Abstract
Background
European Society of Cardiology (ESC) and European Respiratory Society (ERS) guidelines include thermodilution cardiac index (TDCI) and mixed venous oxygen saturation (SvO2) as two of the three hemodynamic determinations used in risk assessment of patients with pulmonary arterial hypertension (PAH). SvO2 may be a better measurement than TDCI to assess prognosis in patients with either idiopathic or heritable PAH.
Research Question
What is the concordance between TDCI and SvO2 ESC/ERS risk group allocation and their prognostic value in patients with PAH?
Study Design and Methods
In this retrospective study, we assessed the correlation between SvO2 and TDCI in patients with idiopathic and heritable PAH. We determined concordance in the ESC/ERS risk group allocation and association with survival, both at baseline and follow-up.
Results
A total of 158 patients (mean age, 58 ± 17 years; 72% women) with idiopathic (91%) and heritable (9%) PAH were included. There was moderate association between TDCI and SvO2 (r = 0.50; 95% CI, 0.37-0.62). Weighted kappa revealed a fair agreement between TDCI and SvO2 (κ = 0.30; 95% CI, 0.18-0.42), with concordance in risk group allocation in 49% of patients. During a median follow-up of 45 months (interquartile range, 23-105), 62 patients (39%) died. Using Kaplan-Meier analysis, survival was impacted by the SvO2 (log rank = 0.002) but not by the TDCI risk group allocation (log-rank = 0.51). Using the Cox proportional hazard model, adjusted for age and sex, SvO2 (but not TDCI) was associated with mortality (hazard ratio per 1% change, 0.94; 95% CI, 0.91-0.97; P < .001).
Interpretation
When using the cutoffs proposed by the ESC/ERS guidelines, we noted poor concordance in risk score allocation between TDCI and SvO2. In patients with idiopathic or heritable PAH, SvO2 measurements are superior to TDCI in predicting long-term mortality.
Key Words: cardiac index, mixed venous oxygen saturation, pulmonary arterial hypertension, pulmonary hypertension
Abbreviations: 6MWD, 6-min walk test distance; ERS, European Respiratory Society; ESC, European Society of Cardiology; HR, hazard ratio; IQR, interquartile range; NT-pro BNP, N-terminal pro B-type natriuretic peptide; PAH, pulmonary arterial hypertension; PH, pulmonary hypertension; RAP, right atrial pressure; RHC, right heart catheterization; RVSP, right ventricular systolic pressure; SVI, stroke volume index; SvO2, mixed venous oxygen saturation; TDCI, thermodilution cardiac index; WHO, World Health Organization
Pulmonary arterial hypertension (PAH) is a condition characterized by narrowing of the small pulmonary arteries that can lead to right heart failure and death.1 Despite remarkable advances in treatment, mortality remains elevated, with a median survival of approximately 7 years from time of diagnosis.2 Given the high mortality of PAH, it is essential to accurately determine prognosis to guide its management.3 For instance, a patient at high mortality risk should be treated with combination therapy that includes parenteral prostacyclin analogs, whereas a patient at low to moderate risk is generally managed with dual oral therapy.3
Right heart catheterization (RHC) is not only an essential tool for diagnosis, but also for prognostication.3, 4, 5 In fact, several hemodynamic determinations, such as right atrial pressure (RAP), cardiac index, and mixed venous oxygen saturation (SvO2), are consistently used clinically. The risk stratification table for pulmonary hypertension (PH), elaborated by the European Society of Cardiology (ESC) and European Respiratory Society (ERS), includes RAP, cardiac index, and SvO2 as the hemodynamic risk variables.4 In practice, cardiac index by thermodilution (TDCI) and SvO2 are routinely measured during RHC; however, not uncommonly, there is conflicting information provided by these determinations.
Several studies investigated the prognostic value of various hemodynamic parameters in PAH.6, 7, 8, 9, 10 Although some studies showed both TDCI and SvO2 to be associated with survival,7,9 others found one or the other.8,10, 11, 12, 13 Although both measurements are important, SvO2 better informs on the adequacy of tissue oxygenation, an essential component in normal organ function.14
No prior studies described the degree of association between TDCI and SvO2 and their individual impact on risk group allocation in idiopathic and heritable PAH.4 We conducted this study to (1) compare TDCI and SvO2 and their prognostic significance in a cohort of mostly incident patients with either idiopathic or heritable PAH; (2) assess the individual effect of TDCI and SvO2 in allocating risk groups, based on the ESC/ERS PH multidimensional assessment table4; and (3) identify factors associated with group allocation discrepancy between TDCI and SvO2. We hypothesize that SvO2 is a better measurement than TDCI to assess prognosis in patients with either idiopathic or heritable PAH.
Methods
Study Subjects and Design
This retrospective study was approved by the Cleveland Clinic institutional review board (study No. 18-1518). Written informed consent was waived given the retrospective design. Patients with either idiopathic or heritable PAH were identified from the Cleveland Clinic Pulmonary Hypertension Registry. We included consecutive patients who underwent diagnostic RHC (incident cases) or had the first RHC at our institution (some of these patients were referred to us on PAH-specific therapies) and had measurements of both TDCI and SvO2. The study was conducted between October 1998 and November 2017. When available, we recorded data at time of the first follow-up RHC.
