Abstract
Background:
Bacterial exposure from house dust has been associated with asthma and atopy in children but whether these relationships are present in adults remains unclear.
Objective:
To examine associations of house dust microbiota with adult asthma, atopy, and hay fever.
Methods:
Vacuumed bedroom dust samples from the homes of 879 participants (average age 62) in the Agricultural Lung Health Study, a case-control study of asthma nested within a farming cohort, were subjected to 16S rRNA amplicon sequencing to characterize bacterial communities. We defined current asthma and hay fever using questionnaires and current atopy by blood specific immunoglobulin E > 0.70 IU/ml to ≥ 1 of ten common allergens. We used linear regression to examine whether overall within-sample bacterial diversity differed by outcome, microbiome regression-based kernel association test (MiRKAT) to evaluate whether between-sample bacterial community compositions differed by outcome, and analysis of composition of microbiomes (ANCOM) to identify differentially abundant bacterial taxa.
Results:
Overall diversity of bacterial communities in house dust was similar by asthma status but was lower (p-value < 0.05) with atopy or hay fever. Many individual bacterial taxa were differentially abundant (false discovery rate < 0.05) by asthma, atopy, or hay fever. Several taxa from Cyanobacteria, Bacteroidetes, and Fusobacteria were more abundant with asthma, atopy, or hay fever. In contrast, several taxa from Firmicutes were more abundant in homes of individuals with adequately controlled asthma (vs inadequately controlled asthma), non-atopics, or individuals without hay fever.
Conclusion:
Microbial composition of house dust may influence allergic outcomes in adults.
Keywords: Bacteria, Microbiome, Host Microbial Interactions, Asthma, Allergy and Immunology
Capsule summary
Chronic exposure to more diverse house dust microbiota was inversely associated with adult allergy outcomes. Differential abundances of specific microbes in house dust are related to a lower or higher likelihood of allergy in adults.
Introduction
Allergic diseases such as asthma and atopy are global public health concerns, affecting 25 million people in the United States alone1. Exposure to bacterial communities inside homes can impact allergic health outcomes2, 3. Studies have shown early life exposure to increased microbial load and diversity is protective against allergic outcomes4. To understand the link between bacterial exposure and such health outcomes, researchers have used endotoxin concentrations as an index of global bacterial burden, reporting associations of house dust endotoxin with allergy outcomes including asthma and atopy5–7. Recent studies using quantitative PCR methods have found several bacteria associated with asthma and atopy in both children and adults8–10.
The advent of high-throughput sequencing technology has allowed a more thorough exploration of associations between bacterial communities in house dust and human health including allergic outcomes11. Although studies using these methods have demonstrated associations between indoor microbial communities and allergy in children12–16, studies examining associations of both overall bacterial diversity and individual bacterial taxa from house dust with asthma and atopy in adults are rare.
In this study, we used high-throughput sequencing to investigate associations of indoor bacterial communities with adult allergic health outcomes in the Agricultural Lung Health Study (ALHS), a case-control study of asthma. We examined bedroom dust samples from 879 independent households to evaluate whether overall bacterial diversity within each sample and bacterial community compositions between samples were associated with occupants’ current asthma and atopy status. Using comprehensive information on indoor bacterial communities, we were able to investigate whether specific bacterial taxa in house dust were differentially abundant by asthma and atopy in adults.
Methods
Study population
The Agricultural Lung Health Study (ALHS) is a nested case-control study of current asthma within Agricultural Health Study (AHS). The AHS is a prospective cohort of farmers and spouses of farmers in North Carolina (NC) and Iowa (IA)17. Details of ALHS participant selection have been described elsewhere (data version P3REL201209.00)18, 19. Briefly, to avoid missing undiagnosed current asthma and to minimize misclassification with chronic obstructive pulmonary disease (COPD), we identified current asthma based on three criteria. Of the 3,301 ALHS participants, there were 1,223 asthma cases: (1) 876 diagnosed asthma cases responded “yes” to “have you ever been diagnosed with asthma?” and “do you still have asthma?”20 and “no” to “have you ever been diagnosed with chronic obstructive pulmonary disease (COPD)?” and “have you ever been diagnosed with emphysema?”; (2) 309 undiagnosed asthma cases responded “no” to the asthma, COPD or emphysema diagnosis questions, but reported current asthma symptoms and use of asthma medications and were never or minimal smokers (< 10 pack-years); and (3) 38 asthma cases responded “yes” to the asthma and COPD or emphysema questions but were never or past smokers. Noncases (N=2,078) were randomly chosen from among individuals not categorized as cases.
Of the 3,301 participants, 2,871 received a home visit at which bedroom dust was collected. From among these, we selected a random sample of 1,000 participants for characterization of house dust microbiota. After excluding 80 individuals whose dust samples had low sequencing quality and 41 individuals whose dust samples came from the same home as another participant, their spouse, the present study included 879 individuals. Of the 879, 480 (54.6%) reported working with crops and 443 (50.4%) reported working with farm animals such as cattle, hogs, and poultry21. The study population (N=879) was representative of the entire ALHS population (N=3301) (Table E1). Workflow of the study, selection criteria, and number of samples can be found in Figure 1. The study was approved by the Institutional Review Board at the National Institute of Environmental Health Sciences. Written informed consent was obtained from all participants.
Figure 1.
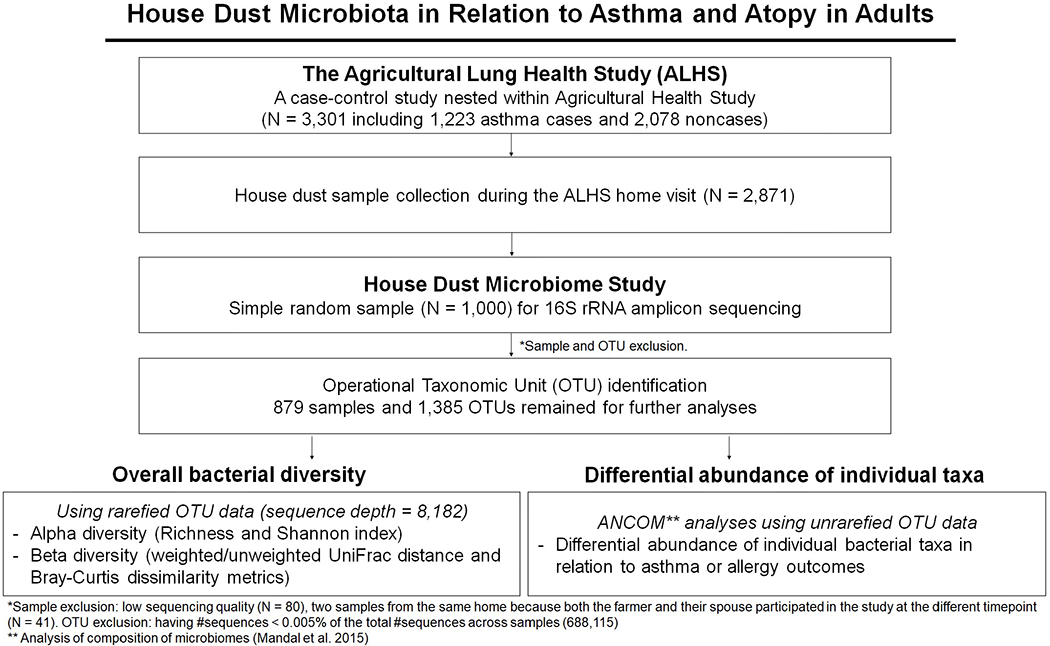
Workflow of our house dust microbiome study. This workflow includes summary of sample selection from the Agricultural Lung Health Study (N=3,301) to the house dust microbiome study (N=879). It also shows association analyses used in this paper: two overall bacterial diversity analyses and differential abundance analysis for asthma and atopy outcomes.
