyme in the complex network of choline pathways and Choline-Mediated 1C metabolism.
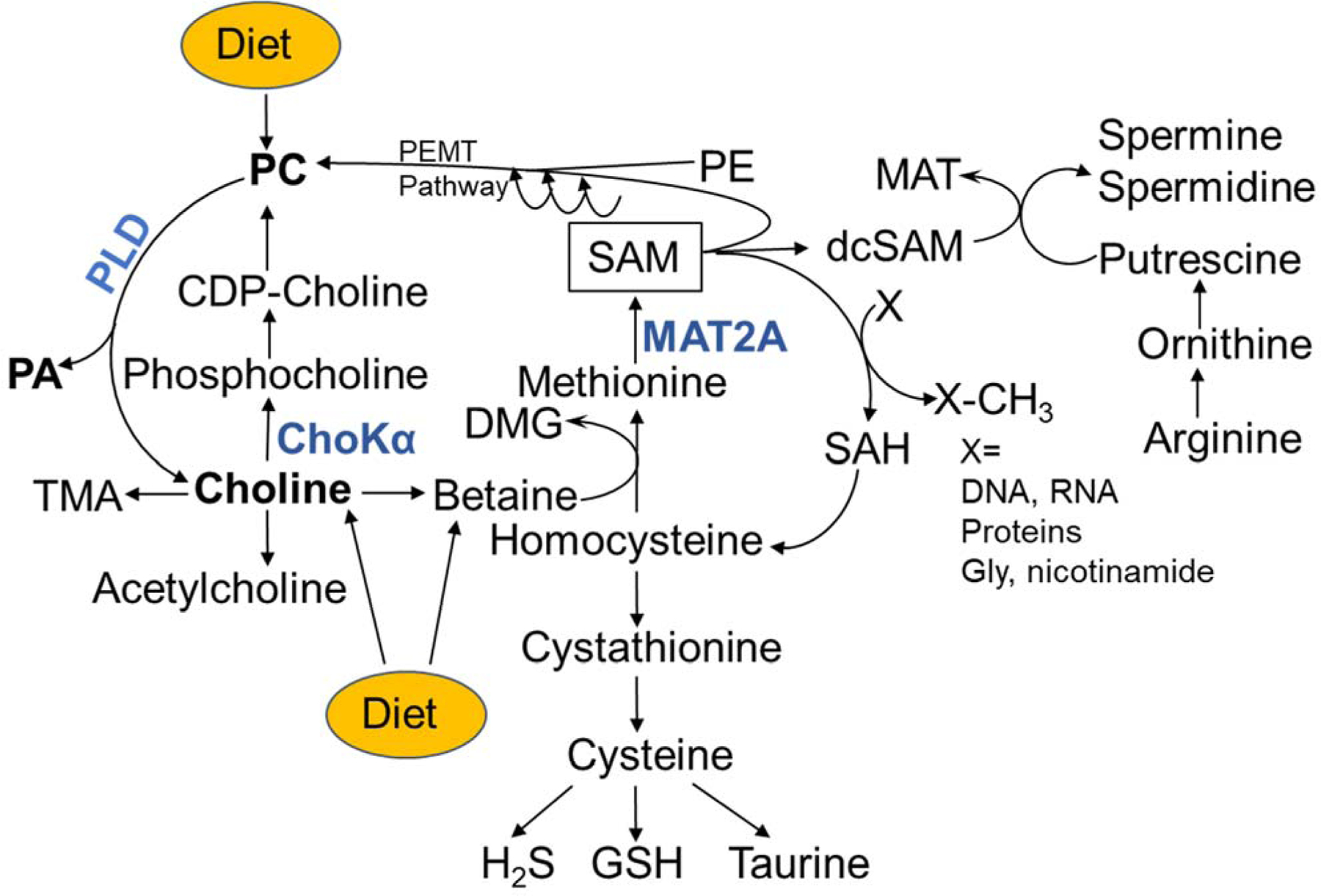
Phospholipase D (PLD) catalyzes release of choline from either diet-derived or endogenously synthesized phosphatidylcholine (PC). The free choline product of PLD activity, or obtained from diet, is a substrate for the synthesis of acetylcholine, trimethylamine (TMA), and phosphatidylcholine (PC) via Cytidine diphosphate choline (CDP-choline). Formation of phosphocholine catalyzed by choline kinase α (ChoKα) is the initial step of de novo PC synthesis. Choline can also be oxidized to betaine. The oxidation of choline to betaine links PLD enzyme activity to 1C metabolism through de novo synthesis of methionine from homocysteine. The byproduct of this reaction dimethylglycine (DMG) is also a potential methyl donor. The methionine product is utilized for synthesis of S-adenocylmethionine (SAM) catalyzed by methionine adenosyltransferase 2A (MAT2A). SAM is the universal methyl donor for a wide range of substrates including phosphatidylethanolamine (PE), DNA, RNA and proteins including histones, and generating S-adenosylhomocysteine (SAH). SAH is hydrolyzed to homocysteine which couples 1C metabolism to the transsulfuration pathway involving sequential synthesis of cystathionine and cysteine and ultimately forming glutathione, taurine and hydrogen sulfide (H2S). SAM can also be decarboxylated to decarboxylated S-adenosylmethionine (dc-SAM) which supports synthesis of the polyamines, spermine and spermidine. The byproduct 50 -methylthioadenosine (MTA) is salvaged back to methionine for SAM generation. The highlighted enzymes PLD, ChoKα and MAT2A are presently attractive therapeutic targets for treatment of cancer.
