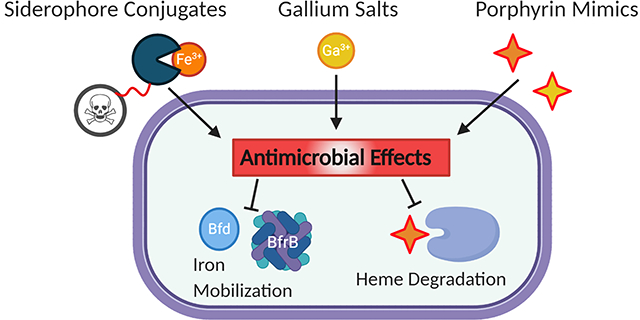
Abstract
Drug-resistant infections pose a significant risk to global health as pathogenic bacteria become increasingly difficult to treat. The rapid selection of resistant strains through poor antibiotic stewardship has reduced the number of viable treatments and increased morbidity of infections, especially among the immunocompromised. To circumvent such challenges, new strategies are required to stay ahead of emerging resistance trends, yet research and funding for antibiotic development lags other classes of therapeutics. Though the use of metals in therapeutics has been around for centuries, recent strategies have devoted a great deal of effort into the pathways through which bacteria acquire and utilize iron, which is critical for the establishment of infection. To target iron uptake systems, siderophore-drug conjugates have been developed that hijack siderophore-based iron uptake for delivery of antibiotics. While this strategy has produced several potential leads, the use of siderophores in infection is diminished over time when bacteria adapt to utilize heme as an iron source, leading to a need for the development of porphyrin mimetics as therapeutics. The use of such strategies as well as the inclusion of gallium, a redox-inert iron mimic, are herein reviewed.
Graphical Abstract
Introduction.
The prevalence of drug-resistant infections, particularly those acquired in hospitals, is of increasing concern and frequently prolongs hospital stays, increasing not only cost, but morbidity. The infections are commonly warned against in national and international reports where common drug-resistance bacteria such as the ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) are listed as high priority, pleading for increased research and improved antibiotic stewardship. Still, the challenges of antibiotic development have largely faced decreased funding and a weak pipeline of new candidates. From an industrial perspective, some of these challenges may be a lack of investment return whereas others report that even large screening campaigns that find successful target inhibitors often fail to have significant activity in cell culture.1,2
The utility of new antibiotics is further dampened by the onset of drug resistance, which has been observed even with new candidates and more advanced delivery methods.3 Overall, the 2019 clinical development pipeline contained 50 compounds, 32 of which targeted WHO priority pathogens and only two that were active against Gram-negative pathogens.4 The paradigm of development has recently evolved to investigate new targets such as virulence and resistance pathways rather than traditional approaches aimed at cell-wall synthesis or protein translation (where development focuses mainly on the improvement of existing molecular classes).5 The targeting of virulence factors is proposed to slow resistance development as it reduces infective potential of bacteria rather than survival.6 This approach has been relatively recent, however, and most progress remains academic.
The Development of Metallotherapeutics.
An interesting subset of antibiotic research has been the resurgence of metallotherapeutics – either new agents containing metal sites or those aimed specifically at bacterial metal utilization.7 Metal-based drugs are not uncommon but are largely underdeveloped as antibiotics and typically favor cancer therapies. The use of metals to prevent microbial growth is centuries old with copper being used as far back as 2600 BCE to sterilize wounds and water.8 Similarly, silver formulations date back to 1500 BCE with similar uses.9 While these approaches may have fallen out of favor in the 1940s and the advent of modern antibiotics, there is renewed interest in metal-based therapeutics with the onset of resistance. Such research has led to copper disinfectants or use as a coating on hospital surfaces, though this practice faces barriers to widespread use.10 New developments in wound dressings, a common site of bacterial infection, have also included silver carriers in the form of hydrogels.11–13 Even beyond infection, silver has woven its way into textiles aimed at reducing bacterial colonization in athletic apparel14–16 Approaches utilizing copper and silver rely on the inherent activity of the ions themselves and yet still have led to reports of emerging resistance.17 Beyond these approaches, the search for metallotherapeutics expands the potential antibiotic repertoire by increasing the landscape of molecular geometries and reactivities beyond purely organic compounds.18 A screen of the Community for Open Antimicrobial Drug Discovery (CO-ADD) library revealed that metal-containing compounds had a much higher hit-rate against bacteria than purely organic molecules (9.9% vs 0.87%, respectively).19 The development of such metallotherapeutics typically relies on the reactivity of the metal site for efficacy. Understanding such mechanisms is critical for the evolution of new therapies against bacteria that are constantly evolving. In addition to the development of new metallotherapeutics, much attention is now given towards understanding the ways in which bacteria sense, acquire and utilize metals from the environment so that these pathways can be exploited for drug development.
Iron and Virulence.
Other developments in metal-based antibiotic development have included interfering with iron uptake and utilization pathways. As these pathways are critical for infection and virulence but not necessarily for survival outside a host, it is less likely that poor antibiotic stewardship will exacerbate resistance.6 The natural redox activity of ferrous iron and the poor solubility of ferric iron already require tightly regulated storage and transport systems in a host. These mechanisms further help to keep labile iron concentrations below what would permit bacterial colonization, leading to the development of complex bacterial iron acquisition systems. Many virulence traits such as siderophore production, biofilm formation and exotoxin secretion are also regulated by iron levels, allowing bacteria to sense and respond to their environment and evade host defenses.20–22 Globally, bacteria are able to respond to changing iron levels through the Ferric Uptake Regulator (Fur), which is a transcription factor that undergoes a conformational change when bound to iron, repressing expression under iron-replete conditions (Figure 1).23,24
Figure 1.
Fur Regulation of Iron-Dependent Genes
Iron Acquisition Mechanisms.
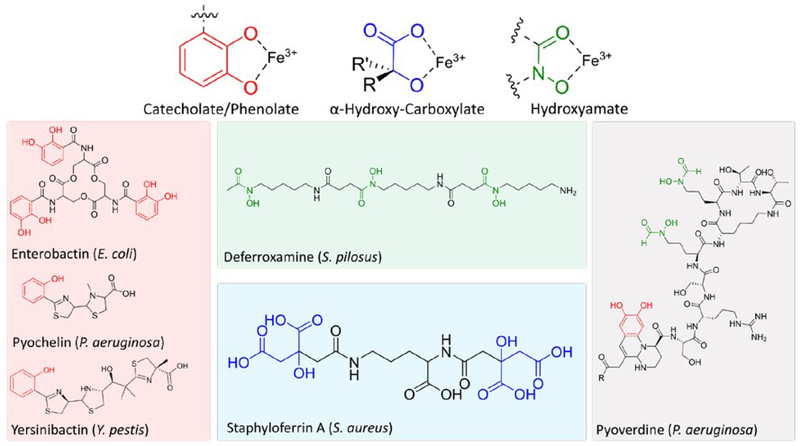
While host iron may be sequestered in storage proteins such as transferrin and lactoferrin, bacteria secrete iron-chelating siderophores (Figure 2). The structures of siderophores vary significantly between bacteria and can include peptidic and non-peptidic features. Regardless of the backbone, siderophores include common iron-coordinating features typically involving oxygen or nitrogen atoms capable of occupying two coordination sites and eventually form octahedral complexes. These molecules have some of the highest known iron binding affinities and are critical to the establishment of infection.25,26 Even still, host defense systems express siderocalin, a siderophore-binding protein meant to inhibit siderophore-based iron acquisition.27,28 Iron-bound siderophores are then transported through outer-membrane receptors. In gram-negative pathogens, ferric iron can be reduced in the periplasm and transported through the Feo system or the siderophore can be transported into the cytoplasm and reduced.29–31 Since the siderophore scaffold typically has a lower affinity for ferrous iron, the reduced form is released and utilized by the cell. In some cases, the siderophore also acts as a signaling molecule, alerting the cell to the availability of iron, and triggering the expression of virulence factors.32 It has also been shown that the expression of xenosiderophore receptors aids bacterial iron uptake and contributes to virulence. Using these receptors, pathogens like P. aeruginosa can utilize iron-chelating molecules other than their native pyoverdine and pyochelin. This is a useful survival trait that also allows for siderophore piracy in co-infections wherein P. aeruginosa can decrease native siderophore production while utilizing secreted siderophores from other bacteria.33,34 The presence of exogenous siderophores has been shown to repress native siderophore production, which is energetically favorable to the cell while maintaining the ability to acquire iron.35
Figure 2.
Siderophore Structures from Various Bacteria. Iron coordinating features shown in color.
Siderophore-Based Approaches.
One of the greatest barriers to efficacy is often permeability through bacterial membranes, often exacerbated by multidrug efflux pumps.36,37 To overcome this challenge, there have been several advancements in targeting iron uptake through siderophore receptors using siderophore-drug conjugates (SDCs) as the cliché Trojan horse approach.38–40 These are bifunctional molecules wherein traditional antibiotics are linked to known siderophore moieties to target them to the site of infection (Table 1). Even before the development of SDCs, bacteria have used this strategy to link toxicophores to secreted siderophores aimed at outcompeting other pathogens as evident by the characterization of naturally-occurring sideromycins such as albomycin, which is secreted by Actinomyces subtropicus in order to inhibit protein synthesis and outcompete neighboring bacteria.41,42 From a therapeutic development perspective, linking antibiotics to siderophores reduces typical resistance profiles such as membrane permeability and efflux since the antibiotic is transported along with a recognized siderophore and can also permit intracellular delivery of Gram-positive antibiotics to Gram-negative pathogens.43,44 The array of siderophore receptors also decreases the need for receptor specificity and allows a degree of structural diversity in SDCs and several siderophore/antibiotic combinations.
