Abstract
Rubinstein-Taybi syndrome (RSTS; Online Mendelian Inheritance in Man® [OMIM®] #180849, #613684; Orpha: 783 ) is a rare plurimalformative autosomal dominant genetic disorder that affects one in 100,000-125,000 newborns with equal male and female distribution. It is characterized by distinctive facial features, short stature, broad and often angulated thumbs and halluces, and moderate-to-severe intellectual disability. In addition to ocular, cardiac, renal, endocrinologic, neurological, and psychomotor abnormalities, RSTS individuals can present with several gastrointestinal symptoms such as feeding difficulties, gastroesophageal reflux, and constipation. Currently, therapeutic strategies for RSTS involves a multi-disciplinary approach focusing mainly on symptomatic management. Here, we present a case of young-onset Barrett's esophagus in a patient with Rubinstein-Taybi syndrome.
Keywords: barrett's esophagus, rubinstein-taybi syndrome, low-grade dysplasia, gastroesophageal reflux disease, esophageal stricture, dysphagia
Introduction
Rubinstein-Taybi syndrome (RSTS) is a rare plurimalformative genetic disorder first described in 1963 by JH Rubinstein and H Taybi [1]. RSTS, also known as the broad thumb-hallux syndrome, is an autosomal dominant disease that affects one in 100,000-125,000 newborns with an equal male:female distribution [2]. It is characterized by distinctive facial features (hypertelorism, microcephaly, protruding beaked nose, downward-slanted eyes, thick and arched eyebrows), talon cusps teeth, short stature, broad and often angulated thumbs and halluces, and moderate-to-severe intellectual disability [3]. The differential diagnosis may include the Genitopatellar syndrome, the Floating-Harbour syndrome, and the Cornelia de Lange syndrome.
There are two types of RSTS. RSTS type 1 is caused by submicroscopic deletion of the cAMP response element-binding protein gene (CREBBP) located on the 156-kb region on chromosome 16p13.3 (50%-60%), and type 2 is caused by a mutation in the EP300 gene on chromosome 22q13.2 (~8%) [4]. However, in some people with RSTS, the cause is unknown. RSTS diagnosis is predominantly based on a physical examination and genetic testing by fluorescence in situ hybridization and genetic sequencing. Family studies and genetic studies suggest that the risk of recurrence of RSTS in siblings is less than one percent.
