Abstract
Osteoarthritis (OA) is the most common chronic musculoskeletal disorder. It can affect any joint and is the most frequent single cause of disability in older adults.
OA is a progressive degenerative disease involving the entire joint structure in a vicious circle that includes the capsule-bursa tissue inflammation, synovial fluid modifications, cartilage breakdown and erosions, osteochondral inflammatory damage leading to bone erosion and distortion.
Research has identified the initial inflammatory-immunologic process that starts this vicious cycle leading to so-called early OA. Research has also identified the role played in the disease advancement by synoviocytes type A and B, chondrocytes, extracellular matrix, local immune-inflammatory mediators and proteases.
This article investigates the joint-resident MSCs that play an essential local homeostatic role and regulate cell turn over and tissue repair. Resident MSCs establish and maintain a local regenerative microenvironment.
The understanding of OA physiopathology clarifies the core mechanisms by which minimally invasive interventions might be able to halt and reverse the course of early stage OA. Interventions employing PRP, MSCs and exosomes are considered in this article.
Keywords: Degenerative osteoarthritis, MSCs, Early OA, Tissue Regeneration, PRP
1. Introduction
Degenerative osteoarthritis (OA) is the most common chronic musculoskeletal disorder.
Whilst OA can affect all joints, the knee, spine, hip, hands, feet, and shoulder are most frequently affected.
Chronic pain leading to disability is the most common direct consequence.
OA is the single most common cause of disability in older adults.
OA risk factors include obesity, sedentary lifestyle, chronic postural defects, bone density, occupational injury, trauma and genetic predisposition.
The 2010 Global Burden of Disease Study estimated that 10%–15% of all adults aged over 60 had some degree of symptomatic OA, with prevalence higher among females than males [1,2].
This millennium has seen significantly deeper understanding of the physiopathology and cellular-molecular biology processes that underlie degenerative OA. This deeper understanding has unveiled new opportunities first for OA management and now for reversal.
Understanding the early biological processes that trigger OA challenges the view of OA as a chronic disease centred on cartilage degeneration.
It is now well recognised that the progression of the disease involves the entire joint structure in a cycle that includes inflammation of the capsule-bursa tissue, changes to the synovial fluid, erosion of the cartilage, osteochondral inflammatory damage and bone distortion.
Initial inflammation and local homeostatic imbalance between the cartilage and the synovial membrane play key roles in early OA.
The ability of resident joint MSCs to modulate local inflammatory and immunologic responses is important to our understanding of how to break the cycle of biological events that lead to OA.
Suitable persistent stimulation of MSCs is the foundation of a promising treatment to halt and reverse both early stage OA and more advanced joint degeneration.
This article examines how the above knowledge may be applied to therapeutic opportunities for tissue regeneration in OA cases.
2. Synovial membrane (SM)
The joint capsule is a connective envelope around a synovial joint or diarthrosis.
The capsule has two main layers:
-
•
The external fibrous membrane is mainly composed of connective fibrous tissue. It is highly innervated by nerves that cross the adjacent muscles and tendons and connect to the joint.
-
•
The internal synovial membrane (SM) is the synovial fluid secreting layer [3].
The synovial membrane or synovium refers to the special mesenchymal tissue lining the joint cavity, tendon sheaths and bursa tissues [4].
The internal surface of synovium may appear flat or folded as “finger-like projections” or villi. This loose structure adapts the membrane to change its form as the joint surfaces move one on another.
The synovium has a dense net of capillary vessels that provide nutrients for synovium and for the avascular cartilage. SM contains vessels that provide cartilage nutrition through synovial fluid production and diffusion [5].
These membranes, like an inner tube, seal the synovial fluid in the joint space and effectively provide a hydropneumatic cushion to sustain the body weight and soften the traumatic impact during movements.
The fibrous membrane structure can vary in different joints: from fibrous dense collagenous in the hip to loose connective tissue (rich of elastin) in the shoulder. In some joints connective adipose tissue is well represented such as Hoffa's fat pad in knee joints [5].
SM consists of a thin sheet of loose connective tissue that forms an Extracellular Matrix (ECM). This hosts different cell types, many of which have multiple functions. A primary function of the synovial membrane is production of synovial fluid [5,6].
The principal roles of the synovial fluid are to reduce friction between the articular cartilages, to nourish the cartilage and remove debrides.
Synovial fluid belongs to the family of transcellular fluids which can be isolated by blood plasma filtration. The fluid is a transudate and viscous in nature. It facilitates the continuous exchange of oxygen, carbon dioxide and metabolites between the intima permeable blood vessels, the synovial cells and the cartilage [6].
Synovial fluid and its nutrients are essential to metabolically support the superficial and middle zones of the cartilage layers. Under normal conditions the synovial fluid contains hyaluronan, lubricin, proteinase, collagenases, prostaglandins and rare <200 to 2000 WBC/mmˆ3.
The external cartilage ring directly in contact with the synovial membrane can receive nutrition directly from the synovium. However, most of the cartilage obtain nutrients through the diffusion of synovial fluid [7].
2.1. Synovial cells
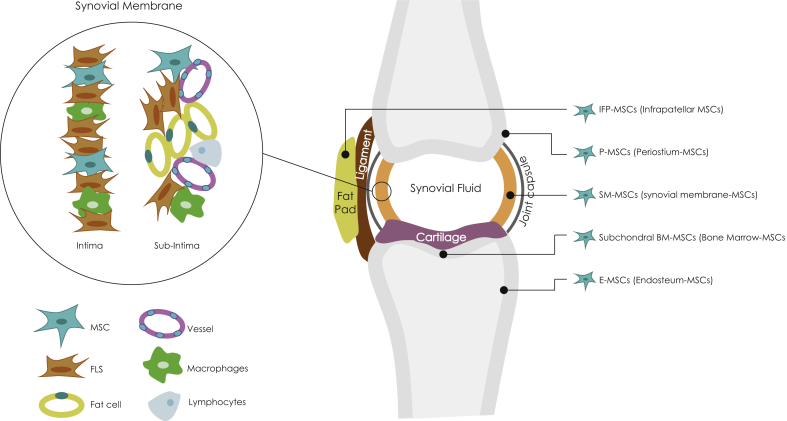
SM consists of two anatomically distinct cell layers: the internal layer named intima and, the underlying supporting tissue subintima. The main intima cell components are macrophages and fibroblasts while the subintima includes blood vessels, adipose cells and fibroblasts with a few lymphocytes and macrophages [4] (Fig. 1).
Fig. 1.
Distribution of various cells in the synovium and joint tissues. FLS = fibroblast-like synoviocytes only represent intima fibroblasts. Joint location of different resident MSCs.
The intima cells are mainly of two types:
Macrophage-like synovial cells known as type A synoviocytes and fibroblast-like synoviocytes (FLS) known as type B synoviocytes.
-
1)
Type A synoviocytes keep the synovial fluid clean by removing wear debris and degraded substances. They account for approximately 25% of cells lining the synovium [3].
Type A synoviocytes have the cell surface markers CD163 and CD68; these identify them as deriving from the macrophage lineage [4].
Smith et al. suggested that macrophage-like synovial cells derive from blood monocytes [8].
-
2)
The fibroblast-like synoviocytes (FLS) or Type B synoviocytes produce a long-chain polymer called hyaluronan which gives the synovial fluid a viscous character, similar to egg-white. Hyaluronan, together with a molecule called lubricin, lubricates the joint surfaces. Water, the main component of synovial fluid, is effectively trapped in the joint space by the strong hydrophilic property of hyaluronan [3].
Type B synoviocytes are typified by surface markers such as CD44 and intercellular adhesion molecule-1 (ICAM-1). These markers classify them as locally derived fibroblasts [4].
The type B synoviocytes possess the morphologic appearence of fibroblasts and have been shown to be capable to generate a large group of molecules including hyaluronic acid, lubricin, proteoglycans, cytokines, arachidonic acid metabolites, and metalloproteinases [9,10].
Recent studies have shown that there is a great difference between synovial intimal fibroblasts and subintimal fibroblasts. Synovial intimal fibroblasts express high levels of UDPGD (Uridine diphosphoglucose dehydrogenase), which is related to the production of hyaluronan. Subintimal fibroblasts do not have this capacity [4,10,11].
Other findings suggested that synovial intimal fibroblasts express several adhesion markers such as VCAM1, ICAM-1, CD44, and β1 integrins, while subintimal fibroblasts and fibroblasts from other sources express lower levels of CD44 and β1 integrins and do not express VCAM-1 [4,8,12].
In 2001, De Bari et al. first isolated MSCs from human synovial membrane (SM) both intima and subintima. He called them Synovium-derived MSCs (SM-MSCs) [13].
SM-MSCs were reported to possess high self-renewal capacity; they can be expanded in vitro over at least ten passages with limited cell senescence.
Furthermore, SM-MSCs often express various markers of synovial fibroblasts in different phases of their differentiation process. This finding suggests that synovial fibroblasts are likely derived from SM-MSCs which are involved in the various stages of fibroblasts maturation and differentiation [13,14].
Li et al. in 2019, confirmed that SM-MSCs have stem cell-like characteristics, immunomodulatory abilities, self-renewal and differentiation properties which lead to the fibroblast lines. Further, they are directly involved in the process of type B synoviocytes turn over and joint connective tissue regeneration [4].
Moreover, other MSC-like cells have been found in other connective tissue of the musculoskeletal system [15].
2.2. MSCs general properties
MSCs were first isolated in bone marrow (BM) and then identified as residential stem-cells in almost all connective tissue. They regulate local homeostasis, turn over of cells and ECM, immune-modulation and tissue regeneration [16].
A well-known in vitro capacity of MSCs is to differentiate in all mesenchymal lineages: fibroblasts, chondroblasts, adipocytes, muscle-cells, osteoblasts and other mesenchymal lineages [17,18].
This stem-cell property might be expressed in vivo directly through the proliferation and differentiation in various mesenchymal cells lineages or by indirectly activating a regenerative microenvironment that facilitates local immune-modulation and trophic changes through the stimulation of local progenitor cells.
These MSCs capabilities operate through autocrine and paracrine effects that play an essential role in tissue repair [16,19].
Caplan AI was the first to locate and identify MSCs as pericytes wrapping the connective tissue microvasculature [20]. While Sacchetti et al. was the first to recognise that CD146+ cells enveloping the micro-vessels in bone marrow are MSCs [21].