Table 1.
Siderophore-Drug Conjugate Examples and Activities
| Structure | Description | Mechanism | Activity |
|---|---|---|---|
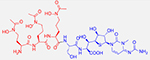 |
Albomycin δ2: | Pyrimidine protein synthesis inhibitor |
K. pneumoniae clinical isolates: (2 μg/mL in vitro) S. dysenteriae clinical isolates: (2 μg/ml in vitro) Y. enterocolitica (8081 serotype O:8) (10 mg/kg mouse) S. pneumoniae (D39/D39T1 albomycin resistant strain) (1 mg/kg mouse)41,42 |
 |
Enterobactin-Ampicillin Conjugate | β-Lactam inhibits cell wall synthesis |
E. coli45 25922 (laboratory): 0.1 μg/mL H9049 (nonpathogenic): 10 μg/mL CFT073 (urinary tract): 0.01 μg/mL UTI89 (urinary tract): 10 μg/mL 35401 (enterotoxigenic): 0.1 μg/mL 43895 (enterohemorrhagic): 1 μg/mL |
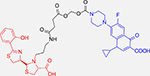 |
Pyochelin-Ciprofloxacin Conjugate | Fluoroquinolone DNA replication inhibitor |
P. aeruginosa46 PAO1: 0.7 μg/mL PAD07 (siderophore deficient): 0.7 μg/mL PAD07 (siderophore, TonB deficient): 0.2 μg/mL |
| Linezolid-Catechol Conjugate | Oxazolidinone protein synthesis inhibitor |
P. aeruginosa (PAO1)47 92 μg/mL (vs 740 for linezolid) |
|
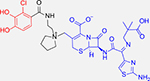 |
Cefiderocol: Cephalosporin-catechol conjugate | β-Lactam inhibits cell wall synthesis |
P. aeruginosa (0.1 –2 μg/mL) A. baumanii (0.1–1 μg/mL) K. pneumoniae (0.1–0.2 μg/mL)48,49 |
The Enterobacter conjugate reported by Zheng and Nolan fused the common β-lactams ampicillin and amoxicillin to the siderophore by functionalizing each piece to enable a copper-mediated azide/alkyne “click” reaction to generate the conjugate.45 It was found that the conjugate had activity against several pathogenic strains of E. coli and demonstrated superior efficacy to the parent drug. The conjugate was also highly specific (>1000-fold) for E. coli relative to K. pneumoniae and P. aeruginosa, which both have enterobactin uptake receptors but are less sensitive to ampicillin. This result highlights that even though bacteria may be able to transport a conjugate, the activity is still largely dependent on the species and strain. Though several strains of pathogenic and non-pathogenic E. coli were used, the uropathogenic CFT073 strain showed the greatest sensitivity to the conjugate, which the authors note is likely due to the greater variety of iron uptake systems. The activity against several strains is encouraging, though the selection away from these types of acquisition over time may decrease efficacy. However, the authors reported no significant resistance development over the course of the assay.
To target P. aeruginosa, Noël and coworkers reported a series of fluoroquinolone conjugates (ciprofloxacin, norfloxacin, N-desmethyl-oflaxacin) linked to pyochelin, one of the siderophores native to P. aeruginosa (and significantly smaller and more accessible than pyoverdine).46 These compounds are recognized by the pyochelin transporter FptA and transported into the cell. The activity, however, required a cleavable linker for all conjugates to release the antibiotic as the stable, non-hydrolyzable linkers showed no activity. Further, the conjugates themselves, though showing the potential for FptA as an SDC transporter, had reduced activity compared to the parent fluoroquinolone. The activity against the siderophore-deficient strains suggests that the linker may be cleaved in the extracellular media and that these conjugates are more pro-drugs than they are Trojan horses. The reduced efficacy of the conjugate, despite binding FptA, demonstrates the importance of linker design and stability. Another development targeting P. aeruginosa is the development of a linezolid conjugate reported by Paulen et. al.47 Oxazolidinone antibiotics are protein synthesis inhibitors with minimal activity against Gram-negative bacteria largely attributed to membrane permeability and efflux. Rather than use a known siderophore, a variety of spacers were used to attach a catechol group to the parent linezolid, again through the copper-catalyzed “click” reaction. As catechol groups are a common siderophore component (such as the previously mentioned enterobactin), the authors believed such a feature would increase transport into the cytoplasm. Indeed, the conjugate displayed improved activity (4–8-fold improvement) to linezolid, though still had MICs in the high micromolar (156–258 μM) range. Importantly, the activity was increased in iron-deficient media, highlighting the importance of iron uptake pathways for SDC transport. Additionally, though it contains a catechol group like enterobactin, the authors found that the enterobactin transporters of P. aeruginosa, PfeA, are not responsible for uptake. Though the resulting activity may still be too low for therapeutic use, the strategy highlights the potential to expand antibiotic efficacy across bacterial species by incorporating iron-chelating groups to target siderophore receptors without the inclusion of native siderophores.
The use of catechol groups in SDC development has also seen recent progress in targeting of iron uptake systems. Recent reports showed that teicoplanin, a glycopeptide that is inactive against Gram-negative bacteria, showed low micromolar activity against several strains of A. baumanii (including multi-drug resistant strains), representing a 60-fold improvement over the parent antibiotic.50 While these results are promising for the enhancement of current antibiotics, the conjugates still showed no improvement over teicoplanin in E. coli and P. aeruginosa and decreased activity in S. aureus.
The characterization of periplasmic or plasma-membrane bound transporters also further aids in our understanding of siderophore transport and the potential of catechol-based conjugates as drug therapies. Campylobacter jejuni, the most common causative agent of foodborne illness, was reported to acquire iron through linear enterobactin hydrolysis products (~100 times more favorably than enterobactin itself) and that these catechol-containing compounds bind to the periplasmic protein CeuE as well as the homologous periplasmic binding proteins in V. cholerae.51 These findings are further supported by the characterization of PiuA in S. pneumoniae, which binds the catechol-containing stress hormone norepinephrine as well as enterobactin hydrolysis products in a manner consistent with that reported in C. jejuni.52 Most importantly, in the context of drug design, these findings highlight the importance and potential for catechol-based drug conjugates. The structure of the binding regions of such proteins is largely solvent exposed and the conformation is minimally impacted by ligand binding. Such features imply that the binding of the iron-coordination complex is critical, but a greater structural variability is tolerated beyond the ferric center, providing a potential for derivatization and the generation of a wide range of conjugates.
Perhaps the most successful catechol-based SDC so far is the FDA-approved cefiderocol – a cephalosporin antibiotic with no natural siderophore attached (Table 1).53 Instead, like the linezolid conjugate, cefiderocol utilizes the iron-chelating catechol moiety to bind extracellular iron and is then transported through the outer-membrane through siderophore uptake pathways.54 Conversion of one hydroxyl group on the catechol moiety to a methoxy group significantly decreased activity, further suggesting the importance of iron-chelation as a mechanism of uptake.55 Luscher et. al also reported that bacteria such as P. aeruginosa express a suite of outer-membrane receptors, namely PiuA, Piera and PiuD, that contribute to siderophore uptake and piracy, and that these receptors were upregulated in the presence of cefiderocol as well as other conjugates.34 Deletion of identified receptors decreased susceptibility to cefiderocol treatment and constitutive expression of such receptors increased susceptibility by an order of magnitude. The promiscuity of such receptors confers susceptibility to siderophore-based drugs and thus slows the evolution of resistance. It is presently approved for the treatment of urinary tract infections and is active against E. coli, K. pneumoniae, Proteus mirabilis, P. aeruginosa, and Enterobacter cloacae.56
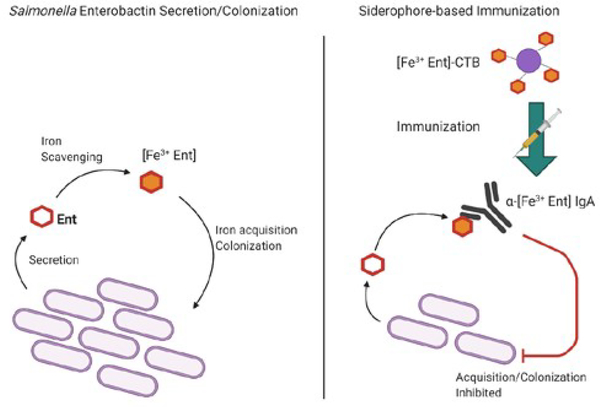
Despite a wide range of structures and siderophore/drug combinations, the SDC approach all follows the same strategy wherein holo-siderophores aid the penetration of an antibiotic conjugate into the cell. While some SDCs maintain affinity to cytoplasmic targets, the activity is typically much lower than that of the free drug, thus complicating linker design.57,58 As demonstrated in Table 1, the activity of the conjugate also varies significantly between bacteria, confounded by the respective combination of siderophore and drug. Though compounds with favorable activity are reported, the SDC approach seems unlikely to produce compounds with significant broad-spectrum activity. Even when factors such as linker, siderophore, and parent antibiotic are considered, the mechanistic approach of SDCs still involves the transport of chelated iron into the bacterium. In contrast to the trojan horse method, an alternative siderophore-based immunization strategy has been reported.59 As siderophores are not highly immunogenic, Sassone-Corsi and coworkers linked enterobactin to cholera toxin subunit B to produce an immune response against both Fe3+-enterobactin and a glucosylated form of Fe3+-enterobactin that is not recognized by the host siderocalin. Anti-siderophore antibodies were produced in mice following 100 μg/ immunization at zero and 14 days and were shown to reduce intestinal Salmonella colonization (Figure 3).
