Abstract
Background and aims:
Apolipoprotein A-II (apoAII) is the second major apolipoprotein of the high-density lipoprotein (HDL) particle, after apoAI. Unlike apoAI, the biological and physiological functions of apoAII are unclear. We aimed to gain insight into the specific roles of apoAII in lipoprotein metabolism and atherosclerosis using a novel rabbit model.
Methods:
Wild-type (WT) rabbits are naturally deficient in apoAII, thus their HDL contains only apoAI. Using TALEN technology, we replaced the endogenous apoAI in rabbits through knock-in (KI) of human apoAII. The newly generated apoAII KI rabbits were used to study the specific function of apoAII, independent of apoAI.
Results:
ApoAII KI rabbits expressed exclusively apoAII without apoAI, as confirmed by RT-PCR and Western blotting. On a standard diet, the KI rabbits exhibited lower plasma triglycerides (TG, 52%, p<0.01) due to accelerated clearance of TG-rich particles and higher lipoprotein lipase activity than the WT littermates. ApoAII KI rabbits also had higher plasma HDL-C (28%, p<0.05) and their HDL was rich in apoE, apoAIV, and apoAV. When fed a cholesterol-rich diet for 16 weeks, apoAII KI rabbits were resistant to diet-induced hypertriglyceridemia and developed significantly less aortic atherosclerosis compared to WT rabbits. HDL isolated from rabbits with apoAII KI had similar cholesterol efflux capacity and anti-inflammatory effects as HDL isolated from the WT rabbits.
Conclusions:
ApoAII KI rabbits developed less atherosclerosis than WT rabbits, possibly through increased plasma HDL-C, reduced TG and atherogenic lipoproteins. These results suggest that apoAII may serve as a potential target for the treatment of atherosclerosis.
Keywords: apoAII, atherosclerosis, HDL, rabbit, knock-in
Graphical Abstract
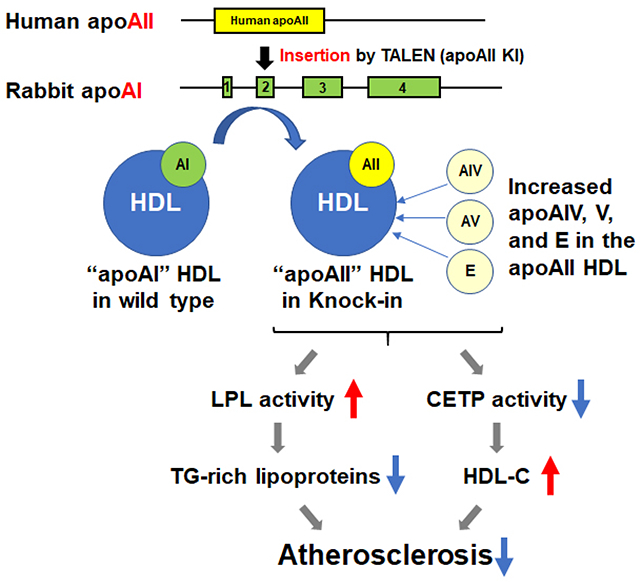
Introduction
High-density lipoprotein (HDL) and apolipoprotein AI (apoAI)1 are well-known plasma markers that are negatively correlated with atherosclerotic disease.2 HDL is known for its anti-atherogenic properties through enhancement of reverse cholesterol transport, anti-inflammatory and anti-oxidative effects as well as improved vascular function.2 The anti-atherogenic properties of HDL are attributed to the biological function of its major apolipoprotein, apoAI. Nevertheless, it remains controversial whether therapeutic elevation of plasma HDL or apoAI can alleviate atherosclerotic disease.3–5 A potential explanation for this controversy is that the protein components of the HDL particles are highly heterogeneous as indicated through proteomics studies.6–12 Furthermore, the biological functions of the major apolipoproteins in the HDL particles are still not fully understood.
Apolipoprotein A-II (apoAII) is the second major constitutive protein in the human HDL particle, accounting for 15-20% of the total proteins.13–15 Unlike apoAI, the biological and physiological functions of apoAII are largely unknown. Epidemiological and genetic studies reported inconsistent results regarding the relationship between apoAII and atherosclerotic disease.16–18 Studies using transgenic mice and knockout (KO) mice also resulted in conflicting findings indicating that apoAII may exert either anti-atherogenic19, 20 or pro-atherogenic effects.21–25 These contradicting findings may be explained by species differences with regards to the apoAII protein structure as well as differences in HDL metabolism in humans and mice. Human apoAII forms a homodimer while mouse apoAII exists as a monomer. Furthermore, the kinetics of HDL and LDL in mice is different from humans due to the lack of cholesteryl ester transfer protein (CETP) in mice.26
To define the functional roles of apoAII, we previously generated transgenic rabbits expressing the human apoAII gene and found that apoAII expression substantially affected lipoprotein metabolism and atherosclerosis.27, 28 Wild-type (WT) rabbits are naturally deficient in the apoAII gene and their HDL contain only apoAI.27 This feature allows a unique opportunity to study the specific biological and physiological functions of apoAII using genetically modified rabbits. Indeed, we previously observed an increased cholesterol efflux capacity of HDL isolated from transgenic rabbits with both apoAI and apoAII in comparison to HDL from WT rabbits with only apoAI.27, 28 These findings are consistent with human studies demonstrating that the presence of apoAII in HDL enhances ATP binding cassette subfamily A member 1 (ABCA1)-mediated cholesterol efflux compared to HDL containing only apoAI.29
In the current study, we aimed to gain insight into the specific roles of apoAII, independent of apoAI. We applied transcription activator-like effector nucleases (TALEN) technology to knock-in (KI) the human apoAII coding sequence in rabbits, replacing the rabbit apoAI. The newly generated apoAII KI rabbits are a unique animal model in which their HDL contains apoAII without apoAI. Comparison of KI rabbits to WT rabbits, in which their HDL contains only apoAI, allowed us to study the specific roles of apoAII in lipoprotein metabolism and atherosclerosis. Using this novel model, we found that replacement of apoAI with apoAII resulted in profound effects on both apoB-containing lipoprotein and HDL metabolism and significantly attenuated cholesterol diet-induced atherosclerosis in rabbits.
Materials and methods
(Extended methods are described in the Supplemental Data).
Animals
New Zealand White rabbits (Covance Inc. Princeton, NJ) were used for this study. All rabbits were housed individually in cages under constant 21°C temperature and 12-hour light/dark cycles. Rabbits were fed a standard diet (Teklad global rabbit diet 2030, Envigo. Indianapolis, IN), unless indicated otherwise. All animal studies were performed in accordance with animal protocols (PRO00008268) approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Michigan and complied with the National Institutes of Health (NIH) Guidelines for the care and use of laboratory animals.
Induction of atherosclerosis with a cholesterol-rich diet
To induce atherosclerosis, we fed the apoAII Kl and WT rabbits a standard diet supplemented with different amounts of cholesterol for 16 weeks. Since the apoAII KI rabbits exhibited an attenuated response to a cholesterol-rich diet (see below), WT rabbits were fed a diet containing 0.3% cholesterol, whereas the apoAII KI rabbits were fed a diet containing ~1% cholesterol. This allowed us to maintain comparable hypercholesterolemia in the two groups. The plasma total cholesterol (TC) levels were monitored weekly and each KI rabbit was fed an adjusted amount of cholesterol in the diet to keep their plasma TC similar to that of WT rabbits (Figure 5 and Supplemental Figure IVA).28, 30, 31
Figure 5.