Dietary sources of choline
Dietary choline can be obtained from a wide variety of foods and supplements. Because de novo synthesis of choline alone is not sufficient to meet human requirements, choline was officially recognized as an essential nutrient by the Institute of Medicine in 19983. It is available in foods as both water-soluble (free choline, phosphocholine, and glycerophosphocholine) and lipid-soluble forms (phosphatidylcholine and sphingomyelin)5. The main dietary sources of choline in the United States are animal products such as meat - especially beef liver, poultry, eggs, fish, and dairy products6. Other sources rich in choline include cruciferous vegetables such as cauliflower and broccoli, nuts, seeds, certain beans and whole grains. Additives and supplements containing choline are also readily available. The most common choline-containing food additive, lecithin, is usually derived from sunflower seeds, eggs, or soybeans. Lecithin is used as an emulsifying agent in routinely-consumed processed foods such as salad dressings, gravies and margarine. It can also be purchased as an over-the-counter supplement. Other forms of supplements high in choline include choline chloride, CDP-choline and alpha-GPC. The choline oxidation product, betaine, is also available as a supplement.
The Food and Nutrition Board of the Institute of Medicine recommends daily intake of choline of 425 and 550 mg for adult females and males respectively3. The Tolerable Upper Intake level for adults is estimated at 3.5 g/day. Dietary choline intake is calculated based on estimates of the free choline and phosphatidylcholine content of foods. There are no reliable estimates of the frequency of use or amount of these dietary supplements consumed by individuals in the United States and Canada. However, there are reports suggesting that the average dietary intake of choline in adults could be higher and is underreported7. According to the US Dietary Guidelines Advisory Committee (DGAC) 2015–2020 report, eggs provide the most abundant source of choline for individuals whose intake is at or above the recommended levels. Egg yolks provide 680 milligrams of choline per 100 grams2.
Choline insufficiency in the diet can lead to liver damage, muscle damage and nonalcoholic fatty liver disease (NAFLD or hepatosteatosis)8. Furthermore, choline deficiency is one of the few single-nutrient deficiencies that causes increased spontaneous carcinogenesis9. In rats and mice, diets low in choline and other methyl-group donors such as folate, methionine, serine, vitamin B12 and betaine result in spontaneous hepatocarcinomas, or sensitize rodents to hepatic carcinogens such as aflatoxin B12610. Oral administration of lecithin (containing esterified choline) or free choline for a prolonged period of time has been shown to increase plasma choline levels11,12. High dietary intake of choline causes adverse effects such as hypotension, fishy body odor, vomiting, excessive sweating and salivation, and liver toxicity13–16. High choline consumption also increases the production of trimethylamine oxide (TMAO), a choline metabolite that has been linked to a higher risk of cardiovascular disease, in a dose-dependent manner in adults17. The association between dietary intake of choline and cancers is less clear. Overall, the recognition of dietary association with cancer incidence has only began to be appreciated. For example, the US DGAC concluded in 2010 that there was not sufficient evidence to acknowledge the effects of dietary patterns and cancer risk18. It was not until very recently in 2015 that DGAC officially recognized diet as a risk factor for colon and postmenopausal breast cancer risk6. However, there is no clear consensus on the effects of high choline consumption on cancer.
Choline and phospholipid synthesis
Choline is an important dietary nutrient that supports the synthesis of the most abundant glycerophospholipid PC via the cytidine diphosphate (CDP)-choline pathway. Up to 95% of the total choline pool in most tissues is used in the synthesis of PC19,20. First described in 1956, Kennedy and Weiss elucidated a pathway for the de novo biosynthesis of PC and PE using rat liver as source of an enzyme activities21. Hence, there are two branches of the Kennedy pathway; CDP-choline pathway and the analogous pathway for PE called CDP-ethanolamine pathway22. The CDP-choline pathway consists of three enzymatic steps. Initially, choline kinase catalyzes the ATP-dependent phosphorylation of choline to form phosphocholine. In the second step, CDP-choline is formed from phosphocholine and CTP catalyzed by CTP:phosphocholine cytidylyltransferase. The final step of PC synthesis involves CDP-choline and a lipid anchor such as diacylglycerol (DAG) or alkyl‐acylglycerol (AAG)22. The Kennedy pathway enzymes are found ubiquitously in eukaryotes23. PC synthesis is required for lipoprotein secretion from the liver24. It is also a major source of the second messengers DAG, phosphatidic acid, lysophosphatidic acid, and arachidonic acid, which can be further metabolized to other signaling molecules24.
Choline and one-carbon metabolism
One-carbon metabolism comprises a complex network of biochemical reactions that facilitates the transfer of 1C moieties in the form of methenyl, formyl, and methyl groups to support the synthesis of molecules that are required for cellular processes25–27. The carbon unit cycle is essential for multiple physiological processes including cellular biosynthesis (purines and thymidine), amino acid homeostasis (glycine, serine, and methionine), methylation (PC biosynthesis, epigenetic maintenance) and regulation of redox status28. Three pathways are involved; the folate cycle, methionine remethylation, and trans-sulfuration pathways. The most important metabolites through which cells refuel one-carbon metabolism are folic acid, serine, glycine and choline. Essentially, two major one-carbon donors are involved in the biosynthetic reactions: tetrahydrofolate (THF) derived from folate and S-adenosylmethionine (SAM) from methionine29.