Allergy outcomes (asthma, atopy, and hay fever)
Of the 879 individuals included in microbiome analyses, 333 were asthma cases and 546 were non-asthma cases. Asthma cases included 239 with a prior diagnosis, 83 undiagnosed cases, and 11 diagnosed cases with overlapping COPD or emphysema diagnosis at enrollment (see full case definitions above). Among individuals with asthma diagnosis, we examined the degree to which asthma was controlled using the Asthma Control Questionnaire (ACQ) which asks participants to rate the frequency or severity of each of six asthma symptoms (night-time waking frequency, night-time waking severity, activity limitation, shortness of breath, wheezing, and bronchodilator use) in the past two weeks on a scale from 0 (no impairment) to 6 (significant impairment)22. Participants’ pre-bronchodilator percent predicted FEV1 values were also categorized on a scale of 0 to 622. An overall ACQ score was then calculated as a mean of the responses to the six questions and percent predicted FEV1. Controlled asthma was defined as ACQ scores < 1.5, while inadequately controlled asthma was defined as ACQ scores ≥ 1.523. Only individuals reporting a prior asthma diagnosis were asked these questions, and calculating the ACQ scores requires complete data, resulting in 234 asthmatics with the scores.
We defined current atopy based on specific immunoglobulin E (IgE) > 0.7 IU/ml24 to at least one of ten common allergens: seasonal (Bermuda grass, ragweed, Timothy grass, and mountain cedar), perennial (Alternaria, dust mite, and cat dander), and food (milk, egg, and wheat) allergens. Serum IgE levels were measured with the Luminex MyAllergyTestTM assay (Luminex Corporation, Austin, TX, USA) at ImmuneTech (Foster City, CA, USA). The assay was approved by the FDA as equivalent to the ImmunoCap24. We identified 163 individuals with atopy and 716 individuals without atopy. In addition, we defined seasonal atopy based on sensitization (IgE > 0. 7) to any seasonal allergen (70 participants sensitized, 809 not), perennial atopy based on sensitization to any perennial allergen (112 sensitized, 767 not), and inhaled atopy based on sensitization to either seasonal or perennial allergens (149 sensitized to either, 730 sensitized to neither).
Self-reported hay fever diagnosis was defined based on response to a question about ever diagnosis of hay fever, seasonal allergies, or allergic rhinitis. Of the 879 participants, 330 (38%) reported hay fever diagnosis.
To examine asthma and atopy together, we generated a combined four-level variable: none (neither asthma nor atopy), atopy only (atopy without asthma), asthma only (asthma without atopy), and both asthma and atopy.
Among these outcomes, we first evaluated associations with current asthma, current atopy, diagnosed hay fever, and a combined four-level asthma and atopy variable. When we found an association with current asthma, we further explored associations with the asthma severity score and atopic status within asthmatics. When we found an association with overall atopy, we additionally analyzed atopy subtypes (seasonal, perennial, and inhaled atopy).
From dust collection to characterization of house dust microbiota
As previously described, trained field technicians collected dust samples from participants’ bedrooms during home visits18. A DUSTREAM™ Collector (Indoor Biotechnologies, Inc., Charlottesville, Virginia, USA) was used to vacuum a one square yard (0.84 m2) area on the sleeping surface and on the floor next to the bed for 2 minutes in each area. Dust samples were sent to Social & Scientific Systems, Inc. (Durham, NC, USA) to be sieved, weighed into aliquots of 50 mg, and frozen at −20 ºC. Details on DNA isolation, 16S rRNA amplicon sequencing, and preprocessing of sequencing reads were also previously described21. In brief, DNA was isolated using the Mo Bio 96 well plate PowerSoil DNA extraction kit (QIAGEN, Inc.). The kit has been reported to be the most effective for soil and environmental samples25. The bead beating step was performed because it is essential to lyse Gram positive and spore-forming bacteria. Each sample was quantified using the NanoDrop™ (A260) (Thermo Fisher Scientific Inc.) and normalized to 5 ng/μL DNA per sample. DNA was amplified using primers targeting the V3-V4 region of the bacterial 16S rRNA gene. The 16S V3-V4 region was chosen because it results in fewer chimeras26 and lower error rates27, provides the best coverage of the domain Bacteria and phylum28, and has been recommended by the Earth Microbiome Project29 as well as in a recent review30 of standardized procedures for microbiome research. The 16S rRNA gene amplicon sequencing was performed using the Illumina High Seq Rapid PE250(500) platform (Illumina, Inc.), which generates paired-end reads of 250 nucleotides. Measurements were made by the Microbiome Core Facility at the University of North Carolina (Chapel Hill, NC, USA).
To obtain bacterial community information from preprocessed sequencing data, we used Quantitative Insights Into Microbial Ecology (QIIME, version 1.9.1, qiime.org)31. For de novo operational taxonomic unit (OTU) picking, we used the script pick_de_novo_otus.py with the default OTU clustering algorithm UCLUST32. To remove chimeric sequences, we applied the ChimeraSlayer algorithm26 using the script identify_chimeric_seqs.py after aligning sequences using the script align_seqs.py with the default alignment method PyNAST33. To obtain taxonomic classification, we used the script assign_taxonomy.py with the default database Greengenes (version 13_5, greengenes.secondgenome.com). A phylogenetic tree was constructed using make_phylogeny.py with the default phylogeny construction algorithm FastTree34. An OTU is a cluster of microbial sequences having more than 97% sequence similarity and serves as a proxy for microbial species. Throughout the paper, we refer to an OTU as a bacterial taxon when appropriate.
As quality control steps for the OTU data, first we removed chimeric sequences, second we excluded samples having sequencing depth < 10,00035, and third we removed OTUs having < 0.005% of the total number of sequence reads36, 37. During these steps, 80 samples, noted above, and 604,569 OTUs (604,569/689,500=87.7%) were excluded after removal of 83,546 OTUs (83,546/689,500=12.1%) of chimeric sequences. For 41 homes where two dust samples were collected because both the farmer and their spouse participated in the study at different time points, we retained results from the farmer for further analyses so that all samples were independent. This left 879 samples with the minimum number of sequence reads across samples of 8,182 for statistical analysis. On average there were 82,895 sequence reads per sample which was highly comparable to an earlier house dust microbiome study of 1100 homes35.
Overall bacterial diversity in relation to the health outcomes
For overall bacterial diversity within each sample (alpha diversity), we calculated two diversity measures: richness (the number of individual bacterial taxa) itself and the Shannon index38, which reflects both richness and the relative abundance of each taxa. We used linear regression to evaluate associations of the diversity measures with asthma and atopy.
To evaluate if bacterial community composition between samples (beta diversity) differs by outcome, we used the microbiome regression-based kernel association test (MiRKAT)39. MiRKAT models the log-odds of the outcome using a mixed model. The fixed effects part of the model is linear in any covariates. The random effects part involves sample-specific random effects whose covariance matrix is derived from a measure of pairwise dissimilarity (or distance) among the samples’ bacterial communities. The test of association between beta diversity and outcome is based on testing whether the component of variance attributable to the random effects is zero. We considered the unique fraction metric (UniFrac)40, a measure of the phylogenetic distance among OTUs in a phylogenetic tree, and the Bray-Curtis dissimilarity metric41 which quantifies compositional dissimilarity between samples by comparing counts at OTUs. Both weighted, taking abundances of OTUs into account, and unweighted UniFrac were included in the analyses. We also considered the three measures simultaneously to provide a more robust association test.
To avoid any bias due to different sequencing depth among samples, the OTU data were rarefied to the minimum number of sequences (8,182) across samples in the bacterial diversity analyses. We used R (version 3.4.1; R Project for Statistical Computing) to summarize characteristics of the study population and perform association analyses of the alpha diversity measures. We used functions specnumber and diversity in the R package vegan version 2.4.342 to calculate richness and Shannon index, respectively. For the beta diversity analysis, we used the R package MiRKAT version 0.0239. We set a threshold of p-value < 0.05 for statistical significance for the diversity analyses.
Abundance of individual bacterial taxa in relation to the health outcomes
To identify specific bacterial taxa (OTUs) whose abundances significantly differ by the health outcomes, we applied analysis of composition of microbiomes (ANCOM)43. ANCOM models the log-ratios of OTU abundances in each sample with a linear model, similarly to an analysis-of-variance model; but, unlike typical analysis of variance, ANCOM’s use of log-ratios accommodates dependencies and correlations among the relative abundances of the OTUs. Such dependencies arise because the relative abundances of OTUs sum to one in each sample and because relative abundances of different OTUs may be positively or negatively correlated. We used un-rarefied OTU data because use of log-ratios accounts for variation in sequencing depth across samples. To correct for multiple testing, we set the Benjamini-Hochberg false discovery rate (FDR) to 0.05. We declared significance by using ANCOM’s W statistic with a threshold of 0.7. For significant bacterial taxa, we calculated relative abundances to quantify the presence of OTUs by the outcome.