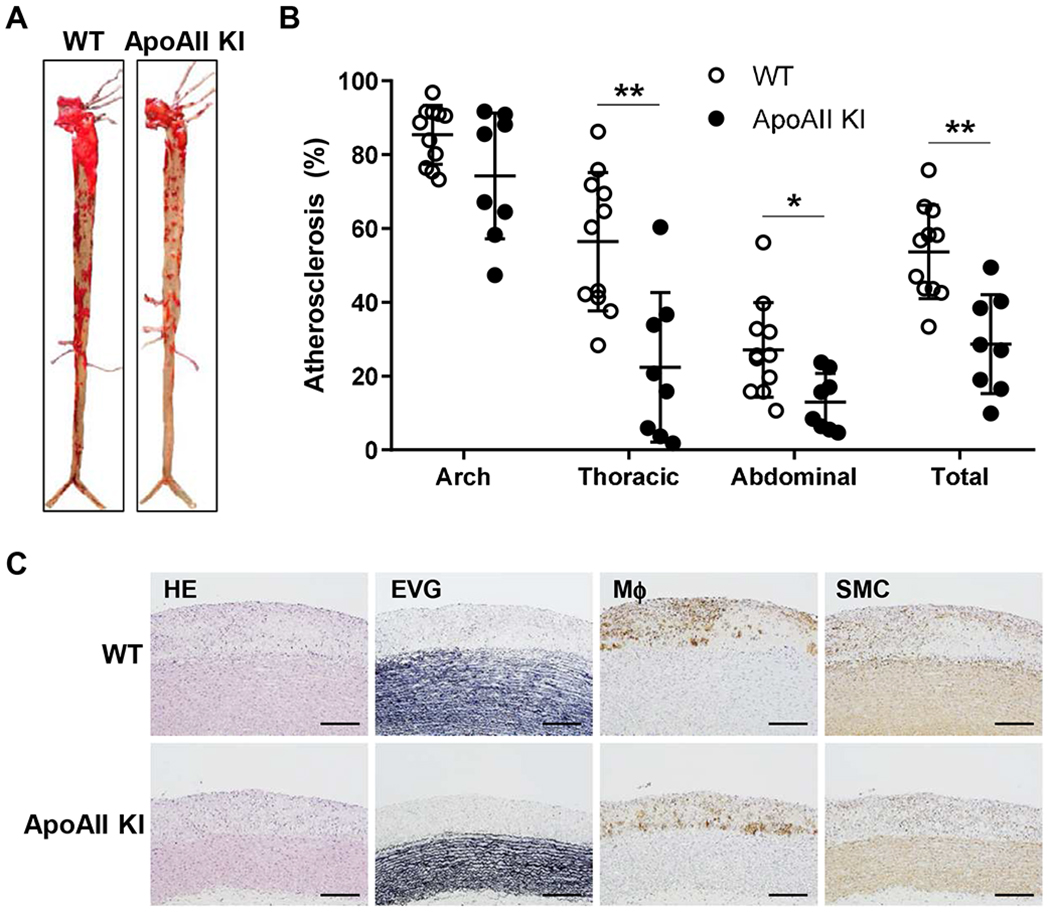
Aortic atherosclerotic lesions after 16 weeks of cholesterol-rich diet feeding.
(A) Representative images of aortic atherosclerosis from WT and apoAII Kl rabbits stained with Sudan IV. (B) The lesion area (defined by sudanophilic staining as red) was quantified using an image analysis system. Each dot represents the lesion area of an individual rabbit. The horizontal bar represents the mean value for each group. n=11 (8 male and 3 female) in WT and n=8 (5 male and 3 female) in Kl rabbits group including male and female rabbits. **p<0.01 or *p<0.05 compared with WT. Statistical analyses were performed using 2-tailed, unpaired Student’s t test. (C) Representative micrographs of the aortic arch lesions from WT and apoAII Kl rabbits. Serial sections of paraffin embedded specimens were stained with hematoxylin-eosin (HE) and elastica van Gieson (EVG) or immunohistochemically using monoclonal antibodies against macrophages (Mϕ) or α-smooth muscle actin for smooth muscle cells (SMCs). The lesions were characterized by intimal accumulation of macrophage-derived foam cells intermingled with smooth muscle cells. Scale bars represent 200 μm.
Statistical analysis
All data are expressed as mean±SEM. Statistical tests performed are detailed in the figure legends and all analyses were conducted using software GraphPad Prism 7.0 (GraphPad Software, San Diego, CA). Each dataset was first assessed for normality using the Shapiro-Wilk test. If data were found not to follow a normal distribution, a nonparametric test was performed as indicated. p<0.05 was considered statistically significant.
Results
Generation of human apoAII knock-in rabbits with TALEN-mediated homologous recombination
The human apoAII coding sequence was designed to be knocked-in the rabbit apoA1 locus by TALEN-mediated homologous recombination, as illustrated in Figure 1A. The TALEN mRNAs to target rabbit apoAI and human apoAII donor DNA vector were injected into 90 rabbit embryos. The embryos were subsequently implanted into 7 surrogate female rabbits, and generated 20 kits. Among them, two founder rabbits carried the human apoAII coding sequences, as determined by PCR and sequencing (Supplemental Figure IB). After breeding with WT rabbits, we established a human apoAII KI rabbit line for the current study as verified by genomic PCR (Figure 1B). Western blot analysis of HDL isolated from rabbit plasma showed that HDL from homozygous apoAII KI rabbits contained only human apoAII without the rabbit apoAI, HDL from the heterozygous apoAI KII rabbits contained both rabbit apoAI and human apoAII and that from WT rabbits contained only abbit apoAI (Figure 1C). ApoAII KI rabbits showed no apparent differences in body weight, and necropsy examination revealed no abnormalities in the heart, kidneys, liver, lungs and other organs (data not shown).
Figure 1.
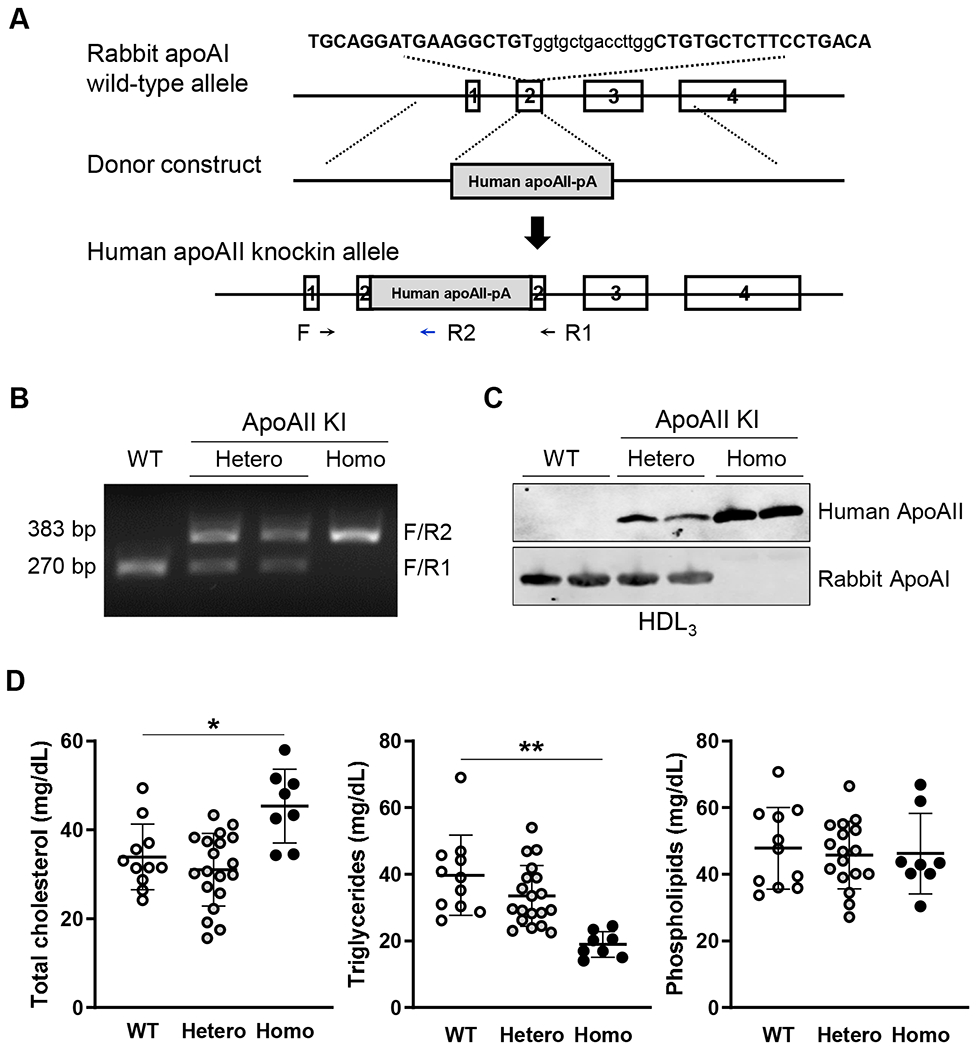
Generation of human apolipoprotein apoAII knock-in rabbits by TALEN genome editing.