Caplan AI and other authors documented that MSCs-pericytes are identified in the perivascular cells. This position is regulated by several factors however, the PDGF receptor is currently considered the main component. Its activation promotes chemotactic and mitogen stimulus for MSCs and facilitates interactions between endothelial cells and MSCs [21,22].
Tissue injury and inflammation activate the resident MSCs which show several local healing and regenerative effects:
-
•
Local immune modulation. MSCs inhibit immune cells surveillance of the injured tissue through the MSCs secretion of HGF (Epatocyte growth factor), TGF-beta (Transforming growth factor-beta) and PGE2 (Prostaglandin E2) and other local mediators. These factors induce lymphocytes to produce IL-10 that balance type 1 T-helper/type 2 T-helper and suppress T-cells activation. The MSCs immunomodulatory effects are so evident that allogenic hMSCs (human-MSCs) infused in the bloodstream for different clinical purposes do not trigger any GVHD (graft versus host disease) and can even protect against xenogeneic rejection when grafts are from different species [[23], [24], [25], [26], [27]].
-
•
Trophic effects. The secretion of an array of bioactive molecules inhibit apoptosis, prevent scar formation and stimulate angiogenesis through VEGF (Vascular Endothelial growth factor) and other mediators. The secretion of mitogenic factors induces the local progenitor cells to promote tissue regeneration [28,29].
For the above reasons, Caplan AI suggested it is more accurate to consider MSCs like “medicinal signalling cells” as MSCs do not directly function in the body as stem-cells for tissues renewal, rather promoting a regenerative microenvironment [19].
2.3. MSCs tissue-specific properties
MSCs are a heterogeneous stem cells population that varies in diverse tissues because they reside in the tissue-specific microenvironment. The tissue-specific MSCs population would include both multipotent and progenitor cells with exclusive capabilities of tissue regeneration and immune response modulation [28].
For example in vitro, dexamethasone stimulates BM-hMSCs to differentiate into osteoblasts but to generate cartilage BM-hMSCs require TGF-beta and a specific medium. Instead, ASCs (Adipose derived-MSCs) requires TGF-beta and BMP-6 (Bone Morphogenetic Protein-6) to differentiate in chondroblasts [30,31].
Therefore, MSCs are not completely the same because they are conditioned by the tissue-specific microenvironment to accomplish their biological role.
2.4. MSCs exosomes
In the last decade, in several experimental models and clinical applications, MSCs have shown local and systemic beneficial effects promoting healing and tissue regeneration.
However, it was very difficult to verify the MSCs survival rate and the specific effects of transplanted cells on the host tissue [32].
Various molecular and biological findings supported the hypothesis that transplanted MSCs did not proliferate and differentiate into tissue-specific mature cells but instead their immune-regenerative actions were supported by the paracrine function [20].
The paracrine action is currently understood to be mostly related to exosomes secretion. Exosomes consist of membrane-enclosed vesicles (EVs) which are generally classified for morphology and size: lipid nanospheres liposome (40–100 nm), MVs or ectosomes which are fragments of plasma membranes (100–1000 nm) and apoptotic bodies (1–5 μm) released by the cell's membrane during apoptosis [20]. Exosomes contain fragments of nucleic acids and various peptides transcription factors, lipids, a large pool of HSPs (the HSP 70 is the most studied) several microRNAs and GFs (VEGF, TGF-beta 1, IL-8, HGF, TCF4) [33].
This vast range of complex molecules locally regulates tissue homeostasis, cell turn over and performance, and tissue regeneration [34].
The exosomes transport and deliver a range of small molecules which mediate the MSCs immunomodulatory effect on local tissue and generate a favourable regenerative microenvironment.
Injured tissue exhibits local hypoxia and lower pH which are optimal conditions for exosome best performance. miRNAs, HSPs and GFs have definitive antiapoptotic and anti-inflammatory effects and promote neovascularization [33,35].
Rani et al. recently reported exosomes not only networking with the local cells; via the blood stream they can reach distant targets [35].
Exosomes, in experimental models, showed significant ability to induce bone regeneration and cartilage repair [36,37].
2.5. Hoffa's MSCs
Adipose tissue is a minor joint tissue component that becomes clinically significant in some conditions such as in the Hoffa's syndrome. The infrapatellar fat pad (Hoffa's fat pad) is an intracapsular but extra-synovial structure and only its deepest portion is covered by the synovial layer.
The infrapatellar fat pad (IFP) with adjacent synovium plays key roles in knee inflammation and OA onset, including the down-regulation of Substance P (SP) which implements the local inflammatory response and nociceptive signal transmission [38].
IFP has a reservoir of tissue-specific MSCs. The isolated IFP-MSCs exhibit increased clonogenicity and in vitro chondrogenic potential compared with BM-MSCs [39].
IFP-MSCs demonstrated plastic-adherence and a fibroblast-like morphology. They also demontrate comparable growth rates to BM-MSCs under basal conditions.
IFP-MSCs sustain high CFU-F (fibroblast colony-forming units) generation through passaging; they express increased chondrogenic differentiation and show a rise in extracellular matrix production compared to BM-MSCs [38,39].
IFP activity is emphasised by the close relationship with intra-articular structures and its essential role played in joint homeostasis and early OA process [38].
The strong immunomodulatory features of IFP-MSCs is confirmed by the high expression of immune suppressive molecule CD10, CD200 and CXCR4, IL-10, and other key immunomodulatory molecules such as IDO, HLA-G and cytokine inhibitors IL6sR and sTNF-RII [38,39].
Moreover, the IFP-MSCs facilitate the degradation of SP which, plays a basic role in the local immune response including T cell proliferation via IL-2 and, stimulation of inflammatory cytokine production [40].
2.6. Joint resident MSCs location
Joint resident MSCs mostly reside in pericytes position spreading together with the microvasculature.
It is currently recognised that there are several types of MSCs contained in the joint structure: SM-MSCs, IFP-MSCs, P-MSCs (Periostium-MSCs), E-MSCs (Endosteum-MSCs) and Subchondral BM-MSCs [13,38,44] (Fig. 1).
Furthermore, stem-cells have been found in other joint structures, for example intervertebral disc stem-cells IVDSCs. These show the capacity to differentiate in adipogenic, osteogenic, and chondrogenic tissues [41].
SM-MSCs lie in niches in the SM intima and subintima. They are mostly packed in the proximity of entheses such as the synovium and the capsule insertion to the periosteum. Similarly, SM-MSCs-like are in tendons and ligaments close to the bone junctions [42,43].
IFP-MSCs are located in the Hoffa's pad and it has been supposed they may reside in adipose tissue other joints, probably in niches close to the bursa-capsule [38].
Periosteum P-MSCs and endosteum E-MSCs play an essential role during fracture repair by becoming bone-forming osteoblasts and chondrocytes that undertake endochondral ossification [44].
Subchondral BM-MSCs exist in a variety of bone medullary cavities, including the mineralized bone matrix and the adipose marrow [44].
Subchondral bone is a highly vascularized tissue allowing the recruitment of MSCs and progenitor cells. It may support cartilage regeneration in particular conditions [45].
Specifically, Subchondral bone consists of the subchondral bone plate (SBP) to which the subchondral trabecular bone (STB) is attached.
Subchondral bone contains at least five different bone cell types – osteocytes, osteoprogenitor cells, bone lining cells, osteoblasts, and osteoclasts. Osteoblasts and osteoclasts form the “bone remodelling unit” that maintain the integrity of the bone and balance between deposited and resorbed bone.
The marrow in the trabecular bone maintains a heterogeneous population of various multipotent mesenchymal stromal cells (BM-MSCs) that provide progenitors for differentiation of osteochondral and other mesenchymal cell lineages [46].
3. Synovial inflammation and early osteoarthritis (EOA)
Historically, OA has been considered a degenerative chronic disease centred on cartilage degeneration. Only recently, research and clinical evidence have indicated that OA involves progressively the entire joint tissues on different timing [47,48].
Rheumatology traditionally defines OA as a condition marked by significant cartilage loss and joint space narrowing and OA classification criteria require the presence of radiographic bony changes or osteophyte formation in accordance with Kellgren and Lawrence standard [49].
These criteria are in agreement with the prevalent physiopathology interpretation that synovitis may be induced by a mechanical injury that generates micro-fissures and micro cartilage fragments. This initial cartilage degradation includes wearing particles, debrides and microcrystals that enter the synovial fluid and trigger the inflammation.
These components, released into the synovial fluid, are phagocyted by synovial macrophages (Type A cells). Type A cells fuel and maintain the inflammation of the synovial membrane through the synthesis of inflammatory mediators and protease which, in turn, diffuse through the synovial fluid into the cartilage generating a vicious circle. This results in progressive cartilage degradation, sustaining and accelerating the vicious circle by producing additional inflammation [48].
Normal articular cartilage is formed by extracellular matrix ECM (water, collagen, proteoglycans and a small amount of calcium salt) and chondrocytes [50]. The normal turnover of the EMC components is managed by the chondrocytes which synthetize these elements and by the proteolytic enzymes responsible for their breakdown.
Chondrocytes are in turn influenced by several factors including synovial fluid composition, GFs and cytokines, joint biomechanics and even by the constituents of the EMC itself [51].
Therefore, EOA results from the failure of chondrocytes and synovial membrane to maintain homeostasis between synthesis and degradation of the ECM components.
Breakdown of ECM collagen and proteoglycans cause the activation of synovial macrophages and the release of proinflammatory cytokines, like TNFα, IL-1 and IL-6. These cytokines push chondrocytes to further release metalloproteinases, inhibit type II collagen production and promote chondrocytes apoptosis; intensifying cartilage degradation [52,53].
In advanced OA, along with articular cartilage damage, chronic inflammation catalyses several changes in the joint capsule and synovium and in synovial fluid, ligaments and tendons.
As the disease progresses, inflammation elevates intra-articular pressure and extensive cartilage destruction allows the synovial fluid to infiltrate the subchondral bone marrow. This causes the production of bone marrow pseudocysts leading to bone erosion and remodelling and the formation of osteophytes [54,55].
Clinically, cartilage debris in the synovial fluid and micro-injuries in articular cartilage may be present for a long time before any degeneration can be noted using MRI or arthroscopy [49].
In 2012 Luyten et al. proposed classification criteria for early knee OA (EKOA), with the aim of identifying patients with signs of initial joint disease in order to initiate early therapeutic approaches [56,57].
A very recent review has focused on early knee OA (EKOA) classification and standardization.