All patients had precapillary PH characterized by mean pulmonary artery pressure ≥ 25 mm Hg, pulmonary artery wedge pressure ≤ 15 mm Hg, and pulmonary vascular resistance > 3 Woods units, based on the definition of the Fifth World Symposium in PH.15 Two PH experts reviewed the information and agreed on PH etiology. Risk groups for TDCI and SvO2 (low, intermediate, and high risk) were allocated based on the cutoff proposed by the ESC/ERS guidelines.4
RHC
Subjects underwent RHC in the outpatient setting under local anesthesia. Oxygen flow was titrated to keep SpO2 ≥ 90% and given continuously at the same flow rate during the entire procedure. In supine position, with the transducer located in the midthoracic line (fourth intercostal space), we measured pulmonary pressures at end expiration. We measured the cardiac output by thermodilution technique,16 averaging at least three measurements with < 15% variation. We then calculated the cardiac index (cardiac output/body surface area).17
Laboratory Determinations
Mixed venous blood for gas analysis was obtained from the distal tip of the pulmonary artery catheter, while freely located in a main pulmonary artery. Catheter position was confirmed by waveform analysis and fluoroscopic evaluation. Then 10 mL of blood were discarded before a sample was collected in a blood gas syringe, rapidly removing any residual air. Blood gas samples were immediately analyzed using cooximetry, which allows precise measurement of the relative concentrations of oxyhemoglobin.
Other Data
We collected data on demographics, World Health Organization (WHO) functional class, and PAH treatment. We recorded the plasma N-terminal pro-B type natriuretic peptide (NT-pro BNP) level, 6-min walk test distance (6MWD), diffusion lung capacity for carbon monoxide, and echocardiographic variables (right ventricular function, right ventricular systolic pressure [RVSP],18 and/or patent foramen oval/intrapulmonary shunt). All these data were collected as close as possible to the date of the diagnostic and follow-up RHC.
Statistical Analysis
Continuous data are presented as mean ± SD. Categorical data are summarized as discrete values and percentages. Continuous and categorical variables were compared across the groups using analysis of variance and χ2 test, respectively. Given the multiple comparisons, P values were adjusted by false discovery rate (q < .05) using the Benjamini and Hochberg method.19 Associations were tested using the Pearson correlation test. After ESC/ERS PAH risk categorization,4 we considered the following cutoffs of TDCI and SvO2: (1) low risk (< 5% 1-year mortality): TDCI ≥ 2.5 L/min/m2 and SvO2 > 65%, (2) intermediate risk (5%-10% 1-year mortality): TDCI: 2 to 2.4 L/min/m2 and SvO2 between 60% and 65%, and (3) high risk (> 10% 1-year mortality): TDCI < 2 L/min/m2 and SvO2 < 60%. Agreement between TDCI and SvO2 in risk group allocation was tested using weighted kappa given ordered scale.20
Survival analysis was performed using Kaplan-Meier and Cox proportional hazards models. Kaplan-Meier survival curves were compared with log-rank test. The Cox model was adjusted by age, sex, and other prespecified variables (WHO functional class, 6MWD, and RAP). The proportional hazards assumption was satisfied. The starting point for the survival analysis was the date of the initial RHC at our institution. Patients were censored at time of lung transplantation and followed until death or end of the study in September 2018. Results of the Cox model are expressed as hazard ratios (HRs) with the corresponding 95% CIs. All P values are two-tailed and a value of < .05 was considered significant. The statistical analyses were performed using the statistical package IBM SPSS version 22 (IBM) and MedCalc version 18.11 (Ostend).
Results
Patient Characteristics
We included a total of 158 patients with a mean age ± SD of 58 ± 17 years, of whom 113 (72%) were women. The etiology of PH was idiopathic in 143 (91%) and heritable in 15 (9%) patients. A total of 38 patients (24%) had the initial RHC while receiving PAH therapies (one agent in 22 patients [14%], two agents in 10 patients [6%], and three agents in six patients [4%]). The rest of the patients’ characteristics are shown in Table 1.
Table 1.
Baseline Patient Characteristics (N = 158)
| Variables | Value |
|---|---|
| Age, y | 57.5 ± 16.6 |
| Female sex | 113 (72) |
| Race | |
| White | 131 (83) |
| Black | 22 (14) |
| Other | 5 (3) |
| BSA, m2 | 1.9 ± 0.3 |
| Etiology of PAH | |
| Idiopathic | 143 (91) |
| Heritable | 15 (9) |
| PAH medications | |
| CCB | 1 (1) |
| PDE5-inh | 24 (15) |
| ERA | 16 (10) |
| Inhaled prostacyclin | 2 (1) |
| Parenteral prostacyclin | 17 (11) |
| WHO functional class | |
| I | 2 (1) |
| II | 49 (31) |
| III | 83 (53) |
| IV | 24 (15) |
| Oxygen supplementation at rest | 53 (36) |
| 6MWD, m (n = 153) | 300 ± 119 |
| NT-pro BNP, pg/mL (n = 76) | 2,075 ± 3,048 |
| Hemoglobin, g/dL | 13.7 ± 2.1 |
| Dlco % predicted (n = 141) | 58 ± 24 |
| Echocardiogram | |
| RVSP, mm Hga | 78 ± 24 |
| RV function | |
| Normal | 35 (24) |
| Mildly dysfunctional | 19 (13) |
| Moderately dysfunctional | 35 (24) |
| Severely dysfunctional | 58 (40) |
| Pericardial effusion | 24 (15) |
| Bubble studyb | |
| Patent foramen ovale | 39 (25) |
| Intrapulmonary shunt | 5 (3) |
| RHC | |
| RAP, mm Hg | 10 ± 6 |
| mPAP, mm Hg | 51 ± 13 |
| PAWP, mm Hg | 10 ± 3 |
| CO by thermodilution, L/min | 4.5 ± 1.3 |
| Cardiac index by thermodilution, L/min/m2 | 2.4 ± 0.6 |
| PVR, Wood units | 10.1 ± 4.6 |
| SvO2, % | 63 ± 9 |
Values are mean ± SD or No. (%). Data on 6MWD, NT-pro BNP, and Dlco were only available in the number of patients indicated in parenthesis. Variables without associated number next to them were obtained in all subjects. For NT-pro BNP, the number of patients is lower because 62 subjects had B-type natriuretic peptide testing instead of NT-pro BNP. 6MWD = 6-min walk test distance; BSA = body surface area; CCB = calcium channel blocker; CO: cardiac output; Dlco = diffusion lung capacity for carbon monoxide; ERA = endothelin receptor antagonist; mPAP = mean pulmonary artery pressure; NT-pro BNP = N-terminal pro B-type natriuretic peptide; PAH = pulmonary arterial hypertension; PAWP = pulmonary artery wedge pressure; PDE5-inh = phosphodiesterase-5 inhibitor; PVR = pulmonary vascular resistance; RAP = right atrial pressure; RHC = right heart catheterization; RV = right ventricle; RVSP = right ventricular systolic pressure; SvO2 = mixed venous oxygen saturation; WHO = World Health Organization.