Gastrointestinal symptoms such as feeding difficulties, gastroesophageal reflux (GERD) (68%), constipation (40-74%), and Hirschsprung disease are prevalent in these patients [3]. If not promptly recognized and managed, these symptoms can lead to secondary complications such as failure to thrive and esophageal strictures.
Case presentation
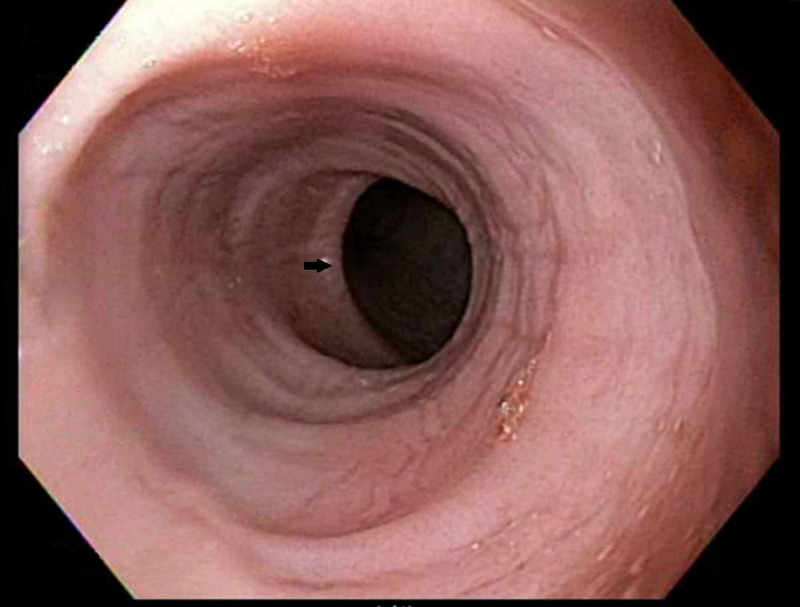
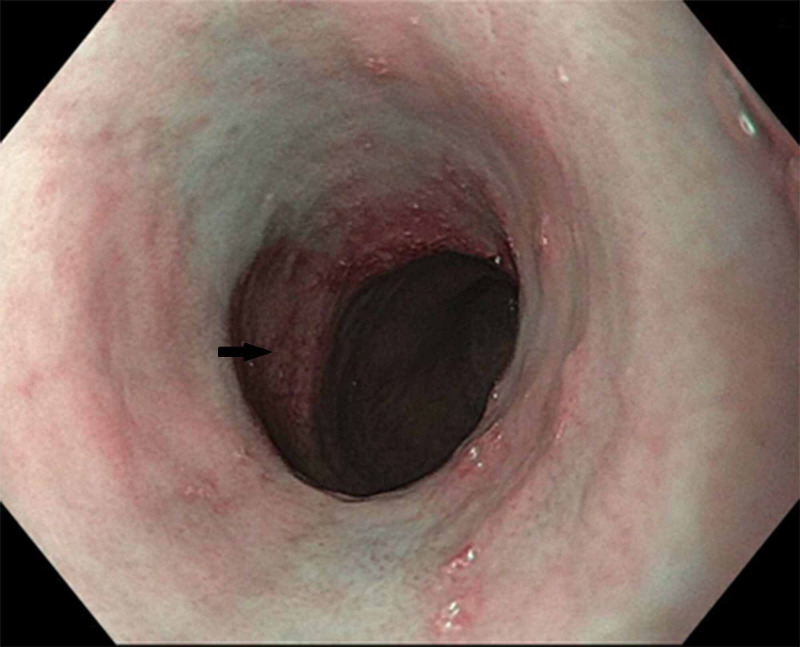
A 26-year-old non-Hispanic white man diagnosed with RSTS presented with dysphagia to solids and was found to have a cervical web on the esophagram. Esophagogastroduodenoscopy (EGD) showed an esophageal stricture extending from 18 to 22 cm, which was treated by endoscopic balloon dilation (Figure 1). A long-segment Barrett's esophagus (BE) extending from 22 to 32 cm was also present (Figure 2). Biopsies showed intestinal metaplasia without any dysplasia. The patient was treated with high dose proton pump inhibitor therapy twice a day. Since then, he was on surveillance for BE and underwent periodic dilations for esophageal stricture. Twelve years after the initial diagnosis of BE, he was found to low-grade dysplasia within the BE segment during periodic surveillance. He underwent one session of radiofrequency ablation therapy after stricture dilation. A follow-up EGD showed a 1 cm segment of BE with complete eradication of dysplasia. Two years later, he presented with recurrent dysphagia. EGD revealed a 5 cm long proximal esophageal stricture, which was dilated with an endoscopic balloon. There was also a 3 cm long BE segment in the distal esophagus with biopsies revealing low-grade dysplasia. The patient was scheduled for a repeat ablation session.
Figure 1. Esophageal stricture.
Figure 2. Barrett's esophagus.
Discussion
RSTS is associated with several gastrointestinal manifestations such as GERD, dysphagia, constipation, and rarely, Hirschsprung disease [3, 5]. Several case reports have illustrated various esophageal disorders such as GERD presenting with recurrent respiratory problems, post cricoid webs, eosinophilic esophagitis, and mediastinal vascular rings [5-8].
Here, we report the first case of young-onset BE in a patient with RSTS. BE is a premalignant condition characterized by replacing more than 1 cm of normal squamous epithelium with specialized intestinal metaplasia in the distal esophagus that often develops as an adaptive response to chronic GERD. The risk factors for BE include Caucasian race, male gender, age >50, severe GERD symptoms for more than five years, smoking, hiatal hernia, and family history of BE or esophageal adenocarcinoma (EAC) [9].