Specifically, two proposals from the Italian Rheumatology Association International and from the First International Early OA Workshop have been recommended.
Various combinations of symptoms, risk factors and minimal X-ray Kellgren & Lawrence (0–1) deviations have been considered for EKOA classification.
International consensus is felt necessary to undertake the OA treatment at an early stage when the disease progression is still reversible.
Discussion about the MRI and USs role and standardisation is still open [58].
Nonetheless, it is still unclear whether the morphological changes that occur in the synovial membrane during OA are the consequence of cartilage degradation and joint inflammation or whether they are primary to these events [59].
Some authors indicated that synovial inflammation may be primary to other structural changes. Abnormalities in joints biomechanics and chronic postural defects certainly play a key role in OA induction.
It has been postulated that excessive or abnormal joint loading and motility disbalance may do more than simply initiate joint tissue damage by wear and tear. Additionally, loading and disbalance may stimulate joint tissue cells to produce pro-inflammatory factors and proteases which ultimately promote OA development [60].
Clinical findings showed that inflammation was present in OA well before the development of significant radiologic changes. Magnetic resonance imaging (MRI), as well as arthroscopy procedures suggested that even at the earliest stage before detectable cartilage degeneration has occurred, OA was already an inflammatory disease in progress [49].
Some studies based on MRI with or without contrast enhancement indicated the presence of synovitis at early stages of OA progression. MRI often showed that thickening of capsule bursa tissue precedes cartilage degradation in OA disease development [[61], [62], [63]].
Other data support the observation of synovial inflammation in the earliest phases of OA.
In 2005 Ayral et al., performed a longitudinal study on 422 patients using diagnostic arthroscopy on knees with symptomatic but no radiographic markers of OA. The research revealed the presence of synovitis well before the development of medial cartilage loss [64].
Scanzello et al. analised a group of patients affected by traumatic meniscal injury without evidence of radiographic OA. During the artrhroscopies performed to repair meniscus, synovial inflammation was noted in 43% of patients and was associated with more severe preoperative pain and poor knee function scores [65].
In one histopathology report based on 70 synovial specimens obtained from arthroscopy, synovial inflammation was present in many patients with the minimal radiographic disease and, in 31% specimens, synovitis was staged as severe [49,66].
Although the synovial membrane is not the only tissue involved in OA-related inflammation, it seems to be a major site of initial inflammatory changes [67].
For histology, cartilage degradation in EOA has been defined and measured by the OARSI (Osteoarthritis Research Society International) scoring system within a range of 1–3 OARSI takes into account the depth of degradation into the articular cartilage layer. These changes involve only the superficial and middle zones of the cartilage.
Therefore, grade 1 is characterised by swelling of the articular cartilage and mild fibrillation in the superficial zone; in grade 2 small areas of the cartilage surface have been lost and chondrocytes have begun to form clusters; in grade 3 vertical fissures have advanced into the middle zone and formation of chondrons may be seen [68,69].
For some authors, Early OA includes a maximal involvement of 50% of the cartilage thickness up to OARSI grade 4 [57].
3.1. Seeking the primum movens
Independent of any discussion whether the synovial or cartilage injury is the starting point of OA; inflammation is the first joint immune-reaction and the main cause of the OA chronic degeneration.
Recently, the Substance P (SP) has been proposed as a trigger factor for both joint pain and inflammatory response.
Substance P (SP) is an undecapeptide (a peptide composed of a chain of 11 amino acid) member of the tachykinin neuropeptide family. It is a neuropeptide, acting as a neurotransmitter and as a neuromodulator.
SP mediates interactions between neurons and immune cells thereby modulating immune cell proliferation and cytokine production. Interestingly, some immune cells have also been found to secrete SP which suggests a central role of Substance P in the local immune response [40].
SP has been found to be secreted by sensory nerve fibres in the synovium and even isolated in the infrapatellar fat pad IFP [40].
SP has been associated with nociceptive pathways promoting and exciting pain; it has also been considered a key modulator of acute local inflammation and chronic fibrotic degeneration.
Specifically, it has been found that SP-secreting sensory nerve fibres predominate over sympathetic ones in clinical presentation of knee pain and increased concentration of SP has been measured in synovial fluid during joint inflammation [70,71].
SP can modulate proliferation of immune cells and promote monocyte activation and migration to sites of inflammation [40].
SP receptor, neurokinin 1 (NK1R), was found highly expressed within synoviocytes where it seems to modulate the expression of cartilage degrading matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs) [40,72].
3.2. Cells and joint tissue inflammatory response
Normally, the synovial membrane is two or three cell layers thick and contains few inflammatory cells. However, during inflammation there is increasing hyperplasia of the Type A synovial cells (macrophages) followed by a rising number of T and B cells, natural killer and mast cells [73,74].
Typically, inflammatory arthritis was defined by the increased numbers of inflammatory cells, mostly leukocytes, which permeate the joint tissues and the synovial fluid. This turns into an inflammatory fluid and loses the main properties of lubrication and cartilage nutrition. This facilitates the degradation and erosion of cartilage. The inflammatory cellular reaction is not prominent in degenerative OA, where the number of leukocytes in the synovial fluid is normally low, and rarely exceeds 1000 to 2000 cells per ml. This is in contrast with inflammatory arthritis such as rheumatoid arthritis (RA), where the number of synovial fluid leukocytes commonly exceed 2000 per ml. The severe leukocytes infiltration of the synovium and the synovial fibroblasts proliferation typical of the RA may result in “pannus” formation [75].
However, some range of synovial inflammation may be evident in OA; in some cases showing hyper immune-inflammatory reaction that can resemble RA.
Benito et al. demonstrated that the degree of leukocytes infiltration was highly heterogeneous in OA. In his study most of the patients exhibited minimally inflammatory and primarily degenerative histopathology while a minority, showed RA-like patterns [76].
In general, macrophages were found predominant in OA synovium, while in RA there were more T cells and B cells [77].
In OA the synovial membrane commonly thickens due to inflammation and leukocyte proliferation. Reports of changes in the synovium that occur at various stages of OA have shown that the amount of fibrin deposited in the synovial membrane and the degree of leukocyte infiltration are correlated with disease severity [78].
Activated Type A synoviocytes and chondrocytes generate large amounts of matrix metalloproteinases (MMPs) including MMP-1, MMP-3, MMP-9 and MMP-13 [79]. Type A synoviocytes are able to secrete not only proteolytic enzymes, but also proinflammatory cytokines (IL-1β, IL-6, TNF-α), which are deemed to be responsible for the inflammation progression and pain associated with the disease [67]. Adipokines, such as resistin are mainly released by the synovium during OA progression accelerating in vitro the ECM degradation [80]. Osteopontin, has been identified as a cytokine expressed by A synoviocytes which, has been correlated with OA prognosis marking the severity of the disease [81].
Therefore the vicious circle seems to be that synovium produces cytokines, chemokines and metalloproteinases which degrade cartilage that in turn catalyses release of the same destructive molecules [82].
The cartilage breakdown products and the exposure of the damaged ECM sustain the release of collagenase and proteolytic enzymes from synovial cells leading to the increased angiogenesis of the synovial membranes [83].
The initial synovial vasodilation is largely caused by Type A synovial cells that secrete inflammatory and pro-angiogenic factors. The latter increase blood vessel permeability and the up-regulation of adhesion molecules supporting the inflammatory response. Specifically, Type A synovial cells release local mediators that stimulate endothelial cells and fibroblasts to produce vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF) and other GFs that support angiogenesis [83,84].
3.3. Role of the joint adipose tissue (Hoffa's fat-pad)
Adipose tissue is a minor joint tissue component that becomes clinically significant in some inflammatory conditions. The best studied joint adipose tissue is the Hoffa's fat-pad also called infrapatellar fat pad (IFP). It plays a key role in Hoffa's syndrome.
Synovium and IFP inflammation describe progressive knee Osteoarthritis, a chronic disease that induces articular cartilage loss and often ends up with a joint replacement.
Interplay between synovium and the adjacent IFP was demonstrated to be fundamental during the onset and progression of OA [38].
Both synovium and IFP host immune cells such as macrophages. When activated these produce pro-inflammatory and damaging molecules including tumour necrosis factor-alpha (TNF-α), interferon-gamma (IFN-γ), interleukin 1-beta (IL-1β) and IL-6 and various adipokines. These together promote cartilage damage [85,86].
4. From early OA to the chronic disease
Early OA features proinflammatory factors that lead to the activation of proteolytic enzymes responsible for the degradation of the extracellular matrix (ECM) resulting in joint tissue damage.
Although destruction and loss of the cartilage is a central component of OA, all joint tissues are progressively affected indicating that OA is a disease of the joint as an “organ” [87].
Current knowledge shows that chronic low-grade inflammation sets the stage for several chronic degenerative diseases. In OA, chronic inflammation is a major driver of the advancement of joint degeneration [49].
Literature indicates that atherosclerosis, chronic periodontitis, age-related macular degeneration, Alzheimer's disease and OA share chronic inflammation as a key factor of the progressive degeneration [[88], [89], [90]].
Joint injury and chronic biomechanical derangement induced by the injury (trauma, overuse, hypermobility, postural misalignments etc) are key factors to induce chronic inflammation in OA. They promote a vicious cycle comprising local tissue damage, inflammation and tissue repair failures resulting in chronic synovitis and fibrosis, cartilage loss and further joint degeneration. These OA events were intriguingly associated by Scanzello et al. to a chronic wound [91].
In the current view of OA progression, mechanical injury is the inducing factor for chronic inflammation.
However, this process is fuelled by continuing disease factors that transform mechanical events into inflammatory signals.
4.1. Factors facilitating the chronic inflammation
Early OA is supported by pro-inflammatory mediators such as cytokines and chemokines that are in turn the consequence of the innate immune response to joint injury.
In advanced stages of OA, additional inflammatory and pro-fibrotic modulators such as connective tissue growth factor (CTGF) together with transforming growth factor-beta (TGF-β) induce synovial and IFP fibrosis, further inducing articular cartilage destruction [49,92].
Innate immunity refers to the immune response induced by invariable pattern-recognition receptors (PRRs) that respond to preserved patterns in nature, including those introduced by invading pathogens such as bacteria, viruses, and fungi so-called pathogen-associated molecular patterns (PAMPs) [93].
Besides microbial patterns, PRRs also recognize multiple endogenous hazard molecules resulting from tissue damage. These group of molecules are known as damage associated molecular patterns (DAMPs). Both PAMPs and DAMPs signal to the immune system the presence of extraneous substances requiring a defensive response [49].