In 11 patients, the RVSP could not be adequately measured and is therefore not reported.
Bubble study was not done in one patient.
Association Between TDCI and SvO2 and Its Impact on Risk Group Allocation
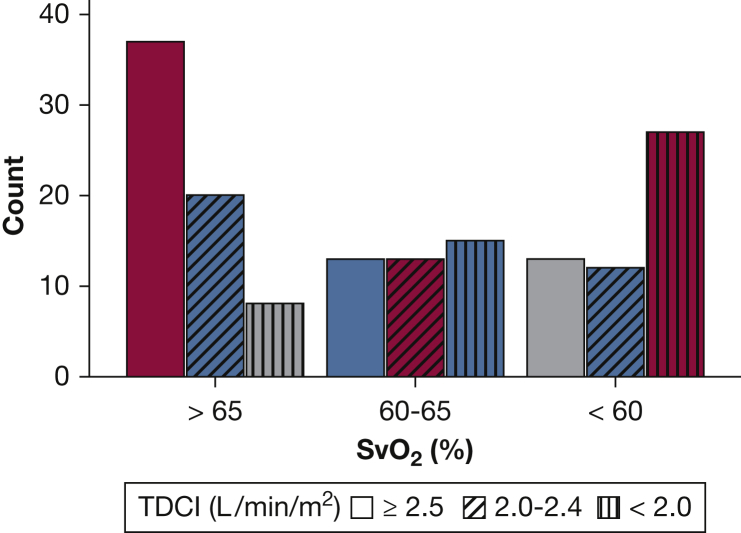
The mean TDCI was 2.4 ± 0.6 L/min/m2, with TDCI ≥ 2.5 L/min/m2 in 63 patients (40%), between 2.0 and 2.4 L/min/m2 in 45 patients (28%), and < 2.0 L/min/m2 in 50 patients (32%). The mean SvO2 was 63% ± 9%, with SvO2 > 65% in 65 patients (41%), between 60% and 65% in 41 patients (26%), and < 60% in 52 patients (33%). There was a significant association between TDCI and SvO2 (r = 0.50; 95% CI, 0.37-0.62). Weighted kappa, testing ERS/ESC risk group allocation agreement by TDCI or SvO2, was 0.30 (95% CI, 0.18-0.42), with risk group concordance in 77 patients (49%), and one and two class discordance in 60 (38%) and 21 (13%) patients, respectively (Fig 1).
Figure 1.
Concordance between SvO2 and TDCI risk groups allocation using the European Society of Cardiology and the European Respiratory Society guideline. SvO2 risk allocation is shown on the x axis. The bars represent the number of patients in each SvO2 and TDCI risk category. Bars shaded with red color represent patients with TDCI and SvO2 risk group concordance (eg, SvO2 > 65% and TDCI ≥ 2.5 L/min/m2). Blue bars represent patients with one risk class discordance (eg, SvO2 between 60% and 65% and TDCI ≥ 2.5 L/min/m2). Gray bars represent patients with two risk class discordance (eg, SvO2 < 60% and TDCI ≥ 2.5 L/min/m2). SvO2 = mixed venous oxygen saturation; TDCI = thermodilution cardiac index.
Factors Associated With Discordance Between TDCI and SvO2 Risk Group Allocation
Use of SvO2 instead of TDCI for ERS/ESC risk group assessment led to lower risk group allocation in 43 patients (27%), same risk group in 77 patients (49%), and higher risk group in 38 patients (24%). When the SvO2 was more severe than the TDCI risk stratification, patients had a lower 6MWD and lower hemoglobin concentration (Table 2).
Table 2.