In mammalian cells, choline contributes to regeneration of methionine from homocysteine via its oxidation product, betaine, catalyzed by the enzyme betaine-homocysteine methyltransferase (BHMT)30. After donating a methyl group to homocysteine, the byproduct of this reaction, dimethylglycine, can also yield additional 1C units through its utilization in the synthesis of THF31. Expression of BHMT is restricted to the liver and kidney. Methionine synthase is expressed globally, thus enabling homocysteine remethylation to occur throughout the body. Methionine is the substrate for S-adenosylmethionine (SAM) synthetase which produces SAM. The reactive methyl carrier, SAM, is the second most common enzymatic cofactor after ATP32. SAM plays a major role in epigenetics; biosynthetic processes including phosphatidylcholine, creatine, and polyamine synthesis; and sulfur metabolism. It is believed that phosphatidylcholine synthesis from phosphoethanolamine using SAM is likely the largest 1C sink in adult mammals33. During the PC synthesis from PE, SAM donates three methyl groups via phosphatidylethanolamine methyltransferase (PEMT), an enzyme expressed principally in liver. The impact of the intake of the 1C donor choline is less studied and the associations between choline consumption and disease is not clear.
Role of PLD in choline metabolism.
Phospholipase D (PLD) and phospholipase C (PLC) are the major enzymes responsible for the release of choline and phosphocholine, respectively, from the most abundant phospholipid PC. Choline can also be released from the lyso-derivative of PC, lysoPC (LPC), by autotaxin, an enzyme that has lysophospholipase D activity. Although no mammalian PC-specific PLC isoforms have been sequenced or cloned, PLD is well characterized and is highly expressed in breast cancers as well as in colorectal, renal, gastric, ovarian, prostate and non-small cell lung cancers34,35. Mammalian PLD constitutes an enzyme superfamily with six members, PLD1–6. Each PLD isoform has unique patterns of sub-cellular localization and roles in cellular processes. PLD1 and PLD2 are the most broadly studied PLD isoforms and are expressed in most tissues at varying basal levels. Human PLD1 and PLD2 share 50% amino acid identity and display a similar protein structure36. The PLD1 (120kDa) isoform is mainly found in the inner membranes of mammalian cells including lysosomes, secretory endosomes, the Golgi complex, and endosomes37. It is readily transported to the plasma membrane subsequent to extracellular stimulation38. PLD2 (106 kDa), on the other hand, most commonly localizes to the plasma membrane39. Whereas PLD1 exhibits low intrinsic activity, i.e. does not transduce extracellular signaling in its basal state, PLD2 has been linked to high basal catalytic activity. However, the available evidence shows that PLD2 displays the same enzymatic activity as PLD1, catalyzing the hydrolysis of PC to produce free choline and phosphatidic acid (PA). A single nucleotide polymorphism with increased risk of non-small cell lung cancer has been observed in PLD1, while polymorphisms in PLD2 are associated with the prevalence of colorectal cancer40,41. Overexpression of PLD1 or PLD2 in murine fibroblasts or in breast cancer xenograft models alters cell growth and leads to primary tumor initiation and metastasis42,43. The signaling lipid PA has pleiotropic effects44. It can facilitate membrane vesicle trafficking, endocytosis/exocytosis and also function as a lipid anchor to recruit PA-binding proteins to localize to sites of signal transduction. PA is also known to activate proteins such as phosphatidylinositol 4-phosphate 5-Kinase (PI4P5K) and mTOR to regulate cellular processes such as cell hypertrophy, survival, and differentiation45. Furthermore, PA can be dephosphorylated by Lipin to produce diacylglycerol (DAG) or hydrolyzed by phospholipase A (PLA) to produce LysoPA (LPA)46. Because of its significance to disease, PLD has been studied both in vitro using cultured cell models and in animal models. Mice with genetic ablation of PLD1 are viable and fertile with no reported abnormalities47. When used in studies on spontaneous intestinal tumorigenesis, PLD1-deficient mice showed significantly reduced intestinal tumorigenesis and increased survival48. In contrast, deficiency of the related isoform PLD2 does not significantly reduce tumorigenesis. Similarly, in a syngeneic melanoma cancer model, loss of PLD1 by genetic knock out or use of the PLD1,2 small molecule inhibitor, 5-fluoro-2-indolyl des-chlorohalopemide (FIPI), decreased tumor size and weight, and decreased metastasis49. These reports suggest that PLD1 could be a promising target for breast cancer therapy. Furthermore, an interdependency has been reported between the PLD1 isoform and choline kinase α enzymes in terms of protein expression50. PLD1 silencing increases CHKA expression and vice versa. Interestingly, novel findings from recent microbiota studies show that several gut microorganisms also express and possibly secrete PLD enzymes that hydrolyze PC as the preferred substrate to produce free choline for downstream metabolism51. Although Chittim et al.51 demonstrated in their work that the released choline is utilized in the production of trimethylamine (TMA) by other gut microorganisms, these findings support the idea that gut microbiota PLD-released choline can be a substrate for choline kinase.