(A) Strategy for knocking-in the human apoAII coding sequence into the rabbit apoAI locus. The rabbit apoAI gene structure, donor vector and the apoAI locus after knock-in are shown in the scheme. The sequence shown at the very top is the TALEN targeting sequence. (B) Electrophoresis result showing the PCR products of rabbit genotyping. The location of the genotyping primers is shown at the bottom of panel A, which results in a 270bp and 383bp amplicon from WT and homozygous apoAII knock-in allele, respectively. (C) Rabbit apoAI and human apoAII proteins detected by Western blot of the HDL3 fraction using antibodies against rabbit apoAI and human apoAII. 4 μl of the HDL3 fraction was loaded into each lane. (D) Total cholesterol (TC, n=8-19 in each group, 5-12 male and 3-7 female rabbits), triglycerides (TG, n=8-19 in each group, 5-12 male and 3-7 female rabbits) and phospholipids (n=8-17 in each group, 5-10 male and 3-7 female rabbits) in the plasma from 4 month-old WT and apoAII KI rabbits. Plasma samples were collected from both male and female rabbits.
Plasma lipid and lipoprotein profiles of apoAII knock-in rabbits fed a standard diet
On a standard diet, homozygous (but not heterozygous) 4-6 month-old apoAII KI rabbits showed higher plasma TC (1.3-fold increase over WT, p<0.05), but significantly lower TG (52% reduction, p<0.01) compared with the WT littermates, with no sex differences observed. Plasma phospholipids were comparable between genotypes (Figure 1D). Next, we compared the plasma lipoprotein profile of homozygous apoAII KI rabbits with that of WT rabbits, including both male and female rabbits. Agarose gel electrophoresis of lipoprotein fractions revealed that pre-β migrating lipoproteins (VLDL) were prominently decreased whereas α-migrating lipoproteins (HDL1-2) were increased in apoAII KI rabbits compared to WT rabbits (Figure 2A). These findings were confirmed by quantifying the TC content in each density fraction, which indicated a significant increase in HDL1 and HDL2 (d=1.06-1.08 and 1.08-1.10 g/mL) but a reduction in the apoB-containing particles in the apoAII KI rabbits. The reduced apoB protein contents in the plasma of apoAII KI rabbits was also identified by proteomics assay (Supplemental Figure II). The TC content in the VLDL and IDL fractions was reduced by 81% and 71%, respectively. The TC content in the HDL1, HDL2(1) and HDL2(2) fractions was increased by 3.2-fold, 3.2-fold and 2.1-fold, respectively (Figure 2B, upper panel). Quantification of the TG content in each fraction revealed a significant reduction in the majority of lipoproteins isolated from apoAII KI rabbits compared to WT rabbits, particularly in the TG-rich lipoproteins (VLDL and IDL fractions in d<1.006 and d=1.006-1.02 g/mL, respectively) and the HDL fractions. The TG content in all fractions except for sLDL/HDL1 was significantly reduced by 76%, 59%, 58%, 32%, 42% and 75% in the VLDL, IDL, LDL, HDL2(1), HDL2(2) and HDL3 fractions, respectively (Figure 2B, lower panel). We further analyzed the apolipoprotein composition in each fraction using SDS-PAGE and Western blotting. We confirmed that rabbit apoAI was replaced by human apoAII in the HDL fractions isolated from apoAII KI rabbits, which was accompanied by several remarkable changes in the HDL and apoB-containing particles. Unlike the WT rabbits, the HDL2-3 (d=1.06-1.21g/mL) isolated from apoAII KI rabbits was rich in apoE. Also, the apoB-100 content in the small LDL (d=1.04-1.08 g/ml) was increased in apoAII KI rabbits (Figure 2C). Furthermore, Western blot analysis of the HDL3 fraction revealed a larger size apoE in apoAII KI rabbits. ApoAIV and apoAV were detected only in HDL3 isolated from the apoAII KI rabbits (Figure 2D).
Figure 2.
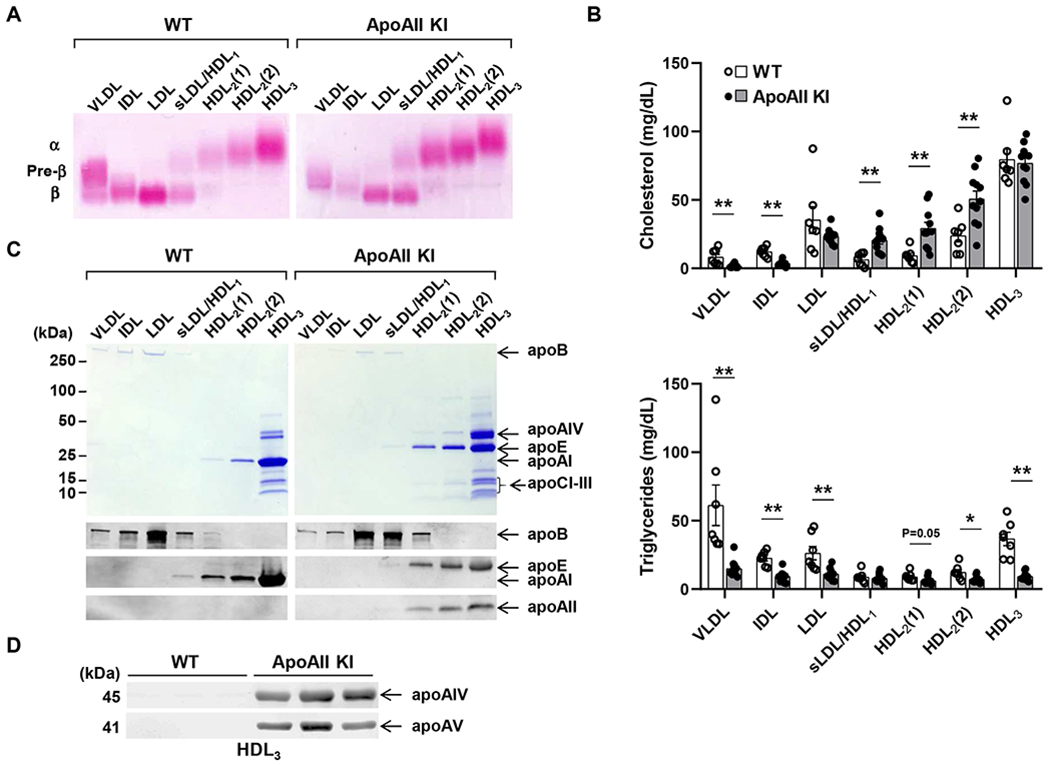
Plasma lipid and lipoprotein profiles of apoAII knock-in and WT rabbits on a standard diet.