Characteristics of Patients With Concordant and Discordant Risk Group Assessment Based on SvO2 and Cardiac Index
| Variables | SvO2 Better Than Cardiac Index (n = 43) | Equal Risk Group (n = 77) | SvO2 Worse Than Cardiac Index (n = 38) | P Value (ANOVA/χ2)a |
|---|---|---|---|---|
| Age, y | 60 ± 16 | 56 ± 16 | 58 ± 18 | .66 |
| Female sex | 32 (74) | 59 (77) | 22 (58) | .26 |
| Race | .09 | |||
| White | 37 (86) | 67 (87) | 27 (71) | |
| Black | 5 (12) | 6 (8) | 11 (29) | |
| Others | 1 (2) | 4 (5) | 0 (0) | |
| BSA, m2 | 1.9 ± 0.3 | 1.9 ± 0.3 | 1.9 ± 0.3 | .98 |
| Type of PAH | ||||
| Idiopathic PAH | 38 (88) | 68 (88) | 37 (97) | .38 |
| SpO2, % | 94 ± 5 | 95 ± 3 | 93 ± 5 | .34 |
| Oxygen supplementation at rest (yes) | 18 (43) | 23 (32) | 12 (34) | .64 |
| WHO functional class | 2.8 ± 0.7 | 2.7 ± 0.8 | 2.9 ± 0.9 | .79 |
| 6MWD, m (n = 153) | 312 ± 127 | 324 ± 113 | 235 ± 99 | .007 |
| Dlco % predicted (n = 141) | 57 ± 27 | 63 ± 23 | 49 ± 18 | .07 |
| NT-pro BNP, pg/mL (n = 76) | 1,481 ± 1,756 | 1,602 ± 3,771 | 2,999 ± 2,391 | .35 |
| Hemoglobin, g/dL | 14.4 ± 2.2 | 13.7 ± 1.9 | 12.9 ± 2.3 | .03 |
| Carboxyhemoglobin, % (n = 80) | 1.2 ± 0.3 | 1.4 ± 0.7 | 1.4 ± 0.7 | .39 |
| Methemoglobin, % (n = 79) | 0.9 ± 0.4 | 1.0 ± 0.5 | 0.9 ± 0.5 | .66 |
| Echocardiography | ||||
| RV function (moderate/severe) | 26 (62) | 45 (63) | 22 (65) | .97 |
| RVSP, mm Hgb | 76 ± 25 | 75 ± 22 | 84 ± 25 | .35 |
| Pericardial effusion (mild or more) | 4 (10) | 14 (19) | 6 (16) | .59 |
| PFOc | 13 (30) | 14 (18) | 12 (32) | .15 |
| Intrapulmonary shuntc | 2 (4.7) | 0 (0) | 3 (8) | |
| RHC | ||||
| Heart rate, beats/min | 78 ± 17 | 80 ± 13 | 81 ± 16 | .69 |
| RAP, mm Hg | 8 ± 5 | 10 ± 7 | 11 ± 5 | .37 |
| mPAP, mm Hg | 47 ± 12 | 52 ± 13 | 53 ± 11 | .20 |
| PAWP, mm Hg | 9 ± 3 | 10 ± 3 | 10 ± 3 | .40 |
| CO, L/min | 3.8 ± 0.7 | 4.6 ± 1.6 | 4.9 ± 0.9 | < .001 |
| Cardiac index, L/min/m2 | 2.0 ± 0.3 | 2.4 ± 0.7 | 2.6 ± 0.3 | < .001 |
| SvO2, % | 67 ± 4 | 63 ± 10 | 57 ± 6 | < .001 |
| PVR, Wood units | 11 ± 4 | 10 ± 5 | 9 ± 3 | .49 |
Values are mean ± SD, No. (%), or as otherwise indicated. Data on 6MWD, NT-pro BNP, DLCO, carboxyhemoglobin, and methemoglobin were only available in the number of patients indicated in parenthesis. Variables without associated number were obtained in all subjects. ANOVA = analysis of variance; PFO = patent foramen oval. See Table 1 legend for expansion of other abbreviations.
P value adjusted by false discovery rate correction for multiple comparisons using Benjamini and Hochberg methodology.
In 11 patients, the RVSP could not be adequately measured and is therefore not reported.
Bubble study was not done in one patient.
Comparison of TDCI and SvO2 Risk Group Allocation With Other ESC/ERS Risk Variables
When TDCI was compared with WHO functional class, 6MWD, NT-pro BNP, and RAP, a total of 63 (40%), 46 (29%), 56 (35%), and 66 (42%) patients had concordance in the ESC/ERS risk group allocation, respectively (Table 3). The weighted kappa for WHO functional class, 6MWD, NT-pro BNP, and RAP were 0.18 (95% CI, 0.07-0.30), 0.06 (95% CI, −0.04 to 0.16), 0.17 (95% CI, 0.04-0.30), and 0.19 (95% CI, 0.07-0.031), respectively.
Table 3.
Risk Assessment Comparison Between Cardiac Index and Other ESC/ERS Risk Variables
| Variable | ESC/ERS Risk Category4 |
P Value (χ2) |
||
|---|---|---|---|---|
| Low |
Intermediate |
High |
||
| TDCI ≥ 2.5 L/min/m2 (n = 63) | TDCI 2-2.4 L/min/m2 (n = 45) | TDCI < 2 L/min/m2 (n = 50) | P Value (χ2) | |
| WHO functional class | .01 | |||
| I/II | 28 (44)a | 14 (31) | 9 (18) | |
| III | 31 (49) | 23 (51)a | 29 (58) | |
| IV | 4 (6) | 8 (18) | 12 (24)a | |
| 6MWD, m (n = 153) | .06 | |||
| > 440 | 10 (17)a | 6 (14) | 1 (2) | |
| 165-440 | 44 (73) | 28 (64)a | 40 (82) | |
| < 165 | 6 (10) | 10 (23) | 8 (16)a | |
| NT-pro BNP, pg/mL (n = 136) | .04 | |||
| < 300 | 24 (43)a | 13 (35) | 6 (14) | |
| 300-1,400 | 15 (27) | 13 (35)a | 18 (42) | |
| > 1,400 | 17 (30) | 11 (30) | 19 (44)a | |
| RAP, mm Hg | .03 | |||
| < 8 | 33 (53)a | 17 (38) | 14 (28) | |
| 8-14 | 23 (37) | 18 (40)a | 21 (42) | |
| > 14 | 6 (10) | 10 (22) | 15 (30)a | |
| Pericardial effusion | 8 (13) | 6 (14) | 10 (21)a | .51 |
Values are No. (%) or as otherwise indicated. ESC/ERS = European Society of Cardiology and the European Respiratory Society; TDCI = thermodilution cardiac index. See Table 1 legend for expansion of other abbreviations.