In the OA physiopathology DAMPs appear to be an essential component sustaining the inflammation pattern.
Unlike RA, OA does not appear to be associated with a significant acquired immune response. However, activation of the innate immune system is a common factor for both diseases.
Studies have reported several DAMPs contributing to the chronic inflammation in OA:
-
•
Extracellular matrix (ECM) damage-associated molecules including fibronectin and hyaluronan [94].
-
•
Plasma protein becomes elevated in the synovial fluid secondary to vascular leak and exudation of plasma proteins such as fibrin [95].
-
•
Intracellular alarmins release from damaged and necrotic cells: HMGB-1 (high-mobility group box 1 protein) and the S100 proteins are the most studied [96,97].
-
•
Microscopic inorganic crystals, including basic calcium phosphate (BCP) and calcium pyrophosphate dihydrate (CPPD) crystals, are frequently observed in osteoarthritic synovial fluids and synovial tissue [98].
-
•
Cellular mediators of innate immunity. Besides macrophages, Type B synoviocytes and chondrocytes have a direct role in promoting local inflammation through molecular mediators such as cytokines TNFα, IL-1β, and proteolytic enzymes such as MMPs [81,92].
-
•
Complement activation. This promotes EMC and cartilage damage which in turn, maintains the cycle of complement activation [99].
-
•
Mechanical stress-induced immune activation. This refers to the capacity of mechanical forces to directly stimulate production of inflammatory mediators from cartilage and synovium [49].
5. Joint tissue regeneration
In recent years several regenerative medicine procedures have been introduced for OA treatment. These treatments have the common aim of improving symptoms through tissue regeneration and restoring homeostasis to local cells.
Unlike bone, articular cartilage is rather recalcitrant to regenerate.
Normal adult cartilage shows poor vascularization and innervation; it is prone to produce high concentrations of proteases but unable to make pro-inflammatory inhibitors. These biological features may partially account for the cartilage poor regenerative performance [100].
Furthermore, it is well known that cartilage tissue due to its lack of vasculature and innervation is not capable by itself of producing inflammation or pain at least in the early stages of degenerative OA [100].
5.1. Platelet-rich plasma (PRP)
Platelet-rich plasma (PRP), also known as autologous conditioned plasma, is a concentrate of platelet-rich plasma proteins derived from centrifuged whole blood.
PRP contains several growth factors including Platelet derived growth factor (PDGF); Vascular endothelial growth factor (VEGF); Transforming growth factor beta-1 (TGF-b1); Epidermal growth factor (EGF); Basic fibroblast growth factor (FGF-ß) and Insulin-like growth factor-1 (IGF-1) [101].
Preparations have been categorised by content into four main groups; PPRP (pure PRP), L-PRP (leukocyte and PRP), P-PRF (pure platelet-rich fibrin) and, L-PRF (leukocyte and platelet-rich fibrin) [101].
Depending on the centrifugation technique PRP may be manufactured with or without leukocytes. The role of leukocytes is controversial. Some authors advance hypothesis that leukocytes produce metalloproteinases and cytokines which may be detrimental for joint inflammation and pain. Other researchers believe that PRP-leukocytes release local inhibitor mediators that are crucial for the cartilage repair process [102].
The mechanism by which PRP can stimulate an antiinflammatory, proliferative and remodelling response in cartilage is unknown. It was suggested to be the result of cell stimulation, migration and proliferation from the adjacent bone marrow [103].
Literature showed PRP has chondrogenic differentiating properties found in vitro as well as in vivo in mice and rabbits indicating a major role in regeneration of osteoarthritic cartilage [[104], [105], [106], [107]].
Further in vitro studies demonstrated that PRP was capable of inducing mesenchymal stem cells to proliferate and increasing chondrogenic differentiation. PRP increased gene expression for chondrogenic and osteogenic differentiation. The chondrogenic proliferation was associated with increased collagen synthesis [[108], [109], [110]].
PRP has been delivered usually through intraarticular injection with or without hyaluronic acid. Some orthopaedics combined microfracture arthroscopy injecting PRP into the cartilage defects to speed the cartilage re-growth [111].
Görmeli et al. compared the efficacy of PRP intra-articular injections against hyaluronic acid (HA) in the knee OA. Mostly in the EKOA (early knee OA) group, PRP showed a significant improvement of clinical outcomes (EQ-VAS and IKDC score) at six months after injections compared with HA [112].
As previously reported, the blood components and humoral factors of PRP vary significantly depending on the preparation method used.
The leucocyte content classifies PRPs as leucocyte-poor PRP (LP-PRP), or leucocyte-rich PRP (LR-PRP). Newly, autologous protein solution (APS), a version of LR-PRP with a higher concentration of humoral factors, has been examined [113].
A very recent study has investigated the differences of the humoral factors present in two types of PRP using the Autologous Protein Solution (APS) kit (LR-PRP) and the Cellaid Serum Collection Set P-type (LP-PRP).
The study was designed to establish a relationship between the levels of humoral factors in the preparations and clinical outcomes.
In the APS group, both anti-inflammatory and inflammatory cytokines were highly enhanced. Specifically, the concentrations of TNF-α, PDGF, FGF, sTNF-R2, sFas, TGF-β1 were higher than in LP-PRP group.
After treatment, there was no significant difference in the KOOS (knee injury and OA outcome score) score between ASP (LR-PRP) and LP-PRP groups.
However, after 3 months follow up KOOS total score and QOL (quality of life) score showed a significant improvement in the ASP group.
The study emphasised the role of interleukin-1 receptor antagonist (IL-1RA) which was positively related to clinical outcome improvements [114].
The clinical effectiveness of PRP for osteoarthritis treatment is still under debate.
In recent systematic reviews and meta-analyses were examined the latest randomized controlled trials to test PRP products as intra-articular treatment for knee osteoarthritis and compared with control (mostly low and high molecular-weight hyaluronic acid). Results supported the PRP use over other intra-articular treatments. PRP quite often resulted to improve pain in short and medium-term (up to 6–12 months). However, the overall level of evidence is still low. The reasons may be found in the weak standardization of PRP products and, sometimes in poor trial design and unconsidered bias which significantly affect results [115,116].
5.2. MSCs therapy
In 2001 the FDA issued a set of regulations governing cellular and tissue-based therapies. The basic principle of these guidelines was that cellular and tissue therapies that require minimal to no manipulation of autologous tissue do not required FDA approval, whereas those that involve any significant modification or treatment, including genetic manipulation, require approval.
Allograft tissue containing allogenic cells are classified as transplant tissue and are subject to different regulations [117].
In general, Cell-based therapies include adult differentiated cells, progenitor cells, multipotent stem cells (e.g. MSCs) and pluripotent stem cells (e.g. ESCs; iPSCs). However, MSCs are the most frequently used in clinical practice.
In OA treatments, the arthroscopy microfracture technique was the first surgical technique used to stimulate the healing of small hyaline-cartilage defects. The aim was to induce bleeding from the osteochondral layer to activate migration of reparative stem cells from the bone marrow [118].
The result was the filling of the defect with a neo-generate fibro-cartilage susceptible to deteriorate and fragment into the synovia. To improve the procedure autologous chondrocyte implantation (ACI) has been injected to fill the defect. ACI is a two-stage procedure encompassing chondrocyte isolation and in vitro expansion, followed by implantation. ACI is currently the only FDA approved cell-based treatment for local cartilage defect. Nonetheless, this technique did not show any obvious improvement against simple microfracture [117,119].
MSCs isolated from different sources such as bone marrow, adipose tissue, cord blood, amniotic fluid, omentum, peripheral blood etc, showed in vitro capacity to differentiate in all mesenchymal lineages: fibroblasts, chondroblasts, adipocytes, muscle-cells, osteoblasts and other mesenchymal lineages [17,120].
The process of proliferation and differentiation of MSCs in vitro is well defined. For example BM-MSCs are in vitro differentiated to chondrocites using TGF-β in define medium while adipose-derived stem cells ASCs requires TGF-β + BMP-6 to differentiate to chondrocites.
However, in vivo the local “disease environment” is completely different from in vitro studies. Animal models are generally not able to strictly replicate the human OA. OA is a long course chronic condition mostly affecting aged patients whereas most animal models have been applied to young, healthy animals in which iatrogenic damage is acutely induced and then the experimental intervention performed almost immediately [117].
The most common MSCs procedures in OA have been performed using autologous or allogeneic stem cells.
For autologous MSCs treatments in OA the use of adipose tissue-derived stem cells (ASCs) is the most common and the most studied. The use of peripheral blood stem cells (PBSCs) is becoming popular.
After liposuction collection, ASCs can be culture-expanded and then injected into the joint. However, ASCs are often abundant in fat samples hence they can be directly injected as stromal vascular fraction SVF. The procedure to make SVF is fairly simple: the fat sample is dispersed as a homogeneous slurry and incubated with collagenase to free the MSCs from the rest of tissue. Then after a saline wash the mixture is centrifuged. From the centrifuge is obtained the so called stromal vascular fraction (SVF) containing the MSCs. SVF has been injected directly with well-reported therapeutic effects [28,121,122].
As with other MSCs it has been supposed that ASCs delivered into an injured tissue induce repair and regeneration via paracrine effects. The ASCs stimulate the resident stem cells and promote their differentiation along the required lineage pathway. Furthermore, ASCs similar to BM-MSCs can deliver new mitochondria to damaged cells, thereby rescuing aerobic metabolism [123].
MSCs has been used as mixed injections in combination with GFs, cytokines and scaffolds in order to improve their effectiveness. The most commune scaffolds used are hyaluronic acid and fibrin gel. PRP has been applied as a nutrient fluid containing chondrogenic and osteogenic growth factors (e.g., TGF-β and PDGF) to support the survival and differentiation of transplanted MSCs [124,125].
Several clinical trials are registered at Clinical Trials. gov using MSCs to treat cartilage defects. Some case-control studies reported significant improvement simply by injecting MSCs into the affected joint while others used MSCs in conjunction with arthroscopy subchondral drilling and hyaluronic acid [115]. Some authors have treated OA at various stages injecting MSCs/PRP suspensions into the articular cavity with encouraging results [126].
It is currently becoming more important to consider the potential use of both autologous and allogeneic ASCs. Autologous ASCs offer advantages from regulatory, histocompatibility, and infectious perspectives.