Additional analyses
We conducted analyses of current asthma restricted to the 239 diagnosed asthmatics with no overlapping diagnosis of COPD or emphysema to examine whether the definition of current asthma influences the results. We also performed analyses of current atopy (149 atopics vs 716 non-atopics) after excluding the 14 atopics sensitized only to food allergens.
We examined whether our findings were independent of age, sex, state of residence (283 from NC and 596 from IA), house dust endotoxin concentrations44 [geometric mean (standard error) 38.42 (1.05)], and home condition. Home condition (cleanliness) was rated by field technicians at the time of the visit using a five-point scale used in an earlier home allergen study45. The five levels were: (a) “Extremely poor: lack of organization,” (b) “Poor,” (c) “Average: clean with moderate clutter,” (d) “Above average,” and (e) “Good: organized, clean all over.” We dichotomized responses into lower (levels a-b) or higher home condition (levels c-e). Of the participating homes, 78% were rated as having higher home condition. Further, we evaluated whether exposures previously related to house dust microbiota21 in this population were associated with the allergy outcomes of interest (asthma, atopy, or hay fever). Of the exposures, working with crops (exposure prevalence 55%) was associated with atopy, and presence of an indoor dog (exposure prevalence 31%) was associated with asthma. We did not adjust for farming factors in asthma analyses because they were not related to asthma status. Accordingly, we evaluated whether crop farming influenced results for atopy and whether the presence of a dog influenced our results for asthma.
Results
Characteristics of study participants and house dust microbiota
Bedroom dust samples from 879 independent homes were from NC (32%) and IA (68%). The average age of participants was 62 years, and about 59% were male (Table 1). 333 (38%) participants had current asthma, 163 (19%) were atopic, and 330 (38%) reported hay fever diagnosis. After quality control steps, we identified 1,385 OTUs that were at least 0.005% of the total number of sequence reads in our house dust microbiota. At the taxonomic level of kingdom, 1,346 (97.2%) OTUs were assigned to Bacteria, seven (0.5%) to Archaea, and 32 (2.3%) were unassigned. At the phylum level, 1,353 OTUs were assigned to 18 distinct phyla and 32 were unassigned (Figure E1). The vast majority of the OTUs were from four phyla: Proteobacteria (27%), Firmicutes (23%), Actinobacteria (17%), and Bacteroidetes (17%).
Table 1.
Characteristics of the 879 study participants
| Characteristics | N (%) or mean ± SD |
|---|---|
| Age, years | 62 ± 11 |
| Sex | |
| Male | 517 (58.8) |
| Female | 362 (41.2) |
| Smoking status | |
| Never | 566 (64.4) |
| Former | 263 (29.9) |
| Current | 50 (5.7) |
| Current asthma | |
| Non-asthmatic | 546 (62.1) |
| Asthmatic | 333 (37.9) |
| Current atopy | |
| Non-atopic | 716 (81.5) |
| Atopic | 163 (18.5) |
| Seasonal atopy | |
| Without | 809 (92.0) |
| With | 70 (8.0) |
| Perennial atopy | |
| Without | 767 (87.3) |
| With | 112 (12.7) |
| Inhaled atopy | |
| Without | 730 (83.0) |
| With | 149 (17.0) |
| Diagnosed hay fever | |
| Without | 549 (62.5) |
| With | 330 (37.5) |
| Current asthma and atopy | |
| None | 476 (54.2) |
| Atopy only | 70 (8.0) |
| Asthma only | 240 (27.3) |
| Both Asthma and Atopy | 93 (10.6) |
Note: SD, standard deviation; Percentages may not add to exactly 100 due to rounding.
Overall bacterial diversity in relation to the allergy outcomes
No significant differences were observed for the overall (alpha and beta) bacterial diversity measures by current asthma status; however, both overall bacterial diversity within each sample (alpha diversity) and bacterial community composition between samples (beta diversity) were associated with atopy status and diagnosed hay fever. The two overall (alpha) diversity measures - richness (the number of individual bacterial taxa) and the Shannon index (H, a combined measure of richness and relative abundance of each bacterial taxon) - were significantly lower in homes of atopics (p-value < 0.05). Average richness was 670 for homes of non-atopics and 650 for atopics; average Shannon index was 4.607 for homes of non-atopics and 4.475 for atopies (Figure 2). Diagnosed hay fever was associated with lower overall bacterial diversity (Figure 2). When we examined combinations of asthma and atopy, we found lower bacterial diversity in homes of individuals with atopy only (i.e. atopy without asthma) compared to individuals with neither atopy nor asthma (p-value = 0.09 for richness; p-value = 0.04 for Shannon index). The bacterial community compositions between samples (beta diversity) also differed by atopy when using unweighted UniFrac distance metric and the omnibus optimization approach (Table E2). Pairwise distances between each pair of samples were associated with current atopy: p-value=0.03 when considering three different distance metrics all together (omnibus optimization approach). Bacterial community composition between dust samples from homes of participants with diagnosed hay fever was significantly different (p-value < 0.05) from bacterial community composition between samples from homes of participants without hay fever whether we used the weighted/unweighted UniFrac distance metrics, the Bray-Curtis dissimilarity metric, or all three metrics combined (Table E2).
Figure 2.
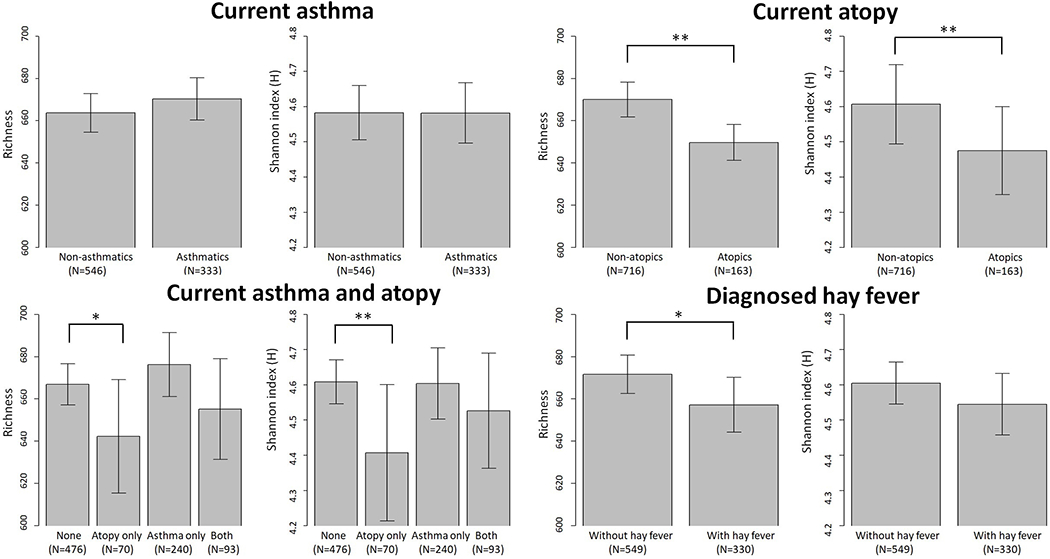
Overall bacterial (alpha) diversity in relation to asthma, atopy, and hay fever. Richness and Shannon index (H) were calculated using rarefied (minimum number of sequences across samples=8,182) OTU data. Error bars represent 95% confidence interval. Mean (95% CI) of alpha diversity measures by health outcomes are as follows: current asthma – richness 663.74 (654.60-672.89) and Shannon index 4.58 (4.51-4.66) for non-asthmatics, richness 670.35 (660.35-680.36) and Shannon index 4.58 (4.50-4.67) for asthmatics; current atopy – richness 670.02 (661.81-678.23) and Shannon index 4.61 (4.49-4.72) for non-atopics, richness 649.67 (641.18-658.16) and Shannon index 4.48 (4.35-4.6) for atopics; current atopy and asthma – richness 666.90 (657.20-676.60) and Shannon index 4.61 (4.55-4.67) for none, richness 642.30 (615.43-669.17) and Shannon index 4.41 (4.21-4.6) for atopy only, richness 676.22 (661.05-691.39) and Shannon index 4.60 (4.50-4.70) for asthma only, and richness 655.22 (631.4-679.03) and Shannon index 4.53 (4.36-4.69) for both; diagnosed hay fever – richness 671.68 (662.62-680.75) and Shannon index 4.60 (4.55-4.66) for individuals without hay fever, richness 657.20 (644.25-670.15) and Shannon index 4.54 (4.46-4.63) for individuals with hay fever. *indicates p-value<0.1; **indicates p-value<0.05.