Risk category that are concordant between the cardiac index and the comparator risk variable.
When SvO2 was compared with WHO functional class, 6MWD, NT-pro BNP, and RAP, a total of 75 (47%), 59 (37%), 74 (47%), and 72 (46%) patients had concordance in risk group allocation, respectively (Table 4). The weighted kappa for WHO functional class, 6MWD, NT-pro BNP, and RAP were 0.29 (95% CI, 0.17-0.40), 0.18 (95% CI, 0.08-0.28), 0.38 (95% CI, 0.25-0.51) and 0.27 (95% CI, 0.15-0.39), respectively.
Table 4.
Risk Assessment Comparison Between SvO2 and Other ESC/ERS Risk Variables
| Variable | ESC/ERS Risk Category4 |
P Value (χ2) |
||
|---|---|---|---|---|
| Low |
Intermediate |
High |
||
| SVO2 > 65% (n = 65) | SVO2 60%-65% (n = 41) | SVO2 < 60% (n = 52) | P Value (χ2) | |
| WHO class | < .001 | |||
| I/II | 35 (54)a | 8 (20) | 8 (15) | |
| III | 25 (39) | 27 (66)a | 31 (60) | |
| IV | 5 (8) | 6 (15) | 13 (25)a | |
| 6MWD, m (n = 153) | .001 | |||
| > 440 | 15 (24)a | 2 (5) | 0 (0) | |
| 165-440 | 40 (64) | 33 (83)a | 39 (78) | |
| < 165 | 8 (13) | 5 (13) | 11 (22)a | |
| NT-pro BNP, pg/mL (n = 136) | < .001 | |||
| < 300 | 30 (55)a | 9 (24) | 4 (9) | |
| 300-1,400 | 14 (26) | 18 (49)a | 14 (32) | |
| > 1,400 | 11 (20) | 10 (27) | 26 (59)a | |
| RAP, mm Hg | < .001 | |||
| < 8 | 37 (58)a | 18 (44) | 9 (17) | |
| 8-14 | 20 (31) | 17 (41)a | 25 (48) | |
| > 14 | 7 (11) | 6 (15) | 18 (35)a | |
| Pericardial effusion | 6 (10) | 7 (18) | 11 (22)a | .19 |
Survival Impact of TDCI and SvO2 Risk Group Allocation
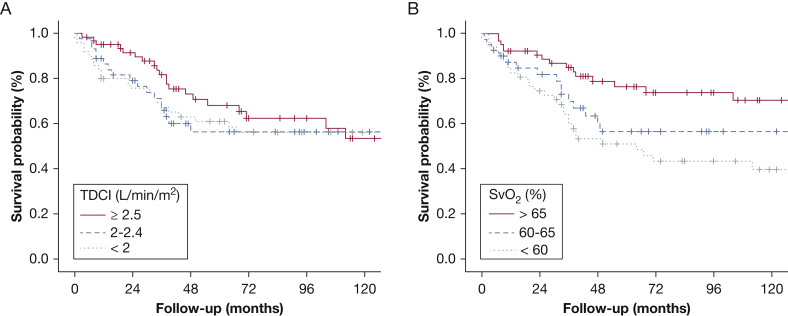
Patients were followed for a median of 45 months (interquartile range [IQR], 23-105). During follow-up, 62 patients (39%) died and eight patients (5%) underwent lung transplantation. Using Kaplan-Meier analysis, the survival varied by the SvO2 (log-rank = 0.002) (Fig 2B) but not by the TDCI risk group allocation (log-rank = 0.51) (Fig 2A). Using a Cox proportional hazard model adjusted for age and sex, SvO2 as a continuous variable was associated with mortality (HR per 1% increase, 0.94; 95% CI, 0.91-0.97; P < .001); in contrast, TDCI was not (HR per 1 L/min/m2 increase, 0.68; 95% CI, 0.43-1.08; P = .10).
Figure 2.
A-B, Kaplan-Meier analysis showing association between survival and European Society of Cardiology and the European Respiratory Society risk categories based on (A) TDCI and (B) SvO2 variables. See Figure 1 legend for expansion of abbreviations.
When adjusted by age, sex, WHO functional class, 6MWD, and RAP, SvO2 did not reach traditional statistical threshold of significance for predicting mortality (HR per 1% increase, 0.97; 95% CI, 0.93-1.00; P = .06). TDCI was not associated with mortality when adjusted by the same variables (HR per 1 L/min/m2 increase, 1.11; 95% CI, 0.65-1.89; P = .71). When both SvO2 and TDCI were included in the previous model, only SvO2 (and not TDCI) was a significant predictor of survival (HR per 1% increase, 0.96; 95% CI, 0.93-0.99; P = .03).