Figure 3.
Siderophore-based Immunization Against Salmonella using Ferric Enterobactin Conjugates. Left: Salmonella secretes enterobactin or a glucosylated form, which is not recognized by host defenses, and acquires iron. Right: conjugates fused to Cholera toxin subunit B produce antibodies against enterobactin, inhibiting further colonization and enteric inflammation.
Gallium as an Iron Mimic.
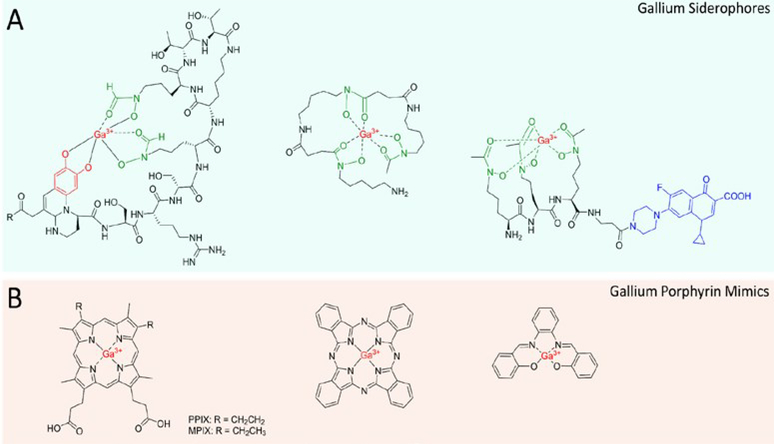
To circumvent the design and synthetic challenges of drug conjugates, significant research has been devoted towards the use of gallium. Ga3+ is an effective mimic of ferric iron due to its similar size and charge. Under physiological conditions, however, it cannot be reduced and does not allow the critical redox activity of iron which disrupts important metabolic pathways.60,61 Ganite (Ga(NO3)3)), an FDA-approved treatment for hypercalcemia in cancer patients, is currently in clinical trials for efficacy as an antibiotic.62,63 While the uptake mechanisms are under investigation, it is largely believed that gallium enters the cell through iron-siderophore uptake pathways, leading to the development of gallium-siderophores as a new antibacterial strategy. It is currently believed that gallium salts such as gallium nitrate target the bacteria through siderophore pathways whether administered as a salt of siderophore-chelate. Uncomplexed gallium is largely found in the iron-transporting transferrin, where it can be pirated by siderophores.64 Indeed, it was reported that the gallium-pyoverdine complex (Figure 4A) delivers gallium to the site of infection with favorable pharmacokinetics and high gallium delivery relative to gallium citrate, further establishing the potential of gallium-siderophores as potential therapeutics.65 By labeling the Ga-PVD complex with 68Ga, the infection could also be localized and imaged using positron-emission tomography. We cannot presently reconcile the necessity of iron reduction for siderophore-release with the redox-inert properties of gallium, but the mechanisms for such uptake pathways are under active investigation.66,67 Guo et al. proposed the periplasmic iron-binding protein HitA of P. aeruginosa as a potential pathway through which gallium is internalized.68 They found that gallium binds HitA in the same site as iron with low micromolar affinity (though weaker than ferric iron) and that genetic deletion of HitA conferred significant resistance to gallium toxicity.
Figure 4.
Gallium Compounds Discussed in this Review. A) Gallium siderophore strategies including Ga-Pyoverdine, Ga-Desferrioxamine and GaD2-Ciprofloxacin conjugate. B) Gallium porphyrins including GaPPIX/GaMPIX, GaPhthalocyanine and GaSalophen
Banin et. al reported the use of desferrioxamine-galllium as an anti-Pseudomonas agent, combining the toxicity of gallium with the chelation therapy of desferrioxamine, an iron-chelating siderophore from Streptomyces pilosus, in what was described as a “push-pull” mechanism.69 Mechanistically, this may be due to extracellular gallium release and iron sequestration by DFO or the use of DFO as a gallium delivery vehicle. The complex had an MIC of 32 μM against planktonic cells, in line with the activity of gallium alone. Gallium and the Ga-DFO complex also blocked biofilm formation at 10 μM, whereas DFO and gentamicin had minimal effect.
Diagnostic Applications of Gallium.
Combining the SDC approach with the antibacterial activity and radioactivity of 67Ga, the development of a ciprofloxacin conjugate as a therapeutic and diagnostic agent was reported with activity against Gram-negative P. aeruginosa (3.8 μM) and K. pneumoniae (0.94 μM) as well as Gram-positive S. aureus (12.5 μM).70 The gallium complex showed similar potency of the conjugate compared to ciprofloxacin (0.9–3.1 μM), better activity than the apo- (8–100 μM) or iron-bound (30 μM) conjugate and further allowed non-invasive pharmacokinetic tracking of the complex and its stability. As the complexes were determined to be largely stable, it is possible that the 67Ga center, which has a longer half-life albeit lower-resolution for imaging, could be substituted for 68Ga, which has a much shorter half-life but can be used for PET imaging.
So far, targeting iron uptake has shown encouraging results in the laboratory and can be seen in the clinic in the forms of chelation therapy and more recently, cefiderocol. These approaches have typically shown improved efficacy under iron-limiting conditions, but are relatively recent and have not been evaluated exhaustively against typical resistance phenotypes beyond varying strains.45–47,71,72 Additionally, exploiting iron uptake with gallium still results in iron deficiency, which can still trigger virulence pathways and may eventually counteract gallium toxicity.73
Perhaps the greatest barriers to targeting iron uptake are the dependence on antibiotic choice as well as linker design, highlighted by several examples in Table 1. Siderophore conjugates and the potential of gallium have been investigated and reviewed extensively with promising results yet the approval of cefiderocol and repurposing trials for Ganite remain the most significant advances. Future success must include conjugates with optimized physicochemical and pharmacological properties and improved activity over their parent antibiotics. This is largely dependent on the siderophore/drug combination and once again suggests the need to pursue such research with the same vigor as more “hot-button” diseases.
Additionally, a long-term approach with greater potential may be to target and inhibit the sensing and regulatory pathways that control iron homeostasis rather than just the iron acquisition pathway itself. As bacteria have extensive evolutionary experience in iron acquisition, such approaches must also constantly adapt to new pathways to circumvent resistance. Further, in chronic infection, the adaptation away from siderophores towards heme acquisition in a host is detrimental to the long-term efficacy of non-heme iron uptake inhibitors.
Heme Acquisition Mechanisms.
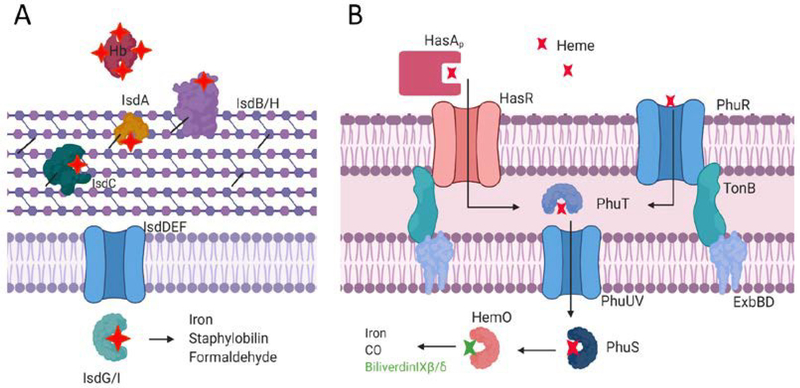
The secretion of high-affinity iron chelators is a critical evolutionary trait that allows bacteria to acquire iron from their environment, significantly predating pathogenicity. While the solubilization and acquisition of ferric iron in a host is important for infection, 75–90% of iron in a human host is found in hemoproteins.74 In host infections, bacteria have shown preference for heme as an iron source and pathways for sensing and uptake of exogenous heme have been identified in Gram-positive and Gram-negative pathogens.75–78 In Gram-positive bacteria such as Staphylococcus aureus, the iron-regulated surface determinant (Isd) family of proteins has been identified (Figure 5A). In this system, heme is acquired from host hemoglobin or haptoglobin by IsdB/H, transferred through a cascade of surface-attached proteins IsdA-C-E and transported into the cell via IsdD/F where it can be degraded by IsdG/I to release iron.79–81
Figure 5.
Representative Heme Uptake Systems in Gram-positive (A) and Gram-negative (B) Bacteria.
Heme uptake systems in Gram-negative bacteria are also well-studied and must include periplasmic and inner-membrane pathways in addition to outer-membrane receptors (Figure 5B).82 Bacteria such as Haemophilus influenzae, Yersinia pestis, Serratia marcescens and P. aeruginosa also secrete hemophores to capture host heme.83–87 Of note, Y. pestis, S. marcescens and P. aeruginosa all secrete the structurally similar HasA, which captures host heme through a dual ligation and conformational change described as a “fish biting heme.” In P. aeruginosa, transcriptomic experiments revealed that hasAp is the most upregulated gene in infection compared to lab cultures.88 Additionally, the persistence of P. aeruginosa biofilms in chronic infections show dependence on HasAp.89 It has also been shown that the hemophore system (Has; Heme assimilation system) in P. aeruginosa is distinct from the Phu (Pseudomonas heme uptake) system. While both the Has and Phu systems acquire extracellular heme, they have been characterized as non-redundant systems for sensing and primary uptake, respectively.90,91 Once transported into the cell, P. aeruginosa degrades heme via HemO to the metabolites biliverdin IXβ and biliverdin IXδ, which is distinct from classical α-producing heme oxygenases and suggests separate pathways for endogenous and exogenous heme.92,93 The BVIXβ isomer specifically has been shown to positively regulate the translation of HasAp, which adds another degree of tunability to respond to heme levels.94,95 The complex interplay between these acquisition and utilization systems and their roles in infection is further evidence of the tightly regulated pathways bacteria have evolved to adapt to their environments.