Given that atopy overall was associated with house dust microbiota, we examined whether indoor bacterial communities differed by atopy subtypes: seasonal, perennial, or inhaled. Associations were similar for overall atopy and subtypes. Specifically, less diverse bacterial communities were found in homes of participants with perennial atopy (vs those without perennial atopy), with seasonal atopy (vs those without seasonal atopy), and with inhaled atopy (vs those without inhaled atopy) (Table E3). Bacterial community composition between samples were associated with seasonal and inhaled atopy when considering the unweighted UniFrac distance metric and all three metrics together (weighted and unweighted UniFrac distance and Bray-Curtis dissimilarity metrics) (Table E2).
Abundance of individual bacterial taxa in relation to the allergy outcomes
Using ANCOM, we found a number of specific bacterial taxa differentially abundant (FDR < 0.05) according to the outcomes: four for current asthma (Figure 3), seven for current atopy (Figure 4), and 28 for diagnosed hay fever (Figure 5). The four asthma-associated bacterial taxa were from the phyla Bacteroidetes (one from the genus Porphyromonas, one from the genus Bacteroides) and Fusobacteria (two from the genus Fusobacterium). When we evaluated relative abundances of those taxa by asthma status, they were more abundant in homes of asthmatics (vs non-asthmatics), suggesting a possible harmful association between those taxa and asthma. The seven atopy-associated bacterial taxa were from the phyla Proteobacteria (one from the family Methylocystaceae), Bacteroidetes (one from the genus Spirosoma), Cyanobacteria (one each from the order Chlorophyta, the family Xenococcaceae, the class Oscillatoriophycideae), and Firmicutes (one from the order of Clostridiales, one from the genus of Clostridium). Of the seven taxa, the two from the phylum Firmicutes showed higher relative abundances in homes of non-atopics (vs atopics), suggesting a potentially protective association. The remaining five taxa from the phyla Cyanobacteria, Proteobacteria, and Bacteroidetes showed higher relative abundances in homes of atopics (vs non-atopics), suggesting a potentially harmful association. The 28 bacterial taxa differentially abundant by hay fever were from the phyla Bacteroidetes (nine taxa including five from the genus Porphyromonas), Firmicutes (eight taxa including six from the order Clostridiales), Proteobacteria (five taxa including three from the class Gammaproteobacteria), Actinobacteria (four taxa from the order Actinomycetales), or Fusobacteria (two taxa from the genus Fusobacterium). Of the 28 taxa, 19 showed higher relative abundances in homes of individuals with hay fever, suggesting a potentially deleterious association. Thirteen of the 19 were from the phyla Bacteroidetes (eight taxa) or Proteobacteria (five taxa). The remaining nine taxa, including six from the phylum Firmicutes, showed higher relative abundances in homes of individuals without hay fever, suggesting potentially inverse associations. There were 35 taxa differently abundant by at least one of the allergy outcomes (asthma, atopy, and hay fever). All 35 were present in > 30% of the dust samples (Table E4).
Figure 3.
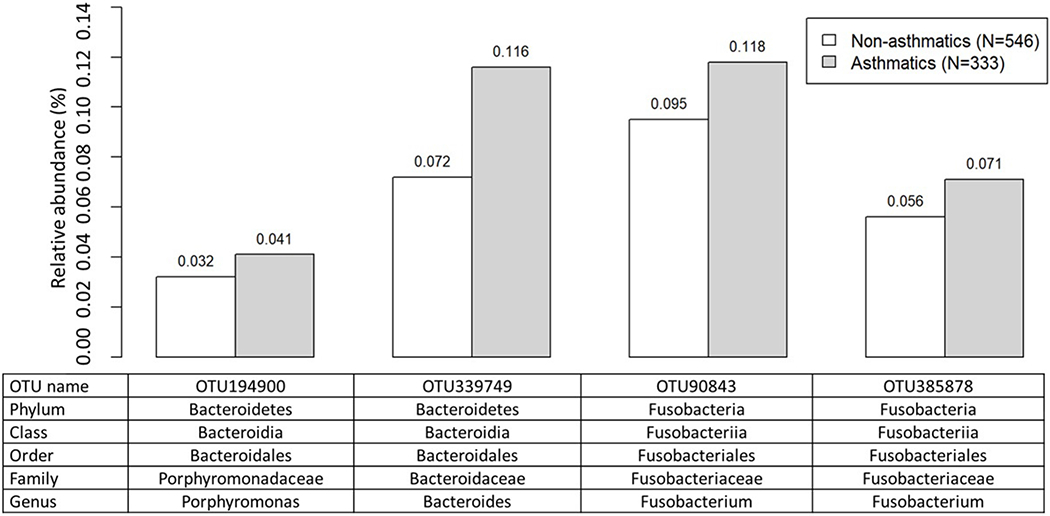
Average relative abundance of bacterial taxa associated with current asthma (FDR<0.05). Differentially abundant taxa by current asthma were identified by using ANCOM43. Average relative abundance (%) of the taxa for non-asthmatics and asthmatics were calculated using rarefied (minimum number of sequences across samples=8,182) OTU data.
Figure 4.
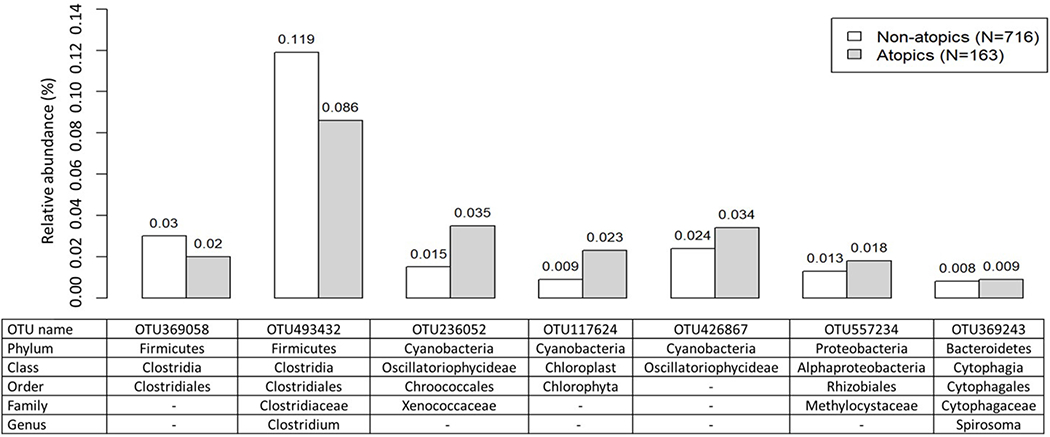
Average relative abundance of bacterial taxa associated with current atopy (FDR<0.05). Differentially abundant taxa by current atopy were identified by using ANCOM43. Average relative abundance (%) of the taxa for non-atopics and atopics were calculated using rarefied OTU data.
Figure 5.