Sensitivity Analyses on Survival
We performed several sensitivity analyses to assess the impact of PAH-specific treatment at time of the initial RHC, hemoglobin concentration, and year of RHC. In treatment-naïve patients (n = 120), the P values of the log-rank test for the SvO2 and TDCI risk groups were .002 and .72, respectively. In patients with hemoglobin < 14 g/dL (n = 79), the P values for the log-rank test for the SvO2 and TDCI risk groups were .005 and 0.08, respectively. Meanwhile, in patients with hemoglobin ≥ 14 g/dL, the P values of the log-rank test for the SvO2 and TDCI risk groups were .23 and .97, respectively. However, hemoglobin concentration as a continuous variable had no impact on the significance of SvO2 (HR per 1% increase, 0.94; 95% CI, 0.92-0.97; P < .001) in a Cox model adjusted by age and sex. Patients who had the RHC done after January 2011 (n = 77) had P values for the log-rank test for SvO2 and TDCI of .003 and .43, respectively. In addition, when the year of RHC was added to a Cox model adjusted by age and sex, only SvO2 (HR per 1% increase, 0.94; 95% CI, 0.91-0.97; P < .001), but not cardiac index, predicted survival.
We tested the performance of stroke volume index (SVI) in the cohort. When added to a Cox model adjusted by age and sex, SVI predicted mortality (HR per 1 mL/m2 increase, 0.97; 95% CI, 0.94-0.99; P = .03). When both SVI and SvO2 were included in this model (after confirming the absence of significant collinearity), only SvO2 remained a significant predictor of mortality (HR per 1% increase, 0.93; 95% CI, 0.90-0.96; P < .001). When adjusted by age and sex, the overall model fit for SVI and SvO2 showed χ2 values of 9.1 (P = .028) and 23.1 (P < .001), respectively.
In addition, we tested the impact of SvO2 on survival in another cohort of patients who also had direct Fick cardiac index.21 Briefly, the cohort consisted of 75 patients with PAH (idiopathic and heritable in 72%) who had determinations of cardiac index by thermodilution and direct Fick. A total of 27 of these patients died during a median follow-up of 43 months (IQR, 15-71). Log-rank tests for SvO2 and cardiac index by thermodilution and direct Fick risk group allocation showed P values of .01, .06, and .77, respectively. SvO2 was significantly associated with mortality (HR per 1% increase, 0.90; 95% CI, 0.85-0.96; P < .001) in a Cox survival analysis adjusted by age and sex, in contrast with cardiac index by direct Fick that was not (HR per 1 L/min/m2 increase, 0.75; 95% CI, 0.45-1.26; P = .27).
Hemodynamic Determinations at Follow-up RHC
RHC was repeated in 80 patients at a median interval of 22 months (IQR, 10-49). The mean TDCI was 3.0 ± 0.7 L/min/m2, with TDCI ≥ 2.5 L/min/m2 in 59 patients (74%), between 2.0 and 2.4 L/min/m2 in 15 patients (19%), and < 2.0 L/min/m2 in six patients (7%). The mean SvO2 was 65% ± 10% with SvO2 > 65% in 44 patients (55%), between 60% and 65% in 14 patients (18%), and < 60% in 22 patients (28%). When compared with baseline, the TDCI increased by a mean of 0.7 ± 0.9 L/min/m2 (P < .001); however, the increase in SvO2 was not statistically significant (1.7% ± 10%, P = .11). The TDCI risk category remained unchanged, improved, and worsened in 32 (40%), 42 (53%), and six (8%) patients, respectively. Meanwhile, the SvO2 risk category remained unchanged, improved, and worsened in 37 (46%), 29 (36%), and 14 (18%) patients, respectively. Risk group concordance between TDCI and SvO2 was noted in 42 patients (53%), and one and two class discordance in 27 (34%) and 11 (14%) patients, respectively. Weighted kappa between the TDCI and SVO2 risk groups was 0.20 (95% CI, 0.05-0.35).
In 27 patients (34%), we noted concordance between TDCI and SvO2 risk group allocation during both the baseline and follow-up RHC. In 16 patients (20%), we noted the same type of discordance [SvO2 risk either better or worse that TDCI], while in the rest (n = 37 [46%]), the discordance between SvO2 and TDCI risk group allocation was different between baseline and follow-up RHC, supporting a lack of systematic error in the measurements.
After the second RHC, patients were followed for a median of 42.5 months (IQR, 16.2-89.0). A total of 25 patients (31.3%) died and four patients (5%) underwent lung transplantation. Similar to prior survival analysis, SvO2 (log-rank < 0.001) but not TDCI risk allocation (log-rank = 0.43) was associated with survival. Similarly, in a Cox model adjusted by age and sex, SvO2 (HR per 1% increase in SvO2, 0.88; 95% CI, 0.84-0.93; P < .001) but not TDCI was significantly associated with mortality. The change (follow-up minus baseline value) in SvO2 or TDCI as continuous variables or the ERS/ESC risk group change was not associated with survival, even after adjusting for age and sex.
Discussion
In a cohort of patients with well-characterized idiopathic and heritable PAH, mostly at the time of diagnostic RHC, we found a moderate degree of association between TDCI and SvO2 and fair agreement in the ESC/ERS risk group allocation. Risk group allocation concordance between TDCI and SvO2 was noted in about one-half the patients. Factors that help explain the variability included a lower hemoglobin concentration. SvO2 had a stronger association compared with TDCI, with other ESC/ERS risk parameters such as RAP, WHO functional class, 6MWD, and NT-pro BNP. Furthermore, SvO2 but not TDCI was associated with long-term survival after the initial RHC. Interestingly, the change in SvO2 and TDCI or the change in risk group allocation during follow-up RHC was not associated with survival.