Dysregulated choline metabolism in Cancer Cells
Cancer is a dynamic disease with phenotypic and functional heterogeneity arising from genetic changes, environmental differences and reversible changes in cellular properties52. Common pathways are rare in cancer. However, dysregulated choline metabolism, a fairly new metabolic hallmark of cancer, is a common feature in nearly all cancers53,54. Elevated levels of choline, phosphocholine and glycerophosphocholine have been consistently observed in cancer cells and tumor tissues55. Comparative studies in cancer cells and rapidly proliferating non-cancerous epithelial cells have identified malignant transformation rather than just cell proliferation as the cause of abnormal choline metabolism in cancer56,57. Enzymes involved in choline metabolism are proving to be an attractive and effective strategy for cancer treatment. For example, choline kinase α (CHKA), the first enzyme in the CDP-pathway, is expressed in a large diversity of human tumors including breast, lung, colorectal, bladder, prostate, ovary, endometrial, and T-cell lymphoma58,59. Overexpression of CHKA has oncogenic potential and synergizes with other known oncogenes. As a result, several groups have made attempts to design small molecules as new inhibitors of its enzymatic activity and characterize their activity as anticancer drugs under in vitro and in vivo conditions60,61. Among these, TCD-717, a second generation of CHKA inhibitors, has completed a first-in-human, Phase I clinical trial (http://clinicaltrials.gov/ct2/show/NCT01215864)62. An interdependency has been reported between the PLD1 isoform and choline kinase α enzymes in terms of protein expression that suggests compensatory effects between these two main choline-regulating enzymes50. Numerous studies with cells, animal models and humans have established magnetic resonance spectroscopy (MRS) measurements for total choline-containing compounds (tCho) for detection of cancers and determining response to therapy57. In addition, dietary manipulations that may impact on choline metabolism, especially methionine restriction, have been shown to attenuate tumor development, suggesting a viable approach to cancer management through nutritional intervention63–65. Choline metabolites have also been implicated in oncogenesis and tumor progression through roles in phospholipid synthesis and 1C metabolism53.
Pharmacological targeting of PLD and 1C metabolism.
Because of their roles in generating phosphatidic acid (PA), which regulates many cellular processes and is also a precursor to many bioactive lipid signaling molecules, PLD1 and PLD2 have emerged as drug targets for various diseases such as cancer, cardiovascular diseases, infectious diseases and neurodegenerative conditions (Parkinson’s and Alzheimer’s disease). Initially, primary alcohols (for example n-butanol) were the most commonly used molecules to inhibit PLD-catalyzed production of PA by promoting the formation of phosphatidyl alcohols which are presumed to be biologically inactive66,67. This approach generally results in only incomplete attenuation of PLD dependent PA production. High throughput screening approaches enabled discovery of small molecules that could be used to target the two PLD isoenzymes. The first compounds described were halopemide and its derivatives notably 5-fluoro-2-indolyl des-chlorohalopemide (FIPI)49,68. Halopemide and its derivatives were initially reported to be PLD2 inhibitors but were later found to also inhibit PLD1 with even greater potency49,69. Using halopemide as a base, isoenzyme-selective PLD inhibitors with improved ancillary pharmacology and drug metabolism and pharmacokinetics profiles were later developed. A series of highly selective PLD1 inhibitors such as VU0359595 were prepared70. VU0359595 is approximately 1,700 times more selective for PLD1 compared to PLD2. On the other hand, the first highly selective PLD2 inhibitor identified by the same group, VU0364739, has 75-fold selectivity for PLD2 versus PLD171. In attempts to further develop even more selective PLD2 inhibitors, ML298 and ML395 were synthesized using triazaspirone as the core of the molecules72. The halopemide-derived and triazaspirone-based series were potent inhibitors of mammalian PLD enzymes but were inactive against both the bacterial Pseudomonas aeruginosa PLD (PldA) and the endocannabinoid regular NAPE-PLD73. This prompted the use of selective oestrogen receptor modulators (SERMs) which had previously displayed off-target inhibition of PLD enzymes74. One of the best characterized SERM, desketoraloxifene, has comparable inhibitory activity against mammalian PLD1 and PLD2, and bacterial PldA73.
As mentioned elsewhere in this review and shown in Fig. 1, the free head group (choline) generated by PLD enzymes can be used for de novo synthesis of phosphatidylcholine or enter 1C metabolism via its oxidative product, betaine. Therefore inhibitors of PC biosynthetic pathway or 1C metabolism might be complementary or synergistic with PLD inhibitors. Two of the enzymes targeted with potential anti-cancer agents are choline kinase α (ChoKα) and methyl adenosyltransferase (MAT2A). ChoKα catalyzes the phosphorylation of choline which is the first step in the choline pathway for synthesis of PC. As with PLD inhibitors, the first inhibitors of ChoKα included alcohols such as propanol and butanol75. Other earlier inhibitors of ChoKα to be synthesized were methyl-substituted derivatives of choline. Hemicholinium-3 (HC-3), structural homologs of choline, were later developed and demonstrated potent antitumor effects57,76. This was followed by a series of bis-quinolinium compounds which are less toxic derivatives of HC-3. The first generation of these compounds was MN58b77. With further improvement for tolerability, RSM-932A, a more potent bis-quinolinium with better therapeutic window and improved safety profile was developed62. RSM-932A (also called TCD-717) became the first ChoKα inhibitor to be tested in humans when in entered phase I clinical trials62. MAT2A catalyzes the biosynthesis of the universal methyl-donor S-adenosylmethionine (SAM) from methionine and ATP. SAM is required for many methyl transfer reactions and polyamine biosynthesis. Inhibitors of MAT2A have been developed that bind to either an allosteric site or to the catalytic subunit of MAT2A. A family of fluorinated N,N-dialkylaminostilbene (FIDAS) agents and their analogs have been developed that directly target the catalytic subunit of MAT2A78. On the other hand Pfizer developed a MAT2A inhibitor, PF-9366, that binds and allosterically regulates the activity of MAT2A79. The MAT2A inhibitor AG-270 developed by Agios Pharmaceuticals as a first-in-class MAT2A inhibitor that is being tested in patients with advanced solid tumors or lymphoma characterized by the homozygous deletion of the MTAP gene which plays a critical role in methionine salvage and consequently when absent renders cells highly dependent on MT2A dependent methionine synthesis80. Collectively, continued development of the classes of inhibitors discussed above is expected to result in compounds with improved specificity, potency and tolerability. These compounds will continue to be valuable tools for analyzing the role of PLD, ChoKα and MAT2A enzymes in normal physiology and disease. These tools can be used to complement genetic knockout approaches in studies targeting distinct PLD isoenzymes and other pathways that utilize products of PLD activity. In addition, these small molecules can be utilized in cases where genetic ablation would be difficult to achieve or in settings where deletion of the aforementioned enzymes would have effects distinct from those of short-term inhibition. Although presently not used in the clinics, it is likely that small molecule inhibitors of PLD isoenzymes will be tested for efficacy in diseases, especially those where currently available therapeutics are limited. PLD inhibitors could be used in combination with novel ChoKα and MAT2A inhibitors, or with standard chemotherapy to target pathways of 1C metabolism that are known to be critical for development and progression of some cancers.