(A) Representative agarose gel electrophoresis of lipoproteins stained with Fat red 7B. An equal volume of each fraction was resolved by electrophoresis in a 1% agarose gel. α and β indicate electrophoretic mobility. (B) Quantification of cholesterol and triglyceride content in lipoproteins. n=7-11 per group (wt: 3 males and 4 females; apoAII KI: 6 males and 5 females), 4-5 month-old rabbits. Each fraction corresponds to the densities of <1.006 (VLDL), 1.006-1.02 (−1.02, IDL), 1.02-1.04 (−1.04, LDL), 1.04-1.06 (−1.06, small LDL and HDL1), 1.06-1.08 [−1.08, HDL2(1)], 1.08-1.10 [−1.10, HDL2(2)], 1.10-1.21 (−1.21, HDL3) g/ml. (C) SDS-PAGE gel (upper panel) and immunoblot using apoB, apoE, apoAI and apoAII antibodies (bottom panel). Lipoproteins were isolated from WT and apoAII KI rabbits by sequential density ultracentrifugation according to the density ranges shown below the gels. (D) ApoAIV and apoAV were detected in the HDL3 fraction from WT rabbits and apoAII KI rabbits by Western blot. Data are expressed as mean±SEM. *p<0.05, **p<0.01 compared with WT group. Statistical analyses were performed using ANOVA for comparison among 3 groups and 2-tailed, unpaired Student’s t test between 2 groups.
Catabolism of triglyceride-rich lipoproteins in apoAII knock-in rabbits
We next sought to explore potential mechanisms underlying the reduction in TG-rich lipoproteins observed in the apoAII KI rabbits. First, we performed a lipid clearance test by intravenous lipid loading. As shown in Figure 3A, the rate of TG clearance from the circulation was significantly accelerated in the apoAII KI rabbits compared to the WT rabbits. In addition, post-heparin lipoprotein lipase (LPL) activity was about 2-fold higher in KI rabbits than WT rabbits (Figure 3B), suggesting that apoAII may increase LPL activity, which in turn enhances the catabolism of TG-rich particles. To examine the effects of apoAII KI on VLDL metabolism, we fed the KI rabbits a diet containing 3% soybean oil and 1% cholesterol. We found that the TG-rich lipoproteins in the apoAII KI rabbits remained low even after feeding the rabbits a cholesterol-rich diet for 6 hours (Supplemental Figure IIIA and B). Both the TG and TC contents in the VLDL fractions (d<1.006 g/mL) isolated from the KI rabbits were significantly lower compared with the WT rabbits (Figure 3C). In addition to plasma lipids, the abundance of both apoB100 and apoB48 in the VLDL fractions at the postprandial state was lower in apoAII KI rabbits compared with WT rabbits, suggesting that apoAII KI rabbits are less responsive to a cholesterol-rich diet (Figure 3D). This notion was further confirmed by feeding the cholesterol-rich diet for a longer period. After one week on cholesterol-rich diet, plasma TC was consistently lower in apoAII Kl rabbits compared to WT rabbits (Figure 3E). The hepatic expression of microsomal triglyceride transfer protein (MTTP) was examined by quantitative real time PCR (data not shown) and Western blotting (Supplemental Figure IIIC) and was not significantly different between the two groups, excluding the possibility that lower VLDL levels in apoAII KI rabbits were caused by reduced hepatic VLDL synthesis.
Figure 3.
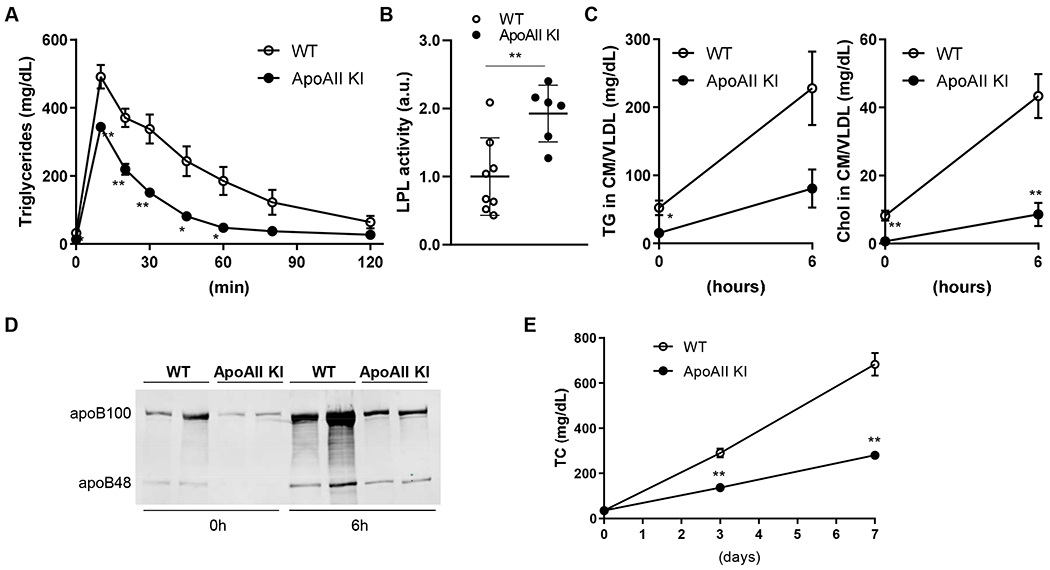
Increased lipid clearance in apoAII knock-in rabbits.
(A) Rabbits were fasted for 16 hours followed by a lipid clearance test performed by intravenous injection of intralipid emulsion (2 ml/kg). The blood was collected sequentially after the injection at the indicated times, and TG levels in each blood sample were quantified. n=4 for each group (2 male and 2 female rabbits). (B) Plasma post-heparin lipoprotein lipase (LPL) activity was measured with an LPL activity assay kit. n=8 (5 male and 3 female) in WT and n=6 (4 male and 2 female) in KI rabbits group. (C) TC and TG levels in the TG-rich lipoprotein fraction (density; < 1.006 g/mL) isolated by ultracentrifugation before and 6 hours after feeding a 1% cholesterol diet. n=7 in WT (4 male and 3 female) and n=5 (3 male and 2 female) in KI rabbits group. (D) Western blot analysis of apoB100 and apoB48 protein in the TG-rich lipoprotein fraction before and after 6 hours of feeding a 1% cholesterol diet. (E) Plasma TC monitored for one week after feeding a cholesterol rich diet (1% cholesterol). n=8 (5 male and 3 female) in WT and n=5 (3 male and 2 female) in KI rabbits group. Data are expressed as mean±SEM. *p<0.05, **p<0.01 compared with WT. Statistical analyses were performed using 2-tailed, unpaired Student’s t test.