To our knowledge, for the first time in PAH, we described the degree of concordance between SvO2 and TDCI and compared them with other ESC/ERS risk variables of the PH multidimensional assessment tool.4 We noted ESC/ERS risk group concordance between TDCI and SvO2 in about one-half of the patients both at baseline and follow-up, likely explained by a moderate degree of association between measurements, measurement errors inherent to TDCI,21 and the arbitrary cutoffs used in the ESC/ERS risk stratification tool, that add simplicity but overlooks subtle variations. For instance, TDCI could be 2.5 L/min/m2 (low risk) but SvO2 could be 65% (moderate risk). To our surprise, we saw risk discordance of two ESC/ERS risk groups in 13% and 14% of the patients, during initial and follow-up RHC, respectively, a finding that could carry important clinical implications. One of the factors that could explain this discrepancy is blood hemoglobin concentration. In fact, Jain et al22 implicated hemoglobin concentration (involved in oxygen delivery) and oxygen consumption as factors affecting the variability between SvO2 and cardiac index.
The cardiac index is a measure of ventricular output and is an indicator of right ventricular function in patients with PAH and normal left ventricular function. Cardiac index can be measured or estimated by several invasive and noninvasive methods,23, 24, 25, 26, 27 with the direct Fick method being the criterion standard.21 Because measuring the direct Fick method is technically demanding and the indirect Fick method is subject to errors in the estimation of oxygen consumption, TDCI is commonly done. We have recently shown that both TDCI and indirect Fick method have wide limits of agreement when compared with direct Fick method, which can impact ERS/ESC risk group allocation.21
Mixed venous blood is sampled from the pulmonary artery.28 The SvO2 reflects the balance between oxygen delivery and extraction, factors affected by cardiac output, oxygen consumption, arterial oxygen saturation, and hemoglobin concentration. Oxygen delivery is dictated by cardiac output and arterial oxygen content, whereas oxygen extraction results from the difference between arterial and venous oxygen content. A decrease in SvO2 indicates that the cardiac index is not enough to meet the tissue oxygen needs, and tissues needed to increase oxygen extraction. In contrast, a cardiac index determination does not specify whether the patient’s oxygen needs at a tissue level are met. In fact, in some subjects, a low cardiac index may be adequate to meet the tissue metabolic demands, while insufficient for others, a difference that can be gauged with SvO2.
We noted that SvO2 may be a better marker of prognosis than TDCI, supported by a stronger association with other ESC/ERS risk factors and overall survival. A few studies in PAH8,10,11,13 and cardiac diseases29 have shown that SvO2 but not cardiac index is associated with survival. We performed several sensitivity analyses to strengthen our findings. Our results were not affected when considering treatment-naïve patients, considering year of RHC, or using the direct Fick method instead of TDCI. SvO2 as a continuous variable remained a significant predictor of mortality when hemoglobin concentration was added to our Cox survival models. However, when patients were divided in two groups based on hemoglobin concentration, we noted that the impact on survival of the SvO2 risk allocation was only significant in the subgroup with hemoglobin < 14 g/dL, in whom oxygen transport to tissues may be more compromised.
Our study has limitations that include a single-center setting and retrospective design. Nevertheless, our novel findings displayed the pronounced degree of discordance between ESC/ERS risk scoring using recommended cutoffs for TDCI and SvO2. Furthermore, even when both TDCI and SvO2 are placed at equal level in the ESC/ERS risk scoring table, SvO2 better tracks with disease severity and survival. Our observations should alert physicians managing PAH about the importance of routinely obtaining SvO2 measurements for risk assessment because it may signal inadequate tissue oxygenation.
Conclusions
There is a moderate degree of association between TDCI and SvO2 in patients with idiopathic or heritable PAH. Given the significant association with survival and greater concordance with other risk variables, SvO2, as opposed to TDCI, appears to be superior in assessing severity of disease and predicting survival.
Acknowledgments
Author contributions: A. R. T. is the guarantor of the paper, taking responsibility for the integrity of the work, from inception to published article. G. K. and A. A. participated in the design of the study, data collection, statistical analysis, interpretation of the results, writing and critical revision of the manuscript for important intellectual content, and final approval of the manuscript submitted. T. N. and B. A. participated in data collection, writing and critical revision of the manuscript for important intellectual content, and final approval of the manuscript submitted. R. A. D. and G. A. H. participated in the design of the study, interpretation of the results and critical revision of the manuscript for important intellectual content, and final approval of the manuscript submitted. A. R. T. participated in the design of the study, statistical analysis and interpretation of the results, writing and critical revision of the manuscript for important intellectual content, and final approval of the manuscript submitted.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: G. A. H. received personal fees for being a member in Bayer HealthCare – Advisory Board and Speaking. None declared (G. K., A. A., T. N., B. A., R. A. D., A. R. T.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Footnotes
Drs Khirfan and Almoushref contributed equally to this manuscript.Part of this article has been presented in abstract form at the American Thoracic Society 2019 International Conference, May 17-22, 2019, Dallas, TX.
FUNDING/SUPPORT: Dr Tonelli is supported by the NIH [Grant R01HL130307].