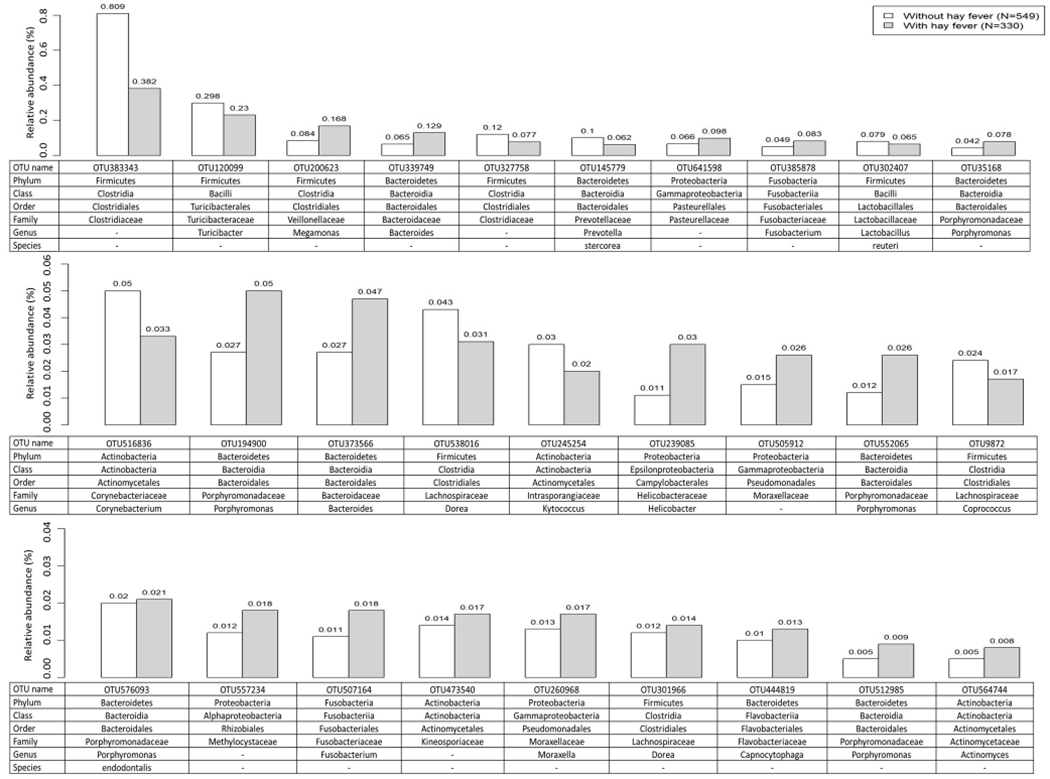
Average relative abundance of bacterial taxa associated with diagnosed hay fever (FDR<0.05). Differentially abundant taxa by diagnosed hay fever were identified by using ANCOM43. Average relative abundance (%) of the taxa for individuals without or with hay fever were calculated using rarefied OTU data.
Notably, some taxa were differentially abundant (FDR < 0.05) in relation to more than one allergy outcome with same direction of associations (Table E5). Three taxa, two from the phylum Bacteroidetes (genera Bacteroides and Porphyromonas) and one from the phylum Fusobacteria (genus Fusobacterium), showed higher relative abundances in homes of asthmatics (versus non-asthmatics) and in homes of participants with hay fever (versus without hay fever). One taxon from the phylum Proteobacteria (family Methylocystaceae) showed higher relative abundance in homes of atopics (versus non-atopics) and in homes of participants with hay fever (versus without hay fever).
When we categorized asthma according to atopy, we identified eleven taxa more abundant in homes of atopic asthmatics versus non-atopic asthmatics (Figure 6.A). Of the 11, five were from Proteobacteria, four were from Cyanobacteria, and two were from Acidobacteria. Notably, of the 11 three taxa (OTU557234 from Proteobacteria; OTU236052 and OTU117624 from Cyanobacteria) were also differentially abundant (FDR < 0.05) by atopy status overall (atopics vs non-atopics) with higher relative abundances in homes of atopics. With respect to degree of asthma control with asthmatics, we found two taxa from Firmicutes that were differentially abundant by degree of asthma control within asthmatics and that showed higher relative abundances in homes of asthmatics with adequately controlled versus inadequately controlled asthma (Figure 6.B).
Figure 6.
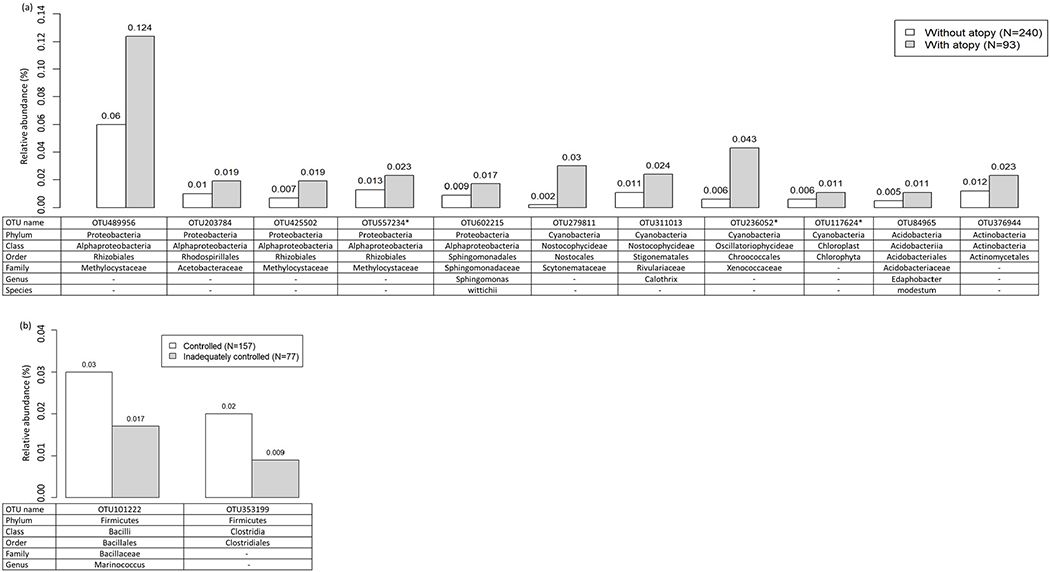
Average relative abundance of bacterial taxa associated with (a) atopy status and (b) degree of asthma control within asthmatics (FDR<0.05). Differentially abundant taxa by atopy status or degree of asthma control within asthmatics were identified by using ANCOM43. Rarefied (minimum number of sequences across samples=8,182) OTU data were used to calculate average relative abundance (%) of the taxa by atopic status (non-atopic asthmatics versus atopic asthmatics) or degree of asthma control status (asthmatics with adequately controlled asthma versus asthmatics with inadequately controlled asthma) within asthmatics. *OTUs also differentially abundant (FDR<0.05) by atopy status in analysis of all participants regardless of asthma status.
When we divided atopy into subtypes (seasonal, perennial, and inhaled), even though numbers are reduced within subcategories, we still found that (1) there were less diverse bacterial communities inside homes of atopics and (2) six of the seven overall atopy-associated (FDR < 0.05) taxa were also differentially abundant (FDR < 0.05) by atopy subtypes. Despite the smaller numbers of cases in each subgroup, the six taxa showed the same directional associations (Table E6). For example, one taxon from the phylum Firmicutes (order Clostridiales) showed higher relative abundance in homes of participants without inhaled atopy (vs those with inhaled atopy), homes of participants without seasonal atopy (vs those with seasonal atopy), and homes of participants without perennial atopy (vs those with perennial atopy). Three taxa, from the phyla Cyanobacteria (family Xenococcaceae), Firmicutes (genus Clostridium) and Proteobacteria (family Methylocystaceae), were differentially abundant by seasonal and inhaled atopy, again with same direction of associations.
Additional analyses
Results from the additional analyses of current asthma restricted to the 239 diagnosed asthmatics with no overlap with COPD or emphysema compared to the 546 non-asthmatic individuals were similar to those from analyses with all asthma cases: no significant difference of overall bacterial diversity by current asthma status (Table E7) and the four previously identified taxa showing similarly higher abundances in homes of asthmatics (Table E8). Associations between indoor microbiota and current atopy in the 149 atopics remaining after removing the 14 sensitized only to food allergens were similar to those for all atop ies. Specifically, we found lower diversity of bacterial communities inside homes of these 149 atopies (versus 716 non-atopics) (Table E7) and bacterial community compositions between samples still differed by atopy when we considered the unweighted UniFrac distance and all three metrics together (Table E2). Of the seven atopy-associated (FDR < 0.05) taxa, five still showed significant differential abundances (FDR < 0.05), with consistent directions of association (Table E6). The remaining two that no longer achieved statistical significance also showed consistent direction of associations: higher relative abundances in homes of atopics (vs non-atopics). Further, we confirmed that adjusting for age, sex, state of residence, house dust endotoxin concentrations, and home condition (cleanliness) did not change our findings on overall (alpha) bacterial diversity (Table E9). Additional adjustment for exposures associated with both the indoor microbiota and the health outcome in question (working with crops for atopy and presence of an indoor dog for asthma) showed the same directional associations as the original analysis: less diverse bacterial communities in homes of atopics (vs non-atopics) and no differences in overall bacterial diversity by asthma (Table E9). In the investigation of individual bacterial taxa associated with health outcomes, adding the above-mentioned factors in the ANCOM model did not change our results; differential abundances of the asthma-, atopy, and hay fever-associated taxa in the original analyses remained significant (FDR < 0.05) after adjusting for the exposures (Table E4).