Concluding comments.
Although substantial advances have been made in understanding the molecular mechanisms that drive the aberrant choline metabolism in cancer, the role of diet in this process is unclear. A number of epidemiologic studies examining the relationship between dietary choline intake and cancer have yielded conflicting findings. Therefore, investigating the idea that high consumption of dietary lipids, rich in esterified choline lipids, promotes the abnormal choline metabolism leading to cancer progression and metastasis would be interesting. This can be done using mouse models of cancer and rodent diets formulated with exogenous phosphatidylcholine as the major source of dietary choline. PLD-deficient animal models have been generated and characterized. Development of small molecule inhibitors targeting PLD is on-going. With the interest and rapid increase in gut microbiome research over the last several years, the discovery of widespread PLD activity among gut bacteria provides additional opportunities for therapeutic intervention. Together, these tools present an excellent opportunity to unravel the missing link between PLD and diet in contributing to choline and one-carbon metabolism. It would be possible, for example, to test the hypothesis that PLD expression and higher consumption of dietary PC supports the abnormal choline metabolism in cancer.
Acknowledgments:
Research in the Authors Laboratories is supported by grants from the NIH and the Department of Veterans Affairs. FOO is the recipient of an NIH/NCI Mentored Research Scientist Development Award K01CA197073.
References
- 1.Gibellini F & Smith TK The Kennedy pathway--De novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUBMB life 62, 414–428, doi: 10.1002/iub.337 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Zeisel SH & da Costa KA Choline: an essential nutrient for public health. Nutrition reviews 67, 615–623, doi: 10.1111/j.1753-4887.2009.00246.x (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Institute of Medicine Standing Committee on the Scientific Evaluation of Dietary Reference, I., its Panel on Folate, O. B. V. & Choline in Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline (National Academies Press (US) National Academy of Sciences, 1998). [PubMed] [Google Scholar]
- 4.Zeisel SH Importance of methyl donors during reproduction. The American journal of clinical nutrition 89, 673s–677s, doi: 10.3945/ajcn.2008.26811D (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeisel SH A brief history of choline. Annals of nutrition & metabolism 61, 254–258, doi: 10.1159/000343120 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGuire S Scientific Report of the 2015 Dietary Guidelines Advisory Committee. Washington, DC: US Departments of Agriculture and Health and Human Services, 2015. Advances in nutrition 7, 202–204, doi: 10.3945/an.115.011684 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer LM et al. Ad libitum choline intake in healthy individuals meets or exceeds the proposed adequate intake level. The Journal of nutrition 135, 826–829, doi: 10.1093/jn/135.4.826 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sherriff JL, O’Sullivan TA, Properzi C, Oddo JL & Adams LA Choline, Its Potential Role in Nonalcoholic Fatty Liver Disease, and the Case for Human and Bacterial Genes. Advances in nutrition 7, 5–13, doi: 10.3945/an.114.007955 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeisel SH Dietary choline deficiency causes DNA strand breaks and alters epigenetic marks on DNA and histones. Mutation research 733, 34–38, doi: 10.1016/j.mrfmmm.2011.10.008 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schrager TF, Newberne PM, Pikul AH & Groopman JD Aflatoxin-DNA adduct formation in chronically dosed rats fed a choline-deficient diet. Carcinogenesis 11, 177–180, doi: 10.1093/carcin/11.1.177 (1990). [DOI] [PubMed] [Google Scholar]
- 11.Buchman AL et al. Lecithin increases plasma free choline and decreases hepatic steatosis in long-term total parenteral nutrition patients. Gastroenterology 102, 1363–1370 (1992). [PubMed] [Google Scholar]
- 12.Buchman AL et al. Choline deficiency: a cause of hepatic steatosis during parenteral nutrition that can be reversed with intravenous choline supplementation. Hepatology 22, 1399–1403 (1995). [PubMed] [Google Scholar]
- 13.Davis KL, Berger PA & Hollister LE Letter: Choline for tardive dyskinesia. The New England journal of medicine 293, 152, doi: 10.1056/nejm197507172930317 (1975). [DOI] [PubMed] [Google Scholar]
- 14.Growdon JH, Hirsch MJ, Wurtman RJ & Wiener W Oral choline administration to patients with tardive dyskinesia. The New England journal of medicine 297, 524–527, doi: 10.1056/nejm197709082971002 (1977). [DOI] [PubMed] [Google Scholar]
- 15.Lawrence CM, Millac P, Stout GS & Ward JW The use of choline chloride in ataxic disorders. Journal of neurology, neurosurgery, and psychiatry 43, 452–454, doi: 10.1136/jnnp.43.5.452 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wood JL & Allison RG Effects of consumption of choline and lecithin on neurological and cardiovascular systems. Federation proceedings 41, 3015–3021 (1982). [PubMed] [Google Scholar]