Porphyrin Therapeutic Development.
Perhaps the largest use of porphyrins and heme-like molecules is photodynamic therapy (PDT) wherein porphyrins are used as photosensitizers that can generate radicals locally following their uptake into the cell and photoactivation.96 These applications have used both metal-containing and metal-free porphyrins to varying degrees of success and are typically praised for their ability to target infection sites when the proper light source is applied.97,98 Advantageously, porphyrin derivatives are frequently synthesized by condensation of pyrrole with benzaldehyde derivatives to produce meso-substituted porphyrin derivatives.98 This allows significant variability in tuning the optical and physicochemical properties of the final product.98–100 To avoid additional photosensitizers, the development of antimicrobial blue-light PDT seeks to utilize endogenous bacterial porphyrins as photosensitizers leading to bacterial inactivation. Though limited by the penetrating power of the laser, this strategy has been tested against surface-level infections such as burn wounds that are commonly infected by drug-resistant pathogens such as P. aeruginosa and A. baumanii.101,102 Repeated cycles of sub-lethal blue light inactivation of bacteria did not appear to produce resistant phenotypes while significantly reducing bacterial burden in skin abrasions.
Much like the siderophore counterparts, gallium has also been used to target heme utilization pathways in bacteria (Figure 4B).103 Since heme-dependent processes rely on the redox activity of the iron center, gallium protoporphyrin IX (GaPPIX) effectively inhibits vital cellular processes with no structural perturbation to the tetrapyrrole macrocycle. In P. aeruginosa, GaPPIX enters the cell via the heme receptors HasR and PhuR. While it likely binds to HemO and prevents heme degradation and iron utilization, it can also target cytochromes and inhibit respiration.104 In several strains of A. baumanii, including clinical isolates classified as multi-drug resistant, GaPPIX reduced bacterial viability (MIC 20 μg/mL).105 In line with this activity, GaPPIX and Ga-mesoporphyrinIX (GaMPIX) were also more efficacious than Ga(NO3)3 at reducing growth in both planktonic (0.5–64 μM vs 64–256 μM) and biofilm models (32 μM vs no activity), supporting the adaptation towards heme in later-stage infections as well as the decreased susceptibility of biofilms to treatment.106 Further exploration of GaPPIX and GaMPIX nanoparticles demonstrated efficacy against P. aeruginosa and A. baumanii cultured in macrophages, biofilms and in infected Caenorhabditis elegans nematodes.107
To combine the effects of photodynamic therapy with the targeting of heme uptake in P. aeruginosa, Shisaka et.al investigated gallium-phthalocyanine (GaPc) as an antimicrobial (Figure 6A).108 In this case, the gallium center was used to generate singlet oxygen species following irradiation with near-infrared light, thus effectively eliminating viability in vitro (<0.1%). This work highlights the utility of antimicrobial delivery through the heme uptake systems as well as the ability of the heme scavenger HasAp to solubilize larger, hydrophobic macrocycles such as phthalocyanine. However, it is likely that because the macrocycle is transported through HasR, that the signaling effects of the Has system will be activated and lead to an increase in HasAp transcription that could potentially increase virulence in the long term. This result also conflicts with reports of FePc as blocking heme uptake as it is more likely that the macrocycle is transported but cannot be broken down to release iron as a mechanism of inhibition. Other work by this group has also expanded on the porphyrin structures tolerated by HasAp, providing a useful structural basis for the development of molecules targeting bacterial heme uptake by hemophores.109–111 While the large structural diversity of ligands accommodated by HasAp is encouraging from a design perspective, hemophore-targeting molecules must be able to compete with heme, which typically binds with low micromolar to nanomolar affinity.95,112,113 This competition may be more favorable under heme-limited conditions maintained by the host or when high levels of apo-HasAp are present. To aid in this competition, porphyrin mimics can be designed to include specific structural features known to contribute to heme binding, which is largely based on hydrophobic interactions in the heme binding site that lead to rapid ligand association rates.114,115
Figure 6.
Gallium Phthalocyanine (A) and Gallium Salophen (B) use Hemophore Systems for Efficacy.
Recently, it was reported that a gallium-salophen compound was able to target hemophore-based heme acquisition as well as siderophore uptake pathways of P. aeruginosa (Figure 6B).113 The planar, aromatic features of the salophen molecule permitted binding to HasAp in the heme binding site. While the iron-salophen molecule bound to HasAp, it was able to act as an iron source, in contrast with its previous characterization as a heme-uptake inhibitor.109 Switching the metal to gallium showed toxicity to cultures despite uptake mechanisms independent of HasR and PhuR. These results demonstrated that the gallium-salophen complex was able to target iron uptake pathways and bind to HasAp, inhibiting activation of the Has system. Simultaneously targeting iron and heme uptake pathways is more difficult to circumvent through traditional resistance mechanisms. Disrupting heme sensing, given its importance in infection, is likely to disrupt intracellular iron homeostasis and virulence at large. As exogenous siderophores repress pyoverdine and pyochelin production, whether this molecule is capable of repressing siderophore synthesis when internalized would also be a useful metric to determine its future success as an inhibitor.35
Alternative Strategies.
Though we have focused largely on metal-containing siderophore and porphyrin-based metallotherapeutics and how the use of gallium has intersected both strategies, it remains important to consider other critical findings related to the role of iron in bacterial pathogenesis.
Chelation Strategies.
Like siderophore-based strategies, iron chelators have been investigated as antibacterial agents. Rather than seek to improve permeability of linked drugs or deliver toxic gallium, chelators act by sequestering available iron away from bacteria. Though this can be done using deferoxamine (previously mentioned), which is approved by the FDA for chelation therapy, and enhances the activity of tobramycin against P. aeruginosa biofilms in a cystic fibrosis co-culture model.71 Beyond siderophores, the use of iron chelators has shown similar effects against biofilms. Chan and co-workers screened a variety of antibiotics for iron-binding activity through visual and spectrophotometric inspection.116 Through this approach, they sought antibiotic combinations that would enhance the activity of thiostrepton, a peptide antibiotic that enters P. aeruginosa through pyoverdine receptors. Notably, selected chelates as well as gallium nitrate were bacteriostatic but showed enhanced bactericidal activity in combination with thiostrepton against clinical isolates of P. aeruginosa and A. baumanii. The authors proposed such “adjuvants” as ways to limit iron availability and spur the upregulation of bacterial iron acquisition systems that leave them more susceptible to thiostrepton (and by extension, other iron uptake-based approaches). The results of these studies also show significant dependence on the aerobic/anaerobic nature of the system and could be largely attributable to the chelation preferences and availability of Fe3+ over Fe2+.72
It is also important to note that such metal-coordinating molecules are not unique to iron. Metallophores such as staphylopine (S. aureus) and pseudopaline (P. aeruginosa) are secreted to capture other essential transition metals (Mn, Co, Cu, Zn) are also critical to survival of bacteria, though are not as extensively characterized as siderophores.117,118 For example, the characterization of staphylopine-mediated acquisition is relatively recent but is still an important step in applying many of the iron-based therapeutics herein mentioned towards new metals and pathways.119 We direct the reader towards notable examples of pro-chelators and strategies for the interruption of metal homeostasis beyond the initial scope of this review.7,120,121
Heme Degradation.
Other notable strategies targeting bacterial iron homeostasis, though not directly involved in the coordination of metals, include the disruption of heme degradation and iron trafficking in P. aeruginosa. Previous studies have shown the P. aeruginosa heme binding and degradation proteins PhuS and HemO, respectively, are critical for driving extracellular heme internalization.94,122 The inhibition of enzymatic heme degradation and the resultant lack of the heme metabolites biliverdin IXβ/δ will subsequently decrease not only heme flux, but prevent the utilization of heme-bound iron.95 Since the metabolites of heme degradation also play a role in the upregulation of the heme-sensing Has system and expression of the hemophore HasAp, the decreased biliverdin levels are likely to further dampen heme sensing abilities.94 To this extent, several approaches targeting HemO have been reported and are under current development.123–125
Iron Mobilization.
Beyond the acquisition and degradation of vital extracellular heme, targeting iron mobilization is also a new method of interrupting iron homeostasis. BfrB, the main iron storage protein in P. aeruginosa, requires interaction with the ferredoxin Bfd to mobilize stored iron for use by the cell. Consequently, the inhibition of this interaction prohibits iron release leading to irreversible iron storage and an iron-deficient cytosol.126 This strategy was initially uncovered using ΔbfrB and ΔbfD mutants but has more recently been interrogated with the development of small-molecule isoindoline BfrB/Bfd inhibitors, leading to an iron-starvation response.127 Further, the inhibitors had improved activity in combination with the commercial antibiotic ciprofloxacin relative to either the inhibitor or ciprofloxacin alone. Most recently, the inhibition of the interaction also showed encouraging disruption of P. aeruginosa biofilms, a common and recalcitrant form of infection.128 The activity, irrespective of environmental iron availability, suggests that such a strategy holds merit in several stages of infection whereas the inhibition of iron uptake may be more suited to the early stages.
Conclusions.