Discussion
To our knowledge, ours is the first large-scale house dust microbiome study to examine associations of indoor bacterial communities with adult allergy outcomes using the 16S rRNA gene amplicon sequencing method. Our findings suggest that the house dust microbiota differs by occupants’ asthma, atopy, and hay fever. Findings of significantly less diverse bacterial communities in homes of adults with atopy and hay fever are consistent with the hygiene hypothesis; namely, a greater likelihood of having allergic diseases with childhood exposure to less diverse bacterial communities. What is additionally novel in this study is that we found specific bacterial taxa differentially represented according to the allergy outcomes in adults. Notably, the associations were independent of house dust endotoxin concentrations. If our results are corroborated in future studies, the identified bacterial taxa may shed light on disease pathogenesis or have potential therapeutic applications.
Our finding of lower bacterial diversity in homes of adults with atopy and hay fever extends our knowledge of associations between bacterial communities inside homes and health outcomes from early-life (childhood) to later-life (adulthood). Prior studies have shown that lower microbial diversity in house dust is associated with allergy outcomes in children8, 16. Decreased bacterial diversity was also observed in skin microbiome from atopic dermatitis patients compared to noncases46. Our study suggests similar associations between indoor microbiota and allergy in adults; whether these associations are causal remains unknown.
At the phylum level, the top three phyla (Proteobacteria, Cyanobacteria, and Bacteroidetes) showing an inverse association with the allergy outcomes in this study have been previously implicated in human health. Proteobacteria, a phylum of Gram-negative bacteria, includes bacteria found in the normal human microbiota as well as pathogens such as Salmonella, Vibrio, and Helicobacter. Exposure to Cyanobacteria, a phylum of photosynthetic bacteria, has been associated with skin irritation and allergic responses47. A fecal microbiome study found associations between Bacteroidetes, also a phylum of Gram-negative bacteria, and childhood eczema48. The phylum Firmicutes, including many taxa showing a potentially protective association with current atopy, degree of asthma control in asthmatics, and diagnosed hay fever in our study, is Gram-positive and comprises the largest portion of human gut microbiota49. A study using house dust samples reported that lower exposure to specific Firmicutes in early life was associated with atopy in children50.
Our findings for house dust are consistent with previous studies of human microbiota in relation to allergy and asthma. A recent study of adult bronchial microbiota reported that bacterial species from the genus Fusobacterium, within the phylum Fusobacteria, were positively associated with asthma51. We found the same directional associations of bacterial taxa from the same genus with asthma: higher abundance of Fusobacterium in homes of asthmatics. A study of eczema and skin microbiota reported that Roseomonas mucosa has therapeutic potential and was the predominant Gram-negative bacteria, identified by their culture methods, in people with healthy skin52. There was one taxon assigned to R. mucosa in our study; that taxon showed higher relative abundance in homes of non-atopics (0.059%) versus atopics (0.044%). In the study, Staphylococcus aureus was more prevalent in skin of individuals with eczema. Of four bacterial taxa assigned to the genera in our study, all four showed higher relative abundances in asthmatics compared to non-asthmatics (Table E10). Birzele and colleagues found inverse associations of childhood asthma with relative abundances of bacteria from the genus Prevotella in nasal swabs12. Of 27 bacterial taxa assigned to that genus in our study, 17 showed the same directional associations in our dust data: higher relative abundances in homes of non-asthmatics compared to asthmatics (Table E11). A study in children14 found having lower abundance of Streptococcaceae in house dust to be protective. We found identical directions of associations: higher relative abundance in homes of asthmatics versus non-asthmatics (Table E12).
Because allergic individuals might take cautionary measures of cleaning or dust reduction that could influence our results, we examined whether hygiene practices, including home condition (cleanliness) and presence of carpet, were associated with our allergy outcomes. The field technician who collected the dust sample documented the presence of carpet versus smooth floor in the room where the dust sample was collected21. Nearly all (93%) of the 879 participants had carpet in the room vacuumed. Not surprisingly given the nearly universal presence of carpeting, we did not find an association with either bacterial communities21 or allergy outcomes (Table E13). Thus, we did not adjust for carpeting. Although home condition (cleanliness) did not differ by allergy outcomes (asthma, atopy, or hay fever) (Table E13), we did adjust for home condition because we previously found that it was related to indoor bacterial communities21.
Given that homes of atopics had significantly less diverse bacterial communities, we examined whether number of allergens to which an atopic individual was sensitized was related to bacterial communities inside homes. Dust from homes of participants sensitized to two or more allergens (N=59) did not contain significantly less diverse bacterial communities than homes of participant sensitized only to a single allergen (N=90) (Table E7). A much larger study might be needed to identify a gradient in association by number of sensitized allergens.
While we do not know the specific age at onset for all cases, we had information on whether asthma started during childhood and identified 66 current asthmatics reporting diagnosis during childhood. Results were similar when we removed these 66 (20% of cases) from the analyses; no association was observed between current asthma and overall alpha bacteria diversity (Table E7), and bacterial community composition between samples did not differ by current asthma except when using the Bray-Curtis dissimilarity metric (Table E2). With the smaller sample size after excluding the 66 asthmatics reporting childhood onset, we still found that one of the four asthma associated taxa was differentially abundant (FDR < 0.05) with same direction of association as before exclusion. Associations with the remaining three taxa with current asthma did not reach statistical significance but the directions of associations were consistent (Table E14).
Childhood asthma differs from adult asthma in various aspects that may reflect variation in etiologic factors. Some adult asthma results from occupational exposures and adult asthma is more likely to be non-atopic53. Nevertheless, asthma across the life-course has common outdoor and indoor environmental triggers54. Both adults and children spend most of their time indoors and exposures to indoor bacterial communities may be related to asthma etiology or persistence. However, there are few data addressing diversity of bacterial communities or specific bacteria in relation to childhood asthma and even fewer examining adult asthma. Therefore, it is informative to examine whether microbiota previously associated with childhood asthma are also related to adult asthma in this study.
Inferences about differential abundance in this study were carried out using analysis of composition of microbiomes (ANCOM)43. ANCOM analyzes relative abundance through its log-ratio-transformation of observed counts. Mandal et al. mathematically proved that inferences about differential relative abundances between two groups using ANCOM are equivalent to inferences about differential absolute abundances per unit volume43. The only assumption required is that two taxa have absolute abundance values (per unit volume) that are the same in both groups; the assumption does not require that those taxa be known. This assumption should hold in most analyses of microbiome data43. Thus, by performing ANCOM we are able to infer not only differential relative abundance but also differential abundance (per unit volume) between two groups.
Morton et al.55 reported a scenario of a potential false positive result generated by ANCOM. In that example, a cutoff of the W statistic of 0.6 was used. In the current study, we used the stricter cutoff of W statistic of 0.7 when reporting differentially abundant taxa which will reduce the chance of false positives. However, in view of Morton et al..55 one needs to be cautious in using methods for differential abundance analysis. As demonstrated in simulation studies56, 57 and others, ANCOM does control FDR reasonably well under a variety of scenarios. In all those simulation studies the authors allow for differential sampling fractions across samples. Thus, at least in a sample of simulation studies reported in the literature, ANCOM seems to control FDR reasonably well. We note the scenarios tested involve gut microbiome; it is possible that dust microbiota could behave differently. In light of these issues, to complement analyses of differential abundance from 16S rRNA sequencing data, quantitative information on total microbial load might help better understand differences in microorganisms that contribute to disease risk.