- 17.Wang Z et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472, 57–63, doi: 10.1038/nature09922 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Horn L Development of the 2010 US Dietary Guidelines Advisory Committee Report: perspectives from a registered dietitian. Journal of the American Dietetic Association 110, 1638–1645, doi: 10.1016/j.jada.2010.08.018 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Zeisel SH & Blusztajn JK Choline and human nutrition. Annual review of nutrition 14, 269–296, doi: 10.1146/annurev.nu.14.070194.001413 (1994). [DOI] [PubMed] [Google Scholar]
- 20.Zeisel SH, Mar MH, Howe JC & Holden JM Concentrations of choline-containing compounds and betaine in common foods. The Journal of nutrition 133, 1302–1307, doi: 10.1093/jn/133.5.1302 (2003). [DOI] [PubMed] [Google Scholar]
- 21.Kennedy EP & Weiss SB The function of cytidine coenzymes in the biosynthesis of phospholipides. The Journal of biological chemistry 222, 193–214 (1956). [PubMed] [Google Scholar]
- 22.Sundler R & Akesson B Regulation of phospholipid biosynthesis in isolated rat hepatocytes. Effect of different substrates. The Journal of biological chemistry 250, 3359–3367 (1975). [PubMed] [Google Scholar]
- 23.Lykidis A Comparative genomics and evolution of eukaryotic phospholipid biosynthesis. Progress in lipid research 46, 171–199, doi: 10.1016/j.plipres.2007.03.003 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Li Z & Vance DE Phosphatidylcholine and choline homeostasis. Journal of lipid research 49, 1187–1194, doi: 10.1194/jlr.R700019-JLR200 (2008). [DOI] [PubMed] [Google Scholar]
- 25.Shane B & Stokstad EL Vitamin B12-folate interrelationships. Annual review of nutrition 5, 115–141, doi: 10.1146/annurev.nu.05.070185.000555 (1985). [DOI] [PubMed] [Google Scholar]
- 26.Stipanuk MH Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine. Annual review of nutrition 24, 539–577, doi: 10.1146/annurev.nutr.24.012003.132418 (2004). [DOI] [PubMed] [Google Scholar]
- 27.Stover PJ Physiology of folate and vitamin B12 in health and disease. Nutrition reviews 62, S3–12; discussion S13, doi: 10.1111/j.1753-4887.2004.tb00070.x (2004). [DOI] [PubMed] [Google Scholar]
- 28.Ducker GS & Rabinowitz JD One-Carbon Metabolism in Health and Disease. Cell metabolism 25, 27–42, doi: 10.1016/j.cmet.2016.08.009 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mentch SJ & Locasale JW One-carbon metabolism and epigenetics: understanding the specificity. Annals of the New York Academy of Sciences 1363, 91–98, doi: 10.1111/nyas.12956 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pajares MA & Perez-Sala D Betaine homocysteine S-methyltransferase: just a regulator of homocysteine metabolism? Cellular and molecular life sciences : CMLS 63, 2792–2803, doi: 10.1007/s00018-006-6249-6 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ueland PM Choline and betaine in health and disease. Journal of inherited metabolic disease 34, 3–15, doi: 10.1007/s10545-010-9088-4 (2011). [DOI] [PubMed] [Google Scholar]
- 32.Su X, Wellen KE & Rabinowitz JD Metabolic control of methylation and acetylation. Current opinion in chemical biology 30, 52–60, doi: 10.1016/j.cbpa.2015.10.030 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stead LM, Brosnan JT, Brosnan ME, Vance DE & Jacobs RL Is it time to reevaluate methyl balance in humans? The American journal of clinical nutrition 83, 5–10, doi: 10.1093/ajcn/83.1.5 (2006). [DOI] [PubMed] [Google Scholar]
- 34.McDermott M, Wakelam MJ & Morris AJ Phospholipase D. Biochemistry and cell biology = Biochimie et biologie cellulaire 82, 225–253, doi: 10.1139/o03-079 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Morris AJ Regulation of phospholipase D activity, membrane targeting and intracellular trafficking by phosphoinositides. Biochemical Society symposium, 247–257, doi: 10.1042/bss0740247 (2007). [DOI] [PubMed] [Google Scholar]
- 36.Pettitt TR, McDermott M, Saqib KM, Shimwell N & Wakelam MJ Phospholipase D1b and D2a generate structurally identical phosphatidic acid species in mammalian cells. The Biochemical journal 360, 707–715, doi: 10.1042/0264-6021:3600707 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nanjundan M & Possmayer F Pulmonary phosphatidic acid phosphatase and lipid phosphate phosphohydrolase. American journal of physiology. Lung cellular and molecular physiology 284, L1–23, doi: 10.1152/ajplung.00029.2002 (2003). [DOI] [PubMed] [Google Scholar]
- 38.Choi WS, Chahdi A, Kim YM, Fraundorfer PF & Beaven MA Regulation of phospholipase D and secretion in mast cells by protein kinase A and other protein kinases. Annals of the New York Academy of Sciences 968, 198–212, doi: 10.1111/j.1749-6632.2002.tb04336.x (2002). [DOI] [PubMed] [Google Scholar]