Lipid metabolism in apoAII knock-in rabbits after prolonged feeding with a cholesterol-rich diet
To investigate the role of apoAII in the development of atherosclerosis, we fed the rabbits a cholesterol-rich diet for 16 weeks. Since hypercholesterolemia is the major determinant for the lesion size, we kept the apoAII KI rabbits with similar hypercholesterolemia as the WT rabbits by adjusting the dietary cholesterol content as described in the Materials and methods section. This approach allowed us to examine the effects of apoAII KI on atherosclerosis development without the confounding effect of different TC levels. At similar hypercholesterolemia (Figure 4A and Supplemental Figure IVB, left panel), we found considerable differences in the levels of plasma TG and cholesterol distribution to lipoproteins between the two genotypes. Although the plasma TC was maintained at similar levels, the TG levels were consistently lower in the KI rabbits (Figure 4B and Supplemental Figure IVB, right panel). In WT rabbits, cholesterol was mainly carried by the more atherogenic apoB-containing particles (β-VLDL and LDL, d<1.006 to 1.04 g/mL), whereas in apoAII KI rabbits, the cholesterol content in all HDL fractions was increased (Figure 4C and Supplemental Figure II). Trends towards reduced TG content in all the apoB-containing particles were found, without reaching statistical significance due to the limited sample size and large variation. The relative large variation in lipid contents in each lipoprotein, especially for VLDL and IDL, may be partially caused by the recovery rate of samples after ultracentrifugation. In addition, the rabbit is not an inbred animal, which also contributes to the variation in plasma lipid contents among rabbits. No significant differences in TG content in HDLs were found between the genotypes (Figure 4D). Although the plasma TC levels were maintained at similar levels between WT and apoAII Kl rabbits, the HDL-C levels were higher in apoAII KI rabbits (Figure 4C right). We also measured the CETP activity in rabbits fed the cholesterol-rich diet. In line with increased HDL-C, plasma CETP activity in apoAII KI rabbits was significantly lower than in WT rabbits (Figure 4E).
Figure 4.
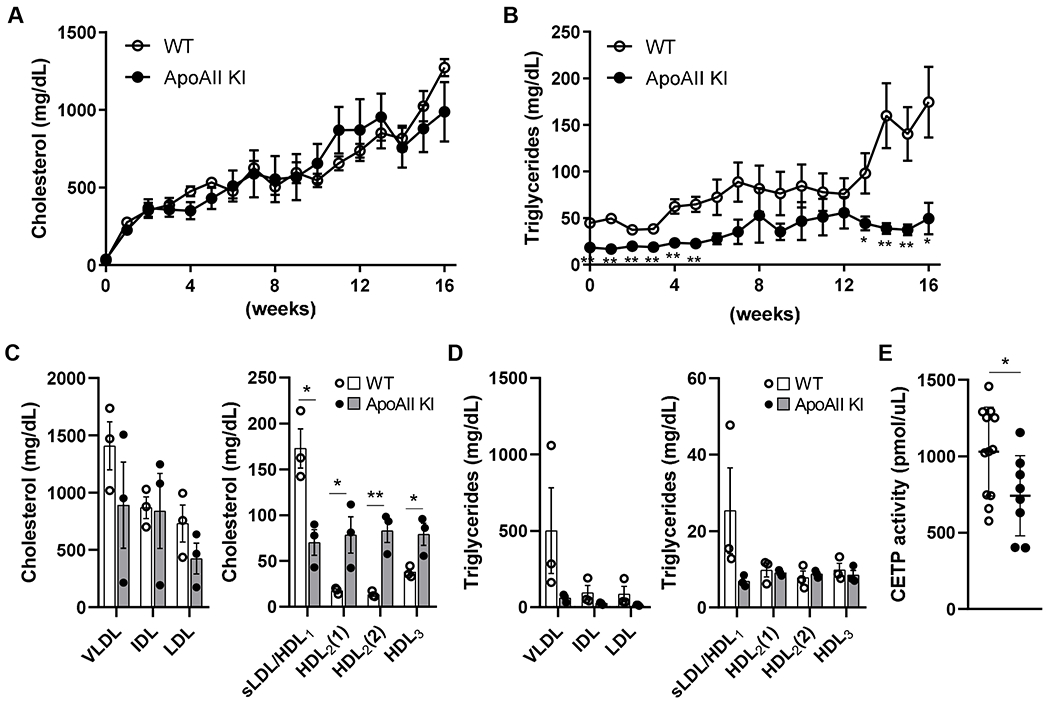
Plasma lipid profiles of WT and knock-in rabbits fed a cholesterol-rich diet.
Plasma TC (A) and TG (B) levels monitored during the 16 weeks of cholesterol-rich diet treatment. TC in WT and apoAII KI rabbits was maintained at similar levels by adjusting the cholesterol content of the diet. n=11 (8 male and 3 female) in WT and n=8 (5 male and 3 female) in KI rabbits group. Quantification of TC (C) and TG (D) content in each lipoprotein fraction. Lipoproteins were isolated from rabbits at 12 weeks on the cholesterol-rich diet. n=3 (male) for each group. (E) Plasma CETP activity was measured after feeding the cholesterol-rich diet for 16 weeks. n=12 (7 male and 5 female) in WT and n=8 (5 male and 3 female) in KI rabbits group. Data are expressed as mean±SEM. *p<0.05, **p<0.01 compared with WT rabbits. Statistical analyses were performed using 2-tailed, unpaired Student’s t test.
In vitro functional analyses of HDL from apoAII knock-in rabbits
To explore functional differences in HDL from WT rabbits and apoAII KI rabbits, we compared their cholesterol efflux capacity and anti-inflammatory effects in vitro. As shown in Supplemental Figure VA, no significant differences in HDL-mediated cholesterol efflux were found between the two genotypes using either apoB-depleted serum as HDL or using HDL3. Furthermore, HDL isolated from WT and apoAII KI rabbits exhibited a similar anti-inflammatory response, and suppressed tumor necrosis factor alpha (TNFα)-induced overexpression of pro-inflammatory genes (Supplemental Figure VB). These results indicate that apoAII in HDL isolated from apoAII KI rabbits has a similar function as apoAI in HDL in WT rabbits.
Atherosclerosis in apoAII KI rabbits after feeding a cholesterol-rich diet
We next analyzed the aortic atherosclerotic lesions in WT and apoAII KI rabbits fed a cholesterol-rich diet for after 16 weeks. En face analysis of aortic lesions visualized by Sudan IV staining revealed that the aortic lesions of apoAII KI rabbits were significantly less in the thoracic (60% decrease), abdominal (52% decrease) and total (47% decrease) aortic area than those of WT littermates (Figure 5A and B). Pathologic analysis of aortic atherosclerosis showed that the proportions of lesion-associated macrophages and smooth muscle cells were not significantly different between WT and apoAII KI rabbits (Figure 5C and Supplemental Figure VI).
Discussion
In the current study, we generated apoAII KI rabbits in which their HDL contains the human apoAII instead of the rabbit apoAI. This novel rabbit model allowed us to investigate the specific roles of apoAII in lipoprotein metabolism and atherosclerosis, independent of apoAI. The apoAII KI rabbits presented remarkable differences in plasma lipid profile compared to WT rabbits including increased HDL, reduced TG and a marked reduction in pro-atherogenic lipoproteins. Furthermore, a significant increase in LPL activity and a decrease in CETP activity were found in apoAII KI rabbits. In line, the apoAII KI rabbits showed substantially lower atherosclerotic burden than the WT rabbits, albeit with no significant differences in the cellular compositions of the plaques.
Replacement of apoAI with apoAII in the KI rabbits resulted in several prominent changes in HDLs. First, compared with apoAI-HDLs, apoAII-HDL particles were rich in apoE along with apoAIV and apoAV, which are known to play important roles in the metabolism of TG-rich lipoproteins. However, replacement of apoAI with apoAII did not alter the HDL function as cholesterol efflux capacity and anti-inflammatory effects were similar in HDL isolated from apoAII KI rabbits and WT rabbits. These findings support the notion that apoAII is equally important as apoAI with regards to HDL function. It is noteworthy that the plasma levels of HDL-C were higher in apoAII KI rabbits than WT rabbits, which may explain the observed anti-atherogenic effects.