What is unusual in this case is the young age at the diagnosis of BE. Our patient has chronic GERD symptoms for more than 15 years before BE diagnosis. A review of prior literature shows that the prevalence of GERD among institutionalized individuals with an intellectual disability is about 50%, with 70% of these GERD patients having reflux esophagitis and 14% having BE. GERD has also been shown to be associated with cerebral palsy, an intelligence quotient (IQ) < 35, scoliosis, and the use of anticonvulsant drugs or benzodiazepines. To establish the diagnosis, 24-h pH measurement, or EGD, should be used in intellectually disabled individuals in whom GERD clinically is suspected [10]. Individuals with RSTS have varying degrees of intellectual disability; hence these individuals should be carefully screened to prevent any progression to GERD complications such as BE and EAC. Once diagnosed with BE, they should undergo periodic surveillance as per guidelines [9].
Conclusions
Since RSTS individuals can present with GERD symptoms and have a varying degree of intellectual disability, physicians should be vigilant about the risk of complications of GERD, such as esophageal strictures and BE. Hence, a prompt evaluation with EGD is indicated when these patients present with esophageal symptoms. Future studies are needed to assess the risk of EAC in these patients.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained by all participants in this study. N/a issued approval waived. Case reports do not require IRB approval in our institution
References
- 1.Broad thumbs and toes and facial abnormalities. A possible mental retardation syndrome. Rubinstein JH, Taybi H. Am J Dis Child. 1963;105:588–608. doi: 10.1001/archpedi.1963.02080040590010. [DOI] [PubMed] [Google Scholar]
- 2.Ultra-rare syndromes: the example of Rubinstein-Taybi syndrome. Spena S, Gervasini C, Milani D. J Pediatr Genet. 2015;4:177–186. doi: 10.1055/s-0035-1564571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stevens CA. GeneReviews®. Seattle : University of Washington; 2002. Rubinstein-Taybi syndrome. [PubMed] [Google Scholar]
- 4.Rubinstein-Taybi syndrome caused by submicroscopic deletions within 16p13.3. Breuning MH, Dauwerse HG, Fugazza G, et al. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1682202/ Am J Hum Genet. 1993;52:249–254. [PMC free article] [PubMed] [Google Scholar]
- 5.Gastroesophageal reflux in Rubinstein-Taybi syndrome. Grunow JE. J Pediatr Gastroenterol Nutr. 1982;1:273–274. doi: 10.1097/00005176-198201020-00019. [DOI] [PubMed] [Google Scholar]
- 6.Post-cricoid web associated with Rubinstein-Taybi syndrome. Scott AR, Proops DW, Kunick TK. J Laryngol Otol. 2000;114:637–638. doi: 10.1258/0022215001906363. [DOI] [PubMed] [Google Scholar]
- 7.Eosinophilic esophagitis and gastritis in Rubinstein-Taybi syndrome. Noble A, Drouin E, Faure C. J Pediatr Gastroenterol Nutr. 2007;44:498–500. doi: 10.1097/MPG.0b013e31802c41cd. [DOI] [PubMed] [Google Scholar]
- 8.Vascular ring leading to tracheoesophageal compression in a patient with Rubinstein-Taybi syndrome. Shashi V, Fryburg JS. Clin Genet. 1995;48:324–327. doi: 10.1111/j.1399-0004.1995.tb04119.x. [DOI] [PubMed] [Google Scholar]
- 9.Shaheen NJ, Falk GW, Iyer PG, Gerson LB. Am J Gastroenterol. Vol. 111. Gastroenterol: 2016 . ACG clinical guideline: diagnosis and management of Barrett's Esophagus; pp. 30–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The prevalence of gastroesophageal reflux disease in institutionalized intellectually disabled individuals. Böhmer CJ, Niezen-de Boer MC, Klinkenberg-Knol EC, Devillé WL, Nadorp JH, Meuwissen SG. Am J Gastroenterol. 1999;94:804–810. doi: 10.1111/j.1572-0241.1999.00854.x. [DOI] [PubMed] [Google Scholar]