- 39.Zhao C, Du G, Skowronek K, Frohman MA & Bar-Sagi D Phospholipase D2-generated phosphatidic acid couples EGFR stimulation to Ras activation by Sos. Nature cell biology 9, 706–712, doi: 10.1038/ncb1594 (2007). [DOI] [PubMed] [Google Scholar]
- 40.Ahn MJ et al. A Single Nucleotide Polymorphism in the Phospholipase D1 Gene is Associated with Risk of Non-Small Cell Lung Cancer. International journal of biomedical science : IJBS 8, 121–128 (2012). [PMC free article] [PubMed] [Google Scholar]
- 41.Yamada Y et al. Association of a polymorphism of the phospholipase D2 gene with the prevalence of colorectal cancer. Journal of molecular medicine 81, 126–131, doi: 10.1007/s00109-002-0411-x (2003). [DOI] [PubMed] [Google Scholar]
- 42.Henkels KM, Boivin GP, Dudley ES, Berberich SJ & Gomez-Cambronero J Phospholipase D (PLD) drives cell invasion, tumor growth and metastasis in a human breast cancer xenograph model. Oncogene 32, 5551–5562, doi: 10.1038/onc.2013.207 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Min DS et al. Neoplastic transformation and tumorigenesis associated with overexpression of phospholipase D isozymes in cultured murine fibroblasts. Carcinogenesis 22, 1641–1647, doi: 10.1093/carcin/22.10.1641 (2001). [DOI] [PubMed] [Google Scholar]
- 44.Cruchaga C et al. Rare coding variants in the phospholipase D3 gene confer risk for Alzheimer’s disease. Nature 505, 550–554, doi: 10.1038/nature12825 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cho JH & Han JS Phospholipase D and Its Essential Role in Cancer. Molecules and cells 40, 805–813, doi: 10.14348/molcells.2017.0241 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baba T et al. Phosphatidic acid (PA)-preferring phospholipase A1 regulates mitochondrial dynamics. The Journal of biological chemistry 289, 11497–11511, doi: 10.1074/jbc.M113.531921 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y & Frohman MA Cellular and physiological roles for phospholipase D1 in cancer. The Journal of biological chemistry 289, 22567–22574, doi: 10.1074/jbc.R114.576876 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dall’Armi C et al. The phospholipase D1 pathway modulates macroautophagy. Nature communications 1, 142, doi: 10.1038/ncomms1144 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Su W et al. 5-Fluoro-2-indolyl des-chlorohalopemide (FIPI), a phospholipase D pharmacological inhibitor that alters cell spreading and inhibits chemotaxis. Molecular pharmacology 75, 437–446, doi: 10.1124/mol.108.053298 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gadiya M et al. Phospholipase D1 and choline kinase-alpha are interactive targets in breast cancer. Cancer biology & therapy 15, 593–601, doi: 10.4161/cbt.28165 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chittim CL, Martinez Del Campo A & Balskus EP Gut bacterial phospholipase Ds support disease-associated metabolism by generating choline. Nature microbiology 4, 155–163, doi: 10.1038/s41564-018-0294-4 (2019). [DOI] [PubMed] [Google Scholar]
- 52.Meacham CE & Morrison SJ Tumour heterogeneity and cancer cell plasticity. Nature 501, 328–337, doi: 10.1038/nature12624 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glunde K, Bhujwalla ZM & Ronen SM Choline metabolism in malignant transformation. Nature reviews. Cancer 11, 835–848, doi: 10.1038/nrc3162 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheng M, Bhujwalla ZM & Glunde K Targeting Phospholipid Metabolism in Cancer. Frontiers in oncology 6, 266, doi: 10.3389/fonc.2016.00266 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Glunde K, Penet MF, Jiang L, Jacobs MA & Bhujwalla ZM Choline metabolism-based molecular diagnosis of cancer: an update. Expert review of molecular diagnostics 15, 735–747, doi: 10.1586/14737159.2015.1039515 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aboagye EO & Bhujwalla ZM Malignant transformation alters membrane choline phospholipid metabolism of human mammary epithelial cells. Cancer research 59, 80–84 (1999). [PubMed] [Google Scholar]
- 57.Daly PF, Lyon RC, Faustino PJ & Cohen JS Phospholipid metabolism in cancer cells monitored by 31P NMR spectroscopy. The Journal of biological chemistry 262, 14875–14878 (1987). [PubMed] [Google Scholar]
- 58.Ramirez de Molina A et al. Increased choline kinase activity in human breast carcinomas: clinical evidence for a potential novel antitumor strategy. Oncogene 21, 4317–4322, doi: 10.1038/sj.onc.1205556 (2002). [DOI] [PubMed] [Google Scholar]
- 59.Ramirez de Molina A et al. Overexpression of choline kinase is a frequent feature in human tumor-derived cell lines and in lung, prostate, and colorectal human cancers. Biochemical and biophysical research communications 296, 580–583 (2002). [DOI] [PubMed] [Google Scholar]
- 60.Al-Saffar NM et al. Noninvasive magnetic resonance spectroscopic pharmacodynamic markers of the choline kinase inhibitor MN58b in human carcinoma models. Cancer research 66, 427–434, doi: 10.1158/0008-5472.Can-05-1338 (2006). [DOI] [PubMed] [Google Scholar]
- 61.Lacal JC Choline kinase: a novel target for antitumor drugs. IDrugs : the investigational drugs journal 4, 419–426 (2001). [PubMed] [Google Scholar]