Another important feature of the apoAII KI rabbits is a significant reduction in plasma TG both on a standard diet and on a cholesterol-rich diet. These findings indicate that apoAII has profound effects on the metabolism of apoB-containing particles. The lower TG levels found in the KI rabbits suggest that apoAII, and/or apoAIV, apoAV, and apoE, which were increased in their HDL particles, accelerate the catabolism of VLDL in the circulation. This notion was supported by our findings in which apoAII KI rabbits showed accelerated clearance of TG and were resistant to hypertriglyceridemia induced by a cholesterol-rich diet. Importantly, these effects were independent of altered VLDL production in the liver since the hepatic expression of MMTP was comparable between apoAII KI and WT rabbits.
In plasma from apoAII KI rabbits, CETP activity was significantly lower than that of WT rabbits. This finding is in agreement with previous reports indicating that CETP can bind to apoAI, which extends the half-life of CETP in the plasma as compared to unbound CETP.32, 33 The TG-lowering effects of apoAII can also be explained by enhanced LPL activity that was observed in apoAII KI rabbits (2-fold increase over the control). In our previous studies using transgenic rabbits expressing human apoAII together with apoAI, LPL activity was found to be lower. 26, 27 In the current study, replacing apoAI with apoAII in the KI rabbits enhanced LPL activity. Our findings, together with previous reports demonstrating that apoAI itself can inhibit LPL activity,34 suggest that apoAII may act as an activator of LPL, although this remains to be verified. The enhanced LPL activity and reduction in plasma TG found in the apoAII KI rabbits can also be explained by increased abundance of apoAIV and apoAV in their HDL fractions. ApoAIV is known to enhance the activity of LPL,35 and apoAV lowers plasma TG by enhancing TG clearance through binding to LPL and/or heparin sulfate proteoglycans and interaction with LDL receptors.36–38 Although we did not find an increase in abundance of apoAIV, apoAV, and apoE in TG-rich lipoproteins, apolipoproteins in HDL particles can interact with apoB in LDL through bridging molecules like CETP.
With regards to lipoprotein metabolism and atherogenicity, our findings from studying the apoAII KI rabbit model indicate an overall beneficial effect of apoAII. To examine the role of apoAII on atherogenesis, we fed KI and WT rabbits a cholesterol-rich diet. To investigate the effects on atherosclerosis without the confounding effects of differences in hypercholesterolemia, we fed the rabbits diets with different amount of cholesterol and maintained similar levels of plasma TC throughout the study. Importantly, we found that the whole aortic lesion area was significantly lower in apoAII KI rabbit than WT littermates, even when maintaining similar hypercholesterolemia. The findings of the current work, together with our previous studies in apoAII transgenic rabbits,28 clearly indicate the potent anti-atherogenic effects of apoAII in the presence or absence of apoAI.
The anti-atherogenic effects of apoAII may be mediated by multiple mechanisms that are independent of plasma cholesterol levels. First, as we found in apoAII KI rabbits, apoAII expression reduced plasma TG and pro-atherogenic TG-rich lipoproteins including β-VLDLs, IDLs and LDLs through enhancing LPL activity, the latter is also known to reduce atherosclerosis in rabbits.39 Second, although the cholesterol efflux capacity and anti-inflammatory effects of HDL isolated from apoAII KI and WT rabbits were comparable, a net increase in HDL particles was found in apoAII KI rabbits. HDL is known to display anti-antioxidant activities,40 maintain endothelial integrity,41 regulate immunity and T cell functions,42, 43 that may further contribute to the atheroprotective effects observed in apoAII KI rabbits. Although apoAII KI rabbits exhibited lower atherosclerosis, it should be noted that rabbits have low activity of hepatic lipase,44, 45 which may affect the function of apoAII observed in KI rabbits. The interaction between apoAII and hepatic lipase could be addressed in future studies by crossbreeding apoAII KI rabbits with hepatic lipase transgenic rabbits.
Whereas the whole aortic lesion area was significantly lower in apoAII KI rabbits, the presence of lesion-associated macrophages and smooth muscle cells was similar to comparable to WT rabbits. These findings are consistent with our previous findings using human apoAII transgenic rabbits and suggest that apoAII does not directly influence the lesion morphological and cellular characteristics rather the overall atherosclerotic burden.28 Our findings suggest a therapeutic potential for HDL-associated apoAII against atherosclerotic disease; however, the beneficial roles of HDL in clinical settings remain controversial. A recent report shows that extremely high HDL-C levels in humans were found to increase all-cause mortality.46 Furthermore, to date, there is no pharmaceutical treatment for cardiovascular diseases that is based on HDL, apoAI or apoAII.3–5 Moreover, genetic variants associated with increased47 or decreased48 plasma HDL-cholesterol did not affect the risk of cardiovascular diseases. These observations highlight the considerable discrepancies between humans and animal models and emphasize the need for further studies to define the role of HDL and that of apoAII in physiological and pathophysiological conditions.
In conclusion, using newly generated apoAII KI rabbits, we uncovered the specific roles of human apoAII in lipoprotein metabolism and atherosclerosis. ApoAII plays multiple roles in modulating apoB-containing and HDL particles and reduces atherosclerosis in rabbits. Our findings suggest that apoAII may serve as a novel therapeutic target for the treatment of atherosclerosis.
Supplementary Material
Highlights.
We generated a human apoAII knock-in rabbit through TALEN technology, in which the human apoAII replaced the endogenous rabbit ApoAI gene.
On a chow diet, apoAII knockin rabbits exhibited lower plasma triglycerides (TG) levels due to faster clearance of TG-rich particles and higher lipoprotein lipase activity than WT.
ApoAII knockin rabbits had higher HDL-C levels and HDL particles rich in apoE, apoAIV, and apoAV protein.
On a cholesterol-rich diet, apoAII knockin rabbits showed consistently lower TG and higher HDL-C levels and developed significantly less aortic atherosclerosis.
These results underscore a better protective function for ApoAII-containing HDL particles compared with ApoAI, suggesting a potential for targeted therapies.