While under active research, the development of metal-based or metal-targeting therapeutics for antimicrobial purposes lags that of other fields and behind traditional antibiotic strategies. Presently, many new developments are showing promise but are often more effective in combination with existing drugs, leading to more complicated treatment routines. The history of drug discovery also hinders new paradigms in antibiotic development wherein specific, targeted therapies are desired for cancer while antibiotics have hitherto been cheap, broad-spectrum agents.129,130 Nonetheless, significant progress has been made in the development of antimicrobials targeting iron and heme utilization pathways. The combination of siderophores with existing antibiotics presents opportunities for customizable molecules based on the bacterial species and their antibiotic susceptibility and are likely to be useful for initial stages of infection. The utility and evolution of porphyrin therapies has also led to a variety of potential therapies and mechanisms. The dual presence of iron and heme utilization pathways and the ability to shift between the two is likely the largest barrier to long-term success, and strategies that account for these pathways and their role in infection are the best suited for further exploration. While we have focused mainly on iron-targeting approaches, the exploration of other essential metals and therapeutic strategies is also critical to a deeper understanding of virulence and resistance development. Ultimately, resistance is unavoidable, and researchers must be adequately prepared to constantly develop new strategies. Certainly, acknowledging this aids the drug development process and where we can predict or avoid potential resistance mechanisms with clever design, but we must also recognize that we remain vastly outnumbered by the microbial world with millennia of experience evolving to survive.
Acknowledgements
G.C. acknowledges the Chemistry/Biology Interface Training Program (NIGMS/NIH T32GM066706) and a Department of Pharmaceutical Sciences Fellowship. A. W. acknowledges NIH award AI134886. F.X and A.W. aided manuscript conceptualization and construction. Figures 1, 3, 5,6 and TOC image created with BioRender.
Footnotes
Conflicts of interest
There are no conflicts to declare.
Publisher's Disclaimer: This is an Accepted Manuscript, which has been through the Royal Society of Chemistry peer review process and has been accepted for publication.
References
- 1.Bettiol E, Wetherington JD, Schmitt N and Harbarth S, Challenges and solutions for clinical development of new antibacterial agents: Results of a survey among pharmaceutical industry professionals, Antimicrob. Agents Chemother, 2015, 59, 3695–3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tommasi R, Brown DG, Walkup GK, Manchester JI and Miller AA, ESKAPEing the labyrinth of antibacterial discovery, Nat. Rev. Drug Discov, 2015, 14, 529–542. [DOI] [PubMed] [Google Scholar]
- 3.Lee HH and Collins JJ, Microbial Environments Confound Antibiotic Efficacy, Nat. Chem. Biol, 2012, 8, 6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.W. H. Organization, 2019 Antibacterial Agents in Clinical Development: an analysis of the antibacterial clinical development pipeline, Geneva, 2019. [Google Scholar]
- 5.Ruer S, Pinotsis N, Steadman D, Waksman G and Remaut H, Virulence-targeted Antibacterials: Concept, Promise, and Susceptibility to Resistance Mechanisms, Chem. Biol. Drug Des, 2015, 86, 379–399. [DOI] [PubMed] [Google Scholar]
- 6.Dickey SW, Cheung GYC and Otto M, Different drugs for bad bugs: antivirulence strategies in the age of antibiotic resistance, Nat. Rev. Drug Discov, 2017, 16, 457–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunsaker EW and Franz KJ, Emerging Opportunities to Manipulate Metal Trafficking for Therapeutic Benefit, Inorg. Chem, 2019, 58, 13528–13545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dollwet HHA and Sorenson JRJ, Historic uses of copper compounds in medicine, Trace Elem. Med, 1985, 2, 80–87. [Google Scholar]
- 9.Möhler JS, Sim W, Blaskovich MAT, Cooper MA and Ziora ZM, Silver bullets: A new lustre on an old antimicrobial agent, Biotechnol. Adv, 2018, 36, 1391–1411. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt MG, Von Dessauer B, Benavente C, Benadof D, Cifuentes P, Elgueta A, Duran C and Navarrete MS, Copper surfaces are associated with significantly lower concentrations of bacteria on selected surfaces within a pediatric intensive care unit, Am. J. Infect. Control, 2016, 44, 203–209. [DOI] [PubMed] [Google Scholar]
- 11.Zakia M, Koo JM, Kim D, Ji K, Huh P, Yoon J and Il Yoo S, Development of silver nanoparticle-based hydrogel composites for antimicrobial activity, Green Chem. Lett. Rev, 2020, 13, 34–40. [Google Scholar]
- 12.Diniz FR, Maia RCAP, Rannier Andrade L, Andrade LN, Vinicius Chaud M, da Silva CF, Corrêa CB, de Albuquerque Junior RLC, Pereira da Costa L, Shin SR, Hassan S, Sanchez-Lopez E, Souto EB and Severino P, Silver Nanoparticles-Composing Alginate/Gelatine Hydrogel Improves Wound Healing In Vivo, Nanomaterials, 2020, 10, 390–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Souza A, Yoon J, Beaman H, Gosavi P, Lengyel-Zhand Z, Sternisha A, Centola G, Marshall LR, Wehrman MD, Schultz KM, Monroe M and Makhlynets OV, Nine-residue peptide self-assembles in the presence of silver to produce a self-healing, cytocompatible, antimicrobial hydrogel, ACS Appl. Mater. Interfaces, 2020, 12, 17091–17099. [DOI] [PubMed] [Google Scholar]
- 14.Gao Yuan and Cranston R, Recent Advances in Antimicrobial Treatments of Textiles, Text. Res. J, 2008, 78, 60–72. [Google Scholar]
- 15.Gerba CP, Sifuentes LY, Lopez GU, Abd-Elmaksoud S, Calabrese J and Tanner B, Wide-spectrum activity of a silver-impregnated fabric, Am. J. Infect. Control, 2016, 44, 689–690. [DOI] [PubMed] [Google Scholar]
- 16.Sim W, Barnard RT, Blaskovich MAT and Ziora ZM, Antimicrobial silver in medicinal and consumer applications: A patent review of the past decade (2007–2017), Antibiotics, DOI: 10.3390/antibiotics7040093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hosny AEDMS, Rasmy SA, Aboul-Magd DS, Kashef MT and El-Bazza ZE, The increasing threat of silver-resistance in clinical isolates from wounds and burns, Infect. Drug Resist, 2019, 12, 1985–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrison CN, Prosser KE, Stokes RW, Cordes A, Metzler-Nolte N and Cohen SM, Expanding medicinal chemistry into 3D space: Metallofragments as 3D scaffolds for fragment-based drug discovery, Chem. Sci, 2020, 11, 1216–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frei A, Zuegg J, Elliott AG, Baker M, Braese S, Brown C, Chen F, Dowson CG, Dujardin G, Jung N, King AP, Mansour AM, Massi M, Moat J, Mohamed HA, Renfrew AK, Rutledge PJ, Sadler PJ, Todd MH, Willans CE, Wilson JJ, Cooper MA and Blaskovich MAT, Metal complexes as a promising source for new antibiotics, Chem. Sci, 2020, 11, 2627–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ochsner UA, Johnson Z, Lamont IL, Cunliffe HE and Vasil ML, Exotoxin A production in Pseudomonas aeruginosa requires the iron-regulated pvdS gene encoding an alternative sigma factor, Mol. Microbiol, 1996, 21, 1019–1028. [DOI] [PubMed] [Google Scholar]
- 21.Oglesby AG, Farrow JM, Lee JH, Tomaras AP, Greenberg EP, Pesci EC and Vasil ML, The influence of iron on Pseudomonas aeruginosa physiology: A regulatory link between iron and quorum sensing, J. Biol. Chem, 2008, 283, 15558–15567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oglesby-Sherrouse AG, Djapgne L, Nguyen AT, Vasil AI and Vasil ML, The complex interplay of iron, biofilm formation, and mucoidy affecting antimicrobial resistance of Pseudomonas aeruginosa, Pathog. Dis, 2014, 70, 307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bags A and Neilands JB, Ferric Uptake Regulation Protein Acts as a Repressor, Employing Iron(II) as a Cofactor To Bind the Operator of an Iron Transport Operon in Escherichia coli, Biochemistry, 1987, 26, 5471–5477. [DOI] [PubMed] [Google Scholar]
- 24.Escolar L, Pérez-Martín J and De Lorenzo V, Opening the iron box: Transcriptional metalloregulation by the fur protein, J. Bacteriol, 1999, 181, 6223–6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saha R, Saha N, Donofrio RS and Bestervelt LL, Microbial siderophores: A mini review, J. Basic Microbiol, 2013, 53, 303–317. [DOI] [PubMed] [Google Scholar]
- 26.Miethke M and Marahiel MA, Siderophore-Based Iron Acquisition and Pathogen Control, Microbiol. Mol. Biol. Rev, 2007, 71, 413–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN and Strong RK, The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition, Mol. Cell, 2002, 10, 1033–1043. [DOI] [PubMed] [Google Scholar]
- 28.Holmes MA, Paulsene W, Jide X, Ratledge C and Strong RK, Siderocalin (Lcn 2) also binds carboxymycobactins, potentially defending against mycobacterial infections through iron sequestration, Structure, 2005, 13, 29–41. [DOI] [PubMed] [Google Scholar]