- 62.Lacal JC & Campos JM Preclinical characterization of RSM-932A, a novel anticancer drug targeting the human choline kinase alpha, an enzyme involved in increased lipid metabolism of cancer cells. Molecular cancer therapeutics 14, 31–39, doi: 10.1158/1535-7163.Mct-14-0531 (2015). [DOI] [PubMed] [Google Scholar]
- 63.Hanley MP, Kadaveru K, Perret C, Giardina C & Rosenberg DW Dietary Methyl Donor Depletion Suppresses Intestinal Adenoma Development. Cancer prevention research (Philadelphia, Pa.) 9, 812–820, doi: 10.1158/1940-6207.capr-16-0042 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kadaveru K, Protiva P, Greenspan EJ, Kim YI & Rosenberg DW Dietary methyl donor depletion protects against intestinal tumorigenesis in Apc(Min/+) mice. Cancer prevention research (Philadelphia, Pa.) 5, 911–920, doi: 10.1158/1940-6207.capr-11-0544 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lamming DW et al. Restriction of dietary protein decreases mTORC1 in tumors and somatic tissues of a tumor-bearing mouse xenograft model. Oncotarget 6, 31233–31240, doi: 10.18632/oncotarget.5180 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brown HA, Thomas PG & Lindsley CW Targeting phospholipase D in cancer, infection and neurodegenerative disorders. Nature reviews. Drug discovery 16, 351–367, doi: 10.1038/nrd.2016.252 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Selvy PE, Lavieri RR, Lindsley CW & Brown HA Phospholipase D: enzymology, functionality, and chemical modulation. Chemical reviews 111, 6064–6119, doi: 10.1021/cr200296t (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Monovich L et al. Optimization of halopemide for phospholipase D2 inhibition. Bioorganic & medicinal chemistry letters 17, 2310–2311, doi: 10.1016/j.bmcl.2007.01.059 (2007). [DOI] [PubMed] [Google Scholar]
- 69.Scott SA et al. Design of isoform-selective phospholipase D inhibitors that modulate cancer cell invasiveness. Nature chemical biology 5, 108–117, doi: 10.1038/nchembio.140 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lewis JA et al. Design and synthesis of isoform-selective phospholipase D (PLD) inhibitors. Part I: Impact of alternative halogenated privileged structures for PLD1 specificity. Bioorganic & medicinal chemistry letters 19, 1916–1920, doi: 10.1016/j.bmcl.2009.02.057 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lavieri RR et al. Design, synthesis, and biological evaluation of halogenated N-(2-(4-oxo-1-phenyl-1,3,8-triazaspiro[4.5]decan-8-yl)ethyl)benzamides: discovery of an isoform-selective small molecule phospholipase D2 inhibitor. Journal of medicinal chemistry 53, 6706–6719, doi: 10.1021/jm100814g (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O’Reilly MC et al. Development of dual PLD1/2 and PLD2 selective inhibitors from a common 1,3,8-Triazaspiro[4.5]decane Core: discovery of Ml298 and Ml299 that decrease invasive migration in U87-MG glioblastoma cells. Journal of medicinal chemistry 56, 2695–2699, doi: 10.1021/jm301782e (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Scott SA et al. Discovery of desketoraloxifene analogues as inhibitors of mammalian, Pseudomonas aeruginosa, and NAPE phospholipase D enzymes. ACS chemical biology 10, 421–432, doi: 10.1021/cb500828m (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eisen SF & Brown HA Selective estrogen receptor (ER) modulators differentially regulate phospholipase D catalytic activity in ER-negative breast cancer cells. Molecular pharmacology 62, 911–920, doi: 10.1124/mol.62.4.911 (2002). [DOI] [PubMed] [Google Scholar]
- 75.Mehendale HM, Dauterman WC & Hodgson E Phosphatidyl carnitine: a possible intermediate in the biosynthesis of phosphatidyl beta-methylcholine in Phormia regina (Meigen). Nature 211, 759–761, doi: 10.1038/211759b0 (1966). [DOI] [PubMed] [Google Scholar]
- 76.Hamza M, Lloveras J, Ribbes G, Soula G & Douste-Blazy L An in vitro study of hemicholinium-3 on phospholipid metabolism of Krebs II ascites cells. Biochemical pharmacology 32, 1893–1897, doi: 10.1016/0006-2952(83)90055-2 (1983). [DOI] [PubMed] [Google Scholar]
- 77.Hernandez-Alcoceba R, Fernandez F & Lacal JC In vivo antitumor activity of choline kinase inhibitors: a novel target for anticancer drug discovery. Cancer research 59, 3112–3118 (1999). [PubMed] [Google Scholar]
- 78.Zhang W et al. Fluorinated N,N-dialkylaminostilbenes repress colon cancer by targeting methionine S-adenosyltransferase 2A. ACS chemical biology 8, 796–803, doi: 10.1021/cb3005353 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Quinlan CL et al. Targeting S-adenosylmethionine biosynthesis with a novel allosteric inhibitor of Mat2A. Nature chemical biology 13, 785–792, doi: 10.1038/nchembio.2384 (2017). [DOI] [PubMed] [Google Scholar]
- 80.Marjon K et al. MTAP Deletions in Cancer Create Vulnerability to Targeting of the MAT2A/PRMT5/RIOK1 Axis. Cell reports 15, 574–587, doi: 10.1016/j.celrep.2016.03.043 (2016). [DOI] [PubMed] [Google Scholar]