A limitation of our study is the single dust sample from each house. Thus, we assume this sample reflects the usual home condition. To the extent that a single sample is not a good reflection of the usual exposure, this limitation would tend to bias our results toward the null rather than result in false positive associations. Although we measured only bedroom dust, since most individuals spend a large portion of their day in the bedroom, we believe this a highly relevant single location to sample. Although the 16S rRNA amplicon sequencing method does not allow us to quantify absolute levels of bacteria, we were able to quantify relative abundance of individual bacterial taxa to evaluate directionality of associations. With the 16S rRNA method, we were not able to characterize functional gene compositions of microbial communities; future studies using metagenomics can provide further insights. Due to the unique characteristics of our study, house dust microbiota (characterized by using the 16S rRNA amplicon sequencing) and asthma/allergy outcomes in adults, we were unable to identify a replication population. Therefore, we performed look-up replication analyses and found associations consistent in direction between our study and previous studies of human and house dust microbiome12, 14, 51, 52. Our results will serve as a replication set for future microbiome studies just as we provide replication of findings from earlier microbiome studies. Lastly, this study was cross-sectional, limiting our ability to draw causal inferences. A prospective study would be ideal; however, such a study would be logistically challenging, even prohibitive. Because the incidence of asthma in adults is low, on the order of 3 per 1,000 people per year58, to accrue 330 cases of asthma as in our study, one would need to visit the homes of 22,000 individuals to collect dust samples and then wait 5 years for incident cases. Only a few studies have linked indoor microbiota to asthma initiation, and those were in children14, 59. To our knowledge, no large-scale study has investigated associations of asthma initiation in adults with indoor microbiota measured by using the state-of-the-art 16S rRNA amplicon sequencing. In addition, we acknowledge that identifying differences in overall bacterial diversity and differential abundance of individual bacterial taxa does not provide a causal mechanism. Although our findings are consistent with our suggested interpretation of differences in microbiota potentially influencing health outcomes; there is a possibility of reverse causation. However, given that it is difficult to conduct a study examining the role of indoor microbiota on asthma or atopy initiation in adults, findings of this study are valuable for understanding indoor microbiota in relation to adult allergy outcomes.
A strength of our study is the large sample size compared to earlier studies examining associations of house dust microbiota with allergy outcomes which ranged from 86 and 196 participants12, 13. Another strength is the comprehensive microbiome data generated by high throughput sequencing; the 1,385 bacterial taxa identified allowed us to perform an exhaustive search of bacterial taxa associated with asthma and atopy outcomes in adults. Our definition of current atopy was objective based on specific IgE with a strict threshold of 0.70. In addition to dichotomized current asthma and atopy outcomes, we analyzed asthma and atopy together and the degree of asthma control within asthmatics to better evaluate asthma subtypes.
In this study, we found that bacterial communities in house dust were associated with asthma, atopy, and hay fever outcomes in adults. Specifically, we noted less diverse bacterial communities inside homes of atopics (versus non-atopics) and identified specific bacterial taxa differentially abundant by current asthma or atopy outcomes. The more abundant bacterial taxa in healthy individuals could shed light on specific mechanisms underlying the hygiene hypothesis and the more abundant taxa in diseased individuals could inform mechanisms of pathogenesis or exacerbation. This comprehensive investigation of house dust microbiota in relation to asthma, atopy, and hay fever extends our understanding of contributions of indoor microbiota to adult health outcomes.
Supplementary Material
Key Messages.
This is the first large-scale study showing associations of adult allergic outcomes with both overall bacterial diversity and individual bacterial taxa using a high-throughput sequencing method.
Exposure to more diverse bacterial communities inside homes was inversely associated with adult atopy and hay fever and exposure to higher/lower abundances of specific bacterial taxa was associated with asthma, atopy, and hay fever in adults.
Acknowledgements
We appreciate all of the study participants for their contribution to this research. We thank Drs. Frank Day of NIEHS for expert computational assistance and Jane Hoppin, ScD (North Carolina State University, Raleigh NC) for her important contribution to the Agricultural Lung Health Study during her tenure at NIEHS.
Funding
This work was supported by the Intramural Research Program of the National Institutes of Health, the National Institute of Environmental Health Sciences (Z01-ES049030 and Z01-ES102385 and for ABW contract no. HHSN273201600003I) and the National Cancer Institute (Z01-CP010119B) and by American Recovery and Reinvestment Act funds. The Microbiome Core Facility at the University of North Carolina is supported in part by NIH National Institute of Diabetes and Digestive and Kidney Diseases grant P30 DK34987. P.S.T. was supported by PHR-SUPS2-S-10-00179 and NIH P30 ES005605.
Abbreviations used:
- ACQ
Asthma Control Questionnaire
- ANCOM
analysis of composition of microbiomes
- COPD
chronic obstructive pulmonary disease
- FDR
false discovery rate
- IgE
immunoglobulin E
- MiRKAT
microbiome regression-based kernel association test
- OTU
operational taxonomic unit
- QIIME
Quantitative Insights Into Microbial Ecology
- UniFrac
unique fraction metric
Footnotes
Disclosure of potential conflict of interest
All authors have nothing to disclose.
References
- 1.2017 National Health Interview Survey (NHIS) Data. National Health Interview Survey, National Center for Health Statistics, CDC.] Available from https://www.cdc.gov/asthma/nhis/2017/table3-1.htm. [Google Scholar]
- 2.Ownby DR, Johnson CC, Peterson EL. Exposure to dogs and cats in the first year of life and risk of allergic sensitization at 6 to 7 years of age. JAMA 2002; 288:963–72. [DOI] [PubMed] [Google Scholar]
- 3.von Mutius E, Vercelli D. Farm living: effects on childhood asthma and allergy. Nat Rev Immunol 2010; 10:861–8. [DOI] [PubMed] [Google Scholar]
- 4.Ege MJ. The Hygiene Hypothesis in the Age of the Microbiome. Ann Am Thorac Soc 2017; 14:S348–S53. [DOI] [PubMed] [Google Scholar]
- 5.Williams LK, Ownby DR, Maliarik MJ, Johnson CC. The role of endotoxin and its receptors in allergic disease. Ann Allergy Asthma Immunol 2005; 94:323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thorne PS, Mendy A, Metwali N, Salo P, Co C, Jaramillo R, et al. Endotoxin Exposure: Predictors and Prevalence of Associated Asthma Outcomes in the United States. Am J Respir Crit Care Med 2015; 192:1287–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smit LA, Heederik D, Doekes G, Blom C, van Zweden I, Wouters IM. Exposure-response analysis of allergy and respiratory symptoms in endotoxin-exposed adults. Eur Respir J 2008; 31:1241–8. [DOI] [PubMed] [Google Scholar]
- 8.Valkonen M, Wouters IM, Taubel M, Rintala H, Lenters V, Vasara R, et al. Bacterial Exposures and Associations with Atopy and Asthma in Children. PLoS One 2015; 10:e0131594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valkonen M, Taubel M, Pekkanen J, Tischer C, Rintala H, Zock JP, et al. Microbial characteristics in homes of asthmatic and non-asthmatic adults in the ECRHS cohort. Indoor Air 2018; 28:16–27. [DOI] [PubMed] [Google Scholar]
- 10.Pekkanen J, Valkonen M, Taubel M, Tischer C, Leppanen H, Karkkainen PM, et al. Indoor bacteria and asthma in adults: a multicentre case-control study within ECRHS II. Eur Respir J 2018; 51. [DOI] [PubMed] [Google Scholar]
- 11.Stephens B What Have We Learned about the Microbiomes of Indoor Environments? mSystems 2016; 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Birzele LT, Depner M, Ege MJ, Engel M, Kublik S, Bernau C, et al. Environmental and mucosal microbiota and their role in childhood asthma. Allergy 2017; 72:109–19. [DOI] [PubMed] [Google Scholar]