- 29.Neilands JB, Siderophores: Structure and function of microbial iron transport compounds, J. Biol. Chem, 1995, 270, 26723–26726. [DOI] [PubMed] [Google Scholar]
- 30.Lau CKY, Krewulak KD and Vogel HJ, Bacterial ferrous iron transport: The Feo system, FEMS Microbiol. Rev, 2016, 40, 273–298. [DOI] [PubMed] [Google Scholar]
- 31.Sestok AE, Linkous RO and Smith AT, Toward a mechanistic understanding of Feo-mediated ferrous iron uptake, Metallomics, 2018, 10, 887–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ravel J and Cornelis P, Genomics of pyoverdine-mediated iron uptake in pseudomonads, Trends Microbiol, 2003, 11, 195–200. [DOI] [PubMed] [Google Scholar]
- 33.Ghysels B, Ochsner U, Möllman U, Heinisch L, Vasil M, Cornelis P and Matthijs S, The Pseudomonas aeruginosa pirA gene encodes a second receptor for ferrienterobactin and synthetic catecholate analogues, FEMS Microbiol. Lett, 2005, 246, 167–174. [DOI] [PubMed] [Google Scholar]
- 34.Luscher A, Moynié L, Saint Auguste P, Bumann D, Mazza L, Pletzer D, Naismith JH and Köhler T, TonB-Dependent Receptor Repertoire of Pseudomonas aeruginosa for Uptake of Siderophore-Drug Conjugates., Antimicrob. Agents Chemother, DOI: 10.1128/AAC.00097-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perraud Q, Cantero P, Roche B, Gasser V, Normant VP, Kuhn L, Hammann P, Mislin GLA, Ehret-Sabatier L and Schalk IJ, Phenotypic adaption of Pseudomonas aeruginosa by hacking siderophores produced by other microorganisms, Mol. Cell. Proteomics, 2020, 19, 589–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winkler ML, Papp-Wallace KM, Hujer AM, Domitrovic TN, Hujer KM, Hurless KN, Tuohy M, Hall G and Bonomo RA, Unexpected challenges in treating multidrug-resistant Gram-negative bacteria: resistance to ceftazidime-avibactam in archived isolates of Pseudomonas aeruginosa., Antimicrob. Agents Chemother, 2015, 59, 1020–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alcalde-Rico M, Hernando-Amado S, Blanco P and Martinez JL, Multidrug efflux pumps at the crossroad between antibiotic resistance and bacterial virulence, Front. Microbiol, 2016, 7, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ji C, Juárez-Hernández RE and Miller MJ, Exploiting bacterial iron acquisition: Siderophore conjugates, Future Med. Chem, 2012, 4, 297–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ji C, Miller PA and Miller MJ, Iron transport-mediated drug delivery: Practical syntheses and in vitro antibacterial studies of tris-catecholate siderophore-aminopenicillin conjugates reveals selectively potent antipseudomonal activity, J. Am. Chem. Soc, 2012, 134, 9898–9901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kong H, Cheng W, Wei H, Yuan Y, Yang Z and Zhang X, An overview of recent progress in siderophore-antibiotic conjugates, Eur. J. Med. Chem, DOI: 10.1016/j.ejmech.2019.111615. [DOI] [PubMed] [Google Scholar]
- 41.Gause GF and Biol D, Recent studies on albomycin, a new antibiotic, Br. Med. J, 1955, 2, 1177–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pramanik A, Stroeher UH, Krejci J, Standish AJ, Bohn E, Paton JC, Autenrieth IB and Braun V, Albomycin is an effective antibiotic, as exemplified with Yersinia enterocolitica and Streptococcus pneumoniae, Int. J. Med. Microbiol, 2007, 297, 459–469. [DOI] [PubMed] [Google Scholar]
- 43.Braun V, Active transport of siderophore-mimicking antibacterials across the outer membrane, Drug Resist. Updat, 1999, 2, 363–369. [DOI] [PubMed] [Google Scholar]
- 44.Liu R, Miller PA, Vakulenko SB, Stewart NK, Boggess WC and Miller MJ, A Synthetic Dual Drug Sideromycin Induces Gram-Negative Bacteria to Commit Suicide with a Gram-Positive Antibiotic, J. Med. Chem, 2018, 61, 3845–3854. [DOI] [PubMed] [Google Scholar]
- 45.Zheng T and Nolan EM, Enterobactin-mediated delivery of ß-lactam antibiotics enhances antibacterial activity against pathogenic Escherichia coli, J. Am. Chem. Soc, 2014, 136, 9677–9691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Noël S, Gasser V, Pesset B, Hoegy F, Rognan D, Schalk IJ and Mislin GLA, Synthesis and biological properties of conjugates between fluoroquinolones and a N3′′-functionalized pyochelin, Org. Biomol. Chem, 2011, 9, 8288–8300. [DOI] [PubMed] [Google Scholar]
- 47.Paulen A, Gasser V, Hoegy F, Perraud Q, Pesset B, Schalk IJ and Mislin GLA, Synthesis and antibiotic activity of oxazolidinone-catechol conjugates against Pseudomonas aeruginosa, Org. Biomol. Chem, 2015, 13, 11567–11579. [DOI] [PubMed] [Google Scholar]
- 48.Negash KH, Norris JKS and Hodgkinson JT, Siderophore-antibiotic conjugate design: New drugs for bad bugs?, Molecules, 2019, 24, 3314–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu JY, Srinivas P and Pogue JM, Cefiderocol: A Novel Agent for the Management of Multidrug-Resistant Gram-Negative Organisms, Infect. Dis. Ther, 2020, 9, 17–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghosh M, Miller PA and Miller MJ, Antibiotic repurposing: bis-catechol- and mixed ligand (bis-catechol-mono-hydroxamate)-teicoplanin conjugates are active against multidrug resistant Acinetobacter baumannii, J. Antibiot. (Tokyo)., 2020, 73, 152–157. [DOI] [PubMed] [Google Scholar]
- 51.Raines DJ, Moroz OV, Blagova EV, Turkenburg JP, Wilson KS and Duhme-Klair AK, Bacteria in an intense competition for iron: Key component of the Campylobacter jejuni iron uptake system scavenges enterobactin hydrolysis product, Proc. Natl. Acad. Sci. U. S. A, 2016, 113, 5850–5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y, Edmonds KA, Raines DJ, Murphy BA, Wu H, Guo C, Nolan EM, VanNieuwenhze MS, Duhme-Klair AK and Giedroc DP, The Pneumococcal Iron Uptake Protein A (PiuA) Specifically Recognizes Tetradentate FeIIIbis- and Mono-Catechol Complexes, J. Mol. Biol, 2020, 432, 5390–5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.U. S. F. and D. Administration, FDA approves new antibacterial drug to treat complicated urinary tract infections as part of ongoing efforts to address antimicrobial resistance, https://www.fda.gov/news-events/press-announcements/fda-approves-new-antibacterial-drug-treat-complicated-urinary-tract-infections-part-ongoing-efforts, (accessed 14 July 2020).
- 54.Ito A, Nishikawa T, Matsumoto S, Yoshizawa H, Sato T, Nakamura R, Tsuji M and Yamano Y, Siderophore cephalosporin cefiderocol utilizes ferric iron transporter systems for antibacterial activity against Pseudomonas aeruginosa, Antimicrob. Agents Chemother, 2016, 60, 7396–7401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aoki T, Yoshizawa H, Yamawaki K, Yokoo K, Sato J, Hisakawa S, Hasegawa Y, Kusano H, Sano M, Sugimoto H, Nishitani Y, Sato T, Tsuji M, Nakamura R, Nishikawa T and Yamano Y, Cefiderocol (S-649266), A new siderophore cephalosporin exhibiting potent activities against Pseudomonas aeruginosa and other gram-negative pathogens including multi-drug resistant bacteria: Structure activity relationship, Eur. J. Med. Chem, 2018, 155, 847–868. [DOI] [PubMed] [Google Scholar]
- 56.FETROJA- cefiderocol sulfate tosylate injection, powder, for solution, https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=75c0c785-38e0-4049-a6fb-b77581f5b35c, (accessed 15 July 2020).
- 57.Wencewicz TA, Möllmann U, Long TE and Miller MJ, Is drug release necessary for antimicrobial activity of siderophore-drug conjugates? Syntheses and biological studies of the naturally occurring salmycin ‘trojan Horse’ antibiotics and synthetic desferridanoxamine- antibiotic conjugates, BioMetals, 2009, 22, 633–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Neumann W and Nolan EM, Evaluation of a reducible disulfide linker for siderophore-mediated delivery of antibiotics, J. Biol. Inorg. Chem, 2018, 23, 1025–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sassone-Corsi M, Chairatana P, Zheng T, Perez-Lopez A, Edwards RA, George MD, Nolan EM and Raffatellu M, Siderophore-based immunization strategy to inhibit growth of enteric pathogens, Proc. Natl. Acad. Sci. U. S. A, 2016, 113, 13462–13467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Apseloff G, Therapeutic Uses of Gallium Nitrate: Past, Present, and Future, Am. J. Ther, 1999, 6, 327–339. [DOI] [PubMed] [Google Scholar]
- 61.Kaneko Y, Thoendel M, Olakanmi O, Britigan BE and Singh PK, The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity., J. Clin. Invest, 2007, 117, 877–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goss CH, Kaneko Y, Khuu L, Anderson GD, Ravishankar S, Aitken ML, Lechtzin N, Zhou G, Czyz DM, McLean K, Olakanmi O, Shuman HA, Teresi M, Wilhelm E, Caldwell E, Salipante SJ, Hornick DB, Siehnel RJ, Becker L, Britigan BE and Singh PK, Gallium disrupts bacterial iron metabolism and has therapeutic effects in mice and humans with lung infections., Sci. Transl. Med, DOI: 10.1126/scitranslmed.aat7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pena A, In CF Lung Infections, Gallium Nitrate Shows Promise in Phase 1 Trial, https://cysticfibrosisnewstoday.com/2018/10/09/gallium-nitrate-shows-promise-cf-lung-infections-phase-1-trial/, (accessed 13 July 2020).