Recent studies support the immunologic safety of allogeneic ASCs transplantation. Three independent studies determined that human culture-expanded ASCs reduce their expression of surface histocompatibility antigens and no longer stimulate a mixed lymphocyte reaction when co-cultured with allogeneic peripheral blood monocytes. Like BM-MSCs, the ASCs actually suppress immunoreactions. This indicates that the ASCs may not cause any cytotoxic T-cell response in vivo [127].
Allogeneic MSCs from bone marrow, umbilical cord UC-MSCs and adipose tissue have been used in OA animal models. Some pharmaceutical laboratories are working to achieve FDA approval for allogeneic MSCs clinical use.
Allogeneic MSCs are quite well tolerated by the host due to their immune-modulatory properties. Different methods have been tried to isolate homogeneous MSCs colonies and to enable a sterile and purified expansion so that allogenic MSCs can be injected in the receiving bloodstream with no major complications.
In experimental models, allogenic MSCs when intraarticularly injected showed improvement in cartilage healing and regeneration. Specifically, ASCs demonstrated a definitive reparative potential in cartilage regeneration [[128], [129], [130]].
In a recent review, Wang et al. analysed last three years preclinical and clinical trials results of intra-articular MSCs injection for knee OA. They found that generally MSCs improve pain indexes, joint mobility and increase the volume of cartilage.
This systematic review suggested that MSCs are safe enough for both intravascular and intra-articular injection. Furthermore, allogenic UC-MSCs showed the capacity of tissue regeneration in the implanted site and can be used as an allogenic stem cell drug.
Despite these studies showed promising therapeutic effects in the short-mid-term OA treatment, the long-term results need to be further investigated. Moreover, larger randomized studies are needed to provide higher levels of evidence [131].
In conclusion, MSCs showed in vitro definitive capacity to generate chondrocytes and produce cartilage tissue. Although in vivo, there is substantial evidence that MSCs produce a wide range of secreted factors that induce a local regeneration through immune modulation and tissue repair. MSCs paracrine anti-inflammatory-immunosuppressive properties and tissue regeneration capacity are currently considered central by the most. Depending on the number of transplanted cells, the mode of administration and behaviour after implantation, MSCs can variously decrease local inflammation, prevent chondrocyte apoptosis as well as differentiate into cartilage-forming chondrocytes.
Modern therapeutic strategies rely on the combination of MSCs and tissue engineering so that MSCs suspensions combined with biodegradable materials may improve cells survival and tissue regeneration capacity [132].
Therefore, against the initial assumption that MSCs might in vivo reproduce the in vitro findings, evidence increasingly suggests that the main therapeutic function of MSCs could be the paracrine effect to induce a local regenerative microenvironment [19,133].
5.3. MSCs exosomes
In some experimental studies, MSCs exosomes promoted tissue repair and regeneration by restoring homeostasis after injury or induced heart disease. Specifically, it was reported that exosomes contain a large amount of ECM proteins and enzymes regulating and restoring the normal ECM turn-over [134].
In 2016 Toh et al. reported that exosomes micro-RNA fractions 22, 23b, 92a, 125b, 221,320 and 145, were involved in the regulation of chondrogenesis and cartilage homeostasis [135].
Afterwards, Tao et al. reported that exosomes derived from human synovial MSCs overexpressed with micro-RNA-140-5p can promote cartilage regeneration and reverse OA in rat models [136].
Human embryonic MSCs derived exosomes were tested in an animal model of cartilage repair. Osteochondral defects were made in adult rats on both distal femurs. The treatment consisted of intraarticular injections of exosomes in one side and phosphate-buffered saline (PBS) in, the other side. The MSCs exosomes and PBS were injected after surgery and afterwards weekly for 12 weeks. Histological findings showed that the exosome treated defects displayed greater improvements than the contralateral PBS-treated defects. In the exosome treated defects, cartilage and subchondral bone exhibited complete restoration and complete integration to adjacent cartilage as well as an ECM deposition resembling the healthy control [37].
However, additional studies are needed to define exosomes safety and effectiveness, as well as methods to standardize extraction and purification should be implemented.
5.4. Stimulation of resident MSCs
Most joint tissue regeneration treatments have targeted the cartilage tissue. The physiopathology of early OA has shown the essential role of articular cartilage together with the synovial membrane and synovial fluid modifications.
Joint pain is firstly triggered by changes to non-cartilaginous components such as the joint capsule, synovial membrane, tendons and ligaments where there is a significant innervation. In addition Substance P was found to be secreted by sensory nerve fibres and was identified in the synovium, synovial fluid and, even isolated in the infrapatellar fat pad IFP [40,137].
In 2012 the author published an article describing a new procedure based on stimulation of joint resident MSCs.
The aim of the study was to show long-term symptoms control through the recovery of local cell homeostasis and tissue repair.
The study examined an individual medication comprised of polydeoxyribonucleotides (PDRN), a specific set of Heat Shock Proteins (HSPs) and a glycerol scaffold. The HSPs were derived from the patient's own blood through a process of thermic stress.
This product named Gel repair (GR) was shown to be a biological activator able to stimulate the resident MSCs located in the capsule and synovial tissue.
An observational clinical trial with 3 years follow up showed a persistent improvement of symptoms together with a radiologic structural recovery of the joints involved in the degenerative osteoarthritis.
Lower pain level and increased joint mobility were observed in almost 80% treated patients and quite often imaging showed a downgrading of the degenerative OA [43,138].
6. Discussion and conclusion
Joint and back pain are often the result of degenerative OA and are one of the most frequent causes of patient visits for assessment.
Most of the current medical treatments are palliative and work on pain management and symptom control.
In advanced OA, total knee and hip replacement are the current standards to alleviate pain and improve function. However, the risks associated with the operation and the finite life span of joint prosthesis exclude this option for a significant group of patients.
Joint replacement cannot often be offered to frail, elderly patients (ASA III-IV) due to high risks of complications and increase mortality. Likewise, surgeons are reluctant to offer younger patients joint replacement due to the limited life span of a prosthesis and chronic postural imbalance induced by the new joint.
The result is the increasing consumption of chronic medications such as NSAIDs and opioids in so-called pain management. This palliation has a severe systemic backlash on patient care and health systems due to the complications related to chronic NSAIDs and opioids intake and addiction.
Dilemmas can arise when we seek to offer patients the greatest benefits along with minimal risks. The basic medical principles primum non nocere, secundum cavere, tertium sanare (first do not harm, second be careful, third cure) should always guide and inspire medical practice.
The latest knowledge about the biological processes involved in the early OA, has given general awareness that this process can be curbed and reversed if addressed at the early stage.
The development of regenerative medicine procedures has now enabled a major change in OA treatments.
Timely diagnostic investigations to tackle early OA such as MRI with or without contrast enhancement can give all the information about disease onset and progression and its impact on joint structure and tissues.
Moreover, US-scan has had an increasing interest because is a low-cost examination and can be performed by the same physician who will deliver the treatment. Specifically, USs can visualise synovium, ligaments and tendons and some cartilage modifications. The 3D USs increases the accuracy and improve the quality of information.
USs has shown to be reliable to detect progressive joint changes induced by OA including details of the articular cartilage damages and demonstration of the inflammation involving the synovial membrane and adjacent soft tissue. For this reason, is a sensible investigation tool to follow up OA progression [139].
USs guidance has been used to inject substances or therapeutic cells into joints and accurately infiltrate the injured site.
Further, USs has been proposed to follow up regenerative medicine procedures and test their effectiveness on joint structures.
Many advancements have been made for the treatment of early OA and even in more advanced stages of OA disease. The direct stimulation of joint resident MSCs is one of the most promising procedure together with the autologous or allogeneic MSCs transplant and MSCs exosomes application. These and other regenerative medicine treatments may become, in the near future, a new frontier for the care of OA using minimally invasive procedures.
Declaration of competing interest
The author declares that he does not have financial or nonfinancial competing interests.
Acknowledgments
The author sincerely compliments Ms Jordana Lyden-Swift for drafting images.
The author is grateful to Thomas Richard Swift for the linguistic revision of the manuscript and his passionate interest in the Regenerative Surgery field.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.WHO Department of Chronic Diseases and Health Promotion. http://www.who.int/chp/topics/rheumatic/en.