Acknowledgments
Financial support
This work was supported in part by National Institutes of Health (NIH) grant (HL147527, HL134569 and HL129778 to Y.E. Chen; HL150233 to O. Rom; HL138139 to J. Zhang), and JSPS KAKENHI Grant (15H04718 to JF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References:
- 1.Asztalos BF, Schaefer EJ. High-density lipoprotein subpopulations in pathologic conditions. Am J Cardiol 2003;91:12E–17E. [DOI] [PubMed] [Google Scholar]
- 2.Av Eckardstein, Kardassis D, SpringerLink (Online service) High Density Lipoproteins From Biological Understanding to Clinical Exploitation. Cham: Springer International Publishing : Imprint: Springer, 2015. [Google Scholar]
- 3.Tall AR, Rader DJ. Trials and Tribulations of CETP Inhibitors. Circ Res 2018;122:106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schandelmaier S, Briel M, Saccilotto R, Olu KK, Arpagaus A, Hemkens LG, Nordmann AJ. Niacin for primary and secondary prevention of cardiovascular events. Cochrane Database Syst Rev 2017;6:CD009744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rader DJ. Apolipoprotein A-I Infusion Therapies for Coronary Disease: Two Outs in the Ninth Inning and Swinging for the Fences. JAMA Cardiol 2018;3:799–801. [DOI] [PubMed] [Google Scholar]
- 6.Shao B, Heinecke JW. Quantifying HDL proteins by mass spectrometry: how many proteins are there and what are their functions? Expert Rev Proteomics 2018;15:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pamir N, Pan C, Plubell DL, Hutchins PM, Tang C, Wimberger J, Irwin A, Vallim TQA, Heinecke JW, Lusis AJ. Genetic control of the mouse HDL proteome defines HDL traits, function, and heterogeneity. J Lipid Res 2019;60:594–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furtado JD, Yamamoto R, Melchior JT, Andraski AB, Gamez-Guerrero M, Mulcahy P, He Z, Cai T, Davidson WS, Sacks FM. Distinct Proteomic Signatures in 16 HDL (High-Density Lipoprotein) Subspecies. Arterioscler Thromb Vasc Biol 2018;38:2827–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon SM, Deng J, Lu LJ, Davidson WS. Proteomic characterization of human plasma high density lipoprotein fractionated by gel filtration chromatography. J Proteome Res 2010;9:5239–5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoofnagle AN, Heinecke JW. Lipoproteomics: using mass spectrometry-based proteomics to explore the assembly, structure, and function of lipoproteins. J Lipid Res 2009;50:1967–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rotllan N, Ribas V, Calpe-Berdiel L, Martin-Campos JM, Blanco-Vaca F, Escola-Gil JC. Overexpression of human apolipoprotein A-II in transgenic mice does not impair macrophage-specific reverse cholesterol transport in vivo. Arterioscler Thromb Vasc Biol 2005;25:e128–132. [DOI] [PubMed] [Google Scholar]
- 12.Vaisar T, Pennathur S, Green PS, Gharib SA, Hoofnagle AN, Cheung MC, Byun J, Vuletic S, Kassim S, Singh P, Chea H, Knopp RH, Brunzell J, Geary R, Chait A, Zhao XQ, Elkon K, Marcovina S, Ridker P, Oram JF, Heinecke JW. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest 2007;117:746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.James RW, Hochstrasser D, Tissot JD, Funk M, Appel R, Barja F, Pellegrini C, Muller AF, Pometta D. Protein heterogeneity of lipoprotein particles containing apolipoprotein A-I without apolipoprotein A-II and apolipoprotein A-I with apolipoprotein A-II isolated from human plasma. J Lipid Res 1988;29:1557–1571. [PubMed] [Google Scholar]
- 14.Asztalos BF, Demissie S, Cupples LA, Collins D, Cox CE, Horvath KV, Bloomfield HE, Robins SJ, Schaefer EJ. LpA-I, LpA-I:A-II HDL and CHD-risk: The Framingham Offspring Study and the Veterans Affairs HDL Intervention Trial. Atherosclerosis 2006;188:59–67. [DOI] [PubMed] [Google Scholar]
- 15.Kido T, Kurata H, Kondo K, Itakura H, Okazaki M, Urata T, Yokoyama S. Bioinformatic Analysis of Plasma Apolipoproteins A-I and A-II Revealed Unique Features of A-I/A-II HDL Particles in Human Plasma. Sci Rep 2016;6:31532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alaupovic P, Mack WJ, Knight-Gibson C, Hodis HN. The role of triglyceride-rich lipoprotein families in the progression of atherosclerotic lesions as determined by sequential coronary angiography from a controlled clinical trial. Arterioscler Thromb Vasc Biol 1997;17:715–722. [DOI] [PubMed] [Google Scholar]
- 17.Birjmohun RS, Dallinga-Thie GM, Kuivenhoven JA, Stroes ES, Otvos JD, Wareham NJ, Luben R, Kastelein JJ, Khaw KT, Boekholdt SM. Apolipoprotein A-II is inversely associated with risk of future coronary artery disease. Circulation 2007;116:2029–2035. [DOI] [PubMed] [Google Scholar]
- 18.Deeb SS, Takata K, Peng RL, Kajiyama G, Albers JJ. A splice-junction mutation responsible for familial apolipoprotein A-II deficiency. Am J Hum Genet 1990;46:822–827. [PMC free article] [PubMed] [Google Scholar]
- 19.Weng W, Breslow JL. Dramatically decreased high density lipoprotein cholesterol, increased remnant clearance, and insulin hypersensitivity in apolipoprotein A-II knockout mice suggest a complex role for apolipoprotein A-II in atherosclerosis susceptibility. Proc Natl Acad Sci U S A 1996;93:14788–14794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tailleux A, Bouly M, Luc G, Castro G, Caillaud JM, Hennuyer N, Poulain P, Fruchart JC, Duverger N, Fievet C. Decreased susceptibility to diet-induced atherosclerosis in human apolipoprotein A-II transgenic mice. Arterioscler Thromb Vasc Biol 2000;20:2453–2458. [DOI] [PubMed] [Google Scholar]
- 21.Forgez P, Chapman MJ, Rall SC Jr., Camus MC. The lipid transport system in the mouse, Mus musculus: isolation and characterization of apolipoproteins B, A-I, A-II, and C-III. J Lipid Res 1984;25:954–966. [PubMed] [Google Scholar]
- 22.Puppione DL, Donna LD, Laganowsky AD, Bassilian S, Souda P, Ryder OA, Whitelegge JP. Mass spectral analyses of the two major apolipoproteins of great ape high density lipoproteins. Comp Biochem Physiol Part D Genomics Proteomics 2009;4:305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scanu AM, Edelstein C, Lim CT. Effect of disulfide cleavage on the molecular weight of one of the major polypeptides of human serum high density lipoprotein. FEBS Lett 1971;18:305–307. [DOI] [PubMed] [Google Scholar]
- 24.Schultz JR, Verstuyft JG, Gong EL, Nichols AV, Rubin EM. Protein composition determines the anti-atherogenic properties of HDL in transgenic mice. Nature 1993;365:762–764. [DOI] [PubMed] [Google Scholar]
- 25.Warden CH, Hedrick CC, Qiao JH, Castellani LW, Lusis AJ. Atherosclerosis in transgenic mice overexpressing apolipoprotein A-II. Science 1993;261:469–472. [DOI] [PubMed] [Google Scholar]