- 64.Chitambar CR, Biochim. Biophys. Acta - Mol. Cell Res, 2016, 1863, 2044–2053. [DOI] [PubMed] [Google Scholar]
- 65.Petrik M, Umlaufova E, Raclavsky V, Palyzova A, Havlicek V, Haas H, Novy Z, Dolezal D, Hajduch M and Decristoforo C, Imaging of Pseudomonas aeruginosa infection with Ga-68 labelled pyoverdine for positron emission tomography, Sci. Rep, 2018, 8, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cox CD, Iron reductases from Pseudomonas aeruginosa., J. Bacteriol, 1980, 141, 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ganne G, Brillet K, Basta B, Roche B, Hoegy F, Gasser V and Schalk IJ, Iron Release from the Siderophore Pyoverdine in Pseudomonas aeruginosa Involves Three New Actors: FpvC, FpvG, and FpvH, ACS Chem. Biol, 2017, 12, 1056–1065. [DOI] [PubMed] [Google Scholar]
- 68.Guo Y, Li W, Li H and Xia W, Identification and Characterization of a Metalloprotein Involved in Gallium Internalization in Pseudomonas aeruginosa, ACS Infect. Dis, 2019, 5, 1693–1697. [DOI] [PubMed] [Google Scholar]
- 69.Banin E, Lozinski A, Brady KM, Berenshtein E, Butterfield PW, Moshe M, Chevion M, Greenberg EP and Banin E, The potential of desferrioxamine-gallium as an anti-Pseudomonas therapeutic agent, Proc. Natl. Acad. Sci. U. S. A, 2008, 105, 16761–16766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pandey A, Savino C, Ahn SH, Yang Z, Van Lanen SG and Boros E, Theranostic Gallium Siderophore Ciprofloxacin Conjugate with Broad Spectrum Antibiotic Potency, J. Med. Chem, 2019, 62, 9947–9960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moreau-Marquis S, O’Toole GA and Stanton BA, Tobramycin and FDA-approved iron chelators eliminate Pseudomonas aeruginosa biofilms on cystic fibrosis cells, Am. J. Respir. Cell Mol. Biol, 2009, 41, 305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mettrick K, Hassan K, Lamont I and Reid D, The Iron-chelator, N,N’-bis (2-hydroxybenzyl) Ethylenediamine-N,N’-diacetic acid is an Effective Colistin Adjunct against Clinical Strains of Biofilm-Dwelling Pseudomonas aeruginosa, Antibiotics, 2020, 9, 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.García-Contreras R, Pérez-Eretza B, Lira-Silva E, Jasso-Chávez R, Coria-Jiménez R, Rangel-Vega A, Maeda T and Wood TK, Gallium induces the production of virulence factors in Pseudomonas aeruginosa, Pathog. Dis, 2014, 70, 95–98. [DOI] [PubMed] [Google Scholar]
- 74.Otto BR, Verweij-van Vught AMJJ and Maclaren DM, Transferrins and Heme-Compounds as Iron Sources for Pathogenic Bacteria, Crit. Rev. Microbiol, 1992, 18, 217–233. [DOI] [PubMed] [Google Scholar]
- 75.Skaar EP, Humayun M, Bae T, DeBord KL and Schneewind O, Iron-Source Preference of Staphylococcus aureus Infections, Science, 2004, 305, 1626–1628. [DOI] [PubMed] [Google Scholar]
- 76.Nguyen AT, O’Neill MJ, Watts AM, Robson CL, Lamont IL, Wilks A and Oglesby-Sherrouse AG, Adaptation of iron homeostasis pathways by a Pseudomonas aeruginosa pyoverdine mutant in the cystic fibrosis lung., J. Bacteriol, 2014, 196, 2265–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marvig RL, Damkiær S, Khademi SMH, Markussen TM, Molin S and Jelsbak L, Within-Host Evolution of Pseudomonas aeruginosa Reveals Adaptation toward Iron Acquisition from Hemoglobin, MBio, DOI: 10.1128/MBIO.00966-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marvig RL, Sommer LM, Molin S and Johansen HK, Convergent evolution and adaptation of Pseudomonas aeruginosa within patients with cystic fibrosis, Nat. Genet, 2015, 47, 57–64. [DOI] [PubMed] [Google Scholar]
- 79.Muryoi N, Tiedemann MT, Pluym M, Cheung J, Heinrichs DE and Stillman MJ, Demonstration of the iron-regulated surface determinant (Isd) heme transfer pathway in Staphylococcus aureus, J. Biol. Chem, 2008, 283, 28125–28136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Torres VJ, Pishchany G, Humayun M, Schneewind O and Skaar EP, Staphylococcus aureus IsdB is a hemoglobin receptor required for heme iron utilization, J. Bacteriol, 2006, 188, 8421–8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reniere ML, Ukpabi GN, Harry SR, Stec DF, Krull R, Wright DW, Bachmann BO, Murphy ME and Skaar EP, The IsdG-family of haem oxygenases degrades haem to a novel chromophore, Mol. Microbiol, 2010, 75, 1529–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang W and Wilks A, Extracellular Heme Uptake and the Challenge of Bacterial Cell Membranes, Annu. Rev. Biochem, DOI: 10.1146/annurev-biochem-060815-014214. [DOI] [PubMed] [Google Scholar]
- 83.Zambolin S, Clantin B, Chami M, Hoos S, Haouz A, Villeret V and Delepelaire P, Structural basis for haem piracy from host haemopexin by Haemophilus influenzae, Nat. Commun DOI: 10.1038/ncomms11590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kumar R, Lovell S, Matsumura H, Battaile KP, Moënne-Loccoz P and Rivera M, The hemophore HasA from Yersinia pestis (HasAyp) coordinates hemin with a single residue, Tyr75, and with minimal conformational change, Biochemistry, 2013, 52, 2705–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arnoux P, Haser R, Izadi N, Lecroisey A, Delepierre M, Wandersman C and Czjzek M, The crystal structure of HasA, a hemophore secreted by Serratia marcescens, Nat. Struct. Biol, 1999, 6, 516–520. [DOI] [PubMed] [Google Scholar]
- 86.Létoffé S, Redeker V and Wandersman C, Isolation and characterization of an extracellular haem-binding protein from Pseudomonas aeruginosa that shares function and sequence similarities with the Serratia marcescens HasA haemophore, Mol. Microbiol, 1998, 28, 1223–1234. [DOI] [PubMed] [Google Scholar]
- 87.Alontaga AY, Rodríguez JC, Schönbrunn E, Becker A, Funke T, Yukl ET, Hayashi T, Stobaugh J, Moënne-Loccoz P and Rivera M, Structural Characterization of the Hemophore HasAp from Pseudomonas aeruginosa : NMR Spectroscopy Reveals Protein−Protein Interactions between Holo-HasAp and Hemoglobin, Biochemistry, 2009, 48, 96–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Damron FH, Oglesby-Sherrouse AG, Wilks A and Barbier M, Dual-seq transcriptomics reveals the battle for iron during Pseudomonas aeruginosa acute murine pneumonia, Sci. Rep, 2016, 6, 39172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thöming JG, Tomasch J, Preusse M, Koska M, Grahl N, Pohl S, Willger SD, Kaever V, Müsken M and Häussler S, Parallel evolutionary paths to produce more than one Pseudomonas aeruginosa biofilm phenotype, npj Biofilms Microbiomes, 2020, 6, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ochsner UA, Johnson Z and Vasil ML, Genetics and regulation of two distinct haem-uptake systems, phu and has, in Pseudomonas aeruginosa, Microbiology, 2000, 146, 185–198. [DOI] [PubMed] [Google Scholar]
- 91.Smith AD and Wilks A, Differential contributions of the outer membrane receptors PhuR and HasR to heme acquisition in Pseudomonas aeruginosa., J. Biol. Chem, 2015, 290, 7756–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ratliff M, Zhu W, Deshmukh R, Wilks A and Stojiljkovic I, Homologues of neisserial heme oxygenase in gram-negative bacteria: degradation of heme by the product of the pigA gene of Pseudomonas aeruginosa., J. Bacteriol, 2001, 183, 6394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wegele R, Tasler R, Zeng Y, Rivera M and Frankeinberg-Dinkel N, The heme oxygenase(s)-phytochrome system of Pseudomonas aeruginosa, J. Biol. Chem, 2004, 279, 45791–45802. [DOI] [PubMed] [Google Scholar]
- 94.Mouriño S, Giardina BJ, Reyes-Caballero H and Wilks A, Metabolite-driven Regulation of Heme Uptake by the Biliverdin IXβ/δ-Selective Heme Oxygenase (HemO) of Pseudomonas aeruginosa., J. Biol. Chem, 2016, 291, 20503–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dent AT, Mouriño S, Huang W and Wilks A, Post-transcriptional regulation of the Pseudomonas aeruginosa heme assimilation system (Has) fine-tunes extracellular heme sensing., J. Biol. Chem, 2019, 294, 2771–2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Castano AP, Demidova TN and Hamblin MR, Mechanisms in photodynamic therapy: Part one - Photosensitizers, photochemistry and cellular localization, Photodiagnosis Photodyn. Ther, 2004, 1, 279–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dai T, Huang YY and Hamblin MR, Photodynamic therapy for localized infections-State of the art, Photodiagnosis Photodyn. Ther, 2009, 6, 170–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Amos-Tautua BM, Songca SP and Oluwafemi OS, Application of porphyrins in antibacterial photodynamic therapy, Molecules, DOI: 10.3390/molecules24132456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Titov DV, Gening ML and Tsvetkov YE, Porphyrins and phthalocyanines: promising molecules for light-triggered antibacterial nanoparticles Related content Glycoconjugates of porphyrins with carbohydrates: methods of synthesis and biological activity, Adv. Nat. Sci Nanosci. Nanotechnol, 2014, 5, 33002–330015. [Google Scholar]