- 2.United Nations . 2004. World population to 2300.http://www.un.org/esa/population/publications/.../WorldPop2300final.pdf [Google Scholar]
- 3.Junqueira's basic histology: text and atlas. 13th ed. McGraw-Hill Education/Medical; 2013. ISBN 9780071780339. [Google Scholar]
- 4.Li F., Tang Y., Song B., Yu M., Li Q., Zhang C. Nomenclature clarification: synovial fibroblasts and synovial mesenchymal stem cells. Stem Cell Res Ther. 2019;10:260. doi: 10.1186/s13287-019-1359-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young B., James S., Lowe B., MedSci Bmbs D.M., Stevens A., Heath J.W. 5 ed. Churchill Livingstone; 2006. Wheater's functional histology: a text and colour atlas; p. 5e. ISBN 9780443068508. [Google Scholar]
- 6.Seidman A.J., Limaiem F. StatPearls Publishing; Treasure Island (FL): Jan 22, 2019. Synovial fluid analysis. StatPearls [Internet] [PubMed] [Google Scholar]
- 7.Jay G.D., Waller K.A. The biology of lubricin: near frictionless joint motion. Matrix Biol. 2014;39:17–24. doi: 10.1016/j.matbio.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Smith M.D. The normal synovium. Open Rheumatol J. 2011;5:100–106. doi: 10.2174/1874312901105010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barland P., Novikoff A.B., Hamerman D. Electron microscopy of the human synovial membrane. J Cell Biol. 1962;14:207–220. doi: 10.1083/jcb.14.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilkinson L.S., Pitsillides A.A., Worrall J.G., Edwards J.C. Light microscopic characterization of the fibroblast-like synovial intimal cell (synoviocyte) Arthritis Rheum. 1992;35:1179–1184. doi: 10.1002/art.1780351010. [DOI] [PubMed] [Google Scholar]
- 11.Pitsillides A.A., Wilkinson L.S., Mehdizadeh S., Bayliss M.T., Edwards J.C. Uridine diphosphoglucose dehydrogenase activity in normal and rheumatoid synovium: the description of a specialized synovial lining cell. Int J Exp Pathol. 1993;74:27–34. [PMC free article] [PubMed] [Google Scholar]
- 12.Morales-Ducret J., Wayner E., Elices M.J., Alvaro-Gracia J.M., Zvaifler N.J., Firestein G.S. Alpha 4/beta 1 integrin (VLA-4) ligands in arthritis. Vascular cell adhesion molecule-1 expression in synovium and on fibroblast-like synoviocytes. J Immunol. 1992;149:1424–1431. [PubMed] [Google Scholar]
- 13.De Bari C., Dell'Accio F., Tylzanowski P., Luyten F.P. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928–1942. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 14.Sakaguchi Y., Sekiya I., Yagishita K., Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 15.Camernik K., Barlic A., Drobnic M., Marc J., Jeras M., Zupan J. Mesenchymal stem cells in the musculoskeletal system: from animal models to human tissue regeneration? Stem Cell Rev. 2018;14:346–369. doi: 10.1007/s12015-018-9800-6. [DOI] [PubMed] [Google Scholar]
- 16.Gazit Z., Pelled G., Sheyn D., Yacubovich D.C., Gazit D. Principle of regenerative medicine ch 14. 3rd ed. Elsevier Inc.; 2019. Mesenchymal stem cells. [Google Scholar]
- 17.Javazon E.H., Begges Kj, Flake A.W. Mesenchymal stem sells: paradoxes of passaging. Exp Hematol. 2004;32(5):414–425. doi: 10.1016/j.exphem.2004.02.004. 18. [DOI] [PubMed] [Google Scholar]
- 18.Prockop D.J. Repair of tissues by adult/stem progenitor cells (MSCs): controversies, myths, and changing paradigsms. Mol Ther. 2009;17(6):939–946. doi: 10.1038/mt.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caplan A.I., Correa D. The MSCs: an injury drugstore. Cell Stem Cell. 2011;9(1):11–15. doi: 10.1016/j.stem.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caplan A.I. All MSCs are pericytes? Cell Stem Cell. 2008;3:229–230. doi: 10.1016/j.stem.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Sacchetti B., Funari A., Michienzi S., Di Cesare S., Piersanti S., Saggio I. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007 Oct 19;131(2):324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 22.Gerhardt H., Semb H. Pericytes: gatekeepers in tumour cell metastasis? J Mol Med. 2008;86:135–144. doi: 10.1007/s00109-007-0258-2. [DOI] [PubMed] [Google Scholar]
- 23.Gao F., Chiu S.M., Motan D.A., Zhang Z., Chen L., Ji H.L. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis. 2016 Jan 21;7 doi: 10.1038/cddis.2015.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Najar M., Raicevic G., Crompot E., Fayyad-Kazan H., Bron D., Toungouz M. The immunomodulatory potential of mesenchymal stromal cells: a story of a regulatory network. J Immunother. 2016 Feb-Mar;39(2):45–59. doi: 10.1097/CJI.0000000000000108. [DOI] [PubMed] [Google Scholar]
- 25.Rasmusson I., Ringdén O., Sundberg B., Le Blanc K. Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Exp Cell Res. 2005 Apr 15;305(1):33–41. doi: 10.1016/j.yexcr.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 26.Yen B.L., Yen M.L., Hsu P.J., Liu K.J., Wang C.J., Bai C.H. Multipotent human mesenchymal stromal cells mediate expansion of myeloid-derived suppressor cells via hepatocyte growth factor/c-met and STAT3. Stem Cell Rep. 2013 Jul 25;1(2):139–151. doi: 10.1016/j.stemcr.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Najar M., Raicevic G., Fayyad-Kazan H., De Bruyn C., Bron D., Toungouz M. Bone marrow mesenchymal stromal cells induce proliferative, cytokinic and molecular changes during the T cell response: the importance of the IL-10/CD210 Axis. Stem Cell Rev Rep. 2015 Jun;11(3):442–452. doi: 10.1007/s12015-014-9567-3. [DOI] [PubMed] [Google Scholar]
- 28.Caplan A.I. Principle of regenerative medicine ch 15. 3nd ed. Elsevier Inc.; 2019. Mesenchymal stem cells in regenerative medicine. [Google Scholar]
- 29.Caplan A.I., Dennis J.E. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 30.Solchaga L.A., Penick K., Porter J.D., Goldberg V.M., Caplan A.I., Welter J.F. FGF-2 enhances the mitotic and chondrogenic potentials of human adult bone marrow-derived mesenchymal stem cells. J Cell Physiol. 2005 May;203(2):398–409. doi: 10.1002/jcp.20238. [DOI] [PubMed] [Google Scholar]
- 31.Estes B.T., Wu A.W., Guilak F. Potent induction of chondrocytic differentiation of human adipose-derived adult stem cells by bone morphogenetic protein 6. Arthritis Rheum. 2006 Apr;54(4):1222–1232. doi: 10.1002/art.21779. [DOI] [PubMed] [Google Scholar]
- 32.Li L., Chen X., Wang W.E., Zeng C. How to improve the survival of transplanted mesenchymal stem cell in ischemic heart? Stem Cell Int. 2016;2016:9682757. doi: 10.1155/2016/9682757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai R.C., Yeo R.W., Lim S.K. Mesenchymal stem cell exosomes. Semin Cell Dev Biol. 2015 Apr;40:82–88. doi: 10.1016/j.semcdb.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Lai R.C., Arslan F., Tan S.S., Tan B., Choo A., Lee M.M. Derivation and characterization of human fetal MSCs: an alternative cell source for large-scale production of cardioprotective microparticles. J Mol Cell Cardiol. 2010 Jun;48(6):1215–1224. doi: 10.1016/j.yjmcc.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 35.Rani S., Ryan A.E., Griffin M.D., Ritter T. Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Mol Ther. 2015 May;23(5):812–823. doi: 10.1038/mt.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamura Y., Miyaki S., Ishitobi H., Matsuyama S., Nakasa T., Kamei N. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett. 2015 May 8;589(11):1257–1265. doi: 10.1016/j.febslet.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 37.Zhang S., Chu W.C., Lai R.C., Lim S.K., Hui J.H., Toh W.S. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthritis Cartilage. 2016 Dec;24(12):2135–2140. doi: 10.1016/j.joca.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 38.Kouroupis D., Bowles A.C., Willman M.A., Perucca Orfei C., Colombini A., Best T.M. Infrapatellar fat-pad derived MSC response to inflammation and fibrosis induces an immunomodulatory phenotype involving CD10-mediated Substance P degradation. Sci Rep. 2019 Jul 26;9(1):10864. doi: 10.1038/s41598-019-47391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tangchitphisut P., Srikaew N., Numhom S., Tangprasittipap A., Woratanarat P., Wongsak S. Infrapatellar fat pad: an alternative source of adipose-derived mesenchymal stem cells. Arthritis. 2016:4019873–4019910. doi: 10.1155/2016/4019873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mashaghi A., Marmalidou A., Tehrani M., Grace P.M., Pothoulakis C., Dana R. Neuropeptide substance P and the immune response. Cell Mol Life Sci. 2016 Nov;73(22):4249–4264. doi: 10.1007/s00018-016-2293-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu B., He R., Ma K., Wang Z., Cui M., Hu H. Intervertebral disc-derived stem/progenitor cells as a promising cell source for intervertebral disc regeneration. Stem Cell Int. 2018:7412304. doi: 10.1155/2018/7412304. 2018 Dec 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Russell T., Watad A., Bridgewood C., Khan A., Millner P., Loughenbury P. FRI0520 The human enthesis contains populations of Mesenchymal stem cells with distinct functional characteristics. Ann Rheum Dis. 2019;78:955. [Google Scholar]
- 43.Di Nicola V., Pierpaoli W. Biological baseline of joint self-repair procedures. Curr Aging Sci. 2013 Jul;6(2):206–214. doi: 10.2174/18746098112059990029. [DOI] [PubMed] [Google Scholar]
- 44.Knight M.N., Hankenson K.D. Mesenchymal stem cells in bone regeneration. Adv Wound Care. 2013 Jul;2(6):306–316. doi: 10.1089/wound.2012.0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tarafder S., Lee C.H. 2016. In situ tissue regeneration: host cell recruitment and biomaterial design; pp. 253–273. Ch. 14. [Google Scholar]
- 46.Säämänen A.-M., Arokoski J.P.A., Jurvelin J.S., Kiviranta Ilkka. The structure and regenerative capacity of synovial joint tissues. In: Archer C., Ralphs J., editors. Regenerative medicine and biomaterials for the repair of connective tissues. Woodhead publishing; 2010. [Google Scholar]
- 47.Dieppe P. Developments in osteoarthritis. Rheumatology. 2011;50:245–247. doi: 10.1093/rheumatology/keq373. [DOI] [PubMed] [Google Scholar]
- 48.Martel-Pelletier J., Pelletier J.P. Is osteoarthritis a disease involving only cartilage or other articular tissues? Eklem Hastalik Cerrahisi. 2010;21:2–14. [PubMed] [Google Scholar]
- 49.Sokolove J., Lepus C.M. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis. 2013 Apr;5(2):77–94. doi: 10.1177/1759720X12467868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goldring M.B., Marcu K.B. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res Ther. 2009;11:224. doi: 10.1186/ar2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wise C. Osteoarthritis. In: Dale D.C., Federman D.D., editors. ACP medicine section 15, chap. 10. WebMD; New York: 2010. pp. 1–12. compiler. [Google Scholar]