References
- 1.Tonelli A.R., Arelli V., Minai O.A. Causes and circumstances of death in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2013;188(3):365–369. doi: 10.1164/rccm.201209-1640OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benza R.L., Miller D.P., Barst R.J. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest. 2012;142(2):448–456. doi: 10.1378/chest.11-1460. [DOI] [PubMed] [Google Scholar]
- 3.Galie N., Channick R.N., Frantz R.P. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. 2019;53(1) doi: 10.1183/13993003.01889-2018. 1801889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galiè N., Humbert M., Vachiery J.L. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Respir J. 2015;46(4):903–975. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 5.Swiston J.R., Johnson S.R., Granton J.T. Factors that prognosticate mortality in idiopathic pulmonary arterial hypertension: a systematic review of the literature. Respir Med. 2010;104(11):1588–1607. doi: 10.1016/j.rmed.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 6.van Wolferen S.A., Marcus J.T., Boonstra A. Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur Heart J. 2007;28(10):1250–1257. doi: 10.1093/eurheartj/ehl477. [DOI] [PubMed] [Google Scholar]
- 7.D'Alonzo G.E., Barst R.J., Ayres S.M. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115(5):343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 8.Raymond R.J., Hinderliter A.L., Willis P.W. Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. J Am Coll Cardiol. 2002;39(7):1214–1219. doi: 10.1016/s0735-1097(02)01744-8. [DOI] [PubMed] [Google Scholar]
- 9.Sandoval J., Bauerle O., Palomar A. Survival in primary pulmonary hypertension. Validation of a prognostic equation. Circulation. 1994;89(4):1733–1744. doi: 10.1161/01.cir.89.4.1733. [DOI] [PubMed] [Google Scholar]
- 10.Hoeper MM, Kramer T, Pan Z, et al. Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J. 2017;50(2):1700740. [DOI] [PubMed]
- 11.Higenbottam T.W., Spiegelhalter D., Scott J.P. Prostacyclin (epoprostenol) and heart-lung transplantation as treatments for severe pulmonary hypertension. Br Heart J. 1993;70(4):366–370. doi: 10.1136/hrt.70.4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee W.T., Ling Y., Sheares K.K. Predicting survival in pulmonary arterial hypertension in the UK. Eur Respir J. 2012;40(3):604–611. doi: 10.1183/09031936.00196611. [DOI] [PubMed] [Google Scholar]
- 13.Higenbottam T., Butt A.Y., McMahon A. Long-term intravenous prostaglandin (epoprostenol or iloprost) for treatment of severe pulmonary hypertension. Heart. 1998;80(2):151–155. doi: 10.1136/hrt.80.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walley K.R. Use of central venous oxygen saturation to guide therapy. Am J Respir Crit Care Med. 2011;184(5):514–520. doi: 10.1164/rccm.201010-1584CI. [DOI] [PubMed] [Google Scholar]
- 15.Simonneau G., Gatzoulis M.A., Adatia I. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 suppl):D34–D41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 16.Ganz W., Donoso R., Marcus H.S. A new technique for measurement of cardiac output by thermodilution in man. Am J Cardiol. 1971;27(4):392–396. doi: 10.1016/0002-9149(71)90436-x. [DOI] [PubMed] [Google Scholar]
- 17.Du Bois D., Du Bois E.F. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition. 1989;5(5):303–311. [PubMed] [Google Scholar]
- 18.Ahmed M., Dweik R.A., Tonelli A.R. What is the best approach to a high systolic pulmonary artery pressure on echocardiography? Cleve Clin J Med. 2016;83(4):256–260. doi: 10.3949/ccjm.83a.14186. [DOI] [PubMed] [Google Scholar]
- 19.Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57(1):289–300. [Google Scholar]
- 20.Cohen J. Weighted kappa: nominal scale agreement with provision for scaled disagreement or partial credit. Psychol Bull. 1968;70(4):213–220. doi: 10.1037/h0026256. [DOI] [PubMed] [Google Scholar]
- 21.Khirfan G., Ahmed M.K., Almaaitah S. Comparison of different methods to estimate cardiac index in pulmonary arterial hypertension. Circulation. 2019;140(8):705–707. doi: 10.1161/CIRCULATIONAHA.119.041614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jain A., Shroff S.G., Janicki J.S. Relation between mixed venous oxygen saturation and cardiac index. Nonlinearity and normalization for oxygen uptake and hemoglobin. Chest. 1991;99(6):1403–1409. doi: 10.1378/chest.99.6.1403. [DOI] [PubMed] [Google Scholar]
- 23.Hoeper M.M., Maier R., Tongers J. Determination of cardiac output by the Fick method, thermodilution, and acetylene rebreathing in pulmonary hypertension. Am J Respir Crit Care Med. 1999;160(2):535–541. doi: 10.1164/ajrccm.160.2.9811062. [DOI] [PubMed] [Google Scholar]
- 24.Schwaiblmair M., Faul C., von Scheidt W. Differences of cardiac output measurements by open-circuit acetylene uptake in pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: a cohort study. Respir Res. 2012;13(1):18. doi: 10.1186/1465-9921-13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tonelli A.R., Alnuaimat H., Li N. Value of impedance cardiography in patients studied for pulmonary hypertension. Lung. 2011;189(5):369–375. doi: 10.1007/s00408-011-9299-y. [DOI] [PubMed] [Google Scholar]
- 26.Yung G.L., Fedullo P.F., Kinninger K. Comparison of impedance cardiography to direct Fick and thermodilution cardiac output determination in pulmonary arterial hypertension. Congest Heart Fail. 2004;10(2 suppl 2):7–10. doi: 10.1111/j.1527-5299.2004.03406.x. [DOI] [PubMed] [Google Scholar]
- 27.Tonelli A.R., Conci D., Tamarappoo B.K. Prognostic value of echocardiographic changes in patients with pulmonary arterial hypertension receiving parenteral prostacyclin therapy. J Am Soc Echocardiogr. 2014;27(7):733–741.e732. doi: 10.1016/j.echo.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kandel G., Aberman A. Mixed venous oxygen saturation. Its role in the assessment of the critically ill patient. Arch Intern Med. 1983;143(7):1400–1402. doi: 10.1001/archinte.143.7.1400. [DOI] [PubMed] [Google Scholar]
- 29.Sumimoto T, Takayama Y, Iwasaka T, et al. Mixed venous oxygen saturation as a guide to tissue oxygenation and prognosis in patients with acute myocardial infarction. Am Heart J. 1991;122(1 pt 1):27-33. [DOI] [PubMed]