- 100.Moylan C, Scanlan E and Senge M, Chemical Synthesis and Medicinal Applications of Glycoporphyrins, Curr. Med. Chem, 2015, 22, 2238–2348. [DOI] [PubMed] [Google Scholar]
- 101.Amin RM, Bhayana B, Hamblin MR and Dai T, Antimicrobial blue light inactivation of Pseudomonas aeruginosa by photo-excitation of endogenous porphyrins: In vitro and in vivo studies, Lasers Surg. Med, 2016, 48, 562–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang Y, Zhu Y, Gupta A, Huan Y, Murray CK, Vrahas MS, Sherwood DG, Baer Margaret E., Hamblin MR and Dai T, Antimicrobial blue light therapy for multidrug-resistant Acinetobacter baumannii infection in a mouse burn model: implications for prophylaxis and treatment of combat-related wound infections, J. Infect. Dis, 2014, 209, 1963–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stojiljkovic I, Kumar V and Srinivasan N, Non-iron metalloporphyrins: potent antibacterial compounds that exploit haem/Hb uptake systems of pathogenic bacteria, Mol. Microbiol, 1999, 31, 429–442. [DOI] [PubMed] [Google Scholar]
- 104.Hijazi S, Visca P and Frangipani E, Gallium-Protoporphyrin IX Inhibits Pseudomonas aeruginosa Growth by Targeting Cytochromes., Front. Cell. Infect. Microbiol, 2017, 7, 12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Arivett BA, Fiester SE, Ohneck EJ, Penwell WF, Kaufman CM, Relich RF and Actis LA, Antimicrobial activity of gallium protoporphyrin IX against Acinetobacter baumannii strains displaying different antibiotic resistance phenotypes, Antimicrob. Agents Chemother, 2015, 59, 7657–7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chang D, Garcia RA, Akers KS, Mende K, Murray CK, Wenke JC, Sanchez CJ Jr. and, Activity of Gallium Meso- and Protoporphyrin IX against Biofilms of Multidrug-Resistant Acinetobacter baumannii Isolates., Pharmaceuticals (Basel)., DOI: 10.3390/ph9010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Choi S-R, Britigan BE and Narayanasamy P, Iron/Heme Metabolism-Targeted Gallium(III) Nanoparticles Are Active against Extracellular and Intracellular Pseudomonas aeruginosa and Acinetobacter baumannii., Antimicrob. Agents Chemother, 2019, 63, e02643–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shisaka Y, Iwai Y, Yamada S, Uehara H, Tosha T, Sugimoto H, Shiro Y, Stanfield JK, Ogawa K, Watanabe Y and Shoji O, Hijacking the Heme Acquisition System of Pseudomonas aeruginosa for the Delivery of Phthalocyanine as an Antimicrobial, ACS Chem. Biol, 2019, 14, 1637–1642. [DOI] [PubMed] [Google Scholar]
- 109.Shirataki C, Shoji O, Terada M, Ozaki S, Sugimoto H, Shiro Y and Watanabe Y, Inhibition of Heme Uptake in Pseudomonas aeruginosa by its Hemophore (HasAp) Bound to Synthetic Metal Complexes, Angew. Chemie Int. Ed, 2014, 53, 2862–2866. [DOI] [PubMed] [Google Scholar]
- 110.Uehara H, Shisaka Y, Nishimura T, Sugimoto H, Shiro Y, Miyake Y, Shinokubo H, Watanabe Y and Shoji O, Structures of the Heme Acquisition Protein HasA with Iron(III)-5,15-Diphenylporphyrin and Derivatives Thereof as an Artificial Prosthetic Group, Angew. Chemie Int. Ed, 2017, 6, 15279–15283. [DOI] [PubMed] [Google Scholar]
- 111.Sakakibara E, Shisaka Y, Onoda H, Koga D, Xu N, Ono T, Hisaeda Y, Sugimoto H, Shiro Y, Watanabe Y and Shoji O, Highly malleable haem-binding site of the haemoprotein HasA permits stable accommodation of bulky tetraphenylporphycenes, RSC Adv, 2019, 9, 18697–18702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cescau S, Cwerman H, Létoffé S, Delepelaire P, Wandersman C and Biville F, Heme acquisition by hemophores, BioMetals, 2007, 20, 603–613. [DOI] [PubMed] [Google Scholar]
- 113.Centola G, Deredge DJ, Hom K, Ai Y, Dent AT, Xue F and Wilks A, Gallium(III)-Salophen as a Dual Inhibitor of Pseudomonas aeruginosa Heme Sensing and Iron Acquisition., ACS Infect. Dis, 2020, 6, 2073–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yukl ET, Jepkorir G, Alontaga AY, Pautsch L, Rodríguez JC, Rivera M and Moënne-Loccoz P, Kinetic and Spectroscopic Studies of Hemin Acquisition in the Hemophore HasAp from Pseudomonas aeruginosa, Biochemistry, 2010, 49, 6646–6654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jepkorir G, Rodríguez JC, Rui H, Im W, Lovell S, Battaile KP, Alontaga AY, Yukl ET, Moënne-Loccoz P and Rivera M, Structural, NMR Spectroscopic, and Computational Investigation of Hemin Loading in the Hemophore HasAp from Pseudomonas aeruginosa, J. Am. Chem. Soc, 2010, 132, 9857–9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chan DCK, Guo I and Burrows LL, Forging new antibiotic combinations under iron-limiting conditions, Antimicrob. Agents Chemother, DOI: 10.1128/AAC.01909-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Laffont C and Arnoux P, The ancient roots of nicotianamine: diversity, role, regulation and evolution of nicotianamine-like metallophores, Metallomics, 2020, 12, 1480–1493 [DOI] [PubMed] [Google Scholar]
- 118.Lhospice S, Gomez NO, Ouerdane L, Brutesco C, Ghssein G, Hajjar C, Liratni A, Wang S, Richaud P, Bleves S, Ball G, Borezée-Durant E, Lobinski R, Pignol D, Arnoux P and Voulhoux R, Pseudomonas aeruginosa zinc uptake in chelating environment is primarily mediated by the metallophore pseudopaline, Sci. Rep, 2017, 7, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Song L, Zhang Y, Chen W, Gu T, Zhang SY and Ji Q, Mechanistic insights into staphylopine-mediated metal acquisition, Proc. Natl. Acad. Sci. U. S. A, 2018, 115, 3942–3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jackson AC, Zaengle-Barone JM, Puccio EA and Franz KJ, A Cephalosporin Prochelator Inhibits New Delhi Metallo-β-lactamase 1 without Removing Zinc, ACS Infect. Dis, 2020, 6, 1264–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zaengle-Barone JM, Jackson AC, Besse DM, Becken B, Arshad M, Seed PC and Franz KJ, Copper Influences the Antibacterial Outcomes of a β-Lactamase-Activated Prochelator against Drug-Resistant Bacteria, ACS Infect. Dis, 2018, 4, 1019–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.O’Neill MJ and Wilks A, The P. aeruginosa Heme Binding Protein PhuS is a Heme Oxygenase Titrateable Regulator of Heme Uptake, ACS Chem. Biol, 2013, 6, 1794–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hom K, Heinzl GA, Eakanunkul S, Lopes PEM, Xue F, MacKerell AD and Wilks A, Small Molecule Antivirulents Targeting the Iron-Regulated Heme Oxygenase (HemO) of P. aeruginosa, J. Med. Chem, 2013, 56, 2097–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liang D, Robinson E, Hom K, Yu W, Nguyen N, Li Y, Zong Q, Wilks A and Xue F, Structure-based design and biological evaluation of inhibitors of the Pseudomonas aeruginosa heme oxygenase (pa-HemO), Bioorganic Med. Chem. Lett, 2018, 28, 1024–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Heinzl GA, Huang W, Yu W, Giardina BJ, Zhou Y, MacKerell AD, Wilks A and Xue F, Iminoguanidines as Allosteric Inhibitors of the Iron-Regulated Heme Oxygenase (HemO) of Pseudomonas aeruginosa, J. Med. Chem, 2016, 59, 6929–6942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Eshelman K, Yao H, Punchi Hewage AND, Deay JJ, Chandler JR and Rivera M, Inhibiting the BfrB:Bfd interaction in: Pseudomonas aeruginosa causes irreversible iron accumulation in bacterioferritin and iron deficiency in the bacterial cytosol, Metallomics, 2017, 9, 646–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Punchi Hewage AND, Yao H, Nammalwar B, Gnanasekaran KK, Lovell S, Bunce RA, Eshelman K, Phaniraj SM, Lee MM, Peterson BR, Battaile KP, Reitz AB and Rivera M, Small Molecule Inhibitors of the BfrB–Bfd Interaction Decrease Pseudomonas aeruginosa Fitness and Potentiate Fluoroquinolone Activity, J. Am. Chem. Soc, 2019, jacs.9b00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Soldano A, Yao H, Chandler JR and Rivera M, Inhibiting Iron Mobilization from Bacterioferritin in Pseudomonas aeruginosa Impairs Biofilm Formation Irrespective of Environmental Iron Availability, ACS Infect. Dis, 2020, acsinfecdis.9b00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Powell K, Narrowing in on species-specific antibiotics | Novartis, https://www.novartis.com/stories/discovery/narrowing-species-specific-antibiotics, (accessed 24 June 2020).
- 130.Melander RJ, Zurawski DV and Melander C, Narrow-spectrum antibacterial agents, Medchemcomm, 2018, 9, 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]