- 52.Stannus O., Jones G., Cicuttini F. Circulating levels of IL-6 and TNF-alpha are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthtitis Cartilage. 2010;18:1441–1447. doi: 10.1016/j.joca.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 53.Buckwalter J.A., Mankin H.J., Grodzinsky A.J. Articular cartilage and osteoarthritis. Instr Course Lect. 2005;54:465–480. [PubMed] [Google Scholar]
- 54.Aigner T., Schmitz N. Pathogenesis and pathology of osteoarthritis. In: Hochberg M., Silman A., Smolen J., Weinblatt M., Weisman M., editors. Rheumatology. 5th ed. Mosby Elsevier; Philadelphia: 2011. pp. 1741–1759. compilers. [Google Scholar]
- 55.Goldring M.B. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann NY Acad Sci. 2010;1192:230–237. doi: 10.1111/j.1749-6632.2009.05240.x. [DOI] [PubMed] [Google Scholar]
- 56.Luyten F.P., Denti M., Filardo G., Kon E., Engebretsen L. Definition and classification of early osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. 2012;20(3):401–406. doi: 10.1007/s00167-011-1743-2. [DOI] [PubMed] [Google Scholar]
- 57.Madry H., Kon E., Condello V., Peretti G.M., Steinwachs M., Seil R. Early osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. 2016;24:1753–1762. doi: 10.1007/s00167-016-4068-3. [DOI] [PubMed] [Google Scholar]
- 58.Kanamoto T., Mae T., Yokoyama T., Tanaka H., Ebina K., Nakata K. Significance and definition of early knee osteoarthritis. Ann Joint. 2020;5:4. [Google Scholar]
- 59.Sutton S., Clutterbuck A., Harris P. The contribution of the synovium, synovial derived inflammatory cytokines and neuropeptides to the pathogenesis of osteoarthritis. Vet J. 2009;179:10–24. doi: 10.1016/j.tvjl.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 60.Vina E.R., Kwoh C.K. Epidemiology of osteoarthritis: literature update. Curr Opin Rheumatol. 2018;30:160. doi: 10.1097/BOR.0000000000000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krasnokutsky S., Belitskaya-Levy I., Bencardino J., Samuels J., Attur M., Regatte R. Quantitative magnetic resonance imaging evidence of synovial proliferation is associated with radiographic severity of knee osteoarthritis. Arthritis Rheum. 2011;63:2983–2991. doi: 10.1002/art.30471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roemer F., Guermazi A., Felson D., Niu J., Nevitt M., Crema M. Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: the MOST study. Ann Rheum Dis. 2011;70:1804–1809. doi: 10.1136/ard.2011.150243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Di Nicola V., Di Pietrantonio M. MRI in degenerative joint disease (DJD): a proposal for imaging standardization in regenerative medicine. SM Musculoskelet Disord. 2016;1(2):1006. [Google Scholar]
- 64.Ayral X., Pickering E., Woodworth T., Mackillop N., Dougados M. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis – results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis Cartilage. 2005;13:361–367. doi: 10.1016/j.joca.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 65.Scanzello C., McKeon B., Swaim B., DiCarlo E., Asomugha E., Kanda V. Synovial inflammation in patients undergoing arthroscopic meniscectomy: molecular characterization and relationship to symptoms. Arthritis Rheum. 2011;63:391–400. doi: 10.1002/art.30137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haywood L., McWilliams D., Pearson C., Gill S., Ganesan A., Wilson D. Inflammation and angiogenesis in osteoarthritis. Arthritis Rheum. 2003;48:2173–2177. doi: 10.1002/art.11094. [DOI] [PubMed] [Google Scholar]
- 67.Sellam J., Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010;6:625–635. doi: 10.1038/nrrheum.2010.159. [DOI] [PubMed] [Google Scholar]
- 68.Pritzker K.P., Gay S., Jimenez S.A., Ostergaard K., Pelletier J.-P., Revell P.A. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14(1):13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 69.Favero M., Ramonda R., Goldring M.B., Goldring Steven R., Punzi Leonardo. Early knee osteoarthritis. RMD Open. 2015;1 doi: 10.1136/rmdopen-2015-000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lehner B., Koeck F.X., Capellino S., Schubert T.E.O., Hofbauer R., Straub R.H. Preponderance of sensory versus sympathetic nerve fibers and increased cellularity in the infrapatellar fat pad in anterior knee pain patients after primary arthroplasty. J Orthop Res. 2008;26:342–350. doi: 10.1002/jor.20498. [DOI] [PubMed] [Google Scholar]
- 71.Koeck F.X., Schmitt M., Baier C., Stangl H., Beckmann J., Grifka J. Predominance of synovial sensory nerve fibers in arthrofibrosis following total knee arthroplasty compared to osteoarthritis of the knee. J Orthop Surg Res. 2016;11:25. doi: 10.1186/s13018-016-0359-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spitsin S., Meshki J., Winters A., Tuluc F., Benton T.D., Douglas S.D. Substance P-mediated chemokine production promotes monocyte migration. J Leukoc Biol. 2017;101(4):967-973. doi: 10.1189/jlb.1AB0416-188RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bondeson J., Blom A., Wainwright S., Hughes C., Caterson B., Van Den Berg W. The role of synovial macrophages and macrophage-produced mediators in driving inflammatory and destructive responses in osteoarthritis. Arthritis Rheum. 2010;62:647–657. doi: 10.1002/art.27290. [DOI] [PubMed] [Google Scholar]
- 74.Dean G., Hoyland J., Denton J., Donn R., Freemont A. Mast cells in the synovium and synovial fluid in osteoarthritis. Br J Rheumatol. 1993;32:671–675. doi: 10.1093/rheumatology/32.8.671. [DOI] [PubMed] [Google Scholar]
- 75.Brandt K.D., Dieppe P., Radin E. Etiopathogenesis of osteoarthritis. Med Clin. 2009;93:1–24. doi: 10.1016/j.mcna.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 76.Benito M., Veale D., FitzGerald O., van den Berg W., Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis. 2005;64:1263–1267. doi: 10.1136/ard.2004.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wood M.J., Leckenby A., Reynolds G., Spiering R., Pratt A.G., Rankin K.S. Macrophage proliferation distinguishes 2 subgroups of knee osteoarthritis patients. JCI Insight. 2019;4(2) doi: 10.1172/jci.insight.125325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Loeuille D., Chary-Valckenaere I., Champigneulle J. Macroscopic and microscopic features of synovial membrane inflammation in the osteoarthritic knee: correlating magnetic resonance imaging findings with disease severity. Arthritis Rheum. 2005;52:3492–3501. doi: 10.1002/art.21373. [DOI] [PubMed] [Google Scholar]
- 79.Yuan G.H., Tanaka M., Masuko-Hongo K. Characterization of cells from pannus-like tissue over articular cartilage of advanced osteoarthritis. Osteoarthritis Cartilage. 2004;12:38–45. doi: 10.1016/j.joca.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 80.Lee J.H., Ort T., Ma K. Resistin is elevated following traumatic joint injury and causes matrix degradation and release of inflammatory cytokines from articular cartilage in vitro. Osteoarthritis Cartilage. 2009;17:613–620. doi: 10.1016/j.joca.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 81.Hasegawa M., Segawa T., Maeda M. Thrombin-cleaved osteopontin levels in synovial fluid correlate with disease severity of knee osteoarthritis. J Rheumatol. 2011;38:129–134. doi: 10.3899/jrheum.100637. [DOI] [PubMed] [Google Scholar]
- 82.Krasnokutsky S., Attur M., Palmer G. Current concepts in the pathogenesis of osteoarthritis. Osteoarthritis Cartilage. 2008;16:S1–S3. doi: 10.1016/j.joca.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 83.Ashraf S., Walsh D.A. Angiogenesis in osteoarthritis. Curr Opin Rheumatol. 2008;20:573–580. doi: 10.1097/BOR.0b013e3283103d12. [DOI] [PubMed] [Google Scholar]
- 84.Bonnet C.S., Walsh D.A. Osteoarthritis, angiogenesis and inflammation. Rheumatology. 2005;44:7–16. doi: 10.1093/rheumatology/keh344. [DOI] [PubMed] [Google Scholar]
- 85.Kalaitzoglou E., Griffin T.M., Humphrey M.B. Innate immune responses and osteoarthritis. Curr Rheumatol Rep. 2017;19(8):45. doi: 10.1007/s11926-017-0672-6. [DOI] [PubMed] [Google Scholar]
- 86.Gross J.B., Guillaume C., Gegout-Pottie P., Reboul P., Jouzeau J.Y., Mainard D. The infrapatellar fat pad induces inflammatory and degradative effects in articular cells but not through leptin or adiponectin. Clin Exp Rheumatol. 2017;35(1):53-60. [PubMed] [Google Scholar]
- 87.Loeser R.F., Goldring S.R., Scanzello C.R., Goldring M.B. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012 Jun;64(6):1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 89.Telander D. Inflammation and age-related macular degeneration (AMD) Semin Ophthalmol. 2011;26:192–197. doi: 10.3109/08820538.2011.570849. [DOI] [PubMed] [Google Scholar]
- 90.Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med. 2006;12:1005–1015. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- 91.Scanzello C., Plaas A., Crow M. Innate immune system activation in osteoarthritis: is osteoarthritis a chronic wound? Curr Opin Rheumatol. 2008;20:565–572. doi: 10.1097/BOR.0b013e32830aba34. [DOI] [PubMed] [Google Scholar]
- 92.Remst D.F., Blaney Davidson E.N., van der Kraan P.M. Unravelling osteoarthritis-related synovial fibrosis: a step closer to solving joint stiffness. Rheumatology. 2015;54(11):1954-1963. doi: 10.1093/rheumatology/kev228. [DOI] [PubMed] [Google Scholar]
- 93.Kawai T., Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 94.Okamura Y., Watari M., Jerud E., Young D., Ishizaka S., Rose J. The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem. 2001;276:10229–10233. doi: 10.1074/jbc.M100099200. [DOI] [PubMed] [Google Scholar]
- 95.Sohn D., Sokolove J., Sharpe O., Erhart J., Chandra P., Lahey L. Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via toll-like receptor 4. Arthritis Res Ther. 2012;14:R7. doi: 10.1186/ar3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu-Bryan R., Terkeltaub R. Emerging regulators of the inflammatory process in osteoarthritis. Nat Rev Rheumatol. 2015 Jan;11(1):35–44. doi: 10.1038/nrrheum.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Van Lent P., Blom A., Schelbergen R., Sloetjes A., Lafeber F., Lems W. Active involvement of alarmins S100A8 and S100A9 in the regulation of synovial activation and joint destruction during mouse and human osteoarthritis. Arthritis Rheum. 2012;64:1466–1476. doi: 10.1002/art.34315. [DOI] [PubMed] [Google Scholar]