- 13.Dannemiller KC, Gent JF, Leaderer BP, Peccia J. Indoor microbial communities: Influence on asthma severity in atopic and nonatopic children. J Allergy Clin Immunol 2016; 138:76–83 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirjavainen PV, Karvonen AM, Adams RI, Taubel M, Roponen M, Tuoresmaki P, et al. Farm-like indoor microbiota in non-farm homes protects children from asthma development. Nat Med 2019; 25:1089–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stein MM, Hrusch CL, Gozdz J, Igartua C, Pivniouk V, Murray SE, et al. Innate Immunity and Asthma Risk in Amish and Hutterite Farm Children. N Engl J Med 2016; 375:411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ege MJ, Mayer M, Normand AC, Genuneit J, Cookson WO, Braun-Fahrlander C, et al. Exposure to environmental microorganisms and childhood asthma. N Engl J Med 2011; 364:701–9. [DOI] [PubMed] [Google Scholar]
- 17.Alavanja MC, Sandler DP, McMaster SB, Zahm SH, McDonnell CJ, Lynch CF, et al. The Agricultural Health Study. Environ Health Perspect 1996; 104:362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carnes MU, Hoppin JA, Metwali N, Wyss AB, Hankinson JL, O’Connell EL, et al. House Dust Endotoxin Levels Are Associated with Adult Asthma in a U.S. Farming Population. Ann Am Thorac Soc 2017; 14:324–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.House JS, Wyss AB, Hoppin JA, Richards M, Long S, Umbach DM, et al. Early-life farm exposures and adult asthma and atopy in the Agricultural Lung Health Study. J Allergy Clin Immunol 2017; 140:249–56 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferris BG. Epidemiology Standardization Project (American Thoracic Society). Am Rev Respir Dis 1978; 118:1–120. [PubMed] [Google Scholar]
- 21.Lee MK, Carnes MU, Butz N, Azcarate-Peril MA, Richards M, Umbach DM, et al. Exposures Related to House Dust Microbiota in a U.S. Farming Population. Environ Health Perspect 2018; 126:067001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J 1999; 14:902–7. [DOI] [PubMed] [Google Scholar]
- 23.Juniper EF, Bousquet J, Abetz L, Bateman ED, Committee G. Identifying ‘well-controlled’ and ‘not well-controlled’ asthma using the Asthma Control Questionnaire. Respir Med 2006; 100:616–21. [DOI] [PubMed] [Google Scholar]
- 24.Van Hoeyveld E, Nickmans S, Ceuppens JL, Bossuyt X. Defining thresholds of specific IgE levels to grass pollen and birch pollen allergens improves clinical interpretation. Clin Chim Acta 2015; 450:46–50. [DOI] [PubMed] [Google Scholar]
- 25.Mahmoudi N, Slater GF, Fulthorpe RR. Comparison of commercial DNA extraction kits for isolation and purification of bacterial and eukaryotic DNA from PAH-contaminated soils. Can J Microbiol 2011; 57:623–8. [DOI] [PubMed] [Google Scholar]
- 26.Haas BJ, Gevers D, Earl AM, Feldgarden M, Ward DV, Giannoukos G, et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res 2011; 21:494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol 2013; 79:5112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klindworth A, Pruesse E, Schweer T, Peplies J, Quast C, Horn M, et al. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res 2013; 41:e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 2012; 6:1621–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lear G, Dickie I, Banks J, Boyer S, Buckley HL, Buckley TR, et al. Methods for the extraction, storage, amplification and sequencing of DNA from environmental samples. New Zealand Journal of Ecology 2018; 42:10-+. [Google Scholar]
- 31.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 2010; 7:335–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010; 26:2460–1. [DOI] [PubMed] [Google Scholar]
- 33.Caporaso JG, Bittinger K, Bushman FD, DeSantis TZ, Andersen GL, Knight R. PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics 2010; 26:266–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Price MN, Dehal PS, Arkin AP. FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Mol Biol Evol 2009; 26:1641–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barberan A, Dunn RR, Reich BJ, Pacifici K, Laber EB, Menninger HL, et al. The ecology of microscopic life in household dust. Proc Biol Sci 2015; 282:212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bokulich NA, Subramanian S, Faith JJ, Gevers D, Gordon JI, Knight R, et al. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat Methods 2013; 10:57–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Navas-Molina JA, Peralta-Sanchez JM, Gonzalez A, McMurdie PJ, Vazquez-Baeza Y, Xu Z, et al. Advancing our understanding of the human microbiome using QIIME. Methods Enzymol 2013; 531:371–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shannon CE. A Mathematical Theory of Communication. Bell System Technical Journal 1948; 27:379–423. [Google Scholar]
- 39.Zhao N, Chen J, Carroll IM, Ringel-Kulka T, Epstein MP, Zhou H, et al. Testing in Microbiome-Profiling Studies with MiRKAT, the Microbiome Regression-Based Kernel Association Test. Am J Hum Genet 2015; 96:797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol 2005; 71:8228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bray JR, Curtis JT. An Ordination of the Upland Forest Communities of Southern Wisconsin. Ecological Monographs 1957; 27:326–49. [Google Scholar]
- 42.Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, et al. vegan: Community Ecology Package. R package 2016. [Google Scholar]
- 43.Mandal S, Van Treuren W, White RA, Eggesbo M, Knight R, Peddada SD. Analysis of composition of microbiomes: a novel method for studying microbial composition. Microb Ecol Health Dis 2015; 26:27663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thorne PS, Kulhankova K, Yin M, Cohn R, Arbes SJ Jr., Zeldin DC. Endotoxin exposure is a risk factor for asthma: the national survey of endotoxin in United States housing. Am J Respir Crit Care Med 2005; 172:1371–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arbes SJ Jr., Sever M, Archer J, Long EH, Gore JC, Schal C, et al. Abatement of cockroach allergen (Bla g 1) in low-income, urban housing: A randomized controlled trial. J Allergy Clin Immunol 2003; 112:339–45. [DOI] [PubMed] [Google Scholar]
- 46.Kim MH, Rho M, Choi JP, Choi HI, Park HK, Song WJ, et al. A Metagenomic Analysis Provides a Culture-Independent Pathogen Detection for Atopic Dermatitis. Allergy Asthma Immunol Res 2017; 9:453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stewart I, Schluter PJ, Shaw GR. Cyanobacterial lipopolysaccharides and human health - a review. Environ Health 2006; 5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nylund L, Satokari R, Nikkila J, Rajilic-Stojanovic M, Kalliomaki M, Isolauri E, et al. Microarray analysis reveals marked intestinal microbiota aberrancy in infants having eczema compared to healthy children in at-risk for atopic disease. BMC Microbiol 2013; 13:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 2006; 124:837–48. [DOI] [PubMed] [Google Scholar]
- 50.Lynch SV, Wood RA, Boushey H, Bacharier LB, Bloomberg GR, Kattan M, et al. Effects of early-life exposure to allergens and bacteria on recurrent wheeze and atopy in urban children. J Allergy Clin Immunol 2014; 134:593–601 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Durack J, Lynch SV, Nariya S, Bhakta NR, Beigelman A, Castro M, et al. Features of the bronchial bacterial microbiome associated with atopy, asthma, and responsiveness to inhaled corticosteroid treatment. J Allergy Clin Immunol 2017; 140:63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Myles IA, Williams KW, Reckhow JD, Jammeh ML, Pincus NB, Sastalla I, et al. Transplantation of human skin microbiota in models of atopic dermatitis. JCI Insight 2016; 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arbes SJ Jr., Calatroni A, Mitchell HE, Gergen PJ. Age-dependent interaction between atopy and eosinophils in asthma cases: results from NHANES 2005-2006. Clin Exp Allergy 2013; 43:544–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dharmage SC, Perret JL, Custovic A. Epidemiology of Asthma in Children and Adults. Front Pediatr 2019; 7:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morton JT, Marotz C, Washburne A, Silverman J, Zaramela LS, Edlund A, et al. Establishing microbial composition measurement standards with reference frames. Nat Commun 2019; 10:2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weiss S, Xu ZZ, Peddada S, Amir A, Bittinger K, Gonzalez A, et al. Normalization and microbial differential abundance strategies depend upon data characteristics. Microbiome 2017; 5:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin H, Peddada SD. Analysis of Compositions of Microbiomes with Bias Correction. Nat Commun 2020; In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Nijs SB, Venekamp LN, Bel EH. Adult-onset asthma: is it really different? European Respiratory Review 2013; 22:193-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karvonen AM, Kirjavainen PV, Taubel M, Jayaprakash B, Adams Rl, Sordillo JE, et al. Indoor bacterial microbiota and development of asthma by 10.5 years of age. J Allergy Clin lmmuno 2019; 144:1402–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