- 26.Yin W, Carballo-Jane E, McLaren DG, Mendoza VH, Gagen K, Geoghagen NS, McNamara LA, Gorski JN, Eiermann GJ, Petrov A, Wolff M, Tong X, Wilsie LC, Akiyama TE, Chen J, Thankappan A, Xue J, Ping X, Andrews G, Wickham LA, Gai CL, Trinh T, Kulick AA, Donnelly MJ, Voronin GO, Rosa R, Cumiskey AM, Bekkari K, Mitnaul LJ, Puig O, Chen F, Raubertas R, Wong PH, Hansen BC, Koblan KS, Roddy TP, Hubbard BK, Strack AM. Plasma lipid profiling across species for the identification of optimal animal models of human dyslipidemia. J Lipid Res 2012;53:51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koike T, Kitajima S, Yu Y, Li Y, Nishijima K, Liu E, Sun H, Waqar AB, Shibata N, Inoue T, Wang Y, Zhang B, Kobayashi J, Morimoto M, Saku K, Watanabe T, Fan J. Expression of human apoAII in transgenic rabbits leads to dyslipidemia: a new model for combined hyperlipidemia. Arterioscler Thromb Vasc Biol 2009;29:2047–2053. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Niimi M, Nishijima K, Waqar AB, Yu Y, Koike T, Kitajima S, Liu E, Inoue T, Kohashi M, Keyamura Y, Yoshikawa T, Zhang J, Ma L, Zha X, Watanabe T, Asada Y, Chen YE, Fan J. Human apolipoprotein A-II protects against diet-induced atherosclerosis in transgenic rabbits. Arterioscler Thromb Vasc Biol 2013;33:224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melchior JT, Street SE, Andraski AB, Furtado JD, Sacks FM, Shute RL, Greve EI, Swertfeger DK, Li H, Shah AS, Lu LJ, Davidson WS. Apolipoprotein A-II alters the proteome of human lipoproteins and enhances cholesterol efflux from ABCA1. J Lipid Res 2017;58:1374–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duverger N, Kruth H, Emmanuel F, Caillaud JM, Viglietta C, Castro G, Tailleux A, Fievet C, Fruchart JC, Houdebine LM, Denefle P. Inhibition of atherosclerosis development in cholesterol-fed human apolipoprotein A-I-transgenic rabbits. Circulation 1996;94:713–717. [DOI] [PubMed] [Google Scholar]
- 31.Ichikawa T, Kitajima S, Liang J, Koike T, Wang X, Sun H, Okazaki M, Morimoto M, Shikama H, Watanabe T, Yamada N, Fan J. Overexpression of lipoprotein lipase in transgenic rabbits leads to increased small dense LDL in plasma and promotes atherosclerosis. Lab Invest 2004;84:715–726. [DOI] [PubMed] [Google Scholar]
- 32.Dergunov AD, Shabrova EV, Dobretsov GE. Cholesteryl ester diffusion, location and self-association constraints determine CETP activity with discoidal HDL: excimer probe study. Arch Biochem Biophys 2014;564:211–218. [DOI] [PubMed] [Google Scholar]
- 33.McPherson R, Lau P, Kussie P, Barrett H, Tall AR. Plasma kinetics of cholesteryl ester transfer protein in the rabbit. Effects of dietary cholesterol. Arterioscler Thromb Vasc Biol 1997;17:203–210. [DOI] [PubMed] [Google Scholar]
- 34.Chroni A, Kan HY, Kypreos KE, Gorshkova IN, Shkodrani A, Zannis VI. Substitutions of glutamate 110 and 111 in the middle helix 4 of human apolipoprotein A-I (apoA-I) by alanine affect the structure and in vitro functions of apoA-I and induce severe hypertriglyceridemia in apoA-I-deficient mice. Biochemistry 2004;43:10442–10457. [DOI] [PubMed] [Google Scholar]
- 35.Goldberg IJ, Scheraldi CA, Yacoub LK, Saxena U, Bisgaier CL. Lipoprotein ApoC-II activation of lipoprotein lipase. Modulation by apolipoprotein A-IV. J Biol Chem 1990;265:4266–4272. [PubMed] [Google Scholar]
- 36.Lookene A, Beckstead JA, Nilsson S, Olivecrona G, Ryan RO. Apolipoprotein A-V-heparin interactions: implications for plasma lipoprotein metabolism. J Biol Chem 2005;280:25383–25387. [DOI] [PubMed] [Google Scholar]
- 37.Merkel M, Loeffler B, Kluger M, Fabig N, Geppert G, Pennacchio LA, Laatsch A, Heeren J. Apolipoprotein AV accelerates plasma hydrolysis of triglyceride-rich lipoproteins by interaction with proteoglycan-bound lipoprotein lipase. J Biol Chem 2005;280:21553–21560. [DOI] [PubMed] [Google Scholar]
- 38.Fruchart-Najib J, Bauge E, Niculescu LS, Pham T, Thomas B, Rommens C, Majd Z, Brewer B, Pennacchio LA, Fruchart JC. Mechanism of triglyceride lowering in mice expressing human apolipoprotein A5. Biochem Biophys Res Commun 2004;319:397–404. [DOI] [PubMed] [Google Scholar]
- 39.Fan J, Unoki H, Kojima N, Sun H, Shimoyamada H, Deng H, Okazaki M, Shikama H, Yamada N, Watanabe T. Overexpression of lipoprotein lipase in transgenic rabbits inhibits diet-induced hypercholesterolemia and atherosclerosis. J Biol Chem 2001;276:40071–40079. [DOI] [PubMed] [Google Scholar]
- 40.Brites F, Martin M, Guillas I, Kontush A. Antioxidative activity of high-density lipoprotein (HDL): Mechanistic insights into potential clinical benefit. BBA Clin 2017;8:66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calabresi L, Gomaraschi M, Franceschini G. Endothelial protection by high-density lipoproteins: from bench to bedside. Arterioscler Thromb Vasc Biol 2003;23:1724–1731. [DOI] [PubMed] [Google Scholar]
- 42.Catapano AL, Pirillo A, Bonacina F, Norata GD. HDL in innate and adaptive immunity. Cardiovasc Res 2014;103:372–383. [DOI] [PubMed] [Google Scholar]
- 43.Creasy KT, Kane JP, Malloy MJ. Emerging roles of HDL in immune function. Curr Opin Lipidol 2018;29:486–487. [DOI] [PubMed] [Google Scholar]
- 44.Fan J, Wang J, Bensadoun A, Lauer SJ, Dang Q, Mahley RW, Taylor JM. Overexpression of hepatic lipase in transgenic rabbits leads to a marked reduction of plasma high density lipoproteins and intermediate density lipoproteins. Proc Natl Acad Sci U S A 1994;91:8724–8728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clay MA, Hopkins GJ, Ehnholm CP, Barter PJ. The Rabbit as an Animal-Model of Hepatic Lipase Deficiency. Biochim Biophys Acta 1989;1002:173–181. [DOI] [PubMed] [Google Scholar]
- 46.Madsen CM, Varbo A, Nordestgaard BG. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: two prospective cohort studies. Eur Heart J 2017;38:2478–2486. [DOI] [PubMed] [Google Scholar]
- 47.Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, Hindy G, Holm H, Ding EL, Johnson T, Schunkert H, Samani NJ, Clarke R, Hopewell JC, Thompson JF, Li M, Thorleifsson G, Newton-Cheh C, Musunuru K, Pirruccello JP, Saleheen D, Chen L, Stewart A, Schillert A, Thorsteinsdottir U, Thorgeirsson G, Anand S, Engert JC, Morgan T, Spertus J, Stoll M, Berger K, Martinelli N, Girelli D, McKeown PP, Patterson CC, Epstein SE, Devaney J, Burnett MS, Mooser V, Ripatti S, Surakka I, Nieminen MS, Sinisalo J, Lokki ML, Perola M, Havulinna A, de Faire U, Gigante B, Ingelsson E, Zeller T, Wild P, de Bakker PI, Klungel OH, Maitland-van der Zee AH, Peters BJ, de Boer A, Grobbee DE, Kamphuisen PW, Deneer VH, Elbers CC, Onland-Moret NC, Hofker MH, Wijmenga C, Verschuren WM, Boer JM, van der Schouw YT, Rasheed A, Frossard P, Demissie S, Willer C, Do R, Ordovas JM, Abecasis GR, Boehnke M, Mohlke KL, Daly MJ, Guiducci C, Burtt NP, Surti A, Gonzalez E, Purcell S, Gabriel S, Marrugat J, Peden J, Erdmann J, Diemert P, Willenborg C, Konig IR, Fischer M, Hengstenberg C, Ziegler A, Buysschaert I, Lambrechts D, Van de Werf F, Fox KA, El Mokhtari NE, Rubin D, Schrezenmeir J, Schreiber S, Schafer A, Danesh J, Blankenberg S, Roberts R, McPherson R, Watkins H, Hall AS, Overvad K, Rimm E, Boerwinkle E, Tybjaerg-Hansen A, Cupples LA, Reilly MP, Melander O, Mannucci PM, Ardissino D, Siscovick D, Elosua R, Stefansson K, O’Donnell CJ, Salomaa V, Rader DJ, Peltonen L, Schwartz SM, Altshuler D, Kathiresan S. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet 2012;380:572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frikke-Schmidt R, Nordestgaard BG, Stene MC, Sethi AA, Remaley AT, Schnohr P, Grande P, Tybjaerg-Hansen A. Association of loss-of-function mutations in the ABCA1 gene with high-density lipoprotein cholesterol levels and risk of ischemic heart disease. JAMA 2008;299:2524–2532. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