- 98.Rosenthal A. Crystals, inflammation, and osteoarthritis. Curr Opin Rheumatol. 2011;23:170–173. doi: 10.1097/BOR.0b013e3283432d1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang Q., Rozelle A., Lepus C., Scanzello C., Song J., Larsen D. Identification of a central role for complement in osteoarthritis. Nat Med. 2011;17:1674–1679. doi: 10.1038/nm.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ayhan E., Kesmezacar H., Akgun I. Intraarticular injections (corticosteroid, hyaluronic acid, platelet rich plasma) for the knee osteoarthritis. World J Orthoped. 2014;5(3):351–361. doi: 10.5312/wjo.v5.i3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhu Y., Yuan M., Meng H.Y., Wang A.Y., Guo Q.Y., Wang Y. Basic science and clinical application of platelet -rich plasma for cartilage defects and osteoarthritis: a review. Osteoarthritis Cartilage. 2013;21(11):1627–1637. doi: 10.1016/j.joca.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 102.Rayegani S., Raeissadat S., Sanei Taheri M., Babaee M., Rayegani S.M., Eliaspour D. Does intra articular platelet rich plasma injection improve function, pain and quality of life in patients with osteoarthritis of the knee? A randomized clinical trial. Orthop Rev. 2014;6:5405. doi: 10.4081/or.2014.5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Laudy A.B., Bakker E.W., Rekers M., Moen M.H. Efficacy of platelet -rich plasma injections in osteoarthritis of the knee: a sistematic review and meta-analysis. Br J Sports Med. 2015;49(10):657–672. doi: 10.1136/bjsports-2014-094036. [DOI] [PubMed] [Google Scholar]
- 104.Mishra A., Tummala P., King A., Lee B., Kraus M., Tse V. Buffered platelet-rich plasma enhances mesenchymal stem cell proliferation and chondrogenic differentiation. Tissue Eng C Methods. 2009;15:431–435. doi: 10.1089/ten.tec.2008.0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Krüger J., Hondke S., Endres M., Pruss A., Siclari A., Kaps C. Human platelet-rich plasma stimulates migration and chondrogenic differentiation of human subchondral progenitor cells. J Orthop Res. 2011;30:845–852. doi: 10.1002/jor.22005. [DOI] [PubMed] [Google Scholar]
- 106.Mifune Y., Matsumoto T., Takayama K., Ota S., Li H., Meszaros L.B. The effect of platelet-rich plasma on the regenerative therapy of muscle derived stem cells for articular cartilage repair. Osteoarthritis Cartilage. 2013;21:175–185. doi: 10.1016/j.joca.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 107.Bendinelli P., Matteucci E., Dogliotti G., Corsi M., Banfi G., Maroni P. Molecular basis of anti-inflammatory action of platelet-rich plasma on human chondrocytes: mechanisms of NF-κB inhibition via HGF. J Cell Physiol. 2010;225:757–766. doi: 10.1002/jcp.22274. [DOI] [PubMed] [Google Scholar]
- 108.Kilian O., Flesch I., Wenisch S., Taborski B., Jork A., Schnettler R. Effects of platelet growth factors on human mesenchymal stem cells and human endothelial cells in vitro. Eur J Med Res. 2004;9:337–344. [PubMed] [Google Scholar]
- 109.Nakagawa K., Sasho T., Arai M., Ogino S., Wada Y., Moriya H. P181 Effects of autologous platelet-rich plasma on the metabolism of human articular chondrocytes. Osteoarthritis Cartilage. 2007;15:B134. [Google Scholar]
- 110.Huang Q., Wang Y.D., Wu T., Jiang S., Hu Y.L., Pei G.X. Preliminary separation of the growth factors in platelet-rich plasma: effects on the proliferation of human marrow-derived mesenchymal stem cells. Chin Med J. 2009;122(1):83-87. [PubMed] [Google Scholar]
- 111.Marmotti A., Rossi R., Castoldi F., Roveda E., Michielon G., Peretti G.M. PRP and articulat cartilage: a clinical update. BioMed Res Int. 2015;2015:542502. doi: 10.1155/2015/542502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Görmeli G., Görmeli C.A., Ataoglu B., Çolak C., Aslantürk O., Ertem K. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. 2017;25(3):958–965. doi: 10.1007/s00167-015-3705-6. [DOI] [PubMed] [Google Scholar]
- 113.O'Shaughnessey K., Matuska A., Hoeppner J., Farr J., Klaassen M., Kaeding C. Autologous protein solution prepared from the blood of osteoarthritic patients contains an enhanced profile of anti-inflammatory cytokines and anabolic growth factors. J Orthop Res. 2014;32(10):1349–1355. doi: 10.1002/jor.22671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wasai S., Sato M., Maehara M., Toyoda E., Uchiyama R., Takahashi T. Characteristics of autologous protein solution and leucocyte-poor platelet-rich plasma for the treatment of osteoarthritis of the knee. Sci Rep. 2020;10:10572. doi: 10.1038/s41598-020-67099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gato-Calvo L., Magalhaes J., Ruiz-Romero C., Blanco F.J., Burguera E.F. Platelet-rich plasma in osteoarthritis treatment: review of current evidence. Ther Adv Chronic Dis. 2019 doi: 10.1177/2040622319825567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Baloch Naveed, Hasan Obada, Zain Baig Umme-e-Hani Abdullah, Atif Mohammad, Ohuchi Hiroshi. Use of intraarticular injections of platelet-rich plasma in the treatment of knee osteoarthritis: a review article. Orthop Rev. 2019 Sep 24;11(3):7747. doi: 10.4081/or.2019.7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rothrauff B.J., Pirosa A., Lin H., Sohn J., Langhans M.T., Tuan R.S. Principle of regenerative medicine ch 54. 3rd ed. Elsevier Inc.; 2019. Stem cells for musculoskeletal diseases. [Google Scholar]
- 118.Farr J., Cole B., Dhawan A., Kercher j, Sherman S. Clinical cartilage restoration: evolution and overview. Clin Orthop Relat Res. 2011;469(10):2696–2705. doi: 10.1007/s11999-010-1764-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Makris E.A., Gomoll A.H., Malizos K.N., Hu J.C., Athanasiou K.A. Repair and tissue engineering technique for articular cartilage. Nat Rev Rheumatol. 2015;11(1):21–34. doi: 10.1038/nrrheum.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Di Nicola V. Omentum a powerful biological source in regenerative surgery. Regen Ther. 2019;11:182-191. doi: 10.1016/j.reth.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Iwashima S., Ozaki T., Maruyama S., Saka Yousuke, Kobori Masato, Omae Kaoru. Novel culture system of mesenchymal stromal cells from human subcutaneous adipose tissue. Stem Cell Dev. 2009;18(4):533-543. doi: 10.1089/scd.2008.0358. [DOI] [PubMed] [Google Scholar]
- 122.Riordan N.H., Ichim T.E., Min W.P., Wang H., Solano F., Lara F. Non-expanded adipose stromal vascular fraction cell therapy for multiple sclerosis. J Transl Med. 2009;7:29. doi: 10.1186/1479-5876-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Spees J.L., Olson S.D., Whitney M.J., Prockop D.J. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci U S A. 2006;103:1283–1288. doi: 10.1073/pnas.0510511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Freitag J., Bates D., Boyd R., Shah K., Barnard A., Huguenin L. Mesenchymal stem cell therapy in the treatment of osteoarthritis: reparative pathways, safety and efficacy - a review. BMC Muscoskel Disord. 2016;17:230. doi: 10.1186/s12891-016-1085-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Koh Y.G., Jo S.B., Kwon O.R., Suh D.S., Lee S.W., Park S.H. Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy. 2013;29:748–755. doi: 10.1016/j.arthro.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 126.Xie X., Wang Y., Zhao C., Guo S., Liu S., Jia W. Comparative evaluation of MSCs from bone marrow and adipose tissue seeded in PRP-derived scaffold for cartilage regeneration. Biomaterials. 2012;33:7008–7018. doi: 10.1016/j.biomaterials.2012.06.058. [DOI] [PubMed] [Google Scholar]
- 127.Gimble Jeffrey M., Katz Adam J., Bunnell Bruce A. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007 May 11;100(9):1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lee K.B., Hui J.H., Song I.C., Ardany L., Lee E.H. Injectable mesenchymal stem cell therapy for large cartilage defects--a porcine model. Stem Cell. 2007;25(11):2964-2971. doi: 10.1634/stemcells.2006-0311. [DOI] [PubMed] [Google Scholar]
- 129.Murphy J.M., Fink D.J., Hunziker E.B., Barry F.P. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48(12):3464–3474. doi: 10.1002/art.11365. [DOI] [PubMed] [Google Scholar]
- 130.Bielli A., Scioli M.G., Gentile P., Cervelli V., Orlandi A. Adipose-derived stem cells in cartilage regeneration: current perspectives. Regen Med. 2016;11(7):693-703. doi: 10.2217/rme-2016-0077. [DOI] [PubMed] [Google Scholar]
- 131.Wang A.T., Feng Y., Jia H.H., Zhao M., Yu H. Application of mesenchymal stem cell therapy for the treatment of osteoarthritis of the knee: a concise review. World J Stem Cell. 2019;11(4):222–235. doi: 10.4252/wjsc.v11.i4.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Maumus M., Pers Y.M., Ruiz M., Jorgensen C., Noël D. Cellules souches mésenchymateuses et médecine régénératrice - quel avenir pour l’arthrose ? [Mesenchymal stem cells and regenerative medicine: future perspectives in osteoarthritis] Med Sci. 2018;34(12):1092–1099. doi: 10.1051/medsci/2018294. [DOI] [PubMed] [Google Scholar]
- 133.Pittenger M. Sleuthing the source of regeneration by MSCs. Cell Stem Cell. 2009;5(1):8-10. doi: 10.1016/j.stem.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 134.Lai R.C., Chen T.S., Lim S.K. Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regen Med. 2011;6:481–492. doi: 10.2217/rme.11.35. [DOI] [PubMed] [Google Scholar]
- 135.Toh W.S., Lai R.C., Hui J.H.P., Lim S.K. MSC exosome as a cell-free MSC therapy for cartilage regeneration: implications for osteoarthritis treatment. Semin Cell Dev Biol. 2017;67:56–64. doi: 10.1016/j.semcdb.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 136.Tao S.C., Yuan T., Zhang Y.L., Yin W.J., Guo S.C., Zhang C.Q. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics. 2017;7:180–195. doi: 10.7150/thno.17133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mora J.C., Przkora R., Cruz-Almeida Y. Knee osteoarthritis: pathophysiology and current treatment modalities. J Pain Res. 2018;11:2189-2196. doi: 10.2147/JPR.S154002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Di Nicola V., Di Nicola R. Self-repair in degenerative joint disease. Curr Aging Sci. 2012;5(3):273-287. doi: 10.2174/1874609811205030015. [DOI] [PubMed] [Google Scholar]
- 139.Kaeley G.S., Bakewell C., Deodhar A. The importance of ultrasound in identifying and differentiating patients with early inflammatory arthritis: a narrative review. Arthritis Res Ther. 2020;22:1. doi: 10.1186/s13075-019-2050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]